Abstract
Background:
Manganism is a central nervous system (CNS) disorder caused by toxic exposure to manganese (Mn). Manganism has been related to occupational exposures, liver diseases, prolonged parenteral nutrition and abuse of illicit drugs. Initially manifested by a reversible neuropsychiatric syndrome (locura manganica), the main symptoms and signs of manganism are emotional lability, compulsive behavior and visual hallucinations. Locura manganica is followed by an irreversible extrapyramidal syndrome, the onset of which occurs years after chronic exposure.
Objectives:
To characterize the regional distribution of Mn in the rat brain after subchronic exposure to Mn. This animal model holds special clinical relevance, reflecting the earlier clinical stages of manganism, before chronic exposure to Mn exerts its irreversible effects.
Methods:
Sprague-Dawley rats were intravenously injected with MnCl2 weekly, for a total of 14 weeks – approx. 1/10 of the lifetime of the rat. T1-weighted MRI was employed as a sensitive tool for detecting the distribution of Mn deposition in brain tissues, as evidenced by areas of T1-weighted hyperintense signals.
Results:
A consistent region-specific pattern of T1-weighted hyperintensities was observed in the brains of Mn treated rats. Cortical hyperintensities were prominent in the hippocampus and dentate gyrus. Hyperintensities were also observed in the olfactory bulbs, pituitary gland, optic nerves and chiasma, pons, midbrain tegmentum, habenula, lentiform and caudate nuclei, thalamus, chorioid plexus and cerebellar hemispheres.
Conclusions:
Prominent Mn depositions, evidenced by T1-weighted hyperintensities in the hippocampus after subacute exposure to Mn, are compatible with the clinical picture of manganism during its early stages; and may explain its pathophysiology.
Keywords: manganese, MRI, rodent studies, subchronic exposure, neurotoxicity
Introduction:
Manganism:
Neurotoxicity of manganese (Mn) assumes particular clinical relevance in both occupational and environmental medicines. Clinically, manganism is a central nervous system (CNS) disease, which includes a broad spectrum of neurological deficits [1]. The strongest correlation between any type of environmental exposure and increased susceptibility to Parkinson’s disease (PD) is observed in Mn-exposed populations [2]. It has been estimated that 68,000–185,000 workers in the US may be exposed to the potential health hazards of Mn and its compounds [3]. There are also numerous reports of Mn intoxication related to long-term total parenteral nutrition (TPN) [4], and from long-term liver failure [5]. Recently, manganism has been observed in intravenous (I.V.) methcathinone abusers in Eastern Europe, where this substance is illicitly produced by potassium permanganate oxidation process [6]. Methcathinone is an increasingly widely abused and potent synthetic derivative of cathinone [7], the natural amphetamine-like monoamine alkaloid found in leaves of the plant khat (Catha edulis), which has long been used in Israel, the Arabian Peninsula, North and Eastern Africa as a psychostimulant.. Initially, patients may complain of anorexia, lassitude and excessive fatigue, apathy, muscle and joint pain. These are followed by signs and symptoms of organic psychosis including impairment of judgment, disorientation, memory loss, compulsive behavior, emotional lability, flight of ideas, visual hallucinations, illusions and delusions. The medical term, locura manganica or manganese madness, describes this initial neuropsychiatric syndrome [1]. Psychomotor slowing and cognitive decline evolve thereafter. This organic brain syndrome is later followed by an extrapyramidal movement disorder with excessive salivation, a disorder clinically resembling PD [8], but with distinct neurological features [9]. The organic psychosis often fades as extrapyramidal signs emerge. Manganism may be reversible if diagnosed and treated in its early stages. The onset of the extrapyramidal signs denotes irreversible damage to the CNS, but these signs appear only after many years of exposure [10]. Occupational exposure to Mn for more than 20 years, or combined long-term exposures to Mn and Al for more than 30 years are associated with increased prevalence of PD [2]. Unlike the full-blown syndrome of manganism, which is well characterized by extrapyramidal signs [1, 2], little is known about the relationship between manganese and its neuropsychological effects which appear earlier and develop insidiously [11]. The peculiar pattern of delayed toxicity with biphasic clinical appearance presents a serious challenge for the design of animal studies addressing this issue.
Magnetic resonance imaging (MRI) and Mn – relevant principles:
In order to be measured by MRI, the direction of the net magnetization needs to be tipped away from its equilibrium direction (parallel to the static magnetic field, z-axis). This is carried out by briefly exposing the object to pulses of electromagnetic energy in the form of weak magnetic fields that oscillate at a specific radio frequency (RF) which is produced by a transmitter coil. The rotating magnetization produces an oscillating signal within a receiving coil wrapped around the sample. After applying such an RF pulse, the magnetization tends to return to the direction of the original static magnetic field. The time that is associated with the return of longitudinal magnetization to its equilibrium condition is referred to as the spin lattice relaxation time, T1; it reflects the strength and nature of magnetic interactions between the spins and their atomic neighborhood. Divalent Mn ions (Mn +2 oxidation state) have been used as a paramagnetic contrast agent. Given its ability to shorten the T1 time of hydrogen nuclei in water molecules [14] it can be detected by T1-weighted imaging, as tissues with higher Mn levels have shorter relaxation times and demonstrate higher signal intensity in T1-weighted images.
Quantification of the absolute tissue Mn levels is nevertheless problematic, since the relaxivity, a determinant of the relaxation rate, is not an intrinsic property of the Mn ion alone and is subject to change that is dependent on the molecular form of Mn in tissues. It is also likely dependent upon the form in which it is bound [13]. Thus, determining the exact concentrations of Mn in tissues with MRI is problematic. The pallidal index (PI), a comparison of the T1 signal intensity in the globus pallidus to the frontal white matter has been used to correct for Mn brain depositions [12], however, the relevance of this calculation to quantitative Mn brain levels must be carefully considered, since Mn also accumulates in brain regions used where the denominator is factored in the PI calculation [14].
MRI findings in human brain in Manganism:
Mn may cause T1- weighted signal hyperintensities in the globus pallidus, striatum and, to a lesser extent, the substantia nigra [15], while idiopathic PD affects mainly the substantia nigra pars compacta and does not cause MRI abnormalities [1]. MRI can also be used to evaluate both the efficacy of various treatment regimes and the effect of cessation of exposure to Mn compounds. Chelation therapy reversed the increases in T1-weighted signal intensity [16], while decreases in the PI have been reported in patients no longer exposed to Mn in occupational settings [13]. There are no reports on MRI abnormalities in humans during the first clinical stages of manganism - the initial stage of non-specific complaints and behavioral changes and the following stage of neuropsychiatric syndrome (locura manganica).
Mn neurotoxicity and MRI use in rodents:
There have been relatively few MRI studies of Mn neurotoxicity. Hyperintensities in rats appear in the choroids plexus and ventricles as the immediate acute effects of exposure to Mn [17]. In mice, dose-dependent Mn-induced hyperintensities were observed in the pituitary gland, olfactory bulb and the hippocampus between 2 and 14 hours after acute exposure [18]. Rat studies demonstrated hyperintensities on T1-weighted MR images after a week of treatment with Mn fortified parenteral nutrition (PN) formula. Cessation of PN treatment for 4 weeks leads to a decrease in these signal intensities [19]. These dynamics are consistent with those observed in human brain MRI following treatment with Mn fortified PN [12].
The aim of this MRI study on Mn brain deposition:
Review of the pertinent medical literature indicates that MRI abnormalities have been observed first and foremost in the basal ganglia and only in patients with full-blown manganism, clinically manifested by extrapyramidal movement disorder during the late stage of chronic Mn intoxication. On the other hand, MRI studies of manganism in rodent models have focused on the initial acute stages of Mn neurotoxicity and showed a different and possibly reversible pattern of Mn distribution in the CNS. The present MRI study aimed to shed light on the natural progression of Mn neurotoxicity during its early stages, after lengthy exposure to Mn, but still before full blown Mn neurotoxicity is manifested. In order to characterize the pattern of the regional distribution of Mn in the CNS, rat brains were imaged using T1-weighted MRI after subchronic treatment of the animals with repeated significant I.V. doses of Mn. The duration of exposure was 14 weeks - approximately 1/10 of the lifetime of the rat. This animal model holds special clinical relevance, as manganism is clinically manifested following lengthy periods of exposure to Mn compounds. This rat model of subchronic exposure to Mn may reflect human exposure to Mn for 5–10 years in occupational settings like mining, grinding operations and, possibly, welding. Although 5–10 years is a lengthy period, the full blown syndrome of manganism is manifested in human only after much longer period of exposure to Mn – up to 2–3 decades. Therefore, it is conceivable that the animal model used in this study may reflect earlier stages of Mn neurotoxicity.
Materials and Methods:
The present manuscript focuses on clinical features of Mn-induced neurotoxicity in the rat as part of a multipronged MRI study conducted at Vanderbilt University Medical Center. The original study was designed to test the hypothesis that Fe supplementation in the presence of increased Mn exposure leads to decreased brain Mn accumulation [20]. Excerpts of the methodology are briefly recapitulated herein.
Animals:
All animal protocols were approved by the Vanderbilt University Medical Center Institutional Animal Care and Use Committee (IACUC). Male Sprague-Dawley rats (ordered from Harlan, Indianapolis, IN), 10–12 weeks old (240–250 g) were supplied with food and water ad libitum during the 14-week experimental protocol. Control (n = 5) and Mn-treated (n = 6) rats received normal rat chow. In addition, animals from the treatment groups received weekly intravenous tail vessel injections of a sterile, isotonic Mn solution (3 mg Mn/kg body mass) of MnCl2 for a total of 14 weeks. Control rats received injections of similar volumes of sterile, isotonic saline.
I.V. Injected Mn was fully and consistently delivered to the systemic blood circulation, as can be judged by the measured Mn levels, which were similar in each of the treated rats.
Blood collection and analysis of Mn RBC levels:
Blood was collected from tail vessels at the conclusion of the study to verify Mn levels. It was collected in heparanized tubes, centrifuged (1000 × g) at 4 °C for 30 min to separate red blood cells (RBCs) from plasma and stored at −80 °C until Mn analysis by atomic absorption spectroscopy.
For RBCs, a 400 μL aliquot was vortexed with 100 μL 0.5% Triton-X for 30 sec. This was brought up to 1 mL total volume with 2% nitric acid for analysis. The mixture was then centrifuged and the clear supernatant was used for analysis (100 μL aliquot brought up to a 1mL volume with 2% nitric acid). Bovine liver (10 μg Mn/L) was digested in ultra-pure nitric acid and used as an internal standard for analysis.
Magnetic Resonance Imaging (MRI):
Upon arrival at Vanderbilt, rats were allowed to adjust to their new environment for 4–6 days prior to any handling or testing. Following this, all animals were imaged prior to the beginning of their respective treatment and again at week 14, the last week of the study. In order to maintain consistency during the study, animals were always imaged 24 hours after that week’s injection.
For the scans, animals were initially anesthetized with 2% isoflurane and placed in a stereotaxic support cradle with their head secured with tape. The cradle was put in the volume coil to ensure the rat’s head was located in the coil’s center. Isoflurane was then lowered to 1.5–1.75% and maintained for the duration of the scanning protocol, usually 1.5 hours. During the scan, body temperature was maintained at 37 °C with warm air controlled by a rectal temperature probe (SA Instruments). Respiration was monitored throughout, and maintained at 50–70 breaths per min.
All experiments were acquired using a 4.7 T, 31 cm bore Varian INOVA magnet with actively shielded gradients (40 G/cm, rise time full amplitude of 130 US) and 63 mm transmit/receive quadrature imaging volume coil. Rat brains were scanned from both horizontal (field of view (FOV) = 40 × 40 mm, 30 slices) and coronal directions (FOV = 40 × 50 mm, 20 slices) with 0.75 mm slice thickness. T1 was measured using 2-D Fast Low Angle Shot sequence (FLASH) with parameters as following: TR/TE = 489/6.59 ms; flip angles = 10, 30, 55 or 70°; 2 acquisitions; image matrix = 256 × 256.
Image Analysis:
In this study, only raw data are demonstrated, as emphasis is on the clinical attributes associated with the noted effects of Mn accumulation.
All animals were scanned at time 0 prior to Mn administration. Furthermore, each animal was used as its own control.
Results:
Blood RBC Mn levels were consistent in all the treated animals, ranging ~ 20 ug/L. The findings on T1-weighted images are consistent in all the examined animals and examples are presented in both coronal and horizontal brain sections (Figures 1–2 and Figures 3–4, respectively). In the Mn treated group, multiple T1 hyperintensities were evident in specific anatomical structures. The outlines of these structures are well demarcated from their surroundings. Areas showing Mn-associated hyperintensities included hyperintensities (in decreasing order of brightness) the olfactory bulbs (Fig. 3), the pituitary gland (Fig. 1B), the optic nerves and optic chiasm (Fig. 1B), the hippocampus (Fig.1B) and dentate gyrus (Fig. 2B), the pons and midbrain tegmentum (Fig. 3), the habenula (Fig.2B), the lentiform nucleus which is composed of the globus pallidus and the putamen (Fig. 3), the caudate nucleus (Figures 2B and 3), the thalamus (Fig. 3), the chorioid plexus (Figures 2B and 3) and the folia of the cerebellar hemispheres (neocerebellum) (fig. 3). No locomotor impairment was observed in the animals during the 14 weeks of Mn exposure (data not shown).
Figure 1:
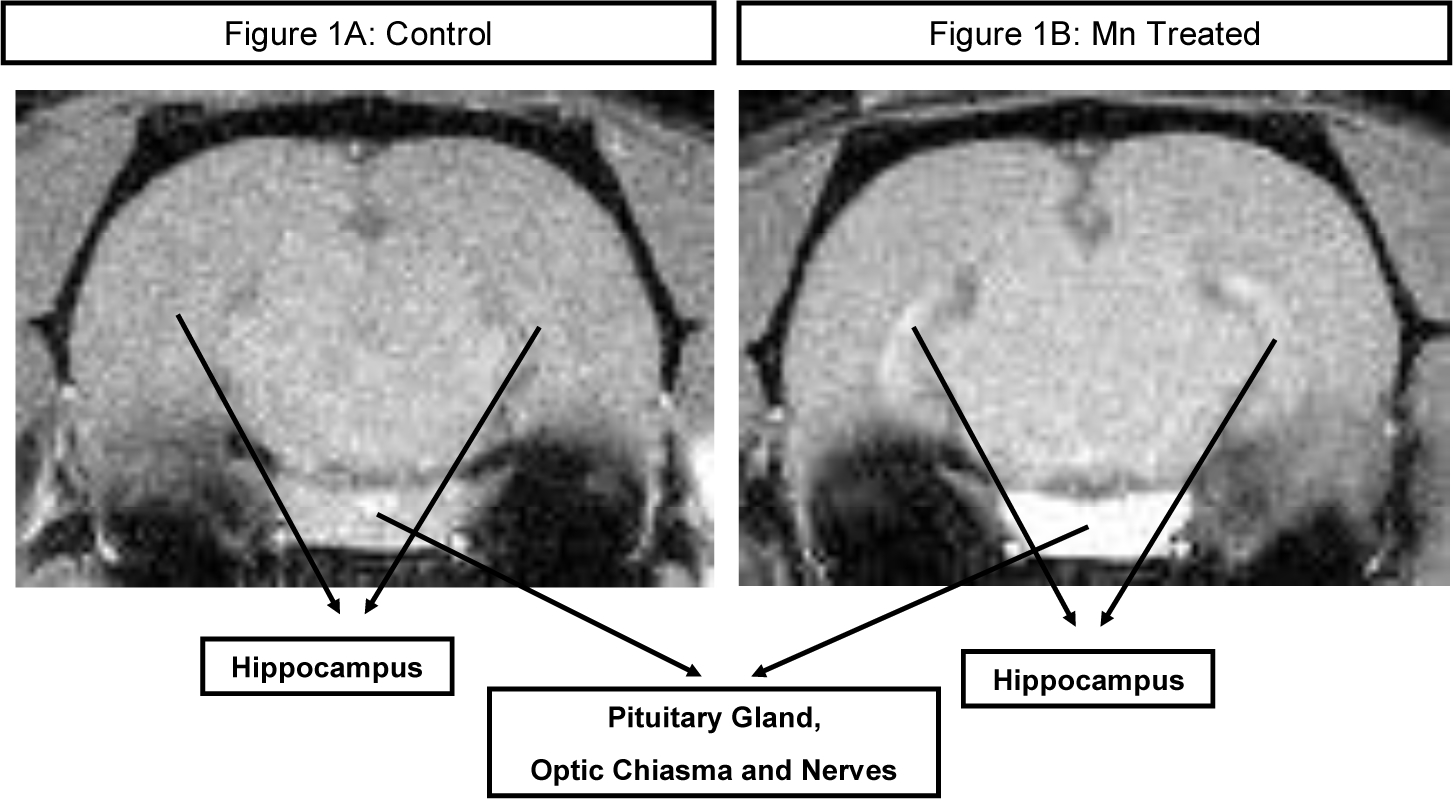
T1-weighted MRI (Figure 1A) presents a coronal section of a rat brain from the intact control group. No high intensity signals are observed in the brain parenchyma. Tiny foci of high intensity are shown in major blood vessels, out of the brain tissue.
T1-weighted MRI hyperintense findings (Figure 1B) are observed at the same level of a rat brain from the Mn treated group, which received weekly intravenous injections of MnCl2 solution (3 mg Mn/kg body mass) for 14 weeks: bilateral symmetrical high intensity signals sharply demarcate the hippocampi, pituitary gland, optic chiasma and nerves.
Figure 2:
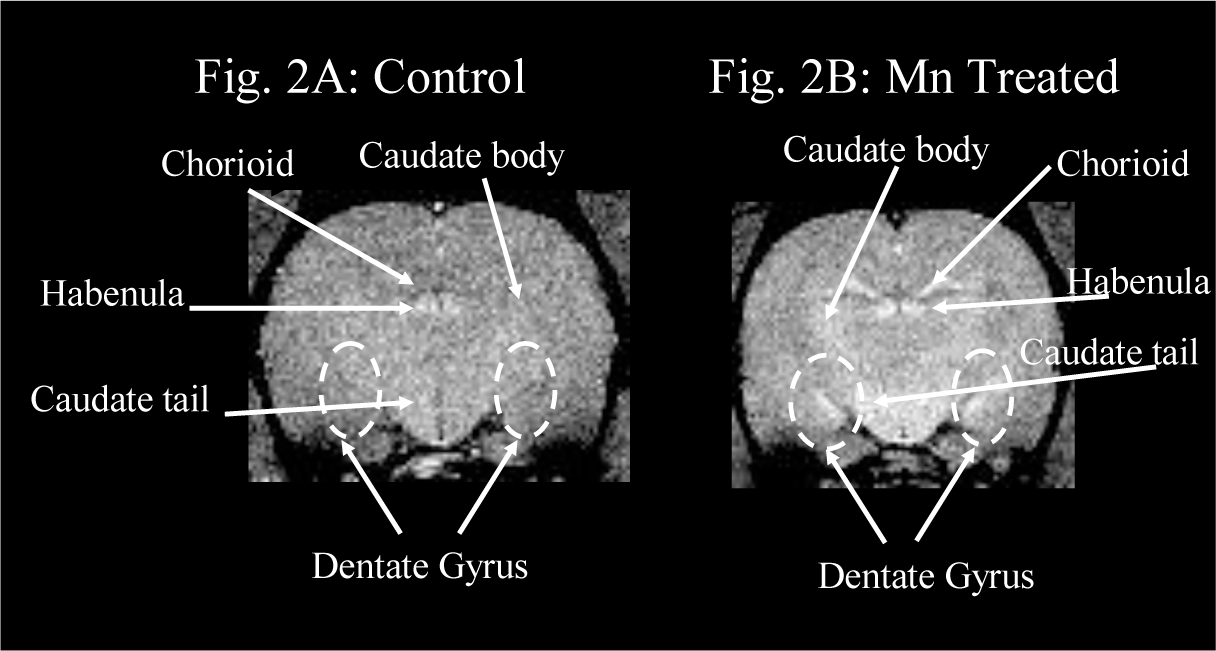
T1-weighted MRI (Figure 2A) presents a coronal section of a rat brain from the intact control group. No high intensity signals are observed in the brain parenchyma. Tiny foci of high intensity are shown in major blood vessels including the chorioid plexus; and in the habenulae.
T1-weighted MRI hyperintense findings (Figure 2B) are observed at the same level of a rat brain from the Mn treated group, which received weekly intravenous injections of MnCl2 solution (3 mg Mn/kg body mass) for 14 weeks: bilateral symmetrical high intensity signals sharply demarcate the dentate gyri and caudate nuclei. Hyperintense signals are more extensively evidenced in the habenulae and the chorioid plexuses of the Mn treated rat brain.
Figure 3:
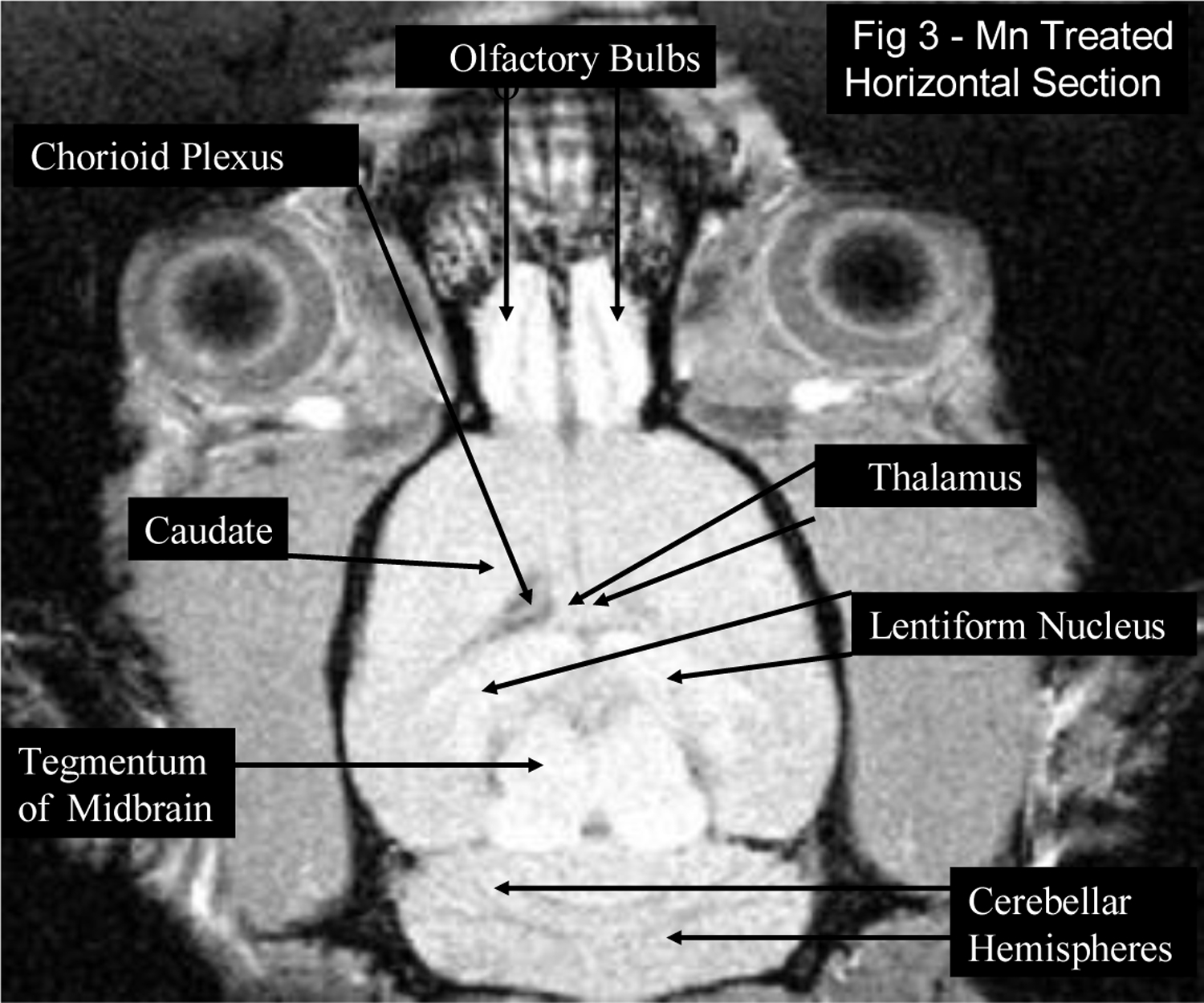
T1-weighted MRI hyperintense findings are observed in a horizontal section of a rat brain from the Mn treated group, which received weekly intravenous injections of MnCl2 solution (3 mg Mn/kg body mass) for 14 weeks: bilateral symmetrical high intensity signals in the olfactory bulbs, the midbrain tegmentum, the lentiform nucleus which is composed of the globus pallidus and the putamen, the caudate nucleus, the thalamus, the chorioid plexus and the folia of the cerebellar hemispheres (neocerebellum).
Figure 4:
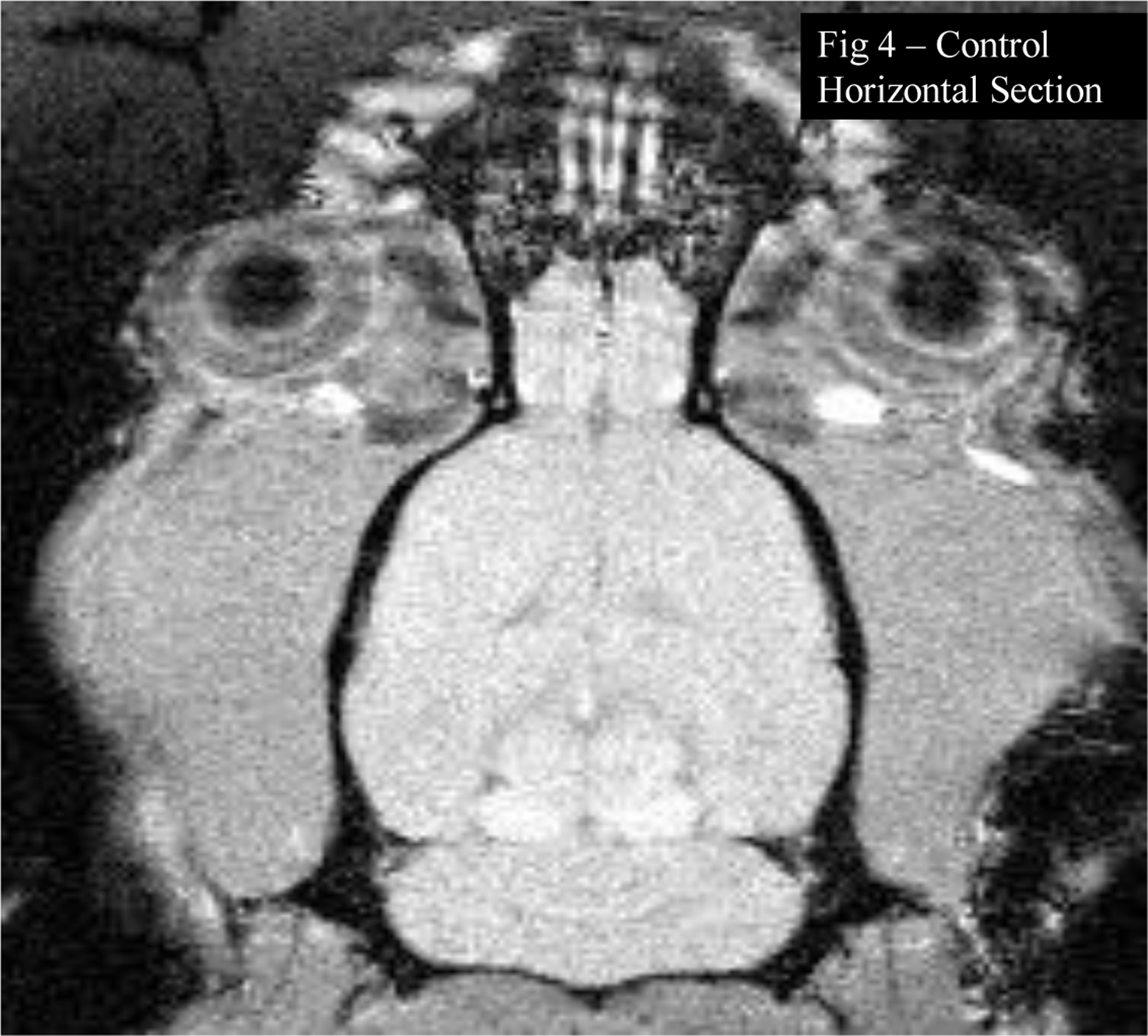
T1-weighted MRI presents a horizontal section of a rat brain from the intact control group, at the same level shown in Figure 3. High intensity signals are observed in the midbrain tegmentum, but less extensively than in the Mn treated rat brain. Tiny focus of high intensity is shown in the pineal gland in both Figures 3 and 4.
In rat brains from the intact control group, at the same levels, high intensity T1-weighted signals are observed in the midbrain tegmentum (Figure 4), but less extensively than in the Mn treated rat brains. Small foci of high intensity were also evident in the pineal gland (Figure 4), in major blood vessels (Figure 1A), including the chorioid plexus (Figure 2A) and in the habenulae (Figure 2A) - but less extensively than in the Mn treated rat brains.
Discussion:
Mn blood levels in the Mn treated group reached ~20 ug/L. This level, which is considered 2–3 folds higher than “normal” values [19], corroborates the concurrent exposure of the rats to Mn. However, it should be emphasized that Mn blood levels may serve merely as an indicator of recent exposure but do not reflect the total body burden of Mn or its intracerebral concentrations.
The T1-weighted MRI images in this study indicate high Mn concentrations in the hippocampal cortex, as well as in other crucial subcortical structures – the brain stem and midbrain, basal ganglia and thalamus. This study also points to significant concentrations of Mn in the olfactory bulbs and the chorioid plexus. The general dispersion of T1 hyperintensities observed throughout the entire brain of Mn treated rats is similar to the findings of a previous study, performed 1 and 2 weeks after a single I. V. injection of MnCl2, in which radioactive Mn was found ubiquitously throughout the CNS, but with the heaviest labeling in hippocampus, thalamus, colliculi, amygdala, olfactory lobe and cerebellum [21]. This likely implies a consistent pattern of distribution of Mn in the CNS, in which the hippocampus and amygadala are significant targets for Mn deposition, at least in the subacute and subchronic stages of exposure.
All animals were scanned at time 0 prior to Mn administration. Furthermore, each animal was used as its own control, so we do have a baseline on which to base our conclusions that Mn treatment led to accumulation of Mn in specific brain regions.
As noted above, Mn was readily deposited in the olfactory bulb area, in spite of the fact that it was administered by I.V infusion. This likely reflects the abundance of divalent metal transporter (DMT-1) in this area [12]. DMT-1 consists of 17 exons, spanning more than 36 kb and encoding two proteins of 561 and 570 amino acids. DMT-1 has been implicated in the transport of Mn (2+ oxidation state) as well as other divalent metals. In rats, DMT1 expression is highest in astrocytes and endothelial cells within the striatum, granule and Purkinje cells of the cerebellum, in neurons within the hippocampus and thalamus and in ependymal cells lining the third ventricle, with moderate staining in the substantia nigra [21, 22].
The deposition of Mn in the hippocampus and dentate gyrus, indicated by increased T1-weighted signals, may be relevant to the clinical aspects of manganism. In a recent neurobehavioral study, young adult rats exposed subchronically to MnCl2 for 10 weeks demonstrated a dose-dependent increase in glial fibrillary acidic protein (GFAP) immunoreactivity in the hilar part of the dentate gyrus, as well as a impairments in spatial memory, exploratory activity and pre-pulse inhibition and diminished sensorimotor reaction. All these neurobehavioral tasks are subserved by the septohippocampal system, suggesting a causal link between Mn-associated deposition and damage to this system. The memory function of initial acquisition, short term (4 hr) and long-term working memory were significantly decreased in the treated animals [23]. Both septohippocampal system and dentate gyrus play a major role in the acquisition of new information and possibly are an integral neural substrate for spatial reference and spatial working memory [24]. Indeed, these functions might be impaired pursuant to Mn-induced decrease in the activity of the septohippocampal cholinergic system [1]. The septohippocampal cholinergic system also controls spontaneous locomotor activity, as observed in rodents which are deficient for M1 muscarinic receptors [25]. The involvement of the hippocampus in the early clinical stages of manganism is crucial, initially in the non-specific complaints and behavioral changes [24] and consequently in the affective disorder of locura manganica [1].
The temporal relationship between hyperintensities in the basal ganglia and the onset of subclinical Mn neurotoxicity has yet to be defined. This is important in determining when metal deposition is actually indicative of a clinical manifestation. This question may be better addressed using non-human primate models of manganism, in which extrapyramidal signs and symptoms are clinicallly expressed. Additionally, groundwork for this issue already exists in the present rodent model. A longitudinal MRI study of chronic exposure to Mn will provide information on the long-term dynamics of Mn deposition in the CNS, where the data from human studies suggest that a shift in the pattern of distribution, with increasing basal ganglia basal ganglia hyperintenisities on T1, might presage the emergence of the clinical extrapyramidal stage of manganism.
These observations may assume relevance in the daily clinical toxicology of khat–related substances, which became a drug epidemic. Methcathinone was initially patented (but never marketed) by the pharmaceutical firm Parke-Davis in 1957. Subsequently, not later than 1982, it was manufactured by Soviet chemists and became illicitly used worldwide. As estimated, 20 percent of drug abusers in Russia are addicted to methcathinone [7]. This drug, which is clandestinely synthesized from ephedrine, usually contains Mn as an adulterant from incomplete processing. Therefore, the experimental findings in this animal model may be relevant to numerous I.V. abusers of methcathinone who are prone to manganism.
In conclusion, this work establishes T1- weighted MRI as a useful tool for determining the relative changes in the deposition of this paramagnetic metal throughout the entire brain. Further work is needed to examine the relationships between the transport of Mn into the brain, the creation of region specific Mn depositions and the clinical manifestations of manganism.
Acknowledgements:
This review was partially supported by grants from NIEHS 10563, DoD W81XWH-05-1-0239, and the Gray E.B. Stahlman Chair of Neuroscience.
References:
- 1.Finkelstein Y, Milatovic D, Aschner M. Modulation of cholinergic systems by manganese. Neurotoxicology 2007;28:1003–14. [DOI] [PubMed] [Google Scholar]
- 2.Gorell JM, Johnson CC, Rybicki BA, et al. Occupational exposure to manganese, copper, lead, iron, mercury and zinc and the risk of Parkinson’s disease. Neurotoxicology 1999;20:239–47. [PubMed] [Google Scholar]
- 3.Tanaka S Manganese and its compounds. In: Zenz C, ed. Occupational Medicine. 32nd ed. St. Louis: Mosby Press. 1994:213–7. [Google Scholar]
- 4.Reimund JM, Dietemann JL, Warter JM, Baumann R, Duclos B. Factors associated to hypermanganesemia in patients receiving home parenteral nutrition. Clin Nutr 2000;19:343–8. [DOI] [PubMed] [Google Scholar]
- 5.Spahr L, Butterworth RF, Fontaine S, et al. Increased blood manganese in cirrhotic patients: relationship to pallidal magnetic resonance signal hyperintensity and neurological symptoms. Hepatology 1996;24:1116–20. [DOI] [PubMed] [Google Scholar]
- 6.Stepens A, Logina I, Liguts V, et al. A Parkinsonian syndrome in methcathinone users and the role of manganese. N Engl J Med 2008;358:1009–17. [DOI] [PubMed] [Google Scholar]
- 7.Gahlinger PM. Illegal Drugs. Plume, Penguin Books. 2004:231–7. [Google Scholar]
- 8.McMillan DE. A brief history of the neurobehavioral toxicity of manganese: some unanswered questions. Neurotoxicology 1999;20:499–507. [PubMed] [Google Scholar]
- 9.Bleich S, Degner D, Sprung R, Riegel A, Poser W, Rüther E. Chronic manganism: fourteen years of follow-up. J Neuropsychiatry Clin Neurosci 1999;11:117. [DOI] [PubMed] [Google Scholar]
- 10.Barbeau A, Inoué N, Cloutier T. Role of manganese in dystonia. Adv Neurol 1976;14:339–52. [PubMed] [Google Scholar]
- 11.Mergler D, Baldwin M. Early manifestations of manganese neurotoxicity in humans: an update. Environ Res 1997;73:92–100. [DOI] [PubMed] [Google Scholar]
- 12.Fitsanakis VA, Zhang N, Avison MJ, Gore JC, Aschner JL, Aschner M. The use of magnetic resonance imaging (MRI) in the study of manganese neurotoxicity. Neurotoxicology 2006;27:798–806. [DOI] [PubMed] [Google Scholar]
- 13.Kim Y High signal intensities on T1-weighted MRI as a biomarker of exposure to manganese. Ind Health 2004;42:111–5. [DOI] [PubMed] [Google Scholar]
- 14.Silva AC, Lee JH, Aoki I, Koretsky AP. Manganese-enhanced magnetic resonance imaging (MEMRI): methodological and practical considerations. NMR Biomed 2004;17:532–43. [DOI] [PubMed] [Google Scholar]
- 15.Kang YS, Gore JC. Studies of tissue NMR relaxation enhancement by manganese. Dose and time dependences. Invest Radiol 1984;19:399–407. [DOI] [PubMed] [Google Scholar]
- 16.Discalzi G, Pira E, Herrero Hernandez E, Valentini C, Turbiglio M, Meliga F. Occupational Mn parkinsonism: magnetic resonance imaging and clinical patterns following CaNa2-EDTA chelation. Neurotoxicology 2000;21:863–6. [PubMed] [Google Scholar]
- 17.London RE, Toney G, Gabel SA, Funk A. Magnetic resonance imaging studies of the brains of anesthetized rats treated with manganese chloride. Brain Res Bull 1989;23:229–35. [DOI] [PubMed] [Google Scholar]
- 18.Lee JH, Silva AC, Merkle H, Koretsky AP. Manganese-enhanced magnetic resonance imaging of mouse brain after systemic administration of MnCl2: dose-dependent and temporal evolution of T1 contrast. Magn Reson Med 2005;53:640–8. [DOI] [PubMed] [Google Scholar]
- 19.Chaki H, Furuta S, Matsuda A, et al. Magnetic resonance image and blood manganese concentration as indices for manganese content in the brain of rats. Biol Trace Elem Res 2000;74:245–57. [DOI] [PubMed] [Google Scholar]
- 20.Fitsanakis VA, Zhang N, Anderson JG, Erikson KM, Avison MJ, Gore JC, Aschner M. Measuring brain manganese and iron accumulation in rats following 14 weeks of low-dose manganese treatment using atomic absorption spectroscopy and magnetic resonance imaging. Toxicol Sci. 2008. 103:116–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gallez B, Baudelet C, Geurts M. Regional distribution of manganese found in the brain after injection of a single dose of manganese-based contrast agents. Magn Reson Imaging 1998;16:1211–5. [DOI] [PubMed] [Google Scholar]
- 22.Burdo JR, Menzies SL, Simpson IA, et al. Distribution of divalent metal transporter 1 and metal transport protein 1 in the normal and Belgrade rat. J Neurosci Res 2001;66:1198–207. [DOI] [PubMed] [Google Scholar]
- 23.Vezér T, Kurunczi A, Náray M, Papp A, Nagymajtényi L. Behavioral effects of subchronic inorganic manganese exposure in rats. Am J Ind Med 2007;50:841–52. [DOI] [PubMed] [Google Scholar]
- 24.Finkelstein Y, Koffler B, Rabey JM, Gilad GM. Dynamics of cholinergic synaptic mechanisms in rat hippocampus after stress. Brain Res 1985;343:314–9. [DOI] [PubMed] [Google Scholar]
- 25.Mattsson A, Pernold K, Ogren SO, Olson L. Loss of cortical acetylcholine enhances amphetamine-induced locomotor activity. Neuroscience 2004;127:579–91. [DOI] [PubMed] [Google Scholar]


