Abstract
Context:
Characteristics of dental pulp capping agents may influence its interaction with the pulpal cells and can impact the treatment outcome.
Aims:
This study aims to microscopically characterize various pulp capping agents following hydration.
Settings and Design:
Original research.
Materials and Methods:
Disk-shaped specimens of five calcium silicate-based materials, i.e., mineral trioxide aggregate (MTA) Angelus, Biodentine, TheraCal LC, ApaCal ART, and Endocem MTA were prepared. After final set, the materials were immersed in 10 mL of deionized water for 14 days at 37°C. The set materials were characterized by scanning electron microscopy (SEM), X-ray energy dispersive analysis (EDX), and X-ray diffraction (XRD) analysis along with pH analysis of the storage solution using pH meter.
Results:
On SEM analysis, all the materials showed crystalline deposition on the cement surface with Biodentine exhibiting the most dense and homogenous microstructure. Calcium-silicate-hydrate and calcium hydroxide (CH) were observed as dark-grey and light-grey matrix material, respectively. EDX analysis revealed a high concentration of calcium. The other major elements were oxygen and carbon. The surface calcium concentration in the tested specimens was as follows: Biodentine (42.59 wt.%) > MTA Angelus (38.51wt.%) > Endocem MTA (30.24wt.%) > TheraCal LC (27.51wt.%) > ApaCal ART (22.02wt.%). On XRD analysis, all the materials exhibited peaks for tricalcium silicate and CH, after 14 days of hydration.
Conclusions:
The higher surface calcium level in Biodentine and MTA Angelus may enhance reparative dentin formation. The surface calcium concentration of Endocem MTA and ApaCal ART was found to be lesser than that of MTA Angelus, but with the added advantage of fast-setting property. Hence, they are potential alternative materials for vital pulp therapy.
Keywords: Biodentine, dental pulp capping, Endocem, mineral trioxide aggregate, TheraCal
INTRODUCTION
Vital pulp therapy is a promising alternative to root canal treatment for permanent teeth with inflamed but vital pulp.[1,2] It involves the placement of a biocompatible capping material over exposed vital pulp after hemorrhage control or in deep dentinal caries, in an attempt to preserve the pulp vitality/health. Calcium silicate-based cements (CSBC) have gained attention as a capping material in vital pulp therapy procedures due to their superior biological properties and sealing ability.[2,3,4] Since the introduction of first-generation CSBC, i.e., Mineral Trioxide Aggregate (MTA) in 1993, newer CSBC with minor modifications or additional components have been developed in an attempt to improve the biological and mechanical properties.[5,6,7,8,9,10]
A light-curable CSBC can be advantageous in several clinical scenarios such as a protective liner under composite restoration. Theracal LC and Apacal ART are light-curable resin modified calcium silicate and calcium phosphate-based pulp capping agent, respectively, which were introduced in the past decade. Apacal ART is a novel light-curable pulp capping agent containing tricalcium phosphate and nano-hydroxypatite. The tricalcium phosphate may serve as a phosphate reservoir and increase the reactivity of cement by nucleation of calcium phosphate nanoapatite which may induce pulpal cells and assist in dentin bridge formation.[10,11] Endocem MTA is a newer modification of MTA with a chemical composition comparable to that of MTA. It was developed mainly to overcome the disadvantage of the long setting time of conventional MTA. Thus, it is a fast setting, pozzolan-based CSBC with a setting time of 4 min, and easier manipulation properties.[12,13] A pozzolan is an amorphous material composed predominantly of silica or silica and aluminum which reacts with calcium hydroxide (CH) to form cementitious material.
Even small changes in the chemical composition can significantly affect the characteristics of the set material and its future interaction with tooth tissues. Hence, in this study, a combination of microscopic, elemental, and phase analysis in standardized in vitro conditions was used to characterize and assess the hydrated pulp capping agents, namely MTA Angelus, Biodentine, Theracal LC, Apacal ART, and Endocem MTA.
MATERIALS AND METHODS
Five pulp capping agents were investigated in this study, namely MTA Angelus (Angelus Solucões Odontológicas, Londrina PR, Brazil), Biodentine (Septodont, Saint-Maur-des-fossés Cedex, France), Theracal LC (Bisco, Schaumburg, IL, USA), Apacal ART (Prevest DenPro, Jammu, India) and Endocem MTA (Maruchi, Wonju-si, Korea). Disk-shaped specimens measuring six mm in diameter and two mm in height were prepared for each material, using custom-designed acrylic molds. The materials were manipulated and allowed to be set according to the manufacturer's recommendations. Mechanical vibrations were used before setting to avoid voids and obtain a flat and regular surface. Once set, the materials were immersed in 10 mL of deionized water for 14 days at 37°C. The set materials were characterized by scanning electron microscopy (SEM), X-ray energy dispersive analysis (EDX), and X-ray diffraction (XRD) analysis along with pH analysis of the storage solution using pH meter.
Microscopy and elemental analysis
The set cement disks were retrieved from deionized water and dried using a vacuum-desiccator. Then the specimens were polished using fine diamond disks, and the polished specimens were mounted on separate aluminum stubs, carbon-coated, and viewed under SEM (Zeiss Merlin Field Emission SEM; Carl Zeiss NTS GmbH, Germany) in backscatter electron mode. The disks were observed at ×2000, ×3000 and ×4000 magnification to visualize the microstructural components of set/hydrated material. EDX was also done for elemental analysis of set cement disks and various elemental peaks were obtained for each material.
X-ray diffraction analysis
XRD was used to identify the various crystalline phases in the set/hydrated materials. Disks were dried in a vacuum-desiccator and crushed into a very fine powder using mortar and pestle. The diffractometer (Panalytical X'pert Pro, Malvern Panalytical Ltd, Malvern, UK) used Cu Kα radiation at 40 mA and 45 kV, and the detector was set to rotate between 15° and 45°, with a sampling width of 0.05° and scan speed of 1°/min. Various phases were identified and ascertained using a search-match software which uses International Centre for Diffraction Database.
Evaluation of pH of leachate
Specimen of each material was immersed separately in 10 mL of deionized water. The pH readings of the respective storage solutions were recorded using a pH meter (Sartorius Stedim Biotech S.A) prior to immersion of the material and after 14 days of retrieving the disks.
RESULTS
Results of SEM analysis of hydrated cements
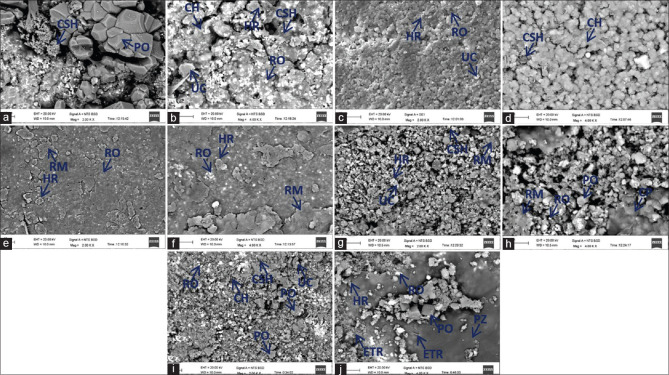
On SEM analysis, all the materials showed crystalline deposition on the cement surface following hydration [Figure 1a-j]. Calcium silicate hydrate and CH were seen as dark-grey and light-grey matrix material respectively. Numerous residual unhydrated particles were observed with a rim of hydration product. Among the tested materials, the microstructure of MTA Angelus was the most porous, whereas Biodentine depicted the most dense and homogenous microstructure [Figure 1c and d]. Radiopacifiers were seen as bright shiny particles in all the materials.
Figure 1.
Back scatter scanning electron micrographs of hydrated (a and b) MTA Angelus, (c and d) Biodentine, (e and f) Theracal LC, (g and h) Apacal ART, (i and j) Endocem MTA at ×2000 and ×4000 magnification respectively. PO: Portlandite, CH: Calcium hydroxide matrix, CSH: Calcium silicate hydrate, RO: Radio-opacifier, UC: Unreacted cement, HR: Hydration rim, RM: Resin matrix, CP: Calcium phosphate, PZ: Pozzolan, ETR: Ettringite
SEM analysis of MTA Angelus showed larger sized rhombohedral and hexagonal portlandite crystals [Figure 1a and b]. Radial acicular-shaped crystals of calcium silicate hydrate were also observed [Figure 1a]. Biodentine showed homogeneous distribution of smaller-sized crystals with fewer bright white particles of unhydrated residual cement [Figure 1c and d]. Radiopacifier particles were scarce and pinpoint in size [Figure 1c and d]. Theracal LC microstructure was predominantly composed of amorphous resin matrix [Figure 1e and f]. On the other hand, Apacal ART had less prominent resin matrix, and showed dense microstructure with small-to-medium-sized rhombohedral portlandite crystals within the matrix [Figure 1g and h]. At ×4000, characteristic calcium phosphate globules were also seen in Apacal ART [Figure 1h]. Endocem MTA revealed medium-sized cuboidal and rhombohedral portlandite crystals. Multiple needle-shaped ettringite crystals were also observed [Figure 1j]. Darker grey amorphous material representing pozzolan was detected only in Endocem MTA [Figure 1i and j].
Results of X-ray energy dispersive analysis of hydrated cements
Results of EDX analysis are summarized in Table 1. EDX analysis of crystals present in SEM images of all the materials revealed a high concentration of calcium. The other major elements observed were oxygen and carbon. Chlorine was observed only in Biodentine. Endocem MTA contained higher percentages of aluminum compared with the other materials [Table 1]. In Apacal ART, higher phosphate concentration was present corresponding to the globular crystals seen in SEM image.
Table 1.
Major elemental composition of mineral trioxide aggregate angelus, Biodentine, Theracal LC, Apacal ART and Endocem mineral trioxide aggregate on energy dispersive X-Ray analysis
| Element | Weight (%) concentration | ||||
|---|---|---|---|---|---|
|
| |||||
| MTA angelus | Biodentine | Theracal LC | Apacal ART | Endocem MTA | |
| Calcium | 38.51 | 42.59 | 27.51 | 22.02 | 31.24 |
| Carbon | 10.30 | 14.04 | 8.30 | 2.78 | 6.06 |
| Silicon | 8.60 | 1.41 | 6.93 | 2.65 | 11.45 |
| Phosphorus | 1.00 | 0.15 | 5.63 | 0.15 | |
| Chlorine | 6.06 | ||||
| Oxygen | 29.63 | 34.28 | 55.63 | 58.12 | 32.22 |
| Aluminium | 0.92 | 0.33 | 0.29 | 1.88 | |
| Bismuth | 11.93 | 19.95 | |||
| Barium | 1.33 | 6.51 | |||
MTA: Mineral trioxide aggregate
Ca2+concentration as seen in X-ray energy dispersive analysis
The amount of calcium at the surface of the tested specimens were as follows: Biodentine (42.59 wt.%) > MTA Angelus (38.51 wt.%) > Endocem MTA (31.24 wt.%) > Theracal LC (27.51 wt.%) > Apacal ART (22.02 wt.%).
X-ray diffraction analysis
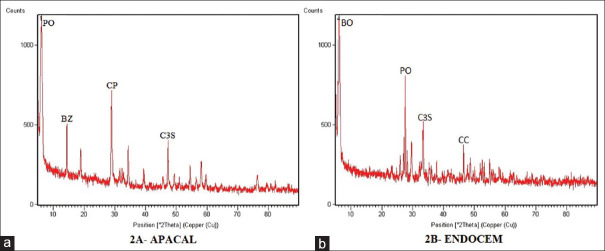
After 14 days of hydration, the tested materials exhibited peaks for tricalcium silicate and CH on XRD analysis. The tricalcium silicate peak was absent in Apacal ART whereas it was found to be of low intensity in rest of the materials. CH peak was not evident for Theracal LC and it was less intense for Apacal ART and Endocem MTA [Figure 2a and b]. The peak of the radiopacifier phases was also observed namely bismuth oxide in MTA Angelus and Endocem MTA [Figure 2b], zirconium oxide in Biodentine and barium zirconate in Theracal LC and Apacal ART [Figure 2a]. Radioopacifier peak in Biodentine was less intense compared to the other materials. A peak of calcium carbonate was observed in Biodentine. Apacal ART [Figure 2a] and Theracal LC exhibited peaks of calcium phosphate and calcium sulfate, respectively.
Figure 2.
X-ray diffraction patterns of hydrated cement (a) Apacal ART and (b) Endocem MTA showing various phases present, after 14 days of hydration. PO: Portlandite, BZ: Barium zirconate, CP: Calcium phosphate, C3S: Tricalcium silicate, BO: Bismuth oxide, CC: Calcium carbonate
Result of pH analysis of leachate
pH of deionized water before immersion of the experimental materials was 6.91. All cements exhibited an alkaline pH of leachate on hydration. Biodentine resulted in the most alkaline leachate followed by MTA Angelus, Endocem MTA, Apacal ART and Theracal LC. The pH of leachate after 14 days of immersion of respective cement specimens in deionized water is shown in Table 2.
Table 2.
pH of leachate after immersion of tested materials in deionized water for 14 days
| MTA angelus | Biodentine | Theracal LC | Apacal ART | Endocem MTA | |
|---|---|---|---|---|---|
| pH of leachate | 10.45 | 11.28 | 8.43 | 8.86 | 9.52 |
MTA: Mineral trioxide aggregate
DISCUSSION
Vital pulp therapy results in the formation of reparative dentin, a physical barrier that functions as a biological seal to protect the underlying pulp tissues and maintain pulp vitality.[14] One of the prerequisites for vital pulp therapy is a biologically active capping material. Over the past few years, CSBCs have increasingly gained popularity due to their biocompatibility and bioactivity. They have also been shown to result in more dense and homogenous dentin bridge formation compared to CH.[15] However, though hydroxyapatite comprises calcium phosphate, the calcium phosphate-based materials have not been extensively studied as pulp capping agents. Identifying the microstructural components of pulp-capping agents is critically important as it may regulate or influence the formation of reparative dentin by the dental pulp stem cells. SEM/EDX analysis is a suitable technique with which hydration of cement and elemental composition can be assessed. XRD is useful to characterize crystalline materials and analyze the crystalline phases within the test material.[16,17]
SEM analysis revealed that hydration of the tested materials resulted in the formation of two major phases, namely, calcium silicate hydrate and CH which is in accordance with other studies.[17,18] Denser microstructure was seen in Biodentine which agrees with studies done by Camilleri et al.[19,20] The low porosity might be attributed to the presence of water-reducing agent in the liquid component of Biodentine. It may translate to better biological properties and mechanical performance for Biodentine. MTA Angelus was found to be the most porous. This is in accordance with a previous in vitro study.[19] This might be attributed to the irregular and large crystal shape and size and due to the use of distilled water along with hand-mixing. Radiopacifiers were seen as bright shiny particles in SEM images.[18] Radiopacifier quantity per unit area was greater in MTA Angelus and Endocem MTA whereas it was least in Biodentine, thus making it less radiopaque on radiographs. Amorphous resin matrix was evident in Theracal LC and Apacal ART which is in accordance with the resin-based composition of these materials. The resin component may be responsible for the lower calcium levels in these materials. Apacal ART also showed characteristic calcium phosphate globules. Calcium phosphate may enhance the reaction rate as it provides phosphate in addition to that from biological fluid for hydroxyapatite formation as stated by Prati and Gandolfi.[3] Darker grey amorphous material representing pozzolan was detected only in Endocem MTA. This may be attributed to its chemical composition and setting reaction which employs chemical reaction of pozzolan with CH resulting in reduced setting time. A previous study by Han et al. showed that unreacted Endocem MTA powder contained a high concentration of aluminum, which may be partly derived from pozzolan.[21] This high concentration of aluminum may have led to the formation of multiple needle-shaped ettringite (hexacalcium aluminate sulphate) crystals seen on SEM.
EDX analysis of crystals present in SEM images of all the materials revealed a high concentration of calcium. The amount of calcium at the surface was highest in Biodentine (42.59 wt.%) and least in Apacal ART (22.02 wt.%). Emerging evidence supports the role of calcium ions released from biomaterials as a key factor in actively inducing reparative dentin formation by eliciting intracellular Ca2+ signaling pathways. ORAI1, an essential pore subunit of store-operated Ca2+ entry (SOCE), regulates intracellular Ca2+ level and Ca2+-mediated signaling pathway in most nonexcitable cells such as the dental pulp stem cells. Sohn et al. recently reported that ORAI1 plays a key role in odontogenic differentiation and mineral deposition by mediating the influx of calcium released from biomaterials.[22] EDX analysis also revealed that Biodentine had the lowest Si/Ca ratio which was due to the abundance of calcium in Biodentine. This would result in a greater deposition of hydroxyapatite when the material is in contact with a physiological solution.
XRD analysis showed smaller tricalcium silicate and dicalcium silicate peaks in comparison to CH peak indicating an active hydration process. CH phase was not seen in XRD analysis of Theracal LC which signifies that calcium release was not in hydroxide form. This may be due to the resin component which binds to and prevents the release of calcium. This observation was similar to the findings of a previous study by Camilleri et al.[23] XRD analysis revealed the lowest radioopacifier peak in Biodentine which confirms the finding of SEM analysis. In Apacal ART, a lower peak of CH was seen which may be due to the utilization of calcium in the formation of calcium–silicate–phosphate–hydrate gel. The lower calcium may also be owed to the resin composition as in Theracal LC. The resin component may also lead to leaching of cytotoxic monomers which may hamper the biocompatibility of these materials. Endocem MTA also showed a lower peak of CH which may be due to the pozzolanic reaction which results in gradual decrease in the amount of free CH, as the calcium is increasingly consumed in the formation of stable crystals of calcium silicate hydrate and calcium aluminate hydrate. These crystals may be associated with improved strength of the material.[13]
pH analysis demonstrated an alkaline pH of leachate for all the materials. Biodentine resulted in the most alkaline leachate followed by MTA Angelus, Endocem MTA, Apacal ART, and Theracal LC. High pH results in antibacterial action by damaging the cytoplasmic membrane and DNA of bacterial microorganisms. Alkaline environment is also known to promote alkaline phosphatase activity which thereby increases the local concentration of inorganic phosphate and leads to osteogenic differentiation and bone formation.[15] High pH may also aid in odontogenic differentiation, but it needs further investigation.
In the present study, simulated body fluid was not used for specimen immersion. Therefore, the formation of hydroxyapatite could not be assessed and is a limitation of our study. However, the higher amount of calcium correspondingly results in a greater deposition of hydroxyapatite when the material is in contact with a physiological fluid which is the physiochemical basis of bioactivity of CSBCs. Hence, materials with higher calcium concentration are expected to provide better clinical results by enhancing the formation of dentin bridge. However, clinical trials must be performed to confirm the clinical implications/outcomes. Overall, this study data would help in understanding the biological impact of the material characteristics of these pulp capping agents.
CONCLUSIONS
The results of this in vitro study showed that Biodentine had the highest surface calcium concentration followed by MTA Angelus, which can potentially enhance dentin bridge formation when used as a pulp capping material. The surface calcium concentration of Endocem MTA was lesser than that of MTA angelus, nevertheless, it offers the added advantage of fast-setting and easier manipulation. Although ApaCal ART was found to have the lowest calcium concentration in this study, yet it has the benefit of being light-curable allowing direct placement under composite resin restoration. However, due to the resin component within Apacal ART and Thercal LC, these materials may not be as favorable as Biodentine, MTA Angelus, or Endocem MTA when in direct contact with pulp tissue such as in direct pulp capping and pulpotomies.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Simon S, Perard M, Zanini M, Smith AJ, Charpentier E, Djole SX, et al. Should pulp chamber pulpotomy be seen as a permanent treatment. Some preliminary thoughts? Int Endod J. 2013;46:79–87. doi: 10.1111/j.1365-2591.2012.02113.x. [DOI] [PubMed] [Google Scholar]
- 2.Schmalz G, Smith AJ. Pulp development, repair, and regeneration: Challenges of the transition from traditional dentistry to biologically based therapies. J Endod. 2014;40:S2–5. doi: 10.1016/j.joen.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 3.Prati C, Gandolfi MG. Calcium silicate bioactive cements: Biological perspectives and clinical applications. Dent Mater. 2015;31:351–70. doi: 10.1016/j.dental.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Taha NA, Khazali MA. Partial pulpotomy in mature permanent teeth with clinical signs indicative of irreversible pulpitis: A randomized clinical trial. J Endod. 2017;43:1417–21. doi: 10.1016/j.joen.2017.03.033. [DOI] [PubMed] [Google Scholar]
- 5.Parirokh M, Torabinejad M. Mineral trioxide aggregate: A comprehensive literature review-part III: Clinical applications, drawbacks, and mechanism of action. J Endod. 2010;36:400–13. doi: 10.1016/j.joen.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Gandolfi MG, Taddei P, Tinti A, Prati C. Apatite-forming ability (bioactivity) of ProRoot MTA. Int Endod J. 2010;43:917–29. doi: 10.1111/j.1365-2591.2010.01768.x. [DOI] [PubMed] [Google Scholar]
- 7.Guven Y, Tuna EB, Dincol ME, Aktoren O. X-ray diffraction analysis of MTA-plus, MTA-angelus and DiaRoot bioaggregate. Eur J Dent. 2014;8:211–5. doi: 10.4103/2278-344X.130603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaur M, Singh H, Dhillon JS, Batra M, Saini M. MTA versus biodentine: Review of literature with a comparative analysis. J Clin Diagn Res. 2017;11:ZG01–5. doi: 10.7860/JCDR/2017/25840.10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zanini M, Sautier JM, Berdal A, Simon S. Biodentine induces immortalized murine pulp cell differentiation into odontoblast-like cells and stimulates biomineralization. J Endod. 2012;38:1220–6. doi: 10.1016/j.joen.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 10.Gandolfi MG, Siboni F, Prati C. Chemical-physical properties of TheraCal, a novel light-curable MTA-like material for pulp capping. Int Endod J. 2012;45:571–9. doi: 10.1111/j.1365-2591.2012.02013.x. [DOI] [PubMed] [Google Scholar]
- 11.Bryan TE, Khechen K, Brackett MG, Messer RL, El-Awady A, Primus CM, et al. In vitro osteogenic potential of an experimental calcium silicate-based root canal sealer. J Endod. 2010;36:1163–9. doi: 10.1016/j.joen.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 12.Silva EJ, Carvalho NK, Guberman MR, Prado M, Senna PM, Souza EM, et al. Push-out bond strength of fast-setting mineral trioxide aggregate and pozzolan-based cements: ENDOCEM MTA and ENDOCEM Zr. J Endod. 2017;43:801–4. doi: 10.1016/j.joen.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Choi Y, Park SJ, Lee SH, Hwang YC, Yu MK, Min KS. Biological effects and washout resistance of a newly developed fast-setting pozzolan cement. J Endod. 2013;39:467–72. doi: 10.1016/j.joen.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 14.Cohenca N, Paranjpe A, Berg J. Vital pulp therapy. Dent Clin North Am. 2013;57:59–73. doi: 10.1016/j.cden.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Song M, Yu B, Kim S, Hayashi M, Smith C, Sohn S, et al. Clinical and molecular perspectives of reparative dentin formation: Lessons learned from pulp-capping materials and the emerging roles of calcium. Dent Clin North Am. 2017;61:93–110. doi: 10.1016/j.cden.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Camilleri J. Characterization and hydration kinetics of tricalcium silicate cement for use as a dental biomaterial. Dent Mater. 2011;27:836–44. doi: 10.1016/j.dental.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Camilleri J. Hydration mechanisms of mineral trioxide aggregate. Int Endod J. 2007;40:462–70. doi: 10.1111/j.1365-2591.2007.01248.x. [DOI] [PubMed] [Google Scholar]
- 18.Camilleri J. Characterization of hydration products of mineral trioxide aggregate. Int Endod J. 2008;41:408–17. doi: 10.1111/j.1365-2591.2007.01370.x. [DOI] [PubMed] [Google Scholar]
- 19.Camilleri J, Sorrentino F, Damidot D. Investigation of the hydration and bioactivity of radiopacified tricalcium silicate cement, Biodentine and MTA Angelus. Dent Mater. 2013;29:580–93. doi: 10.1016/j.dental.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 20.Camilleri J. Hydration characteristics of Biodentine and Theracal used as pulp capping materials. Dent Mater. 2014;30:709–15. doi: 10.1016/j.dental.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 21.Han L, Kodama S, Okiji T. Evaluation of calcium-releasing and apatite-forming abilities of fast-setting calcium silicate-based endodontic materials. Int Endod J. 2015;48:124–30. doi: 10.1111/iej.12290. [DOI] [PubMed] [Google Scholar]
- 22.Sohn S, Park Y, Srikanth S, Arai A, Song M, Yu B, et al. The role of ORAI1 in the odontogenic differentiation of human dental pulp stem cells. J Dent Res. 2015;94:1560–7. doi: 10.1177/0022034515608128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Camilleri J, Laurent P, About I. Hydration of Biodentine, Theracal LC, and a prototype tricalcium silicate-based dentin replacement material after pulp capping in entire tooth cultures. J Endod. 2014;40:1846–54. doi: 10.1016/j.joen.2014.06.018. [DOI] [PubMed] [Google Scholar]