Abstract
Background
In view of the theoretical possibility of beneficial effects of DHEA retarding age‐associated deterioration in cognitive function, we have reviewed studies in this area.
Objectives
To establish whether administration of DHEA improves cognitive function or quality of life or reduces the rate of decline of cognitive function in normal older adults.
Search methods
The Specialized Register of the Cochrane Dementia and Cognitive Improvement Group (CDCIG), The Cochrane Library, MEDLINE, EMBASE, PsycINFO, CINAHL and LILACS were searched on 18 March 2008 using the terms: dhea*, prasterone, dehydroepiandrosterone*. The CDCIG Specialized Register contains records from all major health care databases (The Cochrane Library, MEDLINE, EMBASE, PsycINFO, CINAHL, LILACS) as well as from many clinical trials registries and grey literature sources.
Relevant journals, personal communications and conference abstracts were searched for randomized controlled trials investigating the effects of DHEA/S on cognition in older adults.
Selection criteria
All randomized placebo‐controlled trials enrolling people aged over 50 without dementia and to whom DHEA in any dosage was administered for more than one day were considered for inclusion in the review.
Data collection and analysis
Data for the specified outcomes were independently extracted by two reviewers (JGE and RM) and cross‐checked. Any discrepancies were discussed and resolved. No data pooling was undertaken owing to the lack of availability of the relevant statistics.
Main results
Five studies provided results from adequate parallel‐group data. Barnhart 1999 and Dayal 2006 enrolled perimenopausal women with complaints of decreased well being and, using three cognitive measures, found no significant effect of DHEA compared with placebo at 3 months. Wolf 1998b enrolled 75 healthy volunteers (37 women and 38 men aged 59‐81) in a study of the effect of DHEA supplements on cognitive impairment induced by stress; after two weeks of treatment, placebo group performance deteriorated significantly on a test of selective attention following a psychosocial stressor (p<0.05), while deterioration was not evident in the DHEA group (p=0.85). However, when compared with placebo, DHEA was associated with significant impairment on a visual memory recall test (p<0.01) following the stressor. No significant effects were found on a third cognitive task. Effects were not found on tasks when administered in the absence of a stressor. van Niekerk 2001 found no effect on cognitive function in 46 men aged 62‐76 from three months of DHEA supplementation. Nair 2006, enrolled 57 women and 87 men with low level of sulphated DHEA in a 24‐month study, no significant changes in quality of life measures for either sex were found. In Von Muhlen 2008 DHEA for one year showed no benefit on cognition performance in 225 healthy older people. Reduced performance in a visual memory recall test observed in one trial and a significant drop‐out rate in favour of placebo emerged in another trial.
Authors' conclusions
What little evidence there is from controlled trials does not support a beneficial effect of DHEA supplementation on cognitive function of non‐demented middle‐aged or elderly people. There is inconsistent evidence from the controlled trials about adverse effects of DHEA.
In view of growing public enthusiasm for DHEA supplementation, particularly in the USA, and the theoretical possibility of long‐term neuroprotective effects of DHEA there is a need for further high quality trials in which the duration of DHEA treatment is longer than one year, and the number of participants is large enough to provide adequate statistical power. Cognitive outcomes should be assessed in all trials.
Plain language summary
No current evidence for an improvement in memory or other aspects of cognitive function of non‐demented older people following DHEA supplements
The adrenal hormone dehydroepiandrosterone (DHEA) and its sulphated ester (DHEAS) are together the most abundant of steroid hormones in both sexes. Blood levels are high in young adults and decrease with advancing age. There is some epidemiological evidence that relatively high serum DHEAS levels in males may protect against heart disease and be associated with increased longevity. In the USA there is growing public enthusiasm for DHEA supplementation as a means of retarding ageing and age‐associated cognitive impairment but there is very little evidence from controlled trials. In two trials DHEA was associated with a deleterious effect on visual memory after a psychosocial stressor and quality of life measures, but there is inconsistent systematic evidence of adverse effects from DHEA. Longer‐term randomized placebo controlled trials are needed for low and high doses.
Background
The adrenal hormone dehydroepiandrosterone (DHEA) and its sulphated ester (DHEAS) are together the most abundant steroid hormones in both sexes. They occur in high blood levels in young adults and decrease with advancing age (Guazzo 1996; Orentreich 1992). DHEA/S are neurosteroids, i.e. they can be synthesized in neurons and glial cells as well as in the adrenal glands (Baulieu 1998). They increase the effects of the excitatory neurotransmitter, glutamate (Debonnel 1996), and decrease the effects of the inhibitory neurotransmitter, GABA (Majewska 1995). DHEA may also act as a neuroprotective agent (Kimonides 1998; Kimonides 1999) and boost the immune system (Casson 1993). There is some clinical and experimental evidence that administration of DHEA stimulates the activity of natural killer cells (Casson 1993) and might be effective in the treatment of immunological disease (Ebeling 1994; Van Vollenhoven 1994).
Both the immunological and neural effects of DHEA/S may be related to its powerful antiglucocorticoid action (Blauer 1991; Kimonides 1999). High levels of cortisol cause damage to neurones, particularly in the hippocampus (Kimonides 1999; Lupien 1998), and suppress immune function. In young adults, the adverse effects of cortisol (e.g., during stress or trauma) may be opposed by the high levels of DHEA/S. Older adults may be rendered more vulnerable to the damaging effects of cortisol (Herbert 1997) as a result of their low levels of DHEA/S. In one study (Morales 1994) supplements of DHEA produced an increase in IGF‐1, a marker of growth hormone activity, and this might also have beneficial effects on age‐associated physiological changes.
Since DHEA/S levels decrease with age it has been postulated that various age‐associated changes might be due to DHEA/S deficiency and reversible or preventable by supplements. A community‐based cohort study, in 940 participants followed for 27 years, indicated that DHEA/S level may be a predictor of longevity in men, independent of age, blood pressure, and plasma glucose (Enomoto 2008). There is also some epidemiological evidence which suggests that the DHEA/S concentration is independently and inversely related to death from any cause and death from cardiovascular disease in men over age 50 (Barrett‐Connor 1986). Some studies showed lower than normal levels of DHEA/S in the serum of patients diagnosed with dementia (Hillen 2000; Nasman 1991; Rudman 1990; Sunderland 1989), while others reported no difference (Berr 1996; Schneider 1992). A prospective observational study of 394 women aged 65 and over and followed for four to six years showed no relation between serum DHEAS levels and cognitive performance or decline (Yaffe 1998). In contrast, in a cross‐sectional study of 295 women, aged 21‐77 years, higher endogenous DHEAS levels were independently and favourably associated with better executive function, concentration, and working memory ( Davis 2008).
DHEA taken by mouth is absorbed into the blood stream. Supplements are sold in large quantities in health food stores, particularly in the USA, as a general anti‐ageing agent claimed to improve well being as well as reducing the risk of dementia and having a beneficial effect on cognitive function. The US supplement market in 2000 was estimated at US$16·7 billion, as reported in the Financial Times newspaper (Maughan 2004 ); an important factor behind this growth was the availability of DHEA over‐the‐counter. Since DHEA is biotransformable into other hormones, including androgens and oestrogens, its effects might be indirect, complex, and individually variable. Hirshman 2004, for example, found evidence that following DHEA supplements, oestrogen levels were positively and androgen levels negatively associated with measures of recognition memory but the associations were reversed in perceptual identification tests. DHEA/S is often described as a "weak androgen" but one of its physiological effects in older men is to increase oestrogen levels (Arlt 1999). Dosage recommendations vary; most studies used 50 to 100 mg/day of DHEA, but some have used doses up to 1600 mg/day, and a dose of 50 mg/day has been shown to increase the serum level of older people to that of young adults (Arlt 1999).
The giving of DHEA is sometimes referred to as "treatment" or "replacement", but since the aim is to increase body levels of a natural chemical that is already present, "supplementation" is the most correct term.
In view of the possibility of beneficial effects of DHEA on the cognitive function of ageing adults, we have reviewed the results of relevant studies. This review is concerned with older people not suffering from dementia or depression.
Objectives
To determine whether there is evidence for a beneficial effect of supplemental DHEA on the cognitive function of middle‐aged or older people who are not suffering from dementia or depression, and to identify adverse effects associated with the administration of DHEA.
Methods
Criteria for considering studies for this review
Types of studies
Unconfounded, double‐blind, randomized, placebo‐controlled, parallel‐group trials.
Types of participants
Older adults not diagnosed as suffering from dementia or other relevant disease (including depression).
Types of interventions
DHEA or DHEA/S at any dose, for any duration, and by any route of administration.
Types of outcome measures
The outcomes of interest are performance on objective tests of cognitive function and quality of life measures (e.g. memory, concentration, name‐finding, speed of processing, or global measures).
Search methods for identification of studies
See Cochrane Dementia and Cognitive Improvement Group methods used in reviews.
The Specialized Register of the Cochrane Dementia and Cognitive Improvement Group (CDCIG) was searched on 18 March 2008 for all years up to December 2005. This register contains records from the following major healthcare databases The Cochrane Library, MEDLINE, EMBASE, PsycINFO, CINAHL and LILACS, and many ongoing clinical trial databases and other grey literature sources. The following search terms were used: dhea* OR prasterone OR dehydroepiandrosterone
The Cochrane Library, MEDLINE, EMBASE, PsycINFO, CINAHL and LILACS were searched separately on 18 March 2008 for records added to these databases after December 2005 to March 2008. The search terms used to identify relevant controlled trials on dementia, Alzheimer's disease and mild cognitive impairment for the Group's Specialized Register can be found in the Group's module on The Cochrane Library. These search terms were combined with the following search terms and adapted for each database, where appropriate: dhea* OR prasterone OR dehydroepiandrosterone
On 18 March 2008, the Specialized Register consisted of records from the following databases:
Healthcare databases
The Cochrane Library: (2006, Issue 1);
MEDLINE (1966 to 2006/07, week 5);
EMBASE (1980 to 2006/07);
PsycINFO (1887 to 2006/08, week 1);
CINAHL (1982 to 2006/06);
SIGLE (Grey Literature in Europe) (1980 to 2005/03);
LILACS: Latin American and Caribbean Health Science Literature (http://bases.bireme.br/cgi‐bin/wxislind.exe/iah/online/?IsisScript=iah/iah.xis&base=LILACS&lang=i&form=F) (last searched 29 August 2006).
Conference proceedings
ISTP (http://portal.isiknowledge.com/portal.cgi) (Index to Scientific and Technical Proceedings) (to 29 August 2006);
INSIDE (BL database of Conference Proceedings and Journals) (to June 2000);.
Theses
Index to Theses (formerly ASLIB) (http://www.theses.com/) (UK and Ireland theses) (1716 to 11 August 2006);
Australian Digital Theses Program (http://adt.caul.edu.au/): (last update 24 March 2006);
Canadian Theses and Dissertations (http://www.collectionscanada.ca/thesescanada/index‐e.html): 1989 to 28 August 2006);
DATAD ‐ Database of African Theses and Dissertations (http://www.aau.org/datad/backgrd.htm);
Dissertation Abstract Online (USA) (http://wwwlib.umi.com/dissertations/gateway) (1861 to 28 August 2006)
Ongoing trials
UK
National Research Register (http://www.update‐software.com/projects/nrr/) (last searched issue 3/2006);
ReFeR (http://www.refer.nhs.uk/ViewWebPage.asp?Page=Home) (last searched 30 August 2006);
Current Controlled trials: Meta Register of Controlled trials (mRCT) (http://www.controlled‐trials.com/) (last searched 30 August 2006) :
ISRCTN Register ‐ trials registered with a unique identifier
Action medical research
Kings College London
Laxdale Ltd
Medical Research Council (UK)
NHS Trusts Clinical Trials Register
National Health Service Research and Development Health Technology Assessment Programme (HTA)
National Health Service Research and Development Programme 'Time‐Limited' National Programmes
National Health Service Research and Development Regional Programmes
The Wellcome Trust
Stroke Trials Registry (http://www.strokecenter.org/trials/index.aspx) (last searched 31 August 2006);
Netherlands
Nederlands Trial Register (http://www.trialregister.nl/trialreg/index.asp) (last searched 31 August 2006);
USA/International
ClinicalTrials.gov (http://www.ClinicalTrials.gov) (last searched 31 August 2006) (contains all records from http://clinicalstudies.info.nih.gov/);
IPFMA Clinical trials Register: www.ifpma.org/clinicaltrials.html. The Ongoing Trials database within this Register searches http://www.controlled‐trials.com/isrctn, http://www.ClinicalTrials.gov and http://www.centerwatch.com/. The ISRCTN register and Clinicaltrials.gov are searched separately. Centerwatch is very difficult to search for our purposes and no update searches have been done since 2003.
The IFPMA Trial Results databases searches a wide variety of sources among which are:
http://www.astrazenecaclinicaltrials.com (seroquel, statins)
http://www.centerwatch.com
http://www.clinicalstudyresults.org
http://clinicaltrials.gov
http://www.controlled‐trials.com
http://ctr.gsk.co.uk
http://www.lillytrials.com (zyprexa)
http://www.roche‐trials.com (anti‐abeta antibody)
http://www.organon.com
http://www.novartisclinicaltrials.com (rivastigmine)
http://www.bayerhealthcare.com
http://trials.boehringer‐ingelheim.com
http://www.cmrinteract.com
http://www.esteve.es
http://www.clinicaltrials.jp
This part of the IPFMA database is searched and was last updated on 4 September 2006;
Lundbeck Clinical Trial Registry (http://www.lundbecktrials.com) (last searched 15 August 2006);
Forest Clinical trial Registry (http://www.forestclinicaltrials.com/) (last searched 15 August 2006).
The search strategies used to identify relevant records in MEDLINE, EMBASE, PsycINFO, CINAHL and LILACS can be found in the Group's module on The Cochrane Library.
Other sources that were searched by the reviewers included personal communications and conference abstracts. Hand searching of journals was also performed.
It was not anticipated that pharmaceutical companies would have data to contribute at present since DHEA is classified and marketed as a dietary supplement rather than a drug.
Data collection and analysis
Selection of studies
Irrelevant citations were discarded by reviewers on the basis of the title of a publication or its abstract. In the presence of any suggestion that the article could be relevant, it was retrieved for further assessment. The reviewers independently selected the trials for inclusion in this review from the culled citation list.
Quality assessment
The reviewers assessed the methodological quality of each trial, using ratings described in the Cochrane Collaboration guidelines (Mulrow 1997):
Trial design
Category A (adequate) is where the report describes allocation of treatment by: (i) some form of centralized randomized scheme, such as having to provide details of an enrolled participant to an office by telephone to receive the treatment group allocation; (ii) some form of randomization scheme controlled by a pharmacy; (iii) numbered or coded containers, such as in a pharmaceutical trial in which capsules from identical‐looking numbered bottles are administrated sequentially to enrolled participants; (iv) an on‐site or coded computer system, given that the allocations were in a locked, unreadable file that could be accessed only after inputting the characteristics of an enrolled participant; or (v) if assignment envelopes were used, the report should at least specify that they were sequentially numbered, sealed, opaque envelopes; (vi) other combinations of described elements of the process that provides assurance of adequate concealment.
Category B (intermediate) is where the report describes allocation of treatment by: (i) use of a "list" or "table" to allocate assignments; (ii) use of "envelopes" or "sealed envelopes"; (iii) stating the study as "randomized" without further detail.
Category C (inadequate) is where the report describes allocation of treatment by: (i) alternation; (ii) reference to case record numbers, dates of birth, day of week, or any other such approach; (iii) any allocation procedure that is entirely transparent before assignment, such as an open list of random numbers or assignments.
Trials were included if they conformed to categories A or B; those falling into category C were excluded.
Other aspects of trial design were not assessed by a scoring system although details were noted of blinding, whether intention‐to‐treat analyses were extractable from the published data, and the number of participants lost to follow‐up.
Outcome measures
Trials were included if outcome assessments included cognitive functions as measured by psychometric tests of recognised reliability and validity.
Data extraction
Data were independently extracted by two reviewers (JGE and RM) and cross‐checked. Any discrepancies were discussed and resolved.
For continuous variables, the main outcomes of interest are the final assessment and the change from baseline at final assessment.
In a generic inverse variance method the 95% confidence interval or an exact P‐value was used to estimate the treatment effect and Standard Error between the two groups in the study.
Studies may include a titration period prior to the randomization phase of the study. The data from these titration periods were not used to assess safety or efficacy since patients are not usually randomized, nor are treatment or dose allocation concealed.
Data analysis
For studies using a parallel‐groups design, differences from baseline are compared between the treatment and placebo groups. In studies using a cross‐over design, data from the first treatment period only are used where such data are available. The half life of DHEA/S is short, and wash‐out periods can reduce carry‐over effects. With healthy participants the problem of shifting baselines associated with progressive disorders such as dementia is also less. However, study designs in which participants experience both treatment and placebo, and, in the context of informed consent, know that what they are allocated to in the second phase of a study will be the opposite of what they have already experienced in the first may introduce complex problems from positive and negative placebo effects. Although it might be reasonable to pool biochemical or some types of physiological data from the first and second phases of a cross‐over study, it is not safe to do the same for measures of cognitive function or mood.
Results
Description of studies
Excluded studies
There have been several studies of the effect of DHEA on age‐associated phenomena such as bone loss (Kahn 2002) and sarcopenia (Percheron 2003) and in specific diseases such as fibromyalgia and primary Sjogren syndrome (Finckh 2005;Hartkamp 2008 ), and in people with type 2 diabetes (Brignardello 2007).
A cross‐over study of 30 men and women aged 42 to 70 by Morales 1994 included self‐reported libido and well being in outcome measures but no specific assessment of cognitive function.
Five trials (Hirshman 2003; Hirshman 2004; Wolf 1997a; Wolf 1998a; Alhaj 2006 ) included measures of cognitive function but used cross‐over designs without separate publication of first‐phase data. Wolf 1997a enrolled 40 healthy men and women of mean age 69, and gave DHEA 50 mg or placebo daily for two weeks. Changes in blood levels of DHEA/S were observed but there were no effects on memory as measured by tests of concentration, visual short‐ and long‐term memory, the Stroop test, digit span, psychometric speed, or auditory verbal learning. Hirshman 2003 claimed from a study of 30 postmenopausal women that DHEA was associated with enhanced memory recognition and fewer false positive recognitions, but this was not seen under all conditions of test presentation. Hirshman 2004 carried out a within‐subject cross‐over study of six postmenopausal women and claimed that oestrogenic metabolites of DHEA had positive effect and androgens a negative effect on visual memory. A reverse pattern was seen with perceptual identification. These results should perhaps be seen as hypothesis‐generating rather than hypothesis‐testing. Wolf 1998a studied 17 healthy elderly men aged 59‐81 given DHEA or placebo for two weeks. No effects on visual, spatial, or semantic memory were observed. In Alhaj 2006 24 healthy young men were treated with a seven‐day course of oral DHEA (150 mg/day). Subjective mood and memory were measured using visual analogue scales (VASs). DHEA treatment improved memory recollection and mood, and decreased trough cortisol levels.
Authors of these five studies are being contacted to seek first‐phase data suitable for inclusion in meta‐analysis.
In two trials (Villareal 2006 and Yang 2005), DHEA supplementation was confounded with physical exercise training, and no measures of cognition were included.
Included studies
Six studies provided usable observations based on reliable psychometric tests of memory or other cognitive functions:
Wolf 1998b tested the hypothesis that an antiglucocorticoid action of DHEA would result in an enhanced declarative memory performance after stress‐exposure. (Declarative memory is for facts, as distinct from procedural memory which underpins learnt skills). Seventy‐five men and women aged 59 to 81, without serious memory complaints and with normal scores on a depression index, took part in the study comparing two weeks of DHEA 50 mg/day with two weeks of placebo. At the end of the two weeks, tests of visual and verbal memory, selective‐attention memory and spatial memory were administered before and after an episode of social stress (making a speech and performing mental arithmetic in front of an audience). Biochemical variables were also measured. This study was concerned with possible modulation by DHEA reactions to psychological stress, and is therefore relevant to the cognitive performance of healthy older individuals for whom such stresses are common in daily life.
Barnhart 1999 enrolled 66 perimenopausal women (60 completers) aged 45 to 55 "with symptoms of fatigue, lack of energy, anxiety, tension, irritability, depression, insomnia, forgetfulness, concentration difficulties, decreased libido, or global sense of reduced wellbeing". Exclusion criteria included a current diagnosis of major psychiatric disorder or the taking of antidepressant medication. The study compared the effects of three months of DHEA 50 mg/day with three months of placebo. Main outcome measures were of health‐related quality of life, mood, and depression, but memory and some other aspects of cognitive function were assessed using standardized tests. Given the inter‐relationships between DHEA/S and other steroid hormones, the mechanism of any effect of DHEA/S in women with perimenopausal symptoms might differ from that in men or in women of other ages or without perimenopausal symptoms. Results could not therefore be regarded as necessarily extrapolatable to middle‐aged and older people in general.
Limited information was available from a third study reported by van Niekerk 2001. Forty‐six male volunteers aged 60 to 80 were enrolled in a double‐blind, placebo‐controlled randomized cross‐over study aimed primarily at correlating physiological levels of circulating DHEA with measures of well‐being, mood and cognition. Exclusion criteria included current psychiatric disorder, use of psychotropic medication, or history of cardiovascular or neurological disorder. The investigators found significant period effects in cognitive outcomes and a significant statistical interaction between treatment effect and period. In order to avoid consequent bias in assessing treatment effects they reported on a separate analysis of the first‐phase data only, and no significant treatment effects of DHEA were observed. Detailed data suitable for inclusion in a meta‐analysis were not presented. The statistical interactions in this study exemplify the possible bias in studies with a cross‐over design.
Dayal 2005 enrolled 50 postmenopausal women, aged 44‐70 years, who were randomized for a 12‐week trial into four treatment groups: DHEA 50 mg/day; conjugated equine oestrogen (CEE) 0.65 mg/day; DHEA 50 mg plus CEE 0.65 mg daily; and placebo. Main outcome measures of lower extremity muscle (calf) mass, muscle function, serum hormone and lipid levels, and quality of life (QOL) were obtained at baseline and after treatment. Hormone levels included measures of DHEA, cortisol, total testosterone, total oestrogen, androstenedione,and sex hormone binding globulin (SHBG). Total cholesterol, triglycerides, HDA, LDL, LP, apoA, and apoB were also measured.
Nair 2006 report a two‐year trial involving 87 men and 57 women aged 60 and over. Eligibility criteria for men included low levels of bioavailable testosterone (< 3.6 nmol/L) plus a sulphated DHEA level less than 4.3 mcmol/L; eligibilty for women included a sulphated DHEA level below 2.6 mcmol/L. The male participants were randomized between three treatment groups: DHEA tablet (75 mg/day) plus a transdermal placebo patch; placebo tablet and a transdermal testosterone patch (5 mg/day); or a placebo tablet and a placebo transdermal patch. Women received DHEA (50 mg/day) or a placebo tablet. Primary outcomes measures were physical performance, peak aerobic capacity, body composition, bone mineral density (BMD), plasma insulin and glucose. Other assessments were body weight, the proportion of body fat, insulin‐sensitivity index, levels of various hormones and alkaline phosphate level, haemoglobin, quality of life and monitoring for adverse events.
In von Muhlen 2007, the effect of 50 mg/day DHEA was studied in a community setting trial involving 225 participants aged 55 to 85 years (110 men and 115 women) over one year. Outcome measures included bone mineral density and metabolism, body composition, muscle strength, immune function, cardiovascular risk factors, steroid hormone levels, bone markers, cytokines, the IGF‐1IGF binding protein system, changes in mood and well being. Cognitive function and sexuality were also assessed.
Cognitive tests and quality of life employed
Wolf 1998b: Parallel versions of the following tests: Visual‐verbal memory. Two‐minute delayed free recall of pictures of fourteen everyday objects presented for two seconds (Oswald 1994); Selective attention: On a piece of paper subject required to cross out a specified target item among several similar looking distractor items (Gatterer 1990); Spatial memory: subject given two minutes to remember a description of a scene with seven objects around them. Then asked to recall the positions of the objects as originally described and as they would be after a 90º rotation (Kirschbaum 1996); Stress paradigm. A five‐minute free speech and mental arithmetic test in front of an audience (Kirschbaum 1993).
Barnhart 1999: Buschke Immediate Recall and Delayed Recall tests (Buschke 1974); Symbol copying, and digit‐symbol substitution tests. In digit‐symbol substitution tests the participant is given a paper listing numbers 1 to 9 each with a different abstract symbol alongside. The participant is then required to write the corresponding symbols to match a list of random numbers (Freeman 1992; Petturrson 1983). The SmithKline Beecham Quality of Life Self Report Questionnaire (SKQOL) (Stoker 1992 ) was used: it provides a comprehensible coverage to the physical, mental and social dimensions. It contains 23 predetermined constructions and three fixed elements: self now, ideal self, and sick now.
van Niekerk 2001: Word list memory. Ten common words presented for three seconds each. The subject is required to read each aloud and to remember it. Three parallel lists were used to avoid long‐term learning effects in repeated testing (Mohs 1997); Object location memory. Simple line drawings of familiar objects located in 10 of 30 squares in a grid presented for 30 seconds. Subject then presented with the drawings and required to place them in a blank grid (van Niekerk 2001); Choice reaction time. Subject required to press one of four keys in response to one of four digits appearing on a screen. Time to respond correctly is recorded in milliseconds and wrong responses also recorded (Cox 1993); Visual search. Participants given one minute to cross out all the letters P and W on a sheet of paper presenting rows of random letters (MRC CFAS 1998).
Dayal 2005 employed the Women's Health Questionnaire (WHQ) as a tool to examine well being and quality of life. The WHQ seeks details of minor psychological and somatic symptoms experienced by pre‐ and post‐menopausal women. Symptoms are clustered in nine domains to assess: somatic symptoms, depressed mood, cognitive difficulties, anxiety/fear, sexual functioning, vasomotor symptoms, sleep problems, menstrual symptoms, and self‐perceived attractiveness (Wiklund 1992).
In Nair 2006 the Health Status Questionnaire (HSQ) was used to evaluate quality of life. The HSQ adds three questions to the medical outcomes Study 36‐item Short General Form Survey (SF‐36) to provide a further assessment of physical and mental health constructs. The SF‐36 is an assessment for both physical and psychological facets of health containing 36 multiple‐choice questions requiring less than 10 minutes to complete. It provides a global health score and separate scores for: physical health, role performance, emotional, social and mental health, bodily pain, vitality and general health perceptions.
von Muhlen 2007 used the following standardized batteries to measure changes in cognitive function:
Modified Mini‐Mental Status Examination (3MS) (Teng 1987). This is a general cognitive battery with components for concentration, language, praxis, orientation, and immediate and delayed memory. The test takes10‐15 minutes and scoring ranges from 0‐100.
The Trail Making Test Part B (Fromm‐Auch1983) is a measure of speed mental operation, attention, visual scanning, visual sequential abilities, and mental flexibility. A maximum of 300 seconds is allowed. Scoring depends on the time needed to complete the task, with higher scores indicating poorer performance.
Verbal Fluency (Borkowski 1967) is a test of semantic memory, verbal production and language. The test requires two minutes for completion and test measures the number of different animals named in one minute. Scores depend on level of education.
The Modified Boston Naming Test (Kapalan 1982) is a 15‐item test of language function based on visual confrontation naming. The task lasts three minutes and scoring is based on the number of correct answers. Maximum score is 15 with higher scores indicating better performance.
Word List Memory (Morris 1989) tests ability to remember newly learned information. Ten common words are presented in printed form and the task is to recall as many as possible. Total administration time is four minutes and the maximum score is 30.
Word List Recall (Welsh 1994) tests memory from the Word List Memory test after a 30‐minutes delay. A maximum time of 90 seconds is allowed and scores are the sum of words correctly recalled from the three trials. The maximum score is 10.
In von Muhlen 2007 three instruments were applied to assess changes in quality of life.
For general life satisfaction assessment two standardised measures were used:
The Life Satisfaction Index‐Z (LSI‐Z) (Wood 1969) consists of 13 statements for which respondents are asked to indicate if they agree, disagree, or do not know. Two points are given for satisfaction response, 1 point for do not know, and 0 for dissatisfaction. Several items were reverse scored, and scores for all items were summed to yield a general measure of life satisfaction. Scoring range from 0 to 26, with higher scores indicating better life satisfaction.
The Satisfaction with Life Scale (SWLS) (Diener 1985) consists of five items for which respondents rate the extent to their agreement from strongly disagree (1 point) to strongly agree (7 points). Overall scores range from 5 to 35, with higher scores indicating better life satisfaction.
The Medical Outcomes Study Short‐Form 36 (SF‐36) is a reliable and well‐validated questionnaire addressing both physical and psychological facets of health. It comprises 36 multiple‐choice questions, is easy to administer and takes less than 10 minutes to complete. It provides both a global health status score and a separate score for each of eight domains: physical functioning, role functioning (physical), role functioning (emotional), social functioning, mental health, bodily pain, vitality, and general health perceptions (Ware 1992).
Risk of bias in included studies
Three included studies with eligible data (Barnhart 1999; Wolf 1998b; Dayal 2005, ) were described as double‐blind and randomized but without further information on procedures. Wolf 1998b used random allocation stratified for age and body mass index. von Muhlen 2007 stratified randomization by sex and age group using a computer package based on random number tables. The method of randomization was not stated in Dayal 2005 or Nair 2006. All studies described exclusion criteria applied before randomization. Barnhart 1999 and Nair 2006, described the trial withdrawal rate and reasons for trial withdrawal, and specified whether withdrawal occurred while participants were taking DHEA or placebo. In Barnhart 1999, three participants withdrew owing to adverse effects, one taking DHEA and two taking placebo. In Nair 2006 trial five participants did not complete the study, four in the DHEA groups and one in the placebo, the reasons for dropping out were unspecified. Wolf 1998b reported exclusions of participants from analysis, and described the number of participants excluded and the reasons for exclusion. van Niekerk 2001, Dayal 2005 and Nair 2006 used a computer‐generated randomization procedure and blinding supervised by providing identical DHEA and placebo tablets. All six studies used standardised psychometric tests as outcome measures.
Effects of interventions
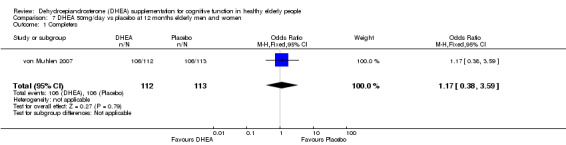
Data for intention‐to‐treat analysis were not available for either of the two studies newly eligible for inclusion, but Barnhart 1999 reported that an intention‐to‐treat analysis of their study produced similar findings to those of the published completers' analysis.
There were few significant findings.
In an analysis of variance, Wolf 1998b found that placebo group performance deteriorated significantly on a test of selective attention following the psychosocial stressor (P < 0.05), while deterioration was not evident in the DHEA group (P = 0.85). However, when compared with placebo, DHEA was associated with a significant impairment on a visual memory test (P < 0.01) following the stressor. No significant effect was found on a third cognitive task. Effects were not found on tasks when administered in the absence of a stressor. There is no obvious unifying interpretation of these results and they may simply reflect the play of chance. No data were extractable for meta‐analysis.
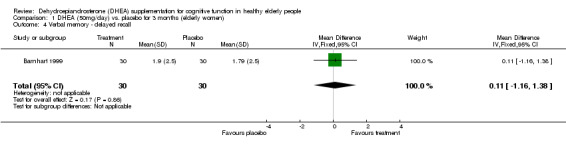
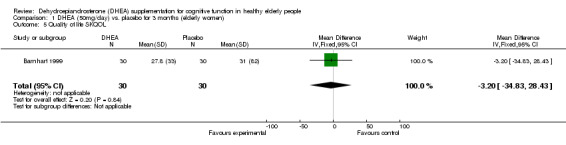
Barnhart 1999, studying women with a variety of perimenopausal symptoms, employed three cognitive measures and found no significant effect of DHEA on any of them. Data have been extracted in the Table of comparisons. For the quality of life using SKQOL no significant difference between DHEA 50 mg/day for 12 weeks over placebo; WMD ‐3.20 (95%CI ‐34.83 to 28.43, P=0.84)
In all the studies DHEA replacement was well tolerated and apart from the effect on post‐stressor visual memory observed by Wolf 1998b, there were no significant adverse effects.
In Dayal 2005, results revealed no significant difference of DHEA 50 mg/day at week 12 over placebo on measures of cognition or quality of life of postmenopausal women. The data were summarized as percentage change from baseline: for cognition (DHEA 9.00 vs ‐8.00 placebo); for quality of life (DHEA ‐1.00 vs ‐6.00 placebo).
For both elderly men and women, the 24‐month daily supplementation with DHEA in Nair 2006 was associated with no significant changes in physical or mental component scales of the HSQ. For elderly women (50 mg/day), quality of life (mental component) showed WMD 0.77 (95% CI ‐2.56 to 4.10, P = 0.61) and for physical components WMD was 0.56 (95% CI ‐2.51 to 3.63, P = 0.72). For elderly men taking DHEA, 75 mg/day quality of life (mental) component showed WMD ‐0.25 (95% CI ‐2.72 to 2.22, P = 0.84) and the physical components WMD ‐1.43 (95% CI ‐4.05 to 1.19, P = 0.29).
The 12‐month results of von Muhlen 2007 showed no significant differences between 50 mg/day of DHEA and placebo in cognitive function measures or quality of life assessments in either elderly men or women. The cognition results were reported as medians with interquartile range and percentage change from baseline. Changes in quality of life were evaluated at 3 and 12 months:
50 mg/day DHEA efficacy on MMSE at 12 months:
Women: median (interquartile range) 96 (4), % Change from baseline 0 (5.3), P = 0.31
Men: median (interquartile range) 96 (5), % Change from baseline ‐1.01 (4.4), P = 0.66
50 mg/day DHEA efficacy on Word List at 12 months:
Women: median (interquartile range) 23 (4), % Change from baseline 0 (22.0), P = 0.25
Men: median (interquartile range) 21(5), % Change from baseline 0(22.0), P = 0.64
50 mg/day DHEA efficacy on Word List Recall at 12 months:
Women: median (interquartile range) 8 (2), % Change from baseline 11.11(28.6), P = 0.23
Men: median (interquartile range) 7(3), % Change from baseline 11.11 (57.1), P = 0.85
50 mg/day DHEA efficacy on Verbal Fluency at 12 months:
Women: median (interquartile range) 19(5.5), % Change from baseline 5.72(31.8), P = 0.19
Men: median (interquartile range) 20(6), % Change from baseline 5.26 (30.7), P = 0.07
50 mg/day DHEA efficacy on Boston Naming at 12 months:
Women: median (interquartile range) 15(1), % Change from baseline 0 (0), P = 0.68
Men: median (interquartile range) 15(1), % Change from baseline 0 (0), P = 0.36
50 mg/day DHEA efficacy on Trail B Test at 12 months:
Women: median (interquartile range) 94 (53), % Change from baseline ‐1.54 (24.9), P = 0.26
Men: median (interquartile range) 86(29), % Change from baseline ‐2.78(23.6), P = 0.36
50 mg/day DHEA efficacy on SF‐36 Physical
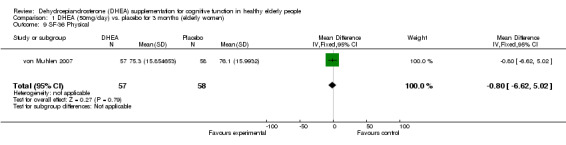
Women: for 3 months WMD ‐0.80 (95% CI ‐6.62 to 5.02, P = 0.79)
Women: for 12 months WMD 0.50 (95% CI ‐5.32 to 6.32, P = 0.87)
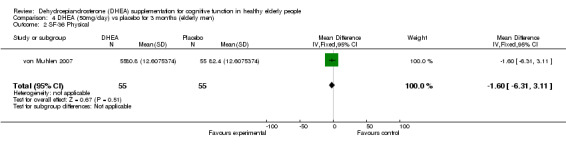
Men: for 3 months WMD ‐1.60 (95% CI ‐6.31to 3.11, P = 0.51)
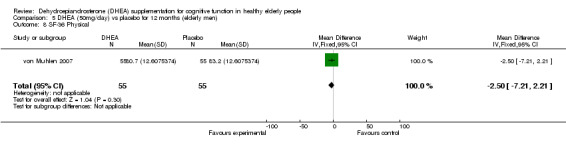
Men: for 12 months WMD ‐2.50 (95% CI ‐7.21 to 2.2, P = 0.30)
50 mg/day DHEA efficacy on SF‐36 Mental
Women: for 3 months WMD ‐2.70 (95% CI ‐7.41 to 2.01, P = 0.26)
Women: for 12 months WMD ‐1.10 (95% CI ‐5.81to 3.61, P = 0.65)
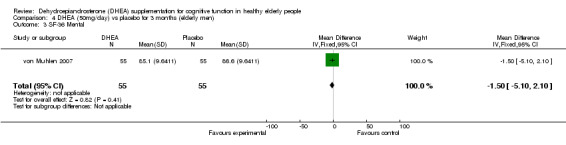
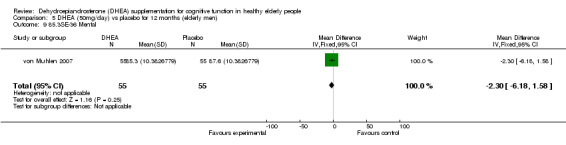
Men: for 3 months WMD ‐1.50 (95%CI ‐5.10 to 2.10, P=0.41)
Men: for 12 months WMD ‐2.30 (95%CI ‐6.18 to 1.58, P=0.25)
50 mg/day DHEA efficacy on Life Satisfaction Index‐Z
Women: for 3 months WMD ‐0.10 (95% CI ‐1.76 to 1.56, P = 0.91)
Women: for 12 months WMD ‐0.50 (95% CI ‐2.16 to 1.16, P = 0.56)
Men: for 3 months WMD ‐1.30 (95% CI ‐2.69 to 0.09, P = 0.07)
Men: for 12 months WMD ‐1.00 (95% CI ‐2.39 to 0.39, P = 0.16)
50 mg/day DHEA efficacy on Satisfaction with life Scale
Women: for 3 months WMD 1.10 ( 95% CI ‐1.12 to 3.32, P = 0.33)
Women: for 12 months WMD ‐1.40 (95% CI ‐3.89 to 1.09, P = 0.27)
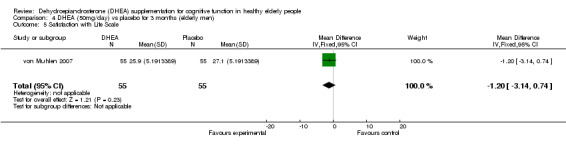
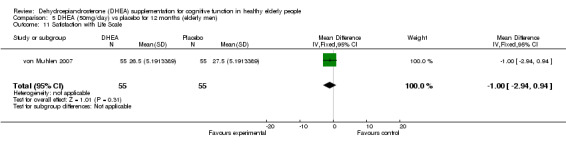
Men: for 3 months WMD ‐1.20 (95% CI ‐3.14 to 0.74, 0.23)
Men: for 12 months WMD ‐1.00 (95% CI ‐2.94 to 0.94, P = 0.16)
Adverse effects
DHEA supplements were well tolerated in all the studies and apart from the effect on post‐stressor visual memory observed by Wolf 1998b, there were no significant adverse effects.
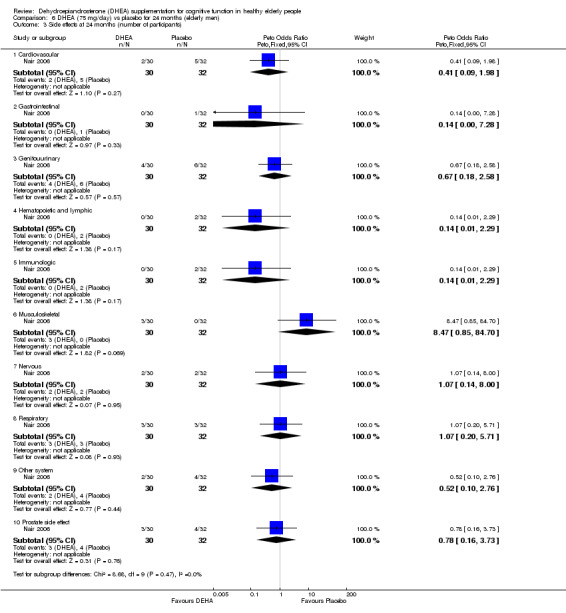
Nair 2006, reported ten different adverse events. The data illustrated no significant differences in incidence between DHEA 50 mg/day or 75 mg/day and placebo groups over 24 months:
Cardiovascular:
Women: 50 mg/day DHEA versus placebo at 24 months (OR 3.65 , 95% CI 0.67 to19.85, P = 0.13)
Men: 75 mg/day DHEA versus placebo at 24 months (OR 0.39, 95% CI 0.07 to 2.16, P = 0.28)
Gastrointestinal:
Women: 50 mg/day DHEA versus placebo at 24 months (OR 5.55, 95% CI 0.25 to120.64, P = 0.28)
Men: 75 mg/day DHEA versus placebo at 24 months (OR 0.34, 95% CI 0.01 to 8.78, P = 0.52)
Genitourinary:
Women: 50mg/day DHEA versus placebo at 24 months (OR 0.32, 95% CI 0.03 to 3.28, P = 0.34)
Men: 75 mg/day DHEA versus placebo at 24 months (OR 0.67, 95% CI 0.17 to 2.64, P = 0.56)
Haematopoietic and lymphatic:
Women: 50mg/day DHEA versus placebo at 24 months (OR 3.35, 95% CI 0.33 to 34.19, P = 0.31)
Men: 75 mg/day DHEA versus placebo at 24 months (OR 0.20, 95% CI 0.01 to 4.34, P= 0.17)
Immunological:
Women: 50 mg/day DHEA versus placebo at 24 months (OR 3.35, 95%CI 0.33 to 34.19, P = 0.31)
Men: 75 mg/day DHEA versus placebo at 24 months (OR 3.35, 95%CI 0.33 to 34.19, P = 0.3)
Musculoskeletal:
Women: 50 mg/day DHEA versus placebo at 24 months (OR 0.33, 95%CI 0.01 to 8.52, P = 0.51)
Men: 75 mg/day DHEA versus placebo at 24 months (OR 8.27, 95%CI 0.41 to167.23, P = 0.17)
Neurological:
Women: 50 mg/day DHEA versus placebo at 24 months (OR 1.04, 95%CI 0.06 to17.38, P = 0.98)
Men: 75 mg/day DHEA versus placebo at 24 months (OR1.07, 95%CI 0.14 to 8.13, P = 0.95)
Respiratory:
Women: 50 mg/day DHEA versus placebo at 24 months ( OR 2.15, 95% CI 0.18 to 25.07, P = 0.54)
Men: 75 mg/day DHEA versus placebo at 24 month (OR1.07, 95% CI 0.20 to 5.79, P = 0.92)
Other:
Women: 50 mg/day DHEA versus placebo at 24 months ( OR 5.55, 95%CI 0.25 to120.64, P = 0.24)
Men: 75 mg/day DHEA versus placebo at 24 month (OR 0.50, 95% CI 0.08 to 2.95, P = 0.24)
Adverse events associated with the prostate gland:
75 mg/day DHEA versus placebo at 24 months (OR 0.78, 95% CI 0.16 to 3.73, P = 0.76)
No difference in numbers with elevated PSR > 1.4 ng/mL between placebo and DHEA.
50 mg/day DHEA versus placebo at 12 months (OR 2.65, 95% CI 0.49 to14.29, P = 0.26)
Drop‐out due to adverse effects:
Compared with their placebo group, von Muhlen 2007 report significantly larger numbers of participants discontinuing 50 mg/day DHEA because of adverse effects: OR at 12 months 2.66 (95% CI 1.20 to 5.89, P = 0.02)
Other known side effects not reported in the trials included in this review are the following: headache; depression; anxiety; irritability; nervousness; changes in libido; gynaecomastia; polycythaemia; hypertension; electrolyte disturbances; wight gain; Increase bone growth; hirsutism; acne; androgenic effects; sleep apneas; liver tumours (BNF 2008).
Drop‐outs
No significant difference in the number of completers between DHEA at 50 mg/day and 75 mg/day versus placebo at 24 months.
Women: 50 mg/day DHEA OR 7.76 (95% CI 0.38 to 157.14, P = 0.18)
Men: 75 mg/day DHEA OR 1.07 (95% CI 0.06 to 17.89, P = 0.96)
Discussion
Despite the growing popularity of DHEA supplementation among older adults, there is very little empirical evidence for beneficial effects. There is also evidence that any effects of DHEA might be mediated by other hormones into which the exogenous DHEA is transformed; these effects might therefore vary between individuals, with age, between sexes, and according to emotional status of stress or arousal. It has also been suggested that oestrogenic and androgenic derivatives of DHEA might have different effects on different cognitive functions (Hirshman 2004).
Although data from only five trials have been included in this review, it is noteworthy that of the four non‐included cross‐over trials two did not show any effect of DHEA on cognitive function and no consistent effects were observed in the others. The few significant effects reported by Wolf 1998b could have arisen by chance in the multiple comparisons undertaken in the analysis. Longer‐term supplementation with DHEA was evaluated by Nair 2006 with administration of two doses (50 mg/day and 75 mg/day) over two years, and by von Muhlen 2007 giving 50 mg/day DHEA for one year. One of the entry criteria for Nair 2006 was low baseline DHEA levels, this criterion was not applied in von Muhlen 2007 in order to enrol a more generally representative sample of older people. Results for men and women were analysed separately and revealed no evidence that either dosage of DEHA (50 mg/day, 75 mg/day) improved cognitive function or quality of life, physical or mental. This trial involved rather small sample sizes. The other two studies also involved small numbers of participants and the duration of supplementation was brief at 12 weeks. Moreover, all the cognitive tests used in these studies had been developed to evaluate the function of impaired individuals as opposed to cognitively intact individuals, also all these tests had the floor and ceiling effect. If DHEA has neuroprotective effects, these may take longer than three months to become evident. The DHEA safety profile was inconsistent for low dosage of DHEA in two trials; longer‐term studies are needed to assess risks and benefits from low and high doses.
Authors' conclusions
Implications for practice.
What little evidence there is from controlled trials does not support a beneficial effect of DHEA supplementation on cognitive function of normal middle‐aged or elderly people. Evidence from controlled trials on adverse effects of DHEA is inconsistent.
Implications for research.
Further research into the effects of DHEA supplements on cognitive function of middle‐aged and elderly people is desirable for two reasons:
1. It is a reasonable hypothesis that DHEA supplements might have beneficial effects on some age‐associated changes including, possibly, age‐associated memory impairment (AAMI).
2. DHEA is widely sold and promoted as a health food supplement; it is in the public interest that its beneficial and adverse effects should be adequately documented.
Future studies need to incorporate the following features:
1. Trials should be of parallel‐group, randomized, double‐blind, and placebo‐controlled design, with clear prior specification of outcome measures of interest.
2.Subgroup analysis based on age, gender and DHEA levels should be considered in order to identify groups which might benefit from DHEA supplementation.
3. Treatment periods should be at least 12 months. 4. Tests of cognitive function used should have good psychometric properties (avoiding floor and ceiling effects and providing a good range of scores) and be sensitive to change. 5. Sample sizes should be large enough to provide adequate power and to allow for statistical correction for multiple outcome comparisons.
What's new
| Date | Event | Description |
|---|---|---|
| 18 March 2008 | New search has been performed | A new update search was performed on 18 March 2008. Three double‐blind trials, with randomized parallel placebo‐controlled design, were included: two long‐term trials involved elderly men and women; and another short‐term trial lasting for 12 weeks recruited only elderly women. There is no beneficial effect of DHEA supplementation on cognitive function of older people. DHEA was well tolerated, but the long‐term safety of low and high doses of DHEA for older people is still uncertain. Further large double‐blind, randomized parallel‐group placebo‐controlled trials are needed. |
History
Review first published: Issue 4, 2006
| Date | Event | Description |
|---|---|---|
| 4 August 2006 | New citation required and conclusions have changed | Substantive amendment |
| 10 October 2005 | New search has been performed | The search of October 2005 found no studies for inclusion |
Acknowledgements
The authors thank the original authors of the review, Felicia Huppert and Jan van Niekerk for their contribution. The authors thank Brigitte Kudielka, Denise von Muhlenand and Oliver Wolf for their generous assistance in providing manuscripts of studies and supplying additional information.
Data and analyses
Comparison 1. DHEA (50mg/day) vs. placebo for 3 months (elderly women).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Psychomotor performance (Symbol Copying Test ‐ no. copied in 90sec) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 4.9 [‐1.69, 11.49] |
| 2 Attention / speed of processing (Digit Symbol Substitution Test ‐ no. replicated in 90sec) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐9.10, 6.90] |
| 3 Verbal memory ‐ immediate recall | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐0.77, 1.57] |
| 4 Verbal memory ‐ delayed recall | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐1.16, 1.38] |
| 5 Quality of life SKQOL | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐3.20 [‐34.83, 28.43] |
| 6 Cognitive symptoms (WHQ) | Other data | No numeric data | ||
| 7 Quality of life | Other data | No numeric data | ||
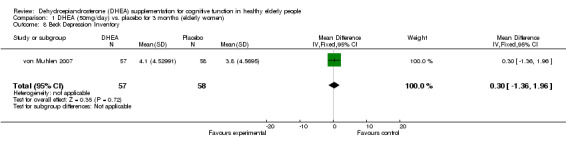
| 8 Beck Depression Inventory | 1 | 115 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐1.36, 1.96] |
| 9 SF‐36 Physical | 1 | 115 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐6.62, 5.02] |
| 10 SF‐36 Mental | 1 | 115 | Mean Difference (IV, Fixed, 95% CI) | ‐2.70 [‐7.41, 2.01] |
| 11 Life Satisfaction Index‐Z | 1 | 115 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐1.76, 1.56] |
| 12 Satisfaction with life scale | 1 | 115 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [‐1.07, 3.27] |
1.1. Analysis.

Comparison 1 DHEA (50mg/day) vs. placebo for 3 months (elderly women), Outcome 1 Psychomotor performance (Symbol Copying Test ‐ no. copied in 90sec).
1.2. Analysis.

Comparison 1 DHEA (50mg/day) vs. placebo for 3 months (elderly women), Outcome 2 Attention / speed of processing (Digit Symbol Substitution Test ‐ no. replicated in 90sec).
1.3. Analysis.

Comparison 1 DHEA (50mg/day) vs. placebo for 3 months (elderly women), Outcome 3 Verbal memory ‐ immediate recall.
1.4. Analysis.
Comparison 1 DHEA (50mg/day) vs. placebo for 3 months (elderly women), Outcome 4 Verbal memory ‐ delayed recall.
1.5. Analysis.
Comparison 1 DHEA (50mg/day) vs. placebo for 3 months (elderly women), Outcome 5 Quality of life SKQOL.
1.6. Analysis.
Comparison 1 DHEA (50mg/day) vs. placebo for 3 months (elderly women), Outcome 6 Cognitive symptoms (WHQ).
| Cognitive symptoms (WHQ) | ||
|---|---|---|
| Study | DHEA 50 mg/day at week 12 % change | Placebo |
| Dayal 2005 | 9.000 | ‐8.000 |
1.7. Analysis.
Comparison 1 DHEA (50mg/day) vs. placebo for 3 months (elderly women), Outcome 7 Quality of life.
| Quality of life | ||
|---|---|---|
| Study | DHEA % change | placebo % change |
| Dayal 2005 | ‐1.00 | ‐6.00 |
1.8. Analysis.
Comparison 1 DHEA (50mg/day) vs. placebo for 3 months (elderly women), Outcome 8 Beck Depression Inventory.
1.9. Analysis.
Comparison 1 DHEA (50mg/day) vs. placebo for 3 months (elderly women), Outcome 9 SF‐36 Physical.
1.10. Analysis.

Comparison 1 DHEA (50mg/day) vs. placebo for 3 months (elderly women), Outcome 10 SF‐36 Mental.
1.11. Analysis.
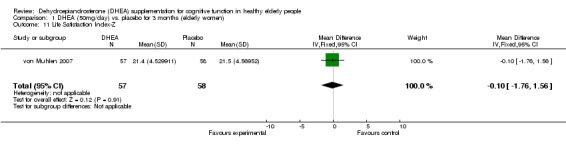
Comparison 1 DHEA (50mg/day) vs. placebo for 3 months (elderly women), Outcome 11 Life Satisfaction Index‐Z.
1.12. Analysis.
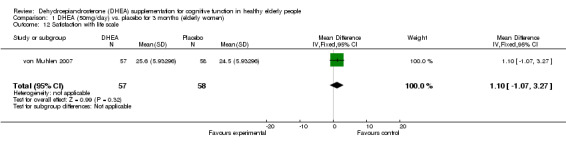
Comparison 1 DHEA (50mg/day) vs. placebo for 3 months (elderly women), Outcome 12 Satisfaction with life scale.
Comparison 2. DEHA (50mg/day) vs placebo for 12 months (elderly women).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 MMSE % Change | Other data | No numeric data | ||
| 2 Word List % Change | Other data | No numeric data | ||
| 3 Word List Recall % Change | Other data | No numeric data | ||
| 4 Verbal Fluency % Change | Other data | No numeric data | ||
| 5 Boston Naming % Cahnge | Other data | No numeric data | ||
| 6 Trials B % Change | Other data | No numeric data | ||
| 7 Beck Depression Inventory | 1 | 115 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐1.13, 2.13] |
| 8 SF‐36 Pysical | 1 | 115 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐5.19, 6.19] |
| 9 SF‐ Mental | 1 | 110 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐5.81, 3.61] |
| 10 Life Satisfaction Index‐Z | 1 | 115 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐2.13, 1.13] |
| 11 Satisfaction with Life Scale | 1 | 115 | Mean Difference (IV, Fixed, 95% CI) | ‐1.40 [‐3.84, 1.04] |
2.1. Analysis.
Comparison 2 DEHA (50mg/day) vs placebo for 12 months (elderly women), Outcome 1 MMSE % Change.
| MMSE % Change | ||||
|---|---|---|---|---|
| Study | Placebo at 12 months(Median and Interquartile) | DHEA at 12 months (Median and Interquartile) | % change (Median and Interquartile) | P‐value |
| von Muhlen 2007 | 96(6) | 96(4) | Placebo 1.01(5.1) DEHA 0 (5.3) |
0.31 |
2.2. Analysis.
Comparison 2 DEHA (50mg/day) vs placebo for 12 months (elderly women), Outcome 2 Word List % Change.
| Word List % Change | ||||
|---|---|---|---|---|
| Study | Placebo at 12 months Median (Interquartile range) | DEHA at 12 months Median (Interquartile Range) | % change | P value |
| von Muhlen 2007 | 22(5) | 23(4) | Placebo0(22.0) DEHA 0 (22.0) |
0.25 |
2.3. Analysis.
Comparison 2 DEHA (50mg/day) vs placebo for 12 months (elderly women), Outcome 3 Word List Recall % Change.
| Word List Recall % Change | ||||
|---|---|---|---|---|
| Study | Placebo at 12 months Median (Interquartile Range) | DHEA at 12 months Median (Interquartile Range) | % Change | P |
| von Muhlen 2007 | 7(3) | 8(2) | Placebo 0(37.5) DEHA 11.11(28.6) |
0.23 |
2.4. Analysis.
Comparison 2 DEHA (50mg/day) vs placebo for 12 months (elderly women), Outcome 4 Verbal Fluency % Change.
| Verbal Fluency % Change | ||||
|---|---|---|---|---|
| Study | Placebo Median (Interquartile Range) | DEHA Median (Interquartile Range) | % Change | P |
| von Muhlen 2007 | 19 (6) | 19 (5.5) | Placebo 0(29.7) DEHA 5.72 (31.8) |
0.19 |
2.5. Analysis.
Comparison 2 DEHA (50mg/day) vs placebo for 12 months (elderly women), Outcome 5 Boston Naming % Cahnge.
| Boston Naming % Cahnge | ||||
|---|---|---|---|---|
| Study | Placebo at months 12 Median (Interquartile Range) | DEHA at months Median (Interquartile Range) | %Change | P |
| von Muhlen 2007 | 15(1) | 15(1) | Placebo 0(0) DEHA 0(0) |
0.68 |
2.6. Analysis.
Comparison 2 DEHA (50mg/day) vs placebo for 12 months (elderly women), Outcome 6 Trials B % Change.
| Trials B % Change | ||||
|---|---|---|---|---|
| Study | Placebo at 12 months Median (Interquartile Range) | DEHA at 12 months Median (Interquartile Range) | % Cahnge | P |
| von Muhlen 2007 | 96 (40) | 94 (53) | Placebo 3.82(36.2) DHEA ‐1.54 (24.9) |
0.26 |
2.7. Analysis.
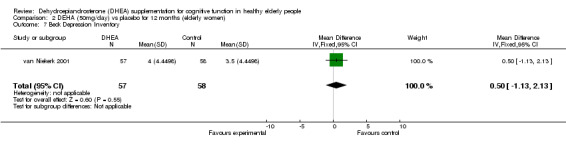
Comparison 2 DEHA (50mg/day) vs placebo for 12 months (elderly women), Outcome 7 Beck Depression Inventory.
2.8. Analysis.
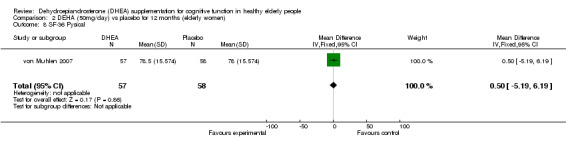
Comparison 2 DEHA (50mg/day) vs placebo for 12 months (elderly women), Outcome 8 SF‐36 Pysical.
2.9. Analysis.
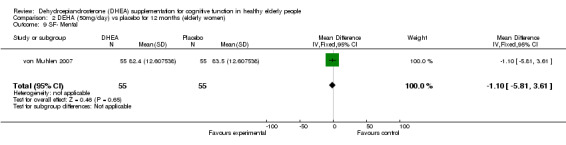
Comparison 2 DEHA (50mg/day) vs placebo for 12 months (elderly women), Outcome 9 SF‐ Mental.
2.10. Analysis.
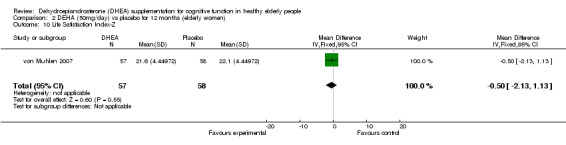
Comparison 2 DEHA (50mg/day) vs placebo for 12 months (elderly women), Outcome 10 Life Satisfaction Index‐Z.
2.11. Analysis.

Comparison 2 DEHA (50mg/day) vs placebo for 12 months (elderly women), Outcome 11 Satisfaction with Life Scale.
Comparison 3. DHEA (50 mg/day) vs placebo for 24 months (elderly women).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Quality of life (HSQ SF‐36) mental component score | 1 | Mean Difference (Fixed, 95% CI) | 0.77 [‐2.56, 4.10] | |
| 2 Quality of life (HSQ Sf‐36) physical component score | 1 | Mean Difference (Fixed, 95% CI) | 0.56 [‐2.51, 3.63] | |
| 3 Side effects at 24 months (number of participants) | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 3.1 Cardiovascular | 1 | 59 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.24 [0.74, 14.22] |
| 3.2 Gastrointestinal | 1 | 59 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.93 [0.48, 129.88] |
| 3.3 Genitourinary | 1 | 59 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.36 [0.05, 2.70] |
| 3.4 Hematopoietic and lymphatic | 1 | 59 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.98 [0.40, 22.28] |
| 3.5 Immunologic | 1 | 59 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.98 [0.40, 22.28] |
| 3.6 Musculoskeletal | 1 | 59 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.00, 7.06] |
| 3.7 Nervous | 1 | 59 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.04 [0.06, 16.96] |
| 3.8 Respiratory | 1 | 59 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.07 [0.21, 20.68] |
| 3.9 Other system | 1 | 59 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.93 [0.48, 129.88] |
| 4 Drop‐out | 1 | 60 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.93 [0.79, 79.26] |
3.1. Analysis.

Comparison 3 DHEA (50 mg/day) vs placebo for 24 months (elderly women), Outcome 1 Quality of life (HSQ SF‐36) mental component score.
3.2. Analysis.

Comparison 3 DHEA (50 mg/day) vs placebo for 24 months (elderly women), Outcome 2 Quality of life (HSQ Sf‐36) physical component score.
3.3. Analysis.
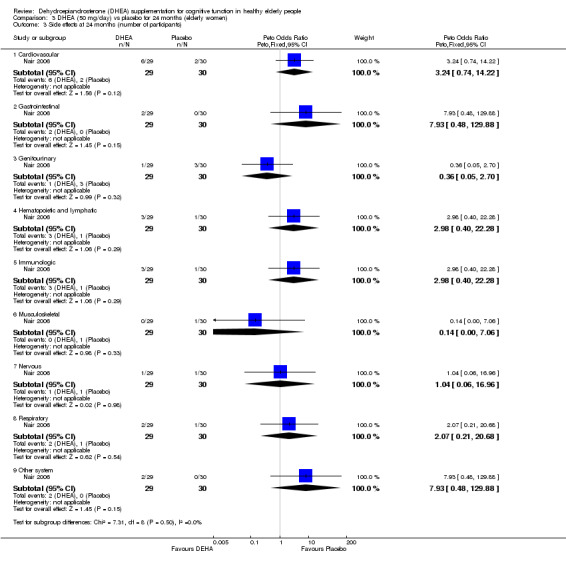
Comparison 3 DHEA (50 mg/day) vs placebo for 24 months (elderly women), Outcome 3 Side effects at 24 months (number of participants).
3.4. Analysis.
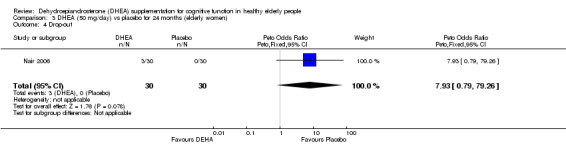
Comparison 3 DHEA (50 mg/day) vs placebo for 24 months (elderly women), Outcome 4 Drop‐out.
Comparison 4. DHEA (50mg/day) vs placebo for 3 months (elderly men).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Beck Depression Inventory | 1 | 110 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐1.81, 0.41] |
| 2 SF‐36 Physical | 1 | 110 | Mean Difference (IV, Fixed, 95% CI) | ‐1.60 [‐6.31, 3.11] |
| 3 SF‐36 Mental | 1 | 110 | Mean Difference (IV, Fixed, 95% CI) | ‐1.5 [‐5.10, 2.10] |
| 4 Life Satisfaction Index‐Z | 1 | 110 | Mean Difference (IV, Fixed, 95% CI) | ‐1.30 [‐2.69, 0.09] |
| 5 Satisfaction with Life Scale | 1 | 110 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐3.14, 0.74] |
4.1. Analysis.

Comparison 4 DHEA (50mg/day) vs placebo for 3 months (elderly men), Outcome 1 Beck Depression Inventory.
4.2. Analysis.
Comparison 4 DHEA (50mg/day) vs placebo for 3 months (elderly men), Outcome 2 SF‐36 Physical.
4.3. Analysis.
Comparison 4 DHEA (50mg/day) vs placebo for 3 months (elderly men), Outcome 3 SF‐36 Mental.
4.4. Analysis.

Comparison 4 DHEA (50mg/day) vs placebo for 3 months (elderly men), Outcome 4 Life Satisfaction Index‐Z.
4.5. Analysis.
Comparison 4 DHEA (50mg/day) vs placebo for 3 months (elderly men), Outcome 5 Satisfaction with Life Scale.
Comparison 5. DHEA (50mg/day) vs placebo for 12 months (elderly men).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 MMSE % Change | Other data | No numeric data | ||
| 2 Word List % Change | Other data | No numeric data | ||
| 3 Word List Recall % Change | Other data | No numeric data | ||
| 4 Verbal Fluency % Change | Other data | No numeric data | ||
| 5 Boston Naming %Change | Other data | No numeric data | ||
| 6 Trials B % Change | Other data | No numeric data | ||
| 7 Beck Depression Inventory | 1 | 110 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐0.99, 1.79] |
| 8 SF‐36 Physical | 1 | 110 | Mean Difference (IV, Fixed, 95% CI) | ‐2.5 [‐7.21, 2.21] |
| 9 85.3SE‐36 Mental | 1 | 110 | Mean Difference (IV, Fixed, 95% CI) | ‐2.30 [‐6.18, 1.58] |
| 10 Life Satisfaction Index‐Z | 1 | 110 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐2.39, 0.39] |
| 11 Satisfaction with Life Scale | 1 | 110 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐2.94, 0.94] |
| 12 Elveted in PSA | 1 | 110 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.65 [0.49, 14.29] |
5.1. Analysis.
Comparison 5 DHEA (50mg/day) vs placebo for 12 months (elderly men), Outcome 1 MMSE % Change.
| MMSE % Change | ||||
|---|---|---|---|---|
| Study | Placebo at 12 months Median (Interquartile Range) | DEHA at 12 months Median (Interquartile Range) | %Change | P |
| van Niekerk 2001 | 96(5) | 96(5) | Placebo ‐1.01(5.1) DHEA ‐1.01 (4.4) |
0.66 |
5.2. Analysis.
Comparison 5 DHEA (50mg/day) vs placebo for 12 months (elderly men), Outcome 2 Word List % Change.
| Word List % Change | ||||
|---|---|---|---|---|
| Study | Placebo at 12 months Median (Interquartile Range) | DEHA at 12 months Median (Interquartile Range) | %Change | P |
| von Muhlen 2007 | 20(6) | 21(5) | Placebo 5.56 (24.0) DHEA 0 (22.0) |
0.64 |
5.3. Analysis.
Comparison 5 DHEA (50mg/day) vs placebo for 12 months (elderly men), Outcome 3 Word List Recall % Change.
| Word List Recall % Change | ||||
|---|---|---|---|---|
| Study | Placebo at months 12 Median (Interquartile Range) | DEHA at months 12 Median (Interquartile Range) | %Cahnge | P |
| von Muhlen 2007 | 6(3) | 7(3) | Placebo 1111(41.7) DHEA11.11 (57.1) |
0.85 |
5.4. Analysis.
Comparison 5 DHEA (50mg/day) vs placebo for 12 months (elderly men), Outcome 4 Verbal Fluency % Change.
| Verbal Fluency % Change | ||||
|---|---|---|---|---|
| Study | Placebo at months 12 Median (Interquartile Range) | DHEA at months 12 Median (Interquartile Range) | %Change | P |
| von Muhlen 2007 | 19(7) | 20(6) | Placebo ‐6.64(19.2) DHEA 5.26(30.7) |
0.07 |
5.5. Analysis.
Comparison 5 DHEA (50mg/day) vs placebo for 12 months (elderly men), Outcome 5 Boston Naming %Change.
| Boston Naming %Change | ||||
|---|---|---|---|---|
| Study | Placebo at months 12 Median (Interquartile Range) | DHEA at months 12 Median (Interquartile Range) | %Change | P |
| von Muhlen 2007 | 15(0) | 15(1) | Placebo 0(0) DHEA 0(0) |
0.36 |
5.6. Analysis.
Comparison 5 DHEA (50mg/day) vs placebo for 12 months (elderly men), Outcome 6 Trials B % Change.
| Trials B % Change | ||||
|---|---|---|---|---|
| Study | Placebo at months 12 Median (Interquartile Range) | DHEA at months 12 Median (Interquartile Range) | %Change | P |
| von Muhlen 2007 | 90(42) | 86(29) | Placebo 1.47(39.0) DHEA ‐2.78(23.6) |
|
5.7. Analysis.

Comparison 5 DHEA (50mg/day) vs placebo for 12 months (elderly men), Outcome 7 Beck Depression Inventory.
5.8. Analysis.
Comparison 5 DHEA (50mg/day) vs placebo for 12 months (elderly men), Outcome 8 SF‐36 Physical.
5.9. Analysis.
Comparison 5 DHEA (50mg/day) vs placebo for 12 months (elderly men), Outcome 9 85.3SE‐36 Mental.
5.10. Analysis.

Comparison 5 DHEA (50mg/day) vs placebo for 12 months (elderly men), Outcome 10 Life Satisfaction Index‐Z.
5.11. Analysis.
Comparison 5 DHEA (50mg/day) vs placebo for 12 months (elderly men), Outcome 11 Satisfaction with Life Scale.
5.12. Analysis.

Comparison 5 DHEA (50mg/day) vs placebo for 12 months (elderly men), Outcome 12 Elveted in PSA.
Comparison 6. DHEA (75 mg/day) vs placebo for 24 months (elderly men).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Quality of life (HSQ SF‐36) mental component score | 1 | Mean Difference (Fixed, 95% CI) | ‐0.25 [‐2.72, 2.22] | |
| 2 Quality of life (HSQ Sf‐36) physical component score | 1 | Mean Difference (Fixed, 95% CI) | ‐1.43 [‐4.05, 1.19] | |
| 3 Side effects at 24 months (number of participants) | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 3.1 Cardiovascular | 1 | 62 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.41 [0.09, 1.98] |
| 3.2 Gastrointestinal | 1 | 62 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.00, 7.28] |
| 3.3 Genitouurinary | 1 | 62 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.67 [0.18, 2.58] |
| 3.4 Hematopoietic and lymphic | 1 | 62 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.01, 2.29] |
| 3.5 Immunologic | 1 | 62 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.01, 2.29] |
| 3.6 Musculoskeletal | 1 | 62 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 8.47 [0.85, 84.70] |
| 3.7 Nervous | 1 | 62 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.07 [0.14, 8.00] |
| 3.8 Respiratory | 1 | 62 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.07 [0.20, 5.71] |
| 3.9 Other system | 1 | 62 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.52 [0.10, 2.76] |
| 3.10 Prostate side effect | 1 | 62 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.78 [0.16, 3.73] |
| 4 Drop‐out | 1 | 62 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.06, 17.89] |
6.1. Analysis.

Comparison 6 DHEA (75 mg/day) vs placebo for 24 months (elderly men), Outcome 1 Quality of life (HSQ SF‐36) mental component score.
6.2. Analysis.

Comparison 6 DHEA (75 mg/day) vs placebo for 24 months (elderly men), Outcome 2 Quality of life (HSQ Sf‐36) physical component score.
6.3. Analysis.
Comparison 6 DHEA (75 mg/day) vs placebo for 24 months (elderly men), Outcome 3 Side effects at 24 months (number of participants).
6.4. Analysis.

Comparison 6 DHEA (75 mg/day) vs placebo for 24 months (elderly men), Outcome 4 Drop‐out.
Comparison 7. DHEA 50mg/day vs placebo at 12 months elderly men and women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Completers | 1 | 225 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.38, 3.59] |
| 2 Drop‐out due to adverse events (number of patients) | 1 | 225 | Odds Ratio (Peto, Fixed, 95% CI) | 2.66 [1.20, 5.89] |
| 3 Chest pain due to drop‐out | 1 | 225 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.42 [0.58, 20.08] |
7.1. Analysis.
Comparison 7 DHEA 50mg/day vs placebo at 12 months elderly men and women, Outcome 1 Completers.
7.2. Analysis.
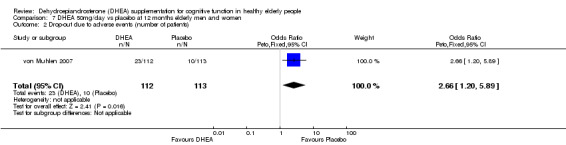
Comparison 7 DHEA 50mg/day vs placebo at 12 months elderly men and women, Outcome 2 Drop‐out due to adverse events (number of patients).
7.3. Analysis.
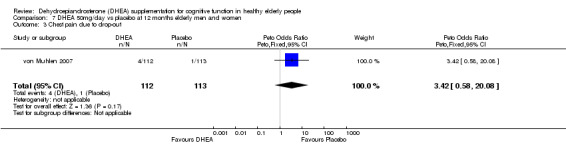
Comparison 7 DHEA 50mg/day vs placebo at 12 months elderly men and women, Outcome 3 Chest pain due to drop‐out.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barnhart 1999.
| Methods | Double‐blind: randomization and "blinding" methods not described. Parallel‐groups. Randomised: method not described. Duration: 3 months. | |
| Participants | Country: USA. Diagnosis: none. No. randomized: 66. Sex: women. Age range: 45 ‐ 55. All were perimenopausal with symptoms of fatigue, lack of energy, anxiety, tension, irritability, depression, insomnia, forgetfulness, concentration difficulties, decreased libido, or global decreased well‐being. Exclusion criteria: any contraindication to hormonal replacement therapy, exposure to an injected / implanted sex steroid / DHEA for 6 months pre‐trial, or a systemic steroid for 90 days pre‐trial; antidepressant / anxiolytic use; current psychiatric disorder; diabetes mellitus; hypercholesterolemia; cardiovascular disease; or abnormal renal or liver function. Drop outs: 6 in total, 3 due to adverse events and 3 withdrew their consent during the trial. In the adverse events group, 1 experienced parasthesia and numbness of an upper extremity while taking DHEA, 2 experienced a rash, and abdominal pain and fatigue, respectively, while taking placebo. | |
| Interventions | 1. DHEA 50 mg/day. 2. Placebo. | |
| Outcomes | 1. Symbol Copying Test: psychomotor performance. 2. Digit Symbol Substitution Test: attention / speed of processing / psychomotor performance. 3. Buschke Immediate Recall and Delayed Recall Tests: verbal memory. | |
| Notes | No significant effects on cognitive function. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomization method was not stated |
| Allocation concealment? | Unclear risk | Method of allocation concealment was not stated |
| Blinding? All outcomes | Low risk | Using identical appearance placebo |
Dayal 2005.
| Methods | Randomised, double‐blind, placebo‐controlled study Duration: 12 weeks |
|
| Participants | Country : USA 50 women aged, 44‐70 years. Inclusion criteria: 1‐Menopausal (no menstrual cycle for at least 1 year or at least 6 months of amenorrhea with FSH >40 mIU/ml. 2‐ Not on hormone treatment for at least 60 days before study enrolment. 3‐Normal Pap smear and mammogram within the last year 4‐Normal liver and renal function 5‐Normal cholesterol and triglyceride (TG) levels 4‐Healthy Exclusion criteria: 1‐Any contraindication for ET treatment ( breast cancer, endometrial hyperplasia/carcinoma 2‐Active thromboembolic disorders, cerebrovascular or coronary artery disease. 3‐Diabetes, uncontrolled hypertension 4‐Major psychiatric disorder (major depression, bipolar disorder, psychotic disorder, drug addition) 2‐Any contra‐indication of the use of MRI (pacemaker, magnetic aneurysm clip, claustrophobia) |
|
| Interventions | 1‐DHEA 50 mg/day for 12 weeks 2‐CEE 0.625 mg/day for 12 weeks 3‐DHEA 50 mg+ CEE 0.625 mg /day for 12 weeks 4‐Placebo |
|
| Outcomes | 1‐Well being 2‐Quality of live (QOL) |
|
| Notes | FSH: Follicle‐stimulating hormone ET: Estrogen Treatment CEE: Conjugated Equine Estrogen |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomization using a computerized program generating random number. |
| Allocation concealment? | Unclear risk | The treatment concealment method was not clarified. |
| Blinding? All outcomes | Low risk | Double‐blind |
Nair 2006.
| Methods | Randomised, double‐blind, placebo‐controlled study Duration: 24 months |
|
| Participants | Country : Italy 60 women aged at least 60 92 men aged at least 60 38 young healthy control women 37 young healthy control men Inclusion criteria for women: 1‐A sulfated DHEA level less than 0.95 mcg/ml (2.6 mcmol/L) 2‐Healthy Inclusion criteria for men: 1‐Testosterone level < 103 ng /dcL (3.6 nmol/L) and a sulfated DHEA level < 1.57 mcg/mL (2.6 mcmol /L) 2‐Healthy |
|
| Interventions | For women: 1‐DHEA (50 mg/day) tablet 2‐Placebo tablet For men: 1‐DHEA (75 mg/day) + transdermal placebo patch 2‐A placebo tablet + transdermal testosterone patch ( 5 mg/day) 3‐Placebo tablet + placebo transdermal patch |
|
| Outcomes | Quality of life using HSQ | |
| Notes | HSQ: Health Status Questionnaire | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomization method prepared by the study statisticians. |
| Allocation concealment? | Unclear risk | The allocation concealment method was not reported. |
| Blinding? All outcomes | Low risk | Identical appearing placebo and DHEA capsules were used. |
van Niekerk 2001.
| Methods | Randomised, double‐blind, placebo‐controlled, cross‐over. First phase data only quoted. Duration:13 weeks |
|
| Participants | Country: UK 46 men aged 60 to 80 recruited volunteers. |
|
| Interventions | 1. DHEA 50 mg/day. 2. Placebo. | |
| Outcomes | 1. Wordlist memory (ADAS). 2. Object location memory. 3. Choice reaction time. 4. Letter cancellation. 5. Measures of mood and well‐being. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomization using a computer generating random numbers |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Blinding? All outcomes | Low risk | Identical appearance placebo and DHEA |
von Muhlen 2007.
| Methods | Randomized double‐blind, placebo‐controlled trial Duration: one year |
|
| Participants | Country: USA 110 men and 115 women, aged 55 to 85 years. Inclusion criteria: 1‐For women, ceased menstruation at least 6 months prior the study entry. 2‐Not taking any hormones by any route. 3‐Healthy Exclusion criteria: 1‐Use of any medications may affect the outcomes or use of DHEA supplements in the last 6 months 2‐Presence of any of the followings: smoking, women with breasts cancer, men with prostate cancer, diabetes, myocardial infarction in the last 6 months, women < 60 with FSH<23 in the past year. 3‐The following lab abnormality: non‐fasting glucose >200 mg/dl; non‐fasting plasma triglycerid >400 mg/dl; heatocrit<30% 4‐Uncontrolled hypertension |
|
| Interventions | 1‐DHEA 50 mg/day 2‐Placebo |
|
| Outcomes | Mood and quality of life; Modified Mini‐Mental Status Examination; Trial B test for speed mental operation, attention and visual scanning; verbal fluency; Modified Boston naming test ; Word list memory; word list recall. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Using block randomization scheme |
| Allocation concealment? | Low risk | According to age and gender |
| Blinding? All outcomes | Low risk | By using identical appearing placebo |
Wolf 1998b.
| Methods | Double‐blind: randomization and "blinding" methods not described. Parallel‐groups. Randomised: stratified by age and body mass index; method not described. Duration: 2 weeks; psychosocial laboratory stressor administered at end of 2‐week period. | |
| Participants | Country: Germany. Diagnosis: none. No. randomized: 81. Sex: 38 men, 37 women (excluding 6 excluded from analyses). Age of 75 included in analyses: men: 67.5 +/‐ 5.5, women: 67.4 +/‐ 4.9 (M +/‐ SD), range: 59 ‐ 81. Exclusion criteria: cardiovascular and endocrine disorders that constitute a medical risk for stress exposure. Participants took medication typically used by an elderly population (cardiac drugs, hypotensives, andrenergics, etc.). Excluded from analyses: 6 in total, 3 due to elevated depression scores and 3 did not participate in cognitive testing. | |
| Interventions | 1. DHEA: 50mg/day. 2. Placebo. | |
| Outcomes | 1. Symbol cancellation test: attention / speed of processing. 2. Immediate and delayed free recall of 14 pictures of everyday objects: visual memory. 3. Mental rotation task: spatial memory. (All outcomes assessed pre‐ and post‐stressor). | |
| Notes | 1. Symbol cancellation test performance (number of errors subtracted from the number of targets successfully crossed out) of DHEA group significantly better than placebo group post‐stress. 2. Visual memory of DHEA group significantly impaired vs. placebo group post‐stress. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomization: method was not reported |
| Allocation concealment? | Low risk | stratified by age and body mass index; method not described. |
| Blinding? All outcomes | Low risk | Method was not reported |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alhaj 2006 | Randomized trial with a cross‐over design including 24 young males aged 18‐40. No data from first phase was available. |
| Brignardello 2007 | Randomized, placebo‐controlled trial in type two diabetes patients. |
| Finckh 2005 | Cognition only a secondary outcome in a group of patients with fibromyalgia. (Primary outcome well being). Fibromyalgia may be associated with depressive effect. |
| Forrest 1960 | The study was undertaken on a vaguely defined group of patients with "vulnerable personalities" or "depressive psychopathy" in a younger age group than the target group of older adults. |
| Hartkamp 2008 | Randomised placebo controlled trial on women with primary Sjogren syndrome. |
| Hirshman 2003 | Study was of cross‐over design and no first‐phase data were not available for analysis. |
| Hirshman 2004 | Study enrolled six postmenopausal women and was of intra‐subject cross‐over design to seek differing hormonal effects. No first‐phase data were available for analysis |
| Kahn 2002 | Placebo‐controlled double‐blind cross‐over study of effect of 90 mg/day of DHEA in 43 health men aged 56‐80 years for 6 months. No measure of cognitive function included in reported study design. |
| Martina 2006 | Randomized, double‐blind, placebo‐controlled study of DHEA 50 mg/day at bedtime for two months in 24 year aged males. Main outcome measures were biochemicals. |
| Morales 1994 | Randomized, double‐blind, placebo‐controlled, cross‐over study of 3 months nightly DHEA 50 mg in 13 men and 17 women volunteers aged 40‐70. Main outcome measures biochemical but a global open‐ended self‐assessment of well‐being (strongly positive) and of libido (little effect) included. Data from first phase only not published. |
| Muller 2006 | Randomized, double‐blind, controlled study of 36 weeks nightly 50 mg/day DHEA and placebo, atamenstane 100 mg/d and placebo, or two placebo tablets. Main outcomes measures were the physical activity. |
| Rabijewski 2007 | Study was of cross‐over design and on men with heart disease, and no first‐phase data were available for analysis |
| Villareal 2006 | Randomized, double‐blind, controlled study of 10 months of DHEA plus weightlifting exercise versus placebo plus exercise. The DHEA intervention was confounded with exercise. No cognition measures were performed. |
| Wolf 1997a | Study was of cross‐over design and no first‐phase data were available for analysis. |
| Wolf 1997b | This study was undertaken on a group of university students average age 25.1 rather than the target group of older adults, and used a very high dose of DHEA administered on a single occasion. |
| Wolf 1998a | Study was of cross‐over design and no first‐phase data were available for analysis |
| Yang 2005 | This study was not randomized controlled trial, the main purpose of the trial was to determine the effect of acute bout of resistance exercise plus DHEA on glucose tolerance and serum lipid. |
Contributions of authors
JGE all correspondence; updating review RM updating review in 2008 and all correspondence in 2008
Contact editor: Leon Flicker Consumer editors: Anne Fonfa (2008 update); David Krassner; Caroline Marshall.
This review has been peer reviewed in June 2006 and October 2008.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Barnhart 1999 {published data only}
- Barnhart KT, Freeman E, Grisso JA, Rader DJ, Sammel M, Kapoor S, Nestler JE. The effect of dehydroepiandrosterone supplementation to symptomatic perimenopausal women on serum endocrine profiles, lipid parameters, and health‐related quality of life. Journal of Clinical Endocrinology and Metabolism 1999;84(11):3896‐3902. [DOI] [PubMed] [Google Scholar]
Dayal 2005 {published data only}
- Dayal M, Sammel MD, Zhao J, Hummel AC, Vandenbourne K, Barnhart KT. Supplementation with DHEA: effect on muscle size, strength, quality of life, and lipids. Journal of Womens Health 2005;14(5):391‐400. [DOI] [PubMed] [Google Scholar]
Nair 2006 {published data only}
- Nair KS, Rizza RA, O'Brien P, Dhatariya, K, Short KR, Nehra A, et al. DHEA in elderly women and DHEA or testosterone in elderly men. New England Journal of Medicine 2006;355(16):1647‐59. [DOI] [PubMed] [Google Scholar]
van Niekerk 2001 {published data only}
- Niekerk JK, Huppert FA, Herbert J. Salivary cortisol and DHEA: association with measures of cognition and well‐being in normal older men, and effects of three months of DHEA supplementation. Psychoneuroendocrinology 2001;26(6):591‐612. [DOI] [PubMed] [Google Scholar]
von Muhlen 2007 {published data only}
- Kritz‐Silverstein D, Muhlen D, Laughlin GA, Bettencourt R. Effects of dehydroepiandrosterone supplements on cognition function and quality of life: The DHEA and well‐ness (DAWN) trial. Journal of American Geriatrics Society 2008. [DOI] [PMC free article] [PubMed]
- Muhlen D, Laughlin GA, Kritz‐Silverstein D, Barrett‐Connor E. The Dehydroepiandrosterone And WellNess (DAWN) study: research design and methods. Contemp Clin Trials 2007;28(2):153‐68. [DOI] [PubMed] [Google Scholar]
Wolf 1998b {published data only}
- Wolf OT, Kudielka BM, Hellhammer DH, Hellhammer J, Kirschbaum C. Opposing effects of DHEA replacement in elderly subjects on declarative memory and attention after exposure to a laboratory stressor. Psychoneuroendocrinology 1998;23(6):617‐629. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alhaj 2006 {published data only}
- Alhaj HA, Massey AE, McAllister‐Williams RH. Effects of DHEA administration on episodic memory, cortisol and mood in healthy young men: a double‐blind, placebo‐controlled study. Psychopharmacology (Berl) 2006;188(4):541‐51. [DOI] [PubMed] [Google Scholar]
Brignardello 2007 {published data only}
- Brignardello E, Runzo C, Aragno M, Catalano MG, Cassader M, Perin PC, Boccuzzi G. Dehydroepiandrosterone administration counteracts oxidative imbalance and advanced glycation end product formation in type 2 diabetic patients.. Diabetes Care 2007 Nov;30(11):2922‐7. [DOI] [PubMed] [Google Scholar]
Finckh 2005 {published data only}
- Finckh A, Berner IC, Aubry Rozier B, So AK. A randomized controlled trial of dehydroepiandrosterone in postmenopausal women with fibromyalgia. Journal of Rheumatology 2005;32(7):1336‐40. [PubMed] [Google Scholar]
Forrest 1960 {published data only}
- Forrest AD, Drewery J, Fotherby K, Laverty SG. A clinical trial of dehydroepiandrosterone (diandrone). Journal of Neurology, Neurosurgery and Psychiatry 1960;23:52‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hartkamp 2008 {published data only}
- Hartkamp A, Geenen R, Godaert GL, Bootsma H, Kruize AA, Bijlsma JW, Derksen RH. Effect of dehydroepiandrosterone administration on fatigue, well‐being, and functioning in women with primary Sjogren syndrome: a randomised controlled trial. Annals of the Rheumatic Diseases 2008;67(1):91‐7. [DOI] [PubMed] [Google Scholar]
Hirshman 2003 {published data only}
- Hirshman E, Wells E, Wierman ME, Anderson B, Butler A, Senholzi M, Fisher J. The effect of dehydroepiandrosterone (DHEA) on recognition memory decision processes and discrimination in postmenopausal women. Psychonomic Bulletin & Review 2003;10(1):125‐34. [DOI] [PubMed] [Google Scholar]
Hirshman 2004 {published data only}
- Hirshman E, Merritt P, Wang CC, Wierman M, Budescu DV, Kohrt W, Templin JL, Bhasin S. Evidence that androgenic and estrogenic metabolites contribute to the effects of dehydroepiandrosterone on cognition in postmenopausal women. Hormones and Behavior 2004;45(2):144‐55. [DOI] [PubMed] [Google Scholar]
Kahn 2002 {published data only}
- Kahn AJ, Halloran B, Wolkowitz O, Brizendine L. Dehydroepiandrosterone supplementation and bone turnover in middle‐aged to elderly men. Journal of Clinical Endocrinology and Metabolism 2002;87(4):1544‐9. [DOI] [PubMed] [Google Scholar]
Martina 2006 {published data only}
- Martina V, Benso A, Gigliardi VR, Masha A, Origlia C, Granata R Ghigo E. Short‐term dehydroepiandrosterone treatment increases platelet cGMP production in elderly male subjects. Clinical Endocrinology 2006;64(3):260‐4. [DOI] [PubMed] [Google Scholar]
Morales 1994 {published data only}
- Morales AJ, Nolan JJ, Nelson JC, Yen SSC. Effects of replacement dose of dehydroepiandrosterone in men and women of advancing age. Journal of Clinical Endocrinology and Metabolism 1994;78:1360. [DOI] [PubMed] [Google Scholar]
Muller 2006 {published data only}
- Muller M, Beld AW, Schouw YT, Grobbee DE, Lamberts SW. Effects of dehydroepiandrosterone and atamestane supplementation on frailty in elderly men. Journal of Clinical Endocrinology and Metabolism 2006;91(10):3988‐91. [DOI] [PubMed] [Google Scholar]
Rabijewski 2007 {published data only}
- Rabijewski M, Zgliczynski W. Dehydroepiandrosterone therapy in men with angiographically verified coronary heart disease: the effects on plasminogen activator inhibitor‐1 (PAI‐1), tissue plasminogen activator (tPA) and fibrinogen plasma concentrations. Endokrynol Pol 2007;58(3):213‐9. [PubMed] [Google Scholar]
Villareal 2006 {published data only}
- Villareal DT, Holloszy JO. DHEA enhances effects of weight training on muscle mass and strength in elderly women and men. Am J Physiol Endocrinol Metab 2006;291(5):1003‐8. [DOI] [PubMed] [Google Scholar]
Wolf 1997a {published data only}
- Wolf OT, Neumann O, Hellhammer DH, Geiben AC, Strasburger CJ, Dressendorfer RA, Pirke K, Kirschbaum C. Effects of a two‐week physiological dehydroepiandrosterone substitution on cognitive performance and well‐being in healthy elderly women and men. Journal of Clinical Endocrinology and Metabolism 1997;82(7):2363‐7. [DOI] [PubMed] [Google Scholar]
Wolf 1997b {published data only}
- Wolf OT, Koster B, Kirschbaum C, Pietrowsky R, Kern W, Hellhammer DH, Born J, Fehm HL. A single administration of dehydroepiandrosterone does not enhance memory performance in young healthy adults but immediately reduces cortisol levels. Biological Psychiatry 1997;42(9):845‐848. [DOI] [PubMed] [Google Scholar]
Wolf 1998a {published data only}
- Wolf OT, Naumann E, Hellhammer DH, Kirschbaum C. Effects of dehydroepiandrosterone (DHEA) replacement in elderly men on event related potentials (ERPs), memory and well‐being. Journal of Gerontology (Medical Sciences) 1998;53(5):M385‐390. [DOI] [PubMed] [Google Scholar]
Yang 2005 {published data only}
- Yang SC, Chen CY, Liao YH, Lin FC, Lee WC, Cho YM, Chen MT, Chous CH, Kuo CH. Interactive effect of an acute bout of resistance exercise and dehydroepiandrosterone administration on glucose tolerance and serum lipids in middle‐aged women. Chinese Journal of Physiology 2005;48(1):23‐9. [PubMed] [Google Scholar]
Additional references
Arlt 1999
- Arlt W, Haas J, Callies F, Reincke M, Hubler D, Oettel M, et al. Biotransformation of oral dehydroepiandrosterone in elderly men: significant increase in circulating estrogens. Journal of Clinical Endocrinology and Metabolism 1999;84(6):2170‐6. [DOI] [PubMed] [Google Scholar]
Barrett‐Connor 1986
- Barrett‐Connor E, Khaw KT, Yen SSC. A prospective study of dehydroepiandrosterone sulfate, mortality and cardiovascular disease. New England Journal of Medicine 1986;315:1519‐24. [DOI] [PubMed] [Google Scholar]
Baulieu 1998
- Baulieu EE. Neurosteroids: A novel function of the brain. Psychoneuroendocrinology 1998;23(8):963‐987. [DOI] [PubMed] [Google Scholar]
Berr 1996
- Berr C, Lafont S, Debuire B, Dartigues J, Baulieu E. Relationships of dehydroepiandrosterone sulfate in the elderly with functional, psychological, and mental status, and short‐term mortality: A French community‐based study. Proceedings of the National Academy of Sciences USA 1996;93:13410‐13415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Blauer 1991
- Blauer KL, Poth M, Rogers WM, Bernton EW. Dehydroepiandrosterone antagonises the suppressive effects of dexamethasone on lymphocyte proliferation. Endocrinology 1991;129:3174‐9. [DOI] [PubMed] [Google Scholar]
BNF 2008
- bnf.org. British National Formulary. London: BMJ Publishing Group Ltd, 2008. [Google Scholar]
Borkowski 1967
- Borkowski JG, Benton AL, Spreen O. Word fluency and brain damage. Neuropsychologia 1967;5:135–40. [Google Scholar]
Buschke 1974
- Buschke K, Fuld PA. Evaluating storage, retention, retrieval in disordered memory and learning. Neurology 1974;24:1019‐25. [DOI] [PubMed] [Google Scholar]
Buvat 2003
- Buvat J. Androgen therapy with dehydroepiandrosterone. World Journal of Urology 2003;21(5):346‐55. [DOI] [PubMed] [Google Scholar]
Casson 1993
- Casson PR, Andersen RN, Herrod HG, et al. Oral dehydroepiandrosterone in physiologic doses modulates immune function in postmenopausal women. American Journal of Obstetrics and Gynecology 1993;169:1536‐9. [DOI] [PubMed] [Google Scholar]
Chalmers 1983
- Chalmers TC, Celano P, Sacks HS, Smith H, Junior. Bias in Treatment Assignment in Controlled Clinical Trials. New England Journal of Medicine 1983;309:1358‐61. [DOI] [PubMed] [Google Scholar]
Cox 1993
- Cox BD, Huppert FA, Whichelow MJ. The Health and Lifestyle Survey: seven years on. Aldershot: Dartmouth, 1993. [Google Scholar]
Davis 2008
- Davis SR, Shah S, McKenzie D, Kulkarni J, Davison S, Bell R. Dehydroepiandrosterone Sulfate Levels Are Associated with More Favorable Cognitive Function in Women. Clinical Endocrinology and Metabolism 2008;93(3):801‐08. [DOI] [PubMed] [Google Scholar]
Debonnel 1996
- Debonnel G, Bergeron R, Montigny C. Potentiation by dehydroepiandrosterone of the neuronal response to N‐methyl‐D‐aspartate in the CA3 region of the rat dorsal hippocampus: an effect mediated via sigma receptors. Journal of Endocrinology 1996;150 (Supplement):S33‐42. [PubMed] [Google Scholar]
Diener 1985
- Diener E, Emmons RA, Larson RJ eta al. The satisfaction with life scale. J Pers Assess 1985;49:71‐5. [DOI] [PubMed] [Google Scholar]
Ebeling 1994
- Ebeling P, Kiovisto VA. Physiological importance of dehydroepiandrosterone. Lancet 1994;343:1479‐81. [DOI] [PubMed] [Google Scholar]
Enomoto 2008
- Enomoto M, Adachi H, Fukami A, Furuki K, Satoh A, Otsuka M, Kumagae S, Nanjo Y, Shigetoh Y, Imaizumi T. Serum dehydroepiandrosterone sulfate levels predict longevity in men: 27‐year follow‐up study in a community‐based cohort (Tanushimaru study). J Am Geriatr Soc 2008 Apr 18;56(6):994‐8. [DOI] [PubMed] [Google Scholar]
Freeman 1992
- Freeman E, Weinstock L, Rickels K, Sondheimer S, Coutifaris C. A placebo‐controlled study of effects of oral progesterone on performance and mood. British Journal of Clinical Pharmacology 1992;333:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fromm‐Auch1983
- Fromm‐Auch D, Yeudall LT. Normative data for the Halstead–Reitan neuropsychological tests. Journal of Clinical Neuropsychology 1983;5(3):221–38. [DOI] [PubMed] [Google Scholar]
Gatterer 1990
- Gatterer G. Alters‐Konzentratrions‐Test. Gottingen: Hogrefe, 1990. [Google Scholar]
Guazzo 1996
- Guazzo EP, Kirkpatrick PJ, Goodyer IM, Shiers HM, Herbert J. Cortisol, dehydroepiandrosterone (DHEA), and DHEA sulfate in the cerebrospinal fluid of man: Relation to blood levels and the effects of age. Journal of Clinical Endocrinology and Metabolism 1996;81(11):3951‐3960. [DOI] [PubMed] [Google Scholar]
Herbert 1997
- Herbert, J. Stress, the brain, and mental illness. British Medical Journal 1997;315:530‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hillen 2000
- Hillen T, Lun A, Reischies FM, Borchelt M, Steinhagen TE, Schaub RT. DHEA‐S plasma levels and incidence of Alzheimer's disease. Biological Psychiatry 2000;47(2):161‐163. [DOI] [PubMed] [Google Scholar]
Kapalan 1982
- Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. Philadelphia, Pa: Lea and Febiger; 1982.. Philadelphia, Pa: Lea and Febiger. 1982. [Google Scholar]
Kimonides 1998
- Kimonides VG, Khatibi NH, Svendsen CN, Sofroniew MV, Herbert J. Dehydroepiandrosterone (DHEA) and DHEA‐sulfate (DHEAS) protect hippocampal neurons against excitatory amino acid‐induced neurotoxicity. Proceedings of the National Academy of Sciences USA 1998;95:1852‐1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kimonides 1999
- Kimonides VG, Spillantini MG, Sofroniew MV, Fawcett JW, Herbert J. Dehydroepiandrosterone (DHEA) antagonises the neurotoxic effects of corticosterone and translocation of SAPK3 in hippocampal primary cultures. Neuroscience 1999;89:429‐436. [DOI] [PubMed] [Google Scholar]
Kirschbaum 1993
- Kirschbaum C, Pirke KM, Hellhammer DH. The "Trier Social Stress Test" ‐ a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology 1993;28:76‐81. [DOI] [PubMed] [Google Scholar]
Kirschbaum 1996
- Kirschbaum C, Wolf OT, May M, Wippich W, Hellhammer DH. Stress‐ and treatment‐induced elevation of free cortisol levels associated with impaired declarative memory in healthy adults. Life Sciences 1996;58:1475‐1483. [DOI] [PubMed] [Google Scholar]
Legrain 2003
- Legrain S, Girard L. Pharmacology and therapeutic effects of dehydroepiandrosterone in older subjects. Drugs and Aging 2003;20(13):949‐67. [DOI] [PubMed] [Google Scholar]
Lupien 1998
- Lupien SJ, Leon M, Santi S, Convit A, Tarshish C, Nair NPV. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nature Neuroscience 1998;1(1):69‐73. [DOI] [PubMed] [Google Scholar]
Majewska 1995
- Majewska MD. Neuronal actions of dehydroepiandrosterone. Annals of the New York Academy of Sciences 1995;774:111‐120. [DOI] [PubMed] [Google Scholar]
Maughan 2004
- Maughan RJ, King DS, Lea T. Dietary supplements. Journal of Sport Sciences 2004;22:95–113. [DOI] [PubMed] [Google Scholar]
Mohs 1997
- Mohs RC, Knopman D, Peterson RC, Ferris SH, Erenesto C, Grundman M, Sano M, Bielauskas L, Geldmacher D, Clark C, Thal LJ. Developmeent of cognitive instruments for use in clinical trials of antidementia drugs: additions to the Alzheimer's Disease Assessment Scale that broaden its scope. The Alzheimer's Disease Cooperative Study.. Alzheimer's Disease and Associated Disorders 1997;11 (Supp2):S13‐S21. [PubMed] [Google Scholar]
Morris 1989
- Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology 1989;39(9):1159–65. [DOI] [PubMed] [Google Scholar]
MRC CFAS 1998
- The Medical Research Council Cognitive Function and Ageing Study (MRC CFAS). Cognitive function and dementia in six areas of England and Wales: the distribution of MMSE and prevalence of GMS organicity level in the MRC CFA Study. Psychological Medicine 1998;28(2):319‐335. [DOI] [PubMed] [Google Scholar]
Mulrow 1997
- Mulrow CD, Oxman AD. Cochrane Collaboration Handbook [updated 9 December 1996] Available in The Cochrane Library [database on disk and CDROM], Issue 1. Updated quarterly. Oxford: Update Software, 1997. [Google Scholar]
Nasman 1991
- Nasman B, Olsson T, Backstrom T, Eriksson S, Grankvist K, Viitanen M, Bucht G. Serum dehydroepiandrosterone sulfate in Alzheimer's disease and in multi‐infarct dementia. Biological Psychiatry 1991;30(7):684‐690. [DOI] [PubMed] [Google Scholar]
Orentreich 1992
- Orentreich N, Brind JL, Vogelman JH, Andres R, Baldwin H. Long‐term longitudinal measurements of plasma dehydroepiandrosterone sulfate in normal men. Journal of Clinical Endocrinology and Metabolism 1992;75(4):1002‐1004. [DOI] [PubMed] [Google Scholar]
Oswald 1994
- Oswald WD, Fleischmann UM. Nurnberger Alters‐Inventar. Gottingen: Hogrefe, 1994. [Google Scholar]
Percheron 2003
- Percheron G, Hogrel JY, Denot Ledunois S, Fayet G, Forette F, Baulieu EE, Fardeau M, Marini JF. Effect of 1‐year oral administration of dehydroepiandrosterone to 60‐ to 80‐year‐old individuals on muscle function and cross‐sectional area: a double‐blind placebo‐controlled trial. Archives of Internal Medicine 2003;163(6):720‐7. [DOI] [PubMed] [Google Scholar]
Petturrson 1983
- Petturrson H, Gudjonsson G, Lader M. Psychometric performance during withdrawal from long‐term, benzodiazepine treatment. Psychopharmacology 1983;31:345‐9. [DOI] [PubMed] [Google Scholar]
Rudman 1990
- Rudman D, Shetty KR, Mattson DE. Plasma dehydroepiandrosterone sulfate in nursing home men. Journal of the American Geriatrics Society 1990;38:421‐7. [DOI] [PubMed] [Google Scholar]
Schneider 1992
- Schneider LS, Hinsey M, Lyness S. Plasma dehydroepiandrosterone sulphate in Alzheimer's Disease. Biological Psychiatry 1992;31:205‐8. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. Journal of the American Medical Association 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
Stoker 1992
- Stoker MJ, Dunbar GC, Beaumont G. The SmithKline Beecham 'Quality of Life' Scale: A Validation and Reliability Study in Patients with Affective Disorder. Quality of Life Research 1992;1(6):385‐95. [DOI] [PubMed] [Google Scholar]
Sunderland 1989
- Sunderland T, Merril CR, Harrington MG, Lawlor BA, Molchan SE, Martinez R, Murphy DL. Reduced plasma dehydroepiandrosterone concentrations in Alzheimer’s disease. Lancet 1989;2(8662 2(8662): 570. 2(8662): 570.):570. [DOI] [PubMed] [Google Scholar]
Teng 1987
- Teng EL, Chui HC. The Modified Mini‐Mental State (3MS) examination. Journal of Clinical Psychiatry 1987;48(8):314–8.. [PubMed] [Google Scholar]
Thompson 2001
- Thompson EM. DHEA supplementation in postmenopausal women: Effects on endogenous hormone levels and cognition. Dissertation Abstracts International: Section B: The Sciences and Engineering 2001;62(3‐B):1602. [Google Scholar]
Van Vollenhoven 1994
- Vollenhoven RF, Engleman EG, McGuire JL. An open study of dehydroepiandrosterone in systemic lupus erythematosus. Arthritis and Rheumatism 1994;37:1305‐10. [DOI] [PubMed] [Google Scholar]
Ware 1992
- Ware Jr JE, Sherbourne CD. THe MOS 36‐item Short‐Form Health Survey (SF‐36).I. Conceptual framework and item secliction. Medical Care 1992;30(6):473‐83. [PubMed] [Google Scholar]
Welsh 1994
- Welsh KA, Butters N, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part V. A normative study of the neuropsychological battery. Neurology 1994;44(4):609–14. [DOI] [PubMed] [Google Scholar]
Wiklund 1992
- Wiklund I, Holst J, Karlberg J, et al. A new methodological approach to the evaluation of quality of life in postmenopausal women. Maturitas 1992;14:211. [DOI] [PubMed] [Google Scholar]
Wood 1969
- Wood V, Wylie ML, Sheafor B. An analysis of a short self‐report measure of life satisfaction: Correlation with rater judgments. Journal of the Gerontology 1969;24(4):465‐9. [DOI] [PubMed] [Google Scholar]
Yaffe 1998
- Yaffe K, Ettinger B, Pressman A, Seeley D, Whooley M, Schaefer C, Cummings S. Neuropsychiatric function and dehydroepiandrosterone sulfate in elderly women: a prospective study. Biological Psychiatry 1998;43(9):694‐700. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Grimley Evans 2006
- Grimley Evans J, Malouf R, Huppert F, Niekerk JK. Dehydroepiandrosterone (DHEA) supplementation for cognitive function in healthy elderly people. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD006221] [DOI] [PMC free article] [PubMed] [Google Scholar]


