Summary
Background
High testing rates and rapid contact tracing have been key interventions to control COVID-19 in Victoria, Australia. A mobile laboratory (LabVan), for rapid SARS-CoV-2 diagnostics, was deployed at sites deemed critical by the Victorian State Department of Health as part of the response. We describe the process of design, implementation, and performance benchmarked against a central reference laboratory.
Methods
A BSL2 compliant laboratory, complete with a class II biological safety cabinet, was built within a Mercedes-Benz Sprinter Panel Van. Swabs were collected by on-site collection teams, registered using mobile internet-enabled tablets and tested using the Xpert® Xpress SARS-CoV-2 assay. Results were reported remotely via HL7 messaging to Public Health Units. Patients with negative results were automatically notified by mobile telephone text messaging (SMS).
Findings
A pilot trial of the LabVan identified a median turnaround time (TAT) from collection to reporting of 1:19 h:mm (IQR 0:18, Range 1:03–18:32) compared to 9:40 h:mm (IQR 8:46, Range 6:51–19:30) for standard processing within the central laboratory. During deployment in nine rural and urban COVID-19 outbreaks the median TAT was 2:18 h:mm (IQR 1:18, Range 0:50–16:52) compared to 19:08 h:mm (IQR 5:49, Range 1:36–58:52) for samples submitted to the central laboratory. No quality control issues were identified in the LabVan.
Interpretation
The LabVan is an ISO15189 compliant testing facility fully operationalized for mobile point-of-care testing that significantly reduces TAT for result reporting, facilitating rapid public health actions.
Funding
This work was supported by the Department of Health, Victoria State Government, Australia.
Keywords: Mobile testing, SARS-CoV-2, Real-time PCR, Turnaround-time, Rapid testing, Point-of-care
Research in context.
Evidence before this study
A key intervention for spread of SARS-CoV-2 has been rapid testing and contact tracing. Although there have been several publications of deployable mobile laboratories for infectious pathogens such as influenza, melioidosis, arboviruses and SARS-CoV-2 along with media reports in the US, UK and China data is sparse assessing the process of implementation, performance and impact of rapid mobile molecular point of care SARS-CoV-2 testing.
Added value of this study
We describe the process of design, development and deployment of LabVan and our initial experiences with implementation, performance benchmarking against a central laboratory, challenges and use cases.
Implications of all the available evidence
Deployment of mobile laboratories results in reduction in turnaround time for result reporting which is instrumental in rapid contact tracing and therefore control of local outbreaks. We describe mobile laboratory testing optimization strategies and an implementation outline to support jurisdictions considering the introduction of mobile laboratory testing. As the pandemic evolves, with increasing vaccination rates, future research will need to explore the changing use cases to optimize the impact of mobile laboratory testing.
Alt-text: Unlabelled box
Introduction
Until September 2021, the Australian state of Victoria had one of the lowest SARS-CoV-2 infection rates globally due mostly to closure of the Australian international border, and interventions such as mask wearing and physical distancing, in conjunction with high rates of diagnostic testing and isolation of positive cases and their contacts.1 Highly sensitive reverse-transcription PCR (RT-PCR) assays performed in clinical laboratories have been the cornerstone of diagnostic testing for SARS-CoV-2 in Australia. However, depending on the setting, RT-PCR results have taken approximately 24–48 h to return, and in some cases longer: this has led to delays in contact tracing and therefore preventable transmission of disease.2 Rapid point of care (POC) molecular tests may decrease test turnaround time for effective COVID-19 control.3,4
At the time of development of this initiative, the Xpert® Xpress SARS-CoV-2 assay (Cepheid, Sunnyvale, USA) was the main Therapeutic Goods Administration (TGA) rapid POC molecular assay available for use in Australia. A March 2021 Cochrane review of rapid SARS-CoV-2 tests found that for Xpert® Xpress SARS-CoV-2 assay, the average sensitivity was 100% (95% CI: 88.1-100%) and average specificity 97.2% (95% CI: 89.4- 99.3%).5
There have been several reports of deployable mobile laboratories for infectious pathogens such as influenza, melioidosis and arboviruses.6, 7, 8, 9 More recently, mobile laboratory vans have been adapted and validated for the purpose of SARS-CoV-2 testing,10,11 and larger truck-based mobile laboratories evaluated for performance in large scale screening using novel technologies.12,13 Despite this, data is sparse assessing the process of implementation, regulatory accreditation, performance, impact and reduction in testing turnaround time of rapid mobile molecular POC SARS-CoV-2 testing in a setting with a low prevalence of SARS-CoV-2.14
In the Australian state of Victoria, the COVID-19 pandemic has been characterised by three peaks of transmission - the first occurring between March and April 2020 (maximum 622 active cases), the second between July and September 2020 (maximum 7,880 active cases) and the third beginning in August 2021 and ongoing (24,899 peak active cases on 23/11/2021). Public health interventions to control the pandemic have primarily focused on extensive testing and contact tracing accompanied by extended lockdowns. Whilst vaccination is playing an increasing role in pandemic management, at the time of initial LabVan deployments only 29.63% of Australians aged >16 years old had received one dose of a SARS-CoV-2 vaccine and 7.92% had received a second dose.15 The Victorian Department of Health (DHV) recognised the potential role for mobile and rapid diagnostic testing in the Victorian public health response to the pandemic and provided funding and support for the development of a mobile laboratory in a van (LabVan). Plans for establishment of the LabVan commenced in late September 2020 and the LabVan was first deployed in July 2021 for rapid SARS-CoV-2 testing response at locations deemed critical by the Department of Health.
We describe the process of design, development and deployment of LabVan and our initial experiences with implementation, performance benchmarking against a central laboratory, challenges and use cases.
Methods
Sample collection and LabVan workflow
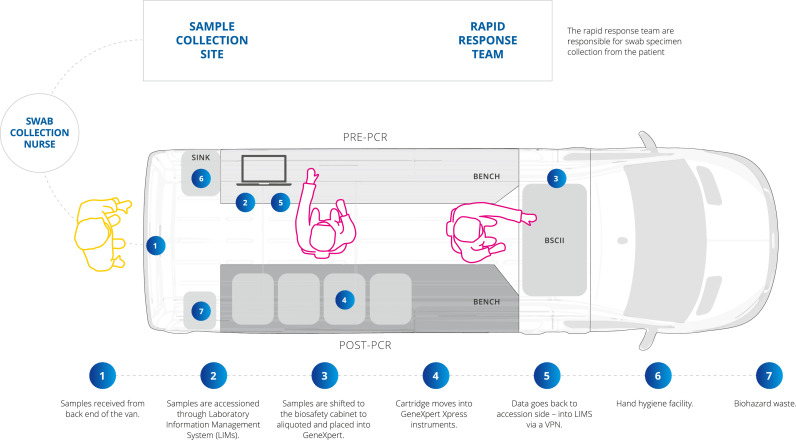
A Mercedes-Benz Sprinter Panel Van (model: 516 CDI VS30 LWB 4.49T RWD 2019) was acquired in October 2020 and the internal cargo section modified as a BSL2 compliant COVID-19 testing unit (Bell Environmental, Victoria) inclusive of a Class II Biosafety Cabinet (Euroclone, Pero) (Figure 1, Supplementary Fig. 1). The internal fit-out and modifications are described in Supplementary material. For deployment within a vehicle the size of the allowable BSC was based on physical limitations of van model (size and configuration) and limited by availability (of both van and BSC) in Australia at the time of design. The choice of Class II BSC provided flexibility for potential future use with other pathogens.
Figure 1.
Sample collection and LabVan workflow.
A sample collection and LabVan workflow was developed (Figure 1). Specimens were collected by host site collection teams. Patient registration was completed using mobile Internet enabled tablet computers (Apple iPad 7th generation, software version 14.2) with a Bluetooth enabled barcode scanner (POS-mate, Adelaide, Australia) using the Victorian Department of Health (DHV) Test Tracker electronic COVID test registry.16 Patient samples were allocated a tracking number (D-Number), which was available in the form of a Quick Response (QR) code that provided a web link to the registration page for that specific sample (e-order).
Nursing staff collected a combined throat and bilateral deep nasal swab (using a single swab stick that is sequentially inserted into the throat and nose) which was immediately placed in 3 ml of Universal Transport Medium (UTM). Samples were labelled with their D-Number, patient name, date of birth and collection date to meet National Pathology Accreditation Advisory Council (NPAAC) regulations. Samples were placed in biohazard bags labelled with the DHV QR code then brought to the LabVan on foot, either singly or in batches up to 16 samples. The entire sample collection and test request process was paperless.
Samples were aliquoted into the Xpert® Xpress SARS-CoV-2 assay cartridge in the Class II biosafety cabinet before testing in one of four Xpert® Xpress systems within the van. The Xpert® Xpress SARS-CoV-2 assay targets both the E-gene and the N2 gene and includes an internal sample processing control and probe check control to ensure adequate sample processing, monitor for sample inhibition and confirm all reaction components are performing. In addition, on arrival at deployments a systems check was performed for all Xpert® Xpress instruments as per the operator manual and a control sample run to ensure all systems were functional. Xpert® Xpress SARS-CoV-2 assay cartridges were stored at room temperature within the LabVan during deployments and at the MainLab between deployments. An internal environmental temperature of 15–28 °C was maintained within the LabVan and testing ceased if temperatures exceeded this range in accordance with the instrument and assay's operational requirements.
Staff performing testing were trained medical laboratory technicians/scientists experienced in performing SARS-CoV-2 testing at the central laboratory, Microbiological Diagnostic Unit Public Health Laboratory (MainLab). Additional training in the LabVan power and data management systems as well as advanced driver training was provided. Due to the inability to physically distance in the small space staff were required to wear fit-tested N95 masks and eye protection in addition to disposable gowns and double gloves. All staff were vaccinated as per mandatory health-care worker vaccination requirements in place at the dates of deployment. Two operators worked in the van, one on sample reception, accessioning and result entry and one operator on sample aliquoting and testing. A unidirectional workflow was followed at all times. Daily operating hours varied with deployments, staff operated on 8–10 h shifts, inclusive of travelling time, meal and rest breaks.
Environmental sampling to monitor decontamination processes and amplicon contamination in the LabVan was performed on a weekly basis in accordance with practice at the MainLab. Decontamination procedures were performed at the end of each day also in accordance with practice at the MainLab (i.e. using 70% ethanol followed by DNA-Erase™). Biohazardous waste was transferred back to the MainLab for discard through routine laboratory processes, or removed by the host site if possible.
Data and result management
Data management in the LabVan was via 4G WiFi VPN access to a custom-built module of Sample Manager™, a Laboratory Information Management System (LIMS) hosted at the MainLab. The LabVan module comprised all data screens for sample accessioning, data entry and result reporting. Electronic test orders (e-order) comprising patient metadata were downloaded into LIMS using the patient allocated D-number.
Sample result data from the Xpert® Xpress systems were printed and manually transcribed into LIMS with transcription cross-checks performed by the second operator. Printed reports for positive results were scanned back into LIMS for viewing by staff at the MainLab. Result reporting was managed back at the MainLab by accessing the LabVan LIMS module (Figure 2). Results were reported to DHV electronically via HL7 messaging. Positive results were phoned through to the medical liaison team for further patient management. Patients with negative results were notified by SMS using a custom-built application that inputs an xml data file containing message data generated by LIMS with a RedCoal Email to a mobile telephone text messaging service (Optus).
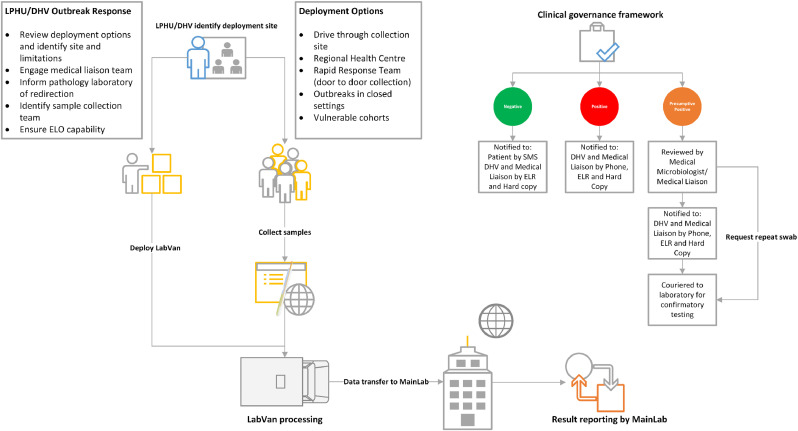
Figure 2.
Mobile laboratory outbreak response. LPHU, Local Public Health Unit; DHV, Department of Health, Victoria; ELO, Electronic test order; ELR, Electronic laboratory report.
Positive samples were transferred by courier to the MainLab at the completion of the LabVan's deployment for that day for confirmatory testing utilising the Aptima® SARS-CoV-2 assay (Hologic, Marlborough, MA, USA). The Aptima® assay amplifies and detects two conserved regions of the ORF1ab gene and is a TGA registered in vitro diagnostic test intended for the qualitative detection of RNA from SARS-CoV-2 in respiratory samples.
Data analysis and statistics
Turnaround times (TATs) from collection to report for sample processing were determined using time stamps obtained from e-orders generated at sample collection, and within LIMS at sample accessioning and reporting. Samples were excluded from TAT calculations where collection time stamps were missing, for example samples submitted with paper request forms. Where there was a delay to accessing the e-order staff would proceed to test, finalising the e-order prior to reporting. All samples with a received to report TAT </= 45 min or a collect to report TAT <50 min were excluded from calculations as this is the minimum run time for the Xpert® Xpress SARS-CoV-2 assay; a 5 min buffer was applied for sample collection.
Median, minimum, maximum and interquartile ranges (IQR) of TATs for deployments were calculated using Excel 2019 version 1808. Box and whisker plots were drawn in Excel 2019 and chosen to graphically demonstrate the distribution of TATs and highlight outliers that differed significantly from the rest of the data set. Boxes extended from the 25th to the 75th percentile, whilst whiskers mark the upper and lower bounds that are 1.5 times the IQR.
Clinical governance framework
Sample collection, testing and reporting complied with ISO15189, Medical Laboratories-Requirements for quality and competence and National Pathology Accreditation Advisory Council (NPAAC) standards of the Royal College of Pathologists of Australasia (RCPA). The LabVan cargo space complied with requirements for Biological Safety Level 2 (BSL2) certification. Testing within the LabVan was audited by the National Association of Testing Authorities (NATA), Australia, for compliance with ISO15189, NPAAC standards and BSL2 certification and formal accreditation was achieved for both the LabVan as a laboratory site and for the performance of the Xpert® Xpress SARS-CoV-2 assay within the LabVan under the MainLab's scope of accreditation.17 Testing and reporting was performed with oversight by a medically qualified Fellow of the Royal College of Pathologists of Australasia providing a Clinical Governance structure (Figure 2).
Risk assessments and mitigation measures were developed to cover staff safety when working with respiratory pathogens in a confined environment, and remote location environments that covered operational hours, driver training and management of staff fatigue.
Ethics
Data were collected in accordance with the Victorian Public Health and Wellbeing Act 2008. Ethical approval was received from the University of Melbourne Human Research Ethics Committee (study number 1954615.3). All authors vouch for the integrity and completeness of data and analyses.
Use case and deployment
Initial use cases were developed to enable targeted deployment and mobilisation of the LabVan into environments where there was an identifiable public health benefit. These included (1) Critical public health testing in rural/regional outbreaks where access to rapid PCR testing is limited to the degree that it will cause delays in result notifications and thus negatively impact timely public health decisions; (2) Outbreaks in closed settings (such as, but not limited to, high-rise apartment buildings, residential aged care and residential disability services) throughout Victoria to provide rapid PCR testing to designated critical public health cases that require urgent identification or clearance.
A centralised request process for deployment through the Victorian Department of Health (DHV) COVID Pathology team was developed (Figure 2). Requests were accepted from the Outbreak Management Team (OMT) or the Local Public Health Unit (LPHU). The Rapid Response Team (RRT) was responsible for coordinating swab collection and ensuring a pathway for medical management and notification of positive results. A standardized deployment form documented expected hours of operation, address for deployment and site access inclusive of any limitations to the site with regards to height/space for parking of the vehicle and power supply; the LabVan operated off mains power where provided by the site or on diesel generator provisioned within the LabVan in the absence of external mains supply. In addition, the deployment form required identification of the medical liaison team and LPHU/OMT reporting contacts for sample management and result notification.
To maximise the benefit of the LabVan it was essential to articulate the purpose of each specific deployment and corresponding criteria for samples to be accepted for testing in the LabVan to provide the greatest benefit of rapid testing for public health decision making. Samples collected at deployments not processed by the LabVan were sent to a variety of local pathology service providers as per predefined pathways. Local pathology service providers in the deployment region were advised of the deployment to ensure triaging of samples to the LabVan and adjustments could be made to local resourcing and flow of samples between the two services and avoid disruption to high throughput and routine pathology provisions. Deployment was identified and discussed during the twice daily OMT meetings, as either outbreaks of public health concern or the LPHU had identified testing issues or outbreak concerns with the public health team.
Role of funding
This work was supported by the Department of Health, Victoria State Government, Australia (DHV). DHV assisted with building and staffing the LabVan, study design, data collection, data analyses and writing of the report. No private commercial company or pharmaceutical company contributed funding to this study.
Results
Initially, a LabVan pilot trial was carried out by deployment to a metropolitan health clinic that was already referring their samples to the MainLab for testing. Samples from symptomatic primary case contacts were diverted directly to the LabVan, whilst all remaining samples collected during the trial were couriered back to the MainLab for testing on the Aptima® SARS-CoV-2 assay (Hologic).
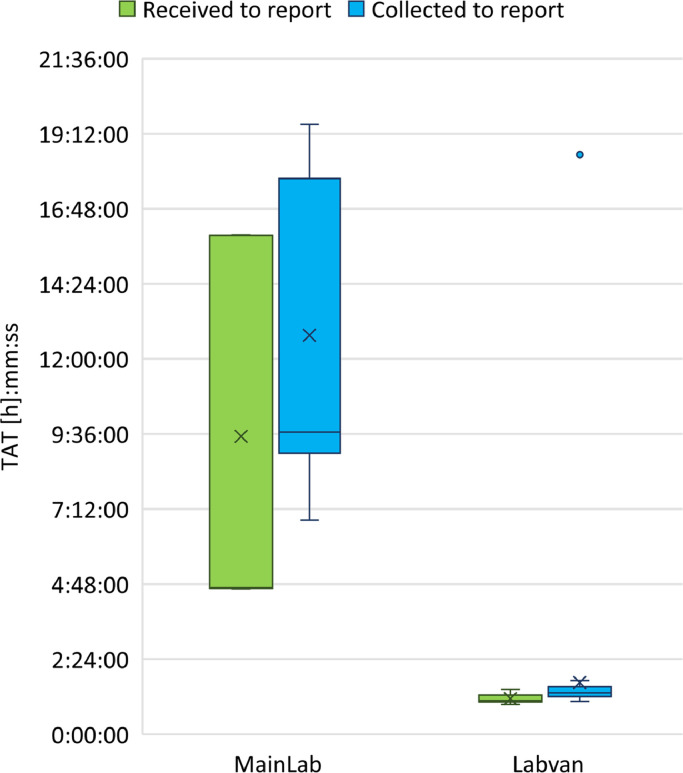
A total of 54 samples were tested in the LabVan compared to 95 samples tested in MainLab (Figure 3; Supplementary Table 1). The median TAT for processing samples in the Labvan was 1:04 h:mm (IQR 0:12, Range, 0:57–1:26 h:mm) compared to the MainLab 4:41 h:mm (IQR 11:17, Range, 4:39–15:58 h:mm). The median TAT from sample collection to reporting in the Labvan was 1:19 h:mm (IQR 0:18, Range, 1:03–18:32 h:mm) compared to 9:40 h:mm (IQR 8:46, Range, 6:51–19:30 h:mm) for testing at the MainLab. There was a single outlier with a TAT of 18:32 (h:mm) in the Labvan due to collection of a sample at the trial site late in the evening the day preceding the trial that was held over for testing in the LabVan on arrival.
Figure 3.
Box plot of turnaround time for sample testing in the pilot trial comparing samples tested in the laboratory with those tested in the LabVan. Boxes extend from the 25th to the 75th percentile, and within each box, horizontal lines denote the median and the cross (X) denotes the mean. The whiskers mark the upper and lower bounds that are 1.5 times the interquartile range (IQR); values beyond these upper and lower bounds were considered outliers and are marked with coloured dots.
Between July and September 2021, the LabVan was used for nine deployments of varying distances from the MainLab, where it was garaged (Figure 4; Supplementary Tables 2 and 3). Deployment types included large drive-through specimen collection sites, walk in collection sites, regional community hospital clinics and high-rise apartment blocks where samples are collected door-to-door by DHV Rapid Response teams. Five of these deployments were to regional areas in Victoria for outbreak management where access to rapid PCR was limited, two deployments were to closed settings and one deployment was to test a vulnerable homeless cohort in metropolitan Melbourne. Existing pathology services were in place for 8 of the deployments comprising a mixture of public and private laboratory services. The two occasions where there were no existing pathology services in place were for settings where there was a contained cohort that required rapid testing, such as Deployment 5 where the LabVan conducted rapid clearance of a small apartment block (24 tests conducted) due to concerns of spread within the building through shared spaces and ventilation and Deployment 6 (86 tests conducted) that targeted a vulnerable homeless cohort.
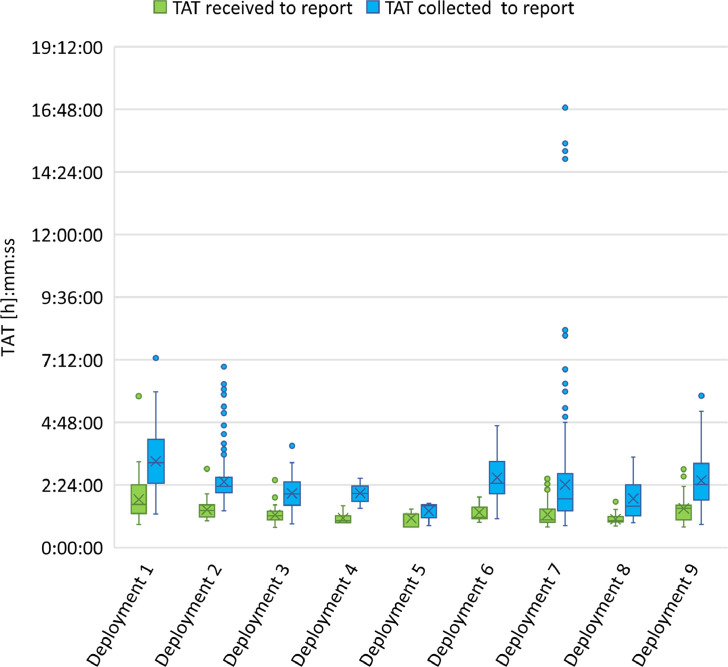
Figure 4.
Box plot of the comparison of TAT for sample processing at LabVan deployments. Boxes extend from the 25th to the 75th percentile, and within each box, horizontal lines denote the median and the cross (X) denotes the mean. The whiskers mark the upper and lower bounds that are 1.5 times the interquartile range (IQR); values beyond these upper and lower bounds were considered outliers and are marked with coloured dots.
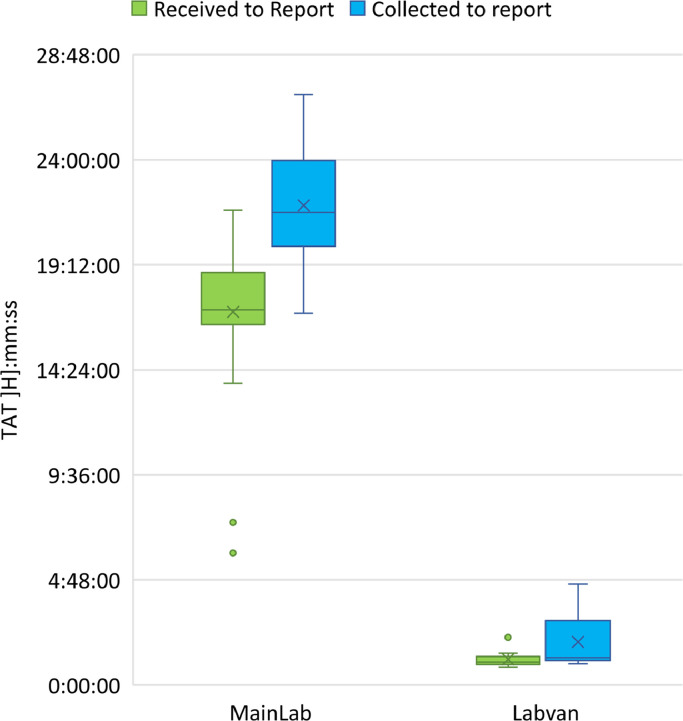
There were 1518 samples collected for testing in the LabVan across the nine deployments (Figure 4). The median TAT from sample receipt to reporting of results was 1:18 h:mm (IQR 0:35, Range, 0:46–5:48 h:mm) for 1489 samples with valid time stamps (Supplementary Table 2). Across the different deployments the median TAT ranged from 1:01 to 1:39 h:mm with the longest TAT observed with the first operationalised deployment. The median TAT from sample collect to reporting of results was 2:18 h:mm (IQR 1:18, Range, 0:50–16:52 h:mm) for 1491 samples with valid time stamps (Supplementary Table 3). In contrast, at the MainLab the median TAT for 12,156 samples tested on the Hologic Panther over the same days as the LabVan deployments was 14:37 h:mm (IQR 4:44, Range, 1:08–48:53 h:mm) from sample receipt to report (Supplementary Table 2) and 19:08 h:mm (IQR 5:49, Range, 1:36–58:52 h:mm) from sample collection to report (Supplementary Table 3).
The period between sample collection and receipt by either the LabVan or MainLab is critical to TAT. Collection sites referring samples to the MainLab were within a 20 km radius and couriers delivered samples over three time frames, 9–11 am, 12 midday-2 pm and 5–7 pm. Samples were tested and reported during the operational hours of 8 am and 7:30 pm. In contrast, samples tested in the LabVan were delivered on demand, processed and reported in real-time; the LabVan's operational hours were aligned with sample collection. Anecdotally, LabVan staff noted that on occasion they needed to retrieve samples that collection staff ‘forgot’ or were ‘too busy’ to transfer to the LabVan. This was particularly noticeable for Deployments 1 and 2, which were large drive-through testing sites and Deployment 7 which was a regional hospital experiencing a large community-based COVID-19 outbreak. The large range with TAT observed with Deployment 7 was also due to sample collection commencing the evening preceding the LabVan arrival on site, as occurred for the pilot deployment. In contrast, Deployments 4 and 5 demonstrated very short TATs (Figure 4). These two sites were managed by a Rapid Response team, moving door-to-door in high rise apartment blocks, rapidly testing and transferring samples to the LabVan. In general, TAT within the van was impacted by the quantity of specimens delivered to LabVan each hour, with smaller, more frequent deliveries (<16 samples/h) leading to faster TATs.
A comparison of TAT for reporting SARS-CoV-2 positive results of samples tested in the LabVan versus the MainLab over the same days of deployments was performed to assess the potential impact on public health responses. Collection teams prioritised symptomatic or high-risk patient samples to LabVan testing resulting in a positivity rate of (0.66%, 10/1518 samples) over the nine deployments, whilst the samples received in the MainLab (encompassing symptomatic and asymptomatic patient samples) reported a positivity rate of 0.39% (47/12,156 samples) during the same time period (Figure 5; Supplementary Table 4). All samples testing positive in the LabVan were subsequently confirmed as positive when retested at the MainLab. The median TAT from sample receipt to report for 9/10 positive results was 1:03 h:mm (IQR 0:09, Range, 0:49–2:11 h:mm) in the LabVan compared to 17:09 h:mm (IQR 2:19, Range, 6:02–21:42 h:mm) for 47/47 samples in the MainLab. One sample was removed from analysis as it was processed in 49 min, just outside the 5 min buffer allowed for sample processing. The median TAT from sample collect to report for 10/10 positive results was 1:14 h:mm (IQR 1:13, Range, 0:53–4:37) in the LabVan compared to 21:36 h:mm (IQR 3:33, Range, 16:59–26:59) for 47/47 samples in the MainLab.
Figure 5.
Box plot of the comparison of TAT for detection of SARS-CoV-2 positive samples in the LabVan compared to the main laboratory. Boxes extend from the 25th to the 75th percentile, and within each box, horizontal lines denote the median and the cross (X) denotes the mean. The whiskers mark the upper and lower bounds that are 1.5 times the interquartile range (IQR); values beyond these upper and lower bounds were considered outliers and are marked with coloured dots.
Public health impact
To maximise the benefit of the LabVan it was essential to articulate the purpose of each specific deployment and corresponding criteria for samples that could be accepted for testing to provide the greatest benefit of rapid analysis for public health decision making. For example, Deployment 1 was into a regional location with zero previous SARS-CoV-2 infections. An outbreak cluster occurred within a school and there was significant concern that there may be undetected infections in a largely unvaccinated population. The LabVan was deployed to an existing pop-up specimen collection site and a Rapid Response Team (RRT) conducted sample collection. Priority testing was allocated to anyone who was symptomatic or an identified primary close contact without impacting the maximum throughput of the site. The median TAT from collection to reporting by the LabVan at Deployment 1 was 3:15 h:mm (IQR 1:40, Range, 1:17–7:16) in comparison to 15 h (IQR 10, Range 1–33) for fixed pathology providers servicing this site (Supplementary Table 3 and Supplementary Table 5). Triaging of priority tests enabled a positive case to be identified within the first 3 h of testing, and expedited contact tracing of the second and third ring contacts of this case.
Deployment 7 was into a regional location with a growing outbreak associated with community transmission. Health care resources and community services were constrained and the LabVan was deployed for the purpose of rapid ‘test and release’ and staff clearance to ensure continuation of services. The median TAT from collection to reporting by the LabVan at Deployment 7 was 1:54 h:mm (IQR 1:27, Range, 0:50–16:52). In contrast pathology providers servicing the community were experiencing a significant increase in testing burden due to the expansion in cases across the state leading to TATs of up to 95 h (Supplementary Table 3 and Supplementary Table 5).
Discussion
We have shown that rapid, flexible deployment of a mobile laboratory in a van can be associated with a dramatic reduction in TAT of results. Median TAT LabVan result from specimen receipt in our evaluation closely approximated the time taken to perform the particular test utilized in the van. With our streamlined process we found minimal additional time to result was required for pre-analytical components of the process such as sample accessioning. This was supported by cohesive alignment of sample collection with the testing capacity of the LabVan. Reduction in TAT was crucial to management of the pandemic locally where small numbers of cases of transmission could potentially lead to population-wide lockdowns in our unvaccinated/susceptible population.
Several alternative options may exist for test type used in a mobile laboratory but factors that need to be considered include safety for testing staff, physical space for instrumentation and staff, complexity of assay, test TAT, testing capacity of the assay, cold chain requirements, performance characteristics and test supply constraints. Consideration of all these issues need to be included for any institution planning on implementing a mobile laboratory. Whilst the LabVan was designed to be platform-agnostic and a range of test choices were considered (Cepheid Xpert® Xpress, Roche cobas Liat®, BD Max™ SARS-CoV-2 assay, in-house developed assay) low complexity equipment was considered more suitable due to physical and environmental constraints. In this respect the Xpert® Xpress instrument and Xpert® Xpress SARS-CoV-2 assay was chosen for speed and ease of use, lack of cold-chain requirements (compared to Roche cobas Liat® for example which requires reagent refrigeration) and ability to contain amplicons in a confined environment as well as for it's compact size still allowing moderate throughput testing capacity. Importantly the assay had already received regulatory approval in Australia and demonstrated exceptional performance characteristics.5 Throughput capability is determined by choice of instrument and assay, and number of instruments that can be placed in the vehicle. Whilst a more complex in-house assay may be able to achieve higher throughput in mobile laboratories,12,14 challenges with amplicon control may arise managing zone separation of extraction and amplification in a confined space.11,12 A secondary consideration with throughput was the number of staff that can operate safely within a vehicle, taking into account social distancing and size of the vehicle.
Several countries have implemented mobile laboratories for SARS-CoV-2 testing within vehicles as reported on media websites and by mobile laboratory manufacturer's,18, 19, 20, 21, 22, 23, 24 however, there are very few published peer-reviewed studies that describe the implementation process, test performance and public health impact of these mobile laboratories. Xing et al.11 described the design and proof of concept of a stand alone automated mobile laboratory inclusive of an automated sampling robot and data management system built into the back of a mobile van, whilst Guo et al.12 demonstrated the use of a truck-based mobile laboratory for large scale screening that mimicked the setup of a fixed site laboratory. The logistical feasibility of these mobile laboratory options was impacted by local regulatory requirements,11 and ease of use/accessibility (van versus a truck).12,13,21, 22, 23, 24
The design and scale of the LabVan (refer to Supplementary material) was a compromise between physical requirements, availability of equipment and ease of use (e.g. no requirement for a heavy vehicle driver's licence, low complexity assay). The LabVan conforms to Australian regulatory requirements including TGA listing of the assay, National Association of Testing Authorities (NATA) accreditation and BSL2 certification of the LabVan as a pathology testing site. In general the feasibility and design of mobile laboratories depends on local regulatory requirements within a given jurisdiction globally. However, integration within the MainLab's quality management systems, LIMS and reporting structures were key to implementation, regulatory accreditation and rapid communication of results, in addition to ensuring the mobile laboratory was embedded within the DHV pandemic response.
Challenges that we encountered included slow or interrupted digital result data transfer when the LabVan was deployed to locations with poor internet access. Regional areas not accustomed to e-orders required mobilisation of a RRT with capacity to collect samples using the DHV COVID test registry. Other potential limitations for LabVan use include locations with weather extremes that exceed the operational capacity of the vehicle's climate control system, essential to maintain equipment function, or the ability to manoeuvre the van (e.g. locations with snow, wet or slippery conditions). There are also limitations to operational hours to accommodate staff availability for rostering in remote locations and staff fatigue when driving long distances and operating in unfamiliar environments for extended deployments.
The longer TAT for “specimen collection to report” than “specimen receipt to report” indicate an opportunity to improve TAT by implementing a process where samples are brought to the LabVan more often (as they are collected) rather than in batches – in order to optimize the impact of the LabVan.
Limitations of our evaluation include the relatively low local prevalence of COVID-19 at the time of deployment and how the LabVan process would perform when the majority of the samples tested positive. However, we anticipate that with delegation of reporting to staff located at the MainLab (rather than to staff located within the LabVan) the performance of the LabVan should continue to result in dramatic reduction of result TAT due to minimal disruption of the testing process with this strategy.
As the pandemic evolves, with increasing community transmission and vaccination and a shift away from tracking and tracing every single case, the use cases for the LabVan continue to evolve. While the focus remains on providing additional laboratory capacity where there are gaps in rapid testing, there is a growing need to focus on the protection of those most vulnerable to serious disease through the early identification of cases of epidemiological significance or clearance of residents in closed settings such as aged-care homes and residential disability services.
Contributors
All authors contributed to and have read and approved the final version of the manuscript. SB, MG and DD were responsible for drafting the manuscript, data analysis, design development, project administration, supporting experiments and manuscript revisions. TH, AD, AM, and SY were responsible for funding acquisition, project administration, resources, manuscript drafting and revisions. MS, NI, MB, and DD were responsible for design development, performing experiments, data verification and manuscript revisions. SB, TS, TH and BH were responsible for conceptualisation, data verification, experimental design and development.
Data sharing statement
Deidentified time stamps and LabVan design and development guidelines will be provided on request to the corresponding author. No patent application applies to the LabVan.
Declaration of interests
AM and SY are employees of Department of Health, Victoria State Government and were involved with funding acquisition, project administration, resources for sample collection, manuscript drafting and revisions. They declare there is no conflict of interest.
All remaining authors declare there are no conflicts of interest.
Acknowledgments
The authors acknowledge the swab collection staff, LPHUs and technical expertise of the Microbiological Diagnostic Unit Public Health Laboratory for generating the laboratory data used in this study. We are grateful to Rebecca Houghton, Stephen Kidd and Nick Cortes from Hampshire Hospitals Foundation Trust (United Kingdom) for their helpful advice during initial planning of the LabVan.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ebiom.2022.103983.
Appendix. Supplementary materials
References
- 1.Chang S.L., Harding N., Zachreson C., Cliff O.M., Prokopenko M. Modelling transmission and control of the COVID-19 pandemic in Australia. Nat Commun. 2020;11(5710) doi: 10.1038/s41467-020-19393-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kretzschmar M.E., Rozhnova G., Bootsma M.C., van Boven M., van de Wijgert J.H., Bonten M.J. Impact of delays on effectiveness of contact tracing strategies for COVID-19: a modelling study. Lancet Public Health. 2020;5(8):e452–e459. doi: 10.1016/S2468-2667(20)30157-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mina M.J., Parker R., Larremore D.B. Rethinking COVID-19 test sensitivity - a strategy for containment. N Engl J Med. 2020;383:e120. doi: 10.1056/NEJMp2025631. [DOI] [PubMed] [Google Scholar]
- 4.Larremore D.B., Wilder B., Lester E., et al. Test sensitivity is secondary to frequency and turnaround time for COVID-19 surveillance. Sci Adv. 2021;7:eabd5393. doi: 10.1126/sciadv.abd5393. 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dinnes J., Deeks J.J., Berhane S., et al. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev. 2021;3(3) doi: 10.1002/14651858.CD013705.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inglis T.J.J., Merritt A.J., Levy A., et al. Deployable laboratory response to influenza pandemic; PCR assay field trials and comparison with reference methods. PLoS One. 2011;6:e25526. doi: 10.1371/journal.pone.0025526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marr I., Francis J.R., Stephens D.P., et al. Development of a mobile laboratory for sudden onset disasters. Disaster Med Public Health Prep. 2020:1–11. doi: 10.1017/dmp.2019.128. [DOI] [PubMed] [Google Scholar]
- 8.Inglis T.J.J., Bradbury R.S., McInnes R.L., et al. Deployable molecular detection of arboviruses in the Australian outback. Am J Trop Med Hyg. 2016;95:633–638. doi: 10.4269/ajtmh.15-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inglis T.J.J., Merritt A., Montgomery J., Jayasinghe I., Thevanesam V., McInnes R. Deployable laboratory response to emergence of melioidosis in central Sri Lanka. J Clin Microbiol. 2008;46:3479–3481. doi: 10.1128/JCM.01254-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Touron P., Siatka C., Pussiau A., et al. A mobile DNA laboratory for forensic science adapted to coronavirus SARS-CoV-2 diagnosis. Eur J Clin Micro Infect Dis. 2021;40:197–200. doi: 10.1007/s10096-020-03989-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xing W., Wang J., Zhao C., et al. A highly automated mobile laboratory foron-site molecular diagnostics in the COVID-19 pandemic. Clin Chem. 2021;67:672–683. doi: 10.1093/clinchem/hvab027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo Z., Li L., Song Y., Xu J., Huang J. Screening high-risk groups and the general population for SARS-CoV-2 nucleic acids in a mobile biosafety laboratory. Front Public Health. 2021;9 doi: 10.3389/fpubh.2021.708476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clinical evaluation confirms high accuracy of highly mobile LamPORE test.. Accessed August 12 August 2021). https://www.gov.uk/government/news/clinical-evaluation-confirms-high-accuracy-of-highly-mobile-lampore-test
- 14.Paton T.F., Marr I., O'Keefe Z., Inglis T.J.J. Development, deployment and in-field demonstration of mobile coronavirus SARS-CoV-2 Nucleic acid amplification test. J Med Microbiol. 2021;70(4) doi: 10.1099/jmm.0.001346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.https://www.health.gov.au/sites/default/files/documents/2021/07/covid-19-vaccine-rollout-update-1-july-2021.pdf. Accessed 18 October 2021
- 16.Department of Health and Human Services, Victoria. Test tracker [Internet]. [Cited 2021 January 29]. Available from: https://testtracker.covid19.dhhs.vic.gov.au/
- 17.https://nata.com.au/news/nata-accredits-first-australian-lab-for-both-mobile-rapid-antigen-tests-and-reverse-transcription-polymerase-chain-reaction-testing.
- 18.Hampshire hospitals NHS foundation trust: HHFT microbiology team on the move. https://www.hampshirehospitals.nhs.uk/news-events/news/lab-van-supporting-hampshire-communities-fight-against-covid-19. Accessed 9 September 2021.
- 19.MAN coronavirus diagnostic vehicle (Germany). https://www.man.eu/de/en/van/business-solutions/man-coronavirus-diagnostic-vehicle/man-coronavirus-diagnostic-vehicle.html#. Accessed 9 September 2021.
- 20.China launches mobile COVID-19 testing lab (020-10-23). https://www.chinadaily.com.cn/a/202010/23/WS5f92909ea31024ad0ba80977.html. Accessed 9 September 2021.
- 21.Florida COVID-19 mobile testing lab. https://flcovidtest-floridadisaster.hub.arcgis.com. Accessed 9 September 2021.
- 22.A COVID Testing Collaboration (Washington D.C.). https://www.insidehighered.com/news/2021/02/25/dc-universities-collaborate-establishing-mobile-covid-testing-lab-will-also-serve. Accessed 9 September 2021.
- 23.CentroTruckTM (Centogene, Germany). https://www.centogene.com/covid-19/testing/centotrucktm.html. Accessed 19 February 2022.
- 24.Mobile laboratory and field-deployable equipment for SARS-CoV-2 analysis (SGS Galson, USA). https://www.sgsgalson.com/mobile-laboratory-for-sars-cov-2. Accessed 18 February 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.