Abstract
Background
Propofol is increasingly used for sedation during colonoscopy, with many recent reports of randomised controlled trials (RCTs) and large non‐randomized case series.
Objectives
The primary objective was to identify, analyze and summarize RCTs comparing the relative effectiveness, patient acceptance and safety of propofol for colonoscopy, to traditional sedatives (narcotics and/or benzodiazepines). The secondary objective was to synthesize the studies comparing propofol administration by anaesthesiologists to that by non‐anaesthesiologists for sedation during colonoscopy.
Search methods
We searched Pubmed, Cancerlit, EMBASE, CINAHL, LILACS, Biological Abstracts, Web of Science and the Cochrane Controlled Trials Registry database between January 1980 and June 2007; and conference proceeding abstracts for DDW, EUGW and ACG between 1990 and June 2007. There were no language restrictions. For this update, searches were repeated for articles and abstracts published between July 2007 and December 2010.
Selection criteria
Randomized controlled trials comparing use of propofol and traditional agents or administration of propofol by anaesthesiologists to that by non‐anesthesiologists for sedation during colonoscopy.
Data collection and analysis
Two reviewers independently extracted the data. The data were pooled using the Cochrane Collaborations' methodology and statistical software RevMan 5.1.
Main results
Twenty two studies met the inclusion criteria for the primary objective in this updated review, in which we have included results from three additional publications. Many of the studies had a potential of moderate to high risk of bias and combing data for some of the outcomes was problematic. Most studies included only healthy out‐patients. Recovery (11 studies; 776 patients) and discharge times (7 studies; 542 patients) were shorter with use of propofol. There was higher patient satisfaction with use of propofol (10 studies, 819 patients; OR for dissatisfaction 0.35, 95% CI 0.23, 0.53). There was no difference in procedure time (9 studies; 736 patients) or complication rates. There was no difference in pain control with non‐ patient controlled sedation (PCS) use of propofol as compared to the traditional agents (5 studies, 396 patients; OR 0.90; 95% CI 0.58, 1.39).
There was only one study (94 patients) comparing administration of propofol by anaesthesiologists to that by non‐anesthesiologists for sedation during colonoscopy, with no difference in procedure time or patient satisfaction.
Authors' conclusions
Propofol for sedation during colonoscopy for generally healthy individuals can lead to faster recovery and discharge times, increased patient satisfaction without an increase in side‐effects. For the comparison of propofol administration by anaesthesiologists to that by non‐anesthesiologists, we found insufficient high quality evidence. There is a need for better quality studies, with double blind randomizations, reporting of allocation concealment and more standardized reporting of outcomes.
Plain language summary
Propofol for sedation during colonoscopy can lead to faster recovery after the procedure and higher patient satisfaction.
Irrespective of the initial screening test, colonoscopy is the final step in colorectal cancer screening. With the advent of the colorectal cancer screening programs in many countries, an increasing number of colonoscopies are being performed each year. Sedation for colonoscopy can improve patients' tolerance of the procedure and enhance colonoscopy completion rates. There is no consensus on the preferred drugs for sedation during colonoscopy. This review found that use of propofol for sedation during colonoscopy can lead to faster recovery after the procedure and higher patient satisfaction, without any increase in side‐effects as compared to the use of drugs traditionally used (narcotics and/or benzodiazepines) for sedation during colonoscopy.
Background
Most patients prefer the use of sedation and analgesia during colonoscopy (Subramanian 2005). The use of sedatives can improve the performance of colonoscopy and enhance colonoscopy completion and colonic polyp detection rates (Radaelli 2008). Traditionally, sedation during colonoscopy has been provided by the combination of a narcotic and a benzodiazepine. In recent years propofol (2, 6‐di‐isopropylphenol) has increasingly been utilized as an alternative method of sedation in endoscopy suites (Faulx 2005). Propofol was initially introduced in 1989 and has since then been widely used in critical care units and emergency departments for providing sedation. Although, propofol is associated with a more rapid onset of action, its use for sedation during endoscopy by non‐anesthesiologists in many parts of the world (particularly North America) has been limited by concerns of potential side‐effects. Large case series suggest that nurses and endoscopists can safely administer propofol for endoscopic procedures (Rex 2005; Walker 2003). There have been several small randomised controlled trials (RCTs) comparing propofol with traditional agents for sedation during colonoscopy (Bright 2003; Hansen 2004; Kostash 1994; Kulling 2001; Lee 2002; Lee 2002a; Moerman 2003; Ng 2001; Paspatis 2002; Reimann 2000; Roseveare 1998; Rudner 2003; Sipe 2002; Ulmer 2003). However these trials could have individually failed to detect significant differences in side‐effects, due to lack of adequate sample size and power. Moreover, although individual trials demonstrated faster recovery with propofol, the magnitude of the benefit varied in different trials. A recent systematic review summarized the potential benefits of use of propofol for sedation during colonoscopy and esophagogastroduodenoscopy (EGD) (McQuaid 2008). However, this review was limited to full publications in Medline and Embase and hence may have excluded negative studies, due to the publication bias. The prior review had several other exclusion criteria and the authors did not perform meta‐analysis for sedation for colonoscopy alone. There is no prior systematic review of studies comparing the administration of propofol by anaesthesiologists to that by non‐anesthesiologists for sedation during endoscopy.
Objectives
The primary aim of this review is to assess the efficacy and safety of propofol to traditional agents (narcotics and/or benzodiazepines) for sedation during colonoscopy.
Secondary, to identify and summarize the data from randomised controlled trials comparing the administration of propofol by anaesthesiologists to non‐anesthesiologists for sedation during colonoscopy.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled studies comparing 1) Use of propofol vs. traditional agents for sedation during colonoscopy OR 2) Delivery of propofol by anaesthesiologists vs. non‐anesthesiologists for sedation during colonoscopy
Types of participants
All adult patients undergoing colonoscopy
Types of interventions
1) Propofol vs. traditional agents for sedation during colonoscopy 2) Anesthesiologist vs. non‐anaesthesiologist administration of propofol for sedation during colonoscopy.
Types of outcome measures
Technical performance of colonoscopy: cecal intubation rate, time required for performing the procedure, post procedure recovery and discharge time and sedation level Patient satisfaction and pain control Complication rates: cardio respiratory events (hypoxia, apnea, hypoxia requiring intervention, hypotension, arrhythmias), colonic perforations and hospital admission rate after procedure (when procedure performed in ambulatory care setting) and death.
Search methods for identification of studies
This review is an update of the previously published Cochrane Collaboration systematic review (First published in the Cochrane Library 2008 Issue 4). The following electronic databases were searched: PubMed (National Library of Medicine), EMBASE, CINAHL, LILACS, Biological Abstracts, Web of Science and the Cochrane Central Register of Controlled Trials from January 1980 to June 2007 for the initial review. The year 1980 was selected as the cut‐off as propofol was discovered in 1970's and introduced in clinical practice in 1980's (Marik 2004). For the update, the search was repeated for articles published between July 2007 and December 2010. The following search strategy was used for PubMed and adapted to suit the other databases: 1. Colonoscopy [MeSH] 2. colonoscop* OR sigmoidoscop* 3. #1 OR #2 4. Propofol [MeSH] 5. propofol OR diisoprofol OR diprivan OR disoprivan OR disoprofol OR rapinovet OR recofol OR diisopropylphenol 6. #4 OR #5 7. #3 AND #6
Owing to the relatively small number of articles in this field, we omitted using an automated RCT filter in the search strategy; instead we chose to use the more sensitive approach of confirming RCTs manually.
Using the same terms, we hand searched the indices of conference abstracts from Digestive Diseases Week, the American College of Gastroenterology and the United European Gastroenterology Week between 1990 and 2007, published in the journals Gastroenterology, Gastrointestinal Endoscopy, American Journal of Gastroenterology, Gut and Endoscopy. Conference abstracts for the United European Gastroenterology Week for the years 1994 and 1996 could not be found and are not included. We also searched the reference lists of eligible RCTs. For the update, the search was repeated for the abstracts published between July 2007 and December 2010.
Data collection and analysis
Two investigators (HS, NC for the initial review and HS, EI for the update) reviewed the titles and abstracts of retrieved studies to identify randomised controlled trials involving use of propofol during colonoscopy that met the inclusion criteria for the type of interventions and participants listed above. Full text manuscripts of potentially relevant studies were then reviewed and data abstracted by two (HS, NC for the initial review and HS, EI for the update) authors working independently of each other. The potentially relevant non‐English articles were translated by other health care professionals at University of Manitoba. Any discrepancy between the reviewers was resolved by joint re‐review of the studies and discussion with a third author (ST). All potentially eligible, but ultimately excluded trials are listed in the final report, along with the reasons for exclusion. The 2010 search of electronic databases identified 145 citations, from which two additional publications (Mandel 2008a, Liu 2009) were identified for inclusion in the review. One of these two additional publications was the full report of a study previously included as an abstract in the prior review (Mandel 2006a). In addition, an additional abstract was identified and included in the review (Amornyotin 2010)
Data abstraction: The following data were recorded using a customized data extraction form:
1) Interventions evaluated
2) Sample size, number of participants randomised
3) Study site(s), including whether the colonoscopy was performed as an in‐patient or in an ambulatory care setting
4) Inclusion and exclusion criteria
5) Baseline characteristics of the study groups
6) Mode of administration of the drugs‐‐‐bolus, infusion or mixture of both
7) Use of supplementary oxygen as part of the study protocol for all participants in the trial
8) Technical performance of colonoscopy
a) Recovery from sedation
i) How the recovery time was defined; mean time and standard deviation in each group
ii) If a measurement scale was used, the scale used was recorded; mean and standard deviation on the scale in each group
b) Discharge from the endoscopy unit: How the discharge time was defined; mean time and standard deviation in each group
c) Procedure duration: mean and standard deviation in each group
d) Procedural completion: Number of patients in whom cecum could be reached in each group
9) Overall patient satisfaction outcome‐ Mean score in each group and standard deviation of score in each group
10) Patient pain control outcome‐Mean pain score in each group and standard deviation of pain score in each group
11) Complication rates: Number in each group who had the following complication
a) Hypoxia (oxygen saturation less than 90%)
b) Respiratory depression requiring an intervention other than an increase in the rate of oxygen administered
c) Apnea
d) Hypotension (systolic blood pressure less than 90). Mean (and standard deviation) drop in blood pressure or lowest blood pressure during the procedure in each group was noted
e) Arrhythmia (author defined, heart rate < 50/min or > 120/min)
f) Colonic perforations
g) Hospital admission after procedure (when procedure performed in ambulatory care setting)
h) Death
12) Sedation level/scores‐ For the maximum levels of sedation in each group, the mean score as well as the standard deviation.
The description of the measurement scales used was extracted. In the studies where the standard deviation was not recorded, standard deviation was calculated from other measures of variance using the methodology suggested by the Cochrane collaboration.
Study quality assessment: 1) Allocation concealment: adequate, unclear, inadequate or not used 2) Blinding: whether the patients, endoscopists and outcome assessors were unaware of assigned intervention? 3) Was there a description of withdrawals and dropouts?
Data analysis: Overall patient satisfaction, patient pain control, procedural time and recovery time after the procedure were evaluated as continuous outcomes. Standardized mean difference (SMD) was calculated for the overall patient satisfaction, pain control and sedation level, as different measurement scales were used for these outcomes in the different studies. Weighted mean differences (WMD) were calculated for the procedural and recovery times and the maximum drops in blood pressure. Both WMD and SMD were calculated for discharge times. Both fixed effect method (Inverse Variance approach) and random effects method (DerSimonian and Laird approach ‐ DerSimonian 1986) were used.
Odds ratios (ORs) were calculated for the dichotomous variables: procedural completion rate and individual complications. Both fixed effects model (Mantel‐Haenszel ‐ Mantel 1959) and random effects model (DerSimonian and Laird method‐ DerSimonian 1986) were examined.
Some studies reported patient satisfaction and pain control as categorical events; others on continuous measurement scales, such as visual analogue scales (VAS). When possible, the categorical outcomes were dichotomized. The studies with dichotomous and continuous outcomes were initially analysed separately, and then combined to calculate aggregate measures; generic inverse variance method was used to pool the data and results expressed as odds ratios, using the methodology suggested by the Cochrane collaboration.
Results are reported using random effect methods.
Forest plots are used to display the mean difference and ORs of individual studies.
Heterogeneity between the studies was evaluated with the Q statistic. Significant heterogeneity was defined as p<0.10. Possible sources of the encountered heterogeneity including differences in the conduct of trials were explored. Pre‐specified sensitivity analysis was performed by excluding studies published in only abstract form and by excluding studies which utilized patient‐controlled sedation (PCS). Sensitivity analysis was also performed excluding the single study, in which anaesthesia was induced with propofol (Moerman 2003). The alternate effect measure of SMD was considered for the outcomes (such as discharge times) defined differently in different studies.
Pre‐specified sub group analysis, was performed for the studies which involved propofol alone in one of the arms of the study and studies which evaluated propofol in combination with other agents. Review Manager Software version 4.2.10 was used for data management and analysis for the initial review and 5.1.2 for the update. Review Manager Software excludes studies with no events in both groups of a study from the meta‐analysis; as such studies do not provide any information about relative probabilities of the events.
Results
Description of studies
Of the 412 citations, 22 studies met the inclusion criteria for our primary objective. There was only one study, reported in abstract form comparing propofol administration by anaesthesiologists to that by gastroenterologists (Laquiere 2006). Eight studies evaluated propofol as a single agent. Seven studies have been published in only abstract format, including studies from 1994, 2000 and 2003 and the largest study (n=7,286 patients), which reported on different rates of colonic perforation. The abstract for this largest study (Jimenez‐Perez 2000), which included more patients than all the other studies combined, did not provide information on our other outcomes of interest; we contacted the authors but were unable to obtain additional information.
One of the studies reported higher patient satisfaction, higher colonoscopy completion rate and shorter recovery time with the use of propofol, but did not provide any measures of variance (Martinez‐Palli 2005). Therefore the results from this study were not pooled with other studies. The recovery time from this study is listed in the additional tables, along with recovery times reported in three other studies, which also reported recovery times in a format which could not be combined with the other studies (Kulling 2001, Paspatis 2002,Liu 2009 ). Two of these studies reported on the extent of recovery at specific time intervals after colonoscopy and found faster recovery with use of propofol.
The abstract by Amornyotin 2010 reported less procedural pain, shorter recovery time and higher patient and endoscopist satisfaction, but more cardiovascular and respiratory adverse events with the use of propofol in combination with meperidine than with the use of midazolam and fentanyl. However since the effect size estimates for these secondary outcome measures were not reported, the secondary outcomes from this study could not be pooled with the other studies. Of note, this study reported that all cardiovascular and respiratory adverse events were transient and with no sequelae.
Two studies had three study arms. For the purpose of the meta‐analysis, the results from the two study arms with of use of traditional sedative agents were combined from the first study (Kostash 1994); similarly the results from the two study arms with use of propofol in second study (Kulling 2001) were combined.
Two studies were published in non‐English literature (Germain 1989; Pohlmann 1993). Only one of the studies was performed at more than one facility (Bright 2003). Six studies included patient‐controlled sedation (PCS); propofol was used in combination with another agent in five of these (Kulling 2001; Bright 2003; Roseveare 1998; Liu 2000; Mandel 2006) and as a single agent in the sixth (Ng 2001).
The inclusion criteria varied. However, almost all studies included only healthy out‐ patients. ASA class IV patients were included in two studies (Martinez‐Palli 2005; Paspatis 2002), of which one could not be included in the pooled results (Martinez‐Palli 2005) (as above). One study included hospitalized in‐patients (Pohlmann 1993).
The drugs administered in the traditional sedative comparative arms included benzodiazepines alone (diazepam, midazolam) or a combination of a benzodiazepine and a narcotic (pethidine, fentanyl, remifentanil or alfentanil). One study included only a narcotic (remifentanil) (Moerman 2003) and all patients in the traditional sedative arm of this study remained awake throughout the procedure. The dosage of the drugs used varied.
The intended level of sedation when stated was defined in most studies as that needed for patients tolerance of the procedure. Few studies used an objective scale for the intended level of sedation (Akcaboy 2006; Kostash 1994). The goal was stated as deep sedation with use of propofol in the study, which reported on the incidence of colonic perforations (Jimenez‐Perez 2000). Among the studies included for our primary objective, propofol was used for anaesthesia in a single study (Moerman 2003). In the only study included for our secondary objective of comparing propofol administration by anaesthesiologists to that by gastroenterologists, the anaesthetists administered propofol with the goal of attaining general anaesthesia (Laquiere 2006).
The patients in most studies received supplemental oxygen at a rate of two to four liters per minute, administered through face mask or nasal cannula. In three studies, no oxygen was provided at baseline (Kostash 1994; Ng 2001; Pohlmann 1993).
In the studies for the primary outcome, propofol was administrated by an endoscopist or a nurse supervised by an endoscopist in six (Kulling 2001; Heuss 2003;Liu 2009; Pohlmann 1993; Sipe 2002; Ulmer 2003), anaesthesiologists in five (Akcaboy 2006; Germain 1989; Martinez‐Palli 2005; Moerman 2003; Paspatis 2002), physicians trained in critical care in one (Reimann 2000), PCS in six (Kulling 2001; Bright 2003; Roseveare 1998; Liu 2000; Mandel 2006; Ng 2001) and the person administering the drug was not stated in remaining five (Jimenez‐Perez 2000; Munoz‐Navas 1994; Kostash 1994; Heuss 2005; Amornyotin 2010).
The definition of the outcomes varied. The criteria for determining post procedure recovery varied from the use of objective scoring scales (Alderete score in Akcaboy 2006 and Kostash 1994; Steward score in Moerman 2003; Number connection test in Bright 2003 and Roseveare 1998) to determination of time to ambulate (Reimann 2000). The criteria for determining readiness for discharge was often subjective and at the discretion of the recovery room nurses (Sipe 2002; Ulmer 2003).
The included studies did not report on hospitalization rates or mortality and hence these outcomes were not considered further in the meta‐analysis. Only one study reported colonic perforations among the study subjects (Jimenez‐Perez 2000).
Risk of bias in included studies
Only one of the included studies was double blinded RCT, where all patients as well as all those involved in administering the medications and assessing the outcomes were not aware of the intervention in different arms of the study (Mandel 2006). Quality of allocation concealment was not reported in most studies. Three studies had adequate concealment of allocation (Ulmer 2003; Liu 2009; Mandel 2006). In another study from the same group as the Ulmer 2003, the concealment of allocation may have been inadequate as blocks of four were used and could have been too small to prevent deciphering of sequence (Sipe 2002). Many of the outcomes were subjective and hence there is an increased potential for bias, in absence of double blinded randomizations and concealment of allocation.
Four studies reported on calculation of sample size, a priori (Moerman 2003; Kulling 2001; Liu 2009; Mandel 2006).
The number of eligible patients was not reported in most studies; this could affect the generalizability, if a large number of the eligible patients did not enroll. Five studies reported on the number of eligible patients (Bright 2003; Kulling 2001; Laquiere 2006; Sipe 2002; Liu 2009). One‐ fifth of the eligible patients in one of the three studies comparing propofol to the traditional agents did not enroll (Bright 2003). One‐third did not enroll in the only RCT comparing propofol administration by anaesthesiologists to that by gastroenterologists (Laquiere 2006)
Other than the study interventions, the study groups were treated identically in all studies.
Effects of interventions
Recovery time
Propofol alone
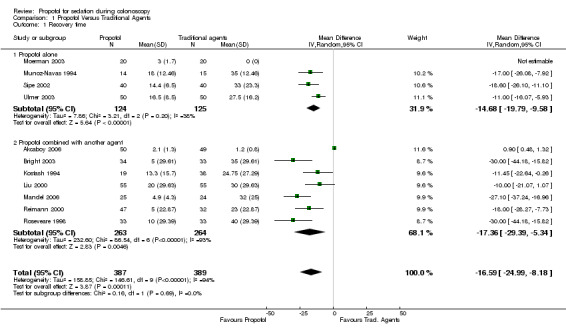
Four studies with 249 patients reported on recovery time with use of propofol as a single agent. Three studies found a shorter mean recovery time with propofol with pooled WMD of ‐14.7 minutes (95% CI ‐ 19.8, ‐9.6) and no significant heterogeneity among the studies (p=0.20). The mean recovery time in the traditional sedative arm of the fourth study was 0 minutes, as the patients were awake through out the procedure; this study in which propofol was administered for anaesthesia (Moerman 2003) could not be combined with the others in the meta‐analysis. In sensitivity analysis, excluding the study reported only in abstract form did not alter the WMD (Munoz‐Navas 1994).
Propofol used in combination with another agent
Six out of the seven studies (527 patients) in this category reported shorter recovery times with use of propofol. The pooled WMD was ‐17.4 minutes (95% CI ‐29.4, ‐5.4); however there was significant heterogeneity (p<0.00001). The study (Akcaboy 2006) which reported shorter recovery times with the traditional agents had very short recovery times in both arms of the study. From the description of this study, the starting point for the calculation of recovery time is not clear, as although the recovery time was defined as the time to reach an Aldrete score of nine in the recovery room, the patients were transferred to recovery unit after achieving an Aldrete score of nine. Excluding this study (hereafter referred to as an outlier) the heterogeneity was reduced, although was still significant (p=0.05) and WMD was ‐20.5 minutes (95% CI ‐27.6,‐13.4). Excluding studies reported in abstract format along with the outlier study, there were five studies with pooled WMD of ‐22.6 minutes (95% CI ‐29.8,‐15.4) and no heterogeneity (p=0.12). In sensitivity analysis, excluding the studies with PCS along with the outlier study, the WMD was ‐15.0 minutes (95% CI ‐22.6,‐7.4) with no heterogeneity (p=0.40) among the two remaining two studies (Kostash 1994; Reimann 2000).
Propofol was administered with another agent in all studies of PCS included in the meta‐analysis for recovery time. Three of the four studies with PCS use of propofol reported significantly shorter recovery times with use of propofol; the WMD for the pooled recovery time from all four was ‐23.8 minutes (95% CI ‐33.6,‐13.9), but with significant heterogeneity (p=0.05). In sensitivity analysis, excluding the PCS study reported in abstract form, the WMD was ‐28.6 minutes (95% CI ‐35.7,‐21.4), with no heterogeneity (p=0.92).
Five studies reported recovery times in formats which could not be combined with the other studies in the meta‐analysis; the recovery time was shorter with use of propofol in combination with another agent in three and with traditional agents in the fourth. Most patients in the fourth study (Liu 2009) were fully conscious during the colonoscopy with traditional agents and hence did not need any time for recovery; the median difference in recovery time in the two groups was 2.5 minutes.
All studies
There was significant heterogeneity when the results from all studies were combined (p<0.0001), which persisted when excluding studies reported in abstract form (p<0.0001). However excluding studies with PCS and the outlier study (Akcaboy 2006), the WMD was ‐14.2 minutes (95% CI ‐17.6,‐10.8) shorter with propofol, with no significant heterogeneity (p=0.41).
Discharge time Propofol alone
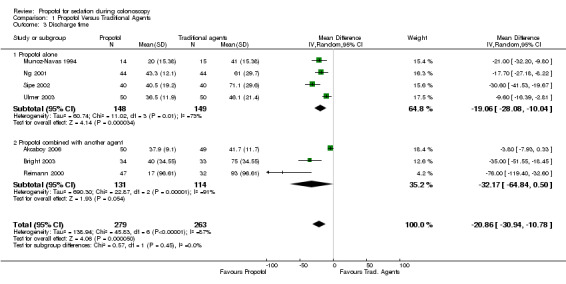
All four studies (297 patients) reporting discharge time, with the use of propofol as single agent in one of the arms of the study, reported shorter discharge times with use of propofol. The WMD was ‐19.1 minutes (95% CI ‐28.1,‐10.0) but with significant heterogeneity (p=0.01). Excluding the studies reported in abstract form (Munoz‐Navas 1994) or with PCS (Ng 2001), did not alter the pooled results or the heterogeneity.
However, as the different trials defined discharge time differently, a more appropriate effect measure may be the Standardized Mean Difference (SMD). The SMD favoured propofol (‐0.90; 95% CI ‐1.24, ‐0.55), with non‐significant heterogeneity (p=0.12).
Propofol in combination with another agent The discharge time was significantly shorter with propofol in two studies and similar in one study (total of 245 patients in three studies); however the pooled WMD was not statistically significant at ‐32.2 minutes (95% CI ‐64.8, 0.50) and with significant heterogeneity (P<0.0001). Excluding the PCS study (Bright 2003) had no effect. Using the possibly more appropriate effect measure of SMD, there was a significantly lower discharge time with propofol containing combinations (SMD ‐0.69; 95% CI: ‐1.07,‐0.31), with insignificant heterogeneity (p=0.13).
All studies
When results from all studies were combined, the discharge time was shorter for propofol (WMD ‐20.9 minutes; 95% CI ‐30.9, 10.8), with significant heterogeneity (p< 0.0001) and no change when excluding the studies reported in abstract form or using PCS. The SMD was ‐0.80 (95% CI ‐1.04,‐0.55) with significant heterogeneity (p=0.08). Excluding the two studies with PCS, the SMD was ‐0.78 (95% CI ‐1.12,‐0.44), with significant heterogeneity in the remaining four studies (p=0.04). Excluding the study reported in the abstract form, SMD was ‐0.76 (95% CI ‐1.00,‐0.56), implying faster discharge with use of propofol as a single agent or in combination with another agent, with borderline heterogeneity (p=0.10).
Procedure duration
Propofol alone
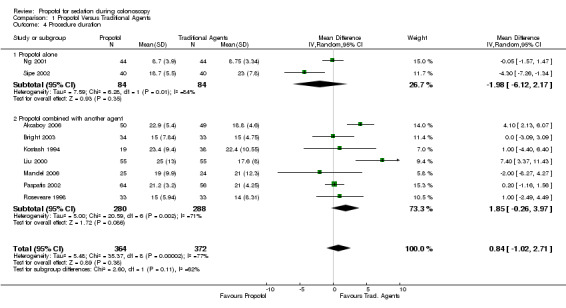
The procedure time was shorter with use of propofol in one study and no different in the other. There was no significant difference on pooling the results from these two studies (168 patients; WMD: ‐1.98 minutes; 95% CI ‐6.12, 2.17 and SMD: ‐0.32; 95% CI ‐0.92, 0.29). There was significant heterogeneity between the two studies (WMD p=0.01; SMD p=0.05).
Propofol in combination with another agent
The procedure time was shorter with traditional agents in two studies and no different in the other five. Another study, which did not provide a measure of variance and hence was not included in the meta‐analysis, also did not find a difference in the procedure times (propofol 18 minutes vs. traditional agents 20 minutes) (Reimann 2000). A second study not included in the meta‐analysis also did not report a difference in the procedure times (median times: propofol 17.5 minutes vs. traditional agents 15.5 minutes; p=0.38) (Liu 2009). On pooling the results, there was no difference in procedure time (WMD 1.85 minutes; 95% CI: ‐0.26, 3.97 and SMD 0.25; 95% CI: ‐0.03, 0.54), with significant heterogeneity (WMD p=0.002 and SMD p=0.09). Sensitivity analysis excluding studies reported in abstract format or using PCS did not alter the pooled results.
All studies
There was no difference in procedure time pooling all studies (WMD 0.84 minutes; 95% CI ‐1.02, 2.71 and SMD 0.12; 95% CI ‐0.18, 0.41), with significant heterogeneity in the results (WMD p =0.0001; SMD p<0.0001). Sensitivity analysis, excluding studies reported in abstract format or using PCS did not alter the pooled results or the heterogeneity.
Cecal intubation/Procedure completion
Propofol alone
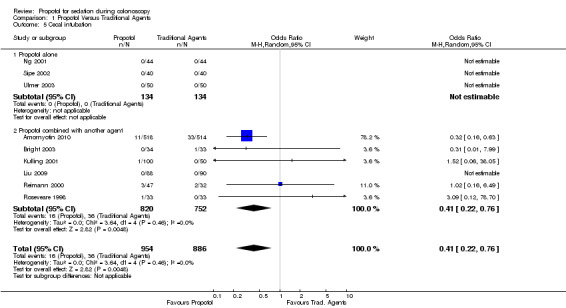
Three studies with 264 patients reported on this outcome and all procedures were completed in both groups.
Propofol in combination with another agent
The procedure completion rate was not significantly different in five of the six studies which reported this outcome, but was significantly higher in the sixth study. On pooling the results, there was a significantly higher completion rate with use of propofol (1,572 patients; OR for failure to complete colonoscopy with use of propofol: 0.41; 95% CI 0.22, 0.76). However on exclusion of the study reported only in the abstract format (1, 032 patients), there was no significant difference in the procedure completion rate with use of propofol in the remaining five studies (540 patients; OR 1.08; 95% CI 0.29, 4.01), with no significant heterogeneity (p=0.80).
All studies When results from all studies were pooled, colonoscopy completion rate was higher with use of propofol (OR for failure to complete colonoscopy with use of propofol: 0.41; 95% CI 0.22, 0.76). However on exclusion of the largest study (n=1,032) reported recently in the abstract format, there was no difference in the procedure completion rate (OR 1.08; 95% CI 0.29, 4.01) with no significant heterogeneity (p=0.80).
Patient satisfaction
Patient satisfaction extracted as dichotomous outcome
Propofol alone
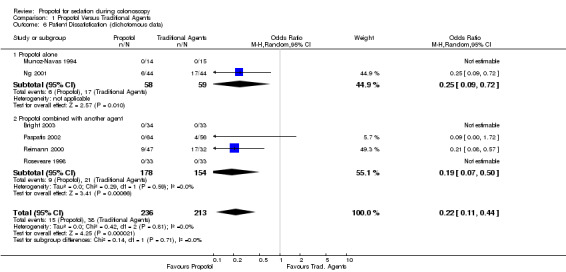
All patients (n=29) were satisfied with the sedation in one study (Munoz‐Navas 1994). In the other (PCS study) a lower proportion of patients were dissatisfied with propofol sedation than with sedation with traditional agents (6/44 vs. 17/44; OR 0.25; 95% CI 0.09, 0.72) (Ng 2001).
Propofol in combination with another agent There were four studies in this category with 332 patients. In two studies, all of the patients were satisfied (PCS studies). In the other two, more patients were satisfied with propofol sedation than with traditional agents (Reimann 2000; Paspatis 2002), with pooled OR for dissatisfaction 0.19 (95% CI: 0.07, 0.50) and no significant heterogeneity (p=0.59). PCS administered propofol was not used in either of these two studies. .
All studies When the results from all studies were pooled, a higher proportion of patients were satisfied with propofol (OR for dissatisfaction: 0.22; 95% CI 0.11, 0.44, with no heterogeneity (p=0.81))
Continuous outcome
Propofol alone
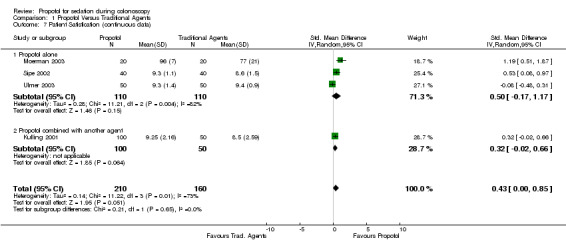
Three studies (220 patients) reported on patient satisfaction using visual analogue scale (VAS). Two reported higher patient satisfaction with propofol and the third found no difference. In the meta‐analysis, there was no difference in patient satisfaction (SMD 0.50; 95% CI: ‐0.17, 1.17), with significant heterogeneity (p=0.004). In sensitivity analysis, excluding the study in which propofol was used for anaesthesia, there was no significant effect on the pooled outcome (SMD 0.21; 95% CI ‐0.39, 0.81) or heterogeneity (p=0.04). All three studies used non‐PCS administration of drugs and have been published as full reports.
Propofol in combination with another agent
A single study with 150 patients did not find a difference in patient satisfaction (Kulling 2001). This study included PCS. All studies
When the results from all studies were pooled, there was no difference in patient satisfaction (SMD 0.43; 95% CI 0.00, 0.85) with significant heterogeneity (p=0.01). The sensitivity analysis, excluding the study where goal of propofol use was anaesthesia, reduced the heterogeneity (p=0.11), with little effect on estimate of patient satisfaction (SMD 0.25; 95% CI ‐0.09, 0.58). Combined dichotomous and continuous outcomes
Propofol alone
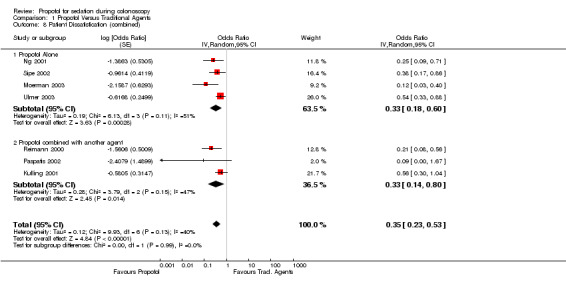
Combining results from studies reporting patient satisfaction as categorical outcome and those reporting as a continuous outcome, pooled results suggested a higher patient satisfaction with propofol (OR for dissatisfaction 0.33; 95% CI 0.18, 0.60), with no significant heterogeneity (p=0.11). In sensitivity analysis, excluding the study, where goal of propofol use was anaesthesia, the patient satisfaction continued to be higher with use of propofol (OR for dissatisfaction 0.45; 95% CI 0.30, 0.66), with little heterogeneity in the remaining studies (p=0.38).
Propofol in combination with another agent
There was higher patient satisfaction with use of propofol (OR for dissatisfaction 0.33; 95% CI 0.14, 0.80), with no heterogeneity (p=0.15).
All studies
Pooled results (7 studies; 657 patients) suggest higher satisfaction with use of propofol (OR for dissatisfaction 0.35, 95% CI 0.23, 0.53) with no heterogeneity (p=0.13). Excluding the two studies with PCS, the satisfaction was higher with propofol (OR 0.19; 95% CI 0.16, 0.55) with borderline heterogeneity among the remaining five studies (p=0.10). The patient satisfaction continued to be higher with use of propofol, even after excluding the study where goal of propofol use was anaesthesia (OR 0.42; 95% CI 0.29, 0.59), with no heterogeneity (p=0.33). Pooled results from the two studies with PCS suggest higher satisfaction with use of propofol (OR for dissatisfaction 0.42, 95% CI 0.20, 0.89) with no significant heterogeneity (p=0.19).
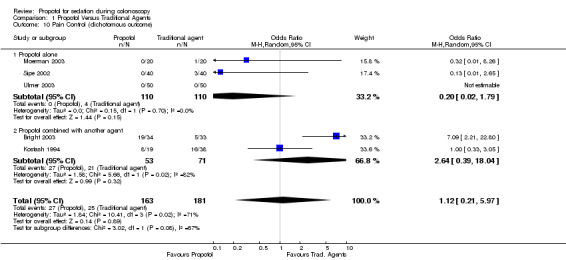
Pain control Dichotomous outcome
Propofol alone
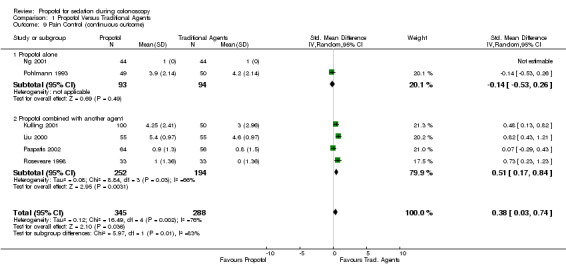
In three studies with 220 patients, the pain control was similar with propofol and traditional agents. There was no significant difference in the pooled results (OR 0.20; 95% CI 0.02,1.79) and no heterogeneity (p=0.70).
Propofol in combination with another agent
The pain control was better with traditional agents, in one study, where propofol was administered by PCS and equivalent in the other, where propofol was administered by bolus dosing. Combining the results from these two studies gave equivalent pain control from propofol and traditional agents (OR 2.64; 95% CI 0.39, 18.04), but as can be expected, there was significant heterogeneity between these two studies (p=0.02). All studies
When the results were pooled from all studies reporting pain control as a dichotomous outcome, there was no difference in the pain control between propofol and traditional agents (OR 1.12; 95% CI 0.21, 5.97), but there was significant heterogeneity (p=0.02). However this heterogeneity disappeared, on excluding the results from the study, in which propofol was given as PCS (OR 0.72; 95% CI 0.27, 1.94) with p for heterogeneity =0.40).
Continuous outcome
Propofol alone In the two studies with 187 patients, pain control was equivalent with propofol and traditional agents; the results could not be combined in meta‐analysis.
Propofol in combination with another agent
In this category, pain control was reported in four studies with 446 patients. Pain control was better with the traditional agents in the three studies (Kulling 2001; Liu 2000; Roseveare 1998), in which propofol was administered as PCS and equivalent in the fourth, in which propofol was administered by bolus injections (Paspatis 2002). In pooled analysis, the pain control was better with traditional agents (SMD 0.51; 95% CI 0.17, 0.84), but with significant heterogeneity (p=0.03). There was no heterogeneity among the three studies in which propofol was administered by PCS (p=0.41), with pooled SMD 0.65 (95% CI 0.04, 0.89) and better pain control with traditional agents. All studies When the results were pooled from all studies reporting pain control as a continuous outcome, the pain control was better with traditional agents (SMD 0.38; 95% CI 0.03, 0.74), but with significant heterogeneity (p=0.002). On restricting to studies with propofol administration by PCS, there was no heterogeneity (p=0.41) and better pain control with traditional agents (SMD 0.65; 95% CI 0.04, 0.89). On excluding studies with PCS, the pain control was equivalent in pooled results from the two remaining studies (SMD ‐0.02; 95% CI ‐0.29, 0.24) with no heterogeneity (p=0.44).
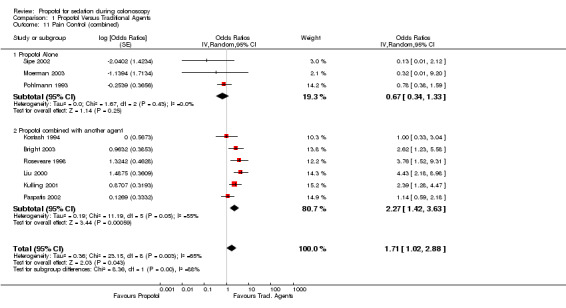
Combined dichotomous and continuous outcomes
Propofol alone
Combined pooled results suggested there was no difference in pain control with propofol as compared to the traditional agents (OR 0.67; 95% CI 0.34,1.33) with no heterogeneity (p=0.43).
Propofol in combination with another agent
Pain control was better with traditional agents than with propofol used in combination with another agent (OR 2.27; 95% CI 1.42, 3.63); however there was significant heterogeneity (p=0.05). In the two studies (Kostash 1994; Paspatis 2002), where propofol was not used as PCS, there was no difference in the pain control with propofol containing combination, as compared to the traditional agents (pooled OR 1.10; 95% CI 0.63,1.93) with no heterogeneity (p=0.85).
In the four studies (Bright 2003; Kulling 2001; Liu 2000; Roseveare 1998) using PCS for propofol administration, there was better pain control with traditional agents than with propofol combination (OR 3.09; 95% CI 2.15‐4.46), with no heterogeneity (p=0.57).
All studies
Pooling results from all studies, that could be pooled together for this outcome (9 studies), there was better pain control with the traditional agents (OR 1.71; 95% CI 1.02,2.88), but with significant heterogeneity (p=0.0006). In sub‐group analysis of studies not involving PCS (five studies), there was no difference in pain control with propofol as compared with the traditional agents (OR 0.90; 95% CI 0.58, 1.39), with no heterogeneity (p=0.58). In studies using PCS (four studies), there was better pain control with traditional agents than with propofol (OR 3.09; 95% CI 2.15, 4.46), with no heterogeneity (p=0.57).
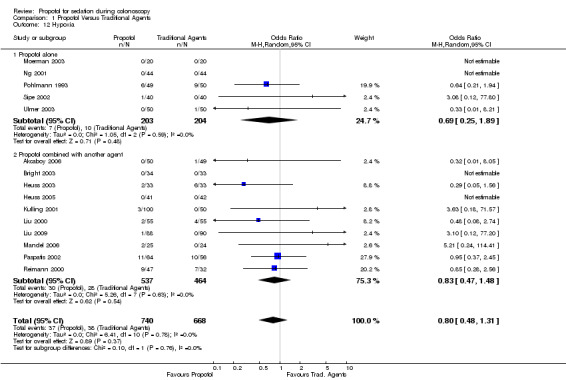
Hypoxia, apnea and respiratory depression requiring intervention
Propofol alone In five studies involving 407 patients, there was no significant difference in the number of patients developing hypoxia (OR 0.69; 95% CI 0.25, 1.89), with no heterogeneity among the studies (p=0.59).
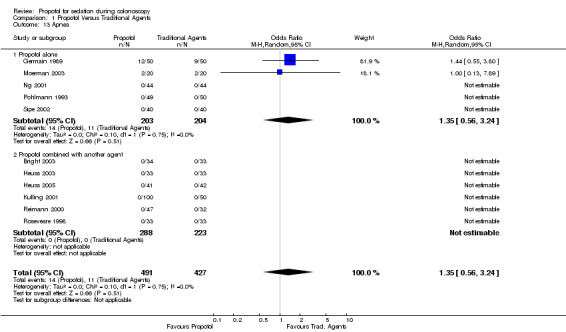
Patients in two studies developed apnea (Germain 1989; Moerman 2003), with no difference among those administered propofol and those administered traditional agents (OR 1.35; 95% CI 0.56, 3.24) with no heterogeneity among these two studies (p=0.75).
One patient, among the three studies reporting on need for a respiratory intervention (other than increased oxygen administration) needed an intervention; there was no statistical significant difference in this study (Ulmer 2003).
Propofol in combination with another agent
In ten studies involving 1001 patients, there was no significant difference in developing hypoxia among those given propofol in combination with another agent, as compared to the traditional sedatives (OR 0.83; 95% CI 0.47,1.48), with no heterogeneity among the studies (p=0.63).
No patient developed apnea in this category of studies.
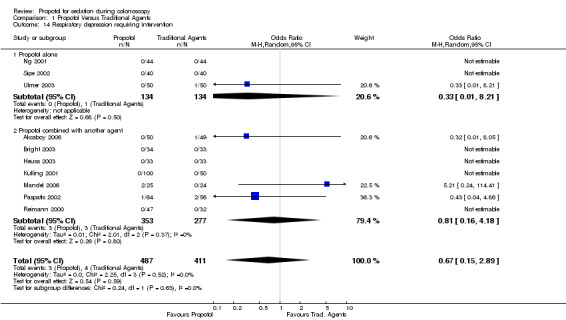
Seven studies (630 patients) collected data on the need for an intervention for respiratory depression. Patients in three studies needed an intervention, with pooled OR of 0.81 (95% CI 0.16, 4.18) with no heterogeneity (p=0.37).
All studies
Pooling the results from 15 studies, involving 1408 patients, there was no significant difference in the number of patients developing hypoxia among those administered propofol as compared to those administered traditional agents (OR 0.80; 95% CI 0.48,1.1) with no significant heterogeneity (p=0.78). Results from only two studies could be combined in the meta‐analysis for the outcome of apnea. There was no difference in the risk of apnea (OR 1.35; 95% CI 0.56, 3.24) with no heterogeneity among the two studies (p=0.75).
There was also no difference in the need for an intervention for hypoxia/apnea (pooled OR 0.67;95% CI 0.15,2.89) with no heterogeneity among the studies (p=0.52).
Arrhythmias
Propofol alone
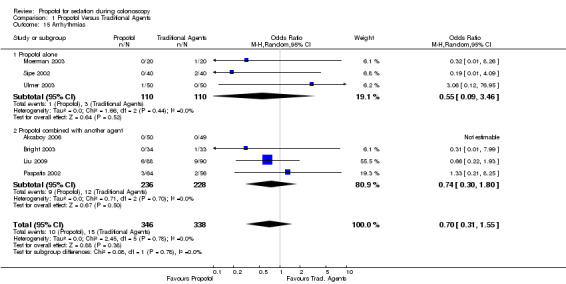
Three studies with 220 patients reported on arrhythmias developing among the study participants. There was no difference in number of patients developing arrhythmias among those administered propofol and those administered the traditional sedatives (OR 0.55; 95% CI 0.09, 3.46), with no heterogeneity among the studies (p=0.44).
Propofol in combination with another agent
Four studies (464 patients) collected data on arrhythmias and arrhythmias were recorded in three. There was no difference in proportion of individuals developing arrhythmias between those administered propofol in combination with another agent as compared to those administered the traditional agents (OR 0.74; 95% CI 0.30,1.80). There was no heterogeneity among the studies (p=0.70).
All studies
The overall pooled OR for arrhythmias with use of propofol was 0.70 (95% CI 0.31, 1.55) with no heterogeneity among the studies (p=0.78).
Hypotension and drop in blood pressure during the procedure
Some studies reported on episodes of hypotension, while others reported on the mean drop in blood pressure (or the lowest blood pressure) during the procedure. These outcomes were abstracted and analysed separately.
Propofol alone
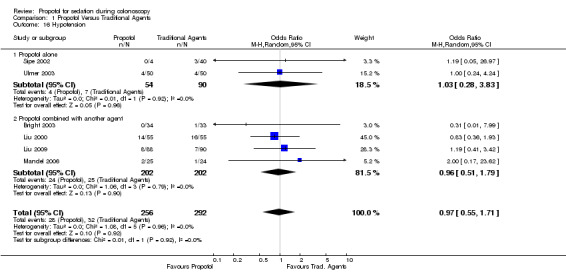
Hypotension was recorded and reported in two studies (144 patients) (Sipe 2002; Ulmer 2003). There was no difference between those given propofol and those given traditional sedatives (pooled OR 1.03; 95% CI 0.28, 3.83), with no heterogeneity among the studies (p=0.92).
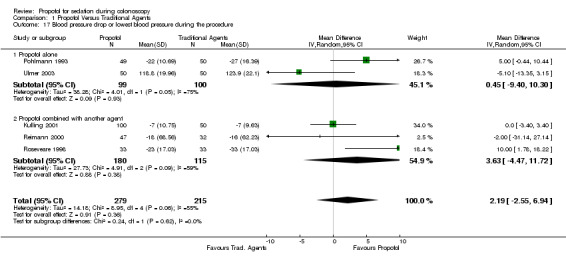
There was no difference in the blood pressure drop (WMD 0.45; 95% CI ‐9.40, 10.30), but significant heterogeneity (p=0.05) between the two studies, involving 199 patients. Propofol in combination with another agent
There was no difference in the hypotension episodes among those given propofol containing combinations and those given traditional sedatives (four studies, 404 patients; pooled OR 0.96; 95% CI: 0.51, 1.79 with no heterogeneity (p=0.79).
In one of the three studies (295 patients) reporting blood pressure drop, there was higher blood pressure drop with the traditional sedatives (Roseveare 1998). On pooling the results, there was no significant difference between the propofol and the traditional sedative use (WMD 3.63; 95% CI ‐4.47, 11.72), but with significant heterogeneity (p=0.09). In sensitivity analysis, limiting to the two PCS studies, there was little change in the observed difference in blood pressure drop (WMD 4.27; 95% CI ‐5.42, 13.97) or the heterogeneity (p=0.03). None of these three studies were reported in abstract format.
All studies On pooling results from studies using propofol as a single agent and those using propofol in combination with another agent, there was no difference in the occurrence of hypotension (pooled OR 0.97; 95% CI 0.55,1.71) and no heterogeneity among the six studies with 548 patients ( p=0.96).
There was no difference in the blood pressure drop (WMD 2.19; 95% CI ‐2.55, 6.94), but there was significant heterogeneity (p=0.06) among the five studies with 494 patients. Pooling the results from the two PCS studies, there was no difference in blood pressure drop (WMD 4.27; 95% CI ‐5.42, 13.97), with significant heterogeneity (p=0.03). In sensitivity analysis, excluding the two studies with PCS, there continued to be no difference in blood pressure drop (WMD 0.45; 95% CI ‐7.61, 8.51), but there was no significant heterogeneity among the remaining three studies (p=0.13).
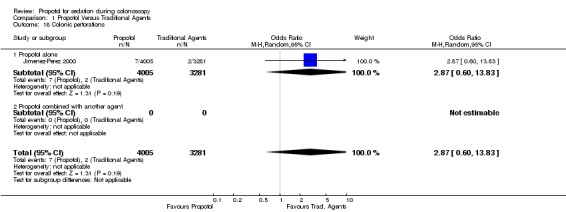
Colonic perforations
One large study (7,286 patients) published in abstract format only (Jimenez‐Perez 2000), reported on the rate of colonic perforations with no difference between the use of propofol and traditional sedatives.
Level of sedation
Level of sedation extracted as dichotomous outcome
Propofol alone
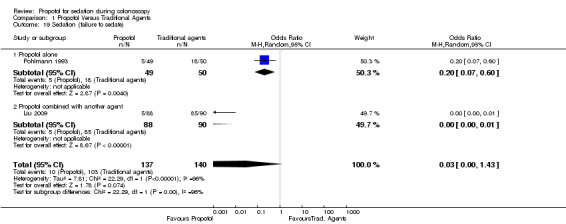
A single study reported that a lower proportion of patients could not be sedated with propofol (Pohlmann 1993).
Propofol in combination with another agent
In a single study, most patients (85 out of 90) administered traditional sedative agents remained conscious (i.e. maintained their highest level of consciousness on the OSSA score scale) as compared with a small minority (5 out of 88) administered propofol in combination with another agent (Liu 2009) (OR 0.0; 95% CI: 0.00‐0.01)
Continous outcome
Propofol alone
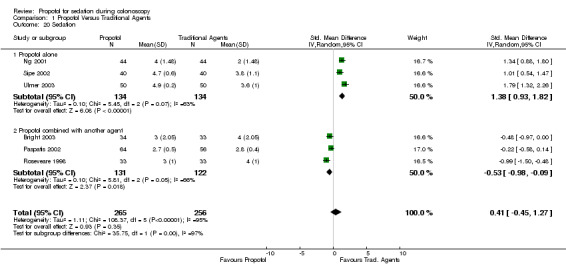
The mean sedation level was higher with use of propofol in all three studies (268 patients), reporting sedation levels. The pooled SMD was 1.38 (95% CI 0.93, 1.82), with significant heterogeneity (p=0.07) among the three studies. Excluding the study with PCS, did not alter the results (SMD 1.40; 95% CI 0.63, 2.16. Heterogeneity p‐value = 0.02). Propofol in combination with another agent
In one out of the three studies (253 patients) the mean level of sedation was lower with use of propofol and similar in the other two. The pooled SMD was ‐0.53 (95% CI ‐0.98,‐0.09), suggesting a lighter level of sedation when propofol was used in combination with another agent compared to the use of traditional sedatives; however there was significant heterogeneity (p=0.05). Sensitivity analysis was performed, excluding the single study which used bolus propofol administration; the remaining two studies (PCS administration) had insignificant heterogeneity (p=0.16) and continued to demonstrate lower level of sedation with the propofol combination (SMD ‐0.73; 95% CI ‐1.22,‐0.23).
All studies
Pooling results from all studies, there was significant heterogeneity (p<0.000001), which persisted when the PCS studies were excluded. The pooled difference in sedation level was not significant (SMD 0.41; 95% CI ‐0.45, 1.27), as the direction of effect was opposite in the two set of studies ‐higher sedation level with propofol in studies with propofol administered as a single agent and lower sedation level with propofol in studies with propofol used in combination with another agent.
Anesthesiologist vs. non‐anaesthesiologist administration of propofol
We found only one study which compared administration of propofol by anaesthesiologist to that administered by non‐anesthesiologists (Laquiere 2006). In this study, which has been published in abstract form only, the non‐anesthesiologists were endoscopists and the number of patients randomised was 94. The goal of propofol administration by anaesthesiologists was anaesthesia and that by endoscopists was sedation. There was no difference in procedure time or patient satisfaction. A higher proportion of patients administered propofol by an anaesthesiologist experienced hypoxia and hypotension, but no patient required an intervention.
Discussion
From the presented meta‐analyses, we found shorter recovery and discharge times and higher patient satisfaction, with no increase in complications with use of propofol.
The study quality of the included studies was limited in the areas of double blinding and/or concealment of allocation. There was a wide variation in the conduct of the studies and determination of the study outcomes.
We have presented meta‐analysis of all outcomes; those with heterogeneity should be viewed with some caution. However the heterogeneity among the studies for many of the outcomes (recovery time, discharge time, pain control and sedation level) could be largely explained by sub‐group and sensitivity analyses. The sub‐group and sensitivity analyses were planned a priori. The heterogeneity was often due to pooling of results from studies with PCS, use of propofol in combination with another agent and/or studies reported in abstract format. However there was no heterogeneity for patient satisfaction, procedure completion or for most of the complications, with higher patient satisfaction with use of propofol and no difference in the procedure completion or complication rates. The heterogeneity among the studies for procedure duration could not be explained; hence it is unclear in what setting the procedure duration would be different with propofol compared to traditional agents. There was one study each for colonic perforations and anaesthesiologist vs. non‐anesthesiologist administration of propofol; and therefore more data are needed for definitive conclusions.
Although it is generally suggested that sub‐group and sensitivity analysis should be viewed with caution, we had planned a priori sensitivity and sub‐group analysis to separate out the effects of propofol use as a single agent and propofol use as PCS. Therefore we believe the results of these sub‐group analyses are valid. We had also planned to perform sensitivity analysis excluding studies reported in abstract form. The results reported in abstract form are often preliminary and can be different from the final results reported in full publications. However, the Cochrane Collaboration traditionally recommended including abstracts in the main analyses to avoid one of the potential sources of publication bias; negative studies may never get published in full format due to publication bias.
There were no language restrictions in this meta‐analysis and we also attempted to obtain all studies published in abstract format. This supplements one previous systematic review,(McQuaid 2008) which was limited to fully published studies in English literature and sedative agents currently available in US. The search strategy of this previous review and meta‐analysis was limited to articles in Embase and Medline. More‐over the previous review did not perform meta‐analysis for sedation for colonoscopy alone and reported most outcomes combined for colonoscopy and EGD. The previous review did list three studies for the outcome of recovery time after colonoscopy, with use of propofol alone. Two of these three studies were from the same group of investigators and the third did not report recovery time separately for colonoscopies (McQuaid 2008; Riphaus 2006). There are no prior systematic reviews comparing propofol administration by anaesthesiologists and non‐anesthesiologists.
The recovery time in this review was about 15 minutes shorter with propofol as a single agent. The recovery time with use of propofol in combination with another agent was shorter than with traditional agents in all but two studies‐‐one of these two studies had exceedingly short recovery times for all patients and in the second study, most patients administered the traditional agents remained conscious throughout the procedure (and hence needed no time to recover). The recovery time was 15 minutes shorter with propofol (as a single agent or in combination with another agent), when the studies using propofol for PCS were excluded. The pooled recovery time was also shorter for PCS use of propofol in the three studies reported as complete publications.
The discharge time was often defined subjectively and reported differently. Hence SMD is a more appropriate measure for determining the difference in discharge times. We initially calculated WMD, as WMD is an easier measure to understand and provides an idea of the absolute difference in the discharge times. Using SMD, the discharge was shorter with use of propofol as a single agent or in combination with another agent, when these two groups of studies were analysed separately.
The procedure time was comparable in most of the studies. The heterogeneity among the studies may have been due to the different experiences of the endoscopists or due to differences in the conduct of the studies. However the cecal intubation rate was close to 99% in these studies, suggesting that all involved endoscopists were experts. The higher colonoscopy completion rate with the use of propofol reported in a recent large study from Thailand needs further follow‐up; this study has so far been reported only in the abstract format.
Based on the results for recovery, discharge and procedure times, propofol use may provide a distinct advantage to endoscopy units, where the throughput of procedures is limited by the availability of recovery room resources. Faster turnover of patients through such endoscopy suites using propofol may help meet some of the increasing demands for endoscopy.
On pooling the results from all studies, patient satisfaction was higher with propofol. Patient satisfaction was higher irrespective of whether propofol was used as a single agent or propofol was administered with another agent. Patient satisfaction was also higher with use of propofol for PCS, as compared to the use of traditional agents. Higher patient satisfaction with sedation and endoscopy may lead to higher patient acceptance and compliance with subsequent endoscopies. This would be of particular importance for colorectal cancer screening and surveillance, where the participants often need multiple endoscopies. However additional studies are needed to determine the predictors of patient acceptance of repeat endoscopy (Condon 2008).
The studies included in this review, assessed and reported pain control. However propofol provides no analgesia. The pain control in studies using propofol as single agent may have been a reflection of patients' inability to remember pain (propofol has amnestic properties). Alternatively deeper levels on sedation/anaesthesia will suppress physiological brain functions, including ability to register pain (Shafer 2008). If the pain control is due to amnestic properties, the autonomic responses to pain may still manifest during the procedure itself.
We found no difference in pain control in the non‐PCS studies. In studies with PCS, the pain control was better with traditional agents. In all of the PCS studies reporting on pain control, propofol was administered by PCS, where as traditional sedatives were administered by bolus injections. Pain during colonoscopy is often transient and worse pain control with PCS could be secondary to a lockout period (minimum time between doses). Lockout period could prevent delivery of the medications during the episode of pain. However, three of the four PCS studies reporting on pain control did not have any lockout period. Alternatively patients could experience more pain with PCS, as patients administer the drug in response to a stimulus and therefore by definition experience the pain prior to PCS. We did not evaluate directly the comparison of PCS administration to bolus administration of a particular drug.
There was no difference in the complication rates between patients administered propofol and those administered traditional sedatives. However we would like to point out the patients in the included studies were generally selected to be healthy. The results of this meta‐analysis in this regard are similar to the low complication rates reported in large patient series with propofol involving healthy patients (Rex 2005; Walker 2003). A study involving sicker patients had a higher complication rate than reported in these earlier series (Wehrmann 2007).
The intended level of sedation in most studies was determined by the patient tolerance of the procedure. Although this is reflective of the clinical practice, this goal is a subjective endpoint. In the absence of double blind randomizations, this subjective goal for sedation may introduce a conscious or subconscious bias into the amount of drug(s) administered.
The mean level of sedation was higher when propofol was used as a single agent, than with the traditional agents. On the other hand the mean level of sedation was lower when propofol was used in combination with another agent, as compared to the traditional agents. We did not directly compare the sedation level between those administered propofol as a single agent and those administered propofol in combination with another agent.
Most large observational case series have reported on use of propofol as a single agent for sedation during endoscopy. However propofol is often used in combination with another agent for other indications. Our meta‐analysis suggests that the benefit of propofol in terms of shorter recovery and discharge times and higher patient satisfaction persists, when it is used in combination with other agents. Propofol has limited analgesic effect and higher doses are often required, when it is used as a single agent for colonoscopy, resulting in higher sedation levels. Thus use of propofol in combination with other agents may be preferable to propofol alone. The combination may be easier to manage due to lower sedation levels and ability to reverse some of the sedation with the use of reversal agents for narcotics and/or benzodiazepines. However propofol used in combination with another agent and propofol alone is more objectively compared in direct head‐to‐head randomised studies.
We found only one study for our secondary objective, comparison of propofol administration by anaesthesiologists to that by endoscopists. This is a hotly debated area among anaesthesiologists and endoscopists, at least in North America. More data directly comparing and evaluating the risks and benefits are needed. Ideally such studies should aim for a similar end‐point for sedation administration by anaesthesiologists and endoscopists. In the only reported study anaesthesiologists aimed for general anaesthesia and endoscopists for sedation. Although this may be reflective of usual clinical practice at many places, this does not provide direct comparison of outcomes due to the difference in the personnel administering the drug.
Sedation for endoscopy has been a largely neglected area in terms of research until very recently. There were no guidelines for training. Increasing acceptance of propofol for sedation for endoscopy has prompted renewed interest in sedation for endoscopy, as propofol has a relatively narrow window between efficacy and side‐effects. Adequate training in administration of the sedative agents, monitoring during sedation and the ability to recover patients from deeper levels of sedation have received increasing attention.
One of the limitations of the current meta‐analysis is the inability to determine the effect of individual traditional agents. There were a limited number of studies for each specific agent administered in similar manner. Some of the unexplained heterogeneity among the studies may be due to the differences in the specific traditional agents. Agents such as diazepam and meperidine have longer duration of effect than midazolam and fentanyl respectively (Cohen 2007).
Authors' conclusions
Implications for practice.
Propofol for sedation during colonoscopy for generally healthy individuals can lead to faster recovery and discharge times, increased patient satisfaction without an increase in side‐effects. Propofol is a reasonable option for sedation during colonoscopy for generally healthy individuals. Propofol may provide an advantage to endoscopy units, where the throughput of procedures is limited by the availability of recovery room resources. Faster turnover of patients through such endoscopy suites using propofol may help meet some of the increasing demands for endoscopy. Moreover higher patient satisfaction when propofol is used for sedation during colonoscopy may also lead to higher patient compliance with subsequent endoscopies. Differences in patient outcomes depend upon not only on the choice of the sedative agent, but also on how the particular sedative agent is used
Implications for research.
There is a need for better quality studies, with double blind randomizations, reporting of allocation concealment and more standardized reporting of outcomes. There are little direct comparative data on administration of propofol by anaesthesiologists to that by non‐anesthesiologists, in terms of efficacy, efficiency, cost or side‐effect profiles.The higher colonoscopy completion rate with the use of propofol reported in a recent large study from Thailand needs further follow‐up to determine the generalizability to other settings.
What's new
| Date | Event | Description |
|---|---|---|
| 11 June 2011 | New search has been performed | In this update we have included results from three additional publications. One of these is a full publication of a study which was previously included as an abstract in the original review published in 2008. |
History
Protocol first published: Issue 4, 2006 Review first published: Issue 4, 2008
| Date | Event | Description |
|---|---|---|
| 18 August 2008 | Amended | Converted to new review format. |
| 8 September 2006 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The authors thank Drs Alain Demers, Piotr Czaykowski and Klaus Wrogemann for translating the non‐English language articles. The initial review was supported in part by Department of Internal Medicine, University of Manitoba start‐up research grant to Dr. Singh. Dr. Singh was supported in part by Dr. F.W. Du Val Clinical Research Professorship Award and by a ACG junior faculty development award.
Data and analyses
Comparison 1. Propofol Versus Traditional Agents.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Recovery time | 11 | 776 | Mean Difference (IV, Random, 95% CI) | ‐16.59 [‐24.99, ‐8.18] |
| 1.1 Propofol alone | 4 | 249 | Mean Difference (IV, Random, 95% CI) | ‐14.68 [‐19.79, ‐9.58] |
| 1.2 Propofol combined with another agent | 7 | 527 | Mean Difference (IV, Random, 95% CI) | ‐17.36 [‐29.39, ‐5.34] |
| 2 Recovery time (minutes) in studies, which reported recovery time in formats which could not be meta‐analyzyed | Other data | No numeric data | ||
| 3 Discharge time | 7 | 542 | Mean Difference (IV, Random, 95% CI) | ‐20.86 [‐30.94, ‐10.78] |
| 3.1 Propofol alone | 4 | 297 | Mean Difference (IV, Random, 95% CI) | ‐19.06 [‐28.08, ‐10.04] |
| 3.2 Propofol combined with another agent | 3 | 245 | Mean Difference (IV, Random, 95% CI) | ‐32.17 [‐64.84, 0.50] |
| 4 Procedure duration | 9 | 736 | Mean Difference (IV, Random, 95% CI) | 0.84 [‐1.02, 2.71] |
| 4.1 Propofol alone | 2 | 168 | Mean Difference (IV, Random, 95% CI) | ‐1.98 [‐6.12, 2.17] |
| 4.2 Propofol combined with another agent | 7 | 568 | Mean Difference (IV, Random, 95% CI) | 1.85 [‐0.26, 3.97] |
| 5 Cecal intubation | 9 | 1840 | Odds Ratio (M‐H, Random, 95% CI) | 0.41 [0.22, 0.76] |
| 5.1 Propofol alone | 3 | 268 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Propofol combined with another agent | 6 | 1572 | Odds Ratio (M‐H, Random, 95% CI) | 0.41 [0.22, 0.76] |
| 6 Patient Dissatisfication (dichotomous data) | 6 | 449 | Odds Ratio (M‐H, Random, 95% CI) | 0.22 [0.11, 0.44] |
| 6.1 Propofol alone | 2 | 117 | Odds Ratio (M‐H, Random, 95% CI) | 0.25 [0.09, 0.72] |
| 6.2 Propofol combined with another agent | 4 | 332 | Odds Ratio (M‐H, Random, 95% CI) | 0.19 [0.07, 0.50] |
| 7 Patient Satisfication (continuous data) | 4 | 370 | Std. Mean Difference (IV, Random, 95% CI) | 0.43 [‐0.00, 0.85] |
| 7.1 Propofol alone | 3 | 220 | Std. Mean Difference (IV, Random, 95% CI) | 0.50 [‐0.17, 1.17] |
| 7.2 Propofol combined with another agent | 1 | 150 | Std. Mean Difference (IV, Random, 95% CI) | 0.32 [‐0.02, 0.66] |
| 8 Patient Dissatisfication (combined) | 7 | Odds Ratio (Random, 95% CI) | 0.35 [0.23, 0.53] | |
| 8.1 Propofol Alone | 4 | Odds Ratio (Random, 95% CI) | 0.33 [0.18, 0.60] | |
| 8.2 Propofol combined with another agent | 3 | Odds Ratio (Random, 95% CI) | 0.33 [0.14, 0.80] | |
| 9 Pain Control (continuous outcome) | 6 | 633 | Std. Mean Difference (IV, Random, 95% CI) | 0.38 [0.03, 0.74] |
| 9.1 Propofol alone | 2 | 187 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.53, 0.26] |
| 9.2 Propofol combined with another agent | 4 | 446 | Std. Mean Difference (IV, Random, 95% CI) | 0.51 [0.17, 0.84] |
| 10 Pain Control (dichotomous outcome) | 5 | 344 | Odds Ratio (M‐H, Random, 95% CI) | 1.12 [0.21, 5.97] |
| 10.1 Propofol alone | 3 | 220 | Odds Ratio (M‐H, Random, 95% CI) | 0.20 [0.02, 1.79] |
| 10.2 Propofol combined with another agent | 2 | 124 | Odds Ratio (M‐H, Random, 95% CI) | 2.64 [0.39, 18.04] |
| 11 Pain Control (combined) | 9 | Odds Ratios (Random, 95% CI) | 1.71 [1.02, 2.88] | |
| 11.1 Propofol Alone | 3 | Odds Ratios (Random, 95% CI) | 0.67 [0.34, 1.33] | |
| 11.2 Propofol combined with another agent | 6 | Odds Ratios (Random, 95% CI) | 2.27 [1.42, 3.63] | |
| 12 Hypoxia | 15 | 1408 | Odds Ratio (M‐H, Random, 95% CI) | 0.80 [0.48, 1.31] |
| 12.1 Propofol alone | 5 | 407 | Odds Ratio (M‐H, Random, 95% CI) | 0.69 [0.25, 1.89] |
| 12.2 Propofol combined with another agent | 10 | 1001 | Odds Ratio (M‐H, Random, 95% CI) | 0.83 [0.47, 1.48] |
| 13 Apnea | 11 | 918 | Odds Ratio (M‐H, Random, 95% CI) | 1.35 [0.56, 3.24] |
| 13.1 Propofol alone | 5 | 407 | Odds Ratio (M‐H, Random, 95% CI) | 1.35 [0.56, 3.24] |
| 13.2 Propofol combined with another agent | 6 | 511 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Respiratory depression requiring intervention | 10 | 898 | Odds Ratio (M‐H, Random, 95% CI) | 0.67 [0.15, 2.89] |
| 14.1 Propofol alone | 3 | 268 | Odds Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.21] |
| 14.2 Propofol combined with another agent | 7 | 630 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.16, 4.18] |
| 15 Arrhythmias | 7 | 684 | Odds Ratio (M‐H, Random, 95% CI) | 0.70 [0.31, 1.55] |
| 15.1 Propofol alone | 3 | 220 | Odds Ratio (M‐H, Random, 95% CI) | 0.55 [0.09, 3.46] |
| 15.2 Propofol combined with another agent | 4 | 464 | Odds Ratio (M‐H, Random, 95% CI) | 0.74 [0.30, 1.80] |
| 16 Hypotension | 6 | 548 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.55, 1.71] |
| 16.1 Propofol alone | 2 | 144 | Odds Ratio (M‐H, Random, 95% CI) | 1.03 [0.28, 3.83] |
| 16.2 Propofol combined with another agent | 4 | 404 | Odds Ratio (M‐H, Random, 95% CI) | 0.96 [0.51, 1.79] |
| 17 Blood pressure drop or lowest blood pressure during the procedure | 5 | 494 | Mean Difference (IV, Random, 95% CI) | 2.19 [‐2.55, 6.94] |
| 17.1 Propofol alone | 2 | 199 | Mean Difference (IV, Random, 95% CI) | 0.45 [‐9.40, 10.30] |
| 17.2 Propofol combined with another agent | 3 | 295 | Mean Difference (IV, Random, 95% CI) | 3.63 [‐4.47, 11.72] |
| 18 Colonic perforations | 1 | 7286 | Odds Ratio (M‐H, Random, 95% CI) | 2.87 [0.60, 13.83] |
| 18.1 Propofol alone | 1 | 7286 | Odds Ratio (M‐H, Random, 95% CI) | 2.87 [0.60, 13.83] |
| 18.2 Propofol combined with another agent | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Sedation (failure to sedate) | 2 | 277 | Odds Ratio (M‐H, Random, 95% CI) | 0.03 [0.00, 1.43] |
| 19.1 Propofol alone | 1 | 99 | Odds Ratio (M‐H, Random, 95% CI) | 0.20 [0.07, 0.60] |
| 19.2 Propofol combined with another agent | 1 | 178 | Odds Ratio (M‐H, Random, 95% CI) | 0.00 [0.00, 0.01] |
| 20 Sedation | 6 | 521 | Std. Mean Difference (IV, Random, 95% CI) | 0.41 [‐0.45, 1.27] |
| 20.1 Propofol alone | 3 | 268 | Std. Mean Difference (IV, Random, 95% CI) | 1.38 [0.93, 1.82] |
| 20.2 Propofol combined with another agent | 3 | 253 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐0.98, ‐0.09] |
1.1. Analysis.
Comparison 1 Propofol Versus Traditional Agents, Outcome 1 Recovery time.
1.2. Analysis.
Comparison 1 Propofol Versus Traditional Agents, Outcome 2 Recovery time (minutes) in studies, which reported recovery time in formats which could not be meta‐analyzyed.
| Recovery time (minutes) in studies, which reported recovery time in formats which could not be meta‐analyzyed | ||||
|---|---|---|---|---|
| Study | Group A | Group B | Group C | Comments |
| Kulling 2001 | 45 min: 0 (IQR ‐0.5 ‐0.5) 90 min: 0 (IQR ‐ 1.0 ‐0.5) | In between A and C Not significantly different from either A or C | 45 min: 1.0 (IQR 0.0 ‐9.0) 90 min: 0.25 (IQR 0.0 ‐ 1.5) | Difference from baseline of the score on Triegger dot‐joining test. Less difference, better recovery. Recovery‐‐therefore better recovery in Group A (PCS propofol), as compared with C at 45 and 90 mins |
| Liu 2009 | 2.5 (0‐15.0) | 0 (0‐7.5) | No group C in this study | Recovery time was reported as median (minutes) and range. Recovery time was s ignificantly longer in group A (p<0.0001) |
| Martinez‐Palli 2005 | 24 | 38 | 32 | No measures of variance provided |
| Paspatis 2002 | 5 min: 9.5±0.6 10 min: 9.8±0.3 30 min: 9.9±0.1 | 5 min: 8.3±1.3 10 min: 8.5±1 30 min: 7.4±0.9 | No group C in this study | Significantly higher Aldrete scores at 5, 10 and 30 minutes in Group A (Propofol) |
1.3. Analysis.
Comparison 1 Propofol Versus Traditional Agents, Outcome 3 Discharge time.
1.4. Analysis.
Comparison 1 Propofol Versus Traditional Agents, Outcome 4 Procedure duration.
1.5. Analysis.
Comparison 1 Propofol Versus Traditional Agents, Outcome 5 Cecal intubation.
1.6. Analysis.
Comparison 1 Propofol Versus Traditional Agents, Outcome 6 Patient Dissatisfication (dichotomous data).
1.7. Analysis.
Comparison 1 Propofol Versus Traditional Agents, Outcome 7 Patient Satisfication (continuous data).
1.8. Analysis.
Comparison 1 Propofol Versus Traditional Agents, Outcome 8 Patient Dissatisfication (combined).
1.9. Analysis.
Comparison 1 Propofol Versus Traditional Agents, Outcome 9 Pain Control (continuous outcome).
1.10. Analysis.
Comparison 1 Propofol Versus Traditional Agents, Outcome 10 Pain Control (dichotomous outcome).
1.11. Analysis.
Comparison 1 Propofol Versus Traditional Agents, Outcome 11 Pain Control (combined).
1.12. Analysis.
Comparison 1 Propofol Versus Traditional Agents, Outcome 12 Hypoxia.
1.13. Analysis.
Comparison 1 Propofol Versus Traditional Agents, Outcome 13 Apnea.
1.14. Analysis.
Comparison 1 Propofol Versus Traditional Agents, Outcome 14 Respiratory depression requiring intervention.
1.15. Analysis.
Comparison 1 Propofol Versus Traditional Agents, Outcome 15 Arrhythmias.
1.16. Analysis.
Comparison 1 Propofol Versus Traditional Agents, Outcome 16 Hypotension.
1.17. Analysis.
Comparison 1 Propofol Versus Traditional Agents, Outcome 17 Blood pressure drop or lowest blood pressure during the procedure.
1.18. Analysis.
Comparison 1 Propofol Versus Traditional Agents, Outcome 18 Colonic perforations.
1.19. Analysis.
Comparison 1 Propofol Versus Traditional Agents, Outcome 19 Sedation (failure to sedate).
1.20. Analysis.
Comparison 1 Propofol Versus Traditional Agents, Outcome 20 Sedation.
Comparison 2. Non‐anesthesiologist Versus Anesthesiologist.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patient Satisfication | Other data | No numeric data | ||
| 2 Procedure duration (minutes) | Other data | No numeric data | ||
| 3 Hypoxia | Other data | No numeric data | ||
| 4 Hypotension | Other data | No numeric data |
2.1. Analysis.
Comparison 2 Non‐anesthesiologist Versus Anesthesiologist, Outcome 1 Patient Satisfication.
| Patient Satisfication | |||
|---|---|---|---|
| Study | Gastroenterologist | Anesthesiologist | Comments |
| Laquiere 2006 | Average score on VAS= 90.8 | Average score on VAS= 89 | Not significantly different |
2.2. Analysis.
Comparison 2 Non‐anesthesiologist Versus Anesthesiologist, Outcome 2 Procedure duration (minutes).
| Procedure duration (minutes) | |||
|---|---|---|---|
| Study | Gastroenterologist | Anesthesiologist | Comment |
| Laquiere 2006 | 16.7 | 17.7 | No significant difference |
2.3. Analysis.
Comparison 2 Non‐anesthesiologist Versus Anesthesiologist, Outcome 3 Hypoxia.
| Hypoxia | |||
|---|---|---|---|
| Study | Gastroenterologist | Anesthesiologist | Comment |
| Laquiere 2006 | 6.6% | 35.5% | "Desaturation" not defined No intervention required p<0.008 |
2.4. Analysis.
Comparison 2 Non‐anesthesiologist Versus Anesthesiologist, Outcome 4 Hypotension.
| Hypotension | |||
|---|---|---|---|
| Study | Gastroenterologist | Anesthesiologist | Comment |
| Laquiere 2006 | 24.4% | 44% | Hypotension not defined No intervention required p<0.008 |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Akcaboy 2006.
| Methods | Single center, randomised, controlled trial 1) Allocation concealment: Unclear/Not stated 2) Blinding of patients: Yes 3) Blinding of outcome assessors: Yes‐all staff, other than anaesthetist No‐anesthetist 4) A priori calculation of sample size: Unclear/Not stated 5) Groups treated identical other than the named intervention: Yes | |
| Participants | Number of eligible patients: Not stated Number of patients enrolled: 100 Drop outs/Withdrawals: 1 Incl. Criteria: 1) Patients scheduled for complete colonoscopy 2) Age 17‐75 years 3) ASA class I‐III Excl. Criteria: 1) Patients with pulmonary, neurological and metabolic diseases 2) History of allergic reaction to any of the study drugs 3) Chronic use of opioid analgesics 4) Pregnancy Country: Turkey Setting: Not stated Age(median(range) )(yrs): Group A: 40 (17‐74) Group B: 48 (18‐75) N (%) males: Group A: 28 (56) Group B: 26(53) ASA score (I/II/III): Group A: 15/15/20 Group B: 17/12/20 Groups comparable at baseline: Yes (age, sex ratio, body weight, height, ASA score, baseline anxiety score) | |
| Interventions | Group A (n=50): Propofol (Mean (SD) dose: 161.06 (52.2) mg) and Midazolam 2 mg (pre‐medication) Group B (n=49): Remifentanil(Mean (SD) dose: 98.7 (45.05) micrograms) and Midazolam 2 mg (pre‐medication) Mode of administration: Intial bolus, followed by continuous infusion and supplementary boluses Administered by: Anesthesiologist Goal level of sedation: Observer's Assessment of Alterness/Sedation scale score of 3 All received supplemental oxygen (3‐4 L/min) through face mask | |
| Outcomes | Patients' level of sedation, pain, recovery time, discharge time, patient satisfaction, vital signs, gastroenterologist satisfaction | |
| Notes | Definitions: 1) Recovery time: time to reach Aldrete score of nine or more in the recovery room. The paper also states that patients were admitted to recovery unit, after they achieved an Aldrete score of nine or more. 2) Discharge time: Stable vital signs, able to tolerate oral fluids, no nausea, vomiting or itching, and able to walk unaided 3) Sedation level: assessed on Observer's Assessment of Alertness/Sedation scale 4) Pain: evaluated during the procedure on a 11 point scale 5) Patient satisfaction: patients contacted via telephone the day after colonoscopy and asked about their satisfaction, which was assessed on a 4‐point scale | |
Amornyotin 2010.
| Methods | Randomized study 1) Allocation concealment: Unclear/Not stated 2) Blinding of patients: Not stated 3) Blinding of outcome assessors: Unclear/Not stated 4) A priori calculation of sample size: Unclear/Not stated 5) Groups treated identical other than the named intervention: Unclear/Not stated |
|
| Participants | Number of eligible patients: Not stated Number of patients enrolled: 1,032 Drop outs/Withdrawals: Not stated Incl. Criteria: Not stated Excl. Criteria: Not stated Country: Thailand Setting: Not stated Age(mean)(yrs): Not stated (%) males: Not stated ASA score :Not stated Groups comparable at baseline: Not stated | |
| Interventions | Group A (n=518): Propofol (mean total dose: 5.98 (2.67) mg/kg/hr) and Meperidine (mean total dose: 1.74 (1.05) mg/kg/hr) Group B (n=514): Midazolam (mean total dose: 0.08 (0.05) mg/kg/hr) and Fentanyl (mean total dose: 0.003 (0.002) mg/kg/hr There is mention of additional dosing with propofol in both groups‐‐which we ascribed as an error in the abstract Mode of administration: Not stated Administered by: Not stated Goal level of sedation: Not stated Supplemental oxygen: Not stated | |
| Outcomes | Primary outcome: colonoscopy completion rate Secondary outcomes: patient tolerance, discomfort during insertion, patient and endoscopist satisfaction, hemodynamic responses, as well as complications during and immediately after procedure | |
| Notes | For the secondary outcomes, no scores or values are provided; only p values provided for some of the secondary outcomes. Hence results for the secondary outcomes from this study were not included in the meta‐analysis. | |
Bright 2003.
| Methods | Multi center, randomised, controlled trial 1) Allocation concealment: Unclear/Not stated 2) Blinding of patients: No 3) Blinding of outcome assessors: Unclear/Not stated 4) A priori calculation of sample size: Unclear/Not stated 5) Groups treated identical other than the named intervention: Yes | |
| Participants | Number of eligible patients: 82 Number of patients enrolled: 67 Drop outs/Withdrawals: 0 Incl. Criteria: Consecutive patients presenting for outpatient colonoscopy Excl. Criteria: 1) Age less than 18 years 2) Patients with significant cardiorespiratory disease 3) previous colonic surgery 4) those judged unable to use PCS Country: UK Setting: Outpatient Age(median(range))(yrs): Group A:49 (21‐84) Group B: 58 (23‐77) N (%) males: Group A: 19 (56) Group B: 12 (36) ASA score: Not stated Groups comparable at baseline: Yes (age, body weight); more women in group B | |
| Interventions | Group A (n=34): PCS Propofol (Median (range) dose: 5.5 (1.9‐12.8) mg/kg/hr) and Alfentanil (Median (range) dose: 13.8 (4.8‐32) micrograms/kg/hr) Group B (n=33): Midazolam (Median (range) dose: 0.06 (0.03‐0.12) mg/kg) and Pethidine: single bolus dose of 50 mg Mode of administration: Group A: PCS boluses Group B: single bolus of pethidine at beginning of procedure, followed by 2.5 mg of midazolam boluses at endoscopist's discretion Administered by: Group A: PCS Group B: endoscopist; an anaesthetist present in observational capacity Goal level of sedation: Group A (PCS): relieve patient discomfort Group B: Not stated All received supplemental oxygen (2 L/min) through nasal cannula | |
| Outcomes | Procedure time, patients' level of sedation, pain, complications, recovery time, discharge time, patient satisfaction, impact on normal activities | |
| Notes | Definitions: 1) Patients' level of sedation, measured by a single nurse specialist during the procedure on a 5 point scale 2) Pain score: Patients' recollection, after recovery on a 4 point scale, measured by a single nurse specialist during the procedure. This outcome was dichomotomized for meta‐analysis as Moderate/Severe Pain vs. No/Mild Pain. 3) Recovery time: Removal of colonoscope to the patient's completion of a number connection test (NCT) to within 10% of their pre procedure time 4) Discharge time: When patient judged to be mobile with a steady gait 5) Complications: Determined by monitoring of blood pressure, heart rate and oxygen saturation 6) Patient Satisfaction: Patients completed a questionnaire concerning their satisfaction with the type of sedation received. No details or definitions provided | |
Germain 1989.
| Methods | Single center, randomised, controlled trial 1) Allocation concealment: Unclear/Not stated 2) Blinding of patients: No 3) Blinding of outcome assessors: No 4) A priori calculation of sample size: Unclear/Not stated 5) Groups treated identical other than the named intervention: Yes | |
| Participants | Number of eligible patients: Not stated Number of patients enrolled: 100 Drop outs/Withdrawals: 0 Incl. Criteria: Not stated Excl. Criteria: Not stated Country: France Setting: Unclear/Not stated Age(mean)(yrs): Group A: 54 Group B: 53.5 N (%) males: Group A: 23 (46) Group B: 27(54) ASA score: Not stated Groups comparable at baseline: Yes (age, gender, body weight) | |
| Interventions | All patients were premedicated with 800mg of Cimetidine prior to the procedures Group A (n=50): Propofol; Dose: Age < 65 yrs‐‐Induction: 1.5mg/kg Maintenance : 6 mg/kg/hr; Age >65 yrs‐‐Induction: 1.25 mg/kg Maintenance : 5 mg/kg/hr Group B (n=50): Midazolam; Dose: Age < 65 yrs‐‐Induction: 0.15 mg/kg Maintenance: 0; Age > 65 yrs‐‐Induction: 0.1 mg/kg Maintenance: 0 Alfentanil ; Dose: Age < 65 yrs‐‐Induction: 0.01 mg/kg Maintenance: 0.06 mg/kg/hr; Dose: Age > 65 yrs‐‐Induction: 0.008 mg/kg Maintenance: 0.05 mg/kg/hr Mode of administration: Both groups‐bolus induction, followed by maintenance infusion Administered by: Anesthesiologist Goal level of sedation: Not stated All received supplemental oxygen (50%) through face mask | |
| Outcomes | Recovery at different time points, after end of sedation Vital signs at different time points Apnea during induction | |
| Notes | French language article High induction dose Definitions: 1) Recovery at different time points, after end of sedation judged by ability to complete Newman's 38 points connection test 2) Level of sedation judged subjectively by anaesthesiologists on a 5 point scale. No measures of significance or variation given‐‐hence not used in analysis 3) Apnea not defined, but numbers during induction given‐‐‐only outcome used in meta‐analysis | |
Heuss 2003.
| Methods | Single center, randomised, controlled trial 1) Allocation concealment: Unclear/Not stated 2) Blinding of patients: Not stated 3) Blinding of outcome assessors: Not stated 4) A priori calculation of sample size: Unclear/Not stated 5) Groups treated identical other than the named intervention: Unclear/Not stated | |
| Participants | Number of eligible patients: Not stated Number of patients enrolled: 66 Drop outs/Withdrawals: 0 Incl. Criteria: Not stated Excl. Criteria: Not stated Country: Switzerland Setting: Not stated Age(mean)(yrs): Group A: 61 Group B: 62 (%) males: Group A: 50 Group B: 52 ASA score >II: Group A: 34% Group B: 25% Groups comparable at baseline: Yes (age, gender, ASA score) | |
| Interventions | Group A (n=33): Propofol (Mean (SD) dose: 117 (65) mg) Group B (n=33): Midazolam (Mean(SD) dose: 5 (1.6) mg) All patients also received alfentanil Mode of administration: Not stated Administered by: nurse, supervised by endoscopist Goal level of sedation: "clinical need" All received supplemental oxygen (4 L/min) through nasal cannula | |
| Outcomes | Drops in oxygen saturation, increase in transcutaneous PCO2, patient satisfaction, endoscopist satisfaction | |
| Notes | No major adverse events occurred during sedation. Definitions: 1) Patient and endoscopist satisfaction were evaluated on a 10 cm VAS 2) Oxygen desaturations less than 90% reported | |
Heuss 2005.
| Methods | Single center, randomised, controlled trial 1) Allocation concealment: Unclear 2) Blinding of patients: Not stated 3) Blinding of outcome assessors: Not stated 4) A priori calculation of sample size: Unclear/Not stated 5) Groups treated identical other than the named intervention: Unclear | |
| Participants | Number of eligible patients: Not stated Number of patients enrolled: 83 Drop outs/Withdrawals: 0 Incl. Criteria: Not stated Excl. Criteria: Not stated Country: Switzerland Setting: Unclear Age: Not stated N (%) males: Not stated ASA score: Not stated Groups comparable at baseline: Not stated | |
| Interventions | Group A (n=41): Propofol (dose: not stated) Group B (n=42): Midazolam (dose: not stated) All patients also received 4 micrograms/ Kg Alfentanil Mode of administration: Not stated Administered by: Not stated Goal level of sedation: Not stated All received supplemental oxygen (3 L/min) | |
| Outcomes | Hypoventilation as measured by changes in the transcutaneous carbon dioxide tension; hypoxemia (SpO2<85%); apnea | |
| Notes | Definitions 1. Hypoxia: SpO2<85% | |
Jimenez‐Perez 2000.
| Methods | Single center, randomised, controlled trial 1) Allocation concealment: Unclear/Not stated 2) Blinding of patients: Not stated 3) Blinding of outcome assessors: Unclear/Not stated 4) A priori calculation of sample size: Unclear/Not stated 5) Groups treated identical other than the named intervention: Unclear/Not stated | |
| Participants | Number of eligible patients: Not stated Number of patients enrolled: 7,286 Drop outs/Withdrawals: Unclear/Not stated Incl. Criteria: Consecutive diagnostic colonoscopies performed during a 3 year period Excl. Criteria: Therapeutic colonoscopies Country: Spain Setting: Not stated Age: Not stated N (%) males: Not stated ASA score: Not stated Groups comparable at baseline: Unclear | |
| Interventions | Group A (n=4,005): Propofol Group B (n=3,281): Diazepam Dosage: Not stated Mode of administration: Not stated, other than that both drugs were given I.V. Administered by: Not stated Goal level of sedation: Deep sedation with propofol. Conscious sedation with diazepam. Supplemental oxygen: Unclear | |
| Outcomes | Incidence of colonic perforations | |
| Notes | It is unclear whether only adult patients were included in the study | |
Kostash 1994.
| Methods | Single center, randomised, controlled trial 1) Allocation concealment: unclear 2) Blinding of patients: Yes 3) Blinding of outcome assessors: Yes 4) A priori calculation of sample size: Unclear/Not stated 5) Groups treated identical other than the named intervention: Yes | |
| Participants | Number of eligible patients: Not stated Number of patients enrolled: 60 Drop outs/Withdrawals: 3 (one in each group due to intolerance to the procedure) Incl. Criteria: Consectutive patients scheduled for elective colonoscopy Excl. Criteria: 1) Age <18 years 2) Allergy to any study medication 3) Patient desire to have colonoscopy without sedation 4) Pregnancy 5) Inability to complete a questionnaire in English following the procedure Country: Canada Setting: Elective colonoscopy (? Outpatients) Age(mean(SD) ) (yrs): Group A: 45.8 (18.4) Group B: 48.3 (18.2) Group C: 40.9 (15.1) N males: Group A: 9 Group B: 10 Group C: 9 ASA score: Not stated Groups comparable at baseline: Yes (age, sex ratio, body weight, baseline oxygen saturation, blood pressure, pulse rate) | |
| Interventions | Group A (n=19: Propofol (Bolus: mean (SD) dose: 1.3 (0.29) mg/kg; Infusion mean (SD) dose:76.5 (30) micro gm/kg/min) and Fentanyl (mean (SD) dose: 2.2 (0.48) micro gm/kg) Group B (n=19): Diazepam (mean(SD) dose: 0.12 (0.04) mg/kg) and Meperidine: (mean (SD) dose: 2.0 (0.50) mg/kg) Group C (n=19: Midazolam (mean(SD) dose: 0.07 (0.02) mg/kg) and Fentanyl: (mean (SD) dose: 2.2 (0.56) mg/kg) For meta‐analysis, the results of group B and C were combined. Mode of administration: Propofol: bolus and infusion; Other drugs: bolus Administered by: Not stated Goal level of sedation: Level 3 or 4 on 5‐point MacKenzie sedation score Initially all patients breathed room air. Oxygen was administered if SpO2 dropped below 85% for longer than 15 s. | |
| Outcomes | Reliability of sedation Recovery time Complications | |
| Notes | Definitions: 1) Recovery time: multiple definitions used in the study (time to eye opening, response to command, orientation, return to level 1 sedation and Aldrete score of 10). We abstracted the time to achieving Aldrete score of 10. 2) Sedation level: adequate or inadequate by endoscopist; assessment by patients was also assessed‐however results are not given for each group separately. The paper does not mention apnea or requirement of any intervention other increasing oxygen to maintain SpO2. | |
Kulling 2001.
| Methods | Single center, randomised, controlled trial 1) Allocation concealment: Unclear/Not stated 2) Blinding of patients: Yes 3) Blinding of outcome assessors: Unclear/Not stated 4) A priori calculation of sample size: Yes 5) Groups treated identical other than the named intervention: Yes | |
| Participants | Number of eligible patients: 166 Number of patients enrolled: 150 (numbers in each group obtained from the first author) Drop outs/Withdrawals: 0 Incl. Criteria: Consectutive patients scheduled for colonoscopy Excl. Criteria: 1) Age below 18 or above 75 2) History of colonic resection 3) Allergy to study medication 4) Severe cardiorespiratory disease (ASA class III or higher) 5) Renal or hepatic impairment 6) Seizure disorder 7) Psychiatric disease 8) Substance abuse 9) Pregnancy or breast feeding 10) Refusal to receive any medication for the forthcoming colonoscopy Country: Switzerland Setting: Outpatient Age(median(IQR) )(yrs): Group A: 55 (43‐63) Group B: 55 (40‐65) Group C: 48 (35‐64) % males: Group A: 56 Group B: 44 Group C: 54 ASA score: I or II Groups comparable at baseline: Yes (age, sex ratio, education, prior colonoscopy with or without sedation, pre‐colonoscopy anxiety) | |
| Interventions | Group A (n=50): PCS with Propofol (median(IQR) dose: 78 (57‐119) mg) and Alfentanil ( 198 (144‐300) micrograms) Group B (n=50): Continous infusion of Propofol (median(IQR) dose: 90 (68‐131) mg) and Alfentanil ( 227 (173‐331) micrograms) Group C (n=50): Midazolam (median(IQR) dose: 2.7 (2.3‐3.0) mg) and Meperidine ( 27(23‐30)mg) Mode of administration: Groups A and C:bolus Group B: continuous infusion Administered by: nurse, supervised by endoscopist Goal level of sedation: Patient comfort All received supplemental oxygen (2 L/min) through nasal cannula | |
| Outcomes | Primary outcome: patient satisfaction with the degree of sedation during colonoscopy Secondary outcomes: pain, procedure time, amnesia, safety, recovery | |
| Notes | Definitions: 1) Patient satisfaction with the degree of sedation during colonoscopy: was measured 90 minutes after the procedure on a 10 cm VAS 2) Pain during procedure: 90 minutes after the procedure, patients asked to rate their pain during the procedure on a 10 cm VAS 3) Recovery from sedation: judged by Trieger dot‐joining test before, as well as 15, 45 and 90 minutes after colonoscopy Data extraction combined for both the propofol groups | |
Laquiere 2006.
| Methods | Single center, randomised, controlled trial 1) Allocation concealment: Unclear 2) Blinding of patients: Not stated 3) Blinding of outcome assessors: Unclear/Not stated 4) A priori calculation of sample size: Unclear/Not stated 5) Groups treated identical other than the named intervention: Unclear | |
| Participants | Number of eligible patients: 148 Number of patients enrolled: 94 Drop outs/Withdrawals: 0 Incl. Criteria: Patients with ASA class I or II, who were scheduled to undergo colonoscopy between November 2004 and March 2005 Excl. Criteria: Not stated Country: France Setting: Outpatient Age(mean(SD) )(yrs): Group A: 55.6 (11.2) Group B: 55.3 (11.8) N (%) males: Group A: 29 (58) Group B: 25(50) ASA score (mean(SD)): Group A: 1.36 (0.45) Group B: 1.31(0.60) Groups comparable at baseline: Yes (age, sex ratio, body weight, race, education, alcohol intake, smoking, handedness, prior colonoscopy, ASA score) | |
| Interventions | Group A (n=Not stated): Propofol dose: 94 (30‐210) mg Group B (n=Not stated): Propofol dose: 260 (60‐670) mg Mode of administration: Gropu A: bolus; Group B: Intravenous administration with an objective of concentration Administered by: Group A: endoscopist (gastroenterologist) Group B: anaesthesiologist Goal level of sedation: Group A: Sedation Group B: Anesthesia All received supplemental oxygen (5 L/min) through nasal cannula | |
| Outcomes | Primary outcome: Patient satisfaction scores, measured on a VAS, 4 hours after colonoscopy Secondary outcomes: Patients' level of sedation, acceptance, procedure time, cecal intubation time, episodes of desaturation and hypotension | |
| Notes | Numbers enrolled in each group not provided. Measure of variation and exact p value not provided for average satisfaction scores or procedure times Definitions: 1) Patients' level of satisfaction scores, measured on a VAS, 4 hours after colonoscopy 2) No other definitions provided | |
Liu 2000.
| Methods | Single center, randomised, controlled trial 1. Allocation concealment: Unclear/Not stated 2. Blinding of patients: Unclear/Not stated 3. Blinding of outcome assessor: Unclear/Not stated 4. A priori calculation of sample size: Unclear/Not stated 5. Groups treated identical other than the named intervention: Unclear/Not stated | |
| Participants | Number of eligible patients: Not stated Number of patients enrolled: 110 Drop outs/Withdrawals: 0 Incl. Criteria:Patients undergoing colonoscopy as an out patient between July 1999 and October 1999 Excl. Criteria: Not stated Country: Hong Kong Setting: Outpatient Age: Not stated N (%) males: Not stated Groups comparable at baseline: Yes (age, sex ratio and body weight) | |
| Interventions | Group A (n=55): Mixture of Propofol 400 mg and Alfentanil 1mg delivered by a PCA pump in bolus of 2mg/ml (lockout period of 3 minutes) Group B (n=55): Intravenous injection of Diazemuls 0.1 mg/ Kg and Pethidine 0.5mg/ Kg Mode of administration: Gropu A: PCA pump bolus, with lockout of 3 mins.; Group B: Intravenous injection Administered by: Not stated Goal level of sedation: Not stated Supplemental oxygen: Not stated | |
| Outcomes | Procedure time; Recovery time Pain score (un‐scaled 10 cm visual analogue score) Oxygen desaturation<90% Hypotension (systolic BP < 90 mm Hg) | |
| Notes | Recovery time not defined Conference proceeding abstract (DDW 2000) | |
Liu 2009.
| Methods | Single center, randomised, controlled trial 1. Allocation concealment: Adequate 2. Blinding of patients: Unclear/Not stated 3. Blinding of outcome assessor: No for the primary outcome, as the level of sedation was recorded by the designated nurse responsible for drug delivery (which was not blinded). For other outcomes Unclear/Not stated‐likely no blinding. 4. A priori calculation of sample size: Yes 5. Groups treated identical other than the named intervention: Yes | |
| Participants | Number of eligible patients: 186
Number of patients enrolled:178
Drop outs/Withdrawals: 0 Incl. Criteria: 1) Patients scheduled for out patient colonoscopy only 2) Age 18‐65 years 3) ASA class I or II Excl. Criteria: 1) ASA class III or above 2) Pregnancy 3) Allergy to any sedative agents 4) Inpatient status 5) History of colonic or rectal resection 6) Cognitive deficit or intellectual disability 6) History of difficult endotracheal intubation 7) Inability or reluctance to give consent. Country: Hong Kong Setting: Outpatient Age (median(range))(yrs): Group A: 48.5 (20‐64) Group B: 49.5 (18‐65) N (%) males: Group A: 43 (49) Group B: 55(61) Groups comparable at baseline: Yes (age, gender, ASA class, previous history of colonoscopy, indications for colonoscopy and baseline hemodynamic parameters) |
|
| Interventions | Group A (n=88): Nurse administered propofol sedation. A loading dose of 40‐60 mg or 0.8 mg/kg propofol, whichever was the higher, was given to patients 1 minute before the commencement of the procedure.. Then a PCA pump was used by the designated nurse to deliver a bolus dose of 1,5 ml mixture containing 14.3 mg propofol admixed with 35 microgram of alfentanil, with zero lock‐out for a goal OSSA score of 3 (assessed every 30 seconds). Median (range) dose; propofol 165 mg (52‐292); alfentanil 0.175 mg (0.035‐0.595) Group B (n=90): Intravenous injection of Diazemuls 0.1 mg/ Kg and Pethidine 0.5mg/ Kg per bolus 1 minute before the commencement of the procedure. Additional drugs administered as half‐dosage bolus, upon request of the endoscopist. Median (range) dose; diazemuls 5 mg (5‐10); pethidine 25 mg (25‐50) Mode of administration: Group A: PCA pump bolus; Group B: Intravenous bolus injections Administered by: Five designated nurses Goal level of sedation: Group A: OSSA 4 Gropu B: as requested by the endoscopist Supplemental oxygen: All patients received supplemental oxygen (2 L/min) through nasal cannula | |
| Outcomes | The primary end‐point of the study was the patient's level of sedation (measured by OSSA score) during colonoscopy intubation and when the cecum was reached. Secondary outcomes: 1) Time to cecal intubation and total procedure time 2) Patient, endoscopist and nurse satisfaction scores with regard to sedation 3) Overall pain score 4) Patients' willingness to repeat colonoscopy with the same sedation 5) Complications related to sedation 6) Cost comparison |
|
| Notes | Nurses and endoscopists involved in the study were first trained by an anaesthesiologist regarding propofol delivery, patient monitoring using OSSA score and airway management. Concealment of allocation is judged to be adequate as first written informed consent was obtained and then randomizations carried out by a designated nurse, using computer generated numbers inside concealed envelopes. Definitions: 1) Patients' level of sedation, measured by OSSA score 2) Complications related to sedation: Hypotension (systolic BP < 90 mm Hg); Oxygen desaturation<90%; Bradycardia (pulse< 50/min). 3) Full recovery= Fully alert (able to calculate serial subtraction in 7s from 100), hemodynamically stable (BP and HR within 20% of baseline and oxygen saturation >90% at room air) and ambulant. 4) Patient, nurse and endoscopist satisfaction: After full recovery of the patient, satisfaction scores regarding sedation from the patient, nurse and endoscopist were documented, using a 10 cm VAS, ranging from 0 (very unsatisfied) to 10 (very satisfied) 5) Pain: as reported by the patients on a similar scale. The continuous outcome variables (procedure time, recovery time, patient satisfaction, pain score, level of sedation) were reported as median and range. As per the Cochrane collaboration handbook, ranges should not be used to calculate SDs and hence these were not included in the meta‐analyses. For the review, we extracted the level of sedation as dichotomous outcome, with "failure to sedate" considered as those with OSSA score of 5 during colonoscopy intubation. |
|
Mandel 2006.
| Methods | Single center, randomised, controlled trial 1) Allocation concealment: Adequate 2) Blinding of patients: Yes 3) Blinding of outcome assessors: Yes 4) A priori calculation of sample size: Yes 5) Groups treated identical other than the named intervention: Unclear/Not stated | |
| Participants | Number of eligible patients: Not stated
Number of patients enrolled: 50
Drop outs/Withdrawals: 1(noncompliance with bowel preparation)
Incl. Criteria: Patients scheduled for elective outpatient colonoscopy with conscious sedation
Excl. Criteria: Not stated
Country: USA
Setting: Outpatient
Age(mean(SD) )(yrs): Group A: 60.5 (9.6) Group B: 57.7 (10.8)
N (%) males: Group A: 13 (52) Group B: 11(46) ASA score : Not provided for all patients (ASA score III: Groups A:2 and group B:1) Groups comparable at baseline: Yes (age, gender, BMI and number of ASA III patients) |
|
| Interventions | Group A (n=25): Propofol (10 mg/ml) and Remifentanil (10 micrograms/ml): Initial bolus of 2.5 ml with demand of 0.75 ml at zero lockout Group B (n=24): Midazolam (0.5 mg/ml) and Fentanyl (12.5 micrograms/ml): Initial bolus of 4 ml with demand of 1ml at 1' lockout Mode of administration: Both groups‐PCS boluses Administered by: PCS; anaesthesiologist present to intervene Goal level of sedation: Not stated All patients received supplemental oxygen (2 L/min) through nasal cannula | |
| Outcomes | Procedure time, time to sedation, recovery time, SaO2 < 85% for 60", patient satisfaction | |
| Notes | Block size of 10 for first ten cases and then 40 for the rest of the cases was used. No mention, whether the study was performed on adult patients, but the mean age and use of PCS suggests that patients were adults. Anesthesiologist intervention for oxygen desaturation recorded Definitions: 1) Patient, gastroenterologist and nurse satisfaction was assessed on a 7‐point Likert scale; however the results section does not provide the recorded scores. 2) Recovery time= time to ambulation‐time at removal of colonoscope; however the time reported in the study and abstracted by us is the "recovery room time", which was not explicitly defined in the study. 3) Procedure time=time between colonoscope removal and insertion. 4) Safety endpoints included: arterial desaturation (85% for more than 60 s); hypotension (90 mmHg systolic or 20% decrease from baseline persisting on repeat determination 1 min later )or inability to tolerate the procedure. 5) Time to sedation: time between insertion of the colonoscope and initiation of the sedation. |
|
Martinez‐Palli 2005.
| Methods | Randomized, controlled trial 1) Allocation concealment: Unclear/Not stated 2) Blinding of patients: Not stated 3) Blinding of outcome assessors: Not stated 4) A priori calculation of sample size: Not stated 5) Groups treated identical other than the named intervention: Unclear/Not stated | |
| Participants | Number of eligible patients: Not stated Number of patients enrolled: 299 Drop outs/Withdrawals: Unclear Incl. Criteria: Not stated Excl. Criteria: Previously difficult and poorly tolerated colonoscopies Country: Spain Setting: Unclear Age(yrs): Not stated N (%) males: Not stated ASA score: Not stated. ASA IV patients were included Groups comparable at baseline: Yes (age, gender, ASA classification, previous experience in colonoscopy and incidence of polypectomy) | |
| Interventions | Group A (n=103): Remiphentanyl (0.1 micrograms/ Kg/min) followed by bolus of Propofol (0.5 mg/ Kg) Group B (n=109): Phentanyl (100 micrograms) and Midazolam (2 mg) Group C (n=87): Meperidine (100 mg) and Midazolam (2 mg) ASA IV patients in group B and C, initially received half the doses. Mode of administration:Remiphentanyl infusion; Propofol bolus; Administered by: Group A: Anesthesiologists; Group B and C: endoscopists Goal level of sedation: Not stated Administration of supplemental oxygen: Not stated | |
| Outcomes | Procedure time, recovery time, colonoscopy completion rate, endoscopist assessment of procedure difficulty and adequacy of sedation, and patient assessment of sedation. | |
| Notes | Age of patients not given in the abstract. Definitions: 1) Patient discharge: Ten points on Aldrete test were needed for patient discharge. | |
Moerman 2003.
| Methods | Single center, randomised, controlled trial 1) Allocation concealment: Unclear/Not stated 2) Blinding of patients: No 3) Blinding of outcome assessors: No 4) A priori calculation of sample size: Yes 5) Groups treated identical other than the named intervention: Yes | |
| Participants | Number of eligible patients: Not stated Number of patients enrolled: 40 Drop outs/Withdrawals:1 in remifentanil group Incl. Criteria: 1) Age: 18‐65 years 2) Outpatients 3) Scheduled for complete colonoscopy Excl. Criteria: 1) Pulmonary, cardiovascular, hepatic, renal, haematological, neurological or metabolic diseases 2) History of allergic reactions to any of the study drugs 3) Patients chronically receiving opoid analgesics or sedative medication 3) General anaesthesia within 7 days before the study Country: Belgium Setting: Outpatient Age(mean(SD) )(yrs): Group A: 41 (15) Group B: 40 (11) N (%) males: Group A: 5 (25) Group B: 3(15) ASA score (I/II): Group A: 16/4 Group B: 18/2 Groups comparable at baseline: Yes (age, sex ratio, body weight, height, indication for colonoscopy, ASA score) | |
| Interventions | Group A (n=20): Propofol (Mean (range) dose: 273 (153‐490) mg) Group B (n=20): Remifentanil (Mean(range) dose: 246 (125‐460) micrograms) Mode of administration: Both groups‐bolus, followed by infusion and supplemental doses Administered by: anaesthesiologist Goal level of sedation: Group A: Supplemental doses given, when lightening of anaesthesia observed Group B: Supplemental doses given, when patients experienced pain All received supplemental oxygen (5 L/min) through face mask | |
| Outcomes | Primary outcome: Recovery time Secondary outcomes: recovery characteristics (neuropsychological test ing scores, before and after the colonoscopy), adverse events (hypoventilation, apnoea, airway obstruction, hypotension, hypertension, dysrhythmias), patient comfort (patient well being 30 mins. after end of anaesthesia, satisfaction with anaesthesia, severe abdominal pain) | |
| Notes | Definitions: 1) Recovery time: immediate recovery assessed using the Steward Post Recovery Score (SPRS) | |
Munoz‐Navas 1994.
| Methods | Single center, randomised, controlled trial 1) Allocation concealment: Unclear/Not stated 2) Blinding of patients: No 3) Blinding of outcome assessors: Yes 4) A priori calculation of sample size: Not stated 5) Groups treated identical other than the named intervention: Not stated | |
| Participants | Number of eligible patients: Not stated Number of patients enrolled: 29 Drop outs/Withdrawals: 0 Incl. Criteria: Not stated Excl. Criteria: Not stated Country: Spain Setting: Ambulatory colonoscopy Age(yrs):45‐75 N (%) males: Not stated ASA score: Not stated Groups comparable at baseline: Not stated | |
| Interventions | Group A (n=14): Propofol (Mean dose: 191.79 mg) Group B (n=15): Midazolam (Mean dose: 18.93 mg) and Flumazenil (Mean dose: 0.28 mg). All patients in this group received incremental doses of flumazenil, until awakening Mode of administration: Both groups‐bolus Administered by: Not stated Goal level of sedation: Not stated Administration of supplemental oxygen: Not stated | |
| Outcomes | Recovery time, discharge time, post procedure amnesia, patient satisfaction, endoscopist satisfaction Vitals and level of sedation during the procedure also measured | |
| Notes | Abstract DDW 1994 Definitions: 1) Recovery time: time to sit, walk and get dressed measured. Time to get dressed used in the meta‐analysis. 2) Methodology for measuring other outcomes not given | |
Ng 2001.
| Methods | Single center, randomised, controlled trial 1) Allocation concealment: Unclear/Not stated 2) Blinding of patients: No 3) Blinding of outcome assessors: Yes 4) A priori calculation of sample size: Unclear/Not stated 5) Groups treated identical other than the named intervention: Yes | |
| Participants | Number of eligible patients: Not stated Number of patients enrolled: 88 Drop outs/Withdrawals: 0 Incl. Criteria: 1) Adult patients scheduled for elective colonoscopy 2) ASA class I or II Excl. Criteria: 1) Pregnancy 2) Patients assessed as being unable to understand or operate the PCS pump Country: Singapore Setting: Patients scheduled for elective colonoscopy Age(mean(SD) )(yrs): Group A: 54 (15) Group B: 49 (13) N (%) males: Group A: 27 (61) Group B: 21(48) ASA score (I/II): Group A: 24/20 Group B: 22/22 Groups comparable at baseline: Yes (age, sex ratio, body weight, ASA score) | |
| Interventions | Group A (n=44): Propofol (Mean (SD) dose: 98.20(36.74) mg) Group B (n=44): Midazolam (Mean(SD) dose: 4.33 (2.08) mg) Mode of administration: Both groups‐bolus; propofol given as PCS Administered by: Group A: PCS Group B: anaesthesiologist Goal level of sedation: Patients in Group B given additional boluses when they experienced discomfort All received supplemental oxygen through nasal cannula, if SaO2 fell below 94% | |
| Outcomes | Primary outcomes: Endoscopist satisfaction, patient cooperation, deepest sedation score, sedation score 30 minutes after surgery and time to discharge Secondary outcomes: Patient overall satisfaction, Patients' level of pain | |
| Notes | 1) Discharge time: Patients deemed fit according to existing protocol in endoscopy suite. 2) Sedation: Patients level of sedation measured on a 6 point scale 3) Pain: Nursing staff assessed pain scores 30 minutes after the procedure on a 4 point scale (post operative pain‐‐therefore not included in the meta‐analysis) 4) Patient satisfaction: assessed on a 4 point scale; results expressed as very/ mostly satisfied vs. mildly satisfied/ quite dissatisfied | |
Paspatis 2002.
| Methods | Single center, randomised, controlled trial 1) Allocation concealment: Unclear/Not stated 2) Blinding of patients: Unclear/Not stated 3) Blinding of outcome assessors: No 4) A priori calculation of sample size: Unclear/Not stated 5) Groups treated identical other than the named intervention: Yes | |
| Participants | Number of eligible patients: Not stated Number of patients enrolled: 120 Drop outs/Withdrawals: Unclear/Not stated Incl. Criteria: Consecutive patients who underwent total colonoscopy Excl. Criteria: 1) Age under 18 or over 80 yrs 2) Cases with incomplete colonoscopy 3) Liver cirrhosis 4) Regular dialysis 5) Critically ill patients (ASA grade V) 6) Pregnant women 7) Cases with chronic use of benzodiazepines or opiates. Country: Greece Setting: Unclear/Not stated Age(mean(SD) )(yrs): Group A: 61.4 (11) Group B: 60.2 (11.5) N (%) males: Group A: 33 (52) Group B: 29(52) ASA grade (n (%): < or = II Group A : 52 (81) Group B: 46(82) > II Group A : 12 (19) Group B: 10(18) Groups comparable at baseline: Yes (age, sex ratio, ASA grade) | |
| Interventions | Group A (n=64): Propofol (Median (range) dose: 80 (40‐150) mg) and Midazolam ( 2‐3 mg) Group B (n=56): Midazolam (Median (range) dose: 5 (3‐7) mg) and Pethidine: (Median (range) dose: 75 (50‐125) mg) Mode of administration: Both groups‐bolus Administered by: Anesthesiologist Goal level of sedation: Group A: adequate level of sedation Group B: Given additional boluses of pethidine, if patients did not tolerate the procedure All received supplemental oxygen (2 L/min) through nasal cannula | |
| Outcomes | Complications (a decline in oxygen saturation <90% for more than 10 s, change in MABP > 10 mm Hg, change in HR < or > 20%) Patients' level of sedation and pain Recovery from sedation | |
| Notes | Number of withdrawals is unclear, as only complete colonoscopies were included. There might have been some patients with incomplete colonoscopy, who were withdrawn after enrolment. Definitions: 1) Recovery from sedation assessed at 5,10,15 and 20 mins., after the procedure, using Aldrete score. 2) Sedation: Patients level of sedation measured on a 5 point scale by anaesthesiologists during procedures and 4 point scale by endoscopists after the procedure 3) Pain: Patients asked (2 hr after the procedure) to rate their pain during the procedure on a 0 to 10 VAS 4) Patient satisfaction: Patients asked (24 hr after the procedure) to rate their evaluation of colonoscopy on a 4 point comfort level scale (data extracted for meta‐analysis dichotomized as No discomfort/Slightly Uncomfortable vs. Extremely uncomfortable/Unacceptable) | |
Pohlmann 1993.
| Methods | Single center, randomised, controlled trial 1) Allocation concealment: Unclear/Not stated 2) Blinding of patients: Yes 3) Blinding of outcome assessors: No 4) A priori calculation of sample size: Unclear/Not stated 5) Groups treated identical other than the named intervention: Unclear/Not stated | |
| Participants | Number of eligible patients: Not stated Number of patients enrolled: 102 Drop outs/Withdrawals: 3 Incl. Criteria: In‐patients, who were 18 years of age or older Excl. Criteria: Serious unrelated medical diseases Country: Germany Setting: Inpatient Age(mean)(yrs): Group A: 55 Group B: 62 N (%) males: Group A: 21 (43) Group B: 25(50) ASA score: Not stated Groups comparable at baseline: Yes (age, sex ratio) | |
| Interventions | Group A (n=49): Propofol: Initial bolus 2.5 mg/kg body weight (for participants 18‐50 years age), 1.7 mg/kg (51‐74 years) or 1.1 mg/kg (75‐93 years). Subsequent infusion 6 mg/kg/hr (18‐50 years ), 4.5 mg/kg/hr (51‐74 years) or 3.8 mg/kg/hr (75‐93 years) Group B (n=50): Midazolam: Maximum dose 12.5 mg Mode of administration: Propofol: bolus followed by infusion Midazolam: bolus Administration directed by endoscopist Goal level of sedation: Slurred speech or lack of reaction (no response to verbal commands) Supplemental oxygen (2 L/min through nasal cannula) administered if oxygen saturation dropped to <90% for more than 1 min | |
| Outcomes | Patients' level of sedation, pain, ability to cooperate, acceptability of procedure, antegrade amnesia, complications (a decline in oxygen saturation, drop in systolic and diastolic pressure, apnea) | |
| Notes | German language article Patients likely blinded, although not explicitly stated in the study. It was stated that the study was single blinded. Withdrawals: Data from three patients (2 in propofol group and 1 in midazolam) were not analysed in the study as they had to be given more than the prescribed medications Definitions: 1) Patients' level of sedation: assessed after the initial bolus injection as awake, somnolent (reaction to verbal command) and asleep 2) Patients' ability to cooperate and acceptability of procedure graded by the endoscopists, immediately after the colonoscopy, on a 10 point scale 3) Pain: Patients asked ( 4 hrs after the procedure) to rate their pain during the procedure on a 10 point scale 4) Mean (and range) drop in systolic, diastolic pressures and O2 saturation provided | |
Reimann 2000.
| Methods | Single center, randomised, controlled trial 1) Allocation concealment: Unclear/Not stated 2) Blinding of patients: No 3) Blinding of outcome assessors: Yes for a) examiners who assessed cognitive patterns and recovery time No for a)endoscopy team 4) A priori calculation of sample size: Unclear/Not stated 5) Groups treated identical other than the named intervention: Yes | |
| Participants | Number of eligible patients: Not stated Number of patients enrolled: 79 Drop outs/Withdrawals: 0 Incl. Criteria: Patients admitted for elective, diagnostic colonoscopies Excl. Criteria: 1) Age less than 18 years or more than 60 years 2) Chronic use of benzodiazepines 3) Known hypersensitivity to midazolam, propofol, or nalbuphine 4) Mental incompetence 5) Pregnancy 6) Severe heart failure 7) Liver cirrhosis 8) Regular dialysis 9) Previous colonic resections Country: Germany Setting: Outpatient Age(mean(SE) )(yrs): Group A: 44 (12) Group B: 41 (12) (%) males: Group A: 57 Group B: 53 ASA score : Not stated Groups comparable at baseline: Yes (age, sex ratio, body weight, height, alcohol intake, anxiety level and indications for procedures) | |
| Interventions | Group A (n=47): Propofol (Median (IQR) dose: 100 (53‐145) mg) and initially 2 mg of midazolam Group B (n=32): Midazolam (Median(IQR) dose: 9 (6‐12) mg) and Nalbuphine: (Median(IQR) dose: 20(10‐20) mg) for the 62%, who did receive nalbuphine Ketamine allowed in both groups, in cases of poor sedation Mode of administration: Both groups‐bolus Administered by: physician trained in critical care medicine Goal level of sedation: Group A: "adequate sedation level" Group B: patient tolerance of the procedure Not stated, whether all patients received supplemental oxygen at baseline | |
| Outcomes | Recovery time, Patients' cognitive function (before and after the colonoscopy), procedure time, patient satisfaction, endoscopist assessment of quality of sedation, severe oxygen desaturation (SaO2 <85%), change in BP (MAP), HR or RR | |
| Notes | Although " the study was planned to include 150 patients", no information is provided on the sample size calculation Definitions: 1) Patient satisfaction:Patients asked (after the procedure) to rate their experience during the colonoscopy: distressing, slightly uncomfortable, or comfortable (results dichotomised as uncomfortable vs. comfortable for meta‐analysis) 2) Recovery time: time to open eyes, speak, shake hands, walk (extracted for meta‐analysis), street fit 3) Fit for discharge: not defined 4) Complications: Determined by monitoring of blood pressure, heart rate and oxygen saturation ; also recorded‐severe oxygen desaturation (SaO2 <85%), "clinically unacceptable changes in BP, HR or RR" | |
Roseveare 1998.
| Methods | Single center, randomised, controlled trial 1) Allocation concealment: Unclear/Not stated 2) Blinding of patients: No 3) Blinding of outcome assessors: No 4) A priori calculation of sample size: Unclear/Not stated 5) Groups treated identical other than the named intervention: Yes | |
| Participants | Number of eligible patients: 86 Number of patients enrolled: 66 Drop outs/Withdrawals: 0 Incl. Criteria: Consecutive patients undergoing routine day‐case colonoscopy Excl. Criteria: 1) Age less than 18 years 2) Severe pre‐existing cardiovascular or respiratory disease 3) Those felt to be unable to use the PCS system Country: UK Setting: Outpatient Age(median(range) )(yrs): Group A: 50 52 (23‐74) Group B: (29‐73) N (%) males: Not stated ASA score: Not stated Groups comparable at baseline: Yes (age, body weight, performance of pre‐procedure number connection test) | |
| Interventions | Group A (n=33): PCS Propofol (Median dose: 105 mg) and Alfentanil (Median dose: 0.13 mg) Group B (n=33): Diazemul (Median (range) dose: 15 (10‐20) mg) and Pethidine: single bolus dose of 50 mg Mode of administration: Group A: PCS boluses Group B: single bolus at beginning of procedure Administered by: Group A: PCS Group B: endoscopist; All patients monitored by anaesthetists Goal level of sedation: Group A (PCS): relieve any discomfort Group B: Not stated All received supplemental oxygen (2 L/min) through nasal cannula | |
| Outcomes | Procedure time, patients' level of sedation, pain, complications, recovery time, patient satisfaction | |
| Notes | Definitions: 1) Patients' level of sedation, measured by a single nurse specialist during the procedure on a 5 point scale 2) Pain score: Patients' recollection, after recovery on a 4 point scale (also measured by a single nurse specialist during the procedure) 3) Recovery time: measured by comparison of number connection tests (NCTs) completed at 10‐min intervals after completion of the procedure with the NCT undertaken prior to sedation. 4) Complications: Maximum fall in systolic blood pressure, difference in O2 saturation, O2 desaturation less than 95% 5) Overall patient satisfaction: not defined | |
Sipe 2002.
| Methods | Single center, randomised, controlled trial 1) Allocation concealment: Inadequate 2) Blinding of patients: Yes 3) Blinding of outcome assessors: Yes for a) examiners performing the neuropsychological testing and assessing patient wakefulness b) recovery room nurses No for endoscopists and nurses administrating sedation 4) A priori calculation of sample size: Unclear/Not stated 5) Groups treated identical other than the named intervention: Yes | |
| Participants | Number of eligible patients: 82 Number of patients enrolled: 80 Drop outs/Withdrawals: 0 Incl. Criteria: Consectutive patients undergoing colonoscopy as out patients who were 1) 18 years of age or older 2) ASA class I or II 3) Scheduled to undergo colonoscopy alone Excl. Criteria: 1) Patient desire to have colonoscopy without sedation 2) ASA class III or more 3) History of colonic or rectal resection 4) Sleep apnea 5) Enlarged tongue 6) Inability to widely open the mouth 7) Short thick neck 8) Known delayed gastric emptying 9) Acute gastrointestinal bleeding 10) Any neurological deficit 11) Pregnancy 12) Inability or unwillingness to give consent 13) Inpatient status 14) An allergy to either eggs or soy products or any of the sedative agents Country: USA Setting: Outpatient Age(mean(SD) )(yrs): Group A: 51.7 (11.3) Group B: 54.2 (14.2) N (%) males: Group A: 21 (52.5) Group B: 19 (47.5) ASA score (mean(SD)): Group A: 1.25 (0.44) Group B: 1.3(0.46) Groups comparable at baseline: Yes (age, sex ratio, body weight, race, education, alcohol intake, smoking, handedness, prior colonoscopy, ASA score) | |
| Interventions | Group A (n=40): Propofol (Mean (SD) dose: 214 (94) mg) Bolus administration Group B (n=40): Midazolam (Mean(SD) dose: 4.7 (1.5) mg) and Meperidine: (Mean (SD) dose: 89.7(29.1)mg) Mode of administration: Both groups‐bolus Administered by: nurse, supervised by endoscopist Goal level of sedation: Nurses instructed to induce a state that allowed patients to tolerate the procedure with minimal to mild pain, while maintaining adequate cardiorespiratory function All received supplemental oxygen (4 L/min) through nasal cannula | |
| Outcomes | Primary outcomes: sedation time, recovery time, discharge time, patient satisfaction scores (10 cm VAS) Secondary outcomes: procedure time, patients' level of sedation, pain, ability to cooperate, Complications (a decline in oxygen saturation <85%, HR< 50, BP < 90/50, need for mechanical ventilation), neuropsychological testing scores (before and after the colonoscopy) | |
| Notes | Before starting the study, the sequence of sedation treatments was created with a coin toss and blocks of 4. The block size of 4 may have been too small to prevent deciphering of sequence. Definitions: 1) Full recovery defined as: systolic/diastolic BP and HR within 20% of baseline, O2 saturation >90% while breathing room air and ability to stand at bedside without instability or assistance Recovery also defined as time to reach score of 5 on Observer's Assessment of Alertness/Sedation scale (data presented in figure only) 2) Discharge time: Recovery room nurses determined when patients could be discharged. The general criteria were: systolic/diastolic BP and HR within 20% of baseline, O2 saturation >90% while breathing room air, ability to walk without instability and ability to drink liquids 3) Sedation: Patients level of sedation measured on a 5 point scale. Nurses instructed to induce a state that allowed patients to tolerate the procedure with minimal to mild pain, while maintaining adequate cardiorespiratory function 4) Pain: Patients asked (after the procedure) to rate their pain during the procedure: None, Mild, Moderate, Severe. Data abstracted for meta‐analysis as Moderate/Severe Pain vs. No/Mild Pain 5) Patient Satisfaction: Patients completed a satisfaction questionnaire by using a visual 10‐cm analogue scale for "overall satisfaction with colonoscopy procedure" | |
Ulmer 2003.
| Methods | Single center, randomised, controlled trial 1) Allocation concealment: Adequate 2) Blinding of patients: Yes 3) Blinding of outcome assessors: Yes for a) examiners performing the neuropsychological testing and assessing patient wakefulness b) recovery room nurses No for a)endoscopists b) nurses administrating the sedation c) endoscopy technicians d) examiners recording intra procedure time points 4) A priori calculation of sample size: Unclear/Not stated 5) Groups treated identical other than the named intervention: Yes | |
| Participants | Number of eligible patients: Not stated Number of patients enrolled: 100 Drop outs/Withdrawals: 0 Incl. Criteria: Patients who presented for outpatient colonoscopy and were 1) 18 years of age or older 2) ASA class I or II (unless they were class III on the basis of renal insufficiency alone) 3) Scheduled to undergo colonoscopy alone Excl. Criteria: 1) Patient desire to have colonoscopy without sedation 2) ASA class III or more (unless they were class III on the basis of renal insufficiency alone) 3) History of colonic or rectal resection 4) Obstructive sleep apnea 5) Anticoagulation 6) Inability to widely open the mouth 7) Short thick neck 8) Known delayed gastric emptying 9) Acute gastrointestinal bleeding 10) Neurological deficit 11) Pregnancy 12) Inability or unwillingness to give consent 13) Inpatient status 14) Known hypersensitivity to any of the study medications or to either soy or egg products Country: USA Setting: Outpatient Age(mean(SD) )(yrs): Group A: 55.6 (11.2) Group B: 55.3 (11.8) N (%) males: Group A: 29 (58) Group B: 25(50) ASA score (mean(SD)): Group A: 1.36 (0.45) Group B: 1.31(0.60) Groups comparable at baseline: Yes (age, sex ratio, body weight, race, education, alcohol intake, smoking, handedness, prior colonoscopy, ASA score) | |
| Interventions | Group A (n=50): Propofol (Mean (SD) dose: 277 (105) mg) Bolus administration Group B (n=50): Midazolam (Mean(SD) dose: 7.2 (2.6) mg) and Fentanyl: (Mean (SD) dose: 117(30) micrograms) Mode of administration: Both groups‐bolus Administered by: nurse, supervised by endoscopist Goal level of sedation: A state that allowed patients to tolerate the procedure with minimal to mild pain, while maintaining adequate cardiorespiratory function All received supplemental oxygen (4 L/min) through nasal cannula | |
| Outcomes | Primary outcomes: sedation time, recovery time, discharge time, patient satisfaction scores (10 cm VAS) Secondary outcomes: procedure time, patients' level of sedation, pain, ability to cooperate, Complications (a decline in oxygen saturation <85%, HR< 50, BP < 90/50, need for mechanical ventilation), neuropsychological testing scores (before and after the colonoscopy) | |
| Notes | Definitions: 1) Full recovery defined as: BP and HR within 20% of baseline, O2 saturation >90% while breathing room air and ability to stand at bedside without assistance Recovery also defined as proportion of patients reaching a score of 5 on Observer's Assessment of Alertness/Sedation scale, 15 mins after arrival to the recovery area 2) Discharge time: Patients met criteria for full recovery, able to drink fluids and judged subjectively to be stable by recovery room nurse. 3) Sedation: Patients level of sedation measured on a 5 point scale 4) Pain: Patients asked (after the procedure) to rate their pain during the procedure: None, Mild, Moderate, Severe. Data abstracted for meta‐analysis dichotomized as Moderate/Severe Pain vs. No/Mild Pain 5) Patient satisfaction: "overall satisfaction with the colonoscopy procedure" on a 10 cm VAS | |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Gasparovic 2003 | Non‐randomised study. " The first group consisted of 102 consecutive patients". |
| Hansen 2004 | Duplicate report. Patients in RCT study, reported in Ulmer 2003 |
| Kostash 1992 | Duplicate reference. Conference abstract. Full article published in Canadian Journal of Gastroenterology 1994 |
| Mandel 2010 | An Anesthesiologist was present in the endoscopy room for all patients. All patients received propofol. |
| Masklekar 2007 | Comparison of use of propofol to inhalational anaesthesia with nitrous oxide (Entonox). |
| Riphaus 2006 | Study randomised patients undergoing upper or lower use gastrointestinal endoscopy. Results for those undergoing colonoscopy not reported separately. |
| Theodorou 2001 | Comparative group was not traditional sedation. Comparison of combination of propofol/fentanyl/midazolam to inhalational anaesthesia with sevoflurane/nitrous oxide |
| Wang 2006 | Retrospective Study |
| Yadav 2000 | Study randomised patients undergoing upper or lower gastrointestinal endoscopy. Results for those undergoing colonoscopy not reported separately. |
Differences between protocol and review
None
Contributions of authors
Intial Review
HS: Protocol writing, Abstraction of data, Analysis, Writing final manuscript WP: Literature search, revision of protocol and final manuscript MC: Statistical analysis, revision of protocol and final manuscript NC: Abstraction of data, revision of final manuscript KB: Revision of protocol and final manuscript ST: Revision of protocol and final manuscript, Abstraction of data, Analysis All authors edited and approved the final manuscript
The 2011 updated review was performed by HS, WP, EI and ST.
Sources of support
Internal sources
University of Manitoba Department of Internal Medicine, Canada.
External sources
No sources of support supplied
Declarations of interest
None known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Akcaboy 2006 {published data only}
- Akcaboy ZN, Akcaboy EY, Albayrak D, Altinoren B, Dikmen B, Gogus N. Can remifentanil be a better choice than propofol for colonoscopy during monitored anesthesia care?. Acta Anaesthesiologica Scandinavica 2006;50(6):736‐741. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Amornyotin 2010 {published data only}
- Amornyotin S, Srikureja W, Chalayonnavin W, Kongphlay S. Intravenous sedation with midazolam and fentanyl versus propofol and meperidine in colonoscopy: a prospective randomized study. Endoscopy 2010;42(Suppl 1):A149. [Google Scholar]
Bright 2003 {published data only}
- Bright E, Roseveare C, Dalgleish D, Kimble J, Elliott J, Shepherd H. Patient‐controlled sedation for colonoscopy: a randomized trial comparing patient‐controlled administration of propofol and alfentanil with physician‐administered midazolam and pethidine. Endoscopy 2003;35(8):683‐687. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Germain 1989 {published data only}
- Germain JL, Levacher S, Casteran R, Bourdiau S, Bernasconi C, Blaise M, Hermant JL. Comparison of 2 anesthetic protocols in colonoscopy on a series of 100 patients. Agressologie: Revue Internationale de Physio‐Biologie et de Pharmacologie Appliquees aux Effets de l'agression 1989;30(4):171‐174. [MEDLINE: ] [PubMed] [Google Scholar]
Heuss 2003 {published data only}
- Heuss LT, Hayoz J, Schnieper P, Hirt T, Beglinger C. Influence of propofol ‐ or midazolam sedation on transcutaneous PCO2 values during colonoscopies ‐ a prospective randomized study. Gut 2003;52(Suppl VI):A115. [MEDLINE: ] [Google Scholar]
Heuss 2005 {published data only}
- Heuss LT, Schnieper P, Hirt T, Beglinger C. Reduced hypoventilation time with propofol sedation compared to midazolam during colonoscopy: A prospective randomized trial. Gastrointestinal endoscopy 2005;61(5):AB114. [MEDLINE: ] [Google Scholar]
Jimenez‐Perez 2000 {published data only}
- Jimenez‐Perez J, Pastor G, Aznarez R, Carral D, Rodriguez C, Borda F. Iatrogenic perforation in diagnostic colonoscopy related to the type of sedation. DDW Abstracts. 2000:3351. [MEDLINE: ]
Kostash 1994 {published data only}
- Kostash MA, Johnston R, Bailey RJ, Konopad EM, Guthrie LP. Sedation for colonoscopy: A double‐blind comparison of diazepam /meperidine, midazolam/fentanyl and propofol/fentanyl combinations. CAN J GASTROENTEROL 1994;8(1):27‐31. [MEDLINE: ] [Google Scholar]
Kulling 2001 {published data only}
- Kulling D, Fantin AC, Biro P, Bauerfeind P, Fried M. Safer colonoscopy with patient‐controlled analgesia and sedation with propofol and alfentanil. Gastrointestinal endoscopy 2001;54(1):1‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Laquiere 2006 {published data only}
- Laquiere A, Poincloux L, Monzy F, Artigues F, Bonny C, Dapoigny M, Bazin J, Bommelaer G. Sedation using propofol under the gastroenterologist's supervision during a colonoscopy: Results of a prospective randomized and controlled study. UEGW 2006 Abstract Database. 2006. [MEDLINE: ]
Liu 2000 {published data only}
- Liu GM, Ching JY, Chan SK, Lau JY, Sun LC. A randomized trial comparing patient‐controlled sedation using propofol/alfentanil to diazemul/pethidine in patients undergoing outpatient colonoscopy. Gastrointestinal endoscopy 2000;51(4):AB62. [MEDLINE: ] [Google Scholar]
Liu 2009 {published data only}
- Liu SY, Poon CM, Leung TL, Wong CW, Chan YL, Leung TC, et al. Nurse‐administered propofol‐alfentanil sedation using a patient‐controlled analgesia pump compared with opioid‐benzodiazepine sedation for outpatient colonoscopy. Endoscopy 2009;41(6):522‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mandel 2006 {published data only}
- Mandel JE, Tanner J, Lichtenstein GR, Metz DC, Katzka DA, Ginsberg G, Kochman ML. A randomized controlled double blind trial of patient‐controlled sedation for colonoscopy with propofol/remifentanil vs. midazolam. American Journal of Gastroenterology 2006;101(9):S520‐S521. [MEDLINE: ] [Google Scholar]
- Mandel JE, Tanner JW, Lichtenstein GR, Metz DC, Katzka DA, Ginsberg GG, et al. A randomized, controlled, double‐blind trial of patient‐controlled sedation with propofol/remifentanil versus midazolam/fentanyl for colonoscopy. Anesth Analg 2008;106(2):434‐9, table. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Martinez‐Palli 2005 {published data only}
- Martinez‐Palli G, Bordas JM, Llach J, Gines A, Lopez A, Gambus P, Pellise M, Mondelo F, Fernandrz‐Esparrach G, Ubres M, Pique JM. Sedo‐analgesia in colonoscopy: Prospective study comparing propofol‐remiphentanyl (PR), phentanyl‐midazolam (PM) and meperidine‐midazolam (MM). Gastrointestinal endoscopy 2005;61(5):AB109. [MEDLINE: ] [Google Scholar]
Moerman 2003 {published data only}
- Moerman AT, Foubert LA, Herregods LL, Struys MM, Wolf DJ, Looze DA, Vos MM, Mortier EP. Propofol versus remifentanil for monitored anaesthesia care during colonoscopy. European journal of anaesthesiology 2003;20(6):461‐466. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Munoz‐Navas 1994 {published data only}
- Munoz‐Navas M, Garcia‐Pedrajas F, Panadero A, Macias E, Corella C, Lopez L, Pueyo J. Midazolam‐flumazenil (MF) versus propofol (P) for ambulatory colonoscopy (C). Preliminary results of a randomized single blinded study.. Gastrointestinal Endoscopy 1994;40(2):P29. [MEDLINE: ] [Google Scholar]
Ng 2001 {published data only}
- Ng JM, Kong CF, Nyam D. Patient‐controlled sedation with propofol for colonoscopy. Gastrointestinal endoscopy 2001;54(1):8‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Paspatis 2002 {published data only}
- Paspatis GA, Manolaraki M, Xirouchakis G, Papanikolaou N, Chlouverakis G, Gritzali A. Synergistic sedation with midazolam and propofol versus midazolam and pethidine in colonoscopies: a prospective, randomized study. The American Journal of Gastroenterology 2002;97(8):1963‐1967. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pohlmann 1993 {published data only}
- Pohlmann S, Herden HN, Hagenmuller F. Propofol narcosis for endoscopy‐‐more dangerous than midazolam?. Bildgebung = Imaging 1993;60 Suppl 1:61‐63. [MEDLINE: ] [PubMed] [Google Scholar]
Reimann 2000 {published data only}
- Reimann FM, Samson U, Derad I, Fuchs M, Schiefer B, Stange EF. Synergistic sedation with low‐dose midazolam and propofol for colonoscopies. Endoscopy 2000;32(3):239‐244. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Roseveare 1998 {published data only}
- Roseveare C, Seavell C, Patel P, Criswell J, Kimble J, Jones C, Shepherd H. Patient‐controlled sedation and analgesia, using propofol and alfentanil, during colonoscopy: a prospective randomized controlled trial. Endoscopy 1998;30(9):768‐773. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sipe 2002 {published data only}
- Sipe BW, Rex DK, Latinovich D, Overley C, Kinser K, Bratcher L, Kareken D. Propofol versus midazolam/meperidine for outpatient colonoscopy: Administration by nurses supervised by endoscopists. Gastrointestinal endoscopy 2002;55(7):815‐825. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ulmer 2003 {published data only}
- Ulmer BJ, Hansen JJ, Overley CA, Symms MR, Chadalawada V, Liangpunsakul S, Strahl E, Mendel AM, Rex DK. Propofol versus midazolam/fentanyl for outpatient colonoscopy: administration by nurses supervised by endoscopists. Clin Gastroenterol Hepatol 2003;1(6):425‐432. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Gasparovic 2003 {published data only}
- Gasparovic S, Rustemovic N, Opacic M, Bates M, Petrovecki M. Comparison of colonoscopies performed under sedation with propofol or with midazolam or without sedation. Acta Medica Austriaca 2003;30(1):13‐16. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hansen 2004 {published data only}
- Hansen JJ, Ulmer BJ, Rex DK. Technical performance of colonoscopy in patients sedated with nurse‐administered propofol. The American Journal of Gastroenterology 2004;99(1):52‐56. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kostash 1992 {published data only}
- Kostash MA, Johnston R, Bailey RJ. Sedation for colonoscopy: A double‐blind comparison of diazepam, midazolam and propofol. Canadian Journal of Anaesthesia 1992;39(5 II SUPPL.):A124. [MEDLINE: ] [Google Scholar]
Mandel 2010 {published data only}
- Mandel JE, Lichtenstein GR, Metz DC, Ginsberg GG, Kochman ML. A prospective, randomized, comparative trial evaluating respiratory depression during patient‐controlled versus anesthesiologist‐administered propofol‐remifentanil sedation for elective colonoscopy. Gastrointest Endosc 2010;72(1):112‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Masklekar 2007 {published data only}
- Masklekar S, Balaji P, Hartely J, Culbert B, Duthie G. Randomized controlled trial of patient controlled sedation for colonoscopy: Entonox versus patient maintained target controlled propofol. Gastrointestinal endoscopy 2007;65(5):AB115. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Riphaus 2006 {published data only}
- Riphaus A, Gstettenbauer T, Frenz MB, Wehrmann T. Quality of psychomotor recovery after propofol sedation for routine endoscopy: a randomized and controlled study. Endoscopy 2006;38(7):677‐683. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Theodorou 2001 {published data only}
- Theodorou T, Hales P, Gillespie P, Robertson B. Total intravenous versus inhalational anaesthesia for colonoscopy: a prospective study of clinical recovery and psychomotor function. Anaesthesia and Intensive Care 2001;29(2):124‐136. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wang 2006 {published data only}
- Wang AH, Mourad WA, Suvock E, Komar MJ. Duration of screening colonoscopies: Comparing sedation with propofol versus meperidine and midazolam. American Journal of Gastroenterology 2006;101(9):S520. [MEDLINE: ] [Google Scholar]
Yadav 2000 {published data only}
- Yadav D, Tatli Y, Sachan P, Kiyici N, Koshy G. Propofol is a safe, effective agent to be used by gastroenterologists for conscious sedation in GI endoscopy: a randomized double blind study. AJG: American Journal of Gastroenterology 2000;95(9):2504. [MEDLINE: ] [Google Scholar]
Additional references
Cohen 2007
- Cohen LB, Delegge MH, Aisenberg J, Brill JV, Inadomi JM, Kochman ML, Piorkowski JD, Jr. AGA Institute review of endoscopic sedation. Gastroenterology 2007;133(2):675‐701. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Condon 2008
- Condon A, Graff L, Elliot L, Ilnyckyj A. Acceptance of colonoscopy requires more than test tolerance. Can J Gastroenterol 2008;22(1):41‐47. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials.. Controlled Cinical Trials 1986;7:177‐188. [DOI] [PubMed] [Google Scholar]
Faulx 2005
- Faulx AL, Vela S, Das A, Cooper G, Sivak MV, Isenberg G, Chak A. The changing landscape of practice patterns regarding unsedated endoscopy and propofol use: a national web survey.. Gastrointestinal Endoscopy 2005;62(1):9‐15. [DOI] [PubMed] [Google Scholar]
Lee 2002
- Lee DW, Chan AC, Sze TS, Ko CW, Poon CM, Chan KC, Sin KS, Chung SC. Patient‐controlled sedation versus intravenous sedation for colonoscopy in elderly patients: a prospective randomized controlled trial.. Gastrointestinal Endoscopy 2002;56(5):629‐632. [DOI] [PubMed] [Google Scholar]
Lee 2002a
- Lee DW, Chan KW, Poon CM, Ko CW, Chan KH, Sin KS, Sze TS, Chan AC. Relaxation music decreases the dose of patient‐controlled sedation during colonoscopy: a prospective randomized controlled trial.. Gastrointestinal Endoscopy 2002;55(1):33‐36. [DOI] [PubMed] [Google Scholar]
Mandel 2006a
- Mandel JE, Tanner J, Lichtenstein GR, Metz DC, Katzka DA, Ginsberg G, Kochman ML. A randomized controlled double blind trial of patient‐controlled sedation for colonoscopy with propofol/remifentanil vs. midazolam. American Journal of Gastroenterology 2006;101(9):S520‐S521. [MEDLINE: ] [Google Scholar]
Mandel 2008a
- Mandel JE, Tanner JW, Lichtenstein GR, Metz DC, Katzka DA, Ginsberg GG, et al. A randomized, controlled, double‐blind trial of patient‐controlled sedation with propofol/remifentanil versus midazolam/fentanyl for colonoscopy. Anesth Analg 2008;106(2):434‐9, table. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mantel 1959
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease.. Journal of the National Cancer Institute 1959;22:719‐748. [PubMed] [Google Scholar]
Marik 2004
- Marik PE. Propofol: Therapeutic indications and side‐effects.. Current Pharmaceutical Design 2004;10(29):3639‐3649. [DOI] [PubMed] [Google Scholar]
McQuaid 2008
- McQuaid KR, Laine L. A systematic review and meta‐analysis of randomized, controlled trials of moderate sedation for routine endoscopic procedures. Gastrointest Endosc 2008;67(6):910‐923. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Radaelli 2008
- Radaelli F, Meucci G, Sgroi G, Minoli G. Technical performance of colonoscopy: the key role of sedation/analgesia and other quality indicators. Am J Gastroenterol 2008;103(5):1122‐1130. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rex 2005
- Rex DK, Heuss LT, Walker JA, Qi R. Trained registered nurses/ endoscopy teams can administer propofol safely for endoscopy.. Gastroenterology 2005;129(5):1384‐1391. [DOI] [PubMed] [Google Scholar]
Rudner 2003
- Rudner R, Jalowiecki P, Kawecki P, Gonciarz M, Mularczyk A, Petelenz M. Conscious analgesia/sedation with remifentanil and propofol versus total intravenous anesthesia with fentanyl, midazolam and propofol for outpatient colonoscopy.. Gastrointestinal Endoscopy 2003;57(6):657‐663. [DOI] [PubMed] [Google Scholar]
Shafer 2008
- Shafer SL, Stanski DR. Defining depth of anesthesia. Handb Exp Pharmacol 2008;182:409‐423. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Subramanian 2005
- Subramanian S, Liangpunsakul S, Rex DK. Preprocedure patient values regarding sedation for colonoscopy.. Journal of Clinical Gastroenterology 2005;39(6):516‐519. [DOI] [PubMed] [Google Scholar]
Walker 2003
- Walker JA, McIntyre Rd, Schleinitz PF, Jacobson KN, Haulk AA, Adesman P, Tolleson S, Parent R, Donnelly R, Rex DK. Nurse‐administered propofol sedation without anesthesia specialists in 9152 endoscopic cases in an ambulatory surgery center.. American Journal of Gastroenterology 2003;98(8):1744‐1750. [DOI] [PubMed] [Google Scholar]
Wehrmann 2007
- Wehrmann T, Riphaus A. Sedation with propofol for interventional endoscopic procedures: A risk factor analysis. Scand J Gastroenterol 2007:1‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]


