Abstract
Background:
Acute myocarditis (AM) is thought to be a rare cardiovascular complication of COVID-19, although minimal data are available beyond case reports. We aim to report the prevalence, baseline characteristics, in-hospital management, and outcomes for patients with COVID-19–associated AM on the basis of a retrospective cohort from 23 hospitals in the United States and Europe.
Methods:
A total of 112 patients with suspected AM from 56 963 hospitalized patients with COVID-19 were evaluated between February 1, 2020, and April 30, 2021. Inclusion criteria were hospitalization for COVID-19 and a diagnosis of AM on the basis of endomyocardial biopsy or increased troponin level plus typical signs of AM on cardiac magnetic resonance imaging. We identified 97 patients with possible AM, and among them, 54 patients with definite/probable AM supported by endomyocardial biopsy in 17 (31.5%) patients or magnetic resonance imaging in 50 (92.6%). We analyzed patient characteristics, treatments, and outcomes among all COVID-19–associated AM.
Results:
AM prevalence among hospitalized patients with COVID-19 was 2.4 per 1000 hospitalizations considering definite/probable and 4.1 per 1000 considering also possible AM. The median age of definite/probable cases was 38 years, and 38.9% were female. On admission, chest pain and dyspnea were the most frequent symptoms (55.5% and 53.7%, respectively). Thirty-one cases (57.4%) occurred in the absence of COVID-19–associated pneumonia. Twenty-one (38.9%) had a fulminant presentation requiring inotropic support or temporary mechanical circulatory support. The composite of in-hospital mortality or temporary mechanical circulatory support occurred in 20.4%. At 120 days, estimated mortality was 6.6%, 15.1% in patients with associated pneumonia versus 0% in patients without pneumonia (P=0.044). During hospitalization, left ventricular ejection fraction, assessed by echocardiography, improved from a median of 40% on admission to 55% at discharge (n=47; P<0.0001) similarly in patients with or without pneumonia. Corticosteroids were frequently administered (55.5%).
Conclusions:
AM occurrence is estimated between 2.4 and 4.1 out of 1000 patients hospitalized for COVID-19. The majority of AM occurs in the absence of pneumonia and is often complicated by hemodynamic instability. AM is a rare complication in patients hospitalized for COVID-19, with an outcome that differs on the basis of the presence of concomitant pneumonia.
Keywords: cardiac, MRI, COVID-2019, MRI, myocarditis, outcome, SARS-CoV-2
Clinical Perspective.
What Is New?
Estimation of definite/probable acute myocarditis prevalence among patients with COVID-19 is 2.4 per 1000 hospitalizations.
Thirty-nine percent of patients with COVID-19 acute myocarditis had a fulminant presentation requiring inotropic support or temporary mechanical circulatory support, and 70.4% were admitted to the intensive care unit.
Mortality or temporary mechanical circulatory support during the hospitalization was 20.4%. At 120 days, among patients with COVID-19 acute myocarditis, estimated mortality was 6.6%.
Among patients with COVID-19 acute myocarditis, those with concurrent pneumonia compared with those without pneumonia had a mortality of 15.1% versus 0% (P=0.044), respectively.
What Are the Clinical Implications?
Hospitalized patients with acute myocarditis associated with COVID-19 have a need for intensive care unit admission in up to 70.4%, despite a median age of 38 years.
Fifty-seven percent of patients with acute myocarditis had no significant acute lung injury caused by COVID-19, but patients with concurrent pneumonia were more likely to develop hemodynamic instability, require temporary mechanical circulatory support, and die compared with those without pneumonia.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection or coronavirus disease 2019 (COVID-19) is a multisystem disease that predominately results in an acute respiratory illness and, accordingly, the majority of deaths are secondary to respiratory failure.1 However, a sizeable minority of patients with COVID-19 develop cardiovascular complications as evidenced by an acute cardiac injury with elevated troponin,2–4 de novo systolic heart failure (HF),5 pericardial effusion, and rarely, acute myocarditis (AM).6–8 It was initially speculated that cardiac injury may be mediated by direct cardiomyocyte infection with cardiomyocyte death,9,10 but this mechanism has not been consistently supported by subsequent data.11,12 Alternatively, cardiac injury might be mediated by indirect mechanisms, such as ischemia, fever, adrenergic hyperactivity, or inflammation secondary to cytokine storm and COVID-19 hyperinflammatory reaction.10 A recent study in patients with COVID-19 complicated by acute respiratory distress syndrome has demonstrated that, in many cases, cardiac injury is associated with baseline comorbidities, and underlying multisystemic organ dysfunction during critical illness.13
Nevertheless, it has been recognized that AM can be a distinct manifestation associated with COVID-19 or with SARS-CoV-2 exposure.6,14,15 Current literature suggests that AM associated with COVID-19 is rare. To date, the largest systematic review on published cases of suspected COVID-19 AM reported data for 38 patients, and only 8 had available histology.8 Two small case series have reviewed adults with COVID-19 multisystemic inflammatory syndrome (MIS) associated with AM (in 7 and 11 patients, respectively),16,17 or AM with delayed onset (9 patients).15 However, many of these patients had presumed myocarditis without meeting the contemporary diagnostic criteria for AM. Thus, the prevalence, characteristics, disease course, and outcomes of COVID-19–associated AM remain uncertain.
Therefore, we conducted an international retrospective study with the following aims: (1) estimate the prevalence of AM in patients hospitalized for COVID-19; (2) describe the clinical presentation and hospital course including treatments and outcomes; and (3) compare the outcome in patients presenting with AM and COVID-19 pneumonia with those without pneumonia.
Methods
The data, analytical methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure because the current ethics approval does not allow us to share sensitive patient data with other researchers without local Institutional Review Board approval.
Study Population
This is a multicenter, multinational, retrospective, observational study involving 23 cardiology centers from Italy (n=10), France (n=4), Spain (n=3), Belgium (n=1), the United Kingdom (n=1), Finland (n=1) and the United States (n=3). To be included in our study, the center must have admitted patients with COVID-19 from February 1, 2020, to April 30, 2021, and have cardiac catheterization and cardiac magnetic resonance imaging (MRI) facilities (Supplemental Methods). A complete list of centers and recruited patients is provided in Table S1 (Supplemental Methods). This study complied with the Declaration of Helsinki and was approved by the investigational review board of Spedali Civili di Brescia, Brescia, Italy, and by other local investigational review boards. As a retrospective observational study of deidentified data, local ethics committees granted waivers for consent.
To be included into our study, patients had to fulfill the following criteria:
Definite myocarditis defined by the following criteria: (1) presence of symptoms consistent with AM (chest pain, dyspnea, palpitation, syncope, fatigue, or abdominal symptoms); (2) evidence of COVID-19 shown by positive results of nasopharyngeal swabs or elevated specific antibodies associated with recent symptoms consistent with COVID-19 infection; (3) diagnosis confirmation by European Society of Cardiology criteria on an endomyocardial biopsy (EMB) or autopsy (presence of inflammatory cells ≥14 cells/mm2 of which CD3+T-lymphocytes ≥7 cells/mm2),18 or by the coexistence of positive biomarkers (troponin >99th upper reference limit or elevated creatine kinase myocardial band) and cardiac MRI findings consistent with AM according to the 2018 updated Lake Louise Criteria (these criteria include at least 1 T2-based criterion [global or regional increase of myocardial T2 relaxation time or an increased signal intensity in T2-weighted images], and at least 1 T1-based criterion [increased myocardial T1, extracellular volume, or late gadolinium enhancement {LGE}]),19 or the combination of increased myocardial T1 and LGE.20
Probable myocarditis: patients in whom cardiac MRI was not performed in the acute phase of COVID-19 because of critical clinical conditions or hospital policy, but who had cardiovascular symptoms, positive biomarkers, and electrocardiographic or echocardiographic changes consistent with AM during hospitalization and follow-up cardiac MRI performed within 9 months from the hospital admission because of COVID-19 consistent with active myocarditis or with nonischemic LGE consistent with previous AM.
Among suspected cases of AM, the following cases were excluded: (1) patients with a previous diagnosis of ischemic heart disease or cardiomyopathy without histological evidence of active myocarditis; (2) pediatric patients younger than 10 years; (3) men older than 50 years and women older than 55 years without a demonstration of the absence of obstructive coronary artery disease on the basis of coronary angiography, coronary computed tomography angiography, or myocardial perfusion imaging; and (4) patients older than 70 years without histological confirmation of AM, because potential confounders could not be completely ruled out at cardiac MRI (ie, Takotsubo cardiomyopathy or other conditions associated with the aging heart). The study cohort for this analysis was the definite/probable AM group (n=54). Furthermore, the 54 patients with COVID-19–associated AM were divided into 2 groups on the basis of the presence of concurrent pneumonia (typical signs and symptoms with radiographic confirmation).
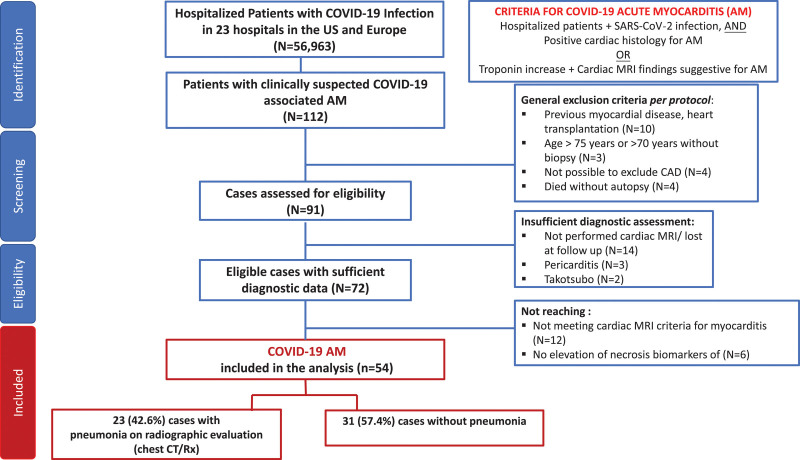
Each center provided the total number of patients with COVID-19 hospitalized during the period they screened for cases of COVID-19–associated AM. These data were used to estimate the proportion of AM cases compared with the total number of patients with COVID-19 who were admitted. We also identified possible cases (n=43) among the suspected AM cases, where a final diagnosis of AM was not supported by histology or cardiac MRI (MRI not performed, or the MRI performed after discharge could not support the diagnosis of recent myocarditis), or there was no troponin elevation, or coronary artery disease was not excluded in men older than 50 years and women older than 55 years (Table S1, Supplemental Methods, and Figure 1).
Figure 1.
Flow diagram illustrating screening and inclusion criteria.
AM indicates acute myocarditis; CAD, coronary artery disease; CT, computed tomography; MRI, magnetic resonance imaging; and Rx‚ radiograph.
We provided a comparator group of patients with AM from a previous large retrospective AM cohort (Lombardy registry), with inclusion criteria comparable with those used for COVID-19–associated AM to assess differences between COVID-19 and non–COVID-19 myocarditis (Supplemental Methods).
Data Collection and Definitions
Demographic, clinical, laboratory, instrumental, treatment, and outcome data were extracted from the in-hospital medical records (Supplemental Methods). Transthoracic echocardiograms were performed and analyzed according to the last recommendations from the American Society of Echocardiography and the European Association of Cardiovascular Imaging.21 Cardiac MRI evaluation was considered acceptable if performed in the acute phase or within 6 months from hospitalizations and included conventional techniques of cine, LGE imaging, and T2-weighted short-tau inversion recovery imaging, as well as parametric mapping of myocardial T1 and T2.22 Information about EMB or autopsy results was collected when available.
Last, hospitalization length of stay, intensive care unit admission, complications (HF, cardiogenic shock, arrhythmias, venous or arterial thromboembolism, sepsis, acute respiratory distress syndrome), and death events were collected. The term fulminant myocarditis was used to describe severe forms of AM, with fast evolution and hemodynamic compromise requiring inotropes or temporary mechanical circulatory support (t-MCS).23–25 The definition of shock was generally based on the requirement of vasopressor, inotropic, or mechanical support, but was not standardized and relied on site-level characterization. Furthermore, the type of shock (cardiogenic, distributive/septic, or mixed) was defined clinically by each center’s physicians. The severity of shock in these patients was classified according to the Society for Cardiovascular Angiography and Interventions classification.26
Statistical Analysis
Continuous variables were reported as median and quartile (Q) 1 through Q3. The Mann-Whitney U test was used to compare continuous variables. The Wilcoxon matched-paired signed-rank test was used to analyze paired data at different time points. Categorical variables were compared with the Fisher exact test, and the relative risk was calculated. The 95% CI was calculated with the method of Katz et al.27 Kaplan-Meier curves were compared with the use of the log-rank statistic. We assessed the association between patient outcome (death or need for t-MCS) and AM with or without concurrent pneumonia using univariate Cox regression analysis. All analyses were 2-tailed. Differences with values of P<0.05 were considered statistically significant. Software packages used were IBM SPSS Statistics (version 20) and GraphPad Prism (version 6). Table S1 (Supplemental Methods) reports the origin and the figures of included cases (definite/probable) and suspected cases, which included a subcohort of possible cases. Hence, the possible cases (n=43) represented a subcohort of suspected cases of COVID-19–associated AM that were excluded from the definite/probable cohort because the diagnosis was supported by neither histology nor cardiac MRI or there was no troponin elevation, or coronary artery disease was not excluded in men older than 50 years and women older than 55 years. Merely suspected cases (n=15) included both patients that had a previous ischemic heart disease or cardiomyopathy diagnosis without histological evidence of active myocarditis, or with a final diagnosis of Takotsubo cardiomyopathy or pericarditis on cardiac MRI.
We used different approaches to estimate the prevalence of COVID-19 am in the attempt to mitigate the uncertainty caused by misdiagnosis/underreporting of AM and the uncertainty associated with the recruitment or exclusion of different medical centers in the study. For the appraisal of COVID-19 am prevalence (defined as COVID-19–associated AM divided by hospitalized COVID-19 cases in a center) among hospitalized patients with COVID-19, we consider 2 types of uncertainty, which are presented in the Supplemental Methods.
Results
Estimation of Prevalence of COVID-19 am
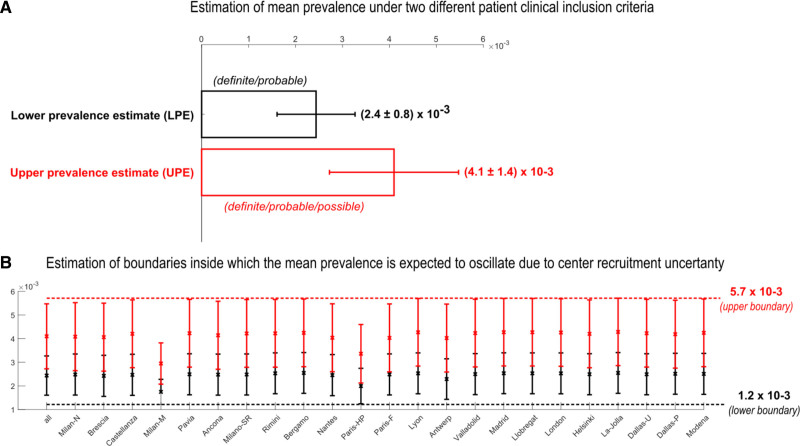
Including only definite/probable cases (lower prevalence estimate) among hospitalized patients, we found a mean prevalence of 0.0024 COVID-19 am (2.4 cases among 1000 hospitalized patients with COVID-19), and also including possible cases (the most permissive prevalence, termed upper prevalence estimate), we found a mean prevalence of 0.0041 (4.1 cases among 1000 hospitalized patients with COVID-19 (Figure 2A). Then we used a second method to estimate prevalence, the leave-1-out procedure, and we found that the boundaries inside which the estimation of the sample mean prevalence was expected to occur were between 0.0012 and 0.0057 (Figure 2B).
Figure 2.
Estimation of lower prevalence estimate (LPE) and upper prevalence estimate (UPE) of acute myocarditis among hospitalized patients with COVID-19. A, Mean LPE and mean UPE computed with the respective CIs on the 23 centers. B, Mean LPE and mean UPE with the respective CIs iteratively computed on 22 centers (by means of leave-1-out procedure). The red dashed line at the top (which is the maximum level reached by the CIs) and the black dashed line at the bottom (which is the minimum level reached by the CIs) represent the boundaries inside which the estimation of the sample mean prevalence is expected to occur. Dallas-P indicates Dallas-Parkland health & Hospital System; Dallas-U, Dallas-University of Texas Southwestern Medical Center; Milan-M, Milan Monzino; Milan-N, Milan Niguarda hospital; Milano-SR, Milano San Raffaele hospital; Paris-F, Paris-Foch; and Paris-HP, Paris-Hôpital Pitié–Salpêtrière.
Clinical Presentation
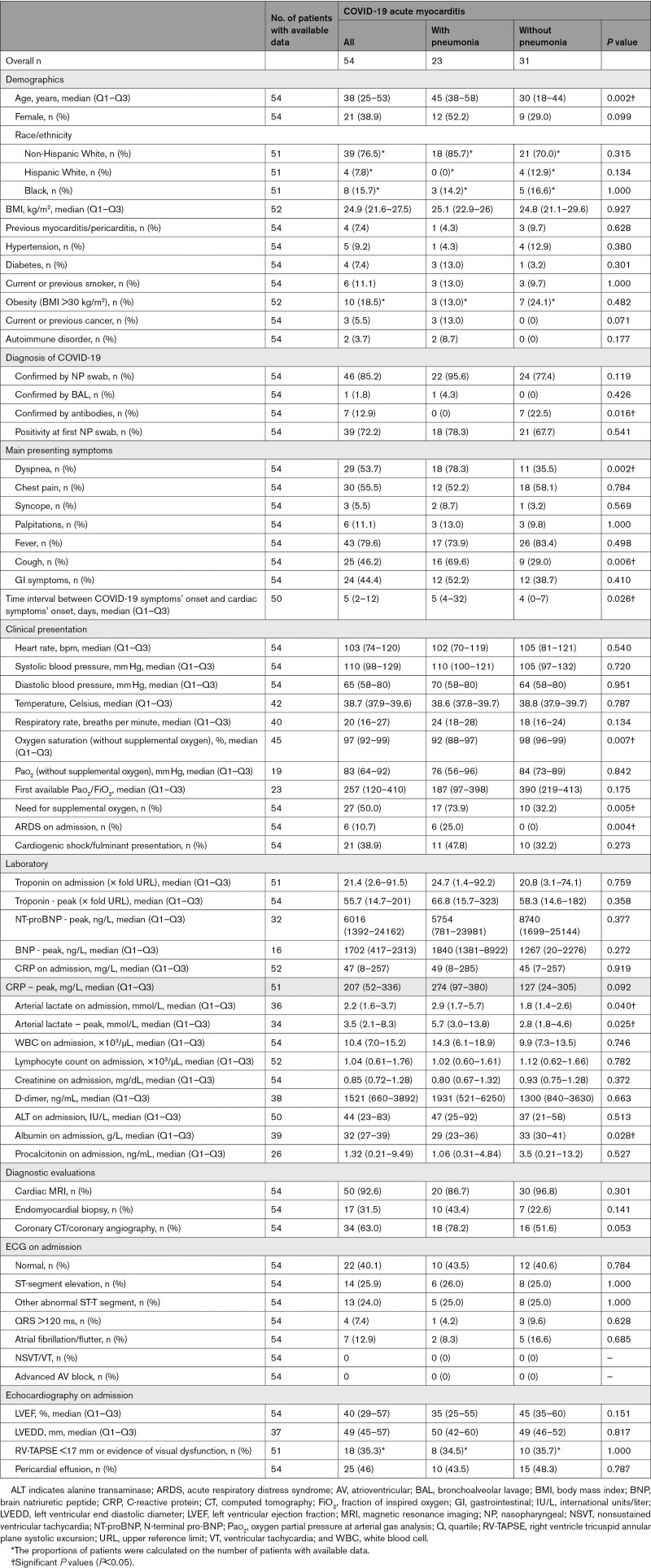
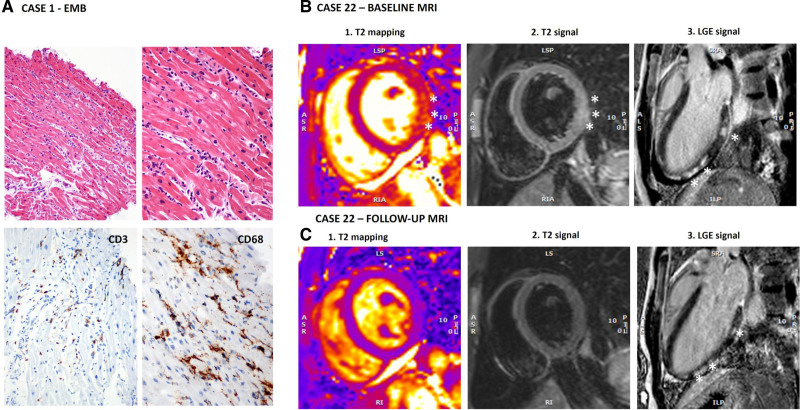
Clinical characteristics of the 54 patients with definite/probable COVID-19–associated AM are reported in Table 1. Median age at presentation was 38 years (Q1–Q3: 25–53), 38.9% of the patients were female, and 76.5% were non-Hispanic White. Thirty-two cases (59.3%) occurred between February 2020 and August 2020, and the remaining 22 cases (40.7%) occurred between September 2020 and April 2021. None received COVID-19 vaccination before myocarditis onset. The exposure to SARS-CoV-2 was confirmed by positive real-time polymerase chain reaction (RT-PCR) either on nasopharyngeal swab (85.2%) or bronchoalveolar lavage (1.8%), or by the presence of SARS-CoV-2–specific antibodies (12.9%). Further details on laboratory and echocardiographic data are presented in the Supplemental Results. Twenty-one patients developed fulminant myocarditis; their characteristics and clinical course are reported in Table S2 (Supplemental Results). In 49 out of 54 patients (90.7%), EMB or cardiac MRI was performed during the index hospitalization confirming the diagnosis of AM (definite AM), whereas in 5 of 54 (9.3%) patients, the diagnosis was supported by the initial clinical presentation with increased troponin levels and findings on follow-up cardiac MRI consistent with recent myocarditis (probable AM), performed at a median of 47 days (Q1–Q3: 21–221) after hospital admission. EMB was performed in 17 patients (31.5%) with positive European Society of Cardiology criteria for myocarditis found in 14 cases (82.3%). The 3 patients with nondiagnostic histology had MRI findings consistent with AM. Detailed histological findings are described in Table S3 (Supplemental Results) and Figure 3A and 3B. Viral RT-PCR performed on myocardial tissue found the SARS-CoV-2 genome in 4 (26.7%) and parvovirus B19 in 5 (33.3%) out of 15 cases in which a viral search was performed, with low replicative status (<500 copies/µg of extracted DNA from the heart) in all available data (n=3). No patients showed microvascular changes or microthrombi on histology. Overall, 50 patients (92.5%) underwent cardiac MRI, and AM diagnosis according to 2018 updated Lake Louise Criteria19 was fulfilled in 45 cases (90.0%), 4 (8.0%) had a combination of increased myocardial T1 and nonischemic LGE, and a remaining case who underwent cardiac MRI only during follow-up had a nonischemic LGE pattern consistent with myocarditis (Table S4 and Figure 3B and 3C). Only 1 patient had a coexisting apical transmural left ventricular LGE consistent with ischemic lesion.
Table 1.
Clinical Presentation and Initial Diagnostic Findings in Patients Admitted With COVID-19–Associated Acute Myocarditis With Pneumonia or Without Pneumonia
Figure 3.
Histological findings and cardiac magnetic resonance imaging (MRI) of 2 patients with COVID-19–associated acute myocarditis. A, Endomyocardial biopsy (EMB) findings from a 20-year-old male patient (case 1 in Table S2) show inflammatory infiltrates in the myocardium (upper images, hematoxylin and eosin images at 100× and 200× magnification) and positive CD3 and CD68 immunohistochemical stains (lower images, images at 200× magnification) revealing CD3+T-lymphocytes ≥7 cells/mm2 and CD68+macrophages ≥4 cells/mm2 consistent with myocarditis on the basis of European Society of Cardiology criteria. B, Baseline and follow-up cardiac MRI images at 1.5 Tesla of a 16-year-old boy (case 22) admitted to the hospital with acute myocarditis without pneumonia. Cardiac MRI at baseline fulfilled the 2018 Lake Louise Criteria for myocarditis because signs of both myocardial edema and nonischemic myocardial injury were present. T2-weighted images showed patchy areas of increased T2 signal intensity. In B, both T2 mapping (1) and short-tau inversion recovery (STIR) T2-weighted imaging (2) showed an area of increased signal intensity (SI) in the basal inferolateral wall (asterisks); in this area, T2 mapping value was 58 ms, whereas it was 43 ms in the septum, and the ratio between myocardial and skeletal muscle SI was elevated at 2.5. Postcontrast images (3) showed patchy late gadolinium enhancement (LGE) with nonischemic pattern in the inferolateral wall (asterisks). Pericardial effusion was also evident at cine images, whereas global systolic function was preserved (left ventricular ejection fraction [LVEF] 63%, Video S1). In C, follow-up images of the same patient at 6 months are shown. Compared with the scan acquired in the acute phase, there were no signs of myocardial edema: in the basal inferolateral wall, T2 mapping value decreased to 44 ms (1), and SI ratio between the myocardium and skeletal muscle at STIR T2-weighted images was <2 (2). Patchy LGE (asterisks) of the inferolateral wall persisted, although reduced (3). The pericardial effusion was still present, and systolic ventricular function improved (LVEF 69%; see Video S2). Of note, indexed myocardial mass also reduced from baseline to follow-up from 85 to 78 g/m2. Increased mass in the acute phase can represent an indirect sign of myocardial edema.
Twenty-three patients (42.6%) had AM with pneumonia, and the remaining 31 patients (57.4%) had AM without pneumonia (Figure 1). Patients with AM and pneumonia were significantly older compared with patients without pneumonia (median age 45 versus 30 years, P=0.002); they more frequently reported cough (69.6% versus 29%, P=0.006) and dyspnea (78.3% versus 35.5%, P=0.002). Patients with COVID-19–associated AM with pneumonia compared with those without pneumonia had a lower oxygen saturation on admission (median 92% versus 98%, P=0.007), and more commonly needed supplemental oxygen (73.9% versus 32.2%, P=0.005). The occurrence of cardiogenic shock was similar in the 2 groups of patients with COVID-19 am with pneumonia (47.8%) and without pneumonia (32.2%, P=0.27). Last, 6 patients with am with pneumonia (10.7%) had acute respiratory distress syndrome on admission. Seven patients without pneumonia (12.9%) had a negative nasopharyngeal swab but positive SARS-CoV-2 antibodies and a recent episode consistent with SARS-CoV-2 infection, whereas in all patients with pneumonia, COVID-19 diagnosis was confirmed by RT-PCR on nasopharyngeal swab or bronchoalveolar lavage (Table 1). No differences were observed in histological and MRI findings between patients with AM with and without pneumonia (Tables S3 and S4, Supplemental Results), although patients with pneumonia underwent cardiac MRI after a median time of 12 days compared with 5 days among patients without pneumonia (P=0.01).
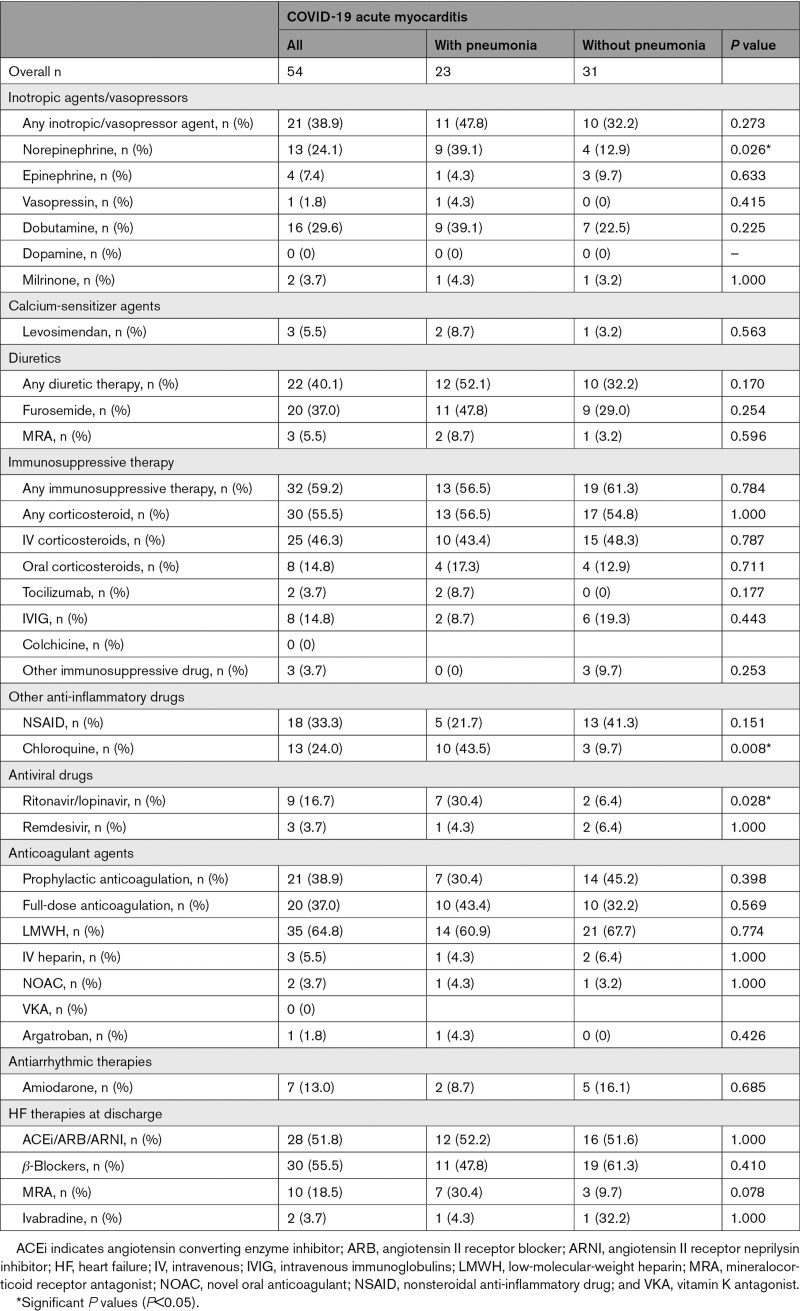
In-Hospital Medications
Complete data about in-hospital medications are shown in Table 2 and in the Supplemental Results.
Table 2.
In-Hospital Medications in COVID-19–Associated Acute Myocarditis With Pneumonia or Without Pneumonia
Hospital Course and Follow-Up
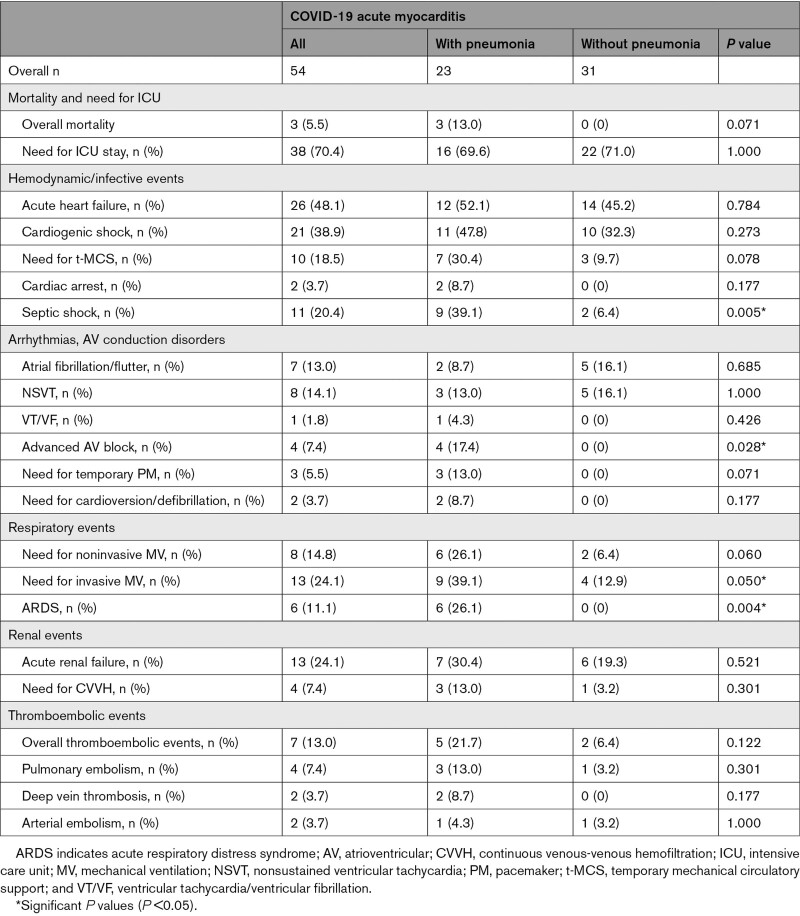
Median hospital stay was 13 days (Q1–Q3: 9–23) with a maximum stay of 131 days. Overall, 38 patients (70.4%) were admitted to the intensive care unit for a median time of 6 days (Q1–Q3: 4–12). Two patients experienced cardiac arrest, 1 because of ventricular fibrillation before hospitalization, effectively treated with cardiopulmonary resuscitation and electric cardioversion, and the other because of complete atrioventricular block. Three patients died (5.5%) during the index hospitalization, all in the AM group with pneumonia (Supplemental Results).
A follow-up visit was available for 47 of 54 patients (87.0%) after a median of 88 days (Q1–Q3: 45–183), with a maximum follow-up of 351 days. No cardiac events occurred after discharge.
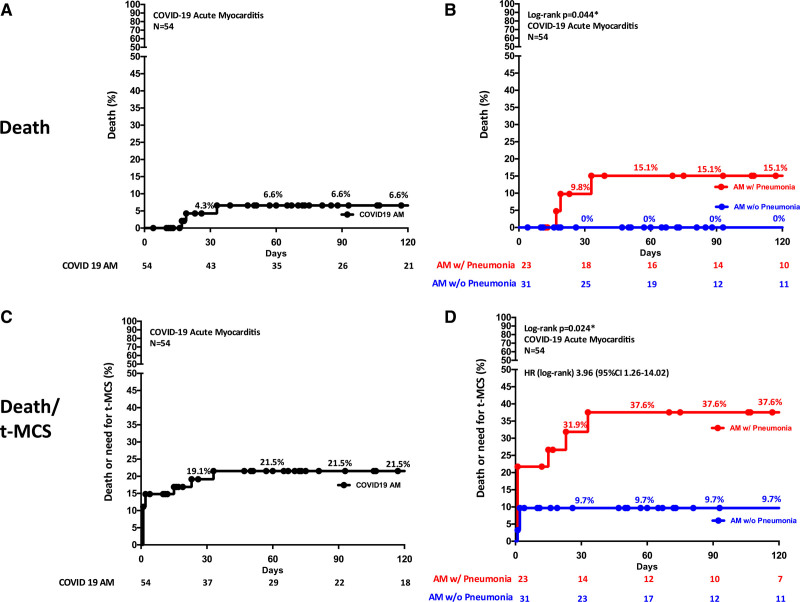
Estimated mortality at 120 days was 6.6% among all COVID-19 AM (Figure 4A). As shown in Figure 4B, overall mortality at 120 days was 15.1% in the group with AM with pneumonia and 0% in those without pneumonia (log-rank P=0.044). No cardiac deaths or heart transplantation occurred, despite 21 (38.9%) patients experiencing a fulminant presentation, which occurred on the day of hospital admission in 13 of 21 patients (61.9%).
Figure 4.
Kaplan
-Meier estimates of 120-day overall mortality and mortality or need for temporary mechanical circulatory support (t-MCS) in patients with COVID-19–associated acute myocarditis (AM). A, Mortality in the whole study population with COVID-19 am and (B) Mortality in patients with COVID-19 am with (w/) pneumonia on the basis of radiographic examinations compared with patients with COVID-19 am without (w/o) pneumonia. Three noncardiac deaths occurred: 2 deaths caused by septic shock and 1 caused by hemorrhagic stroke. C, Mortality or need for t-MCS in the whole study population with COVID-19 am and (D) Mortality or need for t-MCS in patients with COVID-19 am with pneumonia compared with patients with COVID-19 am without pneumonia. Among 10 patients who received a t-MCS, 2 died on support (1 septic shock and 1 hemorrhagic stroke). HR indicates hazard ratio. *Indicates statistically significant with P<0.05.
t-MCS was used in 10 patients (18.5%) for a median time of 5 days (Q1–Q3: 4–10). Specifically, venoarterial extracorporeal membrane oxygenation was used in 6 patients, intra-aortic balloon pump in 5 patients, and the Impella device (Abiomed‚ Danvers‚ MA) and Levitronix CentriMag (Levitronix LLC‚ Waltham‚ MA), respectively, in 2 and 1 patients. Four patients were treated with 2 different types of t-MCS. Estimated mortality or need for t-MCS at 120 days was 21.5% among all the patients (Figure 4C). As shown in Figure 4D, overall mortality or need for t-MCS at 120 days was 37.6% in the group with COVID-19 AM with pneumonia and 9.7% in those without pneumonia (log-rank P=0.024; hazard ratio, 3.96 [95% CI, 1.26–14.02]).
Other in-hospital events are shown in Table 3, described in the Supplemental Results and Table S5.
Table 3.
In-Hospital Events That Occurred in Patients Admitted With COVID-19–Associated Acute Myocarditis With Pneumonia or Without Pneumonia
Among 411 patients with AM from a previous large registry, the proportion of patients who died or required a t-MCS was 5.6%, a figure significantly lower compared with 20.4% in patients with COVID-19–associated AM (P=0.0007). Main characteristics and events in patients with AM versus COVID-19–associated AM are presented in Table S6 (Supplemental Results).
No myocarditis recurrence was reported in 15 of 51 (29.4%) patients with available data on vaccination against SARS-CoV-2 (Supplemental Results).
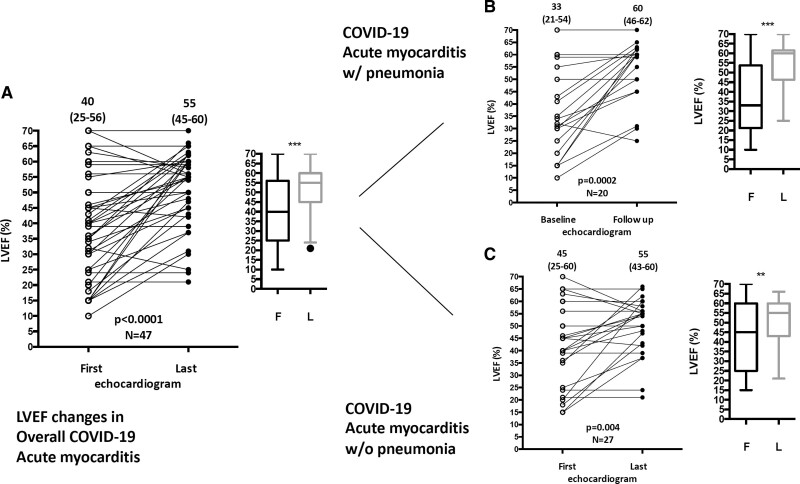
Changes in Left Ventricular Ejection Fraction During Hospitalization and Follow-Up
Data about left ventricular ejection fraction changes are shown in Figure 5, the Supplemental Results, and Table S7.
Figure 5.
Changes in left ventricular ejection fraction (LVEF) during hospitalization in patients with COVID-19 acute myocarditis. A, Echocardiographic data of LVEF at admission and discharge in the entire population of COVID-19 acute myocarditis (available data, n=47 of 54). B, COVID-19 acute myocarditis with (w/) pneumonia (available data, n=20 of 23), and (C) COVID-19 acute myocarditis without (w/o) pneumonia (available data, n=27 of 31). Wilcoxon matched-pair signed-rank test was used for comparisons. The dot plots indicate the median and first and third quartile LVEF at baseline and at follow-up in each group. F indicates first; and L, last. **Indicates P<0.01; ***, P<0.001.
Discussion
This international multicenter study allowed estimation of the prevalence of clinically manifest AM in patients with COVID-19 infection, its clinical characteristics, and association with outcomes: AM mean prevalence was 2.4 in 1000 hospitalized patients with COVID-19 considering definitive/probable AM cases and 4.1 in 1000 also considering possible AM cases. Fifty-seven percent of patients with AM had no significant acute lung injury caused by COVID-19. AM with fulminant presentation was frequent, occurring in 38.9% of the study population. Last, patients with pneumonia and COVID-19 am were more likely to develop hemodynamic instability, require t-MCS, and die compared with those without pneumonia and COVID-19.
The reported prevalence of definite/probable myocarditis among patients hospitalized with COVID-19 has varied markedly depending on the criteria used for diagnosis.14,28–32 The population of the present study was identified using strict criteria. It consists of patients with clinically relevant AM mostly requiring intervention and high care as highlighted by the fact that 70.4% of patients were admitted to the intensive care unit. In a study among 6439 patients with COVID-19 from a single-center registry,5 8 patients (0.12%) had new HF without known cardiovascular disease or cardiovascular risk factors, suggestive of myocarditis. Furthermore, the median age of patients with definitive/probable COVID-19 am in our study was 38 years, consistent with the median age in contemporary non–COVID-19 registries on AM, ranging between 30 and 45 years.25,33 Inclusion criteria used to identify patients with COVID-19 with definite myocarditis were comparable with those used previously in the Lombardy registry that collected data of 443 patients with AM, where the median age of patients was 34 years.34 Similar criteria have been also proposed recently by Bonaca et al35 to diagnose myocarditis in the context of cancer therapeutics. Furthermore, histological diagnosis cannot be reached in all cases of COVID-19–associated AM, because most centers followed the consensus documents suggesting that EMB should be performed primarily in those cases with ventricular arrhythmias, conduction abnormalities, or advanced HF,36,37 or because of the impossibility to perform EMB related to the critical condition of patients or logistic reasons.
Our reported prevalence is lower compared with studies that performed universal cardiac MRI screening during the convalescent COVID-19 period.28,29 An underestimation of the prevalence of mild or subclinical AM is likely in this cohort because of the retrospective nature of the registry, the lack of systematic cardiac MRI, and the possibility of missing some diagnoses, particularly during the first pandemic wave when cardiac MRI and EMB were less frequently performed. Even including cases of possible AM in which a characterization by cardiac MRI or histology was not systematically available, the estimated prevalence was 0.41%. Alternatively, using the boundaries inside which the estimation of the sample mean prevalence was expected to occur, the estimated prevalence ranged between 0.12% and 0.57%. These figures were similar to that reported by Daniels et al in a previous multicenter study that diagnosed 9 (0.56%) patients with a clinically suspected myocarditis among 1597 athletes with cardiac MRI screening after COVID-19 infection.28 Similarly, in the ORCCA registry (Outcomes Registry for Cardiac Conditions in Athletes), which evaluated the SARS-CoV-2 cardiac involvement among collegiate athletes in the United States (mean age, 20 years), the prevalence of definite/probable or possible SARS-CoV-2 cardiac involvement was 0.5% among 2820 athletes who underwent clinically indicated cardiac MRI if at least 1 of the following cardiac tests had an abnormal result: ECG, troponin levels, or transthoracic echocardiography.29 In a third study by Martinez et al that included 789 professional athletes (mean age, 25 years) who underwent a similar cardiac screening after COVID-19, myocarditis was detected in 0.38% of individuals by cardiac MRI.38 It must be noted that in a second population of 198 athletes in the ORCCA registry, a primary screening cardiac MRI identified a higher prevalence of SARS-CoV-2 cardiac involvement in up to 3%,29 suggesting that also among hospitalized patients with COVID-19, a systematic cardiac MRI could have led to a higher prevalence of AM. Furthermore, more mild cases among hospitalized patients may not be easily recognized without systematic biomarker assessment, thus contributing to an underestimation of the complete spectrum of myocarditis caused by COVID-19. As an example, universal cardiac MRIs in older adults who recovered from COVID-19 have demonstrated a high prevalence of myocarditis-like injuries ranging from 26% to 60%,30,31 although the current study in which clinical, laboratory, and instrumental data were integrated seems to suggest that clinically manifest myocarditis associated with COVID-19 is likely less frequent than observed when relying on cardiac MRI. In addition, autopsy series of patients with COVID-19 have observed evidence of myocarditis in 0% to 14%.14,32,39
The in-depth characterization of these cases of AM associated with COVID-19 is underpinned by the fact that 31.5% of patients underwent EMB, compared with 12.6% in another large registry on AM.34 Of note, we point out the occurrence of isolated COVID-19 am in younger patients compared with those with pneumonia. Patients with isolated COVID-19 am still had a fulminant presentation with cardiogenic shock in 10 of 31, (32.3%) highlighting the severity of myocarditis triggered by SARS-CoV-2 independently of the occurrence of viral pneumonia. A direct cardiac injury could be mediated by SARS-CoV-2 that infects cardiomyocytes, pericytes, and fibroblasts via the angiotensin-converting enzyme–2 pathway leading to myocarditis.9 Alternatively, SARS-CoV-2 could trigger an AM by mechanisms that include a nonspecific innate inflammatory response or a molecular mimicry mechanism between viral proteins and cardiomyocytes,25 even if for the latter mechanism, at least a 2-week lapse should be postulated between viral exposure and cardiac injury. Studies that specifically investigated the presence of SARS-CoV-2 in the myocardium found no differences in the inflammatory infiltrates compared with patients without evidence of SARS-CoV-2 in the heart, questioning if the presence of the virus in the heart can trigger an influx of inflammatory cells causing an AM.11 Four out of 15 (26.7%) of our patients with histological evidence of AM had evidence of the SAR-CoV-2 genome in the heart, suggesting that the presence of virus might not be as common as observed in previous studies.11,12 Independently of the presence of SARS-COV-2 in the myocardium, patients with COVID-19–associated AM had a significant improvement of left ventricular ejection fraction during hospitalization.
Furthermore, we found that isolated COVID-19 am appears more frequently than AM associated with concurrent pneumonia. Nevertheless, this is in line with the evidence that respiratory viruses like influenza viruses and other subtypes of human coronavirus can trigger AM also without causing pneumonia.40–43 Although there is evidence that corticosteroids can improve the prognosis of patients with pneumonia requiring respiratory support,44 no evidence-based data are available about immunosuppression in patients with isolated COVID-19–associated AM. In this cohort, an immunosuppressive agent (primarily corticosteroids and intravenous immunoglobulin) was used in 19 out of 31 (61.3%) patients without pneumonia, at least demonstrating the safety of their use because there were no deaths.
Another clinically relevant observation from this study is that a fulminant presentation occurred in a high proportion of patients infected by SARS-CoV-2, in up to 38.9%, a proportion that is higher compared with a subset of 411 patients with non–COVID-19 am from the Lombardy registry, where a fulminant presentation occurred in 8.3%. Nevertheless, in patients with available EMB, diffuse inflammatory infiltrates were less frequently observed in COVID-19–associated AM compared with the subset of non–COVID-19 am (35.3% versus 71.1%; P=0.018), in accordance with other studies.14,32 In addition, COVID-19–associated AM compared with non–COVID-19 am showed a lower left ventricular ejection fraction on first echocardiogram (median 40% versus 55%) and a higher number of patients who died or required a t-MCS (20.4% versus 5.6%) among COVID-19–associated AM. An intuitive explanation can be that the COVID-19 population could be skewed toward the most severe cases of AM, because patients with mild myocarditis can be underrecognized and missed, particularly during the first pandemic wave. Nevertheless, a prospective series of patients with AM and detection of respiratory viruses by RT-PCR on nasopharyngeal swab had a fulminant presentation in up to 55.6% of cases.40 Coronaviruses could trigger immune-mediated reactions that can play a major role in determining cardiac injury in a susceptible host with a permissive genetic background.25,43 Furthermore, hemodynamic instability could be also caused by an associated distributive shock caused by the hyperinflammatory state observed in some of the patients with COVID-19–associated AM,16,17 also called MIS in adults.45 MIS has been observed in COVID-19 more frequently in children, and for this reason, patients younger than 10 years were excluded in the attempt to describe a more defined adult population with AM. Previously described cases of MIS in adults were associated with delayed-onset AM and negative SARS-CoV-2 RT-PCR, suggesting a postinfectious immunologic nature of this COVID-19 complication.15–17 On the contrary, in our series, SARS-CoV-2 was detected by RT-PCR in 87.0% of cases, suggesting that at least in the majority of cases, AM was a concurrent event associated with COVID-19. Yet it must be acknowledged that among patients without pneumonia, SARS-CoV-2 was detected by RT-PCR in a lower proportion of cases (77.4%), thus more resembling the previously described cases of delayed-onset AM in MIS. Nonetheless, the mechanism that leads to cardiac injury can be an immune-mediated response triggered by SARS-CoV-2 both during COVID-19 florid infection and postexposure.
Furthermore, we observed that patients with COVID-19 am with pneumonia had a higher risk of death or t-MCS. In our cohort, patients with associated pneumonia were significantly older compared with patients without pneumonia, and this could partially explain the worse outcome in this subset of patients. Furthermore, these patients more frequently developed acute respiratory distress syndrome and septic shock that can further compromise the hemodynamic stability. In addition, patients with COVID-19 am with pneumonia had lower oxygen saturation and higher lactate levels on admission compared with isolated COVID-19–associated AM. It must be noted that‚ it might be challenging, at least in some cases, to distinguish the diffuse radiographic infiltrate occurring in pulmonary edema from true COVID-19 pneumonia. Nevertheless, the fact that natriuretic peptides, systolic blood pressure, and heart rate on admission were similar between patients with and without pneumonia suggests that the group defined as having pneumonia had a real associated pneumonia and are not simply patients with AM and a more severe pulmonary congestion. In addition, our in-hospital mortality was 5.5%, a figure that is lower compared with the 13.2% mortality reported in a metanalysis of 38 published cases of suspected myocarditis with COVID-19,8 where a publication bias could have overestimated mortality. Nevertheless, the mortality found in our series appears slightly higher compared with the figure of in-hospital mortality plus heart transplantation (1.2%) in the non-COVID-19 AM from the Lombardy registry.34
Last, this study provides results that can be compared with the recent data on AM after mRNA COVID-19 vaccination,46,47 showing that hospitalized patients with AM associated with COVID-19 have a need for intensive care unit admission in up to 70.5%, despite a median age of 38 years, whereas with COVID-19 vaccines, most patients have milder forms of AM.46,47 With regard to the prevalence of AM after vaccination, among 2.8 million doses of mRNA COVID-19 vaccine in the armed forces, only 23 male individuals (median age of cases was 25 years) had evidence of AM, suggesting a prevalence of <1 case of AM among 100 000 mRNA COVID-19 vaccine doses.47 At the time of another report of 8 cases of AM after mRNA COVID-19 vaccine,46 there were 5166 reports of chest pain and 399 reports of myocarditis among 129 million fully vaccinated individuals with BNT162b2 or mRNA-1273 received by the Centers for Disease Control Vaccine Adverse Event Reporting System (www.wonder.cdc.gov/vaers.html). These figures appear reassuring compared with the prevalence of clinically manifest AM observed in this study among hospitalized patients with COVID-19.47
Study Limitations
The retrospective nature of this registry may have introduced potential selection bias. We further account for at least 43 patients with possible AM that were excluded because of the impossibility of performing a cardiac MRI or because of the lack of histological or imaging confirmation of myocarditis. Furthermore, we excluded patients older than 70 years, because the risk of nonspecific inflammatory cardiac injury detected by cardiac MRI has been perceived as higher as well as those older than 50 years without coronary angiography. Nevertheless, in the present study and in the Lombardy registry of AM that had superimposable inclusion criteria, the proportion of patients excluded because of lack of coronary angiography above older than 50 years or older than 70 years was similar (ie, 6.3% and 5.2%, respectively).34 Furthermore, looking at previous large registries of patients with AM, cases in persons older than 70 years were rarely observed.25 Moreover, asymptomatic patients with AM and those without typical symptoms and signs may not have been correctly identified. This approach with stricter diagnostic criteria might have reduced the sensitivity and generalizability of our findings, but it allowed us to describe a more precise and specific population of adults with AM.
We also recognize that in some of the contributing centers heavily hit by the first pandemic wave (for instance, Bergamo in Italy) where no eligible cases of AM were found, some patients with AM could have been missed, even if an autoptic series of patients with COVID-19 from the same hospital found no myocarditis in accordance with our findings.39
Indeed, not all patients were screened for other potential viral causes besides SARS-CoV-2 in nasopharyngeal swab or in myocardial tissue. Furthermore, 33.3% of biopsy samples demonstrated evidence of parvovirus B19. Parvovirus B19 is increasingly considered a bystander because it is frequently found in the normal heart.48,49 However, we cannot exclude that parvovirus B19 could be a contributing cause of AM. Furthermore, just more than half of the patients underwent specific tests to rule out immunologic causes of myocarditis, even if SARS-COV-2 infection per se can be seen as a plausible cause of myocarditis.
Last, the absence of centralized review of pathology and cardiac MRI findings and the use of different MRI hardware and software may have led to a difference in the assessment of qualitative and quantitative parameters, although a high interobserver reproducibility has been reported.50
Conclusions
Our study pointed out that COVID-19–associated AM occurred between 2 and 4 per 1000 hospitalizations for COVID-19. COVID-19–associated AM presented both with and without concomitant pneumonia and was frequently complicated by shock (38.9%). Patients with associated pneumonia had a worse prognosis compared with isolated AM. Furthermore, the use of corticosteroids in patients with AM appeared safe, and in most cases, a rapid increase in the left ventricular ejection fraction was observed, even if no causality can be inferred from our data. Last, among discharged patients with AM, we reported an excellent short-term prognosis without occurrence of cardiovascular events.
Article Information
Sources of Funding
E.A. received an Italian Ministry Grant (GR-2019-12368506). C.B. is supported by the Registry for Cardio-Cerebro-Vascular Pathology, Veneto Region, Italy.
Disclosures
Dr Leonardi reports grants and personal fees from AstraZeneca, Daiichi Sankyo, Bayer, Pifezer/BMS, ICON, Chiesi, and Novonordisk, all outside the submitted work. Dr Farina received personal fees from Bayer for a presentation at an international congress and from Bracco for the production of a non–peer-reviewed publication. E.D. Adler is a consultant for Ancora, AstraZeneca, Cytokinetics, Endotronix, Edwards, Ionis, Lexeo, Medtronic, and Novartis, and a shareholder of Lexeo, and Rocket Pharmaceuticals. Dr de Lemos is a paid consultant for Lexeo Therapeutics, Inc, in New York, NY. Dr Metra received the following personal fees of minimal amounts in the last three years: from Actelion, Amgen, Livanova, Servier and Vifor pharma as member of Executive or Data Monitoring Committeees of sponsored clinical trials; from Astra-Zeneca, Abbott vascular, Bayer, Boheringer Ingelhelm and Edwards Therapeutics for participation to advisory boards and/or speeches at sponsored meetings. The other authors report no conflicts.
Supplemental Material
Supplemental Methods
Supplemental Results
Tables S1–S7
Videos S1 and S2
Supplementary Material
Nonstandard Abbreviations and Acronyms
- AM
- acute myocarditis
- EMB
- endomyocardial biopsy
- HF
- heart failure
- LGE
- late gadolinium enhancement
- MIS
- multisystemic inflammatory syndrome
- MRI
- magnetic resonance imaging
- Q
- quartile
- RT-PCR
- real-time polymerase chain reaction
- t-MCS
- temporary mechanical circulatory support
E. Ammirati and L. Lupi contributed equally.
Circulation is available at www.ahajournals.org/journal/circ
This manuscript was sent to Stephane Heymans, Guest Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material, the podcast, and transcript are available with this article at https://www.ahajournals.org/doi/suppl/10.1161/CIRCULATIONAHA.121.056817.
For Sources of Funding and Disclosures, see page 1137.
Continuing medical education (CME) credit is available for this article. Go to http://cme.ahajournals.org to take the quiz.
Contributor Information
Laura Lupi, Email: lalli.lupi@gmail.com.
Matteo Palazzini, Email: matteo.palazzini@ospedaleniguarda.it.
Nicholas S. Hendren, Email: nicholas.hendren@phhs.org.
Justin L. Grodin, Email: Justin.Grodin@utsouthwestern.edu.
Carlo V. Cannistraci, Email: kalokagathos.agon@gmail.com.
Matthieu Schmidt, Email: matthieuschmidt@yahoo.fr.
Guillaume Hekimian, Email: guillaume.hekimian@aphp.fr.
Giovanni Peretto, Email: peretto.giovanni@hsr.it.
Thomas Bochaton, Email: thomas.bochaton@chu-lyon.fr.
Ahmad Hayek, Email: ahmad.hayek0@gmail.com.
Nicolas Piriou, Email: nicolas.piriou@chu-nantes.fr.
Sergio Leonardi, Email: s.leonardi@smatteo.pv.it.
Stefania Guida, Email: s.rizzo@unipd.it.
Annalisa Turco, Email: A.Turco@smatteo.pv.it.
Simone Sala, Email: sala.simone@hsr.it.
Aitor Uribarri, Email: auribarrig@gmail.com.
Caroline M. Van de Heyning, Email: caroline.vandeheyning@uza.be.
Massimo Mapelli, Email: massimo.mapelli@ccfm.it.
Jeness Campodonico, Email: Jeness.Campodonico@cardiologicomonzino.it.
Patrizia Pedrotti, Email: patrizia.pedrotti@ospedaleniguarda.it.
Albert Ariza Sole, Email: aariza@bellvitgehospital.cat.
Marco Marini, Email: metramarco@libero.it.
Maria Vittoria Matassini, Email: mavimatassini@gmail.com.
Mickael Vourc’h, Email: Mickael.VOURCH@chu-nantes.fr.
Antonio Cannatà, Email: antonio.cannata@kcl.ac.uk.
Daniel I. Bromage, Email: daniel.bromage@kcl.ac.uk.
Daniele Briguglia, Email: daniele.briguglia@gmail.com.
Jorge Salamanca, Email: jsalamancaviloria@gmail.com.
Pablo Diez-Villanueva, Email: pablo_diez_villanueva@hotmail.com.
Jukka Lehtonen, Email: jukka.lehtonen@hus.fi.
Florent Huang, Email: florenth27@hotmail.com.
Stéphanie Russel, Email: s.russel@hopital-foch.com.
Francesco Soriano, Email: francesco.soriano@ospedaleniguarda.it.
Fabrizio Turrini, Email: turrini.fabrizio@aou.mo.it.
Manlio Cipriani, Email: manlio.cipriani@ospedaleniguarda.it.
Manuela Bramerio, Email: manuela.bramerio@ospedaleniguarda.it.
Mattia Di Pasquale, Email: m.dipa91@gmail.com.
Aurelia Grosu, Email: agrosu@asst-pg23.it.
Michele Senni, Email: msenni@asst-pg23.it.
Davide Farina, Email: davide.farina@unibs.it.
Piergiuseppe Agostoni, Email: piergiuseppe.agostoni@cardiologicomonzino.it.
Stefania Rizzo, Email: s.rizzo@unipd.it.
Monica De Gaspari, Email: monica.deg1@gmail.com.
Francesca Marzo, Email: francesca.marzo@auslromagna.it.
Jason M. Duran, Email: jaduran@health.ucsd.edu.
Eric D. Adler, Email: eradler@ucsd.edu.
Cristina Giannattasio, Email: cristina.basso@unipd.it.
Cristina Basso, Email: cristina.basso@unipd.it.
Theresa McDonagh, Email: theresa.mcdonagh@kcl.ac.uk.
Mathieu Kerneis, Email: mathieu.kernis@gmail.com.
Alain Combes, Email: combes001@gmail.com.
Paolo G. Camici, Email: camici.paolo@hsr.it.
James A. de Lemos, Email: james.delemos@utsouthwestern.edu.
References
- 1.Berlin DA, Gulick RM, Martinez FJ. Severe Covid-19. N Engl J Med. 2020;383:2451–2460. doi: 10.1056/NEJMcp2009575 [DOI] [PubMed] [Google Scholar]
- 2.Guo J, Huang Z, Lin L, Lv J. Coronavirus disease 2019 (COVID-19) and cardiovascular disease: a viewpoint on the potential influence of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers on onset and severity of severe acute respiratory syndrome coronavirus 2 infection. J Am Heart Assoc. 2020;9:e016219. doi: 10.1161/JAHA.120.016219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lala A, Johnson KW, Januzzi JL, Russak AJ, Paranjpe I, Richter F, Zhao S, Somani S, Van Vleck T, Vaid A, et al. ; Mount Sinai COVID Informatics Center. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol. 2020;76:533–546. doi: 10.1016/j.jacc.2020.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li C, Jiang J, Wang F, Zhou N, Veronese G, Moslehi JJ, Ammirati E, Wang DW. Longitudinal correlation of biomarkers of cardiac injury, inflammation, and coagulation to outcome in hospitalized COVID-19 patients. J Mol Cell Cardiol. 2020;147:74–87. doi: 10.1016/j.yjmcc.2020.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alvarez-Garcia J, Jaladanki S, Rivas-Lasarte M, Cagliostro M, Gupta A, Joshi A, Ting P, Mitter SS, Bagiella E, Mancini D, et al. New heart failure diagnoses among patients hospitalized for COVID-19. J Am Coll Cardiol. 2021;77:2260–2262. doi: 10.1016/j.jacc.2021.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D, Cani DS, Cerini M, Farina D, Gavazzi E, et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5:819–824. doi: 10.1001/jamacardio.2020.1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hendren NS, Drazner MH, Bozkurt B, Cooper LT, Jr. Description and proposed management of the acute COVID-19 cardiovascular syndrome. Circulation. 2020;141:1903–1914. doi: 10.1161/CIRCULATIONAHA.120.047349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castiello T, Georgiopoulos G, Finocchiaro G, Claudia M, Gianatti A, Delialis D, Aimo A, Prasad S. COVID-19 and myocarditis: a systematic review and overview of current challenges. Heart Fail Rev. 2022;27:251–261. doi: 10.1007/s10741-021-10087-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen L, Li X, Chen M, Feng Y, Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res. 2020;116:1097–1100. doi: 10.1093/cvr/cvaa078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akhmerov A, Marbán E. COVID-19 and the heart. Circ Res. 2020;126:1443–1455. doi: 10.1161/CIRCRESAHA.120.317055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindner D, Fitzek A, Bräuninger H, Aleshcheva G, Edler C, Meissner K, Scherschel K, Kirchhof P, Escher F, Schultheiss HP, et al. Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol. 2020;5:1281–1285. doi: 10.1001/jamacardio.2020.3551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bearse M, Hung YP, Krauson AJ, Bonanno L, Boyraz B, Harris CK, Helland TL, Hilburn CF, Hutchison B, Jobbagy S, et al. Factors associated with myocardial SARS-CoV-2 infection, myocarditis, and cardiac inflammation in patients with COVID-19. Mod Pathol. 2021;34:1345–1357. doi: 10.1038/s41379-021-00790-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Metkus TS, Sokoll LJ, Barth AS, Czarny MJ, Hays AG, Lowenstein CJ, Michos ED, Nolley EP, Post WS, Resar JR, et al. Myocardial injury in severe COVID-19 compared with non-COVID-19 acute respiratory distress syndrome. Circulation. 2021;143:553–565. doi: 10.1161/CIRCULATIONAHA.120.050543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Basso C, Leone O, Rizzo S, De Gaspari M, van der Wal AC, Aubry MC, Bois MC, Lin PT, Maleszewski JJ, Stone JR. Pathological features of COVID-19-associated myocardial injury: a multicentre cardiovascular pathology study. Eur Heart J. 2020;41:3827–3835. doi: 10.1093/eurheartj/ehaa664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bajaj R, Sinclair HC, Patel K, Low B, Pericao A, Manisty C, Guttmann O, Zemrak F, Miller O, Longhi P, et al. Delayed-onset myocarditis following COVID-19. Lancet Respir Med. 2021;9:e32–e34. doi: 10.1016/S2213-2600(21)00085-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chau VQ, Giustino G, Mahmood K, Oliveros E, Neibart E, Oloomi M, Moss N, Mitter SS, Contreras JP, Croft L, et al. Cardiogenic shock and hyperinflammatory syndrome in young males with COVID-19. Circ Heart Fail. 2020;13:e007485. doi: 10.1161/CIRCHEARTFAILURE.120.007485 [DOI] [PubMed] [Google Scholar]
- 17.Hékimian G, Kerneis M, Zeitouni M, Cohen-Aubart F, Chommeloux J, Bréchot N, Mathian A, Lebreton G, Schmidt M, Hié M, et al. Coronavirus disease 2019 acute myocarditis and multisystem inflammatory syndrome in adult intensive and cardiac care units. Chest. 2021;159:657–662. doi: 10.1016/j.chest.2020.08.2099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, Fu M, Heliö T, Heymans S, Jahns R, et al. ; European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636–2648, 2648a. doi: 10.1093/eurheartj/eht210 [DOI] [PubMed] [Google Scholar]
- 19.Ferreira VM, Schulz-Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U, Kindermann I, Gutberlet M, Cooper LT, Liu P, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72:3158–3176. doi: 10.1016/j.jacc.2018.09.072 [DOI] [PubMed] [Google Scholar]
- 20.Lurz P, Luecke C, Eitel I, Föhrenbach F, Frank C, Grothoff M, de Waha S, Rommel KP, Lurz JA, Klingel K, et al. Comprehensive cardiac magnetic resonance imaging in patients with suspected myocarditis: the MyoRacer-Trial. J Am Coll Cardiol. 2016;67:1800–1811. doi: 10.1016/j.jacc.2016.02.013 [DOI] [PubMed] [Google Scholar]
- 21.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 22.Messroghli DR, Moon JC, Ferreira VM, Grosse-Wortmann L, He T, Kellman P, Mascherbauer J, Nezafat R, Salerno M, Schelbert EB, et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: a consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J Cardiovasc Magn Reson. 2017;19:75. doi: 10.1186/s12968-017-0389-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kociol RD, Cooper LT, Fang JC, Moslehi JJ, Pang PS, Sabe MA, Shah RV, Sims DB, Thiene G, Vardeny O; American Heart Association Heart Failure and Transplantation Committee of the Council on Clinical Cardiology. Recognition and initial management of fulminant myocarditis: a scientific statement from the American Heart Association. Circulation. 2020;141:e69–e92. doi: 10.1161/CIR.0000000000000745 [DOI] [PubMed] [Google Scholar]
- 24.Ammirati E, Cipriani M, Lilliu M, Sormani P, Varrenti M, Raineri C, Petrella D, Garascia A, Pedrotti P, Roghi A, et al. Survival and left ventricular function changes in fulminant versus nonfulminant acute myocarditis. Circulation. 2017;136:529–545. doi: 10.1161/CIRCULATIONAHA.117.026386 [DOI] [PubMed] [Google Scholar]
- 25.Ammirati E, Frigerio M, Adler ED, Basso C, Birnie DH, Brambatti M, Friedrich MG, Klingel K, Lehtonen J, Moslehi JJ, et al. Management of acute myocarditis and chronic inflammatory cardiomyopathy: an expert consensus document. Circ Heart Fail. 2020;13:e007405. doi: 10.1161/CIRCHEARTFAILURE.120.007405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baran DA, Grines CL, Bailey S, Burkhoff D, Hall SA, Henry TD, Hollenberg SM, Kapur NK, O’Neill W, Ornato JP, et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock: this document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter Cardiovasc Interv. 2019;94:29–37. doi: 10.1002/ccd.28329 [DOI] [PubMed] [Google Scholar]
- 27.Katz D, Baptista J, Azen SP, Pike MC. Obtaining confidence intervals for the risk ratio in cohort studies. Biometrics. 1978;34:469–474. [Google Scholar]
- 28.Daniels CJ, Rajpal S, Greenshields JT, Rosenthal GL, Chung EH, Terrin M, Jeudy J, Mattson SE, Law IH, Borchers J, et al. ; Big Ten COVID-19 Cardiac Registry Investigators. Prevalence of clinical and subclinical myocarditis in competitive athletes with recent SARS-CoV-2 infection: results from the Big Ten COVID-19 Cardiac Registry. JAMA Cardiol. 2021;6:1078–1087. doi: 10.1001/jamacardio.2021.2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moulson N, Petek BJ, Drezner JA, Harmon KG, Kliethermes SA, Patel MR, Baggish AL; Outcomes Registry for Cardiac Conditions in Athletes Investigators. SARS-CoV-2 cardiac involvement in young competitive athletes. Circulation. 2021;144:256–266. doi: 10.1161/CIRCULATIONAHA.121.054824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kotecha T, Knight DS, Razvi Y, Kumar K, Vimalesvaran K, Thornton G, Patel R, Chacko L, Brown JT, Coyle C, et al. Patterns of myocardial injury in recovered troponin-positive COVID-19 patients assessed by cardiovascular magnetic resonance. Eur Heart J. 2021;42:1866–1878. doi: 10.1093/eurheartj/ehab075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, Shchendrygina A, Escher F, Vasa-Nicotera M, Zeiher AM, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5:1265–1273. doi: 10.1001/jamacardio.2020.3557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Halushka MK, Vander Heide RS. Myocarditis is rare in COVID-19 autopsies: cardiovascular findings across 277 postmortem examinations. Cardiovasc Pathol. 2021;50:107300. doi: 10.1016/j.carpath.2020.107300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ammirati E, Veronese G, Brambatti M, Merlo M, Cipriani M, Potena L, Sormani P, Aoki T, Sugimura K, Sawamura A, et al. Fulminant versus acute nonfulminant myocarditis in patients with left ventricular systolic dysfunction. J Am Coll Cardiol. 2019;74:299–311. doi: 10.1016/j.jacc.2019.04.063 [DOI] [PubMed] [Google Scholar]
- 34.Ammirati E, Cipriani M, Moro C, Raineri C, Pini D, Sormani P, Mantovani R, Varrenti M, Pedrotti P, Conca C, et al. ; Registro Lombardo delle Miocarditi. Clinical presentation and outcome in a contemporary cohort of patients with acute myocarditis: multicenter Lombardy registry. Circulation. 2018;138:1088–1099. doi: 10.1161/CIRCULATIONAHA.118.035319 [DOI] [PubMed] [Google Scholar]
- 35.Bonaca MP, Olenchock BA, Salem JE, Wiviott SD, Ederhy S, Cohen A, Stewart GC, Choueiri TK, Di Carli M, Allenbach Y, et al. Myocarditis in the setting of cancer therapeutics: proposed case definitions for emerging clinical syndromes in cardio-oncology. Circulation. 2019;140:80–91. doi: 10.1161/CIRCULATIONAHA.118.034497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bozkurt B, Colvin M, Cook J, Cooper LT, Deswal A, Fonarow GC, Francis GS, Lenihan D, Lewis EF, McNamara DM, et al. ; American Heart Association Committee on Heart Failure and Transplantation of the Council on Clinical Cardiology; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Epidemiology and Prevention; and Council on Quality of Care and Outcomes Research. Current diagnostic and treatment strategies for specific dilated cardiomyopathies: a scientific statement from the American Heart Association. Circulation. 2016;134:e579–e646. doi: 10.1161/CIR.0000000000000455 [DOI] [PubMed] [Google Scholar]
- 37.Cooper LT, Baughman KL, Feldman AM, Frustaci A, Jessup M, Kuhl U, Levine GN, Narula J, Starling RC, Towbin J, et al. ; American Heart Association; American College of Cardiology; European Society of Cardiology. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Circulation. 2007;116:2216–2233. doi: 10.1161/CIRCULATIONAHA.107.186093 [DOI] [PubMed] [Google Scholar]
- 38.Martinez MW, Tucker AM, Bloom OJ, Green G, DiFiori JP, Solomon G, Phelan D, Kim JH, Meeuwisse W, Sills AK, et al. Prevalence of inflammatory heart disease among professional athletes with prior COVID-19 infection who received systematic return-to-play cardiac screening. JAMA Cardiol. 2021;6:745–752. doi: 10.1001/jamacardio.2021.0565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pellegrini D, Kawakami R, Guagliumi G, Sakamoto A, Kawai K, Gianatti A, Nasr A, Kutys R, Guo L, Cornelissen A, et al. Microthrombi as a major cause of cardiac injury in COVID-19: a pathologic study. Circulation. 2021;143:1031–1042. doi: 10.1161/CIRCULATIONAHA.120.051828 [DOI] [PubMed] [Google Scholar]
- 40.Ammirati E, Varrenti M, Veronese G, Fanti D, Nava A, Cipriani M, Pedrotti P, Garascia A, Bottiroli M, Oliva F, et al. Prevalence and outcome of patients with acute myocarditis and positive viral search on nasopharyngeal swab. Eur J Heart Fail. 2021;23:1242–1245. doi: 10.1002/ejhf.2247 [DOI] [PubMed] [Google Scholar]
- 41.Veronese G, Cipriani M, Bottiroli M, Garascia A, Mondino M, Pedrotti P, Pini D, Cozzi O, Messina A, Droandi G, et al. Fulminant myocarditis triggered by OC43 subtype coronavirus: a disease deserving evidence-based care bundles. J Cardiovasc Med (Hagerstown). 2020;21:529–531. doi: 10.2459/JCM.0000000000000989 [DOI] [PubMed] [Google Scholar]
- 42.Bratincsák A, El-Said HG, Bradley JS, Shayan K, Grossfeld PD, Cannavino CR. Fulminant myocarditis associated with pandemic H1N1 influenza A virus in children. J Am Coll Cardiol. 2010;55:928–929. doi: 10.1016/j.jacc.2010.01.004 [DOI] [PubMed] [Google Scholar]
- 43.Tschöpe C, Ammirati E, Bozkurt B, Caforio ALP, Cooper LT, Felix SB, Hare JM, Heidecker B, Heymans S, Hübner N, et al. Myocarditis and inflammatory cardiomyopathy: current evidence and future directions. Nat Rev Cardiol. 2021;18:169–193. doi: 10.1038/s41569-020-00435-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, et al. ; RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Belhadjer Z, Méot M, Bajolle F, Khraiche D, Legendre A, Abakka S, Auriau J, Grimaud M, Oualha M, Beghetti M, et al. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142:429–436. doi: 10.1161/CIRCULATIONAHA.120.048360 [DOI] [PubMed] [Google Scholar]
- 46.Larson KF, Ammirati E, Adler ED, Cooper LT, Jr, Hong KN, Saponara G, Couri D, Cereda A, Procopio A, Cavalotti C, et al. Myocarditis after BNT162b2 and mRNA-1273 vaccination. Circulation. 2021;144:506–508. doi: 10.1161/CIRCULATIONAHA.121.055913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bozkurt B, Kamat I, Hotez PJ. Myocarditis with COVID-19 mRNA vaccines. Circulation. 2021;144:471–484. doi: 10.1161/CIRCULATIONAHA.121.056135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Verdonschot J, Hazebroek M, Merken J, Debing Y, Dennert R, Brunner-La Rocca HP, Heymans S. Relevance of cardiac parvovirus B19 in myocarditis and dilated cardiomyopathy: review of the literature. Eur J Heart Fail. 2016;18:1430–1441. doi: 10.1002/ejhf.665 [DOI] [PubMed] [Google Scholar]
- 49.Hjalmarsson C, Liljeqvist JÅ, Lindh M, Karason K, Bollano E, Oldfors A, Andersson B. Parvovirus B19 in endomyocardial biopsy of patients with idiopathic dilated cardiomyopathy: foe or bystander? J Card Fail. 2019;25:60–63. doi: 10.1016/j.cardfail.2018.07.466 [DOI] [PubMed] [Google Scholar]
- 50.Kawel-Boehm N, Hetzel SJ, Ambale-Venkatesh B, Captur G, Francois CJ, Jerosch-Herold M, Salerno M, Teague SD, Valsangiacomo-Buechel E, van der Geest RJ, et al. Reference ranges (“normal values”) for cardiovascular magnetic resonance (CMR) in adults and children: 2020 update. J Cardiovasc Magn Reson. 2020;22:87. doi: 10.1186/s12968-020-00683-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.