Abstract
Background
Extra-abdominal desmoid tumors often occur in the necks, shoulder, chest wall, back, arm, buttock, thigh and leg. Multicentric extra-abdominal desmoids are rather rare and seem to have other clinical features. The aim of our study was to investigate clinical features, especially multicentric occurrence of extra-abdominal desmoid tumors.
Patients and Methods
A total of 135 patients diagnosed with extra-abdominal desmoid were enrolled in this study from January 2005 to December 2019 at the Cancer Institute Hospital of The Japanese Foundation for Cancer Research. The operative procedure was principally wide excision. The clinicopathological factors [e.g., age, gender, pain, restriction of range of motion (ROM), tumor site, tumor size, surgical margin, multicentric occurrence, local recurrence, tumoral regression] were collected and assessed by univariate analysis. We assessed how multicentric occurrence influenced clinicopathological factors of desmoid tumors.
Results
The median follow-up was 39.9 months (range=0.29-259 months). Among 135 patients, 20 had multicentric occurrence. Multicentric extra-abdominal desmoids occurred in the neck in six cases, shoulder in four, chest wall in three, back in three, thigh in two and leg in two. In the case of multicentric occurrence on thighs and legs, tumors arose not in the anterior compartment but in the posterior compartment. Univariate analysis showed association of multicentric extra-abdominal desmoids with high local recurrence (p=0.0003), restriction of ROM (p=0.0012) and tumor size larger than 5 cm (p=0.04) but surgical margins were not correlated with local recurrence (p=0.37).
Conclusion
Surgery should be performed in those who have severe pain or restriction of ROM. A 'Wait and see' policy is a first-line management, especially for those with multicentric extra-abdominal desmoids.
Keywords: Desmoid, multicentric occurrence, local recurrence
Desmoids are rare tumors which are considered locally invasive but non-metastasizing, with a reported incidence of 2-4 per million population and account for 0.03% of all neoplasms (1-4). The World Health Organization has characterized desmoids as intermediate, locally aggressive tumors (5).
Histologically, desmoids are characterized by the proliferation of uniform spindle cells resembling myofibroblasts, in a background of abundant collagenous stroma and vascular network (6). On immunohistochemistry, desmoids stain positively for nuclear beta-catenin, vimentin, cyclo-oxygenase-2, tyrosine kinase, platelet-derived growth factor receptor B, androgen receptor, and estrogen receptor beta. Desmoids stain negatively for desmin, S-100, H-caldesmon, CD34, and c-KIT (7). Nuclear beta-catenin positivity supports the diagnosis of desmoid.
They are classified according to their location: extra-abdominal, abdominal wall or intra-abdominal. Extra-abdominal desmoid tumors often occur in the neck, chest wall, shoulder, back, arm, buttock, thigh and leg. Of these tumors, multicentric extra-abdominal desmoids are rather rare and seem to have other clinical features. Previous reports showed that multicentric extra-abdominal desmoids arose in the same extremity (8-11). The aim of our study was to investigate clinical features of extra-abdominal desmoid tumors, especially their multicentric occurrence.
Patients and Methods
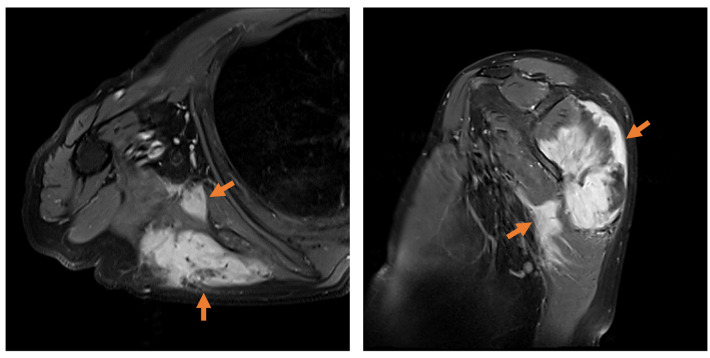
A total of 135 patients diagnosed with extra-abdominal desmoids were enrolled in this study from January 2005 to December 2019 at the Cancer Institute Hospital of The Japanese Foundation for Cancer Research. Clinical and pathological data were collected by reviewing medical records at our Institution, with the last follow-up conducted in December 2019. All patients consented to use of their medical records in articles or meetings and all patient data were strictly anonymized. The inclusion criterion was a confirmed pathological diagnosis by a pathologist at our Institution through histological characteristics of the biopsy specimen. Immunohistochemistry for nuclear beta-catenin positivity was applied in all cases retrospectively to confirm the pathological diagnosis. Patients with no beta-catenin translocation to the nucleus or with hereditary disease such as familial adenomatous polyposis or Gardner syndrome were excluded. The tumors were classified by their location as extra-abdominal, of the abdominal wall, or intra-abdominal. The following demographic data and treatment factors were examined retrospectively for multicentric occurrence: Age at diagnosis (<60 or ≥60 years), gender, pain, restriction of range of motion (ROM), tumor site, tumor size (<5 or ≥5 cm), surgical margin, multicentric occurrence, local recurrence and tumoral regression. Tumoral regression was defined as the disappearance of enhancement on gadolinium-enhanced magnetic resonance imaging (MRI). The surgical margin was pathologically confirmed through surgical specimens by a pathologist. Multicentric occurrence was defined as tumoral extension to several muscles by gadolinium-enhanced MRI at the patient’s first visit (orange arrows in Figure 1). Whether tumoral extension was to muscles adjacent to each other was not considered. The operative procedure was principally wide excision.
Figure 1. Multicentric occurrence was defined as tumoral extension to several muscles by gadolinium-enhanced magnetic resonance imaging (orange arrows).
Statistical analysis. Comparisons between groups for measurement data with normal distributions were analyzed by the chi-squared test. All statistical tests were bilateral. Univariate analysis was performed on demographic data and treatment factors for multicentric occurrence and recurrence. Univariate comparisons were performed using the log-rank test. The data were analyzed using JMP 14.2 (SAS, Tokyo, Japan). p-Values below 0.05 were considered statistically significant.
Results
Baseline information of patients. There were 135 patients included in this study, with a median age of 30 years (range=14-80 years). The median follow-up time was 39.9 months (range=0.29-258 months). At the last follow-up, 133 patients were still alive; two patients had died due to other causes. Overall, most tumors (74 cases) were of the abdomen, neck or back, and the vast majority of patients (n=85) had pain at their tumor site; 30 patients had ROM restriction. The median size of tumors was 7.4 cm (range=1.4-18.1 cm), 105 patients (77.7%) had tumors larger than 5 cm. Approximately one in seven had multicentric occurrence at their first visit. The evaluation of surgical specimens showed most had positive margins (71.4%). Twenty-two patients had tumoral regression. Local recurrence after surgery occurred in just over one-third of patients. Local recurrence occurred at a median of 7.2 months (range=1.9-34.9 months) after surgery. Baseline information of patients is shown in Table I.
Table I. Baseline characteristics of patients (n=135).
ROM: Range of motion.
Multicentric extra-abdominal occurrence. Multicentric extra-abdominal desmoids occurred in the neck in six cases, shoulder in four, chest wall in three, back in three, thigh in two, and leg in two. It was notable that multicentric occurrence in thighs and legs seemed to arise in a specific part, the posterior. The frequency of multicentric extra-abdominal desmoids according to location is shown in Table II.
Table II. The location of multicentric extra-abdominal occurrence (the numbers in parentheses refer to multicentric extra-abdominal occurrence).
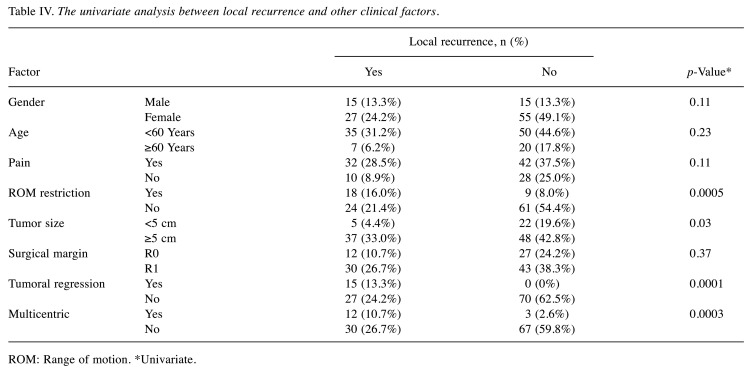
Factors influencing multicentric occurrence. Univariate analysis showed the association of multicentric extra-abdominal desmoids with high local recurrence (p=0.0003), restriction of ROM (p=0.0012) and tumor size larger than 5 cm (p=0.04) (Table III). Univariate analysis also showed local recurrence was positively correlated with restriction of ROM (p=0.0005) and tumor size (p=0.03), however, surgical margin status did not have any correlation with local recurrence (p=0.37) (Table IV).
Table III. The univariate analysis between multicentric occurrence and other clinical factors.
ROM: Range of motion. *Univariate.
Table IV. The univariate analysis between local recurrence and other clinical factors.
ROM: Range of motion. *Univariate.
Discussion
Extra-abdominal desmoid tumors often occur in the neck, shoulder, axilla, chest wall, arm, back, buttock, thigh and leg. Of these tumors, multicentric extra-abdominal desmoids are rather rare and seem to have other clinical features. In this study, we investigated clinical features of extra-abdominal desmoid tumors.
Multicentric extra-abdominal desmoids generally arise in the same extremity (8-11). Previous studies referred to multicentric extra-abdominal desmoids but the definition of multicentric extra-abdominal desmoids was not standardized (11,12).
In this study, we defined multicentric occurrence as tumoral extension to several muscles by gadolinium-enhanced MRI at first presentation. Whether tumoral extension of muscles are adjacent to each other was not considered. Hereditary disease like familial adenomatous polyposis or Gardner syndrome were excluded.
Multicentric extra-abdominal desmoids were associated with a high local recurrence rate (p=0.0003) by univariate analysis. It was notable that multicentric occurrence of both thighs and legs seemed to arise specifically in the posterior compartment. Univariate analysis showed multicentric extra-abdominal desmoids were related to restriction of ROM (p=0.0012), tumor size larger than 5 cm (p=0.04), which were consistent with the definition of multicentric extra-abdominal desmoids. Surgical margin was not correlated with local recurrence (p=0.37), which was consistent with previous studies (13-15).
Surgery should be performed in patients who have severe pain or joint contracture. Given the high local-recurrence rate after surgery and the fact that surgical margin status had no correlation with local recurrence, a 'Wait and see' policy is an appropriate first-line management, especially for those with multicentric extra-abdominal desmoids.
Conflicts of Interest
None declared.
Authors’ Contributions
Yusuke Minami designed the study, and wrote the initial draft of the article. Yusuke Minami also contributed to analysis and interpretation of data, and assisted in the preparation of the article. All other Authors have contributed to data collection and interpretation, and critically reviewed the article. All Authors approved the final version of the article, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Acknowledgements
The Authors thank members of the Department of Orthopedic Oncology at the Cancer Institute Hospital of the Japanese Foundation for Cancer Research for helpful discussions.
References
- 1.PDQ Pediatric Treatment Editorial Board Childhood Soft Tissue Sarcoma Treatment (PDQ®): Health Professional Version. 2021 [Google Scholar]
- 2.Wang Z, Wu J, Lv A, Tian X, Hao C. En bloc resection for intra-abdominal/retroperitoneal desmoid-type fibromatosis with adjacent organ involvement: A case series and literature review. Biosci Trends. 2018;12(6):620–626. doi: 10.5582/bst.2018.01285. [DOI] [PubMed] [Google Scholar]
- 3.Cassidy MR, Lefkowitz RA, Long N, Qin LX, Kirane A, Sbaity E, Hameed M, Coit DG, Brennan MF, Singer S, Crago AM. Association of MRI T2 signal intensity with desmoid tumor progression during active observation: A retrospective cohort study. Ann Surg. 2020;271(4):748–755. doi: 10.1097/SLA.0000000000003073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nieuwenhuis MH, Casparie M, Mathus-Vliegen LM, Dekkers OM, Hogendoorn PC, Vasen HF. A nation-wide study comparing sporadic and familial adenomatous polyposis-related desmoid-type fibromatoses. Int J Cancer. 2011;129(1):256–261. doi: 10.1002/ijc.25664. [DOI] [PubMed] [Google Scholar]
- 5.The WHO Classification of Tumours Editorial Board . Lyon, IARC Press. 2020. WHO Classification of Tumours Soft Tissue and Bone Tumours, 5th Edition. [Google Scholar]
- 6.Muller E, Castagnaro M, Yandel DW, Wolfe HJ, Alman BA. Molecular genetic and immunohistochemical analysis of the tumor suppressor genes Rb and p53 in palmar and aggressive fibromatosis. Diagn Mol Pathol. 1996;5(3):194–200. doi: 10.1097/00019606-199609000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Kotiligam D, Lazar AJ, Pollock RE, Lev D. Desmoid tumor: a disease opportune for molecular insights. Histol Histopathol. 2008;23(1):117–126. doi: 10.14670/HH-23.117. [DOI] [PubMed] [Google Scholar]
- 8.Wagstaff MJ, Raurell A, Perks AG. Multicentric extra-abdominal desmoid tumours. Br J Plast Surg. 2004;57(4):362–365. doi: 10.1016/j.bjps.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 9.Antal I, Szendröi M, Kovács G, Nagykálnai T, Entz L. Multicentric extraabdominal desmoid tumour: a case report. J Cancer Res Clin Oncol. 1994;120(8):490–493. doi: 10.1007/BF01191803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watanabe K, Ogura G, Tajino T, Suzuki T. Extra-abdominal desmoid fibromatosis: two familial cases with synchronous and metachronous multicentric hyalinizing nodules. Histopathology. 2002;41(2):118–121. doi: 10.1046/j.1365-2559.2002.01430.x. [DOI] [PubMed] [Google Scholar]
- 11.Shimoyama T, Hiraoka K, Shoda T, Hamada T, Fukushima N, Nagata K. Multicentric extra-abdominal desmoid tumors arising in bilateral lower limbs. Rare Tumors. 2010;2(1):e12. doi: 10.4081/rt.2010.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Röpke M, Kalinski T, Wördehoff H, Aumann V, Bürger T. Multicentric extra-abdominal fibromatosis: a rare case. Z Orthop Ihre Grenzgeb. 2006;144(2):223–227. doi: 10.1055/s-2005-836751. [DOI] [PubMed] [Google Scholar]
- 13.Melis M, Zager JS, Sondak VK. Multimodality management of desmoid tumors: how important is a negative surgical margin. J Surg Oncol. 2008;98(8):594–602. doi: 10.1002/jso.21033. [DOI] [PubMed] [Google Scholar]
- 14.Turner B, Alghamdi M, Henning JW, Kurien E, Morris D, Bouchard-Fortier A, Schiller D, Puloski S, Monument M, Itani D, Mack LA. Surgical excision versus observation as initial management of desmoid tumors: A population based study. Eur J Surg Oncol. 2019;45(4):699–703. doi: 10.1016/j.ejso.2018.09.015. [DOI] [PubMed] [Google Scholar]
- 15.Cates JM, Stricker TP. Surgical resection margins in desmoid-type fibromatosis: a critical reassessment. Am J Surg Pathol. 2014;38(12):1707–1714. doi: 10.1097/PAS.0000000000000276. [DOI] [PubMed] [Google Scholar]