Summary
Inherited genetic variation is increasingly identified as an important factor predisposing to a variety of cancers; in this issue of Cancer Discovery, Pareja and colleagues developed a method of reliably detecting mosaic cancer susceptibility mutations in patients that have been sequenced as part of the MSK-IMPACT tumor profiling platform. This led to the identification of a number of mosaic mutations in cancer susceptibility alleles that are generally found in the germline, suggesting that many predisposition variants may be missed through conventional testing.
In 1981, it was shown that DNA isolated from multiple human cancers had the capability to transform cells into a cancerous state, providing concrete evidence that all the necessary ingredients of cancer existed within the genome (1). This led to the recognition that acquired somatic mutations within the genome can drive nearly all cancers - a finding bolstered in the subsequent years through innumerable cancer genomic studies (2). Germline inheritance of specific mutations can also predispose to cancer acquisition. For instance, germline variants in the RB1 tumor suppressor can undergo loss of heterozygosity and generate retinoblastoma (3), while germline loss-of-function in BRCA1 and BRCA2 can predispose individuals to acquiring breast and ovarian cancer (4). While these and other germline mutations have been thoroughly defined and have led to testing of individuals with familial cancer syndromes, it is likely that some cancer predisposing mutations have been missed. Cancer predisposing alleles can be overlooked due to low penetrance in families and because of technical limitations in mutation detection. In this latter category, an often underappreciated limitation is mosaicism in which only a subset of cells harbor a mutation, which typically result when a mutation arises during early development. Because of limitations in our ability to detect mosaic mutations, it is unclear to what extent these mutations contribute to cancer predisposition. This is not only a question of mosaic mutation frequency, but as the human body has evolved numerous mechanisms for eliminating mutant cells from heterogeneous populations, it is unclear how cancer predisposition is affected when both nonmutant and mutant cells are present (5). Detecting mosaic mutations reliably, particularly in samples from individuals with cancer who harbor a variety of somatic driver mutations, can be challenging and therefore we do not know how widespread this risk mechanism is. Pareja and colleagues therefore developed a method of reliably detecting mosaic cancer predisposition mutations in order to provide an understanding of rates at which these mosaic events occur and their associated consequences to cancer risk (6).
Identification of germline predisposing alleles has become relatively routine, as established sequencing methods are sufficiently sensitive to detect them. However, mosaic mutations can exist at a range of allele frequencies and can even be inconsistent across tissues in a given individual. These mutations can be difficult to distinguish from sequencing errors, or clonally expanded populations of cells harboring somatic mutations, such as those that occur in premalignancy and in tumors. To understand the contributions of mosaic cancer susceptibility mutations, Pareja and colleagues employed a cohort of 35,310 cancer patients that had undergone sequencing of both tumor and matched peripheral blood samples using the Memorial Sloan Kettering Integrated Mutation Profiling of Actionable Targets (MSK-IMPACT) assay which employs massively parallel sequencing to search over 500 genes for pathogenic mutations (7). From this panel, the authors focused on 61 genes harboring known germline predisposition alleles, and searched for mosaic mutations that may have been missed when calling conventional germline variants. In order to be classified as mosaic, variants were required to exist at an allele frequency between 1.5%-30% in blood samples and be detectable at an allele frequency above 10% in clonal tumor tissue (Figure 1). Because the authors were searching for mutations that contribute to tumorigenesis when present in a fraction of the cells within individuals, they also required that tumors must carry these candidate variants at an allele frequency at least 1.5 times higher than in the blood, as this would increase confidence of a functional role in a cancer derived from a clonal population that harbored the mosaic mutation. This approach allowed the authors to identify 53 mosaic variants that appeared to contribute to tumor development. Interestingly, while 36 of the discovered variants have been previously classified as pathogenic or likely pathogenic, 17 were variants of uncertain significance (VUS), suggesting the author's approach could help to define a potential pathogenic role for many variants (8). When the authors compared the allele frequencies of mosaic mutations in healthy cells to tumor tissue, there was a striking increase in allele frequency from a mean of 8% to 50%. This provided strong support that the author's method of identifying mosaic mutations was robust enough to detect mutations that may have contributed to cancer risk in a clonal population that is found in the tumor. To further confirm the accuracy of their method, the authors used the MSK-IMPACT platform to confirm the presence of mosaic variants within banked formalin-fixed paraffin embedded (FFPE) tissue spanning multiple organs, including tumor samples from 10 patients.
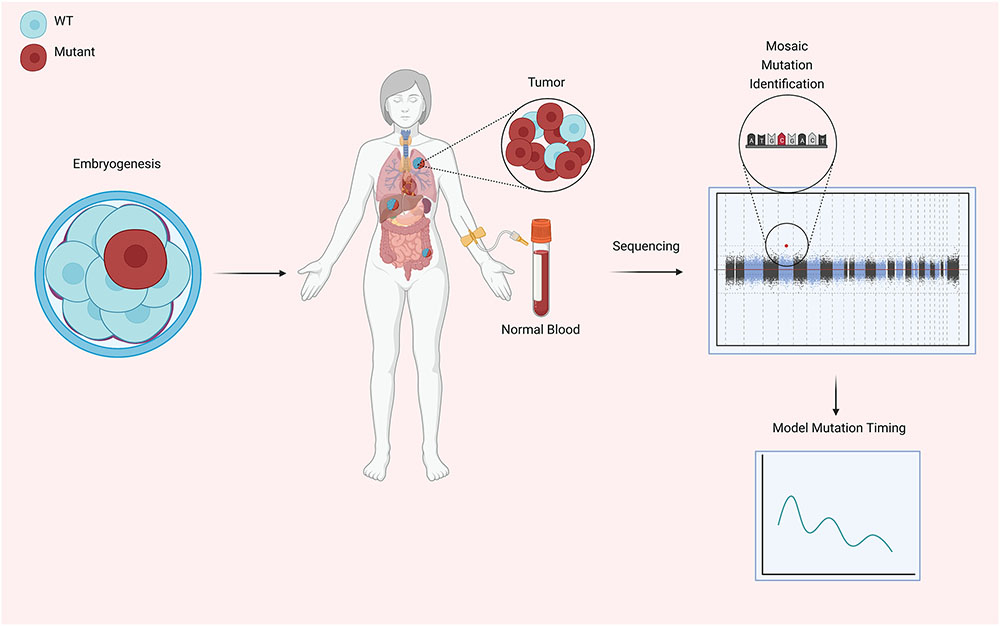
Figure 1.
Early during embryogenesis, mosaicism can develop through fixation of acquired mutations in cancer susceptibility genes (CSGs). Pareja et al. report evidence of this phenomenon occurring as early as the first cell division. In mature organisms mosaic tissues can develop tumors that are driven by the selective advantage of cells carrying mutations in CSGs, and typically tumors have an increased presence of mutant cells than healthy tissue, consistent with their clonal origins. Blood and tumor tissue were sequenced to identify individuals that developed cancer as a result of mutant CSG mosaicism and used to characterize the cancer development driven by mutant CSG mosaicism and model the developmental timing of the original mutation. This figure was created with Biorender.
The pathogenic mosaic mutations found within cancer patients spanned a range of genes; TP53 harbored mutations in nearly half of the cases. 75% of all mosaic mutations were loss of function, and are therefore likely to have important functional consequences on cells during embryogenesis and later during tumor formation. It is unclear why there are such a large number of mosaic TP53 mutations detected in this study, but it may be that mosaicism for these variants is better tolerated than in the context of germline alleles, at least among the set of 61 genes examined in this study (9). Overlapping the observed mosaic mutations with previously defined mutation signatures, the mosaic mutations appeared to be consistent with an age-dependent clocklike signature, suggesting that they spontaneously arise with cell divisions during development without the need for extraordinary sources of DNA damage (10). The cancers that arose as a result of pathogenic mutation mosaicism were surprisingly variable and affected tissues involving all three germ layers. Though breast cancers were the most likely cancer to arise as a result of being mosaic for cancer susceptibility mutations, cancers involving 17 different tissues were found to have these mutations. Using tumor sequencing data, the authors investigated the underlying mechanism behind which mosaic cancer susceptibility mutations gave rise to cancers, and found that in nearly all cases, tumors were composed of cells that biallelically inactivated the gene harboring the mosaic mutation - a mechanism that is common for many germline predisposition alleles, particularly among those genes screened in this study. This provides a fascinating glimpse into the evolution of cancers in general, as mosaicism for cancer predisposing mutations may serve to facilitate a second hit mutation and increase the probability that a cell will inactivate both copies of a tumor suppressor gene.
Finally, the data obtained by sequencing through MSK-IMPACT was used to model the developmental history of the cancers found within patients. To do this, the allele frequencies of mosaic variants were used to predict the number of cells present within each embryo at the time that the mosaic mutation occurred. The authors initially use a simple model that assumes all cells before the first five cell divisions evenly contribute to all three germ layers. Surprisingly, this simple model of embryogenesis did not match the observed allele frequencies of the mosaic mutations identified. To adjust the model, the authors allowed any two early cell divisions to occur asymmetrically and contribute unevenly to the different germ layers, and found this to be consistent with the observed mosaic allele frequencies. This modeling provides compelling evidence that even as early as the 2-4 cell stage, embryonic cells exhibit some degree of lineage bias and may often unevenly contribute to the different germ layers. Furthermore, the authors are able to reliably time many of the observed mosaic mutations to a point in embryogenesis before the fifth cell division.
The author's approach for identifying mosaic cancer predisposition alleles could provide substantial benefit to screening practices and suggest that many individuals who have cancer-predisposing mosaicism may often be overlooked. Indeed, these findings suggest that predisposition alleles may play a broader role in cancer development than currently appreciated. Additionally, as the observed mosaic mutations appear to play a causal role in the development of patient tumors, it is fascinating to imagine how pathogenic clones can be maintained throughout the lives of otherwise healthy individuals and promote the development of cancers only decades after birth. In addition, it will be interesting to examine how cell competition may play a role in enabling the mosaic mutation-harboring clones to survive. It is also possible that mosaicism was the result of reversion of mutations found in the germline, and acted as a protective mechanism either against developmental defects arising from these mutations or the development of cancer. Combining the author's approach to screening with other comorbidities and life history may teach us about important contributing factors that shape the evolution of cancers. In many ways, the work described may only represent the tip of the iceberg, as the authors have only examined genes that are reliably associated with cancer predisposition and as more genes harboring cancer risk variants are identified, the importance of mosaicism for these alleles will need to be fully assessed.
Acknowledgments
The Sankaran lab is supported by the New York Stem Cell Foundation, a gift from the Lodish Family to Boston Children's Hospital, and National Institutes of Health Grants R01 DK103794 and R01 HL146500. L.A.L. is supported by National Institutes of Health Grant T32 HL007574. V.G.S. is a New York Stem Cell-Robertson Investigator.
REFERENCES
- 1.Perucho M, Goldfarb M, Shimizu K, Lama C, Fogh J, Wigler M. Human-tumor-derived cell lines contain common and different transforming genes. Cell. 1981;27:467–76. [DOI] [PubMed] [Google Scholar]
- 2.Stratton MR, Campbell PJ, Futreal PA. The cancer genome. Nature. 2009;458:719–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friend SH, Bernards R, Rogelj S, Weinberg RA, Rapaport JM, Albert DM, et al. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature. 1986;323:643–6. [DOI] [PubMed] [Google Scholar]
- 4.Roy R, Chun J, Powell SN. BRCA1 and BRCA2: different roles in a common pathway of genome protection. Nat Rev Cancer. 2011;12:68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kon S, Ishibashi K, Katoh H, Kitamoto S, Shirai T, Tanaka S, et al. Cell competition with normal epithelial cells promotes apical extrusion of transformed cells through metabolic changes. Nat Cell Biol. 2017;19:530–41. [DOI] [PubMed] [Google Scholar]
- 6.Pareja F Cancer Causative Mutations Occurring in Early Embryogenesis. Cancer Discov. 2022; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng DT, Mitchell TN, Zehir A, Shah RH, Benayed R, Syed A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. J Mol Diagn. 2015;17:251–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Federici G, Soddu S. Variants of uncertain significance in the era of high-throughput genome sequencing: a lesson from breast and ovary cancers. J Exp Clin Cancer Res. 2020;39:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vousden KH, Prives C. P53 and prognosis: new insights and further complexity. Cell. 2005;120:7–10. [DOI] [PubMed] [Google Scholar]
- 10.Nik-Zainal S, Alexandrov LB, Wedge DC, Van Loo P, Greenman CD, Raine K, et al. Mutational processes molding the genomes of 21 breast cancers. Cell. Elsevier; 2012;149:979–93. [DOI] [PMC free article] [PubMed] [Google Scholar]