Abstract
Background
Our objective was to describe day-to-day evolution and variations in patient-reported stent-associated symptoms (SAS) in the STudy to Enhance uNderstanding of sTent-associated Symptoms (STENTS), a prospective multicenter observational cohort study, using multiple instruments with conceptual overlap in various domains.
Methods
In a nested cohort of the STENTS study, the initial 40 participants having unilateral ureteroscopy (URS) and stent placement underwent daily assessment of self-reported measures using the Brief Pain Inventory short form, Patient-Reported Outcome Measurement Information System measures for pain severity and pain interference, the Urinary Score of the Ureteral Stent Symptom Questionnaire, and Symptoms of Lower Urinary Tract Dysfunction Research Network Symptom Index. Pain intensity, pain interference, urinary symptoms, and bother were obtained preoperatively, daily until stent removal, and at postoperative day (POD) 30.
Results
The median age was 44 years (IQR 29,58), and 53% were female. The size of the dominant stone was 7.5 mm (IQR 5,11), and 50% were located in the kidney. There was consistency among instruments assessing similar concepts. Pain intensity and urinary symptoms increased from baseline to POD 1 with apparent peaks in the first 2 days, remained elevated with stent in situ, and varied widely among individuals. Interference due to pain, and bother due to urinary symptoms, likewise demonstrated high individual variability.
Conclusions
This first study investigating daily SAS allows for a more in-depth look at the lived experience after URS and the impact on quality of life. Different instruments measuring pain intensity, pain interference, and urinary symptoms produced consistent assessments of patients’ experiences. The overall daily stability of pain and urinary symptoms after URS was also marked by high patient-level variation, suggesting an opportunity to identify characteristics associated with severe SAS after URS.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12894-022-01004-9.
Keywords: Urinary stone disease, Ureteroscopy, Ureteral stent, Stent-associated symptoms
Introduction
Patients who receive a ureteral stent often experience debilitating symptoms, including pain, urinary urgency and frequency, hematuria, and incontinence. Researchers have struggled to understand and predict the severity and range of stent-associated symptoms (SAS) following ureteroscopy (URS) for stone disease. While most patients will have some degree of SAS, it is unknown who will suffer from severe symptoms that significantly affect quality of life. Furthermore, a clear understanding of the daily variation of SAS after URS remains elusive; having this information is critical to patient counseling, serving as a building block for stent-related research, and employing strategies to mitigate these symptoms.
Investigation of SAS is a research priority as URS has become the most common procedure performed for renal and ureteral stones, and stents are used in most cases [1–3]. Contrary to popular belief, some studies have shown that stents are not associated with increased hospital returns [4] and may actually reduce unplanned visits [3, 5, 6]. Therefore, despite SAS, ureteral stents continue to play an important role in reducing complications such as urinary obstruction, and may slightly reduce ureteral stricture formation [5]. Given the limited insight into drivers of SAS and lack of characterization of the patient’s daily experience after URS, the Urinary Stone Disease Research Network is conducting the STudy to Enhance uNderstanding of sTent-associated Symptoms (STENTS), a prospective observational cohort study.
A key consideration for improving understanding of SAS is assessment of patient-reported symptoms. Patient-reported measures provide important information about the impact of a condition and/or treatment from the patient’s perspective. Within the overall STENTS study, an initial nested cohort study was designed as a unique opportunity to gain a better understanding of patients’ daily experiences after URS for stone treatment. Our goal was to determine the trajectory of day-to-day variations in pain intensity, pain interference, urinary symptoms, and bother, identify a peak day of symptoms, and provide guidance for future studies in choosing outcome measures.
Materials and methods
Study design and nested cohort
STENTS is a multi-institutional prospective observational cohort study of individuals undergoing URS and ureteral stent placement for treatment of a ureteral or renal stone. A complete description of the STENTS protocol has been published [7]. The initial 40 participants made up the nested cohort. A minimum number of participants based on age and gender were prespecified to ensure inclusion of specific age and gender groups in this nested cohort: the minima required were 8 individuals aged 12–25, 12 males aged > 25 years, and 12 females aged > 25 years.
In brief, the nested cohort study was designed such that participants would complete all study procedures performed in the subsequent larger STENTS observational cohort. Unique to the nested cohort, these 40 participants completed daily questionnaires that assessed pain intensity, pain interference, urinary symptoms, and bother, and also participated in a semi-structured interview to further characterize the patient experience. The prespecified objectives of the nested cohort were to generate knowledge about the daily variation of pain intensity, pain interference, urinary symptoms, and bother; identify a peak day of symptoms; and evaluate overlap in experience assessment among the instruments. These analyses informed the selection of instruments and the timing of their administration for the main STENTS cohort.
Study population
Individuals aged 12 and older with a planned unilateral URS for stone treatment were recruited from four clinical centers. All participants were prospectively enrolled after institutional review board approval. Participants aged 17 and under provided their informed assent, and their parents provided parental permission. Exclusion criteria were an indwelling ureteral stent, receipt of a stent in the preceding 60 days, concomitant shockwave lithotripsy or percutaneous nephrolithotomy, conditions resulting in neurogenic bladder dysfunction, anatomic urological abnormality resulting in abnormal bladder sensation, or renal transplantation; bedridden and vulnerable populations were also excluded.
Study procedures
Participants completed baseline questionnaires that recorded individual characteristics, medical and stone history, and medication use. Participants with a history of a ureteral stent reported whether they had severe pain or urinary symptoms with their prior stent. Intraoperative data, including stone features, details of ureteral instrumentation, irrigation type, and stent characteristics, were prospectively collected at the time of URS.
Patient experiences
All participants in the nested STENTS cohort were administered the following questionnaires that assessed pain, urinary symptoms, and the manner and degree to which these symptoms impact the patient’s life (interference and bother): (1) the Brief Pain Inventory (BPI) short form [8], which has been used in pain studies widely, assessed pain intensity and pain interference; (2) Patient-Reported Outcome Measurement Information System (PROMIS) [9] measures of pain intensity and pain interference allowed for comparing scores to population norms; (3) the Urinary Score of the Ureteral Stent Symptom Questionnaire (USSQ) [10], an instrument developed for SAS over a 1-month recall period, assessed urinary symptoms and bother; and (4) Symptoms of Lower Urinary Tract Dysfunction Research Network Symptom Index (LURN SI-10) assessed urinary symptoms and bother [11]. We intentionally administered questionnaires that assessed the same construct in order to determine potential differences in the measured experience. We acknowledge that the USSQ covers other domains besides urinary symptoms, but given the importance of characterizing pain intensity and pain interference as accurately as possible, being able to compare to population norms, and our desire for a comprehensive body map that incudes genitalia, it was decided to incorporate specific pain instruments that are used in the broader community of medicine. This was after several discussions with a multidisciplinary field including experts within pain medicine, pain psychology, and psychometricians well versed in development of patient reported outcome measures. Table 1 shows the instruments listed by SAS domain.
Table 1.
Self-reported measures used in the study by stent-associated symptoms domain
| Instrument | Domain | |||
|---|---|---|---|---|
| Pain Intensity | Pain Interference | Urinary Symptoms | Urinary Bother | |
| BPI | • | • | ||
| LURN SI-10 | • | • | ||
| PROMIS | • | • | ||
| USSQ-U | • | • | ||
BPI = Brief Pain Inventory; LURN SI-10 = Symptoms of Lower Urinary Tract Dysfunction Research Network Symptom Index; PROMIS = Patient-Reported Outcome Measurement Information System; USSQ-U = urinary score of the Ureteral Stent Symptom Questionnaire
Data collection
Participants completed the above questionnaires preoperatively (baseline), on postoperative day (POD) 1, daily until stent removal, including day of stent removal, and 30 days after stent removal (Table 1). Questionnaires were self-administered and completed each day via electronic format, or paper copies if preferred. Participants received an electronic link each day reminding them to complete the forms.
Participants were contacted by study staff to record any adverse events. Prescribed medications following surgery were assessed using a medication diary. Finally, participants were asked about any health care utilization during the 30 days following stent removal.
Statistical analysis
Data are summarized as medians (25th, 75th percentiles) and means (SDs) for continuous variables and as counts (percentages) for categorical variables. Due to multiple outcomes assessed at many time points and the small sample size, we did not test for differences in symptom severity across instruments or days. Summary statistics of daily patient-reported symptoms were calculated, and data are graphically depicted in box-and-whisker plots. All statistical summary data were generated using SAS statistical software version 9.4 (SAS Institute, Inc., Cary, NC).
Results
Forty participants were included, comprising 9 individuals (3 male and 6 female) aged 12–25 years, 16 males aged > 25 years, and 15 females aged > 25 years. The median age was 44 years (IQR 29, 58), and 53% were female. Over half (58%) of the participants had a prior history of kidney stones. Of the 13 (33%) who had a ureteral stent in the past, 8 reported having severe pain and 6 reported having severe urinary symptoms with their previous stent. Participant characteristics are listed in Table 2, and intraoperative data are shown in Table 3. Most participants had more than one stone treated, with the dominant stone location evenly split between renal and ureteral. Stone sizes and location represent stones treated during surgery.
Table 2.
Participant characteristics at baseline
| Characteristic | Participants (n = 40) |
|---|---|
| Sex: female | 21 (53%) |
| Race | |
| White | 38 (95%) |
| Black | 2 (5%) |
| Ethnicity: Hispanic/Latinx | 3 (8%) |
| Age (years) | |
| Median (IQR) | 43.5 (28.5, 57.5) |
| Mean (SD) | 43.1 (17.6) |
| Medical history | |
| Depression | 9 (23%) |
| Anxiety | 8 (20%) |
| Mood disorder (other) | 1 (3%) |
| Chronic pain condition | 7 (18%) |
| Previous stone history | 23 (58%) |
| Prior ureteroscopy | 12 (30%) |
| Prior ureteral stent placement | 13 (33%) |
| Severe pain with prior ureteral stent | 8 (20%) |
| Severe urinary symptoms with prior ureteral stent | 6 (15%) |
| Medication use in past 30 days | |
| Opioids | 11 (28%) |
| NSAIDs | 20 (50%) |
| Tamsulosin (or alpha blocker) | 16 (40%) |
| Oxybutynin (or anticholinergic) | 3 (8%) |
Data shown are n (%) except where indicated. IQR = 25th, 75th percentiles; SD = standard deviation; NSAIDs = nonsteroidal anti-inflammatory drugs
Table 3.
Intraoperative data
| Variable | Participants (n = 40) |
|---|---|
| Side of treatment | |
| Right | 19 (48%) |
| Left | 21 (52%) |
| Dominant stone size (renal, mm) | |
| Median (IQR) | 7.5 (5.0, 10.5) |
| Mean (SD) | 7.5 (3.9) |
| Dominant stone size (ureteral, mm) | |
| Median (IQR) | 6.0 (5.0, 7.0) |
| Mean (SD) | 6.2 (2.2) |
| Dominant stone location* | |
| Renal | 20 (50%) |
| Ureter (proximal) | 7 (18%) |
| Ureter (distal) | 13 (33%) |
| Number of stones treated (renal) | |
| Median (IQR) | 2 (1, 4) |
| Mean (SD) | 5.6 (10.6) |
| Number of stones treated (ureteral) | |
| Median (IQR) | 1 (1, 2) |
| Mean (SD) | 1.3 (0.5) |
| Operative time (min) | |
| Median (IQR) | 51 (36, 80) |
| Mean (SD) | 59.1 (28.6) |
| Ureteroscopy time (min) | |
| Median (IQR) | 32.5 (20, 58.5) |
| Mean (SD) | 40.1 (26.3) |
| Ureteroscope type* | |
| Flexible | 24 (60%) |
| Semirigid | 7 (18%) |
| Both | 9 (23%) |
| Ureteral access sheath use | 19 (48%) |
| Basket extraction | 33 (82%) |
| Irrigation | |
| Manual | 20 (50%) |
| Constant pressure | 20 (50%) |
| Ureteral stent diameter | |
| 4.7 French | 6 (15%) |
| 6 French | 34 (85%) |
| Ureteral stent length* (cm) | |
| 22 | 1 (3%) |
| 24 | 18 (45%) |
| 26 | 14 (35%) |
| 28 | 5 (13%) |
| 30 | 2 (5%) |
| Stent dwell time (days) | |
| Median (IQR) | 8 (6.5, 11) |
| Mean (SD) | 10.1 (6.8) |
*Note: percentages may not sum to 100 due to rounding
Data shown are n (%) except where indicated
IQR = 25th, 75th percentiles; SD = standard deviation
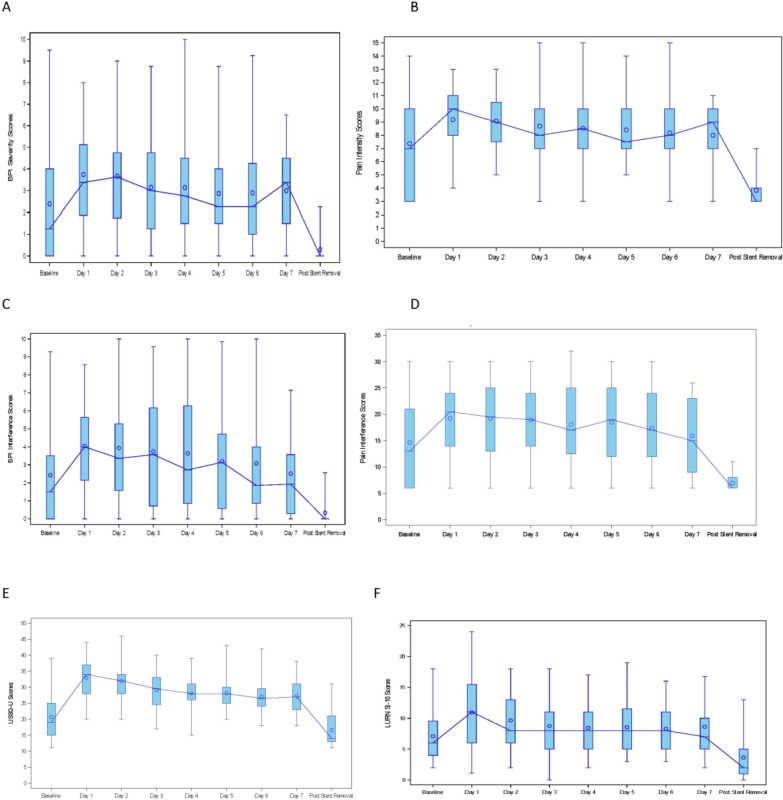
Daily assessments of pain intensity, pain interference, and urinary symptoms are displayed in Fig. 1. The percentage of completed questionnaires was very high, ranging from 84.6% to 98.8% completion on any given day. Similar patterns in daily pain severity were seen using both BPI and PROMIS. Pain intensity increased from baseline to POD 1, remained elevated over the duration of the stent, and varied widely among individuals. Median pain intensity scores were highest during the first 2 days after URS, with variable changes thereafter, but remained persistently elevated compared to baseline.
Fig. 1.
Daily stent-associated symptoms in each domain measured with various instruments displayed in box and whisker plots. The range (whiskers) is shown for each timepoint while the box represents the interquartile range. Circles depict mean values and the median values are connected by a line: A BPI pain severity, B PROMIS pain intensity, C BPI pain interference, D PROMIS pain interference, E Urinary score of USSQ (USSQ-U), F LURN SI-10 urinary symptoms
Pain interference was noted to have a similar pattern as pain intensity early in the postoperative course. Pain interference scores increased versus baseline on POD 1, with diminution over time, approaching baseline after POD 5. As seen with pain intensity, there was also wide inter-participant variation in reported interference due to pain.
Urinary symptoms increased from baseline and appeared to peak the day after surgery as measured by both instruments. Urinary symptoms remained persistently elevated versus baseline, without substantial decrease until after stent removal. Finally, bother due to urinary symptoms increased and was most pronounced immediately after URS. While median bother score mirrored the baseline value by POD 3, there was not definitive resolution until after stent removal. Wide variation among individuals was again noted.
Discussion
This prospective cohort study was the first to measure the daily lived experiences of patients after URS for stone disease. We found that there was wide variation of self-reported pain and urinary symptoms daily, stent symptoms peaked within the first 2 days after surgery but remained elevated throughout stent dwell time, and interference with daily activities secondary to pain persisted longer compared to bother due to urinary symptoms. We also found consistency among various instruments that assessed similar concepts. These results inform the selection of times at which stent symptoms should be measured after URS and, given the variation in symptoms among participants, reveal the importance of identifying characteristics that may help understand which patients are at greatest risk of severe stent symptoms.
Most importantly, this study identified days of the most intense SAS following URS. This information is important in determining the days at which SAS should be measured in research studies and also for counseling patients about the expected experience after URS. We found that the magnitude of adverse symptoms occurred within the first 2 days, a finding consistent among various instruments we used. This is in accord with a previous report by Lingeman and colleagues, who suggested that symptoms peaked on the first day based on pain medication use alone, although stent symptoms were not formally measured until POD 4 [12]. Our findings from daily assessment of SAS informed decisions for the main STENTS cohort. We chose POD 1, 3, and 5 (in addition to day of stent removal and 30 days after removal) in order to capture the peak symptoms and further analyze the trajectory of symptoms after URS. Our results indicate that there is no definitive time point in which symptom assessment is not relevant to measure, since the symptoms persist until stent removal.
The substantial variation in the reported pain intensity, pain interference, urinary symptoms, and urinary bother among participants across our study period highlights the heterogeneity in the experience of individuals undergoing URS for stones. To date, little is known about risk factors for severe SAS, and further investigation is needed. Younger age has been reported to be associated with more severe pain and unplanned hospital visits [13–15]. Krambeck and colleagues, in a subset analysis, reported decreased analgesic use in young males who had a ketorolac-loaded stent, suggesting potential gender differences in stent tolerance [16]. In our study, while bother due to urinary symptoms was most pronounced on POD 1 and 2, median bother was similar to baseline on subsequent days. In a recent study comparing stent designs, Wiseman and colleagues postulated that the effect of surgery on urinary symptoms may improve sooner than pain [17]. A detailed investigation of the effects of patient characteristics, stone factors, operative instrumentation, and medication use on SAS is the aim of the full STENTS study, which will elucidate the potential causes of SAS heterogeneity, identify patients at highest risk for severe SAS, and may allow for the creation of a prediction model [7], all of which would be useful in counseling patients in preemptive therapeutic decision-making and identifying populations to study in future trials.
An important finding of this study is that there was consistency observed among instruments that assessed similar domains of the patient experience. We intentionally chose to perform a comprehensive assessment of SAS using various instruments with conceptual overlap. For example, pain intensity and pain interference were measured using the BPI and PROMIS. Pain interference refers to pain that limits the patient’s ability to engage in daily activities, such as social-, work-, or school-related functions. BPI has been used in pain-related studies widely, contains a complete body map, and measures both the intensity of pain as well as the interference of pain in one’s life. This is the first study, to our knowledge, to use BPI following URS and stent placement. Pain interference was seen to approach baseline after several days as measured by the BPI. The potential significance of this finding is unclear, but there could be some element of adjustment to SAS despite continuing to have pain, as measured by pain severity. Pain intensity and interference were also measured with individual PROMIS instruments, allowing for comparison with population norms. Recently, PROMIS measures have been used to characterize stone patients in various settings [18–20]. In our study, we noted consistency among instruments in ascertaining peak symptoms, the evolution of daily symptoms, and the heterogeneity of the lived experiences following URS and stent placement.
Our study is the first to determine the daily pattern of pain intensity, pain interference, urinary symptoms, and bother after URS with stent placement for the entire postoperative period; however, the following limitations indicate opportunities to build on these findings. The large interindividual variation in symptoms coupled with a relatively small sample size precluded making multiple comparisons of the different domains of pain intensity, pain interference, urinary symptoms, and bother. These comparisons will be addressed in the main STENTS cohort. Additionally, participants were recruited from four clinical centers, and treatment of SAS post-URS differed among urologists. Lastly, we did not consider clustering by surgeon or institution.
Conclusion
In this first study measuring the daily lived experiences of patients after URS for stone disease, daily experiences of pain intensity, pain interference, urinary symptoms, and bother were highly variable but seemed to peak in the first 2 days and remained elevated while the stent was in place. These findings may inform more counseling of patients, shared decision making, and serve as groundwork to facilitate additional research for evaluating therapies and understanding mechanisms and risk factors for adverse experiences after ureteroscopy.
Supplementary Information
Additional file 1. USDRN members and affiliations.
Acknowledgements
We thank Peter Hoffmann for manuscript preparation and submission. Mr. Hoffmann did not receive compensation beyond his employment by Duke University.
NIDDK Urinary Stone Disease Research Network (USDRN): A full list of members and their affiliations appears in Additional file 1.
Author contributions
JDH, ACD, JAA, GET, JBZ, HRA-K, HHL, NMM, PPR, HBW, ZK, and CDS contributed to the analysis design and oversight, manuscript conception and drafting, statistical analysis, and/or editorial review. All authors read and approved the final manuscript.
Funding
This research was supported by the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases, as follows: U01DK110961 (UPenn/CHOP – Reese, Tasian), U01KD110986 (Washington Univ. St Louis – Desai, Lai), U01DK110994 (UT Southwestern - Maalouf), U01DK110954 (University of Washington – Harper, Wessells), and U01DK110988 (Duke University – Scales, Al-Khalidi).
Availability of data and materials
Request for data and materials will be reviewed and addressed according to the network’s policy for ancillary studies. Requests can be e-mailed to USDRN@dm.duke.edu.
Declarations
Ethics approval and consent to participate
The institutional review boards of each clinical center (University of Washington School of Medicine, University of Texas Southwestern Medical Center, Washington University School of Medicine, Children’s Hospital of Philadelphia, and University of Pennsylvania) reviewed and approved the study. All procedures involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants age 18 or above included in the study. Parents provided informed consent for all minor participants (age < 18 years, per the Institutional Review Boards governing this study), along with the assent of each adolescent participant.
Consent for publication
Not applicable.
Competing interests
Dr. Antonelli has relationships with the following entities: Boston Scientific Corp, Cook Medical (Advisory Board). Dr. Tasian has relationships with the following entities: Dicerna (advisory board, scientific study), Alnylam (advisory board), Novome (advisory board), and Allena Pharmaceuticals (consultant). No other authors reported disclosures relevant to this study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jonathan D. Harper and Alana C. Desai have contributed equally to this work.
Contributor Information
Jonathan D. Harper, Email: jdharper@uw.edu
Alana C. Desai, Email: desaia@wustl.edu
Jodi A. Antonelli, Email: Jodi.antonelli@UTSouthwestern.edu
Gregory E. Tasian, TasianG@email.chop.edu
Justin B. Ziemba, Email: Justin.Ziemba@pennmedicine.upenn.edu
Hussein R. Al-Khalidi, Email: hussein.al-khalidi@duke.edu
H. Henry Lai, Email: laih@wudosis.wustl.edu.
Naim M. Maalouf, Email: Naim.Maalouf@UTSouthwestern.edu
Peter P. Reese, Email: peter.reese@uphs.upenn.edu
Hunter B. Wessells, Email: wessells@uw.edu
Ziya Kirkali, Email: ziya.kirkali@nih.gov.
Charles D. Scales, Jr., Email: chuck.scales@duke.edu
References
- 1.Chapter 4.3 Surgical Procedures. In: Feinstein L, Matlaga B. Urologic Diseases in America. National Institute of Diabetes and Digestive and Kidney Diseases. Washington, DC: US Government Printing Office, 2018; NIH Publication No. 12-7865; 9–10.
- 2.Matlaga BR, Meckley LM, Kim M, et al. Management patterns of medicare patients undergoing treatment for upper urinary tract calculi. J Endourol. 2014;28:723–728. doi: 10.1089/end.2013.0580. [DOI] [PubMed] [Google Scholar]
- 3.Mittakanti HR, Conti SL, Pao AC, et al. Unplanned emergency department visits and hospital admissions following ureteroscopy: do ureteral stents make a difference? Urology. 2018;117:44–49. doi: 10.1016/j.urology.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 4.Makarov DV, Trock BJ, Allaf ME, et al. The effect of ureteral stent placement on post-ureteroscopy complications: a meta-analysis. Urology. 2008;71:796–800. doi: 10.1016/j.urology.2007.10.073. [DOI] [PubMed] [Google Scholar]
- 5.Ordonez M, Hwang EC, Borofsky M, et al. Ureteral stent versus no ureteral stent for ureteroscopy in the management of renal and ureteral calculi. Cochrane Database Syst Rev. 2019;2:CD012703. [DOI] [PMC free article] [PubMed]
- 6.Pais VM, Jr, Smith RE, Stedina EA, et al. Does omission of ureteral stents increase risk of unplanned return visit? a systematic review and meta-analysis. J Urol. 2016;196:1458–1466. doi: 10.1016/j.juro.2016.05.109. [DOI] [PubMed] [Google Scholar]
- 7.Scales CD, Jr, Lai HH, Desai AC, et al. Study to Enhance Understanding of Stent-Associated Symptoms: rationale and study design. J Endourol. 2021;35:761–768. doi: 10.1089/end.2020.0776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singap. 1994;23:129–138. [PubMed] [Google Scholar]
- 9.Cella D, Yount S, Rothrock N, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Med Care. 2007;45:S3–11. doi: 10.1097/01.mlr.0000258615.42478.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joshi HB, Newns N, Stainthorpe A, et al. Ureteral stent symptom questionnaire: development and validation of a multidimensional quality of life measure. J Urol. 2003;169:1060–1064. doi: 10.1097/01.ju.0000049198.53424.1d. [DOI] [PubMed] [Google Scholar]
- 11.Cella D, Smith AR, Griffith JW, et al. A new brief clinical assessment of lower urinary tract symptoms for women and men: LURN SI-10. J Urol. 2020;203:164–170. doi: 10.1097/JU.0000000000000465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lingeman JE, Preminger GM, Goldfischer ER, et al. Comfort Study Team: assessing the impact of ureteral stent design on patient comfort. J Urol. 2009;181:2581–2587. doi: 10.1016/j.juro.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gul Z, Alazem K, Li I, et al. Predicting procedural pain after ureteroscopy: does hydrodistention play a role? Int Braz J Urol. 2016;42:734–739. doi: 10.1590/S1677-5538.IBJU.2015.0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Irani J, Siquier J, Pires C, et al. Symptom characteristics and the development of tolerance with time in patients with indwelling double-pigtail ureteric stents. BJU Int. 1999;84:276–279. doi: 10.1046/j.1464-410x.1999.00154.x. [DOI] [PubMed] [Google Scholar]
- 15.Lee FC, Holt SK, Hsi RS, et al. Preoperative belladonna and opium suppository for ureteral stent pain: a randomized, double-blinded, placebo-controlled study. Urology. 2017;100:27–32. doi: 10.1016/j.urology.2016.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krambeck AE, Walsh RS, Denstedt JD, et al. A novel drug eluting ureteral stent: a prospective, randomized, multicenter clinical trial to evaluate the safety and effectiveness of a ketorolac loaded ureteral stent. J Urol. 2010;183:1037–1042. doi: 10.1016/j.juro.2009.11.035. [DOI] [PubMed] [Google Scholar]
- 17.Wiseman O, Ventimiglia E, Doizi S, et al. Effects of silicone hydrocoated double loop ureteral stent on symptoms and quality of life in patients undergoing flexible ureteroscopy for kidney stone: a randomized multicentre clinical study. J Urol. 2020;204:769–777. doi: 10.1097/JU.0000000000001098. [DOI] [PubMed] [Google Scholar]
- 18.Borofsky MS, Lane GI, Neises SM, et al. Patient-Reported Outcomes Measurement System (PROMIS((R))) for patients with urolithiasis: initial report. J Urol. 2017;198:1091–1097. doi: 10.1016/j.juro.2017.05.080. [DOI] [PubMed] [Google Scholar]
- 19.Patel N, Brown RD, Sarkissian C, et al. Quality of life and urolithiasis: the patient-reported outcomes measurement information system (PROMIS) Int Braz J Urol. 2017;43:880–886. doi: 10.1590/s1677-5538.ibju.2016.0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Portis JL, Neises SM, Portis AJ. Pain is independent of stone burden and predicts surgical intervention in patients with ureteral stones. J Urol. 2018;200:597–603. doi: 10.1016/j.juro.2018.04.075. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. USDRN members and affiliations.
Data Availability Statement
Request for data and materials will be reviewed and addressed according to the network’s policy for ancillary studies. Requests can be e-mailed to USDRN@dm.duke.edu.