Summary
Sorghum bicolor (L.) Moench, the fifth most important cereal worldwide, is a multi‐use crop for feed, food, forage and fuel. To enhance the sorghum and other important crop plants, establishing gene function is essential for their improvement. For sorghum, identifying genes associated with its notable abiotic stress tolerances requires a detailed molecular understanding of the genes associated with those traits. The limits of this knowledge became evident from our earlier in‐depth sorghum transcriptome study showing that over 40% of its transcriptome had not been annotated. Here, we describe a full spectrum of tools to engineer, edit, annotate and characterize sorghum’s genes. Efforts to develop those tools began with a morphogene‐assisted transformation (MAT) method that led to accelerated transformation times, nearly half the time required with classical callus‐based, non‐MAT approaches. These efforts also led to expanded numbers of amenable genotypes, including several not previously transformed or historically recalcitrant. Another transformation advance, termed altruistic, involved introducing a gene of interest in a separate Agrobacterium strain from the one with morphogenes, leading to plants with the gene of interest but without morphogenes. The MAT approach was also successfully used to edit a target exemplary gene, phytoene desaturase. To identify single‐copy transformed plants, we adapted a high‐throughput technique and also developed a novel method to determine transgene independent integration. These efforts led to an efficient method to determine gene function, expediting research in numerous genotypes of this widely grown, multi‐use crop.
Keywords: Sorghum, Agrobacterium, engineering, CRISPR/Cas9 editing, morphogene‐assisted transformation, altruistic morphogene‐assisted transformation
Introduction
For many crops, efficient transformation methods (hereafter referring to both engineering and editing) are not available. An example is sorghum [Sorghum bicolor (L.) Moench], a cereal crop with multiple abiotic stress tolerances (Paterson, 2008; Paterson et al., 2009). Because of its use for fuel, feed, forage and food, sorghum has new market demands worldwide (Duff et al., 2019; Mundia et al., 2019). To provide for these needs, the function of its genes must be understood. Our large transcriptomic data sets for sorghum, grown under drought conditions in the field (Varoquaux et al., 2019; Xu et al., 2018), revealed that, although 44% of expressed genes were affected by drought, 43% of the transcriptome was not annotated (Varoquaux et al., 2019). This lack of foundational knowledge left questions about the function of genes involved in drought responses; however, lack of efficient transformation systems slows necessary experimentation to validate their function.
Engineering and editing are being used to study gene function, by overexpressing or turning off genes to modify traits in plants. However, using these methods for sorghum is challenging (Altpeter et al., 2016; Che et al., 2018). Since the first plant transformation success in tobacco (Bevan et al., 1983; Biotechnology and Biological Sciences Research Council, 2013; Fraley et al., 1983; Herrera‐Estrella et al., 1983), progress has been realized in crop and model plants. However, cereal crops have classically been more recalcitrant to Agrobacterium‐mediated transformation than dicots, largely due to the natural host of the pathogen being dicots (De Cleene and De Ley, 1976). Because of this, early maize success was accomplished using biolistics (Gordon‐Kamm et al., 1990).
Barriers to Agrobacterium‐mediated monocot transformation were eventually overcome in select species, rice being the first successful target (Raineri et al., 1990). However, Agrobacterium‐mediated transformation continues to be stymied in monocots by genotype dependence. Only specific cultivars within a species are amenable to transformation, limiting exploration of gene function across a species’ pangenome. Thus, elite agricultural varieties often cannot be transformed directly to modify traits. However, progress has been made in the once recalcitrant crop, maize (Frame et al., 2002; Shrawat and Lörz, 2006; Songstad et al., 1996). More recent efforts utilized specific morphogenes to significantly improve transformation efficiency (Lowe et al., 2016), a technique we call morphogene‐assisted transformation (MAT). Morphogenes used were Zea mays Baby Boom (Zm‐Bbm, Opd2), an AP2/ERF transcription factor that promotes cell proliferation during embryogenesis (Boutilier et al., 2002), and Wuschel2 (Wus2), a transcription factor that maintains stem cells in the shoot meristem (Laux et al., 1996). Their overexpression, under strict regulatory control, generally sped transformation and overcame genotype dependence (Lowe et al., 2016), including recalcitrant maize varieties (Lowe et al., 2018; Mookkan et al., 2017) and several African sorghum varieties (Che et al., 2018).
Continued overexpression of morphogenes, however, causes negative phenotypic and reproductive outcomes, making excision or silencing of these genes necessary after inducing embryogenesis. In one approach morphogenes were embedded between locus of crossing (x) over, P1 (loxP) sites and expression of CRE recombinase was triggered, causing excision of the morphogenes (Sauer and Henderson, 1988). Another approach in maize involved using two Agrobacterium strains, one with a gene‐of‐interest (GOI) construct and the other with a construct containing a morphogene. Using this strategy, termed altruistic, most transformants had the GOI but lacked morphogenes (Hoerster et al., 2020).
These successes led to the present study in sorghum, where Zm‐Bbm and Wus2 were used in a MAT approach. This resulted in faster generation of transformants and more transformable U.S. sorghum genotypes: rapid‐cycling SC187 (Rosenow et al., 1997), stay‐green variety, BTx642, (Rosenow et al., 2002), BTx623 with the first sequenced genome (Frederiksen and Miller, 1972) (Paterson et al., 2009), and recalcitrant sweet sorghum Ramada (Freeman, 1974). To generate GOI‐containing plants, while simultaneously eliminating morphogenes to prevent aberrant phenotypes, necessitated a modified approach, termed altruistic MAT. In this approach an Agrobacterium with the morphogene construct was used to simultaneously infect with another Agrobacterium containing the GOI construct. Thus, altruistic MAT can be used to enhance efficiency of existing transformation constructs, as demonstrated in our study by plants transformed with only the RFP reporter plasmid, pANIC10A. Copy number determination was performed and, because of sorghum’s tendency to tiller, novel molecular genotyping methods were developed to assess transgene independent integration (TII). Finally, to facilitate gene function through targeted gene editing, morphogenes were included in a construct with a Clustered Regularly Interspaced Palindromic Repeats/CRISPR‐associated protein 9 (CRISPR/Cas9) cassette to edit an exemplary GOI, phytoene desaturase (pds). Together, advancements in this study provide a straightforward path to determine gene function in sorghum.
Results
Morphogene‐assisted transformation of multiple sorghum genotypes
To increase transformation speed and efficiency, we first used a MAT approach, using a single construct, pPHP81814 (Chu et al., 2019), containing the acetolactate synthase selection gene (ALS), the GOI for ZsGreen‐1 (ZsG‐1), and the morphogenes, Zm‐Bbm and Wus2. This resulted in transformation of five genotypes, RTx430, SC187, BTx642, BTx623 and Ramada. ZsG‐1 expression was visualized using fluorescence of immature embryos (IEs) between three and ten days post‐transformation, while tissues were on embryo maturation medium (EMM) (Figures 1a,b). Many somatic embryos strongly expressed ZsG‐1 (Figure 1b).
Figure 1.

RTx430 tissues from morphogene‐assisted transformation (MAT) and altruistic MAT. RTx430 IEs, 1.5‐2mm, were transformed with Agrobacterium LBA4404 Thy‐ strain, containing appropriate constructs depending on the experiment. IEs were cultured for one week each on co‐cultivation then resting medium. For MAT, using pPHP81814 (ZSG), images by brightfield (a) and by fluorescence (b) were taken while tissues were on EMM. For MAT RTx430 tissues were moved to EMM with 0.05 mg/L imazapyr (IMZ) (c, d, e). For altruistic MAT 20 mg/L hygromycin was used as a selection agent for RTx430 (f, g, h). Images were taken using brightfield (i, k) and fluorescence microscopy (j, l). For altruistic MAT, using pGL190 (ZSG) and pANIC10A (RFP), IEs expressed ZSG and RFP (j). For comparison a brightfield image of the same tissue is shown (i). As a control, pGL190 was used to transform RTx430 IEs and imaged using brightfield (k) and fluorescence (l); ZSG expression in transformed tissue is shown (l).
Imazapyr (IMZ), a systemic herbicide targeting ALS was used for selection on EMM and rooting medium (RM). RTx430 and BTx642 responded differently to selection, especially on EMM. RTx430 tissues, not killed on EMM with IMZ, formed more somatic embryos (Figures 1d,e), continuously forming tissue pieces about 1 cm in diameter. In contrast, BTx642 tissues on the same medium formed smaller numbers and sizes of somatic embryos (Figures S1a,b). Additionally, most RTx430 tissue under IMZ selection had some healthy tissues that were not brown or necrotic (Figures 1c,d,e) compared to BTx642 that did not (Figures S1a,b). On RM with IMZ, most untransformed plantlets became necrotic and died. When using IMZ selection, more escapes were observed relative to selection on hygromycin (hyg), used for altruistic MAT (see Altruistic morphogene‐assisted transformation).
Genotyping of morphogene‐assisted transformation plants and transformation efficiency
After transfer to RM, putative transformants were genotyped using polymerase chain reaction (PCR). While transformation was successful for all genotypes, transformation efficiency, the number of T0 plants positive only for ALS without morphogenes, varied among genotypes (Table 1). Highest efficiency was for the most commonly transformed variety, RTx430 (9.4%), followed by rapid‐cycling SC187 (2.0%) and stay‐green variety, BTx642 (0.9%), neither previously reported as transformable. Also successful was BTx623 (0.1%), the first genotype with a complete genome sequence. One of the most recalcitrant varieties, sweet sorghum Ramada, has a published transformation efficiency of 0.09% (Raghuwanshi and Birch, 2010). In the present study, a 1.2% efficiency was achieved, an approximate 10‐fold improvement. Also noteworthy is the reduced time to produce T0 plants. The typical 18–21 weeks for RTx430 using conventional transformation procedures (Gurel et al., 2009; Wu et al., 2014) was reduced to 10–12 weeks.
Table 1.
Morphogene‐assisted transformation efficiency in T0 plants of five sorghum genotypes transformed using pPHP81814 and IMZ selection
| Genotype | # replications | # IEs used | # Regnerants | Escapes (%) † | + for only ALS ‡ | + for only ZSG ‡ | + for ALS & ZSG ‡ | Transformation efficiency (%) § | TII ¶ plants + for only ALS ‡ | Transformation efficiency based on TII (%) †† |
|---|---|---|---|---|---|---|---|---|---|---|
| RTx430 | 2 | 297 | 70 | 47.1 | 28 | 0 | 9 | 9.4 | 26 | 8.8 |
| SC187 | 4 | 591 | 64 | 51.6 | 12 | 0 | 19 | 2.0 | 9 | 1.5 |
| BTx623 | 2 | 783 | 25 | 84.0 | 1 | 2 | 1 | 0.1 | 1 | 0.1 |
| BTx642 | 2 | 420 | 53 | 88.7 | 4 | 1 | 1 | 0.9 | 4 | 0.9 |
| Ramada | 3 | 850 | 60 | 76.7 | 10 | 2 | 2 | 1.2 | 5 | 0.6 |
Escapes % = percentage of regenerated plants without the transgene (T‐DNA).
+ indicates plants PCR‐positive for ALS and/or ZSG.
Transformation efficiency (%) = (plants + for only ALS divided by # IEs used) ×100.
TII indicates transgene independent integration.
Transformation efficiency based on TII (%) = (TII plants + for only ALS divided by # IEs used) ×100.
While conventional sorghum transformation typically generates a single independent event per IE, use of Zm‐Bbm and Wus2 led to numerous transgenic somatic embryos per IE, leading to T0 plants with unique transgene insertions. To confirm this, a method was devised to calculate efficiency, based on transgene independent integration (TII). This assured that multiple ALS‐positive plants with identical integration sites were not included when calculating efficiency. An inexpensive, high‐throughput method was used to determine the plants with TII. This was based on an adaptor ligation‐mediated PCR method, previously used for high‐throughput mapping in Arabidopsis (O’Malley et al., 2007). To modify this method, genomic DNA (gDNA) amounts were increased from 30 to 900 ng to account for sorghum’s larger genome size. To test this method, eleven transformed plants were chosen from six different IEs; one wild‐type plant was used as a negative control (Figure 2). Every sample, including the wild‐type (Lane 12), displayed two identical bands, possibly due to the number of amplification cycles, reinforcing the need to use genomic DNA without a T‐DNA insert as a negative control. The difference between the two methods of calculating transformation efficiencies for each genotype was small (Table 1), perhaps indicating efficiency of this transformation strategy.
Figure 2.

Transgene independent integration (TII) was determined with adaptor‐ligation‐mediated PCR. Examples of T‐DNA/gDNA junctions in the sorghum genome from the left border (LB, a‐f). Transgenic lines derived from the same immature embryo are labelled with alphabetic letters at the bottom; wild‐type genomic DNA (WT) is the negative control. Right border (RB) assay was performed if left border assay failed to amplify bands (e). DNA ladder (DL) is on the right. Dots indicate bands from T‐DNA/gDNA junctions. Plants from the same immature embryo in a and b are independent; plants in c, d and e are the same event.
When studying gene function, transgene copy number is also important for stable transgene expression. Digital droplet PCR (ddPCR) was used to quantify the number of inserted gene copies. Values were close to integer values (Table 2) and most were single‐copy: 63.2% of RTx430, 75.0% of SC187, and 77.8% of Ramada plants. After determining amplicon numbers for MAT transformants were mostly single‐copy, that analysis was found to be consistent with copy number analysis. This method generates multiple plants with independent insertions from numerous genotypes, critical for studying gene function and for complementing mutations.
Table 2.
Transgene copy number in T0 plants of various sorghum genotypes using digital droplet PCR
| Genotype | Construct | # of TII plants tested † | # single‐copy plants ‡ | # multi‐copy plants § | % single‐copy plants ¶ |
|---|---|---|---|---|---|
| RTx430 |
pPHP81814 |
14 | 10 | 4 | 71.4 |
| SC187 | 11 | 9 | 2 | 81.8 | |
| Ramada | 9 | 7 | 2 | 77.8 | |
| RTx430 |
pGL190 + pANIC10A |
40 | 25 | 15 | 62.5 |
# of TII plants tested = number of tested plants with transgene independent integration (TII).
# single‐copy plants = number of plants with a single copy of ALS gene.
# multi‐copy plants = number of plants with multiple copies of ALS gene.
% single‐copy plants = (# single‐copy plants divided by # T0 TII plants tested) × 100.
Altruistic morphogene‐assisted transformation
Abnormal phenotypes due to introduced morphogenes (see Phenotypic characterization of putative transformed plants) prompted development of a new transformation strategy, modified from a maize method termed altruistic transformation (Hoerster et al., 2020). In this approach IEs were transformed simultaneously with two Agrobacterium strains, containing either pANIC10A (Mann et al., 2012), with the red fluorescent protein (RFP) GOI and the hygromycin phosphotransferase (hph) selection gene, or pPHP81814, in which WUS expression is driven by the relatively weak auxin‐inducible AXIG1 promoter (Jones et al., 2018).
The first altruistic MAT experiment in RTx430 did not yield plantlets with the GOI. To improve this outcome in a second experiment another construct containing morphogenes was used. The first Agrobacterium strain contained pGL190 (Figure 3a), which has Zm‐Bbm and Wus2 and the ZsG‐1 marker gene. Expression of WUS is driven by a stronger maize promoter, PLTP (phospholipid transferase protein), compared to the promoter in pPHP81814 (Jones et al., 2018). The second strain had pANIC10A, with the RFP GOI and hph. The two strains were mixed 1:9 v:v, pGL190:pANIC10A, and applied simultaneously to sorghum IEs. Tissues were cultured on EMM plus hyg (Figures 1f–h) to select for hph expression; no selection was used on RM. Expression of ZsG‐1 from pGL190 and RFP from pANIC10A was visualized using brightfield (Figure 1i) and fluorescence microscopy (Figure 1j). Most IEs showed both ZsG and RFP expression 3 d post‐transformation. Transformations with only pGL190 (Figures 1k,l) or pANIC10A were used as controls. Transformants with the GOI were regenerated. Unlike IMZ selection, hygromycin selection in the altruistic MAT approach had more visible effects; growth of non‐transformed tissues was strongly inhibited, leadi ng eventually to necrosis. Effects of strong selection were also reflected in genotyping results (Table 3) where only 21.6% escapes were observed despite no selection on RM. The main difference between pGL190 and pPHP81814 constructs is the promoter driving Wus2. Since identical selection schemes were used, lack of success generating plants containing the GOI, when using pPHP81814 compared with pGL190, was likely due to the weaker promoter, Zm‐Axig1pro, driving Wus2 in pPHP81814.
Figure 3.
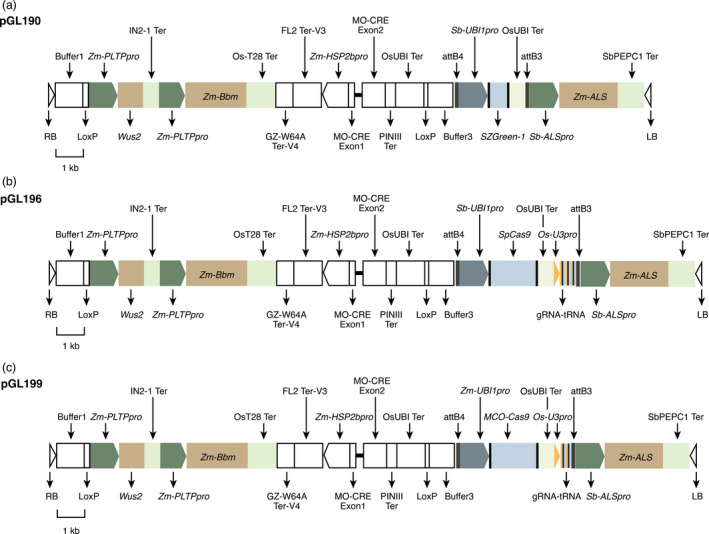
Construct maps for transformation. (a) pGL190 for altruistic morphogene‐assisted transformation (MAT). (b) pGL196 for MAT‐mediated editing of phytoene desaturase genes. The SpCas9 is driven by Sb‐Ubi1pro. Black rectangles indicate terminator regions. (c) pGL199 for MAT‐mediated editing of phytoene desaturase genes. The maize codon‐optimized SpCas9 is driven by Zm‐Ubi1pro. Triangles indicate T‐DNA borders; rectangles with arrows indicate promoters; blank rectangles indicate genes or buffer regions; Black rectangles indicate terminator regions. All three constructs were created from pPHP85425, as a Gateway‐compatible destination vector.
Table 3.
Efficiency of altruistic morphogene‐assisted transformation using pGL190 and pANIC10A in T0 plants
| Genotype | # replications | # IEs used | # Regenerants | Escapes (%) † | + for only RFP & hph ‡ | + for only Bbm ‡ | + for RFP, hph & Bbm ‡ | Transformation efficiency (%) § | TII ¶ plants + for only RFP & hph ‡ | Transformation efficiency based on TII (%) †† |
|---|---|---|---|---|---|---|---|---|---|---|
| RTx430 | 4 | 379 | 232 | 21.6 | 113 | 18 | 51 | 29.8 | 47 | 12.4 |
| SC187 | 1 | 83 | 20 | 0 | 10 | 0 | 10 | 12.0 | 9 | 10.8 |
Escapes (%) = percentage of regenerated plants without transgene (T‐DNA).
+ indicates plants PCR‐positive for RFP & hph (pANIC10A) and/or Bbm (pGL190).
Transformation efficiency (%) = (+ for only RFP & hph divided by # IEs used) × 100.
TII indicates transgene independent integration.
Transformation efficiency based on TII (%) = (TII plants + for only RFP & hph divided by # IEs used) × 100
Genotyping of altruistic morphogene‐assisted transformation plants and transformation efficiency
Similar to MAT, two different methods were used to calculate transformation efficiency, one reflecting the number of transformed plants and one reflecting the number of TII events. This permitted determination of whether transformants from the same IE were truly independent or were clonal events via tillering. For RTx430, four altruistic MAT replications were conducted using a total of 379 IEs (Table 3); for SC187, one replicate of 83 IEs was performed. PCR was used to detect RFP, hph and/or Zm‐Bbm. Transformation efficiency, the number of RTx430 T0 plants, positive only for RFP and hph, was 29.8% (113 of 379) and 12.0% (10 of 83) for SC187. Efficiency, based on TII, was 12.4% (47 of 379) for RTx430 and 10.8% (9 of 83) for SC187. For transgenic plants, 28.0% (51 of 182) of RTx430 and 50.0% (10 of 20) of SC187 plants contained RFP, hph and Zm‐Bbm (Table 3). As with MAT, if amplicons from T‐DNA left borders were not seen, junctions from right borders were used (Figure 2, sample e).
Phenotypic characterization of putative transformed plants
Morphogenes were included in constructs to be able to transform more genotypes and to accelerate the process. But to regenerate plants with normal phenotypes and seed set, those genes must be removed from transformed tissues. For MAT with pPHP81814, putative transformed plants were tested using appropriate PCR primers (Table S1) to confirm CRE‐mediated excision of ZsG‐1, Zm‐Bbm and Wus2, accomplished using loxP sites flanking those genes, generating T0 plants positive only for ALS (Table 1). Genotypes showed different percentages of morphogene excision, with the highest rates for RTx430 (75.7%), Ramada (71.4%) and BTx642 (66.7%). Rates for BTx623 and SC187 were 25.0% and 38.7%, respectively. Plants negative for introduced morphogenes had normal growth and seed set; plants testing positive showed abnormal phenotypes, like short stature, twisted leaves and/or poor seed set (Figures S2a,b). For altruistic MAT with RTx430 and SC187, 62.1% and 50.0% of plants, respectively, had no morphogene insertion (Table 3) and normal phenotypes. Abnormal phenotypes were observed in plants with morphogenes.
Genome editing mediated by MAT
In previous reports of sorghum editing, Agrobacterium‐mediated CRISPR/Cas9 delivery resulted in low efficiencies (Char et al., 2020; Ping Che et al., 2018; Li et al., 2018; G. Liu et al., 2019). Given the highly efficient sorghum transformation methods described in our study, use of MAT methods was combined with CRISPR/Cas9 editing to improve genome‐editing efficiency. Both MAT and altruistic MAT methods were considered to introduce the CRISPR/Cas9 cassette. The attempt chosen focused on MAT due to ease of construct manipulation and ability for T‐DNA segregation.
To create the CRISPR/Cas9 cassette, pGL196, the sorghum ubiquitin1 promoter (Sb‐Ubi1pro) was used to drive Streptococcus pyogenes Cas9 (SpCas9). Oryza sativa U3 promoter (Os‐U3pro) was used to drive three gRNAs, targeting the two sorghum pds genes (Figures 4a, S4). The pGL196 construct also contained Zm‐Bbm, Wus2 and the ALS selection gene (Figure 3b). pGL196 was introduced into RTx430. Transformed tissue was heat‐treated to trigger expression of heat‐shock‐inducible CRE, causing excision of morphogenes. Two replications using 171 IEs yielded 58 plants positive for gRNAs, a 33.9% transformation efficiency (Figure 4c). Of regenerants, no chimeric or biallelic albino phenotypes from a pds knockout were observed; however, genotyping revealed 9 of the 58 gRNA+ plants were edited, a 15.5% editing efficiency (Figure 4b).
Figure 4.

Editing of pds genes using CRISPR/Cas9 system. (a) Four guide RNAs (gRNAs) targeting pds. Dark blue rectangles indicate untranslated regions; yellow rectangles indicate exons; light grey lines indicate intron regions. gRNA1.1 is located in the third exon of pds. gRNA1.2 is located in the fifth exon of pds. These two gRNA are in pGL196. gRNA2.1 is located in the second exon of pds. gRNA2.2 is located in the fourth exon of pds. These two gRNAs are in pGL199. (b) Sanger sequencing of edited pds genes are shown with the main editing versions predicted by Synthego. gRNAs are underlined and PAM sites for gRNAs are in bold black font. Deletions are shown with dashes. (c) Summary of transformation efficiency and CRISPR/Cas9‐induced genome‐editing efficiency in T0 generation from MAT‐mediated editing in RTx430 using pGL196 and pGL199. (d) Albino phenotype from pds knockout, as frameshift mutations, in CRISPR/Cas9 edited plants. Phenotypic characterization of chimeric (i, ii) and fully albino (iii) mutants in T0 generation. Scale bar, 1 cm.
To improve editing efficiency, Cas9 expression was increased as this likely impacted editing efficiency (Liu et al., 2019) and the maize, rather than the sorghum, ubiquitin1 promoter (Zm‐Ubi1pro) was used to drive maize codon‐optimized Cas9. This cassette was coupled in pGL199 with a cassette containing two pds gRNAs (Figures 3c, 4a). In two replications pGL199 was introduced into 125 IEs from RTx430 and 124 putative transformants were generated. PCR indicated that 66 plants were gRNA positive, giving a transformation efficiency of 52.8% (66/125) (Figure 4c). From gRNA‐positive plants, a pds fragment was amplified and analysed using Sanger sequencing and the Synthego Inference of CRISPR Edits (ICE) tool. Successful editing was detected in 15 of the 66 gRNA+ plants, yielding an editing efficiency of 22.7% (Figure 4c). Thus changes in pGL199 versus pGL196 resulted in higher editing efficiencies (22.7% versus 15.5%), increasing the probability of generating loss‐of‐function mutations (Figures 4c,d). Eleven of 15 edited plants had frame‐shift mutations, a knockout efficiency of 16.7% of the gRNA+ transformants (11 of 66) (Figure 4c). Of the 11 plants, six had a chimeric albino phenotype and five plants had a complete albino phenotype (Figure 4d); however, four did not survive after three weeks on RM. One chimeric plant survived and set seed.
Discussion
To accelerate gene function identification in crops, transformation improvements are needed (Altpeter et al., 2016). This became obvious from our transcriptome data of drought‐stressed, field‐grown sorghum that revealed 44% of expressed genes were affected by drought, but only 57% of sorghum’s transcriptome is annotated (Varoquaux et al., 2019) and few annotated genes have validated functions. Major impediments exist in validating function in sorghum using classical methods of transformation, namely genotype dependence and the long times for selection, regeneration and generation advance. Efforts in this study aimed to address these issues.
Morphogene‐assisted transformation reduces genotype dependence, improves efficiency and speed
In this study, morphogene‐containing constructs were used to demonstrate more sorghum varieties amenable to transformation. These include the first successful transformation of the prototype stay‐green variety, BTx642, allowing gene function studies on this important trait. Sweet sorghum Ramada transformation is reported at a rate approximately ten‐times that previously published (Raghuwanshi and Birch, 2010), enabling gene function studies in stem sugar production. Also notable was success with the fast‐cycling variety, SC187, for which T1 seeds are generated in the greenhouse ten weeks faster than with the standard transformation variety, RTx430. Newly published high‐quality BTx642 and SC187 Pac‐Bio sequences (Phytozome v13; Goodstein et al., 2012) will further increase success in gene function studies.
Classical sorghum transformation, requiring extended callus culturing, takes 15–18 weeks to generate RTx430 plantlets (Gurel et al. 2009; Wu et al., 2014). Implementing MAT strategies reported here accelerates regeneration of RTx430; T0 plants mature eight to nine weeks faster. This reduced time can be attributed to morphogenes directly inducing somatic embryogenesis in transformed tissue, as shown in maize where directed morphogene expression led to somatic embryos forming directly from surface cells of IEs (Lowe et al., 2018). This eliminates extended callus phases, which lengthen regeneration, leading to increased somaclonal variation (Bregitzer et al., 1998; Lemaux et al., 1999).
Optimizing altruistic morphogene‐assisted transformation improves efficiency of existing transformation constructs
One challenge of MAT was generating transformed plants with the GOI, but without morphogenes, causing developmental abnormalities and poor seed set (Figure S2). This was addressed in maize using altruistic transformation (Hoerster et al., 2020). In this strategy high Wus2 expression in cells proximal to those with only the GOI yielded GOI‐positive transgenic plants without morphogenes. The Zea mays phospholipid transferase protein promoter (Zm‐PLTPpro) (Garnaat et al., 2002) drove strong Wus2 expression in scutellar epithelial cells of IEs (Lowe et al., 2018), such that sufficient Wus2 diffused into neighbouring cells, triggering developmental progression and GOI‐only transformed plants.
When pPHP81814 was used in our altruistic MAT method, the promoter driving Wus2 expression, Zm‐AXIG1pro, was weak and did not provide sufficiently strong expression to trigger developmental progression in neighbouring cells. Out of 192 IEs used, no plantlets containing the GOI were regenerated. Successful sorghum altruistic MAT necessitated using Zm‐PLTPpro to drive Bbm and Wus2 expression. In our efforts, presence in pANIC10A of the RFP GOI and hph required optimizing hyg selection. For altruistic MAT using pGL190 and pANIC10A, transformed RFP plants without morphogenes were generated; however, efficiencies varied among replicates. Many plants were also generated having both morphogenes from pGL190 and hyg and GOI from pANIC10A (Table 3). In maize, viral enhancers were used to increase Wus2 expression to levels likely hindering regeneration of plantlets with the morphogene (Hoerster et al., 2020). In our study, Wus2 expression likely was not sufficiently high to be toxic, leading to plants with morphogenes both with or without RFP and hph. Using this strategy, however, can increase transformation speed because previously completed GOI constructs can be used without having to insert morphogenes into those constructs. This allows researchers to use existing constructs to immediately improve transformation efficiency, expand transformable genotypes and expedite regeneration.
Modified molecular genotyping techniques provide faster and more informative results
For transformed plants to provide definitive proof of gene function, copy number and TII must also be determined. ddPCR was used to determine copy number and, using MAT technology, notably over 60% of independent transformants were single‐copy. Determining TII in sorghum is also critical with engineering due to sorghum’s tendency to tiller, making it unclear if plants from the same IE are clonal or independent. Independence of events is less critical with CRISPR/Cas9 editing, however, because multiple plants from the same IE can have independent edits whether the original insertion is the same or not. Because previous methods to determine TII, like Southern hybridization and Southern‐by‐Sequencing (Zastrow‐Hayes et al., 2015), can be time‐ and resource‐intensive, a high‐throughput TII assay was created by modifying a transgene insertion mapping method developed for Arabidopsis (O’Malley et al., 2007). Using PCR‐based strategies to determine both copy number and TII, the large number of transformed plants generated by MAT can be genotyped at a higher throughput rate with reduced material and personnel costs.
Integrating morphogene‐assisted transformation and CRISPR/Cas9 gene editing produces biallelic and chimeric T0 knockouts
Previous reports in sorghum described success with editing endogenous genes using both biolistic bombardment and Agrobacterium‐mediated transformation (Char et al., 2020; Che et al., 2018; Li et al., 2018; G. Liu et al., 2019). To improve both transformation and editing efficiencies in sorghum, MAT was combined with CRISPR/Cas9, using multiple gRNAs to edit the exemplary GOI, pds. Based on pGL196 editing experiments (Figure 4c), the altruistic MAT strategy yielded tissues with fewer somatic embryos that proliferated more slowly than when using MAT alone. This led to the decision not to use an altruistic MAT approach for editing, opting instead to combine morphogene cassettes with a CRISPR/Cas9 cassette.
Using pGL196, numerous transformants were identified in which morphogenes were not integrated; however, albino phenotypes due to biallelic pds knockouts were not observed. Low editing efficiency with pGL196 (Figure 4c) was likely due to low‐level expression of Cas9 driven by Sb‐Ubi1pro, compared to the maize ubiquitin1 promoter (Zm‐Ubi1pro) (I. Godwin, personal communication). Replacing Sb‐Ubi1pro and SpCas9 with Zm‐Ubi1pro and maize codon‐optimized SpCas9, respectively, in pGL199 (Figure 3c) likely contributed to increased editing efficiency due to higher expression levels, leading to regeneration of albino plantlets, due to biallelic editing in the T0 generation (Figure 4d). This is the first report of biallelic edits in T0 sorghum plants using CRISPR/ Cas9.
Introducing a single T‐DNA, including gRNAs, Cas9, LoxP and morphogenes (pGL199) led to 16.7% knockout efficiency (Figure 4c); however, there are strategies to increase rates of heritable editing mutations, for example, using a meiosis‐specific promoter, like dmc1, to drive Cas9, as demonstrated in maize (Feng et al., 2018). Another approach might be to use Cas9 orthologues or variants, like Streptococcus thermophilus Cas9 (StCas9) (Müller et al., 2016) and Staphylococcus aureus Cas9 (SaCas9) (Ran et al., 2015). Despite our demonstrated success, integrating MAT and CRISPR/Cas9 into a single construct is not without disadvantages. The resulting plasmid is very large, ~36 kb, and creation of plasmids this size and their introduction into Agrobacterium can be difficult (Table S4). Also having the GOI and morphogenes on the same T‐DNA necessitated removing introduced morphogenes using heat shock to generate plants with normal phenotypes and seed sets (Table 1). Despite these challenges, the considerable improvements in transformation efficiency and the reduced regeneration time observed with the MAT approach, compared to classical transformation, demonstrate its efficacy in facilitating CRISPR/ Cas9 gene editing experiments in sorghum.
Conclusion
Certain steps outlined in this study facilitated development of a pathway from creation and introduction of target genes via engineering and editing to a means to determine gene function. Use of morphogenes led to more sorghum genotypes amenable to transformation and a faster time to generate transformed plants. Another challenge facing researchers with limited resources is garnering the technological resources necessary to get from gene identification to transformed plants ready for field trials. A path to achieving this goal is described in the present study by bringing together existing and newly created constructs, culturing approaches, and analytical methods. Details are provided on these resources and methods – including transformation of new sorghum genotypes, identification of single‐copy, independent events, and creation of MAT‐generated editing constructs. These advancements provide a straightforward means to validate gene function in sorghum for the many uncharacterized genes (Phytozome v13), including those involved in drought responses (Varoquaux et al., 2019; Xu et al., 2018).
Methods
Plant materials
Seeds from sorghum varieties RTx430 (Miller, 1984), BTx623 (Frederiksen and Miller, 1972), BTx642 (Rosenow et al., 2002), SC187 (Rosenow et al., 1997) and sweet sorghum Ramada (Freeman, 1974) were from GRIN (United States Department of Agriculture, Agricultural Research Service, n.d.), planted in three‐gallon pots with SuperSoil 1 (Rod McClellan Co., South San Francisco, CA) and grown in the greenhouse at 28 °C with 16 h light/ 8 h dark photoperiod. IEs from panicles were collected at 12–14 days post‐anthesis.
Preparation of Agrobacterium
LBA4404 Thy‐ is an auxotrophic (THY‐) A. tumefaciens strain (Anand et al., 2017) into which helper plasmid, pPHP71539 (Anand et al., 2017), was introduced. For other constructs, strains were streaked on YEP agar medium (per L: 10 g yeast extract, 10 g Bacto Peptone, 5 g NaCl, 15 g Bacto Agar, pH 7.0, 100 mg/L thymidine, 50 mg/L gentamicin) from stocks stored at −80 °C at 25% final concentration glycerol. After dark incubation for 3 days at 28 °C, 3–5 colonies were used to make overnight cultures on YEP; suspension cultures from those cultures were adjusted to OD550 0.7, using PHI‐I medium (Wu et al., 2014) with 0.005% silwet and 0.2 mM acetosyringone.
Transformation constructs
Primers for creating constructs are in Table S2. pPHP81814 (Chu et al., 2019), used for MAT, has Zm‐Bbm (Boutilier et al., 2002) and Wus2 (Nardmann and Werr, 2006) plus screenable marker gene ZsGreen‐1 (ZsG‐1), driven by Zm‐Axig1pro, Zm‐PLTPpro, and Sb‐Ubi1pro, respectively. These three genes are within loxP sites; P4 recombinase gene causing recombination (cre) is driven by the maize late embryogenesis‐stage Zm‐GLB1pro (Belanger and Kriz, 1989). ALS is driven by the Sorghum bicolor acetolactate synthase promoter (Sb‐ALSpro). Helper plasmid, pPHP71539, contains virB, virC, virD, virE and virG and a bacterial gentamicin resistance gene (Anand et al., 2018).
To create altruistic MAT construct, pGL190 (Figure 3a), pPHP83911 (Figure S3a), contained Sb‐Ubi1pro and the first intron driving uidA and the sorghum gamma kafirin terminator, linearized between attL3 and attL4, using pPL01 and pPL04 primers. The expression cassette, including the Sb‐Ubi1pro and first intron, ZsG‐1, and the rice Ubi1 terminator, was inserted using pPL02 and pPL03 primers via Gibson Assembly (New England BioLabs, Ipswich MA). Entry vector was verified by Sanger sequencing with primers pPL02 and pPL05, and M13 reverse primers. To complete pGL190 construction, the entry vector was recombined with the destination vector, pPHP85425 (Figure S3b), confirmed by BamHI restriction and electroporated into LBA4404 Thy‐. Plasmid was confirmed by complete plasmid sequencing (Center for Computational and Integrative Biology, Massachusetts General Hospital, Boston MA).
For pGL193 (Figure S3c), pPHP83911 (Figure S3a) was linearized with pPL08 and pPL09 primers. SpCas9 was amplified from pRGEB32 (Xie et al., 2015) with pPL06 and pPL07 primers and the two fragments ligated via Gibson Assembly. The gRNA expression cassette, amplified from pRGEB32 with pPL10 and pPL11 primers, was inserted via Gibson Assembly into SmaI‐linearized plasmid. The guide RNAs (gRNAs) polycistronic cassette, targeting two sorghum pds exons (Figures 4a, S4), created with primers pPL12 to pPL17 according to the published protocol (Xie et al., 2015). Using Golden Gate Assembly, the gRNA‐tRNA polycistronic cassette was digested with FokI, and ligated into pGL193. pGL196 (Figures 3b, S5) was generated by recombining the pds gRNA‐tRNA polycistronic cassette and the SpCas9 gene from pGL193 with pPHP85425). For pGL198, maize Ubi1 promoter and maize codon‐optimized SpCas9 were amplified from pBUN411 (Xing et al., 2014) with primers pPL27 and pPL28, replacing by Gibson Assembly the Sb‐Ubi1pro and SpCas9 gene in pGL193 (Figure S3c). Two gRNAs, targeting pds genes in the gRNA‐tRNA polycistronic cassette, were assembled into pGL198 using primers pPL29 to pPL32, with the method described above. pGL199 (Figures 3c, S5) was generated by recombining the pds gRNA‐tRNA polycistronic cassette and maize codon‐optimized SpCas9, with destination vector, pPHP85425. All constructs were confirmed by Sanger sequencing. pGL190 (Figure 3a), pGL196 (Figure 3b), and pGL199 (Figure 3c) from Agrobacterium were confirmed by complete plasmid sequencing (Center for Computational and Integrative Biology, Massachusetts General Hospital, Boston MA).
Morphogene‐assisted transformation of sorghum
All media was modified from (Jones et al., 2019) (Table S3). General construct descriptions and plant and bacterial selection genes and their purpose are in Table S4. Immature seeds of RTx430, BTx623, BTx642, SC187 and Ramada from greenhouse‐grown plants were surface‐sterilized twice for two mins with 75% ethanol, then 20% bleach plus 0.2% Tween20 for 20 mins. IEs (1.5–2 mm) were isolated and put into PHI‐I liquid medium. When all IEs were isolated, PHI‐I was removed, 1 mL of Agrobacterium suspension was added and mixed 5 mins at medium shaker speed; suspension was removed by pipetting. IEs, placed on co‐cultivation medium (CCM) scutellum side up, were kept in the dark for 7 days at 24 °C. IEs were transferred to resting medium for 7 days to halt Agrobacterium growth; tissues were then moved to embryo maturation media (EMM) with 0.05 mg/L IMZ (Sigma Aldrich Chemicals, St. Louis MO) until shoots formed (Figures S1a,b). Plantlets (2–3 cm) were moved to RM with 0.05 mg/L IMZ under 16‐h photoperiod at 26 °C (Figure S1c). Plants with well‐established roots and shoots (10‐12 cm) were moved to soil in growth chambers at 26 °C with 16‐h light and 8‐h dark photoperiod for two weeks of conditioning before transferring to the greenhouse. Per cent transformation efficiency was calculated in two ways. First, efficiency was based on the number of T0 plants PCR‐positive for the target gene, divided by the number of IEs used, times 100. The second method was based on the number of PCR‐positive, TII T0 plants.
Altruistic morphogene‐assisted transformation of sorghum
Immature RTx430 seeds were harvested, sterilized and IEs excised as described for the MAT approach. For transformation, Agrobacterium LBA4404 Thy‐, containing either pGL190/pPHP81814 or pANIC10A at OD550 0.7, were mixed in a 9:1 (pANIC10A: pGL190/pPHP81814) volume: volume ratio. IEs were cultured as described for MAT except EMM selection was on 20 mg/L hygromycin (hyg; PhytoTechnology Laboratories, Lenexa KS); no selection in RM. Brightfield and fluorescence microscopy images were taken. pGL190 and pANIC10A alone were used as transformation controls.
DNA extraction
Leaf samples, ~3 cm, frozen in liquid nitrogen and ground in an MM300 bead beater (Retsch GmbH, Haan Germany) for 1.5 mins at 25 cps, were refrozen and ground again. 700 μl of urea buffer (2 M urea, 0.35 mM NaCl, 20 mM Tris‐HCl pH 8, 20 mM EDTA, 1% sarkosyl) was added and vortexed for 30 secs. 10 μl of RNase A was added, incubated 10 mins at room temperature and 700 μl of phenol:chloroform:isoamyl alcohol (25:24:1) was added, vortexed 15 mins, and centrifuged 13 800 × g for 15 mins. 55 μl of 3 M sodium acetate (pH 5.2) and 367 μl of isopropanol were added to 550 μl of the aqueous phase; to precipitate DNA, tubes were inverted at −20 °C overnight or −80 °C for 1 h. After centrifugation at 13 800 × g for 5 mins, supernatant was removed and pellet rinsed in 500 μl 70% ETOH. Samples were centrifuged 5 mins at 13 800 × g, supernatant removed and samples centrifuged 1 min. Excess ethanol was removed, pellets air‐dried 5–10 mins in laminar flow hood, resuspended in sterile distilled water and stored at −20 °C.
Fluorescence visualization
Zeiss Lumar v12 epifluorescence stereomicroscope and a QImaging Retiga SRV camera were used to observe IEs on EMM expressing RFP and ZsG‐1 between 3 and 10 days post‐infection and while tissues were on EMM. RFP and ZsG‐1 fluorescence was detected using Texas Red and Endow GFP filter sets, respectively.
PCR analysis of putative transformed plants
Polymerase chain reaction was performed on gDNA from leaf tissue of putative T0 plants. For putative, MAT‐generated, PCR primers (Table S1) were used to confirm ZsG‐1 and ALS, reinstated after CRE‐mediated excision of Zm‐Bbm and Wus2. For the altruistic MAT approach with pGL190 and pANIC10A, PCR was performed using hph, RFP and Bbm primers (Tables S1, S5).
Transgene copy number analysis
For transgene copy number, primers and HEX™‐labelled probes were designed to detect the endogenous reference gene, protein phosphatase 2A (PP2A) (Table S6), chosen as one of the most stable in sorghum in different tissues and stress treatments (Sudhakar Reddy et al., 2016). This was used for RTx430, SC187 and Ramada. A different primer set (Table S6) was used to detect ALS, using probes labelled with FAM™ and double‐quenched with ZEN™ and Iowa Black Hole Quencher® (Integrated DNA Technologies, Inc., Coralville, IA). Probes and primers were designed according to the manufacturer's protocol (BioRad Laboratories, Hercules CA); droplets were generated using QX200 droplet generator. Samples included a no template control (ddH20), negative control (wild‐type sorghum gDNA), positive plasmid DNA and gDNA from transformed plants. HindIII, which does not cut the transgene, was used to digest gDNA. Samples were transferred to 96‐well plates; a thermal cycler was used for PCR amplification. To optimize quantification of transgene copy number, gradient PCR (55–65 °C) and dilution gradient of sorghum DNA (6.8–55 ng) was used to determine optimal annealing temperatures and DNA amounts; 55 °C and 27.5 ng gDNA were selected because they gave the highest fluorescence amplitude difference between negative and positive droplets, specific amplification and clear separation between negative and positive droplets. PCR started with 95 °C for 10 mins, 94 °C for 30 secs and 55 °C for 1 min; each step was 40 cycles and 2 °C/second ramp rate. Reactions were stopped by 98 °C enzyme deactivation for 10 mins. Fluorescence was measured using BioRad QX200 Droplet reader; more than 10 000 droplets were generated per sample. Samples were performed in duplicate; data was analysed using BioRad QuantaSoft™ Software.
TII determination
All primers in this section are in Table S2. The TII method was modified from (O’Malley et al., 2007). Adaptors for HindIII (adpH3) and BamHI (adpB1) were annealed with pPL18 and pPL19, plus pPL18 and pPL20, respectively. Oligonucleotides were heated at 98 °C, then cooled to room temperature by turning the water bath off. 900 ng of sorghum gDNA was added to adpH3 and adpB1 adapters, using HindIII and BamHI and T4 ligase; the reaction was at 25 °C for 16 h in a thermocycler (BioRad C1000 Touch™). To analyse gDNA‐tDNA junctions on the left border for MAT‐generated transformants, PCR amplification was performed using pPL21 and pPL23 primers at 55 °C for annealing; elongation was for 4 min. If distinct PCR bands were not seen, PCR products were used for nested‐PCR amplification. 1 μl of PCR product was amplified at 60 °C for annealing, elongation for 4 min using pPL24 and pPL26 primers. PCR products were analysed in 1% agarose and read using Image Lab Software (BIO‐RAD, Hercules CA). If the Transfer‐DNA (T‐DNA)/genomic DNA (T‐DNA/gDNA) junctions could not be amplified from left borders, ligates were amplified from right border with primers pPL22 and pPL23 for first‐round PCR, pPL25 and pPL26 for nested PCR, if needed. Left or right border data was used to determine TII (Figure S6). For MAT‐generated transformants, TII was determined as for the samples from altruistic MAT by amplifying T‐DNA/gDNA junctions using primers pPL23 and pPL33 for first round PCR, primers pPL26 and pPL34 for nested PCR. For TII, it is not mandatory to first verify plants are single‐copy; however, amplification and visualization of gel images are easier.
CRISPR/Cas9 editing mediated by morphogene‐assisted transformation
To demonstrate editing efficiency, three gRNAs targeting pds, Sobic.006G232600 on chromosome 6, and a homologue, Sobic.001G480550 on chromosome 1 [88%, 90% identity, respectively, to rice pds LOC_Os03g08570 coding sequence (Phytozome v13)], were designed according to CRISPR‐P 2.0 (H. Liu et al., 2017) (Figures 4a, S4). The first gRNA targeted the third exon of both genes; the second and third gRNAs targeted the fifth exon of both pds genes. Three gRNAs were assembled into pGL193 (Figure S3c) for LR recombination (Figure S5). For gRNA optimization, two gRNA sequences targeting the second and fourth exons of pds, Sobic.006G232600 (Figure 4a), were from Dr. Ian Godwin (personal communication). gRNAs were incorporated in pGL198 (Figure S3d) for subcloning (Figure S5). For MAT‐mediated editing, SpCas9 expression in the expression cassette was driven by Sb‐UBi1pro or Zm‐Ubi1pro. respectively, in pGL193 or pGL198; gRNA‐tRNA polycistronic expression was driven by Os‐snoRNAU3pro on the pENTR vectors (Figures S3c,d). After incorporating gRNAs targeting pds (Figures 4a, S4) into both cassettes they were introduced into the destination vector, pPHP85425 (Figure S3b), yielding, pGL196 and pGL199 (Figures 3b,c, S5), which were transformed into RTx430 using Agrobacterium tumefaciens LBA4404 Thy‐. Before moving to EMM, IEs on resting media were heat‐shocked at 45°C, 75% humidity, for 2 hrs to trigger CRE‐mediated excision. From each IE, 3‐4 plantlets were moved to RM.
Genotyping putative CRISPR/Cas9 edited plants
Total gDNA was extracted from leaves of seedlings on RM. To identify putative edited plants, PCR was performed using primers for the gRNA‐tRNA polycistronic cassette and Zm‐Bbm (Table S1). Knockout of Sobic.006G232600 leads to albino phenotypes (G. Liu et al., 2019), so the target pds fragment, including the two gRNAs, was amplified to analyse by Sanger sequencing. Targeted pds genes were amplified in PCR‐positive plants, using PDS‐06g‐1 primers, to detect putative edits with pGL196, PDS‐06g‐2 primers for putative edits with pGL199 (Table S1). Amplicons were visualized in 1% agarose, purified with QIAquick® PCR purification kit (QIAgen, Redwood City CA), and prepared for Sanger sequencing (UC Berkeley DNA Sequencing Facility). Sequencing results were analysed using the Inference of CRISPR Edits (ICE) analysis tool (Hsiau et al., 2018).
Conflict of interest statement
pPHP vectors are available for research purposes; using the vectors for commercial applications requires a paid non‐exclusive license from Corteva Agriscience. Manuscript authors have no relationship with Corteva Agriscience.
Author contributions
Kiflom Aregawi: Investigation, Transformation Methodology, Writing ‐ Original Draft ‐ Review & Editing. Jianqiang Shen: Investigation, Transformation Methodology, Constructs, Writing ‐ Review & Editing. Grady Pierroz: Investigation, Transformation Methodology, Writing – Review & Editing. Manoj Sharma: Transformation Methodology, Writing ‐ Review & Editing. Jeffery Dahlberg: Methodology. Judith Owiti: Transformation Methodology, Writing ‐ Review & Editing. Peggy G. Lemaux: Conceptualization, Formal analysis, Supervision, Project administration and funding acquisition, Writing – Original Draft –Review & Editing.
Supporting information
Figure S1. (a, b) BTx642 tissues on EMM with IMZ selection following transformation with pPHP81814. (c) Plantlets growing on rooting media with IMZ.
Figure S2. Plants from (a) RTx430 and (b) BTx642 transformed with pPHP81814. In both, plants null for Zm‐Bbm and Wus2 (right) and plants retaining introduced Zm‐Bbm and Wus2 (left) have shorter stature, twisted leaves and poorer seed set, compared to wild‐type.
Figure S3. Schematic representation of the molecular components of constructs. (a) pPHP83911, used for generating pGL193 and pGL198, is a pENTR vector. (b) pPHP85425 is a destination vector for morphogene‐assisted transformation (MAT). Molecular components between left and right borders are shown. (c) pGL193 is a pENTR vector for MAT‐mediated editing with gRNAs. The remaining backbone part is from pPHP83911. (d) pGL198 is a pENTR vector for MAT‐mediated editing with the maize ubiquitin promoter and maize codon‐optimized SpCas9 for carrying gRNAs. The remaining backbone part is also from pPHP83911.
Figure S4. Two gRNAs targeting pds in pGL196; yellow rectangles indicate exons; light grey lines indicate intron regions. gRNA1.1 is located in the third exon of pds genes. gRNA1.3 is located in the fifth exon of pds genes.
Figure S5. Molecular cloning strategy for constructing sorghum CRISPR/Cas9 vectors. Cloning vector for gRNAs containing BsaI sites for sequential insertion of two or three gRNAs with tRNAs that forms polycistronic cassettes (gRNA‐tRNA), driven by rice U3 promoter (Os‐U3pro). The gRNA‐tRNA and Cas9 expression cassettes were mobilized into pPHP85425 through Gateway recombination, generating pGL196 and pGL199 for Agrobacterium‐mediated sorghum transformation.
Figure S6. Determination of transgene independent integration (TII) with adapter ligation‐mediated PCR. Genomic DNA with a T‐DNA insert (i) was digested with EcoRI and HindIII (ii). Adapters are ligated to digested sites creating adapter‐flanked templates (iii). Only the longer arm of the adapter contains a sequence exactly matching the adapter primers. Adapter‐to‐adapter amplification does not occur due to lack of primer match sites on the shorter arm of adapters. If T‐DNA is present in the template (black line, Figure 4a.i), T‐DNA primers (yellow arrow, left border; dark blue arrow, right border, Figure 4a.iii) will bind to their corresponding sites and initiate synthesis of a complementary strand (iv). PCR products will contain adapter primer‐binding sites derived from the complement of the longer arm of the adapter.
Table S1. Primers for genotyping putative transformed plants
Table S2. Primers for constructs, gRNAs and determining transgene independent integration (TII)
Table S3. Medium composition for sorghum transformation
Table S4. Constructs used for sorghum transformation
Table S5. PCR programmes for genotyping putative transformed plants
Table S6. Primers and probes for digital droplet PCR
Table S7. Description and references for construct elements used for transformation
Acknowledgements
Funding was from the Department of Energy, Biological and Environmental Research Program, Competitive Grant DE‐SC0014081. M.K.S. acknowledges support from DBT India and IUSSTF as a B‐ACER fellowship; present address, School of Biotechnology, Jawaharlal Nehru University, New Delhi India. PGL and JD were supported by the US Cooperative Extension Service through the Division of Agriculture and Natural Resources of the University of California. We thank Drs. William Gordon‐Kamm and Ping Che at Corteva Agriscience for helpful conversations and for constructs pPHP71539, pPHP81814, pPHP83911 and pPHP85425, Dr. Ian Godwin at the University of Queensland for two gRNA sequences targeting pds, Dr. Ronan O’Malley for discussions on his transgene insertion mapping technique and Dr. Frank Harmon for field data on RTx430 and BTx642. We are grateful for help from the staff of the UC Berkeley Biological Imaging Facility and the UC Berkeley Oxford Tract Greenhouse Facility. We thank Barbara Alonso for managing the reference list and Claudia Bucheli for helpful discussions.
Aregawi, K. , Shen, J. , Pierroz, G. , Sharma, M. K. , Dahlberg, J. , Owiti, J. and Lemaux, P. G. (2022) Morphogene‐assisted transformation of Sorghum bicolor allows more efficient genome editing. Plant Biotechnol. J., 10.1111/pbi.13754
Footnotes
[Correction added on 24 January 2022, after first online publication: The full reference detail of Gurel et al. 2009 has been inserted in this version.]
Data availability statement
As available, accession numbers and gene names are provided in text and Table S7. Methods for generating different constructs and transformation protocols are described in the text, Supplementary Tables and Supplementary Figures. PHP vectors are freely available for research; commercial applications require a paid non‐exclusive license from Corteva Agriscience. The sequences of pGL193 and pGL198 were deposited to GeneBank, with the accession numbers, OK017460 and OK017461 respectively. Both of them will be deposited and available on Addgene as well.
References
- Altpeter, F. , Springer, N.M. , Bartley, L.E. , Blechl, A.E. , Brutnell, T.P. , Citovsky, V. , Conrad, L.J. et al. (2016) Advancing crop transformation in the era of genome editing. Plant Cell, 28(7), 1510–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand, A. , Arling, M.L. , da Silva Conceicao, A. , Gordon‐Kamm, W.J. , Hastings, C.E. , Hoerster, G.M. , Klein, T.M. , et al. (2017) Methods and compositions for rapid plant transformation (USPTO Patent No. 20170121722:A1). In US Patent (No. 20170121722:A1). https://patentimages.storage.googleapis.com/9a/69/72/9925b2e096f458/US20170121722A1.pdf
- Anand, A. , Bass, S.H. , Cho, H.‐J. , Klein, T.M. , Lassner, M. and McBridge, K.E. (2017). Methods and Compositions of Improved Plant Transformation (WIPO Patent No. 2017/078836 Al). In World Patent (2017/078836 Al).
- Anand, A. , Bass, S.H. , Wu, E. , Wang, N. , McBride, K.E. , Annaluru, N. , Miller, M. et al. (2018) An improved ternary vector system for Agrobacterium‐mediated rapid maize transformation. Plant Mol. Biol. 97(1–2), 187–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanger, F.C. and Kriz, A.L. (1989) Molecular characterization of the major maize embryo globulin encoded by the glb1 gene. Plant Physiol. 91(2), 636–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan, M.W. , Flavell, R.B. and Chilton, M.‐D. (1983) A chimaeric antibiotic resistance gene as a selectable marker for plant cell transformation. Nature, 304(5922), 184–187. [PubMed] [Google Scholar]
- Biotechnology and Biological Sciences Research Council . (2013) Thirty years of plant transformation: A case study exploring the impact of plant transformation technology on plant science research and the global agricultural biotechnology industry. Swindon: Biotechnology and Biological Sciences Research Council. [Google Scholar]
- Boutilier, K. , Offringa, R. , Sharma, V.K. , Kieft, H. , Ouellet, T. , Zhang, L. , Hattori, J. et al. (2002) Ectopic expression of BABY BOOM triggers a conversion from vegetative to embryonic growth. Plant Cell, 14(8), 1737–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bregitzer, P. , Halbert, S.E. and Lemaux, P.G. (1998) Somaclonal variation in the progeny of transgenic barley. Theor. Appl. Genet. 96(3–4), 421–425. [DOI] [PubMed] [Google Scholar]
- Char, S.N. , Wei, J. , Mu, Q. , Li, X. , Zhang, Z.J. , Yu, J. and Yang, B. (2020) An Agrobacterium‐delivered CRISPR/Cas9 system for targeted mutagenesis in sorghum. Plant Biotechnol. J. 18(2), 319–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu, U.C. , Adelberg, J. , Lowe, K. and Jones, T.J. (2019) Use of DoE methodology to optimize the regeneration of high‐quality, single‐copy transgenic Zea mays L. (maize) plants. In Vitro Cell Dev. Biol. Plant, 55(6), 678–694. [Google Scholar]
- De Cleene, M. and De Ley, J. (1976) The host range of crown gall. Bot. Rev. 42(4), 389–466. 10.1007/bf02860827. [DOI] [Google Scholar]
- Duff, J. , Bice, D. , Hoeffner, I. and Weinheimer, J. (2019) The sorghum industry and its market perspective. In Agronomy Monographs, pp. 503–514. Soil Science Society of America. [Google Scholar]
- Feng, C. , Su, H. , Bai, H. , Wang, R. , Liu, Y. , Guo, X. , Liu, C. et al. (2018) High‐efficiency genome editing using a dmc1 promoter‐controlled CRISPR/Cas9 system in maize. Plant Biotechnol. J. 16(11), 1848–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraley, R.T. , Rogers, S.G. , Horsch, R.B. , Sanders, P.R. , Flick, J.S. , Adams, S.P. , Bittner, M.L. et al. (1983) Expression of bacterial genes in plant cells. Proc. Natl Acad. Sci. USA, 80(15), 4803–4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frame, B.R. , Shou, H. , Chikwamba, R.K. , Zhang, Z. , Xiang, C. , Fonger, T.M. , Pegg, S.E.K. et al. (2002) Agrobacterium tumefaciens‐mediated transformation of maize embryos using a standard binary vector system. Plant Physiol. 129(1), 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiksen, R.A. and Miller, F. (1972) Proposal for release and increase: ATx622, BTx622, ATx623, BTx623, ATx624, BTx624. TAES Form, 96–72
- Freeman, K.C. (1974) Ramada: A New Variety of Sweet Sorghum for Potential Sugar Production in South Texas. S‐41 New Orleans LA: Agricultural Research Service National Agricultural Library, 1974, 4p. https://archive.org/details/ramadanewvariety41free [Google Scholar]
- Garnaat, C. , Lowe, K. and Roth, B. (2002) Zm‐AXIG1‐specific polynucleotides and methods of use. In Patent Number WO2002006499.
- Goodstein, D.M. , Howson, R. , Shu, S. Neupane, R. , Hayes, R.D. , Fazo, J. , Mitros, T. (2012) Phytozome: a comparative platform for green plant genomics. Nucleic Acids Research 40(D1), D1178–D1186. 10.1093/nar/gkr944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon‐Kamm, W.J. , Spencer, T.M. , Mangano, M.L. , Adams, T.R. , Daines, R.J. , Start, W.G. , O’Brien, J.V. et al. (1990) Transformation of Maize Cells and Regeneration of Fertile Transgenic Plants. Plant Cell, 2(7), 603–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurel, S. , Gurel, E. , Kaur, R. , Wong, J. , Meng, L. , Tan, H.‐Q. and Lemaux, P.G. (2009) Efficient, Reproducible Agrobacterium‐mediated Transformation of Sorghum Using Heat Treatment of Immature Embryos. Plant Cell Reports 28, 429–444.[Correction added on 24 January 2022, after first online publication: The full reference detail of Gurel et al. 2009 has been inserted in this version.] [DOI] [PubMed] [Google Scholar]
- Herrera‐Estrella, L. , Depicker, A. , Van Montagu, M. and Schell, J. (1983) Expression of chimaeric genes transferred into plant cells using a Ti‐plasmid‐derived vector. Nature, 303(5914), 209–213. [PubMed] [Google Scholar]
- Hoerster, G. , Wang, N. , Ryan, L. , Wu, E. , Anand, A. , McBride, K. , Lowe, K. et al. (2020) Use of non‐integrating Zm‐Wus2 vectors to enhance maize transformation: Non‐integrating WUS2 enhances transformation. In Vitro Cell Dev. Biol. Plant, 17, 43. [Google Scholar]
- Hsiau, T. , Maures, T. , Waite, K. , Yang, J. , Kelso, R. and Holden, K. (2018) Inference of CRISPR edits from Sanger trace data. BioRxiv, https://www.biorxiv.org/content/ 10.1101/251082v1.abstract [DOI] [PubMed] [Google Scholar]
- Jones, T. , Lowe, K. , Hoerster, G. , Anand, A. , Wu, E. , Wang, N. , Arling, M. et al. (2019) Maize Transformation Using the Morphogenic Genes Baby Boom and Wuschel2. Methods Mol. Biol. 1864, 81–93. [DOI] [PubMed] [Google Scholar]
- Laux, T. , Mayer, K.F. , Berger, J. and Jürgens, G. (1996) The WUSCHEL gene is required for shoot and floral meristem integrity in Arabidopsis. Development, 122(1), 87–96. [DOI] [PubMed] [Google Scholar]
- Lemaux, P.G. , Cho, M.‐J. , Zhang, S. and Bregitzer, P. (1999). Transgenic Cereals: Hordeum vulgare L. (barley). In Vasil, I.K. , Molecular Improvement Of Cereal Crops Advances in Cellular and Molecular Biology of Plants. (5, pp. 255–316). Dordrecht: Springer, 10.1007/978-94-011-4802-3_9 [DOI] [Google Scholar]
- Li, A. , Jia, S. , Yobi, A. , Ge, Z. , Sato, S.J. , Zhang, C. , Angelovici, R. et al. (2018) Editing of an alpha‐kafirin Gene family increases, digestibility and protein quality in sorghum. Plant Physiol. 177(4), 1425–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, G. , Li, J. and Godwin, I.D. (2019) Genome Editing by CRISPR/Cas9 in Sorghum Through Biolistic Bombardment. Methods Mol. Biol. 1931, 169–183. [DOI] [PubMed] [Google Scholar]
- Liu, H. , Ding, Y. , Zhou, Y. , Jin, W. , Xie, K. and Chen, L.‐L. (2017) CRISPR‐P 2.0: An Improved CRISPR‐Cas9 Tool for Genome Editing in Plants. Molecular Plant, 10(3), 530–532. [DOI] [PubMed] [Google Scholar]
- Lowe, K. , La Rota, M. , Hoerster, G. , Hastings, C. , Wang, N. , Chamberlin, M. , Wu, E. et al. (2018) Rapid genotype “independent” Zea mays L. (maize) transformation via direct somatic embryogenesis. In Vitro Cell. Dev. Biol. Plant, 54(3), 240–252. 10.1007/s11627-018-9905-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe, K. , Wu, E. , Wang, N. , Hoerster, G. , Hastings, C. , Cho, M.‐J. , Scelonge, C. et al. (2016) Morphogenic Regulators Baby boom and Wuschel Improve Monocot Transformation. Plant Cell, 28(9), 1998–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann, D.G.J. , LaFayette, P.R. , Abercrombie, L.L. , King, Z.R. , Mazarei, M. , Halter, M.C. , Poovaiah, C.R. et al. (2012) Gateway‐compatible vectors for high‐throughput gene functional analysis in switchgrass (Panicum virgatum L.) and other monocot species. Plant Biotechnol. J. 10(2), 226–236. [DOI] [PubMed] [Google Scholar]
- Miller, F.R. (1984) Registration of RTx430 sorghum parental line. Crop Sci., 24, 1224. [Google Scholar]
- Mookkan, M. , Nelson‐Vasilchik, K. , Hague, J. , Zhang, Z.J. and Kausch, A.P. (2017) Selectable marker independent transformation of recalcitrant maize inbred B73 and sorghum P898012 mediated by morphogenic regulators BABY BOOM and WUSCHEL2. Plant Cell Rep. 36(9), 1477–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller, M. , Lee, C.M. , Gasiunas, G. , Davis, T.H. , Cradick, T.J. , Siksnys, V. , Bao, G. et al. (2016) Streptococcus thermophilus CRISPR‐Cas9 systems enable specific editing of the human genome. Mol. Ther. 24(3), 636–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundia, C.W. , Secchi, S. , Akamani, K. and Wang, G. (2019) A regional comparison of factors affecting global sorghum production: The case of North America, Asia and Africa’s Sahel. Sustain.: Sci. Pract. Policy, 11(7), 2135. [Google Scholar]
- Nardmann, J. and Werr, W. (2006) The shoot stem cell niche in angiosperms: expression patterns of WUS orthologues in rice and maize imply major modifications in the course of mono‐ and dicot evolution. Mol. Biol. Evol. 23(12), 2492–2504. [DOI] [PubMed] [Google Scholar]
- O’Malley, R.C. , Alonso, J.M. , Kim, C.J. , Leisse, T.J. and Ecker, J.R. (2007) An adapter ligation‐mediated PCR method for high‐throughput mapping of T‐DNA inserts in the Arabidopsis genome. Nat. Protoc. 2(11), 2910–2917. [DOI] [PubMed] [Google Scholar]
- Paterson, A.H. (2008) Genomics of sorghum. Int. J. Plant Genom. 2008,362451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson, A.H. , Bowers, J.E. , Bruggmann, R. , Dubchak, I. , Grimwood, J. , Gundlach, H. , Haberer, G. et al. (2009) The Sorghum bicolor genome and the diversification of grasses. Nature, 457(7229), 551–556. [DOI] [PubMed] [Google Scholar]
- Raghuwanshi, A. and Birch, R.G. (2010) Genetic transformation of sweet sorghum. Plant Cell Rep. 29(9), 997–1005. [DOI] [PubMed] [Google Scholar]
- Raineri, D.M. , Bottino, P. , Gordon, M.P. and Nester, E.W. (1990) Agrobacterium‐mediated transformation of rice (Oryza sativa L.). Biotechnology, 8(1), 33–38. [Google Scholar]
- Ran, F.A. , Cong, L. , Yan, W.X. , Scott, D.A. , Gootenberg, J.S. , Kriz, A.J. , Zetsche, B. et al. (2015) In vivo genome editing using Staphylococcus aureus Cas9. Nature, 520(7546), 186–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenow, D.T. , Clark, L.E. , Dahlberg, J.A. , Frederiksen, R.A. , Odvody, G.N. , Peterson, G.C. , Miller, F.R. et al. (2002) Release of four A/B sorghum parental lines ATx642 through ATx645. Int Sorghum Millets Newsl, 43, 24–30. [Google Scholar]
- Rosenow, D.T. , Dahlberg, J.A. , Stephens, J.C. , Miller, F.R. , Barnes, D.K. , Peterson, G.C. , Johnson, J.W. et al. (1997) Registration of 63 Converted Sorghum Germplasm Lines from the Sorghum Conversion Program. Crop Sci. 37(4), 1399–1400. [Google Scholar]
- Sauer, B. and Henderson, N. (1988) Site‐specific DNA recombination in mammalian cells by the Cre recombinase of bacteriophage P1. Proc. Natl Acad. Sci. USA, 85(14), 5166–5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrawat, A.K. and Lörz, H. (2006) Agrobacterium‐mediated transformation of cereals: A promising approach crossing barriers. Plant Biotechnol. J. 4(6), 575–603. [DOI] [PubMed] [Google Scholar]
- Songstad, D.D. , Armstrong, C.L. , Petersen, W.L. , Hairston, B. and Hinchee, M.A.W. (1996) Production of transgenic maize plants and progeny by bombardment of hi‐II immature embryos. In Vitro Cell. Dev. Biol. Plant, 32(3), 179–183. [Google Scholar]
- Sudhakar Reddy, P. , Srinivas Reddy, D. , Sivasakthi, K. , Bhatnagar‐Mathur, P. , Vadez, V. and Sharma, K.K. (2016) Evaluation of sorghum [Sorghum bicolor (L.)] reference genes in various tissues and under abiotic stress conditions for quantitative real‐time PCR data normalization. Front. Plant Sci. 7, 529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Department of Agriculture, Agricultural Research Service . (n.d.). GRIN. Germplasm Resources Information Network. Retrieved 2007‐2017, from http://www.ars‐grin.gov/
- Varoquaux, N. , Cole, B. , Gao, C. , Pierroz, G. , Baker, C.R. , Patel, D. , Madera, M. et al. (2019) Transcriptomic analysis of field‐droughted sorghum from seedling to maturity reveals biotic and metabolic responses. Proc. Natl. Acad. Sci. USA, 116(52), 27124–27132. 10.1073/pnas.1907500116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilardell, J. , Mundy, J. , Stilling, B. , Leroux, B. , Pla, M. , Freyssinet, G. and Pagès, M. (1991) Regulation of the maizerab17 gene promoter in transgenic heterologous systems. Plant Mol. Biol. 17(5), 985–993. 10.1007/bf00037138. [DOI] [PubMed] [Google Scholar]
- Wu, E. , Lenderts, B. , Glassman, K. , Berezowska‐Kaniewska, M. , Christensen, H. , Asmus, T. , Zhen, S. et al. (2014) Optimized Agrobacterium‐mediated sorghum transformation protocol and molecular data of transgenic sorghum plants. In Vitro Cell. Dev. Biol. Plant, 50(1), 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, K. , Minkenberg, B. and Yang, Y. (2015) Boosting CRISPR/Cas9 multiplex editing capability with the endogenous tRNA‐processing system. Proc. Natl Acad. Sci. USA, 112(11), 3570–3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing, H.‐L. , Dong, L. , Wang, Z.‐P. , Zhang, H.‐Y. , Han, C.‐Y. , Liu, B. , Wang, X.‐C. et al. (2014) A CRISPR/Cas9 toolkit for multiplex genome editing in plants. BMC Plant Biol. 14, 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, L. , Naylor, D. , Dong, Z. , Simmons, T. , Pierroz, G. , Hixson, K.K. , Kim, Y.‐M. et al. (2018) Drought delays development of the sorghum root microbiome and enriches for monoderm bacteria. Proc. Natl Acad. Sci. USA, 115(18), E4284–E4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zastrow‐Hayes, G.M. , Lin, H. , Sigmund, A.L. , Hoffman, J.L. , Alarcon, C.M. , Hayes, K.R. , Richmond, T.A. et al.(2015) Southern‐by‐sequencing: A robust screening approach for molecular characterization of genetically modified crops. Plant Genome, 8(1), lantgenome2014.08.0037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. (a, b) BTx642 tissues on EMM with IMZ selection following transformation with pPHP81814. (c) Plantlets growing on rooting media with IMZ.
Figure S2. Plants from (a) RTx430 and (b) BTx642 transformed with pPHP81814. In both, plants null for Zm‐Bbm and Wus2 (right) and plants retaining introduced Zm‐Bbm and Wus2 (left) have shorter stature, twisted leaves and poorer seed set, compared to wild‐type.
Figure S3. Schematic representation of the molecular components of constructs. (a) pPHP83911, used for generating pGL193 and pGL198, is a pENTR vector. (b) pPHP85425 is a destination vector for morphogene‐assisted transformation (MAT). Molecular components between left and right borders are shown. (c) pGL193 is a pENTR vector for MAT‐mediated editing with gRNAs. The remaining backbone part is from pPHP83911. (d) pGL198 is a pENTR vector for MAT‐mediated editing with the maize ubiquitin promoter and maize codon‐optimized SpCas9 for carrying gRNAs. The remaining backbone part is also from pPHP83911.
Figure S4. Two gRNAs targeting pds in pGL196; yellow rectangles indicate exons; light grey lines indicate intron regions. gRNA1.1 is located in the third exon of pds genes. gRNA1.3 is located in the fifth exon of pds genes.
Figure S5. Molecular cloning strategy for constructing sorghum CRISPR/Cas9 vectors. Cloning vector for gRNAs containing BsaI sites for sequential insertion of two or three gRNAs with tRNAs that forms polycistronic cassettes (gRNA‐tRNA), driven by rice U3 promoter (Os‐U3pro). The gRNA‐tRNA and Cas9 expression cassettes were mobilized into pPHP85425 through Gateway recombination, generating pGL196 and pGL199 for Agrobacterium‐mediated sorghum transformation.
Figure S6. Determination of transgene independent integration (TII) with adapter ligation‐mediated PCR. Genomic DNA with a T‐DNA insert (i) was digested with EcoRI and HindIII (ii). Adapters are ligated to digested sites creating adapter‐flanked templates (iii). Only the longer arm of the adapter contains a sequence exactly matching the adapter primers. Adapter‐to‐adapter amplification does not occur due to lack of primer match sites on the shorter arm of adapters. If T‐DNA is present in the template (black line, Figure 4a.i), T‐DNA primers (yellow arrow, left border; dark blue arrow, right border, Figure 4a.iii) will bind to their corresponding sites and initiate synthesis of a complementary strand (iv). PCR products will contain adapter primer‐binding sites derived from the complement of the longer arm of the adapter.
Table S1. Primers for genotyping putative transformed plants
Table S2. Primers for constructs, gRNAs and determining transgene independent integration (TII)
Table S3. Medium composition for sorghum transformation
Table S4. Constructs used for sorghum transformation
Table S5. PCR programmes for genotyping putative transformed plants
Table S6. Primers and probes for digital droplet PCR
Table S7. Description and references for construct elements used for transformation
Data Availability Statement
As available, accession numbers and gene names are provided in text and Table S7. Methods for generating different constructs and transformation protocols are described in the text, Supplementary Tables and Supplementary Figures. PHP vectors are freely available for research; commercial applications require a paid non‐exclusive license from Corteva Agriscience. The sequences of pGL193 and pGL198 were deposited to GeneBank, with the accession numbers, OK017460 and OK017461 respectively. Both of them will be deposited and available on Addgene as well.


