Abstract
Background
Populations such as healthcare workers (HCWs), injection drug users (IDUs), and people engaging in unprotected sex are all at risk of being infected with the human immunodeficiency virus (HIV). Animal models show that after initial exposure, HIV replicates within dendritic cells of the skin and mucosa before spreading through lymphatic vessels and developing into a systemic infection (CDC 2001). This delay in systemic spread leaves a "window of opportunity" for post‐exposure prophylaxis (PEP) using antiretroviral drugs designed to block replication of HIV (CDC 2001). PEP aims to inhibit the replication of the initial inoculum of virus and thereby prevent establishment of chronic HIV infection.
Objectives
To evaluate the effects of antiretroviral PEP post‐occupational exposure to HIV.
Search methods
The Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, AIDSearch, and the Database of Abstracts of Reviews of Effectiveness were searched from 1985 to January 2005 to identify controlled trials. There were no language restrictions. Because no controlled clinical trials were retrieved, the search was repeated on 31 May 2005 in MEDLINE, AIDSearch and EMBASE using a search strategy to identify analytic observational studies. Handsearches of the reference lists of all pertinent reviews and studies found were also undertaken. Experts in the field of HIV prevention were contacted.
Selection criteria
Types of studies: All controlled trials (including randomized clinical trials and controlled clinical trials). If no controlled trials were found, analytic studies (e.g. cohort and case‐control studies) were considered. Descriptive studies (i.e. studies with no comparison groups) were excluded.
Types of participants included: HCWs exposed to any known or potentially HIV contaminated product; anyone exposed to a needlestick contaminated by known or potentially HIV‐infected blood or other bodily fluid in an occupational setting; and anyone exposed through the mucous membranes to an HIV‐infected or potentially infected substance in occupational setting.
Excluded: Sex workers (PEP post‐sexual exposure is addressed in another Cochrane review (Martín 2005)).
Types of interventions: Any intervention that administered single or combinations of antiretrovirals as PEP to people exposed to HIV through percutaneous injuries and/or occupational mucous membrane exposures when the HIV status of the source patient was positive or unknown. Studies comparing two types of PEP regimens were considered, as were studies comparing PEP with no intervention.
Types of outcome measures: Incidence of HIV infection in those given PEP versus those given placebo or a different PEP regimen; Adherence to PEP; Complications of PEP
Types of outcome measures: Incidence of HIV infection in those given PEP versus those given placebo or a different PEP regimen; Adherence to PEP; Complications of PEP
Data collection and analysis
Data concerning outcomes, details of the interventions, and other study characteristics were extracted by two independent authors (TY and JA) using a standardized data extraction form (Table 04). A third author (GK) resolved disagreements. The following information was gathered from each included study: location of study, date, publication status, demographics (e.g. age, gender, occupation, risk behavior, etc.) of participants/exposure modality, form of PEP used, duration of use, and outcomes.
Odds ratios with a 95% confidence interval (CI) were used as the measure of effect. A meta‐analysis was performed for adverse events where two‐drug regimens were compared with three‐drug regimens. Due to overlap between Puro 2000 and Puro 2005, the former was not included in the combined analysis.
Main results
Effect of PEP on HIV seroconversion No randomized controlled trials were identified. Only one case‐control study was included. HIV transmission was significantly associated with deep injury (OR 15, 95% CI 6.0 to 41), visible blood on the device (OR 6.2, 95% CI 2.2 to 21), procedures involving a needle placed in the source patient's blood vessel (OR 4.3, 95% CI 1.7 to 12), and terminal illness in the source patient (OR 5.6, 95% CI 2.0 to 16). After controlling for these risk factors, no differences were detected in the rates at which cases and controls were offered post‐exposure prophylaxis with zidovudine. However, cases had significantly lower odds of having taken zidovudine after exposure compared to controls (OR 0.19, 95%CI 0.06 to 0.52). No studies were found that evaluated the effect of two or more antiretroviral drugs for occupational PEP.
Adherence to and complications with PEP Eight reports from observational comparative studies confirmed findings that adverse events were higher with a three‐drug regimen, especially one containing indinavir. However, discontinuation rates were not significantly different.
Authors' conclusions
The use of occupational PEP is based on limited direct evidence of effect. However, it is highly unlikely that a definitive placebo‐controlled trial will ever be conducted, and, therefore, on the basis of results from a single case‐control study, a four‐week regimen of PEP should be initiated as soon as possible after exposure, depending on the risk of seroconversion. There is no direct evidence to support the use of multi‐drug antiretroviral regimens following occupational exposure to HIV. However, due to the success of combination therapies in treating HIV‐infected individuals, a combination of antiretroviral drugs should be used for PEP. Healthcare workers should be counseled about expected adverse events and the strategies for managing these. They should also be advised that PEP is not 100% effective in preventing HIV seroconversion. A randomized controlled clinical trial is neither ethical nor practical. Due to the low risk of HIV seroconversion, a very large sample size would be required to have enough power to show an effect. More rigorous evaluation of adverse events, especially in the developing world, are required. Seeing that current practice is partly based on results from individual primary animal studies, we recommend a formal systematic review of all relevant animal studies.
Keywords: Humans; Health Personnel; Infectious Disease Transmission, Patient‐to‐Professional; HIV; HIV Infections; HIV Infections/prevention & control; HIV Infections/transmission; Occupational Diseases; Occupational Diseases/prevention & control; Occupational Exposure
Plain language summary
Antiretroviral post‐exposure prophylaxis (PEP) for occupational HIV exposure
This review evaluated the effects of antiretroviral post‐exposure prophylaxis (PEP) for preventing HIV infection following occupational exposure. No randomized controlled trials were identified. Only one case‐control study provides evidence for using zidovudine monotherapy. The study found that, in the occupational setting, HIV transmission was significantly associated with deep injury, visible blood on the sharp instrument, procedures involving a needle placed in the source patient's blood vessel, and terminal illness in the source patient. After taking these into account, it was found that those who became infected with HIV had significantly lower odds of having taken zidovudine after exposure, compared to those who did not seroconvert. There is no direct evidence to support the use of multi‐drug antiretroviral regimens following occupational exposure to HIV. However, due to the success of combination therapies in treating HIV‐infected individuals, a combination of drugs should be used for PEP. Eight reports from other studies confirmed the findings that adverse events were higher with a three‐drug regimen; however, discontinuation rates were not significantly different. A four‐week regimen of post‐exposure prophylaxis should be initiated as soon as possible after exposure, depending on the risk of seroconversion. Healthcare workers should be counseled about expected adverse events and given strategies for managing these events. They should also be advised that PEP is not 100% effective in preventing HIV seroconversion.
Background
Populations such as healthcare workers (HCWs), injection drug users (IDUs), and people engaging in unprotected sex are all at risk of being infected with the human immunodeficiency virus (HIV). Animal models show that after initial exposure, HIV replicates within dendritic cells of the skin and mucosa before spreading through lymphatic vessels and developing into a systemic infection (CDC 2001). This delay in systemic spread leaves a "window of opportunity" for post‐exposure prophylaxis (PEP) using antiretroviral drugs designed to block replication of HIV (CDC 2001). PEP aims to inhibit the replication of the initial inoculum of virus and thereby prevent establishment of chronic HIV infection.
PEP Guidelines in use PEP is recommended for occupational exposure to HIV in many countries (UNAIDS 1998). These countries have set out algorithms regarding who should receive PEP and which drugs should be administered. These vary depending on the level of risk to which the HCW has been exposed and any known or potential antiretroviral resistance in the index patient.
Drug regimens Multiple‐drug (two or more) PEP regimens are recommended, depending on the level of risk for HIV transmission (CDC 2005). The choice of regimen should factor in possible drug interactions, allergies, and pregnancy. Recommended duration of PEP is four weeks (CDC 2005). Two drugs are recommended for most HIV exposures (CDC 2005; Table 1). An expanded regimen that includes the addition of a third drug (Table 1) is advised for exposures that pose an increased risk of HIV transmission (exposure to large volumes of blood, deep injuries, blood containing particularly high levels of HIV, and detectable viral load in a source patient currently on treatment (CDC 2001)).
1. CDC recommendations for Antiretroviral Postexposure Prophylaxis Regimens.
| Basic two drug | Expanded: Basic plus | ||
| Preferred | Preferred | ||
| Zidovudine (ZDV) + Lamivudine (3TC) available as Combivir | ZDV 300 mg twice daily or 200mg three times daily + 3TC 300 mg once daily or 150 mg twice daily (Combivir one tablet twice daily) | Lopinavir/ritonavir (Kaletra) | 400/100 mg twice daily |
| Zidovudine (ZDV) + Emtrictabine (FTC) | ZDV as above + FTC 200 mg once daily | Alternate | |
| Tenofovir (TDF) + Lamivudine (3TC) | TDF 300 mg once daily + 3TC as above | Atazanavir (ATV) +‐ Ritonavir (RTV) | ATV 400mg once daily unless used in combination with TDF in which cae ATV should be boosted with RTV 100mg once daily |
| Tenofovir (TDF) + Emtricitabine (FTC) | TDF as above + FTC as above | Fosamprenavir (FOSAPV) +‐ Ritonavir (RTV) | FOSAPV 1400mg twice daily (without RTV) or FOSAPV 1400mg once daily + RTV 200mg once daily or FOSAPV 700mg twice daily + RTV 100mg twice daily |
| Alternate | Indinavir (IDV) +‐ Ritonavir (RTV) | IDV 800mg + RTV 100mg twice daily or IDV 800mg every 8 hours on an empty stomach | |
| Lamivudine (3TC) + Stavudine (d4T) | 3TC as above + d4T 40 mg twice (30 mg twice daily if weight < 60 kg) | Saquinavir (SQV) + Ritonavir (RTV) | SQV 1000mg + RTV 100mg twice daily |
| Emtricitabine (FTC) + Stavudine (d4T) | FTC as above + d4T as above | Nelfinavir (NFV) | NFV 1250mg twice daily |
| Lamivudine (3TC) + Didanosine (ddI) | 3TC as above + ddI 200 mg twice daily or 400 mg once daily | Efavirenz (EFV) | EFV 600mg daily |
| Emtricitabine (FTC) + Didanosine (ddI) | FTC as above + ddI as above | ||
There is no direct evidence for improved effectiveness using combination therapies for prophylaxis (CDC 2001). However, combination therapies are more efficacious in HIV‐infected patients and in preventing perinatal transmission than mono therapies, so it is theorized that a combination of drugs would enhance the effectiveness of PEP (CDC 2001; Parkin 2000). The addition of nucleoside reverse transcriptase inhibitors (NRTI) may help to reduce emergence of antiretroviral resistance and may also add to efficacy when treating zidovudine‐resistant strains.
Due to toxicity, nevirapine, delavirdine, abacavir and zalcitabine are generally not recommended for use as PEP (CDC 2005). Despite this, nevirapine is used in around 19% of PEP prescriptions in New South Wales (Kaldor 2000 personal communication).
Timing of drugs given In animal models PEP has been shown to work best when given within hours of initial exposure. These models also demonstrate that PEP confers no benefit when started greater than 24‐36 hours post‐exposure (Bottinger 1997; Tsai 1998). However, this time limit has not been proven in humans, so it is recommended to administer PEP even if exposure occurred up to 72 hours previously (CDC 2005).
When to use PEP The estimated risk for HIV infection depends on the site of exposure as well as the nature of the exposure. The risk of HIV infection through a percutaneous needlestick from a known HIV‐infected source is approximately 0.3% (CDC 2005). Several factors have been shown to influence the risk of seroconversion after percutaneous exposure, including, (1) an increased volume of blood contaminant due to a deep injury or procedures involving needle placed directly in vein or artery; (2) an increased HIV viral load in the source patient; and (3) failure to administer a form of PEP after exposure (Cardo 1997).
In several countries, PEP is offered after all occupational percutaneous exposure injuries and mucosal exposures to blood when the HIV status of the source is positive or unknown. The occupational percutaneous injuries for which PEP is indicated are mainly needlesticks but can include any injury with a sharp object that breaks the skin (e.g. a scalpel). The role of PEP in other occupational injuries, such as bites and mucosal exposure to sputum, remains unclear, as it does for needlesticks during injection drug use (CDC 1998b; Miller 1996; Torbati 1999). Guidelines, after taking into consideration the infection status of the source, include assessment of exposures and probability of transmission risk. CDC recommendations divide exposure type into less and more severe for percutaneous injuries or small and large volume for mucous membrane and non‐intact skin exposures (CDC 2005).
Although all of the published guidelines addressing the use of PEP are based on occupational exposures, a far greater amount of HIV exposure occurs sexually. A wide range of scenarios exist for the use of PEP after sexual contact, such as in the case of sexual assaults and for any unprotected sexual encounter with a known HIV‐infected individual or an individual at high risk for transmitting HIV. However, the use of PEP for these types of exposure remains controversial (Low‐Beer 2000; Pinkerton 1998; Pinkerton 2000; Wiebe 2000). The US Public Health Service states that for all non‐occupational exposures the "PHS is unable to recommend for or against this therapeutic approach" (CDC 1998b). PEP for non‐occupational exposures is addressed in another Cochrane review (Martín 2005).
Limitations with PEP There are several problems associated with PEP. The drugs involved give unwanted side effects, such as gastrointestinal upset (nausea and diarrhoea) with NRTIs and protease inhibitors (PIs) (Parkin 2000). PIs also have more serious side effects, such as dangerous drug interactions, diabetic exacerbation, and nephrolithiasis (CDC 2001). These problems increase non‐adherence, which can be harmful to the patient and can lead to the development of drug‐resistant strains if the patient has been infected (Parkin 2000). Failure of PEP to prevent HIV infection may be due to drug‐resistant HIV strains. Other factors that might contribute to PEP failure include delayed initiation, large inoculum, short duration of PEP, and some host factors (CDC 2001).
Objectives
To evaluate the effects of antiretroviral PEP post‐occupational exposure to HIV.
Methods
Criteria for considering studies for this review
Types of studies
All controlled trials (including randomized clinical trials and controlled clinical trials). If no controlled trials were found, analytic studies (e.g. cohort and case‐control studies) were considered. Descriptive studies (i.e. studies with no comparison groups) were excluded.
Types of participants
Included: HCWs exposed to any known or potentially HIV contaminated product; anyone exposed to a needlestick contaminated by known or potentially HIV‐infected blood or other bodily fluid in an occupational setting; and anyone exposed through the mucous membranes to an HIV‐infected or potentially infected substance in occupational setting.
Excluded: Sex workers (PEP post‐sexual exposure is addressed in another Cochrane review (Martín 2005)).
Types of interventions
Any intervention that administered single or combinations of antiretrovirals as PEP to people exposed to HIV through percutaneous injuries and/or occupational mucous membrane exposures when the HIV status of the source patient was positive or unknown.
Studies comparing two types of PEP regimens were considered, as were studies comparing PEP with no intervention.
Types of outcome measures Incidence of HIV infection in those given PEP versus those given placebo or a different PEP regimen Adherence to PEP Complications of PEP
Types of outcome measures
Incidence of HIV infection in those given PEP versus those given placebo or a different PEP regimen Adherence to PEP Complications of PEP
Search methods for identification of studies
The Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, AIDSearch, and the Database of Abstracts of Reviews of Effectiveness were searched from 1985 to January 2005 to identify controlled trials. There were no language restrictions. Because no controlled clinical trials were retrieved, the search was repeated on 31 May 2005 in MEDLINE, AIDSearch and EMBASE using a search strategy to identify analytic observational studies. Handsearches of the reference lists of all pertinent reviews and studies found were also undertaken. Experts in the field of HIV prevention were contacted. See Table 2 and Table 3 for our MEDLINE search strategies.
2. MEDLINE search strategy for controlled trials.
| #1 | Search HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tw] OR hiv‐1*[tw] OR hiv‐2*[tw] OR hiv1[tw] OR hiv2[tw] OR hiv infect*[tw] OR human immunodeficiency virus[tw] OR human immunedeficiency virus[tw] OR human immuno‐deficiency virus[tw] OR human immune‐deficiency virus[tw] OR ((human immun*) AND (deficiency virus[tw])) OR acquired immunodeficiency syndrome[tw] OR acquired immunedeficiency syndrome[tw] OR acquired immuno‐deficiency syndrome[tw] OR acquired immune‐deficiency syndrome[tw] OR ((acquired immun*) AND (deficiency syndrome[tw])) |
| #2 | Search Antiretroviral Therapy, Highly Active[MeSH] OR Anti‐Retroviral Agents[MeSH] OR Antiviral Agents[MeSH:NoExp] OR ((anti) AND (hiv[tw])) OR antiretroviral*[tw] OR ((anti) AND (retroviral*[tw])) OR HAART[tw] OR ((anti) AND (acquired immunodeficiency[tw])) OR ((anti) AND (acquired immunedeficiency[tw])) OR ((anti) AND (acquired immuno‐deficiency[tw])) OR ((anti) AND (acquired immune‐deficiency[tw])) OR ((anti) AND (acquired immun*) AND (deficiency[tw])) |
| #3 | Search randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized controlled trials [mh] OR random allocation [mh] OR double‐blind method [mh] OR single‐blind method [mh] OR clinical trial [pt] OR clinical trials [mh] OR ("clinical trial" [tw]) OR ((singl* [tw] OR doubl* [tw] OR trebl* [tw] OR tripl* [tw]) AND (mask* [tw] OR blind* [tw])) OR ( placebos [mh] OR placebo* [tw] OR random* [tw] OR research design [mh:noexp] OR comparative study [mh] OR evaluation studies [mh] OR follow‐up studies [mh] OR prospective studies [mh] OR control* [tw] OR prospectiv* [tw] OR volunteer* [tw]) NOT (animals [mh] NOT human [mh]) |
| #4 | Search POST‐EXPOSURE PROPHYLAXIS OR POSTEXPOSURE PROPHYLAXIS OR POST EXPOSURE PROPHYLAXIS |
| #5 | Search POST‐EXPOSUREPROPHYLAXIS OR POSTEXPOSUREPROPHYLAXIS OR POST EXPOSUREPROPHYLAXIS |
| #6 | Search #4 OR #5 |
| #7 | Search #1 AND #2 AND #3 AND #6 |
| #8 | Search #7 NOT #8 Field: All Fields, Limits: Publication Date from 1985 to 2005 |
3. MEDLINE search strategy for analytic observational studies.
| #1 | Search HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tw] OR hiv‐1*[tw] OR hiv‐2*[tw] OR hiv1[tw] OR hiv2[tw] OR hiv infect*[tw] OR human immunodeficiency virus[tw] OR human immunedeficiency virus[tw] OR human immuno‐deficiency virus[tw] OR human immune‐deficiency virus[tw] OR ((human immun*) AND (deficiency virus[tw])) OR acquired immunodeficiency syndrome[tw] OR acquired immunedeficiency syndrome[tw] OR acquired immuno‐deficiency syndrome[tw] OR acquired immune‐deficiency syndrome[tw] OR ((acquired immun*) AND (deficiency syndrome[tw])) |
| #2 | Search Antiretroviral Therapy, Highly Active[MeSH] OR Anti‐Retroviral Agents[MeSH] OR Antiviral Agents[MeSH:NoExp] OR ((anti) AND (hiv[tw])) OR antiretroviral*[tw] OR ((anti) AND (retroviral*[tw])) OR HAART[tw] OR ((anti) AND (acquired immunodeficiency[tw])) OR ((anti) AND (acquired immunedeficiency[tw])) OR ((anti) AND (acquired immuno‐deficiency[tw])) OR ((anti) AND (acquired immune‐deficiency[tw])) OR ((anti) AND (acquired immun*) AND (deficiency[tw])) |
| #3 | Search POST‐EXPOSURE PROPHYLAXIS OR POSTEXPOSURE PROPHYLAXIS OR POST EXPOSURE PROPHYLAXIS |
| #4 | Search POST‐EXPOSUREPROPHYLAXIS OR POSTEXPOSUREPROPHYLAXIS OR POST EXPOSUREPROPHYLAXIS |
| #5 | Search #3 OR #4 |
| #6 | Search #1 AND #2 AND #5 |
| #7 | Search #1 AND #2 AND #5 Field: All Fields, Limits: Publication Date from 1985 to 2005 |
| #8 | Search COHORT OR CASE‐CONTROL OR CASE CONTROL OR CROSS‐SECTIONAL OR CROSS SECTIONAL OR CASECONTROL OR CROSSSECTIONAL |
| #9 | Search #7 AND #8 |
| #10 | Search ANIMALS [MH] NOT HUMAN [MH] |
| #11 | Search #9 NOT #10 |
| #12 | Search #7 NOT #10 |
Data collection and analysis
1. Selection of studies Two authors (TY and JA) independently reviewed the titles, abstracts, and descriptor terms of search outputs for relevance based on the criteria for considering studies for the review. Only studies with a comparison group were included. Where necessary, the full text was obtained to determine eligibility of studies for inclusion.
2. Quality assessment No randomized controlled trials were identified. Two authors (TY and JA) independently evaluated the methodological quality of the case‐control study. The likelihood of selection bias was assessed by evaluating the method of participant selection. Information bias was assessed by evaluating the method of outcome ascertainment. The presence of and adjustment for confounders were assessed and attrition bias was assessed by looking at the follow up to see if at least 80% of participants in all groups were included in the final analysis and also to see if the description of those not included was suggestive of bias.
Comparative studies that reported only adverse events were assessed by reviewing the rigor of the methods used to detect adverse events and the quality of reporting (Loke 2005).
3. Data extraction Data concerning outcomes, details of the interventions, and other study characteristics were extracted by two independent authors (TY and JA) using a standardized data extraction form (Table 4). A third author (GK) resolved disagreements. The following information was gathered from each included study: location of study, date, publication status, demographics (e.g. age, gender, occupation, risk behavior, etc.) of participants/exposure modality, form of PEP used, duration of use, and outcomes.
4. Data extraction form.
| Administration of antiretroviral post‐exposure prophylaxis (PEP) to decrease HIV infection in individuals exposed to HIV in occupational setting. | |||
| Study ID | Reviewer ID | ||
| Authors | Journal/yr/vol/iss/pgg | ||
| Title | Location | ||
| Date | |||
| METHODS | |||
| Risk of selection bias | |||
| Risk of information bias Risk of information bias: | |||
| Risk of confounding | |||
| Risk of loss to follow‐up | |||
| Ethics Approval obtained | YES | NO | |
| PARTICIPANTS | |||
| Inclusion criteria | Exclusion criteria | ||
| Number enrolled | |||
| Number completed study | |||
| INTERVENTIONS | |||
| Placebo | YES | NO | |
| Control | YES | NO | |
| No treatment | YES | NO | |
| INTERVENTION | INTERVENTION | CONTROL/ PLACEBO | |
| Description | |||
| Dosage | |||
| Duration of treatment | |||
| Duration of follow‐up | |||
| DESCRIPTION OF OUTCOMES | |||
| Primary outcomes | |||
| Secondary outcomes | |||
| RESULTS | INTERVENTION | INTERVENTION | CONTROL/PLACEBO |
| Total number Total number | |||
| Male | |||
| Female | |||
| Age (median and range or mean and standard deviation) | |||
| Occupation | |||
| HIV infection | |||
| Adherence | |||
| Side effects | |||
| Notes | |||
4. Data analysis Odds ratios with a 95% confidence interval (CI) were used as the measure of effect. A meta‐analysis was performed for adverse events where two‐drug regimens were compared with three‐drug regimens. Due to overlap between Puro 2000 and Puro 2005, the former was not included in the combined analysis.
Results
Description of studies
Effect of PEP on HIV seroconversion Only one case‐control study conducted to identify risk factors for the transmission of HIV to a HCW after percutaneous exposure to HIV‐infected blood fulfilled the selection criteria (Cardo 1997). HCWs from the United States of America (USA), France, Italy, and the United Kingdom were included. Cases were HCWs who had a documented occupational percutaneous exposure to HIV‐infected blood by a needlestick or a cut with a sharp object, HIV seroconversion temporally associated with the exposure, and no other reported concurrent exposure to HIV. Controls were HCWs with documented occupational percutaneous exposure to HIV‐infected blood who were HIV seronegative at the time of exposure and at least six months later. All case patients reported in the USA by August 1994 who were exposed after 1987, and all controls exposed after 1987 whose six‐month follow‐up evaluation was completed as of August 1994 were studied. Case patients reported in France and Italy after 1989 and in the United Kingdom after 1987 were included. Thirty‐three cases and 679 controls were included in the study.
Zidovudine post‐exposure prophylaxis was evaluated. Of those who took zidovudine, 66% of controls and 89% of cases had their first dose within four hours after exposure. Sixty‐six percent of controls and 44% of cases continued taking zidovudine for at least four weeks. The majority of cases and controls took at least 1000 mg of zidovudine per day. In addition, of approximately 70% of cases and controls who took zidovudine, the source patients were taking zidovudine at the time of the HCW's exposure.
No studies were found that evaluated the effect of two or more antiretroviral therapies for occupational PEP.
Adherence to and complications with PEP Eight reports from observational comparative studies, mainly surveillance data comparing various PEP regimens, were included. Srivastava 1998 analyzed data reported to the National Surveillance System for Hospital Health Care Workers. From June 1995 to December 1997, 188 HCWs reported occupational exposures to HIV. One hundred and fourteen HCW had a least one follow‐up visit, and of these, 58 took PEP, 53 did not, and three had missing information. Antiretroviral regimens included zidovudine alone (28%), zidovudine and one other antiretroviral (34%), a combination of three drugs (34%) and four antiretrovirals (4%).
Swotinsky 1998 examined a hospital‐based occupational health clinic's experience with combination antiretroviral therapy for PEP. From September 1996 to September 1997, 235 workers reported occupational exposures to blood. Workers who chose to take PEP were advised to undertake a four‐week regimen of either two or three drugs depending on the risk from the exposure. Sixty‐eight of the 235 workers started PEP, 23 on two drugs and 45 on three drugs.
Two observational studies using the Italian PEP Registry that compared side effects and discontinuation rates with various PEP regimens were identified. Puro 2005 reported results from the Italian PEP Registry to June 2004. They compared 356 subjects who were prescribed two NRTIs with 915 subjects receiving two NRTIs plus a PI. Subjects who dropped out of the study, as well as those who withdrew or discontinued PEP because the source person tested HIV negative, were excluded. Discontinuation of PEP was defined as duration of PEP that was less than 28 days. Puro 2000 reviewed the data prospectively collected by the Italian PEP Registry until December 1999. The study included 647 healthcare workers on zidovudine, 341 on combination PEP (115 receiving zidovudine and lamivudine and the other various combinations of two NRTIs), and 218 receiving two NRTIs plus a PI.
Puro 2003 evaluated the features of hepatotoxicity using data collected from August 1996 to September 2002 in the Italian PEP Registry. Subjects were divided into three groups according to their PEP regimen: two NRTIs (n=207), two NRTIs plus PI (n=429) and one non‐NRTI (n=19). Only individuals who had taken PEP for at least five days and for whom at least two values of plasma level of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were available were included. AST and ALT changes from baseline to highest values were categorized according to the toxicity grading used by the AIDS Clinical Trials Group, modified by Sulkowski 2000. Patients with pre‐treatment serum AST and ALT levels within normal range (AST < 35 U/L and ALT < 31 U/L) were classified based on changes relative to the upper limit of normal (ULN): grade 0 (< 1.25 ULN), grade 1 (1.25‐2.50 X ULN), grade 2 (2.60‐5.00 X ULN), grade 3 (5.10‐10.00 X ULN) and grade 4 (> 10 X ULN). The median duration of PEP was 30 days (range five to 60) in two NRTIs group, 30 days (range five to 57) in two NRTIs plus PI group and 25 days (range five to 32) in the one non‐NRTI group.
ItalianRegistry 2000 reviewed data collected by the Italian PEP Registry on HCWs who received a combination of two NRTIs (47 HCWs) or two NRTIs plus a PI (86 HCWs) after reporting occupational exposure to HIV. All HCWs for whom triglyceride levels were available at baseline and at 10, 20 and 30 days of treatment were included. Individuals who had a triglyceride level greater than 220mg/dl at baseline were excluded. HCWs who discontinued the PI at or before the sixth day of follow up were analyzed with those receiving two NRTIs. Mild to severe hypertriglyceridaemia was defined as triglyceride level greater than 220 and 500 mg/dl respectively.
Parkin 2000 studied retrospectively the use of PEP for occupational HIV‐1 exposure in three London hospitals from 1996 to January 1999. Twenty‐eight HCW received PEP. Eighteen received zidovudine, lamivudine and indinavir. Other triple regimens were used in six HCW. Four received treatment with mono or dual nucleoside analogue regimens containing zidovudine, didanosine or lamivudine. Fifteen of the 28 completed the course of PEP.
Wang 2000 reviewed data from the HIV PEP Registry, established by the Centers for Disease Control and Prevention, Glaxo Wellcome Inc., and Merck & Co Inc. Healthcare providers enrolled HCWs on a voluntary basis for a six‐month observation period. Epidemiological and laboratory data were sent to the registry at baseline, six weeks post‐treatment initiation (data collected included PEP regimen, modifications to the regimen, reports of adverse events or laboratory abnormalities and HIV antibody test results) and at six months post‐exposure. From October 1996 to December 1998 492 HCWs were enrolled. Fifty‐nine percent received three drugs, 36% two drugs, 1% one drug, 3% four drugs and 1% more than five drugs.
Risk of bias in included studies
Effect of PEP on HIV seroconversion
Selection bias Cases and controls were identified from different sources. Cases were identified through reports to national surveillance systems for occupationally acquired HIV infection. Controls were identified through reports to a voluntary CDC surveillance project, Prospective Evaluation of Health Care Workers Exposed to Blood of Patients Infected with HIV, which enrolled HCWs from approximately 300 healthcare institutions in the USA.
Information bias Different sources were used for the risk‐factor information. Incident reports were reviewed to obtain information on case patients. Controls were reported to the CDC at the time of exposure and information was collected with a standardized protocol. Information about the healthcare worker, source patient, and injury were collected.
Confounding Severity of the exposure was a confounder and this was taken into account in the analysis.
Attrition bias Fourteen controls (2%) were not included in the final analysis because of missing values.
Adherence to and complications with PEP
Selection bias The included studies were based on surveillance data. There were no formal sampling or selection procedures making the studies prone to selection bias.
Rigor of methods used in detecting adverse events Only two of the eight comparative studies on adverse events reported rigorous methods for detecting the reported adverse events (Puro 2003; ItalianRegistry 2000). See Table 5.
5. Quality assessment of studies reporting adverse events.
| Quality measure | Puro 2005 | Puro 2000 | Puro 2003 | Italian Reg 2000 | Parkin 2000 | Srivastava 1998 | Wand 2000 | Swotinsky 1998 |
| Conduct | ||||||||
| Are definitions of reported adverse events given? | No | No | Yes | Yes | No | No | Serious adverse events are clearly defined | No |
| How were adverse events data collected? | Not reported | Not reported | Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were measured | Fasting triglyceride levels were anlaysed enzymatically in hospital laboratories. | Not reported | Not reported | Laboratory tests. Collection of symptom data not clear. | Interviews with HCWs and laboratory tests |
| Reporting | ||||||||
| Were any patients excluded from the adverse events analysis? | Subjects who dropped out of the study as well as those who withdrew or discontinued PEP because the source person tested HIV negative were excluded. | HCW who withdrew or discontinued post exposure prophylaxis because the source person tested negative for HIV‐1 were excluded. | Only individuals who had taken PEP for at least five days and for whom at least two values of plasma level of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were available were included. | HCW who discontinued the PI at or before the sixth day of follow‐up were analysed with those receiving two NRTIs. | No | Only HCW with one follow‐up visit were included | Only those who had 6 week follow‐up data were included | Only those who returned for follow‐up were included |
| Were the methods used for monitoring adverse events reported? | No | No | Yes | Yes | No | No | No | No |
| Did the report provide numerical data by intervention group? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Which categories of adverse events were reported by investigators? | Only overall side effects were reported | Only overall side effects were reported | Only AST and ALT | Only one adverse event reported | General adverse events | General adverse events | General and serious adverse events | General adverse events |
| Did the investigators report on all important or serious adverse events? | No | No | No | No | Yes | No | Yes | Yes |
Quality of reporting ItalianRegistry 2000 and Puro 2003 clearly defined reported adverse events. See Table 5.
Attrition bias Five of the eight studies did not include the total number of HCWs at the start in the final analysis (ItalianRegistry 2000; Puro 2003; Srivastava 1998; Swotinsky 1998; Wang 2000).
Effects of interventions
Effect of PEP on HIV seroconversion There was no significant difference between the cases and controls with respect to the year of exposure. For both cases and controls, injuries were mainly from needlesticks, with the majority from hollow bore needles and the rest from other sharp objects. HIV transmission was significantly associated with deep injury (OR 15, 95% CI 6.0 to 41), visible blood on the device (OR 6.2, 95% CI 2.2 to 21), procedures involving a needle placed in the source patient's blood vessel (OR 4.3, 95% CI 1.7 to 12), and terminal illness in the source patient (OR 5.6, 95% CI 2.0 to 16). After controlling for these risk factors associated with HIV infection, no differences were detected in the rates at which cases and controls were offered post‐exposure prophylaxis with zidovudine. However, cases had significantly lower odds of having taken zidovudine after exposure than controls (OR 0.19, 95%CI 0.06 to 0.52).
Adherence to and complications with PEP In Srivastava 1998 71% of the 58 HCWs taking PEP reported one or more side effects. The most common reported symptoms were nausea (24%), fatigue (22%), emotional distress (13%), and headache (9%). In comparison to HCW who did not take PEP, those who received PEP were reporting symptoms more frequently (RR 2.88, 95% CI 1.75 to 4.75).
Results from the Italian PEP Registry (Puro 2005) showed that a significantly higher proportion of individuals in the three‐drug group reported adverse events (OR 1.53, 95%CI 1.19 to 1.95) (Comparison 01‐01) but the difference in discontinuation rates was not statistically significant (OR 1.26, 95%CI 0.92 to 1.73) (Comparison 01‐02). In the three‐drug group, 104 individuals discontinued receipt of the PI alone because of adverse events. In Wang 2000 43% of 449 HCWs completed their PEP regimen, while 44% discontinued all PEP drugs and did not complete the regimen and 13% discontinued one or more drugs, modified drug dosage, or added a drug but did complete the regimen. Of the 197 (44%) who discontinued all PEP drugs, 95 (48%) did so because the source patient tested HIV‐negative. The main reason for discontinuation or modification of regimens was adverse events. Overall, 76% reported some symptoms or adverse events. The most common symptoms were nausea, fatigue or malaise, headache, vomiting, diarrhoea and myalgias. Only eight percent had laboratory abnormalities, but most of these only varied slightly from normal values. Similar proportions of HCWs who took zidovudine plus lamivudine compared to those who took two drugs and indinavir completed their regimens (OR 1.07, 95% CI 0.71 to 1.61). However, significantly more HCWs taking the three‐drug regimen reported adverse events that those taking the two‐drug regimen (OR 2.83, 95%CI 1.76 to 4.54). The median time from start of PEP to onset of each of the five most frequent reported symptoms was three to four days (range one to 44 days). Six HCWs experienced serious adverse events. In Swotinsky 1998 the mean duration of PEP use for 66 workers who returned for follow up was 13.2 ± 10.2 days. Thirty one workers (47%) discontinued because the source patient was HIV negative and 15 discontinued due to adverse events. The most common adverse events were nausea, malaise and fatigue, and headache. Comparing two‐drug with three‐drug regimens (Puro 2005; Swotinsky 1998; Wang 2000), the combined OR for discontinuation was 1.21, 95% CI 0.94 to 1.55 with no significant heterogeneity (I2 0%; Chi2 0.93 with P = 0.63) while the combined OR for adverse events was OR 1.75, 95% CI 1.41 to 2.17 (I2 61.0%; Chi2 5.24 with P = 0.07).
In comparing one‐drug, two‐drug and three‐drug regimens, Puro 2000 found no significant differences in the proportions of HCWs experiencing adverse events (Comparisons 02‐01 and 03‐01) and discontinuing prophylaxis (Comparisons 02‐02 and 03‐02) among the three groups.
In Puro 2003, grade 1 AST/ALT alteration occurred in six cases receiving two NRTIs within 10‐15 days and all HCWs completed PEP. In the two NRTIs plus PI group grade 3 AST/ALT alterations occurred in two HCWs after 20 days, grade 2 developed in six HCWs between 10 and 30 days, and grade 1 in eight cases between 10 and 20 days. Four individuals discontinued PEP. Of the 19 HCWs who received one non‐NRTI seven receiving an efavirenz‐containing regimen developed no alterations however of the 12 receiving nevirapine two cases of grade 2 AST/ALT alterations were observed after 11 and 28 days respectively. One subject required hospitalization. In addition, one grade 2 and one grade 1 alteration developed. In all groups, AST/ALT levels returned to within the normal range, regardless of whether PEP was discontinued or completed.
Four HCWs receiving two NRTIs plus a PI developed triglyceride levels greater than 220 mg/dl at a 30‐day interval (Italian Registry 2000). In all cases the levels returned to normal after 10 days from drug discontinuation. No difference was found between males and females.
In Parkin 2000 13 HCW (46%) stopped or changed therapy, four because the injury was reassessed as low risk and nine because of intolerable side effects. All were on regimens that included indinavir. The reasons for stopping or changing were uncontrolled vomiting, nausea (despite anti‐emetics), or reflux (seven HCWs); urticaria temporally related to indinavir (one HCW); and galactorrhoea with hyperprolactinaemia (one HCW) (Comparisons 04‐01 and 04‐02). The majority of these side effects resolved when indinavir alone was stopped. In addition, six of the 19 who started with indinavir needed more than two weeks sick leave, whereas only one HCW on the other regimens required more than seven days sick leave.
Discussion
No controlled clinical trials comparing two types of PEP regimens or comparing PEP with no intervention after occupational exposure to HIV were found. Evidence of an association between taking zidovudine post‐exposure and reduced HIV seroconversion comes from only one analytic case‐control study (Cardo 1997). Ideally, a randomized controlled trial would provide the best evidence of effectiveness; however, in this case it would neither be ethical nor feasible. Indirect evidence for reduced seroconversion following the use of post‐exposure prophylaxis can be drawn from individual animal studies (Mori 2000; Tsai 1998) and prevention of mother‐to‐child‐transmission of HIV studies (Brocklehurst 2002; Taha 2003).
Adherence to PEP could be influenced by drug tolerability, drug interactions, and the duration of treatment. Reports on adherence and adverse events come mainly from descriptive studies, primarily case studies, case series, and surveillance data, with no comparison groups (Lee 2001). Due to the risk of bias, the ideal study design would have been a randomized controlled trial or alternatively a rigorous analytic study. This review included eight reports from observational comparative studies, mainly surveillance data comparing various PEP regimens, which confirmed findings that adverse events were higher with a three‐drug regimen (Gerberding 2003; Lee 2001), especially containing indinavir. However, discontinuation rates were not significantly different.
Three‐drug regimens are advocated for treatment because of the theoretical higher antiretroviral activity and reduced occurrence of resistance (Yerly 1999). However, using decision modeling, a two‐drug regimen might lead to fewer cases of HIV transmission after a high‐risk occupational exposure because it is almost as effective and is better tolerated (Bassett 2004). The model also suggested that the background prevalence of drug resistance is an important predictor of the optimal PEP regimen, with three drugs being favoured if the prevalence is more than 15%. This review, however, showed that even though a three‐drug regimen was tolerated less well, the discontinuation rates were similar to that of a two‐drug regimen.
All studies were conducted in the developed‐world setting and results of adverse events cannot be generalised to the developing world.
Considering all of the above, this review found evidence to support an effect with zidovudine monotherapy and evidence that PEP is not without adverse effects. There is no direct evidence to support the use of HAART following occupational exposure to HIV. However, due to the success of combination therapies in treating HIV‐infected individuals, a combination of antiretroviral drugs could be used for PEP.
Authors' conclusions
Implications for practice.
The use of occupational PEP is based on limited direct evidence of effect. However, it is highly unlikely that a definitive placebo‐controlled trial will ever be conducted, and, therefore, on the basis of results from a single case‐control study, a four‐week regimen of PEP should be initiated as soon as possible after exposure, depending on the risk of seroconversion. There is no direct evidence to support the use of multi‐drug antiretroviral regimens following occupational exposure to HIV. However, due to the success of combination therapies in treating HIV‐infected individuals, a combination of antiretroviral drugs should be used for PEP. Healthcare workers should be counseled about expected adverse events and the strategies for managing these. They should also be advised that PEP is not 100% effective in preventing HIV seroconversion.
Implications for research.
A randomized controlled clinical trial is neither ethical nor practical. Due to the low risk of HIV seroconversion, a very large sample size would be required to have enough power to show an effect. More rigorous evaluation of adverse events, especially in the developing world, are required. Seeing that current practice is partly based on results from individual primary animal studies, we recommend a formal systematic review of all relevant animal studies.
What's new
| Date | Event | Description |
|---|---|---|
| 12 April 2012 | Amended | Fixed table link. |
| 12 April 2012 | Review declared as stable | No longer being updated. |
History
Protocol first published: Issue 4, 2000 Review first published: Issue 1, 2007
| Date | Event | Description |
|---|---|---|
| 8 February 2010 | Review declared as stable | This review is stable and no longer being updated. |
| 29 October 2008 | Amended | Converted to new review format. |
| 13 November 2006 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We wish to acknowledge Prof Gary Maartens for his advice and information on relevant studies, and Nandi Siegfried for her guidance.
Data and analyses
Comparison 1. Two drugs vs three drugs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse events | 3 | 1717 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.75 [1.41, 2.17] |
| 2 Discontinuation | 3 | 1717 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.94, 1.55] |
1.1. Analysis.
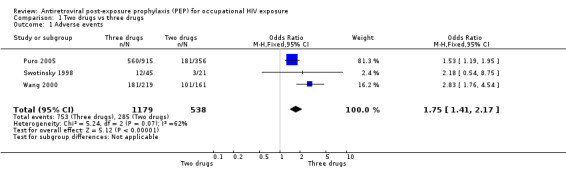
Comparison 1 Two drugs vs three drugs, Outcome 1 Adverse events.
1.2. Analysis.
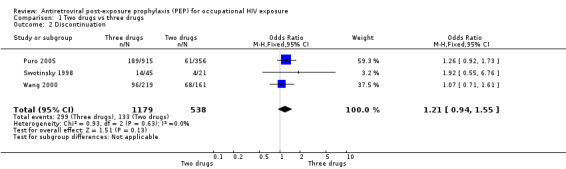
Comparison 1 Two drugs vs three drugs, Outcome 2 Discontinuation.
Comparison 2. One drug vs two drugs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse events | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Discontinuation | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
2.1. Analysis.
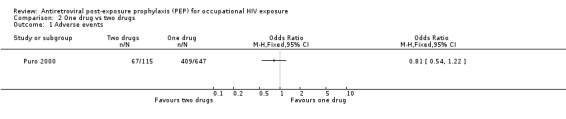
Comparison 2 One drug vs two drugs, Outcome 1 Adverse events.
2.2. Analysis.
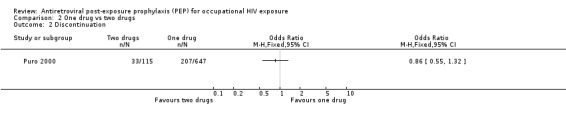
Comparison 2 One drug vs two drugs, Outcome 2 Discontinuation.
Comparison 3. One drug vs three drugs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse events | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Discontinuation | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
3.1. Analysis.
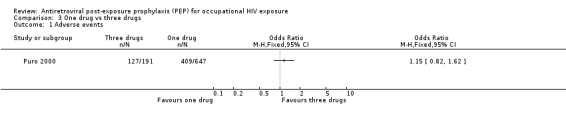
Comparison 3 One drug vs three drugs, Outcome 1 Adverse events.
3.2. Analysis.
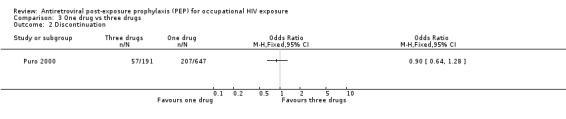
Comparison 3 One drug vs three drugs, Outcome 2 Discontinuation.
Comparison 4. Indinavir vs non‐indinavir containing regimens.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Nausea and vomiting | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Fatigue | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
4.1. Analysis.
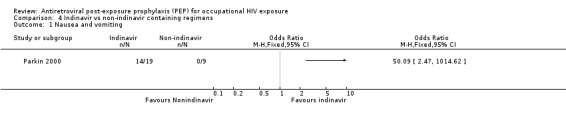
Comparison 4 Indinavir vs non‐indinavir containing regimens, Outcome 1 Nausea and vomiting.
4.2. Analysis.
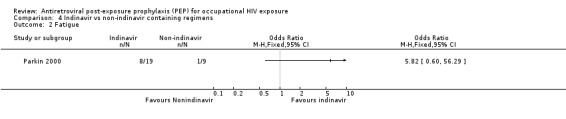
Comparison 4 Indinavir vs non‐indinavir containing regimens, Outcome 2 Fatigue.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cardo 1997.
| Methods | Cases and controls were selected and data on exposure and risk factors were collected retrospectively. Incident reports were reviewed to obtain information on case patients. Controls were reported to the CDC at the time of exposure and information was collected with a standardized protocol. Information about the health care worker, source patient, and injury were collected. | |
| Participants | Cases: HCW who had documented occupational percutaneous exposure to HIV‐infected blood by a needlestick or a cut with a sharp object, HIV serovonversion temporally associated with the exposure and no other reported concurrent exposure to HIV. Controls: HCW with a documented occupational percutaneous exposure to HIV‐infected blood who were HIV seronegative at the time of exposure and at least six months later. All case patients reported in the USA by August 1994 who were exposed after 1987 and all controls exposed after 1987 whose six month follow‐up evaluation was completed as of August 1994 were studied. Case patients reported in France and Italy after 1989 and in the United Kingdom after 1987 were included. Thirty three cases and 679 controls were included in the study. |
|
| Interventions | Zidovudine post‐exposure prophylaxis was used. The majority of cases and controls took at least 1000 mg of zidovudine per day. | |
| Outcomes | Risk factors for transmission of HIV | |
| Notes | Setting: USA, France, Italy and United Kingdom | |
ItalianRegistry 2000.
| Methods | Data collected by the Italian PEP Registry was reviewed. Triglyceride levels were measured at baseline and at 10, 20 and 30 days of treatment. Mild to severe hypertriglyceridaemia was defined as triglyceride level greater than 220 and 500 mg/dl respectively. | |
| Participants | HCW who reported occupational exposure to HIV. All HCWs for whom triglyceride levels were available at baseline and at 10, 20 and 30 days of treatment were included. Individuals who had a triglyceride level greater than 220mg/dl at baseline were excluded. HCW who discontinued the PI at or before the sixth day of follow‐up were analysed with those receiving two NRTIs. Two NRTIs ‐ 47 HCWs (33 women) Two NRTIs plus a PI ‐ 86 HCWs (48 women) |
|
| Interventions | HCWs who received a combination of two NRTIs or two NRTIs plus a PI were compared. In the two NRTI group 43 HCW received zidovudine plus lamivudine and 4 received other combinations of NRTI. In the three drug group 81 HCW received zidovudine, lamivudine plus indinavir and other regimens included indinavir in 6 cases, nelfinavir in 5 and saquinavir in 3 and ritonavir in 1. All drugs were prescribed at the standard dosage for adults. | |
| Outcomes | Serum triglycerides | |
| Notes | Setting: Italy | |
Parkin 2000.
| Methods | Studied retrospectively the use of PEP for accupational HIV‐1 exposure in three London Hospitals from 1996 to January 1999. Adverse events were not defined and methods for measuring adverse events were not reported. | |
| Participants | Twenty eight HCW who received PEP for occupational HIV‐1 exposure in three London Hospitals (St Bartholomew's, Royal London and Homerton) from 1996 to January 1999. | |
| Interventions | Eighteen received zidovudine, lamivudine and indinavir. Other triple regimens were used in six HCW (zidovudine, didanosine and indinavir in one; zidovudine, saquinavir and lamivudine in two; zidovudine, saquinavir and stavudine in one; zidovudine, saquinavir and didanosine in one; and zidovudine, lamivudine and nelfinavir in one). Four received treatment with mono or dual nucleoside analogue regimens containing zidovudine, didanosine or lamivudine. | |
| Outcomes | Side effects Discontinuing prophylaxis | |
| Notes | Setting: United Kingdom | |
Puro 2000.
| Methods | Data prospectively collected by the Italian PEP Registry was reviewed until December 1999. Adverse events were not defined and methods for measuring adverse events were not reported. | |
| Participants | HCW registered with the Italian PEP Registry. HCW who withdrew or discontinued post exposure prophylaxis because the source person tested negative for HIV‐1 were excluded. | |
| Interventions | The study compared ‐ 647 HCW on zidovudine (1000 ‐ 1250 mg/day), ‐ 341 HCW on combination PEP (115 receiving Zidovudine (500‐600 mg/day) and lamivudine and the others various combinations of two NRTIs), ‐ 218 HCW receiving two NRTIs plus a PI (191 received zidovudine, lamivudine plus indinavir and other regimens included indinavir in 14 cases, nelfinavir in 6, saquinavir in 5 and ritonavir in 2). All drugs were prescribed at the standard dose for adults. | |
| Outcomes | Side effects Discontinuing prophylaxis | |
| Notes | Setting: Italy | |
Puro 2003.
| Methods | Data collected from August 1996 to September 2002 in the Italian PEP Registry was evaluated. AST and ALT changes from baseline to the highest values were catergorized according to the toxicity grading used by the AIDS Clinical Trials Group. | |
| Participants | Subjects were divided into three groups according to their PEP regimen: two NRTIs, two NRTIs plus PI and one non‐NRTI. Only individuals who had taken PEP for at least five days and for whom at least two values of plasma level of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were available were included. Two NRTIs: 207 Two NRTIs plus PI: 429 One non‐NRTI: 19 |
|
| Interventions | Compared two NRTIs, two NRTIs plus PI and one non‐NRTI. | |
| Outcomes | Hepatoxicity using aminotransferase levels as the marker. Patients with pretreatment serum AST and ALT levels within normal range (AST < 35 U/L and ALT < 31 U/L) were classified based on changes relative to the upper limit of normal (ULN): grade 0 (< 1.25 ULN), grade 1 (1.25‐2.50 X ULN), grade 2 (2.60‐5.00 X ULN), grade 3 (5.10‐10.00 X ULN) and grade 4 (> 10 X ULN). |
|
| Notes | Setting: Italy | |
Puro 2005.
| Methods | Longitudinal open study conducted by prospective collection of data in the National registry of PEP. Subjects received either two NRTIs or two NRTIs plus a PI. Subjects who dropped out of the study as well as those who withdrew or discontinued PEP because the source person tested HIV negative were excluded. Discontinuation of PEP was defined as duration of PEP less than 28 days. Adverse events were not defined and methods for measuring adverse events were not reported. | |
| Participants | Health care workers and other persons consenting to be treated with post exposure prophylaxis post exposure to HIV ‐ 356 subjects who were prescribed two NRTIs with 915 subjects receiving two NRTIs plus a PI | |
| Interventions | Those receiving two NRTIs were compared to those receiving two NRTIs plus a PI | |
| Outcomes | Adverse events Discontinuation rates | |
| Notes | Setting: Italy | |
Srivastava 1998.
| Methods | Analysed data reported to the National Surveillance System for Hospital Health Care Workers. | |
| Participants | From June 1995 to December 1997, 188 HCWs reported occupational exposures to HIV. One hundred and fourteen HCW had a least one follow‐up visit and of these 58 took PEP, 53 did not, and 3 had missing information. | |
| Interventions | Antiretroviral regimens included zidovudine alone (28%), zidovudine and one other antiretroviral (34%), a combination of three drugs (34%) and four antiretrovirals (4%). | |
| Outcomes | Side effects | |
| Notes | ||
Swotinsky 1998.
| Methods | Hospital‐based occupational health clinic's experiences were examined. Adverse events were not defined. Interviews with HCWs and laboratory monitoring were used to detect adverse events. | |
| Participants | From September 1996 to September 1997, 235 HCWs reported occupational exposures to blood. Only 68 started PEP. | |
| Interventions | Four‐week regimen of either two drugs (zidovudine 200 mg orally three times a day and lamivudine 150 mg orally two times a day) or three drugs (zidovudine, lamivudine and indinavir 800 mg orally three times a day). | |
| Outcomes | Use of PEP Adverse events Costs | |
| Notes | Setting: Boston | |
Wang 2000.
| Methods | Reviewed data from the HIV PEP Registry, established by the Centers for Disease Control and Prevention, Glaxo Wellcome Inc and Merck & Co Inc. At six weeks post treatment initiation data was collected on the PEP regimen, modifications to the regimen, reports of adverse events or laboratory abnormalities and HIV antibody test results. Serious adverse events were clearly defined. | |
| Participants | Healthcare providers enrolled HCW on a voluntary basis for a six month observation period. From October 1996 to December 1998 492 HCWs were enrolled. Six week follow‐up data was available for 449 (91%). | |
| Interventions | 59% received three drugs, 36% two drugs, 1% one drug, 3% four drugs and 1% more than five drugs. | |
| Outcomes | Adverse events Discontinuation rates | |
| Notes | ||
Contributions of authors
John Laurie conceptualised the protocol. Taryn Young and James Arens reviewed search outputs, selected studies for inclusion, located copies of study reports, and extracted data. Taryn Young wrote the review. All authors reviewed and provided input to the writing of the report.
Sources of support
Internal sources
Institute for Global Health, USA.
External sources
No sources of support supplied
Declarations of interest
None known
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Cardo 1997 {published data only}
- Cardo DM, Culver DH, Ciesielski CA, et al. A case‐control study of HIV seroconversion in health care workers after percutaneous exposure. NEJM 1997;337(21):1485‐90. [DOI] [PubMed] [Google Scholar]
ItalianRegistry 2000 {published data only}
- Italian Registry of Antiretroviral Post‐exposure Prophylaxis. Effects of short‐course of antiretroviral agents on serum triglycerides of healthy individuals. AIDS 2000;14:2407‐8. [DOI] [PubMed] [Google Scholar]
Parkin 2000 {published data only}
- Parkin JM, Murphy M, Anderson J, et al. Tolerability and side‐effects of post‐exposure prophylaxis for HIV infection. Lancet 2000;355(9205):722‐3. [DOI] [PubMed] [Google Scholar]
Puro 2000 {published data only}
- Puro V for the Italian Registry of Post‐Exposure Prophylaxis. Post‐exposure prophylaxis for HIV infection. Lancet 2000;355:1556‐7. [DOI] [PubMed] [Google Scholar]
Puro 2003 {published data only}
- Puro V, Soldani F, Carli G, Lazarevic Z, Mattioli F, Ippolito G on behalf of the Italian Registry of Antiretroviral Post‐Exposure Prophylaxis. Drug‐induced aminotransferase alterations during antiretroviral HIV post‐exposure prophylaxis. AIDS 2003;17:1988‐90. [DOI] [PubMed] [Google Scholar]
Puro 2005 {published data only}
- Puro V, CArli G, Ippolito G for the Italian Registry of Antiretroviral Post‐Exposure Prophylaxis. Postexposure HIV prophylaxis regimen. Clin Infect Dis 2005;40:205‐6. [DOI] [PubMed] [Google Scholar]
Srivastava 1998 {published data only}
- Srivastava P, Cardo DM, Panlilio A, Campbell S. Tolerability of antiretroviral agents used by health‐care workers (HCWs) as post‐exposure prophylaxis (PEP) for occupational exposures to HIV. Int Conf AIDS, 12:626 (abstract no. 246/33171). 1998.
Swotinsky 1998 {published data only}
- Swotinsky RB, Steger KA, Sulis C, Snyder S, Craven DE. Occupational exposure to HIV: experience at a tertiary care center. J Occup and Environ Med 1998;40:1102‐9. [DOI] [PubMed] [Google Scholar]
Wang 2000 {published data only}
- Wang SA, Panlilio AL, Doi PA, White AD, Stek M, Saah A and the HIV PEP Registry Group. Experience of Healthcare Workers Taking Postexposure Prophylaxis after Occupational exposures: findings of the HIV Postexposure Prophylaxis Registry. Infect Control Hosp Epidemiology 2000;21:780‐5. [DOI] [PubMed] [Google Scholar]
Additional references
Bassett 2004
- Bassett IV, Freedberg KA, Walensky RP. Two drugs or three? Balancing efficacy, toxicity, and resistance in postexposure prophylaxis for occupational exposure to HIV. Clin Infect Dis 2004;39:395‐401. [DOI] [PubMed] [Google Scholar]
Bottinger 1997
- Bottinger D, Johansson NG, Samuelsson B, et al. Prevention of simian immunodeficiency virus, SIVsm, or HIV2 infection in cynomolgus monkeys by pre‐ and postexposure administration of BEA‐005. AIDS 1997;11(2):157‐62. [DOI] [PubMed] [Google Scholar]
Brocklehurst 2002
- Brocklehurst P, Volmink J. Antiretrovirals for reducing the risk of mother‐to‐child transmission of HIV infection. The Cochrane Database of Systematic Reviews 2002, Issue 2. [DOI] [PubMed] [Google Scholar]
CDC 1998b
- Center for Disease Control and Prevention. Management of possible sexual, injecting‐drug‐use, or other nonoccupational exposure to HIV, including considerations related to antiretroviral therapy. MMWR 1998;47(RR‐17):1‐18. [PubMed] [Google Scholar]
CDC 2001
- Center for Disease Control and Prevention. Updated U.S Public health service guidelines for the management of occupational exposures to HBV, HCV and HIV and recommendations for postexposure prophylaxis. MMWR 2001; Vol. 50, issue RR‐11. [PubMed]
CDC 2005
- Center for Disease Control and Prevention. Updated U.S. Public health service guidelines for the management of occupational exposures to HIV and recommendations for postexposure prophylaxis. MMWR 2005; Vol. 54, issue RR‐9. [PubMed]
Gerberding 2003
- Gerberding JL. Occupational exposure to HIV in health care settings. N Engl J Med 2003;348:826‐33. [DOI] [PubMed] [Google Scholar]
Higgins 2005
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.5 [updated May 2005]. www.cochrane.org/resources/handbook/hbook.htm (accessed 31 May 2005).
Kaldor 2000
- Kaldor J. Peer review comments on PEP proposal. e‐mail 2000.
Lee 2001
- Lee L, Henderson D. Tolerability of postexposure antiretroviral prophylaxis for occupational exposures to HIV. Drug Safety 2001;24(8):587‐97. [DOI] [PubMed] [Google Scholar]
Loke 2005
- Loke YK, Price D, Herxheimer A on behalf of the Cochrane Adverse Effects Subgroup. Including adverse events. In: Higgins JPT, Green S, editors. www.cochrane.org/resources/handbook/hbook.htm (accessed 31 May 2005).
Low‐Beer 2000
- Low‐Beer S, Weber AE, Bartholomew K, et al. A reality check: the cost of making post‐exposure prophylaxis available to gay and bisexual men at high sexual risk. Lancet 2000;14:325‐6. [DOI] [PubMed] [Google Scholar]
Martín 2005
- Martín NV, Almeda J, Casabona J. Effectiveness and safety of HIV post‐exposure prophylaxis after sexual, injecting‐drug‐use or other non‐occupational exposure (Protocol). Cochrane Database of Systematic Reviews 2005, Issue 2. [Google Scholar]
Miller 1996
- Miller S, Lo B, Lurie P, et al. Non‐occupational post‐exposure HIV prophylaxis: guidelines for clinicians. Writing Seminar 1996.
Mori 2000
- Mori K, Yasutomi Y, Sawada S, Villinger F, Sugama K, Rosenwith B, et al. Suppression of acute viremia by short‐term postexposure prophylaxis of simian/human immunodeficiency virus SHIV‐RT‐infected monkeys with a novel reverse transcriptase inhibitor (GW420867) allows for development of potent antiviral immune responses resulting in efficient containment of infection. J Virol 2000;74:5747‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pinkerton 1998
- Pinkerton SD, Holtgrave DR, Bloom FR. Cost‐effectiveness of post‐exposure prophylaxis following sexual exposure to HIV. AIDS 1998;12:1067‐1078. [PubMed] [Google Scholar]
Pinkerton 2000
- Pinkerton SD, Holtgrave DR, Kahn JG. Is post‐exposure prophylaxis affordable?. AIDS 2000;14:325. [DOI] [PubMed] [Google Scholar]
Sulkowski 2000
- Sulkowski MS, Thomas DL, Chaisson RE, Moore RD. Hepatoxicity associated with antiretroviral therapy in adults infected with human immunodeficiency virus and the role of hepatitis C or B virus infection. JAMA 2000;283:74‐80. [DOI] [PubMed] [Google Scholar]
Taha 2003
- Taha T, Kumwenda N, Gibbons A, Broadhead R, Fiscus S, Lema V, et al. Short postexposure prophylaxis in newborn babies to reduce mother‐to‐child transmission of HIV‐1: NVAZ randomised clinical trial. Lancet 2003;362:1171‐7. [DOI] [PubMed] [Google Scholar]
Torbati 1999
- Torbati SS, Guss DA. Emergency department management of occupational exposures to HIV‐infected fluids. J of Emerg Med 1999;17(2):261‐4. [DOI] [PubMed] [Google Scholar]
Tsai 1998
- Tsai C, Emau P, Follis K, Beck T, Benveniste R, Bischofberger N, et al. Effectiveness of postinoculation (R)‐9‐(2‐phosphonylmethoxypropyl) adenine treatment for prevention of persistent simian immunodeficiency virus SIVmne infection depends critically on timing of initiation and duration of treatment. J Virol 1998;72(4265‐73). [DOI] [PMC free article] [PubMed] [Google Scholar]
UNAIDS 1998
- Laporte A, Aggleton P. HIV/AIDS prevention in the context of new therapies. Report of a meeting organized by UNAIDS and the AIDS Research Institute of the University of California at San Francisco. UNAIDS best practice collection 1998:1‐24. [Google Scholar]
Wiebe 2000
- Weibe ER, Comay SE, McGregor M, et al. Offering HIV prophylaxis to people who have been sexually assaulted: 16 months' experience in a sexual assault service. Canad Med Assoc J 2000;162(5):641‐5. [PMC free article] [PubMed] [Google Scholar]
Yerly 1999
- Yerly S, Kaiser L, Race E, Bru J‐P, Clavel F, Perrin L. Transmission of antiretroviral‐drug‐resistent HIV‐1 variants. Lancet 1999;354:729‐33. [DOI] [PubMed] [Google Scholar]


