Abstract
Adaptive thermogenesis in small mammals and infants takes place in brown adipose tissue (BAT). Heat is produced via uncoupling protein 1 (UCP1)-mediated uncoupling between oxidation of energy substrates and adenosine 5′-triphosphate synthesis. Thyroid hormone (TH) signaling plays a role in this process. The deiodinases activate thyroxine (T4) to 3,5,3′-triiodothyronine (T3) (D2) or inactivate T4 and T3 to 3,3,5′-triiodothyronine and T2 (D3), respectively. Using a mouse model with selective inactivation of Dio3 in BAT (flox-Dio3 × UCP1-cre = BAT-D3KO), we now show that knocking out D3 resulted in premature exposure of developing brown adipocytes (embryonic days 16.5-18.5) to T3 signaling, leading to an earlier expression of key BAT genes, including Cidea, Cox8b, Dio2, Ucp1, and Pgc1α. Adult BAT-D3KO mice exhibited increased expression of 1591 genes as assessed by RNA sequencing, including 19 gene sets related to mitochondria, 8 related to fat, and 8 related to glucose homeostasis. The expression of 243 genes was changed by more than 1.5-fold, 36 of which play a role in metabolic/thermogenic processes. BAT-D3KO mice weigh less and exhibit smaller white adipocyte area, but maintain normal energy expenditure at room temperature (22 °C) and in the cold (4 °C). They also defend their core temperature more effectively and do not lose as much body weight when exposed to cold. We conclude that the coordinated actions of Dio3 in the embryonic BAT define the timing and intensity of T3 signaling during brown adipogenesis. Enhanced T3 signaling during BAT embryogenesis (Dio3 inactivation) results in selective life-long modifications in the BAT transcriptome.
Keywords: deiodinase, thyroid hormone, brown fat, metabolism, thyroid, brown adipose tissue, deiodinase, T3, D3
Thyroid hormones (THs) enhance the brown adipose tissue (BAT) thermogenic capacity by stimulating uncoupling protein 1 (UCP-1) expression and responsiveness to norepinephrine, processes that are mediated by TH receptor β (TRβ) and TRα, respectively. TH signaling in BAT is locally controlled through TH deiodination. Thyroxine (T4) is taken up by brown adipocytes and rapidly activated to 3,5,3′-triiodothyronine (T3) via type 2 deiodinase (D2), a process that is accelerated by sympathetic stimulation of the BAT. Accordingly, cold exposure accelerates BAT D2 activity and increases BAT T3 content, virtually saturating local T3 receptors. Local activation of T4 to T3 is required for the optimal thermogenic function of BAT, which is confirmed after Dio2 inactivation (1).
Dio2 expression in BAT is first seen during embryonic day 17.5 (E17.5), indicating that T3 signaling plays a role in brown adipogenesis (2). The embryos of mice lacking Dio2 have normal serum T3 levels but exhibit decreased expression of genes defining BAT identity (ie, Ucp1, Pgc-1α, and Dio2 [truncated]), which impairs differentiation and reduces the oxidative/thermogenic capacity of adult BAT (2-4). These observations highlight the importance of deiodinase-controlled TH signaling in BAT embryogenesis. Embryonic BAT also expresses type 3 deiodinase (D3), which inactivates T4 and T3, terminating TH signaling (2). Dio3 expression is highest at E16.7 and subsides postnatal day 1 (P1) (2), not to be detected during adulthood (5).
Thus, it is assumed that the coordinated changes in deiodinase expression, that is, a progressive increase in Dio2 expression and a proportional decrease in Dio3 expression during BAT embryogenesis, define the timing and intensity of T3 exposure in developing brown adipocytes (1). This is particularly relevant because plasma T3 levels remain low until P10 in mice (6, 7). Whereas the study of mice with Dio2 inactivation supports this hypothesis (2-4), BAT studies of mice with Dio3 inactivation have not been performed.
The coordinated expression of Dio2 and Dio3 have been shown to play a role in other developing structures and organs. The development of sensory structures in the brain constitutes elegant models in which deiodinase expression exerts time and spatial control of TH signaling (8), affecting the maturation of auditory function (9). During the fetal and neonatal period, there is relatively high D3 activity in the immature cochlea that dampens TH signaling (10). Later, in the postnatal phase, cochlea D3 activity decreases at the same time that D2 activity rises and peaks around P7. The absence of the Dio2 peak causes an auditory phenotype similar to TRβ inactivation (11). Local TH signaling defines the fate of distinct populations of cone photoreceptors in the retina. TH signaling is symmetrically distributed in the retina at birth as S-pigment expression begins, due to minimal TH signaling caused by Dio3 expression. Over time, TH signaling is strengthened in the dorsal retina at the time of M-pigment onset (P10), illustrating how the ratio and patterning of cone types are affected by TH availability during retinal development (12, 13).
The maturation of hepatoblasts into hepatocytes is also affected by local control of TH signaling via D2. During E18.5 to P5 there is transient Dio2 expression in the neonatal mouse liver that accelerates local T3 production and interferes with the epigenetic remodeling of hepatocytes, permanently modifying the liver transcriptome (14). The peak in Dio2 expression during this period preserves a state of unpacked chromatin and avoids permanent DNA hypermethylation in long-distance enhancers, increasing DNA accessibility and the expression of approximately 1400 genes involved in multiple hepatic functions (15).
Here we show that the BAT-selective inactivation of Dio3 causes premature developmental expression of Cidea, Cox8b, Dio2, UCP1, and PGC1α, all genes involved in the BAT thermogenic program. The earlier exposure to T3 signaling during BAT embryogenesis led to an adult BAT with a markedly changed transcriptome, including 35 gene sets related to mitochondria, fat, and glucose homeostasis, as well as 36 metabolic/thermogenic genes affected by more than 1.5-fold. These data illustrate Dio3’s critical role in BAT embryogenesis, the absence of which results in the premature exposure of developing brown adipocytes to T3 signaling with long-term consequences.
Experimental Procedures
Animals
Studies were approved by the local institutional animal care and use committee. A mouse with brown fat–specific Dio3 inactivation (BAT-D3KO) was created by crossing the floxed D3 mouse (Dio3fl/fl) (16) with a mouse expressing Cre-recombinase under the control of the uncoupling protein 1 promoter (Cre-Ucp1) (B6.FVB-Tg [Ucp1-cre]1Evdr/J; Jackson Laboratories) (17). To study interscapular BAT (iBAT) in embryos, timed-pregnant dams of FloxD3+/-Cre Alb+/– pairs were prepared to obtain BAT-D3KO and control littermates. Pregnancy was determined by the presence of a vaginal plug (embryonic day 0.5, E0.5). At the indicated times (E16.5 and E18.5), pregnant dams were euthanized through CO2 inhalation, and embryos were surgically removed and immediately dissected for iBAT isolation.
For adult studies, male, 15- to 35-week-old mice were used throughout the experiments. Unless otherwise specified, all mice were kept at room temperature (22 °C), with a 12-hour dark/light cycle. Mice were fed a chow diet (3.1 kcal/g) (2918 Teklan Global Protein rodent diet). Animals were euthanized by asphyxiation in a CO2 chamber. Immediately after killing, tissues fragments were snap-frozen in liquid nitrogen and stored at –80 °C for protein and messenger RNA (mRNA) analysis.
Indirect Calorimetry
Animals were admitted to a comprehensive laboratory animal monitoring system (CLAMS, Columbus Instruments) kept at 22 °C and studied as described previously (3). In cold induction studies, the temperature was gradually decreased every 4 hours until 4 °C was reached; animals were then kept at 4 °C for 24 hours. Oxygen consumption (VO2), energy expenditure (EE), respiratory coefficient (RQ), ambulatory movement, and food consumption (Feed Scale device, Columbus Instruments) were continuously monitored using Oxymax software.
Histology and Immunohistochemistry
iBAT and subcutaneous fat pads were processed for hematoxylin and eosin staining. Two tissue sections were analyzed for each animal. Immunohistochemical analysis of paraffin-embedded BAT included anti-UCP1 with ab234430 (Abcam catalog No. ab234430, RRID:AB_2905638) or anti-cytochrome-C (Novus catalog No. NBP2-21569, RRID:AB_2891022), both at 1:100 dilution, followed by antigen-antibody binding detected with bond polymer refine detection (Leica Biosystems, DS9800).
To measure the average size of the adipocytes, we used the Adiposoft plugin (version 1.16) in Fiji (Image J) (version 2.1.0/1.53c). After uploading the microscope scans into QuPath (version 0.2.3), we highlighted a region with around 500 to 1500 adipocytes. The region was then sent to Image J and saved as a tiff file. In Fiji, the tiff file was analyzed with the Adiposoft plugin using the following parameters: auto mode on, exclude on edges on, output unit microns, 0.1645 µm per pixel, minimum diameter 10, and maximum diameter 200. The outputted data listed every adipocyte and its size. We combined the data from each slide and calculated an average adipocyte size for control and BAT-D3KO animals.
Gene Expression Analysis
Total RNA was extracted using the RNeasy kit (Qiagen) and quantified with a Nano-Drop spectrophotometer. A total of 1 μg of total RNA was used for complementary DNA (cDNA) synthesis with the First Strand cDNA Synthesis Kit for reverse transcription–polymerase chain reaction (RT-PCR) (Roche). Genes of interest were assessed by RT-quantitative (q)PCR (StepOnePlus real-time PCR system, Applied Biosystems) using PowerUp SYBR Green Master Mix (Applied Biosystems). Standard curves consisting of 4 to 5 points of serially diluted mixed experimental and control group cDNA were included, and the coefficient of correlation was consistently greater than 0.98, with an amplification efficiency of 80% to 110%. The primers used are listed in Supplementary Table S4 (18), with 18S and/or cyclophilin B as an internal control. Amplicon specificity was assessed through the melting curve.
RNA Sequencing and Analysis
RNA was isolated from 4 controls and 4 BAT-D3KO adult mouse iBat for each group using the RNeasy Kit (Qiagen). RNA degradation was monitored using a BioAnalyzer (Agilent). Samples of total RNA with an RNA integrity number greater than 9.3 (all controls and 3 BAT-D3KO) were sent to the Genome Technology Access Center at Washington University in St Louis, Missouri, for library preparation and sequence. Libraries were pair-end sequenced with NovaSeqS4 (Illumina). Base-calls and demultiplexing were performed with Illumina’s bcl2fastq software and a custom python demultiplexing program with a maximum of one mismatch in the indexing read. The FASTQ files were aligned to gencode.vM24 transcriptome with STAR (v.2.7.8a) using the Partek flow platform (Partek Inc). All prealignment and postalignment QA/QC was performed in Partek Flow (Partek Inc). Aligned reads were quantified to the annotation model (Partek E/M) and normalized (absolute value). Following the differential analysis (analysis of variance), the biological significance of the changes was interpreted using gene set enrichment analysis (GSEA; Supplementary Table S6) (18).
Biochemical Analyses
Individual plasma samples were processed for thyrotropin (TSH) plasma, leptin, interleukin-6 (IL-6), and adiponectin using a MILLIPLEX Rat thyroid Panel and Adipokine panel (Millipore Corporation) and read on a Magpix (Millipore Sigma). Total T4 and total T3 were measured at the University of Chicago, as previously described in detail (19). Briefly, total T4 and T3 concentrations were measured in plasma with a coated tube radioimmunoassay kit (Siemens Medical Solutions Diagnostics) adapted for mice using 25 and 50 μL of plasma, respectively. Glucose levels were determined using the Glucose Colorimetric/Fluorometric assay kit (Abcam, catalog No. ab65333).
Statistical Analysis
All data were analyzed by PRISM software (GraphPad). Unless otherwise indicated, data are presented as values ± SD (tables) or as box and whiskers plots (figures); the Mann-Whitney U or t test was used when the experiment contained 2 independent groups. P less than .05 was used to reject the null hypothesis.
Results
Brown Adipose Tissue–selective Dio3 Inactivation Causes Premature Developmental Expression of Key Thermogenic Genes
To study the role played by Dio3 in BAT, we created a mouse with BAT-selective inactivation of Dio3 (BAT-D3KO) by crossing the Flox-Dio3 mouse (16) with the Cre-Ucp1 mouse (17). Throughout the studies, we used cre-UCP1 mice as controls, which express Cre recombinase in the iBAT at room temperature, and in the inguinal and epididymal white adipose depots after cold exposure; no evidence of leakage has been described (17). BAT-D3KO mice were born following the expected mendelian distribution. They developed, grew (Fig. 1B), and reproduced normally (Supplementary Table S1) (18). They were systemically euthyroid, with normal serum TSH, T3, and T4 levels (Table 1).
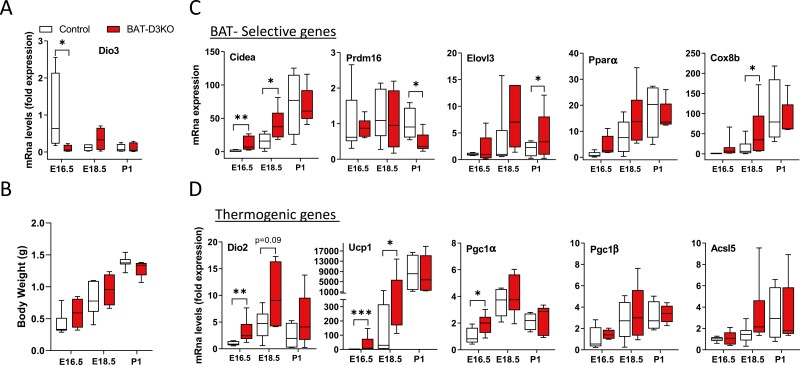
Figure 1.
Interscapular brown adipose tissue (iBAT) gene expression profile of embryonic and neonatal BAT-D3KO mice. A, Relative Dio3 messenger RNA (mRNA) levels in embryonic (E16.5 and E18.5) and neonatal (P1) iBAT; Dio3 mRNA levels are relative to 18S mRNA levels and normalized to E16.5; B, body weight evolution; C, iBAT expression of BAT-selective and D, thermogenic genes at different ages; mRNA data expressed as in A; gene abbreviations are as indicated in Supplementary Table S4 (18); entries are the median and the whiskers indicate the smallest and the largest values of 4 to 9 independent samples; *P less than .05 and **P less than .01 vs same-age control animals.
Table 1.
Plasma biochemistry, body weight, and food intake in adult BAT-D3KO and control mice
| Plasma levels | Control | BAT-D3KO | P |
|---|---|---|---|
| TSH, ng/mL | 0.05 ± 0.06 | 0.30 ± 0.40 | .17 |
| T4, μg/dL | 5.7 ± 1.1 | 5.5 ± 0.6 | .78 |
| T3, μg/dL | 96 ± 15 | 83 ± 13 | .10 |
| Glucose, nmol/μL | 4.5 ± 0.9 | 5.1 ± 0.4 | .24 |
| Leptin, pg/mL | 6105 ± 4406 | 3560 ± 1968 | .16 |
| IL-6, pg/mL | 13 ± 15.1 | 20 ± 7.8 | .33 |
| Adiponectin, pg/mL | 3660 ± 1041 | 5341 ± 2125 | .08 |
| Body weight, g | 34 ± 2.6 | 30 ± 2.8 | .001 |
| Food intake, g | 4.4 ± 1.1 | 4.3 ± 0.6 | .39 |
Values are mean ± SD of 2 to 5 independent samples. Body weight acquired before animals were admitted to the CLAMS. Food intake was the average of 4 days of continuous measuring using Oxymax software in the CLAMS. P value is determined by t test.
Abbreviations: BAT, brown adipose tissue; CLAMS, comprehensive laboratory animal monitoring system; IL-6, interleukin 6; T3, 3,5,3′-triiodothyronine; T4, thyroxine; TSH, thyrotropin.
Dio3 is expressed during BAT embryogenesis (2) (peaks at E16.5) but it is undetectable in newborns and adult mouse iBAT (5). Given that Ucp1 expression in mice first appears on E16.5 and gradually increases until birth (2), the BAT-D3KO mouse is ideal to examine the effects of Dio3 inactivation in the embryonic iBAT. Using RT-qPCR, we confirmed Dio3 mRNA in E16.5 iBAT that subsided on E18.5 and during postnatal life (Fig. 1A). Inactivation of Dio3 in BAT-D3KO mice was associated with an approximately 90% reduction in Dio3 mRNA levels on E16.5 when compared to control iBAT (Fig. 1A). Pups were born with normal body weight (Fig. 1B), and the weight of their iBAT pads was not affected, both in embryos and in P1 (Supplementary Table S2) (18).
Dio3 inactivation led to differences in the timing of expression of some BAT-selective genes. The normal increases in Cidea and Cox8b mRNA levels observed during BAT embryogenesis occurred prematurely in BAT-D3KO iBAT, but both reach a similar plateau level on P1 (Fig. 1C). Elovl3 followed a similar pattern but differences were borderline statistically significant (see Fig. 1C). In contrast, Prdm16 was affected only on P1: iBAT Prdm16 mRNA levels were reduced by 50% (see Fig. 1C). All differences in mRNA levels were dissipated by P1, except for Elov3, which remained borderline increased (see Fig. 1C). Pparα mRNA levels in embryonic and P1 iBAT pads were not affected after Dio3 inactivation (see Fig. 1C).
Similar observations were made when we looked at the mRNA levels of BAT thermogenic genes. There was a premature expression of Dio2, Ucp1, and Pgc1α mRNA levels, which were 2- to 36-fold higher in the embryonic BAT-D3KO iBAT pads compared with respective controls (Fig. 1D). Again, differences had dissipated by P1 (see Fig. 1D). Pgc1β and Acsl5 mRNA levels were unaffected in embryonic and P1 BAT-D3KO iBAT pads (see Fig. 1D).
The Adult BAT-D3KO Mouse Is Seemingly Normal
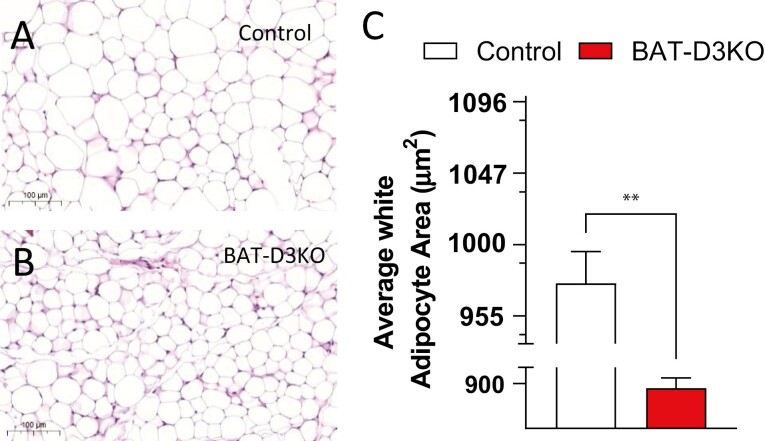
Adult BAT-D3KO mice exhibit approximately 10% lower body weight when compared to control animals, despite eating similar amounts of food daily (see Table 1). Tibial length was not different between BAT-D3KO and controls, suggesting that growth was not affected by BAT-selective Dio3 inactivation (Supplementary Table S3) (18). Furthermore, there were no statistically significant differences in liver, brain, gastrocnemius, heart, or pancreas weight normalized by tibial length, but a minimal increase in kidney weight was observed (see Supplementary Table S3) (18). Similarly, the relative weight of the white fat depots (epididymal, retroperitoneal, and subcutaneous normalized by tibial length) was not different in the BAT-D3KO mice (see Supplementary Table S3) (18). Nonetheless, the analysis of histological sections of the subcutaneous fat revealed a smaller cellular area in BAT-D3KO mice (Fig. 2A and 2C). Other metabolic parameters such as serum levels of glucose, leptin, adiponectin, and IL-6 were not affected by BAT-selective Dio3 inactivation (see Table 1).
Figure 2.
Subcutaneous fat pad histology in adult BAT-D3KO mice. A, Representative microphotographs (×10 magnification) of hematoxylin and eosin–stained sections of subcutaneous fat pads in control mice and B, BAT-D3KO mice; C, subcutaneous adipocyte area in BAT-D3KO and control animals; areas were measured using Image J software from 3 different animals per group (~1000 cells per animal); all values are shown as mean ± SEM; n = 9 per group; **P less than .01 vs control (t test).
Brown Adipose Tissue–selective Dio3 Inactivation Modifies the Transcriptome in the Adult Mouse
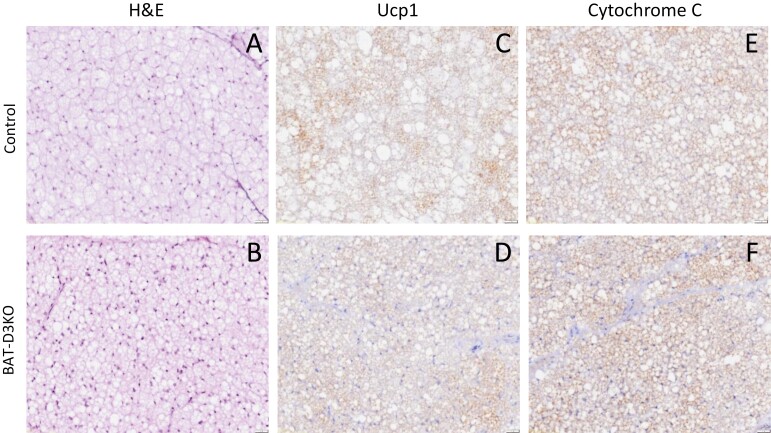
The differences in gene expression detected during embryogenesis indicate that developing brown adipocytes were transiently exposed to premature T3 signaling. We thus hypothesized that this could have triggered epigenetic modifications that led to persistent BAT changes in adulthood. To test if this was the case, we first studied the iBAT of adult BAT-D3KO mice. No differences in iBAT weight were detected (see Supplementary Table S3) (18), but analysis of the cell morphology revealed smaller cell size and reduced content of fat droplets, which are features observed when iBAT is activated (Fig. 3A and 3B).
Figure 3.
Interscapular brown adipose tissue (iBAT) histology in adult BAT-D3KO mice. A and B, Representative microphotographs (×20 magnification) of sections stained with hematoxylin and eosin from control and BAT-D3KO mice as indicated; C and D, immunohistochemical image of BAT sections after staining with UCP1 antibody or with cytochrome-C antibody; E and F, images representative of 4 or 5 independent samples.
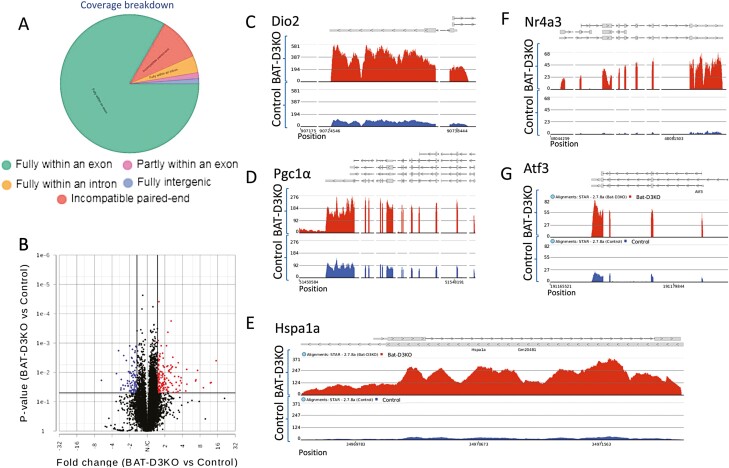
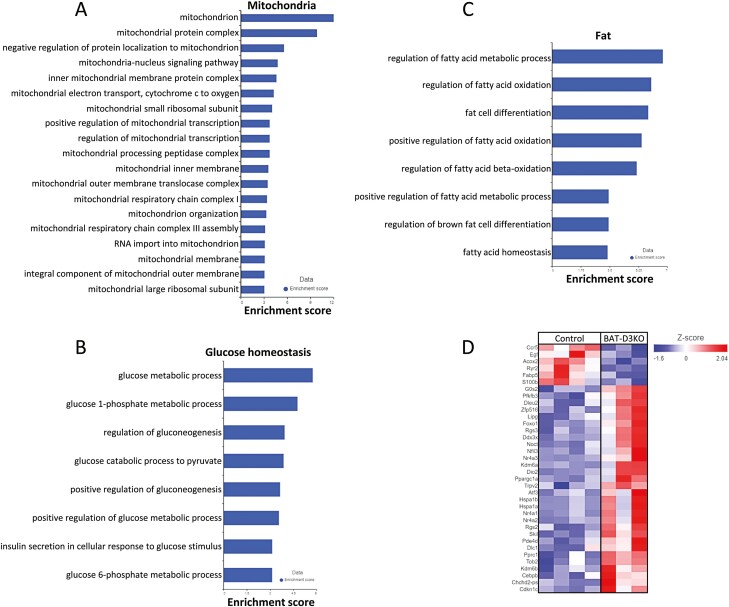
We next performed RNA sequencing analysis of iBAT and identified 1591 genes differentially expressed in the adult BAT-D3KO mouse as compared to controls (P < .05; Fig. 4A and 4B; Supplementary Table S5) (18). The biological interpretation of these gene changes was studied using GSEA, which detected, in the BAT-D3KO iBAT, 1037 statistically significantly enriched gene sets (Supplementary Table S6) (18). Among these, there were 19 gene sets related to mitochondria (Fig. 5A), 8 gene sets related to glucose homeostasis (Fig. 5B), and 8 gene sets related to fat (Fig. 5C). The corresponding pathway analysis revealed 21 statistically significantly enriched pathways in the BAT-D3KO iBAT (Supplementary Table S7) (18), including oxidative phosphorylation, thermogenesis, and glycolysis/gluconeogenesis (see Supplementary Table S7) (18).
Figure 4.
Interscapular brown adipose tissue (iBAT) gene expression profile in adult BAT-D3KO mice. A, RNA sequencing (RNA-seq) coverage breakdown; distribution reflects the average of all samples; B, iBAT differential gene expression (volcano plot) in the BAT-D3KO vs control; each point represents the average of 3 or 4 mice for each transcript; shown in red are 157 genes with fold change greater than 1.5 and P less than .05; shown in blue are 86 genes with fold change less than –1.5 and P less than .05; C to G, chromosome view using Partek flow, indicating the transcript areas of selected genes (5 representative genes out of a total of 243 genes) that are differentially expressed in the BAT-D3KO iBAT; the gene names are indicated at the top of each panel along with the arrow indicating direction of the transcription; the red tracks reflect the coverage for the BAT-D3KO RNA-seq data, and the blue tracks reflect the control mice.
Figure 5.
Gene set enrichment analyses and heat map analysis from adult BAT-D3KO interscapular brown adipose tissue (iBAT). A, Mitochondrial gene sets significantly enriched in BAT-D3KO iBAT; B, fat-related gene sets significantly enriched in BAT-D3KO iBAT; C, glucose metabolism-related gene sets significantly enriched in BAT-D3KO iBAT; D, heat map of 36 genes differentially expressed in BAT-D3KO iBAT that are directly involved in BAT metabolism; gene expression is indicated by the degree of color saturation (red: higher level; blue: lower level).
To capture the most relevant changes in gene expression, we next applied a greater than 1.5-fold change threshold to the set of 1591 genes, which yield 243 genes (Fig. 4B; see Supplementary Table S5) (18). Most of these genes (157/243) were 1.5- to 15-fold upregulated in the BAT-D3KO iBAT, reflecting an overall stimulation of the tissue. Among these genes, we identified 36 that are directly involved in BAT metabolism (Figs. 4C-4E and 5D; Table 2). Notably, the expression of Ucp1, Cycs, Adrb1, Adrb2 and Adrb3 was not affected in the BAT-D3KO mice (Supplementary Fig. S1A-S1E) (18). This was confirmed for Ucp1 and Cycs through immunostaining of iBAT sections (Fig. 3C-3F).
Table 2.
Genes differentially expressed in BAT-D3KO intracapsular BAT of mice involved in BAT metabolism
| Gene | Function | Fold change |
|---|---|---|
| Nr4a3 | ↓ Fabp4 and Ucp3; ↑ Ucp2 in muscle cells (20) | 13 |
| Hspa1a | ↑ Chaperone activity (21) | 11 |
| Hspa1b | ↑ Chaperone activity (21) | 11 |
| Dio2 | ↑ T3 signaling (4) | 6 |
| Nr4a1 | ↑ Ucp1 (22); liver gluconeogenesis (23); skeletal muscle glucose oxidation (24) | 6 |
| Nr4a2 | cAMP-inducible gene (22) | 4 |
| ATF3 | ↑ BAT thermogenesis through Hsf1 induction (25); ↑ browning of white adipose tissue (26) | 4 |
| Pgc1α | ↑ Mitochondriogenesis; ↑ Ucp1 (17) | 3 |
| Noct | ↑ Succinate oxidation in long-term cold exposure (27) | 3 |
| Lipg | ↑ Lipase activity (28) | 3 |
| Nfil3 | ↑ Conversion of white into bright adipocytes (29) | 3 |
| Rgs2 | ↓ GPCR signaling during adaptive thermogenesis (30) | 3 |
| G0s2 | Preserves oxidation of carbohydrates as fuel during fasting (31) | 2 |
| Cebpb | ↑ BAT formation from myoblastic precursors (32) | 2 |
| Zfp516 | ↑ Transcription of UCP1 and other BAT-enriched genes for nonshivering thermogenesis (33) | 2 |
| Kdm6b | ↑ Transcription of BAT-selective genes; ↑ development of beige adipocytes (34) | 2 |
| Trpv2 | ↑ BAT thermogenesis through mediated Ca2 + influx (35) | 2 |
| Pde4d | ↑ Thermogenesis and lipolysis by accumulation of cAMP (36) | 2 |
| Apoe | ↓ Hypercholesterolemia and atherosclerosis (37) | 2 |
| Foxo1 | ↑ Thermogenesis and lipid catabolism by driving SIRT6-mediated FoxO1 deacetylation 9 (38) | 2 |
| Chchd2-ps | ↑ Mitochondrial fission and fusion processes by modulating levels of Opa1 (39) | 2 |
| Kdm6a | ↓ Energy expenditure in obesity (40) | 2 |
| Dleu2 | ↑ Mitochondrial complex IV and sirtuins; decreases oxidative stress (41) | 2 |
| Skil | ↑ Adipocyte differentiation and WAT development by decreasing activity in activin/Smad2 signaling pathway (42) | 2 |
| Pfkfb3 | ↑ Glycolysis (43) | 2 |
| Ddx3x | ↑ Adipocyte development and lipid storage (44) | 2 |
| Tob2 | ↓ Adipogenesis by interfering with Smad signaling (45) | 2 |
| Pprc1 | ↑ Respiratory chain genes (46) | 2 |
| Dlc1 | ↑ Differentiation of white and brown adipocytes (47) | 2 |
| Rgs3 | ↓ cAMP-induced hepatic glucose output (48) | 2 |
| Cdkn1c | ↑ BAT development (49) | 2 |
| Ccr5 | ↓ Adaptive thermogenesis (50) | -2 |
| S100b | ↑ Sympathetic axon growth in BAT (51) | -2 |
| Fabp5 | Supplies fatty acid as substrate for thermogenesis (52) | -2 |
| Egf | ↓ Brown preadipocytes differentiation (53); ↑ Dio3 (54) | -2 |
| Acox2 | ↑ Degradation of the branched-chain fatty acids in peroxisomes (55) | -2 |
| Ryr2 | ↑ UCP-1-independent thermogenesis (56) | -3 |
Abbreviations: BAT, brown adipose tissue; cAMP, 3′,5′-cyclic adenosine 5′-monophosphate; GPCR, G protein–coupled receptor; T3, 3,5,3′-triiodothyronine; UCP, uncoupling protein.
Adaptive Thermogenesis Is Largely Unaffected in the BAT-D3KO Mouse
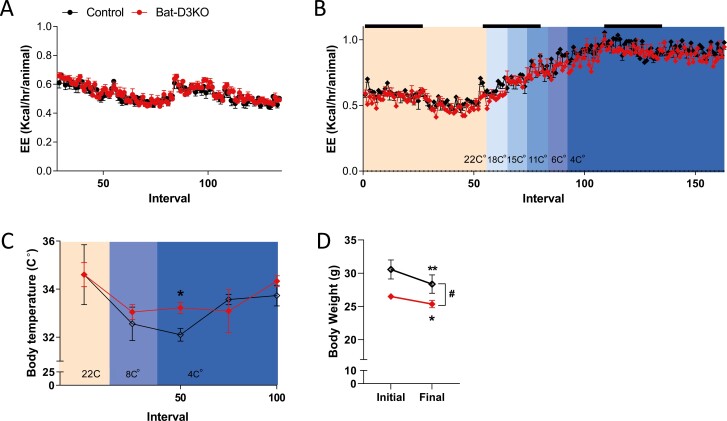
BAT-D3KO and control mice were admitted to the CLAMS for a 48-hour adaptation period followed by continuous observation for the next 4.5 days. At room temperature (22 °C), all mice exhibited the expected circadian rhythmicity in all parameters studied. EE and VO2 were similar between BAT-D3KO and control mice (Fig. 6A and Supplementary Fig. S2A) (18). The RQ tended to be higher in the BAT-D3KO mice, but at only one time point did this difference reach statistical significance (Supplementary Fig. S2B) (18). BAT-D3KO and control mice traveled similar distances, about 1600 and 6400 inches during the light and dark periods, respectively (Supplementary Fig. S2C) (18).
Figure 6.
Comprehensive laboratory animal monitoring system metabolic profile of adult BAT-D3KO mice at room temperature and during cold exposure. A, Energy expenditure (EE) in control and BAT-D3KO mice during 4.5 days, after 48 hours of acclimatization at room temperature; black bars indicate 12 hours of dark cycles; B, EE in control and BAT-D3KO mice exposed to cold; the environment temperature was gradually decreased at each 4-hour interval until it reached 4 °C; where the mice were kept for an additional 24 hours; black bars indicate 12 hours of dark cycles; the color change in the graphic denote the environment temperature decreased; C, core body temperature measured at the indicated intervals and temperature; and D, body weight at the beginning of the experiment (initial) and at the end of the experimental period at 4 °C; values are mean ± SEM; n = 3/group; *P less than or equal to .05 and ** P less than or equal to .01 vs initial respective body weight (paired t test); #P less than or equal to .05 vs control (t test from Δ body weight).
To test whether the adaptive response to cold exposure was affected in BAT-D3KO mice, we gradually decreased the CLAMS temperature at 4-hour intervals until it reached 4 °C, at which point the mice remained under observation for an additional 24 hours. As expected, lowering the temperature progressively accelerated EE and VO2 by approximately 70% in all mice, but no differences were observed between BAT-D3KO and control mice (Fig. 6B and Supplementary Fig. S2D) (18). While there was a tendency for BAT-D3KO mice exposed to cold to exhibit lower RQ values, the drop in RQ was similar between BAT-D3KO and control mice (Supplementary Fig. S2E) (18). Despite the massive thermogenic activation, control mice exhibited the expected 1 to 2 °C drop in core temperature, but the drop in BAT-D3KO mice was less severe, 0 to 1 °C (Fig. 6C). Although all mice lost weight during cold exposure, the loss was approximately 1.1 g (~50%; P < .01) less in the BAT-D3KO mice (Fig. 6D). Control mice reduced ambulatory movements by about 45% to 85% while at 4 °C, in an attempt to reduce heat loss. In contrast, cold-exposed BAT-D3KO mice increased ambulatory movements by 75% during the light period whereas during the dark period ambulatory movements remained stable (Supplementary Fig. S2C) (18). All of these data suggest BAT-D3KO mice are better prepared to withstand exposure to a cold environment.
Discussion
TH deiodinases are known for playing homeostatic roles, adjusting expression and activity levels to preserve TH signaling during iodine deficiency (57, 58). In addition, deiodinases play a developmental role as they define the timing and intensity of TH signaling during the embryogenesis of multiple organs (1). The present studies expand our understanding of the role played by deiodinases in BAT embryogenesis. The BAT-selective inactivation of Dio3 resulted in premature exposure of developing brown adipocytes (E16.5-E18.5) to T3 signaling, leading to an earlier expression of key thermogenic genes Cidea, Cox8b, Dio2, Ucp1, and Pgc1α. The earlier exposure to T3 signaling during BAT embryogenesis led to permanent changes in the iBAT expression of 1591 genes, including 35 gene sets related to mitochondria, fat, and glucose homeostasis. In particular, we identified 36 genes directly related to BAT function with a more than 1.5-fold increased expression in the BAT-D3KO mice. As a result, the adult BAT-D3KO mouse is approximately 10% leaner and exhibits an enhanced capacity to withstand cold exposure.
There are obvious indications that the iBAT has been modified in the adult BAT-D3KO mouse. At room temperature, the cells in the BAT-D3KO iBAT exhibit a smaller size and diameter of the fat droplets, along with upregulation of 1152 genes. These included key thermogenic genes such as Pgc1α, Dio2, Nr4a1-3, and Atf3, which were induced by 3- to 13-fold in the iBAT of BAT-D3KO mice (other important genes are displayed in Table 2). However, it is also obvious that not all key thermogenic BAT genes were stimulated. For example, Ucp1 and Cycs mRNA levels and protein expression were similar in both groups of mice. This is unexpected given that Ucp1 is positively regulated by Dio2-generated T3 and by Pgc1α. These findings suggest that the premature exposure to T3 during embryogenesis selectively affected some, but not all, of the mechanisms involved in BAT thermogenesis. Notwithstanding, the selective changes were sufficient to enhance BAT performance during cold exposure, as documented through the analysis of the core temperature, ambulatory movements, and body weight. These indicate that BAT-D3KO mice are better equipped to endure cold exposure.
The present results are in agreement with the BAT phenotype observed after Dio2 inactivation in mice. Dio2 converts T4 to T3 and is expressed during BAT embryogenesis, enhancing local T3 signaling. Dio2 inactivation leads to an adult mouse with a permanent thermogenic defect in the iBAT that stems from the reduced embryonic expression of genes defining BAT identity (ie, Ucp1, Pgc-1α) (2). Thus, it seems that embryonic T3 signaling, as defined by the timed expression of Dio2 and Dio3, affects the expression of Pgc1α and other key thermogenic genes, which has major consequences for the functioning of the adult iBAT.
The mechanisms through which this happens were recently identified during the embryonic development of hepatocytes. A transient surge in hepatic Dio2 expression on P1 enhances local T3 signaling and affects how the adult mouse liver will respond to feeding on a high-fat diet (14). Hepatocyte-specific Dio2 inactivation reduces local T3 signaling and causes methylation of about 1500 discrete DNA sites during P1 to P5. These sites are associated with reduced chromatin accessibility and expression of about 1400 genes in the adult mouse liver. Thus, it seems logical to assume that the interplay between Dio2 and Dio3 during BAT embryogenesis could act through similar epigenetic mechanisms, which eventually will modulate the expression of key thermogenic genes in the adult iBAT. Future studies should reveal whether DNA methylation is involved in these mechanisms.
In conclusion, the coordinated expression of Dio2 and Dio3 in the embryonic iBAT defines the timing and intensity of T3 signaling during brown fat embryogenesis. Whereas our previous studies indicated that Dio2 inactivation reduces the embryonic expression of Dio3, Cidea, Pgc1α, Ucp1, and Acsl5 (2), and results in an adult iBAT with a reduced thermogenic capacity (2-4), the inactivation of Dio3 induces the expression of Dio2, Cidea, Pgc1α, Ucp1, Elovl3, and Cox8b, and results in the opposite phenotype, that is, an adult mouse with an increased ability to withstand cold exposure.
Acknowledgments
The authors are grateful for the support provided by the National Institute of Diabetes and Digestive and Kidney Diseases.
Glossary
Abbreviations
- BAT
brown adipose tissue
- cDNA
complementary DNA
- CLAMS
comprehensive laboratory animal monitoring system
- D2
type 2 deiodinase
- D3
type 3 deiodinase
- E
embryonic day
- EE
energy expenditure
- GSEA
gene set enrichment analysis
- iBAT
interscapular brown adipose tissue
- IL-6
interleukin 6
- mRNA
messenger RNA
- P
postnatal day
- PCR
polymerase chain reaction
- qPCR
quantitative polymerase chain reaction
- RQ
respiratory coefficient
- RT
reverse transcription
- T3
3,5,3′-triiodothyronine
- T4
thyroxine
- TH
thyroid hormone
- TR
thyroid hormone receptor
- TSH
thyrotropin
- UCP
uncoupling protein;
- VO2
oxygen consumption
Financial Support
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK; grant Nos. DK58538 and DK65055).
Disclosures
A.C.B. is a consultant for AbbVie, Allergan, Synthonics, Sention, and Thyron. The other authors have nothing to disclose.
Data Availability
All data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request. The supplementary information for this manuscript is available as listed in “References” (18).
References
- 1. Bianco AC, Dumitrescu A, Gereben B, et al. Paradigms of dynamic control of thyroid hormone signaling. Endocr Rev. 2019;40(4):1000-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hall JA, Ribich S, Christoffolete MA, et al. Absence of thyroid hormone activation during development underlies a permanent defect in adaptive thermogenesis. Endocrinology. 2010;151(9):4573-4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Castillo M, Hall JA, Correa-Medina M, et al. Disruption of thyroid hormone activation in type 2 deiodinase knockout mice causes obesity with glucose intolerance and liver steatosis only at thermoneutrality. Diabetes. 2011;60(4):1082-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Jesus LA, Carvalho SD, Ribeiro MO, et al. The type 2 iodothyronine deiodinase is essential for adaptive thermogenesis in brown adipose tissue. J Clin Invest. 2001;108(9):1379-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hernandez A, Garcia B, Obregon MJ. Gene expression from the imprinted Dio3 locus is associated with cell proliferation of cultured brown adipocytes. Endocrinology. 2007;148(8):3968-3976. [DOI] [PubMed] [Google Scholar]
- 6. Dussault JH, Labrie F. Development of the hypothalamic-pituitary-thyroid axis in the neonatal rat. Endocrinology. 1975;97(5):1321-1324. [DOI] [PubMed] [Google Scholar]
- 7. Fukuda H, Greer MA. Postnatal development of pituitary-thyroid function in male and female rats: comparison of plasma and thyroid T3 and T4 concentration. J Endocrinol Invest. 1978;1(4):311-314. [DOI] [PubMed] [Google Scholar]
- 8. Rovet J, Daneman D. Congenital hypothyroidism: a review of current diagnostic and treatment practices in relation to neuropsychologic outcome. Paediatr Drugs. 2003;5(3):141-149. [DOI] [PubMed] [Google Scholar]
- 9. Forrest D, Erway LC, Ng L, Altschuler R, Curran T. Thyroid hormone receptor beta is essential for development of auditory function. Nat Genet. 1996;13(3):354-357. [DOI] [PubMed] [Google Scholar]
- 10. Ng L, Hernandez A, He W, et al. A protective role for type 3 deiodinase, a thyroid hormone-inactivating enzyme, in cochlear development and auditory function. Endocrinology. 2009;150(4):1952-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ng L, Goodyear RJ, Woods CA, et al. Hearing loss and retarded cochlear development in mice lacking type 2 iodothyronine deiodinase. Proc Natl Acad Sci U S A. 2004;101(10):3474-3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roberts MR, Srinivas M, Forrest D, Morreale de Escobar G, Reh TA. Making the gradient: thyroid hormone regulates cone opsin expression in the developing mouse retina. Proc Natl Acad Sci U S A. 2006;103(16):6218-6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ng L, Lyubarsky A, Nikonov SS, et al. Type 3 deiodinase, a thyroid-hormone-inactivating enzyme, controls survival and maturation of cone photoreceptors. J Neurosci. 2010;30(9):3347-3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fonseca TL, Fernandes GW, McAninch EA, et al. Perinatal deiodinase 2 expression in hepatocytes defines epigenetic susceptibility to liver steatosis and obesity. Proc Natl Acad Sci U S A. 2015;112(45):14018-14023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fonseca TL, Garcia T, Fernandes GW, Nair TM, Bianco AC. Neonatal thyroxine activation modifies epigenetic programming of the liver. Nat Commun. 2021;12(1):4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dentice M, Ambrosio R, Damiano V, et al. Intracellular inactivation of thyroid hormone is a survival mechanism for muscle stem cell proliferation and lineage progression. Cell Metab. 2014;20(6):1038-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kong X, Banks A, Liu T, et al. IRF4 is a key thermogenic transcriptional partner of PGC-1α. Cell 2014;158(1):69-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fonseca TL, Russo SC, Luongo C, Salvatore D, Bianco AC.Supplementary data for “Inactivation of Type 3 Deiodinase Results in Life-long Changes in Brown Adipose Tissue Transcriptome in the Male Mouse.” Dryad Digital Repository2017. Deposited February 22, 2022. https://zenodo.org/record/6359928#.YjDSQ8ztyUl [DOI] [PMC free article] [PubMed]
- 19. Ferrara AM, Liao XH, Gil-Ibáñez P, et al. Changes in thyroid status during perinatal development of MCT8-deficient male mice. Endocrinology. 2013;154(7):2533-2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maxwell MA, Cleasby ME, Harding A, Stark A, Cooney GJ, Muscat GE. Nur77 regulates lipolysis in skeletal muscle cells. Evidence for cross-talk between the beta-adrenergic and an orphan nuclear hormone receptor pathway. J Biol Chem. 2005;280(13):12573-12584. [DOI] [PubMed] [Google Scholar]
- 21. Matz JM, Blake MJ, Tatelman HM, Lavoi KP, Holbrook NJ. Characterization and regulation of cold-induced heat shock protein expression in mouse brown adipose tissue. Am J Physiol. 1995;269(1 Pt 2):R38-R47. [DOI] [PubMed] [Google Scholar]
- 22. Kumar N, Liu D, Wang H, Robidoux J, Collins S. Orphan nuclear receptor NOR-1 enhances 3’,5’-cyclic adenosine 5’-monophosphate-dependent uncoupling protein-1 gene transcription. Mol Endocrinol. 2008;22(5):1057-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chao LC, Zhang Z, Pei L, Saito T, Tontonoz P, Pilch PF. Nur77 coordinately regulates expression of genes linked to glucose metabolism in skeletal muscle. Mol Endocrinol. 2007;21(9):2152-2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pei L, Waki H, Vaitheesvaran B, Wilpitz DC, Kurland IJ, Tontonoz P. NR4A orphan nuclear receptors are transcriptional regulators of hepatic glucose metabolism. Nat Med. 2006;12(9):1048-1055. [DOI] [PubMed] [Google Scholar]
- 25. Verma N, Perie L, Mueller E. The mRNA levels of heat shock factor 1 are regulated by thermogenic signals via the cAMP-dependent transcription factor ATF3. J Biol Chem. 2020;295(18):5984-5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cheng CF, Ku HC, Cheng JJ, et al. Adipocyte browning and resistance to obesity in mice is induced by expression of ATF3. Commun Biol. 2019;2:389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Onder Y, Laothamatas I, Berto S, et al. The circadian protein nocturnin regulates metabolic adaptation in brown adipose tissue. iScience. 2019;19:83-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schaltenberg N, John C, Heine M, et al. Endothelial lipase is involved in cold-induced high-density lipoprotein turnover and reverse cholesterol transport in mice. Front Cardiovasc Med. 2021;8:628235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Higareda-Almaraz JC, Karbiener M, Giroud M, et al. Norepinephrine triggers an immediate-early regulatory network response in primary human white adipocytes. BMC Genomics. 2018;19(1):794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nunn C, Zhao P, Zou MX, Summers K, Guglielmo CG, Chidiac P. Resistance to age-related, normal body weight gain in RGS2 deficient mice. Cell Signal. 2011;23(8):1375-1386. [DOI] [PubMed] [Google Scholar]
- 31. El-Assaad W, El-Kouhen K, Mohammad AH, et al. Deletion of the gene encoding G0/G 1 switch protein 2 (G0s2) alleviates high-fat-diet-induced weight gain and insulin resistance, and promotes browning of white adipose tissue in mice. Diabetologia. 2015;58(1):149-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kajimura S, Seale P, Kubota K, et al. Initiation of myoblast to brown fat switch by a PRDM16-C/EBP-beta transcriptional complex. Nature. 2009;460(7259):1154-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sambeat A, Gulyaeva O, Dempersmier J, et al. LSD1 interacts with Zfp516 to promote UCP1 transcription and brown fat program. Cell Rep. 2016;15(11):2536-2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pan D, Huang L, Zhu LJ, et al. Jmjd3-mediated H3K27me3 dynamics orchestrate brown fat development and regulate white fat plasticity. Dev Cell. 2015;35(5):568-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sun W, Uchida K, Suzuki Y, et al. Lack of TRPV2 impairs thermogenesis in mouse brown adipose tissue. EMBO Rep. 2016;17(3):383-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kraynik SM, Miyaoka RS, Beavo JA. PDE3 and PDE4 isozyme-selective inhibitors are both required for synergistic activation of brown adipose tissue. Mol Pharmacol. 2013;83(6):1155-1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhou E, Li Z, Nakashima H, et al. Beneficial effects of brown fat activation on top of PCSK9 inhibition with alirocumab on dyslipidemia and atherosclerosis development in APOE*3-Leiden.CETP mice. Pharmacol Res. 2021;167:105524. [DOI] [PubMed] [Google Scholar]
- 38. Jung SM, Hung CM, Hildebrand SR, et al. Non-canonical mTORC2 signaling regulates brown adipocyte lipid catabolism through SIRT6-FoxO1. Mol Cell. 2019;75(4):807-822.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu W, Duan X, Xu L, et al. Chchd2 regulates mitochondrial morphology by modulating the levels of Opa1. Cell Death Differ. 2020;27(6):2014-2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chen J, Xu X, Li Y, et al. Kdm6a suppresses the alternative activation of macrophages and impairs energy expenditure in obesity. Cell Death Differ. 2021;28(5):1688-1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang J, Kay MK, Park MH, Meruvu S, Powell C, Choudhury M. LncRNA DLEU2 regulates sirtuins and mitochondrial respiratory chain complex IV: a novel pathway in obesity and offspring’s health. Int J Obes (Lond). Published online January 20, 2022. doi: 10.1038/s41366-022-01075-6 [DOI] [PubMed] [Google Scholar]
- 42. Zhu Q, Chang A, Xu A, Luo K. The regulatory protein SnoN antagonizes activin/Smad2 protein signaling and thereby promotes adipocyte differentiation and obesity in mice. J Biol Chem. 2018;293(36):14100-14111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Huo Y, Guo X, Li H, et al. Disruption of inducible 6-phosphofructo-2-kinase ameliorates diet-induced adiposity but exacerbates systemic insulin resistance and adipose tissue inflammatory response. J Biol Chem. 2010;285(6):3713-3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang J, Rajbhandari P, Damianov A, et al. RNA-binding protein PSPC1 promotes the differentiation-dependent nuclear export of adipocyte RNAs. J Clin Invest. 2017;127(3): 987-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Takahashi A, Morita M, Yokoyama K, Suzuki T, Yamamoto T. Tob2 inhibits peroxisome proliferator-activated receptor γ2 expression by sequestering Smads and C/EBPα during adipocyte differentiation. Mol Cell Biol. 2012;32(24):5067-5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhai N, Sun C, Gu W, et al. Resistance to high-fat diet-induced obesity in male heterozygous Pprc1 knockout mice. Endocr J. 2015;62(7):633-644. [DOI] [PubMed] [Google Scholar]
- 47. Sim CK, Kim SY, Brunmeir R, et al. Regulation of white and brown adipocyte differentiation by RhoGAP DLC1. PLoS One. 2017;12(3):e0174761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Raab RM, Bullen J, Kelleher J, Mantzoros C, Stephanopoulos G. Regulation of mouse hepatic genes in response to diet induced obesity, insulin resistance and fasting induced weight reduction. Nutr Metab (Lond). 2005;2:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Van De Pette M, Tunster SJ, McNamara GI, et al. Cdkn1c boosts the development of brown adipose tissue in a murine model of Silver Russell syndrome. PLoS Genet. 2016;12(3):e1005916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chan PC, Hung LM, Huang JP, et al. Augmented CCL5/CCR5 signaling in brown adipose tissue inhibits adaptive thermogenesis and worsens insulin resistance in obesity. Clin Sci (Lond). 2022;136(1):121-137. [DOI] [PubMed] [Google Scholar]
- 51. Zeng X, Ye M, Resch JM, et al. Innervation of thermogenic adipose tissue via a calsyntenin 3β-S100b axis. Nature. 2019;569(7755):229-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Syamsunarno MR, Iso T, Yamaguchi A, et al. Fatty acid binding protein 4 and 5 play a crucial role in thermogenesis under the conditions of fasting and cold stress. PLoS One. 2014;9(6):e90825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. García B, Obregón MJ. Growth factor regulation of uncoupling protein-1 mRNA expression in brown adipocytes. Am J Physiol Cell Physiol. 2002;282(1):C105-C112. [DOI] [PubMed] [Google Scholar]
- 54. Hernández A, St. Germain DL, Obregón MJ. Transcriptional activation of type III inner ring deiodinase by growth factors in cultured rat brown adipocytes. Endocrinology. 1998;139(2):634-639. [DOI] [PubMed] [Google Scholar]
- 55. Ferdinandusse S, Denis S, van Roermund CWT, et al. A novel case of ACOX2 deficiency leads to recognition of a third human peroxisomal acyl-CoA oxidase. Biochim Biophys Acta Mol Basis Dis. 2018;1864(3):952-958. [DOI] [PubMed] [Google Scholar]
- 56. Ikeda K, Kang Q, Yoneshiro T, et al. UCP1-independent signaling involving SERCA2b-mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis. Nat Med. 2017;23(12):1454-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Larsen PR, Silva JE, Kaplan MM. Relationships between circulating and intracellular thyroid hormones: physiological and clinical implications. Endocr Rev. 1981;2(1):87-102. [DOI] [PubMed] [Google Scholar]
- 58. Peeters R, Fekete C, Goncalves C, et al. Regional physiological adaptation of the central nervous system deiodinases to iodine deficiency. Am Physiol Endocrinol Metab. 2001;281(1):E54-E61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request. The supplementary information for this manuscript is available as listed in “References” (18).