Abstract
Background. The current study aims to give a scientific origin for employing Habenaria plantaginea Lindl. as a potential candidate against nociception, inflammation, and pyrexia. The pharmacological studies were performed on crude extract and subfractions. In the gas chromatography-mass spectroscopy analysis, a total of 21 compounds were identified. The plant samples were displayed for in vitro anti-inflammatory potentials. The observed IC50 for chloroform against cyclooxygenase-2 and 5-lipoxygenase enzymes was 33.81 and 26.74 μg/mL, respectively. The in vivo activities were prerequisites with the acute toxicity studies. In carrageenan-induced inflammation, the chloroform fraction exhibited 46.15% inhibition similar to that of standard drug diclofenac sodium 47.15%. Likewise, in the acetic acid-induced writhing test, the ethyl acetate fraction displayed 71.42% activity, which was dose-dependent as that of standard drug. In Brewer's yeast-induced antipyretic activity, a significant decrease in rectal volume was observed after 30, 60, and 90 minutes. Moreover, the results of this study indicated that the chloroform and ethyl acetate fractions inhibited nociception, inflammation, and pyrexia dose dependently. Likewise, mechanistic insights indicated that naloxone antagonized the antinociceptive effect of chloroform and ethyl acetate fractions, thereby signifying the involvement of opioidergic mechanisms respectively. These results suggest that these molecules present in this plant have synergistically beneficial potential for the cure and management of analgesia, inflammation, and pyrexia.
1. Introduction
New drug from a natural source with a desirable profile is tremendously an inspiring task in the field of clinical research [1]. Nociceptive pain induces activation of sensory nerve fibers when the stimulus overshoots the noxious intensity [2]. Chemical, thermal, and mechanical stimuli are the various common types of nociceptive pain which might further be alienated into deep, visceral, superficial somatic, and somatic pain [3]. The visceral part of the organ is extremely sensitive to inflammation, ischemia, and stretch but is not sensitive to the other stimuli that induce pain in other parts of the body [4]. Deep somatic pain initiates in blood vessels, muscles, tendons, bones, and ligaments after the stimulations of nociceptors in these organs. This type of pain is dull and poorly localized. Muscle sprain and breaking bones are the most common examples of deep somatic pain [5]. When the nociceptors in superficial and skin tissues activate, they induce superficial pain, which is very sharp. Minor burns and minor wounds are the most common examples of superficial pain [6]. Pain is a sign to propel a person from deleterious conditions to protect a damaged part of the body after healing. After removing the toxic stimuli, most of the pain sensation disappears but in some cases, pain perception remains for a long time after removing the damaging stimuli. Inflammation is a complicated protective response involving immune cells, blood vessels, and molecular mediators of the body to harmful stimuli, damaged cells, or invading microbes [6, 7]. The main role of inflammation is to eliminate of necrotic cells, damaged tissues, and injured cells and to start the tissue repairing process of the body [8]. The immune response of the body is not specific of a body to irritation, infection, and injury, which may be acute or chronic [9].
The clinical hallmarks of inflammations in Latin are rubor (redness), calor (warmth), tumor (swelling), and dolor (pain). The characteristics of inflammation were first described by the Roman physician Aulus Cornelius Celsus (Aurelius) [10]. The body response against the invading microbes or any other foreign substances is the innate immune system that is specific for specific microorganisms that enter into the body [11]. Inflammation is divided into two types: acute and chronic inflammation.
Presently, mild-to-moderate pain, inflammation, and pyrexia are managed with NSAIDs, but there are serious limitations of these therapies [12]. For example, there is well-documented evidence that continuous use of NSAIDs displays toxicity such as GIT ulceration, bleeding, perforation, cardiovascular disorders, and analgesic nephropathy. Hence, across the globe, extensive search is in progress in the area of medical management of pain and inflammation to find out new remedies [13]. This new drug discovery acts as alternative drugs to traditional analgesics including NSAIDs and narcotics.
Hebanaria genus is associated with the Orchidaceae family, which may consist of about 850 genera and 35000 species [14, 15]. Orchids were employed as the basis of conventional remedy for a very long time to cure various disorders such as arthritis, syphilis, stomach problem, acidity, tumor, jaundice, boils, inflammations, piles, hepatitis, malaria, blood dysentery, pyrexia, sexually transmitted ailments, tuberculosis, cholera, wounds, eczema, vermifuge, and diarrhea [16–18]. Bulbophyllum neilgherrense, a traditional therapeutic plant, has lately been assessed as a mediator of inflammation and analgesia [19]. The anti-inflammatory properties of orchids from South Africa have also been studied [20]. No scientific evaluation has been reported for the investigation of pharmacological features of H. plantaginea. In our previous study, we have also explored H. digitata, which belongs to the same family for its anti-inflammatory and analgesic potential [21].
We have deliberated this research work to explore the pyrexia, analgesia, and anti-inflammatory properties of H. plantaginea based on ethnomedicinal exploitation and previous literature assessment. Furthermore, we have indomitable phytochemicals via GC-MS methods. Evaluation of these unexplored plants can potentially lead to the generation of new molecules for drug development and better medicine.
2. Materials and Methods
2.1. Chemicals and Drugs
For anti-inflammatory in vitro assay, the enzyme COX-2 (Catalog no. C0858) and 5-LOX (Catalog no. 437996) were acquired from the Sigma-Aldrich GmbH, USA. The substrate arachidonic acid (CAT no. 150384), linoleic acid (CAS no. 60-33-3), and their cofactor solution materials TMPD (CAS no. 637-01-4), hematin (CAS no. 15489-90-4), and glutathione (CAS no. 70-18-8) were purchased from Sigma-Aldrich, Germany. Carrageenan (CAS no. 9064-67-7), naloxone, and AA (CAS No: 506-32-1) were also acquired from Sigma-Aldrich, Germany. Buffer solution containing KH2PO4 and K2HPO4 and solvents employed were of pure class. Celecoxib and Montelukast was purchased from Pfizer pharmaceutical and Libra (Pvt.) Limited. Tramadol and Diclofenac were purchased from Alliance Pharmaceuticals, Pakistan.
2.2. Plant Material, Collection, and Extraction
The H. plantaginea plant was collected from the Dir (L) KPK, Pakistan, in mid of April and then was identified via Prof. Muhammad Ilyas, Department of Botany, University of Swabi, Swabi KPK, Pakistan. The sample of the said plant was kept and recorded at herbarium having voucher number H.UOS.20-2. The aerial pieces of the plant (15 kg) were soaked with uncontaminated water and were sheltered desiccated for 21 days. The sheltered desiccated pieces of plants were firstly incised into minor pieces and then via grinder changed into coarse fine particles (7.5 kg). The pulverized substance was then macerated in the 26 L methanol (80%) for 21 days. Afterward, the entire substance was filtered using the muslin cloth and consequently by the Whatman filter paper. Then, the filtrate was transferred to a rotary evaporator (40°C) for further extraction [2, 22]. The last gloomy green color hard methanolic extort of H. plantaginea was attained weighing 650 g.
2.3. Fractionation
Methanol extort was decanted quietly in the separating funnel having a closed stopper. The Hp. Cr was mixed with the same amount of 500 ml hexane and water. The separating funnel was shacked vigorously for proper mixing of all the ingredients and then reserved at the correct position via a stand to prepare two separate layers: the n-hexane and water layer. The layer of n-hexane was alienated. A similar process was repeated two times through 500 ml of hexane. The organic layers which were separated three times were then mixed and concentrated in decreased pressure via rotary evaporator having a temperature of 40°C. The obtained concentrated weight of Hex. was 27.6 g. Similarly, the identical protocol was applied with the further solvents via raising the polarization of solvents. Following solvent fractions acquired was basically of ethyl acetate, chloroform, and Bt., having weights of 30, 42, and 94 g correspondingly. In last, the aqueous stratum was concentrated with a weight of 140 g [23].
2.4. GC-MS Analysis (Phytochemistry)
The tandem gas chromatography/mass spectrometry procedure of crude extract was executed by Agilent USB:393752 gas chromatograph (Agilent Technologies, Palo Alto, CA, USA) having an HHP-5MS 5% phenyl methyl siloxane tubular-column (30.0 m × 0.25 mm × 0.25 μm film thickness: Restek, Bellefonte, PA, USA) prepared through the Agilent HP; 5973 mass selective detector having the effective mode of electron (Ionization energy; 70 eV) performance in the parallel investigational ambiance as exemplification, intended for gas chromatography [10, 24].
2.5. In Vitro Pharmacological Activities
2.5.1. COX-2 Activity
The in vitro COX-2 scavenging activity was conceded through the previous explained normal protocol [25]. The COX-2 solution of the enzyme was equipped to have 300 U ml−1 concentrations. For enzyme activation, the enzyme solution (10 μl) was placed on ice for 5 minutes. Likewise, total of 50 μl cofactor mixture with 0.9 mM glutathione, 1 mM hematin, and 0.24 mM TMPD within 0.1 M Tris HCl buffer (pH 8.0) was added to a mixture of the solution of enzyme. Consequently, plant samples (20 μl) including different concentrations (1000–31.25 μg ml−1) along with 60 μl of the solution of the enzyme were placed at room temperature for five minutes. Similarly, 30 mM AA (20 μl) was added for starting the reaction. After that, the solution mixture was incubated for 5 min. After the incubation, the absorbance of the solution mixture was recorded at 570 nm using UV-visible spectrophotometer. The COX-2 enzyme inhibition was indomitable from per unit time of absorbance value. The IC50 values were resolute by plotting the reticence of enzyme beside different test fraction concentrations. Celecoxib was employed as a positive control (standard drug).
2.5.2. 5-LOX Assay
The inhibitory potential of 5-LOX on the H. plantaginea various fractions was carried out as per the previously reported procedure. Firstly, various dilutions were made ranging from 31.25 to 1000 μg/mL. Afterward, the 5-LOX enzyme having 10,000 U ml−1 solutions was prepared. Linoleic acid (80 mM) was used as a substrate in this assay. Likewise, phosphate buffer (50 mM) was ready with 6.3 pH. The various fractions of the plant samples were mixed in phosphate buffer solution and lipoxygenase enzyme (250 μl each) was mixed with it and incubated for 5 minutes at normal room temperature. Then, 0.6 mM of substrate solution (1000 μl) was added with that of the solution containing enzyme and shaken; after that, absorbance was deliberated at 234 nm. The experimental procedures were carried out thrice. In our activity, the standard drug employed was zileuton [26]. The % inhibition was calculated by the following equation:
| (1) |
2.6. In Vivo Pharmacological Activities
2.6.1. Animals
Albino mice of either sex were used in all experimental work and all experiments were conducted in the time range from 8.00 am to 5.00 pm. Food and water were available to all animals. The light-and-dark cycle was conserved with a temperature of 22 ± 2°C and which has an exhaust fan facility. At the end of all experimental processes, animals were sacrificed by scheduled 1 method and appropriately disposed of according to ethical guidelines of the institution. The experimental albino mice were employed as per authorization of the ethical board having letter number: UOS. Ph. 40121 Department of Pharmacy, University of Swabi, Pakistan.
2.6.2. Acute Toxicity
The acute toxicity was carried out on investigational albino mice. All animals were alienated into different sets having test and control groups. Every group has 5 tested animals. The H. plantaginea tested samples were administered per oral at various doses (25 to 2000 mg kg−1) according to body weight. For the dose preparation, Tween-80 was employed. After administration of various doses, the experimental mice were pragmatic for up to 3 days for any minor allergic reactions and abnormal behavior [27].
2.6.3. Antinociceptive Activity
We used two standard models for the evaluation of antinociceptive activity in mice: acetic acid-induced writhes and hot plate models [28, 29].
(1) Acetic Acid-Induced Writhes. Animals were deprived of food and water for 2 hours before starting the experimental procedure. Animals were divided into various groups. The acetic acid at a dose of 10 ml/kg (1%) was administered intraperitoneally into the animal, which leads to constrictions of the abdomen. This constriction was counted for 20 minutes [23]. Diclofenac sodium at a dose of 50 mg/kg was taken as a reference standard. Crude extract and their subsequent fractions were given at doses of 100 mg/kg, while 0.9% sodium chloride was taken as a control group. The standard drug, test drug, and normal saline were administered intraperitoneally to different groups of animals 30 minutes before the administration of acetic acid.
(2) Hot Plate Test. All the animals were habituated to the laboratory environment at least 2 hours before experimental procedures. Analgesiometer was used for the evaluation of analgesic activity on hot plate [30]. The hot plate temperatures were kept at 54.0 ± 0.1°C. Animals were subjected to pretest and all those animals that show latency time less than 30 seconds were selected. The selected animals were then arranged into various groups like normal saline group, standard group, and other potent fractions of the tested samples. The normal saline, standard drug, and various fractions of the test sample were administered intraperitoneally. The latency time of animals on the hot plates at intervals of 30, 60, and 90 minutes was calculated.
(3) Effect of Naloxone on Antinociceptive Activity of Various Fractions on Hot Plate Model Experimental Protocol. For the evaluation of the mechanism of the H. plantaginea, naloxone and tramadol were given to the animals. The animals were divided into ten groups, each group containing six animals (n = 6). All the animals were acclimatized to the laboratory environment 1 hour before the start of experimental work. In this study, albino mice of either sex were used through experiments and are exposed to the hot plate having a temperature of 54.0 ± 0.1°C. All the animals were subjected to pretest. All those animals were selected which shows latency time less than 30 seconds and the other animals were rejected to circumvent tissue damage [29]. After completion of the pretest, tramadol at a dose of 5 mg/kg was injected intraperitoneally and the latency time was recorded at 30, 60, and 90 minutes. In the antagonistic activity, naloxone at a dose of 1 mg/kg was administered subcutaneously 10 minutes before tramadol administrations [31].
2.6.4. Anti-Inflammatory Activity
(1) Carrageenan-Induced Inflammation. Carrageenan-induced paw edema was used for evaluation of anti-inflammatory activity [32]. All the animals of either sex were arranged into five groups each having six animals. All the animals were fasted overnight but have free excess to water. Diclofenac sodium (50 mg/kg) as a standard drug, normal saline as a control group, and various fractions at a dose of 100 mg/kg were administered 30 minutes before administration of 0.05 ml of 1% carrageenan into the subplantar area of the paw. A high-sensitivity instrument digital plethysmometer was used for measuring a small volume change in hid paw developed in the form of edema after injection of carrageenan. Reading was taken on a plethysmometer before and after carrageenan administrations up to 5 hours at an interval of 1 hour.
2.6.5. Antipyretic Activity
(1) Brewer's Yeast-Induced Pyrexia. For the evaluation of antipyretic study, Brewer's yeast model was used in which 20% Brewer's yeast was injected subcutaneously leading to hyperpyrexia [33]. All the animals were fasted overnight having free excess to water only and are arranged into various groups, each group containing 6 animals. With the help of a digital thermometer, rectal temperatures were recorded after 24-hour administration of Brewer's yeast. All those animals were selected which shows the rise in temperature up to 0.3–0.5°C. Group 1 received normal saline at a dose of 10 ml/kg, group 2 received standard drug at a dose of 150 mg/kg, and groups 3, 4, and 5 received test drug at a dose of 100 mg/kg body weight intraperitoneally. Rectal temperature was recorded after administration of all groups at an interval of 30 minutes, 60 minutes, and 90 minutes. Lubricants such as olive oil were used for insertion into the animal rectum and were maintained for 30 seconds for temperature record (Figure 1).
Figure 1.
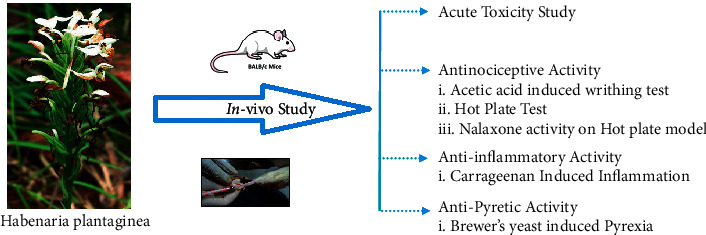
Schematic diagram of the in vivo experimental animal design.
2.6.6. Analysis of Data
The results of all the experimental works were demonstrated as mean ± SEM of all the groups having six animals. The IC50 values were calculated through SPSS software. One-way ANOVA statistical tools were used for the assessment and differences of various means, which follow two-way ANOVA followed by Bonferroni's posttest by using GraphPad Prism version 5. During statistical analysis, the value of P that is less than 0.05 was considered to be statistically significant.
3. Results
3.1. GC-MS Analysis
The GC-MS analysis of Hp. Cr was executed and recognized twenty-two (22) molecules in it. The structure of all the known molecules is exhibited in Figure 2. GC-MS identification depends on the corresponding of spectral peak, the fragmentation pattern of its peak and mass through the identified library is established inside the apparatus. For that reason, occasionally it is probably because of the fact that basic two molecules will have the same fragmentation pattern and mass spectra, but it is not an ordinary case. Furthermore, all the details about the new molecule present inside of plant were not installed in GC-MS library, so there is the probability of not identifying those new molecules. Full details of GC-MS analysis are summarized in Table 1. The compounds 2, 4, 5 were previously reported for their anti-inflammatory potential while compound 6 was reported as antihemorrhagic, analgesic, diuretic, antipyretic, and insecticide activities.
Figure 2.
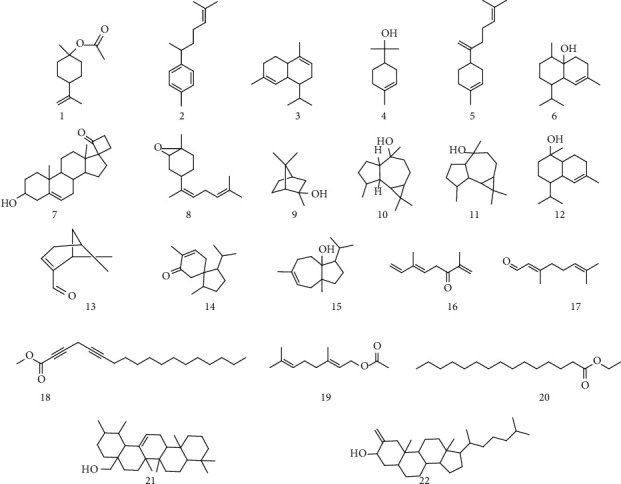
Identified compounds' structures in Habenaria plantaginea.
Table 1.
GC-MS details of identified compounds.
| S. no. | Chemical name | Common name/synonym | Formula |
|---|---|---|---|
| 1 | 1-Methyl-4-(prop-1-en-2yl)cyclohexyl acetate | Beta-terpinyl acetate | C12H20O2 |
| 2 | 1-Methyl-4-(6-methyl hept-5-en-2-yl)benzene | Alpha-curcumene | C15H22 |
| 3 | 1-Isopropyl-4,7-dimethyl-1,2,4a,5,6,8a-hexahydronaphthalene | Not identified | C15H24 |
| 4 | 2-(4-Methylcyclohex-3-enyl)propan-2-0l | Alpha-terpineol | C10H18O |
| 5 | 1-Methyl-4-(6-methylhepta-1,5-dien-2-yl)cyclohex-1-ene | β-Bisabolene | C15H24 |
| 6 | 1-Isopropyl-4,7-dimethyl-1,2,3,4,4a,5,6,8a-octahydronaphthalen-4a-ol | Cadina-1(6),4-diene | C15H26O |
| 7 | Spiro[androst-5-ene-17,1′cyclobutan]-2′one,3-htdroxy- | Not identified | C22H32O2 |
| 8 | (Z)-1-Methyl-4-(6-methylhepta-2,5-dien-2-yl)-7-oxa-bicyclo[4.1.0]heptane | Not identified | C15H24O |
| 9 | 2,7,7-Trimethylbicyclo[2.2.1]heptan-2-ol | Isoborneol | C10H18O |
| 10 | 1,1,4,7-Tetramethyl-decahydro-1H-cyclopropa[e]azulen-4-0l | Viridiflorol | C15H26O |
| 11 | 1,1,4,7-Tetramethyl-decahydro-1H-cyclopropa[e]azulen-4-0l | Globulol | C15H26O |
| 12 | 4-Isopropyl-1,6-dimethyl-1,2,3,4,4a,7,8,8a-octahydronaphthalen-1-ol | δ-Cadinol | C15H26O |
| 13 | 4,6-Dimetylcyclohex-1-enecarbaldehyde | Not identified | C9H14O |
| 14 | Spiro [4.5]dec-6-en-8-0ne, 1,7-dimethyl-4-(1-methylethyl)- | Acorenone 1 | C15H24O |
| 15 | (Z)-3-isoropyl-6,8 a-dimethyl-1,2,3,3a,4,5,8,8a-octahydroazulen-3a-ol | Bullnesol | C15H26O |
| 16 | (E)-3,7-dim ethylocta-1,5,7-trien-3-one | Hotrienol | C10H14O |
| 17 | (E)-3,7-dimethylocta-2,6-dienal | Not identified | C10H16O |
| 18 | Methyl octadeca-2,5-diynoate | Methyl 2,5-octadecadiynoate | C19H30O2 |
| 19 | (E)-3,7-dim ethylocta-2,6-dienyl acetate | 1-Octanol | C12H20O2 |
| 20 | Ethyl pentadecanoate | n-Pentadecanoic acid ethyl ester | C17H34O2 |
| 21 | (1,2,6a,6b,9,9,12a-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-icosahydropicen-4a yl)methanol | Not identified | C30H50O |
| 22 | 10,13-Dimethyl-2-methylene-17-(6-methylheptan-2-yl)-hexadecahydro-1H-cyclopenta[a]phenanthren-3-ol | Not identified | C28H48O |
3.2. In Vitro Pharmacological Activates
3.2.1. Cox-2 Assay
In this assay, various fractions like Hp. Chf and Hp. EtAc exhibited excellent COX-2 inhibition as shown in Table 2. Hp. Chf showed that the highest COX-2 inhibitory potential observed was 77.40 ± 0.25,72.41 ± 0.30, 65.79 ± 1.28, 61.32 ± 0.68, and 56.49 ± 0.73%; activity was monitored for the fraction Hp. Chf at concentration of 1000, 500, 250, 125, and 62.5 μg mL−1, respectively, with IC50 value of 33.81 μg mL−1. Likewise, Hp. EtAc exhibited second highest inhibitory potential observed: 76.38 ± 0.76, 69.37 ± 0.52, 62.90 ± 1.16, 54.48 ± 0.54, and 45.56 ± 0.69 with IC50 value 87.56 μg mL−1, respectively. Celecoxib demonstrated 84.51 ± 0.30, 77.84 ± 0.27, 73.50 ± 2.26, 65.74 ± 0.16, and 61.56 ± 0.28 with IC50 value 23.30 μg mL−1 respectively. All the other fractions displayed good-to-moderate activity in this assay.
Table 2.
Results of in vitro cyclooxygenase and lipoxygenase inhibitory activity.
| Name | Concentration | COX-2% inhibition (mean ± SEM) | COX-2 IC50 (μg/ml) | 5-LOX % inhibition (mean ± SEM) | 5-LOX IC50 (μg/ml) |
|---|---|---|---|---|---|
| Hp. Chf | 1000 | 77.40 ± 0.25∗∗∗ | 33.81 | 81.73 ± 0.37∗∗∗ | 26.74 |
| 500 | 72.41 ± 0.30∗∗ | 75.27 ± 1.37∗∗∗ | |||
| 250 | 65.79 ± 1.28∗∗∗ | 69.62 ± 0.11∗∗∗ | |||
| 125 | 61.32 ± 0.68∗ | 63.81 ± 0.51∗∗∗ | |||
| 62.5 | 56.49 ± 0.73∗∗ | 59.08 ± 0.12∗∗∗ | |||
| Hp. EtAc | 1000 | 76.38 ± 0.76∗∗∗ | 87.56 | 80.47 ± 0.70∗∗∗ | 67.51 |
| 500 | 69.37 ± 0.52∗∗∗ | 73.57 ± 0.43∗∗∗ | |||
| 250 | 62.90 ± 1.16∗∗∗ | 65.12 ± 0.94∗∗∗ | |||
| 125 | 54.48 ± 0.54∗∗∗ | 57.76 ± 1.09∗∗∗ | |||
| 62.5 | 45.56 ± 0.69∗∗∗ | 49.38 ± 0.50∗∗∗ | |||
| Hp. Cr | 1000 | 65.94 ± 0.71∗∗∗ | 200 | 71.50 ± 0.56∗∗∗ | 106.99 |
| 500 | 58.28 ± 0.54∗∗∗ | 65.40 ± 0.55∗∗∗ | |||
| 250 | 52.65 ± 0.91∗∗∗ | 59.36 ± 0.57∗∗∗ | |||
| 125 | 45.30 ± 0.55∗∗∗ | 51.30 ± 0.52∗∗∗ | |||
| 62.5 | 37.63 ± 0.98∗∗∗ | 44.37 ± 0.58∗∗∗ | |||
| Hp. Hex | 1000 | 64.55 ± 0.51∗∗∗ | 217.93 | 66.42 ± 0.46∗∗∗ | 171.05 |
| 500 | 57.55 ± 0.67∗∗∗ | 60.53 ± 0.41∗∗∗ | |||
| 250 | 51.40 ± 0.44∗∗∗ | 52.68 ± 0.64∗∗∗ | |||
| 125 | 45.67 ± 0.55∗∗∗ | 47.46 ± 0.47∗∗∗ | |||
| 62.5 | 37.33 ± 0.62∗∗ | 40.51 ± 0.62∗∗∗ | |||
| Hp. Bt | 1000 | 60.35 ± 0.51∗∗∗ | 438.39 | 63.45 ± 0.59∗∗∗ | 328.34 |
| 500 | 51.27 ± 0.58∗∗∗ | 55.49 ± 0.60∗∗∗ | |||
| 250 | 43.41 ± 0.55∗∗∗ | 46.23 ± 0.44∗∗∗ | |||
| 125 | 34.40 ± 0.76∗ | 37.50 ± 0.61∗∗ | |||
| 62.5 | 27.24 ± 0.80∗ | 31.47 ± 0.46∗ | |||
| Hp. Aq | 1000 | 68.83 ± 1.07∗∗∗ | 141.2 | 72.37 ± 0.54∗∗∗ | 132.27 |
| 500 | 61.39 ± 0.60∗∗∗ | 64.00 ± 0.20∗∗∗ | |||
| 250 | 56.58 ± 0.56∗∗∗ | 57.15 ± 0.91∗∗∗ | |||
| 125 | 49.29 ± 0.43∗∗∗ | 51.15 ± 0.61∗∗∗ | |||
| 62.5 | 41.37 ± 0.58∗∗∗ | 40.40 ± 0.68∗∗∗ | |||
| Celecoxib | 1000 | 84.51 ± 0.30 | 23.20 | — | — |
| 500 | 77.84 ± 0.27 | ||||
| 250 | 73.50 ± 2.26 | ||||
| 125 | 65.74 ± 0.16 | ||||
| 62.5 | 61.56 ± 0.28 | ||||
| Montelukast | 1000 | — | — | 87.66 ± 0.45 | 17.47 |
| 500 | 81.64 ± 0.42 | ||||
| 250 | 76.01 ± 1.61 | ||||
| 125 | 70.46 ± 0.32 | ||||
| 62.5 | 64.50 ± 0.02 |
The values are presented as mean ± SEM (n = 5). The asterisk shows that the significance levels in comparison with that of the negative control. Data were analyzed via two-way ANOVA followed by Bonferroni's posttest. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001.
3.2.2. 5-LOX Assay
In this assay, Hp. Chf exhibited highest activity against with 81.73 ± 0.37, 75.27 ± 1.37, 69.62 ± 0.11, 63.81 ± 0.51, and 59.08 ± 0.12 percent inhibition at concentration of 1000, 500, 250, 125, and 62.5 μg mL−1, respectively. The IC50 value measured from the dose-response curve was 26.74 μg mL−1. Similarly, Hp. EtAc exhibited 80.47 ± 0.70, 73.57 ± 0.43, 65.12 ± 0.94, 57.76 ± 1.09, and 49.38 ± 0.50 with IC50 value 67.51 μg mL−1, respectively. The standard drug linoleic acid displayed 87.66 ± 0.45, 81.64 ± 0.42, 76.01 ± 1.61, 70.46 ± 0.32, and 64.50 ± 0.02 inhibition at concentration ranging from 1000 to 62.5 μg mL−1, respectively, attaining the IC50 value of 17.47 μg mL−1 as shown in Table 2.
3.3. In Vivo Pharmacological Activities
3.3.1. Acute Toxicity Result
In the acute toxicity studies, no mortality and no changes in behavior were noticed in the investigational mice. Based on the observation in the acute toxicity result, a dose of 2000 mg/kg was measured as the safest dose. The information of the doses specified to mice is presented in Table 3.
Table 3.
Group of animals and drug quantities are given for acute toxicity studies with various fractions of H. plantaginea.
| Groups | Animals | Conc. (μg/mL) |
|---|---|---|
| 1 | 5 | 25 |
| 2 | 5 | 50 |
| 3 | 5 | 100 |
| 4 | 5 | 200 |
| 5 | 5 | 300 |
| 6 | 5 | 400 |
| 7 | 5 | 500 |
| 8 | 5 | 1000 |
| 9 | 5 | 2000 |
3.3.2. Evaluation of Antinociceptive Activity
(1) Acetic Acid-Induced Writhing Activity. A significant decrease in the number of writhing was observed in animals treated with standard drug (50 mg/kg I/P) as compared to the normal saline control group. The number of writhes exhibited by the standard group is 10.33 with 74.70% of inhibition, while the Hp. Chf and Hp. EtAc have also reversed the effect of acetic acid and displayed number of writhes 16.17 ± 0.10 and 11.67 ± 0.60 (P < 0.001) with 60.39 ± 0.42, and 71.42 ± 0.55% of inhibition, respectively, at a dose of 100 mg/kg I/P (Figure 3).
Figure 3.
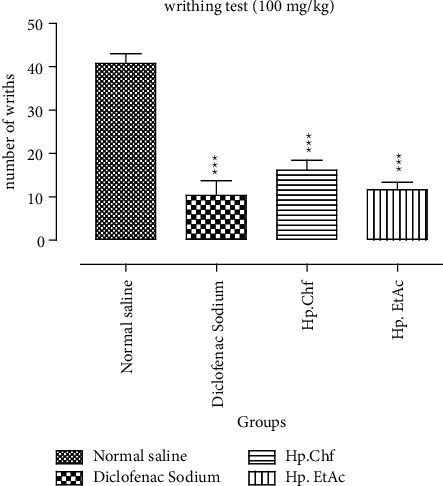
Analgesic activities through acetic acid-induced writhing potential in mice. The data were expressed as means ± SEM (n = 6). Data were analyzed via two-way ANOVA followed by Bonferroni's posttest. ∗∗∗P < 0.001. ###Comparison of standard drug to the normal saline group.
(2) Evaluation of Antinociceptive Activity on Hot Plate Model after 30 Minutes. A significant increase in the latency time was observed in animals treated with standard drug (tramadol 5 mg/kg I/P) as compared to the vehicle control group. The latency time after 30 minutes observed in the positive control group was 17.23 with 47.59 ± 0.71% analgesic activity at 100 mg/kg body weight. The Hp. Chf fraction of the plant has reversed the latency time 14.33 ± 0.44 (P < 0.01) with 36.99 ± 0.94% inhibition of thermal stimuli significantly at the dose of 100 mg/kg I/P. The Hp. EtAc also reversed the effect significantly causing 29.62 ± 0.84 (P < 0.05) % inhibitions at the dose of 100 mg/kg (Table 4).
Table 4.
Antinociceptive activity of H. plantaginea assessed using the hot plate test.
| Treatment | Dose (mg/kg) | Latency time in seconds (mean ± SEM) | ||
|---|---|---|---|---|
| After 30 min | After 60 min | After 90 min | ||
| Normal saline | 10 ml/kg | 9.03 ± 0.40 | 10.05 ± 0.30 | 8.67 ± 0.65 |
| Standard | 5 mg/kg | 17.23 ± 0.33∗∗∗ | 19.56 ± 0.52∗∗∗ | 13.27 ± 0.25∗∗∗ |
| Hp. Chf | 100 mg/kg | 14.33 ± 0.44∗∗ | 16.17 ± 0.42∗∗ | 11.25 ± 0.52∗∗ |
| Hp. EtAc | 100 mg/kg | 12.83 ± 0.52∗ | 14.00 ± 0.10∗∗∗ | 8.83 ± 0.80∗ |
The data represent analgesic activities through hot plate test in mice. The data were expressed as means ± SEM (n = 6) analyzed via two-way ANOVA followed by Bonferroni's posttest. ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001; and ns: not significant.
(3) Evaluation of Antinociceptive Activity on Hot Plate Model Result after 60 Minutes. A significant increase in the latency time was observed in animals treated with standard drug (tramadol 5 mg/kg I/P) as compared to the vehicle control group after 60 minutes (P < 0.001). The fraction Hp. Chf has reversed the analgesic potential causing 44.16 ± 0.70% inhibition (P < 0.01) significantly at the dose of 100 mg/kg I/P. The tested fraction Hp. EtAc having latency time in 14 seconds, which has also reversed the good effect of thermal stimuli with 35.50 ± 0.50% inhibition (P < 0.05) significantly at the dose of 100 mg/kg (Table 4).
(4) Evaluation of Antinociceptive Activity on Hot Plate Model Result after 90 Minutes. A significant increase in the latency time was observed in animals treated with standard drug (tramadol 5 mg/kg I/P) as compared to the vehicle control group with latency time 13.27 (P < 0.001) causing 34.69 ± 0.56 of % inhibition. The Hp. Chf fractions reversed the pain effect significantly with latency time 11.25 (P < 0.01) causing percent analgesic inhibitions 22.93 ± 0.60 at the dose of 100 mg/kg I/P. The Hp. EtAc fraction displayed a latency time of 8.8 causing a very low effect as contrasted to the negative control at the same dose. Results were shown in Table 4.
(5) Mechanistic Antinociceptive Activity of Various Fractions on Hot Plate Model. It depicts the tramadol antinociceptive activity, which shows that standard drug morphine (5 mg/kg) possesses a significant result, 34.10 ± 0.20 (P < 0.001) % inhibition; the tested fraction Hp. Chf at a dose of 100 mg/kg causes 58.23 ± 0.44 (P < 0.001) % inhibition while Hp. EtAc exhibited significant result 43.39 ± 0.52 (P < 0.001). Table 5 represents the effects of naloxone after 30, 60, and 90 minutes in hot plate test. The data were expressed as means ± SEM (n = 6).
Table 5.
Results of analgesic activity following hot plate model and opioid receptors evaluation study.
| Samples | Dose (mg/kg) | Reaction time on hot plate | ||
|---|---|---|---|---|
| 30 min | 60 min | 90 min | ||
| Normal saline | 10 ml/kg | 10.12 ± 0.42 | 10.12 ± 0.57 | 10.12 ± 0.33 |
| Normal saline + NLX | 10 ml/kg + 1 | 10.14 ± 0.71 | 10.14 ± 0.47 | 10.14 ± 0.60 |
| Morphine | 5 | 21.64 ± 0.59 | 23.64 ± 0.70 | 19.64 ± 0.60 |
| Morphine + NLX | 5 + 1 | 14.26 ± 0.94 | 16.60 ± 0.66 | 12.40 ± 0.88 |
| Hp. EtAc | 100 | 19.96 ± 0.05 | 22.26 ± 0.04 | 24.80 ± 0.07 |
| Hp. EtAc + NLX | 100 + 1 | 11.30 ± 0.03 | 13.70 ± 0.09 | 16.60 ± 0.48 |
| Hp. Chf | 100 | 20.42 ± 0.08 | 25.60 ± 0.05 | 28.10 ± 0.55 |
| Hp. Chf + NLX | 100 + 1 | 8.53 ± 0.30 | 9.98 ± 0.59 | 14.40 ± 0.71 |
While after 60 minutes, the standard drug tramadol possesses a significant result (P < 0.01). Hp. Chf and Hp. EtAc fractions displayed dose-dependent result causing 61.01 ± 0.45 (P < 0.001) and 38.45 ± 0.50 (P < 0.01) analgesic effect at the dose of 100 mg/kg. Similarly, after 90 minutes, tramadol again exhibited a significant result (P < 0.01), while tested fraction Hp. EtAc has 33.06 ± 0.33 (P < 0.01)% potential. Likewise, tested fraction Hp. Chf at 100 mg/kg body weight exhibited dose-dependent results as that f the standard drug causing 48.75 ± 0.56 (P < 0.001)% inhibitions.
3.3.3. Evaluation of Anti-Inflammatory Activity Test Results
A significant decrease in paw volume was observed in animals treated with standard drug (diclofenac sodium 10 mg/kg I/P) as compared to the vehicle control group. The percent increase in paw volume in the positive control group is decreased from the first 1st to 5th h with 30.01 ± 0.10 to 23.20 ± 0.40 (P < 0.001) causing 34.76 ± 0.56 to 55.38 ± 0.80% edema volume inhibition from the 1st to 5th hours, respectively. The Hp. Chf displayed 32.61 ± 0.88 percent inhibition at the 1st h and till significant 46.15 ± 0.10 at the 5th h. The next most potent activity was shown by Hp. EtAc at the 4th h (43.40 ± 0.42) after carrageenan induction (Figure 4).
Figure 4.
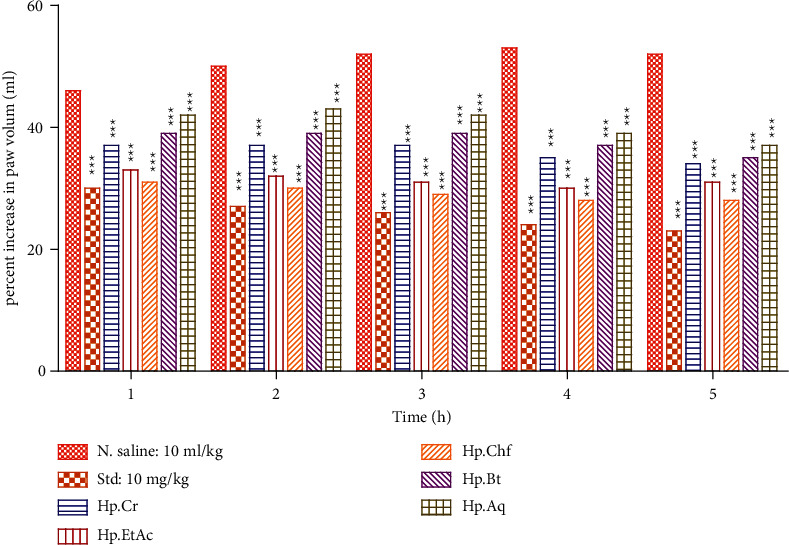
Carrageenan-induced anti-inflammatory potential in mice. The data were expressed as means ± SEM (n = 6). Data were analyzed via two-way ANOVA followed by Bonferroni's posttest. ∗∗∗P < 0.001.
3.3.4. Evaluation of Brewer's Yeast-Induced Antipyretic Activity Result
The subcutaneous injection of yeast suspension noticeably elevated the rectal temperature after administration. A significant decrease in rectal volume was observed after 30, 60, and 90 minutes with 34.20 ± 0.42, 33.02 ± 0.10, and 35.50 ± 0.52°C (P < 0.001) in animals treated with standard drug (50 mg/kg I/P) as compared to the vehicle control group. The tested fraction Hp. Chf also reduced the rectal temperature significantly at 30, 60, and 90 minutes causing 34.10 ± 0.56, 32.88 ± 0.66, and 34.10 ± 0.20°C (P < 0.001) at 100 mg/kg I/p. The other fraction like Hp. EtAc also revealed good reduction in rectal volume after 60 minutes, 36.20 ± 0.40°C (P < 0.01) (Figure 5).
Figure 5.
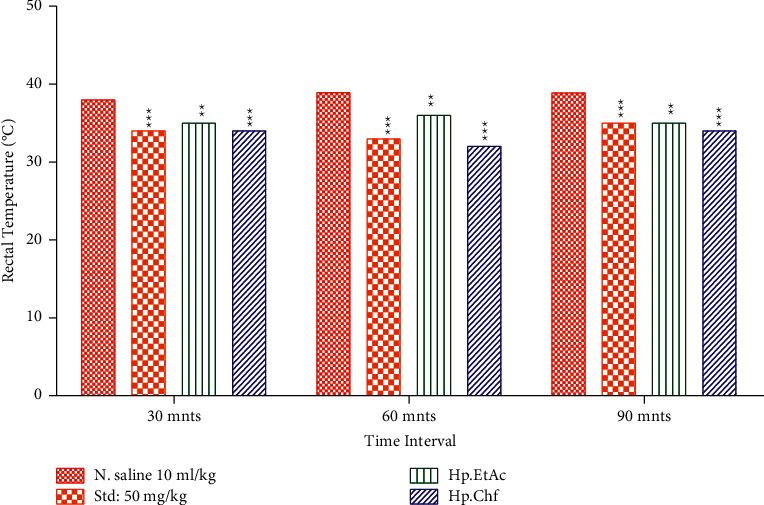
Anti-inflammatory activities through Brewer's yeast-induced pyrexia in mice. The data were expressed as means ± SEM (n = 6). Data were analyzed via two-way ANOVA followed by Bonferroni's posttest. ∗∗∗P < 0.001.
4. Discussion
Currently, several investigational drugs have been developed by scientists to control pain and inflammation but still the therapy is not completely satisfactory [34, 35]. Due to a considerable increase in the field of medicine, disease and disorder treatment with new inventions increase tremendously. The evolutions of synthetic compounds and alternate medicine are searching out in which the possibilities of adverse effects should be minimized as compared to existing drugs. For the treatment and management of pain, inflammation, and pyrexia, the use of NSAIDs leads to severe abnormalities and complications which causes cardiac and kidney abnormality, gastric and intestinal bleeding, and so on (Wolfe et al., 1999) [27]. The human COX enzyme is a dimer of COX-1 and COX-2 subunits and catalyzes the oxidation of AA to generate PGG2 followed by reduction into PGH2 [36]. The prostaglandins PGG2 and PGH2 are the precursors of signaling molecules in various diseases including inflammation, cardiovascular problems, and cancer [37]. The catalytic domain of each subunit of the dimer comprises cyclooxygenase and peroxidase active sites on either side of the heme prosthetic group [38]. The nonselective NSAIDs target the COX-1 and COX-2 enzymes to block the formation of inflammatory signaling precursors leading to acute inflammation, cancer, and cardiovascular diseases [39]. For example, aspirin acetylates Ser-530 on the cyclooxygenase active site and inactivates the enzyme by interfering with the binding of AA to Ser-530 in each of the two subunits [40].
For the evaluation of antinociceptive activity acetic acid-induced writhing model, pain in the peripheral origin is induced through the injection of irritant chemicals, such as acetic acid [41]. In the activity of abdominal constrictions, which are induced by acetic acid, the synthesis of prostaglandins plays a key role [42] through the action of the essential enzymes such as cyclooxygenase-II, which cause an increase in pain perception at sensory nerve endings. Perception of pain through COX pathway and activation of the sensory pathways in the peritoneum of mice encourage abdominal constrictions and a viscerosomatic reflex was observed in response to acetic acid (algesiogenics) [43]. At the terminal of the abdominal peritoneum sensory afferents, neurons possess adrenergic receptors like α-adrenoceptors, β-adrenoceptors, and some opioid receptors. The generations of pain impulses become depressed with the activation of these receptors by the appropriate agonists but in the mice peritoneum, an interaction was found between opioid receptors and α-adrenoceptors [44, 45]. The central and peripheral processes are involved in abdominal constrictions [46].
The inflammatory process along with neurons of the dorsal horn is activated in the later phase of nociception [47]. It was suggested that in hot plate test determination of central pain mechanisms the nociceptive responses are integrated supraspinally [48].
In Figure 1, the study of antinociceptive activity on mice was evaluated through acetic acid induced writhing model, which shows a significant decrease in the number of writhes in animals treated with standard drug being 10.33 with 74.70% of inhibition at a dose of 50 mg/kg I/P. The potent fractions of H. plantaginea like Hp. Chf and Hp. EtAc at the dose of 100 mg/kg body weight I/p show a significant effect with the number of writhes: 16.17 ± 0.10 and 11.67 ± 0.60 causing 60.39 ± 0.42 and 71.42 ± 0.55% of inhibition, respectively (P < 0.001), in acetic acid-induced analgesia. Hot plat test has been performed to distinguish whether the various fractions have a central or peripheral antinociceptive effect. Standard drug shows a significant 47.59 ± 0.71 %inhibition (P < 0.001) resulting in prolonging the latency time at various intervals while in tested fractions Hp. Chf exhibited a significant result causing 36.99 ± 0.94 and 44.16 ± 0.70 (P < 0.01) after 30 and 60 minutes at a dose of 100 mg/kg I/P in hot plate evaluation test. The tramadol antagonistic nociceptive activity was evaluated by utilizing the hot plate test [49]. The antinociceptive effect of tramadol (5 mg/kg) was reversed significantly by opioid antagonist naloxone (1 mg/kg) causing 34.10 ± 0.20 (P < 0.01), and the Hp. EtAc and Hp. Chf fractions have shown a significant result: 43.39 ± 0.52 (P < 0.01) and 29.76 ± 0.66 (P < 0.01) percent inhibition after 30 minutes at a dose of 100 mg/kg, respectively.
For the evaluation of antipyretic activity, fungal pyrogens present in Brewer's yeast induce pyrexia in rodents. Recommended guidelines for the evaluation of antipyretic activities show that Brewer's yeast should be administered subcutaneously [50]. Upon administration of Brewer's yeast, several inflammatory mediators such as transcription factors, cytokines like IL-6 and TNF-, and enzymes involved in the synthesis of PGE2 are released [51]. The retardations of these mediators are accountable for the antipyretic effects (Rawlins, 1973). The cause of pyrexia induced with the administration of Brewer's yeast may be due to the production of prostaglandins [52]. Upon administration of various reactions of the H. plantaginea at high doses such as 100 mg/kg on the base of body weight I/P and standard drug paracetamol the rectal temperature of mice were significantly at 30, 60, and 90 minutes with 34.20 ± 0.42, 33.02 ± 0.10, and 35.50 ± 0.52°C (P < 0.001) reduced, which shows that the tested fraction possesses antipyretic activity. The possible mechanism of the Hp. Chf (34.10 ± 0.56 at 30 minutes) fraction and standard drug paracetamol may be the inhibition of PGs, which shows its antipyretic activities.
The most common model used for the study of inflammation is carrageenan-induced paw edema [53]. The most important parameter of inflammation is the formation of edema, which is considered for the evaluation of anti-inflammatory activity of selected compounds [54]. PGE2 and Bradykinin release at the site of inflammation induced carrageenan [55, 56]. A biphasic response was investigated with the injection of carrageenan in the paw of the mice in which multiple mediators are released which leads inflammation (Cuzzocrea et al., 1999). This study shows that Hp. Chf fraction of H. plantaginea significantly displayed 32.61 ± 0.88 percent inhibition at the 1st h and till significant 46.15 ± 0.10 at the 5th h. The next potent activity was shown by Hp. EtAc at the 4th h (43.40 ± 0.42) after carrageenan induction. The results demonstrate that the both fractions affect the early and late phases of inflammation.
During the pain and inflammatory progression, the release of free radicals like ROS that cause the proinflammatory cytokines (IL1β, TNF-α, and IL-6), production of cell lyses, and COX and LOX expression are responsible for various diseases [57, 58]. The inhibitory result of the various fractions of H. plantaginea against COX-2 and 5-LOX enzyme was assessed that were extensively employed to conclude the anti-inflammatory capacity of the fractions [59] and is generally employed for the evaluation of analgesic and anti-inflammatory potential of various fractions. Based on the result attained from the current study, the COX-2 and 5-LOX scavenging potential of Hp. Chf (IC50 33.81 and 26.74 μg/mL) exhibited comparable and dose-dependent results to that of standard drug celecoxib and montelukast (IC50 23.20 and 17.47 μg/mL) [60]. The in vitro analgesic and anti-inflammatory capacity of various fractions contribute to strengthening the anti-inflammatory, antipyretic, and analgesic potentials.
This study possesses significant importance in the development of new natural product research, which permits us to predict that the said plant has new drugs that contain antinociceptive, antipyretic, and anti-inflammatory properties. The natural products are free from common side effects possessed by traditional NSAIDs, which minimize economic burden and increase patient compliance. The current study possesses a base for the development of new drugs, which require detailed pharmacological study on different animal models.
5. Conclusions
Based on current results, it might be clear that H. plantaginea plant has anti-inflammatory potential and also possesses analgesic and antipyretic compounds. Further, H. plantaginea may be a good candidate for complementary and alternative therapy. This might be free from common side effects passed by traditional NSAIDs. This study also gives investigational and scientific justification for the ethnomedicinal use of H. plantaginea plant as analgesic, anti-inflammatory, and antipyretic.
Acknowledgments
The authors would like to express their gratitude and thanks to the Deanship of Scientific Research at Najran University for funding this paper under the code NU/-/MRC/10/375.
Abbreviations
- AA:
Arachidonic acid
- Aq:
Aqueous
- Bt:
Butanol
- Cr:
Crude extract
- Chf:
Chloroform
- COX:
Cyclooxygenase
- EtAc:
Ethyl acetate
- GC-MS:
Gas chromatography-mass spectroscopy
- GIT:
Gastrointestinal tract
- Hp:
Habenaria plantaginea
- IL-6:
Interleukine-6
- KH2PO4:
Potassium dihydrogen phosphate
- K2HPO4:
Dipotassium hydrogen phosphate
- LOX:
Lipoxygenase
- NSAIDs:
Nonsteroidal anti-inflammatory drugs
- PGH2:
Prostaglandin H2
- ROS:
Reactive oxygen species
- SEM:
Standard error means
- TMPD:
N,N,N-Tetramethyl-p-phenylenediamine dihydrochloride
- TNF-α:
Tumor necrosis factor-alpha.
Data Availability
Data will be available from the corresponding author upon request.
Conflicts of Interest
There are no conflicts of interest declared by all the authors regarding the publication of this paper.
References
- 1.Kalra B., Kalra S., Bajaj S. Vulvodynia: an unrecognized diabetic neuropathic syndrome. Indian journal of endocrinology and metabolism . 2013;17:p. 787. doi: 10.4103/2230-8210.117193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shah S. M. M., Ullah F., Shah S. M. H., Zahoor M., Sadiq A. Analysis of chemical constituents and antinociceptive potential of essential oil of Teucrium Stocksianum bioss collected from the North West of Pakistan. BMC Complementary and Alternative Medicine . 2012;12:p. 244. doi: 10.1186/1472-6882-12-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeb A., Ahmad S., Ullah F., Ayaz M., Sadiq A. Anti-nociceptive activity of ethnomedicinally important analgesic plant Isodon rugosus Wall. ex Benth: mechanistic study and identifications of bioactive compounds. Frontiers in Pharmacology . 2016;7:p. 200. doi: 10.3389/fphar.2016.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ayaz M., Junaid M., Ullah F., et al. Molecularly characterized solvent extracts and saponins from Polygonum hydropiper L. show high anti-angiogenic, anti-tumor, brine shrimp, and fibroblast NIH/3T3 cell line cytotoxicity. Frontiers in Pharmacology . 2016;7:p. 74. doi: 10.3389/fphar.2016.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeb A., Ullah F., Ayaz M., Ahmad S., Sadiq A. Demonstration of biological activities of extracts from Isodon rugosus wall. ex benth: separation and identification of bioactive phytoconstituents by GC-MS analysis in the ethyl acetate extract. BMC Complementary and Alternative Medicine . 2017;17:1–16. doi: 10.1186/s12906-017-1798-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akbar S., Subhan F., Shahid M., et al. 6-Methoxyflavanone abates cisplatin-induced neuropathic pain apropos anti-inflammatory mechanisms: a behavioral and molecular simulation study. European Journal of Pharmacology . 2020;872 doi: 10.1016/j.ejphar.2020.172972.172972 [DOI] [PubMed] [Google Scholar]
- 7.Abdollahi M., Karimpour H., Monsef-Esfehani H. R. Antinociceptive effects of Teucrium polium L total extract and essential oil in mouse writhing test. Pharmacological Research . 2003;48(1):31–35. doi: 10.1016/s1043-6618(03)00059-8. [DOI] [PubMed] [Google Scholar]
- 8.Jan M. S., Shahid M., Ahmad S., et al. Synthesis of pyrrolidine-2, 5-dione based anti-inflammatory drug: in vitro COX-2, 5-LOX inhibition and in vivo anti-inflammatory studies. Latin American Journal of Pharmacy . 2019;38:2287–2294. [Google Scholar]
- 9.Munir A., Khushal A., Saeed K., et al. Synthesis, in-vitro, in-vivo anti-inflammatory activities and molecular docking studies of acyl and salicylic acid hydrazide derivatives. Bioorganic Chemistry . 2020;104 doi: 10.1016/j.bioorg.2020.104168.104168 [DOI] [PubMed] [Google Scholar]
- 10.Ayaz M., Junaid M., Ullah F., et al. GC-MS analysis and gastroprotective evaluations of crude extracts, isolated saponins, and essential oil from Polygonum hydropiper L. Frontiers in Chemistry . 2017;5:p. 58. doi: 10.3389/fchem.2017.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tong X., Li X., Ayaz M., et al. Neuroprotective studies on Polygonum hydropiper L. essential oils using transgenic animal models. Frontiers in Pharmacology . 2021:p. 2290. doi: 10.3389/fphar.2020.580069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jan M. S., Ahmad S., Hussain F., et al. Design, synthesis, in-vitro, in-vivo and in-silico studies of pyrrolidine-2, 5-dione derivatives as multitarget anti-inflammatory agents. European Journal of Medicinal Chemistry . 2020;186 doi: 10.1016/j.ejmech.2019.111863.111863 [DOI] [PubMed] [Google Scholar]
- 13.Khalil A. T., Ovais M., Iqbal J., et al. Seminars in Cancer Biology, Amsterdam, Netherlands: Elsevier; 2021. Microbes-mediated synthesis strategies of metal nanoparticles and their potential role in cancer therapeutics. [DOI] [PubMed] [Google Scholar]
- 14.Ahmad S., Mahnashi M. H., Alyami B. A., et al. Synthesis of michael adducts as key building blocks for potential analgesic drugs: in vitro, in vivo and in silico explorations. Drug Design, Development and Therapy . 2021;15:p. 1299. doi: 10.2147/dddt.s292826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chase M. W., Cameron K. M., Freudenstein J. V., et al. An updated classification of Orchidaceae. Botanical Journal of the Linnean Society . 2015;177:151–174. doi: 10.1111/boj.12234. [DOI] [Google Scholar]
- 16.Hossain M. M. Therapeutic orchids: traditional uses and recent advances—an overview. Fitoterapia . 2011;82:102–140. doi: 10.1016/j.fitote.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Kong J.-M., Goh N.-K., Chia L.-S., Chia T.-F. Recent advances in traditional plant drugs and orchids. Acta Pharmacologica Sinica . 2003;24:7–21. [PubMed] [Google Scholar]
- 18.Ramos P., Colareda G. A., Rosella M. A., Debenedetti S. L., Spegazzini E. D., Consolini A. E. Phytochemical profile and anti-inflammatory effect of the orchid Catasetum macroglossum. Latin American Journal of Pharmacy . 2012;31:62–67. [Google Scholar]
- 19.Nair V. G., Prajapati P. K., Nishteswar K., Unnikrishnan V., Nariya M. B. Analgesic and anti-inflammatory activities of Bulbophyllum neilgherrense wight. pseudobulb: a folklore plant. Ayu . 2018;39:p. 76. doi: 10.4103/ayu.ayu_134_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chinsamy M., Finnie J., Van Staden J. Anti-inflammatory, antioxidant, anti-cholinesterase activity and mutagenicity of South African medicinal orchids. South African Journal of Botany . 2014;91:88–98. doi: 10.1016/j.sajb.2013.12.004. [DOI] [Google Scholar]
- 21.Mahnashi M. H., Alyami B. A., Alqahtani Y. S., et al. Phytochemical profiling of bioactive compounds, anti-inflammatory and analgesic potentials of Habenaria digitata Lindl.: molecular docking based synergistic effect of the identified compounds. Journal of Ethnopharmacology . 2021;273 doi: 10.1016/j.jep.2021.113976.113976 [DOI] [PubMed] [Google Scholar]
- 22.Zeb A., Sadiq A., Ullah F., Ahmad S., Ayaz M. Phytochemical and toxicological investigations of crude methanolic extracts, subsequent fractions and crude saponins of Isodon rugosus. Biological Research . 2014;47:p. 57. doi: 10.1186/0717-6287-47-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sadiq A., Zeb A., Ullah F., et al. Chemical characterization, analgesic, antioxidant, and anticholinesterase potentials of essential oils from Isodon rugosus wall. ex. Benth. Frontiers in Pharmacology . 2018;9:p. 623. doi: 10.3389/fphar.2018.00623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zafar R., Ullah H., Zahoor M., Sadiq A. Isolation of bioactive compounds from Bergenia ciliata (haw.) sternb rhizome and their antioxidant and anticholinesterase activities. BMC Complementary and Alternative Medicine . 2019;19:1–13. doi: 10.1186/s12906-019-2679-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah S. M. M., Sadiq A., Shah S. M. H., Ullah F. Antioxidant, total phenolic contents and antinociceptive potential of Teucrium stocksianum methanolic extract in different animal models. BMC Complementary and Alternative Medicine . 2014;14:1–7. doi: 10.1186/1472-6882-14-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alam F., Din K. M., Rasheed R., et al. Phytochemical investigation, anti-inflammatory, antipyretic and antinociceptive activities of Zanthoxylum armatum DC extracts-in vivo and in vitro experiments. Heliyon . 2020;6 doi: 10.1016/j.heliyon.2020.e05571.e05571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sadiq A., Rashid U., Ahmad S., et al. Treating hyperglycemia from Eryngium caeruleum M. Bieb: in-vitro α-glucosidase, antioxidant, in-vivo antidiabetic and molecular docking-based approaches. Frontiers in Chemistry . 2020;8:p. 1064. doi: 10.3389/fchem.2020.558641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rocha N. F. M., Rios E. R. V., Carvalho A. M. R., et al. Anti-nociceptive and anti-inflammatory activities of (−)-α-bisabolol in rodents. Naunyn-Schmiedeberg’s archives of pharmacology . 2011;384:525–533. doi: 10.1007/s00210-011-0679-x. [DOI] [PubMed] [Google Scholar]
- 29.Subhan F., Abbas M., Rauf K., Arfan M., Sewell R., Ali G. The role of opioidergic mechanism in the activity of Bacopa monnieri extract against tonic and acute phasic pain modalities. Pharmacologyonline . 2010;3:903–914. [Google Scholar]
- 30.Mahmoud A. M., EL-Wekil M. M., Mahnashi M. H., Ali M. F., Alkahtani S. A. Modification of N, S co-doped graphene quantum dots with p-aminothiophenol-functionalized gold nanoparticles for molecular imprint-based voltammetric determination of the antiviral drug sofosbuvir. Microchimica Acta . 2019;186:1–8. doi: 10.1007/s00604-019-3647-7. [DOI] [PubMed] [Google Scholar]
- 31.Ahmad N., Subhan F., Islam N. U., Shahid M., Rahman F. U., Fawad K. A novel pregabalin functionalized salicylaldehyde derivative afforded prospective pain, inflammation, and pyrexia alleviating propensities. Archiv der Pharmazie . 2017;350 doi: 10.1002/ardp.201600365.e201600365 [DOI] [PubMed] [Google Scholar]
- 32.Winter C. A., Risley E. A., Nuss G. W. Anti-inflammatory and antipyretic activities of indo-methacin, 1-(p-chlorobenzoyl)-5-methoxy-2-methyl-indole-3-acetic acid. Journal of Pharmacology and Experimental Therapeutics . 1963;141:369–376. [PubMed] [Google Scholar]
- 33.Al-Harbi M. M., Qureshi S., Ahmed M. M., Raza M., Miana G. A., Shah A. H. Studies on the antiinflammatory, antipyretic and analgesic activities of santonin. The Japanese Journal of Pharmacology . 1994;64:135–139. doi: 10.1254/jjp.64.135. [DOI] [PubMed] [Google Scholar]
- 34.Portenoy R. K., Foley K. M. Chronic use of opioid analgesics in non-malignant pain: report of 38 cases. Pain . 1986;25:171–186. doi: 10.1016/0304-3959(86)90091-6. [DOI] [PubMed] [Google Scholar]
- 35.Rozen D., Grass G. W. Perioperative and intraoperative pain and anesthetic care of the chronic pain and cancer pain patient receiving chronic opioid therapy. Pain Practice . 2005;5:18–32. doi: 10.1111/j.1533-2500.2005.05104.x. [DOI] [PubMed] [Google Scholar]
- 36.Smith W. L., Urade Y., Jakobsson P. J. Enzymes of the cyclooxygenase pathways of prostanoid biosynthesis. Chemical Reviews . 2011;111:5821–5865. doi: 10.1021/cr2002992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smyth E. M., Grosser T., Wang M., Yu Y., Fitzgerald G. A. Prostanoids in health and disease. The Journal of Lipid Research . 2009;50:S423–S428. doi: 10.1194/jlr.r800094-jlr200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malkowski M. G., Ginell S. L., Smith W. L., Garavito R. M. The productive conformation of arachidonic acid bound to prostaglandin synthase. Science . 2000;289:1933–1937. doi: 10.1126/science.289.5486.1933. [DOI] [PubMed] [Google Scholar]
- 39.Vecchio A. J., Simmons D. M., Malkowski M. G. Structural basis of fatty acid substrate binding to cyclooxygenase-2. Journal of Biological Chemistry . 2010;285:22152–22163. doi: 10.1074/jbc.m110.119867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lucido M. J., Orlando B. J., Vecchio A. J., Malkowski M. G. Crystal structure of aspirin-acetylated human cyclooxygenase-2: insight into the formation of products with reversed stereochemistry. Biochemistry . 2016;55:1226–1238. doi: 10.1021/acs.biochem.5b01378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gawade S. Acetic acid induced painful endogenous infliction in writhing test on mice. Journal of Pharmacology and Pharmacotherapeutics . 2012;3:p. 348. doi: 10.4103/0976-500x.103699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matsumoto H., Naraba H., Ueno A., et al. Induction of cyclooxygenase-2 causes an enhancement of writhing response in mice. European Journal of Pharmacology . 1998;352:47–52. doi: 10.1016/s0014-2999(98)00340-9. [DOI] [PubMed] [Google Scholar]
- 43.Ballou L. R., Botting R. M., Goorha S., Zhang J., Vane J. R. Nociception in cyclooxygenase isozyme-deficient mice. Proceedings of the National Academy of Sciences . 2000;97:10272–10276. doi: 10.1073/pnas.180319297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gray A., Spencer P., Sewell R. The involvement of the opioidergic system in the antinociceptive mechanism of action of antidepressant compounds. British Journal of Pharmacology . 1998;124:669–674. doi: 10.1038/sj.bjp.0701882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gray A. M., Nevinson M. J., Sewell R. D. The involvement of opioidergic and noradrenergic mechanisms in nefopam antinociception. European Journal of Pharmacology . 1999;365:149–157. doi: 10.1016/s0014-2999(98)00837-1. [DOI] [PubMed] [Google Scholar]
- 46.Zakaria Z. A., Wen L. Y., Rahman N. I. A., Ayub A. H. A., Sulaiman M. R., Gopalan H. K. Antinociceptive, anti-inflammatory and antipyretic properties of the aqueous extract of Bauhinia purpurea leaves in experimental animals. Medical Principles and Practice . 2007;16:443–449. doi: 10.1159/000107749. [DOI] [PubMed] [Google Scholar]
- 47.Tjølsen A., Lund A., Hole K. Antinociceptive effect of paracetamol in rats is partly dependent on spinal serotonergic systems. European Journal of Pharmacology . 1991;193:193–201. doi: 10.1016/0014-2999(91)90036-p. [DOI] [PubMed] [Google Scholar]
- 48.Hosseinzadeh H., Younesi H. M. Antinociceptive and anti-inflammatory effects of Crocus sativus L. stigma and petal extracts in mice. BMC Pharmacology . 2002;2:p. 7. doi: 10.1186/1471-2210-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Noble F., Smadja C., Valverde O., et al. Pain-suppressive effects on various nociceptive stimuli (thermal, chemical, electrical and inflammatory) of the first orally active enkephalin-metabolizing enzyme inhibitor RB 120. Pain . 1997;73:383–391. doi: 10.1016/s0304-3959(97)00125-5. [DOI] [PubMed] [Google Scholar]
- 50.Thangaraj P. Pharmacological Assays Of Plant-Based Natural Products . Berlin Germany: Springer; 2016. Antipyretic activity. [Google Scholar]
- 51.Dangarembizi R., Erlwanger K., Rummel C., Roth J., Madziva M., Harden L. Brewer’s yeast is a potent inducer of fever, sickness behavior and inflammation within the brain. Brain, Behavior, and Immunity . 2018;68:211–223. doi: 10.1016/j.bbi.2017.10.019. [DOI] [PubMed] [Google Scholar]
- 52.Pittman Q. J., Chen X., Mouihate A., Hirasawa M., Martin S. Arginine vasopressin, fever and temperature regulation. Progress Brain Research . 1998;119:383–392. doi: 10.1016/s0079-6123(08)61582-4. [DOI] [PubMed] [Google Scholar]
- 53.Nantel F., Denis D., Gordon R., et al. Distribution and regulation of cyclooxygenase-2 in carrageenan-induced inflammation. British Journal of Pharmacology . 1999;128:853–859. doi: 10.1038/sj.bjp.0702866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morris C. J. Inflammation Protocols . Berlin, Germany: Springer; 2003. Carrageenan-induced Paw Edema in the rat and mouse. [DOI] [PubMed] [Google Scholar]
- 55.Calixto J. B., Campos M. M., Otuki M. F., Santos A. R. Anti-inflammatory compounds of plant origin. Part II. Modulation of pro-inflammatory cytokines, chemokines and adhesion molecules. Planta Medica . 2004;70:93–103. doi: 10.1055/s-2004-815483. [DOI] [PubMed] [Google Scholar]
- 56.Gallin J. I., Goldstein I. M., Snyderman R. Inflammation: Basic Principles and Clinical Correlates . Philadelphia, PA, USA: Lippincott Williams & Wilkins; 1999. [Google Scholar]
- 57.Ahmad A., Ullah F., Sadiq A., et al. Comparative cholinesterase, α-glucosidase inhibitory, antioxidant, molecular docking, and kinetic studies on potent succinimide derivatives. Drug Design, Development and Therapy . 2020;14:p. 2165. doi: 10.2147/dddt.s237420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alam B., Akter F., Parvin N., et al. Antioxidant, analgesic and anti-inflammatory activities of the methanolic extract of piper betle leaves. Avicenna Journal of Phytomedicine . 2013;3:p. 112. [PMC free article] [PubMed] [Google Scholar]
- 59.Aouey B., Samet A., Fetoui H., Simmonds M., Bouaziz M. Anti-oxidant, anti-inflammatory, analgesic and antipyretic activities of grapevine leaf extract (vitis vinifera) in mice and identification of its active constituents by LC–MS/MS analyses. Biomedicine & Pharmacotherapy . 2016;84:1088–1098. doi: 10.1016/j.biopha.2016.10.033. [DOI] [PubMed] [Google Scholar]
- 60.Choi E.-M., Hwang J.-K. Antiinflammatory, analgesic and antioxidant activities of the fruit of Foeniculum vulgare. Fitoterapia . 2004;75:557–565. doi: 10.1016/j.fitote.2004.05.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be available from the corresponding author upon request.


