Abstract
Objective: This study aimed to evaluate the association between vitamin D receptor (an essential component in the vitamin D signaling pathway) and serum vitamin D as well as its clinical significance in papillary thyroid cancer. Methods: This prospective cohort study comprised patients with thyroid tumors who visited our hospital, from 2017 to 2018. The level of vitamin D receptor expression from thyroid tissue was measured in patients with thyroid tumor and evaluated for correlation with serum vitamin D levels and clinicopathologic characteristics of papillary thyroid cancer. Data from 501 patients with papillary thyroid cancer from The Cancer Genome Atlas database were analyzed. Results: Increased vitamin D receptor protein and mRNA expression were observed in papillary thyroid cancer compared to those in normal and benign tissues. Lower vitamin D receptor protein expression was associated with high TNM stage papillary thyroid cancer and low p21 protein expression. Lower relative vitamin D receptor mRNA expression in papillary thyroid cancer was associated with low serum 25-hydroxyvitamin D level. The Cancer Genome Atlas database showed a positive correlation among mRNA expression of vitamin D receptor, CYP24A1, and p21. Conclusions: An association between decreased vitamin D receptor protein expression and advanced stage papillary thyroid cancer, and a correlation between low vitamin D receptor mRNA expression with low serum 25-hydroxyvitamin D level was observed. Low vitamin D receptor expression in papillary thyroid cancer was shown to positively correlate with low serum vitamin D level and disease aggressiveness.
Keywords: vitamin D, vitamin D receptor, papillary thyroid cancer, surgery
Introduction
Since its discovery in the early 20th century, vitamin D has evolved from being considered as a simple vitamin to a steroid pro-hormone. 1 Previous studies have demonstrated that vitamin D deficiency, which is common worldwide, could be associated with non-skeletal conditions, which include muscle weakness; cardiovascular disorders; and metabolic, autoimmune, and infectious diseases.2,3 The associations between low serum 25-hydroxyvitamin D (25(OH)D), a biomarker of vitamin D status, and several malignancies like colorectal cancer, prostate cancer, and breast cancer have been reported, particularly focusing on the potential anticancer effects of vitamin D.4–6 In thyroid cancer, correlation between serum 25(OH)D level and its clinicopathologic characteristics has been controversial.7–10
Calcitriol (1,25-dihydroxyvitamin D3, 1,25(OH)2D3), a potent activated form of vitamin D, is tightly regulated through a complex process involving the vitamin D-activating enzymes, 1-α-hydroxylase (also named CYP27B1) and 24-hydroxylase (also named CYP24A1). The enzyme 1-α-hydroxylase is responsible for the final hydroxylation step from 25(OH)D to 1,25(OH)2D3, while 24-hydroxylase is the key enzyme in the inactivation of 1,25(OH)2D3. The action of calcitriol occurs mainly through its binding to the nuclear vitamin D receptor (VDR), which acts as a hormone-regulated transcription factor.11,12
Several studies have focused on the dynamics of VDR and calcitriol-related enzymes in cancer tissues,13,14 suggesting that VDR plays a critical role in the anticancer mechanism of calcitriol. VDR expression in cancer tissue has been shown to have an anticancer effect, and its clinical significance and prognostic value in several cancers has been consistently reported.15–19 Meanwhile, there have not been many reports on the relationship among serum vitamin D, VDR, and thyroid cancer. A few studies have discussed the overall vitamin D metabolism in normal, benign, and malignant thyroid tissues.20–22
Based on the studies mentioned above, we aimed to investigate VDR expression in papillary thyroid cancer (PTC) and evaluate its clinical significance. We hypothesized that low VDR expression in PTC may be associated with low serum vitamin D levels and aggressive clinicopathologic features. In this study, we first investigated the expression of VDR, CYP27B1, CYP24A1, and markers of cell proliferation (ie, p21, a cell cycle regulator) and metastasis (ie, E-cadherin, a cell-to-cell adhesion marker) in human thyroid tissues, from normal, benign, to PTC. Secondly, we correlated the expression profiles of VDR in PTC with serum vitamin D levels and other clinicopathologic characteristics.
Materials and Methods
A prospective cohort study was conducted on the consecutive patients with thyroid tumors who had visited our hospital from April 2017 to July 2018. Patients diagnosed with PTC or benign thyroid tumor by fine-needle aspiration biopsy were included in this study. Patients were excluded if they were on medications that might alter vitamin D metabolism; had the following: a disease that could affect serum vitamin D levels, abnormal thyroid function, history of previous neck surgery or irradiation, any prior cancer history; or declined to participate in the study.
Thyroid surgery was determined based on clinical findings and performed by a single surgeon. Blood samples were obtained within one month before surgery. Serum levels of 25(OH)D, 1,25(OH)2D3 were measured simultaneously. According to recent criteria of an Endocrine Society clinical practice guideline, serum 25(OH)D levels <20 ng/mL, 20–29.9 ng/mL, and ≥30 ng/mL are defined as deficient, insufficient, and sufficient, respectively. We categorized the patients into two groups based on their serum vitamin D levels, according to this guideline. 23 A 20 ng/mL cut-off for serum 25(OH)D was used to divide the patients into vitamin D deficient or non-deficient groups,24–26 and a 40 pg/mL cut-off was utilized for serum 1,25(OH)2D3 as a mean value of the reference range (20-60 pg/mL). 27
Normal, benign, and malignant thyroid tissues were obtained from patients for immunohistochemistry (IHC) and real-time quantitative polymerase chain reaction (qPCR). Biopsy samples were collected from a central location in thyroid tumors to obtain a pure tumor sample. Malignant and benign tumors were present simultaneously in four patients, from which both tissues samples were taken. Normal thyroid tissues were taken from the contralateral lobe of the thyroid tumor. Paraffin-embedded tissues from 92 patients and snap-frozen thyroid tissues from 68 patients were collected.
Clinical characteristics and demographic data were reviewed, extracting the following patient data: age at the time of surgery; sex; histologic type of primary tumor; tumor size, multiplicity, and bilaterality; extrathyroidal extension; BRAF (serine/threonine-protein kinase B-Raf) V600E mutations; and the Tumor, Node, Metastasis (TNM) classification. A cut-off value of ≥1 cm was used for tumor size since a tumor size of <1 cm was used in the definition of papillary thyroid microcarcinoma. The TNM classification system of the American Joint Committee on Cancer (AJCC) (eighth edition) was used for the staging system. The T stage was classified into 1/2 or 3/4, the N stage into N0 versus N1, and the tumor stage into I/II or III/IV. This study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of our hospital (4-2016-0657, approved on September 7, 2016). All patients provided written informed consent. Detailed patient information is not included in the manuscript. The reporting of this study conforms to the STROBE guidelines.
IHC staining for VDR, CYP27B1, CYP24A1, p21, and E-cadherin was performed on paraffin-embedded sections from 73 PTC, 23 benign, and 25 normal thyroid tissues. Paraffin-embedded tissue specimens were cut into 4-μm-thick sections and IHC was performed as previously described, 21 using a Ventana Discovery XT Automated Slide Stainer (Ventana Medical System, Tucson, AZ, USA) with the following primary antibodies: anti-VDR antibodies (ab134826; Abcam, Cambridge, UK; 1:200), anti-CYP27B1 antibodies (sc515903; Santa Cruz Biotechnology, TX, USA; 1:200), anti-CYP24 antibodies (sc365700; Santa Cruz Biotechnology; 1:200), anti-p21 antibodies (ab109520; Abcam; 1:200), and anti-E-cadherin antibodies (ab15148; Abcam; 1:50).
IHC findings were interpreted by a single independent investigator (JSKoo), using an Olympus BX41 microscope (Olympus, Tokyo, Japan). Protein expression of nuclear VDR, cytoplasmic VDR, and nuclear p21 were quantified as the percent of stained nuclei per 100 cells. For cytoplasmic p21, CYP24A1, CYP27B1, and E-cadherin, staining intensity was scored as follows: 0 (no staining), 1 (weak), 2 (modest), or 3 (strong) (Fig. S1). In order to classify samples into high or low expression groups, we used the cut-off levels of 50% for nuclear and cytoplasmic VDR expression, 28 and 10% for nuclear p21 expression. For cytoplasmic p21, CYP27B1, CYP24A1, and E-cadherin, a score of 0–1 was categorized as negative, and a score of 2–3 was considered as positive (Figure 1).
Figure 1.
Protein expression profiles of VDR, CYP27B1, CYP24A1, p21, and E-cadherin in normal, benign, and papillary thyroid cancer (400 × )
Tissue samples obtained during thyroidectomy in 68 patients were immediately snap-frozen in liquid nitrogen and stored at −80 °C. Total RNA was extracted using the RNeasy Plus Mini Kit (Qiagen, Hilden, Germany). RNA was quantified using a Spectrophotometer NanoDrop 2000 (Nanodrop Technologies, Wilmington, DE, USA). The acceptable purity as indicated by A260/280 was greater than 1.8, and RNA samples that showed smear or degradation were excluded. The fresh 38 PTC-normal pairs and 10 benign-normal pairs were subjected to qPCR analyses. 29
RNA was reversely transcribed to cDNA using an AccuPower RT Premix Kit (Bioneer Inc., Daejeon, South Korea) for experiments. qPCR was performed with an EvaGreen Q Master Mix (LaboPass, Seoul, Korea). Primer sequences for the target genes VDR, CYP27B1, CYP24A1, p21, and E-cadherin are listed in Table S1. Gene expression was measured by qRT-PCR using a StepOnePlus™ real-time PCR machine (Applied Biosystems, CA, USA). β-Actin was used as an internal control.
In the present study, data is presented as the fold change in target gene expression of the tumors relative to its expression in the counterpart normal tissue. A cut-off level of two (a median value) for the N-fold differential expression of VDR was used to categorize the PTC patients into two groups to evaluate for correlation with clinicopathologic features.
Publicly available mRNA sequencing (RNA-Seq) data of 501 patients with thyroid cancer from The Cancer Genome Atlas (TCGA) database (version 2016_01_28; https://gdac.broadinstitute.org) were analyzed.30,31 RNA-Seq data of VDR, CYP27B1, CYP24A1, p21, and E-cadherin expressions of PTC were retrieved for further evaluation.
The baseline data is presented as the number and percentage for categorical variables and as the mean ± standard deviation for continuous variables, unless otherwise specified. Continuous variables were compared using Student’s t-test for two group comparisons. Categorical variables were compared using the chi-squared (χ2) test or Fisher's exact test. The Pearson correlation coefficient was determined in TCGA RNA-Seq data. P-values < 0.05 were considered statistically significant. All data were processed and statistically analyzed using IBM SPSS Statistics for Windows, version 25.0 (IBM Corp., Armonk, NY, USA).
Results
Immunohistochemistry Staining
Vitamin D metabolism, cell proliferation, and metastasis were evaluated in 73 PTC, 23 benign, and 25 normal samples by comparing protein expressions of VDR, CYP27B1, CYP24A1, p21, and E-cadherin. A total of 25 pairs of cancer-normal tissues from the same patient were first compared (Table 1). Higher nuclear VDR expression was found in 68.0% of PTC, compared to 20.0% of normal thyroid tissues (P = 0.001). Positive CYP27B1 expression was significantly higher in PTC compared to that in normal thyroid tissues (92.0% vs 52.0%; P = 0.004). Nuclear p21 expression was also significantly increased in the PTC group compared to the normal thyroid group (28.0% vs 4.0%; P = 0.049).
Table 1.
Protein expression profiles of 25 paired papillary thyroid cancer and normal tissues.
| Expression | Normal (n = 25) |
Cancer (n = 25) |
P value |
|---|---|---|---|
| VDR - Nuclear | |||
| 0–50 | 20 (80.0) | 8 (32.0) | 0.001 |
| 51–100 | 5 (20.0) | 20 (68.0) | |
| VDR - Cytoplasmic | |||
| 0–50 | 25 (100.0) | 25 (100.0) | NA |
| 51–100 | 0 (0.0) | 0 (0.0) | |
| CYP27B1 | |||
| Negative | 12 (48.0) | 2 (8.0) | 0.004 |
| Positive | 13 (52.0) | 23 (92.0) | |
| CYP24A1 | |||
| Negative | 22 (88.0) | 18 (72.0) | 0.289 |
| Positive | 3 (12.0) | 7 (28.0) | |
| P21 - nuclear | |||
| 0–10 | 24 (96.0) | 18 (72.0) | 0.049 |
| 11–100 | 1 (4.0) | 7 (28.0) | |
| P21 - cytoplasmic | |||
| Negative | 22 (88.0) | 18 (72.0) | 0.289 |
| Positive | 3 (12.0) | 7 (28.0) | |
| E-cadherin | |||
| Negative | 21 (84.0) | 16 (64.0) | 0.196 |
| Positive | 4 (16.0) | 9 (36.0) |
Values are expressed as number (%).
VDR, vitamin D receptor.
Analyses between 73 PTC and 25 normal tissues revealed a higher protein expression in nuclear VDR (57.5% vs 20.0%; P = 0.001) and cytoplasmic VDR (23.3% vs 0%; P = 0.005) in the PTC group (Table 2). There was a significant increase in nuclear p21 (41.0% vs 4.0%; P < 0.001) and E-cadherin expression (52.1% vs 16.0%; P = 0.002) in the PTC group compared to the normal tissue group.
Table 2.
Protein expression profiles of the thyroid tissue samples, stratified by histology.
| Expression | Normal (n = 25) |
Benign (n = 23) |
Cancer (n = 73) |
|---|---|---|---|
| VDR - Nuclear | |||
| 0–50 | 20 (80.0) | 17 (73.9) | 31 (42.5) **/# |
| 51–100 | 5 (20.0) | 6 (26.1) | 42 (57.5) |
| VDR - Cytoplasmic | |||
| 0–50 | 25 (100.0) | 23 (100.0) | 56 (76.7) **/# |
| 51–100 | 0 (0.0) | 0 (0.0) | 17 (23.3) |
| CYP27B1 | |||
| Negative | 12 (48.0) | 11 (47.8) | 24 (32.9) |
| Positive | 13 (52.0) | 12 (52.2) | 49 (67.1) |
| CYP24A1 | |||
| Negative | 22 (88.0) | 21 (91.3) | 62 (84.9) |
| Positive | 3 (12.0) | 2 (8.7) | 11 (15.1) |
| P21 - nuclear | |||
| 0–10 | 24 (96.0) | 22 (95.7) | 43 (58.9) **/## |
| 11–100 | 1 (4.0) | 1 (4.3) | 30 (41.1) |
| P21 - cytoplasmic | |||
| Negative | 22 (88.0) | 22 (95.7) | 64 (87.7) |
| Positive | 3 (12.0) | 1 (4.3) | 9 (12.3) |
| E-cadherin | |||
| Negative | 21 (84.0) | 18 (78.3) | 35 (47.9) **/# |
| Positive | 4 (16.0) | 5 (21.7) | 38 (52.1) |
Values are expressed as number (%).
**p < 0.01 versus normal, #p < 0.05 and ##p < 0.01 versus benign.
VDR, vitamin D receptor.
We then compared 73 PTC and 23 benign thyroid tissue groups. The PTC group demonstrated higher protein expression in nuclear VDR (57.5% vs 33.3%; P = 0.009), cytoplasmic VDR (23.3% vs 0%; P = 0.010), nuclear p21 (41.0% vs 0%; P = 0.001), and E-cadherin (52.1% vs 16.7%; P = 0.011). Comparative analyses between 23 benign and 25 normal samples yielded no significant difference in the expression of target proteins.
The association of VDR protein expression with the clinicopathologic characteristics of 73 PTC patients was investigated (Table 3). High nuclear VDR expression was significantly associated with high nuclear p21 expression (P = 0.023). A significant decrease in nuclear VDR expression was observed in PTC TNM stages 3 and 4 (P = 0.017). There was no significant association between nuclear VDR and other clinicopathologic features, including serum 25(OH)D and 1,25(OH)2D3 levels. Serum vitamin D levels of both 25(OH)D and 1,25(OH)2D3 showed no significant correlation with other clinicopathologic features (Tables S2, S3).
Table 3.
Clinicopathological characteristics of patients with papillary thyroid cancer according to vitamin D receptor expression level.
| Nuclear VDR Expression | P-value | ||
|---|---|---|---|
| 0–50% (N = 31), n (%) | 51–100% (N = 42), n (%) | ||
| Age (yr), mean ± SD | 46.74 ± 14.21 | 43.98 ± 13.22 | 0.395* |
| Age (yr) | |||
| <55 | 20 (64.5) | 34 (81.0) | 0.114† |
| ≥55 | 11 (35.5) | 8 (19.0) | |
| Sex | |||
| Male | 9 (29.0) | 13 (31.0) | 0.860† |
| Female | 22 (71.0) | 29 (69.0) | |
| 25(OH)D (ng/mL) | 19.41 ± 8.40 | 18.00 ± 7.30 | 0.446* |
| 25(OH)D (ng/mL) | |||
| <20 | 22 (71.0) | 30 (71.4) | 0.966† |
| ≥20 | 9 (39.0) | 12 (28.6) | |
| 1,25(OH)2D3 (pg/mL) | 40.39 ± 18.66 | 48.56 ± 22.05 | 0.100* |
| 1,25(OH)2D3 (pg/mL) | |||
| <40 | 16 (51.6) | 15 (35.7) | 0.174† |
| ≥40 | 15 (48.4) | 27 (64.3) | |
| Tumor size (cm) | 1.29 ± 0.98 | 1.25 ± 0.82 | 0.866* |
| Tumor size | |||
| ≤1cm | 16 (51.6) | 22 (52.4) | 0.948† |
| >1cm | 15 (48.4) | 20 (47.6) | |
| Multifocality | |||
| Negative | 23 (74.2) | 30 (71.4) | 0.793† |
| Positive | 8 (25.8) | 12 (28.6) | |
| Bilaterality | |||
| Negative | 27 (87.1) | 36 (85.7) | 1.000† |
| Positive | 4 (12.9) | 6 (14.3) | |
| Extrathyroidal extension | |||
| Negative | 10 (32.3) | 16 (38.1) | 0.607† |
| Positive | 21 (67.7) | 26 (61.9) | |
| T-stage | |||
| T1-T2 | 11 (35.5) | 18 (42.9) | 0.525† |
| T3-T4 | 20 (64.5) | 24 (57.1) | |
| Regional lymph node | |||
| N0 | 17 (54.8) | 25 (59.5) | 0.689† |
| N1 | 14 (45.2) | 17 (40.5) | |
| Distant metastasis | |||
| M0 | 30 (96.8) | 39 (92.9) | 0.632† |
| M1 | 1 (3.2) | 3 (7.1) | |
| TNM stage group | |||
| I-II | 18 (58.1) | 35 (83.3) | 0.017† |
| III-IV | 13 (41.9) | 7 (16.7) | |
| BRAF mutation | |||
| Absent | 6 (19.4) | 7 (16.7) | 0.767† |
| Present | 25 (80.6) | 35 (83.3) | |
| VDR - Cytoplasmic | |||
| Negative to positive 0–50% | 21 (67.7) | 35 (83.3) | 0.119† |
| Strong Positive 51–100% | 10 (32.3) | 7 (16.7) | |
| CYP27B1 | |||
| Negative 0–1 | 10 (32.3) | 14 (33.3) | 0.923† |
| Positive 2–3 | 21 (67.7) | 28 (66.7) | |
| CYP24A1 | |||
| Negative 0–1 | 27 (87.1) | 35 (83.3) | 0.657† |
| Positive 2–3 | 4 (12.9) | 7 (16.7) | |
| P21 - Nuclear | |||
| Negative 0–10% | 23 (74.2) | 20 (47.6) | 0.023† |
| Positive 11–100% | 8 (25.8) | 22 (52.4) | |
| P21 - Cytoplasmic | |||
| Negative 0–1 | 30 (96.8) | 34 (81.0) | 0.069† |
| Positive 2–3 | 1 (3.2) | 8 (19.0) | |
| E-cadherin | |||
| Negative 0–1 | 17 (54.8) | 18 (42.9) | 0.311† |
| Positive 2–3 | 14 (45.2) | 24 (57.1) | |
*P-values were calculated using Student's t-test. Data are expressed as mean ± SD.
P-values were calculated using χ2 test. Data are expressed as number (%).
25(OH)D, 25-hydroxyvitamin D; 1,25(OH)2D3, 1,25-dihydroxyvitamin D3 (calcitriol).
We further investigated the correlation between the protein expression of nuclear VDR and other markers in PTC tissues and found no significant association (Table 3).
Real-Time Quantitative PCR (qPCR)
Comparative analysis was conducted for the relative tumor-normal mRNA expression values in 38 PTC and 10 benign tissues, along with their normal counterparts. The VDR gene showed a higher relative expression in PTC compared to that in benign tumors (20.24 ± 7.63 vs 3.41 ± 1.58; P = 0.037). The other genes yielded no evident difference in relative mRNA expression between PTC and benign tumors (Figure 2).
Figure 2.
Relative tumor-normal mRNA expression levels of the target genes (A) VDR, (B) CYP27B1, (C) CYP24A1, (D) p21, and (E) E-cadherin in papillary thyroid cancer and benign tumor
Data are expressed as the mean ± standard error of the mean; *p < 0.05; VDR, vitamin D receptor.
We examined the correlation of relative cancer-normal mRNA expression of VDR, CYP27B1, CYP24A1, p21, and E-cadherin with VDR protein expression in PTC. Increased mRNA expression of VDR in cancer was observed in patients with higher (> 50) nuclear VDR protein expression (31.21 ± 12.16 vs 3.43 ± 1.04; P = 0.033). The other genes showed no association with nuclear VDR protein expression (Figure 3).
Figure 3.
Correlation between the mRNA expression profile of the target genes (A) VDR, (B) CYP27B1, (C) CYP24A1, (D) p21, and (E) E-cadherin with VDR protein expression
Data are expressed as the mean ± standard error of the mean; *p < 0.05; VDR, vitamin D receptor.
We further investigated the relationship between the relative cancer-normal mRNA expression level of VDR genes and the clinicopathologic characteristics of 38 PTC patients. The group with ≤ 2-fold cancer-normal VDR mRNA expression ratio had a lower mean serum 25(OH)D level (22.42 ± 9.56 vs 16.01 ± 5.56; P = 0.017). However, serum 1,25(OH)2D3 levels were similar, regardless of the relative VDR mRNA expression levels (48.81 ± 25.67 vs 47.50 ± 19.43, P = 0.860) (Table 4).
Table 4.
Clinicopathological characteristics of patients with papillary thyroid cancer according to vitamin D receptor mRNA relative expression level.
| VDR mRNA relative expression level | P-value | ||
|---|---|---|---|
| T/N < 2 (N = 19), n (%) | T/N ≥ 2 (N = 19), n (%) | ||
| Age (yr), mean ± SD | 48.81 ± 12.66 | 42.53 ± 12.61 | 0.731* |
| Age | |||
| <55 | 16 (84.2) | 15 (78.9) | 1.000† |
| ≥55 | 3 (15.8) | 4 (21.1) | |
| Sex | |||
| Male | 4 (21.1) | 6 (31.6) | 0.714† |
| Female | 15 (78.9) | 13 (68.4) | |
| 25(OH)D (ng/mL) | 16.01 ± 5.56 | 22.42 ± 9.56 | 0.017* |
| 25(OH)D (ng/mL) | |||
| <20 | 16 (84.2) | 9 (47.4) | 0.038† |
| ≥20 | 3 (15.8) | 10 (52.6) | |
| 1,25(OH)2D3 (pg/mL) | 40.39 ± 18.66 | 48.56 ± 22.05 | 0.100* |
| 1,25(OH)2D3 (pg/mL) | |||
| <40 | 8 (42.1) | 7 (36.8) | 0.740† |
| ≥40 | 11 (57.9) | 12 (63.2) | |
| Tumor size (cm) | 1.06 ± 0.54 | 1.57 ± 1.28 | 0.117* |
| Tumor size | |||
| ≤1cm | 10 (52.6) | 9 (47.4) | 0.746† |
| >1cm | 9 (47.4) | 10 (52.6) | |
| Multifocality | |||
| Negative | 12 (63.2) | 13 (68.4) | 0.732† |
| Positive | 7 (36.8) | 3 (31.6) | |
| Bilaterality | |||
| Negative | 13 (68.4) | 17 (89.5) | 0.232† |
| Positive | 6 (31.6) | 2 (10.5) | |
| Extrathyroidal extension | |||
| Negative | 7 (36.8) | 7 (36.8) | 1.000† |
| Positive | 12 (63.2) | 12 (63.2) | |
| T-stage | |||
| T1-T2 | 7 (36.8) | 8 (42.1) | 0.740† |
| T3-T4 | 12 (63.2) | 11 (57.9) | |
| Regional lymph node | |||
| N0 | 13 (68.4) | 12 (63.2) | 0.732† |
| N1 | 6 (31.6) | 7 (36.8) | |
| Distant metastasis | |||
| M0 | 18 (94.7) | 18 (94.7) | 1.000† |
| M1 | 1 (5.3) | 1 (5.3) | |
| TNM stage group | |||
| I-II | 13 (68.4) | 13 (68.4) | 1.000† |
| III-IV | 6 (31.6) | 6 (31.6) | |
| BRAF mutation | |||
| Absent | 5 (26.3) | 3 (15.8) | 0.693† |
| Present | 14 (73.7) | 16 (84.2) | |
| VDR - Nuclear | |||
| Negative to positive 0–50% | 7 (36.8) | 8 (42.1) | 0.740† |
| Strong Positive 51–100% | 12 (63.2) | 11 (57.9) | |
| VDR - Cytoplasmic | |||
| Negative to positive 0–50% | 15 (78.9) | 14 (73.7) | 1.000† |
| Strong Positive 51–100% | 4 (21.1) | 5 (26.3) | |
| CYP27B1 | |||
| Negative 0–1 | 5 (26.3) | 5 (26.3) | 1.000† |
| Positive 2–3 | 14 (73.7) | 14 (73.7) | |
| CYP24A1 | |||
| Negative 0–1 | 18 (94.7) | 15 (78.9) | 0.340† |
| Positive 2–3 | 1 (5.3) | 4 (21.1) | |
| P21 - Nuclear | |||
| Negative 0–10% | 9 (47.4) | 10 (52.6) | 0.746† |
| Positive 11–100% | 10 (52.6) | 9 (47.4) | |
| P21 - Cytoplasmic | |||
| Negative 0–1 | 17 (89.5) | 16 (84.2) | 1.000† |
| Positive 2–3 | 2 (10.5) | 3 (15.8) | |
| E-cadherin | |||
| Negative 0–1 | 12 (63.2) | 7 (36.8) | 0.105† |
| Positive 2–3 | 7 (36.8) | 12 (63.2) | |
* P-values were calculated using Student's t-test. Data are expressed as mean ± SD.
P-values were calculated using χ2 test. Data are expressed as number (%).
25(OH)D, 25-hydroxyvitamin D; 1,25(OH)2D3, 1,25-dihydroxyvitamin D3 (calcitriol).
TCGA Thyroid Cancer Data According to the VDR mRNA Expression status
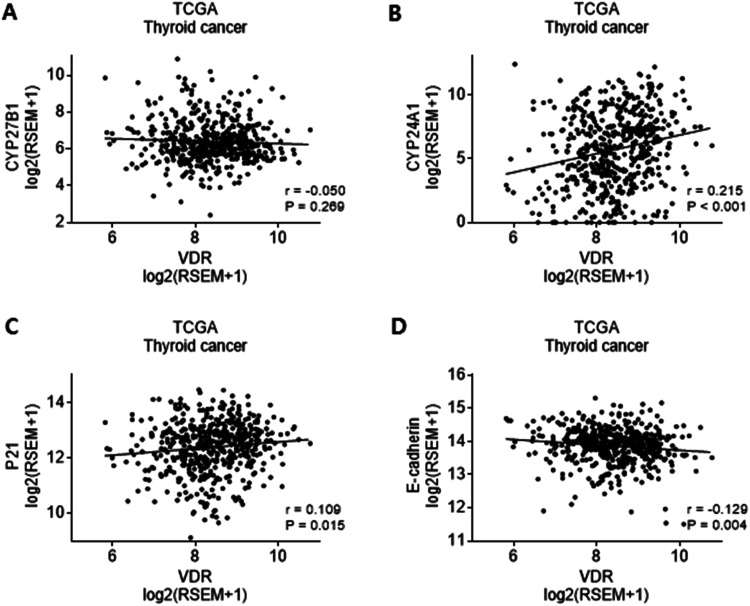
Owing to restricted mRNA expression profiles in our analyses, we obtained RNA-Seq data of 501 PTC patients from the TCGA database. We investigated the relationship of VDR mRNA expression profiles with CYP27B1, CYP24A1, p21, and E-cadherin. VDR mRNA expression demonstrated a positive association with p21 (r = 0.109, P = 0.015) and CYP24A1 (r = 0.215, P < 0.001), and a negative association with E-cadherin (r = −0.129, P = 0.004). VDR mRNA expression showed no significant relationship with CYP27B1 (r = −0.05, P = 0.269) (Figure 4).
Figure 4.
mRNA expression of (A) CYP27B1, (B) CYP24A1, (C) p21, and (D) E-cadherin in the RNA-Seq data of 501 patients with thyroid cancer from The Cancer Genome Atlas database
*p < 0.05; VDR, vitamin D receptor.
Discussion
In this study, we identified relevant components of vitamin D metabolism and their effect in thyroid cancer. The presence of VDR in normal thyroid tissue has been previously described. 32 PTC samples showed enhanced protein and mRNA expressions of VDR and vitamin D related enzymes when compared to normal and benign human thyroid tissue, suggesting a potential antitumor response.20,21 Our study evaluated the protein and mRNA expression profiles of VDR, CYP24A1, and CYP27B1 in normal, benign, and PTC tissues and assessed their anti-proliferation, anti-adhesion, and anti-invasion characteristics in cancer cells.
During IHC analysis, we evaluated VDR protein expression in different cellular compartments (ie, nucleus and the cytoplasm). Only a few studies have evaluated the clinical significance of either nuclear or cytoplasmic VDR expression in cancer.33–35 One study reported that IHC staining was generally cytoplasmic in thyroid cancer and was more intense near the tumor capsule. 20 Another study revealed that nuclear VDR expression in PTC samples negatively correlated with STAT3 hyperphosphorylation, which indicates worse clinicopathologic characteristics. 35
In our study, nuclear VDR expression was higher than cytoplasmic VDR expression in most PTCs, compared to those in normal and benign samples. Cytoplasmic VDR expression was significantly enhanced in PTC than in normal and benign tissues, and it was detected only in few patients. The behavior of VDR seems consistent throughout the vitamin D pathway and may contribute to the anticancer activity of calcitriol.12,36 Furthermore, nuclear VDR expression was higher than cytoplasmic VDR, implying that VDR activity occurs mainly in the nucleus. Nuclear VDR expression was significantly decreased in TNM stage 3 and 4 PTC, indicating that calcitriol-VDR complex had a reduced anticancer effect.
The comparison of 73 PTC with 25 normal samples revealed elevated CYP27B1 and CYP24A1 expression in PTC compared to those in normal tissue, although this was not statistically significant. Previous studies have demonstrated elevated CYP27B1 and VDR expression can lead to a magnified vitamin D action in thyroid cancer.20,21 This study's result of CYP27B1 elevation in PTC compared to that in paired normal tissue is similar to previous studies.
VDR expression was increased in PTC than in normal and benign tissues, both in protein and mRNA levels. The relative mRNA expression level of VDR in PTC was higher than the protein expression level, which might have resulted from the compartmentalization of VDR protein expression in the nucleus and cytoplasm. This could lead to a greater increase in overall VDR mRNA expression. In addition, higher VDR protein expression in PTC was evident in patients with high cancer-normal mRNA expression ratio. This supports the augmented expression of VDR in PTC throughout the experiments. These findings are consistent with findings of previous studies that described the potential anticancer effect of VDR in thyroid cancer.20,21
Calcitriol is identified to regulate specific signaling pathways through which it plays roles in anti-proliferation, pro-apoptosis, de-differentiation, anti-inflammation, anti-angiogenesis, and anti-invasion and anti-metastasis of cancer.12,37,38 Recently, Pang et al suggested that VDR knockdown attenuates the anti-proliferative, pro-apoptotic, and anti-invasive effect of vitamin D in PTC by activating the Wnt/β-catenin signaling pathway. 39 Zhang et al showed that calcitriol enhances doxorubicin-induced apoptosis in PTC cells by regulating VDR/PTPN2/p-STAT3 pathway. 35 We hypothesized that increased VDR expression in PTC tissue is caused by similar mechanisms, which may also be impaired in advanced stage PTC.
Previous literature demonstrated that calcitriol inhibits cell proliferation through cell cycle arrest by activating p21 and p27, particularly in the G0/G1 phase.40,41 p21 has been recognized for its pro-apoptotic activity in the nucleus and anti-apoptotic activity in the cytoplasm.42,43 We evaluated p21 separately according to nuclear and cytoplasmic expression. The human p21 gene contains VDR binding promoter regions and is a transcriptional target of calcitriol-VDR complex.37,44 Liu et al demonstrated that calcitriol-induced p27 activation in thyroid cancer cells was accomplished by VDR-mediated regulation of p27 phosphorylation and degradation. 41 In our study, p21 mRNA expression did not demonstrate notable findings, but the positive correlation of nuclear p21 and nuclear VDR protein expression may develop into potential anti-proliferative effect in thyroid cancer.
Several studies have reported that E-cadherin is involved in the invasion and metastasis of thyroid cancer, but the results were inconsistent. 45 Even though the role of E-cadherin in thyroid cancer remains unclear, its elevated protein expression in PTC suggests its anticancer potential according to our study. Previous studies have shown that the VDR activation by calcitriol induces E-cadherin expression by promoting the translocation of β-catenin from the nucleus to the plasma membrane and inhibiting the Wnt/β-catenin/TCF4 signaling pathway. This also supports its anti-invasion and anti-metastasis roles in thyroid cancer.45,46
Analyses of 501 PTC samples from the TCGA database revealed significant positive correlation between VDR mRNA expression, and CYP24A1 and p21. A previous study reported decreased VDR and CYP24A1 mRNA expression in the PTC N1 stage, accompanied by a decreased p21 expression. 21 Although the TCGA database was based on mRNA expression level, we observed a similar correlation in the protein level analyses of our study, with decreased nuclear VDR and nuclear p21 protein expression and advanced PTC TNM stage. Although the N stage was not significantly correlated, the findings still explain the loss of anti-proliferative, dedifferentiating functions in aggressive thyroid cancer.
Repressed VDR action may be explained by impaired vitamin D and VDR signaling pathways. Studies have investigated the counteraction of cancer cells via multiple mechanisms to restrain VDR expression, such as in colon and breast cancer. These include the snail family transcriptional repressor (SNAIL) which inhibits transcription, and RAS oncogene mutation which suppresses its transcription.47,48 The tumor-suppressor gene, p53, is known to enhance VDR transcription. However, p53 mutant cells can regulate VDR responses by directly binding to VDR and redirecting its transcriptional program to apoptosis.49,50 Others include epigenetic gene silencing, CpG island methylation, and microRNA (miRNA). 51 In thyroid cancer, the mechanism for decreased VDR expression in advanced stages is not clearly defined; hence, further investigation is needed.
In this study, the low VDR mRNA expression in PTC compared to that in normal tissues was associated with a low serum 25(OH)D level. VDR protein expression was not associated with serum vitamin D levels and no clinicopathologic significance in PTC was found with serum vitamin D levels. We assume that this disparity primarily comes from the compartmentalized expression of VDR protein and relative mRNA expression level. Several factors such as unstable environmental factors, unstable co-binding proteins, and patient factor can affect both protein and gene expressions. 14
Nevertheless, the positive correlation between relative VDR mRNA expression and serum 25(OH)D level in PTC implies similar anticancer effect in thyroid cancer. There have been varying results in the correlation between serum vitamin D level and the aggressiveness and prognosis of thyroid cancer.7–10,52 There are only a few reports on the relationship of serum vitamin D levels and VDR in different cancers.53,54 Since serum 25(OH)D is the best biomarker for vitamin D status, the positive correlation between VDR mRNA expression and serum 25(OH)D level can be a convincing indication of our hypothesis.
This study has several limitations. First, a majority of the patients (71.2%, 51/72) were deficient in serum 25(OH)D. Secondly, for the analysis, serum vitamin D levels were not strictly adjusted for BMI and seasonal variation. Thirdly, in the mRNA expression analyses, we excluded a substantial number of tissues during the qualification control procedure for accurate results, resulting in a smaller number of specimens. A small sample size of 10 benign tumors would especially have led to results with selection bias, since surgery is recommended in large symptomatic benign tumors. Fourthly, assessing the association between serum vitamin D, VDR expression and patient prognosis was not feasible in this study.
Conclusion
In this study, we demonstrated elevated protein and mRNA expression of VDR in PTC compared to normal and benign tissues. However, lower protein expression of nuclear VDR was identified in high TNM stage PTC, which was associated with low nuclear p21 protein expression. This study provides further evidence for the potential anti-proliferative effects of VDR in PTC, which is diminished in aggressive thyroid cancer. Moreover, lower VDR mRNA expression in PTC was associated with low serum 25(OH)D levels. Overall, this is the first report to identify the possibility of a positive correlation between low VDR expression, low serum 25(OH)D level, and aggressiveness of thyroid cancer.
We believe that this study will contribute to a better understanding of vitamin D metabolism and the clinical significance of VDR expression in PTC. Furthermore, large prospective studies are needed to validate the potential anticancer effect of VDR in thyroid cancer and establish the use of VDR expression and serum vitamin D levels as a prognostic indicator. The therapeutic potential of vitamin D in thyroid cancer should be further investigated in future research.
Supplemental Material
Supplemental material, sj-tif-1-tct-10.1177_15330338221089933 for Vitamin D Receptor Expression and its Clinical Significance in Papillary Thyroid Cancer by Min Jhi Kim, Daham Kim, Ja Seung Koo, Ju Hee Lee and Kee-Hyun Nam in Technology in Cancer Research & Treatment
Supplemental material, sj-docx-2-tct-10.1177_15330338221089933 for Vitamin D Receptor Expression and its Clinical Significance in Papillary Thyroid Cancer by Min Jhi Kim, Daham Kim, Ja Seung Koo, Ju Hee Lee and Kee-Hyun Nam in Technology in Cancer Research & Treatment
Supplemental material, sj-docx-3-tct-10.1177_15330338221089933 for Vitamin D Receptor Expression and its Clinical Significance in Papillary Thyroid Cancer by Min Jhi Kim, Daham Kim, Ja Seung Koo, Ju Hee Lee and Kee-Hyun Nam in Technology in Cancer Research & Treatment
Supplemental material, sj-docx-4-tct-10.1177_15330338221089933 for Vitamin D Receptor Expression and its Clinical Significance in Papillary Thyroid Cancer by Min Jhi Kim, Daham Kim, Ja Seung Koo, Ju Hee Lee and Kee-Hyun Nam in Technology in Cancer Research & Treatment
Supplemental material, sj-docx-5-tct-10.1177_15330338221089933 for Vitamin D Receptor Expression and its Clinical Significance in Papillary Thyroid Cancer by Min Jhi Kim, Daham Kim, Ja Seung Koo, Ju Hee Lee and Kee-Hyun Nam in Technology in Cancer Research & Treatment
Abbreviations
- 25(OH)D
25-hydroxyvitamin D
- AJCC
American Joint Committee on Cancer
- BRAF
serine/threonine-protein kinase B-Raf
- Calcitriol
1,25-dihydroxyvitamin D3,1,25(OH)2D3
- IHC
immunohistochemistry
- miRNA
microRNA
- RNA-Seq
mRNA sequencing
- PTC
papillary thyroid cancer
- qPCR
quantitative polymerase chain reaction
- SNAIL
snail family transcriptional repressor
- TCGA
The Cancer Genome Atlas
- TNM
Tumor, Node, Metastasis
- VDR
vitamin D receptor.
Footnotes
Ethics Statement: This study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of the Severance Hospital (4-2016-0657). All patients provided written informed consent.
Data Availability Statement: Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
Supplemental material: Supplemental material for this article is available online.
ORCID iD: Min Jhi Kim MD, PhD https://orcid.org/0000-0002-7791-2994
References
- 1.DeLuca HF. Evolution of our understanding of vitamin D. Nutr Rev. Oct 2008;66(10 Suppl 2):S73-S87. doi: 10.1111/j.1753-4887.2008.00105.x [DOI] [PubMed] [Google Scholar]
- 2.Makariou S, Liberopoulos EN, Elisaf M, Challa A. Novel roles of vitamin D in disease: what is new in 2011? Eur J Intern Med. Aug 2011;22(4):355-362. doi: 10.1016/j.ejim.2011.04.012 [DOI] [PubMed] [Google Scholar]
- 3.Bikle D. Nonclassic actions of vitamin D. J Clin Endocrinol Metab. Jan 2009;94(1):26-34. doi: 10.1210/jc.2008-1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma Y, Zhang P, Wang F, Yang J, Liu Z, Qin H. Association between vitamin D and risk of colorectal cancer: a systematic review of prospective studies. J Clin Oncol. Oct 1 2011;29(28):3775-3782. doi: 10.1200/jco.2011.35.7566 [DOI] [PubMed] [Google Scholar]
- 5.Gandini S, Boniol M, Haukka J, et al. Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int J Cancer. Mar 15 2011;128(6):1414-1424. doi: 10.1002/ijc.25439 [DOI] [PubMed] [Google Scholar]
- 6.Bauer SR, Hankinson SE, Bertone-Johnson ER, Ding EL. Plasma vitamin D levels, menopause, and risk of breast cancer: dose-response meta-analysis of prospective studies. Medicine (Baltimore). May 2013;92(3):123-131. doi: 10.1097/MD.0b013e3182943bc2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim JR, Kim BH, Kim SM, et al. Low serum 25 hydroxyvitamin D is associated with poor clinicopathologic characteristics in female patients with papillary thyroid cancer. Thyroid. Nov 2014;24(11):1618-1624. doi: 10.1089/thy.2014.0090 [DOI] [PubMed] [Google Scholar]
- 8.Ahn HY, Chung YJ, Park KY, Cho BY. Serum 25-hydroxyvitamin D level does not affect the aggressiveness and prognosis of papillary thyroid cancer. Thyroid. Mar 2016;26(3):429-433. doi: 10.1089/thy.2015.0516 [DOI] [PubMed] [Google Scholar]
- 9.Zhao J, Wang H, Zhang Z, et al. Vitamin D deficiency as a risk factor for thyroid cancer: a meta-analysis of case-control studies. Nutrition. Jan 2019;57:5-11. doi: 10.1016/j.nut.2018.04.015 [DOI] [PubMed] [Google Scholar]
- 10.Kim D. The role of vitamin D in thyroid diseases. Int J Mol Sci. Sep 12 2017;18(9):1-19. 10.3390/ijms18091949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown AJ. Vitamin D analogues. Am J Kidney Dis. Oct 1998;32(2 Suppl 2):S25-S39. doi: 10.1053/ajkd.1998.v32.pm9808141 [DOI] [PubMed] [Google Scholar]
- 12.Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer. May 2014;14(5):342-357. doi: 10.1038/nrc3691 [DOI] [PubMed] [Google Scholar]
- 13.McCarthy K, Laban C, Bustin SA, et al. Expression of 25-hydroxyvitamin D-1-α-hydroxylase, and vitamin D receptor mRNA in normal and malignant breast tissue. Anticancer Res. 2009;29(1):155-157. [PubMed] [Google Scholar]
- 14.Horváth HC, Lakatos P, Kósa JP, et al. The candidate oncogene CYP24A1: a potential biomarker for colorectal tumorigenesis. J Histochem Cytochem. Mar 2010;58(3):277-285. doi: 10.1369/jhc.2009.954339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hendrickson WK, Flavin R, Kasperzyk JL, et al. Vitamin D receptor protein expression in tumor tissue and prostate cancer progression. J Clin Oncol. Jun. 10 2011;29(17):2378-2385. doi: 10.1200/jco.2010.30.9880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Azhri J, Zhang Y, Bshara W, et al. Tumor expression of vitamin D receptor and breast cancer histopathological characteristics and prognosis. Clin Cancer Res. Jan. 1 2017;23(1):97-103. doi: 10.1158/1078-0432.Ccr-16-0075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Ghafari AB, Balamash KS, Al Doghaither HA. Relationship between Serum vitamin D and calcium levels and vitamin D receptor gene polymorphisms in colorectal cancer. Biomed Res Int. 2019;2019:8571541. doi: 10.1155/2019/8571541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrer-Mayorga G, Gómez-López G, Barbáchano A, et al. Vitamin D receptor expression and associated gene signature in tumour stromal fibroblasts predict clinical outcome in colorectal cancer. Gut. 2017;66(8):1449. doi: 10.1136/gutjnl-2015-310977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu H, Liu Z, Shi H, Wang C. Prognostic role of vitamin D receptor in breast cancer: a systematic review and meta-analysis. BMC Cancer. 2020/11/01;20(1):1051. doi: 10.1186/s12885-020-07559-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khadzkou K, Buchwald P, Westin G, Dralle H, Akerström G, Hellman P. 25-hydroxyvitamin D3 1alpha-hydroxylase and vitamin D receptor expression in papillary thyroid carcinoma. J Histochem Cytochem. Mar 2006;54(3):355-361. doi: 10.1369/jhc.5A6734.2005 [DOI] [PubMed] [Google Scholar]
- 21.Clinckspoor I, Hauben E, Verlinden L, et al. Altered expression of key players in vitamin D metabolism and signaling in malignant and benign thyroid tumors. J Histochem Cytochem. Jul 2012;60(7):502-511. doi: 10.1369/0022155412447296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morand GB, da Silva SD, Hier MP, Alaoui-Jamali MA. Insights into genetic and epigenetic determinants with impact on vitamin d signaling and cancer association studies: the case of thyroid cancer. Front Oncol. 2014;4(309):1-9. doi: 10.3389/fonc.2014.00309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. Jul 2011;96(7):1911-1930. doi: 10.1210/jc.2011-0385 [DOI] [PubMed] [Google Scholar]
- 24.Amrein K, Scherkl M, Hoffmann M, et al. Vitamin D deficiency 2.0: an update on the current status worldwide. Eur J Clin Nutr. Nov 2020;74(11):1498-1513. doi: 10.1038/s41430-020-0558-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ginde AA, Brower RG, Caterino JM, et al. Early high-dose vitamin D(3) for critically ill, vitamin D-deficient patients. N Engl J Med. Dec 26 2019;381(26):2529-2540. doi: 10.1056/NEJMoa1911124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferreira TS, Rocha TM, Klein MR, Sanjuliani AF. Vitamin d deficiency is associated with insulin resistance independent of intracellular calcium, dietary calcium and serum levels of parathormone, calcitriol and calcium in premenopausal women. Nutr Hosp. Apr 1 2015;31(4):1491-1498. doi: 10.3305/nh.2015.31.4.8490 [DOI] [PubMed] [Google Scholar]
- 27.Lips P. Relative value of 25(OH)D and 1,25(OH)2D measurements. J Bone Miner Res. Nov 2007;22(11):1668-1671. doi: 10.1359/jbmr.070716 [DOI] [PubMed] [Google Scholar]
- 28.Chen J, Shao J, Shen A, et al. Enhanced expression of FGF19 predicts poor prognosis in patients with non-small cell lung cancer. J Thorac Dis. Mar 2021;13(3):1769-1784. doi: 10.21037/jtd-21-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim HS, Kim DH, Kim JY, et al. Microarray analysis of papillary thyroid cancers in Korean. Korean J Intern Med. Dec 2010;25(4):399-407. doi: 10.3904/kjim.2010.25.4.399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agrawal N, Akbani R, áArman Aksoy B, et al. Integrated genomic characterization of papillary thyroid carcinoma. Cell. Oct 23 2014;159(3):676-690. doi: 10.1016/j.cell.2014.09.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weinstein JN, Collisson EA, Mills GB, et al. The cancer genome atlas pan-cancer analysis project. Nat Genet. Oct 2013;45(10):1113-1120. doi: 10.1038/ng.2764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al Bookout YJ, Downes M, Yu R, Evans RM, Mangelsdorf DJ. Data from: NURSA Dataset. Tissue-specific expression patterns of nuclear receptors. http://www.nursa.org/10.1621/datasets.02001. 2005.
- 33.Brożyna AA, Jóźwicki W, Slominski AT. Decreased VDR expression in cutaneous melanomas as marker of tumor progression: new data and analyses. Anticancer Res. Jun 2014;34(6):2735-2743. [PMC free article] [PubMed] [Google Scholar]
- 34.Menezes RJ, Cheney RT, Husain A, et al. Vitamin D receptor expression in normal, premalignant, and malignant human lung tissue. Cancer Epidemiol Biomarkers Prev. May 2008;17(5):1104-1110. doi: 10.1158/1055-9965.Epi-07-2713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang T, He L, Wang Z, et al. Calcitriol enhances Doxorubicin-induced apoptosis in papillary thyroid carcinoma cells via regulating VDR/PTPN2/p-STAT3 pathway. J Cell Mol Med. May 2020;24(10):5629-5639. doi: 10.1111/jcmm.15224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lopes N, Sousa B, Martins D, et al. Alterations in vitamin D signalling and metabolic pathways in breast cancer progression: a study of VDR, CYP27B1 and CYP24A1 expression in benign and malignant breast lesions. BMC Cancer. Sep 11 2010;10:483. doi: 10.1186/1471-2407-10-483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer. Sep 2007;7(9):684-700. doi: 10.1038/nrc2196 [DOI] [PubMed] [Google Scholar]
- 38.Jeon SM, Shin EA. Exploring vitamin D metabolism and function in cancer. Exp Mol Med. Apr. 16 2018;50(4):1-14. doi: 10.1038/s12276-018-0038-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pang R, Xu Y, Hu X, Liu B, Yu J. Vitamin D receptor knockdown attenuates the antiproliferative, pro-apoptotic and anti-invasive effect of vitamin D by activating the Wnt/β-catenin signaling pathway in papillary thyroid cancer. Mol Med Rep. Nov 2020;22(5):4135-4142. doi: 10.3892/mmr.2020.11522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhoora S, Punchoo R. Policing cancer: vitamin D arrests the cell cycle. Int J Mol Sci. Dec. 6 2020;21(23):1-20. doi: 10.3390/ijms21239296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu W, Asa SL, Fantus IG, Walfish PG, Ezzat S. Vitamin D arrests thyroid carcinoma cell growth and induces p27 dephosphorylation and accumulation through PTEN/akt-dependent and -independent pathways. Am J Pathol. Feb 2002;160(2):511-519. doi: 10.1016/s0002-9440(10)64870-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shamloo B, Usluer S. P21 in cancer research. Cancers (Basel). Aug. 14 2019;11(8):1-19. doi: 10.3390/cancers11081178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blagosklonny MV. Are p27 and p21 cytoplasmic oncoproteins? Cell Cycle. Nov-Dec 2002;1(6):391-393. doi: 10.4161/cc.1.6.262 [DOI] [PubMed] [Google Scholar]
- 44.Saramäki A, Banwell CM, Campbell MJ, Carlberg C. Regulation of the human p21(waf1/cip1) gene promoter via multiple binding sites for p53 and the vitamin D3 receptor. Nucleic Acids Res. 2006;34(2):543-554. doi: 10.1093/nar/gkj460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou C, Yang C, Chong D. E-cadherin expression is associated with susceptibility and clinicopathological characteristics of thyroid cancer: a PRISMA-compliant meta-analysis. Medicine (Baltimore). Jul 2019;98(30):e16187. doi: 10.1097/md.0000000000016187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Naito A, Iwase H, Kuzushima T, Nakamura T, Kobayashi S. Clinical significance of E-cadherin expression in thyroid neoplasms. J Surg Oncol. Mar 2001;76(3):176-180. doi: 10.1002/jso.1031 [DOI] [PubMed] [Google Scholar]
- 47.Pálmer HG, Larriba MJ, García JM, et al. The transcription factor SNAIL represses vitamin D receptor expression and responsiveness in human colon cancer. Nat Med. Sep 2004;10(9):917-919. doi: 10.1038/nm1095 [DOI] [PubMed] [Google Scholar]
- 48.DeSmet ML, Fleet JC. Constitutively active RAS signaling reduces 1,25 dihydroxyvitamin D-mediated gene transcription in intestinal epithelial cells by reducing vitamin D receptor expression. J Steroid Biochem Mol Biol. Oct 2017;173:194-201. doi: 10.1016/j.jsbmb.2017.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maruyama R, Aoki F, Toyota M, et al. Comparative genome analysis identifies the vitamin D receptor gene as a direct target of p53-mediated transcriptional activation. Cancer Res. May. 1 2006;66(9):4574-4583. doi: 10.1158/0008-5472.Can-05-2562 [DOI] [PubMed] [Google Scholar]
- 50.Stambolsky P, Tabach Y, Fontemaggi G, et al. Modulation of the vitamin D3 response by cancer-associated mutant p53. Cancer Cell. Mar. 16 2010;17(3):273-285. doi: 10.1016/j.ccr.2009.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mohri T, Nakajima M, Takagi S, Komagata S, Yokoi T. MicroRNA regulates human vitamin D receptor. Int J Cancer. Sep. 15 2009;125(6):1328-1333. doi: 10.1002/ijc.24459 [DOI] [PubMed] [Google Scholar]
- 52.Kim D. Low vitamin D status is not associated with thyroid cancer risk. 2016.
- 53.Muralidhar S, Filia A, Nsengimana J, et al. Vitamin D-VDR signaling inhibits Wnt/β-catenin-mediated melanoma progression and promotes antitumor immunity. Cancer Res. Dec 1 2019;79(23):5986-5998. doi: 10.1158/0008-5472.Can-18-3927 [DOI] [PubMed] [Google Scholar]
- 54.Al-Ghafari AB, Balamash KS, Al Doghaither HA. Serum vitamin D receptor (VDR) levels as a potential diagnostic marker for colorectal cancer. Saudi J Biol Sci. Mar 2020;27(3):827-832. doi: 10.1016/j.sjbs.2020.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-tif-1-tct-10.1177_15330338221089933 for Vitamin D Receptor Expression and its Clinical Significance in Papillary Thyroid Cancer by Min Jhi Kim, Daham Kim, Ja Seung Koo, Ju Hee Lee and Kee-Hyun Nam in Technology in Cancer Research & Treatment
Supplemental material, sj-docx-2-tct-10.1177_15330338221089933 for Vitamin D Receptor Expression and its Clinical Significance in Papillary Thyroid Cancer by Min Jhi Kim, Daham Kim, Ja Seung Koo, Ju Hee Lee and Kee-Hyun Nam in Technology in Cancer Research & Treatment
Supplemental material, sj-docx-3-tct-10.1177_15330338221089933 for Vitamin D Receptor Expression and its Clinical Significance in Papillary Thyroid Cancer by Min Jhi Kim, Daham Kim, Ja Seung Koo, Ju Hee Lee and Kee-Hyun Nam in Technology in Cancer Research & Treatment
Supplemental material, sj-docx-4-tct-10.1177_15330338221089933 for Vitamin D Receptor Expression and its Clinical Significance in Papillary Thyroid Cancer by Min Jhi Kim, Daham Kim, Ja Seung Koo, Ju Hee Lee and Kee-Hyun Nam in Technology in Cancer Research & Treatment
Supplemental material, sj-docx-5-tct-10.1177_15330338221089933 for Vitamin D Receptor Expression and its Clinical Significance in Papillary Thyroid Cancer by Min Jhi Kim, Daham Kim, Ja Seung Koo, Ju Hee Lee and Kee-Hyun Nam in Technology in Cancer Research & Treatment