Abstract
Background
Globally, one in three adults has a chronic condition. Many chronic diseases that are not neurological in nature (e.g., diabetes and heart failure) are increasingly associated with cognitive symptoms. However, the instruments used to assess cognitive symptoms in those with nonneurologic chronic illness are heterogeneous, and questions remain as to how cognitive symptoms may be related to demographic and clinical outcome variables, neurocognitive test performance, and other patient-reported outcomes. In this review, we describe associations among self-reported cognitive function, cognitive performance, and additional patient-reported outcomes as well as how cognitive symptoms are measured in nonneurologic chronic illness.
Method
Multiple databases (PubMed, Medline, CINAHL, PsycInfo, EMBASE, SCOPUS, the Cochrane Library, and Academic Search Complete) were searched for studies from 1990 to 2020 that provided data on self-reported cognitive symptoms in those with nonneurological chronic conditions. Initial search yielded 304 articles, of which 32 met inclusion criteria. Quality assessment was conducted using the Critical Appraisal Skills Programme.
Results
Thirty-two total studies were included: twenty cross-sectional, 10 longitudinal, and 2 randomized controlled trials. The tools used to assess self-reported cognitive function in the studies were heterogeneous: 28 unique tools were used. Thirty studies examined associations among self-reported cognitive function and other patient-reported outcomes. In 19 there were significant associations. Six studies showed no significant associations between neuropsychological tests and self-reported cognitive function; another 6 studies found a significant association.
Conclusion
Tools to assess cognitive symptoms were heterogeneous. In most studies, self-reported cognitive symptoms were not correlated with neuropsychological test results, but the majority of studies found a strong association between self-reported cognitive function and other patient-reported outcomes. Implications. Consensus on measuring cognitive symptoms would facilitate cross-study comparisons and facilitate scientific progress in those with nonneurological chronic conditions. Based on these results, there is a need to establish a standardized approach for self-reported cognitive function measurement in patients with nonneurologic chronic illness.
1. Introduction
National surveys suggest that more than 26% of older adults are concerned about a potential diagnosis of Alzheimer's, and more than 50% are concerned about becoming a burden on family because of future cognitive problems [1, 2]. Some cognitive decline is expected in older adults, but cognitive changes that impair one's ability to function in middle to late adulthood are unexpected. These changes are complex and multifaceted, especially in those with nonneurologic chronic conditions with known cognitive risk factors (e.g., diabetes, cardiovascular disease, and cancer) [3, 4]. However, despite risk factors and the prevalence of cognitive changes in those with nonneurologic chronic conditions, less is known about cognitive function in such populations.
The effects and impact of cognitive dysfunction on day-to-day life such as difficulties in memory and deficits in attention are difficult to assess with standard neuropsychological tests [5]. Individuals' perspectives are therefore critical to our understanding of cognitive symptoms, not only because perceived cognitive decline may be a precursor to mild cognitive development and dementia [6], but also because self-reported cognitive function captures the impact of cognitive symptoms on daily function. At least 20% of people 45 years and older with one chronic disease report having cognitive problems, and this prevalence may be higher for those with specific conditions [7]. Those who have had a stroke, a history of heart disease, or chronic obstructive pulmonary disease have a higher occurrence of self-reported cognitive symptoms than do those without those diseases [8]. For example, 27.1% of adults aged 45–65 years who have coronary artery disease report subjective cognitive problems, whereas in healthy adults 65 and older, the prevalence is 18.7% [7]. The presence of midlife self-reported cognitive dysfunction can be a risk for dementia, sometimes presenting before objective impairments are found with neuropsychological tests [9]. In addition, self-reported cognitive dysfunction can impact daily self-management of chronic conditions such as diabetes [10, 11] as well as quality of life [12, 13]. As a result, research on self-reported cognitive dysfunction in persons at risk for mild cognitive impairment has increased [14].
In this review, we describe associations among self-reported cognitive function (SRCF), cognitive performance, and additional patient-reported outcomes as well as how cognitive symptoms are measured in nonneurologic chronic illness.
2. Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement, including the PRISMA 27-item checklist of essential review characteristics, informed the procedures for this review [15] (Supplementary Material A). The review protocol was registered with PROSPERO (CRD4202146706) per guidance from the Cochrane Collaborative [15].
2.1. Data Sources, Search Strategy, and Selection
The following databases were searched for articles related to SRCF in peer-reviewed journals from January 1990 through October 2020: CINAHL, MedLine, PubMed, PsycInfo, EMBASE, SCOPUS, the Cochrane Library, and Academic Search Complete.
Broad search terms (MeSH) and synonyms were used including subjective cognitive complaints, perceived cognitive problems, chronic conditions, and chronic disease (Supplementary Material B). Citations of all relevant studies were also reviewed. MedLine was searched first, and resulting syntax and headings were used to search the other databases. Key inclusion criteria were as follows: (1) use of a self-report measure of cognitive function (e.g., perceived cognitive issues, symptoms of cognitive problems, memory complaints); (2) participants 18 years of age or older; and (3) participants diagnosed with one or more nonneurologic chronic conditions (e.g., type 2 diabetes, coronary artery disease, and obstructive pulmonary disease). All quantitative study designs—randomized controlled trials (RCTs), cross-sectional studies, and longitudinal studies—were included. Exclusion criteria were as follows: (1) study participants diagnosed with neurologic chronic conditions, such as dementia, stroke, HIV-associated cognitive disorders, and central nervous system disorders; (2) publications that were not peer-reviewed or not written in English; and (3) posters, review papers, letters, and conference proceedings. We also excluded studies of those with chemotherapy-related cognitive dysfunction, because comprehensive reviews have examined self-reported cognitive function following chemotherapy treatment [16, 17]. All titles, abstracts, and full texts of the studies were independently screened by two reviewers, and disagreements were settled to ensure the studies' eligibility.
2.2. Data Extraction
Data, extracted by all authors, included author, year of publication, research design, data collection time points, purpose of the study, study setting, sample characteristics, measures of SRCF and primary variables, and associations with objective neuropsychological tests. The first and second reviewers double-verified the extracted data for accuracy. When data needed for extraction were missing, the first author contacted the authors of the study via e-mail to request the data (Supplementary Material C).
2.3. Quality Appraisal
The Critical Appraisals Skills Programme (CASP) [18] was used to assess the quality of the included studies. In this review, randomized controlled trials (RCTs) were assessed using the CASP RCT checklist, and the remaining studies were evaluated using the CASP cohort study checklist.
2.4. Synthesis
Meta-analysis was not feasible, due to the variability among studies in design, measures of self-reported cognitive function, and outcome variables. The data were instead analyzed using Popay et al. [19] methods for narrative synthesis in systematic reviews.
3. Results
3.1. Search
Eight hundred and sixty-six eligible studies were retrieved from the databases. After duplicates (n = 562) were removed, 304 titles remained and were imported to an online platform from Covidence (https://covidence.org) for independent screening and data extraction. A total of 32 studies were included in the final analysis (See Figure 1 PRISMA flow diagram for details of screening.).
Figure 1.
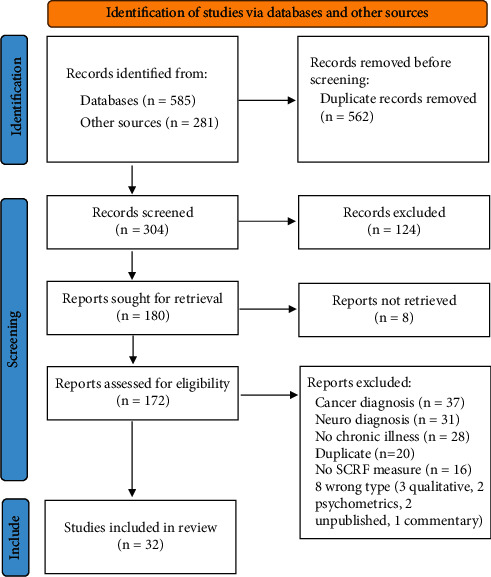
PRISMA flow diagram.
3.2. Risk of Bias/Quality Assessment
Two studies were RCTs; for both studies, all 11 items on the CASP [18] RCT checklist indicated relatively high study quality. Of the remaining studies, a few had high scores, with no quality issues recognized (n = 8) or one or two low (“No”) quality scores (n = 4). Most of the “No” scores were related to confounding factors (n = 3) and bias minimization (n = 1) (Supplementary Material D).
3.3. Characteristics of Included Studies
Of the 32 studies included in the analysis [20–51], 20 (63%) were cross-sectional, 10 (31%) were longitudinal, and two (6%) were RCTs (Table 1). Of the two RCTs, one investigated the effects of a computerized cognitive training intervention in participants with chronic pain [23]. The other examined the effects of cognitive behavioral therapy on cognitive impairment, both objective and subjective [38]. Six studies compared participants with healthy controls [21, 25, 39, 42, 45, 49]. Four other studies used specific comparators: (1) treatment with steroids versus nontreatment [30]; (2) two or more chronic conditions versus one or none [35]; (3) amputation for vascular or nonvascular etiologies [41]; and (4) treatment with erythropoietin versus nontreatment [43]. Follow-up in the longitudinal studies ranged from 1 week to 2 years.
Table 1.
Summary of included studies.
| Author(s), year | Study design | Sample & setting | Assessment schedule | SRCF measure | Other assessment types | SRCF results | Association between SRCF and (1) NP tests (2) other PRO |
|---|---|---|---|---|---|---|---|
| Alonso-Prieto et al. [20] | Longitudinal | Depression N = 36 outpatient psychiatric clinic/Canada | Baseline and 8 weeks | British Columbia Cognitive Complaints Inventory (BC-CCI) | PRO | SRCF improved after treatment with desvenlafaxine (Cohen's d 1.24) | 1. Not reported |
| 2. Significant association with work functioning and depression | |||||||
|
| |||||||
| Avants et al. [21] | Cross-sectional | HIV- v. HIV+N = 120 outpatient methadone maintenance clinic/United States | Baseline | Neuropsychological impairment scale | PRO | HIV +positive more cognitive impairment, cognitive symptoms, and intensity of symptoms (F = 0.6, p < .002; F = 10.7, p < .001; F = 3.8, p < .053) | 1. Not reported |
| 2. SRCF significantly related to affective distress (r .880, p < .001) | |||||||
|
| |||||||
| Baker, Gibson, et al. [22] | Cross-sectional | Chronic pain | Baseline | Cognitive failures questionnaire, Everyday Memory Questionnaire behavior rating BRIEF-A (working memory subscale) | NP PRO | Group means for the SRCF measures indicated higher levels of reported problems (WM: 69.8(12.8) out of 100; CFQ: 51.18(21.56) out of 100; EMQ: 21.56(14.34) out of 52 | 1. SRCF was significantly correlated with NP tests (p < 0.5) |
| 2. Depression and catastrophizing did not moderate the association between subjective and objective performance | |||||||
| N = 41 | |||||||
| Community dwelling adults/Australia | |||||||
|
| |||||||
| Baker, Georgiou-Karistianis, et al. [23] | RCT | Chronic pain | Baseline and 8 weeks | Cognitive failures questionnaire, Everyday Memory Questionnaire Behavior Rating Inventory of Executive Function | NP PRO | Intervention group improved SRCF v. control (ES .43, p = .017) | 1. Not reported |
| N = 39 | 2. Depression, anxiety, and pain interference, not significant | ||||||
| Community dwelling adults/Australia | |||||||
|
| |||||||
| Brück et al. [24] | Longitudinal | PTSD after ICU discharge | Baseline, 3, 6, and 12 months | Cognitive failures questionnaire | NP PRO | Prevalence of cognitive dysfunction 34% at 3 months, 51% at 6 months, and 45% at 12 months | 1. Not significant |
| N = 58 | 2. Not significant | ||||||
| University hospital/Sweden | |||||||
|
| |||||||
| Brunette et al. [25] | Longitudinal | Chronic obstructive pulmonary disease | Baseline and at 3 weeks | Cognitive difficulties scale (CDS) | NP PRO | No significant difference in SRCF between those with and without COPD | 1. Cognitive difficulties were associated with worse performance (p .037) |
| N = 59 | 2. Not significant | ||||||
| Community based/United States | |||||||
|
| |||||||
| Brunmeier et al. [26] | Cross-sectional | Congenital heart disease | Baseline | Functional assessment of cancer therapy (FACT) brain scale | PRO | 34% met criteria based on SRCF for formal neuro cognitive evaluation | 1. Not reported |
| N = 337 | 2. Not reported | ||||||
| Outpatient congenital heart program/United States | |||||||
|
| |||||||
| Cockshell & Mathias [27] | Cross-sectional | Chronic fatigue | Baseline | Cognitive failures questionnaire | NP PRO | 90% of those with chronic fatigue reported cognitive problems v. 12% without | 1. Not significant |
| N = 50 | 2. Depression was significantly positively related to SRCF (p < 0.01) | ||||||
| Outpatient clinics/Australia | |||||||
|
| |||||||
| Duijndam et al. [28] | Longitudinal | Cardiovascular disease | Baseline, 1 month, 12 months | Health complaints scale | PRO | Those with more perceived cognitive problems were younger and had more frequent percutaneous coronary intervention | 1. Not reported |
| N = 385 | 2. Poorer perceived cognition over time was related to poor quality of life (p < 0.01) | ||||||
| Hospital-based/The Netherlands | |||||||
|
| |||||||
| Fazeli et al. [29] | Cross-sectional | HIV | Baseline | Patient's assessment of own functioning inventory | NP PRO | Median SRCF score was 2 (0–9) | 1. Significant association (p < 0.01) |
| N = 100 | 2. Not reported | ||||||
| Community dwelling/United States | |||||||
|
| |||||||
| Frol et al. [30] | Cross-sectional | Asthma and rheumatoid arthritis | Baseline | Global measure of impairment (GMI; patient-rated) | NP PRO | 65% taking corticosteroids had subjective cognitive problems v. 29% not taking corticosteroids | 1. Not significant |
| N = 31 | 2. Not significant | ||||||
| Outpatient clinics/United States | |||||||
|
| |||||||
| Gallo et al. [31] | Longitudinal | Cardiovascular disease | Baseline, 3.5–7.5 months | Cognitive difficulties scale (CDS) | PRO | Emotional distress and SRCF were significantly positively correlated (<.01) | 1. Not reported |
| N = 76 | 2. SRCF predicted emotional symptoms (p < 0.01) | ||||||
| Outpatient cardiology clinics/United States | |||||||
|
| |||||||
| Haley et al. [32] | Longitudinal | Cardiovascular disease | Baseline and 1 year | Cognitive difficulties scale (CDS) | PRO imaging | Higher baseline cognitive complaints were significantly related to lower cognitive ability at 12 months | 1. Not reported |
| N = 83 | 2. Cognitive complaints were significantly positively related to severity of microvascular disease (p = .028) | ||||||
| Outpatient cardiology clinics and cardiac rehab/United States | |||||||
|
| |||||||
| Henry et al. [33] | Longitudinal | End-stage kidney disease | Baseline, daily monitoring for 1 week | Cognitive function subscale of the kidney disease quality of life-short form | NP PRO | Ratings of cognitive impairment were greater on dialysis days when compared to nondialysis days (beta = 0.097, p = .005) | 1. Greater diary-rated cognitive impairment was significantly related to lower working memory (beta = -0.07, p = .022), visual recall scores (beta = -0.05, p = .004), and longer dot tracing times (beta = 0.002, p = .005) |
| N = 26 | 2. Not reported | ||||||
| Dialysis clinic/United States | |||||||
|
| |||||||
| Jackson & Cooper [34] | Cross-sectional | Diabetes, cardiovascular disease, arthritis, chronic obstructive pulmonary disease, obesity | Baseline | Investigator developed item “during the past 12 months, have you experienced confusion or memory loss that is happening more often or is getting worse?” | PRO | 11.5% of the sample had experienced subjective cognitive decline in the preceding 12 months | 1. Not reported |
| N = 4,129 | 2. Those with subjective cognitive decline were significantly more likely to have depression (54.3%, p < 0.0001), be dissatisfied with life (24.7%, p < 0.0001), experience mental distress (37.6%, p < 0.0001), and feel they have inadequate social/emotional support (20%, p < 0.0001) | ||||||
| Telephone survey/United States | |||||||
|
| |||||||
| Jacob et al. [35] | Cross-sectional | Multiple chronic conditions | Baseline | Investigator developed item: “In the past month, have you had any problems with concentrating on what you were doing?” and “have you noticed any problems with forgetting things in the past month?” | PRO | The prevalence (95% CI) of subjective concentration complaints and subjective memory complaints was 22.0% (20.9–23.2%) and 29.9% (28.7–31.1%), respectively | 1. Not reported |
| N = 7,399 | 2. Depression and anxiety were significantly positively related to cognitive complaints (p < .001) | ||||||
| Community based/United Kingdom | |||||||
|
| |||||||
| Kiessling & Henriksson [36] | Cross-sectional | Coronary artery disease | Baseline | Cardiac health profile questionnaire (CHP) | PRO | No significant differences in assessed total SRCF scores between patients with or without a prior myocardial infarction (p = .78) | 1. Not reported |
| N = 253 | 2. SRCF was significantly correlated with quality of life (p < .001) | ||||||
| In- and outpatient medicine departments/Sweden | |||||||
|
| |||||||
| Kiessling & Henriksson [37] | Longitudinal | Coronary artery disease | Baseline, 1 year, 2 years | Cardiac health profile questionnaire (CHP) | PRO | Reduced perceived cognitive function [OR 1.59 (95% CI: 1.12–2.25); p = 0.0087] predicted sick leave or early retirement due to CAD | 1. Not reported |
| N = 169 | 2. Lower perceived cognitive function was associated with lower quality of life (p = .002) | ||||||
| In- and outpatient medicine departments/Sweden | |||||||
|
| |||||||
| Knoop et al. [38] | RCT | Chronic fatigue syndrome | Baseline, 8 months, 14 months | Checklist individual strength-concentration sickness impact profile-alertness behavior | NP PRO | Self-reported cognitive impairment decreased significantly more after CBT than in the control group | 1. Not reported |
| N = 233 | 2. Not reported | ||||||
| Outpatient clinics/The Netherlands | |||||||
|
| |||||||
| Matsuzawa et al. [39] | Cross-sectional | Type 2 diabetes | Baseline | Self-reported questionnaire for subjective complaints of memory and daily functioning: 3 items (yes/no) derived from the Cambridge examination for mental disorders of the elderly | NP PRO | Self-perception of memory dysfunction was not different between diabetic and nondiabetic participants (60.0% v. 60.0%) | 1. Not reported |
| N = 261 | 2. Memory dysfunction noticeable by others (P = 0.018) and impaired activity in taking medication (P = 0.001) predicted dementia | ||||||
| Outpatient clinic/Japan | |||||||
|
| |||||||
| McCracken & Iverson [40] | Cross-sectional | Chronic pain | Baseline | Sickness impact profile (SIP): alertness behavior subscale | PRO | 54% reported at least one cognitive complaint. Most common subjective cognitive complaints: Forgetfulness (23.4%); minor accidents (23.1%); difficulty finishing tasks (20.5) | 1. Not reported |
| N = 275 | 2. Pain-related anxiety and depression were moderately associated with total cognitive complaints (p < .01) | ||||||
| Outpatient clinic/Canada | |||||||
|
| |||||||
| Morgan et al. [41] | Cross-sectional | Lower limb loss (vascular etiology) | Baseline | Quality of life in neurological disorders applied cognition–general concerns v1.0 short form | PRO | Subjective complaints were higher in those with limb loss v. controls | 1. Not reported |
| N = 484 | 2. Worse quality of life significantly associated with more cognitive complaints (p < .001) | ||||||
| Community dwelling/United States | |||||||
|
| |||||||
| Nguyen et al. [42] | Cross-sectional | Hypertension | Baseline | Subset of the memory functioning questionnaire (MFQ): 1-item on overall problems with memory | NP PRO | No significant difference in SRCF in those with hypertension v. those without hypertension | 1. Those with memory complaints and hypertension had greater difficulty on NP tests than those without hypertension (p = .0003) |
| N = 105 | 2. Not significant | ||||||
| Community dwelling adults/United States | |||||||
|
| |||||||
| Ott et al. [43] | Longitudinal | Depression | Baseline, 9 weeks, 14 weeks | Massachusetts general hospital cognitive and physical functioning questionnaire (CPFQ) | NP PRO | Those treated with erythropoietin had reduced cognitive complaints v. those not treated with erythropoietin | 1. Not significant |
| N = 79 | 2. Improvement in SRCF was not significantly associated with quality of life of occupational functioning | ||||||
| Setting not described/Denmark | |||||||
|
| |||||||
| Roth et al. [44] | Cross-sectional | Chronic pain | Baseline | Brief symptom inventory | PRO | 62% reported moderate to severe problems with cognitive function | 1. Not reported |
| N = 222 | 2. Associations with negative affect, negative self, catastrophizing, neck pain, and fatigue were significant (p < 0.5) | ||||||
| Outpatient pain management program/United States | |||||||
|
| |||||||
| Sharma et al. [45] | Cross-sectional | HIV | Baseline | Self-reported cognitive complaints | PRO | 12.5% reported subjective cognitive problems | 1. Not reported |
| N = 2,062 | 2. Subjective cognitive complaints were over twice as likely to report falls than those reporting no cognitive difficulties (AOR 2.19, 95% CI: 1.56–3.08) | ||||||
| Community dwelling/United States | |||||||
|
| |||||||
| Steinbusch et al. [46] | Longitudinal | Cardiovascular disease/cardiac arrest | Baseline, 2 weeks, 3 months, 1 year | Cognitive failures questionnaire | NP | Two weeks after cardiac arrest, SRCF was impaired in 11%, 12% at 3 months, and 14% at 1 year | 1. Not reported |
| N = 141 | 2. Not reported | ||||||
| Inpatient coronary care units/The Netherlands | |||||||
|
| |||||||
| Touradji et al. [47] | Cross-sectional | Lyme disease | Baseline | Questionnaire of neurocognitive complaints | NP | 92% reported problems with cognitive function | 1. Not significant |
| N = 124 | 2. Not reported | ||||||
| Outpatient clinic/United States | |||||||
|
| |||||||
| Vance et al. [48] | Cross-sectional | HIV | Baseline | 2003 AIDS Alabama needs assessment 4 items' assessing cognitive complaints | PRO | Mean cognitive complaints score was 17.63(5.57)–range 4–24 with higher scores indicating better SRCF | 1. Not reported |
| N = 427 | 2. Self-perceived health status and stress predicted SRCF (p < .05) | ||||||
| Community AIDS services organization/United States | |||||||
|
| |||||||
| Wingbermühle et al. [49] | Cross-sectional | Noonan syndrome | Baseline | Symptom checklist-90-revised (SC-90R) | NP PRO | Those with Noonan's reported more cognitive problems than control | 1. There was significant difference in speed information processing (F1,82 = 5.15, p = .026, ηp2 = 0.059) and delayed recall (F1,82 = 4.80, p = .031, ηp2 = .055) |
| N = 42 | 2. There was a significant difference in quality of life between groups (case group mean = 18.4, SD = 7.4; control group mean: 15.0, SD = 4.6; t (66.9) = 2.52, p = .014, d = 0.55) | ||||||
| Medical center-genetics department/The Netherlands | |||||||
|
| |||||||
| Yoon et al. [50] | Cross-sectional | Rheumatoid arthritis | Baseline | Perceived deficits questionnaire | NP PRO | Mean score on the PDQ was 11.8(5.1) | 1. There was no significant relationship between total cognitive function score and SRCF score |
| N = 40 | 2. Depression and sleep quality (β = 0.37, p = .025; β = 0.17, p = .034) were significantly associated with SRCF | ||||||
| Outpatient rheumatology clinic/Korea | |||||||
| Zhu et al. [51] | Cross-sectional | HIV | Baseline | AIDS health assessment questionnaire | PRO | 47.22% reported at least one cognitive impairment in the last month | 1. Not reported |
| N = 324 | 2. Higher levels of perceived discrimination (β = −121, p = .036) were significantly associated with lower levels of SRCF | ||||||
| Community clinic/China | |||||||
3.3.1. Study Populations
Sample sizes ranged from 26 to 11,379 with a mean age range of 30 to 78.5 years. The most common chronic conditions included coronary artery disease (10 studies), HIV (4 studies), and chronic pain (4 studies). Fifteen studies had a majority of females in the sample, 15 were majority males, and 2 were evenly split. Seventeen studies did not report the ethnic makeup of the samples. Sixteen of the studies were conducted in the U.S., and four were conducted in The Netherlands (See Table 1 and Supplementary Material E).
3.3.2. Self-Reported Cognitive Function Measures
Twenty-eight different tools were used to assess SRCF across the 32 studies. The most common were the Cognitive Failures Questionnaire (five studies) and the Cognitive Difficulties Scale (three studies). Other tools included the Everyday Memory Questionnaire, the Cognitive Complaints Inventory, the Behavior Rating Inventory of Executive Function, and the Health Complaints Scale. Four were sets of author-derived questions, with items such as “Do you have any complaints concerning your memory?” (See Supplementary Material F). Three studies did not report the cognitive domains assessed by the SCRF measure [43, 47, 49] and two used “global” SCRF tools [30, 42]. The remaining studies ranged from measuring one domain (e.g., only executive function) [23, 39] to seven domains [26]. Ten studies [21, 25, 28, 31, 32, 36, 37, 44, 48, 51] reported reliability of the SCRF tools with Cronbach's alphas ranging from .67 to .8 for the Health Complaints Scale–Concentration subscale to .96 for the Cognitive Difficulties Scale. Items on each tool ranged from three [39] to 95 [21].
3.3.3. Reporting of Other Patient-Reported Outcomes
Thirty studies included assessment of other self-reported outcomes. The most common were the Beck Depression Inventory (6 studies) and the Hospital Anxiety and Depression Scale (3 studies). Thirteen studies (41%) included objective neuropsychological testing. The neuropsychological tests ranged from screening tests to computerized assessments to comprehensive batteries. Only one study included imaging [32].
3.4. Primary Study Results
Descriptions of study outcomes varied widely in design, SRCF related endpoints, and measures. Therefore, a meta-analysis could not be conducted. In some studies, only descriptive results of self-report measures were reported (e.g., means, percentages). Other studies gave more in-depth results including (1) comparison of symptoms of cognitive problems, usually between participants with a chronic illness and those who did not; (2) cognitive changes over time in longitudinal studies; and (3) differences in cognitive symptoms between groups in RCTs of interventions. Therefore, the number of studies was insufficient to conduct a meta-analysis.
3.4.1. Demographics and Self-Reported Cognitive Function
Three studies [33, 37, 48] focused mainly on participants' demographic characteristics in relation to self-perceived cognitive function. One of these studies [37] investigated the relationship further, using longitudinal data collected at 12 months. Two of the three studies [33, 37] found a significant relationship between unemployment/early retirement/homemaker and increased cognitive complaints. Middle age (45–54 years) was also associated with more cognitive complaints [33], and older age (55 and older) was associated with more cognitive complaints [37, 48].
3.4.2. Self-Reported Cognitive Function and Neuropsychological Tests
Nine studies (28%) compared objective neuropsychological tests of cognitive function with SRCF [22, 24, 25, 27, 29, 32, 34, 42, 46] with Pearson's r correlations ranging from −24 to −27 [25] to .44 to .85 [22]. Two of them found no significant relationships between self-report and objective measures [24, 27]. Four others [22, 25, 29, 32] identified relationships between specific cognitive domains and perceived function. In two of those four, memory concerns were significantly related to objective memory test performance [22, 32]. In another, greater overall perceived problems were associated with worse scores on executive function, processing speed, and language measurements [25]. The last found that higher scores on the Montreal Cognitive Assessment were significantly related to fewer everyday cognitive symptoms [29]. Steinbusch et al. [46] followed cognitive function over time and found that higher baseline cognitive complaints were significantly related to lower cognitive ability at 12 months. Jackson and Cooper [34] found that a diagnosis of hypertension was associated with worse objective cognitive function over time in those with SRCF than in those without. Similarly, Nguyen et al. [42] showed that community dwelling older adults who had hypertension and memory concerns had worse objective performance on cognitive tests than did nonhypertensive participants with memory complaints.
3.4.3. Other Patient-Reported Outcomes and SRCF
A number of additional patient-reported outcomes were associated with SRCF in samples with various nonneurologic chronic conditions. Two studies reported findings from separate samples undergoing interventions for cardiovascular disease—percutaneous coronary interventions (PCIs) and coronary artery bypass surgery (CABG) [28, 31]. For those undergoing PCI, poorer perceived cognitive function was associated with poorer quality of life independently of demographics, fatigue, mood, and other clinical variables [28]. In the sample undergoing CABG, baseline cognitive complaints predicted a higher rate of negative emotional symptoms at 5 months [31]. For those with cardiovascular disease, but not undergoing any cardiac interventions, worse SRCF had a significant association with worse quality of life [36]. For patients with chronic pain, female gender, pain intensity, catastrophizing, posttraumatic stress disorder, depression, location of pain, and fatigue were positively associated with cognitive complaints. Depression and fatigue were most predictive [40, 44]. Depression severity and worse work functioning were significantly associated with poorer SRCF in depressed patients [20]. For rheumatoid arthritis patients, sleep quality was significantly associated with SRCF [50]. Zhu, Hu, Xing, Guo, and Wu [51] reported that increased levels of HIV-related discrimination were associated with higher levels of SCRD even after controlling for demographics, mental health conditions, and social support.
3.4.4. Severity of Chronic Conditions and Self-Reported Cognitive Function
Eleven studies (34%) examined associations between severity of chronic conditions and SRCF [21, 26, 32–35, 39, 41, 46, 47, 49]. Overall, multimorbidity and higher severity of disease were positively associated with greater self-reported cognitive problems. However, in one of these studies [39], those with type 2 diabetes mellitus (T2DM) and diagnosed cognitive impairment did not differ in the number of cognitive complaints when compared with those with T2DM who were cognitively healthy.
4. Discussion
Almost every evaluation of self-reported cognitive symptoms used a unique approach to assess self-reported cognitive function (28 of 32 studies, 88%). This heterogeneity of instrumentation can inhibit data sharing and generalizability of results across diverse populations. The use of common data elements for self-reported cognitive function in persons with nonneurologic chronic illness could contribute to accelerating intervention development and testing [13]. The reviewed studies used 28 measures to assess SRCF, with little overlap among them. A prior review and meta-analysis of studies (n = 53; 20,319 participants) examined the association between objective and subjective cognitive function in normatively aging adults without chronic illnesses and found that self-reported cognitive function accounted for less than 1% of the performance in objective measures [52]. However, that review included studies that used five specific measures for subjective memory and likely excluded a large number of other studies, because there are not any “gold-standard” assessments of self-reported cognitive function. We therefore expanded on that review in two ways: by focusing on self-reported cognitive function and a broader population, adults with at least one chronic condition. Two other meta-analyses of self-reported memory concerns and prediction of mild cognitive impairment and dementia (n = 49 studies) showed that conversion to dementia was 1.5 to 3 times higher in those who had self-reported cognitive complaints than in those who did not [53, 54]. Both reviews also noted the lack of established/standardized measures for self-reported cognitive function and the impact of depression on cognitive symptoms.
In the studies in this review, increased severity of chronic disease was associated with greater subjective cognitive impairment. Chronic diseases are themselves associated with a number of negative consequences such as lower quality of life, increased mortality, and loss of independence [55, 56]. A number of mechanisms dependent on the type of chronic illness may be responsible for this association. For example, in chronic obstructive pulmonary disease, low oxygen levels may directly affect the brain [57]. Or, more generally, physical illness can lead to fatigue and the subjective feeling of “not thinking well” [58]. It may also be that increased severity of disease has led to a decrease in leisure activities, exercise, sleep, or functional independence, which have protective effects on cognition [59]. Additionally, it has been shown that subjective cognitive dysfunction can impact daily self-management of chronic conditions like diabetes [10, 11] and quality of life [13, 59]. For these reasons, qualitative and quantitative research on subjective cognitive dysfunction in persons at risk for dementia are needed.
5. Conclusions
As this review demonstrates, many tools are used to measure self-reported cognitive symptoms, and clinicians should be aware that instrument selection will likely impact results. However, other reviews have found that self-reported cognitive complaints are a valid indicator of cognitive decline [60, 61]. It may be that deciding on a high-quality psychometric tool and using it consistently are important for clinical practice. Confounders for cognitive symptoms may also need to be assessed in clinical settings; anxiety, neuroticism, and dementia-related worry are variables related to increased subjective cognitive symptoms [60, 62].
Jessen et al. [63] provide a framework for investigating subjective cognitive symptoms in clinical settings and research. This framework includes suggested variables to examine (e.g., onset of subjective cognitive decline, believing one's cognitive performance is worse than of those of the same age) and criteria that increase the possibility of the existence of preclinical Alzheimer's disease in people with subjective cognitive decline. Molinuevo et al. [64] also suggest differentiating between “complaints” and “worries,” using a measure appropriate for the target population and including measures of stress, depression, and anxiety. Although the criteria in these two studies, from the Subjective Cognitive Decline Initiative Working Group, indicate that nonneurologic medical issues (e.g., chronic conditions) could underlie self-reported cognitive decline due to poor physical health, the studies do not suggest any recommendation other than to use care in interpreting the results of subjective cognitive complaints. Longitudinal assessment of subjective cognitive complaints may also be important, because changes in SRCF can indicate the functional benefit of prevention interventions.
5.1. Limitations
This review contributes a synthesis of measures and characteristics of self-reported cognitive symptoms in persons with nonneurologic chronic illnesses, but the heterogeneity of studies' effect sizes, outcomes, and measures did not permit data pooling, limiting cross study. The wide range of tests to measure perceived cognitive function likely contributed to variation in findings.
Cognitive impairment and chronic illness are both prevalent and detrimental. Given the present review's heterogeneity in assessment tools and evidence limitations, it is possible that self-management of chronic illness is influenced by the cooccurrence of cognitive symptoms. Future prospective longitudinal studies should examine the relationship of perceived cognitive symptoms and the self-management of chronic conditions, and assessments and interventions for improving cognitive function should be incorporated into care for older adults with chronic conditions.
Acknowledgments
Editorial support was provided by Dr. John Bellquist at the Cain Center for Nursing Research and the Transdisciplinary Precision Health Intervention Methodology Training Program (PI Kim: T32 NR01903520) at The University of Texas at Austin School of Nursing.
Data Availability
The data are available in the submitted supplementary files.
Conflicts of Interest
The authors do not have any conflicts of interest.
Supplementary Materials
Supplementary Materials. Supplementary Material A. Methods. Supplementary Material B. Search Strategy. Supplementary Material C. Data Extraction. Supplementary Material D. Quality Appraisals. Supplementary Material E. Study Demographics. Supplementary Material F. Table of Subjective Cognitive Function Measures.
References
- 1.Mehegan L., Rainville C. 2018 AARP brain health and mental well-being survey. AARP Research Issues and Topics: Health and Health Care . 2018 https://www.aarp.org/research/topics/health/info-2018/brain-health-mental-well-being.html . [Google Scholar]
- 2.Tang W., Kannaley K., Friedman D. B., et al. Concern about developing Alzheimer’s disease or dementia and intention to be screened: An analysis of national survey data. Archives of gerontology and geriatrics . 2017;71:43–49. doi: 10.1016/j.archger.2017.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cuevas H. E., Stuifbergen A. K., Brown S. A., Rock J. L. Thinking about cognitive function: perceptions of cognitive changes in people with type 2 diabetes. The Diabetes Educator . 2017;43(5):486–494. doi: 10.1177/0145721717729806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henneghan A., Stuifbergen A., Becker H., Kesler S., King E. Modifiable correlates of perceived cognitive function in breast cancer survivors up to 10 years after chemotherapy completion. Journal of Cancer Survivorship . 2018;12(2):224–233. doi: 10.1007/s11764-017-0661-9. [DOI] [PubMed] [Google Scholar]
- 5.Illman N. A., Moulin C. J. A., Kemp S. Assessment of everyday memory functioning in temporal lobe epilepsy and healthy adults using the multifactorial memory questionnaire (MMQ) Epilepsy Research . 2015;113:86–89. doi: 10.1016/j.eplepsyres.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 6.Hill N. L., Mogle J., Whitaker E. B., et al. Sources of Response bias in cognitive self-report items: “Which memory are you talking about? Gerontologist . 2019;59(5):912–924. doi: 10.1093/geront/gny087. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Chronic Diseases and Cognitive Decline—a Public Health Issue . 2020, https://www.cdc.gov/aging/pdf/20-03-Chronic-Diseases-and-Cognitive-Decline-Pages-h.pdf. [Google Scholar]
- 8.Taylor C. A., Bouldin E. D., Greenlund K. J., McGuire L. C. Comorbid chronic conditions among older adults with subjective cognitive decline, United States, 2015–2017. Innovation in Aging . 2020;4(1) doi: 10.1093/geroni/igz045.igz045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hess C., Levy B., Hashmi A. Z., et al. Subjective versus objective assessment of cognitive functioning in primary care. The Journal of the American Board of Family Medicine . 2020;33(3):417–425. doi: 10.3122/jabfm.2020.03.190265. [DOI] [PubMed] [Google Scholar]
- 10.Cuevas H. E., Stuifbergen A. K., Brown S. A., Ward C. A nurse-led cognitive training intervention for individuals with type 2 diabetes. Research in Gerontological Nursing . 2019;12(4):203–212. doi: 10.3928/19404921-20190612-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cuevas H. E., Stuifbergen A. K., Ward C. Participant perspectives of cognitive rehabilitation for type 2 diabetes: expectations and impact. Journal of Aging Research . 2018;2018:9. doi: 10.1155/2018/6563457.6563457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dandona P. Minimizing glycemic fluctuations in patients with type 2 diabetes: approaches and importance. Diabetes Technology & Therapeutics . 2017;19(9):498–506. doi: 10.1089/dia.2016.0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rabin L. A., Smart C. M., Crane P. K., et al. Subjective cognitive decline in older adults: an overview of self-report measures used across 19 international research studies. Journal of Alzheimer’s Disease . 2015;48(s1):S63–S86. doi: 10.3233/JAD-150154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liew T. M. Subjective cognitive decline, anxiety symptoms, and the risk of mild cognitive impairment and dementia. Alzheimer’s Research & Therapy . 2020;12 doi: 10.1186/s13195-020-00673-8.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moher D., Liberati A., Tetzlaff J., Altman D. G. Preferred reporting items for systematic reviews and meta-analysis: the PRISMA statement. BMJ . 2009;339 doi: 10.1136/bmj.b2535.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bray V. J., Dhillon H. M., Vardy J. L. Systematic review of self-reported cognitive function in cancer patients following chemotherapy treatment. Journal of Cancer Survivorship . 2018;12(4):537–559. doi: 10.1007/s11764-018-0692-x. [DOI] [PubMed] [Google Scholar]
- 17.Henneghan A. M., Van Dyk K., Kaufmann T., et al. Measuring self-reported cancer-related cognitive impairment: recommendations from the cancer neuroscience initiative working group. JNCI: Journal of the National Cancer Institute . 2021;113(12):1625–1633. doi: 10.1093/jnci/djab027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Critical Appraisal Skills Programme. 2018. https://casp-uk.net .
- 19.Popay J., Roberts H., Sowden A., et al. Guidance on the conduct of narrative synthesis in systematic reviews. A product from the ESRC methods programme . 2006 doi: 10.13140/2.1.1018.4643. [DOI] [Google Scholar]
- 20.Alonso-Prieto E., Rubino C., Lucey M., et al. Relationship between work functioning and self-reported cognitive complaints in patients with major depressive disorder treated with desvenlafaxine. Psychiatry Research . 2019;272:144–148. doi: 10.1016/j.psychres.2018.12.062. [DOI] [PubMed] [Google Scholar]
- 21.Avants S. K., Margolin A., McMahon T. J., Kosten T. R. Association between self-report of cognitive impairment, HIV status, and cocaine use in a sample of cocaine-dependent methadone-maintained patients. Addictive Behaviors . 1997;22(5):599–611. doi: 10.1016/s0306-4603(96)00070-6. [DOI] [PubMed] [Google Scholar]
- 22.Baker K. S., Gibson S. J., Georgiou-Karistianis N., Giummarra M. J. Relationship between self-reported cognitive difficulties, objective neuropsychological test performance and psychological distress in chronic pain. European Journal of Pain . 2018;22(3):601–613. doi: 10.1002/ejp.1151. [DOI] [PubMed] [Google Scholar]
- 23.Baker K. S., Georgiou-Karistianis N., Lampit A., Valenzuela M., Gibson S. J., Giummarra M. J. Computerised training improves cognitive performance in chronic pain: a participant-blinded randomised active-controlled trial with remote supervision. Pain . 2018;159(4):644–655. doi: 10.1097/j.pain.0000000000001150. [DOI] [PubMed] [Google Scholar]
- 24.Brück E., Larsson J. W., Lasselin J., et al. Lack of clinically relevant correlation between subjective and objective cognitive function in ICU survivors: a prospective 12-month follow-up study. Critical Care . 2019;23:p. 253. doi: 10.1186/s13054-019-2527-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brunette A. M., Holm K. E., Wamboldt F. S., et al. Subjective cognitive complaints and neuropsychological performance in former smokers with and without chronic obstructive pulmonary disease. Journal of Clinical and Experimental Neuropsychology . 2018;40(4):411–422. doi: 10.1080/13803395.2017.1356912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brunmeier A., Reis M. P., Earing M. G., et al. Identifying self‐reported neurocognitive deficits in the adult with congenital heart disease using a simple screening tool. Congenital Heart Disease . 2018;13(5):728–733. doi: 10.1111/chd.12646. [DOI] [PubMed] [Google Scholar]
- 27.Cockshell S. J., Mathias J. L. Cognitive functioning in people with chronic fatigue syndrome: a comparison between subjective and objective measures. Neuropsychology . 2014;28(3):394–405. doi: 10.1037/neu0000025. [DOI] [PubMed] [Google Scholar]
- 28.Duijndam S., Denollet J., Nyklíček I., Kupper N. Perceived cognition after percutaneous coronary intervention: association with quality of life, mood and fatigue in the THORESCI study. International Journal of Behavioral Medicine . 2017;24(4):552–562. doi: 10.1007/s12529-016-9624-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fazeli P. L., Casaletto K. B., Paolillo E., Moore R. C., Moore D. J. Screening for neurocognitive impairment in HIV-positive adults aged 50 years and older: montreal cognitive assessment relates to self-reported and clinician-rated everyday functioning. Journal of Clinical and Experimental Neuropsychology . 2017;39(9):842–853. doi: 10.1080/13803395.2016.1273319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frol A. B., Vasquez A., Getahun Y., Pacheco M., Khan D. A., Brown E. S. A comparison of clinician-rated neuropsychological and self-rated cognitive assessments in patients with asthma and rheumatologic disorders. Allergy and Asthma Proceedings . 2013;34(2):170–175. doi: 10.2500/aap.2013.34.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gallo L. C., Malek M. J., Gilbertson A. D., Moore J. L. Perceived cognitive function and emotional distress following coronary artery bypass surgery. Journal of Behavioral Medicine . 2005;28(5):433–442. doi: 10.1007/s10865-005-9010-y. [DOI] [PubMed] [Google Scholar]
- 32.Haley A. P., Hoth K. F., Gunstad J., et al. Subjective cognitive complaints relate to white matter hyperintensities and future cognitive decline in patients with cardiovascular disease. The American Journal of Geriatric Psychiatry . 2009;17(11):976–985. doi: 10.1097/jgp.0b013e3181b208ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henry S. L., Jamner L. D., Choi S. E., Pahl M. V. The effect of the interdialytic interval on cognitive function in patients on haemodialysis. Journal of Renal Care . 2018;44(1):44–51. doi: 10.1111/jorc.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jackson T. L, Cooper T. Subjective cognitive decline and associated health problems among Rhode Island adults. Rhode Island medical journal (2013) . 2017;100(3):35–38. https://www.rimed.org/rimedicaljournal/2017/03/2017-03-35-health-jackson.pdf . [PubMed] [Google Scholar]
- 35.Jacob L., Haro J. M., Koyanagi J. Physical multimorbidity and subjective cognitive complaints among adults in the United Kingdom: a cross-sectional community-based study. Scientific Reports . 2019;9 doi: 10.1038/s41598-019-48894-8.12417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kiessling A., Henriksson P. Perceived cognitive function in coronary artery disease - an unrecognised predictor of unemployment. Quality of Life Research . 2005;14(6):1481–1488. doi: 10.1007/s11136-005-0195-x. [DOI] [PubMed] [Google Scholar]
- 37.Kiessling A., Henriksson P. Perceived cognitive function is a major determinant of health related quality of life in a non-selected population of patients with coronary artery disease ? a principal components analysis. Quality of Life Research . 2004;13(10):1621–1631. doi: 10.1007/s11136-004-7863-0. [DOI] [PubMed] [Google Scholar]
- 38.Knoop H., Prins J. B., Stulemeijer M., van der Meer J. W., Bleijenberg G. The effect of cognitive behaviour therapy for chronic fatigue syndrome on self-reported cognitive impairments and neuropsychological test performance. Journal of Neurology, Neurosurgery, and Psychiatry . 2007;78(4):434–436. doi: 10.1136/jnnp.2006.100974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsuzawa T., Takata T., Yokono K., et al. A warning index used in prescreening for alzheimer’s disease, based on self-reported cognitive deficits and vascular risk factors for dementia in elderly patients with type 2 diabetes. International Journal of Alzheimer’s Disease . 2012;2012:8. doi: 10.1155/2012/124215.124215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCracken L. M., Iverson G. L. Predicting complaints of impaired cognitive functioning in patients with chronic pain. Journal of Pain and Symptom Management . 2001;21(5):392–396. doi: 10.1016/s0885-3924(01)00267-6. [DOI] [PubMed] [Google Scholar]
- 41.Morgan S. J., Kelly V. E., Amtmann D., Salem R., Hafner B. J. Self-reported cognitive concerns in people with lower limb loss. Archives of Physical Medicine and Rehabilitation . 2016;97(6):912–918. doi: 10.1016/j.apmr.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nguyen L. A., Haws K. A., Fitzhugh M. C. Interactive effects of subjective memory complaints and hypertension on learning and memory performance in the elderly. Aging, Neuropsychology, and Cognition . 2016;23(2):154–170. doi: 10.1080/13825585.2015.1063580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ott C. V., Vinberg M., Kessing L. V., Miskowiak K. W. The effect of erythropoietin on cognition in affective disorders—associations with baseline deficits and change in subjective cognitive complaints. European Neuropsychopharmacology . 2016;26(8):1264–1273. doi: 10.1016/j.euroneuro.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 44.Roth R. S., Geisser M. E., Theisen-Goodvich M., Dixon P. J. Cognitive complaints are associated with depression, fatigue, female sex, and pain catastrophizing in patients with chronic pain. Archives of Physical Medicine and Rehabilitation . 2005;86(6):1147–1154. doi: 10.1016/j.apmr.2004.10.041. [DOI] [PubMed] [Google Scholar]
- 45.Sharma A., Hoover D. R., Shi Q., et al. Falls among middle-aged women in the Women’s Interagency HIV Study. Antiviral therapy . 2016;21(8):697–706. doi: 10.3851/IMP3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steinbusch C. V. M., van Heugten C. M., Rasquin S. M. C., Verbunt J. A., Moulaert V. R. M. Cognitive impairments and subjective cognitive complaints after survival of cardiac arrest: a prospective longitudinal cohort study. Resuscitation . 2017;120:132–137. doi: 10.1016/j.resuscitation.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 47.Touradji P., Aucott J. N., Yang T., Rebman A. W., Bechtold K. T. Cognitive decline in post-treatment Lyme disease syndrome. Archives of Clinical Neuropsychology . 2019;34(4):455–465. doi: 10.1093/arclin/acy051. [DOI] [PubMed] [Google Scholar]
- 48.Vance D. E., Okonkwo O., Antia L., et al. Factors of cognitive complaints in adults with HIV: a structural equation model analysis. Occupational Therapy in Mental Health . 2009;25(1):4–25. doi: 10.1080/01642120802644896. [DOI] [Google Scholar]
- 49.Wingbermühle E., Roelofs R. L., van der Burgt I., et al. Cognitive functioning of adults with Noonan syndrome: a case-control study. Genes, Brain and Behavior . 2012;11(7):785–793. doi: 10.1111/j.1601-183x.2012.00821.x. [DOI] [PubMed] [Google Scholar]
- 50.Yoon B. Y., Lee J. H., Shin S. Y. Discrepancy between subjective and objective measures of cognitive impairment in patients with rheumatoid arthritis. Rheumatology International . 2017;37(10):1635–1641. doi: 10.1007/s00296-017-3806-2. [DOI] [PubMed] [Google Scholar]
- 51.Zhu Z., Hu Y., Xing W., Guo M., Wu B. Perceived discrimination and cognitive function in middle-aged and older adults living with HIV in China. AIDS Care . 2019;31(9):1061–1068. doi: 10.1080/09540121.2019.1601674. [DOI] [PubMed] [Google Scholar]
- 52.Crumley J. J., Stetler C. A., Horhota M. Examining the relationship between subjective and objective memory performance in older adults: a meta-analysis. Psychology and Aging . 2014;29(2):250–263. doi: 10.1037/a0035908. [DOI] [PubMed] [Google Scholar]
- 53.Mendonça M. D., Alves L., Bugalho P. From subjective cognitive complaints to dementia: who is at risk? a systematic review. American Journal of Alzheimer’s Disease & Other Dementias . 2016;31(2):105–114. doi: 10.1177/1533317515592331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mitchell A. J., Beaumont H., Ferguson D., Yadegarfar M., Stubbs B. Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: meta-analysis. Acta Psychiatrica Scandinavica . 2014;130(6):439–451. doi: 10.1111/acps.12336. [DOI] [PubMed] [Google Scholar]
- 55.Shellington E. M., Reichert S. M., Heath M., Gill D. P., Shigematsu R., Petrella R. J. Results from a feasibility study of square-stepping exercise in older adults with type 2 diabetes and self-reported cognitive complaints to improve global cognitive functioning. Canadian Journal of Diabetes . 2018;42(6):603–612. doi: 10.1016/j.jcjd.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 56.Yap K. H., Warren N., Allotey P., Reidpath D. D. Chronic disease profiles of subjective memory complaints: a latent class analysis of older people in a rural Malaysian community. Aging & Mental Health . 2020;24(5):709–716. doi: 10.1080/13607863.2018.1550632. [DOI] [PubMed] [Google Scholar]
- 57.van Beers M., Gosker H. R., Janssen D. J. A., et al. Cognitive performance in relation to metabolic disturbances in patients with COPD. Clinical Nutrition . 2021;40(4):2061–2067. doi: 10.1016/j.clnu.2020.09.029. [DOI] [PubMed] [Google Scholar]
- 58.Breuning M., Schäfer-Fauth L., Lucius-Hoene G., Holmberg C. Connecting one’s own illness story to the illness experiences of others on a website—an evaluation study using the think aloud method. Patient Education and Counseling . 2020;103(1):199–207. doi: 10.1016/j.pec.2019.08.014. [DOI] [PubMed] [Google Scholar]
- 59.Fitzpatrick T. R. Play, leisure activities, cognitive health, and quality of life among older cancer survivors. In: Fitzpatrick T. R., editor. Quality of Life Among Cancer Survivors: Challenges and Strategies for Oncology Professionals and Researchers . New York, NY, USA: Springer International; 2018. pp. 7–22. [DOI] [Google Scholar]
- 60.Burmester B., Leathem J., Merrick P. Subjective cognitive complaints and objective cognitive function in aging: a systematic review and meta-analysis of recent cross-sectional findings. Neuropsychology Review . 2016;26(4):376–393. doi: 10.1007/s11065-016-9332-2. [DOI] [PubMed] [Google Scholar]
- 61.Jessen F., Amariglio R. E., Buckley R. F., et al. The characterisation of subjective cognitive decline. The Lancet Neurology . 2020;19(3):271–278. doi: 10.1016/s1474-4422(19)30368-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kinzer A., Suhr J. A. Dementia worry and its relationship to dementia exposure, psychological factors, and subjective memory concerns. Applied Neuropsychology: Adult . 2016;23(3):196–204. doi: 10.1080/23279095.2015.1030669. [DOI] [PubMed] [Google Scholar]
- 63.Jessen F., Amariglio R. E., Boxtel M., et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimer’s & Dementia . 2014;10(6):844–852. doi: 10.1016/j.jalz.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Molinuevo J. L., Rabin L. A., Amariglio R., et al. Implementation of subjective cognitive decline criteria in research studies. Alzheimer’s & Dementia . 2017;13(3):296–311. doi: 10.1016/j.jalz.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Materials. Supplementary Material A. Methods. Supplementary Material B. Search Strategy. Supplementary Material C. Data Extraction. Supplementary Material D. Quality Appraisals. Supplementary Material E. Study Demographics. Supplementary Material F. Table of Subjective Cognitive Function Measures.
Data Availability Statement
The data are available in the submitted supplementary files.


