Abstract
Background:
Although there is some evidence that cannabinoid (CBD) products may provide a therapeutic effect for musculoskeletal pain, little is known about the usage patterns or their prevalence of use in orthopaedic sports medicine patients.
Purpose:
To report the prevalence and perceived self-efficacy of CBD products in patients evaluated in an orthopaedic sports medicine clinic.
Study Design:
Descriptive epidemiology study. Level of evidence, 2.
Methods:
The study population consisted of new patients who visited an orthopaedic surgery sports medicine clinic at a large academic center for consultation with a surgeon between August 2020 and March 2021. All patients were asked to complete a survey that assessed perceived pain and effectiveness of CBD products and other nonsurgical treatment modalities using the Single Assessment Numeric Evaluation score (range, 0-100) and the Numeric Pain Rating Scale (NRS). Descriptive factors were collected via chart review. Descriptive statistics were used to characterize the data.
Results:
Overall, 823 patients completed the survey (45.4% female; mean age, 51 years [range, 18-87 years]; mean body mass index, 28.9 [range, 17.2-58.4]). Body areas involved included 285 shoulders, 44 elbows, 76 hips, 276 knees, 58 ankles, and 77 other. Of these patients, 19% (152/823) endorsed the use of CBD products before their initial evaluation. The mean NRS for pain was significantly different between non-CBD users and CBD users (5.6 vs 6.1; P = .029). CBD users were significantly more likely to have tried other nonoperative modalities compared with nonusers, including nonsteroidal anti-inflammatory drugs (79.6% vs 69.8%; P = .032), bracing (44.7% vs 34.6%; P = .024), steroid injections (38.8% vs 21.6%; P < .001), and physical therapy (54% vs 36.1%; P < .001). In addition, 30.9% of CBD utilizers reported marijuana use compared with 2.8% of non-CBD users (P < .001) for management of their pain.
Conclusion:
In the current study, 19% of patients had used CBD products to manage joint-related issues. Sports medicine providers should be aware of this high incidence of usage and the potential interactions CBD products may have with other treatment modalities. Further studies are needed to assess the effectiveness of CBD as a therapeutic agent and the specific interactions it has with other drugs and other forms of treatment.
Keywords: ankle, CBD, cannabinoid, hemp, hip, knee, marijuana, shoulder, sports medicine, THC
Cannabidiol (CBD) is derived from the plant Cannabis sativa, which is also the source of tetrahydrocannabinol (THC). The legalization of hemp, a variety of C. sativa, in the United States has led to the production and widespread availability of CBD products. 20 CBD has been touted for uses ranging from epilepsy to the treatment of musculoskeletal pain, without the mind-altering effects associated with THC. 31 Although CBD is frequently marketed to treat musculoskeletal pain, no marketing applications are approved by the US Food and Drug Administration (FDA) for cannabis or cannabis-derived compounds. 23,31 While there is early evidence to support these claims, the overwhelming availability and consumer interest in these products exceeds our current knowledge. §
Although evidence regarding these products is limited, our knowledge about usage is even less. Stephen Hahn, the Commissioner of the FDA from 2019-2021, wrote that the “rates of CBD use, and rates of use of specific CBD products, are poorly understood.” 11 A 2019 poll reported a 14% usage rate of CBD products among the US population. 3 At present, the incidence of use of these products is unknown in the sports medicine patient. It is important that orthopaedic surgeons are aware of these products and their patients’ usage patterns, as they have been shown to have drug-drug interactions. 4
This study aimed to characterize the use of CBD products by sports medicine patients and to assess patients’ perceived efficacy of these products. We hypothesized that CBD use would be common (>15% of population) and that patients would report it is effective in treating pain.
Methods
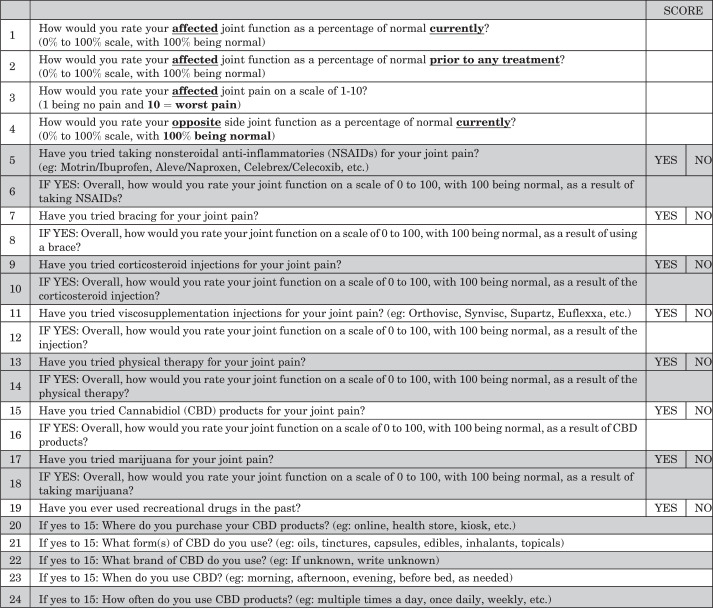
Institutional review board approval was obtained prior to the initiation of this study. All patients aged 18 years and older who visited 1 of 4 sports medicine fellowship-trained orthopaedic surgeons (K.A.P., K.E., J.M.T., A.C.) at a single high-volume academic center for an initial consultation between August 2020 and March 2021 were eligible. As part of the initial intake screening, patients were asked to complete an optional 24-question survey (Appendix Figure A1). Questions concerning function and perceived efficacy of treatments were assessed using Single Assessment Numeric Evaluation (SANE) on a scale of 1 to 100 points, with 100 indicating the highest perceived benefit. 1,28 A Numeric Pain Rating Scale (NRS) was also used to assess pain in the affected joint. 7
Data Collection
After questionnaires were completed, all answers were categorized and tabulated. Forms were excluded if incomplete. A chart review was carried out to obtain descriptive information. The average SANE score was calculated for each of the questions. Question results were either binary (yes/no), numeric (SANE/NRS), or free text (eg, Question 22: What brand of CBD do you use?). Free-text answers were reviewed for each respondent and categorized into nominal reviewable outcomes.
Data Analysis
Descriptive statistics were calculated to characterize the population. Comparisons of variation for continuous variables were scrutinized using the Student t test. Comparison of proportions for sample populations was performed using the z test. All statistical analysis was performed using JMP statistical software (SAS Institute) and Microsoft Excel (Microsoft Corporation). A p-value of .05 was used as the cutoff for significance in all analyses.
Results
Of 1000 consecutive patients initially surveyed, 823 met inclusion criteria and completed the questionnaire. Of those included, there were more men than women (54.6% vs 45.4%). The average age was 51 years (range, 18-87 years). The average body mass index was 28.9 (range, 17.2-58.4). Shoulder (285/823; 34.6%) and knee (276/823; 33.5%) were the 2 most common joint pathologies affected (Table 1).
Table 1.
Characteristics of Orthopaedic Surgery Sports Medicine Clinic Survey Respondents a
| Overall (N = 823) |
CBD Users (n = 152; 18.5%) |
Non-CBD Users (n = 671; 81.5%) |
P | |
|---|---|---|---|---|
| Age, y | 50.9 ± 16.2 | 51.2 ± 16.5 | 50.7 ± 16.0 | .711 |
| Sex | ||||
| Male | 449 (54.6) | 64 (42.1) | 385 (57.4) | <.001 |
| Female | 374 (45.4) | 88 (57.9) | 286 (42.6) | <.001 |
| BMI | 28.9 ± 6.2 | 29.2 ± 6.8 | 28.6 ± 6.0 | .339 |
| Laterality | ||||
| Right | 385 (46.8) | 71 (46.7) | 314 (46.8) | .985 |
| Left | 358 (43.5) | 60 (39.5) | 298 (44.4) | .268 |
| Bilateral | 78 (9.5) | 21 (13.8) | 57 (8.5) | .043 |
| Joint affected | ||||
| Shoulder | 285 (34.6) | 52 (34.2) | 233 (34.7) | .905 |
| Elbow | 44 (5.3) | 7 (4.6) | 37 (5.5) | .653 |
| Hip | 76 (9.2) | 15 (9.9) | 61 (9.1) | .765 |
| Knee | 276 (33.5) | 59 (38.8) | 217 (32.3) | .127 |
| Ankle | 58 (7.0) | 10 (6.6) | 48 (7.2) | .803 |
| Other b | 77 (9.4) | 9 (5.9) | 68 (10.1) | .177 |
a Data are reported as mean ± SD or n (%). Bold indicates statistically significant difference between the study groups (P < .05). BMI, body mass index; CBD, cannabinoid.
b Other: any nonlarge joint (eg, sports injuries to toes, fingers, feet).
Overall, 19% of respondents (152/823) reported use of CBD products before the initial evaluation (Table 2). The mean SANE score for the affected joint at presentation was 50 for CBD users and 53 for non-CBD users (P = .256). The CBD users reported an average SANE score of 56 before receiving any form of conservative treatment before being seen at our clinic compared with 59 for non-CBD users (P = .262). The average affected joint NRS was 6.1 for CBD users and 5.6 for non-CBD users (P = .029). Contralateral joint SANE score was 81 for CBD users and 89 for non-CBD users (P < .001).
Table 2.
Comparison of SANE and NRS Scores Between CBD Users and Non-CBD Users a
| CBD Users (n = 152) |
Non-CBD Users (n = 671) |
P | |
|---|---|---|---|
| Affected joint SANE at initial evaluation | 50.3 ± 21.3 | 52.7 ± 24.4 | .256 |
| Affected joint SANE before any previous treatment | 55.6 ± 28.3 | 58.8 ± 30.3 | .262 |
| Affected joint NRS score | 6.1 ± 2.2 | 5.6 ± 2.2 | .029 |
| Contralateral joint SANE at initial evaluation | 81.0 ± 27.8 | 88.8 ± 21.7 | <.001 |
a Data are reported as mean ± SD. Bold indicates statistically significant difference between groups (P < .05). CBD, cannabinoid; NRS, Numeric Pain Rating Scale; SANE, Single Assessment Numeric Evaluation score.
CBD users had tried all listed forms of nonoperative treatments, including nonsteroidal anti-inflammatory drugs (NSAIDs), bracing, steroid injections, viscosupplementation injections, and physical therapy (PT) at statistically higher rates than non-CBD users (Table 3). In addition, CBD users were significantly more likely to report marijuana usage (30.9%; 47/152) when compared with non-CBD users (2.8%; 19/671; P < .001). This was also true for “other” reported recreational drug use between the 2 cohorts (28.9% vs 13.7%; P < .001; CBD vs non-CBD users, respectively).
Table 3.
Comparison of Previous Nonoperative Treatments Reported by CBD Users and Non-CBD Users a
| CBD Users (n = 152) |
Non-CBD Users (n = 671) |
Pb | |
|---|---|---|---|
| NSAID | 121 (79.6) | 468 (69.8) | .032 |
| Bracing | 68 (44.7) | 232 (34.6) | .024 |
| Steroid injection | 59 (38.8) | 145 (21.6) | <.001 |
| Viscosupplementation injection | 12 (7.9) | 19 (2.8) | .003 |
| Physical therapy | 82 (54.0) | 242 (36.1) | <.001 |
| Marijuana | 47 (30.9) | 19 (2.8) | <.001 |
| Recreational “other” drug use c | 43 (28.9) | 92 (13.7) | <.001 |
a Data are reported as n (%). Bold indicates statistically significant difference between groups (P < .05). CBD, cannabinoid; NSAID, nonsteroidal anti-inflammatory drug.
b Computed using z test for difference in proportions.
c Patients were not asked to specify what “other” recreational drugs they used.
When comparing posttreatment SANE scores between CBD users and nonusers, there was a statistically significant difference in improvement after steroid injection, although this difference was not clinically relevant (6.6 vs 6.8 points of improvement for CBD users vs nonusers, respectively; P < .001) (Table 4). There was also a statistically significant but clinically insignificant decrease from baseline after PT between the 2 groups. Interestingly, after marijuana use, non-CBD users reported a statistically significant increase in SANE score of 15.7 points compared with no change from baseline for CBD users (P = .03).
Table 4.
Comparison of SANE Scores After Nonoperative Treatments Between CBD Users and Non-CBD Users a
| SANE Score | |||
|---|---|---|---|
| CBD Users | Non-CBD Users | P | |
| Baseline | 55.6 ± 28.2 | 58.8 ± 30.3 | .261 |
| After CBD | 52.1 ± 26.1 | n/a | n/a |
| Change from baseline | –3.4 | n/a | n/a |
| After NSAID | 53.5 ± 23.2 | 53.8 ± 27.2 | .932 |
| Change from baseline | –2.1 | –5.0 | .428 |
| After bracing | 52.3 ± 23.5 | 51.4 ± 27.3 | .828 |
| Change from baseline | –3.3 | –7.4 | .059 |
| After steroid injection | 62.2 ± 29.3 | 65.6 ± 29.4 | .467 |
| Change from baseline | 6.6 | 6.8 | <.001 |
| After viscosupplementation | 64.1 ± 20.2 | 54.4 ± 30.5 | .363 |
| Change from baseline | 8.5 | –4.4 | .099 |
| After physical therapy | 55.5 ± 23.4 | 55.5 ± 55.5 | .989 |
| Change from baseline | –0.1 | –3.3 | .003 |
| After marijuana | 55.6 ± 26.1 | 74.5 ± 15.0 | .034 |
| Change from baseline | 0 | 15.7 | .03 |
a Scores are reported as mean ± SD unless otherwise indicated. Bold indicates statistically significant difference between groups (P < .05). CBD, cannabinoid; n/a, not applicable; NSAID, nonsteroidal anti-inflammatory drug; SANE, Single Assessment Numeric Evaluation score.
CBD users most frequently (44%; 53/121) reported once daily usage of these products (Table 5). Eighty percent of patients (122/152 patients) could not recall the name brand of their CBD product. Patients most frequently bought these products online (22%; 34/152 patients) or from a health or grocery store (26%; 40/138 patients). Topical applications, in the form of creams, oils, and lotions, were the most commonly used forms of CBD (75%; 104/138).
Table 5.
Characterization of CBD Use and Procurement in the Study Sample a
| Descriptor | n (%) |
|---|---|
| Frequency of use b | |
| As needed | 37 (31) |
| 1 time daily | 53 (44) |
| 2 times daily | 17 (14) |
| 3 times daily | 3 (2) |
| Discontinued use | 11 (9) |
| Brand c | |
| Recalled brand name | 30 (20) |
| Could not recall brand name | 122 (80) |
| Purchasing location c | |
| Online | 34 (22) |
| CBD store | 6 (4) |
| Health or grocery store | 40 (26) |
| Dispensary | 22 (15) |
| Unsure/could not recall | 50 (33) |
| CBD type d | |
| Capsule | 9 (7) |
| Edible | 18 (13) |
| Topical (cream/oil/lotion) | 104 (75) |
| Inhalant | 7 (5) |
a CBD, cannabinoid.
b n = 121 respondents.
c n = 152 respondents.
d n = 138 respondents.
Discussion
In this prospective cohort of 823 consecutive patients, we report a prevalence of 19% (152/823) of patients who reported use of CBD products before initial evaluation with an orthopaedic sports medicine surgeon. This is the first study to date to characterize the prevalence of CBD use in this patient population, with nearly 50% (53/121) reporting daily use of these products. Although there does not appear to be a perceived benefit of CBD use to nonuse, CBD users reported higher rates of usage of other nonoperative modalities. This may suggest that they believe they have exhausted other methods of relief and have turned to CBD as another option. Interestingly, nearly 30% of CBD users reported marijuana use as well (marijuana was legalized on November 30, 2020, in the state of Arizona). Overall, this is similar to both the 25% prevalence of CBD use in patients with nonoperatively treated hip and knee osteoarthritis 6 and the 16.4% incidence of use in the perioperative period around hip and knee arthroplasty. 26 Overall, the findings presented in this study are comparable with previously reported rates of marijuana use in the United States. 2,14 Given the high rate of use of these products, it is important that orthopaedic sports medicine providers be aware of CBD products and the growing rate of their use.
To provide context, it may be beneficial to review certain key events in recent history. As previously mentioned, in 2014, the United States passed the Agricultural Act of 2014, which technically defined the distinction between hemp and marijuana as “C. sativa L. and any part of such plant, whether growing or not, with a delta-9-THC content of no more than 0.3% on dry weight basis.” 20,31 The Agriculture Improvement Act of 2018 (the Farm Bill 2018) further preserved the ability of the FDA to regulate all cannabis or cannabis-derived products, regardless of whether the product is derived from hemp or not. 5 To date, the FDA has approved only 1 cannabis-derived and 3 cannabis-related drugs. 21 All of the non-FDA approved products that contain CBD and market these for therapeutic purposes are technically doing so illicitly, and many have received warning letters. 11 Moreover, the online sources and information about these products can be misleading and unreliable. 25 Presently, the demand and popularity of these products has outpaced our knowledge, as there is little high-quality evidence to endorse the use of these products. 18
Cannabinoids are hypothesized to augment the endogenous cannabinoid system of the body via the CB1 and CB2 receptors in both the peripheral and central nervous systems. 18 In addition, there is evidence that cannabinoids have anti-inflammatory properties and decrease pain. 37 Zhu et al 36 showed that a cannabinoid receptor agonist (WIN55) attenuates pain behavior in a rat model in the postoperative period without any untoward side effects. In addition, this drug was shown to have no adverse effect on osteogenic differentiation, bone regeneration, or spinal fusion in a rat model. 35 This increases its attractiveness for a potential therapeutic option for orthopaedic surgeons in the postoperative care of patients, where bony union or fracture healing are the desired outcomes. Especially considering the opioid epidemic, a medication that could provide both nociception and anti-inflammatory effects, without a potential for interference with bone formation, such as with NSAIDs, would be of significant utility to the orthopaedic surgeon. Hickernell and colleagues 12 found that, after total hip and knee arthroplasty, patients who were prescribed drocannabinol---a synthetic form of THC---had significantly shorter lengths of stay and required fewer total morphine equivalents.
Although CBD does not have the mind-altering effects that THC is known to have, it is not innocuous. These products have the potential for adverse drug events, as well as drug-drug interactions, which is why providers should be aware that their patients are consuming these products. 4 CBD has been reported to be associated with transaminase elevations, sedation, sleep disturbances, infection, and anemia. 4,10 Moreover, CBD and its active metabolite have been shown to affect a number of the cytochromes P450 enzymes, which are known to play a role in the metabolism of many commonly used prescription drugs. 4 Lui et al 16 showed that preoperative use of cannabinoids was associated with higher pain and worse sleep in the early postoperative period in patients undergoing major orthopaedic surgery. Again, this reinforces the need to know which patients are taking these products preoperatively.
With nearly 20% of patients in the group presented here reporting CBD use, it is important to recognize the growing prevalence of use of these products in patients. While at baseline, CBD users reported similar levels of function in their affected joint compared with nonusers, they did report significantly higher levels of pain, even when using CBD. Moreover, CBD users were significantly more likely to have tried all listed nonoperative treatment modalities compared with nonusers. This may suggest that patients decided to try CBD as they had not found relief in other traditional nonoperative treatment modalities. In addition, it is important to acknowledge that the nearly 30% of CBD users reported concomitant marijuana and/or “other” recreational drug use. Interestingly, neither group responded to common nonoperative forms of management, with worse SANE scores in both groups worsening after NSAIDs, PT, and bracing, likely suggesting disease progression.
CBD users also reported worse function at baseline in their contralateral joint/extremity, with SANE scores of 81 for CBD users and 89 for non-CBD users (P < .001). This does raise a question of whether these patients had (1) a lower pain tolerance, (2) lower resilience, or (3) just worse pain and function at baseline. The question of resilience in orthopaedic patients has been previously studied extensively. 17,19,22,29 Patients with increased resilience report better outcomes and satisfaction after both anterior cruciate ligament reconstruction and arthroplasty. 17,19,22,29
Limitations
Although we believe that this study accurately captured the population in question, some limitations must be acknowledged. Although this was a prospective study, there is significant potential for recall bias. In addition, even though the study reported on >800 patients, this is a limited sample size that may be subject to regional biases or type 2 error when concluding there was no difference in effect between the 2 groups. There is also a potential for underreporting, as our sports medicine practice provides medical care/coverage for the local university. Collegiate athletes may fear that reporting use could affect their eligibility. Interestingly the average age of both groups in this study was >50 years, and, therefore, there is a potential that the captured population includes the weekend warrior population in addition to the younger athlete population. In addition, product effectiveness may be drastically variable, as there are no regulations for manufacturing or production, thus the amount of CBD or other ingredients could be highly variable. Further studies examining the direct effects of CBD in humans are warranted.
Conclusion
This study has defined the prevalence of the use of CBD products in sports medicine patients and the effect of their use on other conservative treatment modalities. Sports medicine providers should be aware of this high incidence of use and the potential interactions they may have with other treatment modalities. Further studies are needed to assess their effectiveness as a therapeutic agent and the specific interactions they have with other drugs and other forms of treatment.
Acknowledgment
The authors thank Jason Alben, RN, for his help administering and collecting surveys. The authors also thank Nina Lara, MD, for her contributions to the original survey design.
APPENDIX
Figure A1.
24-Item CBD sports questionnaire. CBD, cannabidiol; NSAID, nonsteroidal anti-inflammatory drug.
Footnotes
Final revision submitted November 16, 2021; accepted January 21, 2022.
One or more of the authors has declared the following potential conflict of interest or source of funding: D.G.D. has received education payments from Goode Surgical. J.D.H. has received education payments from Arthrex. K.A.P. has received education payments from Arthrex and ImpactOrtho. K.E. has received education payments from Arthrex, consulting fees from Arthrex, speaking fees from Desert Mountain Medical, and hospitality payments from Stryker. J.M.T. has received education payments from Goode Surgical, Peerless Surgical, and Elite Orthopedics and consulting fees from Arthrex, DePuy/Medical Device Business Services, and Zimmer Biomet. J.S.B. has received education payments from Stryker and Arthrex. A.C. has received education payments from Arthrex and Zimmer Biomet and consulting fees from Arthrex, Zimmer Biomet, and Cayenne Medical. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was waived by Mayo Clinic (ref No. 19-010363).
References
- 1. Austin DC, Torchia MT, Werth PM, Lucas AP, Moschetti WE, Jevsevar DS. A one-question patient-reported outcome measure is comparable to multiple-question measures in total knee arthroplasty patients. J Arthroplasty. 2019;34(12):2937–2943. doi:10.1016/j.arth.2019.07.023 [DOI] [PubMed] [Google Scholar]
- 2. Baron EP, Lucas P, Eades J, Hogue O. Patterns of medicinal cannabis use, strain analysis, and substitution effect among patients with migraine, headache, arthritis, and chronic pain in a medicinal cannabis cohort. J Headache Pain. 2018;19(1):37. doi:10.1186/s10194-018-0862-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brenan M. 14% of Americans Say They Use CBD Products. Gallup News Service. Published 2019. Accessed June 26, 2021. https://news.gallup.com/poll/263147/americans-say-cbd-products.aspx
- 4. Brown J, Winterstein A. Potential adverse drug events and drug-drug interactions with medical and consumer cannabidiol (CBD) use. J Clin Med. 2019;8(7):989. doi:10.3390/jcm8070989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Conaway KM. Agriculture Improvement Act of 2018, HR 2, 115th Cong (2017-2018). Accessed June 16, 2021. https://www.congress.gov/bill/115th-congress/house-bill/2
- 6. Deckey DG, Lara NJ, Gulbrandsen MT, Hassebrock JD, Spangehl MJ, Bingham JS. Prevalence of cannabinoid use in patients with hip and knee osteoarthritis. JAAOS Glob Res Rev. 2021;5(2):e20.00172. doi:10.5435/JAAOSGLOBAL-D-20-00172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ferreira-Valente MA, Pais-Ribeiro JL, Jensen MP. Validity of four pain intensity rating scales. Pain. 2011;152(10):2399–2404. doi:10.1016/j.pain.2011.07.005 [DOI] [PubMed] [Google Scholar]
- 8. Fitzcharles MA, Baerwald C, Ablin J, Häuser W. Efficacy, tolerability and safety of cannabinoids in chronic pain associated with rheumatic diseases (fibromyalgia syndrome, back pain, osteoarthritis, rheumatoid arthritis): a systematic review of randomized controlled trials. Schmerz. 2016;30(1):47–61. doi:10.1007/s00482-015-0084-3 [DOI] [PubMed] [Google Scholar]
- 9. Fitzcharles MM-A, Clauw DJ, Hauser W. A cautious hope for cannabidiol (CBD) in rheumatology care. Published online March 7, 2020. Arthritis Care Res (Hoboken). doi:10.1002/acr.24176 [DOI] [PubMed] [Google Scholar]
- 10. Gamble L-J, Boesch JM, Frye CW, et al. Pharmacokinetics, safety, and clinical efficacy of cannabidiol treatment in osteoarthritic dogs. Front Vet Sci. 2018;5:165. doi:10.3389/fvets.2018.00165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hahn S, Abernathy A. Better data for a better understanding of the use and safety profile of cannabidiol (CBD) products. US Food and Drug Administration. Published 2021. Accessed June 16, 2021. https://www.fda.gov/news-events/fda-voices/better-data-better-understanding-use-and-safety-profile-cannabidiol-cbd-products
- 12. Hickernell TR, Lakra A, Berg A, Cooper HJ, Geller JA, Shah RP. Should cannabinoids be added to multimodal pain regimens after total hip and knee arthroplasty? J Arthroplasty. 2018;33(12):3637–3641. doi:10.1016/j.arth.2018.07.027 [DOI] [PubMed] [Google Scholar]
- 13. Klein TW, Newton CA. Therapeutic potential of cannabinoid-based drugs. In: Shurin MR, Smolkin YS, eds. Advances in Experimental Medicine and Biology. Vol 601. Springer; 2007:395–413. doi:10.1007/978-0-387-72005-0_43 [DOI] [PubMed] [Google Scholar]
- 14. Kondrad EC, Reed AJ, Simpson MJ, Nease DE. Lack of communication about medical marijuana use between doctors and their patients. J Am Board Fam Med. 2018;31(5):805–808. doi:10.3122/jabfm.2018.05.170462 [DOI] [PubMed] [Google Scholar]
- 15. Korsh J, Marvil S, Guttmann D. Cannabinoids Can Serve as Alternatives to Narcotic Pain Medication for Fracture Healing. American Academy of Orthopaedic Surgeons. Accessed March 26, 2021. https://www.southpalmorthopedics.com/pdfs/edu-cannabinoids-can-serve-as-alternatives-to-narcotic-pain-medication-for-fracture-healing.pdf [Google Scholar]
- 16. Liu CW, Bhatia A, Buzon-Tan A, et al. Weeding out the problem: the impact of preoperative cannabinoid use on pain in the perioperative period. Anesth Analg. 2019;129(3):874–881. doi:10.1213/ANE.0000000000003963 [DOI] [PubMed] [Google Scholar]
- 17. Lynskey SJ, Ling F, Greenberg AM, Penny-Dimri JC, Sutherland AG. The influence of patient resilience and health status on satisfaction after total hip and knee arthroplasty. Surgeon. 2021;19(1):8–14. doi:10.1016/j.surge.2020.02.007 [DOI] [PubMed] [Google Scholar]
- 18. Madden K, van der Hoek N, Chona S, et al. Cannabinoids in the management of musculoskeletal pain: a critical review of the evidence. JBJS Rev. 2018;6(5):e7. doi:10.2106/JBJS.RVW.17.00153 [DOI] [PubMed] [Google Scholar]
- 19. Magaldi RJ, Staff I, Stovall AE, Stohler SA, Lewis CG. Impact of resilience on outcomes of total knee arthroplasty. J Arthroplasty. 2019;34(11):2620–2623.e1. doi:10.1016/j.arth.2019.06.008 [DOI] [PubMed] [Google Scholar]
- 20. McConnell M. Hemp Farming Act of 2018, HR 5485, 115th Cong (2017-2018). 2018. Accessed March 26, 2021. https://www.congress.gov/bill/115th-congress/house-bill/5485/text
- 21. Mead A. The legal status of cannabis (marijuana) and cannabidiol (CBD) under US law. Epilepsy Behav. 2017;70:288–291. doi:10.1016/j.yebeh.2016.11.021 [DOI] [PubMed] [Google Scholar]
- 22. Nwachukwu BU, Adjei J, Rauck RC, et al. How Much do psychological factors affect lack of return to play after anterior cruciate ligament reconstruction? A systematic review. Orthop J Sport Med. 2019;7(5):2325967119845313. doi:10.1177/2325967119845313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. O’Connor CM, Anoushiravani AA, Adams C, Young JR, Richardson K, Rosenbaum AJ. Cannabinoid use in musculoskeletal illness: a review of the current evidence. Curr Rev Musculoskelet Med. 2020;13(4):379–384. doi:10.1007/s12178-020-09635-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Philpott HT, O’Brien M, McDougall JJ. Attenuation of early phase inflammation by cannabidiol prevents pain and nerve damage in rat osteoarthritis. Pain. 2017;158(12):2442–2451. doi:10.1097/j.pain.0000000000001052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Premkumar A, Almeida BA, Lopez J, Pean CA, Nwachukwu BU, Sculco PK. The quality of online resources available to patients regarding cannabidiol for symptomatic relief of hip or knee arthritis is poor. JAAOS Glob Res Rev. 2021;5(1):1–7. doi:10.5435/jaaosglobal-d-20-00241 [DOI] [PubMed] [Google Scholar]
- 26. Runner RP, Luu AN, Nassif NA, et al. Use of tetrahydrocannabinol and cannabidiol products in the perioperative period around primary unilateral total hip and knee arthroplasty. J Arthroplasty. 2020;35(6):S138–S143. doi:10.1016/j.arth.2020.01.077 [DOI] [PubMed] [Google Scholar]
- 27. Russo EB. Cannabinoids in the management of difficult to treat pain. Ther Clin Risk Manag. 2008;4(1):245–259. doi:10.2147/tcrm.s1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sueyoshi T, Emoto G, Yato T. Correlation between Single Assessment Numerical Evaluation score and Lysholm score in primary total knee arthroplasty patients. Arthroplast Today. 2018;4(1):99–102. doi:10.1016/j.artd.2017.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Trinh JQ, Carender CN, An Q, Noiseux NO, Otero JE, Brown TS. Resilience and depression influence clinical outcomes following primary total joint arthroplasty. J Arthroplasty. 2021;36(5):1520–1526. doi:10.1016/j.arth.2020.11.032 [DOI] [PubMed] [Google Scholar]
- 30. Urits I, Gress K, Charipova K, et al. Use of cannabidiol (CBD) for the treatment of chronic pain. Best Pract Res Clin Anaesthesiol. 2020;34(3):463–477. doi:10.1016/J.BPA.2020.06.004 [DOI] [PubMed] [Google Scholar]
- 31. VanDolah HJ, Bauer BA, Mauck KF. Clinicians’ guide to cannabidiol and hemp oils. Mayo Clin Proc. 2019;94(9):1840–1851. doi:10.1016/j.mayocp.2019.01.003 [DOI] [PubMed] [Google Scholar]
- 32. Ware M, Beaulieu P. Cannabinoids for the treatment of pain: an update on recent clinical trials. Pain Res Manag. 2005;10(suppl A):27A–30A. doi:10.1155/2005/847562 [DOI] [PubMed] [Google Scholar]
- 33. White CM. A review of human studies assessing cannabidiol’s (CBD) therapeutic actions and potential. J Clin Pharmacol. 2019;59(7):923–934. doi:10.1002/jcph.1387 [DOI] [PubMed] [Google Scholar]
- 34. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313(24):2456–2473. doi:10.1001/jama.2015.6358 [DOI] [PubMed] [Google Scholar]
- 35. Yun C, Haleem MS, Jeong S, et al. Effect of postoperative analgesic exposure to the cannabinoid receptor agonist WIN55 on osteogenic differentiation and spinal fusion in rats. J Bone Joint Surg Am. 2021;103(11):984–991. doi:10.2106/JBJS.20.00573 [DOI] [PubMed] [Google Scholar]
- 36. Zhu CZ, Mikusa JP, Fan Y, et al. Peripheral and central sites of action for the non-selective cannabinoid agonist WIN 55,212-2 in a rat model of post-operative pain. Br J Pharmacol. 2009;157(4):645–655. doi:10.1111/j.1476-5381.2009.00184.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zuardi AW. Cannabidiol: from an inactive cannabinoid to a drug with wide spectrum of action. Rev Bras Psiquiatr. 2008;30(3):271–280. doi:10.1590/S1516-44462008000300015 [DOI] [PubMed] [Google Scholar]