Abstract
Currently, there are two safe and effective therapeutic strategies for chronic hepatitis B treatment, namely, nucleoside analogs and interferon alpha (pegylated or non-pegylated). These treatments can control viral replication and improve survival; however, they do not eliminate the virus and therefore require long-term continued therapy. In addition, there are significant concerns about virus rebound on discontinuation of therapy and the development of fibrosis and hepatocellular carcinoma despite therapy. Therefore, the search for new, more effective, and safer antiviral agents that can cure hepatitis B virus (HBV) continues. Anti-HBV drug discovery and development is fundamentally impacted by our current understanding of HBV replication, disease physiopathology, and persistence of HBV covalently closed circular DNA (cccDNA). Several HBV replication targets are the basis for novel anti-HBV drug development strategies. Many of them are already in clinical trial phase 1 or 2, while others with promising results are still in preclinical stages. As research intensifies, potential HBV curative therapies and modalities in the pipeline are now on the horizon.
Keywords: Hepatitis B, Core inhibitor, cccDNA, DAA—directly acting antiviral, Immune therapy, Hepatocellular carcinoma
5.1. Introduction
Chronic hepatitis B virus (HBV) infection affects approximately 300 million people worldwide [1], and while prophylactic vaccines and antiviral therapies are currently in use, they do not provide a cure. Therefore, safe antiviral agents that target the HBV replication cycle and sites of virus persistence are urgently needed to prevent the nearly one million human deaths annually due to liver diseases associated with hepatitis B. HBV is a hepadnavirus that replicates its DNA in the liver through two main steps: formation of covalently closed circular DNA (cccDNA) and the reverse transcription of a pregenomic RNA (pgRNA).
With current available antiviral therapies for chronic hepatitis B, it is possible to control HBV replication. However, treatment is non-curative and therefore requires long-term continued use which has resulted in concerns for the development of antiviral resistance and adverse events, such as renal impairment or gastrointestinal disorders (important issue when considering adherence to treatment) [2–4]. The clinical endpoints now are focused on suppressing viral replication and alanine aminotransferase (ALT) normalization. This desirable endpoint of a functional cure (loss of HBsAg) is unlikely with current nucleoside analogs or pegylated interferons [5]. This may be due to cccDNA that persists in the nuclei of infected hepatocytes where it forms the template for all viral transcripts and HBV integration. New HBV targets and immune therapies are being sought, and we aim to review them according to their stage in clinical development, focusing on medicinal chemistry and/or biochemistry/molecular biology [6]. In addition, this review focuses on the outcomes of antiviral drugs newly developed or in clinical evaluation, as well as novel experimental drugs.
5.2. HBV Pathogenicity (Immunological Background)
HBV is a hepatotropic virus and most of the time does not cause a cytopathic effect [7]. The host immune response determines whether the virus persists (chronic infection) or not (cleared infection). In the natural history of chronic hepatitis B infection, initially there is an immunotolerant phase characterized by the presence of HBeAg, high rates of HBV DNA replication, and absence of inflammatory liver disease progression [3]. In this phase, the innate immune system is poorly activated due to an intrinsic ability of the virus to escape recognition [8].
In contrast, a persistent immune response to HBV-infected hepatocytes is the determinant of chronic liver disease, with inflammation (with or without HBeAg) leading to progression of fibrosis and cirrhosis, and ultimately hepatocellular carcinoma [3, 9, 10]. Individuals who have resolved HBV infection, with HBsAg clearance with or without HBs antibody, undetectable HBV DNA, and normal levels of ALT, are in the so-called functional cure phase [11]. In this phase, HBV is not fully eliminated, with a few hepatocytes remaining with the cccDNA form under a repressed translational control by innate and adaptive immune mechanisms [9].
In this regard, several immune pathways with the potential to suppress HBV replication in infected hepatocytes are currently under consideration as targets for the development of new therapeutic strategies for chronic hepatitis B infection. For example, retinoic acid-inducible gene-I (RIG-I) and apolipoprotein B mRNA editing catalytic polypeptide-like (APOBEC) [9] are among other pathways that will be discussed below.
5.3. HBV Replication
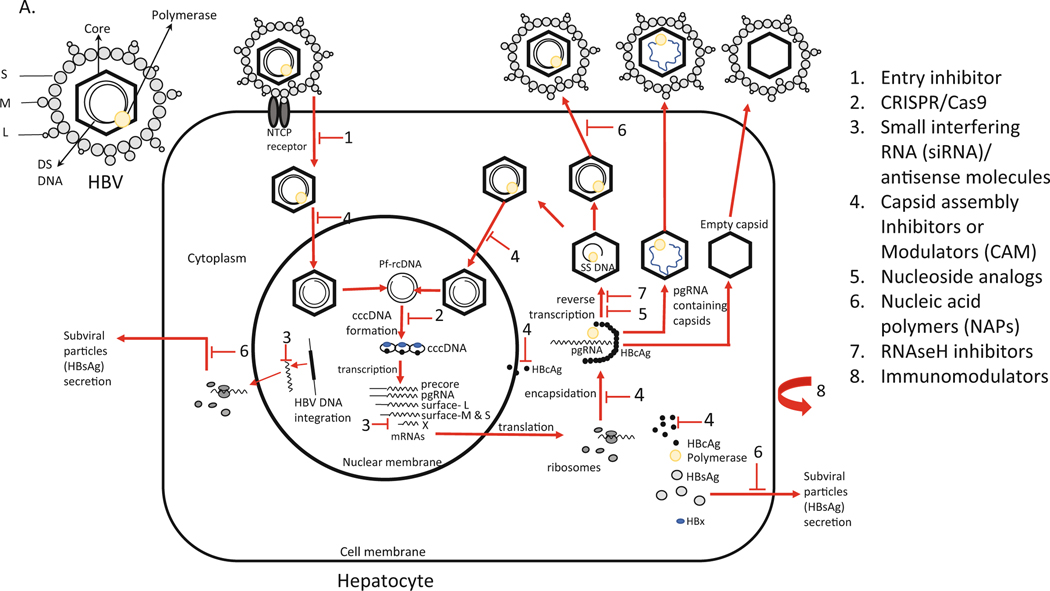
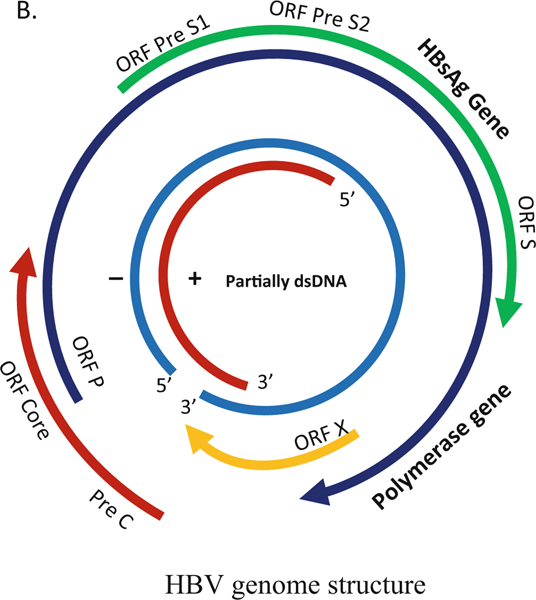
HBV is a 3200 bp partially double-stranded DNA (rcDNA, relaxed circular DNA) from Hepadnaviridae family. Difference greater than 8% in nucleotide sequence across the complete HBV genotype determines ten major genotypes (A to J) with differences in replication, natural history, pathogenesis, and treatment response [12, 13]. HBV genome encodes four overlapping genes. The HBV RNA transcripts are translated into seven proteins: HBsAg (surface large [preS1+preS2+S domains], middle [preS2+S domain], small [S domain]), HBeAg, HBcAg (core), RT-polymerase, and X protein. The HBV virion particles have an outside envelope composed with three forms (large, middle, and small) of surface proteins that encloses the capsid with the double-stranded DNA genome (Fig. 5.1). An important intermediate form (occurring in the nucleus of infected cells) is the covalently closed circular DNA (cccDNA) that is the template for pregenomic RNA (pgRNA) transcription and produces the template for reverse transcription and viral genome replication [14].
Fig. 5.1.
(a, b) HBV replication mechanism, genome structure, and schematic representation of inhibition sites. (a) The HBV has an envelope composed with three forms (large, middle, and small) of surface proteins that encloses the capsid with the double-stranded DNA genome. (b) Replication starts with HBV binding to the hepatocyte at the NTCP receptor. After entry, the viral particles are uncoated, and the nucleocapsid particle goes to the cellular nucleus. HBV protein free rcDNA (Pf-rcDNA) is converted to an episomal cccDNA, which is the transcription template for all four viral RNAs. The pgRNA is encapsidated together with viral polymerase and subsequently reverse-transcribed into viral minus strand DNA, followed by degradation of the RNA by RNAseH. Then, the plus-stranded DNA is synthesized to form the partially double-stranded relaxed circular DNA. Mature nucleocapsid can either be recycled back to the nucleus to maintain the pool of cccDNA or packed with envelope proteins and exported as infectious virions to infect other cells. pgRNA containing nucleocapsid and empty nucleocapsids are also packed with envelope proteins and released. S small, M medium, L large, DS double stranded, NTCP sodium taurocholate cotransporting polypeptide, CRISPR clustered regularly interspaced short palindrome repeats; CAS9 CRISPR associated 9
5.3.1. Replication Cycle
HBV binds to the hepatocyte at the sodium taurocholate cotransporting polypeptide (NTCP) receptor and enters into the cells. HBV attachment is believed to be mediated through the preS1 domain [15]. After entry, the viral particles containing the relaxed circular DNA (rcDNA) are uncoated, and the nucleocapsid particle must be directed into the cellular nucleus. HBV rcDNA is converted to an episomal cccDNA (see detailed information below). HBV cccDNA is the transcription template for all four viral RNAs (Fig. 5.1):
A 2.4-kb mRNA for the large (L) envelope protein, a 2.1-kb mRNA for the middle (M), and major surface (S) proteins
A 0.7-kb mRNA for the X protein
A 3.5-kb pre-core mRNA that encodes the pre-core protein
A 3.5-kb pregenomic RNA (pgRNA) that encodes the core and the polymerase
The pgRNA, upon being exported to the cytoplasm, is encapsidated together with viral polymerase and subsequently reverse-transcribed into viral minus strand DNA. Then, the plus-stranded DNA is synthesized to form the partially double-stranded relaxed circular DNA. The mature nucleocapsid can either be recycled back to the nucleus to maintain the pool of cccDNA or packed with envelope proteins and exported as infectious virions to infect other cells [14, 16] (Fig. 5.1).
5.3.2. Role of cccDNA
Intrahepatic cccDNA is the episomal virus template in the nucleus of HBV-infected hepatocytes. It is considered an important cause of viral persistence and a key obstacle for a cure of chronic hepatitis B [17]. This is especially true because current antiviral therapies including nucleoside analogs do not eliminate HBV mini-chromosome (cccDNA) or integrated HBV; therefore, continued virus gene expression from these templates will drive pathogenesis toward hepatocellular carcinoma, one of the main complications of chronic hepatitis B. Another currently used treatment for chronic hepatitis B, interferon alpha, upregulates the expression of APOBEC3 nuclear deaminase resulting in a modest reduction in cccDNA copy number via deamination [18].
Because cccDNA elimination is a major goal for the future HBV antiviral agents for the treatment of chronic hepatitis B, it is important to monitor and study this particular HBV form. However, the amount of cccDNA compared to pgRNA is very low (median 1.5 copies and 6.5 per cell, respectively) [19]. Therefore, to detect HBV cccDNA unambiguously is a great challenge [17]. Southern blotting is the gold standard test for detection and quantification of HBV intermediates and cccDNA; however, few samples can be tested at a time, and it requires high amounts of infected cells to detect cccDNA. Because it is not a high-throughput system, other tests including cccDNA-specific PCR have been assessed using specific primers located at each side of the gap region of rcDNA together with the appropriate HBV DNA purification or nucleus enrichment and the use of appropriate enzymes to selectively remove HBV rcDNA without degrading cccDNA [20]. Because liver biopsy is required to quantify cccDNA in vivo, measurements of HBV RNA and HBcAg in the serum may serve as surrogate biomarkers for cccDNA [11].
5.4. Overview of Current Therapies
Interferon alpha 2b (FDA approved in 1991) and peginterferon alpha-2a (approved in 2005) are immunomodulators administered subcutaneously, but due to adverse effects treatment duration varies up to 48 weeks (Table 5.1) [26, 27]. There are reports that HBV genotype A may present a higher response rate considering HBeAg seroconversion [28, 29].
Table 5.1.
Antivirals approved for chronic hepatitis B
| Drug | Route | Class | HBV DNA EC50, μM | Company | FDA approval year |
|---|---|---|---|---|---|
| Lamivudine | Oral | Nucleoside analog | 0.56 [21] | GlaxoSmithKline | 1998 |
| Adefovir dipivoxil | Oral | Nucleotide analog | 0.58 [21] | Gilead Sciences | 2002 |
| Entecavir | Oral | Nucleoside analog | 0.00036 [21] | BMS | 2005 |
| Tenofovir disoproxil fumarate | Oral | Nucleotide analog | 0.1 [22] | Gilead Sciences | 2008 |
| Telbivudine | Oral | Nucleoside analog | 1.3 [23] | Novartis | 2006 |
| Tenofovir alafenamide | Oral | Nucleotide analog | 0.0347 [24] | Gilead Sciences | 2016 |
| Interferon alpha 2b | Parenteral | Immunomodulator | Merck | 1991 | |
| Peginterferon alpha 2a | Parenteral | Immunomodulator | Genentech | 2005 | |
| Clevudine | Oral | Nucleoside analog | 0.053 [21] | Bukwang/Esai Pharmaceuticals | 2009 |
| Besifovir (LB80380) | Oral | Nucleotide analog | 0.5 [25] | LG Chem Ltd. | n/a |
| Thymosin alpha-1 | Parenteral | Immunomodulator | SciClone Pharmaceuticals |
EC50 median effective concentration to inhibit HBV DNA replication, n/a not applicable
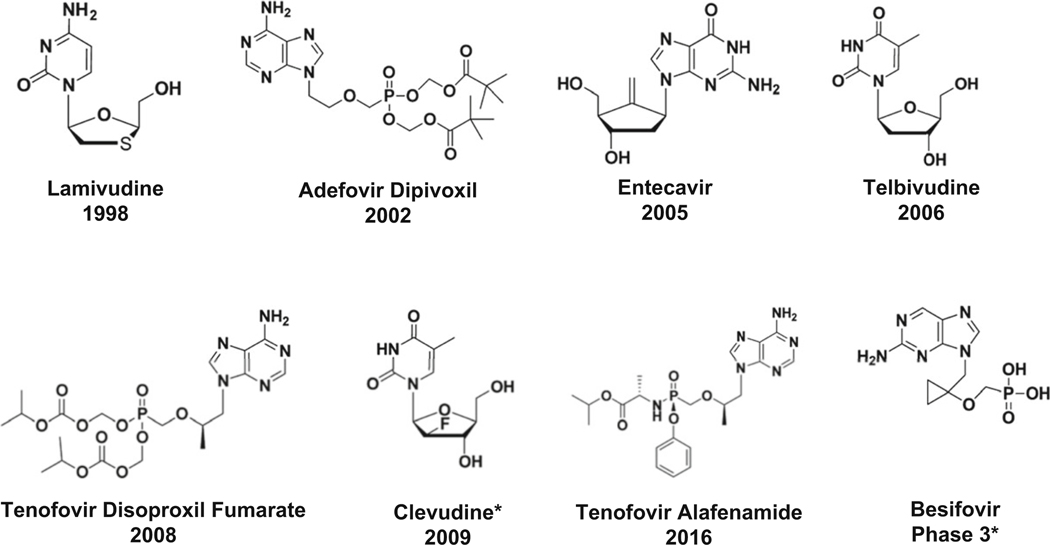
Lamivudine (approved in 1998), adefovir (approved in 2002), entecavir (approved in 2005), telbivudine (approved in 2006), tenofovir (approved in 2008), and tenofovir alafenamide (approved in 2016) are nucleoside analogs used orally, with fewer adverse events compared to immunomodulators and very efficient to reduce viral load (Table 5.1 and Fig. 5.2) [27]. However, a functional cure (loss of HBsAg) is rarely seen with these therapies. Duration of treatment varies, most of the time lasting several years. Because of the long-term need for these medications, adhesion to treatment is a concern, together with the development of drug resistance [21, 30].
Fig. 5.2.
Chemical structures of nucleoside/nucleotide analogs and year of FDA approval. *Approved in South Korea
Other nucleoside analogs approved are clevudine (approved for HBV in South Korea and the Philippines) and besifovir (nucleotide approved in South Korea) [31]. Although clevudine was approved as an antiviral agent for HBV without significant toxicity during the six-month clinical trial, longer therapy (14 months) was found to cause reversible mitochondrial myopathy [32]. This nucleoside analog was one of the few drugs that seemed to have an impact on HBV cccDNA in a woodchuck model (Tennant, personal communication).
Thymosin alpha-1 (Zadaxin) is an immune modulator, administered subcutaneously with minimal side effects approved as monotherapy for chronic hepatitis B in Asian countries [33]. The activity is via an enhancement of T cell differentiation and maturation and is especially effective in settings where there is a reduction in T cell number and/or function [33].
5.5. Drugs in the Pipeline
There are several novel antiviral agents being developed for chronic hepatitis B. The drugs can be divided according to their strategies to eradicate chronic HBV infection (Table 5.2) [34]:
Virologic (direct-acting agents or DAAs)
Host immune approaches (indirect-acting agents or immune therapy)
Table 5.2.
Classes of antiviral agents in the pipeline for HBV
| Direct-acting antiviral agents (DAAs) |
| Capsid assembly inhibitors or modulators (CAM) |
| Entry inhibitor |
| Small interfering RNA (siRNA) |
| Nucleic acid polymers (NAPs) |
| HBsAg inhibitors |
| s-Antigen transport inhibiting oligonucleotide polymers (STOPs) |
| Antisense molecules |
| Nucleoside analogs |
| Indirect-acting agents (immune therapy) |
| Therapeutic vaccines |
| Innate immune stimulation |
| TLR-8 agonist |
| TLR-7 agonist |
| Host acting pathway |
| Apoptosis inducer |
| Cyclophilin inhibitor |
| Gene editing |
| Gene-editing CRISPR/Cas 9 |
| Gene-editing ARCUS platform |
| Other mechanisms |
| Monoclonal antibody |
| FXR agonist |
| Host targeting antisense (LNA) |
| PD-L1 |
| Cell immunotherapy |
| MicroRNA |
| Nucleic acid-directed HBV cell killing |
5.5.1. Direct-Acting Antiviral Agents (DAAs)
Virologic antiviral agents or DAAs are new therapies that could directly target HBV replication steps without killing infected cells [35]. Nucleoside analogs target the viral reverse transcriptase enzyme, thus inhibiting HBV replication. Several nucleoside analogs are approved for chronic hepatitis B treatment as mentioned above, but because they require long-term use and do not completely clear HBV from hepatocytes, new DAAs are being developed, and next we will discuss different strategies used.
5.5.1.1. Capsid Assembly Effectors or Modulators (CAM)
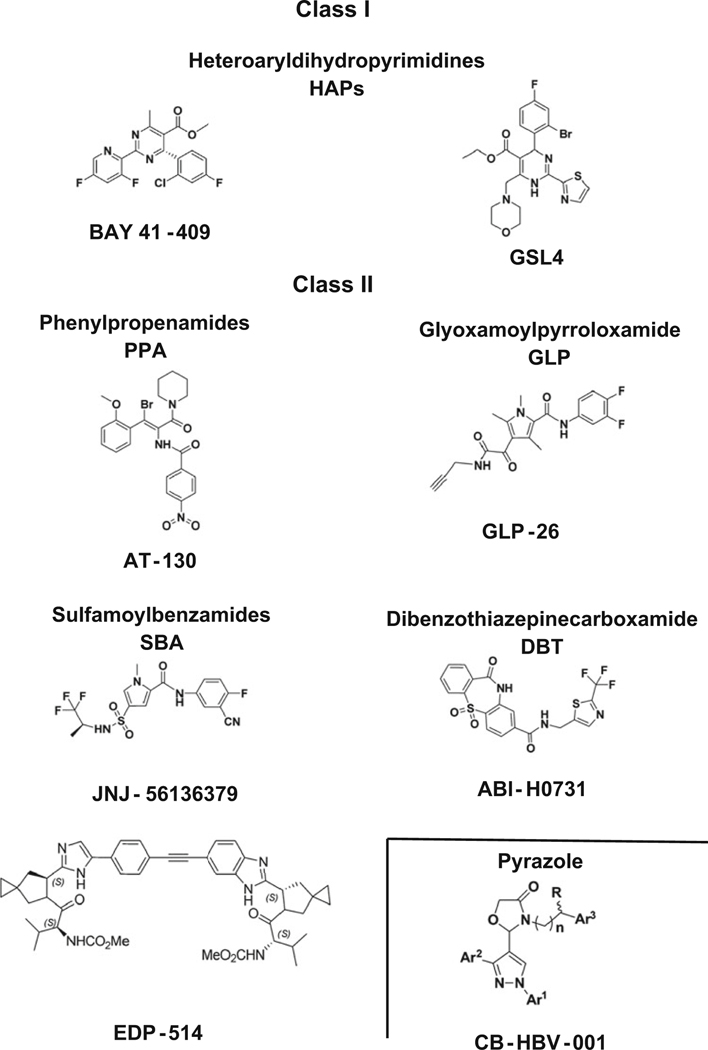
The HBV nucleocapsid plays an essential role in the viral replication cycle that includes HBV genome packaging, reverse transcription, intracellular trafficking of relaxed circular DNA (rcDNA) into the nucleus, and maintenance of chronic infection. Capsid assembly modulators (CAM) are characterized by two types (Table 5.3 and Fig. 5.3): (1) class I or heteroarylpyrimidines (HAP) are core protein allosteric modulators (CpAM) that upon binding to HBV capsids promote their misassembled to aberrant non-capsid core polymers, and (2) class II or phenylpropanamides (PP), sulfamoylbenzamides (SBA), or derivatives are capsid assembly modulators that upon binding to the capsid form normal but empty nonfunctional capsids devoid of pgRNA/rcDNA.
Table 5.3.
Clinical status of capsid assembly effectors or modulators
| Drug/class | Route | HBV DNA Log reductiona | Company | Clinical trial phase | ClinicalTrials.gov identifier |
|---|---|---|---|---|---|
| Morphothiadin (GLS4)/I | Oral | 2.3 | HEC Pharma, PR China | 2 | NCT03638076/NCT04147208/NCT04147208 |
| JNJ 56136379/II | Oral | 2.9 | Janssen, Ireland | 2 | NCT02662712 |
| ABI-H0731/II | Oral | 3.9 | Assembly Biosciences, USA | 2 | NCT03109730/NTC03780543/NTC03577171/NTC03576066/NTC04454567/NTC02908191 |
| ABI-H2158/II | Oral | 2.3 | Assembly Biosciences, USA | 2 | NCT04398134 |
| QL-007/ | Oral | - | Qilu, PR China | 2 | NCT04157257/NCT04157699 |
| RG7907 (RO7049389)/I | Oral | 3–5 | Roche, Switzerland | 1 | NCT02952924 |
| EDP-514/II | Oral | > 4.0b | Enanta Pharma, USA | 1 | NCT04470388 |
| ABI-H3733/II | Oral | 5.0c | Assembly Biosciences, USA | 1 | NCT04271592 |
| ZM-H1505R/pyrazole | Oral | ZhiMeng Biopharma, PR China | 1 | NCT04220801 | |
| ALG-000184/II | Oral | 5.0b | Aligos Therapeutics, USA/Emory University | 1 | NCT04536337 |
| GLP-26/II | Oral | 1–3b | Emory University, Aligos Therapeutics | Preclinical | n/a |
| CB-HBV-001/pyrazole | Oral | 12c | ZhiMeng Biopharma, PR China | Preclinical | n/a |
N/A not applicable
HBV DNA Log10 IU/ml in vivo (data obtained from clinical trials)
Log10 decrease in HBV DNA (data obtained from mice models)
HBV DNA, EC50 (nM) in vitro
Fig. 5.3.
Chemical structures of prototypical capsid assembly effectors or modulators (CAM)
Both classes of HBV capsid effectors can interfere with several steps of HBV replication cycle including pre- and post-capsid formation, prevention of capsid assembly, perturbation of capsid integrity of incoming virus particles, entry of HBV capsid and core particles into the cell nucleus, pregenomic RNA encapsidation, and consequently its reverse transcription. All these changes in the HBV replication cycle may ultimately prime inhibition of cccDNA formation and/or amplification (Fig. 5.1).
There are five capsid effectors in phase 2 clinical trials. Morphothiadin (GLS4) is a class I HAP compound developed from Bay41–4109 that has shown potent in vitro inhibition of HBV DNA replication; nevertheless, in vivo studies with health volunteers have shown that GLS4 needs an extra-booster (ritonavir) to increase its plasma concentration and achieve effective antiviral activity in humans [36]. Two CpAM ABI-H0731 and ABI-H2158 are in phase 2 clinical trials. ABI-H0731 has shown a decline in HBV RNA that correlated with HBV DNA decline in a 4-week therapy [37], and several phase 2 clinical trials are being conducted with this compound in combination with nucleoside analogs, including entecavir or tenofovir. ABI-H2158 has shown in vivo decline of HBV DNA and pgRNA by ~2 log10 IU/ml and is in phase 2 clinical trial in combination with entecavir [38]. JNJ56136379 is an inducer of empty nonfunctional HBV capsids (CAM-N) that was well tolerated by healthy volunteers in phase 1 and has shown reduced HBV DNA and RNA levels; in a 4-week phase 1b monotherapy study, baseline polymorphisms or enrichment of substitutions did not show an impact on virological response, though the emergence of resistance to longer treatments are underway in phase 2 studies [39]. QL-007 (Qilu, PR China) is in phase 2 clinical trials with entecavir or tenofovir for both safety and efficacy evaluation (Table 5.3).
Four capsid effectors are in phase 1 clinical trial including RG7907, EDP-514, ABI-H3733, and ZM-H1505R. RG7907 (RO7049389), a class I CpAM, reduced both HBV DNA and RNA levels at the end of 28-days treatment, with favorable PK profiles [40]. EDP-514 is a class II core inhibitor that has shown to prevent de novo formation of cccDNA in human primary hepatocytes, and it is in phase 1a/1b study with healthy volunteers [41]. ABI-H3733 is a class II capsid inhibitor that has shown to be a potent inhibitor of HBV DNA (EC50 = 5 nM) and cccDNA formation (EC50 = 125 nM) in vitro [42]. ZM-H1505R is a new pyrazole compound that inhibits HBV DNA replication by inhibiting pgRNA encapsidation and cccDNA formation.
Three main capsid effectors are in preclinical studies: GLP-26, ALG-000184, and CB-HBV-001. GLP-26 (Emory University) is a novel class II CAM, with a unique glyoxamidopyrrolo backbone. It showed substantial in vitro effects in HBV DNA replication and HBe antigen with low nanomolar ranges (EC50 = 3 nM for both markers), with >1 log reduction in cccDNA, and no apparent cytotoxicity. Sustained decreases in HBeAg and HBsAg levels were also observed in HBV-infected humanized mouse model treated with GLP-26 in combination with entecavir up to 3 months after drug cessation [43–46]. ALG-000184 (Aligos Therapeutics/Emory University) is the prodrug of ALG-001075, another potent class II CAM that has shown picomolar activity in vitro and substantial effects in HBV DNA replication in mouse model, with no apparent signs of toxicity and markedly improved solubility [47]. This drug is now entering phase 1a/1b clinical trial in New Zealand, Hong Kong and Republic of Moldova. CB-HBV-001 is a new oxazolidinone, pyrazole capsid inhibitor that is being evaluated in preclinical trials (AASLD 2018).
5.5.1.2. Entry Inhibitors
HBV enters the cell by attaching the receptor binding region of pre-S1 to the NTCP receptor at the membrane of the hepatocyte [48] (Fig. 5.1). Bulevirtide (Myrcludex B) binds irreversibly to NTCP inhibiting the HBV entry into the hepatocyte [49]. This drug is administered subcutaneously and is being studied for chronic hepatitis B and delta in phase 2b with or without peginterferon (PEG-IFN) alfa-2a (Table 5.4) [49]. Preliminary results showed that 12/30 (40%) of individuals treated with bulevirtide plus PEG-IFN for 48 weeks had alanine aminotransferase (ALT) normalization and HDV RNA negative. In the follow-up of 24 weeks of treatment, 4 out 15 individuals treated with 2 mg bulevirtide plus PEG-IFN had undetectable HBsAg, and three out four had HBsAg seroconversion [50]. Bulevirtide was well tolerated, with some drug-related adverse events primarily caused by an increase in total bile salts [50]. This is explained because the drug binding to NTCP prevents infection but also inhibits hepatic bile salt uptake leading to the transiently elevated bile salt level [51].
Table 5.4.
Entry Inhibitor in development
| Drug | Route | Dose | Company | Clinical trial phase | ClinicalTrials.gov identifier |
|---|---|---|---|---|---|
| Bulevirtide (myrcludex B) | Parenteral | 2–5 mg SC qd 48 weeks | Hepatera, Russia with MYR GmbH, Germany | 2b | NCT02888106 |
5.5.1.3. Small Interfering RNA (siRNA)
RNA interference (RNAi) is the mechanism through which double-stranded RNAs silence cognate genes (Fig. 5.1). It is characterized by the presence of RNAs about 22 nucleotides homologous to the gene that is being suppressed. Dicer is the cellular nuclease that cleaves double-stranded RNAs and can produce putative guide RNAs or small interfering RNA (siRNA) [52]. After the sense strand is removed and the antisense strand is loaded on the RNA-induced silence complex (RISC), it hybridizes to a complementary region of a target mRNA, which results in its degradation [53]. This phenomenon provides effective agents for inhibiting infectious, metabolic, cancer, and genetic diseases [53]. A critical issue in the development of siRNA-based drugs is to avoid toxicity such as (1) immunogenic reactions to dsRNA (2′-O-methyl base modifications have largely avoided this issue), (2) toxicity of excipients (work continues on developing potent and nontoxic nanoparticles), (3) unintended RNAi activity (avoided by detailed screening target sites against human genome sequences), and (4) on target RNAi activity in nontarget tissues (selection of highly diseased selective genes and delivery routes which reduce accumulation in nontarget tissues) [54]. Previous studies showed that siRNA could significantly inhibit HBV transcripts and cccDNA in vitro in HepG2 cells and in vivo in mice [53, 55, 56]. Currently, several siRNAs are being evaluated in preclinical and phases 1, 1/2, and 2 clinical trials shown in Table 5.5. VIR-2218 has shown dose-dependent HBsAg reductions (mean decline of 1.0 log10) in HBeAg negative or positive patients virally suppressed on nucleos (t)ide analogs without significant fibrosis [58]. Another siRNA drug, JNJ-3989 (ARO-HBV) that is in a phase 2a study, has demonstrated a ≥log10 reduction in HBsAg at nadir was achieved in 98% of patients [59]. In total, 15/38 (39%) of patients who were responders throughout the study were sustained responder at day 392 [59].
Table 5.5.
Small interfering RNA (siRNA) drugs in development
| Drug | Route | Company | Clinical trial phase | ClinicalTrials.gov identifier |
|---|---|---|---|---|
| VIR-2218 (ALN-HBV02) | Parenteral (SC) | Alnylam and Vir Biotech, USA | 2 | NCT03672188 |
| JNJ-3989 (ARO-HBV) | Parenteral (SC) | Arrowhead Pharma with Janssen, USA | 2a | NCT03365947 |
| RG6346 (DCR HBVS) | Parenteral (SC) | Roche, Switzerland | 2/1 | NCT03772249 |
| AB-729 [57] | Parenteral (SC) | Arbutus Biopharma, USA | 1 | NA |
NA not available
5.5.1.4. Nucleic Acid Polymers (NAPs)
NAPs are phosphorothioate oligonucleotides (PS-ONs) that inhibit HBV via a post-entry mechanism blocking the assembly/release of HBV subviral particles (Fig. 5.1). The universal model for NAP pharmacology is based on the interaction of the amphipathic protein domain and the hydrophobic side of NAPs, preventing the conformational changes in the target or its interaction with other amphipathic helices [60]. In this class of antivirals, there are the HBsAg inhibitors and the STOPs (s-antigen transport inhibiting oligonucleotide polymers).
HBsAg Inhibitors
Aside from the ability of HBsAg to sequester anti-HBs from the blood system, HBsAg has direct immunoinhibitory action against both innate and adaptive immune responses (Fig. 5.1). HBsAg loss is infrequently achieved with the current therapy; therefore, antivirals targeting the inhibition of HBsAg are being developed. NAPs have the ability to interact with hydrophobic surfaces of proteins and have emerged as the first therapy to be able to achieve rapid HBsAg loss [61].
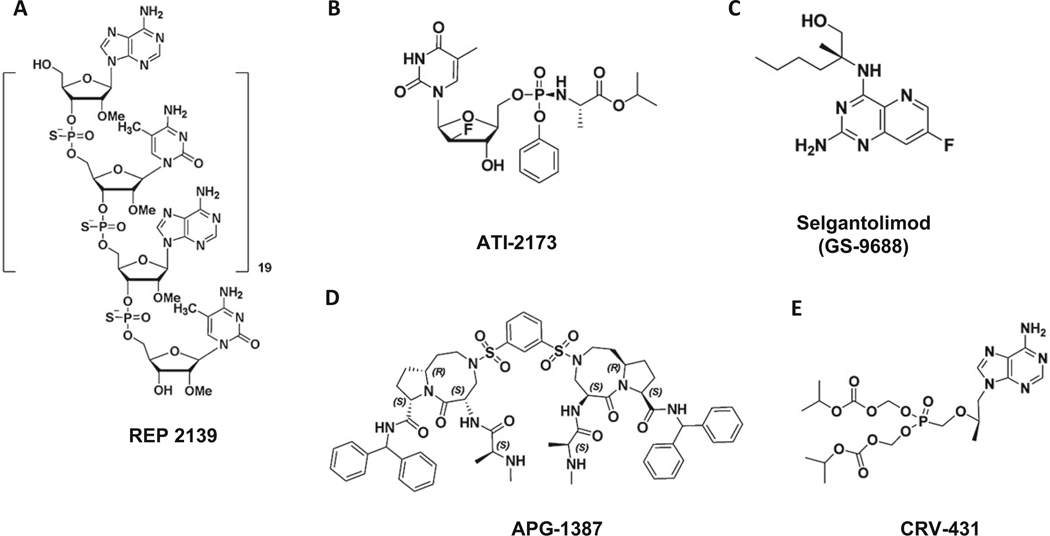
REP 2139 is a phosphorothioate oligodeoxyribonucleotide (PS-ONs) with the sequence (dAdC)20 (Table 5.6 and Fig. 5.4) [62]. Clinical studies of REP 2139 in combination with thymosin or PEG-IFN was well tolerated and resulted in liver flares (without liver dysfunctions) following initial reductions of serum HBsAg and HBV DNA [62]. REP 2165 is a version of REP 2139 which has been shown preclinically to retain antiviral activity with lower accumulation in the liver. The results of phase 2 randomized trial showed that addition of NAPs to tenofovir and PEG-IFN increased functional cure after therapy without altering tolerability [63].
Table 5.6.
HBsAg inhibitors in development
| Drug | Route | Company | Clinical trial phase | ClinicalTrials.gov identifier |
|---|---|---|---|---|
| REP 2139 | Parenteral | Replicor, Canada | 2 | NCT02726789 |
| REP 2165 | Parenteral | Replicor, Canada | 2 | NCT02565719 |
Fig. 5.4.
Chemical structures of HBsAg inhibitor (a), nucleoside analog (b), innate immune stimulation (c), cellular inhibitor of apoptosis proteins (cIAPs) (d), and cyclophilin inhibitor (e)
STOPs (s-Antigen Transport Inhibiting Oligonucleotide Polymers)
STOPs are oligonucleotide aptamers (protein binding) comprised of a repeating poly AC sequence (Fig. 5.1). STOPs share the structural similarity with NAPs but contain several novel chemical features. STOPs can reduce HBsAg secretion by affecting protein trafficking from the infected cell resulting in its degradation [64]. In HepG2.2.15 cells, ALG-10133 reduced HBsAg secretion in nanomolar range and with synergistic effects when combined with class II CAMs [65]. ALG-10133 has been selected as the lead candidate, starting clinical trials on 2020 with projected human efficacious dose of 30–75 mg delivered SC weekly (Table 5.7) [66].
Table 5.7.
STOPs (s-antigen transport inhibiting oligonucleotide polymers) in development
| Drug | EC50 μM | Route | Company | Clinical trial phase | ClinicalTrials.gov identifier |
|---|---|---|---|---|---|
| ALG-10133 | 0.0032 | Parenteral (SC) | Aligos Therapeutics, USA | 1 | NCT04485663 |
EC50 median effective concentration to inhibit HBV DNA replication
5.5.1.5. Antisense Molecules
Antisense oligonucleotides (ASO) (Table 5.8.) are small single-stranded nucleic acid sequences that bind with high selectivity to their target RNAs. This triggers degradation via an RNAse H-dependent pathway [68]. GSK 3228836 is a 2′-O-methoxyethyl free ASO currently in development for the treatment of chronic hepatitis B. It has been tested as a subcutaneous injection in doses up to 120 mg, and no safety concerns were identified [69]. GSK3389404 (GSK404) is a second-generation ASO that showed an acceptable safety profile [70]. GSK3389404 presented platelet dose-dependent declines that plateaued on treatment and started to recover after dose completion [70, 71]. RO7062931 is an N-acetylgalactosamine (GalNAc) conjugated single-stranded oligonucleotide (SSO) with locked nucleic acid (LNA) that is complementary to messenger RNAs (mRNAs) of the HBV genome [72, 73]. Gal-Nac conjugation should reduce ASO renal and platelet toxicities. It was well tolerated in healthy volunteers. Phase 1 studies showed a mean nadir of HBsAg of −0.5 log10 IU/mL, with treatment emergent ALT elevations with transient concurrent HBsAg decline (0.6–0.8 log10 IU/mL) with no changes in liver function [74].
Table 5.8.
Antisense molecules in development
| Drug | Route | Company | Clinical trial phase | ClinicalTrials.gov identifier |
|---|---|---|---|---|
| GSK 3228836 /IONIS-HBVRx/ISIS505358 | Parenteral (SC) [67] | Ionis with GSK, USA | 2a | NCT04449029/NCT02981602 |
| GSK3389404/Ionis-HBV-LRx | Parenteral (SC) | Ionis with GSK | 2a | NCT02647281 |
| RO7062931 | Parenteral (SC) | Roche | 1a | NCT03505190/NCT03038113 |
5.5.1.6. Nucleoside Analogs
ATI-2173 is a novel phosphoramidate prodrug of clevudine in preclinical studies for chronic hepatitis B (Table 5.9 and Fig. 5.4) [67]. Long-term use of clevudine was found to exhibit reversible skeletal myopathy in a small group of individuals and therefore subsequently discontinued from development. ATI-2173 was designed by modifying clevudine that bypasses the first phosphorylation step where the 5′-monophosphate is converted to the active 5′-triphosphate in the liver [67]. ATI-2173 activity was decreased by 25 viral polymerase mutations associated with entecavir, lamivudine, and adefovir resistance, but not capsid inhibitor resistance mutations [67]. It has been claimed that this compound could behave as a non-nucleoside antiviral agent.
Table 5.9.
Nucleoside analog in development
| Drug | EC50 μM | Route | Company | Clinical trial phase | ClinicalTrials.gov identifier |
|---|---|---|---|---|---|
| ATI-2173 | 0.0013 | Oral | Antios Therapeutics, USA | 1 | NCT04248426 |
EC50 median effective concentration to inhibit HBV DNA replication
5.5.1.7. RNAseH Inhibitors
RNAseH is one of the two enzymatically active domains on HBV polymerase and destroys the HBV RNA after it has been copied into DNA by the reverse transcriptase [75]. RNAseH is a potential target for antiviral drugs, and over 150 RNAseH inhibitors are divided in four compound classes: (1) α-hydroxytropolones (αHT), (2) N-hydroxyisoquinolinediones (HID), (3) N-hydroxypyridinediones (HPD), and (4) and N-hydroxynapthyridinones [76–81]. Novel amide αHT were studied with EC50 values from 0.31 to 54 μM [79]. Studies in chimeric mouse showed that an HPD and an αHT suppressed HBV replication to up to 1.4 log10 after two weeks of treatment followed by a rebound in the viral titers [82].
5.5.2. Indirectly Acting Antiviral Agents (Immune Therapy)
Specific immune therapy can maintain the HBV replication under control of a functional host antiviral response [9] (Fig. 5.1). An example of approved immune therapy for chronic hepatitis B is interferon alpha (pegylated or not). Pegylated interferon alpha alone or in combination therapy can achieve sustained off-treatment control but in only a small portion of individuals [26].
Therapeutic restoration of protective immunity is a strategy that can be considered to achieve the functional cure of HBV [83]. Several approaches are being considered such as therapeutic vaccines, innate immune stimulation (TLR-8 and TLR-7 agonists), host acting pathway (apoptosis inducer and cyclophilin inhibitor), gene editing, and many other mechanisms.
5.5.2.1. Therapeutic Vaccines
There is a renewed interest in therapeutic vaccines with the development of novel formulations, suitable immunization routes for designed adequate antigens, and adjuvant strategies (Table 5.10). In addition, it is important to consider adequate strategies, including combination therapy with other antivirals, either concomitant or sequential strategies.
Table 5.10.
Therapeutic vaccines in development
| Drug | Platform | Route | Company | Clinical trial phase | ClinicalTrials.gov Identifier |
|---|---|---|---|---|---|
| NASVAC (ABX203) | HBs and HBc antigen mixed with carboxyl vinyl polymer [84] | Nasal | CIGB, Cuba | 3 | NCT01374308/NCT02249988 |
| HepTcell (FP-02.2) | Synthetic HBV-derived peptides formulated with IC31®, a TLR9based adjuvant [85] | IM [86] | Altimmune, USA | 2 | NCT02496897 |
| AIC 649 | Parapoxvirus (iPPVO) [87] | IV | AiCuris, Germany | 1 | |
| JNJ 64300535 (HB-110) | Plasmids encoding HBsAg, HBcAg, and IL12 [88] | IM Electroporation | Janssen, Ireland/Ichor Medical Genexine, USA | 1 | NCT03463369 |
| TG1050 | Ad5 which encodes truncated HBV core, POL, and two small domains from the ENV [89] | SC | Transgene, France | 1 | NCT04168333/NCT02909023/NCT04168333 |
| MVA-VLP-HBV | Modified vaccinia Ankara-virus-like particle-hepatitis B virus [90] | GeoVax, USA | Preclinical | n/a | |
| Chimigen HBV | Recombinant chimeric fusion protein comprising hepatitis B virus (HBV) S1 and S2 surface antigen fragments, core antigen, and a murine monoclonal antibody heavy chain fragment (Fc) [91] | Akshaya, Canada | Preclinical | n/a | |
| TherVacB | HBsAg, HBcAg, and a boost using a modified vaccinia virus Ankara (MVA) vector [92] | Helmholtz Zentrum Muenchen, Germany | Preclinical | n/a | |
| 3xT2A and Mix2A | VLVs expressing polymerase (Pol), core (HBcAg), and MHBs [93] | CaroGen, USA | Preclinical | n/a | |
| HBV | TheraT® and VaxWave® investigational arenavirus-based immunization technologies [94] | HOOKIPA Pharma, Austria, with Gilead | Preclinical | n/a | |
| VBI-2601 (BRII-179) | Recombinant, protein-based immunotherapeutic [95] | VBI Vaccines, USA | 1b/2a |
ID intradermal, SC subcutaneous, Ad adenovirus, POL polymerase, ENV envelope, VLV virus-like vesicles, N/A not applicable
5.5.2.2. Innate Immune Stimulation
The host immune responses to HBV determine if the individuals will clear (functional cure) or fail to clear the virus (chronic hepatitis B). Toll-like receptor (TLR) family and its functions are one way to modulate the immunological host responses [96]. TLR8 and TLR7 are endosomal TLRs members with a high degree of sequence and function similarity. They recognize pathogen-associated molecular patterns (viral single-stranded RNA fragments) and trigger innate and adaptive immune responses[96, 97]. Agonist ligands of Toll-like receptors 7 and 8 have immune-stimulating activity allowing to intervene several diseases and to be valuable vaccine adjuvant candidates [96].
Selgantolimod (formerly GS-9688) is a small molecular agonist of Toll-like receptor 8 (TLR8) [98]. It sustained reduced intrahepatic RNA and DNA of woodchuck hepatitis virus (WHV) in animal model. With a finite, short duration treatment, the serum WHsAg level reduced with half of animals with levels below the limit of detection [97]. Selgantolimod is an oral drug under phase 2 clinical trial (Table 5.11 and Fig. 5.4). RO7020531 (RG7854) is an oral prodrug of a TLR-7 agonist in phase 2 clinical trial (Table 5.11). Carboxylesterase (mainly CES2) and oxidation by aldehyde oxidase converts RO702053 into the active metabolite RO7011785 [99]. Preclinical data showed that a combination of HBV locked nucleic acid antisense oligonucleotide (HBV-LNA ASO) with RO7020531 reduced HBsAg and HBV DNA with delayed rebound off-treatment in mice [100].
Table 5.11.
Innate immune stimulation in development
| Drug | Class | Route | Company | Clinical trial phase | ClinicalTrials.gov identifier |
|---|---|---|---|---|---|
| GS9688 (Selgantolimod) | TLR-8 agonist | Oral | Gilead Sciences, USA | 2 | NCT03491553/NCT03615066 |
| RO7020531 (RG7854) | TLR-7 agonist | Oral | Roche, Switzerland | 2 | NCT02956850/NCT03530917/NCT04225715 |
5.5.2.3. Host Acting Pathway
Cellular inhibitor of apoptosis proteins (cIAPs) impairs clearance of hepatitis B virus (HBV) infection by preventing TNF-mediated killing/death of infected cells. Animal studies showed that drug inhibitors of cIAPs were able to reduce serum HBV DNA, hepatitis B surface, and core antigens [101]. APG-1387 is an apoptosis inducer; it is a second mitochondria-derived activator of caspase (SMAC) mimetic, and it targets inhibitors of apoptosis proteins (IAPs) [102]. Currently, APG-1387 is under clinical trial phase 1 study for chronic hepatitis B (Table 5.9 and Fig. 5.4).
CRV-431 is an oral cyclophilin inhibitor, non-immunosuppressive analog of cyclosporine A. CRV 431 is a small molecule under clinical development for the treatment of liver diseases including fibrosis and hepatocellular carcinoma [103]. Preclinical studies showed antiviral activity against hepatitis B reducing HBV DNA and HBsAg levels in transgenic mice and a phase 1 is ongoing (Table 5.12 and Fig. 5.4) [104].
Table 5.12.
Host-acting pathway
| Drug | Class | Route | Company | Clinical trial phase | ClinicalTrials.gov identifier |
|---|---|---|---|---|---|
| APG-1387 | Apoptosis inducer | IV | Ascentage Pharma, PR China | 1 | NCT03585322 |
| CRV-431 | Cyclophilin inhibitor | Oral [103] | Hepion, USA (formerly ContraVir) | 1 | NCT03596697 |
IV intravenous
5.5.2.4. Gene Editing
Clustered regularly interspaced short palindrome repeats (CRISPR)/Cas9-based antiviral strategy is one of the most versatile gene-editing tools, discovered as a bacterial adaptive immune system [105]. The CRISPR/Cas9 system can specifically destruct HBV genomes in vitro and in vivo, mediating specific cleavage of cccDNA [106–108] (Fig. 5.1). Several optimal targets in HBV genome have been described, such as the surface and polymerase overlap region; the YMDD RT motif and the HBV enhancer I, II, X protein; and pre-core regions with high efficacy [109]. However, CRISPR/Cas system inevitably targets integrated HBV DNA and induces double-strand breaks (DSBs) of host genome, raising concerns of genome instability and carcinogenicity [108, 110]. To avoid DSBs of the host genome, recently it was described a permanently Cas9-mediated base editors that effectively introduced nonsense mutations that generated premature stop codons of surface gene in both integrated and cccDNA reducing HBsAg secretion [110]. EBT107 is a gene-editing CRISPR/Cas 9 drug that uses a duplex gRNA excision knockout as a candidate for HBV in preclinical studies (Table 5.13) [111].
Table 5.13.
Gene editing
| Drug | Class | Company | Clinical trial phase |
|---|---|---|---|
| EBT107 | Gene-editing CRISPR/Cas 9 | Excision Bio, USA | Preclinical |
| ARCUS nucleases | Gene-editing ARCUS platform | Precision Biosciences, USA | Preclinical |
IV intravenous
ARCUS genome-editing technology is another platform of gene editing being developed for chronic hepatitis B [112]. The ARCUS technology is based on the properties of a naturally occurring gene-editing enzyme – the homing endonuclease I-CreI—and reduces the risk of additional off-target DNA edits [113].
5.5.2.5. Other Mechanisms
Recombinant hepatitis B human monoclonal antibody:
Lenvervimab (GC1102) is a recombinant hepatitis B human monoclonal antibody expected to improve sustained virological response reducing HBsAg levels in individuals with chronic hepatitis B infection [114]. It is under study for HBV-related liver transplant recipients (Table 5.14).
Table 5.14.
Drugs with other mechanisms in development
| Drug | Route | Mechanisms | Company | Clinical trial phase | ClinicalTrials.gov identifier |
|---|---|---|---|---|---|
| Lenvervimab (GC1102) | IV | Monoclonal antibody | Green Cross, South Korea | 2a | NCT03801798/NCT02304315 |
| Vonafexor (EYP001) | Oral | FXR agonist | Enyo Pharma, France | 1 | NCT04365933 |
| ASC22 (KN035) | SC | PD-L1 pathway | Ascletis Pharma, PR China | 2a | NCT04465890 |
| LTCR-H2–1 | IV | T cell immunotherapy | Lion TCR, Singapore | 1 | NCT04745403 |
IV intravenous, SC subcutaneous, N/A not applicable
Farnesoid X receptor (FXR) agonist:
HBV enters the hepatocyte by binding to NTCP, the genome of which contains two active farnesoid X receptor (FXR)α response elements that participate in HBV transcriptional activity [115]. In vitro studies showed that FXR agonists inhibited viral mRNA, DNA, and protein production and reduced the cccDNA pool size [115]. Vonafexor (EYP001) is a farnesoid X receptor (FXR) agonist with anti-HBV effects [116, 117]. It is under study in combination with PEG-IFN, nucleoside analogs in double or triple therapy (Table 5.14).
PD-L1 pathway:
The programmed cell death protein 1 (PD-1)/programmed death-ligand 1(PD-L1) pathway is a key immune checkpoint regulator that controls the induction and maintenance of immune tolerance in chronic hepatitis B infection [118]. ASC22 (KN035) is a novel fusion anti-PDL1 antibody being studied for the treatment of solid tumors and in clinical trials for chronic hepatitis B phase 2a (Table 5.14).
T cell immunotherapy:
LTCR-H2–1 (Table 5.14) is a preclinical drug that boosts adaptive immune response through T cell receptor (TCR) gene transfer [119]. It is engineered to target virus-derived peptides presented on MHC class I on the surface of virus-infected cells. This technology is based on leukapheresis to isolate white blood cells, followed by T cell expansion; HBV targeting TCR are introduced into the activated T cells by viral transduction or electroporation, and then after phenotypic and functional validation, the TCR-engineered T cells are infused back into the individual [120].
5.6. Conclusions
Currently, nucleoside analogs and peginterferon are available for chronic hepatitis B treatment and are quite effective and safe. They can prevent progression of disease, but even persons treated with these drugs can develop hepatocellular carcinoma. The treatments can achieve inhibition of HBV replication; however, few individuals achieve “functional cure” status (HBsAg clearance with or without surface antibody). Several novel drugs are in the pipeline for treatment and elimination of chronic hepatitis B. The drugs are at different stages of development from preclinical to phase 2 clinical trials, and some of them are considered for combination strategies. These drugs will be instrumental for a sustained HBV DNA undetectability with sustained clearance of HBsAg and for preventing liver cancer. Elimination of cccDNA and integrated HBV DNA will be key to eradicate chronic hepatitis B infection. Currently, there are numerous drugs that have the potential to cure HBV, but most do not have the necessary potency to clear all cccDNA. We now know that the half-life of cccDNA (several months and not decades) is shorter than was previously reported [121]. Thus, it may be possible to eliminate cccDNA in approximately 1 year with more potent agents or more likely a combined modality (e.g., capsid effector plus STOPs). As expounded above, a great number of approaches are being tried to eliminate HBV, and it is clear that we are beginning to turn the tide.
Key Points.
Current treatments do not completely clear HBV from hepatocytes leading to the establishment of lifetime chronic infection.
Novel anti-HBV therapies targeting different steps of HBV replication cycle with the potential of curing individuals chronically infected are needed.
Elimination of cccDNA from the nuclei of hepatocytes and clearance of HBV surface antigen (HBsAg) from blood are crucial to achieving a functional and complete cure.
Drug-drug combinations synergistically targeting key steps of HBV replication cycle and immunomodulators boosting the host immune response may lead to a functional cure.
Novel strategies including CRISPR and siRNA technologies which can inactivate persistent HBV cccDNA and also target integrated viral DNA may eliminate HBV from chronically infected human hepatocytes.
Acknowledgments
This work was supported by the funding from NIH (1-RO1-AI-132833 to RFS) (1-RO1-AI-148740 to JC Gumbart) and Emory Center for AIDS Research (5P30-AI-50409 to RFS). SKO would like to thank Fundação de Amparo à Pesquisa de São Paulo (FAPESP 2017/50042-2) and Brazilian Council for Development of Science and Technology (CNPq) for grant PQ 308609/2018-2. The authors thank Dr. James J Kohler (Emory University) for editing the review and Dr. Zhe Chen (Emory University) for drawing the chemical structures.
Abbreviations
- Ad
Adenovirus
- ALT
Alanine aminotransferase
- APOBEC
Apolipoprotein B mRNA editing catalytic polypeptide-like
- ARCUS
Gene-editing platform
- ASO
Antisense oligonucleotide
- CAM
Capsid assembly effectors or modulators
- CAS
CRISPR associated
- cccDNA
Covalently closed circular DNA
- CES
Carboxylesterase
- cIAPS
Cellular inhibitor of apoptosis proteins
- CpAM
Core protein allosteric modulators
- CRISPR
Clustered regularly interspaced short palindrome repeats
- DAAs
Direct-acting agents
- DS
Double stranded
- DSBs
Double-strand breaks
- EC50
Median effective concentration to inhibit HBV DNA replication
- ENV
Envelope
- FXR
Farnesoid X receptor
- GalNAc
N-acetylgalactosamine
- GLS4
Morphothiadin
- HAP
Heteroarylpyrimidines
- HBcAg
HBV core antigen
- HBeAg
HBV e antigen
- HBsAg
HBV surface antigen
- HBV
Hepatitis B virus
- HID
N-hydroxyisoquinolinediones
- HPD
N-hydroxypyridinediones
- IAPs
Inhibitors of apoptosis proteins
- ID
Intradermal
- IM
Intramuscular
- IV
Intravenous
- L
HBV large surface protein
- LNA
Locked nucleic acid
- M
HBV middle surface protein
- MHC
Major histocompatibility complex
- mRNA
Messenger RNA
- n/a
Not applicable
- NAPs
Nucleic acid polymers
- nM
Nanomolar
- NTCP
Sodium taurocholate cotransporting polypeptide
- PD-1
Programed cell death protein 1
- PD-L1
Programed death ligand protein 1
- PEG-IFN
Peginterferon
- pgRNA
Pregenomic RNA
- PK
Pharmacokinetics
- POL
Polymerase
- PP
Phenylpropanamides
- PS-ONs
Phosphorothioate oligonucleotides
- rcDNA
Relaxed circular DNA
- RIG-I
Retinoic acid-inducible gene-I
- RISC
RNA-induced silence complex
- RNAi
RNA interference
- RT
Reverse transcriptase
- S
HBV small surface protein
- SBA
Sulfamoylbenzamides
- SC
Subcutaneous
- siRNA
Small interfering RNA
- SMAC
Second mitochondria-derived activator of caspases
- SSO
Single-stranded oligonucleotide
- STOPs
s-Antigen transport inhibiting oligonucleotide polymers
- TCR
T cell receptor
- TLR
Toll-like receptor
- TNF
Tumor necrosis factor
- VLV
Virus-like vesicles
- WHsAg
WHV surface antigen
- WHV
Woodchuck hepatitis virus
- YMDD RT
Tyrosine, methionine, aspartate, motif aspartate reverse transcriptase motif
- αHT
α-hydroxytropolones
- μM
Micromolar
Footnotes
Declaration of Competing Interest Drs. Schinazi and Bassit along with Emory University are entitled to equity and royalties related to anti-HBV products licensed to Aligos Therapeutics, Inc., being further evaluated in the research described in this review. The terms of this arrangement have been reviewed and approved by Emory University in accordance with its conflict of interest policies.
Contributor Information
Leda Bassit, Center for AIDS Research, Laboratory of Biochemical Pharmacology, Department of Pediatrics, Emory University School of Medicine, and Children’s Healthcare of Atlanta, Atlanta, GA, USA.
Suzane Kioko Ono, Department of Gastroenterology, University of Sao Paulo School of Medicine, Sao Paulo, SP, Brazil.
Raymond F. Schinazi, Center for AIDS Research, Laboratory of Biochemical Pharmacology, Department of Pediatrics, Emory University School of Medicine, and Children’s Healthcare of Atlanta, Atlanta, GA, USA
References
- 1.Razavi H (2020) Global epidemiology of viral hepatitis. Gastroenterol Clin North Am 49:179–189 [DOI] [PubMed] [Google Scholar]
- 2.De Fraga RS, Van Vaisberg V, Mendes LCA et al. (2020) Adverse events of nucleos(t)ide analogues for chronic hepatitis B: a systematic review. J Gastroenterol 55:496–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Easl (2017) EASL 2017 clinical practice guidelineson the management of hepatitis B virus infection. J Hepatol 67:370–398 [DOI] [PubMed] [Google Scholar]
- 4.Zhang X, Chen X, Wei M et al. (2019) Potentialresistant mutations within HBV reverse transcriptase sequences in nucleos(t)ide analogues-experienced patients with hepatitis B virus infection. Sci Rep 9:8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Block TM, Locarnini S, Mcmahon BJ et al. (2017) Use of current and new endpoints in the evaluation of experimental hepatitis B therapeutics. Clin Infect Dis 64:1283–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anonymous (2020) Drug watch - compound in development for chronic hepatitis B. Hepatitis B Foundation. https://www.hepb.org/treatment-and-management/drug-watch/
- 7.Zhang YY, Hu KQ (2015) Rethinking the pathogenesis of hepatitis B virus (HBV) infection. J Med Virol 87:1989–1999 [DOI] [PubMed] [Google Scholar]
- 8.Maini MK, Gehring AJ (2016) The role of innateimmunity in the immunopathology and treatment of HBV infection. J Hepatol 64:S60–S70 [DOI] [PubMed] [Google Scholar]
- 9.Bertoletti A, Le Bert N (2018) Immunotherapy forchronic hepatitis B virus infection. Gut Liver 12:497–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin J, Wu JF, Zhang Q et al. (2014) Virus-related livercirrhosis: molecular basis and therapeutic options. World J Gastroenterol 20:6457–6469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cornberg M, Lok AS, Terrault NA et al. (2020) Guidance for design and endpoints of clinical trials in chronic hepatitis B - report from the 2019 EASLAASLD HBV treatment endpoints conference. J Hepatol 72:539–557 [DOI] [PubMed] [Google Scholar]
- 12.Madejón A, Romero M, Hernández Á et al. (2016) Hepatitis B and D viruses replication interference: influence of hepatitis B genotype. World J Gastroenterol 22:3165–3174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sozzi V, Shen F, Chen J et al. (2018) In vitro studiesidentify a low replication phenotype for hepatitis B virus genotype H generally associated with occult HBV and less severe liver disease. Virology 519:190–196 [DOI] [PubMed] [Google Scholar]
- 14.Fanning GC, Zoulim F, Hou J et al. (2020) Therapeutic strategies for hepatitis B virus infection: towards a cure. Nat Rev Drug Discov 19:291. [DOI] [PubMed] [Google Scholar]
- 15.Eller C, Heydmann L, Colpitts CC et al. (2018) Thefunctional role of sodium taurocholate cotransporting polypeptide NTCP in the life cycle of hepatitis B, C and D viruses. Cell Mol Life Sci 75:3895–3905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu J, Cheng J, Tang L et al. (2019) Virological basisfor the cure of chronic hepatitis B. ACS Infect Dis 5:659–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nassal M (2015) HBV cccDNA: viral persistencereservoir and key obstacle for a cure of chronic hepatitis B. Gut 64:1972–1984 [DOI] [PubMed] [Google Scholar]
- 18.Lucifora J, Xia Y, Reisinger F et al. (2014) Specific and nonhepatotoxic degradation of nuclear hepatitis B virus cccDNA. Science 343:1221–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laras A, Koskinas J, Dimou E et al. (2006) Intrahepatic levels and replicative activity of covalently closed circular hepatitis B virus DNA in chronically infected patients. Hepatology 44:694–702 [DOI] [PubMed] [Google Scholar]
- 20.Qu B, Ni Y, Lempp FA et al. (2018) T5 exonucleasehydrolysis of hepatitis B virus replicative intermediates allows reliable quantification and fast drug efficacy testing of covalently closed circular DNA by PCR. J Virol 92:e01117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ono SK, Kato N, Shiratori Y et al. (2001) The polymerase L528M mutation cooperates with nucleotide binding-site mutations, increasing hepatitis B virus replication and drug resistance. J Clin Invest 107:449–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lada O, Benhamou Y, Cahour A et al. (2004) In vitrosusceptibility of lamivudine-resistant hepatitis B virus to adefovir and tenofovir. Antivir Ther 9:353–363 [PubMed] [Google Scholar]
- 23.Idenix Pharmaceuticals I (2006) NDA 22–011 TYZEKA™. https://www.accessdata.fda.gov/drugsatfda_docs/label/2006/022011lbl.pdf
- 24.Gilead (2016) NDA 208464. https://www.accessdata.fda.gov/drugsatfa_docs/nda/2016/208464Orig1s000MedR.pdf
- 25.Tillmann HL (2008) The treatment of chronic hepatitis B: Focus on adefovir-like antivirals. Ther Clin Risk Manag 4:797–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim V, Abreu RM, Nakagawa DM et al. (2016) Pegylated interferon alfa for chronic hepatitis B: systematic review and meta-analysis. J Viral Hepat 23:154–169 [DOI] [PubMed] [Google Scholar]
- 27.Schinazi RF, Ehteshami M, Bassit L et al. (2018) Towards HBV curative therapies. Liver Int 38 (Suppl 1):102–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janssen HL, Van Zonneveld M, Senturk H et al. (2005) Pegylated interferon alfa-2b alone or in combination with lamivudine for HBeAg-positive chronic hepatitis B: a randomised trial. Lancet 365:123–129 [DOI] [PubMed] [Google Scholar]
- 29.Moucari R, Martinot-Peignoux M, Mackiewicz Vet al (2009) Influence of genotype on hepatitis B surface antigen kinetics in hepatitis B e antigennegative patients treated with pegylated interferonalpha2a. Antivir Ther 14:1183–1188 [DOI] [PubMed] [Google Scholar]
- 30.Abreu RM, Bassit LC, Tao S et al. (2019) Long-termvirological and adherence outcomes to antiviral treatment in a 4-year cohort chronic HBV study. Antivir Ther 24:567–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kasl (2019) KASL clinical practice guidelines formanagement of chronic hepatitis B. Clin Mol Hepatol 25:93–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park SH, Park KS, Kim NH et al. (2017) Clevudineinduced mitochondrial myopathy. J Korean Med Sci 32:1857–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naylor PH, Mutchnick MG (2018) Immunotherapyfor hepatitis B in the direct acting antiviral era: reevaluating the thymosin α1 efficacy trials in the light of a combination therapy approach. J Viral Hepat 25:4–9 [DOI] [PubMed] [Google Scholar]
- 34.Peters MG, Locarnini S (2017) New direct-actingantiviral agents and immunomodulators for hepatitis B virus infection. Gastroenterol Hepatol 13:348–356 [PMC free article] [PubMed] [Google Scholar]
- 35.Martinez MG, Villeret F, Testoni B et al. (2020) Canwe cure hepatitis B virus with novel direct-acting antivirals? Liver Int 40(Suppl 1):27–34 [DOI] [PubMed] [Google Scholar]
- 36.Zhao N, Jia B, Zhao H et al. (2020) A first-in-human trial of GLS4, a novel inhibitor of hepatitis B virus capsid assembly, following single- and multipleascending-oral-dose studies with or without ritonavir in healthy adult volunteers. Antimicrob Agents Chemother 64:e01686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yuen MF, Agarwal K, Gane EJ et al. (2020) Safety,pharmacokinetics, and antiviral effects of ABI-H0731, a hepatitis B virus core inhibitor: a randomised, placebo-controlled phase 1 trial. Gastroenterol Hepatol 5:152–166 [DOI] [PubMed] [Google Scholar]
- 38.Yuen MF, Agarwal K, Gane EJ et al. (2019) Thesecond-generation hepatitis B virus (HBV) core inhibitor (CI) ABI-H2158 is associated with potent antiviral activity in a 14-day monotherapy study in HBeAg-positive patients with chronic hepatitis B (CHB). Hepatology 70:1497A–1498A31661168 [Google Scholar]
- 39.Verbinnen T, Hodari M, Talloen W et al. (2020) Virology analysis of chronic hepatitis B virus– infected patients treated for 28 days with JNJ-56136379 monotherapy. J Viral Hepat 27:1127–1137 [DOI] [PubMed] [Google Scholar]
- 40.Gane E, Yuen MF, Bo Q et al. (2019) RO7049389, acore protein allosteric modulator, demonstrates robust decline in HBV DNA and HBV RNA in chronic HBV infected patients. J Hepatol 70:e491 [Google Scholar]
- 41.Vaine M, Dellisola V, Clugston S et al. (2019) EDP-514, a novel HBV core inhibitor with potent antiviral activity both in vitro and in vivo. J Hepatol 70:474–475 [Google Scholar]
- 42.Huang Q, Haydar S, Zhou Y et al. (2019) Preclinicalprofile of HBV core protein inhibitor, ABI-H3733, a potent inhibitor of cccDNA generation in HBV infected cells. J Hepatol 70:e48 [Google Scholar]
- 43.Amblard F, Boucle S, Bassit L et al. (2020) Novelhepatitis B virus capsid assembly modulator induces potent antiviral responses in vitro and in humanized mice. Antimicrob Agents Chemother 64:e01701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bassit L, Cox B, Ono SK et al. (2018) Novel andpotent HBV capsid modulator reduces HBeAg and cccDNA in core site directed T109I mutant in HepNTCP cells. J Hepatol 68:S16–S17 [Google Scholar]
- 45.Bassit L, Verma K, Ono SK et al. (2019) Novel HBVcapsid assembly modulator inhibits pregenomic RNA encapsidation by accelerating capsid assembly kinetics and disrupting core protein dephosphorylation. J Hepatol 70:e457 [Google Scholar]
- 46.Sari O, Bassit L, Gavegnano C et al. (2017) Synthesisand antiviral evaluation of 2’,2’,3’,3’-tetrafluoro nucleoside analogs. Tetrahedron Lett 58:642–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Debing Y, Jekle A, Vendeville S et al. (2019) Preclinical assessment of a novel CAPSID assembly modulator, ALG-001075, demonstrates best-in-class in vitro potency and in vivo antiviral efficacy. Hepatology 70:437A30791105 [Google Scholar]
- 48.Yan H, Zhong G, Xu G et al. (2012) Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife 1:e00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smolders EJ, Burger DM, Feld JJ et al. (2020) Clinical pharmacology of current and investigational hepatitis B virus therapies. Aliment Pharmacol Ther 51:231–243 [DOI] [PubMed] [Google Scholar]
- 50.Wedemeyer H, Schöneweis K, Bogomolov P et al(2019) Final results of a multicenter, open-label phase 2 clinical trial (MYR203) to assess safety and efficacy of myrcludex B in with PEG-interferon Alpha 2a in patients with chronic HBV/HDV co-infection. J Hepatol 2019:e81 [Google Scholar]
- 51.Donkers JM, Appelman MD, Graaf SFJ (2019) Mechanistic insights into the inhibition of NTCP by myrcludex B. JHEP Rep 1:278–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bernstein E, Caudy AA, Hammond SM et al. (2001) Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 409:363–366 [DOI] [PubMed] [Google Scholar]
- 53.Thongthae N, Payungporn S, Poovorawan Y et al(2014) A rational study for identification of highly effective siRNAs against hepatitis B virus. Exp Mol Pathol 97:120–127 [DOI] [PubMed] [Google Scholar]
- 54.Setten RL, Rossi JJ, Han S-P (2019) The current stateand future directions of RNAi-based therapeutics. Nat Rev Drug Discov 18:421–446 [DOI] [PubMed] [Google Scholar]
- 55.Giladi H, Ketzinel-Gilad M, Rivkin L et al. (2003) Small interfering RNA inhibits hepatitis B virus replication in mice. Mol Ther 8:769–776 [DOI] [PubMed] [Google Scholar]
- 56.Li G, Jiang G, Lu J et al. (2014) Inhibition of hepatitisB virus cccDNA by siRNA in transgenic mice. Cell Biochem Biophys 69:649–654 [DOI] [PubMed] [Google Scholar]
- 57.Collier WH (2020) Arbutus announces positive preliminary phase 1a/1b clinical trial results for AB-729, a proprietary GalNAc delivered RNAi compound in development for people living with chronic hepatitis B. In: GlobeNewswire, https://www.globenewswire.com/news-release/2020/03/26/2007290/0/en/Arbutus-Announces-Positive-Preliminary-Phase-1a-1b-Clinical-Trial-Results-for-AB-729-a-Proprietary-GalNAc-Delivered-RNAi-Compound-in-Development-for-People-Living-with-Chronic-Hepa.html [Google Scholar]
- 58.Gane E, Lim Y-S, Tangkijvanich P et al. (2020) Preliminary safety and antiviral activity of VIR-2218, an X-targeting HBV RNAi therapeutic, in chronic hepatitis B patients. J Hepatol 73:S50–S51 [Google Scholar]
- 59.Gane E, Locarnini S, Lim TH et al. (2020) Short-termtreatment with RNA interference therapy, JNJ-3989, results in sustained hepatitis B surface antigen suppression in patients with chronic hepatitis B receiving nucleos(t)ide analogue treatment. J Hepatol 73:S20 [Google Scholar]
- 60.Vaillant A (2016) Nucleic acid polymers: broad spectrum antiviral activity, antiviral mechanisms and optimization for the treatment of hepatitis B and hepatitis D infection. Antiviral Res 133:32–40 [DOI] [PubMed] [Google Scholar]
- 61.Vaillant A (2019) REP 2139: antiviral mechanismsand applications in achieving functional control of HBV and HDV infection. ACS Infect Dis 5:675–687 [DOI] [PubMed] [Google Scholar]
- 62.Al-Mahtab M, Bazinet M, Vaillant A (2016) Safetyand efficacy of nucleic acid polymers in monotherapy and combined with immunotherapy in treatmentnaive Bangladeshi patients with HBeAg+ chronic hepatitis B infection. PLoS One 11:e0156667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bazinet M, Pântea V, Placinta G et al. (2020) Safetyand efficacy of 48 weeks REP 2139 or REP 2165, tenofovir disoproxil, and pegylated interferon alfa-2a in patients with chronic HBV infection naïve to nucleos(t)ide therapy. Gastroenterology 158:2180–2194 [DOI] [PubMed] [Google Scholar]
- 64.Hong J, Pandey R, Rajwanshi VK et al. (2019) S-antigen traffic-inhibiting oligonucleotide polymers (STOPS) can effectively inhibit hepatitis B surface antigen (HBsAg) secretion from hepatitis B virus (HBV) cell lines. Hepatology 430A:70 [Google Scholar]
- 65.Tan H, Hong J, Hyunsoon K et al. (2020) Combination drug interactions of hepatitis B virus (HBV) S-antigen transport - inhibiting oligonucleotide polymers in vitro. J Hepatol 2020:S868 [Google Scholar]
- 66.Jobe A (2020) Aligos therapeutics doses first subject in phase 1 proof of concept study of oligonucleotide drug candidate ALG-010133. In: GlobeNewswire, https://www.globenewswire.com/news-release/2020/08/18/2080093/0/en/Aligos-Therapeutics-Doses-First-Subject-in-Phase-1-Proof-of-Concept-Study-of-Oligonucleotide-Drug-Candidate-ALG-010133.html [Google Scholar]
- 67.Squires KE, Mayers DL, Bluemling GR et al. (2020) ATI-2173, a novel liver-targeted non-chain terminating nucleotide for HBV cure regimens. Antimicrob Agents Chemother 64:e00836–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Han K, Cremer J, Elston R et al. (2019) A randomized, double-blind, placebo-controlled, firsttime-in-human study to assess the safety, tolerability, and pharmacokinetics of single and multiple ascending doses of GSK3389404 in healthy subjects. Clin Pharmacol Drug Dev 8:790–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Han K, Cremer J, Elston R et al. (2019) A randomized, double-blind, placebo-controlled, firsttime-in-human study to assess the safety, tolerability, and pharmacokinetics of single and multiple ascending doses of GSK3389404 in healthy subjects. Clin Pharmacol Drug Dev 8:790–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kelong H, Hiroshi I, Robert E et al. (2020) Pharmacokinetics of GSK3389404, an antisense oligonucleotide (ASO), is similar in subjects with chronic hepatitis B (CHB) across Asia-Pacific region. Hepatol Int 14:S66 [Google Scholar]
- 71.Yuen MF, Heo J, Kumada H et al. (2019) Results after12 weeks treatment of multiple doses of GSK3389404 in chronic hepatitis B (CHB) subjects on stable nucleos(t)ide therapy in a phase 2a double-blind, placebo-controlled study. Hepatology 70:433A–434A [Google Scholar]
- 72.Feng S, Cheung TT, Luk AOY et al. (2020) Livertargeted single stranded (SSO) oligonucleotide RO7062931 is safe and well tolerated in Chinese healthy volunteers (HVs) with similar pharmacokinetic profile to non-Chinese HVs. Hepatol Int 14:S72 [Google Scholar]
- 73.Gane EJ, Wat C, Das S et al. (2019) Interim results ofa phase 1 study of RO7062931, a novel liver-targeted single-stranded oligonucleotide (SSO) with locked nucleic acid (LNA) that targets HBV transcripts. Hepatology 70:436A [Google Scholar]
- 74.Yuen M-F, Gane E, Kim DJ et al. (2020) RO7062931 antisense oligonucleotide phase 1 study demonstrates target engagement in patients with chronic hepatitis B on established nucleos(t)ide therapy. J Hepatol 2020: S51 [Google Scholar]
- 75.Tavis JE, Zoidis G, Meyers MJ et al. (2019) Chemicalapproaches to inhibiting the hepatitis B virus ribonuclease H. ACS Infect Dis 5:655–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Edwards TC, Lomonosova E, Patel JA et al. (2017) Inhibition of hepatitis B virus replication by N-hydroxyisoquinolinediones and related polyoxygenated heterocycles. Antiviral Res 143:205–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Edwards TC, Mani N, Dorsey B et al. (2019) Inhibitionof HBV replication by N-hydroxyisoquinolinedione and N-hydroxypyridinedione ribonuclease H inhibitors. Antiviral Res 164:70–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Edwards TC, Ponzar NL, Tavis JE (2019) Sheddinglight on RNaseH: a promising target for hepatitis B virus (HBV). Expert Opin Ther Targets 23:559–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li Q, Lomonosova E, Donlin MJ et al. (2020) Amide-containing α-hydroxytropolones as inhibitors of hepatitis B virus replication. Antiviral Res 177:104777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lomonosova E, Daw J, Garimallaprabhakaran AK et al. (2017) Efficacy and cytotoxicity in cell culture of novel α-hydroxytropolone inhibitors of hepatitis B virus ribonuclease H. Antiviral Res 144:164–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lomonosova E, Tavis JE (2017) In vitro enzymaticand cell culture-based assays for measuring activity of HBV RNaseH inhibitors. Methods Mol Biol 1540:179–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Long KR, Lomonosova E, Li Q et al. (2018) Efficacy of hepatitis B virus ribonuclease H inhibitors, a new class of replication antagonists. Antivir Res 149:41–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Boni C, Barili V, Acerbi G et al. (2019) HBV immune-therapy: from molecular mechanisms to clinical applications. Int J Mol Sci 20:2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hiasa Y, Yoshida O, Guillen GE et al. (2019) The HBvaccine containing HBS and HBC antigen (NASVAC) can effectively induce anti-HBS antibody in non-responders to the prophylactic vaccine. Hepatology 70:589A–590A [Google Scholar]
- 85.Brown W (2020) Altimmune announces IND clearance for a phase 2 trial of HepTcell™ immunotherapeutic for the treatment of chronic hepatitis B. In: Altimmune (ed) Globe newswire. Altimmune, Gaithersburg [Google Scholar]
- 86.Lim YS, Mutimer D, Heo J et al. (2019) A phase 1bevaluation of HepTcell HBV-specific immunotherapy in NUC-controlled, eAg negative chronic HBV infection. J Hepatol 70:e50–e51 [Google Scholar]
- 87.Daniela P (2018) AIC649 Innate activation with inactivated parapoxviruses for HBV therapy. In: International HBV Cure Meeting. Toronto [Google Scholar]
- 88.Yoon SK, Seo YB, Im SJ et al. (2015) Safety andimmunogenicity of therapeutic DNA vaccine with antiviral drug in chronic HBV patients and its immunogenicity in mice. Liver Int 35:805–815 [DOI] [PubMed] [Google Scholar]
- 89.Zoulim F, Fournier C, Habersetzer F et al. (2020) Safety and immunogenicity of the therapeutic vaccine TG1050 in chronic hepatitis B patients: a phase 1b placebo-controlled trial. Hum Vaccin Immunother 16:388–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McNally RT (2018) GeoVax and CaroGen to collaborate on development of therapeutic hepatitis B vaccine. https://www.geovax.com/news/geovax-and-carogen-to-collaborate-on-development-of-therapeutic-hepatitis-b-vaccine
- 91.George R, Ma A, Motyka B et al. (2020) A dendriticcell-targeted chimeric hepatitis B virus immunotherapeutic vaccine induces both cellular and humoral immune responses in vivo. Hum Vaccin Immunother 16:779–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Su J, Kosinska A, Brunner L et al. (2019) ExploringTH1/TH2 adjuvants to improve the efficacy of the therapeutic vaccination against chronic hepatitis B. J Hepatol 70:e485–e486 [Google Scholar]
- 93.Yarovinsky TO, Mason SW, Menon M et al. (2019) Virus-like vesicles expressing multiple antigens for immunotherapy of chronic hepatitis B. iScience 21:391–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tg (2018) Hookipa and Gilead ink US$400m deal.In: European Biotechnology - life science and industry magazine. https://european-biotechnology.com/up-to-date/latest-news/news/hookipa-and-gilead-ink-us400m-deal.html [Google Scholar]
- 95.Vaccines V (2019) VBI Vaccines and Brii Biosciences initiate phase 1b/2a study of BRII-179 (VBI-2601) in patients with chronic hepatitis B infection. https://www.vbivaccines.com/wire/vbi-and-brii-initiate-phase-1b-2a-study-of-brii-179-inchronic-hbv/
- 96.Patinote C, Karroum NB, Moarbess G et al. (2020) Agonist and antagonist ligands of toll-like receptors 7 and 8: ingenious tools for therapeutic purposes. Eur J Med Chem 193:112238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Daffis S, Balsitis S, Chamberlain J et al. (2020) Tolllike receptor 8 agonist GS-9688 induces sustained efficacy in the woodchuck model of chronic hepatitis B. Hepatology 73:53–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mackman RL, Mish M, Chin G et al. (2020) Discovery of GS-9688 (Selgantolimod) as a potent and selective oral toll-like receptor 8 agonist for the treatment of chronic hepatitis B. J Med Chem 63:10188–10203 [DOI] [PubMed] [Google Scholar]
- 99.Luk A, Jiang Q, Glavini K et al. (2020) A single andmultiple ascending dose study of toll-like receptor 7 agonist (RO7020531) in Chinese healthy volunteers. Clin Transl Sci 13:985–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Blaising J, Yu Y, Zhou X et al. (2019) Combinationtreatment of liver-targeted HBV locked nucleic acid antisense oligonucleotide and tlr7 agonist RO7020531 leads to prolonged off-treatment antiviral effect in the AAV-HBV mouse model. Hepatology 70:428A–429A [Google Scholar]
- 101.Ebert G, Allison C, Preston S et al. (2015) Eliminatinghepatitis B by antagonizing cellular inhibitors of apoptosis. Proc Natl Acad Sci U S A 112:5803–5808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pan W, Luo Q, Yan X et al. (2018) A novel SMACmimetic APG-1387 exhibits dual antitumor effect on HBV-positive hepatocellular carcinoma with high expression of cIAP2 by inducing apoptosis and enhancing innate anti-tumor immunity. Biochem Pharmacol 154:127–135 [DOI] [PubMed] [Google Scholar]
- 103.Trepanier DJ, Ure DR, Foster RT (2017) In vitrophase I metabolism of CRV431, a novel oral drug candidate for chronic hepatitis B. Pharmaceutics 9:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gallay P, Ure D, Bobardt M et al. (2019) The cyclophilin inhibitor CRV431 inhibits liver HBV DNA and HBsAg in transgenic mice. PLoS ONE 14:e0217433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lee C (2019) CRISPR/Cas9-based antiviral strategy: current status and the potential challenge. Molecules 24:1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dong C, Qu L, Wang H et al. (2015) Targeting hepatitis B virus cccDNA by CRISPR/Cas9 nuclease efficiently inhibits viral replication. Antiviral Res 118:110–117 [DOI] [PubMed] [Google Scholar]
- 107.Liu Y, Zhao M, Gong M et al. (2018) Inhibition ofhepatitis B virus replication via HBV DNA cleavage by Cas9 from Staphylococcus aureus. Antiviral Res 152:58–67 [DOI] [PubMed] [Google Scholar]
- 108.Yang H-C, Chen P-J (2018) The potential and challenges of CRISPR-Cas in eradication of hepatitis B virus covalently closed circular DNA. Virus Res 244:304–310 [DOI] [PubMed] [Google Scholar]
- 109.Kennedy EM, Kornepati AV, Cullen BR (2015) Targeting hepatitis B virus cccDNA using CRISPR/Cas9. Antiviral Res 123:188–192 [DOI] [PubMed] [Google Scholar]
- 110.Yang YC, Chen YH, Kao JH et al. (2020) Permanentinactivation of HBV genomes by CRISPR/Cas9-mediated non-cleavage base editing. Mol Ther 20:480–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Excision (2020) Technology - a novel approach toCRISPR. https://www.excision.bio/technology
- 112.Haskins B (2020) Precision biosciences, Gilead sciences pull plug on potential $445M deal. WRAL TechWire. https://www.wraltechwire.com/2020/07/07/precision-biosciences-gilead-sciences-pull-plugon-potential-445m-deal/
- 113.Biosciences P (2020) ARCUS genome editing. https://precisionbiosciences.com/our-approach/arcus-genome-editing/
- 114.Lee HW, Park JY, Hong T et al. (2019) A prospective,open-label, dose-escalation, single-center, phase 1 study for Lenvervimab (GC1102), a new and safe human monoclonal antibody drug for chronic hepatitis B patients. Hepatol Int 13:S48 [Google Scholar]
- 115.Radreau P, Porcherot M, Ramière C et al. (2016) Reciprocal regulation of farnesoid X receptor α activity and hepatitis B virus replication in differentiated HepaRG cells and primary human hepatocytes. FASEB J 30:3146–3154 [DOI] [PubMed] [Google Scholar]
- 116.Darteil R, Joly S, Radreau P et al. (2019) In vitrocharacterization of EYP001 a novel, potent and selective FXR agonist entering phase 2 clinical trials in chronic hepatitis B. Hepatology 70(suppl 1):60A [Google Scholar]
- 117.Monteiro C, Bruezière L, Laheux S et al. (2019) Anin-silico disease model for the development of FXR agonist EYP001 as a therapy for HBV infection. Hepatology 70:441A–442A [Google Scholar]
- 118.Li B, Yan C, Zhu J et al. (2020) Anti-PD-1/PD-L1 blockade immunotherapy employed in treating hepatitis B virus infection-related advanced hepatocellular carcinoma: a literature review. Front Immunol 11:1037–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ghany MG, Block TM (2018) Disease pathways andmechanisms of potential drug targets. Clin Liver Dis 12:12–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.LionTCR (2019) TCR T cell. http://www.liontcr.com/technology
- 121.Huang Q, Zhou B, Cai D et al. (2020) Rapid turnoverof HBV cccDNA indicated by monitoring emergence and reversion of signature-mutation in treated chronic hepatitis B patients. Hepatology 73:41–52 [DOI] [PMC free article] [PubMed] [Google Scholar]