Abstract
Protein secretion is an essential process for bacterial growth, yet there are few if any antimicrobial agents which inhibit secretion. An in vivo, high-throughput screen to detect secretion inhibitors was developed based on the translational autoregulation of one of the central protein components, SecA. The assay makes use of a SecA-LacZ fusion reporter construct in Escherichia coli which is induced when secretion is perturbed. Several compounds, including two natural product extracts, which had the ability to induce the reporter fusion were identified and the MICs of these compounds for Staphylococcus aureus strain MN8 were found to be ≤128 μg/ml. Enzyme-linked immunosorbent assay, Western blotting, and immunoprecipitation techniques were used to analyze the affects of these compounds on protein secretion. Six representative compounds presented here appear to be bona fide secretion inhibitors but were found to have deleterious effects on membranes. It was concluded that, while the method described here for identifying inhibitors of secretion is valid, screens such as this, which are directed against the membrane-bound portion of a pathway, may preferentially identify compounds which affect membrane integrity.
Bacterial protein secretion is an attractive target for antimicrobial chemotherapy because the secretion machinery is highly conserved among bacterial species but is distinct from its eukaryotic counterparts (10, 11, 14, 15, 27, 42, 43). In Escherichia coli, approximately 20% of the total cellular protein is secreted across the cytoplasmic membrane (30). The Sec-dependent pathway of secretion is a multicomponent system consisting of at least seven proteins, five of which are essential for cell viability (for a review, see reference 8). Homologs for several of the components have been identified in both gram-negative and gram-positive bacteria, and therefore it is considered likely that an inhibitor of this pathway would have broad-spectrum activity.
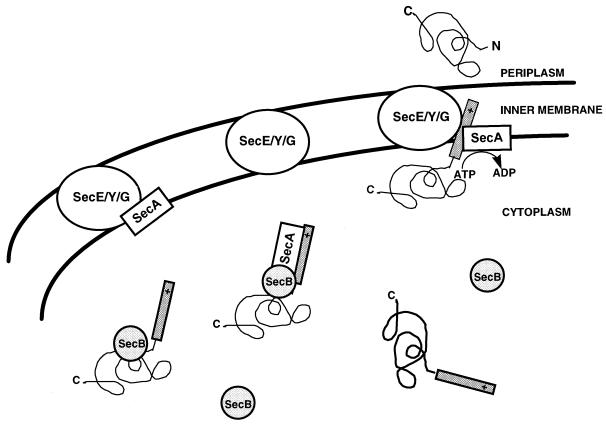
Secreted proteins are produced as precursors containing signal sequences. Those which are secreted via the Sec pathway are accompanied to the membrane by SecB, a chaperone molecule (Fig. 1). SecA, an ATPase required for translocation at the multisubunit translocase SecY-SecE-SecG, is found associated with both the signal sequence of secretable proteins, and also with the membrane at SecE-SecY-SecG. During translocation ATP is hydrolyzed, the protein is inserted across the membrane, the signal sequence is cleaved, and the protein is released.
FIG. 1.
Sec-dependent secretory pathway.
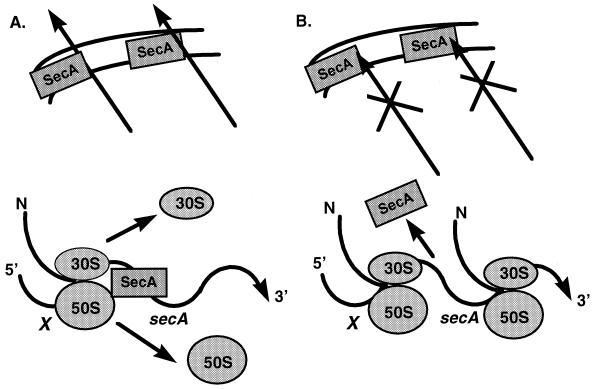
Expression of SecA appears to be a focal point of regulation for this process (20, 24, 33) (Fig. 2). SecA translation is autogenously regulated in response to changes in secretion levels. secA is transcribed as the second gene in an operon after geneX, an open reading frame of unknown function. Under normal secretion conditions, SecA binds to its own mRNA, blocking the ribosomal binding site, thus inhibiting initiation of translation. If secretion is blocked, SecA releases its mRNA and translation levels increase (25).
FIG. 2.
Autogenous regulation of SecA expression. (A) Under conditions of normal secretion, SecA binds its own mRNA and blocks its ribosomal binding site, inhibiting translation. (B) Under conditions in which secretion is blocked, SecA releases its mRNA and translation is increased.
An E. coli cell-based screen was developed in which a SecA-LacZ reporter fusion was used to identify inducers of SecA expression with the presumption that among these inducers would be inhibitors of secretion. Active compounds were tested for antimicrobial activity. Effects on the secretion of the Staphylococcus aureus toxic shock syndrome toxin-1 (TSST-1) were analyzed to further define the effects of these compounds. Immunoprecipitation of pulse-labeled maltose binding protein (MBP) in E. coli was also used to explore the mechanism of action of several of the confirmed inhibitors. Finally, the effect of a subset of the compounds on potassium leakage and precursor utilization in E. coli, and on lysis of red blood cells (RBCs), was determined to identify potential pleiotropic effects on membrane integrity.
MATERIALS AND METHODS
Bacterial strains.
Mc4100 is an E. coli strain (F− araD139 Δ(argF-lac)U169 rpsL 150 (Strr) relA1 flbB5301 deoC 1 ptsF2,5 rbsR) (4).
MM171-2 imp rec is derived from Mc4100 φ(secA-lacZ)f181(Hyb) (λPR9). The secA-lacZ fusion is integrated at the secA locus. λPR9 contains envA, geneX, secA, and the 5′ portion of mutT and provides wild-type secA function. An imp 4214 allele and a rec allele were introduced using P1 transduction (38). MM171-2 was provided by Don Oliver (25, 32).
MN8 is an S. aureus clinical isolate shown to produce TSST-1. MN8 was provided by Patrick Schlievert (36).
Assay for detection of protein secretion inhibitors.
MM171-2 imp rec was grown to an optical density at 650 nm (OD650) of 0.025 (10-mm path length). Ten microliters of test compound at 100 μg/ml was added to 90 μl of culture in 96-well microtiter plates, and the plate was incubated at 37°C with shaking for 60 min. The OD650 was read in a microtiter plate, and 50 μl of ZOB buffer was added. ZOB buffer was prepared by mixing a 4:1 ratio of Z buffer (0.074 M monobasic sodium phosphate, 0.126 M dibasic sodium phosphate, 2 mM magnesium sulfate, 0.4 mM manganese sulfate, hexadecyltrimethylammonium bromide [399 mg/liter], sodium deoxycholate [199.5 mg/liter], and 0.174 M β-mercaptoethanol) with o-nitrophenyl-β-d-galactopyranoside (ONPG) (8 mg/ml) in T-base (15.1 mM ammonium sulfate, 0.08 M dibasic potassium phosphate, 0.044 M monobasic potassium phosphate, and trisodium citrate [1 g/liter]). The OD405 initial was read. Plates were incubated for 1 h at room temperature, and 50 μl of 1 M sodium carbonate–8 M urea was added to stop the reaction. The final OD405 was read and the level of β-galactosidase produced was calculated: (final OD405 − initial OD405/OD650. This number was divided by that obtained with the negative control (i.e., no test compound) to determine the level of SecA induction (a parameter referred to herein as the fold induction value).
MIC determination for strain MN8.
Susceptibility tests were performed by broth microdilution using standard methods (23). Briefly, serial twofold dilutions of compound were made in Mueller-Hinton broth and inoculated with a bacterial suspension to give a final inoculum of 105 CFU/ml. The MIC was defined as the lowest concentration preventing visible growth after 18 h of incubation at 35°C.
Western analysis of TSST-1 secretion.
A stationary culture of S. aureus MN8 was diluted 1:100 in brain heart infusion medium and grown with shaking for 6 h at 37°C in the presence of compounds of interest at concentrations previously determined to approach growth-inhibitory levels. At 6 h culture density was determined at OD650 (10-mm path length) and samples were harvested and quantitatively normalized to the lowest OD. Cell pellets and medium were both retained. Cell pellets were resuspended in 100 μl of 10 mM Tris-HCl–1 mM EDTA (pH 8.0), and lysis was effected by 10 μg of lysostaphin at 37°C for 30 min and then at 65°C for 30 min. Gel loading dye containing sodium dodecyl sulfate (SDS) (37) was added, and samples were boiled prior to gel electrophoresis. Medium was also normalized based on the lowest cell culture OD650, combined with gel loading dye, and boiled. Samples were subjected to SDS–11% polyacrylamide gel electrophoresis (16) and transferred to nitrocellulose using an LKB Multiphor II system as recommended by the manufacturer (Amersham Pharmacia Biotech, Piscataway, N.J.). Western blot analysis was performed using a polyclonal antibody to TSST-1 (Toxin Technologies, Inc., Sarasota, Fla.) at a 1:5,000 dilution and detected by chemiluminescence using the enhanced chemiluminescence Western blotting chemiluminescence detection system (Amersham Pharmacia Biotech).
TSST-1 secretion detection by ELISA.
S. aureus MN8 was diluted and incubated with test compounds, as described above, for 6 h at 37°C. Cell density was measured at OD600, and the culture was centrifuged to harvest the medium. The harvested medium was heated to 95°C for 5 min and then coated onto a protein-binding microtiter plate overnight in 0.2 M sodium carbonate, pH 9.4. The coated plate was assayed by enzyme-linked immunosorbent assay (ELISA) using the anti-TSST-1 antibody and spectrophotometric quantitation of horseradish peroxidase linked to a secondary antibody. The percent of TSST-1 present in the medium was compared with the percent inhibition of growth levels at the different concentrations of inhibitory compounds.
Whole-cell pulse-chase labeling and immunoprecipitation of MBP from Mc4100.
MICs (as estimated by increase in the OD650 of the cell culture in Luria-Bertani medium in the presence or absence of test compound) of each compound were determined for E. coli Mc4100. Cells were grown in maltose-glycerol minimal medium overnight at 37°C, diluted in fresh medium to an OD550 of 0.1, and then grown at 30°C to mid-log phase. The cell cultures were grown in the absence of or in the presence of 0.25 times the MIC of the compound of interest for 15 and 30 min and labeled for 10 s with 60 μCi of [35S]methionine (1,175 Ci/mmol; NEN, Boston, Mass.) (31). A methionine chase was initiated by addition of an equal volume of unlabeled 1% methionine, and samples were taken at 0.25, 0.5, 1, 2, and 5 min. Samples were added to 1/10 volume of 100% trichloroacetic acid (TCA) (wt/vol), precipitated by microcentrifugation, washed in acetone, and solubilized in 1.0% SDS–1 mM EDTA–10 mM Tris, pH 7.5. Samples were boiled, vortexed vigorously, and frozen at −70°C. Thawed samples were then microcentrifuged at 16,000 × g for 5 min, and the supernatants were transferred to a new tube. Twenty microliters of extract was added to 650 μl of 2.0% Triton X-100–0.1 mM EDTA–50 mM Tris (pH 8.0)–0.15 M NaCl. One microliter of anti-MBP antibody (New England Biolabs, Beverly, Mass.) was added and samples were rocked overnight at 4°C. Samples were mixed with 50 μl of Igsorb (The Enzyme Center, Malden, Mass.), washed, resuspended in 2× sample loading buffer, and electrophoresed in an SDS–11% polyacrylamide gel (16). The gel was stained with Coomassie blue, soaked in 1.0% glycerol, dried, and exposed to X-Omat film (Eastman Kodak Company, Rochester, N.Y.) for 3 to 8 days. The following compounds were used at the indicated concentrations cerulenin, 2.5 μg/ml; chloramphenicol, 0.25 μg/ml; polymyxin B, 2.5 μg/ml; compound 3, 16 μg/ml; compound 5, 8 μg/ml; and compound 6, 16 μg/ml. These concentrations were estimated from growth curves to be 0.25 to 0.5 times the MICs, respectively.
Incorporation of radiolabeled precursors.
Macromolecular synthesis by E. coli imp was studied by measuring the incorporation of the appropriate radiolabeled precursors into TCA-precipitable material (40). An E. coli strain carrying an imp mutation was grown at 37°C to an OD600 (10-mm path length) of 0.2 in modified minimal medium. Aliquots of 100 μl were dispensed into microtiter wells containing the compounds of interest (see Table 2), and the plates were incubated for 10 min at 37°C with vigorous agitation. Cells were pulse labeled for 5 min by adding the following radiolabeled precursors at the indicated final concentrations: [3H]thymidine, 2.5 μCi/ml (90 Ci/mM; Amersham Corporation, Arlington Heights, Ill.) with 0.06 μg of unlabeled thymidine/ml; [3H]uridine, 2.5 μCi (49 Ci/mM; Amersham Corporation) with 0.2 μg of unlabeled uridine/ml; or 3H-labeled amino acids, 6.67 μCi/ml (mixture of leucine, lysine, phenylalanine, proline, and tyrosine with specific activities of 135, 83, 123, 103, and 118 Ci/mmol, respectively; Amersham Corporation). One hundred microliters of chilled TCA (10%) supplemented with 0.5 mg of unlabeled precursors per ml was added to each well, and the plate was immediately refrigerated for 1 h. The precipitate was collected on a glass fiber filter (filtermat B; model no. 1205-404; Wallac) using a Skatron Micro-96 cell harvester (model 1118) programmed for a 3-s prewetting with chilled distilled water, followed by a 12-s wash with 5% chilled TCA and a 5-s drying cycle. To assess the effects of the test compounds on cellular uptake of radiolabeled precursors, the addition of TCA to the microtiter plate was eliminated, and the contents of each well were harvested onto a glass fiber filter by the harvester programmed for a 3-s prewetting, a 12-s wash with chilled normal saline (0.9% NaCl in deionized water), and a 5-s drying cycle. Filter mats were dried for 7 min at high power in a microwave oven (700 W; Quasar), solid scintillant (MeltilexB; Pharmacia model no. 1205-402) was applied, and the isotope that was retained on the filter was quantitated in an LKB Betaplate scintillation counter (Wallac model no. 1205). The levels of incorporation of [3H]thymidine, [3H]uridine, and 3H-labeled amino acids were expressed as the percent of that of the untreated control.
TABLE 2.
Incorporation of radiolabeled precursors
| Compound | Concn (μg/ml) | Precursora
|
|||||
|---|---|---|---|---|---|---|---|
| [3H]thymidine
|
[3H]uridine
|
3H-labeled amino acids
|
|||||
| % Uptakeb | % Incorpc | % Uptake | % Incorp | % Uptake | % Incorp | ||
| 3 | 128 | 2 | 6 | 2 | 4 | 11 | 41 |
| 16 | 64 | 60 | 77 | 67 | 50 | 72 | |
| 4 | 128 | 1 | 12 | 3 | 19 | 11 | 42 |
| 16 | 50 | 43 | 52 | 48 | 60 | 45 | |
| 5 | 128 | 2 | 2 | 8 | 1 | 27 | 28 |
| 16 | 1 | 2 | 2 | 1 | 19 | 11 | |
| 6 | 128 | 6 | 8 | 8 | 6 | 16 | 2 |
| 16 | 84 | 52 | 66 | 26 | 48 | 2 | |
| Ciprofloxacin | 0.25 | 60 | 4 | 103 | 90 | 105 | 96 |
| Rifampin | 0.25 | 159 | 113 | 46 | 2 | 85 | 22 |
| Chloramphenicol | 8 | 112 | 85 | 109 | 100 | 50 | 27 |
After 10 min of drug treatment and 5 min of pulse labeling. Results are presented as a percentage of untreated-control results.
Total radiolabeled precursor remaining in the cells after an instant saline wash.
Precursor incorporated into TCA-precipitable material. Incorp, incorporation.
Effect on intracellular K of E. coli imp.
To assess potential membrane disruption by the test compounds, the affects on the intracellular potassium in E. coli imp was studied in a saline buffer (10 mM HEPES [pH 7.0], 150 mM NaCl, and 0.1 mM KCl [pH 7.0]) (1). A log-phase culture was washed twice with saline buffer, and the pellet was resuspended in the same buffer to an OD600 (path length of 10 mm) of 2.00. One milliliter of the bacterial suspension was treated with test compound at various concentrations for 1 h, and cells were pelleted by centrifugation (at 10,000 × g for 2 min). The resulting supernatant was diluted 1:10 in high-performance liquid chromatography-grade water and analyzed for potassium ions by atomic absorption spectrophotometry (Instrumentation Laboratories model 551 spectrophotometer). For the determination of the total potassium level, 1 ml of the culture was hydrolyzed in 2 M sulfuric acid with heat (100°C, for 1 h), chilled for 1 h, and centrifuged (at 10,000 × g for 2 min). The supernatant was then diluted 1:10 and analyzed for potassium content.
Lysis of human RBCs.
One milliliter of freshly pooled human blood was centrifuged (at 10,000 × g for 2 min), and the pellet was washed four times with normal saline by repeated resuspension and centrifugation. The final pellet was resuspended in 1 ml of RBC buffer (10 mM sodium phosphate [pH 7.4], 150 mM NaCl, 1 mM MgCl2) (1). Twenty-five microliters of the RBC suspension was added to the microcentrifuge tube containing 1 ml of drug solution (final concentration ranging from 1 to 128 μg/ml) prepared in duplicate in RBC buffer. After 2 h of treatment, the content of the tube was centrifuged (at 10,000 × g for 2 min) and the OD540 (10-mm pathlength) of the supernatant was measured. To acheive 100% lysis, 25 μl of RBC suspension was added to 1 ml of water.
RESULTS
Design of a SecA–β-galactosidase fusion reporter system and assay protocol.
E. coli strain MM171-2 carries a secA-lacZ fusion at the normal chromosomal secA locus and can be used to detect autoregulation of SecA translation (25, 31). The strain also carries λPR9, which provides wild-type SecA function. When secretion is disrupted, an increase in β-galactosidase activity due to induction of SecA expression can be measured using ONPG as a substrate (21). This system was developed into a high-throughput assay in which compounds and natural product extracts could be screened for protein secretion inhibitory activity (see Materials and Methods).
Synthetic chemical and natural product extract libraries were screened for compounds that derepress SecA translation in MM171-2. Sodium azide, which inhibits the ATPase function of SecA (26), was used as a control. At a concentration of 40 μg/ml, this compound induced β-galactosidase expression three- to fivefold over background levels. Compounds which gave a fold induction value of 1.4-fold or greater (i.e., a ratio of expression in the presence of inhibitor/expression in the absence of inhibitor) were considered potential secretion inhibitors. This value was chosen as a cut-off based on the observed sensitivity and reproducibility of the assay.
Inhibitory activity was confirmed by a dose response assay, and subsequent secondary analysis was carried out as described below.
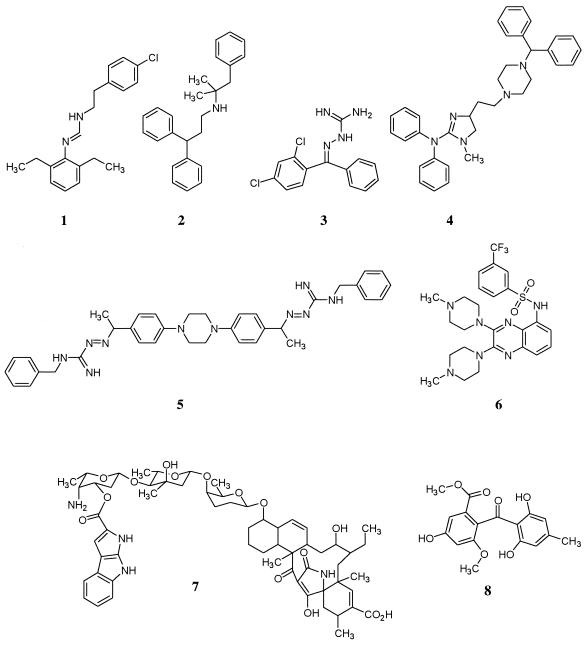
Compounds of interest were identified based on induced expression of the secA-lacZ reporter to a level of 1.4-fold over background. Of the two natural product extracts which caused induction, one contained a previously identified compound, pyrroindomycin (compound 7 [Fig. 3]) which was previously shown to have significant antimicrobial activity in vitro but to lose activity in the presence of serum (39).
FIG. 3.
Structures of potential secretion inhibitors.
A single active compound was purified from a strain of Saprinas radians and was shown to be sulochrin (compound 8 [Fig. 3]), a secondary metabolite previously isolated from several fungal species, Aspergillis wentii, Aspergillus terreus, Penicillium frequentans, and most recently Chrysosporium species (6, 13). This compound has demonstrated relatively weak antifungal and antibacterial activities.
Antibacterial activity was used to prioritize those synthetic compounds which merited further study (Table 1). The MICs of those which were considered of interest for S. aureus strain MN8 were ≤128 μg/ml, and these compounds underwent secondary analyses to study their mechanism of action. Six diverse representative structures are shown in Fig. 3. A structural motif common to structures 1, 3, and 5 is the presence of imino moieties. The presence of lipophilic characteristics appear to be a common structural feature of the entire set. It should be noted that sulochrin bears a gross structural similarity to compounds 2, 3, and 4 in that they all contain geminal diphenyl groups.
TABLE 1.
Identification of potential secretion inhibitors exhibiting antimicrobial activity
| Compound | Induction ratio | MIC (μg/ml) for S. aureus RN 8081 |
|---|---|---|
| 1 | 2.5 | 128 |
| 2 | 1.4 | 64 |
| 3 | 2.3 | 32 |
| 4 | 1.6 | 64 |
| 5 | 3.6 | 2 |
| 6 | 1.8 | 32 |
| 7 | 1.6 | NDa |
| 8 | 1.7 | ND |
ND, not determined.
ELISA and Western analysis demonstrated inhibition of secretion by selected compounds.
Because inhibitors of secretion should decrease the amount of extracellular protein released into the medium in vitro, ELISA and Western analyses were used to quantitate the amount of TSST-1 secreted by strain MN8 in the presence or absence of the compounds of interest. TSST-1 contains a signal sequence, and presumably is secreted via the Sec-dependant pathway (3). To analyze the effect of the potential synthetic secretion inhibitors on TSST-1 secretion, MN8 was grown in the presence of inhibitory concentrations of the compounds for 6 h. Cell extracts and media were examined by Western analysis or by ELISA immunoprecipitation using an antibody to TSST-1 to identify effects on production or localization of the protein. The effects of the compounds can be divided into two categories: those which show a clear inhibition of secretion of TSST-1 and those for which this assay does not give definitive results. For the latter compounds an affect on a metabolic step before secretion, for example, translation, cannot be ruled out. Examples of each category are shown in Fig. 4.
FIG. 4.
ELISA and Western blot analyses of secretion of TSST-1 in S. aureus MN8. ELISA and Western blot analysis were performed to examine the extracellular and intracellular presence of TSST-1 in the presence of various potential secretion inhibitors. Cells were grown in the presence of compounds at the concentrations designated. The mobility of TSST-1 in the Western blot is noted with an arrow, while the mobility of a non-TSST-1 protein nonspecifically recognized by the antibody is noted with an asterisk. In panel D, nonspecific recognition of a marker protein is apparent in the empty middle lane. Percent inhibition is defined as for growth of TSST-1 secretion over 6 h in comparison with an untreated control. Western blots were scanned using a GS-700 Imaging Densitometer (Bio-Rad Laboratories, Hercules, Calif.), and graphics were added with Adobe Photoshop 5.0. (A) cerulenin; (B) compound 3; (C) compound 4; (D) compound 5; (E) compounds 1 (left, Western blot; top, ELISA) and 2 (right, Western blot; bottom, ELISA).
Cerulenin is a fatty acid synthesis inhibitor which reduces protein secretion in bacteria (18, 19, 34) and was used as a control for secondary analyses. Western blot and ELISA analysis showed that the level of TSST-1 secreted into the extracellular medium was greatly reduced in the presence of sub-MIC concentrations of cerulenin (Fig. 4A). In addition, the Western blot suggested that some form of feedback regulation of TSST-1 translation in the presence of this inhibitor exists (see Discussion). General protein synthesis was not inhibited at these concentrations, as demonstrated by the production of a higher-molecular-weight protein present in the cellular extract which fortuitously cross-reacted with the anti-TSST-1 antibody.
Western blot and ELISA data are presented for three compounds, 3, 4, and 5, which clearly caused a significant decrease in the level of TSST-1 secreted into the medium before protein translation was affected (Fig. 4B, C, and D). Additionally, Western blot analysis of TSST-1 secretion in the presence of a derivative of pyrroindomycin also demonstrated a significant decrease in the amount of TSST-1 present in the medium, lending support to the hypothesis that the mechanism of action for this compound acts at the level of secretion (data not shown). For compound 4, it is interesting that the ELISA detected an initial decrease in TSST-1 signal in the medium but an increase at higher concentrations of compound. This may have been due to artifactual presentation of a recognized epitope in some other protein, perhaps protein A, as cellular viability was decreased. It should be noted that, in the cases of compounds 4 and 5, the ELISA still detected intermediate levels of TSST-1 in the medium at concentrations for which the Western blot analysis demonstrated significant inhibition of secretion.
Compounds 1 and 2, which represent the category for which these assays did not provide a definitive conclusion, are presented in Fig. 4E. While the ELISA analyses clearly demonstrated a reduction of TSST-1 present in the media prior to growth inhibition, the Western blot analysis did not indicate a significant inhibition of secretion. Additionally, because in these cases there is no cross-reaction of the antibody with proteins within the cellular extract to use as internal controls for expression of intracellular protein, inhibition of protein translation, or even protein stability, cannot be ruled out. For compound 6, inhibition of secretion and growth occurred simultaneously and could not be separated by this analysis.
Pulse-chase labeling and immunoprecipitation of MBP in E. coli Mc4100 in the presence of potential secretion inhibitors.
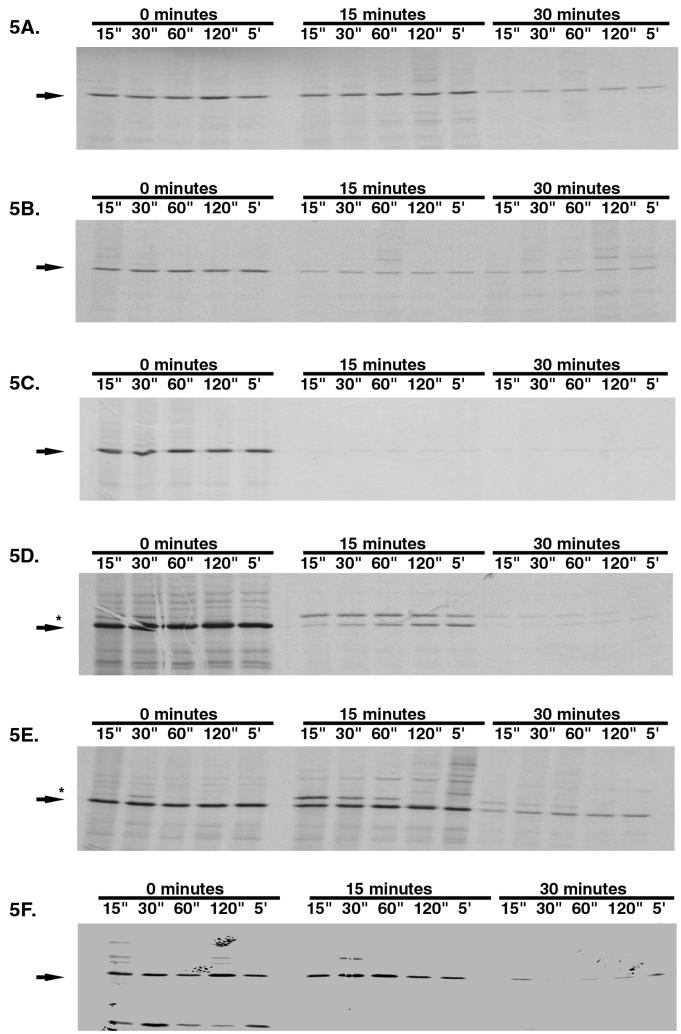
In order to further elucidate the mechanism of action of the putative secretion inhibitors, an examination of the expression and localization of the secreted protein MBP in E. coli was performed. Cells were labeled at 0.25 to 0.5 times the MIC for test compounds, and MBP was detected by immunoprecipitation. Several antimicrobial agents with known mechanisms of action were used as reference compounds. Cerulenin, which inhibits secretion in E. coli as a result of a deleterious effect on fatty acid synthesis (19), causes a decreased expression of MBP at 15 min (Fig. 5A). After 30 min, the level of mature MBP was significantly less than in the absence of drug. There was little if any accumulation of precursor protein, as is often detected in secretion mutants (see discussion). Chloramphenicol, an inhibitor of prokaryotic protein synthesis (17, 28), also caused an inhibition of MBP expression (Fig. 5B). There seemed not to be a specific accumulation of precursor protein but rather an overall decrease in MBP levels by 15 min of exposure. Because secretion occurs at the membrane and membrane-damaging agents could potentially give false-positive results in this secretion inhibitor assay, polymyxin B, which causes bacterial membrane damage (5), was also tested (Fig. 5C). This compound caused a quick and significant decrease in the level of mature MBP detected in the cell.
FIG. 5.
Pulse-chase immunoprecipitation analysis of MBP secretion in E. coli Mc4100. Cells were grown in minimal maltose medium as described in Materials and Methods and were left untreated or were treated at 0.25 times the MIC of each compound (0.5 times the MIC for compound 3) for 15 or 30 min prior to pulse labeling. A chase period with cold methionine was applied for 15 s to 5 min. Mature MBP mobility is designated by an arrow. Nonprocessed MBP is designated by an asterisk. Autoradiograms were scanned using a GS-700 Imaging Densitometer (Bio-Rad Laboratories), and graphics were added using Adobe Photoshop 5.0. (A) Cerulenin; (B) chloramphenicol; (C) polymyxin B; (D) compound 5; (E) compound 6; (F) compound 3.
Pulse-chase immunoprecipitation analysis of three representative secretion inhibitors was performed. For two compounds tested, compounds 5 and 6, a marked increase in precursor protein after 15 min of exposure occurs (Fig. 5D and E). In the presence of compound 6, at 15 min there was no noticeable decrease in mature protein expression. After 30 min, the increased precursor protein level is still apparent and there is a concomitant decrease in overall protein expression. In the case of compound 5, as for compound 6, there was an accumulation of precursor protein after 15 min of exposure, but the effect on total MBP concentration appeared to be more immediate. This analysis demonstrated that at least these two compounds cause an accumulation of nonprocessed precursor protein. Compound 3, on the other hand, has an effect more similar to those of the reference compounds in that no increase in precursor levels occurred before a decreased level of mature protein became apparent (Fig. 5F).
Membrane activity and eukaryotic toxicity of selected putative secretion inhibitors.
Many of the compounds identified in the high-throughput screen for secretion inhibitors were found to cause toxicity in tissue culture: >75% inhibition of growth at 10 μg/ml in more than one cell type, e.g., Vero and human foreskin fibroblast cell lines (data not shown). Therefore, as shown in Table 2, the four compounds believed to inhibit secretion by the previous analyses were assessed for their affect on metabolic precursor uptake and incorporation. Each was found to cause nonspecific decreases in the in vivo labeling of DNA, RNA, and protein. This pattern of inhibition suggests general membrane damage. Moreover, a semisynthetic diacetyl derivative of pyrroindomycin (39), which was positive in the secretion inhibition screen (data not shown), has previously been shown to cause membrane damage.
In order to determine whether the observed secretion inhibition caused in this study might be a potential secondary effect of membrane damage, the membrane integrity of both prokaryotic and eukaryotic cells growing in the presence of the compounds of interest was examined (Table 3). The affect of various compounds on intracellular potassium levels in an E. coli strain carrying an imp mutation was compared with their affect on human RBC membrane integrity (measured in terms of percent unlysed membrane compared to no inhibitor). In the range of concentrations measured, all four compounds exhibited a deleterious effect on eukaryotic and prokaryotic membranes (where tested), suggesting that this screen preferentially identifies compounds which are membrane active.
TABLE 3.
The effects of selected compounds on membrane integrity in an imp mutant E. coli strain and human RBCs
| Compound | Conc of compound | % Intracellular K+a | Concn of compound (μg/ml) | % Human RBC lysis at:
|
|
|---|---|---|---|---|---|
| 2 h | 24 h | ||||
| 3 | 16 μg/ml | 73 | 16 | 1 | 1 |
| 128 | 6 | 98 | |||
| 4 | NDb | 16 | 1 | 1 | |
| 128 | 13 | 76 | |||
| 5 | 4 μg/ml | 18 | 16 | 10 | 34 |
| 128 | 50 | 83 | |||
| 6 | 32 μg/ml | 39 | 16 | 4 | 1 |
| 128 | 50 | 100 | |||
| Polymyxin B | 16 μg/ml | 25 | ND | ND | |
| Water | 1.28% | 100 | ND | ND | |
| Amphotericin B | ND | 1 | 11 | 39 | |
| ND | 8 | 94 | 97 | ||
| DMSOc | ND | 0.5 | 0 | 3 | |
Relative to the level for the untreated control.
Relative to the level for the untreated control.
DMSO, dimethyl sulfoxide.
DISCUSSION
In an era when increased bacterial resistance to existing antibiotics threatens to impede successful chemotherapy, identification of inhibitors of novel antibacterial targets is critical. Such targets should be highly conserved among prokaryotes, not conserved among eukaryotes, essential, and well defined. The secretion pathway meets all of these criteria (22, 41). The method described here takes advantage of a whole-cell system in which several targets of a multicomponent system can be attacked at once. Induced expression of SecA has been documented under both genetic and chemically induced conditions which cause secretion inhibition (9, 24, 25, 35). Therefore, chemical inhibition of any of the Sec proteins besides SecB should give a positive signal using the SecA–β-galactosidase fusion protein.
Western blot and ELISA analyses of TSST-1 secretion in S. aureus were used as the initial secondary assays to identify bona fide secretion inhibitors in this screen. Both of these assays utilize immunological detection of TSST-1. While the ELISA enables quick high-throughput screening, the Western blot technique offers a visual analysis of secreted or retained TSST-1. In both cases, monitoring the growth rate of the cells often allows dissection of the narrow window between inhibition of an essential process, i.e., secretion, and growth inhibition. Interestingly, in at least the case of inhibition by cerulenin, the Western blot suggests a specific feedback inhibition to translation of secreted proteins, as demonstrated by the level of TSST-1 in the cellular compartment. A connection between inhibition of translation and secretion has been documented before in a secA(Am) mutant (24). One shortcoming to these assay techniques is the need for a control for general translation inhibition. In some cases, recognition of a protein other than TSST-1 in the cellular extract by the primary antibody was assumed to be a valid control for protein translation.
A further and more laborious analysis of compounds identified in this screen was pulse-chase labeling and immunoprecipitation. Many examples exist in the literature in which this technique was used to analyze the effects of secretion mutants on the expression and localization of various secreted proteins (7, 24, 25, 29, 32, 37, 44). In particular, accumulation of precursor to the MBP has been demonstrated in mutants of secA, secY, secD, and secF (7, 24, 25, 29, 32, 37, 44). Additionally, sodium azide was previously shown to cause precursor accumulation in E. coli (25). The fact that only a subset of the compounds identified here cause precursor accumulation begs the question as to how their effect differs from those that do not, including cerulenin, which affects secretion as a by-product of its effect on lipid biosynthesis. Previous reports described some mutations in secA and secY that likewise did not cause accumulation (32). It was hypothesized that those mutations might affect the overexpression of SecA without affecting secretory function or, alternatively, that the debilitating effects of the mutations might be overcome by SecA overexpression. Similarly, the compounds described here might act by one of these mechanisms. The possibility exists, however, that the effect on SecA expression caused by these compounds is pleiotropic and that their site of action is unrelated to secretion. It would be interesting to determine if mutants that are resistant to these specific inhibitors can be isolated. If alterations are found in the secretory machinery, it could provide more detailed information regarding the mode of action of these compounds.
All of the compounds presented as secretion inhibitors in this study appear to have nonspecific effects on the integrity of both prokaryotic and eukaryotic membranes. This observation raises some concerns about the utility of targeting membrane-bound proteins without considering effects on membrane integrity. All of the proteins targeted in this assay are at least peripherally membrane associated. It is possible, if not likely, that membrane-damaging agents will negatively affect membrane-associated proteins and be noted as positive in screens for inhibitors of these targets. It is of interest that several of the compounds presented here bear striking structural similarity to some of the hydrophobic tyramine derivatives identified by Barrett et al. as inhibitors of a sensor kinase (2). Although those compounds were identified in vitro, they are also targeted against a membrane protein. There is evidence to suggest that they too have multiple deleterious effects on cellular integrity (12).
The data presented here demonstrate the power of whole-cell screens utilizing reporter fusion proteins to identify inhibitors of different members of a complex pathway. Use of an induced enzyme as a positive signal enables in many cases the dissection of the tight linkage between an inhibitory process and death. While the compounds identified in this screen had pleotropic effects on cell viability, evidence presented here suggests that they are bona fide secretion inhibitors, and thus the secretion pathway is indeed a valid target for antibacterial chemotherapy.
ACKNOWLEDGMENTS
The participation of Amy Ashline, James LaRocque, John Morin, and Pedro Sobers in this study is gratefully acknowledged. We thank Beth Rasmussen, Elizabeth Glasfeld, and Chris Murphy for critical reading of the manuscript.
REFERENCES
- 1.Abbanat D A, Singh M P, Greenstein M. Hongoquercins, new antibacterial agents from the fungus LL-23G227: fermentation and biological activity. J Antibiot. 1998;51:708–714. doi: 10.7164/antibiotics.51.708. [DOI] [PubMed] [Google Scholar]
- 2.Barrett J F, Goldschmidt R M, Lawrence L E, Foleno B, Chen R, Demers J P, Johnson S, Kanojia R, Fernandez J, Bernstein J, Licata L, Donetz A, Huang S, Hlasta D J, Macielag M J, Ohemeng K, Frechette R, Frosco M B, Klaubert D H, Whiteley J M, Wang L, Hoch J A. Antibacterial agents that inhibit two-component signal transduction systems. Proc Natl Acad Sci USA. 1998;95:5317–5322. doi: 10.1073/pnas.95.9.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blomster-Hautamaa D A, Kreiswirth B N, Kornblum J S, Novick R P, Schlievert P M. The nucleotide and partial amino acid sequence of toxic shock syndrome toxin-1. J Biol Chem. 1986;261:15783–15786. [PubMed] [Google Scholar]
- 4.Casadaban M J, Cohen S N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980;138:179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- 5.Chopra I, Hacker K. Uptake of minocycline by Escherichia coli. J Antimicrob Chemother. 1992;29:19–25. doi: 10.1093/jac/29.1.19. [DOI] [PubMed] [Google Scholar]
- 6.Curtis R F, Harries P C, Hassall C H, Levi J D. The biosynthesis of phenols. 5. The relationships of some phenolic metabolites of mutants of Aspergillus terreus Thom, I.M.I. 16043. Biochem J. 1964;90:43–51. doi: 10.1042/bj0900043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danese P N, Murphy C K, Silhavy T J. Multicopy suppression of cold-sensitive sec mutations in Escherichia coli. J Bacteriol. 1995;177:4969–4973. doi: 10.1128/jb.177.17.4969-4973.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Den Blaauwen T, Driessen A J M. Sec-dependent preprotein translocation in bacteria. Arch Microbiol. 1996;165:1–8. doi: 10.1007/s002030050289. [DOI] [PubMed] [Google Scholar]
- 9.Gardel C, Benson S, Hunt J, Michaelis S, Beckwith J. SecD, a new gene involved in protein export in Escherichia coli. J Bacteriol. 1987;169:1286–1290. doi: 10.1128/jb.169.3.1286-1290.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gorlich D, Rapoport T A. Protein translocation into proteoliposomes reconstituted from purified components of the endoplasmic reticulum membrane. Cell. 1993;75:615–630. doi: 10.1016/0092-8674(93)90483-7. [DOI] [PubMed] [Google Scholar]
- 11.Hartmann E, Sommer T, Prehn S, Gorlich D, Jentsch S, Rapoport T A. Evolutionary conservation of components of the protein translocation complex. Nature (London) 1994;367:654–657. doi: 10.1038/367654a0. [DOI] [PubMed] [Google Scholar]
- 12.Hilliard J J, Goldschmidt R, Licata L, Baum E Z, Bush K. Multiple mechanisms of action for inhibitors of histidine protein kinases from bacterial two-component systems. Antimicrob Agents Chemother. 1999;43:1693–1699. doi: 10.1128/aac.43.7.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inamori Y, Kato Y, Kubo M, Kamiki T, Takemato T, Namoto K. Studies on metabolites produced by Aspergillus terreus var. aureus. I. Chemical structures and antimicrobial activities of metabolites isolated from culture broth. Chem Pharm Bull. 1983;31:4543–4548. doi: 10.1248/cpb.31.4543. [DOI] [PubMed] [Google Scholar]
- 14.Klein M, Meens J, Freudl R. Functional characterization of the Staphylococcus carnosus SecA protein in Escherichia coli and Bacillus subtilis secA mutant strains. FEMS Microbiol Lett. 1995;131:271–277. doi: 10.1016/0378-1097(95)00267-9. [DOI] [PubMed] [Google Scholar]
- 15.Koivula T, Palva I, Hemila H. Nucleotide sequence of the secY gene from Lactococcus lactis and identification of conserved regions by comparison of four SecY proteins. FEBS Lett. 1991;288:114–118. doi: 10.1016/0014-5793(91)81015-z. [DOI] [PubMed] [Google Scholar]
- 16.Laemmli U K. Cleavage of structural proteins during the assembly of the head of the bacteriophage T4. Nature (London) 1970;277:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 17.Lessard J L, Pestka S. Studies on the formation of transfer ribonucleic acid-ribosome complexes. XXIII. Chloramphenicol, aminoacyl-oligonucleotides, and Escherichia coli ribosomes. J Biol Chem. 1972;247:6909–6912. [PubMed] [Google Scholar]
- 18.Mantsala P. Inhibition of protein secretion by cerulenin in Bacillus subtilis. J Gen Microbiol. 1982;128:2967–2972. doi: 10.1099/00221287-128-12-2967. [DOI] [PubMed] [Google Scholar]
- 19.Mantsala P, Lehtinen J. Secretion of β-lactamase by Escherichia coli in vivo and in vitro: effect of cerulenin. Antonie Leeuwenhoek. 1982;48:353–364. doi: 10.1007/BF00418288. [DOI] [PubMed] [Google Scholar]
- 20.McNicholas P, Salavati R, Oliver D. Dual regulation of Escherichia coli secA translation by distinct upstream elements. J Mol Biol. 1997;265:128–141. doi: 10.1006/jmbi.1996.0723. [DOI] [PubMed] [Google Scholar]
- 21.Miller J H. A short course in bacterial genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. [Google Scholar]
- 22.Misra R, Silhavy T. Protein secretion in bacteria: a chemotherapeutic target? In: Sutcliffe J, Georgopapadakou N, editors. Emerging targets in antibacterial and antifungal therapy. New York, N.Y: Chapman and Hall; 1992. pp. 163–175. [Google Scholar]
- 23.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A2. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1977. [Google Scholar]
- 24.Oliver D B, Beckwith J. E. coli mutant pleiotropically defective in the export of secreted proteins. Cell. 1981;25:765–772. doi: 10.1016/0092-8674(81)90184-7. [DOI] [PubMed] [Google Scholar]
- 25.Oliver D B, Beckwith J. Regulation of a membrane component required for protein secretion in Escherichia coli. Cell. 1982;30:311–319. doi: 10.1016/0092-8674(82)90037-x. [DOI] [PubMed] [Google Scholar]
- 26.Oliver D B, Cabelli R J, Dolan K M, Jarosik G P. Azide-resistant mutants of Escherichia coli alter the SecA protein, an azide-sensitive component of the protein export machinery. Proc Natl Acad Sci USA. 1990;87:8227–8231. doi: 10.1073/pnas.87.21.8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Overhoff B, Klein M, Spies M, Freudl R. Identification of a gene fragment which codes for the 364 amino-terminal amino acid residues of a SecA homologue from Bacillus subtilis: further evidence for the conservation of the protein export apparatus in gram-positive and gram-negative bacteria. Mol Gen Genet. 1991;228:417–423. doi: 10.1007/BF00260635. [DOI] [PubMed] [Google Scholar]
- 28.Pestka S. Chloramphenicol. In: Corcoran J W, Hahn F E, editors. Antibiotics. Vol. 3. New York, N.Y: Springer-Verlag; 1975. p. 396. [Google Scholar]
- 29.Pogliano K J, Beckwith J. The Cs sec mutants of Escherichia coli reflect the cold sensitivity of protein export itself. Genetics. 1993;133:763–773. doi: 10.1093/genetics/133.4.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pugsley A P. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev. 1993;57:50–108. doi: 10.1128/mr.57.1.50-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rasmussen B A, MacGregor C H, Ray P H, Bassford P J., Jr In vivo and in vitro synthesis of Escherichia coli maltose-binding protein under regulatory control of the lacUV5 promoter-operator. J Bacteriol. 1985;164:665–673. doi: 10.1128/jb.164.2.665-673.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riggs P D, Derman A I, Beckwith J. A mutation affecting the regulation of a secA-lacZ fusion defines a new sec gene. Genetics. 1988;118:571–579. doi: 10.1093/genetics/118.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salavati R, Oliver D. Identification of elements on GeneX-secA RNA of Escherichia coli required for SecA binding and secA auto-regulation. J Mol Biol. 1997;265:142–152. doi: 10.1006/jmbi.1996.0724. [DOI] [PubMed] [Google Scholar]
- 34.Saleh F A K, Freer J H. Inhibition of secretion of staphylococcal alpha toxin by cerulenin. J Med Microbiol. 1984;18:205–216. doi: 10.1099/00222615-18-2-205. [DOI] [PubMed] [Google Scholar]
- 35.Schatz P J, Riggs P D, Jacq A, Fath M J, Beckwith J. The secE gene encodes an integral membrane protein required for protein export in Escherichia coli. Genes Dev. 1989;3:1035–1044. doi: 10.1101/gad.3.7.1035. [DOI] [PubMed] [Google Scholar]
- 36.Schlievert P M, Blomster D A. Production of staphylococcal pyrogenic exotoxin type C: influence of physical and chemical factors. J Infect Dis. 1983;147:236–242. doi: 10.1093/infdis/147.2.236. [DOI] [PubMed] [Google Scholar]
- 37.Shimoike T, Akiyama Y, Baba T, Taura T, Ito K. SecY variants that interfere with Escherichia coli protein export in the presence of normal secY. Mol Microbiol. 1992;6:1205–1210. doi: 10.1111/j.1365-2958.1992.tb01559.x. [DOI] [PubMed] [Google Scholar]
- 38.Silhavy T J, Berman M L, Enquist L W. Experiments with gene fusions. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1984. [Google Scholar]
- 39.Singh M P, Petersen P J, Jacobus N V, Mroczenski-Wildey M J, Maiese W M, Greenstein M, Steinberg D A. Pyrroindomycins, novel antibiotics produced by Streptomyces rugosporus LL-42D005. J Antibiot. 1994;47:1258–1265. doi: 10.7164/antibiotics.47.1258. [DOI] [PubMed] [Google Scholar]
- 40.Singh M P, Petersen P J, Jacobus N V, Maiese W M, Greenstein M, Steinberg D A. Mechanistic studies and biological activity of bioxalomycin alpha 2, a novel antibiotic produced by Streptomyces viridodiastaticus subsp. “litoralis” LL-31F508. Antimicrob Agents Chemother. 1994;38:1808–1812. doi: 10.1128/aac.38.8.1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stephens C, Shapiro L. Bacterial protein secretion—a target for new antibiotics? Chem Biol. 1997;4:637–641. doi: 10.1016/s1074-5521(97)90217-9. [DOI] [PubMed] [Google Scholar]
- 42.Suh J W, Boylan S A, Thomas S M, Dolan K M, Oliver D B, Price C W. Isolation of a secY homologue from Bacillus subtilis: evidence for a common protein export pathway in eubacteria. Mol Microbiol. 1990;4:305–314. doi: 10.1111/j.1365-2958.1990.tb00597.x. [DOI] [PubMed] [Google Scholar]
- 43.Takamatsu H, Fuma S, Nakamura K, Sadaie Y, Shinkai A, Matsuyama S, Mizyshima S, Yamane K. In vivo and in vitro characterization of the secA gene product of Bacillus subtilis. J Bacteriol. 1992;174:4308–4316. doi: 10.1128/jb.174.13.4308-4316.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Traxler B, Murphy C. Insertion of the polytopic membrane protein MalF is dependent on the bacterial secretion machinery. J Biol Chem. 1996;27:12394–12400. doi: 10.1074/jbc.271.21.12394. [DOI] [PubMed] [Google Scholar]