Abstract
Three-dimensional (3D) bioprinting seeks to unlock the rapid generation of complex tissue constructs, but long-standing challenges with efficient in vitro microvascularization must be solved before this can become a reality. Microvasculature is particularly challenging to biofabricate due to the presence of a hollow lumen, a hierarchically branched network topology, and a complex signaling milieu. All of these characteristics are required for proper microvascular – and, thus, tissue – function. While several techniques have been developed to address distinct portions of this microvascularization challenge, no single approach is capable of simultaneously recreating all three microvascular characteristics. In this review, we present a three-part framework that proposes integration of existing techniques to generate mature microvascular constructs. First, extrusion-based 3D bioprinting creates a mesoscale foundation of hollow, endothelialized channels. Second, biochemical and biophysical cues induce endothelial sprouting to create a capillary-mimetic network. Third, the construct is conditioned to enhance network maturity. Across all three of these stages, we highlight the potential for extrusion-based bioprinting to become a central technique for engineering hierarchical microvasculature. We envision that the successful biofabrication of functionally engineered microvasculature will address a critical need in tissue engineering, and propel further advances in regenerative medicine and ex vivo human tissue modeling.
Keywords: 3D printing, extrusion-based bioprinting, microvasculature, endothelial sprouting, vascular structure, vascular function
1. A Multi-Step Approach to Engineering Functional Vascularized Constructs
1.1. The Power and Promise of Extrusion-Based 3D Bioprinting
With applications ranging from on-chip models of disease progression [1,2] and drug efficacy [3], to the generation of tissue replacements and implantables [4] for resolving the long-bemoaned donor organ shortage, creating functional microvasculature at the bench is key to unlocking the promise of tissue engineering and regenerative medicine. Three-dimensional (3D) bioprinting is currently revolutionizing these fields by enabling the rapid generation of complex tissue constructs that aim to ameliorate long-standing challenges associated with efficient in vitro vascularization. Extrusion-based 3D bioprinting has garnered particular recognition largely due to the accessibility and adaptability of this technique. In addition to the growing number of cutting-edge commercial bioprinters available today, custom-manufacturing and operating an extrusion-based 3D bioprinter is accessible thanks to a thriving open-source 3D printing community [5–7]. Further, the design of many extrusion-based bioprinters leverages a wealth of semi-modular hardware, making these bioprinters adaptable for use with various biomaterial inks, commonly hydrogels, that employ diverse gelation strategies. Of particular importance for tissue engineering is the ability to mount additional extruders or custom-built multimaterial extrusion systems onto the bioprinter for simultaneous printing of multiple biomaterial inks [8–10]. Crucially, principles from biomaterials science may be used to engineer these inks to provide user-defined biophysical and biochemical cues within the printed construct for instructing long-term cell behaviors. Taken together, these capabilities make extrusion-based bioprinting a promising technique for representing the complexity of the microvascular niche in vitro.
To date, efforts to bioprint constructs that mirror the nutritive arteriole-capillary-venule networks characteristic of microvasculature have primarily focused on reproducibly creating perfusable structures, such as simplified lattices. As such, less emphasis has been placed on recapitulating other important drivers of vascular function within these printed constructs. This first wave of 3D bioprinting built a strong foundation by identifying the design constraints for printable biomaterial inks and establishing a variety of techniques for solving the unique challenges of additive manufacturing with soft materials. Armed with this knowledge, researchers are more capable than ever of using extrusion-based 3D bioprinting to make elegant and complex shapes reminiscent of native tissues [11] and microvasculature [8] from a variety of different materials. The output of these studies has largely been restricted to the maintenance of cell viability following printing; however, the accurate recapitulation of microvasculature in vitro requires significantly more incorporation of biochemical and biophysical signals known to impact cell phenotype. Recognizing this need [12], there has been increasing interest in engineering further biological complexity and function into bioprinted microvascular constructs to enhance their translational applicability.
1.2. Three Key Characteristics Make Microvasculature Difficult to Replicate
Despite more than three decades of exciting progress in the field of in vitro vascularization, particularly with microfluidic models [13,14] and 3D bioprinting [4], the on-demand creation of truly biomimetic microvasculature at scale remains out of reach. This is largely because functional multiscale microvasculature is inherently challenging to biofabricate from an engineering standpoint. Specifically, three characteristics that allow vascular networks to function in vivo include (1) a hollow, endothelialized lumen; (2) a highly organized and hierarchical network structure; and (3) a complex signaling environment that dynamically regulates and drives vascular function (figure 1(A–C)). Here, we will focus on vessels relevant to nutritive microvascular beds, including arterioles (40–300 μm in diameter), capillaries (10–50 μm in diameter), and post-capillary venules (10–100 μm in diameter) [15,16]. Even within this limited size regime, the three key microvascular characteristics listed above each pose unique biofabrication challenges that make it difficult to recapitulate functional microvasculature through any one fabrication technique alone.
Figure 1. Biofabricating functional microvasculature in three steps.
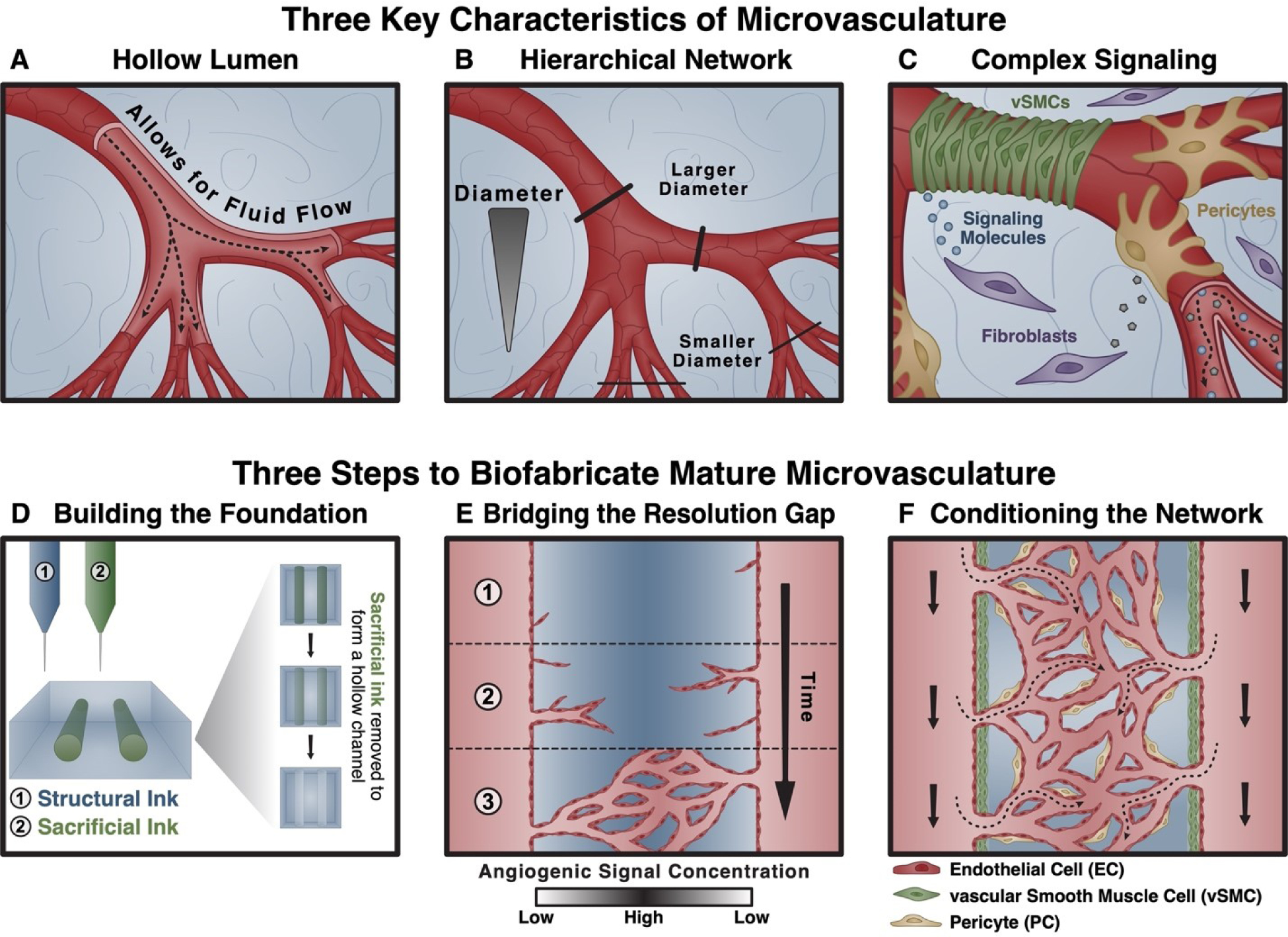
At a high level, three characteristics of microvasculature are both crucial for function and difficult to biofabricate using any one technique alone: (A) the presence of a hollow lumen that enables fluid (blood) flow; (B) a hierarchically branched network topology; and (C) a complex signaling environment composed of biophysical (fluid flow, extracellular matrix mechanics) and biochemical (diverse cell types, signaling molecules, matrix ligands) cues for tissue homeostasis. We propose overcoming this challenge using a three-step approach that leverages existing techniques. (D) First, a foundational, hollow network structure is fabricated using extrusion-based bioprinting. (E) Second, proangiogenic signaling is harnessed to induce endothelial cell sprouting and the formation of a capillary-scale network. (F) Third, the network is matured using signals known to condition and stabilize nascent vascular networks, including the introduction of supporting cell types (vascular smooth muscle cells, pericytes).
1.3. A Three-Part Framework to Solve Current Challenges
In this review, we propose a three-part framework that addresses current obstacles by integrating existing techniques. (1) First, a foundation of mesoscale, hollow, and endothelialized channels is fabricated using extrusion-based 3D bioprinting (figure 1(D)). To that end, we discuss the challenges associated with bioprinting perfusable, endothelial cell (EC)-lined vascular networks and survey their current solutions. (2) Next, we highlight biomaterials- and biofabrication-based strategies to direct the growth of microvascular beds from the bioprinted foundation, thereby bridging a critical resolution gap between bioprinted channels and the capillaries required for function (figure 1(E)). With this goal in mind, we discuss the limitations of current strategies for creating hierarchical networks and the importance of different cues in driving sprouting morphogenesis. We further highlight methods to pattern these signals and discuss considerations for selecting an appropriate EC source and phenotype. (3) Last, we discuss instructive signals that can condition and stabilize the network, thereby enhancing function (figure 1(F)). These include the presence of supporting mural or tissue-specific cells and fluid flow. With an eye towards achieving function, we discuss previous challenges associated with recapitulating the microvascular signaling environment, facile methods for incorporating key drivers of vascular function into bioprinted constructs, and emerging considerations for engineering and assessing the function of biofabricated microvasculature. Marked advances have been made towards each of these three goals, but no single system has yet seamlessly addressed all three to achieve functional, multiscale engineered microvasculature. This review will demonstrate the potential for extrusion-based bioprinting to become a central technique for engineering microvasculature by discussing its compatibility with strategies for accomplishing all three of these stages.
2. Building the Foundation Using Extrusion-Based Bioprinting
One defining characteristic of all healthy vasculature is the presence of an unobstructed, EC-lined lumen through which blood and its constituent cells, nutrients, and signals can flow [17]. The importance of both the lumen geometry and presence of a uniform endothelium cannot be overstated. Alterations to this geometry are characteristic of disease and injury, such as stenosis associated with atherosclerotic plaque accumulation or thrombosis [18]. If severe enough, vessel occlusion can cause rapid tissue damage via ischemia, as in a heart attack or stroke. Meanwhile, the interaction between blood and ECs made possible by the hollow architecture is crucial to vascular development, where the initiation of blood flow helps stabilize the nascent cardiovascular system. This importance continues into adulthood, where dysfunction or disruption of the endothelium can have dire consequences [17,18]. Because of the integral role of this endothelialized lumen in vascular function, early attempts to bioprint microvasculature focused on these two criteria – creating a hollow lumen and forming an endothelial monolayer – as benchmarks for success. While challenging, techniques have been developed to achieve these goals.
2.1. Bioprinting Techniques for Hollow Lumen Formation
Using our three-part framework, the first step in biofabricating functional, multiscale microvasculature requires the establishment of a foundational scaffold of arteriole-scale (40–300 μm in diameter) and venule-scale (10–100 μm in diameter) vessel-like structures from which to build [15,16]. This requires the creation of hollow channels, which are later lined by an EC monolayer (figure 2(A)). For many biofabrication approaches, however, the rapid and reliable creation of void spaces within a 3D construct can be challenging. In free-standing 3D bioprinting, for example, biomaterial inks that simultaneously maintain cell viability and enable cell-mediated matrix remodeling post-print are often too soft to support their own weight and collapse into luminal spaces during the printing process, which precludes their use as models of vascularization. Because a successful biomaterial ink must optimize both structural integrity and cell viability [19], striking a balance between these opposing design constraints is central to the development of biomaterial inks and bioprinting strategies. To that end, several promising techniques have been developed that allow for rapid and reliable creation of complex, vascular-mimetic structures within a defined size regime. In particular, three techniques commonly employed to fabricate perfusable channels include the use of sacrificial inks as support materials, printing into gel-phase support baths, and using coaxial nozzles to directly extrude hollow channels (figure 2(B–D)).
Figure 2. Building the foundation using extrusion-based bioprinting.
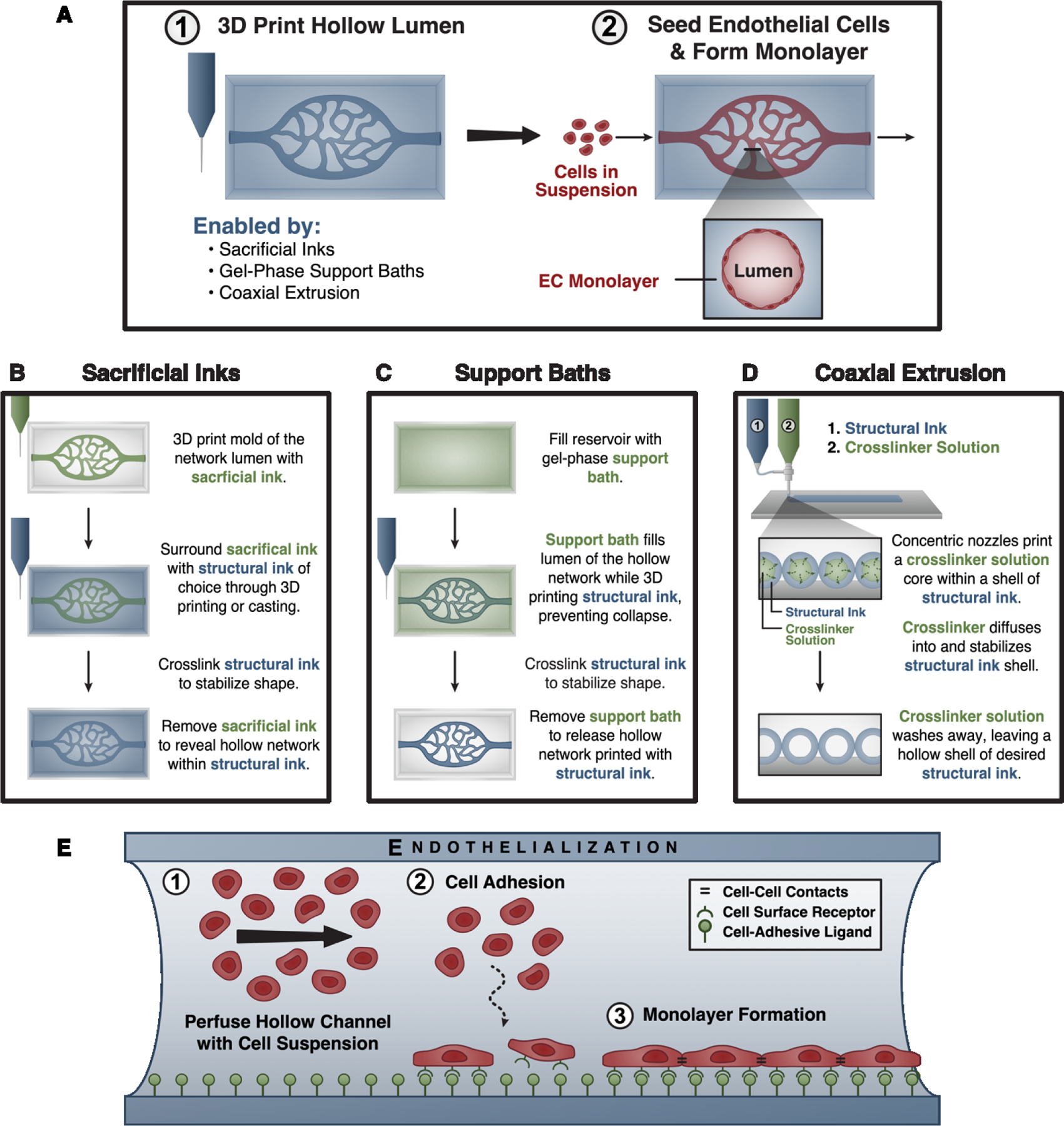
(A, panel 1) Several 3D bioprinting techniques enable the fabrication of hollow networks by using materials or crosslinker solutions that fill and/or physically support the hollow lumen being created. (A, panel 2) Because these techniques often preclude the direct deposition of endothelial cell-laden inks, hollow channels are endothelialized – or seeded with endothelial cells – after fabrication. (B) Sacrificial inks are used to create a mold of the desired hollow space, which physically supports the surrounding ink as it is printed or cast around it. After crosslinking the surrounding ink, the sacrificial ink is removed to form a hollow lumen. (C) Gel-phase support baths similarly fill and physically support a hollow structure during printing. These materials locally fluidize around the printing nozzle as it passes, and rapidly self-heal to confine the deposited ink. After crosslinking the biomaterial ink, the support bath is removed. (D) Coaxial extrusion systems allow for direct printing of hollow channels by extruding both a biomaterial ink and its crosslinker from concentric nozzles to crosslink the material in situ. (E) During endothelialization, the 3D printed lumen is perfused with a suspension of endothelial cells. The cells are allowed to adhere to the construct surface, which is crucially dependent upon factors such as the presentation of cell-adhesive ligands. After removing unattached cells, the construct is cultured to allow endothelial monolayer formation.
2.1.1. Sacrificial Inks for Channel Formation.
Sacrificial inks, also referred to as fugitive inks, are materials that can be directly printed or cast into a defined shape, embedded or co-printed within a surrounding biomaterial ink, and then removed after printing to reveal a desired – often hollow – structure (figure 1(D), figure 2(B)). One of the first demonstrations of sacrificial inks used a wax-based material to form 3D microvascular structures within a microfluidic device [20–22]. Today, a variety of sacrificial inks have been developed that can be broadly categorized by their removal mechanism.
Aqueous Dissolution.
Inspiration for sacrificial inks has stemmed from diverse sources, such as sugar spinning [23]. For example, existing models of glass-fiber drawing have been adapted to thermal extrusion in order to produce carbohydrate-glass lattices that were readily dissolved in aqueous conditions, including cell culture medium [24]. This approach allowed for fusion between filaments by controlling the temperature of the build platform, making an interconnected network that can be challenging to produce with other techniques and materials. To prevent osmotic damage to cells encapsulated in the surrounding material during dissolution, the lattices were briefly coated in poly(D-lactide-co-glycolide) (PLDGA), which ensured that dissolved carbohydrates flowed out of the channels instead of through the bulk, and then similarly dissolved away. Carbohydrate glasses have been used in subsequent applications, such as the creation of vascular patches that, upon implantation, improved reperfusion post-ischemia [4].
Thermal Gelation and Melting.
Another class of sacrificial inks relies on reversible thermal gelation to remove the printed material. Pluronic F-127 is a triblock-copolymer of poly(ethylene oxide) (PEO) and poly(propylene oxide) (PPO) that has seen extensive use as a sacrificial ink for extrusion-based bioprinting [25]. Pluronic F-127 solutions form liquid-phase micelles at low temperatures and a liquid crystalline gel phase at higher temperatures, where the gelation and melting temperatures are highly dependent upon the concentration of the solution [26]. For example, solutions below 20 wt% do not form gels, while those above 40 wt% are always a gel between 0°C and 100°C [27]. The gelation of Pluronic F-127 is also impacted by resident salts [28]. This tunability makes processing and removal of sacrificial Pluronic F-127 inks straightforward, for they are commonly formulated to liquify close to 4°C. In particular, Pluronic F-127 has been used as a sacrificial ink for printing vascular networks in conjunction with a variety of materials and cell types [25,29,30], demonstrating its broad suitability for bioprinting applications. Gelatin, a collagen-derived biomaterial, is another commonly used sacrificial ink that relies upon temperature changes for removal. In contrast to Pluronic F-127, which solidifies when heated, solutions of gelatin will solidify when cooled. While the melting temperature can be tuned through gelatin concentration, molecular weight, and thermal history [31], gelatin-based sacrificial inks are commonly designed to melt at physiological temperatures (37°C) [32]. This makes their removal cell friendly. Furthermore, because gelatin is a derivative of collagen, it is naturally cell-adhesive, and channels made with gelatin will likely contain residual cell-adhesive ligands capable of promoting EC anchoring. The success of gelatin-based sacrificial inks can be seen in their recent uses, such as the printing of gelatin vascular channels within a bioink composed of reconstituted basement membrane, collagen I, and tissue-specific spheroids [33].
Physical Crosslink Disruption by Small Molecules.
A third class of sacrificial inks takes advantage of physical crosslinking for gelation and dissolution. One common example is alginate, a naturally derived, linear polysaccharide composed of guluronic acid and mannuronic acid subunits. Alginate uses a cytocompatible ionic crosslinking mechanism for gelation, wherein guluronic acid monomers complex around divalent cations, such as calcium, and form a gel. Further, these crosslinks are readily disrupted by treatment with calcium chelators like sodium citrate and ethylenediaminetetraacetic acid (EDTA). Because of its ease of use, alginate has been utilized extensively in the biomaterials community as a cell culture platform [34] and, more recently, as a sacrificial ink within various biomaterials to form vascular-mimetic networks [35,36]. Other physical hydrogels, such as those that employ guest-host interactions for crosslinking, have also been used as sacrificial inks. Specifically, a sacrificial hydrogel composed of adamantane- and β-cyclodextrin-conjugated hyaluronic acid (HA) was bioprinted into a similar hydrogel support bath of adamantane-norbornene-HA and β-cyclodextrin-HA [37]. Removal of this guest-host material used an analogous strategy to that of alginate. After printing microvascular structures, the support bath was crosslinked by ultraviolet (UV) light via thiol-ene click chemistry, and the print was flushed with soluble β-cyclodextrin to disrupt the physical crosslinks and remove the sacrificial ink [38]. This creative example of biomaterial ink development encourages future investigation into under-explored crosslinking methods to propel further advances in extrusion-based bioprinting with sacrificial inks [39].
2.1.2. Support Baths for Channel Fabrication.
Gel-phase support baths (also referred to as suspension media) solve the challenge of print collapse encountered in early bioprinting work by physically supporting and confining deposited biomaterial inks during the printing process. When a support bath is used, the biomaterial ink is not printed into air, but rather directly within a reservoir containing the support bath (figure 2(C)). This unlocks three primary benefits [40]. First, support baths allow hollow structures to be directly, continuously, and omnidirectionally printed, thereby expanding the scope of accessible print geometries and resolutions. Unlike printing in air, which can lead to ink spreading and loss of resolution, printing within a support bath helps to keep the ink confined, so that the filament resolution can approach that of the needle diameter [8,40]. Additionally, support baths widen the range of mechanical properties suitable for biomaterial inks, bringing their printable window closer to that required for cell culture and expansion. What is more, support baths keep the printed materials hydrated, which improves post-print cell viability. Support baths must display a few unique mechanical properties, including shear-thinning and self-healing behaviors, to provide these benefits [40–43]. To allow the needle to move through the support bath, it must shear-thin and yield, allowing ink deposition. To confine and physically support the newly extruded filament, the shear-thinned region of the support bath must rapidly self-heal.
Interestingly, jammed microparticles often exhibit these characteristics and, as a result, many support baths are microparticle-based [44,45]. For example, gelatin microparticles have been used for freeform reversible embedding in suspended hydrogels (FRESH) [43]. When jammed, the gelatin microparticle slurry behaves as a shear-thinning Bingham plastic, making it an ideal support bath for 3D bioprinting. Furthermore, the FRESH approach has been shown to simultaneously support multiple different gelation strategies and enable printing of physiologically relevant coronary arterial trees [8]. Microgels of a crosslinked polyacrylic acid copolymer, commonly referred to as carbomer or Carbopol, have also been used as a granular support bath for extrusion-based bioprinting. While this copolymer is primarily marketed as a thickening agent for surfactant systems, such as shampoo and body wash, it has found applications in fields such as drug delivery and bioprinting [46]. The versatility of Carbopol microgels as a support bath for 3D printing and bioprinting was demonstrated by creating complex structures, such as a vascular-mimetic branching tree, from a plethora of different inks, including polyvinyl alcohol (PVA), polyacrylamide, poly(ethylene glycol) (PEG), HA, alginate, and collagen, with fine resolution [42]. This work was recently expanded upon through the bioprinting of vascular channels from synthetic bioelastomer prepolymers into a Carbopol support bath [47], further demonstrating the specific utility of Carbopol support baths for microvascular bioprinting. Pluronic F-127, while commonly used as a sacrificial ink, has also been used as a support bath. In fact, because of the similarity in their purpose, namely physical support and facile removal post-print, many materials used for sacrificial inks are also used as support baths. This crossover is exemplified by the printing of Pluronic F-127 as a sacrificial ink within a photocurable support bath of Pluronic F-127 diacrylate for the formation of microvascular networks [25]. Another example is the use of gelatin microgels, often utilized as a support bath, as a sacrificial component to control void space within a GelMA microgel ink [48]. The granular support baths discussed herein are made from diverse biomaterials using a variety of fabrication methods known to influence microgel properties [49,50]. Yet, because of the shared characteristics inherent to jammed microgels, each satisfies the mechanical requirements for use in 3D bioprinting [44,50]. As a result, the development of new granular hydrogels for both support baths and biomaterial inks has become an area of active research [45,49,51].
2.1.3. Coaxial Extrusion Systems for Channel Fabrication.
Coaxial extrusion systems enable the fabrication of perfusable channels by directly extruding hollow filaments of biomaterial ink from concentric nozzles (figure 2(D)). This often circumvents the need for extraneous sacrificial inks or support baths, but inherently struggles with making networks of branched, interconnected channels. One common approach for coaxial extrusion simultaneously prints alginate-based biomaterial inks with a calcium solution that crosslinks the alginate in situ to form a solid shell [52,53]. Alginate has also been blended with other biomaterials, such as gelatin methacryloyl (GelMA) and poly(ethylene glycol)-tetra-acrylate (PEGTA), to form perfusable geometries through coaxial extrusion [54]. In this system, alginate enabled the creation of hollow channels via coaxial extrusion with calcium, while GelMA and PEGTA allowed for post-print stiffening upon exposure to UV light. Interestingly, because of the shape fidelity conferred by coaxial extrusion via immediate crosslinking, alginate-based sacrificial inks have also leveraged coaxial extrusion with calcium to improve resolution. Meanwhile, sacrificial inks are sometimes used in coaxial extrusion to provide additional support to the hollow filament during printing. Coaxial extrusion systems have additional benefits for vascular biofabrication. For example, coaxial nozzles allow for the direct printing of layered and hollow filaments that better mimic the stratification seen within the wall of higher-order vessels. This is exemplified by recent work that used a coaxial arrangement of catechol-functionalized GelMA and smooth muscle cells surrounding a sacrificial crosslinking slurry of Pluronic F-127, sodium periodate, and ECs [55]. This overlap between techniques demonstrates the adaptability and user-friendly nature of sacrificial inks, gel-phase support baths, and coaxial extrusion systems for creating vascular-mimetic hollow channels using extrusion-based bioprinting.
2.2. Biomaterial Strategies for Lumen Endothelialization
While the techniques discussed above enable the creation of vascular-mimetic (hollow) geometries, these hollow channels themselves are not enough to provide the foundation needed for later function; a key characteristic of the native vascular lumen is a monolayer of homogeneously dispersed ECs. This endothelial monolayer is essential for vascular function [17,18]. Therefore, after successfully creating stable, perfusable channels using extrusion-based bioprinting, it is essential that the channels are not only seeded with ECs, but also biofabricated in a way that promotes the formation of a healthy, cohesive endothelial lining from the seeded cells. The predominant method for endothelializing a print fills the hollow channels with an EC suspension and relies upon cellular adhesion to and proliferation along the luminal surface (figure 2(E)). Other techniques for introducing ECs, such as directly printing cells within a biomaterial ink, have been explored to a lesser extent. Several luminal characteristics like presentation of cell-adhesive ligands, substrate mechanics, and substrate topography – all quasi-2D considerations in this context – play a role in EC adhesion, which is the first step to achieving multicellular function and a stable endothelium.
Ensuring sufficient EC coverage along the bioprinted lumen is crucial to monolayer formation and is influenced by the incorporation of cell-adhesive ligands along the quasi-2D surface of the lumen. To that end, many commonly used biomaterial inks either naturally contain or are modified to present cell-adhesive ligands. For example, the naturally-derived biopolymer gelatin is often used as a biomaterial ink, sacrificial ink [33], and support bath [43], and contains collagen-derived cell-adhesive ligands. In contrast, synthetic materials like PEG and some natural biopolymers, including alginate, do not contain any known cell-adhesion sites. To enable cell adhesion, such materials are chemically conjugated with cell-adhesive peptides, such as the integrin-binding arginine-glycine-aspartic acid (RGD) sequence. Yet even with these cell-adhesive ligands, achieving sufficient cell seeding along vessels on the smaller length scale of our bioprinted spectrum can prove challenging. Recent work using photoablation to create capillary-scale features noted that seeding these small (5–20 μm in diameter) channels via perfusion was challenging and, in some cases, impossible [56]. In the future, ligand affinity and specificity could be tuned to ameliorate this issue. For example, cell-adhesive ligands with higher specificity for endothelial progenitor cells (EPCs) and ECs over other cell types, such as platelets and inflammatory cells, have been developed [57]. This work uncovers an interesting avenue for improving the reliability and success of EC adhesion to bioprinted channels. Additionally, several methods exist for spatiotemporally altering the presentation of cell-adhesive ligands post-print, which may prove useful for synchronizing or preferentially controlling EC binding. For example, spontaneous and site-specific covalent bonding between the small protein domain SpyCatcher and its reciprocal pair SpyTag has been used to temporally control the presentation of RGD within PEG hydrogels [58], and could be modified for bioprinting applications. Alternatively, cell attachment may be coordinated in time through the triggered presentation of pre-existing but previously obscured ligands [59]. Furthermore, it has been shown that integrin-binding ligand clustering can have a marked effect on cell adhesion and motility [60]. This has also been shown with ECs, where ligand clustering enhanced EC proliferation and formation of focal adhesions [61], important steps for endothelial monolayer formation. Additional methods to spatially control cell-adhesive ligand tethering in 3D, with the goal of promoting endothelial sprouting, will be discussed in section 3.1.3.
In addition to ligand presentation, the mechanical properties and topography of the lumen produced via extrusion-based bioprinting can have a lasting impact on not only cellular adhesion but eventual vessel integrity (topographical cues discussed in greater length in section 4.2). For instance, the mechanics of the vascular intima are intimately related to vascular health and disease, and intimal stiffening is known to promote vascular permeability [62] associated with atherogenesis [63]. Likewise, mechanical heterogeneity of the intima has also been implicated in this increased permeability. A study of 2D substrate mechanical heterogeneity using photopatterned methacrylated HA (MeHA) hydrogels found that substrates with heterogeneous stiffness impaired endothelial monolayer formation, increased intercellular gap size, and impaired cell-cell junction integrity [64]. This is not surprising, as 2D mechanical heterogeneity plays a role in other biological contexts, such as stem cell differentiation [65]. In addition to altering EC attachment and monolayer formation, substrate stiffness has been implicated in regulating the interaction between ECs and monocytes by altering endothelial expression of monocyte chemoattractant protein-1 (MCP-1), vascular cell adhesion protein-1 (VCAM-1), and intercellular adhesion molecule-1 (ICAM-1) [66]. Thus, design considerations at this initial stage of biofabrication can influence eventual function of constructed microvasculature. Despite this compelling connection, exploration into inherent mechanical microheterogeneity along a printed lumen remains under-explored to date. We will discuss the better-elucidated effects of topography on function in section 4.2.
3. Bridging the Gap Through Induced Sprouting
Native vasculature exhibits a characteristically hierarchical network architecture that controls blood flow and nutrient transport. As diameter changes along the vascular tree, the primary function of the vessel similarly changes. At one end, larger vessels serve to regulate blood flow, while at the other, beds of small-caliber capillaries maximize contact with the surrounding tissue for nutrient exchange. Even within the narrower scope of microvasculature (10–300 μm in diameter), creating a similarly hierarchical network is rarely possible using a single biofabrication technique. Previous efforts to create microvascular-mimetic structures using extrusion-based bioprinting have focused on the fabrication of constructs similar in size to mid-scale vessels. This is partly due to the range of print resolutions accessible with extrusion-based bioprinting, which is limited by physical constraints including the inner diameter of the printing nozzle and the resolution of the stepper motors on the printer. Because of this, extrusion-based bioprinting is well-equipped to rapidly generate vessel-like structures with diameters similar to arterioles and post-capillary venules (fabricated diameters of 100–300 μm), but struggles to create capillary-like structures (10–50 μm in diameter) [15,16]. Meanwhile, alternative techniques excel at creating capillary-scale vascular features, such as the use of biochemical and biophysical signals known to induce endothelial network formation. This approach relies upon the inherent ability of ECs to undergo sprouting morphogenesis when exposed to the proper signals, and has proven quite powerful for the creation of capillary-scale microvessels [67–69] (overview of angiogenic sprouting shown in figure 3(A–C)). However, achieving larger-caliber vessels, such as those attainable via extrusion-based bioprinting, remains challenging using sprouting-based techniques. The dearth of strategies capable of seamlessly transitioning from arteriole- and venule-scale to capillary-scale vessels represents a crucial rift in feature resolution that the biofabrication community must address to successfully create functional, hierarchical, and scalable microvasculature. Focusing on the second part of our three-part framework (section 1.3), we propose that an integration of mid-range, bioprinted vessel-like channels (section 2) with approaches to guide EC sprouting using biochemical and biophysical cues presents a promising method for bridging this resolution gap.
Figure 3. Biomaterials and biofabrication approaches to bridge the critical resolution gap through induced spouting.

Angiogenic sprouting progresses through well-orchestrated stages. (A) First, local endothelial cells become activated and initiate sprouting in response to an angiogenic signal. In this first step, endothelial basement membrane is degraded, endothelial junctions weaken, and a tip cell is formed. (B) Next, the liberated tip cell directionally migrates toward the angiogenic signal, followed by a leaky nascent vessel composed of proliferating stalk cells. (C) Finally, after sprout fusion, perfusion helps to restore a quiescent phalanx endothelial phenotype, basement membrane is deposited, and mural cells (such as pericytes, shown) mature and stabilize the vessel. (D) A host of biochemical and biophysical signals are known to direct angiogenic sprouting, including proangiogenic growth factor signaling, hypoxia, the presentation of cell-adhesive ligands, matrix stiffness, matrix density, and matrix degradation. Biomaterials and biofabrication-based approaches have been used to spatiotemporally control angioinductive cues (E-G). (E) Photochemistry allows the user to tether one or more bioactive molecules in complex spatial patterns within biomaterial constructs, with the ability to further control release. (F) Microfluidic channels are commonly used to create stable gradients of soluble signaling molecules. (G) Meanwhile, bioprinting may be used for the patterned deposition of multiple materials with different matrix stiffnesses or densities, tethered ligands or growth factors, or degradation profiles.
3.1. Inducing Endothelial Sprouting via Controlled Biochemical Signaling
The role of biochemical signaling in EC sprouting is well known from vascular biology and in vitro microfluidics-based models of vascularization [70–72]. Signals within the microenvironment, including growth factors, oxygen gradients, and cell-adhesive ligands, all play crucial roles in orchestrating vascular sprouting by regulating the signaling pathways responsible for sprouting morphogenesis (figure 3(D)). Incorporating these angiogenic signals into the microvascular biofabrication process can better mimic the physiological conditions conducive to hierarchical sprouting and capillary formation. While most of these strategies have not yet been used with bioprinting, they could, in theory, be readily integrated within the extrusion-based 3D bioprinting workflow.
3.1.1. Angiogenic Growth Factors.
A beautifully complex process, sprouting morphogenesis is mediated by several angiogenic growth factors, which are upregulated under stressful conditions such as hypoxia, inflammation, and ischemia [73]. The most potent of these factors, vascular endothelial growth factor (VEGF) stimulates vascular morphogenesis by upregulating signaling pathways responsible for lumen formation, endothelial and mural cell co-assembly, and tubulogenesis [74]. While these downstream effects are crucial to sprout formation, other processes (previously reviewed in-depth here [75,76]) are necessary for the stabilization and subsequent function of newly-formed vessels. Briefly, during sprouting angiogenesis, local basement membrane degradation allows plasma-derived proteins to form a provisional extracellular matrix (ECM) around the parent vessel that facilitates sprout extension led by a liberated endothelial tip cell (figure 3(A)). As endothelial stalk cells proliferate behind the tip cell, a leaky neo-vessel forms (figure 3(B)). This nascent vessel is then stabilized through perfusion, basement membrane deposition, and the recruitment of mural cells such as pericytes. This allows resident ECs to return to a quiescent phalanx cell phenotype (figure 3(C)). Later, as sprouts mature into a stable network, they are pruned and remodeled to establish the hierarchical structure characteristic of vasculature [73,75,76]. Despite VEGF being considered the master regulator of angiogenesis, delivery of this one factor alone is often insufficient to create stable sprouts and can result in the formation of leaky and disorganized vessels. Other growth factors work in tandem with VEGF to stabilize and mature the developing vessel. Most notably, platelet-derived growth factor (PDGF) [77] directs the recruitment of mural cells, which tighten EC-EC junctions through the production of angiopoietin 1 (ANG-1) [73,75,76]. Furthermore, fibroblast growth factor (FGF) is implicated in myriad angiogenic processes, including basement membrane degradation and EC proliferation in early angiogenesis, and matrix deposition and vessel maintenance in later stages [75]. The potency of these angiogenic signals has translated to in vitro models of vascularization, where methods to spatiotemporally control their signaling have been effectively used to direct sprouting morphogenesis [78]. Several of these methods are described in detail below.
Microfluidic Gradients.
Microfluidic devices are commonly used to form stable biochemical gradients that direct endothelial migration and sprouting (figure 3(F)). Specifically, ECs are known to migrate from low to high concentrations of angiogenic growth factors [79]. The slope of these microfluidic concentration gradients has also been shown to influence EC directionality and pathfinding [80]. Recently, emphasis has been placed on developing microfluidic platforms that allow for three-dimensional control over sprouting within extracellular matrices by establishing growth factor gradients through microfluidic channels [81]. To accomplish this, lithographically defined channels were embedded within hydrogels, where one central channel housed ECs and surrounding parallel channels served as a source and a sink for the selected growth factor [82]. Some of these platforms have been explicitly designed to facilitate image acquisition in order to evaluate sprout formation [83]. Recognizing that one angiogenic signal is often insufficient to achieve stable network formation, microfluidic platforms have been used that combine multiple growth factors. For example, collagen microfluidic channels seeded with ECs and stimulated with a combination of VEGF and FGF formed robust EC cords within 24–48 hours [84]. Furthermore, microfluidic channels have been used to create combined VEGF and ANG-1 gradients to direct sprouting morphogenesis. In this dual-growth factor system, the VEGF gradient played a chemotactic role while the ANG-1 gradient stabilized connections between tip and stalk cells [85]. The diffusion-based approach central to microfluidic signaling gradients is directly translatable to extrusion-based bioprinting. For example, sacrificial inks (see section 2.1.1) have been used to bioprint microfluidic channels with complex curved geometries that allowed for the formation of an angiogenic growth factor gradient [86]. Since it was also found that channel curvature influences the efficacy of growth factor gradients, extrusion bioprinting offers the added benefit of facile design and fabrication of curved channels with circular cross-sections compared to soft lithographic techniques used to fabricate microfluidic channels [86].
Sequestration within the Extracellular Matrix.
Another method for directing sprouting morphogenesis takes advantage of the native affinity between angiogenic growth factors and extracellular matrix (ECM) constituents, such as proteoglycans, glycosaminoglycans (GAGs), and proteins to achieve growth factor immobilization and controlled release. One common method uses heparin, a highly sulfated GAG composed primarily of uronic acid and glucosamine disaccharides that sequesters several growth factors in the ECM largely through electrostatic interactions [87]. Because heparin binding slows growth factor release and degradation, HA-based hydrogels chemically functionalized with heparin have enabled sustained dual-release of VEGF and FGF, which demonstrated an extended angiogenic response in vivo [87]. Sulfation-mediated growth factor sequestration by heparin has been mimicked using sulfated biomaterials, such as uronic acid-containing alginate, to achieve similarly controlled release of FGF [88]. Protein domains commonly found in the ECM, such as the 12th-14th type three repeats of fibronectin (FNIII12–14), have also been shown to bind a host of growth factors, including PDGF, FGF, and VEGF, without inhibiting activity [89]. This opens the door to leveraging protein domains and protein-based biomaterials for generalized growth factor sequestration and release. Additionally, some ECM protein domains bind growth factors with high affinity and greater but still moderate specificity, such as collagen with bone morphogenetic protein (BMP) and fibronectin with VEGF [74]. Many of the examples mentioned use materials commonly employed for bioprinting. Therefore, engineering biomaterial inks to incorporate specific binding domains may provide a more selective method of growth factor sequestration than heparin-functionalized materials, which bind to a wide array of growth factors and other proteins.
Degradable Microspheres.
Controlled growth factor release has also been achieved using degradable microspheres. This is especially relevant for growth factors that have a short half-life such as PDGF, which has a half-life of 30 minutes in circulating blood [90]. To prolong its bioactivity, PDGF has been loaded into poly(lactic-co-glycolic acid) (PLGA) microspheres, which formed a stable concentration gradient as the PLGA microspheres erode [77]. Additional methods for manipulating growth factor release include loading growth factors into microspheres exhibiting different degradation rates to independently tune release kinetics, or using microspheres that photothermally rupture at specific, non-overlapping wavelengths [91]. These methods have demonstrated the importance of temporal control for vascularization, where sequential delivery of growth factors important for early (VEGF and ANG-2) and late (ANG-1 and PDGF) angiogenesis enhanced vascularization over simultaneous delivery [92], presumably through more accurate mimicry of native cues. These methods also allow for remote and spatiotemporal control over local growth factor concentrations known to influence sprouting and vascularization, and can be modularly incorporated into biomaterial inks.
Patterned Immobilization and Release.
Growth factors can also be directly attached to hydrogel scaffolds via chemical immobilization. For example, PDGF and FGF have been simultaneously tethered within 3D PEG hydrogels, which showed synergistic enhancement of cell migration [90]. To confer greater spatial control over where sprouting occurs, photopatterning can be used to direct vascular morphogenesis with angiogenic growth factors (figure 3(E)). In one study, VEGF was covalently immobilized onto the surface of PEG gels in defined micron-scale patterns using laser scanning lithography. In this case, endothelial tubules formed only in areas of patterned VEGF [93]. Interestingly, not only can chemical immobilization enable spatially controlled attachment of growth factors to the ECM, but it can also allow for user-defined, often photolytic release of tethered growth factors. Many of these sophisticated approaches involve direct modification of biomaterials using more intricate chemical methods but offer high returns in the form of both spatial and temporal (termed “4D”) control of patterning in 3D cultures through specific and reversible reactions. One such 4D approach used sortase A, a bacterial-derived enzyme that performs site-specific and reversible transpeptidation at a defined sortase recognition sequence. This enzymatic conjugation allowed for the installation of bioorthogonal reactive handles capable of tethering growth factors to appropriately modified biomaterials in 3D. Using this approach, tethered angiogenic growth factors such as FGF and epidermal growth factor (EGF) were released using multiphoton laser-scanning lithography in a spatially controlled manner, allowing for downstream signaling to encapsulated cells [94]. Another reversible, photomediated reaction involving thiol-ene bioconjugation of signaling proteins to hydrogels allowed for sequential tethering and release of multiple growth factors (such as transforming growth factor, or TGF) with 3D control [95]. There is also potential for photocaged chemistry to be used for enzymatic and site-specific tethering or release of growth factors within hydrogels [96]. While these studies did not explicitly examine vascular morphogenesis, they offer promising proof-of-concept for the potential chemical modification of biomaterial inks to form growth factor patterns that facilitate endothelial sprouting.
Engineered Growth Factors.
In addition to engineering material scaffolds that bind and release growth factors, growth factors themselves can be engineered to have increased affinity to the ECM and controlled release over time. These ‘super affinity’ growth factors have been constructed by fusing a heparin-binding domain from placenta growth factor (PGF-2123–144) that promiscuously binds ECM constituents to VEGF and PDGF-BB. The resulting pro-angiogenic growth factors demonstrated enhanced affinity for the ECM and induced greater tissue healing in a rat wound model than their unaltered counterparts [97]. By flipping the traditional, materials-focused protein delivery paradigm, this approach highlights the methods by which angiogenic growth factor signaling may be precisely engineered in bioprinting-compatible materials.
3.1.2. Oxygen Gradients and Hypoxia.
Hypoxia is a potent biochemical cue that induces the release of pro-angiogenic signals and other regulators of vascularization through hypoxia-inducible factors (HIFs) including HIF-1α and HIF-2α [98]. These transcription factors target genes encoding growth factors such as VEGF, PDGF, and PGF (section 3.1.1) in addition to matrix metalloproteinases (MMPs) and vascular endothelial (VE) cadherin (VE-cadherin), altering their activity in hypoxic conditions and ultimately resulting in increased angiogenic sprouting. Various methods have been used to observe how oxygen gradients influence EC migration, such as microfluidic channels. One study using microfluidic channels that allowed for control of gas exchange found that hypoxic conditions reduced VE-cadherin expression by ECs, disrupting these crucial cell-cell contacts and facilitating cell migration for vascular tube formation [99]. In addition to microfluidic oxygen gradients, other work has used prolyl hydroxylase (PHD) inhibitors to induce hypoxia [98]. These inhibitors stabilize HIF complexes, thus chemically inducing a hypoxic response by encapsulated cells, and could be an avenue for further exploration. Along these lines, a novel class of biomaterials – hypoxia-inducible hydrogels – has been developed with the purpose of introducing finely controlled oxygen gradients within ECM-mimetic material systems [100]. These hypoxia-inducible hydrogels were composed of gelatin and dextran polymer backbones crosslinked via a laccase-mediated reaction that consumed oxygen and allowed the duration of hypoxia to be controlled [101]. Using the laccase-mediated crosslinking was found to upregulate MMP-1 expression, which allowed matrix degradation-mediated EPC cluster formation reminiscent of vasculogenesis [102]. In contrast, biomimetic materials have been designed that release oxygen over time [103]. Incorporating these materials and techniques into bioprinted constructs could potentially enable the spatiotemporal formation of oxygen gradients along which directed sprouting morphogenesis could occur.
3.1.3. 3D Cell-Adhesive Ligand Patterning.
The mechanisms underlying EC sprouting and lumen formation rely not only on growth factor signaling and oxygen tension, but also on cell-matrix interactions. What is more, cellular attachment to the surrounding ECM is necessary for growth and motility of diverse cell types in both 2D and 3D contexts. Often mediated by integrin engagement, this important cell-matrix interaction can be controlled through the presentation of cell-adhesive ligands within a biomaterial [104–106]. The biomaterials community frequently leverages cell-adhesive ligand concentration, location, and identity to guide cellular attachment and migration [107]. For example, techniques have been used to photopattern three-dimensional tracks of the RGD ligand within hydrogels, demonstrating the utility of this approach for guiding cell migration within 3D biomaterials [108,109]. Beyond motility, other processes crucial to sprouting morphogenesis are also guided by cell-adhesive ligand engagement. For example, lumen formation is an integrin-dependent process that progresses via intra- and intercellular endothelial vacuole fusion [110]. While integrin engagement in general is essential for lumen formation, the specific integrin requirements depend on the surrounding matrix. Previous reports demonstrated that engagement of α2β1 integrin was necessary in collagen matrices [111], while αVβ3 and α5β1 engagement was required in fibrin matrices [112]. In more recent work, 3D HA hydrogels containing recombinant fibronectin fragments that preferentially engaged α3β1 and α5β1 integrins produced space-filling, non-leaky microvascular networks, while hydrogels that preferentially engaged αVβ3 produced networks that were leaky and pathological [113]. Interestingly, hydrogels containing RGD ligands without a proline-histidine-serine-arginine-asparagine (PHSRN) synergy domain developed similarly pathological networks. Further, α5β1 has been shown to modulate endothelial response to ANG-1 through interaction with the ANG-1 receptor Tie2 [114]. The context dependent role of integrins in endothelial sprouting highlights a need for further exploration with biomaterials that can precisely control cellular attachment. Taken together, these findings underscore the power of cell-adhesive ligands not as mere sites for cell attachment, but as potent biochemical morphogens that can be leveraged to shape sprouting morphogenesis. Thus, biomaterials – and biomaterial inks – with precise control over cell adhesion could be important in creating functional microvasculature.
Many of the materials and approaches discussed above are made from precursors that have been previously printed via extrusion-based bioprinting or could be used to modify existing biomaterial inks (figure 3(G)). Integration of these ideas for controlled biochemical signaling with extrusion-based bioprinting could be a first step toward bridging the resolution gap in biofabricated microvasculature.
3.2. Modulating Sprouting Using Biophysical Cues
During sprouting morphogenesis, the impact of biochemical cues, such as growth factor signaling and cell-adhesive ligand presentation, is tightly linked to the biophysical context experienced by migrating and proliferating ECs. For example, the interplay between matrix mechanical properties and cell-adhesive ligand identity has been shown to dictate the extent of network formation on 2D substrates [115]. Similarly, EC mechanosensing alters VEGF signaling in vascular development [116]. Matrix mechanics have also been implicated in vascular integrity in vivo [62], and ECs have been shown to stiffen their surrounding matrix during sprouting [117]. Biomaterials-based strategies have been used to explore the role of myriad matrix properties in EC sprouting in vitro. From this work, three primary material physical parameters have been identified as regulators of vascularization, namely matrix mechanics, its counterpart matrix density, and matrix degradation. Knowledge about matrix stiffness, density, and degradation may be leveraged to induce and control sprouting morphogenesis in bioprinted microvascular constructs.
3.2.1. Matrix Mechanics.
Previous work exploring the effect of 2D substrate stiffness on EC network formation identified a clear pattern, wherein compliant substrates generally promoted better sprouting. 3D matrix mechanics, while important to vascular development, have a somewhat more complex role in sprouting morphogenesis. Biomaterials and biofabrication strategies have been employed to control the spatial patterning of matrix mechanics in 3D culture to further elucidate this relationship. Stereolithographic 3D printing has been used to create PEG-based constructs with patterned bulk mechanical stiffness by altering the extent of photocrosslinking [118]. Similar results may be achieved with extrusion-based 3D printing. For example, biomaterial inks possessing different mechanical properties can be simultaneously deposited to pattern matrix mechanics. Alternatively, post-fabrication methods can be used to spatiotemporally alter mechanical properties. In one example, PEG-based hydrogels formed via thioester bonds enabled the photouncaging of a catalyst that accelerated the rate of material stress relaxation in the presence of mesenchymal stem cells (MSCs) [119]. This could provide a non-degradative means to enable patterned, cell-mediated matrix remodeling and highlights the kind of materials that are poised to impact vascularization in the future. Recently, viscoelasticity has been implicated as a driver of many cell responses in 3D culture [120]. While some have recognized the viscoelastic nature of natural biopolymers and begun to explore the role of viscoelasticity in EC sprouting [102,121], this remains under-explored in the context of vascularization.
3.2.2. Matrix Density.
Due to its biological relevance, many groups have explored the density of fibrillar biopolymer-based matrices, such as collagen, as a modulator of sprouting morphogenesis. Results demonstrated that reduced matrix density – to a point – is more permissive to EC proliferation and migration, leading to more robust network formation. If density is too low, however, the material cannot support network formation [81,122,123]. Multimaterial printing can leverage matrix density in two ways. First, using sequential deposition of unique inks through different extruders, it is possible to pattern distinct biomaterials with different matrix densities and mesh sizes, similar to work using molded or cast hydrogels [81,122,123]. Second, altering matrix density in 3D can be achieved through microfluidic mixing of hydrogel precursors [124], which has been adapted to extrusion-based printing using custom-build microfluidic extruders [10,125,126]. Because matrix stiffness and matrix density are tightly interrelated and impact printability, post-printing approaches have also been developed to independently tune matrix stiffness and density, such as the non-enzymatic glycation of collagen matrices [127]. Expanding upon this idea, selective stiffening in 3D fibrin hydrogels has been used to enable user-defined spatial patterning of stiffness independent of matrix density within a pre-formed hydrogel [128]. These techniques demonstrate ways to pattern matrix stiffnesses and densities relevant to endothelial sprouting that are compatible with extrusion-based bioprinting.
3.2.3. Matrix Degradation.
The effects of matrix stiffness and density on sprouting morphogenesis are closely linked to EC-mediated proteolytic degradation of the surrounding ECM. The relationship between these matrix characteristics is readily apparent in materials with sufficiently high matrix density, wherein ECs struggle to degrade the matrix enough to facilitate migration. As a result, the migration of tip cells is slowed and the balance between tip cell migration and stalk cell proliferation is disrupted [122]. This example demonstrates how crucial protease expression and resultant material degradation are for sprouting morphogenesis. Meanwhile, matrix mechanics themselves are thought to regulate the production of matrix metalloproteinases (MMPs), and matrix remodeling has been shown to control the mode of EC migration in 3D [129]. For example, membrane type 1 matrix metalloproteinase (MT1-MMP) has been shown to be required for lumen and tube formation in 3D collagen matrices. Using MT1-MMP, ECs proteolytically carved out vascular guidance tunnels, quasi-2D surfaces along which they could later migrate in an MMP-independent manner [130]. Many synthetic materials exist for controlling cell-mediated matrix degradation in vitro [131]. Using these, methods have been developed to pattern the degradation landscape within 3D materials. For example, hydrogels formed from an MMP-cleavable backbone have been made selectively nondegradable by the patterned crosslinking of a kinetic chain [132,133]. This approach allows the user to define areas that are permissive to cell remodeling and areas that are not. Because the signaling involved in sprouting morphogenesis is so well understood, it provides an excellent application of triggerable and smart biomaterials that can be engineered to degrade in response to combinations of environmental stimuli [134]. While relying upon proteolytic degradation may mimic the native mechanisms of sprouting morphogenesis, materials may be selectively degraded by other means, such as photoablation or photolysis. These light-based approaches are fast and allow for user-defined patterning of otherwise cell-directed morphogenetic processes [56,135,136].
Biophysical properties of the matrix are clearly implicated in vascularization, and this relationship is bidirectional. For example, sprouting morphogenesis has been shown to lead to local ECM stiffening around neovessels in 3D fibrin gels [137]. Interestingly, different transient EC phenotypes have differing contributions to this effect. Tip cells were recently shown to be the primary contractile unit in a sprout and cause mechanical deformations through non-muscle myosin IIA-mediated contractility [138]. It will be important to account for not only the biochemical but also the biophysical signaling landscape experienced by ECs within a bioprinted construct and tune this to achieve the desired effect. Because unique EC phenotypes and sources have similarly unique influences over these signaling landscapes, the source of ECs can play a major role in engineering sprouting morphogenesis within bioprinted microvascular constructs.
3.3. Accounting for Endothelial Cell Heterogeneity
The functional capability and gene expression of ECs used in this phase of sprouting morphogenesis, as well as initial endothelialization (section 2) and later functional maturation (section 4), is crucial for determining success. There is clear evidence of temporal (as in angiogenesis, figure 3(A–C)) and tissue-specific phenotypic heterogeneity between populations of ECs from vascular and developmental biology [139,140] and the mechanism for this heterogeneity is an active area of research [141,142]. While EC phenotype is often overshadowed by biofabrication technologies in the bioprinting community, it is of the utmost importance when using biomaterials- and biofabrication-based approaches to precisely engineer the endothelial niche and align it to the endothelial source. Without this consideration, efforts to induce important stages in microvascular network formation that depend upon transient EC phenotypes, such as stalk and tip cell specification during sprouting morphogenesis or the adoption of a quiescent phalanx phenotype to promote maturation, will be misguided. Recognizing this, studies have explored the response of different EC subsets to microenvironmental cues. We can find some hope in work comparing the response of different EC populations to changes in substrate stiffness, wherein diverse ECs followed similar, though broad, behavioral trends in response to stiffness [143]. For example, ECs of different types generally formed a more cohesive monolayer on physiologically relevant stiffnesses and exerted higher traction forces on stiffer surfaces [144]. Despite this, it remains clear that different EC types exhibit marked differences in gene expression [144]. In one study of immortalized versus primary murine cardiac ECs, it was found that many canonical endothelial markers were downregulated in immortalized EC populations [145].
Much of our understanding of EC biology has relied upon the use of human umbilical vein endothelial cells (HUVEC) due to the accessibility of primary tissue. While HUVEC represent a reliable endothelial model, it is important to consider how thoroughly this fetal, immune-privileged cell source recapitulates the functions under study [146,147]. Work has been done to derive ECs from stem cell sources [148–151], thereby providing a promising alternative cell population for in vitro models of vascularization. While stem cell-derived ECs come with their own set of challenges, they expand the family of different ECs available for in vitro studies [152–154]. For example, different progenitor populations have been used that give rise to not only ECs, but also mural cells such as pericytes and vascular smooth muscle cells (vSMCs) [155]. In one demonstration of this idea, mesodermal progenitors incorporated within tumor or neural spheroids gave rise to blood vessels that were hierarchical and displayed appropriate mural cell populations [156]. The development of stem cell-derived ECs also allows for the use of tissue-specific endothelial phenotypes that not only play crucial roles in tissue development and regeneration in vivo [157–160], but also exhibit different behaviors in vitro [161]. More work is necessary to fully characterize the consequences of endothelial phenotypic heterogeneity for biofabricated microvasculature. Nonetheless, the continued advancement of microvascular models is an important endeavor for tissue engineering and disease modeling. The responsibility lies with the fabricator to match the cell source and microenvironmental cues accordingly.
4. Conditioning of Biofabricated Microvasculature to Promote Maturation
Once a construct has been fabricated (section 2) and a capillary-scale network has been formed through sprouting (section 3), additional tissue conditioning will still be required to mature the nascent vessels and create stable microvasculature. Without this conditioning step, in vitro endothelial sprouts are prone to regress over time [162]. Vascular maturation, like endothelial sprouting, is regulated by the interplay of a host of different biochemical and biophysical signals. To achieve truly mature microvasculature, in vivo drivers of vascular maturation, such as different stromal cell types and external mechanical forces, must be incorporated into biofabricated constructs with precise spatial control. Here, we describe a variety of ways in which these stabilizing components may be engineered into biofabricated microvasculature. The approaches discussed are both compatible with extrusion-based 3D bioprinting and help actualize the final goal of our three-part framework: engineering mature microvasculature with biomimetic function. Specifically, we discuss the addition of mural and other cell types to provide crucial paracrine signaling and heterotypic cell contact (figure 4(A)); sources of substrate heterogeneity, focusing on biomaterial topography (figure 4(B)); and the application of external mechanical forces, notably fluid shear stress, to condition engineered microvasculature with an eye towards the contribution of each of these on vascular integrity and maturation (figure 4(C)).
Figure 4. Conditioning microvascular constructs to promote stability and maturation.

Several methods exist for conditioning microvascular networks, including: (A) the use of co-culture systems that mirror the heterotypic cell-cell signaling inherent within the vascular niche, (B) heterogeneous substrate cues, and (C) the use of fluid flow to mechanically condition the network. Topographic patterns are one aspect of substrate heterogeneity that can be fabricated through a variety of methods (D-F). (D) Soft lithography can be used to make simple gratings, hierarchical gratings, convex microlenses, concave microlenses, pillars, and holes at the micro- and nanoscale. (E) Electrospinning can create nanofibers aligned parallel or perpendicular to the seeded cells, or not aligned at all. (F) Based on print resolution, the printed construct can contain grooves and surface features that influence cell alignment, migration, and adhesion similarly to the patterns formed in (D) and (E).
4.1. Co-Culture Systems to Enhance Microvascular Maturation
In native microvasculature, interactions between endothelial and mural cells, including pericytes and vascular smooth muscle cells (vSMCs), are important for regulating vascular morphogenesis and function (figure 4(A)). Several co-culture systems have been shown to stabilize emergent vascular networks and improve construct function. This later resolution stage of vascularization requires suppression of EC proliferation, stabilization of newly formed vessels, and the deposition of a basement membrane, all of which are critically enhanced by mural cell recruitment (figure 3(C)) [163]. Due to the hierarchical diversity of vasculature, relevant mural cell types differ along the vascular tree. Thus, the specific mural cell type incorporated into a co-culture model can greatly influence sprouting morphogenesis and resolution into a stable network. For example, while MT1-MMP has been shown to play a crucial role in EC network formation in collagen hydrogels [130], different proteolytic mechanisms may be required for capillary formation depending on the presence of either MSCs or fibroblasts [164]. Further, despite the generally enhancing effect of stromal cells on EC-mediated network formation, various populations of stromal cells have been shown to differ in their angioinductive capabilities [165]. Here, we describe several cell populations that have been used to influence vascularization in vitro, with the goal of incorporating multiple cell types into bioprinted vascular constructs to improve network formation and stability.
4.1.1. Pericytes and Pericyte-Like Cells.
Pericytes are the primary mural cell in microcirculation and are crucial to both the maturation and maintenance of microvascular function [166]. As such, pericytes or pericyte-like cells may be incorporated into in vitro models of vascularization to better mimic native microvasculature. Because of the importance of pericytes for vascularization, attempts have been made to create better-defined pericyte populations [167], but debate continues to surround both their post-natal origin and the scope of their functions in vivo. Pericytes are commonly identified through a combination of: (1) the expression of gene products such as PDGF receptor beta (PDGFRβ), neural-glial antigen 2 (NG2; also called chondroitin sulfate proteoglycan, or cspg4), and alpha smooth muscle actin (α-SMA); (2) morphology characterized by the extension of primary, and perpendicular secondary, cytoplasmic processes along the outer surface of EC tubes; and (3) a unique location embedded within the vascular basement membrane [168]. Pericytes, in concert with ECs, are important for neovessel stabilization by inhibiting both further tubulogenesis and tubule regression [169]. Beyond active sprouting morphogenesis, pericytes have been shown to directly influence the permeability and diameter of established vessels [170,171]. The interaction between ECs and pericytes is bidirectional, with recent reports demonstrating that ECs have a significant impact on pericytes, influencing transcriptional and behavioral changes that, in turn, alter EC function [172]. The inclusion of pericytes within engineered microvascular constructs has several advantages, including the promotion of endothelial sprout formation and stabilization. In these co-culture systems, pericytes and ECs are often both distributed randomly throughout the hydrogel, and vasculogenic mechanisms are leveraged to form microvascular networks [173]. This is quite straightforward from a biofabrication standpoint and mimics the co-recruitment of ECs and pericytes seen in native vascular biology. What is more, a similar co-culture could be readily recapitulated using extrusion-based 3D bioprinting by co-printing ECs and pericytes in the same or separate bioinks. Because multimaterial bioprinting with cell-containing inks remains underexplored, this would represent important progress in the field.
A number of pericyte-like cells have been reported to improve angiogenesis in vitro, including mesenchymal stromal cells (MSCs) and adipose-derived stromal cells (ASCs) [174–176]. Similar to pericytes, ASCs and MSCs influence EC sprouting and microvascular function through both paracrine signaling and direct cell-cell contacts [177–180]. Despite uncertainty surrounding the definitive identification of pericytes and their relationship to MSCs and ASCs, it is clear that pericytes and pericyte-like cells can play a powerful role in promoting vessel maturation and subsequent function.
4.1.2. Vascular Smooth Muscle Cells.
Within pre-capillary arterioles and post-capillary venules, the endothelial basement membrane is surrounded by concentrically arranged vSMCs. This close association allows for bidirectional crosstalk between ECs and vSMCs that has been shown to aid vessel function [181,182]. EC-vSMC paracrine signaling underlies healthy vascular function, and models of arterioles and venules often leverage this innate signaling through co-culture to promote in vitro function [183]. Biofabrication techniques can be used to directly mirror the unique arrangement of vSMC and endothelial compartments within the vessel wall. For example, coaxial extrusion systems, previously discussed for hollow channel formation (section 2.1.3), have been used to directly print a shell of vSMC-containing biomaterial ink within which ECs may be seeded [52,55]. But vSMCs do not only respond to paracrine signals. Like ECs, vSMCs are mechanosensitive and respond to matrix stiffness through transforming growth factor beta (TGF-β) [184]. Interestingly, TGF-β is also a primary player in vSMC to EC paracrine signaling in low shear stress conditions [182], highlighting both the potency of vSMCs as function-promoting mural cells and the necessity to carefully tune the vSMC niche in bioprinted microvasculature.
4.1.3. Fibroblasts.
In native vasculature, fibroblasts reside within the adventitia or outer layer of larger diameter vessel walls. Interestingly, there has long been a link between vascular cell populations and adipose tissue, with many positing that adipocyte progenitors (APs) responsible for the expansion of adipocyte populations were derived from one of the several cell types present in the vascular wall. Recent work has shed light on this, identifying adventitial and capsular fibroblasts as the predominant APs in vivo [185]. While intriguing, this raises unanswered questions about the role of fibroblasts, often considered a robust and multifunctional cell type, in vascular biology. Many models exist that allow for these new questions to be explored in a variety of 3D culture platforms [162,186,187] that are complementary to extrusion-based bioprinting [30].
4.1.4. Tissue-Specific Cells.
While many of the previous examples focused on the creation of generalized models of microvasculature, the true goal of tissue engineering is to create the functional units of a specific tissue, on demand. Given the inherent heterogeneity of ECs with respect to physiological location and time (section 3.3), and the myriad tissue-specific cell types with which they interact, the creation of functional and tissue-specific vasculature will require studying the interactions between ECs and the cells of that tissue. This has recently become an area of great interest with the development of more physiologically relevant organotypic culture platforms such as microphysiological systems and organoids. For example, one unique niche that necessitates co-culture for accurate in vitro modeling is the neurovascular unit. This vascular niche exhibits precise spatial coordination of soluble and physical heterotypic cell-cell interactions and uses these to dictate the permeability of the blood-brain barrier (BBB) [170,188]. Because of the need for models that elucidate neurological diseases, and the importance of brain vasculature and the BBB to neurological health and treatment of disease, there has been growing interest in finely characterizing markers for and the localization of vascular cells in the brain [189,190]. What is more, co-cultures of ECs and neural cell types have been explored as models of neurological development and disease [191–193]. The function of skeletal muscle is also closely tied to vascular function and is a co-culture system ripe for exploration. In one study, ECs seeded along the lumen of a microchannel were cultured in soluble contact with myofibers of C2C12 myoblasts. In response to optically triggered muscle training, ECs exhibited enhanced sprouting toward the myofibers while reciprocally enhancing myogenesis [194]. This innate linkage between vascular and tissue function is also exhibited in the lungs. While tissue-specific pulmonary capillary ECs have been shown to guide lung alveolarization [157], pulmonary pericytes have also been shown to impact postnatal lung morphogenesis [195]. These examples highlight the tissue-specific heterogeneity of both ECs and pericytes [196]. With greater ability to engineer more complexity into bioprinted constructs, it becomes increasingly possible to generate tissue-specific vasculature in vitro.
4.2. Material Topography and Bioprinting Features
One form of substrate heterogeneity, material topography (figure 4(B)) is tightly linked to one of the most important markers of vascular health, vessel barrier function [197]. While controlling and fine-tuning material topography can incidentally alter matrix mechanical cues (such as those discussed in section 3.2), there exists an explicit connection between surface topography and EC function. This link originates from the native basement membrane [198], where the presence of micro- and nano-scale features on subendothelial surfaces has been found to influence a range of EC behaviors, including morphology, adhesion, migration, proliferation, and alignment [198]. In the context of vascular function, vessel integrity is compromised under conditions of highly variable topography via disruption of EC-EC adherens junctions [199]. Meanwhile, more ordered topographical features, such as aligned material fibers, enhance monolayer integrity by promoting EC alignment and formation of cell-cell contacts [200]. Crucially, surface topography enhances endothelial response to lateral shear stress from fluid flow [201] (discussed in section 4.3, below), and may play a more influential role in vascularization than certain biochemical cues previously found to direct EC motility [202] (discussed in section 3.1).
4.2.1. Lithographic Patterning.
Lithographic biofabrication techniques have been used to create a variety of micro- and nano-patterned 2D surface topographies that have shown profound effects on vessel barrier function. In particular, soft lithography has been used to generate at least five different classes of topographies on polydimethylsiloxane (PDMS) chips, including gratings (micro- and nanoscale), hierarchical gratings, microlenses, pillars, and holes (figure 4(D)) [203]. Lithography has also been used to control the width of grooves or ridges in the formed patterns, down to the hundred-nanometer scale [204]. To achieve even finer surface features at the ten-nanometer scale, sputter coating and acetone etching of polystyrene sheet molds has been used to create random and anisotropic nanoscale grooves on PDMS [205]. These different patterning techniques affect EC behavior [206], gene expression [204], focal adhesion formation, and actin polymerization [207] – all important aspects of vessel barrier function. What is more, specific topographic classes have been shown to elicit different phenotypic and functional responses from unique EC types. For example, a subtype of hierarchical gratings was shown to skew progenitor cells towards an arterial phenotype and function [203]. Meanwhile, gratings of nano-grooves on silicon wafers with a 400 nm pitch disrupted expression of cell cycle and ECM proteins in HUVEC compared to surfaces with longer pitched grooves [204]. Further, embryonic stem cell-derived ECs have been shown to robustly align on anisotropic nanoscale grooves, but not on random topographies [205]. Taken together, these examples provide powerful evidence that user-defined 2D topography alone can bias engineered vessels toward a desired phenotype.
4.2.2. Electrospun Fiber Alignment.
Manipulating material topography and alignment in a 2D or quasi-3D context is possible through electrospinning (figure 4(E)). Nanoscale features of fibrous electrospun materials have been shown to influence proper function of vascular constructs by directing EC behavior and phenotype. For example, electrospun elastin-like protein (ELP) fabrics with tunable fiber width showed that variable topography disrupted VE-cadherin-based cell-cell contacts and activated EC migration and proliferation [199]. In addition to impacting vascular integrity, this highlights that biomimetic surface topography may modulate the balance between the quiescent phalanx EC phenotype seen in stable vessels and the activated (tip and stalk) phenotypes involved in sprouting and migration. Moreover, HUVEC seeded on electrospun fibers showed directional migration along VEGF gradients parallel to fiber alignment, but not along VEGF gradients that ran perpendicular to the fibers, suggesting that topographically induced signals for EC migration can override chemotactic gradients [202]. Fiber alignment may also enhance the effect of conditioning cues, such as fluid shear stress, and has been shown to promote greater vessel formation than unaligned substrates [200].
4.2.3. Bioprinting Features.
While not often discussed, extrusion-based 3D printing can generate topographical features similar to those introduced by electrospinning and lithographic approaches (figure 4(F)). These features are most apparent on exposed surfaces and result from the physical realities of layer-by-layer deposition with a finite layer height. As such, they are impacted by printing parameters including print speed, vertical and lateral resolution of the printer, size of the printing nozzle, layer height, and properties of the extruded material, to name a few [208–210]. Interestingly, while the effect of print parameters on vertical resolution, surface smoothness, and build time of 3D printed, thermoplastic constructs has been analyzed [209–211], their effect on bioprinted constructs has been largely overlooked. Given the abundance of literature implicating surface topography in altered vascular integrity, the topographical features introduced during printing should be considered when optimizing the function of 3D bioprinted microvasculature. What is more, printed layer properties particularly pertain to inducing vascular morphogenesis, as incomplete fusion between printed layers and filaments may generate spaces or voids into which ECs can infiltrate, adhere, and grow. How this phenomenon relates to EC-EC communication, monolayer formation, barrier integrity, and growth requires further study and consideration.
4.3. Construct Conditioning via Fluid Flow
Hemodynamic forces play an essential role in shaping native vascular development and function (figure 4(C)) [212–215]. The same holds true in vitro, where models of vascularization have helped to characterize EC mechanotransduction in response to fluid shear stress [216,217], hydrostatic pressure [218], interstitial flow [219], and cyclic stretch [220]. Cyclic stretch, for example, has been shown to alter autocrine and paracrine signaling between ECs and surrounding mural cells, such as vSMCs [221]. This finding highlights the immense crosstalk that occurs between microenvironmental signals – such as hemodynamics, matrix cues, and soluble biochemical signals – in driving EC behaviors. In general, interstitial flow and hemodynamic pressure are reported to improve sprouting angiogenesis [218,219], while fluid shear stress is reported to assist with maturation of nascent tubes [216,217,219].
4.3.1. Shear Stress.
Of the mechanical forces exerted on the endothelium as a result of blood circulation, wall shear stress has been the most widely explored. The process of EC mechanosensing in response to shear stress further emphasizes the crosstalk that exists between different signals discussed thus far. For example, while integrins are crucial for vessel endothelialization (section 2.2) and sprouting morphogenesis (section 3.1.3), they also play a large role in EC response to shear stress [222]. Specifically, exposure of ECs to atheroprone flow was demonstrated to activate the integrin subunit α5 by translocation to membrane lipid rafts, which is implicated in the EC dysfunction thought to promote atherosclerosis. Meanwhile, knockdown of integrin α5 has been shown to partially mitigate this EC dysfunction [223]. Upstream of integrin signaling, platelet endothelial cell adhesion molecule-1 (PECAM-1), VE-cadherin, and VEGF receptor 2 (VEGFR2) have been shown to form a mechanosensory complex thought to initiate EC response to shear stress [224]. Beyond ECs in monoculture, fluid flow has been demonstrated to have a drastic effect on the functional outcomes of both vascular networks and tissue-specific cell types within more complex organotypic models. For example, kidney organoids cultured under flow conditions not only developed perfusable, mural cell-lined vascular networks, but also displayed enhanced kidney organoid development and maturation [225]. Fluid flow also promoted vessel stabilization and was shown to markedly enhance vascular barrier function in vitro [226].
The specific effect of shear stress on ECs depends highly on both the developmental and anatomical context. In development, low shear stress promotes sprouting angiogenesis from newly established vessels to induce the vascular plexus formation and subsequent vascular remodeling. The vascular network formed through this process allows increased tissue perfusion and experiences similarly increased shear stress, which promotes EC quiescence [212,213]. But the story in adulthood, as told by in vitro models, is somewhat more nuanced. For example, microfluidic devices have been used to explore the role of both fluid shear stress along the endothelium and interstitial flow across it in either promoting or suppressing vascular sprouting. In line with reports from vascular development, these in vitro models demonstrated that high shear stress suppresses sprout formation in a nitric oxide (NO)-dependent manner, while interstitial flow promoted sprouting [219]. Contradictory reports, however, identified a somewhat higher threshold for both tangential shear stress and interstitial flow that consistently promoted sprouting through MMP-1. This work went a step further, combining complex, microfluidic culture geometries with finite element modeling to predict shear stress and, in keeping with their own findings, pattern sprouting [227].
4.3.2. Recreating Biomimetic Fluid Flow Paradigms with Bioprinting.
While tuning flow-induced shear stress has generally been shown to enhance vascularization [225,228] and vascular integrity [226], more work must be done to elucidate the role of specific fluid flow paradigms on the endothelium. 3D bioprinting may be a promising tool for facilitating these endeavors, by providing a unique way to probe the effects of altered hemodynamics due to changes in vascular structure [229,230], such as curvatures [86] or bifurcations [217,231,232], on endothelial health and disease progression [233]. In addition to being correlated with disease, different flow paradigms have also been used to promote arterial or venous EC specification, particularly for maturation of stem cell-derived ECs. Building on previous studies, microfluidic devices and flow bioreactors have been used to further demonstrate that high shear stress, in addition to promoting a more quiescent, anti-inflammatory EC phenotype, suppresses venous specification in favor of an arterial phenotype [234,235]. Similar applications, and more, may be explored through bioprinting. Cardiovascular medicine is beginning to integrate 3D printing of traditional materials, such as thermoplastics, into the creation of patient-specific vascular models. These 3D printed models can be used for patient education, training for surgery, and in vitro validation of computational fluid dynamics (CFD) simulations that study the effect of hemodynamics on patient outcomes [236].
In addition to apical hemodynamic forces, ECs actively sense and respond to basal signals from the basement membrane, such as the topographical features described above (section 4.2). This leads to a complex interplay of signaling that dictates EC behaviors. Advanced biofabrication and biomaterials strategies have been employed to simultaneously investigate the effects of combinatorial microenvironmental cues on vascular function, such as the response of ECs to shear stress on soft versus stiff substrates [237], to shear stress and interstitial flow [219], or fluid flow and substrate topography [238]. Interestingly, despite the powerful role of fluid flow in vascular development and homeostasis, reports have demonstrated that topographical cues from the basement membrane can dampen the pro-inflammatory effect of disturbed flow [238]. This provides insights into how ECs rapidly integrate and respond to such a vast array of microenvironmental cues, and further highlights the need for biomimetic, bioprinted culture platforms that can deliver these crucial signals.
4.4. Future Requirements for Clinical Translation of Engineered Microvasculature
The description herein is by no means an exhaustive coverage of the requirements for producing functional microvasculature for potential clinical translation, and some key requirements for translation have been under-explored to date. For example, one major barrier to clinical translation is the pre-implantation shelf-life of a bioprinted and conditioned microvascular construct. To investigate shelf-life, a recent study used alginate-based microgels as a bioink to print complex shapes. The microgels could be cryopreserved and, upon thawing, hMSCs encapsulated within maintained activity [239]. Similar types of strategies should be evaluated for their potential feasibility with microvascular biofabrication. Anastomosis, or fusion of implanted and host vasculature, is also required for successful translation. Recently, the maturity of pre-vascularized implants was shown to have a dramatic effect on this graft-host anastomosis. In that work, microvascular network maturity correlated with in vitro culture duration, and implantation of more mature networks markedly increased host vessel invasion, reduced blood clot accumulation, and maintained a proangiogenic secretome [186]. This finding highlights the importance of not only pre-implant maturation, but also blood-EC interactions and thrombogenicity as driving eventual clinical success or failure. As such, hemocompatibility is also a crucial design consideration for engineered microvasculature, but is overlooked in many bioprinting applications.
In the body, the endothelium interacts with a diverse range of circulating blood and immune cells and serves as the gatekeeper to tissues. Thrombosis mediated by platelet activation, in particular, has historically been a key mode of vascular graft failure. Recent work demonstrated a simple way to improve the hemocompatibility and function of engineered microvessels using platelet-rich plasma. Specifically, the work showed that treatment of engineered microvascular networks with platelet-rich plasma improved vascular barrier function and reduced thrombogenicity in 24 hours [240]. Another important interaction that could determine the clinical efficacy of bioprinted vessels is the interaction of the fabricated endothelium and circulating leukocytes. Indeed, inflammation is tightly linked to in vivo angiogenesis and atherosclerosis [76], and work has characterized the role of different macrophage phenotypes in vascularization [241]. Moreover, building on mounting evidence for the role of T cell subsets in contributing to the chronic inflammation that underlies atherosclerosis (recently reviewed here [242]), regulatory T cells have been demonstrated to play a role in the regression of atherosclerosis [243]. While this list of considerations may seem daunting, the fields of biomaterials and 3D bioprinting already possess many promising tools to begin creating more functional, biomimetic microvasculature in vitro, which will undoubtedly speed the progress towards future clinical translation.
5. Conclusion
Though there have been many advancements in tissue engineering and biofabrication, no one comprehensive strategy has emerged to solve the complex problem of creating functional, vascularized volumetric tissue replacements. As such, the creation of functional multiscale vasculature remains a key challenge in tissue engineering. Technologies exist that address incomplete portions of the vascularization problem. For example, extrusion-based 3D bioprinting is uniquely capable of fabricating complex, perfusable network structures on a larger scale, but inherently struggles with creating capillary-scale features. Meanwhile, innate EC biology can be harnessed through biomaterials and biofabrication-based approaches to induce sprouting from EC-lined, bioprinted vessels, but struggles to integrate these features with larger-diameter vessels. Further, bioprinting makes it possible to co-encapsulate stromal cells, to introduce EC-instructive surface topography, and to apply fluid flow that each provide signals important for vessel maturation and stability. In the future, we envision that these disparate strategies can be unified to harness the unique strengths of each. Only through a multifaceted approach will it be possible to bridge the gap between the constraints of current tissue culture and the lofty goal of functionally engineered vasculature.
Acknowledgements
The authors acknowledge Sarah Hull, Bauer LeSavage, and Lucia Brunel for valuable thoughts and comments on an early draft of this work.
Funding
We acknowledge financial support from the National Science Foundation (DMR 1808415 and CBET 2033302 to S.C.H) and the National Institutes of Health (R01 HL142718, R01 EB027666, R01 HL151997 to S.C.H.).
References
- [1].Shin Y, Choi SH, Kim E, Bylykbashi E, Kim JA, Chung S, Kim DY, Kamm RD and Tanzi RE Blood–Brain Barrier Dysfunction in a 3D In Vitro Model of Alzheimer’s Disease. Adv. Sci. 6, 1900962 (2019). doi: 10.1002/advs.201900962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gertler FB, Condeelis JS, Zervantonakis IK, Charest JL, Kamm RD and Hughes-Alford SK Three-dimensional microfluidic model for tumor cell intravasation and endothelial barrier function. Proc. Natl. Acad. Sci. 109, 13515–13520 (2012). doi: 10.1073/pnas.1210182109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wang YI, Abaci HE and Shuler ML Microfluidic blood–brain barrier model provides in vivo-like barrier properties for drug permeability screening. Biotechnol. Bioeng. 114, 184–194 (2017). doi: 10.1002/bit.26045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mirabella T, Macarthur JW, Cheng D, Ozaki CK, Woo YJ, Yang MT and Chen CS 3D-printed vascular networks direct therapeutic angiogenesis in ischaemia. Nat. Biomed. Eng. 1, (2017). doi: 10.1038/s41551-017-0083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Fitzsimmons RE, Aquilino MS, Quigley J, Chebotarev O, Tarlan F and Simmons CA Generating vascular channels within hydrogel constructs using an economical open-source 3D bioprinter and thermoreversible gels. Bioprinting 9, 7–18 (2018). doi: 10.1016/j.bprint.2018.02.001 [DOI] [Google Scholar]
- [6].Pusch K, Hinton TJ and Feinberg AW Large volume syringe pump extruder for desktop 3D printers. HardwareX 3, 49–61 (2018). doi: 10.1016/j.ohx.2018.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].NIH 3D Print Exchange. Available at: https://3dprint.nih.gov/.
- [8].Lee A, Hudson AR, Shiwarski DJ, Tashman JW, Hinton TJ, Yerneni S, Bliley JM, Campbell PG and Feinberg AW 3D bioprinting of collagen to rebuild components of the human heart. Science (80-. ). 365, 482–487 (2019). doi: 10.1126/science.aav9051 [DOI] [PubMed] [Google Scholar]
- [9].Skylar-Scott MA, Mueller J, Visser CW and Lewis JA Voxelated soft matter via multimaterial multinozzle 3D printing. Nature 575, 330–335 (2019). doi: 10.1038/s41586-019-1736-8 [DOI] [PubMed] [Google Scholar]
- [10].Liu W … Khademhosseini A Rapid Continuous Multimaterial Extrusion Bioprinting. Adv. Mater. 29, 1604630 (2017). doi: 10.1002/adma.201604630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kang H-W, Lee SJ, Ko IK, Kengla C, Yoo JJ and Atala AA 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 34, 312–319 (2016). doi: 10.1038/nbt.3413 [DOI] [PubMed] [Google Scholar]
- [12].Sun W … Ozbolat IT The bioprinting roadmap. Biofabrication 12, 022002 (2020). doi: 10.1088/1758-5090/ab5158 [DOI] [PubMed] [Google Scholar]
- [13].Zhang B, Montgomery M, Chamberlain MD, Ogawa S, Korolj A, Pahnke A, Wells LA, Massé S, Kim J, Reis L, Momen A, Nunes SS, Wheeler AR, Nanthakumar K, Keller G, Sefton MV and Radisic M Biodegradable scaffold with built-in vasculature for organ-on-a-chip engineering and direct surgical anastomosis. Nat. Mater. 15, 669–678 (2016). doi: 10.1038/nmat4570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Haase K and Kamm RD Advances in on-chip vascularization. Regen. Med. 12, 285–302 (2017). doi: 10.2217/rme-2016-0152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Traore MA and George SC Tissue Engineering the Vascular Tree. Tissue Eng. Part B Rev. 23, 505–514 (2017). doi: 10.1089/ten.teb.2017.0010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Schöneberg J, De Lorenzi F, Theek B, Blaeser A, Rommel D, Kuehne AJC, Kießling F and Fischer H Engineering biofunctional in vitro vessel models using a multilayer bioprinting technique. Sci. Rep. 8, 1–13 (2018). doi: 10.1038/s41598-018-28715-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cahill PA and Redmond EM Vascular endothelium - Gatekeeper of vessel health. Atherosclerosis 248, 97–109 (2016). doi: 10.1016/j.atherosclerosis.2016.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gimbrone MA and García-Cardeña G Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 118, 620–636 (2016). doi: 10.1161/CIRCRESAHA.115.306301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Malda J, Visser J, Melchels FP, Jüngst T, Hennink WE, Dhert WJA, Groll J and Hutmacher DW 25th Anniversary Article: Engineering Hydrogels for Biofabrication. Adv. Mater. 25, 5011–5028 (2013). doi: 10.1002/adma.201302042 [DOI] [PubMed] [Google Scholar]
- [20].Therriault D, White SR and Lewis JA Chaotic mixing in three-dimensional microvascular networks fabricated by direct-write assembly. Nat. Mater. 2, 265–271 (2003). doi: 10.1038/nmat863 [DOI] [PubMed] [Google Scholar]
- [21].Therriault D, Shepherd RF, White SR and Lewis JA Fugitive inks for direct-write assembly of three-dimensional microvascular networks. Adv. Mater. 17, 395–399 (2005). doi: 10.1002/adma.200400481 [DOI] [Google Scholar]
- [22].Therriault D, White SR and Lewis JA Rheological behavior of fugitive organic inks for direct-write assembly. Appl. Rheol. 17, 10112–1–10112–8 (2007). doi: 10.1515/arh-2007-0001 [DOI] [Google Scholar]
- [23].Bellan LM, Singh SP, Henderson PW, Porri TJ, Craighead HG and Spector JA Fabrication of an artificial 3-dimensional vascular network using sacrificial sugar structures. Soft Matter 5, 1354–1357 (2009). doi: 10.1039/b819905a [DOI] [Google Scholar]
- [24].Miller JS, Stevens KR, Yang MT, Baker BM, Nguyen D-HT, Cohen DM, Toro E, Chen AA, Galie PA, Yu X, Chaturvedi R, Bhatia SN and Chen CS Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat. Mater. 11, 768–774 (2012). doi: 10.1038/nmat3357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wu W, DeConinck A and Lewis JA Omnidirectional Printing of 3D Microvascular Networks. Adv. Mater. 23, H178–H183 (2011). doi: 10.1002/adma.201004625 [DOI] [PubMed] [Google Scholar]
- [26].Schmolka IR Artificial skin I. Preparation and properties of pluronic F-127 gels for treatment of burns. J. Biomed. Mater. Res. 6, 571–582 (1972). doi: 10.1002/jbm.820060609 [DOI] [PubMed] [Google Scholar]
- [27].Pandit NK and Kisaka J Loss of gelation ability of Pluronic® F127 in the presence of some salts. Int. J. Pharm. 145, 129–136 (1996). doi: 10.1016/S0378-5173(96)04748-5 [DOI] [Google Scholar]
- [28].Pandit N, Trygstad T, Croy S, Bohorquez M and Koch C Effect of Salts on the Micellization, Clouding, and Solubilization Behavior of Pluronic F127 Solutions. J. Colloid Interface Sci. 222, 213–220 (2000). doi: 10.1006/jcis.1999.6628 [DOI] [PubMed] [Google Scholar]
- [29].Kolesky DB, Truby RL, Gladman AS, Busbee TA, Homan KA and Lewis JA 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv. Mater. 26, 3124–3130 (2014). doi: 10.1002/adma.201305506 [DOI] [PubMed] [Google Scholar]
- [30].Kolesky DB, Homan KA, Skylar-Scott MA and Lewis JA Three-dimensional bioprinting of thick vascularized tissues. Proc. Natl. Acad. Sci. 113, 3179–3184 (2016). doi: 10.1073/pnas.1521342113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Eldridge JE and Ferry JD Studies of the cross-linking process in gelatin gels. III. Dependence of melting point on concentration and molecular weight. J. Phys. Chem. 58, 992–995 (1954). doi: 10.1021/j150521a013 [DOI] [Google Scholar]
- [32].Lee W, Lee V, Polio S, Keegan P, Lee JH, Fischer K, Park JK and Yoo SS On-demand three-dimensional freeform fabrication of multi-layered hydrogel scaffold with fluidic channels. Biotechnol. Bioeng. 105, 1178–1186 (2010). doi: 10.1002/bit.22613 [DOI] [PubMed] [Google Scholar]
- [33].Skylar-Scott MA, Uzel SGM, Nam LL, Ahrens JH, Truby RL, Damaraju S and Lewis JA Biomanufacturing of organ-specific tissues with high cellular density and embedded vascular channels. Sci. Adv. 5, eaaw2459 (2019). doi: 10.1126/sciadv.aaw2459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Rowley JA, Madlambayan G and Mooney DJ Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials 20, 45–53 (1999). doi: 10.1016/S0142-9612(98)00107-0 [DOI] [PubMed] [Google Scholar]
- [35].Wang XY, Jin ZH, Gan BW, Lv SW, Xie M and Huang WH Engineering interconnected 3D vascular networks in hydrogels using molded sodium alginate lattice as the sacrificial template. Lab Chip 14, 2709–2716 (2014). doi: 10.1039/c4lc00069b [DOI] [PubMed] [Google Scholar]
- [36].Hammer J, Han L-H, Tong X and Yang F A Facile Method to Fabricate Hydrogels with Microchannel-Like Porosity for Tissue Engineering. Tissue Eng. Part C Methods 20, 169–176 (2014). doi: 10.1089/ten.tec.2013.0176 [DOI] [PubMed] [Google Scholar]
- [37].Highley CB, Rodell CB and Burdick JA Direct 3D Printing of Shear-Thinning Hydrogels into Self-Healing Hydrogels. Adv. Mater. 27, 5075–5079 (2015). doi: 10.1002/adma.201501234 [DOI] [PubMed] [Google Scholar]
- [38].Song KH, Highley CB, Rouff A and Burdick JA Complex 3D-Printed Microchannels within Cell-Degradable Hydrogels. Adv. Funct. Mater. 28, 1801331 (2018). doi: 10.1002/adfm.201801331 [DOI] [Google Scholar]
- [39].Lee JB, Wang X, Faley S, Baer B, Balikov DA, Sung HJ and Bellan LM Development of 3D Microvascular Networks Within Gelatin Hydrogels Using Thermoresponsive Sacrificial Microfibers. Adv. Healthc. Mater. 5, 781–785 (2016). doi: 10.1002/adhm.201500792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].McCormack A, Highley CB, Leslie NR and Melchels FPW 3D Printing in Suspension Baths: Keeping the Promises of Bioprinting Afloat. Trends Biotechnol. 38, 584–593 (2020). doi: 10.1016/j.tibtech.2019.12.020 [DOI] [PubMed] [Google Scholar]
- [41].Loebel C, Rodell CB, Chen MH and Burdick JA Shear-thinning and self-healing hydrogels as injectable therapeutics and for 3D-printing. Nat. Protoc. 12, 1521–1541 (2017). doi: 10.1038/nprot.2017.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Bhattacharjee T, Zehnder SM, Rowe KG, Jain S, Nixon RM, Sawyer WG and Angelini TE Writing in the granular gel medium. Sci. Adv. 1, e1500655 (2015). doi: 10.1126/sciadv.1500655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hinton TJ, Jallerat Q, Palchesko RN, Park JH, Grodzicki MS, Shue H, Ramadan MH, Hudson AR and Feinberg AW Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci. Adv. 1, e1500758 (2015). doi: 10.1126/sciadv.1500758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Riley L, Schirmer L and Segura T Granular hydrogels: emergent properties of jammed hydrogel microparticles and their applications in tissue repair and regeneration. Curr. Opin. Biotechnol. 60, 1–8 (2019). doi: 10.1016/j.copbio.2018.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Highley CB, Song KH, Daly AC and Burdick JA Jammed Microgel Inks for 3D Printing Applications. Adv. Sci. 6, 1801076 (2019). doi: 10.1002/advs.201801076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hinton TJ, Hudson A, Pusch K, Lee A and Feinberg AW 3D Printing PDMS Elastomer in a Hydrophilic Support Bath via Freeform Reversible Embedding. ACS Biomater. Sci. Eng. 2, 1781–1786 (2016). doi: 10.1021/acsbiomaterials.6b00170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Savoji H, Davenport Huyer L, Mohammadi MH, Lun Lai BF, Rafatian N, Bannerman D, Shoaib M, Bobicki ER, Ramachandran A and Radisic M 3D Printing of Vascular Tubes Using Bioelastomer Prepolymers by Freeform Reversible Embedding. ACS Biomater. Sci. Eng. 6, 1333–1343 (2020). doi: 10.1021/acsbiomaterials.9b00676 [DOI] [PubMed] [Google Scholar]
- [48].Seymour AJ, Shin S and Heilshorn SC 3D Printing of Microgel Scaffolds with Tunable Void Fraction to Promote Cell Infiltration. Adv. Healthc. Mater. 2100644, 2100644 (2021). doi: 10.1002/adhm.202100644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Muir VG, Qazi TH, Shan J, Groll J and Burdick JA Influence of Microgel Fabrication Technique on Granular Hydrogel Properties. ACS Biomater. Sci. Eng. acsbiomaterials.0c01612 (2021). doi: 10.1021/acsbiomaterials.0c01612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Daly AC, Riley L, Segura T and Burdick JA Hydrogel microparticles for biomedical applications. Nat. Rev. Mater. 5, 20–43 (2020). doi: 10.1038/s41578-019-0148-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Qazi TH and Burdick JA Granular hydrogels for endogenous tissue repair. Biomater. Biosyst. 1, 100008 (2021). doi: 10.1016/j.bbiosy.2021.100008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Dolati F, Yu Y, Zhang Y, Jesus AMD, Sander EA and Ozbolat IT In vitro evaluation of carbon-nanotube-reinforced bioprintable vascular conduits. Nanotechnology 25, (2014). doi: 10.1088/0957-4484/25/14/145101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Gao Q, He Y, Fu J, Liu A and Ma L Coaxial nozzle-assisted 3D bioprinting with built-in microchannels for nutrients delivery. Biomaterials 61, 203–215 (2015). doi: 10.1016/j.biomaterials.2015.05.031 [DOI] [PubMed] [Google Scholar]
- [54].Jia W, Gungor-Ozkerim PS, Zhang YS, Yue K, Zhu K, Liu W, Pi Q, Byambaa B, Dokmeci MR, Shin SR and Khademhosseini A Direct 3D bioprinting of perfusable vascular constructs using a blend bioink. Biomaterials 106, 58–68 (2016). doi: 10.1016/j.biomaterials.2016.07.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Cui H, Zhu W, Huang Y, Liu C, Yu ZX, Nowicki M, Miao S, Cheng Y, Zhou X, Lee SJ, Zhou Y, Wang S, Mohiuddin M, Horvath K and Zhang LG In vitro and in vivo evaluation of 3D bioprinted small-diameter vasculature with smooth muscle and endothelium. Biofabrication 12, 015004 (2020). doi: 10.1088/1758-5090/ab402c [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Arakawa C, Gunnarsson C, Howard C, Bernabeu M, Phong K, Yang E, DeForest CA, Smith JD and Zheng Y Biophysical and biomolecular interactions of malaria-infected erythrocytes in engineered human capillaries. Sci. Adv. 6, eaay7243 (2020). doi: 10.1126/sciadv.aay7243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Hao D, Fan Y, Xiao W, Liu R, Pivetti C, Walimbe T, Guo F, Zhang X, Farmer DL, Wang F, Panitch A, Lam KS and Wang A Rapid endothelialization of small diameter vascular grafts by a bioactive integrin-binding ligand specifically targeting endothelial progenitor cells and endothelial cells. Acta Biomater. (2020). doi: 10.1016/j.actbio.2020.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Hammer JA, Ruta A, Therien AM and West JL Cell-Compatible, Site-Specific Covalent Modification of Hydrogel Scaffolds Enables User-Defined Control over Cell-Material Interactions. Biomacromolecules 20, 2486–2493 (2019). doi: 10.1021/acs.biomac.9b00183 [DOI] [PubMed] [Google Scholar]
- [59].Iturri J, García-Fernández L, Reuning U, García AJ, Campo A. Del and Salierno MJ Synchronized cell attachment triggered by photo-activatable adhesive ligands allows QCM-based detection of early integrin binding. Sci. Rep. 5, 9533 (2015). doi: 10.1038/srep09533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Maheshwari G, Brown G, Lauffenburger D a, Wells, A. and Griffith, L. G. Cell adhesion and motility depend on nanoscale RGD clustering. J. Cell Sci. 113 (Pt 1, 1677–1686 (2000). doi: 10.1083/jcb.144.5.1019 [DOI] [PubMed] [Google Scholar]
- [61].Benitez PL, Mascharak S, Proctor AC and Heilshorn SC Use of protein-engineered fabrics to identify design rules for integrin ligand clustering in biomaterials. Integr. Biol. 8, 50–61 (2016). doi: 10.1039/C5IB00258C [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Wang W, Lollis EM, Bordeleau F and Reinhart-King CA Matrix stiffness regulates vascular integrity through focal adhesion kinase activity. FASEB J. 33, 1199–1208 (2019). doi: 10.1096/fj.201800841R [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Van Popele NM, Grobbee DE, Bots ML, Asmar R, Topouchian J, Reneman RS, Hoeks APG, Van Der Kuip DAM, Hofman A and Witteman JCM Association between arterial stiffness and atherosclerosis: The Rotterdam study. Stroke 32, 454–460 (2001). doi: 10.1161/01.STR.32.2.454 [DOI] [PubMed] [Google Scholar]
- [64].Lampi MC, Guvendiren M, Burdick JA and Reinhart-King CA Photopatterned Hydrogels to Investigate the Endothelial Cell Response to Matrix Stiffness Heterogeneity. ACS Biomater. Sci. Eng. 3, 3007–3016 (2017). doi: 10.1021/acsbiomaterials.6b00633 [DOI] [PubMed] [Google Scholar]
- [65].Yang C, DelRio FW, Ma H, Killaars AR, Basta LP, Kyburz KA and Anseth KS Spatially patterned matrix elasticity directs stem cell fate. Proc. Natl. Acad. Sci. 113, E4439–E4445 (2016). doi: 10.1073/pnas.1609731113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Chen W, Tian B, Liang J, Yu S, Zhou Y and Li S Matrix stiffness regulates the interactions between endothelial cells and monocytes. Biomaterials 221, 119362 (2019). doi: 10.1016/j.biomaterials.2019.119362 [DOI] [PubMed] [Google Scholar]
- [67].Wimmer RA … Penninger JM Human blood vessel organoids as a model of diabetic vasculopathy. Nature 565, 505–510 (2019). doi: 10.1038/s41586-018-0858-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Wang X, Phan DTT, Sobrino A, George SC, Hughes CCW and Lee AP Engineering anastomosis between living capillary networks and endothelial cell-lined microfluidic channels. Lab Chip 16, 282–290 (2016). doi: 10.1039/c5lc01050k [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Kim S, Lee H, Chung M and Jeon NL Engineering of functional, perfusable 3D microvascular networks on a chip. Lab Chip 13, 1489 (2013). doi: 10.1039/c3lc41320a [DOI] [PubMed] [Google Scholar]
- [70].Xu K and Cleaver O Tubulogenesis during blood vessel formation. Seminars in Cell and Developmental Biology 22, 993–1004 (2011). doi: 10.1016/j.semcdb.2011.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Iruela-Arispe ML and Davis GE Cellular and Molecular Mechanisms of Vascular Lumen Formation. Developmental Cell 16, 222–231 (2009). doi: 10.1016/j.devcel.2009.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Davis GE, Stratman AN, Sacharidou A and Koh W Molecular Basis for Endothelial Lumen Formation and Tubulogenesis During Vasculogenesis and Angiogenic Sprouting. International Review of Cell and Molecular Biology 288, (Elsevier Inc., 2011). doi: 10.1016/B978-0-12-386041-5.00003-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Kant RJ and Coulombe KLK Integrated approaches to spatiotemporally directing angiogenesis in host and engineered tissues. Acta Biomaterialia 69, 42–62 (2018). doi: 10.1016/j.actbio.2018.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Bowers SLK, Norden PR and Davis GE Molecular Signaling Pathways Controlling Vascular Tube Morphogenesis and Pericyte-Induced Tube Maturation in 3D Extracellular Matrices. Advances in Pharmacology 77, (Elsevier Inc., 2016). doi: 10.1016/bs.apha.2016.04.005 [DOI] [PubMed] [Google Scholar]
- [75].Carmeliet P and Jain RK Molecular mechanisms and clinical applications of angiogenesis. Nature 473, 298–307 (2011). doi: 10.1038/nature10144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Potente M, Gerhardt H and Carmeliet P Basic and therapeutic aspects of angiogenesis. Cell 146, 873–887 (2011). doi: 10.1016/j.cell.2011.08.039 [DOI] [PubMed] [Google Scholar]
- [77].Akar B, Jiang B, Somo SI, Appel AA, Larson JC, Tichauer KM and Brey EM Biomaterials with persistent growth factor gradients in vivo accelerate vascularized tissue formation. Biomaterials 72, 61–73 (2015). doi: 10.1016/j.biomaterials.2015.08.049 [DOI] [PubMed] [Google Scholar]
- [78].Richardson TP, Peters MC, Ennett AB and Mooney DJ Polymeric system for dual growth factor delivery. Nat. Biotechnol. 19, 1029–1034 (2001). doi: 10.1038/nbt1101-1029 [DOI] [PubMed] [Google Scholar]
- [79].Hasan A, Paul A, Vrana NE, Zhao X, Memic A, Hwang Y-S, Dokmeci MR and Khademhosseini A Microfluidic techniques for development of 3D vascularized tissue. Biomaterials 35, 7308–7325 (2014). doi: 10.1016/j.biomaterials.2014.04.091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Shamloo A, Xu H and Heilshorn S Mechanisms of Vascular Endothelial Growth Factor-Induced Pathfinding by Endothelial Sprouts in Biomaterials. Tissue Eng. Part A 18, 320–330 (2012). doi: 10.1089/ten.tea.2011.0323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Shamloo A and Heilshorn SC Matrix density mediates polarization and lumen formation of endothelial sprouts in VEGF gradients. Lab Chip 10, 3061 (2010). doi: 10.1039/c005069e [DOI] [PubMed] [Google Scholar]
- [82].Verbridge SS, Chakrabarti A, Delnero P, Kwee B, Varner JD, Stroock AD and Fischbach C Physicochemical regulation of endothelial sprouting in a 3D microfluidic angiogenesis model. J. Biomed. Mater. Res. - Part A 101, 2948–2956 (2013). doi: 10.1002/jbm.a.34587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Chung S, Sudo R, MacK PJ, Wan CR, Vickerman V and Kamm RD Cell migration into scaffolds under co-culture conditions in a microfluidic platform. Lab Chip 9, 269–275 (2009). doi: 10.1039/b807585a [DOI] [PubMed] [Google Scholar]
- [84].Raghavan S, Nelson CM, Baranski JD, Lim E and Chen CS Geometrically controlled endothelial tubulogenesis in micropatterned gels. Tissue Eng. - Part A 16, 2255–2263 (2010). doi: 10.1089/ten.tea.2009.0584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Shin Y, Jeon JS, Han S, Jung G-S, Shin S, Lee S-H, Sudo R, Kamm RD and Chung S In vitro 3D collective sprouting angiogenesis under orchestrated ANG-1 and VEGF gradients. Lab Chip 11, 2175 (2011). doi: 10.1039/c1lc20039a [DOI] [PubMed] [Google Scholar]
- [86].Song KH, Highley CB, Rouff A and Burdick JA Complex 3D-Printed Microchannels within Cell-Degradable Hydrogels. Adv. Funct. Mater. 28, 1801331 (2018). doi: 10.1002/adfm.201801331 [DOI] [Google Scholar]
- [87].Pike DB, Cai S, Pomraning KR, Firpo MA, Fisher RJ, Shu XZ, Prestwich GD and Peattie RA Heparin-regulated release of growth factors in vitro and angiogenic response in vivo to implanted hyaluronan hydrogels containing VEGF and bFGF. Biomaterials 27, 5242–5251 (2006). doi: 10.1016/j.biomaterials.2006.05.018 [DOI] [PubMed] [Google Scholar]
- [88].Freeman I, Kedem A and Cohen S The effect of sulfation of alginate hydrogels on the specific binding and controlled release of heparin-binding proteins. Biomaterials 29, 3260–3268 (2008). doi: 10.1016/j.biomaterials.2008.04.025 [DOI] [PubMed] [Google Scholar]
- [89].Martino MM and Hubbell JA The 12th–14th type III repeats of fibronectin function as a highly promiscuous growth factor-binding domain. FASEB J. 24, 4711–4721 (2010). doi: 10.1096/fj.09-151282 [DOI] [PubMed] [Google Scholar]
- [90].Saik JE, Gould DJ, Watkins EM, Dickinson ME and West JL Covalently immobilized platelet-derived growth factor-BB promotes angiogenesis in biomimetic poly(ethylene glycol) hydrogels. Acta Biomater. 7, 133–143 (2011). doi: 10.1016/j.actbio.2010.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Bittner SM, Guo JL and Mikos AG Spatiotemporal control of growth factors in three-dimensional printed scaffolds. Bioprinting 12, e00032 (2018). doi: 10.1016/j.bprint.2018.e00032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Brudno Y, Ennett-Shepard AB, Chen RR, Aizenberg M and Mooney DJ Enhancing microvascular formation and vessel maturation through temporal control over multiple pro-angiogenic and pro-maturation factors. Biomaterials 34, 9201–9209 (2013). doi: 10.1016/j.biomaterials.2013.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Leslie-Barbick JE, Shen C, Chen C and West JL Micron-Scale Spatially Patterned, Covalently Immobilized Vascular Endothelial Growth Factor on Hydrogels Accelerates Endothelial Tubulogenesis and Increases Cellular Angiogenic Responses. Tissue Eng. Part A 17, 221–229 (2011). doi: 10.1089/ten.tea.2010.0202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Shadish JA, Benuska GM and DeForest CA Bioactive site-specifically modified proteins for 4D patterning of gel biomaterials. Nat. Mater. (2019). doi: 10.1038/s41563-019-0367-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Grim JC, Brown TE, Aguado BA, Chapnick DA, Viert AL, Liu X and Anseth KS A Reversible and Repeatable Thiol–Ene Bioconjugation for Dynamic Patterning of Signaling Proteins in Hydrogels. ACS Cent. Sci. 4, 909–916 (2018). doi: 10.1021/acscentsci.8b00325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Mosiewicz KA, Kolb L, Van Der Vlies AJ, Martino MM, Lienemann PS, Hubbell JA, Ehrbar M and Lutolf MP In situ cell manipulation through enzymatic hydrogel photopatterning. Nat. Mater. 12, 1072–1078 (2013). doi: 10.1038/nmat3766 [DOI] [PubMed] [Google Scholar]
- [97].Martino MM, Briquez PS, Guc E, Tortelli F, Kilarski WW, Metzger S, Rice JJ, Kuhn GA, Muller R, Swartz MA and Hubbell JA Growth Factors Engineered for Super-Affinity to the Extracellular Matrix Enhance Tissue Healing. Science (80-. ). 343, 885–888 (2014). doi: 10.1126/science.1247663 [DOI] [PubMed] [Google Scholar]
- [98].Agis H Vascularization for Tissue Engineering and Regenerative Medicine. Vascularization for Tissue Engineering and Regenerative Medicine (Springer International Publishing, 2020). doi: 10.1007/978-3-319-21056-8 [DOI] [Google Scholar]
- [99].Tabata Y, Yoshino D, Funamoto K, Koens R, Kamm RD and Funamoto K Migration of vascular endothelial cells in monolayers under hypoxic exposure. Integr. Biol. (Camb). 11, 26–35 (2019). doi: 10.1093/intbio/zyz002 [DOI] [PubMed] [Google Scholar]
- [100].Park KM and Gerecht S Hypoxia-inducible hydrogels. Nat. Commun. 5, 1–12 (2014). doi: 10.1038/ncomms5075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Blatchley M, Park KM and Gerecht S Designer hydrogels for precision control of oxygen tension and mechanical properties. J. Mater. Chem. B 3, 7939–7949 (2015). doi: 10.1039/c5tb01038a [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Blatchley MR, Hall F, Wang S, Pruitt HC and Gerecht S Hypoxia and matrix viscoelasticity sequentially regulate endothelial progenitor cluster-based vasculogenesis. Sci. Adv. 5, eaau7518 (2019). doi: 10.1126/sciadv.aau7518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Camci-Unal G, Alemdar N, Annabi N and Khademhosseini A Oxygen-releasing biomaterials for tissue engineering. Polym. Int. 62, 843–848 (2013). doi: 10.1002/pi.4502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Avraamides CJ, Garmy-Susini B and Varner JA Integrins in angiogenesis and lymphangiogenesis. Nat. Rev. Cancer 8, 604–617 (2008). doi: 10.1038/nrc2353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Hynes RO Integrins: Bidirectional, Allosteric Signaling Machines. Cell 110, 673–687 (2002). doi: 10.1016/S0092-8674(02)00971-6 [DOI] [PubMed] [Google Scholar]
- [106].Humphries JD, Byron A and Humphries MJ Integrin ligands at a glance. J. Cell Sci. 119, 3901–3903 (2006). doi: 10.1242/jcs.03098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Hersel U, Dahmen C and Kessler H RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials 24, 4385–4415 (2003). doi: 10.1016/S0142-9612(03)00343-0 [DOI] [PubMed] [Google Scholar]
- [108].Lee S-H, Moon JJ and West JL Three-dimensional micropatterning of bioactive hydrogels via two-photon laser scanning photolithography for guided 3D cell migration. Biomaterials 29, 2962–2968 (2008). doi: 10.1016/j.biomaterials.2008.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].DeForest CA and Anseth KS Cytocompatible click-based hydrogels with dynamically tunable properties through orthogonal photoconjugation and photocleavage reactions. Nat. Chem. 3, 925–931 (2011). doi: 10.1038/nchem.1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Kamei M, Brian Saunders W, Bayless KJ, Dye L, Davis GE and Weinstein BM Endothelial tubes assemble from intracellular vacuoles in vivo. Nature 442, 453–456 (2006). doi: 10.1038/nature04923 [DOI] [PubMed] [Google Scholar]
- [111].Davis GE and Camarillo CW An α2β1 integrin-dependent pinocytic mechanism involving intracellular vacuole formation and coalescence regulates capillary lumen and tube formation in three-dimensional collagen matrix. Exp. Cell Res. 224, 39–51 (1996). doi: 10.1006/excr.1996.0109 [DOI] [PubMed] [Google Scholar]
- [112].Bayless KJ, Salazar R and Davis GE RGD-dependent vacuolation and lumen formation observed during endothelial cell morphogenesis in three-dimensional fibrin matrices involves the α(v)β3 and α5β1 integrins. Am. J. Pathol. 156, 1673–1683 (2000). doi: 10.1016/S0002-9440(10)65038-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Li S, Nih LR, Bachman H, Fei P, Li Y, Nam E, Dimatteo R, Carmichael ST, Barker TH and Segura T Hydrogels with precisely controlled integrin activation dictate vascular patterning and permeability. Nat. Mater. 16, 953–961 (2017). doi: 10.1038/nmat4954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Cascone I, Napione L, Maniero F, Serini G and Bussolino F Stable interaction between α5β1 integrin and Tie2 tyrosine kinase receptor regulates endothelial cell response to Ang-1. J. Cell Biol. 170, 993–1004 (2005). doi: 10.1083/jcb.200507082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Califano JP and Reinhart-King CA A Balance of Substrate Mechanics and Matrix Chemistry Regulates Endothelial Cell Network Assembly. Cell. Mol. Bioeng. 1, 122–132 (2008). doi: 10.1007/s12195-008-0022-x [DOI] [Google Scholar]
- [116].Rivron NC, Vrij EJ, Rouwkema J, Gac S. Le, Van Berg A. Den, Truckenmüller RK and Van Blitterswijk CA Tissue deformation spatially modulates VEGF signaling and angiogenesis. Proc. Natl. Acad. Sci. U. S. A. 109, 6886–6891 (2012). doi: 10.1073/pnas.1201626109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Du Y, Herath SCB, Wang QG, Wang DA, Asada HH and Chen PCY Three-Dimensional Characterization of Mechanical Interactions between Endothelial Cells and Extracellular Matrix during Angiogenic Sprouting. Sci. Rep. 6, 1–14 (2016). doi: 10.1038/srep21362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Yin H, Ding Y, Zhai Y, Tan W and Yin X Orthogonal programming of heterogeneous micro-mechano-environments and geometries in three-dimensional bio-stereolithography. Nat. Commun. 9, 4096 (2018). doi: 10.1038/s41467-018-06685-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Brown TE, Carberry BJ, Worrell BT, Dudaryeva OY, McBride MK, Bowman CN and Anseth KS Photopolymerized dynamic hydrogels with tunable viscoelastic properties through thioester exchange. Biomaterials 178, 496–503 (2018). doi: 10.1016/j.biomaterials.2018.03.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Chaudhuri O, Gu L, Klumpers D, Darnell M, Bencherif SA, Weaver JC, Huebsch N, Lee HP, Lippens E, Duda GN and Mooney DJ Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater. 15, 326–334 (2016). doi: 10.1038/nmat4489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Wei Z, Schnellmann R, Pruitt HC and Gerecht S Hydrogel Network Dynamics Regulate Vascular Morphogenesis. Cell Stem Cell 1–15 (2020). doi: 10.1016/j.stem.2020.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Shamloo A, Mohammadaliha N, Heilshorn SC and Bauer AL A Comparative Study of Collagen Matrix Density Effect on Endothelial Sprout Formation Using Experimental and Computational Approaches. Ann. Biomed. Eng. 44, 929–941 (2016). doi: 10.1007/s10439-015-1416-2 [DOI] [PubMed] [Google Scholar]
- [123].Ghajar CM, Chen X, Harris JW, Suresh V, Hughes CCW, Jeon NL, Putnam AJ and George SC The Effect of Matrix Density on the Regulation of 3-D Capillary Morphogenesis. Biophys. J. 94, 1930–1941 (2008). doi: 10.1529/biophysj.107.120774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Pedron S, Pritchard AM, Vincil GA, Andrade B, Zimmerman SC and Harley BAC Patterning Three-Dimensional Hydrogel Microenvironments Using Hyperbranched Polyglycerols for Independent Control of Mesh Size and Stiffness. Biomacromolecules 18, 1393–1400 (2017). doi: 10.1021/acs.biomac.7b00118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Costantini M, Testa S, Mozetic P, Barbetta A, Fuoco C, Fornetti E, Tamiro F, Bernardini S, Jaroszewicz J, Święszkowski W, Trombetta M, Castagnoli L, Seliktar D, Garstecki P, Cesareni G, Cannata S, Rainer A and Gargioli C Microfluidic-enhanced 3D bioprinting of aligned myoblast-laden hydrogels leads to functionally organized myofibers in vitro and in vivo. Biomaterials 131, 98–110 (2017). doi: 10.1016/j.biomaterials.2017.03.026 [DOI] [PubMed] [Google Scholar]
- [126].Colosi C, Shin SR, Manoharan V, Massa S, Costantini M, Barbetta A, Dokmeci MR, Dentini M and Khademhosseini A Microfluidic Bioprinting of Heterogeneous 3D Tissue Constructs Using Low-Viscosity Bioink. Adv. Mater. 28, 677–684a (2016). doi: 10.1002/adma.201503310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Mason BN, Starchenko A, Williams RM, Bonassar LJ and Reinhart-King CA Tuning three-dimensional collagen matrix stiffness independently of collagen concentration modulates endothelial cell behavior. Acta Biomater. 9, 4635–4644 (2013). doi: 10.1016/j.actbio.2012.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Keating M, Lim M, Hu Q and Botvinick E Selective stiffening of fibrin hydrogels with micron resolution via photocrosslinking. Acta Biomater. 87, 88–96 (2019). doi: 10.1016/j.actbio.2019.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Trappmann B, Baker BM, Polacheck WJ, Choi CK, Burdick JA and Chen CS Matrix degradability controls multicellularity of 3D cell migration. Nat. Commun. 8, 371 (2017). doi: 10.1038/s41467-017-00418-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Stratman AN, Saunders WB, Sacharidou A, Koh W, Fisher KE, Zawieja DC, Davis MJ and Davis GE Endothelial cell lumen and vascular guidance tunnel formation requires MT1-MMP–dependent proteolysis in 3-dimensional collagen matrices. Blood 114, 237–247 (2009). doi: 10.1182/blood-2008-12-196451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Lutolf MP, Lauer-Fields JL, Schmoekel HG, Metters AT, Weber FE, Fields GB and Hubbell JA Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: Engineering cell-invasion characteristics. Proc. Natl. Acad. Sci. 100, 5413–5418 (2003). doi: 10.1073/pnas.0737381100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Khetan S and Burdick JA Patterning network structure to spatially control cellular remodeling and stem cell fate within 3-dimensional hydrogels. Biomaterials 31, 8228–8234 (2010). doi: 10.1016/j.biomaterials.2010.07.035 [DOI] [PubMed] [Google Scholar]
- [133].Hanjaya-Putra D, Wong KT, Hirotsu K, Khetan S, Burdick JA and Gerecht S Spatial control of cell-mediated degradation to regulate vasculogenesis and angiogenesis in hyaluronan hydrogels. Biomaterials 33, 6123–6131 (2012). doi: 10.1016/j.biomaterials.2012.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Badeau BA, Comerford MP, Arakawa CK, Shadish JA and DeForest CA Engineered modular biomaterial logic gates for environmentally triggered therapeutic delivery. Nat. Chem. 10, 251–258 (2018). doi: 10.1038/nchem.2917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Applegate MB, Coburn J, Partlow BP, Moreau JE, Mondia JP, Marelli B, Kaplan DL and Omenetto FG Laser-based three-dimensional multiscale micropatterning of biocompatible hydrogels for customized tissue engineering scaffolds. Proc. Natl. Acad. Sci. 112, 12052–12057 (2015). doi: 10.1073/pnas.1509405112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Hushka EA, Yavitt FM, Brown TE, Dempsey PJ and Anseth KS Relaxation of Extracellular Matrix Forces Directs Crypt Formation and Architecture in Intestinal Organoids. Adv. Healthc. Mater. 9, 1–9 (2020). doi: 10.1002/adhm.201901214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Juliar BA, Keating MT, Kong YP, Botvinick EL and Putnam AJ Sprouting angiogenesis induces significant mechanical heterogeneities and ECM stiffening across length scales in fibrin hydrogels. Biomaterials 162, 99–108 (2018). doi: 10.1016/j.biomaterials.2018.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Yoon C, Choi C, Stapleton S, Mirabella T, Howes C, Dong L, King J, Yang J, Oberai A, Eyckmans J and Chen CS Myosin IIA–mediated forces regulate multicellular integrity during vascular sprouting. Mol. Biol. Cell 30, 1974–1984 (2019). doi: 10.1091/mbc.E19-02-0076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Aird WC Phenotypic Heterogeneity of the Endothelium. Circ. Res. 100, 158–173 (2007). doi: 10.1161/01.RES.0000255691.76142.4a [DOI] [PubMed] [Google Scholar]
- [140].Aird WC Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ. Res. 100, 174–190 (2007). doi: 10.1161/01.RES.0000255690.03436.ae [DOI] [PubMed] [Google Scholar]
- [141].Man HSJ, Sukumar AN, Lam GC, Turgeon PJ, Yan MS, Ku KH, Dubinsky MK, Ho JJD, Wang JJ, Das S, Mitchell N, Oettgen P, Sefton MV and Marsden PA Angiogenic patterning by STEEL, an endothelial-enriched long noncoding RNA. Proc. Natl. Acad. Sci. 115, 2401–2406 (2018). doi: 10.1073/pnas.1715182115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Yuan L, Chan GC, Beeler D, Janes L, Spokes KC, Dharaneeswaran H, Mojiri A, Adams WJ, Sciuto T, Garcia-Cardeña G, Molema G, Kang PM, Jahroudi N, Marsden PA, Dvorak A, Regan ER and Aird WC A role of stochastic phenotype switching in generating mosaic endothelial cell heterogeneity. Nat. Commun. 7, (2016). doi: 10.1038/ncomms10160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Wood JA, Shah NM, McKee CT, Hughbanks ML, Liliensiek SJ, Russell P and Murphy CJ The role of substratum compliance of hydrogels on vascular endothelial cell behavior. Biomaterials 32, 5056–5064 (2011). doi: 10.1016/j.biomaterials.2011.03.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Bastounis EE, Yeh Y-T and Theriot JA Subendothelial stiffness alters endothelial cell traction force generation while exerting a minimal effect on the transcriptome. Sci. Rep. 9, 18209 (2019). doi: 10.1038/s41598-019-54336-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Deng L, Pollmeier L, Zhou Q, Bergemann S, Bode C, Hein L and Lother A Gene expression in immortalized versus primary isolated cardiac endothelial cells. Sci. Rep. 10, 2241 (2020). doi: 10.1038/s41598-020-59213-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Tan P ., Chan C, Xue S ., Dong R, Ananthesayanan B, Manunta M, Kerouedan C, Cheshire NJ ., Wolfe J ., Haskard D ., Taylor K . and George AJ . Phenotypic and functional differences between human saphenous vein (HSVEC) and umbilical vein (HUVEC) endothelial cells. Atherosclerosis 173, 171–183 (2004). doi: 10.1016/j.atherosclerosis.2003.12.011 [DOI] [PubMed] [Google Scholar]
- [147].Richardson MR, Lai X, Witzmann FA and Yoder MC Venous and arterial endothelial proteomics: mining for markers and mechanisms of endothelial diversity. Expert Rev. Proteomics 7, 823–831 (2010). doi: 10.1586/epr.10.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D and Yoder MC Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood 104, 2752–2760 (2004). doi: 10.1182/blood-2004-04-1396 [DOI] [PubMed] [Google Scholar]
- [149].Li Z, Hu S, Ghosh Z, Han Z and Wu JC Functional Characterization and Expression Profiling of Human Induced Pluripotent Stem Cell- and Embryonic Stem Cell-Derived Endothelial Cells. Stem Cells Dev. 20, 1701–1710 (2011). doi: 10.1089/scd.2010.0426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Zhang J, Chu L-F, Hou Z, Schwartz MP, Hacker T, Vickerman V, Swanson S, Leng N, Nguyen BK, Elwell A, Bolin J, Brown ME, Stewart R, Burlingham WJ, Murphy WL and Thomson JA Functional characterization of human pluripotent stem cell-derived arterial endothelial cells. Proc. Natl. Acad. Sci. 114, E6072–E6078 (2017). doi: 10.1073/pnas.1702295114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Patsch C … Cowan CA Generation of vascular endothelial and smooth muscle cells from human pluripotent stem cells. Nat. Cell Biol. 17, 994–1003 (2015). doi: 10.1038/ncb3205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Kusuma S, Shen Y-I, Hanjaya-Putra D, Mali P, Cheng L and Gerecht S Self-organized vascular networks from human pluripotent stem cells in a synthetic matrix. Proc. Natl. Acad. Sci. 110, 12601–12606 (2013). doi: 10.1073/pnas.1306562110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Natividad-Diaz SL, Browne S, Jha AK, Ma Z, Hossainy S, Kurokawa YK, George SC and Healy KE A combined hiPSC-derived endothelial cell and in vitro microfluidic platform for assessing biomaterial-based angiogenesis. Biomaterials 194, 73–83 (2019). doi: 10.1016/j.biomaterials.2018.11.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Atchison L, Abutaleb NO, Snyder-Mounts E, Gete Y, Ladha A, Ribar T, Cao K and Truskey GA iPSC-Derived Endothelial Cells Affect Vascular Function in a Tissue-Engineered Blood Vessel Model of Hutchinson-Gilford Progeria Syndrome. Stem Cell Reports 14, 325–337 (2020). doi: 10.1016/j.stemcr.2020.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Chen Q, Zhang H, Liu Y, Adams S, Eilken H, Stehling M, Corada M, Dejana E, Zhou B and Adams RH Endothelial cells are progenitors of cardiac pericytes and vascular smooth muscle cells. Nat. Commun. 7, 12422 (2016). doi: 10.1038/ncomms12422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Wörsdörfer P, Dalda N, Kern A, Krüger S, Wagner N, Kwok CK, Henke E and Ergün S Generation of complex human organoid models including vascular networks by incorporation of mesodermal progenitor cells. Sci. Rep. 9, 1–13 (2019). doi: 10.1038/s41598-019-52204-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Ding B-S, Nolan DJ, Guo P, Babazadeh AO, Cao Z, Rosenwaks Z, Crystal RG, Simons M, Sato TN, Worgall S, Shido K, Rabbany SY and Rafii S Endothelial-Derived Angiocrine Signals Induce and Sustain Regenerative Lung Alveolarization. Cell 147, 539–553 (2011). doi: 10.1016/j.cell.2011.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Lammert E, Cleaver O and Melton D Induction of pancreatic differentiation by signals from blood vessels. Science (80-. ). 294, 564–567 (2001). doi: 10.1126/science.1064344 [DOI] [PubMed] [Google Scholar]
- [159].Matsumoto K, Yoshitomi H, Rossant J and Zaret KS Liver organogenesis promoted by endothelial cells prior to vascular function. Science (80-. ). 294, 559–563 (2001). doi: 10.1126/science.1063889 [DOI] [PubMed] [Google Scholar]
- [160].Butler JM, Nolan DJ, Vertes EL, Varnum-Finney B, Kobayashi H, Hooper AT, Seandel M, Shido K, White IA, Kobayashi M, Witte L, May C, Shawber C, Kimura Y, Kitajewski J, Rosenwaks Z, Bernstein ID and Rafii S Endothelial Cells Are Essential for the Self-Renewal and Repopulation of Notch-Dependent Hematopoietic Stem Cells. Cell Stem Cell 6, 251–264 (2010). doi: 10.1016/j.stem.2010.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Uwamori H, Ono Y, Yamashita T, Arai K and Sudo R Comparison of organ-specific endothelial cells in terms of microvascular formation and endothelial barrier functions. Microvasc. Res. 122, 60–70 (2019). doi: 10.1016/j.mvr.2018.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Nakatsu MN, Sainson RCA, Aoto JN, Taylor KL, Aitkenhead M, Pérez-del-Pulgar S, Carpenter PM and Hughes CCW Angiogenic sprouting and capillary lumen formation modeled by human umbilical vein endothelial cells (HUVEC) in fibrin gels: The role of fibroblasts and Angiopoietin-1. Microvasc. Res. 66, 102–112 (2003). doi: 10.1016/S0026-2862(03)00045-1 [DOI] [PubMed] [Google Scholar]
- [163].Stratman AN, Malotte KM, Mahan RD, Davis MJ and Davis GE Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood 114, 5091–5101 (2009). doi: 10.1182/blood-2009-05-222364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Ghajar CM, Kachgal S, Kniazeva E, Mori H, Costes SV, George SC and Putnam AJ Mesenchymal cells stimulate capillary morphogenesis via distinct proteolytic mechanisms. Exp. Cell Res. 316, 813–825 (2010). doi: 10.1016/j.yexcr.2010.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Newman AC, Chou W, Welch-Reardon KM, Fong AH, Popson SA, Phan DT, Sandoval DR, Nguyen DP, Gershon PD and Hughes CCW Analysis of Stromal Cell Secretomes Reveals a Critical Role for Stromal Cell-Derived Hepatocyte Growth Factor and Fibronectin in Angiogenesis. Arterioscler. Thromb. Vasc. Biol. 33, 513–522 (2013). doi: 10.1161/ATVBAHA.112.300782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Haase K, Gillrie MR, Hajal C and Kamm RD Pericytes Contribute to Dysfunction in a Human 3D Model of Placental Microvasculature through VEGF-Ang-Tie2 Signaling. Adv. Sci. 6, (2019). doi: 10.1002/advs.201900878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Greenwood-Goodwin M, Yang J, Hassanipour M and Larocca D A novel lineage restricted, pericyte-like cell line isolated from human embryonic stem cells. Sci. Rep. 6, 24403 (2016). doi: 10.1038/srep24403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Armulik A, Genové G and Betsholtz C Pericytes: Developmental, Physiological, and Pathological Perspectives, Problems, and Promises. Dev. Cell 21, 193–215 (2011). doi: 10.1016/j.devcel.2011.07.001 [DOI] [PubMed] [Google Scholar]
- [169].Saunders WB, Bohnsack BL, Faske JB, Anthis NJ, Bayless KJ, Hirschi KK and Davis GE Coregulation of vascular tube stabilization by endothelial cell TIMP-2 and pericyte TIMP-3. J. Cell Biol. 175, 179–191 (2006). doi: 10.1083/jcb.200603176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR and Betsholtz C Pericytes regulate the blood–brain barrier. Nature 468, 557–561 (2010). doi: 10.1038/nature09522 [DOI] [PubMed] [Google Scholar]
- [171].Chang WG, Andrejecsk JW, Kluger MS, Saltzman WM and Pober JS Pericytes modulate endothelial sprouting. Cardiovasc. Res. 100, 492–500 (2013). doi: 10.1093/cvr/cvt215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Brandt MM, van Dijk CGM, Maringanti R, Chrifi I, Kramann R, Verhaar MC, Duncker DJ, Mokry M and Cheng C Transcriptome analysis reveals microvascular endothelial cell-dependent pericyte differentiation. Sci. Rep. 9, 15586 (2019). doi: 10.1038/s41598-019-51838-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Zheng Y, Chen J, Craven M, Choi NW, Totorica S, Diaz-Santana A, Kermani P, Hempstead B, Fischbach-Teschl C, Lopez JA and Stroock AD In vitro microvessels for the study of angiogenesis and thrombosis. Proc. Natl. Acad. Sci. 109, 9342–9347 (2012). doi: 10.1073/pnas.1201240109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Bourget J-M, Kérourédan O, Medina M, Rémy M, Thébaud NB, Bareille R, Chassande O, Amédée J, Catros S and Devillard R Patterning of Endothelial Cells and Mesenchymal Stem Cells by Laser-Assisted Bioprinting to Study Cell Migration. Biomed Res. Int. 2016, 1–7 (2016). doi: 10.1155/2016/3569843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Chen YC, Lin RZ, Qi H, Yang Y, Bae H, Melero-Martin JM and Khademhosseini A Functional human vascular network generated in photocrosslinkable gelatin methacrylate hydrogels. Adv. Funct. Mater. 22, 2027–2039 (2012). doi: 10.1002/adfm.201101662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Andrée B, Ichanti H, Kalies S, Heisterkamp A, Strauß S, Vogt P-M, Haverich A and Hilfiker A Formation of three-dimensional tubular endothelial cell networks under defined serum-free cell culture conditions in human collagen hydrogels. Sci. Rep. 9, 5437 (2019). doi: 10.1038/s41598-019-41985-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Rao RR, Peterson AW, Ceccarelli J, Putnam AJ and Stegemann JP Matrix composition regulates three-dimensional network formation by endothelial cells and mesenchymal stem cells in collagen/fibrin materials. Angiogenesis 15, 253–264 (2012). doi: 10.1007/s10456-012-9257-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Marshall J, Barnes A and Genever P Analysis of the intrinsic self-organising properties of mesenchymal stromal cells in three-dimensional co-culture models with endothelial cells. Bioengineering 5, (2018). doi: 10.3390/bioengineering5040092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Traktuev DO, Merfeld-Clauss S, Li J, Kolonin M, Arap W, Pasqualini R, Johnstone BH and March KL A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ. Res. 102, 77–85 (2008). doi: 10.1161/CIRCRESAHA.107.159475 [DOI] [PubMed] [Google Scholar]
- [180].Merfeld-Clauss S, Gollahalli N, March KL and Traktuev DO Adipose tissue progenitor cells directly interact with endothelial cells to induce vascular network formation. Tissue Eng. - Part A 16, 2953–2966 (2010). doi: 10.1089/ten.tea.2009.0635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Ren J, Zhou T, Sekhar Pilli VS, Phan N, Wang Q, Gupta K, Liu Z, Sheibani N and Liu B Novel paracrine functions of smooth muscle cells in supporting endothelial regeneration following arterial injury. Circ. Res. 124, 1253–1265 (2019). doi: 10.1161/CIRCRESAHA.118.314567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Qi YX, Jiang J, Jiang XH, Wang XD, Ji SY, Han Y, Long DK, Shen BR, Yan ZQ, Chien S and Jiang ZL PDGF-BB and TGF-β1 on cross-talk between endothelial and smooth muscle cells in vascular remodeling induced by low shear stress. Proc. Natl. Acad. Sci. U. S. A. 108, 1908–1913 (2011). doi: 10.1073/pnas.1019219108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].DiVito KA, Daniele MA, Roberts SA, Ligler FS and Adams AA Microfabricated blood vessels undergo neoangiogenesis. Biomaterials 138, 142–152 (2017). doi: 10.1016/j.biomaterials.2017.05.012 [DOI] [PubMed] [Google Scholar]
- [184].Tian B, Ding X, Song Y, Chen W, Liang J, Yang L, Fan Y, Li S and Zhou Y Matrix stiffness regulates SMC functions via TGF-β signaling pathway. Biomaterials 221, 119407 (2019). doi: 10.1016/j.biomaterials.2019.119407 [DOI] [PubMed] [Google Scholar]
- [185].Cattaneo P, Mukherjee D, Spinozzi S, Zhang L, Larcher V, Stallcup WB, Kataoka H, Chen J, Dimmeler S, Evans SM and Guimarães-Camboa N Parallel Lineage-Tracing Studies Establish Fibroblasts as the Prevailing In Vivo Adipocyte Progenitor. Cell Rep. 30, 571–582.e2 (2020). doi: 10.1016/j.celrep.2019.12.046 [DOI] [PubMed] [Google Scholar]
- [186].Ben-Shaul S, Landau S, Merdler U and Levenberg S Mature vessel networks in engineered tissue promote graft–host anastomosis and prevent graft thrombosis. Proc. Natl. Acad. Sci. U. S. A. 116, 2955–2960 (2019). doi: 10.1073/pnas.1814238116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Hsu Y-H, Moya ML, Hughes CCW, George SC and Lee AP A microfluidic platform for generating large-scale nearly identical human microphysiological vascularized tissue arrays. Lab Chip 13, 2990 (2013). doi: 10.1039/c3lc50424g [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Abbott NJ, Rönnbäck L and Hansson E Astrocyte–endothelial interactions at the blood–brain barrier. Nat. Rev. Neurosci. 7, 41–53 (2006). doi: 10.1038/nrn1824 [DOI] [PubMed] [Google Scholar]
- [189].Vanlandewijck M, He L, Mäe MA, Andrae J, Ando K, Del Gaudio F, Nahar K, Lebouvier T, Laviña B, Gouveia L, Sun Y, Raschperger E, Räsänen M, Zarb Y, Mochizuki N, Keller A, Lendahl U and Betsholtz C A molecular atlas of cell types and zonation in the brain vasculature. Nature 554, 475–480 (2018). doi: 10.1038/nature25739 [DOI] [PubMed] [Google Scholar]
- [190].Smyth LCD, Rustenhoven J, Scotter EL, Schweder P, Faull RLM, Park TIH and Dragunow M Markers for human brain pericytes and smooth muscle cells. J. Chem. Neuroanat. 92, 48–60 (2018). doi: 10.1016/j.jchemneu.2018.06.001 [DOI] [PubMed] [Google Scholar]
- [191].Wang C, Li J, Sinha S, Peterson A, Grant GA and Yang F Mimicking brain tumor-vasculature microanatomical architecture via co-culture of brain tumor and endothelial cells in 3D hydrogels. Biomaterials 202, 35–44 (2019). doi: 10.1016/j.biomaterials.2019.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Song L, Yuan X, Jones Z, Griffin K, Zhou Y, Ma T and Li Y Assembly of Human Stem Cell-Derived Cortical Spheroids and Vascular Spheroids to Model 3-D Brain-like Tissues. Sci. Rep. 9, 1–16 (2019). doi: 10.1038/s41598-019-42439-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Truong D, Fiorelli R, Barrientos ES, Melendez EL, Sanai N, Mehta S and Nikkhah M A three-dimensional (3D) organotypic microfluidic model for glioma stem cells – Vascular interactions. Biomaterials 198, 63–77 (2019). doi: 10.1016/j.biomaterials.2018.07.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Osaki T, Sivathanu V and Kamm RD Crosstalk between developing vasculature and optogenetically engineered skeletal muscle improves muscle contraction and angiogenesis. Biomaterials 156, 65–76 (2018). doi: 10.1016/j.biomaterials.2017.11.041 [DOI] [PubMed] [Google Scholar]
- [195].Kato K, Diéguez-Hurtado R, Park DY, Hong SP, Kato-Azuma S, Adams S, Stehling M, Trappmann B, Wrana JL, Koh GY and Adams RH Pulmonary pericytes regulate lung morphogenesis. Nat. Commun. 9, 2448 (2018). doi: 10.1038/s41467-018-04913-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Dias Moura Prazeres PH, Sena IFG, Borges I. da T., de Azevedo PO, Andreotti JPP, de Paiva AE, de Almeida VM, de Paula Guerra DA, Pinheiro dos Santos GS, Mintz A, Delbono O and Birbrair A Pericytes are heterogeneous in their origin within the same tissue. Dev. Biol. 427, 6–11 (2017). doi: 10.1016/j.ydbio.2017.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Ding Y, Floren M and Tan W High-Throughput Screening of Vascular Endothelium-Destructive or Protective Microenvironments: Cooperative Actions of Extracellular Matrix Composition, Stiffness, and Structure. Adv. Healthc. Mater. 6, 1601426 (2017). doi: 10.1002/adhm.201601426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Dreier B, Gasiorowski JZ, Morgan JT, Nealey PF, Russell P and Murphy CJ Early responses of vascular endothelial cells to topographic cues. Am. J. Physiol. Physiol. 305, C290–C298 (2013). doi: 10.1152/ajpcell.00264.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Mascharak S, Benitez PL, Proctor AC, Madl CM, Hu KH, Dewi RE, Butte MJ and Heilshorn SC YAP-dependent mechanotransduction is required for proliferation and migration on native-like substrate topography. Biomaterials 115, 155–166 (2017). doi: 10.1016/j.biomaterials.2016.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Whited BM and Rylander MN The influence of electrospun scaffold topography on endothelial cell morphology, alignment, and adhesion in response to fluid flow. Biotechnol. Bioeng. 111, 184–195 (2014). doi: 10.1002/bit.24995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Morgan JT, Wood JA, Shah NM, Hughbanks ML, Russell P, Barakat AI and Murphy CJ Integration of basal topographic cues and apical shear stress in vascular endothelial cells. Biomaterials 33, 4126–4135 (2012). doi: 10.1016/j.biomaterials.2012.02.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Sundararaghavan HG, Saunders RL, Hammer DA and Burdick JA Fiber alignment directs cell motility over chemotactic gradients. Biotechnol. Bioeng. 110, 1249–1254 (2013). doi: 10.1002/bit.24788 [DOI] [PubMed] [Google Scholar]
- [203].Arora S, Lin S, Cheung C, Yim EKF and Toh YC Topography elicits distinct phenotypes and functions in human primary and stem cell derived endothelial cells. Biomaterials 234, 119747 (2020). doi: 10.1016/j.biomaterials.2019.119747 [DOI] [PubMed] [Google Scholar]
- [204].Gasiorowski JZ, Liliensiek SJ, Russell P, Stephan DA, Nealey PF and Murphy CJ Alterations in gene expression of human vascular endothelial cells associated with nanotopographic cues. Biomaterials 31, 8882–8888 (2010). doi: 10.1016/j.biomaterials.2010.08.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Hatano R, Mercurio K, Luna J, Glaser DE, Leppert VJ and McCloskey KE Endothelial cells derived from embryonic stem cells respond to cues from topographical surface patterns. J. Biol. Eng. 7, 18 (2013). doi: 10.1186/1754-1611-7-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Liliensiek SJ, Wood JA, Yong J, Auerbach R, Nealey PF and Murphy CJ Modulation of human vascular endothelial cell behaviors by nanotopographic cues. Biomaterials 31, 5418–5426 (2010). doi: 10.1016/j.biomaterials.2010.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Bettinger CJ, Langer R and Borenstein JT Engineering substrate topography at the Micro- and nanoscale to control cell function. Angewandte Chemie - International Edition 48, 5406–5415 (2009). doi: 10.1002/anie.200805179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Abeykoon C, Sri-Amphorn P and Fernando A Optimization of fused deposition modeling parameters for improved PLA and ABS 3D printed structures. Int. J. Light. Mater. Manuf. 3, 284–297 (2020). doi: 10.1016/j.ijlmm.2020.03.003 [DOI] [Google Scholar]
- [209].Ahn D, Kweon JH, Kwon S, Song J and Lee S Representation of surface roughness in fused deposition modeling. J. Mater. Process. Technol. 209, 5593–5600 (2009). doi: 10.1016/j.jmatprotec.2009.05.016 [DOI] [Google Scholar]
- [210].Taufik M and Jain PK A study of build edge profile for prediction of surface roughness in fused deposition modeling. J. Manuf. Sci. Eng. Trans. ASME 138, 1–11 (2016). doi: 10.1115/1.4032193 [DOI] [Google Scholar]
- [211].Thrimurthulu K, Pandey PM and Reddy NV Optimum part deposition orientation in fused deposition modeling. Int. J. Mach. Tools Manuf. 44, 585–594 (2004). doi: 10.1016/j.ijmachtools.2003.12.004 [DOI] [Google Scholar]
- [212].Baratchi S, Khoshmanesh K, Woodman OL, Potocnik S, Peter K and McIntyre P Molecular Sensors of Blood Flow in Endothelial Cells. Trends Mol. Med. 23, 850–868 (2017). doi: 10.1016/j.molmed.2017.07.007 [DOI] [PubMed] [Google Scholar]
- [213].Souilhol C, Serbanovic-Canic J, Fragiadaki M, Chico TJ, Ridger V, Roddie H and Evans PC Endothelial responses to shear stress in atherosclerosis: a novel role for developmental genes. Nat. Rev. Cardiol. 17, 52–63 (2020). doi: 10.1038/s41569-019-0239-5 [DOI] [PubMed] [Google Scholar]
- [214].Levesque MJ and Nerem RM The Elongation and Orientation of Cultured Endothelial Cells in Response to Shear Stress. J. Biomech. Eng. 107, 341–347 (1985). doi: 10.1115/1.3138567 [DOI] [PubMed] [Google Scholar]
- [215].Kataoka N, Ujita S and Sato M Effect of flow direction on the morphological responses of cultured bovine aortic endothelial cells. Med. Biol. Eng. Comput. 36, 122–128 (1998). doi: 10.1007/BF02522869 [DOI] [PubMed] [Google Scholar]
- [216].Polacheck WJ, Kutys ML, Yang J, Eyckmans J, Wu Y, Vasavada H, Hirschi KK and Chen CS A non-canonical Notch complex regulates adherens junctions and vascular barrier function. Nature 552, 258–262 (2017). doi: 10.1038/nature24998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Bouhrira N, DeOre BJ, Sazer DW, Chiaradia Z, Miller JS and Galie PA Disturbed flow disrupts the blood-brain barrier in a 3D bifurcation model. Biofabrication 12, 025020 (2020). doi: 10.1088/1758-5090/ab5898 [DOI] [PubMed] [Google Scholar]
- [218].Yoshino D, Funamoto K, Sato K, Kenry, Sato M and Lim CT Hydrostatic pressure promotes endothelial tube formation through aquaporin 1 and Ras-ERK signaling. Commun. Biol. 3, 152 (2020). doi: 10.1038/s42003-020-0881-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Song JW and Munn LL Fluid forces control endothelial sprouting. Proc. Natl. Acad. Sci. U. S. A. 108, 15342–15347 (2011). doi: 10.1073/pnas.1105316108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Rosenfeld D, Landau S, Shandalov Y, Raindel N, Freiman A, Shor E, Blinder Y, Vandenburgh HH, Mooney DJ and Levenberg S Morphogenesis of 3D vascular networks is regulated by tensile forces. Proc. Natl. Acad. Sci. U. S. A. 113, 3215–3220 (2016). doi: 10.1073/pnas.1522273113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Yu CY, Chae J, Buehler MJ, Hunter CP and Mooney DJ Cyclic tensile strain triggers a sequence of autocrine and paracrine signaling to regulate angiogenic sprouting in human vascular cells. Proc. Natl. Acad. Sci. U. S. A. 106, 15279–15284 (2009). doi: 10.1073/pnas.0905891106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Jalali S, del Pozo MA, Chen K-D, Miao H, Li Y-S, Schwartz MA, Shyy JY-J and Chien S Integrin-mediated mechanotransduction requires its dynamic interaction with specific extracellular matrix (ECM) ligands. Proc. Natl. Acad. Sci. 98, 1042–1046 (2001). doi: 10.1073/pnas.98.3.1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Sun X, Fu Y, Gu M, Zhang L, Li D, Li H, Chien S, Shyy JYJ and Zhu Y Activation of integrin α5 mediated by flow requires its translocation to membrane lipid rafts in vascular endothelial cells. Proc. Natl. Acad. Sci. 113, 769–774 (2016). doi: 10.1073/pnas.1524523113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H and Schwartz MA A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 437, 426–431 (2005). doi: 10.1038/nature03952 [DOI] [PubMed] [Google Scholar]
- [225].Homan KA, Gupta N, Kroll KT, Kolesky DB, Skylar-Scott M, Miyoshi T, Mau D, Valerius MT, Ferrante T, Bonventre JV, Lewis JA and Morizane R Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nat. Methods 16, (2019). doi: 10.1038/s41592-019-0325-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Faley SL, Neal EH, Wang JX, Bosworth AM, Weber CM, Balotin KM, Lippmann ES and Bellan LM iPSC-Derived Brain Endothelium Exhibits Stable, Long-Term Barrier Function in Perfused Hydrogel Scaffolds. Stem Cell Reports 12, 474–487 (2019). doi: 10.1016/j.stemcr.2019.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Galie PA, Nguyen D-HT, Choi CK, Cohen DM, Janmey PA and Chen CS Fluid shear stress threshold regulates angiogenic sprouting. Proc. Natl. Acad. Sci. 111, 7968–7973 (2014). doi: 10.1073/pnas.1310842111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Zohar B, Blinder Y, Mooney DJ and Levenberg S Flow-Induced Vascular Network Formation and Maturation in Three-Dimensional Engineered Tissue. ACS Biomater. Sci. Eng. acsbiomaterials.7b00025 (2017). doi: 10.1021/acsbiomaterials.7b00025 [DOI] [PubMed] [Google Scholar]
- [229].Abe Y, Watanabe M, Chung S, Kamm RD, Tanishita K and Sudo R Balance of interstitial flow magnitude and vascular endothelial growth factor concentration modulates three-dimensional microvascular network formation. APL Bioeng. 3, 036102 (2019). doi: 10.1063/1.5094735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Helm CLE, Fleury ME, Zisch AH, Boschetti F and Swartz MA Synergy between interstitial flow and VEGF directs capillary morphogenesis in vitro through a gradient amplification mechanism. Proc. Natl. Acad. Sci. U. S. A. 102, 15779–15784 (2005). doi: 10.1073/pnas.0503681102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Dai G, Kaazempur-Mofrad MR, Natarajan S, Zhang Y, Vaughn S, Blackman BR, Kamm RD, Garcia-Cardena G and Gimbrone MA Distinct endothelial phenotypes evoked by arterial waveforms derived from atherosclerosis-susceptible and -resistant regions of human vasculature. Proc. Natl. Acad. Sci. 101, 14871–14876 (2004). doi: 10.1073/pnas.0406073101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Ajami NE, Gupta S, Maurya MR, Nguyen P, Li JY-S, Shyy JYJ, Chen Z, Chien S and Subramaniam S Systems biology analysis of longitudinal functional response of endothelial cells to shear stress. Proc. Natl. Acad. Sci. 114, 10990–10995 (2017). doi: 10.1073/pnas.1707517114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Wang K-C, Yeh Y-T, Nguyen P, Limqueco E, Lopez J, Thorossian S, Guan K-L, Li Y-SJ and Chien S Flow-dependent YAP/TAZ activities regulate endothelial phenotypes and atherosclerosis. Proc. Natl. Acad. Sci. 113, 11525–11530 (2016). doi: 10.1073/pnas.1613121113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Arora S, Lam AJY, Cheung C, Yim EKF and Toh YC Determination of critical shear stress for maturation of human pluripotent stem cell-derived endothelial cells towards an arterial subtype. Biotechnol. Bioeng. 116, 1164–1175 (2019). doi: 10.1002/bit.26910 [DOI] [PubMed] [Google Scholar]
- [235].Sivarapatna A, Ghaedi M, Le AV, Mendez JJ, Qyang Y and Niklason LE Arterial specification of endothelial cells derived from human induced pluripotent stem cells in a biomimetic flow bioreactor. Biomaterials 53, 621–633 (2015). doi: 10.1016/j.biomaterials.2015.02.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Giannopoulos AA, Mitsouras D, Yoo SJ, Liu PP, Chatzizisis YS and Rybicki FJ Applications of 3D printing in cardiovascular diseases. Nat. Rev. Cardiol. 13, 701–718 (2016). doi: 10.1038/nrcardio.2016.170 [DOI] [PubMed] [Google Scholar]
- [237].Kohn JC, Zhou DW, Bordeleau F, Zhou AL, Mason BN, Mitchell MJ, King MR and Reinhart-King CA Cooperative effects of matrix stiffness and fluid shear stress on endothelial cell behavior. Biophys. J. 108, 471–478 (2015). doi: 10.1016/j.bpj.2014.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Nakayama KH, Surya VN, Gole M, Walker TW, Yang W, Lai ES, Ostrowski MA, Fuller GG, Dunn AR and Huang NF Nanoscale Patterning of Extracellular Matrix Alters Endothelial Function under Shear Stress. Nano Lett. 16, 410–419 (2016). doi: 10.1021/acs.nanolett.5b04028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Jeon O, Lee YBB, Hinton TJJ, Feinberg AWW and Alsberg E Cryopreserved cell-laden alginate microgel bioink for 3D bioprinting of living tissues. Mater. Today Chem. 12, 61–70 (2019). doi: 10.1016/j.mtchem.2018.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Nagao RJ, Marcu R, Wang Y, Wang L, Arakawa C, DeForest C, Chen J, López JA and Zheng Y Transforming Endothelium with Platelet-Rich Plasma in Engineered Microvessels. Adv. Sci. 6, (2019). doi: 10.1002/advs.201901725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Spiller KL, Anfang RR, Spiller KJ, Ng J, Nakazawa KR, Daulton JW and Vunjak-Novakovic G The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials 35, 4477–4488 (2014). doi: 10.1016/j.biomaterials.2014.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Saigusa R, Winkels H and Ley K T cell subsets and functions in atherosclerosis. Nature Reviews Cardiology 3050, (2020). doi: 10.1038/s41569-020-0352-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Sharma M, Schlegel MP, Afonso MS, Brown EJ, Rahman K, Weinstock A, Sansbury B, Corr EM, van Solingen C, Koelwyn G, Shanley LC, Beckett L, Peled D, Lafaille JJ, Spite M, Loke P, Fisher EA and Moore KJ Regulatory T Cells License Macrophage Pro-Resolving Functions During Atherosclerosis Regression. Circ. Res. CIRCRESAHA.119.316461 (2020). doi: 10.1161/CIRCRESAHA.119.316461 [DOI] [PMC free article] [PubMed] [Google Scholar]


