Abstract
We recently reported that strain EN1252a, a fluoroquinolone-resistant derivative of Staphylococcus aureus NCTC8325 with mutations in grlA and gyrA, expressed increased levels of fibronectin-binding proteins (FnBPs) and showed a significantly higher attachment to fibronectin-coated polymer surfaces after growth in the presence of subinhibitory concentrations of ciprofloxacin. The present study evaluated the occurrence and frequency of fluoroquinolone-induced FnBP-mediated adhesion in clinical isolates of fluoroquinolone-resistant methicillin-resistant S. aureus (MRSA) and methicillin-susceptible S. aureus (MSSA). Eight of ten MRSA isolates and four of six MSSA isolates with grlA and gyrA mutations exhibited significant increases in attachment to fibronectin-coated surfaces after growth in the presence of one-quarter the MIC of ciprofloxacin. Fluoroquinolone-induced FnBP-mediated adhesion of one clinical MRSA strain and the double mutant strain EN1252a also occurred on coverslips removed from the subcutaneous space of guinea pigs. For strain EN1252a, the regulation of fnb transcription by sub-MICs of ciprofloxacin was studied on reporter plasmids carrying fnb-luxAB fusions. One-quarter of the MIC of ciprofloxacin significantly increased fnbB, but not fnbA, promoter activity of the fluoroquinolone-resistant mutant but not its fluoroquinolone-susceptible parent ISP794. This response was abolished by pretreatment with rifampin, indicating an effect at the level of transcription. Activation of the fnbB promoter was not due to an indirect effect of ciprofloxacin on growth rate and still occurred in an agr mutant of strain EN1252a. These data suggest that sub-MIC levels of ciprofloxacin activate the fnbB promoter of some laboratory and clinical isolates, thus contributing to increased production of FnBP(s) and leading to higher levels of bacterial attachment to fibronectin-coated or subcutaneously implanted coverslips.
The ability of Staphylococcus aureus to recognize several extracellular matrix or plasma proteins that coat indwelling devices promotes bacterial attachment to these artificial surfaces. Clinical and laboratory isolates of S. aureus and many other bacterial species express surface proteins called MSCRAMM (microbial surface components recognizing adhesive matrix molecules) that specifically recognize each of the host matrix or plasma components (37). S. aureus fibronectin-binding activity is exerted by two closely related fibronectin-binding proteins (FnBPs) encoded by two adjacent genes called fnbA and fnbB (11, 19). The structural genes coding for FnBPs are partly redundant since both of them need to be inactivated to suppress bacterial interaction with fibronectin (14).
Previous studies indicate that some cell wall-associated proteins, such as protein A and FnBP(s), may be regulated by the interaction of two global regulons called agr and sar (4, 5, 6, 33). While agr is known to downregulate fnb genes during the postexponential phase (41, 44), the sar locus seems to exert an opposite upregulating effect during the exponential phase, as documented by knockout mutations in each of these regulatory genes (A. L. Cheung and C. Wolz, Abstr. 37th Int. Conf. Antimicrob. Agents Chemother, session 77B, 1997).
Expression of surface proteins may also be affected by exposure to a variety of additional internal and external signals and regulatory factors. Subinhibitory concentrations of antibiotics may downregulate or upregulate specific adhesins as well as other virulence-associated determinants of S. aureus such as FnBPs (39, 40), collagen-binding protein (2), or alpha-toxin (21, 35). On the other hand, the acquisition of single or multiple antibiotic resistance determinants by S. aureus may also modulate expression of surface proteins. In a recent study, we found that introduction of the methicillin resistance determinant into the laboratory strains 8325 and Newman of S. aureus altered the functional expression of their fibrinogen ClfA adhesin and to some extent FnBPs (56).
Shortly after the introduction of the fluoroquinolones into clinical practice, strains expressing resistance to these compounds were detected, in particular among isolates of methicillin-resistant S. aureus (MRSA), which frequently exhibit multiple additional resistance determinants to unrelated antimicrobial agents (51). Fluoroquinolone resistance in S. aureus is chromosomally mediated and commonly associated with point mutations in two intracellular targets: (i) site-specific amino acid substitutions in GrlA (topoisomerase IV, A subunit) found at codons 80 (Ser→Phe or Tyr), 84 (Glu→Lys), and 116 (Ala→Glu or Pro) (9, 10, 25, 32, 48, 52, 59) and/or amino acid substitutions in GrlB (B subunit) (12, 45, 46); and (ii) site-specific amino acid substitutions in GyrA (DNA gyrase, A subunit) found at codons 84 (Ser→Leu) and 88 (Glu→Lys) (9, 18, 31, 32, 38, 49, 50) and/or amino acid substitutions in GyrB (B subunit) (18, 46). Combined mutations in grlA and gyrA confer much higher levels of resistance than single grlA mutations (9, 10, 32). Finally, fluoroquinolone resistance may also result from increased norA-mediated efflux of these antimicrobial agents by the flqB mutation (20, 30, 32, 36, 49).
We recently reported that an isogenic grlA gyrA double mutant of S. aureus grown in the presence of subinhibitory concentrations of ciprofloxacin expressed increased levels of FnBPs and showed a significantly higher attachment to fibronectin-coated polymer surfaces (1). This phenomenon was selectively expressed in fluoroquinolone-resistant grlA gyrA double mutants, but not in either grlA and gyrA single mutants or susceptible parental strains. The aims of this report were: (i) to document the occurrence and frequency of increased FnBP-mediated adhesion in a pilot sample of both methicillin-resistant and methicillin-susceptible quinolone-resistant clinical isolates grown in the presence of subinhibitory concentrations of ciprofloxacin, (ii) to evaluate how quinolone-induced overexpression of FnBPs may contribute to increased attachment of S. aureus to subcutaneously implanted polymer surfaces, and (iii) to study whether sub-MICs of ciprofloxacin might alter transcription of each fnb gene in quinolone-resistant S. aureus by using luciferase fnb-luxAB reporter plasmids.
MATERIALS AND METHODS
Bacterial strains.
The properties of 16 fluoroquinolone-resistant clinical isolates of S. aureus, collected from the University Hospital of Geneva, Geneva, Switzerland, the Massachusetts General Hospital, or the Pasteur Institute (Paris, France), are listed in Table 1. Ten isolates were methicillin-resistant (MRSA) and six were methicillin-susceptible S. aureus (MSSA). Five MRSA isolates from Geneva, collected in February 1994, and one MRSA from Boston were associated with clinically documented infections. Four MRSA isolates from the Pasteur Institute (kindly provided by N. El Solh) were collected from three separate areas in 1989 and 1990 and characterized by an unusual streptogramin resistance pattern recovered in several patients during a short period of time. The presence of this characteristic antibiotic resistance pattern suggested an epidemiological mode of transmission for each of these four MRSA isolates. All six quinolone-resistant MSSA isolates were collected in Boston in 1993 and 1994 and were not epidemiologically linked, and five of them were associated with infections.
TABLE 1.
Clinical strains used in the study
| Strain | Antibiotic resistance patterna | Amino acid change(s) in:
|
Origin | MIC (μg/ml) of ciprofloxacin | % Increase in adhesionb | |
|---|---|---|---|---|---|---|
| GrlA | GyrA | |||||
| MRSA | ||||||
| 4613 | Mcr Gmr Fosr Cipr | S80F, E84K | E88K | Geneva | 64 | 149 |
| 4673 | Mcr Gmr Cipr | S80F, S81P | S84L, E88A | Geneva | 128 | 5 |
| 4753 | Mcr Gmr Fosr Cipr | S80F, S81P | S84L | Geneva | 128 | 17 |
| 4718 | Mcr Gmr Cipr | S80F | S84L, E88A | Geneva | 128 | 61 |
| 4623 | Mcr Gmr Cipr | S80F | E88K | Geneva | 128 | 104 |
| 95067 | Mcr Gmr Fosr SgAr Cipr | S80F | S84A | Paris | 16 | 353 |
| BM10762 | Mcr Gmr Fosr SgAr SgBr Rfr Far Cipr | S80F | S84L | Toulousec | 64 | 206 |
| 95057 | Mcr Gmr Fosr SgAr SgBr Rfr Cipr | S80F, E84K | S84L | Orsayc | 128 | 73 |
| BM10835 | Mcr Gmr Fosr SgAr Rfr Cipr | S80F, E84K | S84L | Rennesc | 128 | 27 |
| We | Mcr Gmr Cipr | S80Y | S84L | Boston | 16 | 29 |
| MSSA | ||||||
| Ch | Cipr | S80F, I45M | None | Boston | 4 | 16 |
| Mc | Cipr | S80F | None | Boston | 8 | 4 |
| Mu | Cipr | E84K | None | Boston | 8 | 46 |
| Av | Cipr | S80F, I45M | S84A | Boston | 8 | 22 |
| Dec | Cipr | S80F | S84L | Boston | 16 | 112 |
| Ki | Cipr | S80F | S84A | Boston | 16 | 66 |
Abbreviations: Mc, methicillin; Gm, gentamicin; Fos, fosfomycin; SgA, streptogramin A; SgB, streptogramin B; Rf, rifampin; Fa, fusidic acid; Cip, ciprofloxacin.
After growth in one-quarter the MIC of ciprofloxacin.
City in France.
The properties of isogenic strains and plasmids used in this study are listed in Table 2. Single grlA and gyrA quinolone-resistant mutant strains MT5224c4 and SS1, respectively, and the double grlA gyrA mutant strain EN1252a of S. aureus have been previously described (1).
TABLE 2.
Isogenic laboratory strains and plasmids used in the studya
| S. aureus strains | Characterization | Notable property(-ies) | Origin or reference(s) | MIC (μg/ml) of ciprofloxacin |
|---|---|---|---|---|
| Laboratory | ||||
| ISP794 | 8325 pig-131 | Parent strain | 7 | 0.25 |
| SS1 | MT5 gyrA | Amino acid change in GyrA (S84L) | 1 | 0.25 |
| MT5224c4 | MT5 flqA (grlA542) | Amino acid change in GrlA (S80F) | 31, 52 | 2.0 |
| EN1252a | MT5 flqA(grlA542) gyrA Ω1051(Erm) Nov+ | Double Amino acid changes in GrlA (S80F) and GyrA (S84L) | 1 | 32.0 |
| RN6911 | RN6390B Δagr::tetM | agr locus has been replaced with tetM | 34 | ND |
| ALC 355 | Newman Δagr::tetM | Newman with Δagr::tetM from strain RN6911 | 58 | ND |
| CB3 | EN1252a Δagr::tetM | Double grlA gyrA mutant with Δagr::tetM from strain ALC355 | This work | 32.0 |
| Plasmids | ||||
| pFNBA6 | Cmr Ampr | pSB327 shuttle vector with fused luxAB and fnbA promoter | 14 | |
| pFNBB6 | CMr Ampr | pSB327 shuttle vector with fused luxAB and fnbB promoter | 14 |
Abbreviations: ND, not determined; Cm, chloramphenicol; Amp, ampicillin.
Plasmids pFNBA6 and pFNBB6 carrying the promoter of fnbA and fnbB, respectively, fused with a luxAB reporter gene (coding for bacterial luciferase) were transduced from strain DU5883 (14) with phage 85 into strains ISP794 and EN1252a. Control experiments verified that the susceptibility to ciprofloxacin of strains ISP794 and EN1252a was unaffected by the presence of plasmids pFNBA6 and pFNBB6 (data not shown).
To obtain a derivative of strain EN1252a, in which the agr locus has been replaced with tetM, as originally developed in strain RN6911 (Δagr::tetM) (34), phage 85 was used to transduce this agr mutation from strain ALC 355 (58) to strain EN1252a, with selection for tetracycline resistance. The presence of the Δagr::tetM mutation in strain CB3, which is a derivative of strain EN1252a, was assessed by Southern blot hybridization, and the Agr− phenotype was confirmed by defective hemolysin production on sheep blood agar plates.
Susceptibility to antimicrobial agents.
Antimicrobial susceptibilities of the clinical isolates were determined by the disk diffusion method (29). The following antibiotics were tested: oxacillin, gentamicin, fosfomycin, rifampin, fusidic acid, and ciprofloxacin and, when indicated, streptogramin A and B.
The MICs of ciprofloxacin for fluoroquinolone-resistant strains were determined by a macrodilution method using Mueller-Hinton broth (MHB) (Difco, Detroit, Mich.) and a standard inoculum of 106 CFU/ml (28).
Characterization of grlA and gyrA point mutations by DNA sequencing.
Point mutations determining fluoroquinolone resistance in MRSA and MSSA clinical isolates were recorded by sequencing the quinolone-resistance determining regions (QRDR) of grlA and gyrA as previously described (12). Chromosomal DNA was purified from bacterial cells lysed with lysostaphin and lysozyme using a previously described phenol-chloroform procedure (24, 43). QRDR of grlA and gyrA were amplified by a standard PCR protocol (Qiagen, Basel, Switzerland), using oligonucleotide primers identical to those described by Schmitz et al. (46) and Fournier and Hooper (12). PCR products were purified with a PCR purification kit (Qiagen), sequenced by a fluorescent-dye terminator method (Abi Prism; Perkin-Elmer), and resolved and automatically analyzed with a 373A Stretch Sequencer (Perkin-Elmer).
In vitro bacterial adhesion assay to fibronectin.
The attachment properties of the different clinical and laboratory strains of S. aureus were measured using a previously described adhesion assay with polymethylmethacrylate coverslips coated in vitro with three different concentrations of purified fibronectin as previously described (1, 14, 15, 55). Adhesion of S. aureus was evaluated by incubating for 1 h at 37°C the fibronectin-coated coverslips with 107 CFU of washed cultures of late-logarithmic-phase cells, metabolically radiolabeled with [3H]thymidine during growth for 5 h at 37°C, as previously described (1, 14, 55). At the end of the attachment period, the fluids containing unbound bacteria were removed, the coverslips were rinsed, and radioactivity on the coverslips was determined. Each of the clinical isolates was tested after continuous incubation of the 5-h cultures with a single subinhibitory concentration equivalent to one-quarter the MIC of ciprofloxacin which was run in parallel with a culture in control quinolone-free MHB.
For the laboratory isolates ISP794 and EN1252a, 5-h cultures of either strain were also continuously incubated with either 1/4, 1/8, or 1/16 the MIC of ciprofloxacin or control MHB, respectively.
Each adhesion experiment was performed at least three times, and the results were expressed as the mean ± standard error of the mean. For each clinical isolate, the significance of differences between exposure and no exposure to ciprofloxacin during growth was evaluated by the nonparametric Wilcoxon signed-rank test as previously described (1). The adhesion profiles of ciprofloxacin-exposed and unexposed cells were considered significantly different from each other when all increases or decreases accumulated for the three coating concentrations of fibronectin yielded P values of <0.05 with a two-tailed significance level (1).
To evaluate the significance of differences in the attachment of strain EN1252a grown under control conditions or in the presence of three different subinhibitory concentrations of ciprofloxacin, we used the Kruskal-Wallis test followed by the Dunn procedure for comparison of specific groups (42).
Bacterial adhesion to explanted coverslips.
We used a previously described animal model of foreign body infection, in which four perforated cylinders termed tissue cages, each containing three polymethylmethacrylate coverslips, were implanted subcutaneously into guinea pigs (54, 60). After an infection-free period of 4 weeks, guinea-pigs were sacrificed and the subcutaneously implanted tissue cages with enclosed coverslips were removed (54). After extensive rinsing in phosphate-buffered saline, each explanted coverslip coated in vivo with extracellular matrix components was tested in the in vitro bacterial adhesion assay as described above. The most highly adhesion-promoted clinical fluoroquinolone-resistant isolate was tested with explanted coverslips and compared to isogenic quinolone-resistant and -susceptible laboratory isolates of S. aureus. For each strain, the significance of cumulated differences between exposure and no exposure to ciprofloxacin during growth was evaluated by the nonparametric Mann-Whitney test (42), using P values of <0.05 with a two-tailed significance level.
SDS-PAGE and Western ligand affinity blotting.
Bacterial protein extracts were prepared from whole cells grown and lysed with lysostaphin as previously described (1). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western ligand affinity blotting were performed on lysostaphin extracts of the different strains to identify and quantify the amount of FnBPs by densitometric analysis as previously described (1).
Luciferase assay.
To analyze the expression of each fnb promoter in the presence or absence of subinhibitory concentrations of ciprofloxacin, the pFNBA6 or pFNBB6 plasmid-containing strains EN1252a or ISP794 were first grown overnight at 35°C in MHB supplemented with chloramphenicol (5 μg/ml). Then, 600-μl aliquots of washed overnight cultures were diluted into 30-ml aliquots of MHB in conical flasks and grown at 35°C for a maximal period of 5 h, in the absence or presence of one-quarter the MIC of ciprofloxacin. At regular intervals, duplicate 1-ml samples were removed, one being assayed for luminescence and the other being for monitoring growth of the culture by the absorbance at 540 nm. Luciferase activity was assayed after adding to a 1-ml portion 10 μl of 1% (vol/vol) dodecanal in ethanol using a LKB Wallac 1250 Luminometer. The specific light units (SLU) were determined by dividing the relative light units by absorbance at 540 nm (14). Each experiment was performed at least three times, and the results were expressed as the means ± standard errors. For each strain and each time point, the significance of differences in SLU expressed by ciprofloxacin-exposed compared to unexposed cultures was evaluated by the paired t test, using P values of <0.05 with a two-tailed significance level.
To evaluate the lag time of fnb promoter responses to subinhibitory concentrations of ciprofloxacin, 38-μl portions of washed overnight cultures of either strain, EN1252a(pFNBB6) or ISP794(pFNBB6), were inoculated into flasks containing 30 ml of antibiotic-free MHB. After 4 h of growth at 35°C, each bacterial culture was split into four subcultures, each growing in a different medium: (i) plain MHB, (ii) MHB containing rifampin (2 μg/ml), (iii) MHB supplemented with either one-quarter or one-eighth the MIC of ciprofloxacin, or (iv) MHB first treated with rifampin (2 μg/ml) for 5 min at 35°C before addition of one-quarter the MIC of ciprofloxacin. The RNA synthesis inhibitor rifampin was used to determine that the ciprofloxacin-promoted increase of luciferase activity occurred at the level of transcription of the fnb genes. At 20 min, samples were processed for SLU measurements as described above. Each experiment was performed at least three times, and the results were expressed as the mean percent changes ± standard errors over the 20-min period. For each strain, the significance of percent changes in SLU expressed by each ciprofloxacin- or/and rifampin-treated group was compared to that of unexposed cultures by one-way variance analysis followed by paired t test for comparison of specific groups, using P values of <0.05 with a two-tailed significance level.
Evaluation of plasmid copy number.
Assays of chloramphenicol acetyltransferase (CAT) activity were used as a control for the copy number of the fusion plasmids in strains EN1252a and ISP794. Lysostaphin extracts of either strain grown in the presence or absence of one-quarter the MIC of ciprofloxacin were prepared as described above. Protein concentrations were determined by the Pierce method. CAT activity was assayed with a commercial kit (CAT-ELISA; Roche, Basel, Switzerland).
RESULTS
Ciprofloxacin-containing growth medium can alter adhesion of clinical quinolone-resistant isolates of S. aureus.
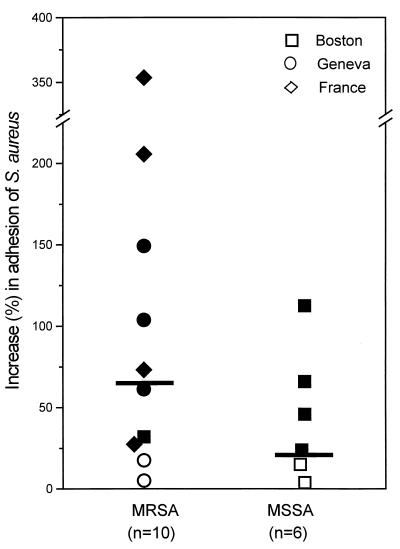
None of the 16 quinolone-resistant clinical isolates showed any reduction in bacterial adhesion after growth in the presence of one-quarter the MIC of ciprofloxacin (Fig. 1). Average adhesion values of the 10 MRSA and 6 MSSA isolates grown in control ciprofloxacin-free medium ranged from 86 × 103 to 394 × 103 CFU/coverslip and 113 × 103 to 297 × 103 CFU/coverslip, respectively. After growth in the presence of one-quarter the MIC of ciprofloxacin, average adhesion values of MRSA and MSSA isolates ranged from 101 × 103 to 769 × 103 CFU/coverslip and 239 × 103 to 360 × 103 CFU/coverslip, respectively. Only four isolates, two MRSA and two MSSA isolates, showed nonsignificant marginal increases in adhesion. All other isolates exhibited significant average increases in attachment to fibronectin-coated surfaces, ranging from 27 to 353% for 8 of 10 MRSA isolates and 22 to 112% for 4 of 6 MSSA isolates (Table 1).
FIG. 1.
Percentage increases in the adhesion to fibronectin-coated coverslips of 10 MRSA or 6 MSSA fluoroquinolone-resistant clinical isolates from either Boston, Mass. (□, ■), Geneva, Switzerland (○, ●), or France (◊, ⧫), promoted by growth in the presence of one-quarter the MIC of ciprofloxacin for each strain. Solid symbols represent strains whose adhesion is significantly promoted by ciprofloxacin-containing growth medium; open symbols represent strains whose adhesion is not significantly promoted.
An important property of the randomly collected clinical MRSA isolates was the wide diversity of amino acid changes in the QRDR of their grlA and gyrA loci (Table 1). This provided indirect evidence that we were not dealing with a single clone in particular for the Geneva subgroup, of which four of five isolates were collected in the Orthopedic Clinic within a 1-month interval.
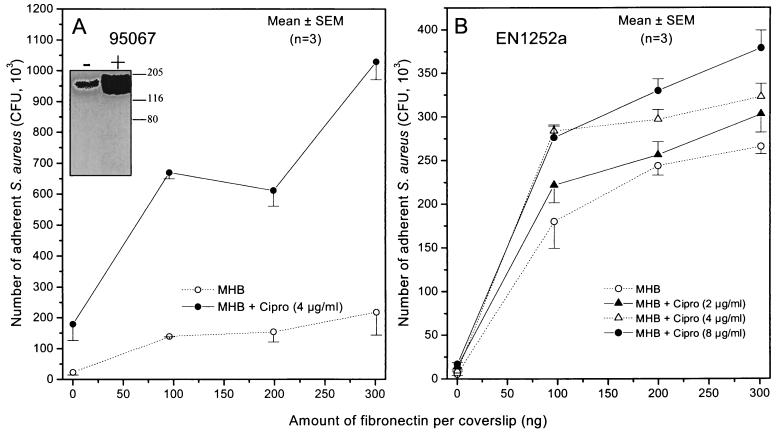
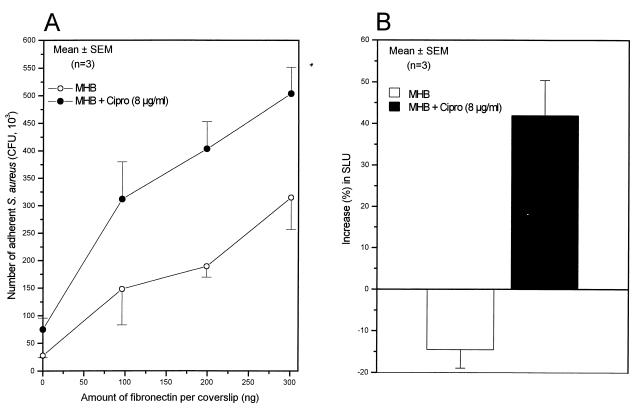
The most remarkable changes in adhesion were found in the subgroup of multidrug-resistant strains collected from the Pasteur Institute, which showed some evidence of epidemic transmission. Among them, strains BM10762 and 95067 exhibited 206 and 353% average increases in attachment, respectively, following growth in the presence of one-quarter the MIC of ciprofloxacin. The adhesion profiles of strain 95067 grown either in the absence or presence of one-quarter the MIC of ciprofloxacin are shown in Fig. 2A. Western fibronectin affinity blots confirmed that increased adhesion of strain 95067 was associated with an 3.5-fold increase in the amount of FnBPs promoted by growth in the ciprofloxacin-containing medium (Fig. 2A, inset). Calibration curves performed with three different amounts of protein extract of strain 95067 loaded on SDS-PAGE and tested by Western affinity blots verified that the ciprofloxacin-promoted increase in FnBPs was within the linear range of the densitometric analysis, as shown previously (1). Further experiments also demonstrated increased amounts of FnBPs, reaching 152 and 90% in clinical strains BM10762 and 95057 grown in the presence of one-quarter the MIC of ciprofloxacin, respectively. Such increases in FnBPs were consistent with the increased adhesion of strains BM10762 and 95057 of 206 and 73%, respectively (Table 1).
FIG. 2.
Adhesion to fibronectin-coated coverslips of the fluoroquinolone-resistant strains 95067 (A) and EN1252a (B) of S. aureus grown in the absence or presence of the indicated concentrations of ciprofloxacin. The insert in panel A shows expression of FnBPs by Western ligand affinity blots in a bacterial lysate of strain 95067 grown in the absence (−) or presence (+) of one-quarter the MIC of ciprofloxacin.
Figure 2B shows the adhesion profiles of strain EN1252a, a well defined quinolone-resistant gyrA grlA double mutant of S. aureus 8325, grown in the absence or presence of increasing subinhibitory concentrations of ciprofloxacin. Attachment of this fluoroquinolone-resistant mutant was previously shown to be significantly promoted by one-quarter the MIC of ciprofloxacin (1). Growth in the presence of 1/16 the MIC of ciprofloxacin (2 μg/ml) led to a marginal, nonsignificant increase in bacterial adhesion compared to growth in ciprofloxacin-free medium. In contrast, growth in the presence of higher subinhibitory concentrations of ciprofloxacin, namely, one-eighth the MIC (4 μg/ml) and one-quarter the MIC (8 μg/ml), led to significant increases in adhesion. Interestingly, the dose responses of strain EN1252a adhesion promoted by growth in the presence of one-eighth MIC and one-quarter the MIC of ciprofloxacin were almost equivalent, representing a plateau of response at these subinhibitory concentrations. Western fibronectin affinity blotting assessed that strain EN1252a grown in the presence of 1/4 and 1/8 the MIC but not 1/16 the MIC of ciprofloxacin exhibited an increased production of FnBPs (data not shown), thus confirming previously reported data recorded with 1/4 the MIC of this fluoroquinolone and further correlating increased FnBP expression and increased adhesion (1). Of note, growth in the presence of one-eighth the MIC of ciprofloxacin did not alter the growth rate of strain EN1252a, which grew at a rate identical to that in control antibiotic-free MHB.
Taken together, the dose response of FnBP production and increased adhesion of strain EN1252a were closely related to each other. Optimal promotion of FnBP-mediated adhesion was obtained by growing fluoroquinolone-resistant organisms in the presence of ciprofloxacin concentrations that are still clinically relevant.
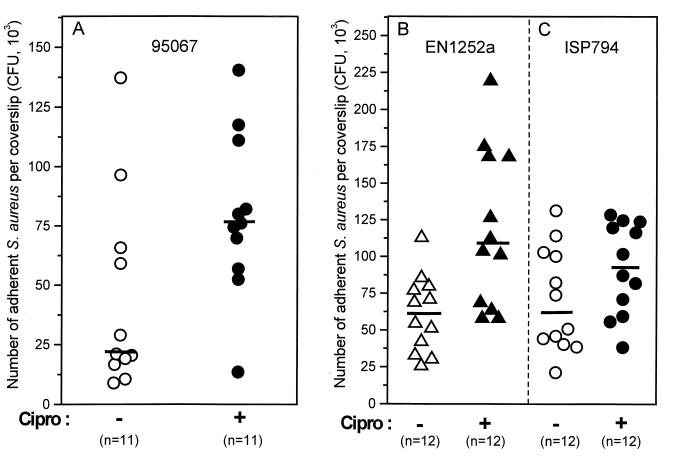
Adhesion of fluoroquinolone-resistant S. aureus to explanted coverslips.
The attachment of clinical strain 95067 to coverslips explanted from guinea pigs increased from 21 × 103 to 76 × 103 CFU/coverslip for organisms grown in ciprofloxacin-containing medium (one-quarter the MIC) compared to those grown in control medium (Fig. 3A). Similarly, attachment of EN1252a grlA gyrA was increased significantly in the presence of one-quarter the MIC of ciprofloxacin (from 62 × 103 to 108 × 103 CFU/coverslip [Fig. 3B]). In contrast, the median attachment increase of the fluoroquinolone-susceptible parental strain ISP794 (from 62 × 103 to 94 × 103 CFU/coverslip [Fig. 3C]) did not reach statistical significance. Finally, neither strains EN1252a nor ISP794 showed increased attachment when grown in the presence of one-eighth the MIC of ciprofloxacin (data not shown). Thus, as was the case for adhesion to fibronectin-coated coverslips, adhesion to explanted coverslips was promoted by ciprofloxacin in a dose-dependent manner.
FIG. 3.
Adhesion to coverslips explanted from guinea pigs of the clinical MRSA strain 95067 (A), the double grlA gyrA mutant strain EN1252a (B), and its parent ISP794 (C) grown in the absence (open symbols) or presence (solid symbols) of one-quarter the MIC of ciprofloxacin for each strain, namely 4 μg/ml for 95067, 8 μg/ml for EN1252a, and 0.06 μg/ml for ISP794.
Activation of fnbA and fnbB promoter by subinhibitory concentrations of ciprofloxacin.
To study whether expression of the fnbA and fnbB promoters was modified by subinhibitory concentrations of ciprofloxacin in fluoroquinolone-resistant or fluoroquinolone-susceptible S. aureus, we used the previously described fnbA-luxAB and fnbB-luxAB reporter plasmids pFNBA6 and pFNBB6 (14) transferred into the double mutant strain EN1252a or its susceptible parent ISP794.
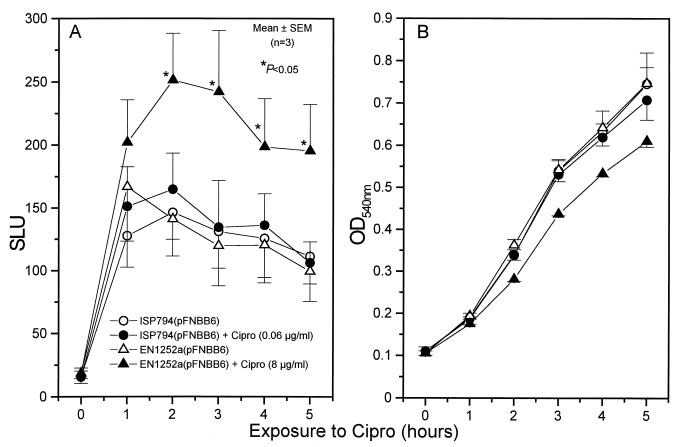
In the first set of experiments, expression of fnbA or fnbB promoters was recorded on EN1252a or ISP794 cells that were continuously exposed to one-quarter the MIC of ciprofloxacin for 5 h. In contrast to the fnbA promoter, which exhibited no specific response to ciprofloxacin (data not shown) and was not further studied, the fnbB promoter of strain EN1252a was significantly more active from 2 to 5 h in the presence of ciprofloxacin than in its absence (Fig. 4A). Of note, the quinolone-induced response of the fnbB-lux fusion observed in the fluoroquinolone-resistant strain EN1252a was not seen with its quinolone-susceptible parent ISP794 (Fig. 4A). The presence of one-quarter the MIC of ciprofloxacin in the medium slightly dampened the growth rate of strain EN1252a but had no effect on strain ISP794 (Fig. 4B).
FIG. 4.
Luciferase activity of the fnbB-luxAB reporter plasmid in strains EN1252a(pFNBB6) and ISP794(pFNBB6) either unexposed or continuously exposed for 5 h to one-quarter the MIC of ciprofloxacin (A) and bacterial growth monitored by measuring the optical density at 540 nm (B). ∗, P < 0.05 for culture grown with ciprofloxacin compared to that without ciprofloxacin.
To verify that the increased luciferase activity was not due to differences in plasmid copy number, CAT activity of the fnbB-lux fusion plasmid, which is not under control of the fnbB promoter, was determined for strains ISP794 and EN1252a. No difference was observed between strains grown in the presence or absence of ciprofloxacin (data not shown).
Further experiments with the parent and grlA gyrA double mutant (strain EN1252a) were carried out to study the initial phase of fnbB activation by one-quarter the MIC of ciprofloxacin. At 20 min after the addition of ciprofloxacin, strain EN1252a(pFNBB6) (Fig. 5A) showed a significant increase in SLU of 39% ± 4% compared to a slight nonsignificant decrease (−2% ± 3%) of cells grown in quinolone-free MHB (Fig. 5A). Control experiments verified that the growth rate of fluoroquinolone-exposed or unexposed strain EN1252a(pFNBB6) monitored by optical density was equivalent to that monitored by CFU counts (data not shown).
FIG. 5.
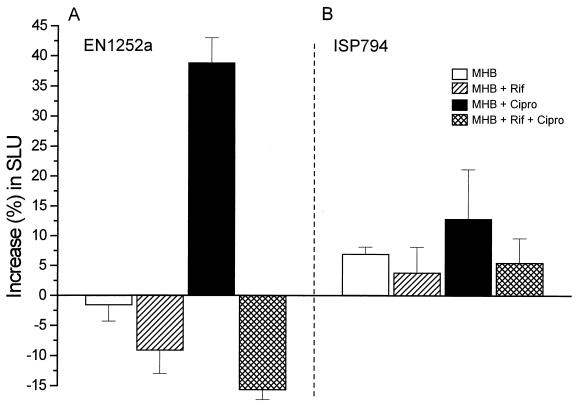
Increase in SLU by the fnbB-luxAB reporter plasmid in strain EN1252a(pFNBB6) or ISP794(pFNBB6) exposed for 20 min to either control MHB or MHB supplemented with rifampin (2 μg/ml), with one-quarter the MIC (8 μg/ml) of ciprofloxacin, or with rifampin (2 μg/ml) followed by ciprofloxacin (8 μg/ml).
Pretreatment of the ciprofloxacin-exposed organisms with rifampin (2 μg/ml) abolished the fluoroquinolone-promoted increase in SLU of strain EN1252a, indicating that this increase was due to increased transcription of the fnbB-lux fusion and not to some other unrelated mechanism. Since the average SLU of rifampin-pretreated organisms decreased at 20 min by 9% ± 4% and 16% ± 2% for cultures incubated without and with ciprofloxacin, respectively (Fig. 5A), this finding indicated that the turnover of the fnbB-lux fusion protein was marginal in these experimental conditions. In contrast to strain EN1252a, the fluoroquinolone-susceptible parental strain ISP794 showed no significant change in SLU at 20 min after the addition of ciprofloxacin (13% ± 8%), rifampin (4% ± 4%), or rifampin plus ciprofloxacin (6% ± 4%). These values were similar to those of control cells whose SLU marginally increased by 7% ± 1% (Fig. 5B). Thus one-quarter the MIC of ciprofloxacin could selectively activate the fnbB promoter of the fluoroquinolone-resistant strain EN1252a but not the fluoroquinolone-susceptible strain ISP794.
To further confirm that the ciprofloxacin-promoted increase in fnbB expression was not an indirect effect of decreased growth rate, we also compared the rates of activation of fnb-lux in strain EN1252a by either one-eighth or one-quarter the MIC of ciprofloxacin in the growth medium. At 20 min after the addition of ciprofloxacin, strain EN1252a(pFNBB6) showed significant increases in SLU of 45% ± 7% and 29% ± 7% when exposed to one-eighth and one-quarter the MIC of ciprofloxacin, respectively, while exhibiting a slight nonsignificant decrease of −4% ± 2% in quinolone-free MHB. During this 20-min period, the increase in optical density of strain EN1252a (30% ± 2%) was not affected by one-eighth the MIC of ciprofloxacin in MHB, being at least equivalent to that in quinolone-free MHB (26% ± 2%) and slightly superior to that in MHB containing one-quarter the MIC of the fluoroquinolone (21% ± 2%). These data ruled out the notion that decreased growth rate is an absolute requirement for the effect of a sub-MIC of ciprofloxacin on fnb gene expression in the quinolone-resistant strain EN1252a.
Activation of the agr locus is not required for ciprofloxacin-promoted fnbB gene expression.
The contribution of agr activation to the ciprofloxacin-mediated fnbB gene activation was tested by comparing strain EN1252a with its agr-defective mutant strain CB3, which showed equivalent ciprofloxacin MICs. Adhesion rates of strains CB3 (Fig. 6A) and EN1252a (not shown) were similarly promoted by growth in the presence of one-quarter the MIC of ciprofloxacin in MHB. Furthermore, activation of fnbB-lux by one-quarter the MIC of ciprofloxacin recorded in the agr-defective strain CB3(pFNBB6) (Fig. 6B) was equivalent to that recorded in strain EN1252a(pFNBB6) (data not shown). Thus, the agr locus was not required for activation of the fnbB promoter by one-quarter the MIC of ciprofloxacin.
FIG. 6.
(A) Adhesion to fibronectin-coated coverslips of the agr-defective fluoroquinolone-resistant strain CB3 of S. aureus grown in the absence or presence of the indicated concentrations of ciprofloxacin. (B) Increase in SLU by the fnbB-luxAB reporter plasmid in strain CB3(pFNBB6) exposed for 20 min to either control MHB or MHB supplemented with one-quarter the MIC (8 μg/ml) of ciprofloxacin.
DISCUSSION
A few recent studies indicate that exposure of S. aureus to subinhibitory concentrations of some antibiotics may trigger in this pathogen a paradoxical increase in some of its virulence factors (1, 21, 35). Sub-MIC levels of β-lactams, in particular nafcillin, were shown to induce hla gene expression and increase alpha-toxin production in S. aureus (21, 35). We recently reported that an isogenic fluoroquinolone-resistant grlA gyrA double mutant of S. aureus NCTC8325 grown in the presence of subinhibitory concentrations of ciprofloxacin expressed increased levels of FnBPs and showed a significantly higher attachment to fibronectin-coated polymer surfaces (1).
In this study, adhesion of a majority (12 of 16) of fluoroquinolone-resistant clinical isolates, including 10 MRSA isolates and 6 MSSA isolates which were not epidemiologically linked, was significantly promoted by growth in the presence of one-quarter the MIC of ciprofloxacin for each strain. These results, which mimic the findings of our previous study with isogenic fluoroquinolone-resistant mutants of NCTC8325, may indicate a common ciprofloxacin-induced upregulation of fibronectin adhesins in both clinical and laboratory strains which may be of epidemiological relevance. The most impressive responses in ciprofloxacin-promoted adhesion were found in two epidemic though geographically distinct multidrug-resistant strains of MRSA. Much larger representative samples of epidemic and nonepidemic strains will have to be tested to document a possible correlation between intensive use of fluoroquinolones and selection of particularly invasive S. aureus isolates with a high potential of epidemic transmission. Interestingly, one recent study evaluating the relationship between the incidence of MRSA and the use of different classes of antimicrobials in Belgian hospitals found a correlation with increasing use of quinolones in a multivariate analysis (7). In another prospective randomized study, outbreaks of MRSA superinfections causing late-onset pneumonia were clearly associated with ciprofloxacin use in intensive care units (23).
Adhesion rates of the most highly promoted clinical strain, 95067, and that of the isogenic fluoroquinolone-resistant mutant EN1252a were also significantly increased on coverslips explanted from guinea pigs after growth in the presence of one-quarter the MIC of ciprofloxacin. This finding indicated that fibronectin, which is associated with several other extracellular matrix components, including collagen, laminin, and glycosaminoglycans (53, 54), was indeed playing a leading role in promoting the attachment of both clinical and laboratory isolates to such subcutaneously implanted biomaterials. Since coverslips explanted from guinea pigs promoted less regular attachment than fibronectin-coated surfaces, this might be due to variable amounts of fibronectin adsorbed on the subcutaneously implanted surfaces and to the potential contribution or antagonistic effects of other extracellular matrix components to S. aureus adhesion. The large variability in quantitative adhesion data recorded on explanted coverslips probably explained why only one-quarter the MIC, but not lower sub-MICs of ciprofloxacin, was able to significantly promote S. aureus attachment to in vivo-coated biomaterials.
To evaluate the potential activation of fnbA and/or fnbB gene by sub-MIC levels of ciprofloxacin, we used luciferase fnb-luxAB reporter plasmids transformed into fluoroquinolone-resistant or -susceptible isogenic strains EN1252a and ISP794, respectively. As previously described in other strains (14), the luciferase activity mediated by the fnbB promoter was two to three times higher than that of the fnbA promoter in both strain EN1252a and ISP794 grown in control antibiotic-free MHB. In this study, continuous incubation of strains EN1252a and ISP794 with one-quarter the MIC of ciprofloxacin demonstrated at 2 to 5 h a specific activation of the fnbB, as opposed to fnbA, promoter which occurred only in the fluoroquinolone-resistant double grlA gyrA mutant but not its fluoroquinolone-susceptible parent. This difference was not attributable to a change in plasmid copy number. In short-term experiments evaluating the activation of the fnbB promoter by sub-MICs of ciprofloxacin, the fluoroquinolone-resistant strain EN1252a but not its fluoroquinolone-susceptible parent ISP794 already expressed increased luciferase activity at 20 min compared to expression in control antibiotic-free MHB. Since pretreatment with the RNA transcription inhibitor rifampin abolished the ciprofloxacin-induced response, this indicated that the increased luciferase activity indeed reflected increased transcription of the fnbB-luxAB reporter plasmid in the fluoroquinolone-resistant strain EN1252a. In addition, the rifampin pretreatment experiments showed a marginal turnover of luciferase under these experimental conditions. This observation, which validated the use of the luxAB reporter plasmid at 35°C, was in contrast to other observations reporting a high instability of luciferase at 37°C (16). Taken together, these observations suggest that ciprofloxacin at sub-MICs can activate by undefined mechanisms the fnbB promoter in fluoroquinolone-resistant grlA gyrA laboratory isolates as well as in a substantial proportion of the clinical isolates of S. aureus that were tested. Our data suggest that this fnbB promoter activation is responsible for increased production of FnBP(s), leading to a higher level of bacterial attachment to fibronectin-coated or subcutaneously implanted coverslips.
Different bacterial response mechanisms may contribute to the ciprofloxacin-activated increase in FnBP-mediated adhesion in fluoroquinolone-resistant mutants of S. aureus. Fluoroquinolones are known to induce the SOS response in gram-negative bacteria stemming from DNA damage thought to be generated by collisions of the DNA replication fork with ternary complexes of drug, DNA, and either topoisomerase IV or DNA gyrase (3, 13, 17, 22, 57). Other studies indicate that fluoroquinolones can also induce heat shock proteins DnaK and GroEL in Escherichia coli, mediated by the sigma 32 transcription factor (26, 27). It is also possible that fluoroquinolones have either direct or indirect effects on some global regulator(s) of S. aureus known to affect the expression of FnBP and other cell surface proteins (41) such as agr (33) or sar (44;; Chung and Wolz, 37th ICAAC). A recent report also indicates that agr- or sar-defective mutants express a somewhat lower level of methicillin resistance, which is associated with a reduction in levels of PBP1 and PBP3 but not PBP2′ (8). While the present study, which was carried out with an agr knockout mutant, indicates that this global regulator is not required for the ciprofloxacin-induced response in fnbB transcription, further studies are in progress to evaluate the potential role of other global regulators, such as the sar locus or the more complex heat shock and SOS response systems.
In conclusion, sub-MIC levels of ciprofloxacin and potentially other fluoroquinolones may upregulate adhesin expression in S. aureus strains resistant to these antimicrobial agents. Further in vitro and in vivo studies are needed to evaluate the molecular mechanisms of this bacterial response and its clinical and epidemiological relevance.
ACKNOWLEDGMENTS
This work was supported by the Swiss National Foundation (grant 3200-045810.95/2 [to P.V.]) and the National Institute of Health (grant AI23988 [to D.C.H.]).
We thank Xiamei Zhang for performing the DNA sequencing of the Boston strains, T. J. Foster for providing the reporter plasmids and A. L. Cheung strain ALC 355, Manuela Bento for technical assistance, and William Kelley for helpful discussion.
REFERENCES
- 1.Bisognano C, Vaudaux P E, Lew D P, Ng E Y W, Hooper D C. Increased expression of fibronectin-binding proteins by fluoroquinolone-resistant Staphylococcus aureus exposed to subinhibitory levels of ciprofloxacin. Antimicrob Agents Chemother. 1997;41:906–913. doi: 10.1128/aac.41.5.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butcher W G, Close J, Krajewska-Pietrasik D, Switalski L M. Antibiotics alter interactions of Staphylococcus aureus with collagenous substrata. Chemotherapy. 1994;40:114–123. doi: 10.1159/000239182. [DOI] [PubMed] [Google Scholar]
- 3.Chen C R, Malik M, Snyder M, Drlica K. DNA gyrase and topoisomerase IV on the bacterial chromosome: quinolone-induced DNA cleavage. J Mol Biol. 1996;258:627–637. doi: 10.1006/jmbi.1996.0274. [DOI] [PubMed] [Google Scholar]
- 4.Cheung A L, Eberhardt K J, Chung E, Yeaman M R, Sullam P M, Ramos M, Bayer A S. Diminished virulence of a sar−/agr− mutant of Staphylococcus aureus in the rabbit model of endocarditis. J Clin Investig. 1994;94:1815–1822. doi: 10.1172/JCI117530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chien Y T, Cheung A L. Molecular interactions between two global regulators, sar and agr, in Staphylococcus aureus. J Biol Chem. 1998;273:2645–2652. doi: 10.1074/jbc.273.5.2645. [DOI] [PubMed] [Google Scholar]
- 6.Chien Y T, Manna A C, Cheung A L. SarA level is a determinant of agr activation in Staphylococcus aureus. Mol Microbiol. 1998;30:991–1001. doi: 10.1046/j.1365-2958.1998.01126.x. [DOI] [PubMed] [Google Scholar]
- 7.Crowcroft N S, Ronveaux O, Monnet D L, Mertens R. Methicillin-resistant Staphylococcus aureus and antimicrobial use in Belgian hospitals. Infect Control Hosp Epidemiol. 1999;20:31–36. doi: 10.1086/501555. [DOI] [PubMed] [Google Scholar]
- 8.Durán S P, Kayser F H, Berger-Bächi B. Impact of sar and agr on methicillin resistance in Staphylococcus aureus. FEMS Microbiol Lett. 1996;141:255–260. doi: 10.1111/j.1574-6968.1996.tb08394.x. [DOI] [PubMed] [Google Scholar]
- 9.Ferrero L, Cameron B, Crouzet J. Analysis of gyrA and grlA mutations in stepwise-selected ciprofloxacin-resistant mutants of Staphylococcus aureus. Antimicrob Agents Chemother. 1995;39:1554–1558. doi: 10.1128/aac.39.7.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrero L, Cameron B, Manse B, Lagneaux D, Crouzet J, Famechon A, Blanche F. Cloning and primary structure of Staphylococcus aureus DNA topoisomerase IV: a primary target of fluoroquinolones. Mol Microbiol. 1994;13:641–653. doi: 10.1111/j.1365-2958.1994.tb00458.x. [DOI] [PubMed] [Google Scholar]
- 11.Flock J I, Froman G, Jonsson K, Guss B, Signäs C, Nilsson B, Raucci G, Höök M, Wadström T, Lindberg M. Cloning and expression of the gene for a fibronectin-binding protein from Staphylococcus aureus. EMBO J. 1987;6:2351–2357. doi: 10.1002/j.1460-2075.1987.tb02511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fournier B, Hooper D C. Mutations in topoisomerase IV and DNA gyrase of Staphylococcus aureus: novel pleiotropic effects on quinolone and coumarin activity. Antimicrob Agents Chemother. 1998;42:121–128. doi: 10.1128/aac.42.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gari E, Figueroa-Bossi N, Blanc-Potard A B, Spirito F, Schmid M B, Bossi L. A class of gyrase mutants of Salmonella typhimurium show quinolone-like lethality and require rec functions for viability. Mol Microbiol. 1996;21:111–122. doi: 10.1046/j.1365-2958.1996.6221338.x. [DOI] [PubMed] [Google Scholar]
- 14.Greene C, McDevitt D, François P, Vaudaux P, Lew D P, Foster T J. Adhesion properties of mutants of Staphylococcus aureus defective in fibronectin binding proteins and studies on the expression of fnb genes. Mol Microbiol. 1995;17:1143–1152. doi: 10.1111/j.1365-2958.1995.mmi_17061143.x. [DOI] [PubMed] [Google Scholar]
- 15.Greene C, Vaudaux P E, François P, Proctor R A, McDevitt D, Foster T J. A low-fibronectin-binding mutant of Staphylococcus aureus 879R4S has Tn918 inserted into its single fnb gene. Microbiology. 1996;142:2153–2160. doi: 10.1099/13500872-142-8-2153. [DOI] [PubMed] [Google Scholar]
- 16.Hill P J, Rees C E, Winson M K, Stewart G S. The application of lux genes. Biotechnol Appl Biochem. 1993;17:3–14. [PubMed] [Google Scholar]
- 17.Howard B M, Pinney R J, Smith J T. Function of the SOS process in repair of DNA damage induced by modern 4-quinolones. J Pharm Pharmacol. 1993;45:658–662. doi: 10.1111/j.2042-7158.1993.tb05673.x. [DOI] [PubMed] [Google Scholar]
- 18.Ito H, Yoshida H, Bogaki-Shonai M, Niga T, Hattori H, Nakamura S. Quinolone resistance mutations in the DNA gyrase gyrA and gyrB genes of Staphylococcus aureus. Antimicrob Agents Chemother. 1994;38:2014–2023. doi: 10.1128/aac.38.9.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jonsson K, Signäs C, Muller H P, Lindberg M. Two different genes encode fibronectin binding proteins in Staphylococcus aureus. The complete nucleotide sequence and characterization of the second gene. Eur J Biochem. 1991;202:1041–1048. doi: 10.1111/j.1432-1033.1991.tb16468.x. [DOI] [PubMed] [Google Scholar]
- 20.Kaatz G W, Seo S M, Ruble C A. Efflux-mediated fluoroquinolone resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1993;37:1086–1094. doi: 10.1128/aac.37.5.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kernodle D S, McGraw P A, Barg N L, Menzies B E, Voladri R K R, Harshman S. Growth of Staphylococcus aureus with nafcillin in vitro induces α-toxin production and increases the lethal activity of sterile broth filtrates in a murine model. J Infect Dis. 1995;172:410–419. doi: 10.1093/infdis/172.2.410. [DOI] [PubMed] [Google Scholar]
- 22.Khodursky A B, Cozzarelli N R. The mechanism of inhibition of topoisomerase IV by quinolone antibacterials. J Biol Chem. 1998;273:27668–27677. doi: 10.1074/jbc.273.42.27668. [DOI] [PubMed] [Google Scholar]
- 23.Manhold C, von Rolbicki U, Brase R, Timm J, von Pritzbuer E, Heimesaat M, Kljucar S. Outbreaks of Staphylococcus aureus infections during treatment of late onset pneumonia with ciprofloxacin in a prospective, randomized study. Intensive Care Med. 1998;24:1327–1330. doi: 10.1007/s001340050770. [DOI] [PubMed] [Google Scholar]
- 24.Martineau F, Picard F J, Roy P H, Ouellette M, Bergeron M G. Species-specific and ubiquitous-DNA-based assays for rapid identification of Staphylococcus aureus. J Clin Microbiol. 1998;36:618–623. doi: 10.1128/jcm.36.3.618-623.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martínez J A, Ortiz G, Segovia M, Alonso M A, Gutiérrez M N, Muñoz J L, García-Rodríguez J A. Analysis of grlA mutations in clinical isolates of Staphylococcus aureus with different levels of quinolone resistance. Antimicrob Agents Chemother. 1998;42:1306–1307. doi: 10.1128/aac.42.5.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mizushima T, Matsuo M, Sekimizu K. Induction of DnaK and GroEL heat shock proteins by fluoroquinolones in Escherichia coli. Antimicrob Agents Chemother. 1997;41:193–195. doi: 10.1128/aac.41.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mizushima T, Ohtsuka Y, Mori H, Miki T, Sekimizu K. Increase in synthesis and stability of sigma 32 on treatment with inhibitors of DNA gyrase in Escherichia coli. Mol Gen Genet. 1996;253:297–302. doi: 10.1007/pl00008596. [DOI] [PubMed] [Google Scholar]
- 28.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 2nd ed. 1990. Approved standard M7-A2. National Committee for Clinical Laboratory Standards, Villanova, Pa. [Google Scholar]
- 29.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial disk susceptibility tests. 4th ed. 1990. Approved standard M2-A4. National Committee for Clinical Laboratory Standards, Villanova, Pa. [Google Scholar]
- 30.Neyfakh A A, Borsch C M, Kaatz G W. Fluoroquinolone resistance protein NorA of Staphylococcus aureus is a multidrug efflux transporter. Antimicrob Agents Chemother. 1993;37:128–129. doi: 10.1128/aac.37.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ng E Y, Trucksis M, Hooper D C. Quinolone resistance mutations in topoisomerase IV: relationship to the flqA locus and genetic evidence that topoisomerase IV is the primary target and DNA gyrase is the secondary target of fluoroquinolones in Staphylococcus aureus. Antimicrob Agents Chemother. 1996;40:1881–1888. doi: 10.1128/aac.40.8.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ng E Y W, Trucksis M, Hooper D C. Quinolone resistance mediated by norA: physiologic characterization and relationship to flqB, a quinolone resistance locus on the Staphylococcus aureus chromosome. Antimicrob Agents Chemother. 1994;38:1345–1355. doi: 10.1128/aac.38.6.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Novick R P, Projan S J, Kornblum J, Ross H F, Ji G, Kreiswirth B, Vandenesch F, Moghazeh S. The agr P2 operon: an autocatalytic sensory transduction system in Staphylococcus aureus. Mol Gen Genet. 1995;248:446–458. doi: 10.1007/BF02191645. [DOI] [PubMed] [Google Scholar]
- 34.Novick R P, Ross H F, Projan S J, Kornblum J, Kreiswirth B, Moghazeh S. Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO J. 1993;12:3967–3975. doi: 10.1002/j.1460-2075.1993.tb06074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ohlsen K, Ziebuhr W, Koller K P, Hell W, Wichelhaus T A, Hacker J. Effects of subinhibitory concentrations of antibiotics on alpha-toxin (hla) gene expression of methicillin-sensitive and methicillin-resistant Staphylococcus aureus isolates. Antimicrob Agents Chemother. 1998;42:2817–2823. doi: 10.1128/aac.42.11.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohshita Y, Hiramatsu K, Yokota T. A point mutation in norA gene is responsible for quinolone resistance in Staphylococcus aureus. Biochem Biophys Res Commun. 1990;172:1028–1034. doi: 10.1016/0006-291x(90)91549-8. [DOI] [PubMed] [Google Scholar]
- 37.Patti J M, Allen B L, McGavin M J, Höök M. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu Rev Microbiol. 1994;48:585–617. doi: 10.1146/annurev.mi.48.100194.003101. [DOI] [PubMed] [Google Scholar]
- 38.Peterson L R, Willard K E, Sinn L M, Fasching C E, Gerding D N. GyrA sequence analysis of Staphylococcus aureus and methicillin-resistant S. aureus strains selected, in vitro, for high-level ciprofloxacin resistance. Diagn Microbiol Infect Dis. 1993;17:97–101. doi: 10.1016/0732-8893(93)90019-4. [DOI] [PubMed] [Google Scholar]
- 39.Proctor R A, Hamill R J, Mosher D F, Textor J A, Olbrantz P J. Effects of subinhibitory concentrations of antibiotics on Staphylococcus aureus interactions with fibronectin. J Antimicrob Chemother. 1983;12(Suppl C):85–95. doi: 10.1093/jac/12.suppl_c.85. [DOI] [PubMed] [Google Scholar]
- 40.Proctor R A, Olbrantz P J, Mosher D F. Subinhibitory concentrations of antibiotics alter fibronectin binding to Staphylococcus aureus. Antimicrob Agents Chemother. 1983;24:823–826. doi: 10.1128/aac.24.5.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Projan S J, Novick R P. The molecular basis of pathogenicity. In: Crossley K B, Archer G L, editors. The staphylococci in human disease. New York, N.Y: Churchill Livingstone; 1997. pp. 55–81. [Google Scholar]
- 42.Rosner B. Analysis of variance. In: Payne M R, Hankinson S, London S, editors. Fundamentals of biostatistics. Belmont, Calif: PWS-KENT Publishing Company; 1990. pp. 474–526. [Google Scholar]
- 43.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 44.Saravia-Otten P, Müller H P, Arvidson S. Transcription of Staphylococcus aureus fibronectin binding protein genes is negatively regulated by agr and an agr-independent mechanism. J Bacteriol. 1997;179:5259–5263. doi: 10.1128/jb.179.17.5259-5263.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmitz F J, Hofmann B, Hansen B, Scheuring S, Lückefahr M, Klootwijk M, Verhoef J, Fluit A, Heinz H P, Köhrer K, Jones M E. Relationship between ciprofloxacin, ofloxacin, levofloxacin, sparfloxacin and moxifloxacin (BAY 12-8039) MICs and mutations in grlA, grlB, gyrA and gyrB in 116 unrelated clinical isolates of Staphylococcus aureus. J Antimicrob Chemother. 1998;41:481–484. doi: 10.1093/jac/41.4.481. [DOI] [PubMed] [Google Scholar]
- 46.Schmitz F J, Jones M E, Hofmann B, Hansen B, Scheuring S, Lückefahr M, Fluit A, Verhoef J, Hadding U, Heinz H P, Köhrer K. Characterization of grlA, grlB, gyrA, and gyrB mutations in 116 unrelated isolates of Staphylococcus aureus and effects of mutations on ciprofloxacin MIC. Antimicrob Agents Chemother. 1998;42:1249–1252. doi: 10.1128/aac.42.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stahl M L, Pattee P A. Confirmation of protoplast fusion-derived linkages in Staphylococcus aureus by transformation with protoplast DNA. J Bacteriol. 1983;154:406–412. doi: 10.1128/jb.154.1.406-412.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tanaka M, Onodera Y, Uchida Y, Sato K, Hayakawa I. Inhibitory activities of quinolones against DNA gyrase and topoisomerase IV purified from Staphylococcus aureus. Antimicrob Agents Chemother. 1997;41:2362–2366. doi: 10.1128/aac.41.11.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tanaka M, Zhang Y X, Ishida H, Akasaka T, Sato K, Hayakawa I. Mechanisms of 4-quinolone resistance in quinolone-resistant and methicillin-resistant Staphylococcus aureus isolates from Japan and China. J Med Microbiol. 1995;42:214–219. doi: 10.1099/00222615-42-3-214. [DOI] [PubMed] [Google Scholar]
- 50.Tankovic J, Duval J, Courvalin P. Construction of a gyrA plasmid for genetic characterization of fluoroquinolone-resistant Staphylococcus aureus. FEMS Immunol Med Microbiol. 1994;9:35–40. doi: 10.1111/j.1574-695X.1994.tb00471.x. [DOI] [PubMed] [Google Scholar]
- 51.Trucksis M, Hooper D C, Wolfson J S. Emerging resistance to fluoroquinolones in staphylococci: an alert. Ann Intern Med. 1991;114:424–426. doi: 10.7326/0003-4819-114-5-424. [DOI] [PubMed] [Google Scholar]
- 52.Trucksis M, Wolfson J S, Hooper D C. A novel locus conferring fluoroquinolone resistance in Staphylococcus aureus. J Bacteriol. 1991;173:5854–5860. doi: 10.1128/jb.173.18.5854-5860.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vaudaux P, Lew D P, Waldvogel F A. Host factors predisposing to foreign body infections. In: Bisno A L, Waldvogel F A, editors. Infections associated with indwelling medical devices. American Society for Microbiology; 1989. pp. 3–26. [Google Scholar]
- 54.Vaudaux P, Suzuki R, Waldvogel F A, Morgenthaler J J, Nydegger U E. Foreign body infection: role of fibronectin as a ligand for the adherence of Staphylococcus aureus. J Infect Dis. 1984;150:546–553. doi: 10.1093/infdis/150.4.546. [DOI] [PubMed] [Google Scholar]
- 55.Vaudaux P, Waldvogel F A, Morgenthaler J J, Nydegger U E. Adsorption of fibronectin onto polymethylmethacrylate and promotion of Staphylococcus aureus adherence. Infect Immun. 1984;45:768–774. doi: 10.1128/iai.45.3.768-774.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vaudaux P E, Monzillo V, Francois P, Lew D P, Foster T J, Berger-Bächi B. Introduction of the mec element (methicillin resistance) into Staphylococcus aureus alters in vitro functional activities of fibrinogen and fibronectin adhesins. Antimicrob Agents Chemother. 1998;42:564–570. doi: 10.1128/aac.42.3.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walker G C. The SOS response of E. coli. In: Neidhardt F C, Ingraham J L, Low K B, Magasanik B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhirium: cellular and molecular biology. Vol. 2. Washington, D.C.: American Society for Microbiology; 1987. pp. 1346–1357. [Google Scholar]
- 58.Wolz C, McDevitt D, Foster T J, Cheung A L. Influence of agr on fibrinogen binding in Staphylococcus aureus Newman. Infect Immun. 1996;64:3142–3147. doi: 10.1128/iai.64.8.3142-3147.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamagishi J I, Kojima T, Oyamada Y, Fujimoto K, Hattori H, Nakamura S, Inoue M. Alterations in the DNA topoisomerase IV grlA gene responsible for quinolone resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1996;40:1157–1163. doi: 10.1128/aac.40.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zimmerli W, Waldvogel F A, Vaudaux P, Nydegger U E. Pathogenesis of foreign body infection: description and characteristics of an animal model. J Infect Dis. 1982;146:487–497. doi: 10.1093/infdis/146.4.487. [DOI] [PubMed] [Google Scholar]