Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-related coronavirus disease 2019 (COVID-19) manifests with symptoms ranging from fully asymptomatic to severe disease. Many risk factors have been identified in the progression of COVID-19 into a severe stage, including old age, male gender, underlying comorbidities, immunodeficiencies, and pregnancy.1 The neutralizing antibodies targeting the Spike protein Casirivimab/Imdevimab2 and Sotrovimab,3 and the protease inhibitor Nirmatrelvir4 have been shown to reduce the risk of COVID-19-related hospitalization and death in patients with mild-to-moderate COVID-19 and who are at high risk for progression. However, those studies conducted between Sept. 2020 and Dec. 2021 (during the Alpha and Delta waves) did not assess the effect of those investigational therapeutic agents on the Omicron BA.1 (B.1.1.529) variant of concern (VOC) that emerged in Nov. 2021 before becoming dominant in France, and worldwide.5 Compared to Delta, Omicron was shown to totally escape the antibody cocktail Casirivimab/Imdevimab, while Sotrovimab, targeting a receptor binding domain epitope outside the ACE2-binding site, retains (although reduced) an in vitro activity.6 Clinical efficiency of Sotrovimab to prevent COVID-19 related complications in high-risk patients with mild-to-moderate COVID-19 Omicron remains unknown. Our aim was to compare the outcome of Omicron and Delta-infected patients who received respectively Sotrovimab and Casirivimab/Imdevimab.
Our study is based on the ANRS 0003S COCOPREV study (NCT04885452), an ongoing multicentric prospective cohort study that includes patients considered to be at high-risk for progression to severe COVID-19, having PCR-proven mild-to-moderate COVID-19 in the first five days of symptoms, and who are treated under an emergency use authorization (EUA) in one of the 32 participating centers. Treatment initiation, based on French Health Authorities recommendation, was left at the treating physician discretion. Delta-infected patients included in the cohort were treated by 600/600 mg or 1200/1200 mg of Casirivimab/Imdevimab IV (from Sept 21th 2021 to Jan 14th 2022), while Omicron-infected patients received 500 mg of Sotrovimab IV (from Jan 24th until Mar 3rd 2022). The primary outcome was the proportion of patients with COVID-19-related hospitalization or death within one month of treatment administration. Secondary outcome was the slope of the change over time in the cycle threshold (Ct) value assessed by nasopharyngeal PCR. Baseline characteristics, clinical and virological outcomes (including the slope of the change in the Ct value) were compared between Delta-infected patients who received Casirivimab/Imdevimab and Omicron-infected patients who received Sotrovimab. Mixed effect models were used to estimate the temporal dynamics of the Ct value. Ethical approval was obtained (Comité de Protection des Personnes SUD-EST IV) and all patients provided a written informed consent.
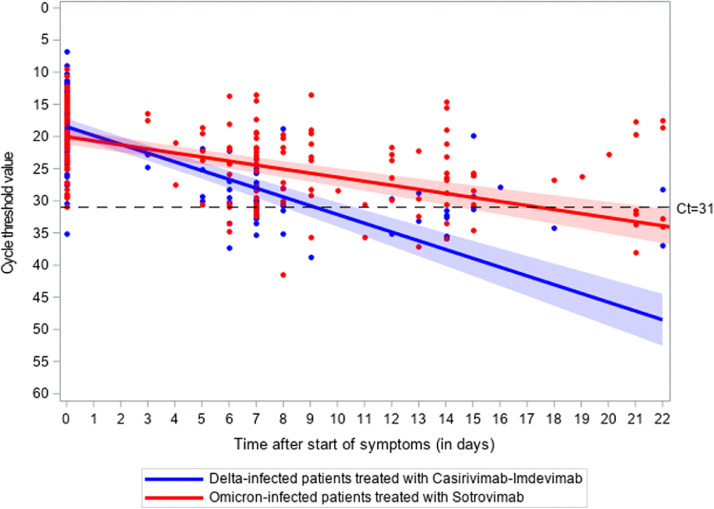
Among the 249 patients analyzed, 133 Delta-infected patients received Casirivimab/Imdevimab (53%) and 116 Omicron-infected patients received Sotrovimab (47%), with a median time between first symptoms and administration of 3 days (Q1-Q3 2–4) (Table 1 ). All had mild-to-moderate COVID-19, and risk factors for severe COVID-19, including being > 65 years-old (76/249, 31%) and being immunocompromised (191/249, 77%). Patients who received Sotrovimab were more often fully vaccinated (≥ 3 doses, 77/116, 77%) than patients who received Casirivimab/Imdevimab (56/133, 54%, p = 0.002), and had higher median IgG anti-Spike serum level (408 BAU/mL (Q1-Q3: 95–3735) versus 148 BAU/mL (Q1-Q3 41–449), p = 0.03). Among patients with available data at the 28th day visit after treatment administration, respectively 3/129 (2% - 95% Clopper-Pearson CI: 1%−7%) and 2/63 (3% - 95% Clopper-Pearson CI: 0%−11%) patients who received Casirivimab/Imdevimab or Sotrovimab were hospitalized due to COVID-19, and none died. All were immunocompromised patients. Median time between administration of Casirivimab/Imdevimab or Sotrovimab and hospitalization was respectively 2 days (Q1-Q3: 1–16) and 4 days (Q1-Q3: 0–7). The slope of Ct values, adjusted on immunosuppressive conditions, was lower in patients who received Sotrovimab (n = 75) than in patients who received Casirivimab/Imdevimab (n = 66) (p <0.0001, Fig. 1 ). No major side effect was reported by the treating physicians.
Table 1.
Baseline characteristics of patients and outcomes at the 28th day visit.
| Baseline characteristics | AllN = 249 | Delta-infected patients who received Casirivimab/Imdevimab N = 133 (53%) | Omicron-infected patients who received Sotrovimab N = 116 (47%) | p-value |
|---|---|---|---|---|
| Age (median, Q1-Q3) | 54 (43–67) | 54 (42–67) | 55 (44–68) | 0.56 |
| ≥65 years (%) | 76 (31) | 41 (31) | 35 (30) | |
| ≥80 years (%) | 20 (8) | 11 (8) | 9 (8) | |
| Male sex (%) | 126 (51) | 71 (53) | 55 (47) | 0.35 |
| BMI (median, Q1-Q3) | 21 (18–25) | 22 (18–25) | 21 (19–25) | 0.73 |
| Immunocompromised patients (%), including: | 191 (77) | 102 (77) | 89 (77) | 0.99 |
| Solid organ transplantation (%) | 77 (40) | 41 (40) | 36 (40) | 0.97 |
| Immunosuppressive therapy including rituximab (%) | 74 (39) | 42 (41) | 32 (36) | 0.46 |
| Ongoing chemotherapy for cancer or hematological malignancies (%) | 33 (17) | 15 (15) | 18 (20) | 0.31 |
| Corticosteroids >10 mg/day for > 2 weeks (%) | 25 (13) | 17 (17) | 8 (9) | 0.12 |
| Systemic lupus or vasculitis with immunosuppressive medications (%) | 11 (6) | 6 (6) | 5 (6) | 0.93 |
| Allogeneic hematopoietic stem cell transplantation (%) | 7 (4) | 2 (2) | 5 (6) | 0.25 |
| Kidney failure with GFR < 30 mL/min or dialysis (%) | 6 (3) | 6 (6) | – | – |
| Other immunosuppressive conditions (%) | 2 (1) | 1 (1) | 1 (1) | – |
| Other risk factors for severe COVID-19 (%) | 106 (43) | 48 (36) | 58 (50) | 0.03 |
| Diabetes (type 1 and type 2,%) | 34 (32) | 18 (38) | 16 (28) | 0.28 |
| Obesity (BMI>30,%) | 30 (28) | 17 (35) | 13 (22) | 0.14 |
| Chronic kidney disease (%) | 24 (23) | 9 (19) | 15 (26) | 0.38 |
| High blood pressure (%) | 24 (22) | 12 (25) | 12 (21) | 0.60 |
| COPD and chronic respiratory failure (%) | 7 (7) | 5 (12) | 2 (3) | 0.24 |
| Congestive heart failure (%) | 5 (5) | 2 (4) | 3 (5) | 0.99 |
| Vaccination status | 0.002 | |||
| Complete (≥ 3 doses) | 133 (66) | 56 (54) | 77 (77) | |
| Incomplete (≤ 2 doses) | 41 (20) | 30 (29) | 11 (11) | |
| Unvaccinated | 29 (14) | 17 (17) | 12 (12) | |
| Known serum IgG anti-Spike status (%) | 106 (43) | 67 (50) | 39 (35) | 0.01 |
| Negative | 57 (55) | 41 (62) | 16 (41) | 0.04 |
| Positive | 48 (45) | 25 (38) | 23 (59) | – |
| Median IgG anti-Spike level (BAU/mL, Q1-Q3) | 169 (79–824) | 148 (41–449) | 408 (95–3735) | 0.03 |
| Gene N Ct value at baseline – median (Q1-Q3) | 19 (15–25) | 17 (15–26) | 23 (18–25) | 0.43 |
| Outcome at day 28 visit | Delta-infected patients who received Casirivimab/Imdevimab N = 129 (97%) | Omicron-infected patients who received SotrovimabN = 63 (54%) | ||
| Changes in symptoms severity | ||||
| Worsening of symptoms (%) | 3 (3) | 3 (5) | ||
| No change in symptoms (%) | 3 (3) | 0 (0) | ||
| Improvement of symptoms (%) | 99 (94) | 55 (95) | ||
| COVID-19–related hospitalization (%) | 3 (2) | 2 (3) | ||
| Death from any cause (%) | 0 | 0 |
Missing values: BMI: n = 28 (25 in the Casirivimab - Imdevimab group and 3 in the Sotrovimab group); Median time between symptoms onset and administration of MAbs: n = 17 (10 in the Casirivimab - Imdevimab group and 7 in the Sotrovimab group); Vaccination status: n = 46 (30 in the Casirivimab - Imdevimab group and 16 in the Sotrovimab group); Results of the serum IgG anti-Spike status: n = 1 (in the Casirivimab - Imdevimab group); Gene N Ct value at baseline: n = 21 (14 in the Casirivimab - Imdevimab group and 7 in the Sotrovimab group); Median IgG anti-Spike: n = 14 (10 in the Casirivimab - Imdevimab group and 4 in the Sotrovimab group); Changes in symptoms severity at day 7: n = 13 (5 in the Casirivimab - Imdevimab group and 8 in the Sotrovimab group); Changes in symptoms severity at day 28: n = 29 (24 in the Casirivimab - Imdevimab group and 5 in the Sotrovimab group).
Fig. 1.
Change in Ct value of gene N in 66 Delta-infected patients treated with Casirivimab/Imdevimab and in 75 Omicron-infected patients treated with Sotrovimab. The p-value for the slope difference is < 0.0001.
In this prospective real-life cohort study that included mainly severely immunocompromised patients with mild-to-moderate COVID-19, early administration of Sotrovimab in Omicron-infected patients was associated with a low rate of COVID-19-related hospitalization within one month after treatment administration, and with no death.
A recent meta-analysis, in which 3309 patients with mild-to-moderate COVID-19 received neutralizing antibodies, and 2397 patients received a placebo, reported a significant reduction of COVID-19-related hospitalization (OR 0.24; 95%CI 0.17−0.34), and death (OR 0.16; 95%CI 0.05−0.58).7 However, most patients were middle-aged non-immunocompromised, having obesity or diabetes as risk factors for severe COVID-19, and at a time period where Omicron did not circulate. Ex vivo inhibition of Omicron and Delta variants by sera obtained from unvaccinated patients treated with Casirivimab/Imdevimab and Sotrovimab showed that only Sotrovimab was active against the Omicron variant, while Casirivimab/Imdevimab neutralized very efficiently the Delta variant. Here we provide evidence that Sotrovimab protected very high-risk Omicron-infected patients from COVID-19 progression to the same extent as did Casirivimab/Imdevimab for Delta-infected patients. However, differences in viral clearance highlight in vivo the lower in vitro neutralization capacity of Sotrovimab on the Omicron variant, compared to Casirivimab/Imdevimab on the Delta variant.6
Two parameters may however mitigate the interpretation of our results. First, compared with the Delta variant, infection with the Omicron variant was associated with a lower severity.8 We would nevertheless argue that our population with a high proportion of severely immunocompromised patients would still exhibit a high likelihood of severe COVID-19 without intervention. Second, the proportion of Omicron-infected patients who received a booster vaccine dose was higher. However, among patients with a known serum IgG anti-Spike status at baseline, 41% were negative, and among those who were positive, the median IgG anti-Spike level might be considered as low.
In conclusion, Sotrovimab was found to effectively protect from progression very high-risk Omicron-infected patients with mild-to-moderate COVID-19. However, emergence of Omicron BA.2 that contains compared to BA.1 eight unique mutations in the spike protein, and which was shown to escape Sotrovimab,9 may abrogate this protection, highlighting the urgent need for availability of therapeutic strategies that could adequately treat all sublineages of the Omicron variant, and future emerging variants.
Funding
The ANRS0003S COCOPREV cohort is conducted with the support of ANRS│MIE and funded by French ministries: Ministère desSolidarités et de la Santé and Ministère de l'Enseignement Supérieur, de la Recherche et de l'Innovation»
Ethical approval
This study received the ethical approval of the Comité de Protection des Personnes SUD-EST IV).
Transparency declaration
GM and YY (the manuscript's guarantors) affirm that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Data sharing statement
Data available upon request for academic researchers
CRediT authorship contribution statement
Guillaume Martin-Blondel: Conceptualization, Data curation, Formal analysis, Writing – original draft. Anne-Genevieve Marcelin: Conceptualization, Data curation, Formal analysis, Writing – original draft. Cathia Soulié: Conceptualization, Data curation, Formal analysis, Writing – original draft. Sofia Kaisaridi: Conceptualization, Data curation, Formal analysis, Writing – original draft. Clovis Lusivika-Nzinga: Conceptualization, Data curation, Formal analysis, Writing – original draft. Céline Dorival: Conceptualization, Data curation, Formal analysis, Writing – original draft. Laura Nailler: Conceptualization, Data curation, Formal analysis, Writing – original draft. Anaïs Boston: Conceptualization, Data curation, Formal analysis, Writing – original draft. Cléa Melenotte: Writing – review & editing. Géraldine Gaube: Writing – review & editing. Christophe Choquet: Writing – review & editing. Roland Liblau: Conceptualization, Writing – review & editing. Fabrice Carrat: Conceptualization, Data curation, Formal analysis, Writing – original draft. Youri Yordanov: Conceptualization, Data curation, Formal analysis, Writing – original draft.
Declaration of Competing Interest
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Acknowledgments
We thank Pr Yazdan Yazdanpanah and all the ANRS-MIE team for their invaluable support and help.
This study would have not been possible without the teams involved in the COCOPREV Study and designated as the COCOPREV Study Group: Magali Garcia, Valentin Giraud, Agathe Metais, France Cazenave-Roblot, Jean-Philippe Martellosio (CHU de Poitiers); Anne-Marie Ronchetti, Thomas Gabas, Naima Hadjadj, Célia Salanoubat, Amélie Chabrol, Pierre Housset, Agathe Pardon, Anne-Laure Faucon, Valérie Caudwell, Latifa Hanafi (CHU Sud Francilien, Corbeil-Essonne); Laurent Alric, Grégory Pugnet, Morgane Mourguet, Eva Bories, Delphine Bonnet, Sandrine Charpentier, Pierre Delobel, Alexa Debard, Colleen Beck, Xavier Boumaza, Stella Rousset (CHU de Toulouse); Fanny Lanternier, Claire Delage, Elisabete Gomes Pires, Morgane Cheminant, Nathalie Chavarot (Hôpital Necker, Paris); Anthony Chauvin, Xavier Eyer; Véronique Delcey (Hôpital Lariboisière, Paris); Simon Bessis, Romain Gueneau (Hôpital du Kremlin Bicêtre); Pelagie Thibaut, Marine Nadal, Martin Siguier, Marwa Bachir, Christia Palacios (Hôpital Tenon, Paris); Valérie Pourcher, Antoine Faycal, Vincent Berot, Cécile Brin, Siham Djebara, Karen Zafilaza, Stephane Marot, Sophie Sayon, Valentin Leducq (Hôpital de la Pitié Salpétrière,Paris); Karine Lacombe, Yasmine Abi Aad, Thibault Chiarabini, Raynald Feliho, Nadia Valin, Fabien Brigant, Julien Boize, Pierre-Clément Thiébaud, Marie Moreau, Charlotte Billard (Hôpital St Antoine, Paris), Nathalie De Castro, Geoffroy Liégeon, Blandine Denis, Jean-Michel Molina, Lucia Etheve (Hôpital Saint Louis, Paris); André Cabié, Sylvie Abel, Ornella Cabras, Karine Guitteaud, Sandrine Pierre-François (CHU de Martinique); Vincent Dubee, Diama Ndiaye, Jonathan Pehlivan, Michael Phelippeau, Rafael Mahieu (CHU d'Angers); Charles Cazanave (CHU de Bordeaux); Alexandre Duvignaud, Thierry Pistone, Arnaud Desclaux, Didier Neau, Jean-François Faucher, Benjamin Festou, Magali Dupuy-Grasset, Véronique Loustaud-Ratti, Delphine Chainier (CHU de Limoges); Nathan Peiffer-Smadja, Olivia Da Conceicao, Michael Thy, Lio Collas, Cindy Godard, Donia Bouzid, Vittiaroat Ing, Laurent Pereira, Thomas Pavlowsky, Camille Ravaut (Hôpital Bichat, Paris); Antoine Asquier-Khati, David Boutoille, Marie Chauveau, Colin Deschanvres, François Raffi (CHU de Nantes); Audrey Le Bot, Marine Cailleaux, François Benezit, Anne Maillard, Benoit Hue, Pierre Tattevin (CHU de Rennes); François Coustilleres, Claudia Carvalho-Schneider, Simon Jamard, Laetitia Petit, Karl Stefic (CHU de Tours); Natacha Mrozek, Clement Theis, Magali Vidal, Leo Sauvat, Delphine Martineau (CHU de Clermond-Ferrand); Benjamin Lefèvre, Guillaume Baronnet, Agnès Didier (CHRU de Nancy); Florence Ader, Thomas Perpoint, Anne Conrad, Paul Chabert, Pierre Chauvelot (CHU de Lyon); Aurélie Martin, Paul Loubet, Julien Mazet, Romaric Larcher, Didier Laureillard (CHU de Nîmes); Mathilde Devaux (Hôpital de Poissy); Jérôme Frey, Amos Woerlen, Aline Remillon, Laure Absensur-Vuillaume, Pauline Bouquet (CHU de Metz); Albert Trinh-Duc, Patrick Rispal (Hôpital d'Agen); Philippe Petua, Julien Carillo (Hôpital de Tarbes); Aurore Perrot, Karen Delavigne, Pierre Cougoul, Jérémie Dion, Odile Rauzy (Oncopole, Toulouse) Yazdan Yazdanpanah, Ventzislava Petrov-Sanchez, Alpha Diallo, Soizic Le Mestre, Guillaume Le Meut (ANRS-MIE); Isabelle Goderel, Frédéric Chau, Brahim Soltana, Jessica Chane Tang (IPLESP), Jeremie Guedj (Université de Paris, IAME, INSERM, Paris), Yvanie Caille (Renaloo)
References
- 1.Laura S., Jérémie B., Jérôme D., François C., Rosemary D.S., Alain W., et al. Chronic diseases, health conditions and risk of COVID-19-related hospitalization and in-hospital mortality during the first wave of the epidemic in France: a cohort study of 66 million people. Lancet Reg Health Eur. 2021;8 doi: 10.1016/j.lanepe.2021.100158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinreich D.M., Sumathi S., Thomas N., Shazia A., Haitao G., Rafia B., et al. REGEN-COV antibody combination and outcomes in outpatients with Covid-19. N Engl J Med. 2021;385(23):e81. doi: 10.1056/NEJMoa2108163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anil G., Yaneicy G.R., Erick J., Manuel C.C., Jaynier M., Diego R.F., et al. Effect of Sotrovimab on hospitalization or death among high-risk patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2022 doi: 10.1001/jama.2022.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jennifer H., Heidi L.T., Annie G., Paula A., Weihang B., Wayne W., et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med. 2022 doi: 10.1056/NEJMoa2118542. null. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.James H., Colin M., Bell Sidney M., John H., Barney P., Charlton C., et al. Nextstrain: real-time tracking of pathogen evolution. Bioinformatics. 2018;34(23):4121–4123. doi: 10.1093/bioinformatics/bty407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delphine P., Nell S., Piet M., Florence G.B., Cyril P., Julian B., et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature. 2022;602(7898):671–675. doi: 10.1038/s41586-021-04389-z. [DOI] [PubMed] [Google Scholar]
- 7.Wei-Ting L., Shun-Hsing H., Chih-Cheng L., Cheng-Yi W., Chao-Hsien C. The impact of neutralizing monoclonal antibodies on the outcomes of COVID-19 outpatients: a systematic review and meta-analysis of randomized controlled trials. J Med Virol. 2022;94(5):2222–2229. doi: 10.1002/jmv.27623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donia B., Benoit V., Christian K., Asma D., Florent F., Christelle H., et al. Comparison of patients infected with Delta versus Omicron COVID-19 variants presenting to Paris emergency departments. Ann Intern Med. 2022 doi: 10.7326/M22-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sho I., Lihong L., Yicheng G., Liyuan L., Chan Jasper F.W., Yiming H., et al. Antibody evasion properties of SARS-CoV-2 Omicron sublineages. Nature. 2022;1 doi: 10.1038/s41586-022-04594-4. [DOI] [PMC free article] [PubMed] [Google Scholar]