Abstract
The reason for increased sleep-disordered breathing with predominance of central apneas in the elderly is unknown. We hypothesized that the propensity to central apneas is increased in older adults, manifested by a reduced carbon-dioxide (CO2) reserve in older compared with young adults during non-rapid eye movement sleep. Ten elderly and 15 young healthy adults underwent multiple brief trials of nasal noninvasive positive pressure ventilation during stable NREM sleep. Cessation of mechanical ventilation (MV) resulted in hypocapnic central apnea or hypopnea. The CO2 reserve was defined as the difference in end-tidal CO2 () between eupnea and the apneic threshold, where the apneic threshold was that demarcated the central apnea closest to the eupneic . For each MV trial, the hypocapnic ventilatory response (controller gain) was measured as the change in minute ventilation (V̇e) during the MV trial for a corresponding change in . The eupneic was significantly lower in elderly vs. young adults. Compared with young adults, the elderly had a significantly reduced CO2 reserve (−2.6 ± 0.4 vs. −4.1 ± 0.4 mmHg, P = 0.01) and a higher controller gain (2.3 ± 0.2 vs. 1.4 ± 0.2 l·min−1·mmHg−1, P = 0.007), indicating increased chemoresponsiveness in the elderly. Thus elderly adults are more prone to hypocapnic central apneas owing to increased hypocapnic chemoresponsiveness during NREM sleep.
NEW & NOTEWORTHY The study describes an original finding where healthy older adults compared with healthy young adults demonstrated increased breathing instability during non-rapid eye movement sleep, as suggested by a smaller carbon dioxide reserve and a higher controller gain. The findings may explain the increased propensity for central apneas in elderly adults during sleep and potentially guide the development of pathophysiology-defined personalized therapies for sleep apnea in the elderly.
Keywords: aging, apneic threshold, CO2 reserve, controller gain, chemoresponsiveness, elderly, hypocapnic ventilatory response, plant gain
INTRODUCTION
Epidemiologic studies reveal increased prevalence of sleep apnea in the elderly compared with young adults (9, 25). Specifically, the prevalence of sleep-disordered breathing (SBD), defined as apnea-hypopnea index greater than or equal to five events per hour in healthy older adults was 39.5% in individuals in the ninth decade and 33.3% in the eighth decade (25). Additionally, in a large community-based sample, Bixler et al. (9) noted that the prevalence of SDB, particularly central apneas, increased with age. The prevalence of central apneas was 12% at age 65–100 yr vs. 0% at age 20–44 yr (odds ratio between older vs. young age groups: 3.9, 95% confidence interval: 1.9, 7.8) (9). The prevalence was higher in males compared with females with a preponderance of central apneas (9, 25). However, the underlying pathophysiology for this age-related increase in SBD remains elusive. While upper-airway anatomy (33), obesity (34), and dilator muscle function (33) explain a portion of the variability, these factors do not entirely account for the higher prevalence of central sleep apnea in the elderly (9), and only a third of the variability in the severity of sleep apnea can be ascribed to differences in mechanical load (26). Pack et al. (39, 40) noted a waxing and waning oscillatory breathing pattern during the lighter stages of sleep in elderly subjects and postulated that ventilatory control mechanisms were implicated. Of note, studies confirm that breathing instability trigger airway collapse (5), i.e., induction of periodic breathing during sleep promotes upper airway obstruction in adult humans (27, 38, 49). Thus it is likely that changes in ventilatory control stability may predispose to age-related breathing instability (50) and, ultimately, promote upper airway obstruction during sleep. However, available physiological studies have not confirmed the above theory. In one study, healthy elderly subjects and elderly obstructive sleep apnea (OSA) patients had reduced loop gains and reduced predisposition to periodic breathing using proportional assisted ventilation (PAV), which determined the propensity for mechanically augmented loop gain (51). In a separate study, steady-state loop gain was also lower in older vs. younger OSA patients (22). Conversely, we have demonstrated that the elderly have higher peripheral chemoresponsiveness than young adults (16), which may contribute to increased periodic breathing. Given the conflicting findings, it is important to clarify the underlying pathophysiology for SDB in the elderly, which may inform targeted therapies of sleep apnea in the elderly. Thus we sought to investigate the underlying ventilatory control mechanisms in healthy elderly adults during sleep and compare them with younger adults. We hypothesized that compared with young adults; older adults have an increased propensity to hypocapnic central apnea as expressed by a reduced carbon-dioxide (CO2) reserve during non-rapid eye movement (NREM) sleep. The CO2 reserve was defined as the difference in the end-tidal CO2 () between eupnea and apneic threshold (AT), where the AT was the that demarcated the first central apnea.
METHODS
Participants
The Human Investigation Committees of Wayne State University School of Medicine and Detroit Veterans Affairs Medical Center approved the experimental protocols. Ten healthy elderly adults participants (age 60 yr or greater) free of sleep apnea or other medical disorders, except for one individual with a history of benign hypertension, participated in the study. The participants had normal pulmonary function tests and electrocardiogram. Fifteen healthy young participants (age 20–50 yr) free of daytime sleepiness, sleep, or medical disorders were studied as controls. Informed written consent was obtained from all participants. All elderly and young participants were screened for sleep apnea with questionnaires and sleep studies; the latter were scored per standard American Academy of Sleep Medicine scoring rules (8). An apnea hypopnea index of <10 events per hour was set as the cut-off to determine the absence of SDB. A full night in-laboratory polysomnography study was available in 8 of the 10 older adults; for the remaining 2 older adults, the apnea hypopnea index was obtained from a baseline period of recording on the night of the experimental study. Sleep apnea in the young group was also ruled out either with full polysomnography (n = 5) or a period of baseline recording at the same night of the AT study (n = 10). Please see Table 1 for the apnea-hypopnea indexes in the two age groups.
Table 1.
Patient characteristics
| Age group | Older (n = 10) | Young (n = 15) |
|---|---|---|
| Age, yr | 66.9 ± 5.4 | 35.9 ± 9.9 |
| BMI, kg/m2 | 26.4 ± 2.5 | 27.3 ± 3.2 |
| Gender | 6F/4M | 8F/7M |
| Apnea-hypopnea index/h | 6.2 ± 3.8 | 1.2 ± 1.3 |
| CO2 production, ml·min−1·kg−1 | 4.1 ± 0.2 | 4.2 ± 0.2 |
Results are means ± SD. BMI, body mass index; F, female; M, male.
Breathing Circuit
The following methodology has been previously described by our group (15, 18, 59). Each participant was connected to the breathing circuit via a nasal mask. An appropriate-sized, airtight silicone nasal mask (Respironics, Murrysville, PA) was glued to the face to prevent mask leaks. The mask was connected to a Plateau Exhalation Valve (Respironics, Pittsburgh, PA), via a heated pneumotachometer. The valve, which provides a continuous leak path in the breathing circuit and serves as an exhaust vent, was connected to the inspiratory line. Participants were restricted to nasal breathing by placing tape over the mouth. As described in Mechanical ventilation protocol, hyperventilation was achieved using a pressure support ventilator [Quantum PSV; Healthdyne Technologies (Marietta, GA) or a bilevel positive airway pressure (PAP) machine; Resmed Sullivan VPAP II ST-A] with a minimum achievable expiratory positive airway pressure of 2–4 cm H2O.
Measurements
Electroencephalograms (EEG), electrooculograms (EOG), and chin electromyograms (EMG) were recorded using the International 10–20 system of electrode placement (EEG: C3-A2 and C4-A1; EOG, O-A2). Inspiratory airflow was measured by a heated pneumotachometer (Model 3700A; Hans Rudolph, Kansas City, MO) that was attached to a pressure transducer (Validyne, Northridge, CA). The tidal volume (VT) was obtained from the electronic integration of the flow signal (Model FV156 Integrator; Validyne, Northridge, CA). To confirm the central etiology of apnea and to ascertain upper airway mechanics, supraglottic pressure (PSG) was measured using a pressure transducer tipped catheter (Model TC-500XG; Millar Instruments, Houston, TX), with the tip positioned in the hypopharynx. The hypopharyngeal position was obtained by advancing the catheter tip for 2 cm after it disappeared behind the tongue. readings were obtained continuously by an infrared analyzer (Model CD-3A; AEI Technologies, Pittsburgh, PA) from tubing placed in the nares via a port in nasal mask. Arterial oxygen saturation () was measured by a pulse oximeter (Biox 3700; Ohmeda). The signals were displayed on a polygraph recorder (Grass Model 15; Astro-Med, West Warwick, RI) and recorded using Powerlab data acquisition software (AD Instruments, Colorado Springs, CO) for detailed analysis.
Experimental Protocol
Overview.
All participants underwent the same experimental protocol during their normal nocturnal sleep period. Study participants were instructed to limit total sleep time to a maximum of 4–5 h on the night preceding the study.
Mechanical ventilation protocol.
Participants assumed a supine position for the entire experimental protocol conducted during stable stage 2 or stage 3 sleep. We used noninvasive positive pressure mechanical ventilation (MV) to determine the AT as described previously (15, 18, 59). MV was applied for 3 min, in the spontaneous-timed mode during stable NREM sleep. Briefly, to achieve this, the inspiratory positive airway pressure was increased gradually in 1 to 2 cm H2O increments starting from 6 to 8 cm H2O at the beginning of each MV trial, while keeping the expiratory positive airway pressure fixed at the minimum 2–4 cm H2O allowed by the machine. MV was terminated after 3 min during expiration by returning the inspiratory positive airway pressure to the baseline expiratory positive airway pressure. The ensuing hypocapnia resulted in either a hypopnea or central apnea. Central apnea was defined as an expiratory time ≥5 s. If an apnea was not induced, additional hyperventilation trials at intervals of 3 min were completed until an apnea was evident. The elderly participants initially underwent MV without zolpidem. However, the elderly participants were consistently unable to maintain sleep through the MV trials; subsequently, the protocol was modified to allowed the elderly patients to receive 10 mg zolpidem, a nonbenzodiazepine GABA receptor agonist, before the study and this allowed sustained stable NREM sleep with successful completion of the experimental protocol. All MV trials were conducted in stable stage N2 or stage N3 sleep. Zolpidem has been used consistently by other authors for similar physiological experiments without altering the AT (54, 57) (see discussion).
Data Analysis
Sleep staging and scoring of arousals were completed using standard criteria, analyzing trials with stable NREM sleep (28). We analyzed MV trials accompanied by a stable stage N2 sleep state for all study patients; stage N3 sleep was rare in our participants due to detailed instrumentation. We analyzed 10 ± 4 trials per individual. During the control period of the study, five breaths recorded immediately before the onset of MV were averaged. Likewise, during the MV period, the last five mechanically ventilated breaths before the return to baseline expiratory positive airway pressure were averaged. The nadir breath immediately following MV was recorded. The data analysis methodology has been previously described (15, 18, 59). The AT was defined as the end-tidal CO2 () that demarcated the central apnea closest to the eupneic . The CO2 reserve was defined as the difference in between eupneic (control) and AT (i.e., Δ). The “hypocapnic ventilatory response” was calculated for each trial as the ratio of change in minute ventilation (V̇e) between control and the post-MV periods to the corresponding Δ associated with the AT or a hypopnea (ΔV̇e/), i.e., this is the slope of the ventilatory response (also known as the controller gain) (15, 21). The plant gain, which is a measure of the background drive to breath (21), was estimated as the ratio of the eupneic to the corresponding eupneic V̇e for each control period of each MV trial. A smaller or reduced CO2 reserve with a higher hypocapnic ventilatory response indicates increased propensity for breathing instability whereas a larger CO2 reserve with a lower hypocapnic ventilatory response indicates reduced propensity for central apneas and periodic breathing (21). We measured steady-state plant gain to ascertain the ease by which a sustained nonchemical ventilatory stimulus lowers Po2 towards the AT. There are also two other determinants of feedback instability that were not assessed in our study, namely plant gain dynamics (e.g., lung volume) and circulatory delay.
The CO2 production was calculated by the usual method as previously described (15, 52) to determine if the observed physiological changes were related to change in metabolic rate. The CO2 production was calculated using the equation: V̇co2 = × (VA/0.863), where VA (alveolar ventilation) = V̇i – VD; VD (dead space; in ml) was estimated as being equal to the participant’s weight (pounds); V̇i was the baseline minute ventilation; and the estimated respiratory quotient was taken as 0.863 (52). The results are expressed as millilters per minute per kilogram of body weight. Although the CO2 reserve has been reported in a few of the young adults from our group (17, 46), we have not previously reported analyses including plant and controller gains and metabolic rate in these individuals.
Statistical Analysis
A commercially available computer statistical package was used to analyze the data (Sigma Stat 3.11.0, SPSS). The level of statistical significance was set at P ≤ 0.05. The measured values obtained during NREM sleep were compared between the two age groups. For normally distributed data, comparisons between the age groups were made using t-test. In addition, two-way ANOVA was conducted for each dependent variable using age groups (elderly/young) and gender (male/female) as the two factors. Linear regression analyses were conducted to determine the coefficients for age, body mass index, and gender for each of the measured (eupneic , eupneic V̇e, AT, and CO2 reserve) and calculated (controller and plant gains) dependent variables. The results are presented as means ± SE of mean unless specified otherwise.
RESULTS
The demographic characteristics of the participants are presented in Table 1.
Effect of Older Age on Eupneic Breathing and the AT
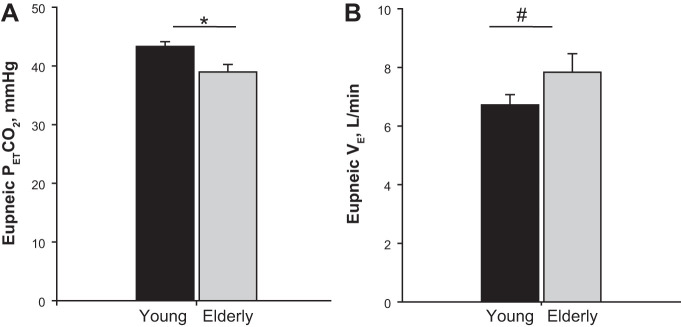
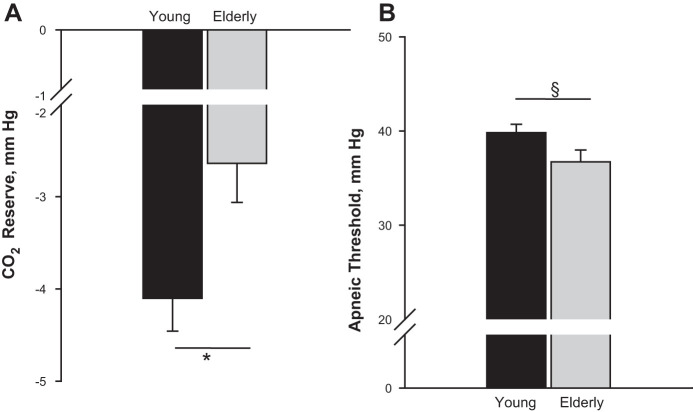
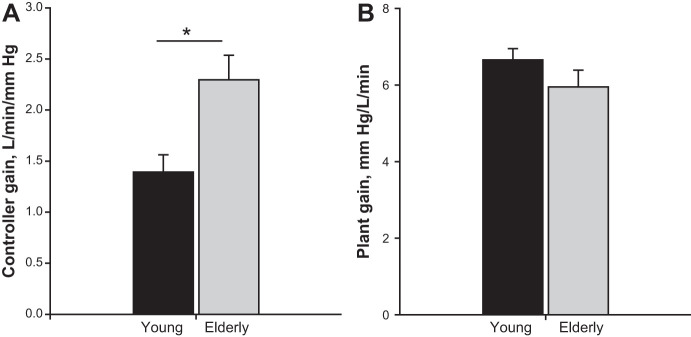
Eupneic was significantly lower in the elderly vs. young (39.0 ± 1.3 vs. 43.3 ± 0.8 mmHg, P = 0.006, Fig. 1A) with a higher eupneic V̇e (7.8 ± 0.6 vs. 6.7 ± 0.4 l/min, P = 0.01, Fig. 1B) after adjusting for gender. The CO2 reserve, i.e., the difference between the AT and the eupneic , was significantly smaller (−2.6 ± 0.4 vs. −4.1 ± 0.4, P = 0.01, Fig. 2A). The narrow CO2 reserve in the elderly was accompanied with a significantly higher hypocapnic ventilatory response (2.3 ± 0.2 vs. 1.4 ± 0.2 l·min−1·mmHg−1, P = 0.007, Fig. 3A), indicating increased chemoresponsiveness. However, plant gain was not significantly different between elderly vs. young adults (5.9 ± 0.4 vs. 6.6 ± 0.3 mmHg·l−1·min, P = 0.18) (Fig. 3B). Due to the lower eupneic in the elderly, the demarcating the AT was significantly lower in the elderly vs. young (36.7 ± 1.3 vs. 39.8 ± 0.9 mmHg, P = 0.049, Fig. 2B). There was no interaction between age and gender for any of these variables. Finally, the CO2 production was also not different between the age groups (Table 1). Regression analyses demonstrated that after adjusting for gender, age was a significant predictor for eupneic , eupneic V̇e, AT, CO2 reserve, and controller gain, respectively (Table 2).
Fig. 1.
Grouped data comparing the eupneic end-tidal CO2 (A) and eupneic V̇e (B) in the 2 age groups: young and elderly. The bars represent averaged data for each age group (black bar = young, n = 15; gray bar = elderly age, n = 10). The eupneic was significantly lower (*P = 0.006), whereas V̇e was significantly higher in the elderly vs. young age groups (#P = 0.01).
Fig. 2.
Grouped data comparing the CO2 reserve (A) and apneic threshold (B). The bars represent averaged data for each age group (black bar = young, n = 15; gray bar = elderly age, n = 10). The CO2 reserve was reduced in elderly vs. young adults, denoting an increased propensity to central apneas (*P = 0.01). The apneic threshold was significantly reduced in the elderly (§P = 0.049) (see text for explanation).
Fig. 3.
Grouped data comparing the controller gain (A) and plant gain (B) in the two age groups: young and elderly age. The bars represent averaged data for each age group (black bar = young; gray bar = elderly age, respectively). The controller gain was significantly higher in the elderly compared with young (*P = 0.007), whereas, the plant gain was not significantly different between the age groups.
Table 2.
Results of linear regression analyses adjusted for BMI
| Dependent Variable | Coefficient for Age groups: Elderly vs. Young (Reference) | Coefficient for Gender: Female vs. Male (Reference) |
|---|---|---|
| Apneic threshold, mmHg | −3.2, P = 0.05* | 0.7, P = ns |
| CO2 reserve, mmHg | −1.3, P = 0.02* | −1.0, P = 0.07 |
| Controller gain, l·min−1·mmHg−1 | 0.9, P = 0.004* | 0.3, P = ns |
| Eupneic CO2, mmHg | −4.2, P = 0.01* | −1.2, P = ns |
| Eupneic V̇e, l/min | 1.5, P = 0.01* | −2.0, P = 0.001* |
| Plant gain, mmHg·l−1·min | −0.9, P = 0.04 | 0.5, P = ns |
Denotes significant coefficient.
DISCUSSION
Summary of Findings
Our study in healthy individuals from old and young age groups revealed the following important findings: first, the CO2 reserve was significantly smaller in older vs. younger adults, demonstrating an age-related increase in the propensity to develop central apnea during NREM sleep, i.e., a smaller decline in during sleep from eupneic breathing was sufficient to induce a central apnea. Second, narrowing of the CO2 reserve was associated with increased hypocapnic ventilatory response in older adults but without differences in plant gain. These findings may explain the increased prevalence of central sleep apnea in older adults.
Mechanism(s) of the Age Effect
We noted narrowing of the CO2 reserve indicative of increased propensity to develop central apnea. To identify potential mechanisms, it is useful to conceptualize the ventilatory control during sleep as a negative feedback closed-loop cycle. Accordingly, a brief hyperventilation leads to hypocapnia and a subsequent ventilatory decline. The magnitude of the decline is determined by the degree of hypocapnia for a given ventilatory increase (“plant” factors) and by the ventilatory response to hypocapnia (chemoreflex sensitivity) (14, 21). Many authors have adopted the engineering concept of “loop gain” as a useful framework to describe susceptibility to central apnea and recurrent periodic breathing (21, 30). Loop gain is an index to express the ventilatory change for a given perturbation, combining two types of gain: plant gain and controller gain, the two main factors that determine the CO2 reserve. We estimated the “steady-state” overall loop gain as the steady-state plant gain multiplied by the controller gain, and found it to be higher in older vs. young adults (14.1 ± 1.9 vs. 9.2 ± 1.2, P = 0.04). Thus the increased propensity to hypocapnic central apnea conveyed by the increased loop gain in older adults is attributed to increased chemoreceptor sensitivity with no differences in steady-state plant gain or metabolic rate. However, two other determinants of feedback instability could not be assessed in our study namely dynamic plant gain and circulatory delay.
The loss of the wakefulness drive to breathe leads to sleep-related hypoventilation and hence increased plant gain. We noted that plant gain was not different between the older and young age groups, suggesting that hypoventilation was not the likely culprit. Instead, we noted increased hypocapnic chemoreflex sensitivity during sleep in elderly adults relative to the younger group, indicative of increased central chemoreceptor activity. These findings corroborate our previous findings demonstrating increased peripheral chemoreceptor activity in older adults (16).
The effect of age on chemoreflex sensitivity remains unclear. Studies during wakefulness have reached conflicting conclusions regarding the effect of age on the CO2 or hypoxic ventilatory response (13, 35, 42, 43). Conversely, studies during sleep have investigated the effect of hypercapnia (10) but not hypocapnia on ventilation in the elderly population. A prior study from our laboratory noted increased central apnea propensity in postmenopausal women, likely related to hormonal changes rather than age (45). Moreover, the study population included only a small number of older adults in the age range of 50 to 60 yr, whereas the present study was carefully designed and powered to detect differences between a well-demarcated young age group from an older population (age range of 60 to 76 yr) of men and postmenopausal women. Nevertheless, our study was not powered to detect additional gender differences within the age groups.
Increased propensity to hypocapnic central apnea provides a physiologic explanation for the marked increase in the prevalence of central apnea in older adults (9, 25). We considered several potential explanations for increased central apnea propensity. While sleep-state instability may contribute to spontaneous central apnea (39), our protocol induced central apnea during stable NREM sleep; thus sleep state oscillations did not account for our findings. In addition, there is no effect of aging on the arousal threshold (23). Upper airway structure and function were unlikely to affect our results. While some studies have noted narrowed upper airway dimensions in the elderly vs. young adults, others have not (11, 12, 33, 34, 48, 53). Data are also conflicting about whether the upper airway is more collapsible in the elderly vs. young (11, 23). Older adults had greater genioglossus EMG activity than young in one study (11), and the length of the airway, an important factor that determines airway stability, was also not greater in older vs. young adults (33).
Cerebrovascular responsiveness to CO2 is a potential contributor to chemoresponsiveness (1, 6). In healthy young adults, a decrease in cerebral blood flow allows accumulation of CO2 that stimulates ventilation, while increased cerebral blood flow allows CO2 removal and depresses ventilation during wakefulness and sleep (2, 29, 55, 56). This was demonstrated in young healthy adults following administration of oral indomethacin, a drug that reduces cerebral blood flow velocity. Indomethacin was associated with reduced cerebrovascular response to hypocapnia (54) leading to increased AT and narrowed CO2 reserve, i.e., indicating that a reduction in cerebrovascular responsiveness increases the propensity for central apneas. Given that aging is associated with reduced vasoregulatory capacity along with decreased cerebral blood flow velocities and reduced reactivity to CO2 (31, 41), potentially the observed age-related breathing instability during sleep may be mediated, in part, by reductions in cerebral vascular reactivity to CO2.
Peripheral chemoreflex sensitivity may also contribute to the development of central apnea. We have previously demonstrated age-related increase in peripheral chemosensitivity, as evidenced by increased isocapnic hypoxic ventilatory response and hyperoxic suppression of ventilation during NREM sleep, compared with young adults (16). Thus the combination of increased chemosensitivity and reduced cerebrovascular responsiveness to CO2 may explain the narrow CO2 reserve and increased propensity for central apneas in elderly individuals during sleep. The aforementioned discussion is a theoretical explanation awaiting empirical proof.
Our finding of increased chemoreflex sensitivity is in contrast to a previous study demonstrating decreased loop gain in older adults. Using proportional assist ventilation (PAV), Wellman et al. (51) noted decreased loop gain in healthy older adults, free of OSA. In fact, loop gain or controller gain is expected to be elevated in patients with OSA compared with non-OSA individuals (46, 50). Methodological differences may account for different findings. For example, the application of PAV may alter plant gain via an effect on lung volume and oxygen stores and may also alter circulatory delay. Individual characteristics, including age, may influence the physiologic effects of PAV. Of note, here are several limitations to using PAV which could have influenced loop gain per se that Wellman et al. (50) alluded to, by inducing periodic breathing, raising questions about the validity of using PAV to determine loop gain. A second study in a group of young and old patients with OSA, using a different methodology of decreasing PAP in a step-wise manner, also found increased collapsibility and decreased loop gain in older adults (22), suggesting that upper airway factors were more important in older adults with OSA. However, unlike our study, this study was restricted to participants with OSA (22) and hence was not designed to test the specific effects of aging on the underlying traits. Moreover, it is possible that the loop gains assessed with PAV (i.e., dynamic loop gain) and the PAP-drop method (i.e., steady-state loop gains) may not be comparable. Using a validated and well-established methodology of measuring CO2 reserve and controller gain, we have demonstrated that in older healthy adults without OSA the controller gain is elevated with a reduction in the CO2 reserve, indicating an increased propensity for central apneas during NREM sleep. We likely need more evidence from larger cohorts of healthy elderly adults to have conclusive evidence on this topic.
Methodological Considerations
Our analysis included only trials with stable non-REM sleep state to ensure that sleep state changes did not influence the AT. The present study utilized noninvasive ventilation as a model to evaluate propensity to hypocapnic central apnea; our laboratory has used and validated this intervention in multiple studies (17, 18, 59). Several considerations may influence the interpretation of the findings. We allowed slight sleep restriction before the study. Our previous experience demonstrated no difference in the findings between subjects who obtained normal vs. curtailed nocturnal sleep (15, 18), and the available literature supports the fact that sleep deprivation per se does not alter ventilatory responsiveness (47). In both the older and young age groups the MV trials and data analysis were completed during stable stage 2 sleep; hence, sleep state per se would not have impacted the results of the experiments.
We used low-dose zolpidem to ensure stable sleep state during the study in older adults. Prior published studies did not find that zolpidem influences ventilatory parameters during sleep (7, 16, 32, 37, 57). Zolpidem is a nonbenzodiazepine GABA receptor agonist. We used zolpidem in older adults to allow completion of the experimental protocol with an adequate number of MV trials during stable sleep. Studies measuring the effect of zolpidem on respiratory control demonstrate that zolpidem does not alter the minute ventilation, oxygen saturation or chemoreflex function. For example, zolpidem does not impair nocturnal respiratory and sleep architecture, pulmonary function tests, occlusion pressure, or chemroesponiveness in young, middle-aged, and older adults (24). Additionally, in healthy elderly adults zolpidem at 10 mg did not increase the incidence of sleep apnea or sleep-related hypoxia compared with placebo (44). Compared with placebo, there was also no impact of zolpidem on SDB in middle-aged and young adults (37), on the ventilatory response to CO2 (20), or on tidal volume or ventilation (32). Due to the absence of effect of zolpidem on control of ventilation or on cerebral perfusion (19), Dempsey and other investigators (54, 57) have used zolpidem to stabilize sleep when determining the AT. Hence, given the established lack of effect of zolpidem on ventilation, nocturnal oxygenation, sleep apnea severity, or ventilatory control breathing during wake and sleep, we believe that zolpidem did not have an effect on the CO2 reserve or on hypocapnic ventilatory responsiveness during sleep in older adults in our study. A sedative agent, if at all, would be expected to reduce ventilatory drive and ventilatory responsiveness; however, in our study in older adults there was an increase in the hypocapnic ventilatory responsiveness, thus it unlikely that zolpidem contributed to changes in ventilatory control in older adults in our study.
Our study was not designed to study other mechanisms of breathing instability, including the relative contributions of peripheral-central chemoreceptors vs. cerebrovascular responsiveness during sleep. This study also only focused on healthy individuals without sleep apnea. The selection of healthy participants was deliberate to determine the association between age and chemoreceptors control without any intervening confounding factors that we consider was the strength of the study. Future studies are planned to investigate chemoreceptor contributions and cerebrovascular responsiveness as components of ventilatory control in older adults.
Implications to the Pathogenesis of SDB in The Elderly
There are significant adverse cardiovascular consequences of sleep apnea in the elderly (3, 4, 36). Our findings are relevant to elderly health as these results may provide the underpinnings for identifying alternative therapies for treating sleep apnea and positively impact the health of elderly with this prevalent disorder. The current limited state of knowledge regarding the pathophysiology of sleep apnea in the elderly has led to limited therapeutic options that are confined to positive airway pressure therapy. Our study allows us to identify specific physiological traits of ventilatory control in this population. Stabilizing central respiratory motor output via prevention of transient hypocapnia will likely prevent obstructive sleep apnea in patients with a high chemosensitivity and a collapsible upper airway (58), We expect that the information gained from these detailed studies will potentially allow us to identify similar traits in a sleep clinic setting and potentially guide the development of pathophysiology-defined personalized therapies for sleep apnea in the elderly.
GRANTS
Support for this work was provided by a Career Development Award-2 from the Department of Veterans Affairs (CDA-2–019–07F to S. Chowdhuri) and a Merit Review Award from the Department of Veterans Affairs (1I01CX000194 to M. S. Badr).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.C. and M.S.B. conceived and designed research; S.C., S.P., H.L.-K., and A.S. performed experiments; S.C., S.P., H.L.-K., A.S., and M.S.B. analyzed data; S.C., S.P., H.L.-K., A.S., and M.S.B. interpreted results of experiments; S.C., S.P., and M.S.B. prepared figures; S.C. and M.S.B. drafted manuscript; S.C. and M.S.B. edited and revised manuscript; S.C., S.P., H.L.-K., A.S., and M.S.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Nicole Nickert and Simran Narula for technical assistance.
REFERENCES
- 1.Ainslie PN, Duffin J. Integration of cerebrovascular CO2 reactivity and chemoreflex control of breathing: mechanisms of regulation, measurement, and interpretation. Am J Physiol Regul Integr Comp Physiol 296: R1473–R1495, 2009. doi: 10.1152/ajpregu.91008.2008. [DOI] [PubMed] [Google Scholar]
- 2.Ainslie PN, Poulin MJ. Ventilatory, cerebrovascular, and cardiovascular interactions in acute hypoxia: regulation by carbon dioxide. J Appl Physiol (1985) 97: 149–159, 2004. doi: 10.1152/japplphysiol.01385.2003. [DOI] [PubMed] [Google Scholar]
- 3.Ancoli-Israel S, DuHamel ER, Stepnowsky C, Engler R, Cohen-Zion M, Marler M. The relationship between congestive heart failure, sleep apnea, and mortality in older men. Chest 124: 1400–1405, 2003. doi: 10.1378/chest.124.4.1400. [DOI] [PubMed] [Google Scholar]
- 4.Ancoli-Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O. Sleep-disordered breathing in community-dwelling elderly. Sleep 14: 486–495, 1991. doi: 10.1093/sleep/14.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Badr MS, Toiber F, Skatrud JB, Dempsey J. Pharyngeal narrowing/occlusion during central sleep apnea. J Appl Physiol (1985) 78: 1806–1815, 1995. [DOI] [PubMed] [Google Scholar]
- 6.Przybyłowski T, Bangash MF, Reichmuth K, Morgan BJ, Skatrud JB, Dempsey JA. Mechanisms of the cerebrovascular response to apnoea in humans. J Physiol 548: 323–332, 2003. doi: 10.1111/j.1469-7793.2003.t01-1-00323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beaumont M, Goldenberg F, Lejeune D, Marotte H, Harf A, Lofaso F. Effect of zolpidem on sleep and ventilatory patterns at simulated altitude of 4,000 meters. Am J Respir Crit Care Med 153: 1864–1869, 1996. doi: 10.1164/ajrccm.153.6.8665047. [DOI] [PubMed] [Google Scholar]
- 8.Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, Marcus CL, Mehra R, Parthasarathy S, Quan SF, Redline S, Strohl KP, Davidson Ward SL, Tangredi MM; American Academy of Sleep Medicine; Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine . Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. J Clin Sleep Med 8: 597–619, 2012. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med 157: 144–148, 1998. doi: 10.1164/ajrccm.157.1.9706079. [DOI] [PubMed] [Google Scholar]
- 10.Browne HA, Adams L, Simonds AK, Morrell MJ. Ageing does not influence the sleep-related decrease in the hypercapnic ventilatory response. Eur Respir J 21: 523–529, 2003. doi: 10.1183/09031936.03.00039002. [DOI] [PubMed] [Google Scholar]
- 11.Burger CD, Stanson AW, Sheedy PF II, Daniels BK, Shepard JW Jr. Fast-computed tomography evaluation of age-related changes in upper airway structure and function in normal men. Am Rev Respir Dis 145: 846–852, 1992. doi: 10.1164/ajrccm/145.4_Pt_1.846. [DOI] [PubMed] [Google Scholar]
- 12.Carlisle T, Carthy ER, Glasser M, Drivas P, McMillan A, Cowie MR, Simonds AK, Morrell MJ. Upper airway factors that protect against obstructive sleep apnoea in healthy older males. Eur Respir J 44: 685–693, 2014. doi: 10.1183/09031936.00177213. [DOI] [PubMed] [Google Scholar]
- 13.Chapman KR, Cherniack NS. Aging effects on the interaction of hypercapnia and hypoxia as ventilatory stimuli. J Gerontol 42: 202–209, 1987. doi: 10.1093/geronj/42.2.202. [DOI] [PubMed] [Google Scholar]
- 14.Chowdhuri S, Badr MS. Control of ventilation in health and disease. CHEST J 151: 917–929, 2016. doi: 10.1016/j.chest.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chowdhuri S, Bascom A, Mohan D, Diamond MP, Badr MS. Testosterone conversion blockade increases breathing stability in healthy men during NREM sleep. Sleep (Basel) 36: 1793–1798, 2013. doi: 10.5665/sleep.3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chowdhuri S, Pranathiageswaran S, Franco-Elizondo R, Jayakar A, Hosni A, Nair A, Badr MS. Effect of age on long-term facilitation and chemosensitivity during NREM sleep. J Appl Physiol (1985) 119: 1088–1096, 2015. doi: 10.1152/japplphysiol.00030.2015. [DOI] [PubMed] [Google Scholar]
- 17.Chowdhuri S, Shanidze I, Pierchala L, Belen D, Mateika JH, Badr MS. Effect of episodic hypoxia on the susceptibility to hypocapnic central apnea during NREM sleep. J Appl Physiol (1985) 108: 369–377, 2010. doi: 10.1152/japplphysiol.00308.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chowdhuri S, Sinha P, Pranathiageswaran S, Badr MS. Sustained hyperoxia stabilizes breathing in healthy individuals during NREM sleep. J Appl Physiol (1985) 109: 1378–1383, 2010. doi: 10.1152/japplphysiol.00453.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clauss RP, Dormehl IC, Oliver DW, Nel WH, Kilian E, Louw WK. Measurement of cerebral perfusion after zolpidem administration in the baboon model. Arzneimittelforschung 51: 619–622, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Cohn MA. Effects of zolpidem, codeine phosphate and placebo on respiration. A double-blind, crossover study in volunteers. Drug Saf 9: 312–319, 1993. doi: 10.2165/00002018-199309040-00009. [DOI] [PubMed] [Google Scholar]
- 21.Dempsey JA. Crossing the apnoeic threshold: causes and consequences. Exp Physiol 90: 13–24, 2005. doi: 10.1113/expphysiol.2004.028985. [DOI] [PubMed] [Google Scholar]
- 22.Edwards BA, Wellman A, Sands SA, Owens RL, Eckert DJ, White DP, Malhotra A. Obstructive sleep apnea in older adults is a distinctly different physiological phenotype. Sleep (Basel) 37: 1227–1236, 2014. doi: 10.5665/sleep.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eikermann M, Jordan AS, Chamberlin NL, Gautam S, Wellman A, Lo YL, White DP, Malhotra A. The influence of aging on pharyngeal collapsibility during sleep. Chest 131: 1702–1709, 2007. doi: 10.1378/chest.06-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Girault C, Muir JF, Mihaltan F, Borderies P, De La Giclais B, Verdure A, Samson-Dollfus D. Effects of repeated administration of zolpidem on sleep, diurnal and nocturnal respiratory function, vigilance, and physical performance in patients with COPD. Chest 110: 1203–1211, 1996. doi: 10.1378/chest.110.5.1203. [DOI] [PubMed] [Google Scholar]
- 25.Hoch CC, Reynolds CF III, Monk TH, Buysse DJ, Yeager AL, Houck PR, Kupfer DJ. Comparison of sleep-disordered breathing among healthy elderly in the seventh, eighth, and ninth decades of life. Sleep 13: 502–511, 1990. doi: 10.1093/sleep/13.6.502. [DOI] [PubMed] [Google Scholar]
- 26.Horner RL. Motor control of the pharyngeal musculature and implications for the pathogenesis of obstructive sleep apnea. Sleep 19: 827–853, 1996. doi: 10.1093/sleep/19.10.827. [DOI] [PubMed] [Google Scholar]
- 27.Hudgel DW, Gordon EA, Thanakitcharu S, Bruce EN. Instability of ventilatory control in patients with obstructive sleep apnea. Am J Respir Crit Care Med 158: 1142–1149, 1998. doi: 10.1164/ajrccm.158.4.9712105. [DOI] [PubMed] [Google Scholar]
- 28.Iber C, Ancoli-Israel S, Chesson A, Quan SF (editors). The American Academy of Sleep Medicine Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications . Westchester, IL: American Academy of Sleep Medicine, 2007. [Google Scholar]
- 29.Kety SS, Schmidt CF. The effects of altered arterial tensions of carbon dioxide and oxygen on cerebral blood flow and cerebral oxygen consumption of normal young men. J Clin Invest 27: 484–492, 1948. doi: 10.1172/JCI101995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khoo MC, Kronauer RE, Strohl KP, Slutsky AS. Factors inducing periodic breathing in humans: a general model. J Appl Physiol Respir Environ Exerc Physiol 53: 644–659, 1982. [DOI] [PubMed] [Google Scholar]
- 31.Lu H, Xu F, Rodrigue KM, Kennedy KM, Cheng Y, Flicker B, Hebrank AC, Uh J, Park DC. Alterations in cerebral metabolic rate and blood supply across the adult lifespan. Cereb Cortex 21: 1426–1434, 2011. doi: 10.1093/cercor/bhq224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maillard D, Thiercelin JF, Fuseau E, Rosenzweig P, Attali P. Effects of zolpidem versus diazepam and placebo on breathing control parameters in healthy human subjects. Int J Clin Pharmacol Res 12: 27–35, 1992. [PubMed] [Google Scholar]
- 33.Malhotra A, Huang Y, Fogel R, Lazic S, Pillar G, Jakab M, Kikinis R, White DP. Aging influences on pharyngeal anatomy and physiology: the predisposition to pharyngeal collapse. Am J Med 119: 72.e9–72.e14, 2006. doi: 10.1016/j.amjmed.2005.01.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin SE, Mathur R, Marshall I, Douglas NJ. The effect of age, sex, obesity and posture on upper airway size. Eur Respir J 10: 2087–2090, 1997. doi: 10.1183/09031936.97.10092087. [DOI] [PubMed] [Google Scholar]
- 35.Martinez D. Effects of aging on peripheral chemoreceptor CO2 response during sleep and wakefulness in healthy men. Respir Physiol Neurobiol 162: 138–143, 2008. doi: 10.1016/j.resp.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 36.Martínez-García MA, Campos-Rodríguez F, Catalán-Serra P, Soler-Cataluña JJ, Almeida-Gonzalez C, De la Cruz Morón I, Durán-Cantolla J, Montserrat JM. Cardiovascular mortality in obstructive sleep apnea in the elderly: role of long-term continuous positive airway pressure treatment: a prospective observational study. Am J Respir Crit Care Med 186: 909–916, 2012. doi: 10.1164/rccm.201203-0448OC. [DOI] [PubMed] [Google Scholar]
- 37.McCann CC, Quera-Salva MA, Boudet J, Frisk M, Barthouil P, Borderies P, Meyer P. Effect of zolpidem during sleep on ventilation and cardiovascular variables in normal subjects. Fundam Clin Pharmacol 7: 305–310, 1993. doi: 10.1111/j.1472-8206.1993.tb00244.x. [DOI] [PubMed] [Google Scholar]
- 38.Onal E, Burrows DL, Hart RH, Lopata M. Induction of periodic breathing during sleep causes upper airway obstruction in humans. J Appl Physiol (1985) 61: 1438–1443, 1986. [DOI] [PubMed] [Google Scholar]
- 39.Pack AI, Cola MF, Goldszmidt A, Ogilvie MD, Gottschalk A. Correlation between oscillations in ventilation and frequency content of the electroencephalogram. J Appl Physiol (1985) 72: 985–992, 1992. [DOI] [PubMed] [Google Scholar]
- 40.Pack AI, Silage DA, Millman RP, Knight H, Shore ET, Chung DC. Spectral analysis of ventilation in elderly subjects awake and asleep. J Appl Physiol (1985) 64: 1257–1267, 1988. [DOI] [PubMed] [Google Scholar]
- 41.Peisker T, Bartoš A, Skoda O, Ibrahim I, Kalvach P. Impact of aging on cerebral vasoregulation and parenchymal integrity. J Neurol Sci 299: 112–115, 2010. doi: 10.1016/j.jns.2010.08.064. [DOI] [PubMed] [Google Scholar]
- 42.Pokorski M, Marczak M. Ventilatory response to hypoxia in elderly women. Ann Hum Biol 30: 53–64, 2003. doi: 10.1080/03014460210162000. [DOI] [PubMed] [Google Scholar]
- 43.Poulin MJ, Cunningham DA, Paterson DH, Kowalchuk JM, Smith WD. Ventilatory sensitivity to CO2 in hyperoxia and hypoxia in older aged humans. J Appl Physiol (1985) 75: 2209–2216, 1993. [DOI] [PubMed] [Google Scholar]
- 44.Rhodes SP, Parry P, Hanning CD. A comparison of the effects of zolpidem and placebo on respiration and oxygen saturation during sleep in the healthy elderly. Br J Clin Pharmacol 30: 817–824, 1990. doi: 10.1111/j.1365-2125.1990.tb05446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rowley JA, Zhou XS, Diamond MP, Badr MS. The determinants of the apnea threshold during NREM sleep in normal subjects. Sleep 29: 95–103, 2006. doi: 10.1093/sleep/29.1.95. [DOI] [PubMed] [Google Scholar]
- 46.Salloum A, Rowley JA, Mateika JH, Chowdhuri S, Omran Q, Badr MS. Increased propensity for central apnea in patients with obstructive sleep apnea: effect of nasal continuous positive airway pressure. Am J Respir Crit Care Med 181: 189–193, 2010. doi: 10.1164/rccm.200810-1658OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spengler CM, Shea SA. Sleep deprivation per se does not decrease the hypercapnic ventilatory response in humans. Am J Respir Crit Care Med 161: 1124–1128, 2000. doi: 10.1164/ajrccm.161.4.9906026. [DOI] [PubMed] [Google Scholar]
- 48.Thurnheer R, Wraith PK, Douglas NJ. Influence of age and gender on upper airway resistance in NREM and REM sleep. J Appl Physiol (1985) 90: 981–988, 2001. [DOI] [PubMed] [Google Scholar]
- 49.Warner G, Skatrud JB, Dempsey JA. Effect of hypoxia-induced periodic breathing on upper airway obstruction during sleep. J Appl Physiol (1985) 62: 2201–2211, 1987. [DOI] [PubMed] [Google Scholar]
- 50.Wellman A, Jordan AS, Malhotra A, Fogel RB, Katz ES, Schory K, Edwards JK, White DP. Ventilatory control and airway anatomy in obstructive sleep apnea. Am J Respir Crit Care Med 170: 1225–1232, 2004. doi: 10.1164/rccm.200404-510OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wellman A, Malhotra A, Jordan AS, Schory K, Gautam S, White DP. Chemical control stability in the elderly. J Physiol 581: 291–298, 2007. doi: 10.1113/jphysiol.2006.126409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.West JB. Respiratory Physiology–the Essentials. Baltimore, MD: Williams & Wilkins, 1974. [Google Scholar]
- 53.Worsnop C, Kay A, Kim Y, Trinder J, Pierce R. Effect of age on sleep onset-related changes in respiratory pump and upper airway muscle function. J Appl Physiol (1985) 88: 1831–1839, 2000. [DOI] [PubMed] [Google Scholar]
- 54.Xie A, Skatrud JB, Barczi SR, Reichmuth K, Morgan BJ, Mont S, Dempsey JA. Influence of cerebral blood flow on breathing stability. J Appl Physiol (1985) 106: 850–856, 2009. doi: 10.1152/japplphysiol.90914.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xie A, Skatrud JB, Khayat R, Dempsey JA, Morgan B, Russell D. Cerebrovascular response to carbon dioxide in patients with congestive heart failure. Am J Respir Crit Care Med 172: 371–378, 2005. doi: 10.1164/rccm.200406-807OC. [DOI] [PubMed] [Google Scholar]
- 56.Xie A, Skatrud JB, Morgan B, Chenuel B, Khayat R, Reichmuth K, Lin J, Dempsey JA. Influence of cerebrovascular function on the hypercapnic ventilatory response in healthy humans. J Physiol 577: 319–329, 2006. doi: 10.1113/jphysiol.2006.110627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xie A, Skatrud JB, Puleo DS, Rahko PS, Dempsey JA. Apnea-hypopnea threshold for CO2 in patients with congestive heart failure. Am J Respir Crit Care Med 165: 1245–1250, 2002. doi: 10.1164/rccm.200110-022OC. [DOI] [PubMed] [Google Scholar]
- 58.Xie A, Teodorescu M, Pegelow DF, Teodorescu MC, Gong Y, Fedie JE, Dempsey JA. Effects of stabilizing or increasing respiratory motor outputs on obstructive sleep apnea. J Appl Physiol (1985) 115: 22–33, 2013. doi: 10.1152/japplphysiol.00064.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou XS, Rowley JA, Demirovic F, Diamond MP, Badr MS. Effect of testosterone on the apneic threshold in women during NREM sleep. J Appl Physiol (1985) 94: 101–107, 2003. doi: 10.1152/japplphysiol.00264.2002. [DOI] [PubMed] [Google Scholar]