Abstract
Background/Aim
According to limited current reports, therapeutic paraaortic lymph node (PALN) dissection with intensive combined therapy for colorectal cancer improves prognosis in select patients. Laparoscopic PALN dissection is a difficult technique that has not yet been established. We applied this procedure using an intraoperative fluorescence navigation technique with a near-infrared ray catheter (NIRC™) fluorescent ureteral catheter (NIRFUC).
Patients and Methods
We evaluated the utility of laparoscopic fluorescence navigation and the short-term outcomes of 6 patients undergoing laparoscopic PALN dissection.
Results
There were 3 surgeries for synchronous metastasis and 3 surgeries for recurrent metastasis. The mean surgical duration, blood loss, and postoperative hospital stay were 677 (range=518-1,090) min, 7.5 (range=3-1,600) ml, and 14 (range=9-33) days, respectively. Postoperative complications (Clavien-Dindo grade >III) occurred in 1 case.
Conclusion
Dissection around the ureter was navigated with a NIRFUC. Fluorescence ureteral navigation facilitated completion of the complex laparoscopic PALN dissection procedure.
Keywords: Laparoscopic surgery, paraaortic lymph node dissection, colorectal cancer, fluorescence navigation, ureter
Approximately 12% of patients with colorectal cancer are diagnosed with distant metastasis (1). The incidence of isolated paraaortic lymph node (PALN) metastasis with colorectal cancer is reported to be 1-2% (2). Surgical prophylactic PALN dissection was first described in 1959 as a modification of the Miles operation for rectal cancer (3). However, prophylactic PALN dissection has been reported to be associated with high surgical morbidity and does not improve prognosis (4). Therefore, routine prophylactic PALN dissection has since been abandoned (5). However, according to the few current reports, therapeutic PALN dissection with intensive combined therapy improve the prognosis of select patients (5-10). The significance of this surgery is still controversial, and it is rarely performed at limited institutions. If improvement of the disease-free survival rate or overall survival rate is expected with resection of the liver and lung metastases, indications of PALN dissection should be more actively considered. On the other hand, for colorectal cancer, the performance of laparoscopic surgery has increased, and minimally invasive surgery is generally preferred (11-13). However, laparoscopic PALN dissection is a difficult technique and has not been well established. We applied this difficult procedure in our institution using an intraoperative fluorescence navigation technique with a near-infrared ray catheter (NIRC™) fluorescent ureteral catheter (NIRFUC; Nippon Covidien, Ltd., Tokyo, Japan).
Patients and Methods
We evaluated the utility of laparoscopic fluorescence navigation and the short-term outcomes of 6 patients treated with PALN dissection at Kawaguchi Municipal Medical Center between September 2020 and April 2021. The study was approved by the Research Ethics Committee of the Kawaguchi Municipal Medical Center (Saitama, Japan) approval number: 2020-3 (https://kawaguchi-mmc.org/wp-content/uploads/clinicalresearch-r02.pdf).
Surgery. The patients underwent NIRFUC insertion before surgery. Laparoscopic procedures were performed with a camera port at the umbilicus and five additional ports located in each abdominal quadrant and at the lower abdominal midline. First, right and left colon mobilization and Kocher mobilization of the duodenum were performed. Next, the duodenum and mesocolon of the left colon were pulled to the abdominal wall with an organ retractor device. Dissection between the mesocolon and ureter was navigated with the NIRFUC. After identification of the ureter with the NIRFUC, the left gonadal vessel was dissected upward to the left renal vein, which is the head-side edge of PALN dissection. The caudal edge of the dissection was the common iliac bifurcation. The left edge of the dissection was the left gonadal vessel and ureter. While visualizing the ureter and avoiding injury, the anterior surface of the psoas muscle was exposed to remove fat from the Gerota fascia. After determining the left-side goal, the PALNs were dissected by an intraside approach from the inferior vena cava. In one case, the left renal artery was visualized with intravenous indocyanine green (ICG) injection.
Results
The mean patient age was 79 (range=51-83 years), including 4 men and 2 women (Table I). In terms of the primary lesion, there was 1 case of ascending colon cancer (Figure 1), 1 case of transverse colon cancer, and 4 cases of rectal cancer (Figure 2 and Figure 3). Three surgeries were performed for synchronous metastasis (2 right hemicolectomies and 1 total pelvic exenteration), and 3 surgeries were performed for recurrent metastasis of rectal cancer (including 1 partial hepatectomy and 1 lateral lymph node dissection).
Table I. Patient characteristics (n=6).
Values are presented as the median [range] or n (%).
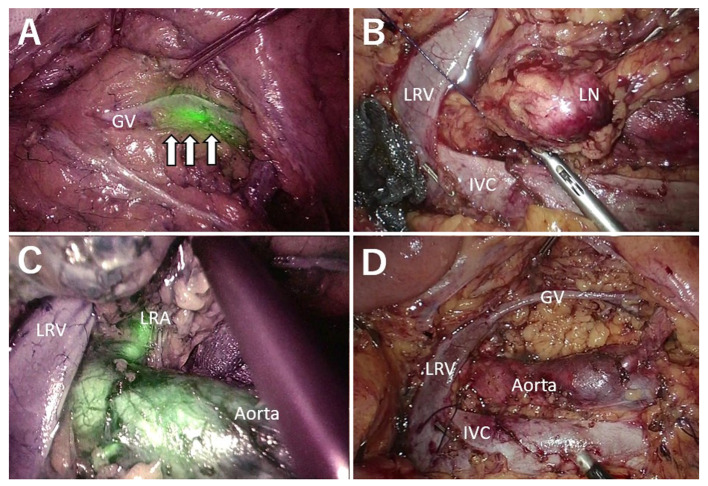
Figure 1. Case of synchronous metastasis. A: The white arrow indicates the ureter with the NIRFUC. B: Lymph node metastasis was dissected from the LRV and IVC. Lymphatic vessels were ligated. C: FVN revealed the left renal artery with fluorescence. D: Surgical field after PALN dissection. IVC: Inferior vena cava; NIRFUC: near-infrared ray catheter (NIRC™) fluorescent ureteral catheter; FVN: fluorescence vessel navigation; PALN: paraaortic lymph node; Aorta: abdominal aorta; LRV: left renal vein; LRA: left renal artery; GV: gonadal vein.
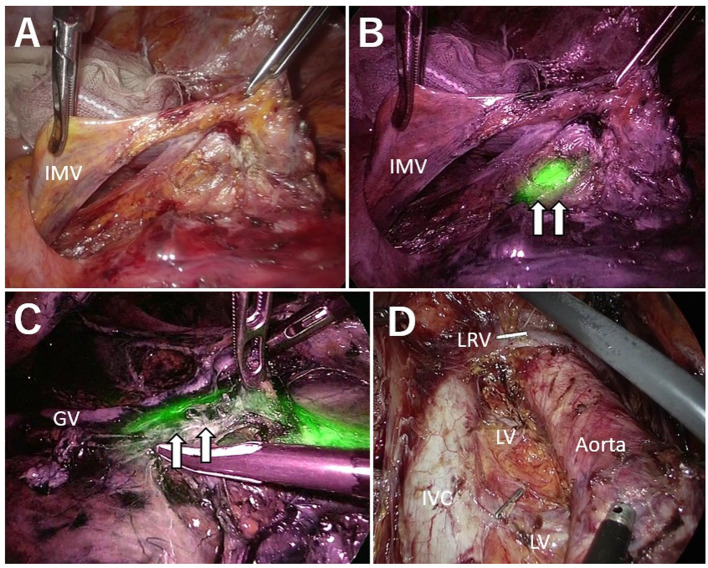
Figure 2. Case of recurrence after low anterior resection. A: Under normal light observation, the ureter was not visible. On the dorsal side, the mesocolon and Gerota fascia were easily dissected. However, on the caudal side, dissection was difficult because of adhesion from a previous surgery. B: NIRFUC allowed visualization of the ureter with fluorescence. C: Lymph node dissection was performed while preserving visualization of the ureter (white arrow). D: Surgical field after paraaortic lymph node dissection. NIRFUC: near-infrared ray catheter (NIRC™) fluorescent ureteral catheter; IMV: inferior mesenteric vein; IVC: Inferior vena cava; Aorta: abdominal aorta; LRV: left renal vein; GV: gonadal vein; LV: lumbar vein.
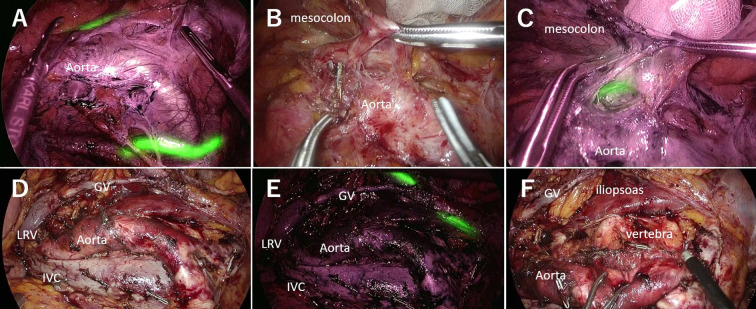
Figure 3. Case of recurrence after the Hartmann procedure. A: Fluorescence observation before paraaortic lymph node (PALN) dissection. NIRFUC allowed visualization of both sides of the ureter with fluorescence. B: Dissection between the mesocolon, ureter and gonadal vein under normal light observation. The ureter was not detected. C: Fluorescence observation using the NIRFUC to visualize the ureter. D: Surgical field after PALN dissection under normal light observation. E: Surgical field after PALN dissection with fluorescence observation. F: Surgical field after PALN dissection. NIRFUC: Near-infrared ray catheter (NIRC™) fluorescent ureteral catheter; IVC: Inferior vena cava; aorta: abdominal aorta; PALN: paraaortic lymph node; LRV: left renal vein; GV: gonadal vein.
The mean surgical duration was 677 (range=518-1,090) min, the mean blood loss was 7.5 (range=3-1,600) ml, and the mean postoperative hospital stay was 14 (range=9-33) days (Table II). Postoperative complications [Clavien-Dindo (CD) grade III and above] occurred in 1 case (pancreatic fistula). Five cases (83%) were pathologically positive.
Table II. Study results (n=6).
Values are presented as the median [range] or n (%). aClavien-Dindo grade III or higher.
Discussion
ICG in living body is excited by irradiation with near infrared (NIR) light at 760 to 780 nm, and it emits NIR fluorescence at 800 to 850 nm (14). This phenomenon is known as fluorescence and specific camera enables visualization of ICG in vivo by its NIR fluorescence.
Recently, intraoperative fluorescence imaging has progressed, and a newly invented NIR fluorescent resin emits NIR fluorescence, as in the case of ICG (15). The NIRFUC (Nippon Covidien, Ltd., Tokyo, Japan) is a Newly developed devices made with this resin (16,17). Before the development of the NIRFUC, visualization of ureters using ICG (18,19), methylene blue (20-22), and lighted stents (23,24) was reported. However, infusing ICG through a ureteral stent and intravenous administration of methylene blue have insufficient and unstable brightness (22). In addition, these techniques have not been approved for clinical use in Japan. Lighted stents are also expected to be useful devices for visualization of the ureter. However, studies on the use of stents have described intraoperative ureteral injury rates of 1.5% (23). In addition, ureteral injuries during preoperative catheter insertion have been indicated to occur at a rate of 1.5% (23). Now lighted stents are not used in general because the ureter can be blocked, causing hydronephrosis, and thermal damage to the ureter due to light emission has been reported (23,25-27). The infrared illumination system (IRIS; Stryker, Tokyo, Japan) is a new lighted stent that could be used in Japan (27). The improvements of the IRIS over conventional lighted stents include that the IRIS does not generate heat because of its use of NIR light (27). However, the luminous stent length from the ureteral orifice is only 20 cm. Thus, its utility is limited to pelvic surgery, and it is not useful for PALN dissection. In this study, we used the NIRFUC, which does not generate heat similar to the IRIS. The length of the NIRFUC is 70 cm, and it reached the renal pelvis. Therefore, ureter navigation is possible in the surgical field of PALN dissection. The outer and inner diameters of the NIRFUC are 2 mm and 1.15 mm, respectively. Urine flows through the lumen of the catheter to prevent hydronephrosis and urinary tract infection. The NIRFUC is excited by irradiation with NIR light at 750 to 810 nm, and it emits NIR fluorescence (16). A fluorescence scope captures NIR fluorescence and enables visualization. The luminance of the NIR fluorescence resin material of the NIRFUC is much high and has been reported to be a 30-fold fluorescence intensity compared with that of ICG (15).
Laparoscopic PALN dissection is a difficult technique and generally has not been performed. There are few reports regarding laparoscopic surgery for PALN dissection for colorectal cancer (28-31). However, technically feasible laparoscopic PALN dissection has been performed with no serious complications (28). Especially for cases of recurrence (Figures 2 and 3), postoperative adhesion caused by colon mobilization performed during the first surgery increases the difficulty of PALN dissection. To prepare for PALN dissection, the left-side mesocolon should be dissected from the ureter and gonadal vessel. The left edge of the PALN dissection is the left gonadal vessel and ureter. Because the peeling layer disappears at those points, there is a risk of ureter injury. If the safety of this surgery is not guaranteed, it is not possible to determine the surgical indication for this high-risk, controversial surgery. PALN dissection carries a risk of massive bleeding in addition to ureter injury. Past studies have described blood loss volumes of 806.3 (range=307-1,929) ml, 1,095 (range=42-4,315) ml, and 628 (range=20-4,900) ml (5,7,31). In addition to dissection around the great vessel, lumbar vein and artery and ascending lumbar vein, the renal artery has an anatomy such that surgeons should take extra care during procedures. In particular, the ascending lumbar vein, which flows into the renal vein, is a critical bleeding point, and the renal artery is a blood vessel that should never be damaged. The ICG fluorescence method enables the visualization of blood vessels (32). Fluorescence vessel navigation (FVN) using ICG enables identification of branching of the intrailiac artery (16). Identification of the inferior mesenteric vein and left colic artery with FVN can shorten the operative time (33).
In one case in which the renal artery was not detected, FVN with ICG injection revealed the course of the renal artery (Figure 1). A lymph node metastasis between the renal artery and renal vein was safely dissected. Laparoscopic PALN dissection comprises a complex procedure performed before lymph node dissection, including the division of adhesions from the first surgery and bilateral colon and duodenal mobilization. Additionally, dissection around the great vessel has a risk of dangerous bleeding, which places a heavy burden of stress on the surgeon. In this study, fluorescence navigation, including ureter and vessel navigation, facilitated completion of the complex laparoscopic PALN dissection procedure. PALN dissection was safely applied at our institution. However, larger studies are needed.
The limitations of this study include its retrospective design, the small number of patients at a single institution, and the possible selection bias.
Conflicts of Interest
Shunjin Ryu MD, PhD, Keigo Hara MD, Keisuke Goto MD, Keisuke Goto MD, Atsuko Okamoto MD, Takahiro Kitagawa MD, Rui Marukuchi MD, PhD, Ryusuke Ito MD, PhD, and Yukio Nakabayashi MD, PhD have no conflicts of interest or financial ties to disclose in relation to this study.
Authors’ Contributions
Shunjin Ryu: Project development, Surgery, Data collection, data analysis, manuscript writing; Keigo Hara: Surgery, Data collection; Keisuke Goto: Data collection; Atsuko Okamoto: Data collection; Takahiro Kitagawa: Data collection; Rui Marukuchi: Data collection; Ryusuke Ito: Data collection; Yukio Nakabayashi: Manuscript editing.
Acknowledgements
The Authors would like to thank Professor Masashi Yoshida (Department of Surgery, International University of Health and Welfare Hospital, Japan) for his invaluable advice regarding fluorescence navigation surgery. The Authors would also like to thank the medical staff of the Kawaguchi Municipal Medical Center, especially Ms. Keika Iijima, for her assistance with the colorectal cancer database. Finally, the Authors especially thank Sho Ohno, Yusuke Sasaki and Taketo Ichinose (Department of Urology, Kawaguchi Municipal Medical Center, Japan).
References
- 1.Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA. Seer cancer statistics review, 1975-2014. Bethesda, MD, National Cancer Institute. Available at: https://seer.cancer.gov/archive/csr/1975_2014/ [Last accessed on June 14, 2021]
- 2.Gagnière J, Dupré A, Chabaud S, Peyrat P, Meeus P, Rivoire M. Retroperitoneal nodal metastases from colorectal cancer: Curable metastases with radical retroperitoneal lymphadenectomy in selected patients. Eur J Surg Oncol. 2015;41(6):731–737. doi: 10.1016/j.ejso.2015.03.229. [DOI] [PubMed] [Google Scholar]
- 3.Stearns MW Jr, Deddish MR. Five-year results of abdominopelvic lymph node dissection for carcinoma of the rectum. Dis Colon Rectum. 1959;2(2):169–172. doi: 10.1007/BF02616711. [DOI] [PubMed] [Google Scholar]
- 4.Leggeri A, Roseano M, Balani A, Turoldo A. Lumboaortic and iliac lymphadenectomy: what is the role today. Dis Colon Rectum. 1994;37(2 Suppl):S54–S61. doi: 10.1007/BF02048433. [DOI] [PubMed] [Google Scholar]
- 5.Yamada K, Tsukamoto S, Ochiai H, Shida D, Kanemitsu Y. Improving selection for resection of synchronous para-aortic lymph node metastases in colorectal cancer. Dig Surg. 2019;36(5):369–375. doi: 10.1159/000491100. [DOI] [PubMed] [Google Scholar]
- 6.Ushigome H, Yasui M, Ohue M, Haraguchi N, Nishimura J, Sugimura K, Yamamoto K, Wada H, Takahashi H, Omori T, Miyata H, Takiguchi S. The treatment strategy of R0 resection in colorectal cancer with synchronous para-aortic lymph node metastasis. World J Surg Oncol. 2020;18(1):229. doi: 10.1186/s12957-020-02007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sakamoto J, Ozawa H, Nakanishi H, Fujita S. Oncologic outcomes after resection of para-aortic lymph node metastasis in left-sided colon and rectal cancer. PLoS One. 2020;15(11):e0241815. doi: 10.1371/journal.pone.0241815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sahara K, Watanabe J, Ishibe A, Suwa Y, Suwa H, Ota M, Kunisaki C, Endo I. Long-term outcome and prognostic factors for patients with para-aortic lymph node dissection in left-sided colorectal cancer. Int J Colorectal Dis. 2019;34(6):1121–1129. doi: 10.1007/s00384-019-03294-2. [DOI] [PubMed] [Google Scholar]
- 9.Min BS, Kim NK, Sohn SK, Cho CH, Lee KY, Baik SH. Isolated paraaortic lymph-node recurrence after the curative resection of colorectal carcinoma. J Surg Oncol. 2008;97(2):136–140. doi: 10.1002/jso.20926. [DOI] [PubMed] [Google Scholar]
- 10.Choi PW, Kim HC, Kim AY, Jung SH, Yu CS, Kim JC. Extensive lymphadenectomy in colorectal cancer with isolated para-aortic lymph node metastasis below the level of renal vessels. J Surg Oncol. 2010;101(1):66–71. doi: 10.1002/jso.21421. [DOI] [PubMed] [Google Scholar]
- 11.Fujii S, Akagi T, Inomata M, Katayama H, Mizusawa J, Ota M, Saito S, Kinugasa Y, Yamaguchi S, Sato T, Kitano S, Japan Clinical Oncology Group Transitional impact of short- and long-term outcomes of a randomized controlled trial to evaluate laparoscopic versus open surgery for colorectal cancer from Japan Clinical Oncology Group Study JCOG0404. Ann Gastroenterol Surg. 2019;3(3):301–309. doi: 10.1002/ags3.12245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jayne DG, Thorpe HC, Copeland J, Quirke P, Brown JM, Guillou PJ. Five-year follow-up of the Medical Research Council CLASICC trial of laparoscopically assisted versus open surgery for colorectal cancer. Br J Surg. 2010;97(11):1638–1645. doi: 10.1002/bjs.7160. [DOI] [PubMed] [Google Scholar]
- 13.Fleshman J, Sargent DJ, Green E, Anvari M, Stryker SJ, Beart RW Jr, Hellinger M, Flanagan R Jr, Peters W, Nelson H, Clinical Outcomes of Surgical Therapy Study Group Laparoscopic colectomy for cancer is not inferior to open surgery based on 5-year data from the COST Study Group trial. Ann Surg. 2007;246(4):655–62. doi: 10.1097/SLA.0b013e318155a762. discussion 662-4. [DOI] [PubMed] [Google Scholar]
- 14.Schaafsma BE, Mieog JS, Hutteman M, van der Vorst JR, Kuppen PJ, Löwik CW, Frangioni JV, van de Velde CJ, Vahrmeijer AL. The clinical use of indocyanine green as a near-infrared fluorescent contrast agent for image-guided oncologic surgery. J Surg Oncol. 2011;104(3):323–332. doi: 10.1002/jso.21943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anayama T, Sato T, Hirohashi K, Miyazaki R, Yamamoto M, Okada H, Orihashi K, Inoue K, Kobayashi M, Yoshida M, Hanazaki K. Near-infrared fluorescent solid material for visualizing indwelling devices implanted for medical use. Surg Endosc. 2020;34(9):4206–4213. doi: 10.1007/s00464-020-07634-0. [DOI] [PubMed] [Google Scholar]
- 16.Ryu S, Ishida K, Okamoto A, Nakashima K, Hara K, Ito R, Nakabayashi Y. Laparoscopic fluorescence navigation for left-sided colon and rectal cancer: Blood flow evaluation, vessel and ureteral navigation, clip marking and trans-anal tube insertion. Surg Oncol. 2020;35:434–440. doi: 10.1016/j.suronc.2020.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Ushimaru Y, Ohigawa A, Yamashita K, Saito T, Tanaka K, Makino T, Takahashi T, Kurokawa Y, Yamasaki M, Mori M, Doki Y, Nakajima K. Real-time ureteral identification with novel, versatile, and inexpensive catheter. Surg Endosc. 2020;34(8):3669–3678. doi: 10.1007/s00464-019-07261-4. [DOI] [PubMed] [Google Scholar]
- 18.Siddighi S, Yune JJ, Hardesty J. Indocyanine green for intraoperative localization of ureter. Am J Obstet Gynecol. 2014;211(4):436.e1–436.e2. doi: 10.1016/j.ajog.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 19.Mandovra P, Kalikar V, Patankar RV. Real-time visualization of ureters using indocyanine green during laparoscopic surgeries: Can we make surgery safer. Surg Innov. 2019;26(4):464–468. doi: 10.1177/1553350619827152. [DOI] [PubMed] [Google Scholar]
- 20.Barnes TG, Hompes R, Birks J, Mortensen NJ, Jones O, Lindsey I, Guy R, George B, Cunningham C, Yeung TM. Methylene blue fluorescence of the ureter during colorectal surgery. Surg Endosc. 2018;32(9):4036–4043. doi: 10.1007/s00464-018-6219-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Taher M, van den Bos J, Schols RM, Bouvy ND, Stassen LP. Fluorescence ureteral visualization in human laparoscopic colorectal surgery using methylene blue. J Laparoendosc Adv Surg Tech A. 2016;26(11):870–875. doi: 10.1089/lap.2016.0264. [DOI] [PubMed] [Google Scholar]
- 22.Matsui A, Tanaka E, Choi HS, Kianzad V, Gioux S, Lomnes SJ, Frangioni JV. Real-time, near-infrared, fluorescence-guided identification of the ureters using methylene blue. Surgery. 2010;148(1):78–86. doi: 10.1016/j.surg.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chahin F, Dwivedi AJ, Paramesh A, Chau W, Agrawal S, Chahin C, Kumar A, Tootla A, Tootla F, Silva YJ. The implications of lighted ureteral stenting in laparoscopic colectomy. JSLS. 2002;6(1):49–52. [PMC free article] [PubMed] [Google Scholar]
- 24.Ryu S, Okamoto A, Nakashima K, Hara K, Ishida K, Ito R, Nakabayashi Y. Ureteral navigation using a fluorescent ureteral catheter during laparoscopic colorectal surgery. Surg Endosc Online ahead of print. 2021 doi: 10.1007/s00464-021-08538-3. [DOI] [PubMed] [Google Scholar]
- 25.Kaku S, Shimizu Y, Wakinoue S, Takebayashi A, Takashima A, Tarumoto Y, Nakagawa T, Kita N, Takahashi K, Murakami T. Prevention of ureteral damage by using ureteral illuminating catheter during laparoscopic surgery. Japanese Journal of Gynecologic and Obstetric Endoscopy. 2021;26(2):541–544. doi: 10.5180/jsgoe.26.541. [DOI] [Google Scholar]
- 26.Lee Z, Kaplan J, Giusto L, Eun D. Prevention of iatrogenic ureteral injuries during robotic gynecologic surgery: a review. Am J Obstet Gynecol. 2016;214(5):566–571. doi: 10.1016/j.ajog.2015.10.150. [DOI] [PubMed] [Google Scholar]
- 27.Uno K, Ueno T, Yamada T, Takeda T, Tano S, Suzuki T, Kishigami Y, Oguchi H. Prevention of ureteral injury during laparoscopic surgery with lymph node dissection using infrared illumination system. Japanese Journal of Gynecologic and Obstetric Endoscopy. 2021;35(2):328–332. doi: 10.5180/jsgoe.35.2_328. [DOI] [Google Scholar]
- 28.Song SH, Park SY, Park JS, Kim HJ, Yang CS, Choi GS. Laparoscopic para-aortic lymph node dissection for patients with primary colorectal cancer and clinically suspected para-aortic lymph nodes. Ann Surg Treat Res. 2016;90(1):29–35. doi: 10.4174/astr.2016.90.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liang JT. Surgical techniques of laparoscopic peritonectomy plus paraaortic lymph node dissection for the treatment of patients with positive lymph node metastasis and peritoneal seeding from rectosigmoid cancer. Surg Endosc. 2012;26(8):2383–2387. doi: 10.1007/s00464-012-2163-1. [DOI] [PubMed] [Google Scholar]
- 30.Liang JT, Lai HS, Wu CT, Huang KC, Lee PH, Shun CT. Laparoscopic prophylactic oophorectomy plus N3 lymphadenectomy for advanced rectosigmoid cancer. Ann Surg Oncol. 2007;14(7):1991–1999. doi: 10.1245/s10434-007-9346-3. [DOI] [PubMed] [Google Scholar]
- 31.Kawasaki M, Kameyama M, Imagawa A, Ueki T, Yamazaki K, Sonoo H, Ogawa M, Demura K, Ooba K, Fujio N. Resection of para-aortic lymph node metastases of colorectal cancer. The Japanese Journal of Gastroenterological Surgery. 2021;44(9):1097–1104. doi: 10.5833/jjgs.44.1097. [DOI] [Google Scholar]
- 32.Kim M, Son SY, Cui LH, Shin HJ, Hur H, Han SU. Real-time vessel navigation using indocyanine green fluorescence during robotic or laparoscopic gastrectomy for gastric cancer. J Gastric Cancer. 2017;17(2):145–153. doi: 10.5230/jgc.2017.17.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ryu S, Suwa K, Kitagawa T, Aizawa M, Ushigome T, Okamoto T, Eto K, Yanaga K. Real-time fluorescence vessel navigation using indocyanine green during laparoscopic colorectal cancer surgery. Anticancer Res. 2019;39(6):3009–3013. doi: 10.21873/anticanres.13433. [DOI] [PubMed] [Google Scholar]