Abstract
Background
The phase 1 cohorts (1c+1d) of CheckMate 143 (NCT02017717) evaluated the safety/tolerability and efficacy of nivolumab plus radiotherapy (RT) ± temozolomide (TMZ) in newly diagnosed glioblastoma.
Methods
In total, 136 patients were enrolled. In part A (safety lead-in), 31 patients (n = 15, methylated/unknown MGMT promoter; n = 16, unmethylated MGMT promoter) received nivolumab and RT+TMZ (NIVO+RT+TMZ) and 30 patients with unmethylated MGMT promoter received NIVO+RT. In part B (expansion), patients with unmethylated MGMT promoter were randomized to NIVO+RT+TMZ (n = 29) or NIVO+RT (n = 30). Primary endpoint was safety/tolerability; secondary endpoint was overall survival (OS).
Results
NIVO+RT±TMZ was tolerable; grade 3/4 treatment-related adverse events occurred in 51.6% (NIVO+RT+TMZ) and 30.0% (NIVO+RT) of patients in part A and 46.4% (NIVO+RT+TMZ) and 28.6% (NIVO+RT) in part B. No new safety signals were detected. In part A, median OS (mOS) with NIVO+RT+TMZ was 33.38 months (95% CI, 16.2 to not estimable) in patients with methylated MGMT promoter. In patients with unmethylated MGMT promoter, mOS was 16.49 months (12.94–22.08) with NIVO+RT+TMZ and 14.41 months (12.55–17.31) with NIVO+RT. In part B, mOS was 14.75 months (10.01–18.6) with NIVO+RT+TMZ and 13.96 months (10.81–18.14) with NIVO+RT in patients with unmethylated MGMT promoter.
Conclusions
CheckMate 143 was the first trial evaluating immune checkpoint inhibition with first-line treatment of glioblastoma. Results showed that NIVO can be safely combined with RT±TMZ, with no new safety signals. Toxicities, including lymphopenia, were more frequent with NIVO+RT+TMZ. OS was similar with or without TMZ in patients with unmethylated MGMT promoter, and differences by MGMT methylation status were observed.
Keywords: nivolumab, newly diagnosed glioblastoma, PD-1, radiotherapy, temozolomide
Key Points.
Nivolumab can be safely combined with RT±TMZ in patients with newly diagnosed GBM.
OS was similar with or without TMZ in patients with unmethylated MGMT promoter.
Differences in OS by MGMT promoter methylation status were observed.
Importance of the Study.
Glioblastomas generate an immunosuppressive environment that allows for escape from immune system surveillance. Immune checkpoint inhibitors have provided clinical benefit in multiple malignancies, and programmed cell death 1 ligand 1 (PD-L1) expression is high in glioblastoma, providing the rationale for exploring anti-programmed cell death 1 (PD-1) therapies in this disease. We report the first study investigating immune checkpoint inhibition in newly diagnosed glioblastoma. Results demonstrated that nivolumab can be safely combined with radiotherapy ± temozolomide, with no new safety signals identified. Toxicities, including lymphopenia, were more frequent with nivolumab + radiotherapy + temozolomide than nivolumab + radiotherapy. Survival results reflected MGMT promoter methylation status, and although these results seemed superior to historical controls, subsequent studies have not shown a survival advantage with nivolumab. The regimens developed in this study are being further investigated in several ongoing or planned trials exploring novel immunotherapies in glioblastoma.
Glioblastoma, the most common malignant primary brain tumor in adults, is associated with a poor prognosis, with 5-year survival rates of 5–10%.1–4 Patients with newly diagnosed disease are most commonly treated with surgical resection followed by radiotherapy (RT) with concomitant and adjuvant temozolomide (TMZ), with or without tumor-treating fields.5–7 However, nearly all patients experience relapse after completion of therapy, and salvage therapies such as TMZ, bevacizumab, lomustine, and tumor-treating fields have limited efficacy in the setting of recurrent disease.5,8,9 New and more active agents that improve clinical outcomes and quality of life are therefore urgently needed.
Chemosensitivity to TMZ has been linked to epigenetic silencing of the DNA repair enzyme O6-methylguanine-DNA methyltransferase (MGMT) via promoter methylation.10–12 Patients with tumor MGMT promoter methylation experience a better prognosis with RT + TMZ than those with an unmethylated MGMT promoter.11,13,14 Due to the lack of treatment alternatives, TMZ is offered to all patients, including those with an unmethylated MGMT promoter,4,6,7 although reassessment of the role of TMZ in that population remains of interest.15
Increasing evidence suggests that immune cells can enter the CNS and function within the tumor microenvironment (TME).16 In glioblastoma, tumor-infiltrating lymphocytes contain a high percentage of exhausted T cells overexpressing immune checkpoint receptors.17 Inhibition of these exhausted T cells may affect the glioblastoma TME and complement the effects of RT by amplifying tumor-specific immune responses to cell death and tumor antigen release.18,19 Programmed cell death 1 ligand 1 (PD-L1) is expressed in primary glioblastoma, and expression levels correlate with glioma grade and clinical outcomes.16,20,21 In murine glioblastoma models, checkpoint pathway inhibition with either single-agent anti-programmed cell death 1 (PD-1), anti-PD-L1, or anti-cytotoxic T-lymphocyte protein 4 (CTLA-4) therapy resulted in long-term tumor-free survival.22,23 Moreover, in murine glioma models, combination of a PD-1 inhibitor with RT improved overall survival (OS) vs either treatment alone.23 Additionally, anti-PD-1 treatment demonstrated local immunomodulatory effects characterized by enhanced expression of cytokine transcripts, higher immune-cell infiltration, and augmented T-cell receptor clonal diversity among tumor-infiltrating T lymphocytes when administered in a neoadjuvant setting, prior to surgery for recurrent tumors.24 Collectively, these findings suggest that immunotherapy may potentiate antitumor response in glioblastoma and support the rationale for combination with standard-of-care RT±TMZ.
Nivolumab, a fully human immunoglobulin G4 monoclonal antibody targeting the PD-1 receptor, is approved as a single agent or in combination with ipilimumab for the treatment of multiple advanced cancers, having shown improved survival or clinical benefit over prior standard-of-care therapies.25
CheckMate 143 is a multicohort trial designed to establish the safety of nivolumab in both newly diagnosed and recurrent glioblastoma in multiple phase 1 cohorts and to compare the safety and efficacy of nivolumab vs bevacizumab in patients with first recurrence of glioblastoma in a phase 3 cohort. Results from the recurrent disease cohorts have been previously reported.26,27 Here we report results from cohorts 1c and 1d, which are the first prospective phase 1 studies evaluating the safety and feasibility of combining nivolumab with standard-of-care, first-line RT with TMZ (NIVO+RT+TMZ) or without TMZ (NIVO+RT) in patients with newly diagnosed glioblastoma.
Methods
Patients
Eligible patients had newly diagnosed, histologically confirmed WHO grade IV malignant glioma (glioblastoma or gliosarcoma); had undergone at least a subtotal resection; and had not received previous treatment with TMZ, RT, or PD-1– and CTLA-4–targeted therapies. Patients had to be ≥18 years of age, have a Karnofsky performance status of ≥70, and be eligible to receive first-line standard-of-care treatment (surgery and RT±TMZ followed by maintenance TMZ). Topical, ocular, intra-articular, intranasal, and inhaled corticosteroids (with minimal systemic absorption) were permitted; systemic corticosteroid use or physiological replacement doses of steroids were permitted, even if >10-mg/day prednisone equivalents, for treatment-related adverse events (TRAEs), sequelae of underlying glioblastoma treatment, or treatment of non-autoimmune conditions. Corticosteroid dose documentation was required within 14 days prior to randomization. Patients requiring escalating or chronic supraphysiological doses of corticosteroids for control of disease at randomization were excluded. Additional exclusion criteria encompassed the following: recurrent or secondary glioblastoma (glioblastomas that progressed from low-grade diffuse astrocytoma or anaplastic astrocytoma); metastatic extracranial or leptomeningeal disease; active, known, or suspected autoimmune disease; and prior treatment with RT, carmustine wafer, chemotherapy, or investigational agent for glioblastoma. Patients with a historical report of an isocitrate dehydrogenase mutation were not included in this study.
Study Design and Treatment
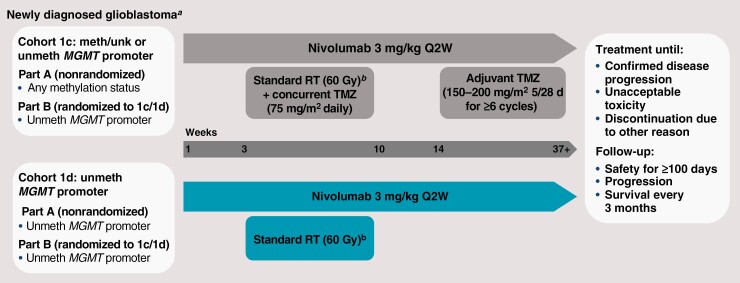
This report describes results (data cutoff: August 8, 2019) from the safety lead-in and expansion phases of the exploratory phase 1 cohorts of CheckMate 143 (NCT02017717), 1c and 1d. This study evaluated the safety and tolerability of nivolumab in combination with RT with or without TMZ in patients with newly diagnosed glioblastoma (Figure 1).
Figure 1.
Study design. Abbreviations: 5/28 d, days 1–5 of every 28-day cycle; meth, methylated; MGMT, O6-methylguanine-DNA methyltransferase; Q2W, every 2 weeks; RT, radiotherapy; TMZ, temozolomide; unk, unknown; unmeth, unmethylated. aPatients in both cohorts had surgical resection prior to starting study treatment. bStandard RT (60 Gy; 2-Gy fractions 5 days per week for ≤7 weeks) was administered starting ≥7 days after the first dose of nivolumab.
Patients were enrolled into cohorts 1c and 1d within 2 study parts: safety lead-in (part A) and randomized expansion (part B). In part A, patients were eligible for cohort 1c regardless of MGMT promoter methylation status (methylated, unknown, or unmethylated) and only patients with unmethylated MGMT promoter were enrolled in cohort 1d. During expansion in part B, only patients with unmethylated MGMT promoter were randomized 1:1 to cohort 1c or 1d.
Patients enrolled in each cohort received nivolumab after full recovery from surgical resection, as defined by no ongoing safety issues and ≥14 days from surgery and ≥7 days prior to first dose of RT. RT should have been initiated within 35 days of surgery. Nivolumab was then continued in combination with RT+TMZ (cohort 1c) or RT (cohort 1d). In cohort 1c, patients received nivolumab 3 mg/kg every 2 weeks with RT (60 Gy; 2-Gy fractions 5 days/week for ≤7 weeks) and TMZ (75 mg/m2 once daily during RT followed by a 4-week treatment break and then 150 mg/m2 [first cycle] followed by 200 mg/m2 [second cycle+] once daily on days 1-5 of every 28-day cycle for ≥6 cycles; NIVO+RT+TMZ). Additional cycles of TMZ were allowed if clinically warranted. In cohort 1d, no TMZ was administered, and patients received nivolumab 3 mg/kg every 2 weeks plus RT (60 Gy; 2-Gy fractions 5 days/week for ≤7 weeks; NIVO+RT). Patients continued receiving nivolumab after RT until unacceptable toxicity or disease progression (Figure 1). Nivolumab treatment could be continued beyond suspected progression until confirmation of progression by follow-up MRI if the investigator determined clinical benefit and tolerance of study drug.
In part A, the safety and tolerability of the treatment in each cohort were evaluated after the first 10 patients in each cohort completed 4 doses of nivolumab or discontinued dosing before completing 4 doses of nivolumab. Upon meeting the safety and tolerability criteria, ≤20 additional patients were enrolled to each cohort. The sample size of the safety lead-in was chosen with the goal of providing an adequate estimate of safety before exposing more patients to treatment. Part B was initiated after completion of part A. Inclusion criteria for part B were prespecified and not based on preliminary evidence from part A.
Assessments
Tumor samples were assessed for MGMT promoter methylation status using a centralized methylation-specific polymerase chain reaction assay. A sample was determined to be MGMT methylated when the ratio of the gene copy numbers of MGMT to control (β-actin) × 1000 was ≥2 and the gene copy numbers of MGMT and control were within the reportable range (β-actin ≥10 copies and MGMT ≥10 copies).
AEs were assessed continually during the study per NCI Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.28 TRAEs were determined by the investigator as related to study treatment. Serious AEs were defined as any events that, at any dose, resulted in death, were considered life threatening, required or prolonged inpatient hospitalization, resulted in persistent or significant disability/incapacity, were congenital anomaly/birth defects, or were important medical events. AEs leading to discontinuation were determined by the investigator.
Tumor assessments were performed by investigators using contrast-enhanced MRI at baseline (postsurgery; as close as possible to first dose of study drug, but no longer than 21 days before), 4 weeks after completion of RT, and then every 8 weeks thereafter per Response Assessment in Neuro-Oncology (RANO) criteria.29 Survival was assessed every 3 months during the follow-up phase.
Outcomes
The primary endpoint was safety and tolerability, and the secondary endpoint was OS, defined as time from date of first dose to date of death from any cause. Exploratory objectives included progression-free survival (PFS) as assessed by the investigator (investigator-reported response based on RANO criteria), defined as time from date of first dose to documented progression or death from any cause. Because corticosteroids suppress the immune response,30 additional ad hoc subanalyses of OS based on whether patients received baseline corticosteroids were performed.
Statistical Analysis
The primary endpoint of safety and tolerability was analyzed based on AEs and laboratory parameters that were graded per CTCAE version 4.0 and summarized using descriptive statistics. OS and PFS curves and medians were estimated using Kaplan-Meier methodology and reported with 95% CIs.
Study Oversight
The study was conducted in accordance with Good Clinical Practice guidelines per the International Council for Harmonization and with ethical principles of the European Union Directive and US Code of Federal Regulations and registered at ClinicalTrials.gov (NCT02017717). The protocol was approved by an institutional review board or independent ethics committee at each site before study activation. All patients provided written informed consent in accordance with the Declaration of Helsinki.
The study was designed by the authors in collaboration with the sponsor (Bristol Myers Squibb). The authors and the sponsor were responsible for data collection, and the sponsor was responsible for data analysis. The authors and sponsor were involved in data interpretation and the development of this report, and they attest that the study was conducted in accordance with the study protocol.
Results
Patients and Treatment
From May 2015 through September 2016, 136 patients were enrolled and 117 were treated. In part A, 31 patients (15 with methylated [n = 12] or unknown [n = 3] MGMT promoter and 16 with unmethylated MGMT promoter) received NIVO+RT+TMZ, and 30 patients with unmethylated MGMT promoter received NIVO+RT. In part B, an additional 59 patients with unmethylated MGMT promoter were randomized 1:1 to NIVO+RT+TMZ (n = 29) or NIVO+RT (n = 30); 28 patients received each treatment (Supplementary Figure S1).
There were no major imbalances in baseline characteristics between study parts and cohorts. All patients had a histopathologic diagnosis of glioblastoma, with the exception of 1 patient with gliosarcoma, and approximately 30% to 45% of patients received corticosteroids at baseline (Table 1).
Table 1.
Patient Demographics and Clinical Characteristics
| Variable | Part A | Part B | ||||
|---|---|---|---|---|---|---|
| Cohort 1c: Nivolumab + RT + TMZ |
Cohort 1d: Nivolumab + RT |
Cohort 1c: Nivolumab + RT + TMZ |
Cohort 1d: Nivolumab + RT |
|||
| Meth/Unk MGMT Promoter (n = 15) No. (%)a |
Unmeth MGMT Promoter (n = 16) No. (%)a |
Combined (n = 31) No. (%)a |
Unmeth MGMT Promoter (n = 30) No. (%)a |
Unmeth MGMT Promoter |
||
| (n = 28) No. (%)a |
(n = 28) No. (%)a |
|||||
| Age | ||||||
| Median (range), years | 55.0 (25–78) | 61.5 (42–72) | 58.0 (25–78) | 58 (28–76) | 59.5 (26–79) | 62.5 (39–78) |
| ≥65 years | 3 (20.0) | 5 (31.3) | 8 (25.8) | 8 (26.7) | 7 (25.0) | 11 (39.3) |
| Sex | ||||||
| Male | 9 (60.0) | 11 (68.8) | 20 (64.5) | 22 (73.3) | 19 (67.9) | 21 (75.0) |
| Female | 6 (40.0) | 5 (31.3) | 11 (35.5) | 8 (26.7) | 9 (32.1) | 7 (25.0) |
| Histopathologic diagnosis | ||||||
| Glioblastoma | 15 (100) | 15 (93.8) | 30 (96.8) | 30 (100) | 28 (100) | 28 (100) |
| Gliosarcoma | 0 | 1 (6.3) | 1 (3.2) | 0 | 0 | 0 |
| KPS | ||||||
| 100 | 2 (13.3) | 2 (12.5) | 4 (12.9) | 5 (16.7) | 2 (7.1) | 5 (17.9) |
| 90 | 6 (40.0) | 6 (37.5) | 12 (38.7) | 14 (46.7) | 17 (60.7) | 16 (57.1) |
| 80 | 6 (40.0) | 5 (31.3) | 11 (35.5) | 7 (23.3) | 8 (28.6) | 7 (25.0) |
| 70 | 1 (6.7) | 3 (18.8) | 4 (12.9) | 4 (13.3) | 1 (3.6) | 0 |
| Patients with ≥1 target lesion | 7 (46.7) | 7 (43.8) | 14 (45.2) | 17 (56.7) | 18 (64.3) | 16 (57.1) |
| Sum of the product of measurable lesion(s), median (range), mm 2 | 1008.0 (143–2200) | 322.0 (110–1260) | 799.5 (110–2200) | 792.0 (140–2226) | 813.0 (126–3105) | 510.0 (0–3696) |
| Site of target lesion(s) | ||||||
| Frontal lobe | 4 (26.7) | 0 | 4 (12.9) | 5 (16.7) | 6 (21.4) | 5 (17.9) |
| Occipital lobe | 1 (6.7) | 0 | 1 (3.2) | 1 (3.3) | 2 (7.1) | 0 |
| Parietal lobe | 2 (13.3) | 2 (12.5) | 4 (12.9) | 5 (16.7) | 1 (3.6) | 3 (10.7) |
| Temporal lobe | 1 (6.7) | 4 (25.0) | 5 (16.1) | 5 (16.7) | 9 (32.1) | 4 (14.3) |
| Other | 0 | 1(6.3) | 1 (3.2) | 1 (3.3) | 2 (7.1) | 4 (14.3) |
| MGMT promoter methylation status | ||||||
| Methylated | 12 (80.0) | 0 | 12 (38.7) | 0 | 0 | 0 |
| Unmethylated | 0 | 16 (100) | 16 (51.6) | 30 (100) | 28 (100) | 28 (100) |
| Unknown | 3 (20.0) | 0 | 3 (9.7) | 0 | 0 | 0 |
| Baseline corticosteroid use | ||||||
| Yes | 7 (46.7) | 7 (43.8) | 14 (45.2) | 9 (30.0) | 12 (42.9) | 9 (32.1) |
| <4 mg/day | 3 (20.0) | 7 (43.8) | 10 (32.3) | 4 (13.3) | 10 (35.7) | 4 (14.3) |
| >4 mg/day | (26.7) | 0 | 4 (12.9) | 5 (16.7) | 2 (7.1) | 5 (17.9) |
| No | 8 (53.3) | 9 (56.3) | 17 (54.8) | 21 (70.0) | 16 (57.1) | 19 (67.9) |
Baseline characteristics across treatment groups in cohort 1c (nivolumab + RT + TMZ, methylated/unknown or unmethylated MGMT promoter) and cohort 1d (nivolumab + RT, unmethylated MGMT promoter) in part A and in cohort 1c (nivolumab + RT + TMZ, unmethylated MGMT promoter) and cohort 1d (nivolumab + RT, unmethylated MGMT promoter) in part B.
Abbreviations: KPS, Karnofsky performance status; meth, methylated; MGMT, O6-methylguanine-DNA methyltransferase; RT, radiotherapy; TMZ, temozolomide; unk, unknown; unmeth, unmethylated.
aAge and sum of the product of measurable lesion(s) are reported as median (range).
At data cutoff (August 8, 2019), most patients had discontinued treatment (part A: NIVO+RT+TMZ, 90.3% [methylated/unknown MGMT promoter, 86.7%; unmethylated MGMT promoter, 93.8%]; NIVO+RT unmethylated MGMT promoter, 100%; part B: NIVO+RT+TMZ, 96.4%; NIVO+RT, 100%). The most common reasons were disease progression, treatment-related toxicity, and patient decision (Supplementary Figure S2).
The minimum duration of follow-up for OS in patients in this study was 33.1 months. Durations of treatments are summarized in Supplementary Table S1.
Safety
The rates of any AEs, neurological AEs, serious AEs, and AEs leading to discontinuation are displayed in Supplementary Table S2. Overall, 93.5% (29/31) of NIVO+RT+TMZ-treated and 83.3% (25/30) of NIVO+RT-treated patients experienced TRAEs in part A, with 51.6% (16/31) and 30.0% (9/30) of patients, respectively, experiencing grade 3/4 TRAEs. Among NIVO+RT+TMZ-treated patients with methylated/unknown and unmethylated MGMT promoter, the rates of grade 3/4 AEs were 46.7% (7/15) and 56.3% (9/16), respectively. In part B, 85.7% (24/28) of NIVO+RT+TMZ-treated and 71.4% (20/28) of NIVO+RT-treated patients experienced TRAEs, with 46.4% (13/28) and 28.6% (8/28) of patients, respectively, experiencing grade 3/4 TRAEs (Table 2). Neurological TRAEs were reported in 38.7% (12/31) of all NIVO+RT+TMZ-treated and 26.7% (8/30) of all NIVO+RT-treated patients in part A and 25.0% (7/28) and 28.6% (8/28), respectively, in part B. In addition to headache, neurological TRAEs occurring in ≥2 patients in any cohort were seizure and dizziness (n = 2/31 each) with NIVO+RT+TMZ in part A and seizure and hemiparesis (n = 2/28 each) with NIVO+RT in part B. Additional neurological TRAEs occurring in more than 1 cohort were aphasia (part A: NIVO+RT+TMZ [n = 1] and NIVO+RT [n = 1]; part B: NIVO+RT+TMZ [n = 1]), dysgeusia (part A: NIVO+RT [n = 1]; part B: NIVO+RT+TMZ [n = 1]), and parosmia (part A: NIVO+RT [n = 1]; part B: NIVO+RT [n = 1]). In part A, 87.1% (27/31) of NIVO+RT+TMZ-treated patients and 73.3% (22/30) of NIVO+RT-treated patients experienced lymphopenia, as assessed by laboratory values. Grade 3/4 events were reported in 41.9% (13/31) and 13.3% (4/30) of patients, respectively. Among NIVO+RT+TMZ-treated patients, grade 3/4 lymphopenia was reported in 40% (6/15) of patients with methylated/unknown MGMT promoter and 43.8% (7/16) of patients with unmethylated MGMT promoter. In part B, most NIVO+RT+TMZ-treated (88.8% [24/28]) and NIVO+RT-treated (59.3% [16/28]) patients had lymphopenia. Among these patients, grade 3/4 lymphopenia was observed in 48.1% (13/27) of those treated with NIVO+RT+TMZ and 18.5% (5/27) of those treated with NIVO+RT (Table 2). The majority of immune-mediated AEs were of low-grade severity (Table 3).
Table 2.
Treatment-Related Adverse Events (≥10% of Patients in Any Cohort)
| Part A | Part B | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cohort 1c: | Cohort 1d: | Cohort 1c: | Cohort 1d: | |||||||||
| Nivolumab + RT + TMZ | Nivolumab + RT | Nivolumab + RT + TMZ | Nivolumab + RT | |||||||||
| Meth/Unk MGMT Promoter (n = 15) No. (%) | Unmeth MGMT Promoter (n = 16) No. (%) | Combined (n = 31) No. (%) | Unmeth MGMT Promoter (n = 30) No. (%) | Unmeth MGMT Promoter | ||||||||
| (n = 28) | (n = 28) | |||||||||||
| No. (%) | No. (%) | |||||||||||
| Any Grade | Grade 3/4 | Any Grade | Grade 3/4 | Any Grade | Grade 3/4 | Any Grade | Grade 3/4 | Any Grade | Grade 3/4 | Any Grade | Grade 3/4 | |
| Any TRAEa,b | 14 (93.3) | 7 (46.7) | 15 (93.8) | 9 (56.3) | 29 (93.5) | 16 (51.6) | 25 (83.3) | 9 (30.0) | 24 (85.7) | 13 (46.4) | 20 (71.4) | 8 (28.6) |
| TRAEs in ≥10% of patients in any cohort | ||||||||||||
| Nervous system disorders | ||||||||||||
| Headache | 7 (46.7) | 0 | 5 (31.3) | 1 (6.3) | 12 (38.7) | 1 (3.2) | 4 (13.3) | 0 | 4 (14.3) | 0 | 5 (17.9) | 1 (3.6) |
| General disorders and administration site conditions | ||||||||||||
| Fatigue | 9 (60.0) | 0 | 7 (43.8) | 1 (6.3) | 16 (51.6) | 1 (3.2) | 9 (30.0) | 0 | 10 (35.7) | 0 | 8 (28.6) | 0 |
| Pyrexia | 1 (6.7) | 0 | 2 (12.5) | 0 | 3 (9.7) | 0 | 3 (10.0) | 1 (3.3) | 0 | 0 | 3 (10.7) | 0 |
| Gastrointestinal disorders | ||||||||||||
| Diarrhea | 4 (26.7) | 0 | 4 (25.0) | 1 (6.3) | 8 (25.8) | 1 (3.2) | 2 (6.7) | 0 | 3 (10.7) | 0 | 4 (14.3) | 1 (3.6) |
| Nausea | 4 (26.7) | 0 | 3 (18.8) | 0 | 7 (22.6) | 0 | 4 (13.3) | 0 | 4 (14.3) | 0 | 3 (10.7) | 1 (3.6) |
| Skin disorders | ||||||||||||
| Dry skin | 3 (20.0) | 0 | 2 (12.5) | 0 | 5 (16.1) | 0 | 0 | 0 | 0 | 0 | 2 (7.1) | 0 |
| Pruritis | 4 (26.7) | 0 | 1 (6.3) | 0 | 5 (16.1) | 0 | 2 (6.7) | 0 | 0 | 0 | 2 (7.1) | 0 |
| Rash | 1 (6.7) | 0 | 3 (18.8) | 1 (6.3) | 4 (12.9) | 1 (3.2) | 1 (3.3) | 0 | 1 (3.6) | 0 | 2 (7.1) | 0 |
| Rash maculopapular | 4 (26.7) | 0 | 0 | 0 | 4 (12.9) | 0 | 4 (13.3) | 1 (3.3) | 1 (3.6) | 0 | 1 (3.6) | 0 |
| Investigations | ||||||||||||
| Lymphopeniac | 14 (93.3) | 6 (40.0) | 13 (81.3) | 7 (43.8) | 27 (87.1) | 13 (41.9) | 22 (73.3) | 4 (13.3) | 24 (88.9) | 13 (48.1) | 16 (59.3) | 5 (18.5) |
| ALT increased | 4 (26.7) | 1 (6.7) | 4 (25.0) | 1 (6.3) | 8 (25.8) | 2 (6.5) | 4 (13.3) | 2 (6.7) | 3 (10.7) | 0 | 2 (7.1) | 1 (3.6) |
| AST increased | 5 (33.3) | 2 (13.3) | 4 (25.0) | 0 | 9 (29.0) | 2 (6.5) | 3 (10.0) | 2 (6.7) | 2 (7.1) | 0 | 2 (7.1) | 0 |
| Lipase increased | 4 (26.7) | 0 | 2 (12.5) | 1 (6.3) | 6 (19.4) | 1 (3.2) | 5 (16.7) | 4 (13.3) | 2 (7.1) | 2 (7.1) | 1 (3.6) | 1 (3.6) |
| Platelet count decreased | 3 (20.0) | 0 | 4 (25.0) | 0 | 7 (22.6) | 0 | 1 (3.3) | 0 | 4 (14.3) | 0 | 1 (3.6) | 0 |
| Amylase increased | 3 (20.0) | 1 (6.7) | 1 (6.3) | 1 (6.3) | 4 (12.9) | 2 (6.5) | 1 (3.3) | 1 (3.3) | 2 (7.1) | 1 (3.6) | 2 (7.1) | 1 (3.6) |
| WBC count decreased | 3 (20.0) | 2 (13.3) | 2 (12.5) | 0 | 5 (16.1) | 2 (6.5) | 1 (3.3) | 0 | 2 (7.1) | 0 | 1 (3.6) | 0 |
| Metabolism and nutrition disorders | ||||||||||||
| Hyperglycemia | 0 | 0 | 2 (12.5) | 0 | 2 (6.5) | 0 | 1 (3.3) | 0 | 3 (10.7) | 0 | 0 | 0 |
| Decreased appetite | 1 (6.7) | 0 | 0 | 0 | 2 (6.5) | 0 | 0 | 0 | 2 (7.1) | 0 | 4 (14.3) | 0 |
| Hyponatremia | 1 (6.7) | 1 (6.7) | 0 | 0 | 1 (3.2) | 1 (3.2) | 3 (10.0) | 2 (6.7) | 1 (3.6) | 0 | 0 | 0 |
| Endocrine disorders | ||||||||||||
| Hyperthyroidism | 1 (6.7) | 0 | 1 (6.3) | 0 | 2 (6.5) | 0 | 4 (13.3) | 0 | 0 | 0 | 0 | 0 |
| Hypothyroidism | 1 (6.7) | 0 | 2 (12.5) | 0 | 3 (9.7) | 0 | 3 (10.0) | 0 | 2 (7.1) | 0 | 2 (7.1) | 0 |
| Neoplasms | ||||||||||||
| Tumor flare | 0 | 0 | 1 (6.3) | 1 (6.3) | 1 (3.2) | 1 (3.2) | 3 (10.0) | 2 (6.7) | 3 (10.7) | 3 (10.7) | 2 (7.1) | 2 (7.1) |
| Blood and lymphatic disorders | ||||||||||||
| Thrombocytopenia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 (14.3) | 2 (7.1) | 0 | 0 |
| Any serious TRAEa,b | 6 (40.0) | 5 (33.3) | 5 (31.3) | 4 (25.0) | 11 (35.5) | 9 (29.0) | 6 (20.0) | 4 (13.3) | 8 (28.6) | 6 (21.4) | 5 (17.9) | 4 (14.3) |
| Serious TRAEs in ≥2 patients in any cohort | ||||||||||||
| Pyrexia | 1 (6.7) | 0 | 2 (12.5) | 0 | 3 (9.7) | 0 | 1 (3.3) | 1 (3.3) | 0 | 0 | 1 (3.6) | 0 |
| ALT increased | 1 (6.7) | 1 (6.7) | 1 (6.3) | 1 (6.3) | 2 (6.5) | 2 (6.5) | 1 (3.3) | 1 (3.3) | 0 | 0 | 1 (3.6) | 1 (3.6) |
| Pneumonia | 1 (6.7) | 1 (6.7) | 1 (6.3) | 1 (6.3) | 2 (6.5) | 2 (6.5) | 0 | 0 | 0 | 0 | 0 | 0 |
| AST increased | 1 (6.7) | 1 (6.7) | 1 (6.3) | 0 | 2 (6.5) | 1 (3.2) | 1 (3.3) | 1 (3.3) | 0 | 0 | 0 | 0 |
| Tumor flare | 0 | 0 | 1 (6.3) | 1 (6.3) | 1 (3.2) | 1 (3.2) | 2 (6.7) | 2 (6.7) | 3 (10.7) | 3 (10.7) | 2 (7.1) | 2 (7.1) |
Treatment-related adverse events in cohort 1c (nivolumab + RT + TMZ, methylated/unknown or unmethylated MGMT promoter) and cohort 1d (nivolumab + RT, unmethylated MGMT promoter) in part A and in cohort 1c (nivolumab + RT + TMZ, unmethylated MGMT promoter) and cohort 1d (nivolumab + RT, unmethylated MGMT promoter) in part B.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; meth, methylated; MGMT, O6-methylguanine-DNA methyltransferase; RT, radiotherapy; TMZ, temozolomide; TRAE, treatment-related adverse event; unk, unknown; unmeth, unmethylated.
aIncludes events reported between first dose and 30 days after last dose of study therapy.
bOne grade 5 event (sudden death, n = 1) in cohort 1c in part B.
cDerived from laboratory assessments. Percentages reported for cohorts 1c and 1d in part B were calculated based on 27 evaluable patients in each cohort.
Table 3.
Immune-Mediated Adverse Events (≥2 Patients in Any Cohort)
| imAE | Part A | Part B | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cohort 1c: Nivolumab + RT + TMZ |
Cohort 1d: Nivolumab + RT |
Cohort 1c: Nivolumab + RT + TMZ |
Cohort 1d: Nivolumab + RT |
|||||||||
| Meth/Unk MGMT Promoter (n = 15) No. (%) |
Unmeth MGMT Promoter (n = 16) No. (%) |
Combined (n = 31) No. (%) |
Unmeth MGMT Promoter (n = 30) No. (%) |
Unmeth MGMT Promoter |
||||||||
| (n = 28) No. (%) |
(n = 28) No. (%) |
|||||||||||
| Any Grade | Grade 3/4 | Any Grade | Grade 3/4 | Any Grade | Grade 3/4 | Any Grade | Grade 3/4 | Any Grade | Grade 3/4 | Any Grade | Grade 3/4 | |
| imAEs in ≥2 patients in any cohort a | ||||||||||||
| Acute kidney injury | 0 | 0 | 0 | 0 | 0 | 0 | 2 (6.7) | 0 | 1 (3.6) | 0 | 2 (7.1) | 0 |
| ALT increased | 5 (33.3) | 1 (6.7) | 4 (25.0) | 1 (6.3) | 9 (29.0) | 2 (6.5) | 5 (16.7) | 2 (6.7) | 5 (17.9) | 2 (7.1) | 2 (7.1) | 1 (3.6) |
| AST increased | 5 (33.3) | 2 (13.3) | 4 (25.0) | 0 | 9 (29.0) | 2 (6.5) | 4 (13.3) | 3 (10.0) | 4 (14.3) | 1 (3.6) | 2 (7.1) | 0 |
| Blood bilirubin increased | 1 (6.7) | 0 | 1 (6.3) | 0 | 2 (6.5) | 0 | 3 (10.0) | 0 | 0 | 0 | 0 | 0 |
| Blood creatinine increased | 0 | 0 | 0 | 0 | 0 | 0 | 2 (6.7) | 2 (6.7) | 3 (10.7) | 1 (3.6) | 0 | 0 |
| Diarrhea | 4 (26.7) | 0 | 4 (25.0) | 1 (6.3) | 8 (25.8) | 1 (3.2) | 4 (13.3) | 0 | 3 (10.7) | 0 | 8 (28.6) | 1 (3.6) |
| Drug eruption | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (7.1) | 0 | 0 | 0 |
| Hyperthyroidism | 1 (6.7) | 1 (6.7) | 1 (6.3) | 0 | 2 (6.5) | 1 (3.2) | 4 (13.3) | 0 | 0 | 0 | 0 | 0 |
| Hypothyroidism | 1 (6.7) | 0 | 2 (12.5) | 0 | 3 (9.7) | 0 | 3 (10.0) | 0 | 2 (7.1) | 0 | 3 (10.7) | 0 |
| Infusion-related reaction | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (7.1) | 0 | 2 (7.1) | 1 (3.6) |
| Rash | 3 (20.0) | 0 | 6 (37.5) | 1 (6.3) | 9 (29.0) | 1 (3.2) | 2 (6.7) | 0 | 2 (7.1) | 0 | 8 (28.6) | 0 |
| Rash maculopapular | 4 (26.7) | 0 | 1 (6.3) | 0 | 5 (16.1) | 0 | 4 (13.3) | 1 (3.3) | 1 (3.6) | 0 | 2 (7.1) | 0 |
Immune-mediated adverse events in cohort 1c (nivolumab + RT + TMZ, methylated/unknown or unmethylated MGMT promoter) and cohort 1d (nivolumab + RT, unmethylated MGMT promoter) in part A and in cohort 1c (nivolumab + RT + TMZ, unmethylated MGMT promoter) and cohort 1d (nivolumab + RT, unmethylated MGMT promoter) in part B.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; imAE, immune-mediated adverse event; meth, methylated; MGMT, O6-methylguanine-DNA methyltransferase; RT, radiotherapy; TMZ, temozolomide; unk, unknown; unmeth, unmethylated.
aIncludes events reported between first dose and 100 days after last dose of study therapy.
Serious TRAEs were reported in 35.5% (11/31) of all NIVO+RT+TMZ-treated and 20.0% (6/30) of all NIVO+RT-treated patients in part A of the study, with grade 3/4 events reported in 9 of 31 NIVO+RT+TMZ-treated and 4 of 30 NIVO+RT-treated patients. Among NIVO+RT+TMZ-treated patients, 40.0% (6/15) of those with methylated/unknown MGMT promoter and 31.3% (5/16) of those with unmethylated MGMT promoter experienced serious TRAEs; most were grade 3/4. Serious TRAEs in ≥2 patients receiving NIVO+RT+TMZ or NIVO+RT in part A included pyrexia, increased alanine aminotransferase (ALT), pneumonia, and tumor flare. In part B, serious TRAEs, including tumor flare (10.7%), were reported in 28.6% (8/28) of patients treated with NIVO+RT+TMZ. Serious TRAEs, including pyrexia (3.6%), increased ALT (3.6%), and tumor flare (7.1%), were reported in 17.9% (5/28) of patients treated with NIVO+RT. One serious TRAE of sudden death was reported with NIVO+RT+TMZ in part B (Table 2).
TRAEs led to discontinuation in 4 of 31 NIVO+RT+TMZ-treated patients (12.9%) (methylated/unknown MGMT promoter, 2/15; unmethylated MGMT promoter, 2/16) and 5 of 30 NIVO+RT-treated patients (16.7%) in part A; 2 patients treated with NIVO+RT discontinued due to tumor flare. In part B, 5 of 28 NIVO+RT+TMZ-treated patients (17.9%) and 4 of 28 NIVO+RT-treated patients (14.3%) discontinued treatment due to TRAEs (see Supplementary Table S3). At the time of analysis, 53 of 61 patients had died in part A (NIVO+RT+TMZ: n = 23/31 [methylated/unknown MGMT promoter, n = 9/15; unmethylated MGMT promoter, n = 14/16]; NIVO+RT: unmethylated MGMT promoter, n = 30/30) and 51 of 56 patients had died in part B (NIVO+RT+TMZ: n = 24/28; NIVO+RT: n = 27/28); none of the deaths were related to treatment.
Survival
Overall survival.—
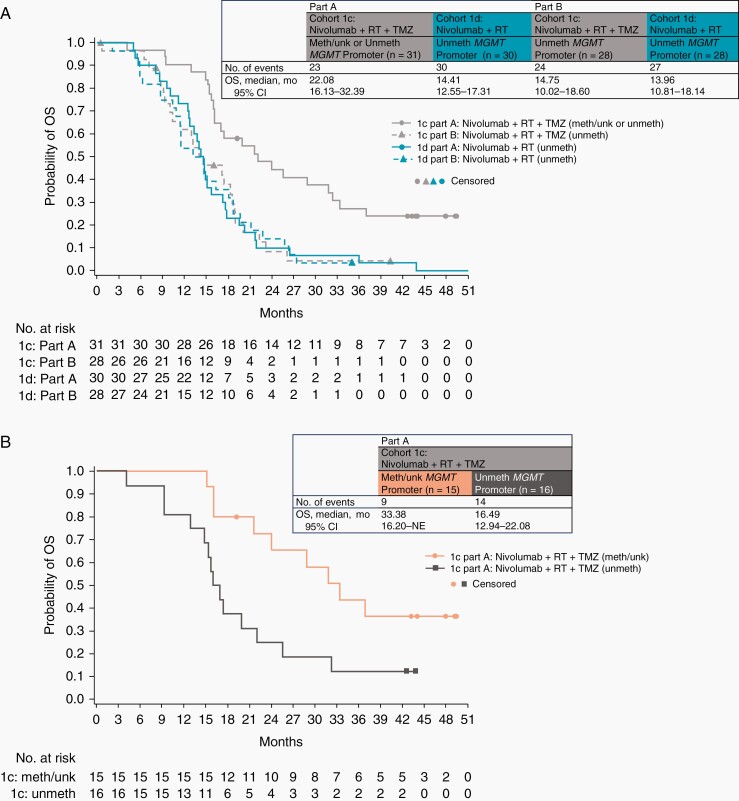
In part A, median OS (mOS) with NIVO+RT+TMZ was 22.08 months (95% CI, 16.13–32.39 months). Among NIVO+RT+TMZ-treated patients, mOS was 33.38 months (95% CI, 16.20 months-not estimable) in patients with methylated/unknown MGMT promoter and 16.49 months (95% CI, 12.94–22.08 months) in patients with unmethylated MGMT promoter. With NIVO+RT, mOS was 14.41 months (95% CI, 12.55–17.31 months) in patients with unmethylated MGMT promoter. In part B, mOS was similar with NIVO+RT+TMZ and NIVO+RT (14.75 months [95% CI, 10.02–18.60 months] and 13.96 months [95% CI, 10.81–18.14 months]) in patients with unmethylated MGMT promoter (Figure 2).
Figure 2.
Kaplan-Meier estimates of OS, with insets demonstrating the number of events and median overall survival. (A) OS in cohort 1c (nivolumab + RT + TMZ, methylated/unknown or unmethylated MGMT promoter) and cohort 1d (nivolumab + RT, unmethylated MGMT promoter) in part A and in cohort 1c (nivolumab + RT + TMZ, unmethylated MGMT promoter) and cohort 1d (nivolumab + RT, unmethylated MGMT promoter) in part B. (B) OS in cohort 1c in part A (nivolumab + RT + TMZ, methylated/unknown MGMT promoter; nivolumab + RT + TMZ, unmethylated MGMT promoter). Symbols indicate censored observations. Abbreviations: meth, methylated; MGMT, O6-methylguanine-DNA methyltransferase; NE, not estimable; OS, overall survival; RT, radiotherapy; TMZ temozolomide; unk, unknown; unmeth, unmethylated.
OS in prespecified subgroups of patients based on baseline corticosteroid use (with vs without) was also evaluated (see Supplementary Figure S2 and Supplementary Table S4).
Progression-free survival.—
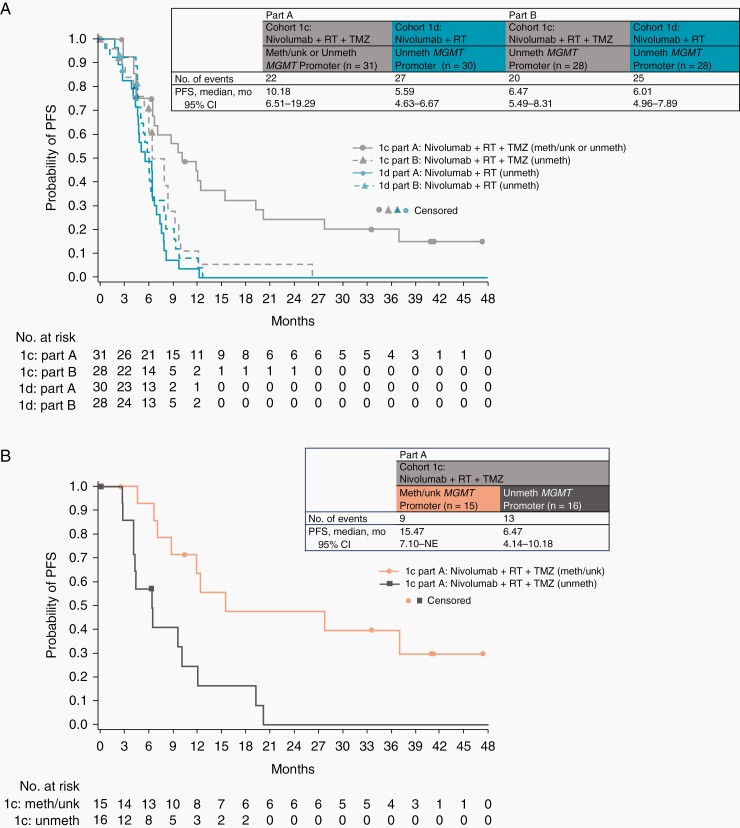
In part A, median PFS (mPFS) was 10.18 months (95% CI, 6.51–19.29 months) with NIVO+RT+TMZ. Among NIVO+RT+TMZ-treated patients, mPFS was 15.47 months (95% CI, 7.10 months-not estimable) in patients with methylated/unknown MGMT promoter and 6.47 months (95% CI, 4.14–10.18 months) in patients with unmethylated MGMT promoter. mPFS was 5.59 months (95% CI, 4.63–6.67 months) with NIVO+RT in patients with unmethylated MGMT promoter. In part B, mPFS was similar with NIVO+RT+TMZ and NIVO+RT (6.47 months [95% CI, 5.49–8.31 months] and 6.01 months [95% CI, 4.96–7.89 months], respectively) (Figure 3).
Figure 3.
Kaplan-Meier estimates of PFS, with insets demonstrating the number of events and median PFS. (A) PFS in cohort 1c (nivolumab + RT + TMZ, methylated/unknown or unmethylated MGMT promoter) and cohort 1d (nivolumab + RT, unmethylated MGMT promoter) in part A and in cohort 1c (nivolumab + RT + TMZ, unmethylated MGMT promoter) and cohort 1d (nivolumab + RT, unmethylated MGMT promoter) in part B. (B) PFS in cohort 1c in part A (nivolumab + RT + TMZ, methylated/unknown MGMT promoter; nivolumab + RT + TMZ, unmethylated MGMT promoter). Symbols indicate censored observations. Abbreviations: meth, methylated; MGMT, O6-methylguanine-DNA methyltransferase; NE, not estimable; PFS, progression-free survival; RT, radiotherapy; TMZ, temozolomide; unk, unknown; unmeth, unmethylated.
Discussion
CheckMate 143 is the first phase 1 prospective clinical trial investigating immunotherapy with an immune checkpoint inhibitor added to RT both with and without chemotherapy in newly diagnosed glioblastoma. Across the lead-in and expansion cohorts (1c and 1d) of CheckMate 143, a total of 136 patients were enrolled and 117 were treated. The rapid accrual observed in this study underscored the urgent unmet need for alternatives to TMZ-free treatment options in unmethylated MGMT glioblastoma. The safety profile of nivolumab was consistent with that previously reported in recurrent glioblastoma,27 with no unexpected neurological TRAEs. Additionally, TMZ in combination with nivolumab and RT did not lead to significant additional safety events, other than those known to occur with each drug alone; the increased toxicity rates with NIVO+RT+TMZ vs NIVO+RT mostly reflected the toxicities due to addition of TMZ. In part B, lymphopenia was more frequent with NIVO+RT+TMZ than with NIVO+RT, with grade 3/4 lymphopenia observed in 48.1% of NIVO+RT+TMZ-treated patients vs 18.5% of NIVO+RT-treated patients. Grade 3/4 lymphopenia was also reported in 41.9% of NIVO+RT+TMZ-treated patients vs 13.3% of NIVO+RT-treated patients in part A, which indicates that TMZ is an important factor in determining the chronic lymphopenia observed in patients with glioblastoma.
Our data add to the growing body of studies seeking to define a potential role for immune checkpoint inhibitors in glioblastoma. Since the completion of this analysis, results in the first-line treatment setting for glioblastoma, both with and without MGMT promoter methylation, have been disappointing.26,31,32 Some studies have suggested that immune checkpoint inhibitors may affect the TME of glioblastomas.24,33,34 A small randomized phase 2 study suggested that the addition of neoadjuvant pembrolizumab prior to salvage surgery followed by continued adjuvant therapy may prolong survival.24 However, the randomized phase 3 portion of CheckMate 143, which compared nivolumab with bevacizumab in patients with recurrent glioblastoma, did not meet its primary endpoint of OS.26 Post hoc analyses suggested longer OS and PFS in patients with methylated MGMT promoter than in those with unmethylated MGMT promoter in both treatment groups; however, no corticosteroid use at baseline was associated with longer mOS only with nivolumab.26 In this study, an exploratory ad hoc subanalysis of OS in patients with no corticosteroid use at baseline was performed; however, due to small patient numbers, no definitive conclusions could be drawn.
More recently, nivolumab was evaluated in 2 phase 3 trials in newly diagnosed glioblastoma. CheckMate 498 (NCT02617589) evaluated NIVO+RT vs RT+TMZ in patients with unmethylated MGMT promoter, and CheckMate 548 (NCT02667587) evaluated nivolumab added to RT+TMZ in patients with methylated MGMT promoter. Those studies were designed and launched before these survival data for CheckMate 143 became available to rapidly address the high unmet medical need in these patients and based on the history of favorable outcomes of nivolumab in PD-L1-expressing tumors at that time. The respective rationale for those studies included TMZ’s association with limited efficacy in patients with unmethylated MGMT promoter (CheckMate 498) and the potential for enhanced benefit via the addition of immunotherapy to chemoradiation in patients with methylated MGMT promoter (CheckMate 548). Findings from those studies have been released; however, neither of those studies achieved their primary endpoints of improved survival outcomes.31,32
Taken together, these results suggest that the prolonged survival observed in our study in comparison to historical controls likely reflects changes in the contemporary management of glioblastoma and that further studies are needed to understand the biological effects of anti-PD-1 therapy in this disease, if any. A limitation of our study is that tumor and blood specimens are not available for further characterization of biomarkers or pharmacodynamic effects of treatment. In addition, the requirement for at least a subtotal tumor resection potentially allowed for the selection of a cohort of patients with a slightly better prognosis than all comers and was taken into consideration in the design of subsequent trials.
In conclusion, our results show that nivolumab can be safely combined with RT with and without TMZ, with no new safety signals observed. Treatment with NIVO+RT+TMZ was associated with more frequent toxicities and higher rates of lymphopenia compared with NIVO+RT. Although recent results have been unfavorable, the combination of RT with anti-PD-1 therapy with and without TMZ pioneered in this study is now being investigated in several ongoing or planned trials exploring novel immunotherapies to overcome immunosuppression and improve the efficacy of first-line therapy for glioblastoma. Additional translational analyses from phase 3 studies (CheckMate 498 and CheckMate 548) may help to elucidate whether nivolumab has a role in glioblastoma.
Supplementary Material
Acknowledgments
We thank the patients and their families who made this study possible; Corina Taitt, MD (formerly of Bristol Myers Squibb), for her contribution to study development and design; investigators and research staff at all study sites; and Ono Pharmaceutical Company Ltd, Osaka, Japan. Editorial assistance was provided by Larra Yuelling, PhD, of SciMentum, Inc, a Nucleus Global company, Hamilton, NJ, funded by Bristol Myers Squibb. Results from this study were presented at the 5th Quadrennial Meeting of the World Federation of Neuro-Oncology Societies, May 4-7, 2017, in Zurich, Switzerland, and the European Society for Medical Oncology 2017 Congress, September 8-12, 2017, in Madrid, Spain.
Funding
Bristol Myers Squibb, Princeton, NJ.
Conflict of interest statement. A.O. has served as a consultant on ad hoc advisory boards for Bristol Myers Squibb, AstraZeneca, Inovio, Merck, Stemline, Novocure, and Alexion, and has received grant funding from Merck and Arcus Biosciences. D.A.R. has received grant funding from Acerta Pharma, Incyte, Midatech, Omniox, and Tragara; grant funding and personal fees from Agenus, Celldex Therapeutics, EMD Serono, and Inovio; and personal fees from Advantagene, Genentech/Roche, Merck, Merck KGaA, Monteris, Novocure, Oncorus, OXiGENE, Regeneron, Stemline, and Taiho Oncology. J.H.S. served as a consultant/advisory board member for Bristol Myers Squibb and Brainlab; has received grant funding and personal fees from and is a patent holder for Celldex Therapeutics; has received grant funding and personal fees from, owns equity/stock in, and is a patent holder for Annias; and owns stock in Istari. J.B. has served as a consultant for Bristol Myers Squibb. S.S. has received grant funding from Merck and Bristol Myers Squibb. T.F.C. is a cofounder, major stock holder, consultant, and board member of Katmai Pharmaceuticals; is a member of the board for the 501c3 Global Coalition for Adaptive Research; holds stock options for Notable Labs; holds stock in Chimerix and receives milestone payments and possible future royalties; is a member of the scientific advisory board for Break Through Cancer and member of the scientific advisory board for Cure Brain Cancer Foundation; has provided paid consulting services to GCAR, Gan & Lee, BrainStorm, Katmai, Sapience, Inovio, Vigeo Therapeutics, DNATrix, Tyme, Sumitomo Dainippon Pharma, Novartis, Roche, Kintara, Bayer, Merck, Boehringer Ingelheim, VBL, Amgen, Kiyatec, Odonate Therapeutics, QED, Medefield, Pascal Biosciences, Bayer, Tocagen, Karyopharm, GW Pharma, AbbVie, VBI, Deciphera, VBL, Agios, Genocea, Celgene, Puma, Lilly, Bristol Myers Squibb, Cortice, Wellcome Trust, Novocure, Novogen, Boston Biomedical, Sunovion, Human Longevity, Insys, ProNai, Pfizer, Notable Labs, Medqia Trizel, and Medscape; and has contracts with UCLA for the Brain Tumor Program with Oncovir, Merck, Oncoceutics, Novartis, Amgen, AbbVie, DNAtrix, Beigene, Bristol Myers Squibb, AstraZeneca, Kazia, Agios, Boston Biomedical, Deciphera, Tocagen, Orbus, AstraZeneca, and Karyopharm. A.-G.C. was an employee of and received stocks from Bristol Myers Squibb. V.P. was an employee of Bristol Myers Squibb. N.B. has received grant funding from KIYATEC, Amgen, Medicenna, Denovo, Karyopharm, Merck, Carthera, Biomimex, Novocure, Oncoceutics, Istari, Stellar Orbus, MimiVax, and CNS Pharma; personal fees for an advisory board from DelMar, QED, and Nativis; and personal fees from VBL Therapeutics, Cellinta, Boehringer Ingelheim, and Carthera. M.L. has received grant funding from Bristol Myers Squibb, Arbor, Accuray, Urogen, Biohaven, and Kyrin-Kyowa and personal fees from Tocagen, Merck, VBI, Stryker, InCephalo Therapeutics, InSightec, Biohaven, Sanianoia, Hemispherian, Black Diamond Therapeutics, Novocure, and Pyramid Bio; and has a patent combining local chemotherapy with immunotherapy pending for Arbor and a patent combining stereotactic radiosurgery with immunotherapy that has been issued.
Authorship statement. Study conception and design: A.O., D.A.R., and J.H.S. Data acquisition: A.O., D.A.R., J.H.S., J.B., S.S., T.F.C., N.B., and M.L. Data analyses: A.-G.C. and V.P. Data interpretation: all authors. Contribution to and approval of manuscript: all authors.
Data Availability
Bristol Myers Squibb’s policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
References
- 1. Stupp R, Hegi ME, Mason WP, et al. . Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 2. Ostrom QT, Gittleman H, Truitt G, et al. . CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2011-2015. Neuro Oncol. 2018;20 (suppl 4):iv1–iv86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Preusser M, Lim M, Hafler DA, Reardon DA, Sampson JH. Prospects of immune checkpoint modulators in the treatment of glioblastoma. Nat Rev Neurol. 2015;11(9):504–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wen PY, Weller M, Lee EQ, et al. . Glioblastoma in adults: a Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro Oncol. 2020;22(8):1073–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stupp R, Mason WP, van den Bent MJ, et al. . Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 6. Weller M, van den Bent M, Tonn JC, et al. . European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 2017;18(6):e315–e329. [DOI] [PubMed] [Google Scholar]
- 7. Stupp R, Taillibert S, Kanner A, et al. . Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA. 2017;318(23):2306–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: a clinical review. JAMA. 2013;310(17):1842–1850. [DOI] [PubMed] [Google Scholar]
- 9. Weller M, Cloughesy T, Perry JR, Wick W. Standards of care for treatment of recurrent glioblastoma—are we there yet? Neuro Oncol. 2013;15(1):4–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weller M, Stupp R, Reifenberger G, et al. . MGMT promoter methylation in malignant gliomas: ready for personalized medicine? Nat Rev Neurol. 2010;6(1):39–51. [DOI] [PubMed] [Google Scholar]
- 11. Hegi ME, Diserens AC, Gorlia T, et al. . MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. [DOI] [PubMed] [Google Scholar]
- 12. Esteller M, Garcia-Foncillas J, Andion E, et al. . Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med. 2000;343(19):1350–1354. [DOI] [PubMed] [Google Scholar]
- 13. Hegi ME, Diserens AC, Godard S, et al. . Clinical trial substantiates the predictive value of O-6-methylguanine-DNA methyltransferase promoter methylation in glioblastoma patients treated with temozolomide. Clin Cancer Res. 2004;10(6):1871–1874. [DOI] [PubMed] [Google Scholar]
- 14. Berghoff AS, Preusser M. Clinical neuropathology practice guide 06-2012: MGMT testing in elderly glioblastoma patients—yes, but how? Clin Neuropathol. 2012;31(6):405–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Weller M. Where does O6-methylguanine DNA methyltransferase promoter methylation assessment place temozolomide in the future standards of care for glioblastoma? Cancer. 2018;124(7):1316–1318. [DOI] [PubMed] [Google Scholar]
- 16. Reardon DA, Freeman G, Wu C, et al. . Immunotherapy advances for glioblastoma. Neuro Oncol. 2014;16(11):1441–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Woroniecka K, Chongsathidkiet P, Rhodin K, et al. . T-cell exhaustion signatures vary with tumor type and are severe in glioblastoma. Clin Cancer Res. 2018;24(17):4175–4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Park J, Kwon M, Kim KH, et al. . Immune checkpoint inhibitor-induced reinvigoration of tumor-infiltrating CD8(+) T cells is determined by their differentiation status in glioblastoma. Clin Cancer Res. 2019;25(8):2549–2559. [DOI] [PubMed] [Google Scholar]
- 19. Tang C, Wang X, Soh H, et al. . Combining radiation and immunotherapy: a new systemic therapy for solid tumors? Cancer Immunol Res. 2014;2(9):831–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nduom EK, Wei J, Yaghi NK, et al. . PD-L1 expression and prognostic impact in glioblastoma. Neuro Oncol. 2016;18(2):195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hao C, Chen G, Zhao H, et al. . PD-L1 expression in glioblastoma, the clinical and prognostic significance: a systematic literature review and meta-analysis. Front Oncol. 2020;10:1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Reardon DA, Gokhale PC, Klein SR, et al. . Glioblastoma eradication following immune checkpoint blockade in an orthotopic, immunocompetent model. Cancer Immunol Res. 2016;4(2):124–135. [DOI] [PubMed] [Google Scholar]
- 23. Zeng J, See AP, Phallen J, et al. . Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J Radiat Oncol Biol Phys. 2013;86(2):343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cloughesy TF, Mochizuki AY, Orpilla JR, et al. . Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med. 2019;25(3):477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Opdivo (nivolumab) [package insert]. Princeton, NJ: Bristol-Myers Squibb. June 2020. [Google Scholar]
- 26. Reardon DA, Brandes AA, Omuro A, et al. . Effect of nivolumab vs bevacizumab in patients with recurrent glioblastoma: the CheckMate 143 phase 3 randomized clinical trial. JAMA Oncol. 2020;6(7):1003–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Omuro A, Vlahovic G, Lim M, et al. . Nivolumab with or without ipilimumab in patients with recurrent glioblastoma: results from exploratory phase I cohorts of CheckMate 143. Neuro Oncol. 2018;20(5):674–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. US Department of Health and Human Services. National Cancer Institute: Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. May 28, 2009. update. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.0_2009-05-29_QuickReference_8.5x11.pdf [Google Scholar]
- 29. Wen PY, Macdonald DR, Reardon DA, et al. . Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 30. Gustafson MP, Lin Y, New KC, et al. . Systemic immune suppression in glioblastoma: the interplay between CD14+HLA-DRlo/neg monocytes, tumor factors, and dexamethasone. Neuro Oncol. 2010;12(7):631–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bristol Myers Squibb. Bristol Myers Squibb announces phase 3 CheckMate-498 study did not meet primary endpoint of overall survival with opdivo (nivolumab) plus radiation in patients with newly diagnosed MGMT-unmethylated glioblastoma multiforme. News release. https://news.bms.com/news/corporate-financial/2019/Bristol-Myers-Squibb-Announces-Phase-3-CheckMate--498-Study-Did-Not-Meet-Primary-Endpoint-of-Overall-Survival-with-Opdivo-nivolumab-Plus-Radiation-in-Patients-with-Newly-Diagnosed-MGMT-Unmethylated-Glioblastoma-Multiforme/default.aspx.
- 32. Bristol Myers Squibb. Bristol Myers Squibb announces update on phase 3 CheckMate-548 trial evaluating patients with newly diagnosed MGMT-methylated glioblastoma multiforme. News release. https://news.bms.com/news/details/2020/Bristol-Myers-Squibb-Announces-Update-on-Phase-3-CheckMate--548-Trial-Evaluating-Patients-with-Newly-Diagnosed-MGMT-Methylated-Glioblastoma-Multiforme/default.aspx#:~:text=PRINCETON%2C%20N.J.%2D%2D(BUSINESS%20WIRE,in%20patients%20with%20newly%20diagnosed.
- 33. Schalper KA, Rodriguez-Ruiz ME, Diez-Valle R, et al. . Neoadjuvant nivolumab modifies the tumor immune microenvironment in resectable glioblastoma. Nat Med. 2019;25(3):470–476. [DOI] [PubMed] [Google Scholar]
- 34. Zhao J, Chen AX, Gartrell RD, et al. . Immune and genomic correlates of response to anti-PD-1 immunotherapy in glioblastoma. Nat Med. 2019;25(3):462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Bristol Myers Squibb’s policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.