Abstract
Background:
Manuka honey (MH) has been shown in vitro to be effective against biofilm-producing bacteria. This study assessed the effectiveness of MH for patients with active chronic rhinosinusitis (CRS) and prior sinus surgery.
Methods:
This prospective single-blinded (clinician only) randomized controlled trial recruited patients with active CRS and prior sinus surgery. Patients received either MH or saline (SAL) sinus irrigations twice daily for 30 days and were offered oral antibiotics and/or oral/topical steroids as indicated. Outcomes were 22-item Sino-Nasal Outcome Test (SNOT-22) change score (primary), culture negativity, and Lund-Kennedy endoscopic change score.
Results:
Forty-two patients were analyzed (MH, n = 20; SAL, n = 22). The SNOT-22 change score achieved a clinically significant improvement in both groups but was similar between MH (median [interquartile range]: −12 [−20, −1]) and SAL (−12.5 [−22, −6]) (p = 0.57). Culture negativity was better on MH (8/19, 42%) compared to SAL (4/21, 19%), nearing statistical significance (p = 0.11). Lund-Kennedy endoscopic change score improved in both groups but was not statistically better on MH (−3 [−5, 0]) compared to SAL (−1 [−2, 0]) (p = 0.20). For patients not receiving oral antibiotics/steroids, culture negativity was statistically better on MH (5/10, 50%) compared to SAL (0/6, 0%) (p = 0.04). MH was well-tolerated. No adverse events were reported.
Conclusion:
In patients with active CRS and prior sinus surgery, both MH and SAL improved outcomes, but there was no statistically significant difference between these groups. However, in the subset that did not receive oral antibiotics/steroids, culture negativity was statistically better on MH, suggesting that MH alone may be effective for acute exacerbations of CRS.
Keywords: biofilm, endoscopic sinus surgery, ESS, patient reported outcome measure, quality of life, SNOT-22, topical therapy for chronic rhinosinusitis
Chronic rhinosinusitis (CRS) is 1 of the most common chronic diseases, affecting 14% to 16% of the United States population.1 In addition to local sinonasal symptoms, such as obstruction, pressure, discharge, and poor smell function, CRS has broader negative implications for physical and social functioning.2 The standard treatment for CRS includes medical therapy, such as oral antibiotics and steroids, and endoscopic sinus surgery (ESS). Approximately 6% to 10% of CRS patients remain symptomatic despite medical therapy and multiple surgeries.3 These patients represent a significant and particularly problematic subset of patients with limited treatment options.
The persistence of pathogenic bacteria is believed to contribute to CRS. This persistence has been attributed to the propensity of these bacteria to form biofilms, well-organized structures of bacterial communities encased in a self-produced polymeric matrix tightly adherent to the sinonasal mucosa, which provide protection from environmental stresses and attack.3,4 Furthermore, they are thought to be a source of recurrent exacerbations via the periodic release of bacteria.5 Staphylococcus aureus and Pseudomonas aeruginosa are the most commonly implicated bacteria in patients with active CRS despite prior sinus surgery and have been associated with biofilm formation, fueling an interest in topical therapies that inhibit biofilm-forming bacteria and thus may be effective in the treatment of CRS.6-10
In patients who have undergone ESS and have open sinuses, topical administration allows for direct delivery to affected tissue, higher local concentrations, and less systemic absorption. High-volume irrigation appears to be more beneficial than other delivery methods.11 Topical antibiotics provide the ability to deliver intravenous antibiotics when compounded into a liquid or powder form that can be added to sinus irrigations. Recent studies have focused on the adjunctive use of mupirocin, which targets Staphylococcus aureus specifically, and demonstrated significant decreases in positive posttreatment culture rates but high relapse rates over time.12-14 Other studies have focused on topical antibiotics to target other biofilm-forming bacteria, such as Pseudomonas aeruginosa, with varying results.11 Recently, 1 study showed modest success in using topical antibiotics for CRS.15 However, given the small number of studies and concerns regarding relapse and development of resistance over time, the routine use of mupirocin and other topical antibiotics remains controversial.11 Alternative topical therapies that inhibit biofilm-forming bacteria are needed to treat CRS.
Manuka honey (MH) has long been used in the treatment of wounds. The mechanism has not been fully elucidated but is likely related to the high sugar content and therefore high osmolarity, acidity, and unique phytochemical compounds, such as methylglyoxal, all of which inhibit bacterial growth.16-19 Numerous in vitro studies have demonstrated a minimum inhibitory concentration (MIC), the lowest concentration that inhibits visible growth after overnight incubation, as low as 2% (vol/vol) for Staphylococcus aureus and 5.5% (vol/vol) for Pseudomonas aeruginosa.16,20-22 Variation does exist, with recent in vitro studies published in the rhinology literature determining MICs only as low as 22% (vol/vol).17,23 This variation may be related to differences in bacterial strains tested, storage time and conditions, and perhaps most significantly, honey sources. Unpublished in vitro pilot data (N. Sorrel, personal communication, July 28, 2014) has demonstrated a MIC of 7% (vol/vol) for the Medihoney® MH. Although increasing the concentration offers greater antibacterial effect, it decreases tolerability for patients primarily due to burning and stinging, presumably related to its acidity. From anecdotal experience (E. Leon, personal communication, July 16, 2014), a 10% (vol/vol) concentration is the upper limit of tolerability for patients while still remaining within the bactericidal range.
MH offers several advantages over topical antibiotics: (1) natural, nontoxic product with decreased likelihood of side effects and allergic reactions; (2) availability over-the-counter; (3) antibacterial effect against a wide range of bacteria, including those most commonly implicated in CRS, Staphylococcus aureus and Pseudomonas aeruginosa; and (4) decreased propensity for the development of resistant bacteria.18,22-24 Only 1 clinical study has evaluated MH for nasal use. This evaluation, however, was limited to allergic fungal sinusitis patients treated with a spray delivery device and lacked a control group.25
The purpose of our study was to determine whether MH sinus irrigations are more effective compared to saline sinus irrigation controls in the treatment of active CRS in patients who have had prior sinus surgery based on subjective and objective outcome measures. In addition, we sought to identify the effect size of MH for our outcome measures to better direct a larger future study. This is the first clinical study to evaluate MH in high-volume sinus irrigation form, and the first randomized controlled trial (RCT) to evaluate MH in any rhinologic context.
Patients and methods
Participants
This study was a prospective, single-blinded (clinician only) RCT conducted at the University of Washington Medical Center. Given the obvious difference in appearance between the honey and saline, and the need for subjects to mix the honey with the saline solution, subject blinding could not be performed. Ethics approval was granted by our local institutional board. Patients were approached for this study between November 2015 and August 2016 and considered for inclusion if they met diagnostic criteria for CRS (as defined by the American Academy of Otolaryngology–Head and Neck Surgery 2015 clinical practice guidelines), had undergone ESS >6 weeks prior to enrollment, and had purulent discharge on nasal endoscopy.26 Exclusion criteria were <18 years of age, terminal illness or significant immune dysfunction, severe or emergent complications from CRS or presence of a sinus tumor, unwillingness to stop topical antibiotic sinus irrigations if receiving them, and allergy to honey. Patients with cystic fibrosis were enrolled in a parallel trial and reported elsewhere. They were excluded from this study.
Randomization and intervention
Patients were block randomized in sets of 6 to receive either 10% (vol/vol) MH (Medihoney®; Derma Sciences; Princeton, NJ) or saline (SAL) control sinus irrigations for 30 days. A blocked randomization list was generated prior to the start of the study using an electronic random number generator tool (Sealed Envelope Ltd, London, UK). When a new patient was enrolled, the member of the research team (V.S.L., J.E.) communicating with the patient was informed of the next assigned irrigation treatment on the list.
Patients were provided with supplies and instructions to make the assigned irrigation treatment at home (Table 1). They were instructed to irrigate with one-half of the bottle twice daily for 30 days. To minimize the possibility of bacterial contamination, patients were instructed to use previously boiled or distilled water, clean the rinse bottle at the end of each day, and use 1 bottle for 15 days and a new bottle for the next 15 days. Each patient was also offered a medical treatment regimen for an acute exacerbation of CRS, consisting of a culture-directed oral antibiotic for up to 3 weeks, and/or oral steroids depending on the presence of polyps/inflammation for up to 3 weeks, and/or high-volume topical steroid sinus irrigations (budesonide 0.5 mg/2 mL vial or 0.6 mg/2 mL capsule, 0.5 bottle to each nasal cavity twice daily) depending on the presence of polyps or inflammation for 30 days.26,27 The dose of budesonide was determined when the patient filled the prescription; if insurance covered it, then the vial version at the 0.5 mg/2 mL dose was used, and if not covered, then the capsule version at the 0.6 mg/2 mL dose was used.
TABLE 1.
Recipe for irrigation treatments
| Treatment | Supplies | Instructions |
|---|---|---|
| 10% Manuka honey | 2 rinse bottlesa Buffered salt packeta Manuka honey pasteb Syringe |
Mix 24 mL manuka honey paste (measured using syringe) with buffered salt packet and 240 mL of waterc in rinse bottle |
| Saline control | 2 rinse bottlesa Buffered salt packeta |
Mix buffered salt packet with 240 mL of waterc in rinse bottle |
NeilMed Pharmaceuticals (Santa Rosa, CA).
Medihoney®, Derma Sciences (Princeton, NJ).
Previously boiled or distilled.
Outcomes
The primary outcome was the 22-item Sino-Nasal Outcome Test score (SNOT-22, consisting of 22 items, each scored from 0 to 5; total score recorded as the sum of all items, 0 to 110) change from baseline.28 A secondary outcome was posttreatment culture negativity, defined as “negative” if no bacterial pathogen was identified on posttreatment culture but at least 1 had been present on pretreatment culture. Cultures were collected with 30-degree rigid endoscopic visualization using a sterile alligator forceps and culture swab (Becton, Dickinson and Company, Franklin Lakes, NJ) or Xomed Sinus Secretion Collector (Medtronic-Xomed, Jacksonville, FL). Another secondary outcome was Lund-Kennedy endoscopic score (consisting of 10 items, each scored from 0 to 2; total score recorded as the sum of all items, 0 to 20) change from baseline.29 Discomfort associated with the sinus irrigations was measured using a visual analog scale (VAS, continuous scale comprised of a 100-mm horizontal line bounded by no pain [0] to worst imaginable pain [100]). Patients were also asked to complete a compliance diary to assess if they were performing the irrigations per the instructed schedule, which was used to calculate the percentage actually received of total scheduled treatments.
Sample size
We powered our study to detect a 9-point difference in the SNOT-22 score change from baseline between the MH and SAL groups, which is considered clinically significant.28 With a power of 0.80, significance level of 0.05, and standard deviation of 10, our desired enrollment was 42 patients to complete the study.
Data collection
The pretreatment time point was defined as the clinic visit at time of enrollment. Descriptive characteristics, including demographics (age, sex, and race), Lund-Mackay computed tomography (CT) score (for the scan closest to the treatment period), relevant comorbidities (nasal polyposis, asthma, inhalant allergies, aspirin sensitivity, and current smoker), concurrent therapies (oral antibiotics or steroids and topical steroid sinus irrigations), whether or not multiple surgeries had been done, and time to follow-up, were recorded.30 At this visit, patients were asked to fill out a SNOT-22 questionnaire, and the score was recorded. A nasal endoscopy was performed, an endoscopically-collected sinus culture obtained, and the culture result recorded. A member of the research team (G.E.D.), blinded to the assigned irrigation treatment, determined the Lund-Kennedy endoscopic score.
Following 30 days of treatment, patients returned to the clinic for assessment. The SNOT-22 score, culture result, and Lund-Kennedy endoscopic score were recorded in an analogous fashion. Patients were also asked to complete a VAS form and bring in their compliance diaries, and the results were recorded.
Statistical analysis
Statistical analyses were performed using Stata/IC 13.1 software (StataCorp LP, College Station, TX). Distribution and summary statistics were evaluated for descriptive characteristics, VAS scores, and compliance results. Data were examined for normality prior to hypothesis testing. To assess for inadequate randomization of known confounders and the need for adjusted analyses of outcome data, a chi-square test for binary variables and a Mann-Whitney U test for continuous variables were performed evaluating differences in descriptive characteristics between treatment groups.
For the unadjusted analysis, comparisons for SNOT-22 score and Lund-Kennedy endoscopic score changes from baseline between the treatment groups were performed using a Mann-Whitney U test. Comparisons for posttreatment culture negativity were performed using a chi-square test. For the adjusted analysis, linear regression was performed to adjust for significantly different descriptive characteristics for the outcomes of SNOT-22 score and Lund-Kennedy endoscopic score changes from baseline. Logistic regression was performed to adjust for significantly different descriptive characteristics for the outcome of posttreatment culture negativity. Median and interquartile range (IQR) are presented unless otherwise specified. A p value <0.05 was considered significant.
Results
Study overview
Participant flow is shown in Figure 1. A total of 49 patients were randomized in this study. In the MH group (n = 25), 3 patients were lost to follow-up and 2 patients had worsening symptoms and requested switching to a culture-directed topical antibiotic (1 mupirocin and the other tobramycin), hence were excluded from the study. In the SAL group (n = 24), 2 patients were lost to follow-up. As the 2 treatment discontinuations were patients that switched to a topical antibiotic rather than simply stopping the assigned treatment, an intention-to-treat analysis was deemed unfavorable as the outcome data would not solely reflect MH. Therefore, these patients in addition to the ones lost to follow-up were excluded, and a per-protocol analysis was performed.
FIGURE 1.
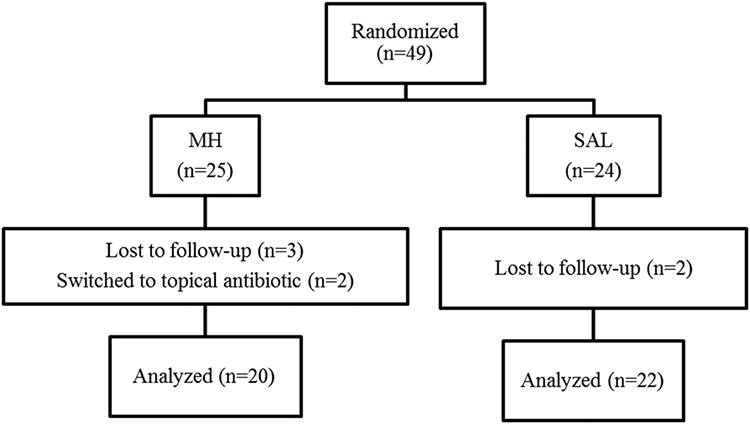
Participant flow. MH = manuka honey; SAL = saline.
Descriptive characteristics
A total of 42 patients were included in the analysis. Table 2 shows descriptive characteristics and p values for the differences in these descriptive characteristics between treatment groups. Significant differences in Lund-Mackay CT score and presence of asthma were found between treatment groups, indicating that randomization may have been inadequate for these descriptive characteristics.
TABLE 2.
Baseline patient demographics and clinical data (n = 42)*
| MH (n = 20) | SAL (n = 22) | P | |
|---|---|---|---|
| Age (years), median (IQR) | 54 (51–64) | 54 (39–61) | 0.38 |
| Female, n | 11 (55) | 14 (64) | 0.57 |
| White, n | 19 (95) | 16 (73) | 0.06 |
| Lund-Mackay CT scorea | 8 (5–12) | 11 (7–17) | 0.05* |
| Lund-Kennedy endoscopy score | 7 (5–9) | 8 (5–10) | 0.74 |
| Nasal polyposis, n | 5 (25) | 10 (45) | 0.17 |
| Asthma, n | 7 (35) | 15 (68) | 0.03* |
| Inhalant allergies, n | 10 (50) | 8 (36) | 0.38 |
| Aspirin sensitivity, n | 1 (5) | 4 (18) | 0.19 |
| Current smoker, n | 0 (0) | 0 (0) | 1.00 |
| Oral antibiotics, n | 10 (50) | 15 (68) | 0.23 |
| Oral steroids, n | 2 (10) | 5 (23) | 0.27 |
| Topical steroid sinus irrigations, n | 15 (75) | 19 (86) | 0.35 |
| Multiple surgeries, n | 5 (25) | 8 (36) | 0.43 |
| Time to follow-up (days), median (IQR) | 37 (31–41) | 38 (30–43) | 0.70 |
Median (IQR) or n (%) of patients is presented.
Radiologic grading of sinus systems, consisting of 6 items for each nasal cavity, each scored from 0 to 2; total score recorded as the sum of all items, 0 to 24. CT = computed tomography; IQR = interquartile range; MH = manuka honey, SAL = saline.
Outcome measures
The covariates in the adjusted analysis were determined from post hoc assessment of imbalance in descriptive characteristics, which may have introduced bias. Therefore, the primary analysis of the outcome data was determined a priori to be the per-protocol unadjusted analysis. A SNOT-22 score with a negative change value represents an improvement, and a more negative value is a better response. A change of −9 is considered a clinically significant improvement.28 Although there was a clinically significant improvement in both groups, SNOT-22 score change from baseline was similar between the MH (median [IQR]: −12 [−20, −1]) and SAL (− 12.5 [−22, −6]) groups (p = 0.57; Fig. 2).
FIGURE 2.
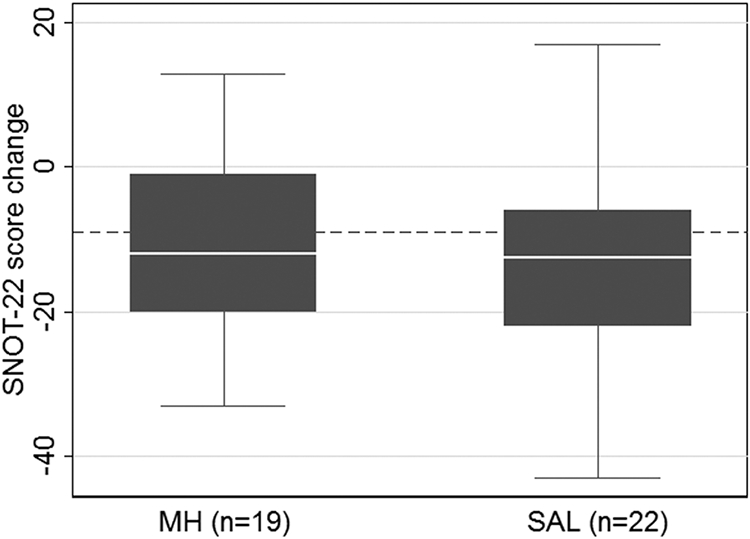
Box plot comparison of SNOT-22 score change from baseline (p = 0.57). Dotted line indicates change of −9, considered a clinically significant improvement. Missing outcome data related to difficulty with clinic logistics and inability to collect data: post-SNOT-22 score in MH group (n = 1). MH = manuka honey; SAL = saline; SNOT-22 = 22-item Sino-Nasal Outcome Test.
A higher posttreatment culture negativity rate is a better response. Posttreatment culture negativity was better on MH (8/19, 42%) compared to SAL (4/21, 19%), but did not quite reach significance (p = 0.11; Fig. 3). Table 3 shows the pretreatment and posttreatment culture results by type of bacterial species for the MH and SAL groups. There was a greater improvement in the proportion of patients with positive Pseudomonas, Streptococcus, and other gram-positive bacteria cultures in the MH group compared to the SAL group. Conversely, there was a greater improvement in the proportion of patients with positive Staphylococcus and other gram-negative bacteria cultures in the SAL group compared to the MH group.
FIGURE 3.
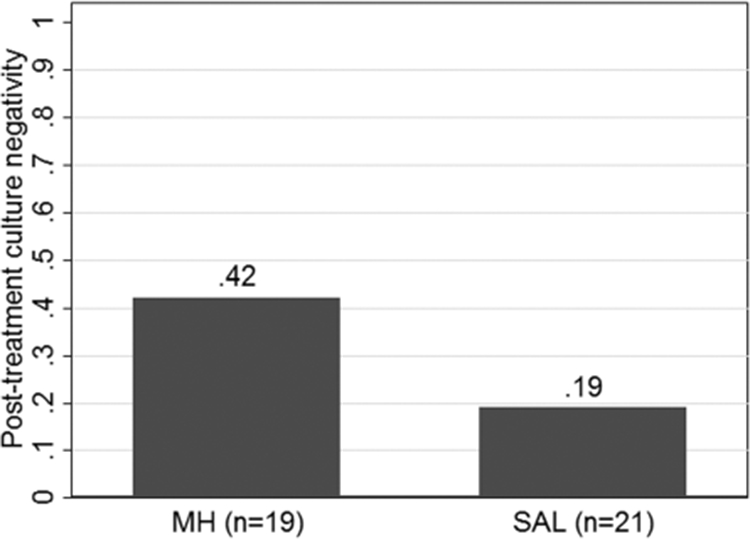
Bar plot comparison of posttreatment culture negativity (p = 0.11). Proportion of negative posttreatment cultures is provided for each treatment group above the respective bar. Missing outcome data related to difficulty with clinic logistics and inability to collect data: pretreatment culture in MH group (n = 1), posttreatment culture in SAL group (n = 1). MH = manuka honey, SAL = saline.
TABLE 3.
Pretreatment and posttreatment culture results by bacterial species*
| MH (n = 20) | SAL (n = 22) | |||
|---|---|---|---|---|
| Pretreatment | Posttreatment | Pretreatment | Posttreatment | |
| Staphylococcus | 8 (40) | 6 (30) | 9 (41) | 3 (14) |
| Pseudomonas | 4 (20) | 3 (15) | 4 (18) | 5 (23) |
| Streptococcus | 4 (20) | 2 (10) | 3 (14) | 5 (23) |
| Other gram-positive speciesa | 1 (5) | 1 (5) | 1 (5) | 2 (9) |
| Other gram-negative speciesb | 8 (40) | 7 (35) | 8 (36) | 5 (23) |
Values are n (%).
Corynebacterium, Microbacterium.
Serratia, Bacillus, Haemophilus, Stenotrophomonas, Achromobacter, Moraxella, Acinetobacter, Ochrobactrum, Enterobacter, Pantoea, Citrobacter.
A Lund-Kennedy endoscopic change score with a negative value is an improvement, and a more negative value is a better response. Although there was improvement in both groups, Lund-Kennedy endoscopic score change from baseline was not significantly better on MH (−3 [−5, 0]) compared to SAL (−1 [−2, 0]) (p = 0.20; Fig. 4). In the per-protocol adjusted analysis for Lund-Mackay CT score and presence of asthma, SNOT-22 score change from baseline (p = 0.33), posttreatment culture negativity (p = 0.40), and Lund-Kennedy endoscopic score change from baseline (p = 0.21) were not significantly different between the treatment groups.
FIGURE 4.
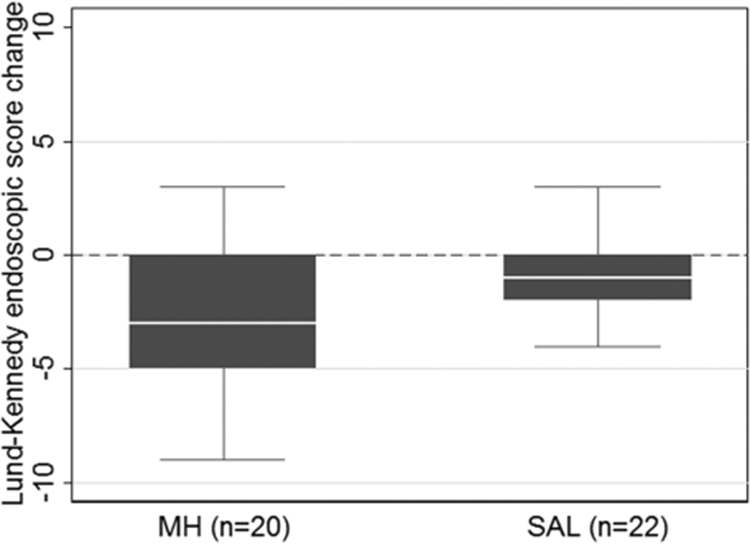
Box plot comparison of Lund-Kennedy endoscopic score change from baseline (p = 0.20). Dotted line indicates change of 0, below which is considered an improvement. MH = manuka honey, SAL = saline.
Subgroup analysis: patients not receiving oral antibiotics or oral steroids
Although randomization theoretically equally distributes patients receiving systemic therapy between the treatment groups, we were interested in a subgroup analysis excluding these patients to ensure testing the impact of MH alone. Concern for enrollment issues prevented us from performing a comparison of MH to SAL without offering any additional systemic therapy. Oral antibiotics and, in certain patients, oral steroids may be considered standard of care for acute exacerbations of CRS. There was, however, a population of subjects who chose not to receive oral antibiotics or oral steroids (n = 16; MH, n = 10; SAL, n = 6).
SNOT-22 score change from baseline was similar in the MH (−3.5 [−13, 4]) and SAL (−5.5 [−18, −3]) groups, and this was not significant (p = 0.70). Posttreatment culture negativity was better on MH (5/10, 50%) compared to SAL (0/6, 0%), and this was significant (p = 0.04). Lund-Kennedy endoscopic score change from baseline was similar in the MH (−2 [−5, 2]) and SAL (−1.5 [−4, 0]) groups, and this was not significant (p = 0.91). Overall, these results were similar to the full analysis with the exception of posttreatment culture negativity, which was significantly better in the MH group in this subgroup analysis.
Tolerability, adverse effects, compliance
Median VAS scores were similar in both treatment groups (MH 12.5 [0, 35] vs SAL 4 [0, 22]), indicating irrigations were well-tolerated. Only 3 patients experienced mild irritation with the MH irrigation. No serious adverse effects were reported. Median compliance rates were also excellent in both treatment groups (MH 100% [90, 100] vs SAL 100% [95, 100]). Three patients in the MH group noted substantial hassle associated with self-compounding the formula as the honey paste was quite viscous.
Discussion
This randomized trial compared topical MH irrigations with saline irrigations as an adjuvant treatment for active CRS in patients who had undergone prior ESS. Both objective posttreatment culture and endoscopic outcomes were better in the MH cohort compared to saline cohort. These differences, however, did not achieve significance. Our study was powered to detect a clinically significant improvement in the SNOT-22 score and therefore may have been underpowered to detect significance in these other outcomes. In addition, the patients in our study may represent a more challenging subset of CRS given that this study occurred at a tertiary rhinology center. Some subjects had comorbid nasal polyposis, asthma, inhalant allergies, and aspirin sensitivity; they typically had received multiple courses of oral antibiotics and oral steroids, and they had to be suffering from an acute exacerbation of CRS despite successful sinus surgery, defined by patent ostia, for inclusion in this study.
In addition, although the SAL group performed relatively poorly, with only a 19% posttreatment culture negativity rate and only a 1-point improvement in the median Lund-Kennedy endoscopic score, the trend was overall improvement compared to the pretreatment baseline. Patients in both arms of the study were offered oral antibiotics and/or oral steroids, as for this initial study we were concerned about enrollment issues with withholding systemic therapy in addition to withholding topical antibiotics for patients with an acute exacerbation of CRS. Now that we understand there is benefit with MH irrigations, future studies may be designed that do not offer systemic therapies during the trial. However, patients may still be less willing to enroll if there is a possibility of receiving only saline sinus irrigations.
Randomization ideally evenly distributes patients receiving systemic therapy between treatment groups. Based on the lack of a significant difference in the proportion of patients receiving oral antibiotics or oral steroids between the treatment groups, the randomization was determined to be adequate. An adjusted analysis therefore was not needed. An additional caveat required to eliminate systemic therapy as a confounder, however, is that its impact is the same in both treatment groups. In our subgroup analysis of patients who did not receive oral antibiotics or steroids, the MH group achieved significantly better culture negativity rates. This finding suggests that, particularly with regard to culture outcome, MH may have benefits and that the relative importance of systemic therapy may be higher in patients receiving saline irrigations alone. The relatively higher benefit of systemic therapy in the SAL group, coupled with the more modest performance of the MH group, may have contributed to the lack of a statistical difference. Considering these observations, these results show promise for MH as an adjuvant treatment for active CRS in patients who have had prior sinus surgery if success is based on culture and perhaps endoscopic outcomes. The key is access to the sinuses. Not only does a patient need to have had sinus surgery, their sinus ostia need to be patent and not obstructed with scar tissue or nasal polyps.
When the culture results were analyzed by bacterial species, an interesting observation was that the MH group appeared to be better at achieving culture negativity for certain species, specifically Pseudomonas, Streptococcus, and non-Staphylococcus/Streptococcus gram-positive bacteria. This suggests that MH may be more effective at eliminating certain bacteria, which is worthy of further investigation, particular in cystic fibrosis patients for which Pseudomonas is prevalent.
Regarding our primary outcome measure, the SNOT-22 score changes from baseline were similar between the treatment groups, suggesting that with regard to patient-reported quality of life, MH may not have much additional benefit compared to saline sinus irrigations. SNOT-22 scores may be less responsive to treatment or perhaps this study was over too short a time interval to observe an improvement in subjective outcomes. Future studies should consider longer follow-up intervals to further evaluate quality of life measures. Interestingly, the SNOT-22 score changes from baseline were clinically significant in both treatment groups, indicating that an aggressive regimen of oral antibiotics, oral or topical steroids, and topical therapy, regardless of whether or not it is effective against biofilm-producing bacteria, has symptomatic benefit.28 In summary, our study suggests that patients prescribed MH should be counseled that there may be greater benefits observed with regard to microbiological and endoscopic outcome, which may not necessarily be reflected in their quality of life.
A foreseeable limitation of MH use is its cost. We used Medhioney® produced by Derma Sciences, which has a base unit cost of $26/tube and is often more expensive when sold on an individual basis. A 30-day treatment requires 8 tubes, and therefore the estimated cost is at least $208. This can be a significant sum for some patients and is not covered by insurance. We used this particular brand because it is a “medical-grade” MH. The company describes “medical-grade” as meeting a rigorous set of standards including the following quality control measures: demonstration of product consistency from batch-to-batch, sterilization by gamma irradiation, destroying any bacterial spores without loss of product effectiveness, and coming from traceable source that is free of pesticides and antibiotics. Other brands are not “medical-grade” but are cheaper and available from a wide variety of distributors. Certainly, it may be worthwhile for future studies to evaluate less expensive options if cost proves to be a limiting factor. It may also be useful to explore the development of a sinus irrigation formula so that patients do not have to self-compound it. The effectiveness of using a non-medical grade form of MH, however, cannot be extrapolated from this study. Furthermore, non-medical grade MH could conceivably contain contaminants or infectious spores as can be found (uncommonly) in any source of standard honey.
Our study has limitations. As previously mentioned, although this is the first study investigating MH sinus irrigations in the treatment of CRS, it is now clear that it was underpowered to detect smaller effect sizes. As previously mentioned with regard to receiving systemic therapies, the purpose of randomization is to minimize confounding by equally distributing confounders among the treatment groups, but with smaller sample sizes, there is the risk of inadequate randomization and residual confounding. We did assess for significant differences in known confounders among the treatment groups, though there may be unknown confounders that we did not account for. Furthermore, it was not possible to blind patients to the irrigation treatment as MH differs from saline in appearance and smell. This lack of blinding may have biased the self-reported SNOT-22 score. There is also no agreed upon frequency or dosing for MH sinus irrigations. The concentration used in our study was based on in vitro studies and tolerability data, but the data is varied (N. Sorrel, personal communication, July 28, 2014; E. Leon, personal communication, July 16, 2014).16-23 Finally, the results may not be generalizable to patients with CRS as a whole. As a tertiary rhinology practice, these patients represent a more comorbid population. The advantage of this, however, is that our study may have captured CRS patients with more difficult to treat disease, and that the more modest results observed in this population may be greater in a population with less severe disease.
Conclusion
This initial novel study tested MH vs SAL sinus irrigations in patients with active CRS who had prior sinus surgery. In this relatively small sample size, MH sinus irrigations did not statistically improve culture negativity rates, endoscopic findings, or quality of life compared to SAL controls; however, there was a trend suggesting MH may improve culture negativity. Interestingly, culture negativity rates were significantly better in the MH compared to SAL group when systemic therapies including oral antibiotics and steroids were not utilized, suggesting that MH by itself may be an effective treatment for acute exacerbations of CRS. Future studies should be more conservatively powered to detect smaller effect sizes, employ longer follow-up intervals when assessing quality of life outcomes, explore cost issues affecting compliance, and evaluate optimal frequency and dosing. In addition, study designs withholding systemic therapies for a shorter period of time and/or treating with topical therapies for a longer period of time are warranted to assess the true impact of topical therapies.
Acknowledgments
We acknowledge Carolyn Bea and Jane Edelson, our research coordinators, for their work in obtaining Institutional Review Board approval for this study and coordinating study logistics, and the Otolaryngology Outcomes Research Group, for valuable feedback. This study was supported by Derma Sciences, which solely provided financial and product support; Derma Sciences was not involved in any other aspect of this study, including design, analysis, and reporting of results.
Funding sources for the study:
Derma Sciences.
Footnotes
Potential conflict of interest: None provided.
Podium presentation at the Annual ARS meeting on September 17, 2016, in San Diego, CA.
References
- 1.Rosenfeld RM. Clinical practice guideline on adult sinusitis. Otolaryngol Head Neck Surg. 2007;137:365–377. [DOI] [PubMed] [Google Scholar]
- 2.Rosenfeld RM, Andes D, Bhattacharyya N, et al. Clinical practice guideline: adult sinusitis. Otolaryngol Head Neck Surg. 2007;137:S1–S31. [DOI] [PubMed] [Google Scholar]
- 3.Fokkens WJ, Lund VJ, Mullol J, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinol Suppl. 2012;(23):3 p preceding table of contents, 1–298. [PubMed] [Google Scholar]
- 4.Ramadan HH, Sanclement JA, Thomas JG. Chronic rhinosinusitis and biofilms. Otolaryngol Head Neck Surg. 2005;132:414–417. [DOI] [PubMed] [Google Scholar]
- 5.Palmer JN. Bacterial biofilms: do they play a role in chronic sinusitis? Otolaryngol Clin North Am. 2005;38:1193–1201, viii. [DOI] [PubMed] [Google Scholar]
- 6.Suh JD, Cohen NA, Palmer JN. Biofilms in chronic rhinosinusitis. Curr Opin Otolaryngol Head Neck Surg. 2010;18:27–31. [DOI] [PubMed] [Google Scholar]
- 7.Suh JD, Ramakrishnan V, Palmer JN. Biofilms. Otolaryngol Clin North Am. 2010;43:521–530, viii. [DOI] [PubMed] [Google Scholar]
- 8.Sanderson AR, Leid JG, Hunsaker D. Bacterial biofilms on the sinus mucosa of human subjects with chronic rhinosinusitis. Laryngoscope. 2006;116:1121–1126. [DOI] [PubMed] [Google Scholar]
- 9.Prince AA, Steiger JD, Khalid AN, et al. Prevalence of biofilm-forming bacteria in chronic rhinosinusitis. Am J Rhinol. 2008;22:239–245. [DOI] [PubMed] [Google Scholar]
- 10.Foreman A, Psaltis AJ, Tan LW, Wormald PJ. Characterization of bacterial and fungal biofilms in chronic rhinosinusitis. Am J Rhinol. 2009;23:556–561. [DOI] [PubMed] [Google Scholar]
- 11.Rudmik L, Hoy M, Schlosser RJ, et al. Topical therapies in the management of chronic rhinosinusitis: an evidence-based review with recommendations. Int Forum Allergy Rhinol. 2013;3:281–298. [DOI] [PubMed] [Google Scholar]
- 12.Jervis-Bardy J, Boase S, Psaltis A, et al. A randomized trial of mupirocin sinonasal rinses versus saline in surgically recalcitrant staphylococcal chronic rhinosinusitis. Laryngoscope. 2012;122:2148–2153. [DOI] [PubMed] [Google Scholar]
- 13.Jervis-Bardy J, Wormald PJ. Microbiological outcomes following mupirocin nasal washes for symptomatic, Staphylococcus aureus–positive chronic rhinosinusitis following endoscopic sinus surgery. Int Forum Allergy Rhinol. 2012;2:111–115. [DOI] [PubMed] [Google Scholar]
- 14.Uren B, Psaltis A, Wormald PJ. Nasal lavage with mupirocin for the treatment of surgically recalcitrant chronic rhinosinusitis. Laryngoscope. 2008;118:1677–1680. [DOI] [PubMed] [Google Scholar]
- 15.Lee VL, Davis GE. Culture-directed topical antibiotic treatment for chronic rhinosinusitis. Am J Rhinol. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooper RA, Molan PC, Harding KG. The sensitivity to honey of gram-positive cocci of clinical significance isolated from wounds. J Appl Microbiol. 2002;93:857–863. [DOI] [PubMed] [Google Scholar]
- 17.Jervis-Bardy J, Foreman A, Bray S, et al. Methylglyoxal-infused honey mimics the anti-Staphylococcus aureus biofilm activity of manuka honey: potential implication in chronic rhinosinusitis. Laryngoscope. 2011;121:1104–1107. [DOI] [PubMed] [Google Scholar]
- 18.Lu J, Turnbull L, Burke CM, et al. Manuka-type honeys can eradicate biofilms produced by Staphylococcus aureus strains with different biofilm-forming abilities. PeerJ. 2014;2:e326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kilty SJ, Duval M, Chan FT, et al. Methylglyoxal: (active agent of manuka honey) in vitro activity against bacterial biofilms. Int Forum Allergy Rhinol. 2011;1:348–350. [DOI] [PubMed] [Google Scholar]
- 20.Cooper RA, Molan PC, Harding KG. Antibacterial activity of honey against strains of Staphylococcus aureus from infected wounds. J R Soc Med. 1999;92:283–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lusby PE, Coombes AL, Wilkinson JM. Bactericidal activity of different honeys against pathogenic bacteria. Arch Med Res. 2005;36:464–467. [DOI] [PubMed] [Google Scholar]
- 22.Molan PC. The evidence supporting the use of honey as a wound dressing. Int J Low Extrem Wounds. 2006;5:40–54. [DOI] [PubMed] [Google Scholar]
- 23.Alandejani T, Marsan J, Ferris W, et al. Effectiveness of honey on Staphylococcus aureus and Pseudomonas aeruginosa biofilms. Otolaryngol Head Neck Surg. 2009;141:114–118. [DOI] [PubMed] [Google Scholar]
- 24.Kilty SJ, AlMutairi D, Duval M, et al. Manuka honey: histological effect on respiratory mucosa. Am J Rhinol. 2010;24:e63–e66. [DOI] [PubMed] [Google Scholar]
- 25.Thamboo A, Philpott C, Javer A, et al. Single-blind study of manuka honey in allergic fungal rhinosinusitis. J Otolaryngol Hed Neck Surg. 2011;40:238–243. [PubMed] [Google Scholar]
- 26.Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. Clinical practice guideline (update): Adult Sinusitis Executive Summary. Otolaryngol Head Neck Surg. 2015;152:598–609. [DOI] [PubMed] [Google Scholar]
- 27.Head K, Chong LY, Piromchai P, et al. Systemic and topical antibiotics for chronic rhinosinusitis. Cochrane Database Syst Rev. 2016;4:CD011994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hopkins C, Gillett S, Slack R, et al. Psychometric validity of the 22-item Sinonasal Outcome Test. Clin Otolaryngol. 2009;34:447–454. [DOI] [PubMed] [Google Scholar]
- 29.Lund VJ, Kennedy DW. Staging for rhinosinusitis. Otolaryngol Head Neck Surg. 1997;117:S35–S40. [DOI] [PubMed] [Google Scholar]
- 30.Lund VJ, Mackay IS. Staging in rhinosinusitus. Rhinology. 1993;31:183–184. [PubMed] [Google Scholar]


