Abstract
Background
Headache is an adverse event of coronavirus 2019 (COVID-19) vaccination. Whether patients with history of headache suffer more from vaccination-induced headaches is unknown. We aimed to uncover if headache patients develop more headaches after COVID-19 mRNA vaccination than healthy controls.
Methods
We performed a questionnaire survey for nursing staff in our hospital from April to May 2021. Based on baseline characteristics, we divided the participants into migraine, non-migrainous headache, and healthy control, and examined the occurrence and features of headache after COVID-19 vaccinations.
Results
We included 171 participants (15.2% migraine and 24.6% non-migrainous headache). Headache incidence after vaccinations was significantly higher in the migraine (69.2%) and non-migrainous headache (71.4%) groups than in the healthy control (37.9%) group. The incidence of headaches was significantly higher after the second dose compared to the first (45.6% vs. 20.5%).
Conclusion
Migraineurs and non-migrainous headache participants developed more headaches compared to the healthy controls after COVID-19 vaccination.
Keywords: COVID-19, vaccination, migraine
Introduction
Coronavirus 2019 (COVID-19) (1) has become a global pandemic with 185 million cases and 4.0 million deaths (8 July 2021, Johns Hopkins COVID-19 Dashboard (2)). COVID-19 vaccines are the most promising approach to prevent infections, and the development of vaccines has progressed worldwide, with vaccination beginning in many parts of the world at the end of 2020.
BNT162b2 (Comirnaty®; BioNTech and Pfizer) is an mRNA vaccine against COVID-19, which has proven to be 85% effective and is expected to prevent the spread of COVID-19 infection (3–5). Vaccines are effective in preventing not only the infection but also for moderate to severe cases including death (4). BNT162b2 is given as two injections usually 3 weeks apart. In clinical trials of BNT162b2, mild to moderate headaches have been reported in 25–52% of patients, with the second dose having higher incidents of headaches than the first dose (39–52% vs. 25–42%) (5).
The prevalence of migraine is reported to be approximately 14% in the general population (6). Headache is a frequent symptom of COVID-19, and studies conducted in the emergency department have shown that up to 68.3–74.6% of COVID-19 patients reported headache (7,8). In COVID-19 patients, 6.1–7.8% of patients without headache had a history of migraine, as opposed to 14.1–25.3% of patients with headache having a history of migraine (7–9).
This suggests that a history of migraine is also associated with headache after COVID-19 infection. Since a history of headache affects headache symptoms during COVID-19 infection, we hypothesized that headache sufferers may be at higher risk after the vaccination.
We compared the incidence of headache after vaccination between migraineurs or non-migrainous headache patients, which do not meet diagnosis of migraine, and healthy controls. We also investigated the features such as onset and duration of headaches after vaccination. We conducted a cross-sectional questionnaire study of nursing staff in our hospital to survey their incidence of usual headaches and headaches after COVID-19 vaccination. This is the first study to focus on headache after COVID-19 vaccination by comparing the difference between migraineurs, non-migrainous headache patients, and healthy controls.
Methods
This was a single-centre, cross-sectional study of nursing staff at Keio University Hospital, Tokyo, Japan. Appropriate consent was obtained from the participants included in the study based on Ethical Guidelines for Medical and Health Research Involving Human Subject issued by the Japanese Ministry of Health, Labour and Welfare. This study was approved by the Ethics Committee of Keio University School of Medicine (20210032).
The study period was from 12 April to 1 May 2021. We conducted a questionnaire survey (89% response rate), and the inclusion criteria were nursing staff who received two doses of the BNT162b2 vaccine at our hospital. There are three COVID-19 vaccines (5,10,11) in Japan as for July 2021, but at the time of survey, only the BNT162b2 vaccine was available. Those with history of headaches were classified as the migraine or non-migrainous headache group. Participants were classified as migraine if meeting diagnosis of migraine with typical aura or migraine without aura using International Classification of Headache, 3rd edition (ICHD-3), and non-migrainous headache if not meeting the diagnosis of the above (12). Those without history of headache were classified as the healthy control group. Monthly headache days (MHD), duration, and the numerical rating scale (NRS) scores as pre-existing headache characteristics were collected from those in the headache groups.
We compared the incidence of headaches after vaccinations among the three groups, and between the first and second vaccination doses. If headaches occurred, the presence of fever (≥37.5°C) was surveyed. Additionally, the features of post-vaccine headaches were evaluated, including the NRS score, time from vaccination to the onset of headache, headache duration, and headache characteristics (laterality, pulsating quality, aggravation by routine physical activity, presence of nausea/vomiting, photophobia and phonophobia). These were compared among the three groups and between the first and second vaccinations in each group. Also, the information on the use of pain killers during post-vaccine headache was collected.
Statistical analysis
The chi-squared test was used to compare the categorical data. The McNemar test was used to compare the incidence of headaches after the first and second vaccinations. For quantitative data, a Kruskal–Wallis test was used for three-group comparisons and a Wilcoxon rank-sum test was used for two-group comparisons and for comparisons of the first and second vaccinations. When there was a significant difference in the three-group comparison, two-group comparisons were performed and then p-values were adjusted with Bonferroni correction. We did not impute missing data. All p-values are two-sided, and p-values of <0.05 were considered statistically significant. Statistical analysis was performed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Baseline characteristics
One participant whose baseline headache status was unknown was excluded, and 171 participants were included in the study. Of the 171 participants, 26 (15.2%) had a history of migraine, 42 (24.6%) had a history of non-migrainous headache, and 103 (60.2%) were healthy controls. Of the participants in the migraine group, 42.3% (95% CI; 23.4%, 63.1%) were migraineurs with typical aura. In the non-migrainous headache group, 24 (57.1%) met the diagnosis of tension-type headache, 13 (31.0%) met the diagnosis of probable migraine, and five (11.9%) were solely probable tension-type headache (12). The overall median age was 31.0 years, and 93.6% were female. There were no significant differences for age and sex between the three groups (p = 0.802 and p = 0.236, respectively). The duration of baseline headaches and the NRS scores were significantly higher in the migraine group than in the non-migrainous headache group (p = 0.002 and p = 0.008, respectively) (Table 1 and Supplementary Table).
Table 1.
Characteristics of participants and the post-vaccination headache in each group. Please refer to Supplementary Table for 95% CI, estimate, and information on missing data.
| Healthy | Migraine | Non-migraine | p-value | |
|---|---|---|---|---|
| n = 103 | n = 26 | n = 42 | ||
| Age, year, median (IQR) | 30.0 (25.0, 41.0) | 31.5 (25.0, 41.0) | 34.5 (25.0, 43.0) | 0.802 |
| Female, n (%) | 94 (91.3) | 26 (100.0) | 40 (95.2) | 0.236 |
| Ordinary headache | ||||
| MHD, median (IQR) | – | 4.0 (2.0, 7.5) | 3.5 (2.5, 6.0) | 0.439 |
| Duration, hours, median (IQR) | – | 6.0 (4.0, 10.0) | 3.5 (2.5, 6.0) | 0.002 |
| NRS, median (IQR) | – | 6.3 (5.0, 7.5) | 5.0 (4.0, 7.0) | 0.008 |
| Post-first vaccination headache, n (%) | 14 (13.6) | 9 (34.6) | 12 (28.6) | 0.019 |
| Onset, hours, median(IQR) | 8.0 (6.0, 20.0) | 12.0 (8.0, 24.0) | 11.0 (6.8, 13.5) | 0.601 |
| Duration, hours, median(IQR) | 4.5 (2.0, 12.0) | 4.5 (2.0, 6.0) | 8.0 (2.8, 42.0) | 0.604 |
| Laterality | ||||
| Unilateral location, n (%) | 4 (28.6) | 3 (33.3) | 4(33.3) | 0.957 |
| Bilateral location, n (%) | 10 (71.4) | 6 (66.7) | 8(66.7) | |
| Quality | ||||
| Pulsating, n (%) | 7 (50.0) | 5 (55.6) | 2(18.2) | 0.164 |
| Non-pulsating, n (%) | 7 (50.0) | 4 (44.4) | 9(81.8) | |
| Aggravation by routine physical activity, n (%) | 5 (35.7) | 4 (44.4) | 5(41.7) | 0.907 |
| Nausea/vomiting, n (%) | 0 (0.0) | 2 (22.2) | 1(8.3) | 0.178 |
| Photophobia, n (%) | 0 (0.0) | 2 (22.2) | 0(0.0) | 0.047 |
| Phonophobia, n (%) | 0 (0.0) | 1 (11.1) | 2(16.7) | 0.303 |
| Headache with fever, n (%) | 1 (7.1) | 3 (33.3) | 1(8.3) | 0.166 |
| Pain killer use, n (%) | 10 (71.4) | 9 (100.0) | 7(58.3) | 0.092 |
| Post-second vaccination headache, n (%) | 33 (32.0) | 16 (61.5) | 29(69.0) | <0.001 |
| Onset, hours, median (IQR) | 12.0 (6.0, 18.0) | 12.0 (8.0, 12.0) | 12.0(8.0, 20.0) | 0.388 |
| Duration, hours, median (IQR) | 8.0 (3.0, 24.0) | 12.0 (4.5, 24.0) | 7.3(4.8, 27.0) | 0.503 |
| Laterality | ||||
| Unilateral location, n (%) | 7 (21.2) | 6 (37.5) | 7(24.1) | 0.460 |
| Bilateral location, n (%) | 26 (78.8) | 10 (62.5) | 22(75.9) | |
| Quality | ||||
| Pulsating, n (%) | 14 (45.2) | 9 (56.3) | 16(64.0) | 0.365 |
| Non-pulsating, n (%) | 17 (54.8) | 7 (43.8) | 9(36.0) | |
| Aggravation by routine physical activity, n (%) | 15 (46.9) | 12 (75.0) | 22(75.9) | 0.036 |
| Nausea/vomiting, n (%) | 3 (9.1) | 6 (37.5) | 5(17.2) | 0.052 |
| Photophobia, n (%) | 1 (3.1) | 2 (12.5) | 3(10.3) | 0.422 |
| Phonophobia, n (%) | 5 (15.6) | 4 (25.0) | 7(24.1) | 0.641 |
| Headache with fever, n (%) | 12 (36.4) | 8 (50.0) | 12(41.4) | 0.660 |
| Pain killer use, n (%) | 28 (84.8) | 12 (75.0) | 28(96.6) | 0.102 |
Healthy: healthy control; non-migraine: non-migrainous headache.
Incidence of headaches after the vaccinations
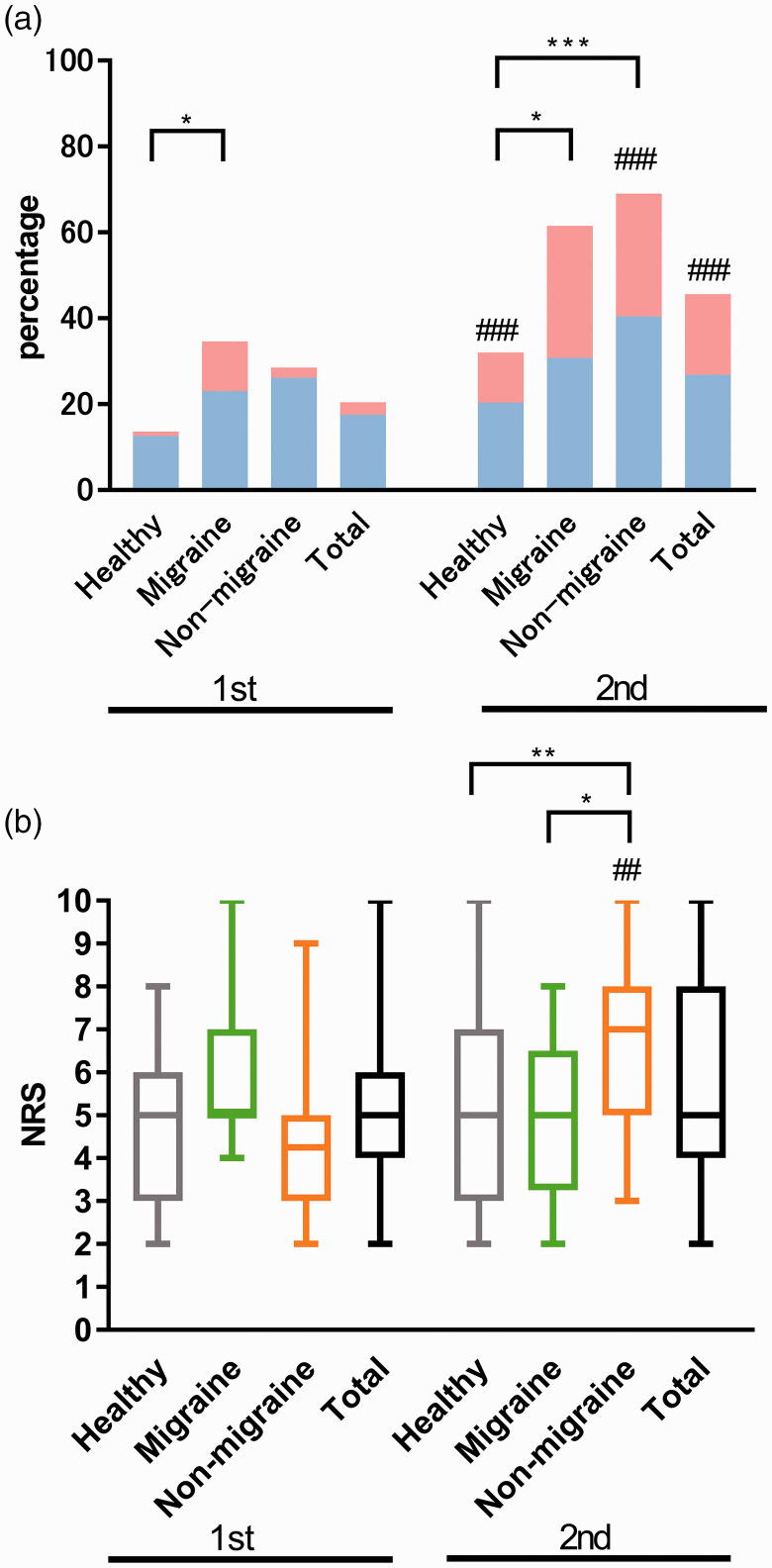
The incidence of participants with headaches after the vaccinations was 69.2% in the migraine group, 71.4% in the non-migrainous headache group, and 37.9% in the healthy control group (p < 0.001). The migraine and non-migrainous headache groups had significantly higher rates of headaches after vaccination than healthy controls (adjusted p = 0.012 and adjusted p < 0.001, respectively). There was no difference between the migraine and non-migrainous headache groups (adjusted p = 1.000). The overall incidence of post-vaccination headaches was significantly higher after the second vaccination dose than the first dose (45.6% vs. 20.5%, p < 0.001). In the migraine group, the frequency of headaches after the second vaccination dose tended to be higher than that after the first vaccination dose (p = 0.065), and in the non-migrainous headache and healthy control groups, the frequency of headaches was significantly higher after the second vaccination dose compared to the first (p < 0.001).
The incidence of headaches after the first vaccination dose was 34.6% in the migraine group, 28.6% in the non-migrainous headache group, and 13.6% in the healthy control group. There was significant difference between the migraine group and healthy control group in the incidence of post-vaccine headache (adjusted p = 0.037). The incidence of headaches after the second vaccination was 61.5% in the migraine group, 69.0% in the non-migrainous headache group, and 32.0% in the healthy control group. The incidence of post-vaccine headache in the migraine group and the non-migrainous headache group were significantly higher than the healthy control group (adjusted p = 0.017 and adjusted p < 0.001, respectively) (Figure 1(a)). There was no significant difference among the three groups in the proportion of people who had headaches with fever after the first and second vaccination (p = 0.166 and p = 0.660, respectively) (Table 1). In the healthy control and non-migrainous headache groups, headache with fever was significantly more common after the second vaccination dose than after the first (p = 0.041 and p = 0.039, respectively).
Figure 1.
Incidence and numerical rating scale (NRS) scores of post-vaccination headaches.(a) The incidence of post-vaccination headaches.
*Significant difference between groups.
#Significant difference between first and second vaccination doses within the group. Blue bars represent headache without fever and red bars represent headache with fever.
(b) NRS scores of post-vaccination headaches. Whiskers, full range; box, interquartile range; horizontal line, median. Grey, green, orange, and black represent healthy control, migraine, non-migrainous headache, and total, respectively.
*Significant difference between groups.
#Significant difference between first and second vaccination doses within the group.
Healthy: healthy control; non-migraine: non-migrainous headache.
*adjusted p < 0.05; **adjusted p < 0.01; ***adjusted p < 0.001; ##p < 0.01; ###p < 0.001.
Headache characteristics
The median NRS scores for headache after the first and second vaccination doses were 5.0 (IQR 4.0, 6.0) and 5.0 (IQR 4.0, 8.0), respectively. In the non-migrainous headache group, the NRS score of the second dose-induced post-vaccination headache was significantly higher than that of the migraine or healthy control groups (adjusted p = 0.018 and adjusted p = 0.008, respectively). In the non-migrainous headache group, the NRS of the second dose-induced post-vaccination headache was significantly higher than that of the headache induced by the first dose (p = 0.003) (Figure 1(b)).
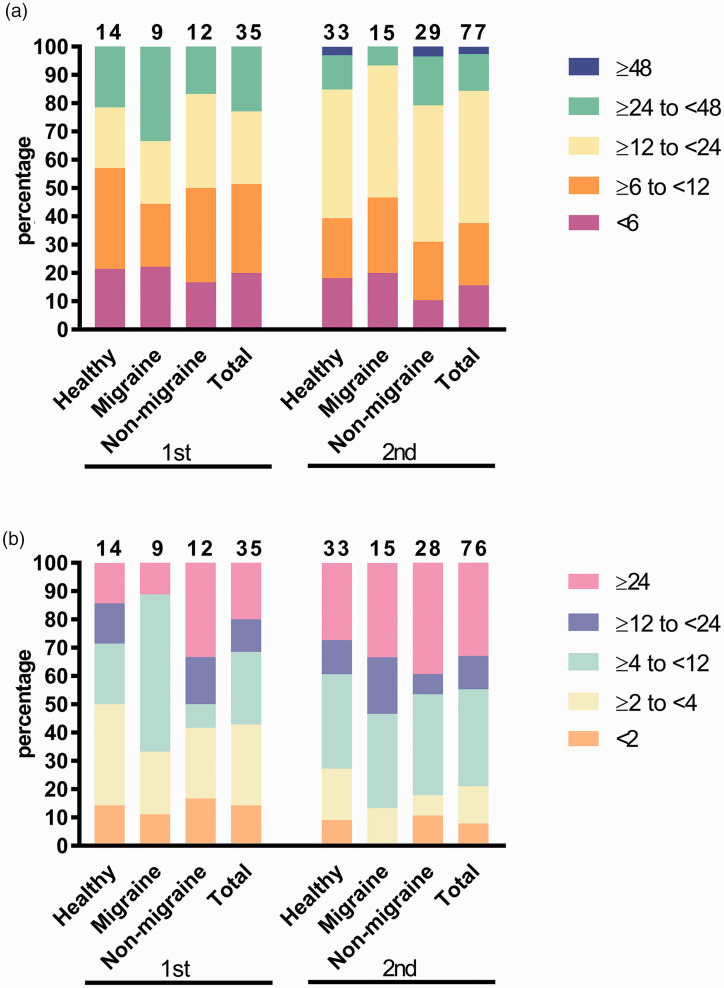
The median onset time of headaches after the first and the second vaccination doses was 10.0 h and 12.0 h, respectively, with no significant difference among the three groups (p = 0.601 and p = 0.388, respectively) (Table 1, Figure 2(a)). There was no significant difference in the onset time of headaches after the first and second vaccination doses in each group (migraine: p = 0.320, non-migrainous headache: p = 0.259, healthy control: p = 0.779).
Figure 2.
Proportion of time of onset and duration of post-vaccination headaches.(a) Onset of post-vaccination headaches. Blue: ≥48 h, Green: ≥24 to <48 h, Yellow: ≥12 to <24 h, Orange: ≥6 to <12 h, Magenta: <6 h. Numbers on the top of the graph represent the number of participants in each group.
(b) Duration of post-vaccination headaches. Pink: ≥24 h, Blue: ≥12 to <24 h, Green: ≥4 to <12 h, Yellow: ≥2 to <4 h, Orange: <2 h. Numbers on the top of the graph represent the number of participants in each group.
Healthy: healthy control; non-migraine: non-migrainous headache.
The median durations of headaches after the first and second vaccination doses were 4.5 h and 8.0 h in total, respectively, which were not significantly different among the three groups (p = 0.604 and p = 0.503, respectively) (Table 1, Figure 2(b)). There was no significant difference in the duration of post-vaccination headaches between the first and second vaccination doses in the non-migrainous headache and healthy control groups (non-migrainous headache: p = 0.416, healthy control: p = 0.321), but the duration in the migraine group tended to be longer after the second vaccination dose compared to the first (p = 0.078). A graph plotting the onset and duration of headaches, starting from the time of vaccination, is shown in the Supplementary Figure.
Characteristics of post-vaccine headache are shown in Table 1 and the Supplementary table. Seventy-four (71.2%) out of 104 post-vaccine headache attacks (excluding nine attacks with missing data) exhibited clinical features of tension-type headache based on ICHD-3 except for the presence of fever (12). Among participants with post-vaccine headache, pain killers were used in 74.3% and 87.2% after the first and second vaccination doses, respectively with no significant difference between the three groups (p = 0.092 and p = 0.102, respectively). In the non-migrainous headache group, the pain killer use was significantly higher after the second dose compared to the first (p = 0.002).
Discussion
This study is the first report to compare the incidence of headache after COVID-19 vaccination in participants with and without pre-existing headaches. The results showed that patients with pre-existing headache were more likely to suffer from headaches after COVID-19 vaccination than those without pre-existing headache. There was no difference in the incidence of headaches after vaccination between the migraine and non-migrainous headache groups. There was no clear difference in the percentage of headaches with fever and the onset time or duration of headaches among the three groups, and the NRS score was higher after the second vaccination dose in the non-migrainous headache group. Of the 171 subjects, 39.8% had a history of headache and 15.2% had migraine. The prevalence of headache and migraine among nursing staff has been reported to be 42–45% and 15–17%, respectively, consistent with our results (13,14).
The strength of this study is that the questionnaire survey was conducted after vaccinations, avoiding the cognitive bias of triggering the anticipation of headaches. In addition, we were able to maintain a high response rate (89%), resulting in little selection bias. The limitation of this study is that, due to the study design, we cannot exclude potential recall bias about information including fever or headache pattern changes after vaccination. The questionnaire survey was conducted only among nursing staff and the majority of the respondents were women (93.6%) and young with a median age of 31. Caution is required when directly applying the data to the general population. Since the number of cases is small, further verification is desirable. Although we found that those with pre-existing headaches were more likely to experience headaches after vaccination than healthy subjects, the mechanism and pathogenesis of the headaches are the subject of future research. Anxiety or stress generated by having to undergo vaccination could be a trigger for headache. Lastly, study participants were able to take a pain killer freely, so data on duration of headaches after vaccination may have been affected.
In conclusion, the incidence of headache after COVID-19 vaccination tended to be higher in those with pre-existing headaches, including migraineurs. It is important to note that headache patients may have higher incidence of headaches after COVID-19 vaccination.
Clinical implications
Migraine and non-migrainous headache were associated with significantly higher incidences of headaches after BNT162b2 vaccination compared to healthy controls (69.2% vs. 71.4% vs. 37.9%).
The incidence of headache after vaccination was significantly higher after the second shot compared to the first shot (45.6% vs. 20.5%).
Supplemental Material
Supplemental material, sj-pdf-1-cep-10.1177_03331024211038654 for Incidence of headache after COVID-19 vaccination in patients with history of headache: A cross-sectional study by Koji Sekiguchi, Narumi Watanabe, Naoki Miyazaki, Kei Ishizuchi, Chisato Iba, Yu Tagashira, Shunsuke Uno, Mamoru Shibata, Naoki Hasegawa, Ryo Takemura, Jin Nakahara and Tsubasa Takizawa in Cephalalgia
Supplemental material, sj-xlsx-2-cep-10.1177_03331024211038654 for Incidence of headache after COVID-19 vaccination in patients with history of headache: A cross-sectional study by Koji Sekiguchi, Narumi Watanabe, Naoki Miyazaki, Kei Ishizuchi, Chisato Iba, Yu Tagashira, Shunsuke Uno, Mamoru Shibata, Naoki Hasegawa, Ryo Takemura, Jin Nakahara and Tsubasa Takizawa in Cephalalgia
Acknowledgement
We would like to thank the nursing staff of Keio University Hospital who participated in this study.
Footnotes
Declaration of conflicting interests: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: HN received research funds and speaker’s fees from Pfizer, but the company has no relation to the study. TT reports being an advisor for Otsuka and Amgen. TT received speaker’s fees from Daiichi Sankyo, Eli Lilly Japan, Kowa, UCB Japan, and research fund from Tsumura outside the submitted work.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The publication of this article was supported by JSPS KAKENHI (grant number 19K16989 to TT).
ORCID iDs
Mamoru Shibata https://orcid.org/0000-0002-2416-4259
Ryo Takemura https://orcid.org/0000-0002-3733-7411
References
- 1.Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med 2020; 382: 1199–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johns Hopkins University of Medicine. Johns Hopkins Coronavirus Resource Center, https://coronavirus.jhu.edu/ (accessed 8 July 2021).
- 3.Hall VJ, Foulkes S, Saei A, et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): A prospective, multicentre, cohort study. Lancet 2021; 397: 1725–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med 2021; 384: 1412–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020; 383: 2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stovner LJ, Nichols E, Steiner TJ, et al. Global, regional, and national burden of migraine and tension-type headache, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2018; 17: 954–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Membrilla JA, de Lorenzo I, Sastre M, et al. Headache as a cardinal symptom of coronavirus disease 2019: A cross-sectional study. Headache 2020; 60: 2176–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caronna E, Ballve A, Llaurado A, et al. Headache: A striking prodromal and persistent symptom, predictive of COVID-19 clinical evolution. Cephalalgia 2020; 40: 1410–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez-de-Las-Penas C, Gomez-Mayordomo V, Cuadrado ML, et al. The presence of headache at onset in SARS-CoV-2 infection is associated with long-term post-COVID headache and fatigue: A case-control study. Cephalalgia. Epub ahead of print 18 June 2021. DOI: 10.1177/03331024211020404. [DOI] [PMC free article] [PubMed]
- 10.Anderson EJ, Rouphael NG, Widge AT, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med 2020; 383: 2427–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021; 397: 99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018; 38: 1–211. [DOI] [PubMed] [Google Scholar]
- 13.Durham CF, Alden KR, Dalton JA, et al. Quality of life and productivity in nurses reporting migraine. Headache 1998; 38: 427–435. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Xie J, Yang F, et al. The prevalence of primary headache disorders and their associated factors among nursing staff in North China. J Headache Pain 2015; 16: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-cep-10.1177_03331024211038654 for Incidence of headache after COVID-19 vaccination in patients with history of headache: A cross-sectional study by Koji Sekiguchi, Narumi Watanabe, Naoki Miyazaki, Kei Ishizuchi, Chisato Iba, Yu Tagashira, Shunsuke Uno, Mamoru Shibata, Naoki Hasegawa, Ryo Takemura, Jin Nakahara and Tsubasa Takizawa in Cephalalgia
Supplemental material, sj-xlsx-2-cep-10.1177_03331024211038654 for Incidence of headache after COVID-19 vaccination in patients with history of headache: A cross-sectional study by Koji Sekiguchi, Narumi Watanabe, Naoki Miyazaki, Kei Ishizuchi, Chisato Iba, Yu Tagashira, Shunsuke Uno, Mamoru Shibata, Naoki Hasegawa, Ryo Takemura, Jin Nakahara and Tsubasa Takizawa in Cephalalgia