Introduction
Unfractionated heparin (UFH) and low molecular weight heparin (LMWH) are mainstays of therapeutic anticoagulation with multiple desirable pharmacologic traits and a wealth of evidence supporting their use [1]. UFH and LMWH have the potential to cause heparin induced thrombocytopenia (HIT), an off-target, immune-mediated adverse drug reaction (ADR). HIT occurs in up to 2.4% of patients treated with heparin anticoagulants, has a greater than 30% mortality rate, and may result in catastrophic thromboembolic complications, including life- and limb-threatening thrombosis [2–5]. Considering the widespread use of these agents and the severity of this reaction, the inability to predict HIT constitutes a considerable liability for heparin anticoagulant treatment. Genetic variation has the potential to constitute clinically implementable biomarkers that predict ADRs such as HIT, improving the safety of heparin anticoagulants [6, 7]. Previous studies aimed at identifying genetic influences on HIT are primarily candidate gene studies that have focused on polymorphisms in FCGR2A and the HLA locus. Genome-wide association studies (GWAS) have also been conducted with conflicting results. Here we review the pathophysiology of HIT, the likely biological pathways influencing HIT risk, and the evidence of genetic associations with HIT. As both UFH and LMWH have the potential to cause HIT, this summary will focus on UFH due to its higher HIT risk [1].
Heparin Induced Thrombocytopenia
Treatment with heparin anticoagulants can result in two types of drug-induced thrombocytopenia. Type I HIT is a non-immune mediated reaction where few clinical implications are present and platelets stabilize quickly. Type I HIT is more common and can occur as early as one day into therapy. Type II HIT is immune-mediated, less common, and typically occurs 5–14 days after initial therapy. For the purposes of this article, HIT is used to refer to Type II HIT.
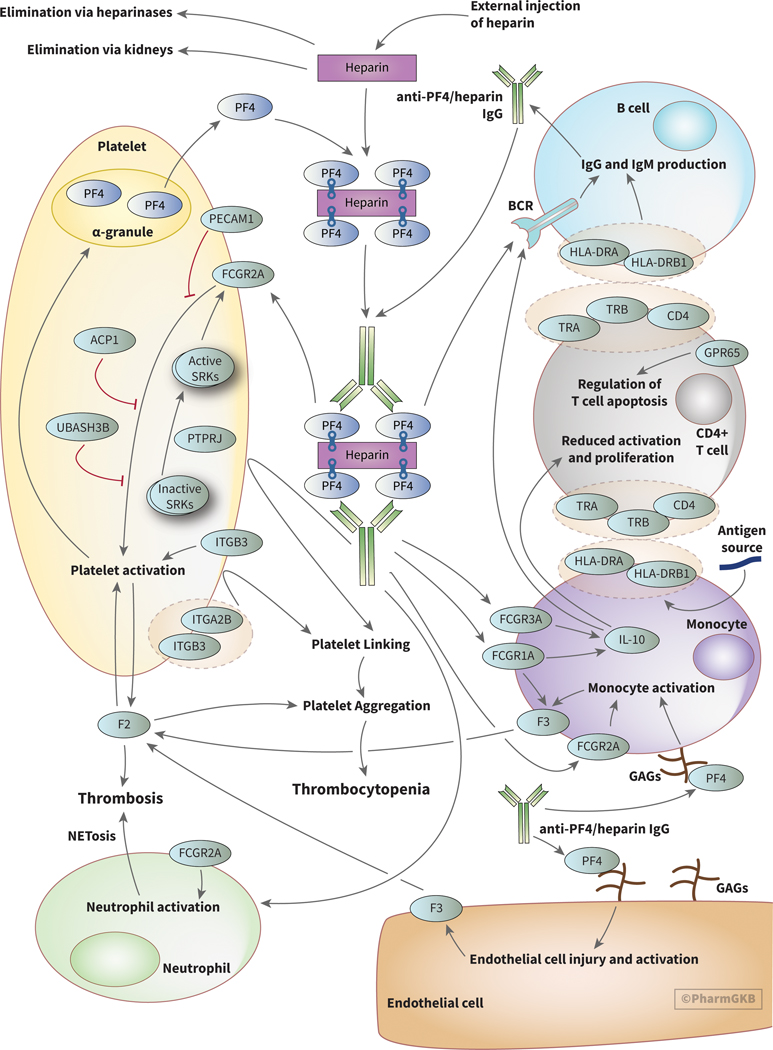
The pathogenesis of HIT involves binding of the linear polyanion heparin to the positively charged protein platelet factor 4 (PF4), an endogenous chemokine stored in alpha granules of platelets (Figure 1). PF4 is involved in bacterial defense through interactions with bacterial lipopolysaccharides suggesting that HIT may be a misdirected response towards exogenous heparins [8–10]. Complexes of PF4 and heparin are recognized by IgG antibodies, forming PF4/heparin-IgG complexes [11]. Interactions between PF4 and heparin are dependent on charge and stoichiometric ratios; excess of either PF4 or heparin will prevent assembly of antibody complexes [12, 13]. These antibody complexes activate platelets and monocytes via the platelet Fcγ-receptor (FcγRIIa), encoded by the gene FCGR2A, resulting in thrombin generation and thrombotic complications [8, 14, 15]. Recent data suggest that release of Neutrophil Extracellular Traps (NETosis) following neutrophil activation via the FcγRIIa receptor is a causative factor in heparin induced thrombocytopenia-associated thrombosis (HITT) [16]. In macrophages, FCGR1A and FCGR3A have been linked to release of IL-10, an important immunoregulatory cytokine that may have relevant downstream effects in HIT [17, 18]. Current models for HIT indicate that a yet unidentified antigen exposure happens prior to heparin exposure, creating a primary immune response that primes production of relevant IgGs.
Figure 1.
PharmGKB pathway for heparin induced thrombocytopenia (HIT) beginning with heparin injection and including interactions with platelets, monocytes, CD4+ T cells, B cells, neutrophils, and endothelial cells that result in thrombocytopenia and thrombosis. An interactive version of this pathway can be found at https://www.pharmgkb.org/pathway/PA166248901. BCR: B cell receptor, GAGs: glycoaminoglycans, SRKs: Src family kinases.
Platelet monitoring is a primary method of detecting HIT during heparin treatment. American College of Chest Physician (CHEST) guidelines recommend for patients with a greater than 1% chance of developing HIT to have their platelet count checked every 2–3 days in the first 14 days of treatment with heparin [1]. Clinical scoring systems are useful in risk stratification of patients based on HIT risk and the most frequently used is the 4Ts score. This scoring system evaluates degree of thrombocytopenia, timing of platelet decrease, thrombosis or other sequelae, and other possible causes of thrombocytopenia to estimate the likelihood of HIT prior to laboratory confirmation [19, 20]. American Society of Hematology recommendations include assay testing for HIT if patients have an intermediate to high 4Ts score [21]. Enzyme-linked immunosorbent assays are used to detect PF4/heparin antibodies and screen for HIT in suspected patients. Up to 50% of heparin-treated patients will develop PF4/heparin antibodies, but only a fraction of those patients will develop HIT and its thromboembolic complications [22–24]. Therefore, platelet reactivity testing using highly sensitive and specific functional assays is necessary to diagnose HIT. Such assays include the Serotonin Release Assay (SRA) and the Heparin Induced Platelet Aggregation Assays (HIPA) [25]. These tests are technically demanding, usually restricted to specialized laboratories, and have the potential to result in significant delays in diagnosis. Clinical approaches to functional assay-confirmed HIT focus on management and treatment of the condition after thrombocytopenia or other signs of HIT are presented. Discontinuation of heparin anticoagulants and initiating a non-heparin anticoagulant is critical to prevent HIT-related thrombosis. HIT patients that manifest thrombotic complications should be transitioned to non-heparinoid anticoagulant such as argatroban, lepirudin, danaparoid, or bivalirudin.
Patients may present with a HIT-like syndrome termed autoimmune HIT without heparin exposure [26]. Like HIT, autoimmune HIT results from platelet activation by PF4/heparin-like antibodies, but in autoimmune HIT these antibodies form in response to PF4 binding polyanionic compounds other than heparin. Other polyanions such as RNA, proteoglycans, polyphosphates, and bacterial cell wall components may bind to PF4 to stimulate an immune response, providing multiple pathways for autoimmune HIT. Treatment often includes immediate therapy with an anticoagulant other than heparin (as heparin can exacerbate the condition) until platelet counts return to normal. Similar clinical features to autoimmune HIT have been observed in cases of vaccine-induced thrombotic thrombocytopenia (VITT) due to adenoviral vector SARS-CoV-2 vaccines[27–32]. VITT is characterized by high levels of antibodies to PF4-polyanion complexes, unusual thrombotic sites, and high mortality, bearing striking similarity to autoimmune HIT. Studies have also observed an unusually high rate of PF4/heparin antibodies and increased risk of HIT in Coronavirus Disease 2019 (COVID-19) patients, indicating a possible link between COVID-19 and HIT [33–39].
Heparin Pharmacology
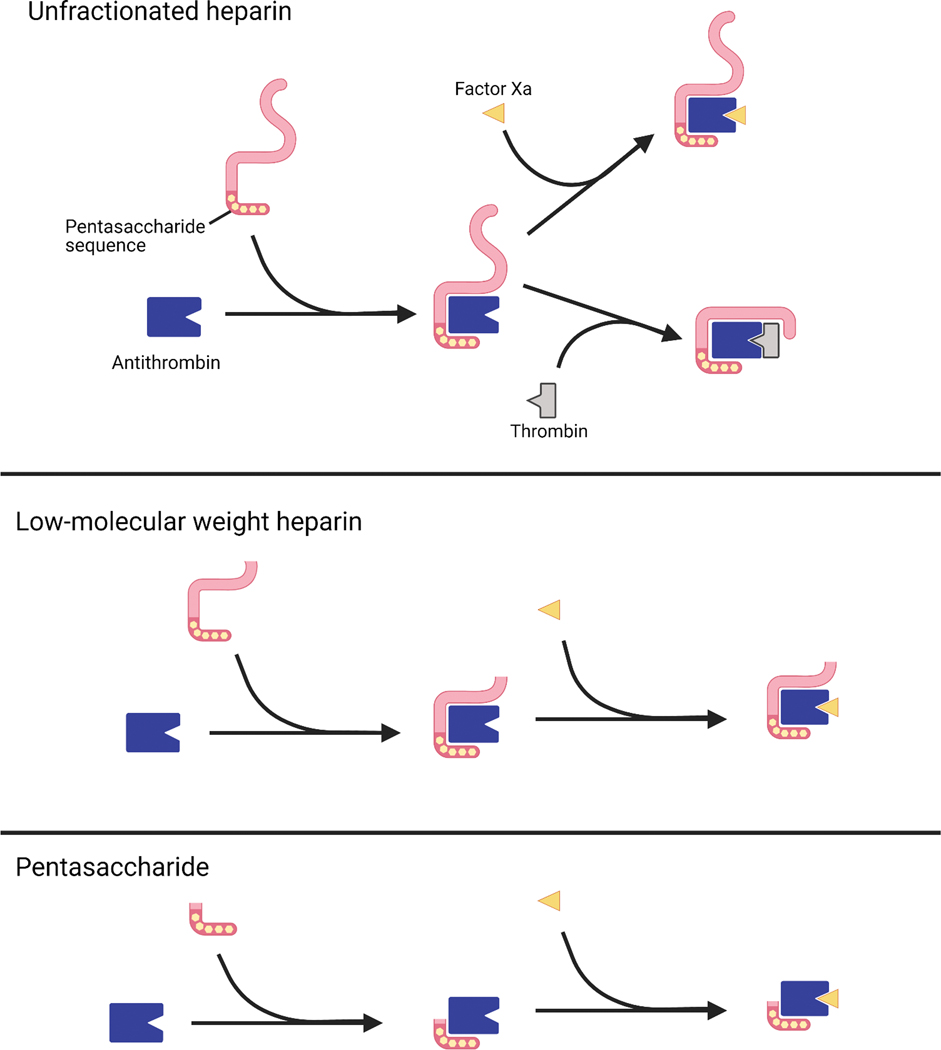
Heparins are produced by all mammals in basophils and mast cells, but exogenous heparins are used for therapeutic and prophylactic anticoagulation. Heparin is often supplied in an unfractionated form, UFH, which is used in the inpatient setting due to ease of administration, low cost, immediate onset of action, short half life, availability of lab monitoring, reversibility, a lack of renal adjustments, and a wealth of evidence supporting its use [6, 7]. Clinical monitoring of UFH can be done via anti-factor Xa testing or by proxy using activated partial thromboplastin time (aPTT) testing [40]. UFH contains sulfated glycosaminoglycans of variable length that bind to antithrombin III, a serine protease. Heparin binding to antithrombin III induces an activating conformational change that allows it to inactivate other clotting factors, including Factors IIa (thrombin) and Xa. Heparins have a minimum five saccharide chain length and simultaneously bind to both antithrombin III and thrombin if the chain length is 18 or greater [41]. LMWHs, such as dalteparin and enoxaparin, have been fractionated to fewer chain lengths and have a lower potential to cause HIT (Figure 2). A synthetic heparin pentasaccharide, fondaparinux, has a still lower potential for HIT.
Figure 2.
The variable chain lengths of (a) unfractionated heparin, (b) enoxaparin, and (c) a pentamer such as fondaparinux offer different pharmacologic profiles, including differences in binding to antithrombin and subsequent inactivation of Thrombin and Factor Xa.
Heparin Pharmacokinetics
UFH and exogenous heparins do not behave as their endogenous counterparts. The pharmacologic profile of UFH is complex due to its nature as a linear glycosaminoglycan of variable length [42]. UFH is typically isolated from bovine lung or porcine intestine and this results in variability in chain lengths, product strengths, and patient response, requiring the use of therapeutic monitoring for UFH. Increasing UFH dose is also a risk factor for HIT [1]. UFH can be modeled using non-linear Michaelis-Menten kinetics. UFH can quickly bind to circulating PF4 in the blood as well as other proteins, including albumin, plasmin, and fibrinogen. UFH distributes throughout the blood compartment in the intravascular space and is protein bound to a clinically relevant extent [43]. For the purposes of modeling, UFH can be considered to have a volume of distribution limited to the intravascular space. Two-compartment models have also been determined [44].
UFH has a half-life of effect on clotting time of approximately 1.5 hours [45, 46]. However, half-life of the molecule is variable depending on the dose given [46, 47]. There appear to be two processes by which heparin is eliminated. A saturable mechanism which predominates at lower doses, and a non-saturable mechanism at higher doses [43]. In the saturable mechanism, heparins are phagocytized by the reticuloendothelial system, which plays a large role in elimination [47, 48]. The non-saturable mechanism of elimination is via renal excretion. Heparins are also capable of being depolymerized via Heparanase-1 and via N-desulfation [45, 49–51].
Pharmacogenetics of Heparin Induced Thrombocytopenia
Pharmacogenomics is an area of great interest in HIT research as predictive genetic markers might have clinical utility. A number of pharmacogenetic associations have been found regarding HIT, but few of these studies have been rigorously replicated (Table 1) [52–64]. Fcγ receptors, especially FcγRIIA and FcγRIIIA (encoded by FCGR2A and FCGR3A, respectively), are frequently discussed in HIT pharmacogenomic research. These receptors are a family of immunoglobulin proteins that bind to antigen-antibody complexes including PF4/heparin–IgG complexes involved in HIT. The H131R polymorphism of the FCGR2A (CD32A) gene (rs1801274) was first associated with increased HITT in a study with 20 HIT patients and 24 healthy controls [56]. This association was further supported in a larger study with HIPA-confirmed HIT patients confirmed and non-HIT thrombocytopenia or thrombosis patients that tested negative for PF4/heparin antibodies (Abneg) [56]. Further research has found that individuals homozygous for the H131R polymorphism were at significantly higher risk of thrombosis, likely due to increased platelet activation by antibodies [62]. The consistent results of these studies have provided strong evidence for a role of FCGR2A-H131R in HITT risk.
Table 1.
Pharmacogenomic associations with heparin induced thrombocytopenia and related outcomes.
| Locus | Variant | Study | Phenotype(s) and sample size | HIT Phenotype Confirmation | Study Design | Replication |
|---|---|---|---|---|---|---|
| FCGR2A | H131R (rs1801274) | Burgess 1995 [57] | 20 HIT patients 24 healthy controls |
PAT or SRA | Candidate gene | No |
| Carlsson 1998 [58] | 389 HIT 351 Abneg 256 healthy blood donors |
HIPA | Candidate gene | No | ||
| Rollin 2015 [63] | 35 HITT 54 HIT 160 Abpos 174 Abneg 206 healthy controls |
SRA and immunoassay | Candidate gene | No | ||
| ACP1 | rs11553742/ rs11553746 haplotypes |
Rollin 2013 [62] | 89 HIT 160 Abpos 179 Abneg |
SRA and immunoassay | Candidate gene | No |
| PTPRJ | Q276P (rs1566734), R326Q (rs1503185) | Rollin 2012 [55] | 97 HIT 160 Abpos 179 Abneg |
SRA and immunoassay | Candidate gene | No |
| FCGR3A | F158V (rs396991) | Gruel 2004 [56] | 102 HIT 84 Abpos 86 Abneg |
SRA and immunoassay | Candidate gene | No |
| FCGR2A, ITGB3, PECAM1 | H131R (rs1801274), HPA-1b (PIA2, rs5918), L125V (rs668) | Pamela 2013 [65] | 53 HITT 50 HIT 51 Abpos 100 blood donors |
HIPA and immunoassay | Candidate gene | No |
| ITGB3 | PIA2 (HPA-1b, rs5918) | Harris 2008 [64] | 27 HITT 39 HIT |
No functional assay | No | |
| IL10 | IL10G G20 microsatellite | Pouplard 2012 [61] | 82 HIT 84 Abpos 85 Abneg |
SRA and immunoassay | Candidate gene | No |
| Chr 5 | rs1433265 | Witten 2018 [53] |
Discovery: 96 HIT 96 heparin-treated controls Replication: 86 HIT 86 heparin-treated controls |
HIPA or immunoassay | GWAS | Yes (discovery p=6.5×10−5, replication p=1.5×10−4) |
| HLA-DR | HLA-DR3 serotype |
Paparella 2001 [59] | 10 Abpos 59 Abneg 860 heparin-treated controls |
Immunoassay | Candidate gene | No |
| HLA-DR | DRB3*01:01 | Karnes 2017 [60] | 65 HIT 350 heparin-treated controls |
Immunoassay | Candidate gene | No |
| GPR65 | rs1887289 rs10782473 | Karnes 2015 [54] |
Discovery: 67 HIT 884 heparin-treated controls Replication: 94 HIT 154 Abpos 178 Abneg |
Discovery: Immunoassay Replication: SRA and immunoassay |
GWAS | Yes (replicated for antibody production but not HIT) |
Abneg: patient with negative PF4/antibody test result; Abpos: patient with positive PF4/antibody test result but negative functional assay result; Chr 5: chromosome 5; GWAS: genome-wide association study; HIPA: heparin-induced platelet aggregation assay; HIT: heparin-induced thrombocytopenia; HITT: heparin-induced thrombocytopenia with thrombosis; PAT: platelet aggregation test; SRA: serotonin release assay.
Research has also expanded into factors that may influence FCGR2A expression, such as low-molecular-weight phosphotyrosine phosphatase (LMW-PTP) and variation in protein tyrosine phosphatase (PTPRJ). ACP1 encodes LMW-PTP, which is involved in phosphorylation of FCGR2A in platelets [65]. In a non-replicated study with confirmed HIT patients, high catalytic efficiency ACP1 haplotypes were more common in HIT patients [61]. The authors reasoned that this may be attributable to the fact LMW-PTP regulates ZAP-70, which plays a critical role in T-cell development and lymphocyte activation. However, there was no difference in genotypic frequency between HIT and Abpos patients, suggesting that APC1 polymorphisms do not play a role in HIT antibody pathogenicity [61]. In one study, patients homozygous for Q276P (rs1566734) and R326Q (rs1503185) in PTPRJ were found to be underrepresented in HIT cases, suggesting that homozygotes are more resistant to HIT development [54]. In addition, individuals homozygous for FCGR3A-F158V (rs396991) were more likely to be diagnosed with HIT [55].
Several other genetic associations with HIT have been identified, although additional research is warranted to confirm a true effect. A polymorphism in ITGB3 (glycoprotein IIIa subunit, GPIIb/IIIa is a fibrinogen receptor on platelets involved in activation) called human platelet antigen 1b (HPA-1b, L33P, rs5918) and PECAM1-L125V (platelet endothelial cell adhesion molecule) have been associated with increased thrombosis in HIT patients [64]. The G20 allele of the IL10G microsatellite was found to influence immune response to heparin and antibody production and an intronic SNP on chromosome 5 with no known function (rs1433265) was strongly associated with HIT in a GWAS [52]. One study determined that Human Leukocyte Antigens (HLA) play a role in HIT pathogenesis, especially HLA-DR, although the study had very small sample size [58]. A later study examined HLA gene variants, as these can be biomarkers of immune-mediated adverse drug reactions and determined that HLA-DRB3*01:01 is a potential risk factor for HIT, although the population examined was mostly of European descent [59]. No replication was performed for any of these studies, so additional research is needed to confirm these associations. A GWAS with a discovery cohort and replication cohort observed a statistically significant association between GPR65, also known as T-Cell Death-Associated Gene 8 (TDAG8), and HIT [53]. In the discovery cohort, GPR65 SNPs were significantly associated with HIT and in the replication cohort, GPR65 SNPs were associated with PF4/heparin antibodies, but not HIT [53]. This may be caused by the small sample size or differences in HIT case diagnosis, as the replication cohort had more stringent criteria for inclusion in the HIT group. Additionally, HIT patients in the discovery cohort were not functionally confirmed and the study was unable to differentiate predictors of antibody response and HIT. GPR65 is connected to regulation of T cell apoptosis and immune-mediated inflammation via reduction of cytokine production in T cells and macrophages, which are both important players in HIT [66, 67]. GPR65 was also shown to reduce the severity of two hypersensitivity models in mice, but the precise role of GPR65 in HIT remains unknown [68].
Future Directions
While multiple studies have observed associations between genetic variation and HIT as well as HITT, several major limitations reduce the potential impact. Limited sample sizes, inconsistent use of functional assay confirmation for HIT/HITT, and an inability to distinguish genetic predictors of antibody production and HIT make confident conclusions from this literature difficult. Future studies should address these limitations and would likely benefit from a focus on genome-wide approaches rather than candidate gene studies [69]. The biological complexity and unusual immunologic presentation of the HIT ADR suggest that agnostic approaches may be more appropriate to determine causal variation, identify potentially translatable biomarkers, and identify new influences on HIT pathogenesis. Such approaches could also be used to evaluate the heritability of HIT, which is currently unknown. Recruitment of sufficient sample sizes will likely remain a problem for such studies due to the rarity of the phenotype, especially with HITT. As a result, uncommon variants and/or variants with low effect sizes are unlikely to be discovered using genome-wide approaches. Overall, well-powered, rigorously replicated GWASs of HIT, which include functional assay confirmation of HIT and can differentiate genetic predictors of antibody production and HIT, are needed to provide a better understanding of HIT and potential prevention using genetic variability.
In addition, many emerging approaches have yet to be applied to HIT, including epigenomics, metagenomics, and single cell RNA-Seq and proteomics. These may provide new insights into HIT pathology and identify further biomarkers that can be used for HIT prevention. Sampling relevant cells before heparin treatment and after HIT develops is likely not feasible due to the sheer number of heparin-treated patients required for one HIT case. However, HIT’s unusual immunologic progression, such that antibodies are often undetectable after 90 days and HIT patients may often be rechallenged with heparin, presents a unique opportunity to study cellular activation during the ADR and after the ADR has subsided. The emergence of large, diverse DNA biorepositories coupled to electronic health records also introduces an opportunity to study the genetics of HIT on a larger scale. However, observational studies of HIT are often plagued by a lack of functional assay confirmation of a HIT diagnosis based in part on the commonly misconception that a positive PF4/heparin antibody test result is diagnostic for HIT [21]. Such patients without functional assay results may be excluded from future studies, exacerbating sample size problems. Future research should also focus on genetic predictors of HITT in particular, since thromboembolic complications have the greatest clinical relevance and prevention of HITT would likely have the greatest clinical impact.
Conclusion
HIT is a rare but potentially fatal condition induced by heparin anticoagulants that are widely used in clinical practice. Genetic polymorphisms have the potential to predict HIT, HITT, and other ADRs, and pharmacogenomic research has associated multiple genes with the condition. FCGRA2-H131R is the most supported by prior studies and has the greatest potential thus far to predict HITT. However, past research has had limitations that may reduce the potential impact of the literature. Limited sample size, inconsistent phenotype definitions, and the inability to distinguish genetic predictors of antibody production and HIT may weaken any new findings. While the potential for preemptive genetic testing to prevent HIT and HITT exists, more research is necessary to confidently discern the influence of genetic variation on this ADR.
Acknowledgments
Funding: JHK is supported by grants from the NIH’s National Heart Lung and Blood Institute (NHLBI) under award number K01HL143137 and R01HL158686, the American College of Clinical Pharmacy, and the Flinn Foundation. PharmGKB is supported by the NIH/NHGRI/NICHD grant U24 HG010615.
Footnotes
Conflicts of Interest: None declared
References
- 1.Linkins LA, Dans AL, Moores LK, Bona R, Davidson BL, Schulman S, et al. Treatment and prevention of heparin-induced thrombocytopenia: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e495S–e530S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Girolami B, Prandoni P, Stefani PM, Tanduo C, Sabbion P, Eichler P, et al. The incidence of heparin-induced thrombocytopenia in hospitalized medical patients treated with subcutaneous unfractionated heparin: a prospective cohort study. Blood. 2003;101(8):2955–9. [DOI] [PubMed] [Google Scholar]
- 3.Greinacher A, Farner B, Kroll H, Kohlmann T, Warkentin TE, Eichler P. Clinical features of heparin-induced thrombocytopenia including risk factors for thrombosis. A retrospective analysis of 408 patients. Thromb Haemost. 2005;94(1):132–5. [DOI] [PubMed] [Google Scholar]
- 4.Franchini M. Heparin-induced thrombocytopenia: an update. Thromb J. 2005;3:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martel N, Lee J, Wells PS. Risk for heparin-induced thrombocytopenia with unfractionated and low-molecular-weight heparin thromboprophylaxis: a meta-analysis. Blood. 2005;106(8):2710–5. [DOI] [PubMed] [Google Scholar]
- 6.Garcia DA, Baglin TP, Weitz JI, Samama MM. Parenteral anticoagulants: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e24S–e43S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karnes JH. Pharmacogenetics to prevent heparin-induced thrombocytopenia: what do we know? Pharmacogenomics. 2018;19(18):1413–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greinacher A. CLINICAL PRACTICE. Heparin-Induced Thrombocytopenia. N Engl J Med. 2015;373(3):252–61. [DOI] [PubMed] [Google Scholar]
- 9.Krauel K, Weber C, Brandt S, Zahringer U, Mamat U, Greinacher A, et al. Platelet factor 4 binding to lipid A of Gram-negative bacteria exposes PF4/heparin-like epitopes. Blood. 2012;120(16):3345–52. [DOI] [PubMed] [Google Scholar]
- 10.Greinacher A, Holtfreter B, Krauel K, Gatke D, Weber C, Ittermann T, et al. Association of natural anti-platelet factor 4/heparin antibodies with periodontal disease. Blood. 2011;118(5):1395–401. [DOI] [PubMed] [Google Scholar]
- 11.Zucker MB, Katz IR. Platelet factor 4: production, structure, and physiologic and immunologic action. Proc Soc Exp Biol Med. 1991;198(2):693–702. [DOI] [PubMed] [Google Scholar]
- 12.Rauova L, Poncz M, McKenzie SE, Reilly MP, Arepally G, Weisel JW, et al. Ultralarge complexes of PF4 and heparin are central to the pathogenesis of heparin-induced thrombocytopenia. Blood. 2005;105(1):131–8. [DOI] [PubMed] [Google Scholar]
- 13.Suvarna S, Espinasse B, Qi R, Lubica R, Poncz M, Cines DB, et al. Determinants of PF4/heparin immunogenicity. Blood. 2007;110(13):4253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelton JG, Sheridan D, Santos A, Smith J, Steeves K, Smith C, et al. Heparin-induced thrombocytopenia: laboratory studies. Blood. 1988;72(3):925–30. [PubMed] [Google Scholar]
- 15.Brandt S, Krauel K, Gottschalk KE, Renne T, Helm CA, Greinacher A, et al. Characterisation of the conformational changes in platelet factor 4 induced by polyanions: towards in vitro prediction of antigenicity. Thromb Haemost. 2014;112(1):53–64. [DOI] [PubMed] [Google Scholar]
- 16.Perdomo J, Leung HHL, Ahmadi Z, Yan F, Chong JJH, Passam FH, et al. Neutrophil activation and NETosis are the major drivers of thrombosis in heparin-induced thrombocytopenia. Nat Commun. 2019;10(1):1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang L, Li W, Sun Y, Kong L, Xu P, Xia P, et al. Antibody-Mediated Porcine Reproductive and Respiratory Syndrome Virus Infection Downregulates the Production of Interferon-alpha and Tumor Necrosis Factor-alpha in Porcine Alveolar Macrophages via Fc Gamma Receptor I and III. Viruses. 2020;12(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ji JD, Tassiulas I, Park-Min KH, Aydin A, Mecklenbrauker I, Tarakhovsky A, et al. Inhibition of interleukin 10 signaling after Fc receptor ligation and during rheumatoid arthritis. J Exp Med. 2003;197(11):1573–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crowther MA, Cook DJ, Albert M, Williamson D, Meade M, Granton J, et al. The 4Ts scoring system for heparin-induced thrombocytopenia in medical-surgical intensive care unit patients. J Crit Care. 2010;25(2):287–93. [DOI] [PubMed] [Google Scholar]
- 20.Cuker A, Gimotty PA, Crowther MA, Warkentin TE. Predictive value of the 4Ts scoring system for heparin-induced thrombocytopenia: a systematic review and meta-analysis. Blood. 2012;120(20):4160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cuker A, Arepally GM, Chong BH, Cines DB, Greinacher A, Gruel Y, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin-induced thrombocytopenia. Blood Adv. 2018;2(22):3360–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warkentin TE, Levine MN, Hirsh J, Horsewood P, Roberts RS, Gent M, et al. Heparin-induced thrombocytopenia in patients treated with low-molecular-weight heparin or unfractionated heparin. N Engl J Med. 1995;332(20):1330–5. [DOI] [PubMed] [Google Scholar]
- 23.Trossaert M, Gaillard A, Commin PL, Amiral J, Vissac AM, Fressinaud E. High incidence of anti-heparin/platelet factor 4 antibodies after cardiopulmonary bypass surgery. Br J Haematol. 1998;101(4):653–5. [DOI] [PubMed] [Google Scholar]
- 24.Pouplard C, May MA, Iochmann S, Amiral J, Vissac AM, Marchand M, et al. Antibodies to platelet factor 4-heparin after cardiopulmonary bypass in patients anticoagulated with unfractionated heparin or a low-molecular-weight heparin : clinical implications for heparin-induced thrombocytopenia. Circulation. 1999;99(19):2530–6. [DOI] [PubMed] [Google Scholar]
- 25.Minet V, Dogne JM, Mullier F. Functional Assays in the Diagnosis of Heparin-Induced Thrombocytopenia: A Review. Molecules. 2017;22(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greinacher A, Selleng K, Warkentin TE. Autoimmune heparin-induced thrombocytopenia. J Thromb Haemost. 2017;15(11):2099–114. [DOI] [PubMed] [Google Scholar]
- 27.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N Engl J Med. 2021;384(22):2092–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vayne C, Rollin J, Gruel Y, Pouplard C, Galinat H, Huet O, et al. PF4 Immunoassays in Vaccine-Induced Thrombotic Thrombocytopenia. N Engl J Med. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scully M, Singh D, Lown R, Poles A, Solomon T, Levi M, et al. Pathologic Antibodies to Platelet Factor 4 after ChAdOx1 nCoV-19 Vaccination. N Engl J Med. 2021;384(23):2202–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGonagle D, De Marco G, Bridgewood C. Mechanisms of Immunothrombosis in Vaccine-Induced Thrombotic Thrombocytopenia (VITT) Compared to Natural SARS-CoV-2 Infection. J Autoimmun. 2021;121:102662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cines DB, Bussel JB. SARS-CoV-2 Vaccine-Induced Immune Thrombotic Thrombocytopenia. N Engl J Med. 2021;384(23):2254–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simpson CR, Shi T, Vasileiou E, Katikireddi SV, Kerr S, Moore E, et al. First-dose ChAdOx1 and BNT162b2 COVID-19 vaccines and thrombocytopenic, thromboembolic and hemorrhagic events in Scotland. Nat Med. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tran M, Sheth C, Bhandari R, Cameron SJ, Hornacek D. SARS-CoV-2 and pulmonary embolism: who stole the platelets? Thromb J. 2020;18:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Warkentin TE, Kaatz S. COVID-19 versus HIT hypercoagulability. Thromb Res. 2020;196:38–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dragonetti D, Guarini G, Pizzuti M. Detection of anti-heparin-PF4 complex antibodies in COVID-19 patients on heparin therapy. Blood Transfus. 2020;18(4):328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang CT, Hsu SY, Chang KW, Huang CG, Yang CT, Cheng MH. Heparin-induced thrombocytopenia and thrombosis in a patient with Covid-19. Thromb Res. 2020;196:11–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nazy I, Jevtic SD, Moore JC, Huynh A, Smith JW, Kelton JG, et al. Platelet-activating immune complexes identified in critically ill COVID-19 patients suspected of heparin-induced thrombocytopenia. J Thromb Haemost. 2021;19(5):1342–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Daviet F, Guervilly C, Baldesi O, Bernard-Guervilly F, Pilarczyk E, Genin A, et al. Heparin-Induced Thrombocytopenia in Severe COVID-19. Circulation. 2020;142(19):1875–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patell R, Khan AM, Bogue T, Merrill M, Koshy A, Bindal P, et al. Heparin induced thrombocytopenia antibodies in Covid-19. Am J Hematol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smythe MA, Priziola J, Dobesh PP, Wirth D, Cuker A, Wittkowsky AK. Guidance for the practical management of the heparin anticoagulants in the treatment of venous thromboembolism. J Thromb Thrombolysis. 2016;41(1):165–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lane DA, Denton J, Flynn AM, Thunberg L, Lindahl U. Anticoagulant activities of heparin oligosaccharides and their neutralization by platelet factor 4. Biochem J. 1984;218(3):725–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mulloy B, Hogwood J, Gray E, Lever R, Page CP. Pharmacology of Heparin and Related Drugs. Pharmacol Rev. 2016;68(1):76–141. [DOI] [PubMed] [Google Scholar]
- 43.Kandrotas RJ. Heparin pharmacokinetics and pharmacodynamics. Clin Pharmacokinet. 1992;22(5):359–74. [DOI] [PubMed] [Google Scholar]
- 44.Jia Z, Tian G, Ren Y, Sun Z, Lu W, Hou X. Pharmacokinetic model of unfractionated heparin during and after cardiopulmonary bypass in cardiac surgery. J Transl Med. 2015;13:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eiber HB, Danishefsky I, Borrelli FJ. Studies made with radioactive heparin in humans. Angiology. 1960;11:40–3. [DOI] [PubMed] [Google Scholar]
- 46.Estes JW. The fate of heparin in the body. Curr Ther Res Clin Exp. 1975;18(1 Pt1):45–57. [PubMed] [Google Scholar]
- 47.McAvoy TJ. Pharmacokinetic modeling of heparin and its clinical implications. J Pharmacokinet Biopharm. 1979;7(4):331–54. [DOI] [PubMed] [Google Scholar]
- 48.Monkhouse FC. Physiological factors concerned with the removal of injected heparin from the circulating blood. Am J Physiol. 1954;178(2):223–8. [DOI] [PubMed] [Google Scholar]
- 49.Vlodavsky I, Ilan N, Nadir Y, Brenner B, Katz BZ, Naggi A, et al. Heparanase, heparin and the coagulation system in cancer progression. Thromb Res. 2007;120 Suppl 2:S112–20. [DOI] [PubMed] [Google Scholar]
- 50.Kjellen L, Pertoft H, Oldberg A, Hook M. Oligosaccharides generated by an endoglucuronidase are intermediates in the intracellular degradation of heparan sulfate proteoglycans. J Biol Chem. 1985;260(14):8416–22. [PubMed] [Google Scholar]
- 51.Thunberg L, Backstrom G, Wasteson A, Robinson HC, Ogren S, Lindahl U. Enzymatic depolymerization of heparin-related polysaccharides. Substrate specificities of mouse mastocytoma and human platelet endo-beta-D-glucuronidases. J Biol Chem. 1982;257(17):10278–82. [PubMed] [Google Scholar]
- 52.Witten A, Bolbrinker J, Barysenka A, Huber M, Ruhle F, Nowak-Gottl U, et al. Targeted resequencing of a locus for heparin-induced thrombocytopenia on chromosome 5 identified in a genome-wide association study. J Mol Med (Berl). 2018;96(8):765–75. [DOI] [PubMed] [Google Scholar]
- 53.Karnes JH, Cronin RM, Rollin J, Teumer A, Pouplard C, Shaffer CM, et al. A genome-wide association study of heparin-induced thrombocytopenia using an electronic medical record. Thromb Haemost. 2015;113(4):772–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rollin J, Pouplard C, Gratacap MP, Leroux D, May MA, Aupart M, et al. Polymorphisms of protein tyrosine phosphatase CD148 influence FcgammaRIIA-dependent platelet activation and the risk of heparin-induced thrombocytopenia. Blood. 2012;120(6):1309–16. [DOI] [PubMed] [Google Scholar]
- 55.Gruel Y, Pouplard C, Lasne D, Magdelaine-Beuzelin C, Charroing C, Watier H. The homozygous FcgammaRIIIa-158V genotype is a risk factor for heparin-induced thrombocytopenia in patients with antibodies to heparin-platelet factor 4 complexes. Blood. 2004;104(9):2791–3. [DOI] [PubMed] [Google Scholar]
- 56.Burgess JK, Lindeman R, Chesterman CN, Chong BH. Single amino acid mutation of Fc gamma receptor is associated with the development of heparin-induced thrombocytopenia. Br J Haematol. 1995;91(3):761–6. [DOI] [PubMed] [Google Scholar]
- 57.Carlsson LE, Santoso S, Baurichter G, Kroll H, Papenberg S, Eichler P, et al. Heparin-induced thrombocytopenia: new insights into the impact of the FcgammaRIIa-R-H131 polymorphism. Blood. 1998;92(5):1526–31. [PubMed] [Google Scholar]
- 58.Paparella D, Micelli M, Favoino B, D’Alo M, Fiore T, de Luca Tupputi Schinosa L. Anti-heparin-platelet factor 4 antibodies after cardiopulmonary bypass: role of HLA expression. Haematologica. 2001;86(3):326–7. [PubMed] [Google Scholar]
- 59.Karnes JH, Shaffer CM, Cronin R, Bastarache L, Gaudieri S, James I, et al. Influence of Human Leukocyte Antigen (HLA) Alleles and Killer Cell Immunoglobulin-Like Receptors (KIR) Types on Heparin-Induced Thrombocytopenia (HIT). Pharmacotherapy. 2017;37(9):1164–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pouplard C, Cornillet-Lefebvre P, Attaoua R, Leroux D, Lecocq-Lafon C, Rollin J, et al. Interleukin-10 promoter microsatellite polymorphisms influence the immune response to heparin and the risk of heparin-induced thrombocytopenia. Thromb Res. 2012;129(4):465–9. [DOI] [PubMed] [Google Scholar]
- 61.Rollin J, Pouplard C, Leroux D, May MA, Gruel Y. Impact of polymorphisms affecting the ACP1 gene on levels of antibodies against platelet factor 4-heparin complexes. J Thromb Haemost. 2013;11(8):1609–11. [DOI] [PubMed] [Google Scholar]
- 62.Rollin J, Pouplard C, Sung HC, Leroux D, Saada A, Gouilleux-Gruart V, et al. Increased risk of thrombosis in FcgammaRIIA 131RR patients with HIT due to defective control of platelet activation by plasma IgG2. Blood. 2015;125(15):2397–404. [DOI] [PubMed] [Google Scholar]
- 63.Harris K, Nguyen P, Van Cott EM. Platelet PlA2 Polymorphism and the risk for thrombosis in heparin-induced thrombocytopenia. Am J Clin Pathol. 2008;129(2):282–6. [DOI] [PubMed] [Google Scholar]
- 64.Pamela S, Anna Maria L, Elena D, Giovanni M, Emanuele A, Silvia V, et al. Heparin-induced thrombocytopenia: the role of platelets genetic polymorphisms. Platelets. 2013;24(5):362–8. [DOI] [PubMed] [Google Scholar]
- 65.Mancini F, Rigacci S, Berti A, Balduini C, Torti M. The low-molecular-weight phosphotyrosine phosphatase is a negative regulator of FcgammaRIIA-mediated cell activation. Blood. 2007;110(6):1871–8. [DOI] [PubMed] [Google Scholar]
- 66.Choi JW, Lee SY, Choi Y. Identification of a putative G protein-coupled receptor induced during activation-induced apoptosis of T cells. Cell Immunol. 1996;168(1):78–84. [DOI] [PubMed] [Google Scholar]
- 67.Onozawa Y, Fujita Y, Kuwabara H, Nagasaki M, Komai T, Oda T. Activation of T cell death-associated gene 8 regulates the cytokine production of T cells and macrophages in vitro. Eur J Pharmacol. 2012;683(1–3):325–31. [DOI] [PubMed] [Google Scholar]
- 68.Onozawa Y, Komai T, Oda T. Activation of T cell death-associated gene 8 attenuates inflammation by negatively regulating the function of inflammatory cells. Eur J Pharmacol. 2011;654(3):315–9. [DOI] [PubMed] [Google Scholar]
- 69.Linskey DW, Linskey DC, McLeod HL, Luzum JA. The need to shift pharmacogenetic research from candidate gene to genome-wide association studies. Pharmacogenomics. 2021;22(17):1143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]