Abstract
A growing body of evidence suggests a role for androgens in asthma and asthma control. This includes a sex discordance in disease rates that changes with puberty, experiments in mice showing androgens reduce airway inflammation, and a reported association between airway androgen receptor (AR) expression and disease severity in asthma patients. We set out to determine whether airway AR expression differs between asthma patients and healthy controls. We analyzed data from 8 publicly available data sets with gene expression profiling from airway epithelial cells obtained both from asthma patients and control individuals. We found that airway AR expression was lower in asthma patients than in controls in both sexes, and that having AR expression below the median in the pooled data set was associated with substantially elevated odds of asthma vs having AR expression above the median (odds ratio 4.89; 95% CI, 3.13-7.65, P < .0001). In addition, our results suggest that whereas the association between asthma and AR expression is present in both sexes in most of the age range analyzed, the association may be absent in prepubescent children and postmenopausal women. Our results add to the existing body of evidence suggesting a role for androgens in asthma control.
Keywords: asthma, androgen, androgen receptor, steroids, sex hormones
In children, asthma is more common among boys than girls. This reverses during puberty, and in adulthood women continue to have higher asthma rates, suggesting sex steroids play a role [1]. Changes in asthma severity during different phases of the menstrual cycle and during pregnancy suggest influences of the female sex hormones, estrogens and progestogens [2]. The decline in male asthma rates after puberty is suggestive of a beneficial effect of male sex hormones.
In mouse models of allergic airway inflammation, castrated males were shown to have increased inflammation and infiltration of eosinophils and lymphocytes, comparable to females [3], and testosterone was shown to reduce type 2 and interleukin-17A–mediated airway inflammation [4]. In humans, recent studies have shown associations between decreased androgen receptor (AR) gene expression in airways and increased disease severity in asthma patients [5] and between a rare inherited AR deficiency and increased risk of asthma [6]. In addition, an inverse association between serum testosterone levels and odds of asthma has been reported [7], and an association between reduced airway inflammation in glucocorticoid-treated asthma patients and a germline variant permitting increased androgen synthesis has been described [8]. To follow along from this expanding body of evidence, we set out to determine whether there is a difference in airway AR expression between asthma patients and healthy controls.
Materials and Methods
We searched the NCBI’s gene expression omnibus [9] and identified 8 asthma data sets with gene expression profiling from airway epithelial cells. Six data sets from adults contained expression data from bronchial epithelia; 2 of the 6 contained data from nasal epithelia. Two data sets from children contained nasal epithelial expression data. In total, bronchial expression data were obtained from 264 adult asthma patients and 146 controls; nasal expression data were obtained from 52 adult asthma patients and 29 controls plus 49 child asthma patients and 58 controls.
We extracted AR expression data for all subjects along with estrogen receptor α (ESR1) and estrogen receptor β (ESR2) expression as comparators. In data sets containing multiple probes for a gene, results from different probes were averaged. Expression was then normalized to the average expression for control individuals in the given data set and tissue. Normalized expression values from the different data sets were pooled and analyzed. Asthma vs control expression was compared by 2-tailed t tests within each sample group (eg, adult bronchial, adult nasal, child nasal, and breakdowns by sex and/or age range in subsequent analyses). Odds ratios and associated CIs and P values were determined using binomial logistic regression of [disease status equals asthma] against [normalized AR expression is less than median normalized AR expression]. Analyses were performed in R under RStudio. Details of the data sets are shown in Table 1.
Table 1.
Characteristics of data sets used in analysis
| Data set | Age group | Age: mean (range), y | No. of participants (asthma, control) | Male participants (asthma, control) | Female participants (asthma, control) | Bronchial samples (asthma, control | Nasal samples (asthma, control) |
|---|---|---|---|---|---|---|---|
| GSE104468 [15] | Adult | 36.4 (24-74) |
24 (12, 12) |
10 (4, 6) |
14 (8, 6) |
24 (12, 12) |
24 (12, 12) |
| GSE41861 [16] | Adult | 35.1 (19-66) |
84 (54, 30) |
40 (29, 11) |
44 (25, 19) |
81 (51, 30) |
57 (40, 17) |
| GSE43696 [17] | Adult | 37.1 (18-62) |
108 (88, 20) |
34 (25, 9) |
74 (63, 11) |
108 (88, 20) |
0 |
| GSE64913 [18] | Adult | 31.6 (19-63) |
36 (13, 23) |
22 (8, 14) |
14 (5, 9) |
36 (13, 23) |
0 |
| GSE67472 [19] | Adult | 35.3 (20-68) |
105 (62, 43) |
51 (28, 23) |
54 (34, 20) |
105 (62, 43) |
0 |
| GSE89809 [20] | Adult | 37.0 (20-67) |
56 (38, 18) |
31 (19, 12) |
25 (19, 6) |
56 (38, 18) |
0 |
| GSE19187 [21] | Child | 11.0 (6-16) |
38 (13, 25) |
20 (6, 14) |
18 (7, 11) |
0 | 38 (13, 25) |
| GSE65204 [22] | Child | 11.0 (9-12) |
69 (36, 33) |
34 (19, 15) |
35 (17, 18) |
0 | 69 (36, 33) |
Results
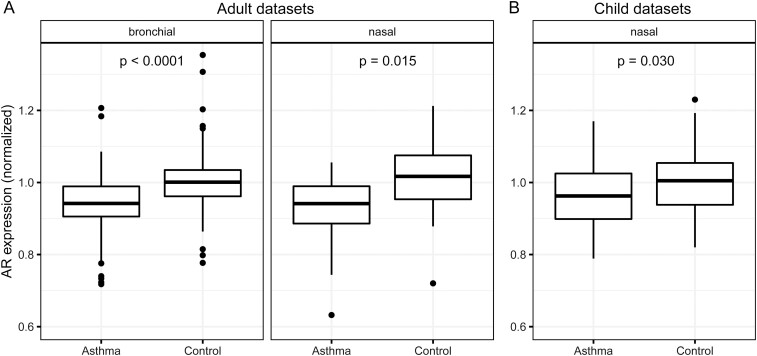
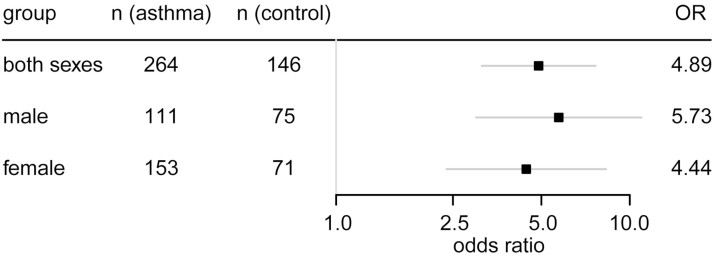
AR expression was lower in asthma patients than controls in bronchial epithelia of adults (normalized expression median [interquartile range; IQR]: 0.94 [0.91-0.99] asthma, 1.00 [0.96-1.03] controls, P < .0001), in nasal epithelia of adults (0.94 [0.89-0.99] asthma, 1.02 [0.95-1.08] controls, P = .015), and in nasal epithelia of children (0.96 [0.90-1.02] asthma, 1.00 [0.94-1.05] controls, P = .030) (Fig. 1); no bronchial data from children were available. No associations were found between asthma and either ESR1 expression (adult bronchial: 0.99 [0.96-1.03] asthma, 1.00 [0.98-1.01] controls, P = .45; adult nasal: 1.01 [0.98-1.03] asthma, 1.00 [0.97-1.04] controls, P = .37; child nasal: 0.99 [0.97-1.00] asthma, 1.00 [0.99-1.02] controls, P = .12) or ESR2 expression (adult bronchial: 0.99 [0.94-1.02] asthma, 0.99 [0.97-1.02] controls, P = .40; adult nasal: 1.01 [0.98-1.04] asthma, 1.00 [0.96-1.01] controls, P = 0.81; child nasal: 1.01 [0.98-1.04] asthma, 1.00 [0.98-1.05] controls, P = .59). Subgroup analyses were performed for bronchial epithelia in adults because of limited sample sizes for nasal epithelia. Having AR expression below the pooled data set’s median was associated with substantially elevated odds of asthma vs having expression above the median: odds ratio 4.89 (95% CI, 3.13-7.65) for both sexes together, 5.73 (95% CI, 3.00-10.95) for male participants, 4.44 (95% CI, 2.38-8.30) for female participants (Fig. 2).
Figure 1.
Androgen receptor (AR) expression is lower in airway epithelial cells of asthma patients than control individuals. Box-and-whisker plots of normalized AR expression from bronchial (left) and nasal (right) epithelia in adults (A) and nasal epithelia in children (B). Center lines indicate median values, boxes indicate first quartile to third quartile range, whiskers indicate values up to 1.5 times interquartile range, and points indicate outlying values. For visual clarity, outliers below 0.6 normalized expression were excluded from the plot: one each from adult bronchial asthma, adult nasal asthma, and adult nasal control. Sample sizes for each group: A, bronchial asthma 264, control 146; nasal asthma 52, control 29; B, nasal asthma 49, control 58 (child data sets contained no bronchial samples). Adult data sets contained participant ages ranging from 18 to 74 years (mean, 35.7 years). Child data sets contained participant ages ranging from 6 to 16 years (mean, 11.0 years). P values from 2-tailed t tests of asthma vs control within each sample group are shown.
Figure 2.
Lower androgen receptor (AR) expression in airways is associated with increased odds of asthma in both sexes. Odds ratios (ORs) with 95% CIs for having disease status asthma among individuals in the bottom 50% vs the top 50% of normalized AR expression in the pooled data set of bronchial epithelial samples. ORs and associated CIs and P values were determined using binomial logistic regression of [disease status equals asthma] against [normalized AR expression is less than median normalized AR expression], performed in R under RStudio. For all 3 comparisons, P is less than .001.
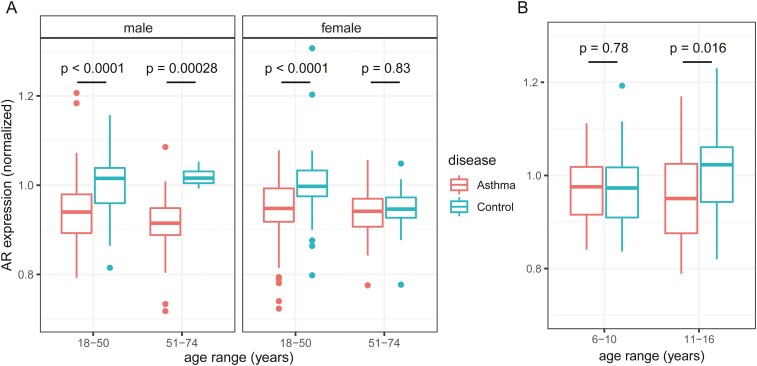
To explore whether associations might change at phases of life characterized by large changes in circulating sex hormone levels, we additionally performed analyses with age breakdowns. In adults, bronchial AR expression was lower in asthma than in controls in men aged younger than 51 years (P < .0001) and 51 years or older (P = .00028), but in women aged 51 or older, AR expression was similar between asthma and controls (P = .83), in contrast to lower expression with asthma in women younger than 51 (P < .0001) (Fig. 3A). In children, nasal AR expression was lower in asthma than controls in ages 11 or older (P = .016), but was similar between asthma and controls in children younger than 11 (P = .78) (Fig. 3B).
Figure 3.
Associations between asthma and airway androgen receptor (AR) expression exist except in postmenopausal women and prepubescent children. Box-and-whisker plots of normalized AR expression from bronchial epithelia in A, adults, and nasal epithelia in B, children, broken down by age and sex in adults and by age in children. Center lines indicate median values, boxes indicate first quartile to third quartile range, whiskers indicate values up to 1.5 times interquartile range, and points indicate outlying values. For visual clarity, one outlier each below 0.7 normalized expression (in female ages 18-50 asthma) and above 1.3 (in female ages 18-50 control) were excluded from plot. Sample sizes for each group: A, male age < 51 asthma 94, control 71; male ≥ 51 asthma 17, control 4; female < 51 asthma 130, control 59; female ≥ 51 asthma 23, control 12. B, age < 11 asthma 20, control 21; age ≥ 11 asthma 29, control 37. P values from 2-tailed t tests of asthma vs control within each sample group are shown.
Discussion
Our results strongly support the conclusion that airway epithelial AR expression is lower in asthma patients than controls in both sexes. Although based on smaller sample sizes, our results also suggest that this association may not be present in prepubescent children or postmenopausal women, groups with much lower circulating sex steroid levels. Whether this association is indeed absent in prepubescent children and postmenopausal women should be verified in cohorts with larger sample sizes in these age ranges. The causative basis for this association between AR expression and asthma is undetermined (ie, does low AR expression cause asthma, is low AR expression a byproduct of having asthma, or is it a combination of the two), but the previously described link between inherited AR deficiency and asthma [6] supports that decreased AR airway expression could increase asthma susceptibility.
Although the direction of causation for the described association is not yet determined, these findings add to previous data suggesting a role for androgens in asthma control [4, 8, 10]. It has been reported that inhaled corticosteroids provide less benefit to female than to male asthma patients [11, 12]. This may in part be attributable to corticosteroids suppressing circulating androgen levels, which could have a bigger effect in women because their circulating androgen levels are lower [8]. A randomized clinical trial found a benefit of inhaled dehydroepiandrosterone (DHEA) sulfate in patients with poorly controlled asthma [13], and a pilot study found that oral DHEA appeared to benefit female asthma patients with low androgen levels [14]. Our findings add to a growing body of work suggesting that androgens should be further studied as a treatment for asthma, including in combination with corticosteroids, and also raise the question of whether AR expression levels in airways could affect the response to asthma treatments.
Glossary
Abbreviations
- AR
androgen receptor
- DHEA
dehydroepiandrosterone
- IQR
interquartile range
Financial Support
This work was supported in part by the National Heart, Lung, and Blood Institute (grant No. P01HL158507 to B.G. and No. K08HL133381 to J.Z.) and the National Cancer Institute (grant Nos. R01CA236780 and R01CA1723382 to N.S.).
Disclosures
The authors have nothing to disclose.
Data Availability
Data analyzed during this study are available from the NCBI’s Gene Expression Omnibus (https://www.ncbi.nlm.nih.gov/geo/).
References
- 1. Naeem A, Silveyra P. Sex differences in paediatric and adult asthma. Eur Med J (Chelmsf). 2019;4(2):27-35. [PMC free article] [PubMed] [Google Scholar]
- 2. Graziottin A, Serafini A. Perimenstrual asthma: from pathophysiology to treatment strategies. Multidiscip Respir Med. 2016;11:30-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hayashi T, Adachi Y, Hasegawa K, Morimoto M. Less sensitivity for late airway inflammation in males than females in BALB/c mice. Scand J Immunol. 2003;57(6):562-567. [DOI] [PubMed] [Google Scholar]
- 4. Fuseini H, Yung JA, Cephus JY, et al. Testosterone decreases house dust mite-induced type 2 and IL-17A-mediated airway inflammation. J Immunol. 2018;201(7):1843-1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zein JG, McManus JM, Sharifi N, et al. Benefits of airway androgen receptor expression in human asthma. Am J Respir Crit Care Med. 2021;204(3):285-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gaston B, Marozkina N, Newcomb DC, Sharifi N, Zein J. Asthma risk among individuals with androgen receptor deficiency. JAMA Pediatr. 2021;175(7):743-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Han YY, Yan Q, Yang G, Chen W, Forno E, Celedón JC. Serum testosterone and asthma outcomes in two large population-based studies. Eur Respir J. 2020;56(Suppl 64):2062. [Google Scholar]
- 8. Zein J, Gaston B, Bazeley P, et al. HSD3B1 genotype identifies glucocorticoid responsiveness in severe asthma. Proc Natl Acad Sci U S A. 2020;117(4):2187-2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barrett T, Wilhite SE, Ledoux P, et al. NCBI GEO: archive for functional genomics data sets—update. Nucleic Acids Res. 2013;41(Database issue):D991-D995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cephus JY, Stier MT, Fuseini H, et al. Testosterone attenuates group 2 innate lymphoid cell-mediated airway inflammation. Cell Rep. 2017;21(9):2487-2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Convery R, Leitch D, Bromly C, Ward R, Bartlett G, Hendrick D. Effect of inhaled fluticasone propionate on airway responsiveness in treatment-naive individuals—a lesser benefit in females. Eur Respir J. 2000;15(1):19-24. [DOI] [PubMed] [Google Scholar]
- 12. Dijkstra A, Vonk JM, Jongepier H, et al. Lung function decline in asthma: association with inhaled corticosteroids, smoking and sex. Thorax. 2006;61(2):105-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wenzel SE, Robinson CB, Leonard JM, Panettieri RA Jr. Nebulized dehydroepiandrosterone-3-sulfate improves asthma control in the moderate-to-severe asthma results of a 6-week, randomized, double-blind, placebo-controlled study. Allergy Asthma Proc. 2010;31(6):461-471. [DOI] [PubMed] [Google Scholar]
- 14. Marozkina N, Zein J, DeBoer MD, et al. Dehydroepiandrosterone supplementation may benefit women with asthma who have low androgen levels: a pilot study. Pulm Ther. 2019;5(2): 213-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang IV, Richards A, Davidson EJ, et al. The nasal methylome: a key to understanding allergic asthma. Am J Respir Crit Care Med. 2017;195(6):829-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. NCBI Gene Expression Omnibus Series GSE41861. https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE41861. Accessed April 8, 2020.
- 17. Li X, Hawkins GA, Moore WC, et al. Expression of asthma susceptibility genes in bronchial epithelial cells and bronchial alveolar lavage in the Severe Asthma Research Program (SARP) cohort. J Asthma. 2016;53(8):775-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Singhania A, Rupani H, Jayasekera N, et al. Altered epithelial gene expression in peripheral airways of severe asthma. PLoS One. 2017;12(1):e0168680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Christenson SA, Steiling K, van den Berge M, et al. Asthma-COPD overlap. Clinical relevance of genomic signatures of type 2 inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;191(7):758-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Singhania A, Wallington JC, Smith CG, et al. Multitissue transcriptomics delineates the diversity of airway T cell functions in asthma. Am J Respir Cell Mol Biol. 2018;58(2):261-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Giovannini-Chami L, Marcet B, Moreilhon C, et al. Distinct epithelial gene expression phenotypes in childhood respiratory allergy. Eur Respir J. 2012;39(5):1197-1205. [DOI] [PubMed] [Google Scholar]
- 22. Yang IV, Pedersen BS, Liu AH, et al. The nasal methylome and childhood atopic asthma. J Allergy Clin Immunol. 2017;139(5):1478-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data analyzed during this study are available from the NCBI’s Gene Expression Omnibus (https://www.ncbi.nlm.nih.gov/geo/).