Abstract
Stress disorders are leading causes of disease burden in the U.S. and worldwide, yet available therapies are fully effective in less than half of all individuals with these disorders. While to date much of the focus has been on neuron-instrinsic mechanisms, emerging evidence suggests that chronic stress can affect a wide range of cell types in the brain and body, which are linked to maladaptive behavioral outcomes. Here we synthesize emerging literature and discuss mechanisms of how non-neuronal cells in limbic regions of brain interface at synapses, the neuro-vascular unit, and other sites of intercellular communication to mediate the deleterious, or adaptive (i.e. pro-resilient), effects of chronic stress in rodent models and in human stress-related disorders. We believe that such an approach may one day allow us to adopt a holistic “whole body” approach to stress disorder research, which could lead to more precise diagnostic tests and personalized treatment strategies.
2. INTRODUCTION
Stress-related disorders, such as major depressive disorder (MDD) and post-traumatic stress disorder (PTSD), are among the world’s greatest public health problems. Yet, their etiology and pathophysiology remain incompletely understood. While there are many effective treatments for these disorders, more than half of affected individuals are not fully treated by available antidepressant medications or other therapies (Gaynes et al., 2009; Rush et al., 2006). All existing antidepressants, which act initially on either the brain’s monoamine or glutamate systems, exert their eventual therapeutic actions through unknown mechanisms: many prominent theories suggest that the drugs’ therapeutic effects are not mediated via changes in monoaminergic or glutamatergic neurotransmission per se but require neuroplastic adaptations to those initial actions (Berton and Nestler, 2006; Duman and Monteggia, 2006; Kavalali and Monteggia, 2020; Pena and Nestler, 2018; Slattery et al., 2004). Challenges in treating these disorders also reflect the fact that they are highly heterogeneous syndromes, diagnosed today solely on the basis of behavioral abnormalities, with no biological endpoints used for diagnosis or to guide treatment (Goldberg, 2011; Zimmermann et al., 2009). Moreover, the exclusive focus on monoaminergic and glutamatergic transmission has ignored decades of unbiased transcriptomic studies pointing to broad perturbations in non-neuronal mechanisms in stress-related disorders beyond changes observed in neurons (Girgenti et al., 2020; Girgenti et al., 2021; Labonte et al., 2017; Pantazatos et al., 2017; Zhu et al., 2019). Our review examines emerging evidence that is providing a fundamentally more complete view of the non-neuronal contributions to stress action in rodent models, related human disorders, and their treatment. We focus on technological advances that have allowed for a multi-scale understanding of unique biological changes in a range of non-neuronal cells that contribute to an individual’s risk—or resilience to—stress over the lifetime.
3. GENERAL OVERVIEW
Considerable evidence supports the view that a series of highly inter-connecting brain structures—referred to as limbic regions—are important in regulating mood, motivation, and related emotional states under normal conditions and the abnormalities in these behavioral domains that characterize stress-related disorders as defined by DSM5 or by RDoC (Dunlop and Mayberg, 2014; Epstein et al., 2006; Price and Drevets, 2010; Sheline et al., 2002). These include the nucleus accumbens (NAc), medial prefrontal cortex (mPFC), hippocampus (HIP), amygdala (AMY), and ventral tegmental area (VTA), among other regions. We focus here largely on NAc and mPFC—based on the robust published data for these two regions—but whenever possible we discuss studies of other regions where evidence is available. In recent years, investigators have focused on molecular abnormalities in these structures that are induced in rodent stress models, with increasing investigation of human postmortem brain tissue as well. Early studies took a candidate gene approach, examining alterations in one or at most a few proteins or mRNAs, mostly of neuronal origin (Barrot et al., 2002; Covington et al., 2009; Golden et al., 2013; Menard et al., 2017; Monteggia et al., 2007; Vialou et al., 2010). The past decades have seen the increasing use of genome-wide methods—first microarrays and more recently RNA-sequencing (RNA-seq)—to provide a global view of alterations in gene expression in limbic brain regions of rodents and humans (Andrus et al., 2012; Bagot et al., 2016; Berton et al., 2006; Chang et al., 2014; Chaudhury et al., 2014; Hernandez et al., 2021; Hodes et al., 2015b; Kronman et al., 2021; Li et al., 2021; Seney et al., 2021; Yoshino et al., 2021). Such global approaches are essential, as they provide an unbiased view of genes most regulated by stress, as opposed to relying on our still very limited knowledge of human stress disorder pathophysiology. These unbiased screens—performed initially on bulk tissue dissections—have shown that some of the most highly regulated genes and molecular pathways are enriched not only in neurons, but also in astrocytes, myeloid cells, endothelial cells, or oligodendrocyte-lineage (OL) cells (Girgenti et al., 2021; Labonte et al., 2017). Limitations of these early data include their analysis of whole tissue extracts without first enriching for a given cell type, and use of a single rodent stress model and small cohorts of human brains, with generally inadequate attention given to sex differences. In this review we discuss established and emerging roles for nonneuronal cells in limbic regions of brain that interface at synapses, the neuro-vascular unit, and other sites of intercellular communication to mediate the deleterious effects, as well as the adaptive, protective (i.e., proresilient) effects, of different forms of chronic stress in rodent models and in human stress-related disorders, with some shared but many distinct mechanisms operating in males vs. females.
Major themes discussed in this review.
The review focuses on the role of 4 distinct non-neuronal cell types in stress action stated above: astrocytes, myeloid cells, endothelial cells, and oligodendrocytes. Though at first they may appear distinct, in reality, we will discuss how non-neuronal cells interact within the central nervous system (CNS) and at particular sites of intercellular communication (e.g., blood brain barrier (BBB)) to ultimately affect neural networks and complex stress-related behaviors (Fig 1). Three major themes related to intercellular communication are woven across the review sections. First, a major focus is on the ability of non-neuronal cells to affect neural connectivity in limbic brain regions by regulating synaptic inputs and myelination of axons. Second, we focus on interactions at the BBB, which is permeabilized in specific brain regions by stress in susceptible individuals, and serves as a critical site of communication between blood-derived signals and the CNS. Third, we focus on how the cross-talk between non-neuronal cells and neurons ultimately controls stress behaviors.
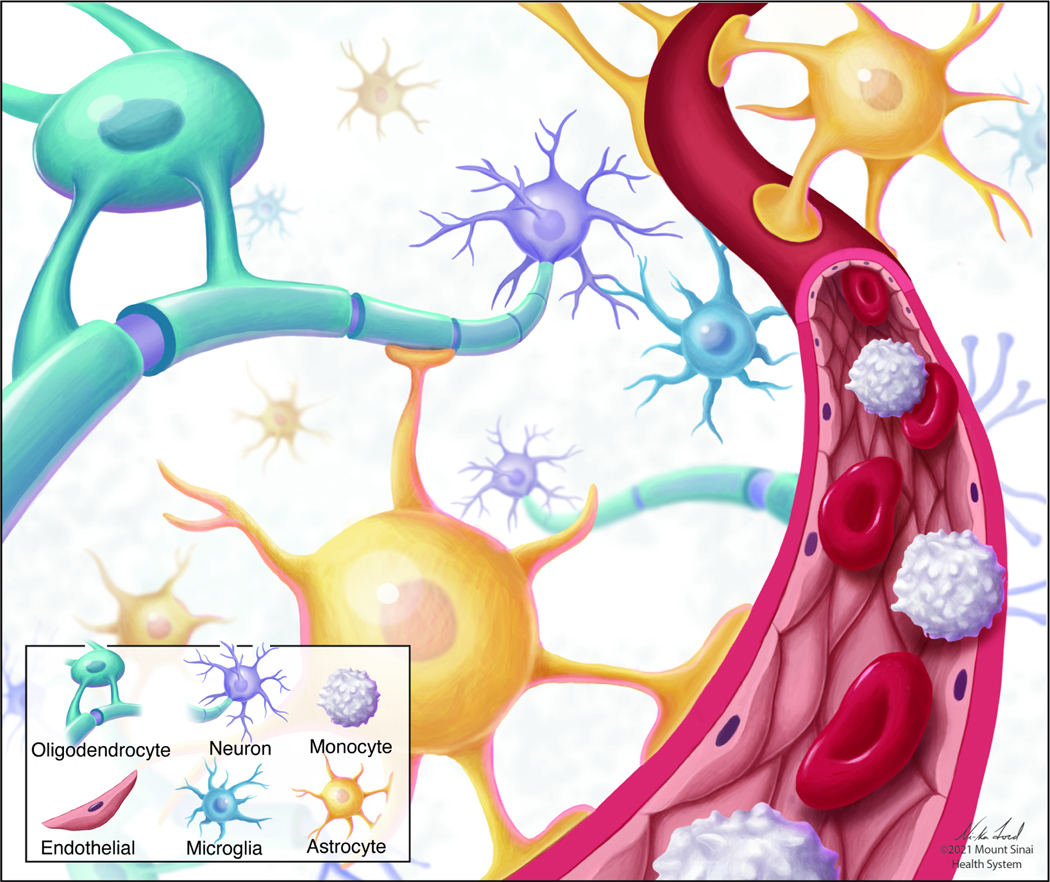
Fig 1. Interactions among non-neuronal cells in brain.
Non-neuronal cells interact at cellular barriers, including the blood brain barrier (BBB), synapses, and other sites of intercellular communication in the brain. Oligodendrocytes (turquoise); astrocytes (yellow); monocytes (white); microglia (blue); endothelial cells (brown); neurons (purple).
Non-neuronal cells in stress action.
Over many decades, stress research has focused almost exclusively on mechanisms of neuronal dysfunction from a cell-intrinsic perspective; that is, to identify a cellular or molecular change within a given neuronal cell type and then test whether that change is causally linked to the deleterious effects of stress on behavior. While this approach has yielded many important insights into stress action, it has largely ignored the contribution of non-neuronal cell types, which exist in high numbers throughout the brain of mammalian and non-mammalian species (Christoffel et al., 2015; Deyama et al., 2019; Donahue et al., 2014; Friedman et al., 2017; Golden et al., 2013; Hokenson et al., 2021; Issler et al., 2020; Tye et al., 2013). There are however considerable differences in the ratio of neuronal vs. non-neuronal cells across species that must be accounted for in basic research (Vasile et al., 2017): For example, the nervous system of C. elegans has more than 5 neurons per glia. In mammals, the glial:neuron ratio varies considerably across brain regions. In the cerebral cortex of humans, the glial:neuron ratio is approximately 4:1, while it is inverse in the cerebellum (Herculano-Houzel, 2014). Adding to the complexity there is increasing evidence that there are sex differences in non-neuronal cells in both rodents and humans (Guneykaya et al., 2018). Here we discuss four major non-neuronal cell types (Fig 1) affected by stress and altered in human stress disorders that interface with neurons in limbic brain regions to mediate the effects of stress on behavior. Decades of research have implicated myeloid cells (monocytes, macrophages and microglia) of the immune system in mediating responses to stress in both mouse models (Gallagher et al., 2019; Kronenberg et al., 2019; Lehmann et al., 2016; Pfau et al., 2019b; Wohleb et al., 2013; Woodburn et al., 2021; Yin et al., 2019) and human stress disorders (Bekhbat et al., 2020; Bottcher et al., 2020; Chiang et al., 2019; Hasselmann et al., 2018; Lago et al., 2020; Syed et al., 2018). Work in mice has shown that stress activates myeloid cells in the periphery, some of which then traffic to the brain to mediate stress-induced behavioral abnormalities. Translational studies in human patients and mouse models show that a subset of individuals with stress disorders exhibit a heightened immune response to stress and neuroimmune interactions with certain brain regions through damaged endothelial cells of the BBB that ultimately affects reward-related behaviors (Cathomas et al., 2022; Hodes et al., 2014; Menard et al., 2017; Pfau et al., 2019a). As well, glial cells in the brain, which have been historically described as support cells, are now being recognized to control many aspects of synaptic and circuit function and serve as a critical link between peripheral organ systems, the BBB, and neuronal signaling and ultimately behavior. The primary classes of glia include astrocytes, microglia, and oligodendrocyte lineage (OL) cells. Astrocytes and microglia are both capable of directly regulating synaptic transmission by shaping neuronal synapses (Liddelow et al., 2020; Schafer et al., 2012; Stevens et al., 2007). Astrocytes further regulate synaptic transmission by controlling extracellular levels of glutamate and can interface with myeloid cells of the periphery via the BBB (Kofuji and Araque, 2021a; Moura et al., 2017; Ross et al., 2020; Wang et al., 2021). Loss of astrocyte integrity can damage the BBB and allow for entry of peripheral factors that impact behavior (Abbott et al., 2006). Oligodendrocytes provide myelin sheathing to facilitate neural conductance, and serve as a metabolic interface with neurons, while oligodendrocyte progenitor cells (OPCs) receive direct synaptic inputs from neurons and facilitate cross-talk with other glial cell types (Domingues et al., 2016; Simons and Nave, 2015). Several recent papers (Bonnefil et al., 2019; Cui et al., 2018; Dudek et al., 2020; Hodes et al., 2014; Liu et al., 2012; Liu et al., 2018; Liu et al., 2020; Menard et al., 2017; Nagy et al., 2020; O’Leary and Mechawar, 2021; Rajkowska and Stockmeier, 2013; Woodburn et al., 2021) have shown that human stress disorders or rodent stress models are associated with alterations in many of these processes including: 1) trafficking of peripheral myeloid cells to the brain, 2) altered myelin content and OL cell dynamics, 3) alterations in glial- and myeloid-mediated synaptic alterations, and 4) impaired BBB integrity.
Animal models of stress disorders.
A major challenge in psychiatry research, more so than in other branches of medicine, is the challenge in generating animal models since all psychiatric syndromes today are still diagnosed solely on the basis of behavioral abnormalities many of which are inaccessible in animals. This challenge holds for stress-related disorders (Bale et al., 2010; Bliss-Moreau and Rudebeck, 2021; Fitzgerald et al., 2021; Nestler and Hyman, 2010; Simmons et al., 2021), including both translationally valid stress paradigms and behavioral readouts of stress exposure with high face and predictive validity. While certain tests (e.g., forced swim test) have proven useful in predicting clinical efficacy of currently available monoamine-acting antidepressants, results from these tests have not yielded major breakthroughs in developing novel antidepressants with better efficacy and faster onset (Nestler and Hyman, 2010). Many stress paradigms used in preclinical stress research typically involve acute stress—or chronic physical stress—in normal rodents, which is very different from the increased vulnerability to stress, typically emotional, seen in most patients with stress-related disorders. That vulnerability is thought to be due to a combination of genetic factors and life experiences. Genetic factors remain an intense area of research and recent GWAS studies have at long last begun to identify potentially interesting genomic loci involved in increasing risk for these disorders (Cai et al., 2020; Wray et al., 2018). However, it is clear that genetic risk for stress disorders is highly polygenic, involving many hundreds of genes acting in synergy, with each gene contributing a minute amount and heredity only accounting for 30–40% of the overall risk for developing an illness. Therefore, human genetic risk for stress disorders cannot be reproduced in rodent models. By contrast, the best-established risk factor for these conditions is a lifetime history of stress exposure, most commonly repeated or chronic emotional stress (Albert and Newhouse, 2019; Belleau et al., 2019; Otte et al., 2016), which is driving the field increasingly to focus on the latter (Kuske and Trainor, 2021; Russo and Nestler, 2013). For a comprehensive discussion on these pre-clinical stress paradigms across the lifespan see (Kuske and Trainor, 2021; Lopez and Bagot, 2021; Nestler and Hyman, 2010; Planchez et al., 2019; Schmidt et al., 2011; Torres-Berrio et al., 2019). In the present review, we discuss emerging evidence that chronic stress across the life cycle, via alterations in neuronal and non-neuronal cell types, affects complex behaviors that tap into translationally relevant domains and brain mechanisms that have been shown or are hypothesized to be disrupted in stress-related mental illnesses such as depression or PTSD.
4. ROLE OF ASTROCYTES IN STRESS DISORDERS
Astrocytes—the most prevalent type of glia in the brain—are best known for their roles in regulating synaptic signaling, metabolic coupling with neurons, and maintaining the BBB. Crucial to astrocyte function is their so-called star-shaped morphology, whereby astrocytes extend multiple primary branches that elongate into increasingly fine peripheral processes (Ben Haim and Rowitch, 2017; Sofroniew and Vinters, 2010). It is at these peripheral processes that astrocytes: 1) contact nerve terminals and dendritic spines to form the “tripartite synapse,” 2) form gap-junctions with other astrocytes, 3) engage with neurons to control their metabolic state and availability of certain neurotransmitters, and 4) form endfeet to enwrap blood vessels. Research in stress disorders shows consistent alterations in astrocyte number and morphology, loss of gap-junction coupling, and regulation of neuronal excitability and synaptic communication both in animal models and in human brain tissue. Recent studies have also highlighted the involvement of astrocytes, along with microglia, in mediating neuroinflammatory-like responses, synaptic dysfunction, and a damaged BBB in stress and MDD.
Changes in astrocyte number and morphology.
One of the most common ways to investigate astrocytic involvement in stress disorder subjects and rodent stress models has been to examine astrocyte density and morphological complexity. Reports demonstrate a reduction in the number and complexity of astrocytes across multiple brain regions, including the PFC and NAc (Banqueri et al., 2019). These studies have largely utilized glial fibrillary acidic protein (GFAP), a cytoskeletal protein specific to astrocytes, to stain and visualize astrocytes. However, it is important to note that not every astrocyte expresses GFAP at appreciable levels, and GFAP expression itself varies by brain region. Thus, there is some debate about whether there is a true reduction in the number of astrocytes, or instead a reduction in the number of GFAP+ astrocytes or in expression levels of GFAP in an unchanged number of total astrocytes. Studies identifying astrocytes by other methods, such as Nissl, S100B, or vimentin staining, have reported mixed results, with some confirming that stress or MDD is associated with a loss of astrocytes, with others reporting no change (Kim et al., 2018; Tynan et al., 2013). Notably, decreased GFAP expression is one of the most consistent findings in MDD patients and across chronic stress models in rodents, including chronic variable stress (CVS), early life stress, and genetic rodent models of anxiety-like behavior. Nevertheless, the utilization of GFAP allows for the concomitant examination of astrocyte morphology, and demonstrates loss of astrocyte branch complexity--including both branch number and arborization in multiple brain regions in both human MDD patients and rodent models (Bender et al., 2016; Kim et al., 2018). No change in branch complexity has been observed in response to acute stress (Bender et al., 2016; Kim et al., 2018). Importantly, treatment with a range of antidepressants has been shown to reverse changes in astrocyte branch morphology in rodents (Wang et al., 2017b).
The majority of astrocyte morphological complexity occurs at peripheral processes. Indeed, the cytoskeletal structure revealed by GFAP staining only encompasses roughly 10–15% of total astrocyte volume (Bushong et al., 2002). Examination of astrocyte peripheral process morphology is technically complex, given that these processes are thinner than the diffraction limit of normal microscopy techniques. Therefore, cellular reconstruction and estimates of astrocyte volume are most commonly used as a proxy for astrocyte complexity. To date, human postmortem studies have utilized sparse Golgi staining to report on overall astrocyte cell soma size, with mixed results. Hypertrophic astrocytes were found in the dorsolateral PFC of MDD patients and in the anterior cingulate cortex (ACC) in MDD patients who committed suicide (Kim et al., 2018). In contrast, no change in astrocyte size was observed in the frontal cortex (Kim et al., 2018).
Influence of astrocytes on synapses and neuronal activity.
Astrocyte peripheral processes enwrap upwards of 90% of synapses in the brain, which ideally positions astrocytes for monitoring and controlling neuronal and circuit activity, including maintaining ionic and neurotransmitter homeostasis, providing structural support, and facilitating elimination of synapses. To our knowledge, only one study has investigated astrocyte peripheral processes and synapse localization in the context of stress. The authors found that acute stress in rats decreased the number of presynaptic nerve terminals within astrocyte domains in the NAc core (Garcia-Keller et al., 2021). Interestingly, this acute stress paradigm did not alter overall astrocyte volume, suggesting that the loss of astrocytic contact of synapses could be driven by retraction of astrocytes from synapses rather than decreased astrocyte morphology per se. Thus, changes in the number of astrocyte peripheral processes may not be required for the loss of astrocytic control over synapses.
Astrocytes passively influence neuronal activity via neurotransmitter and ionic homeostasis. For example, the glutamate transporter SLC1A2 (also referred to as GLT1 or EAAT2), which is highly enriched in astrocytes in rodent and human brain, accounts for upwards of 90% of glutamate recycling at synapses, and loss of SLC1A2 is associated with disruptions of glutamate uptake and subsequent excitation-inhibition imbalances (Bechtholt-Gompf et al., 2010; Tanaka et al., 1997). Glutamate taken up by astrocytes via SLC1A2 is also converted to glutamine and shuttled to neurons to be converted back into glutamate. Importantly, loss of SLC1A2 is consistently found in multiple rodent stress models as well as in human MDD subjects at both the mRNA and protein levels, including in the PFC and NAc (reviewed in (Rajkowska and Stockmeier, 2013; Rappeneau et al., 2016) and Fig 2). Genetic deletion or pharmacological inhibition of SLC1A2 has been shown to elicit or exacerbate depressive-like behavioral phenotypes in rodents (Bechtholt-Gompf et al., 2010; Blacker et al., 2020; Fullana et al., 2020; John et al., 2012; Kofuji and Araque, 2021b). Additionally, imbalances in the glutamate-glutamine cycle are found in both rodent models and human MDD patients (Rappeneau et al., 2016). Given the recent shift in the field from monoamine-based treatments of MDD to ones focused on glutamate (based on the approval of ketamine—an NMDA glutamate receptor antagonist among other actions—for treatment-resistant depression), these findings further support the utility in targeting glutamatergic mechanisms in treating depression, and shed light on the involvement of astrocytes in the pathophysiology of this syndrome (Sanacora et al., 2012).
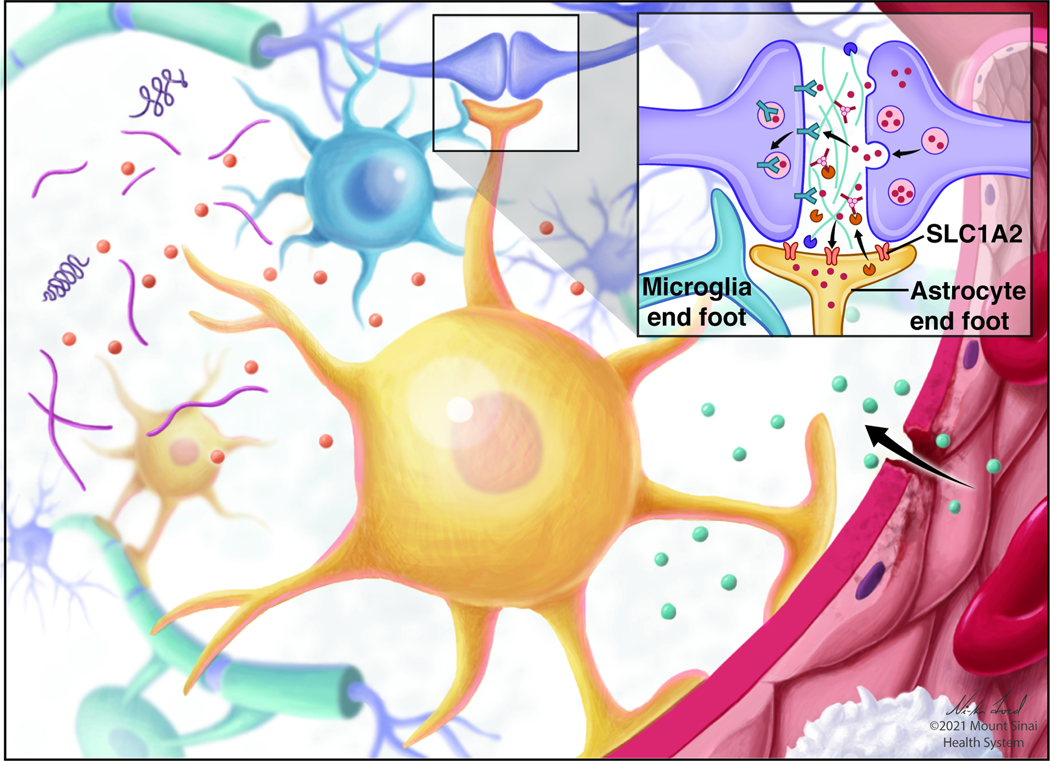
Fig 2. Stress effects on astrocytes.
Stress effects on astrocytes (yellow) and their interaction with the blood-brain barrier (BBB). As shown in the inset, SLC1A2 (also known as GLT1 or EAAT2) removes glutamate from the extracellular space. SLC1A2 is downregulated in both humans and rodent models of stress and depression. Additionally, loss of astrocyte endfeet integrity may loosen the BBB and allow peripheral factors into the brain.
Astrocytes also influence neuronal activity by buffering extracellular K+ after neuronal action potentials. The inward-rectifying K+ channel, KCNJ10 (also referred to as KIR4.1), is thought to be the main mediator of astrocytic extracellular K+ buffering (Nwaobi et al., 2016). Within the lateral habenula of genetically-derived learned helpless rats, KCNJ10 was upregulated and contributed to the modulation of neuronal burst firing. Furthermore, overexpression of KCNJ10 in astrocytes alone was sufficient to induce a depressive-like behavioral phenotype (Cui et al., 2018). KCNJ10 expression was increased as well in the parietal cortex of human MDD subjects (Xiong et al., 2019). Several antidepressants have been shown to inhibit KCNJ10 function, albeit at high, supra-therapeutic concentrations (Furutani et al., 2009; Ohno et al., 2007; Su et al., 2007). While the specific role of Ca2+ in astrocytes is still a topic of debate (Bazargani and Attwell, 2016; Khakh and McCarthy, 2015), Ca2+ signaling is thought to be the main mode of astrocyte communication, including the surveying and integration of neuronal activity, astrocyte-to-astrocyte communication, and release of factors that influence neurons. Ca2+ signaling in astrocytes is largely generated through two mechanisms: extracellular Ca2+ entry via plasma membrane ion channels or ionotropic glutamate receptors or the release of Ca2+ from internal stores (e.g., endoplasmic reticulum). Genetic deletion of IP3R2 in astrocytes, a major mechanism of releasing Ca2+ from internal stores, increased susceptibility to a depressive-like phenotype after chronic social defeat stress (CSDS) (Cao et al., 2013). Additionally, CSDS in rodents impairs Ca2+ responses and subsequent ATP release in the PFC (Cao et al., 2013), and fluoxetine treatment increased ATP release by astrocytes in the hippocampus (Kinoshita et al., 2018). Furthermore, transcranial direct current stimulation reduced chronic restraint stress-induced behavioral abnormalities in mice, as well as increased cortical astrocyte Ca2+ signaling (Monai et al., 2016). Serotonin-selective reuptake inhibitor (SSRI) antidepressants also increase Ca2+ signaling in PFC astrocytes regardless of neuronal activity, suggesting a direct effect of SSRIs on astrocytes which express certain serotonin receptor subtypes (Schipke et al., 2011). Finally, multiple studies of chemogenetic activation or attenuation of Ca2+ signaling in astrocytes have demonstrated an influence on both neuronal synapse activity and complex behaviors—including learning and memory, decision-making, fear conditioning, and goal-directed behavior (Kang et al., 2020; Kofuji and Araque, 2021b; Nagai et al., 2021).
Astrocytes and microglia.
More recently, interactions between astrocytes and other types of non-neuronal cells have been investigated. Most prominent are bidirectional communications between astrocytes and microglia to regulate neuronal synapse number as stated earlier (Han et al., 2021; Vainchtein and Molofsky, 2020). For example, astrocyte-derived interleukin-33 (IL-33) promotes microglia engulfment of hippocampal synapses during normal neurodevelopment (Vainchtein et al., 2018). While this astrocyte-microglia pathway has not been directly examined in the context of stress and depression, IL-33 expression is increased in rat PFC in response to acute footshock stress (Kudinova et al., 2016), and microglia engulfment of synapses is increased after 14 days of CVS (Woodburn et al., 2021). Furthermore, females diagnosed with recurring MDD exhibited higher peripheral levels of IL-33 than females with only one episode or no history of MDD (Kudinova et al., 2016). Conversely, reactive microglia are capable of inducing a reactive astrocyte transcriptional profile (termed A1/A2 astrocytes) in vitro (Liddelow et al., 2017). The microglia-induced effect has been attributed to a combination of cytokines, such as IL-1a, tumor necrosis factor (TNF), and complement C1q (Liddelow et al., 2017). Notably, TNF and IL-1 have both been previously implicated in depression in both MDD patients and rodent models (Dowlati et al., 2010; Goshen and Yirmiya, 2009).
Astrocytes and BBB.
The BBB is tightly maintained via tight junctions between adjacent endothelial cells, surrounded by pericytes, and finally enwrapped by astrocyte endfeet. Indeed, nearly the entire vascular network within the brain is covered by astrocytic endfeet (Abbott et al., 2006; Lundgaard et al., 2014; Mathiisen et al., 2010; Petzold and Murthy, 2011), underscoring that astrocytes contribute importantly to the physical barrier between the periphery and brain. Increasing evidence indicates that this barrier is impaired by chronic stress in rodents and in human MDD. For example, within the orbitofrontal cortex, immunofluorescence of AQP4 (an isoform of aquaporin that is enriched in astrocytes) revealed a 50% decreased colocalization between astrocytes and blood vessels in MDD patients (Rajkowska et al., 2013). Similar results were determined in rodents after chronic stress (Hallof-Bustrich and Di Benedetto, 2019). AQP4 expression itself is decreased in MDD (Rajkowska and Stockmeier, 2013), and single nucleotide polymorphisms (SNPs) in AQP4 have been found in a subset of patients diagnosed with atherosclerotic disease and a comorbid depression diagnosis (Westermair et al., 2018). AQP4 expression may be regulated by pericytes; astrocytic endfeet nearby pericytes exhibit higher levels of AQP4 than those nearby endothelial cells (Gundersen et al.). Astrocytes additionally contribute to the BBB and its permeability via the release of several cytokines, chemokines, and other factors. Acute stress increases the number of astrocytes expressing IL-1β (Sugama et al.). In response to IL-1β stimulation, astrocytes release VEGF, leading to greater BBB permeability and entry of leucocytes into the brain parenchyma (Rudzki and Maes). Additionally, CSDS in mice decreases endothelial cell tight junction protein claudin-5, subsequently increases BBB permeability and the infiltration of peripheral IL-6 into the brain (Menard et al., 2017). Cell culture experiments demonstrate that astrocytes stimulated with IL-6 increase production of cytokines associated with recruitment of T cells to the brain (Meares et al., 2012), which could further increase BBB permeability.
Transcriptomic mapping of astrocytes in stress and depression.
The above discussion highlights several astrocytic genes and functions that have been implicated in depressive-like phenotypes or their treatment. Genome-wide transcriptomic data identify numerous additional genes enriched in astrocytes as being among the most highly regulated in specific brain regions in mouse stress models and human MDD subjects examined postmortem (Bagot et al., 2016; Bagot et al., 2017; Labonte et al., 2017; Pantazatos et al., 2017; Writing Committee for the Attention-Deficit/Hyperactivity et al., 2021). Gene co-expression network analysis, which clusters genes based on coordinated regulation into “modules” and identifies key hub or driver genes deduced to play a coordinating role within a module, found modules in both human MDD and mouse stress models that include large numbers of astrocyte-enriched genes (Bagot et al., 2016; Labonte et al., 2017; Pantazatos et al., 2017). Such a role for astrocyte-enriched modules was particularly prominent in NAc and mPFC. Furthermore, a correlation between differences in cortical structure and cell-type-specific transcriptomics in MDD patients revealed that the highest number of overlapping genes were astrocytic (Li et al.). Changes in astrocyte gene expression occur even after acute stressors, indicating a potential role in the development of depression and other stress-related disorders. For example, microarray analysis after acute footshock demonstrated differentially-expressed genes enriched in astrocytes from rats, with persistent changes out to 20 days post-stressor (Ponomarev et al.). Furthermore, RNA-seq of the “translatome” (RNAs associated with ribosomes) in mouse cortical astrocytes revealed a robust change in astrocytes 90 minutes after a forced swim test (Murphy-Royal et al.). In particular, astrocytic expression of Cxn30—which encodes one of the major connexins for gap junction function—was decreased. Loss of Cxn30 and Cxn43 has also been demonstrated in the dorsolateral PFC of individuals who died by suicide (Ernst et al.). These findings together provide support for the hypothesis that stress alters gene expression in astrocytes within the NAc and mPFC and presumably other brain regions not yet investigated. Current work is focused on delineating the precise mechanisms by which regulation of such genes alters astrocyte function and consequently controls neuronal, synaptic, and circuit function to contribute to stress-related behavioral abnormalities.
5. ROLE OF MYELOID CELLS IN STRESS DISORDERS
Resident immune cells account for ~10% of all CNS cells. Tissue resident macrophages in the CNS, which belong to the mononuclear phagocytic system, can be divided into two subgroups: microglia (whose name stems from their relatively small soma, which are located in the parenchyma of the brain), and CNS-associated macrophages (CAMs), which are located at brain border regions (thus also called border-associated macrophages [BAMs]), such as the meninges, choroid plexus, and perivascular space (Li and Barres, 2018). Of note, while microglia are the only myeloid cells within the CNS parenchyma, several other types of immune cells of both the myeloid (e.g., monocytes, neutrophils) and lymphoid (e.g., B cells, T cells) lineage populate brain border regions (Mrdjen et al., 2018). These immune cells not only interact closely with each other but they also interact with other neuronal and non-neuronal cells of the CNS and play a crucial role in tissue homeostasis under physiological conditions, development, and disease.
Role of brain-resident myeloid cells in stress action.
Microglia and a majority of CAMs originate during embryogenesis from erythromyeloid progenitor cells from the yolk sac beginning around embryonic day 7.5 in mice and gestation week 4.5 in humans (Menassa and Gomez-Nicola, 2018), although the latter is much less well characterized (Bian et al., 2020). They maintain themselves by self-renewal, with little contribution from bone marrow-derived cells in peripheral circulation (Ginhoux et al., 2010). One exception are CAMs of the choroid plexus that are replenished by peripheral monocytes through fenestrated capillaries (Goldmann et al., 2016). Microglia implant in the developing brain around the same time as early neuronal development, consistent with the view that they are important in regulating and guiding embryonic neurogenesis and neuronal migration (Prinz et al., 2021). However, this microglial-neuron interaction is not restricted to prenatal development. Under homeostatic conditions, there is bidirectional communication between the two cell types to maintain neuronal function (Koo and Wohleb, 2021). Neurons release soluble factors such as fractalkine (CX3CL1) (Cardona et al., 2006) or colony-stimulating factor 1 (CSF1) (Elmore et al., 2014), while microglia release cytokines such as IL-1β or TNF-α (Schneider et al., 1998; Stellwagen and Malenka, 2006). Microglial TNF-α has been shown to regulate activity-dependent plasticity at established functioning synapses (Stellwagen and Malenka, 2006). In addition to cytokines, microglia secrete several other factors, including brain derived neurotrophic factor (BDNF) which modulates synaptic plasticity via tropomyosin-related kinase receptor B (TRKB) (Parkhurst et al., 2013). Another important aspect of microglial-neuron interactions is the ability of microglia to phagocytize synapses to shape neuronal plasticity (Wilton et al., 2019).
Microglia also play a role in surveillance and respond to a variety of stimuli indicative of changes in physiological homeostatic conditions. Thus, it is not surprising that they are involved in many pathological conditions, including the response to stress. While dynamic changes of microglia can be both neuroprotective “disease attenuating” and neurotoxic “disease promoting” (Shemer et al., 2015), early studies have shown that chronic stress changes microglia morphology, characterized by increased soma size and shorter and thicker cell processes, and is associated with increased phagocytic activity in limbic brain regions, such as the PFC, hippocampus, and amygdala (McKim et al., 2016; Tynan et al., 2010; Wohleb et al., 2011). While phagocytic removal of apoptotic cells and cellular debris through phagocytosis from the brain is crucial to maintain brain homeostasis (Lauber et al., 2004), recent studies have shown that stress can increase phagocytic activity of microglia and thereby promotes stress-related behaviors (Fig 3). For example, Wohleb et al. (Wohleb et al., 2018) demonstrated that stress-induced anxiety- and depressive-like behaviors were associated with increased expression of CSF1 gene expression in the PFC, which is necessary for development and maintenance of microglia. This increased CSF1 expression was also found in postmortem dorsolateral PFC of MDD patients compared to healthy controls. In addition, the authors described increased phagocytosis of neuronal elements and coinciding decreased dendritic spine density on apical dendrites of pyramidal neurons in the mPFC. In line with this study, CSDS increased the proportion of microglia expressing high levels of CD68, a marker of phagocytic activity, and they displayed increased phagocytic activity in an ex vivo culture preparation (Lehmann et al., 2016).
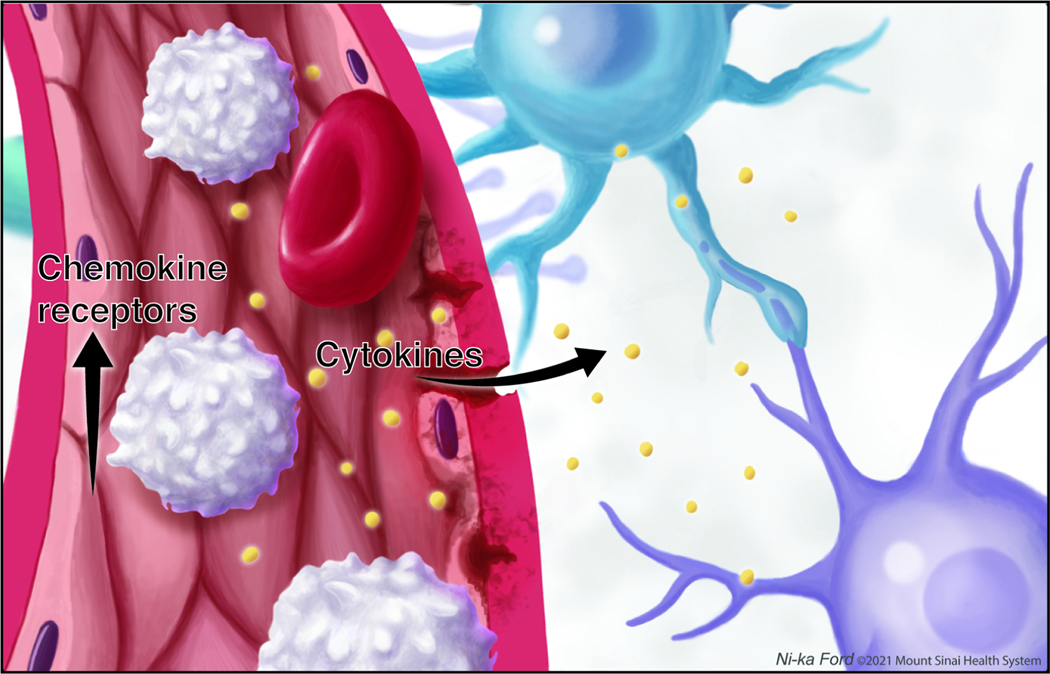
Fig 3. Stress effects on central and peripheral myeloid cells.
Stress results in trafficking of peripheral monocytes (white) to the brain via upregulation of chemokine receptors. In the brain, stress leads to activation of microglia (blue) and increased secretion of cytokines and production of reactive oxygen species.
Stress can also directly increase expression of several cytokines and chemokines or other damage-associated molecules in microglia (Avitsur et al., 2005). For example, a recent study using CSDS showed that microglia can be a source of reactive oxygen species, which have been associated with stress-induced behavioral changes in both rats and mice (Lehmann et al., 2019; Salim, 2014; Seo et al., 2012). Functional changes of microglia can be induced by molecules from both within the brain and from circulation. Chronic stress can activate microglia through local glucocorticoid and noradrenergic signaling (Frank et al., 2012; Iwata et al., 2013) as well as the NLRP3 (nucleotide-binding domain leucine-rich repeat and pyrin domain containing receptor 3) inflammasome. Microglia express pattern recognition receptors (e.g., phylogenetically conserved, germ-line encoded Toll-like receptors) (Qureshi and Medzhitov, 2003), and can thus bind PAMPS (pathogen-associated molecular patterns) and DAMPS (damage-associated molecular patterns), molecules induced in the CNS by stress (Fleshner et al., 2017). This leads to an activation of the NLRP3 inflammasome resulting in activation of the enzyme caspase-1 which proteolytically cleaves pro-inflammatory cytokines such as IL-1β (Wohleb et al., 2016). Several forms of rodent stress have been shown to activate NLRP3 inflammasome, both via DAMPs (such as HMGB1 (high-mobility group box 1) or ATP) and PAMPs, resulting in expression of stress-related behaviors.
One of the more exciting recent findings is that microglia can directly shape synaptic plasticity through synaptic pruning processes (Schafer et al., 2012). Synaptic pruning describes the process during development by which excessive synapses are removed in a controlled and timely manner to achieve a refined mature neural circuitry (Neniskyte and Gross, 2017). Such pruning can be regulated via the complement system, where for example C1q or C3 expressed on immature synapses can be recognized by corresponding receptors on microglia thereby marking them for phagocytosis (Stevens et al., 2007). Synaptic pruning continues throughout life and is thought to be an important dimension of normal synaptic and behavioral plasticity. Abnormalities in synaptic pruning have been reported in both neurodegenerative disorders such as Alzheimer’s disorder and psychiatric disorders such as schizophrenia (Hong et al., 2016). While synaptic pruning has been implicated in developmental changes in limbic brain regions involved in stress disorders such as the NAc (Kopec et al., 2018), if and how stress-induced changes in synaptic pruning is affected across the lifespan still needs to be elucidated.
Role of brain resident myeloid cells in human stress disorders.
Initially, the field hypothesized that human stress disorders, and their resultant behavioral pathologies, were mediated by aberrant microglia activation and increased local production of proinflammatory cytokines (Shelton et al., 2011; Torres-Platas et al., 2014). However, emerging studies of microglia cells in human stress disorders are far more variable, with some showing microglia activation and neuroinflammation and others showing no change or even an anti-inflammatory profile (Holmes et al., 2018; Li et al., 2018; Richards et al., 2018; Setiawan et al., 2015; Steiner et al., 2011; Su et al., 2016; Torres-Platas et al., 2014). Part of the inconsistency stems from multiple factors including the marker used to assess microglia activation, small sample size, and postmortem factors such as cause of death (e.g. suicide vs. non-suicide). Much of what we know regarding microglia activation in human stress disorder subjects comes from studies using positron emission tomography (PET) with radioligands that bind to translocator protein (TSPO), a microglia- and endothelial cell-enriched protein (Albrecht et al., 2016; Enache et al., 2019; Holmes et al., 2018; Li et al., 2018; Richards et al., 2018; Setiawan et al., 2015; Su et al., 2016). While several studies and a recent meta analysis consistently show increased TSPO binding in MDD patients, interpreted as increased microglia activation, the specificity of TSPO to distinguish between activated microglia versus other cell types has come into question (Perry, 2018; Veronese et al., 2018). Postmortem molecular studies have provided important additional information to better understanding the mechanisms of neuroinflammation in depression, although again the studies are mixed and a more nuanced interpretation is warranted (Bayer et al., 1999; Böttcher et al., 2020; Shelton et al., 2011; Snijders et al., 2020). For example, several studies argue that microglia activation and neuroinflammation is not a ubiquitous neuropathological change defining all types of depression, but rather it only occurs in depressed patients experiencing suicidality (Steiner et al., 2011; Torres-Platas et al., 2014). Gene expression analysis of isolated microglia from postmortem brain tissue in largely non-suicide depressed subjects did not find upregulation of markers of immune activation associated with depression (183). In fact, a recent single-cell analysis of microglia activation in MDD subjects suggests that they exhibit a “non-inflammatory” signature compared to healthy control subjects (Böttcher et al., 2020). Given the incomplete and often contradictory results observed in studies of microglia in patients with depression and related stress disorders, far more studies are need to clarify their roles.
Brain- resident myeloid cells and oligodendrocyte interactions.
Microglia also closely interact with oligodendrocytes and are important at various stages of oligodendrocyte development. For example, Nicholas et al. (Nicholas et al., 2001) has shown that microglia produce soluble factors that promote oligodendrocyte development through an effect on the platelet-derived growth factor-alpha (PDGF-α) receptor-signaling pathway. A subpopulation of microglia located in the white matter of the brain contribute to myelination (Hagemeyer et al., 2017). Similar to the synaptic pruning processes described above, microglia can phagocytose OPCs, a process that seems to be dependent on microglia CX3CR1 (Nemes-Baran et al., 2020). Interestingly, while there is clear evidence from both preclinical animal models and human postmortem studies that stress disorders such as MDD associate with impaired myelination (Liu et al., 2012; Lutz et al., 2017), it is still unknown whether this is due in part to increased phagocytosis of OPCs by microglia.
Peripheral myeloid cells in stress disorders.
It is now well accepted that a subset of patients with stress-related disorders show increased peripheral immune system activation (Cathomas et al., 2019; Dantzer et al., 2008). Early studies mainly focused on the role of peripheral cytokines in mediating stress-relevant behaviors, which has, analogous to the “monoamine hypothesis of depression”, led to the “cytokine hypothesis of depression” (Hodes et al., 2015a; Raison et al., 2006). While cytokines are mainly produced by cells of the immune system, several other cell types in the periphery (adipocytes, hepatocytes) and CNS (neurons, astrocytes) also produce cytokines (Turner et al., 2014).
Studies investigating the interaction between peripherally-derived cytokines of the immune system with the brain in preclinical mouse models have mainly used two different strategies: 1) activation of the immune system by administration of exogenous immune system activators such as the bacterial endotoxin lipopolysaccharide (LPS) and 2) mouse models of social stress that produce strong elevations of systemic inflammation. Both preclinical stress studies and studies of human stress disorder patients have pointed towards a major disturbance in peripheral myeloid cells (i.e., monocytes and neutrophils). Social stress mobilizes bone marrow-derived peripheral myeloid cells into circulation (Heidt et al., 2014; Powell et al., 2013). Several studies using transcriptional analysis of peripheral blood in humans with depression or related stress disorders have revealed important transcriptional changes indicative of systemic immune disturbances (Glahn et al., 2012; Jansen et al., 2016; Leday et al., 2018; Mostafavi et al., 2014; Savitz et al., 2013; Spijker et al., 2010; Yi et al., 2012). In the largest RNA-seq study to date (almost 500 cases and controls), Mostafavi et al. (Mostafavi et al., 2014) showed that the interferon α/β pathway was among the most highly regulated in MDD subjects. Leday et al. (Leday et al., 2018) compared data from two different whole blood microarray datasets and showed that 90 genes upregulated in both cohorts were significantly enriched for the gene ontology (GO) term “immune response to infection”. Most genes were affiliated with the gene network specialized for innate immune response including neutrophils, monocytes, and dendritic cells. Interestingly, the downregulated genes were enriched for GO terms related to T cell function and adaptive immunity, and clusters of strongly co-expressed genes were enriched in T cells, B cells, and NK-cells (Leday et al., 2018). These findings indicate that peripheral immune system dysfunction is associated with both activation of the innate immune system and relative suppression of the adaptive immune system.
In addition to these peripheral effects, animal models show that pro-inflammatory monocytes traffic to the brain in a CCL2/CCR2-dependent manner (Fig 3): genetic deletion of Ccr2 prevented the recruitment of monocytes to the brain and associated stress-induced behavioral changes (Wohleb et al., 2013). Importantly, there is a close interaction between CNS microglia, BBB endothelial cells, and peripheral monocytes. In fact, microglia can actively recruit peripheral monocytes, through an IL-1β-dependent mechanism, to stress-sensing brain regions where they regulate anxiety-like behaviors (McKim et al., 2018). Depletion of microglia with the CSF1R inhibitor plexxikon prevents such monocyte trafficking and anxiety-like behavior. A subsequent study confirmed the important role of endothelial IL-1 signaling in mediating sickness behavior: Endothelial IL-1R1 was necessary and sufficient for mediating sickness behavior and monocyte recruitment to the CNS, whereas ventricular IL-1R1 was critical for monocyte recruitment to the CNS (Liu et al., 2019).
Another important cytokine associated with stress-induced behavioral alterations is IL-6. Together with TNF-α, IL-6 is the cytokine most consistently elevated in circulation of patients with stress disorders (Dowlati et al., 2010; Miller et al., 2011). CSDS increases peripheral IL-6 specifically in susceptible mice, with no effect seen in resilient mice that are subjected to the same stress but avoid most behavioral abnormalities (Hodes et al., 2014). Both IL-6 neutralization with a systemically administered antibody and depleting IL-6 from bone marrow-derived leukocytes prevented susceptibility (Hodes et al., 2014). This was one of the first studies causally linking stress-induced cytokine changes in the periphery with behavioral alterations. While cytokines have extensively been shown to be increased in stress disorders, our knowledge of the exact mechanisms are sparser. Most neuronal and non-neuronal cells in the brain express cytokine receptors and several potential pathways have been proposed to link peripheral inflammation and neuronal function. One candidate pathway is tryptophan, the precursor of serotonin, and its catabolites (referred to as TRYCATs) (Savitz, 2020). Cytokines activate several enzymes of the kynurenine pathway which derives from tryptophan, not only depleting tryptophan but also leading to neuroactive catabolites affecting dopamine and glutamate (Savitz, 2020). Song et al. for example demonstrated that systemic administration of IL-6 decreases extracellular levels of dopamine in the NAc (Song et al., 1999). A potential mechanism downstream of IL-6 important for its behavioral effects is the NFκB signalling pathway. Previous studies demonstrated that NFκB signaling is activated by IL-6 in the NAc of susceptible mice following CSDS. Neuronal NFκB is necessary for increased excitatory synaptic plasticity induced by CSDS (Christoffel et al., 2011; Christoffel et al., 2015; Hodes et al., 2016). Pro-inflammatory cytokines such as IL-6, TNF, and IL-1β also have a direct effect on stress-evoked changes in neurogenesis and neuronal differentiation, suggesting that inflammatory signalling may play a broad role across multiple brain regions and neuronal cell types to regulate maladaptive plasticity (Borsini et al., 2015; Levin and Godukhin, 2017). Although beyond the scope of the current review, it is important to note that neurons express a large number of cytokine receptors themselves and thus their function can be directly modulated by cytokines from both within the CNS and from the periphery (Salvador et al., 2021). The importance of such interactions in stress-related disorders was recently described by Disabato et al. (DiSabato et al., 2021) where they showed that IL-1R1 on glutamatergic neurons in the hippocampus were causally linked to stress-induced impairments in social interaction and working memory deficits. Future work to more broadly define additional neuroimmune mechanisms across brain regions and cell types is needed.
6. ROLE OF THE ENDOTHELIAL BARRIER IN STRESS DISORDERS
The CNS has traditionally been viewed as an immune-privileged organ (Galea et al., 2007). However, there is increasing evidence that the brain interacts extensively with the peripheral immune system, both directly and indirectly (Louveau et al., 2015). The BBB tightly controls the bidirectional communication between the CNS and the peripheral circulation and is therefore vital for brain protection and function. This complex selective interface – referred to as the neurovascular unit – consists of several specialized cell types: non-fenestrated brain endothelial cells that are characterized by highly specific tight junctions sealing the para-cellular space, pericytes, and smooth muscle cells that play a major role in controlling the cerebral blood flow, and astrocytic endfeet covering most of the vasculature (Abbott et al., 2010). The immune system and BBB are tightly intertwined (Abbott, 2000). While under physiological conditions most peripheral cytokines or immune cells cannot penetrate the BBB or depend upon specialized transporters regulating their passage, pathological conditions such as acute or chronic inflammatory states can lead to increased BBB leakiness (Abbott, 2000).
The BBB in stress and depression.
Although early studies in mouse models showed that inflammation (e.g., via TNFα) compromises BBB integrity (Danielski et al., 2018), animal studies linking BBB dysfunction to depression-like behaviors have only recently been performed. Menard et al. showed that CSDS in stress-susceptible but not stress-resilient mice downregulated the endothelial tight junction gene and its protein product claudin-5 (Menard et al., 2017). This results in disruption of the BBB, allowing for the influx of potentially neurotoxic proteins such as peripheral IL-6. Claudin-5 gene expression was also shown to be downregulated in postmortem tissue from patients with MDD (Dudek et al., 2020; Menard et al., 2017). Interestingly, there is a sex-specific effect of stress on the BBB, where female mice exhibit endothelial damage and BBB permeability in the frontal cortex and NAc, whereas males only exhibit such damage in the NAc (Dion-Albert et al., 2022). In both cases, however, BBB damage is dependent upon a loss of endothelial integrity via downregulation of claudin-5 (Fig 4). Claudin-5 downregulation exhibits sex-specific effect in postmortem human PFC and NAc of MDD subjects similar to mice following chronic stress. The link between stress, systemic inflammation, and BBB permeability was further substantiated in a study showing that hippocampal BBB permeability was increased in mice that underwent learned helplessness and that BBB permeability and behavioral abnormalities could be reversed by systemic injection of a TNFα inhibitor (Cheng et al., 2018).
Fig 4. Stress impairs function of the blood-brain barrier (BBB).
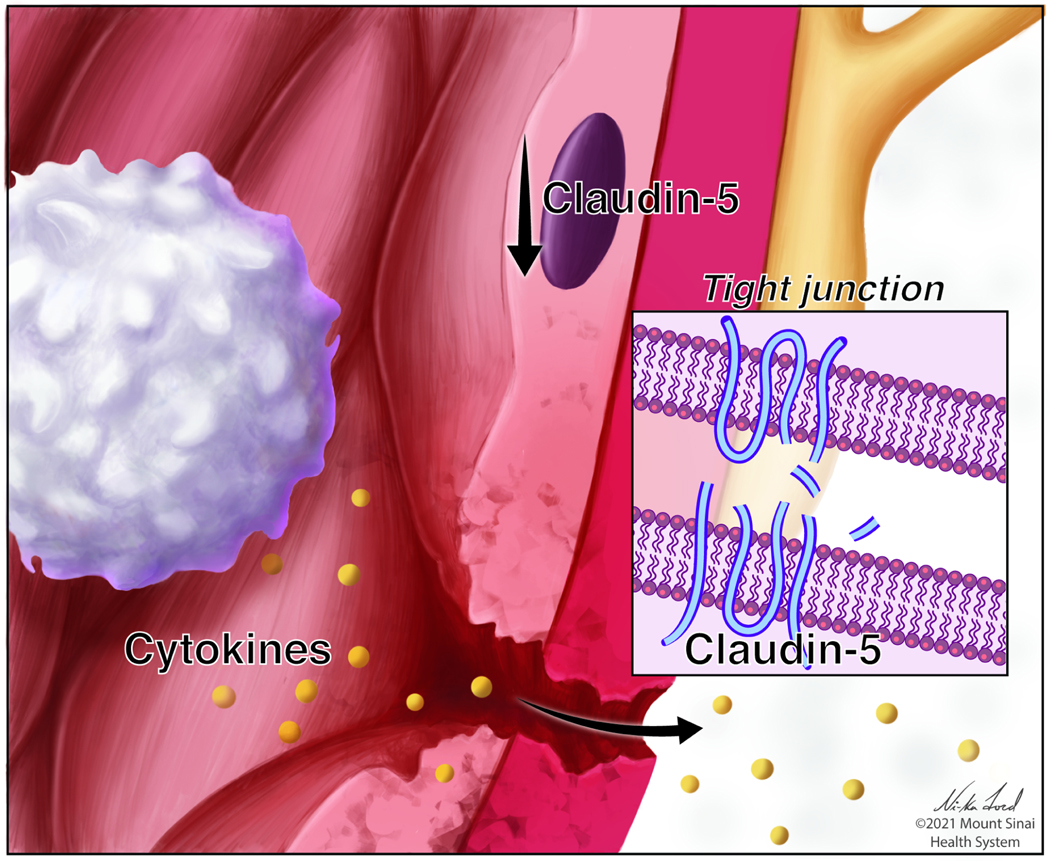
Stress leads to a damage of endothelial cells (brown), including brain-region specific downregulation of the tight junction protein claudin-5 (inset) resulting in increased permeability of the BBB and infiltration of peripheral factors such as cytokines.
In humans, BBB permeability can be assessed by neuroimaging to directly visualize infiltration of contrast dyes into the brain parenchyma or by indirect measures to examine the concentration of plasma vs. cerebrospinal fluid (CSF) proteins (Heye et al., 2014; Marchi et al., 2003). To our knowledge, no study has thus far investigated BBB differences in MDD patients vs. controls using neuroimaging approaches. Therefore, the evidence linking neurovascular dysfunction and MDD stems from studies that have used indirect measures like examining vascular markers in circulation or ratios between blood and CSF proteins, such as the CSF albumin/serum albumin quotient (Andersson et al., 1994). Because albumin is not synthesized centrally, albumin measured in CSF stems from the circulation, and this measure can therefore be used as a proxy to assess blood-CSF or BBB dysfunction. In a study performed in elderly women, those with MDD had a higher CSF/serum albumin ratio (Gudmundsson et al., 2007). Another peripheral marker of BBB dysfunction is S100β. This Ca2+-binding protein, which is mainly expressed in glial cells, is normally not detectable in serum; however, it is elevated in the presence of BBB damage (Kanner et al., 2003). To date, several studies have reported increased levels of S100β in patients with MDD compared to controls, and have shown associations with treatment response to antidepressants (Ambree et al., 2015; Polyakova et al., 2015; Schroeter et al., 2002). The related protein, S100A10 (also known as p11), is highly enriched in endothelial cells in humans and also implicated in MDD and antidepressant action (Milosevic et al., 2017). In a recent prospective cohort study, low-grade inflammation (assessed by CRP, serum amyloid A (SAA), intercellular adhesion molecule 1 (ICAM-1), IL-6, IL-8, and TNF-α), and endothelial dysfunction (assessed by vascular cell adhesion molecule 1 (VCAM-1), E-selectin, von Willebrand factor (VWF), and ICAM-1) were associated with depressive symptoms, while endothelial dysfunction was further associated with chronicity of depressive symptoms (Janssen et al., 2021). In addition, an early study showed increased markers of BBB permeability in MDD compared to controls (Niklasson and Agren, 1984). In summary, since there is a bidirectional interaction between peripheral inflammation and endothelial cell function, it can be hypothesized that both contribute to the etiology and pathophysiology of MDD.
7. ROLE OF OLIGODENDROCYTE LINEAGE CELLS IN STRESS DISORDERS
OL cells include several unique cell subtypes that differ at the transcriptional, morphological, and functional level. OL cells are the only myelinating cell type in the CNS. They are characterized by a specialized membrane, called myelin, with a unique proteolipid composition and the ability to wrap around axons, thereby creating areas of insulated axonal segments called internodes (Baumann and Pham-Dinh, 2001; Simons and Nave, 2015). The discrete regions between internodes, called nodes of Ranvier, are characterized by high expression levels of voltage-gated Na+ channels and other specialized proteins, which together mediate saltatory conduction of action potentials. Myelinating OL cells also serve a main role in providing metabolic support to neurons. During development, OL cells are generated from neonatal OPCs, which continue to persist in the adult brain. OPCs are very dynamic and electrically-responsive, characterized by the ability to proliferate, migrate, and differentiate in response to neural activity. They respond to signals from glutamatergic, GABAergic, and potentially other neuronal subtypes.
OL cells respond to stressful events throughout the lifespan.
There is a large literature, both in humans and rodents, supporting the age-dependent response of myelin and OL cells to distinct types of stressors. Evidence ranges from MRI and histological studies in postmortem human or rodent brains to transcriptional studies using punch biopsies or sorted cells from human brains or animal models. Within the prenatal and neonatal periods, exposure to parental stress has been shown to alter white matter microstructures in the frontal lobe (Dean et al., 2018) and amygdala (Rifkin-Graboi et al., 2013) of infants from mothers with reported depression and anxiety symptoms. Additional reports further validated the concept that developmental myelination of neural tracts is negatively impacted by maternal depression (Posner et al., 2016; Scheinost et al., 2016) or exposure to stress (Lautarescu et al., 2020), highlighting the importance of myelination in favoring the development of connectivity involved in emotional regulation. Important information on the role of early life exposure to stress and the development of psychiatric symptoms in adulthood are also emerging from studies on preterm babies (Lammertink et al., 2020). While recent evidence argues against a generalized effect of prematurity on overall volume of brain structures (Lautarescu et al., 2021), it is becoming clear that prematurity and exposure to the stressful environment of the neonatal intensive care unit interfere with autonomic nervous system development, and especially with myelination of vagal fibers from the nucleus ambiguus (Porges and Furman, 2011; Sachis et al., 1982), which may in part explain the higher propensity for long-term dysregulation of vagal inhibitory control on heart rate and respiration detected in individuals who were born preterm. As well, evidence supporting the critical role of stress during the period of developmental myelination includes studies on maternal separation reporting aberrant PFC myelin formation (Carlyle et al., 2012; Yang et al., 2017).
Childhood and adolescence are periods of significant risk related to the impact of stress on OL cell development and function. Imaging studies in children subjected to the stress of institutionalization revealed correlations between white matter changes detected by MRI and time spent in orphanages prior to adoption (Govindan et al., 2010; Kumar et al., 2014). Studies of adoptees highlighted reduced size of major white matter tracts, such as corpus callosum, as potentially related to behavioral adjustments of children to their new environment (Mehta et al., 2009). The long-term consequences of early life stress on white matter abnormalities have been shown to persist into adulthood in the brains of MDD subjects (Choi et al., 2012; Choi et al., 2009; Hanson et al., 2015; Lutz et al., 2017; Siehl et al., 2018; Tanti et al., 2018). Furthermore, other forms of stressful experience in adolescence such as social isolation or sleep deprivation significantly impact adolescent white matter tract development (Jamieson et al., 2021a; Jamieson et al., 2021b). The functional consequences of these alterations during such a critical period for psychological and emotional development is just beginning to be elucidated, but are likely to be catastrophic, as indicated by high rates of suicide among teenagers associated with early stress exposure (Mayne et al., 2021). In summary, stressful experience during critical developmental periods in humans severely impairs white matter tract development in the PFC, a finding which is consistent with previous reports in rodents (Liu et al., 2012; Makinodan et al., 2012).
Most of the literature on the effect of stress on white matter integrity in the adult brain refers to alterations detected in patients with MDD or other stress-related disorders such as PTSD or anxiety disorders, with results consistently showing decreased myelin content in frontal cortical circuitry (Baur et al., 2011; Murphy and Frodl, 2011; Phan et al., 2009; Sacchet and Gotlib, 2017), as well as in the corpus callosum and thalamic tracts (Gunning-Dixon et al., 2008; Kumar et al., 2004; Miyata et al., 2016; Siehl et al., 2018). Histopathological studies of postmortem brains from stress disorder subjects are largely consistent with in vivo imaging findings: they show reduced myelin content, fewer oligodendrocyte cells, reduced levels of myelin gene transcripts, and in some cases micro-alterations in the length of the internodal segments (see below) (Aston et al., 2005; Boda, 2021; Hamidi et al., 2004; Hayashi et al., 2011; Miyata et al., 2016; Rajkowska et al., 2015; Seney et al., 2018; Tham et al., 2011; Williams et al., 2019). The relationship between demyelination in areas of the limbic system and depressive symptoms was suggested by studies in multiple sclerosis (MS) patients, who display increased risk of depression and anxiety (Habek et al., 2006; Pham et al., 2018; Rocca et al., 2018; Sanders and van Lieshout, 1992; Simpson et al., 2016). Moreover, stress is known to alter the course of MS, including precipitating relapse during symptom remittance and exacerbation (Ackerman et al., 2002; Buljevac et al., 2003; Mitsonis et al., 2008). Exposure of adult mice to CVS, social isolation, or CSDS also revealed overall decreased myelin transcript levels (Bonnefil et al., 2019; Lehmann et al., 2017; Liu et al., 2012; Liu et al., 2018), although important differences were observed between the distinct stress models. The most notable difference is the reported number of neuron glial antigen 2+ (NG2+) OPCs, which was found to be reduced in CVS models (Banasr et al., 2007; Liu et al., 2018; Yang et al., 2016), but increased in susceptible mice following CSDS (Bonnefil et al., 2019). The functional relevance of these differences have yet to be elucidated. OPCs in the brains of stressed mice were also characterized by the presence of abnormal histone marks, suggestive of an overall defective mechanism of epigenetic repression [71]. Interestingly, a recent transcriptomic analysis of single nuclei isolated from the PFC of MDD subjects identified a cluster of immature OPCs with dysregulated gene expression (Nagy et al., 2020). This cluster included a profile indicative of a very immature population of OPCs, displaying higher levels of transcripts which previously were reported to be downregulated during differentiation into OL cells by repressive histone marks (Liu et al., 2015).
Mechanisms of dysregulation in OL cells in response to stress.
As shown in Fig 5, stress affects myelination in three ways: 1) myelin loss or reduced thickness of existing sheath, 2) impaired “de novo” myelination and OPC differentiation, or 3) adjusted internodal length. While these changes may all have been detected in distinct studies, they do not necessarily occur in concert. Human studies provide ample evidence for the integrity of myelin as well as transcriptional alterations in stress disorders, whereas animal studies provide further characterization of the ultrastructural alterations of white matter tracts and dysregulated OL cell population dynamics, thereby suggesting several potential explanations of the effect of stress on this lineage. The mechanisms underlying each type of change in myelin requires investigation.
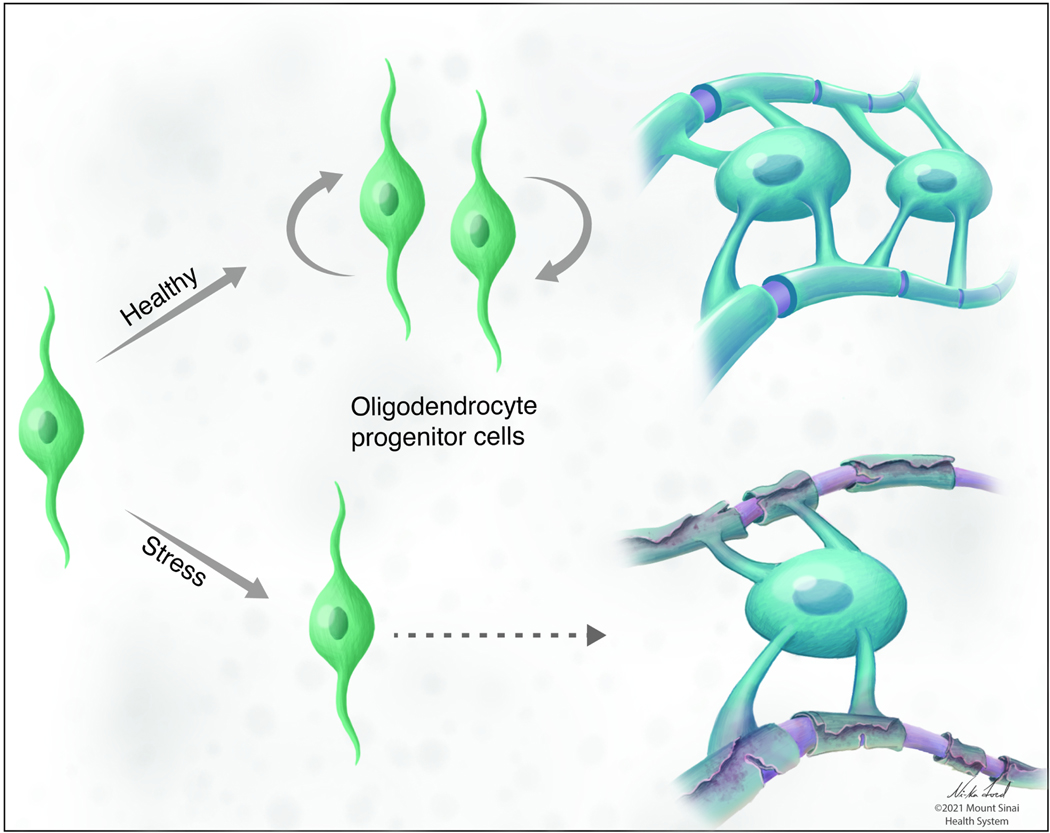
Fig 5. Stress effects on oligodendrocyte lineage (OL) cells.
Stress induces brain-region-specific impairments of myelination that is a result of reduced OL cell differentiation and maturation potentially through mechanisms such as oxidative stress or immune dysregulation in the periphery.
Inflammatory mechanisms of myelin and OL cell loss in response to stress.
The link between demyelinating disorders, such as multiple sclerosis (MS), and depression suggests that dysregulation of immune system and elevated levels of pro-inflammatory cytokines may be a common mechanism between them (Beurel et al., 2020; Pucak et al., 2007). Pro-inflammatory cytokines, released by T cells and astrocytes, such as interferon-gamma (IFNγ) and IL-17, have been shown to inhibit the ability of OPCs to exit from the cell cycle and differentiate (Balabanov et al., 2007; Pucak et al., 2007; Wang et al., 2017a), while promoting their ability to serve as putative antigen-presenting cells and eventually leading to apoptosis (Wang et al., 2017a). An additional signal responsible for decreased progenitor numbers and impaired differentiation into myelinating cells is upregulation of death receptor 6 (DR6), a member of TNF receptor superfamily, in OPCs in mice exposed to chronic stress (Yang et al., 2016). OL cells may also be a direct target for T cell-derived metabolites induced by stress, such as xanthine, a purine derivative elevated in patients with MDD and in mice exposed to chronic stress (Ali-Sisto et al., 2016; Fan et al., 2019). Using metabolic profiling along with single-cell transcriptomics, it was reported that CD4+ T cell-derived xanthine acts directly on OPCs in the amygdala via adenosine A1 receptors whose activation promoted OPC proliferation and resulted in reduced number of mature OL cells (Fan et al., 2019). Silencing A1 receptor specifically in OL cells rescued anxiety-like behavior, even in mice with elevated levels of xanthine, providing a direct link between T cell metabolism and OL cells in stress-driven anxiety-like behavior. Finally, oxidative stress, consequent to microglia activation and inflammation, is frequently detected both in MDD (Spaas et al., 2021; Yirmiya et al., 2015) and in MS (Lassmann and van Horssen, 2016; Schuh et al., 2014) brains. This is of relevance because oxidative DNA damage and protein and lipid peroxidation in OL cells (Giacci and Fitzgerald, 2018; Jana and Pahan, 2007) impair OPC maturation (French et al., 2009), and lead to decreased levels of myelin and lower numbers of oligodendrocytes. As mentioned above, pro-inflammatory cytokines can diffuse into the brain of stressed individuals due to stress-induced neurovascular damage and increased BBB permeability (Menard et al., 2017). Whether or not OL cells play a role in the effects of stress on BBB permeability remains unknown, however, a recent study linked OPC-derived matrix metalloproteinase 9 (MMP9) to BBB opening and neutrophil infiltration in early stages of white matter injury (Seo et al., 2013).
Impaired “de novo” myelination and OPC differentiation in response to stress.
Decreased OL cell number and impaired OPC differentiation are commonly observed in MDD patients and stressed animals (Bonnefil et al., 2019; Hamidi et al., 2004; Liu et al., 2016; Yang et al., 2016) and several mechanisms, including hormonal signals and neuronal activity, have been implicated. Glucocorticoids and their receptors are expressed by both OPC and OL cells (Matsusue et al., 2014), where they have been shown to inhibit OPC proliferation (Alonso, 2000) and induce abnormal branching of OL cells (Miyata et al., 2011). A separate mechanism, possibly relevant to decreased myelin levels in animals and humans subjected to social isolation is decreased neuronal activity, which is a well-known regulator of myelination (de Faria et al., 2019). Neuronal activity induces OPC proliferation, differentiation, and de novo myelination (de Faria et al., 2019; Gibson et al., 2014) and also affects the stability of newly formed myelin sheaths on axons (Gibson et al., 2014; Mensch et al., 2015). Conversely, decreasing neuronal activity with pharmacological treatment or optogenetics leads to reduced OPC proliferation, lower number of myelinating OL cells, and overall decreased de novo myelination (Demerens et al., 1996; Mensch et al., 2015; Wake et al., 2011). The reported loss of excitatory synapses in frontal cortex of MDD patients and animal stress models (Duman and Aghajanian, 2012; Liu et al., 2017) may be related to the reduced OL cells and myelin content in conditions of chronic stress. The concept is perhaps best exemplified in the model of social isolation of juvenile mice, which is associated with reduced excitatory synaptic inputs on PFC pyramidal neurons and with hypomyelinated neurons (Makinodan et al., 2017; Makinodan et al., 2012; Tan et al., 2021; Yamamuro et al., 2018). Conversely, enriched environments that promote stress resilience increase PFC neuronal activity and promote myelinogenesis (Goldstein et al., 2021; Nicholson et al., 2020). However, when stress is experienced during certain critical developmental period, environmental enrichment is not capable of restoring impaired myelination (Makinodan et al., 2012), which might due in part to premature OPC differentiation that exhausts the pool of existing progenitor cells in adulthood (Teissier et al., 2020).
At a molecular level, impaired OPC differentiation as detected in stress disorder patients and in animal stress models can be partly explained by alterations of the epigenome and consequent changes in gene expression. For instance, whole genome DNA methylation profiles in the ACC of MDD individuals with a history of child abuse identified altered DNA methylation in OL lineage genes and a global impairment of the OL-related transcriptional program (Lutz et al., 2017). Aberrant nuclear chromatin structure and altered expression levels of histone modifiers was also reported in OL cells in the PFC of socially isolated mice (Liu et al., 2012), whereas treatment with clemastine, a muscarinic receptor antagonist, promoted histone methyltransferases activity and was sufficient to restore myelination and social behavior (Liu et al., 2016). Reduced heterochromatin content and repressive histone marks was also detected in mice susceptible to CSDS, but not in resilient mice (Bonnefil et al., 2019), suggesting that stress may disrupt the epigenetic program in OL cells thereby promoting maladaptive behavioral consequences in susceptible individuals.
Remodeling of internodes in response to stress
Micro-alterations of myelin structure have been reported in several animal stress models. Shorter internodal length was detected in mice susceptible to CSDS and was significantly correlated with social interaction behavior (Bonnefil et al., 2019). The length of nodes of Ranvier as well as their boundary regions, the paranode, was shortened in mice subject to chronic restraint stress (Miyata et al., 2016). This was characterized by a diffused distribution of contactin-associated protein (Caspr) and the voltage-dependent K+ channel Kv1.1, suggesting a disruption of axon-myelin adhesion. Interestingly, in socially isolated adult mice, expression of neuronal-specific nodal and paranodal genes was unaffected, whereas expression of OL cell-specific paranodal genes was decreased in the PFC (Liu et al., 2012), suggesting a cell-type-specific response.
8. SUMMARY AND FUTURE DIRECTIONS
As discussed throughout this review, important advances have been made recently in uncovering the role of non-neuronal cells in stress-related disorders such as MDD and PTSD. However, it is also clear that the field needs a far better understanding of non-neuronal mechanisms, both under normal and pathological conditions. Available data suggest that non-neuronal mechanisms play very diverse roles in brain function. In astrocytes, studies have confirmed that stress alters their number and morphology and influences their interactions with neurons, microglia, and the BBB. While we continue to learn about the roles of astrocytes in brain function, there remains many unknowns regarding the true extent of their involvement in stress-related illnesses. Both peripheral and central myeloid cells (monocytes and microglia), through interactions with several other non-neuronal cell types, play crucial roles in mediating stress-induced effects on brain and behavior, though much additional research is needed. For example, a majority of the studies that characterize microglia state after stress still use relatively simple markers of activation or classification approaches such as M1 (i.e., activated, pro-inflammatory) vs. M2 (i.e., anti-inflammatory) phenotypes that do not account for the complexity and heterogeneity of these resident immune cells (Borsini et al., 2015). Additionally, we need a better understanding about how peripheral monocytes interact with the brain. While evidence suggests that they are recruited to the brain endothelium and may initiate endothelial-specific inflammatory signaling to the brain, several important questions remain. For example, do they play a direct role in stress-induced disruption of endothelial cells? Are they actively trafficked to specific places within the brain endothelium and is this why stress seems to affect the BBB in some but not all brain regions? We know from recent single cell RNA-sequencing (scRNA-seq) studies that the gene expression signature of endothelial cells is highly dependent on the size and type of blood vessel and brain region. However, we do not know how these translate to the observed differences in BBB leakiness in the context of stress disorders. Finally, the evidence suggests that impaired myelination by oligodendrocytes in response to stress associates with stress-induced behavioral and neuronal alterations, which is consistent with the idea that stress-related signals eventually cause loss of myelin in areas related to emotional regulation and executive function. Those signals may include oxidative stress or cytokine signaling from the periphery, resulting in a myelinotoxic environment in the brain. On the other hand, the regional specificity (i.e., within PFC) of stress-induced myelination defects appears to challenge such an interpretation, given that research has shown little BBB damage or subsequent peripheral cytokine infiltration in the PFC. The recent identification of immature OPC transcriptional signatures in PFC of MDD subjects and stressed mice (Bonnefil et al., 2019; Liu et al., 2012; Nagy et al., 2020; Seo et al., 2013) may suggest that stress affects epigenetic mechanisms intrinsic to OPCs. Whether or not such OL cell dysfunction is causally linked to the onset of depression remains an active area of investigation. In sum, while non-neuronal cells are likely to be key factors in stress-related disorders, there are many gaps in the preclinical and clinical literature, including the need for a much broader understanding of non-neuronal perturbations in both sexes throughout the brain and body. Below we have highlighted important avenues for future investigation:
One important aspect of future research will be the identification of blood and cerebrospinal fluid biomarkers with high sensitivity and specificity to detect and quantify alterations in non-neuronal cells. While a few biomarkers, such as astrocyte derived S100B and endothelial cell adhesion molecules exist, several open questions need to be addressed: Are these markers that have mainly been used in disorders like traumatic brain injury or multiple sclerosis sensitive enough to measure the more subtle changes associated with stress-disorders? How specific are these biomarkers given that although enriched in certain cell types, they could also be derived from sources outside the CNS, e.g. S100B from adipocytes (Goncalves et al., 2010)?
Another important avenue of research is the development of novel in vivo neuroimaging techniques in both rodents and humans to assess non-neuronal cells with far greater precision and specificity. Currently most approaches utilized to date are not able to specifically identify individual cell types; one example is the use of radiolabeled ligands binding to the translocator protein (TSPO) (Notter et al., 2018), which are incapable of distinguishing between astrocytes, microglia and endothelial cells. In addition, existing neuroimaging modalities like PET and MRI could be better integrated with blood based biomarkers and clinical and behavioral phenotyping to obtain much higher resolution clinical information about the role of non-neuronal cells in stress disorders.
Ultimately, the development and application of tools to manipulate non-neuronal cells in pre-clinical animal models is crucial to establish causal mechanisms. Indeed, recent advances have been made to enable the cell-type specific genetic manipulation of non-neuronal cells with transgenic Cre driver lines and viral vectors (Dumas et al., 2021; Galichet et al., 2021; Yu et al., 2020). Studies have also begun to apply optogenetic and chemogenetic approaches to manipulate non-neuronal cells. Much of the work to date has focused on astrocytes, and have utilized Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) to regulate calcium signaling (Yu et al., 2020). Activation of astrocytes using Gq DREADDs in the central amygdala decreased the firing rate of neighboring neurons and reduced fear expression in a fear-conditioning paradigm (Martin-Fernandez et al., 2017).” Though limited, some studies are beginning to apply these approaches to other non-neuronal cells including bone marrow derived immune cells (Bohineust et al., 2020). Such work has shown that optogenetic activation of T cells can alter intracellular Ca2+ signaling, T cell migration, adhesion, and chemokine release. While much more work is needed, such approaches offer promising new avenues for controlling non-neuronal cells throughout the body.
A major need in the field is to understand the extent to which sex differences in non-neuronal cells influences the ~2-fold greater risk for stress disorders in girls and women. Several early lines of evidence from large scale transcriptomic studies described above point to a host of sex differences in non-neuronal cell types throughout the brain and body. For example, as mentioned above, data from rodent stress models point towards sex differences in endothelial dysfunction after stress. The importance of such sex differences in the behavioral response to stress have yet to be elucidated, including whether PFC damage in females is associated with more profound disturbances in PFC-dependent behaviors than observed in males. From a therapeutic perspective, we’ll need to gain a very high-dimensional understanding of non-neuronal mechanisms perturbed in stress disorders if we aim to develop personalized medications optimized for the unique physiology of women vs. men. Novel molecular tools such as brain-region specific scRNA-seq should be applied to appropriately identify stress-related phenotypic signatures. Advanced bioinformatic analyses integrating multimodal “omics” data will be required to elucidate underlying driving mechanisms.
Lastly, the field continues to struggle with development and validation of preclinical models of complex behavior with greater translational relevance to human disease. We need to double down and advance efforts in this area. There has been some early successes in developing cross-species effort-based models that tap into a limited number of key behavioral domains disrupted in human stress disorders, but we need a much broader array of these tests to reflect the highly complex biological and behavioral domains disrupted in MDD and related disorders. Studies should include mutliple stress-paradigms in both sexes to elucidate shared and dissociable mechanisms underlying effects of stress on non-neuronal cell types and pathways. Such advances in combination with a better understanding of the non-neuronal cellular and molecular perturbations that define stress disorders will usher in a new era of discovery efforts to conquer these debilitating illnesses.
Stress is a major risk factor for many psychiatric disorders. Cathomas et al. review new insight into how non-neuronal cells mediate the deleterious effects, as well as the adaptive, protective effects, of stress in rodent models and human stress-related disorders.
Acknowledgements:
Preparation of this review was supported by NIH grants R01MH114882–01, R01MH104559, and R01MH127820 (S.J.R.), P50MH096890 and R01MH051399 (E.J.N.), Swiss National Science Foundation (P400PM_186708) (F.C.) and Brain & Behavior Research Foundation (29104) (F.C.). We would like to thank Ms. Ni-ka Ford, academic medical illustrator for the Icahn School of Medicine at Mount Sinai, for generating the figures for this review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott NJ (2000). Inflammatory Mediators and Modulation of Blood–Brain Barrier Permeability. Cellular and Molecular Neurobiology 20, 131–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott NJ, Patabendige AAK, Dolman DEM, Yusof SR, and Begley DJ (2010). Structure and function of the blood–brain barrier. Neurobiology of disease 37, 13–25. [DOI] [PubMed] [Google Scholar]
- Abbott NJ, Ronnback L, and Hansson E. (2006). Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 7, 41–53. [DOI] [PubMed] [Google Scholar]
- Ackerman KD, Heyman R, Rabin BS, Anderson BP, Houck PR, Frank E, and Baum A. (2002). Stressful life events precede exacerbations of multiple sclerosis. Psychosom Med 64, 916–920. [DOI] [PubMed] [Google Scholar]
- Albert KM, and Newhouse PA (2019). Estrogen, Stress, and Depression: Cognitive and Biological Interactions. Annu Rev Clin Psychol 15, 399–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht DS, Granziera C, Hooker JM, and Loggia ML (2016). In Vivo Imaging of Human Neuroinflammation. ACS chemical neuroscience 7, 470–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali-Sisto T, Tolmunen T, Toffol E, Viinamaki H, Mantyselka P, Valkonen-Korhonen M, Honkalampi K, Ruusunen A, Velagapudi V, and Lehto SM (2016). Purine metabolism is dysregulated in patients with major depressive disorder. Psychoneuroendocrinology 70, 25–32. [DOI] [PubMed] [Google Scholar]
- Alonso G. (2000). Prolonged corticosterone treatment of adult rats inhibits the proliferation of oligodendrocyte progenitors present throughout white and gray matter regions of the brain. Glia 31, 219–231. [DOI] [PubMed] [Google Scholar]
- Ambree O, Bergink V, Grosse L, Alferink J, Drexhage HA, Rothermundt M, Arolt V, and Birkenhager TK (2015). S100B Serum Levels Predict Treatment Response in Patients with Melancholic Depression. The international journal of neuropsychopharmacology 19, pyv103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson M, Alvarez-Cermeño J, Bernardi G, Cogato I, Fredman P, Frederiksen J, Fredrikson S, Gallo P, Grimaldi LM, Grønning M, et al. (1994). Cerebrospinal fluid in the diagnosis of multiple sclerosis: a consensus report. Journal of neurology, neurosurgery, and psychiatry 57, 897–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrus BM, Blizinsky K, Vedell PT, Dennis K, Shukla PK, Schaffer DJ, Radulovic J, Churchill GA, and Redei EE (2012). Gene expression patterns in the hippocampus and amygdala of endogenous depression and chronic stress models. Mol Psychiatry 17, 49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston C, Jiang L, and Sokolov BP (2005). Transcriptional profiling reveals evidence for signaling and oligodendroglial abnormalities in the temporal cortex from patients with major depressive disorder. Mol Psychiatry 10, 309–322. [DOI] [PubMed] [Google Scholar]
- Avitsur R, Kavelaars A, Heijnen C, and Sheridan JF (2005). Social stress and the regulation of tumor necrosis factor-alpha secretion. Brain, behavior, and immunity 19, 311–317. [DOI] [PubMed] [Google Scholar]
- Bagot RC, Cates HM, Purushothaman I, Lorsch ZS, Walker DM, Wang J, Huang X, Schluter OM, Maze I, Pena CJ, et al. (2016). Circuit-wide Transcriptional Profiling Reveals Brain Region-Specific Gene Networks Regulating Depression Susceptibility. Neuron 90, 969–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagot RC, Cates HM, Purushothaman I, Vialou V, Heller EA, Yieh L, LaBonte B, Pena CJ, Shen L, Wittenberg GM, et al. (2017). Ketamine and Imipramine Reverse Transcriptional Signatures of Susceptibility and Induce Resilience-Specific Gene Expression Profiles. Biol Psychiatry 81, 285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balabanov R, Strand K, Goswami R, McMahon E, Begolka W, Miller SD, and Popko B. (2007). Interferon-gamma-oligodendrocyte interactions in the regulation of experimental autoimmune encephalomyelitis. J Neurosci 27, 2013–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Baram TZ, Brown AS, Goldstein JM, Insel TR, McCarthy MM, Nemeroff CB, Reyes TM, Simerly RB, Susser ES, et al. (2010). Early life programming and neurodevelopmental disorders. Biol Psychiatry 68, 314–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banasr M, Valentine GW, Li XY, Gourley SL, Taylor JR, and Duman RS (2007). Chronic unpredictable stress decreases cell proliferation in the cerebral cortex of the adult rat. Biol Psychiatry 62, 496–504. [DOI] [PubMed] [Google Scholar]
- Banqueri M, Mendez M, Gomez-Lazaro E, and Arias JL (2019). Early life stress by repeated maternal separation induces long-term neuroinflammatory response in glial cells of male rats. Stress 22, 563–570. [DOI] [PubMed] [Google Scholar]
- Barrot M, Olivier JD, Perrotti LI, DiLeone RJ, Berton O, Eisch AJ, Impey S, Storm DR, Neve RL, Yin JC, et al. (2002). CREB activity in the nucleus accumbens shell controls gating of behavioral responses to emotional stimuli. Proc Natl Acad Sci U S A 99, 11435–11440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann N, and Pham-Dinh D. (2001). Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev 81, 871–927. [DOI] [PubMed] [Google Scholar]
- Baur V, Hanggi J, Rufer M, Delsignore A, Jancke L, Herwig U, and Beatrix Bruhl A. (2011). White matter alterations in social anxiety disorder. J Psychiatr Res 45, 1366–1372. [DOI] [PubMed] [Google Scholar]
- Bayer TA, Buslei R, Havas L, and Falkai P. (1999). Evidence for activation of microglia in patients with psychiatric illnesses. Neuroscience letters 271, 126–128. [DOI] [PubMed] [Google Scholar]
- Bazargani N, and Attwell D. (2016). Astrocyte calcium signaling: the third wave. Nat Neurosci 19, 182–189. [DOI] [PubMed] [Google Scholar]
- Bechtholt-Gompf AJ, Walther HV, Adams MA, Carlezon WA Jr., Ongur D, and Cohen BM (2010). Blockade of astrocytic glutamate uptake in rats induces signs of anhedonia and impaired spatial memory. Neuropsychopharmacology 35, 2049–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekhbat M, Treadway MT, Goldsmith DR, Woolwine BJ, Haroon E, Miller AH, and Felger JC (2020). Gene signatures in peripheral blood immune cells related to insulin resistance and low tyrosine metabolism define a sub-type of depression with high CRP and anhedonia. Brain Behav Immun 88, 161–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belleau EL, Treadway MT, and Pizzagalli DA (2019). The Impact of Stress and Major Depressive Disorder on Hippocampal and Medial Prefrontal Cortex Morphology. Biol Psychiatry 85, 443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Haim L, and Rowitch DH (2017). Functional diversity of astrocytes in neural circuit regulation. Nat Rev Neurosci 18, 31–41. [DOI] [PubMed] [Google Scholar]
- Bender CL, Calfa GD, and Molina VA (2016). Astrocyte plasticity induced by emotional stress: A new partner in psychiatric physiopathology? Prog Neuropsychopharmacol Biol Psychiatry 65, 68–77. [DOI] [PubMed] [Google Scholar]
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, et al. (2006). Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 311, 864–868. [DOI] [PubMed] [Google Scholar]
- Berton O, and Nestler EJ (2006). New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci 7, 137–151. [DOI] [PubMed] [Google Scholar]
- Beurel E, Toups M, and Nemeroff CB (2020). The Bidirectional Relationship of Depression and Inflammation: Double Trouble. Neuron 107, 234–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian Z, Gong Y, Huang T, Lee CZW, Bian L, Bai Z, Shi H, Zeng Y, Liu C, He J, et al. (2020). Deciphering human macrophage development at single-cell resolution. Nature 582, 571–576. [DOI] [PubMed] [Google Scholar]
- Blacker CJ, Millischer V, Webb LM, Ho AMC, Schalling M, Frye MA, and Veldic M. (2020). EAAT2 as a Research Target in Bipolar Disorder and Unipolar Depression: A Systematic Review. Mol Neuropsychiatry 5, 44–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss-Moreau E, and Rudebeck PH (2021). Animal models of human mood. Neurosci Biobehav Rev 120, 574–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boda E. (2021). Myelin and oligodendrocyte lineage cell dysfunctions: New players in the etiology and treatment of depression and stress-related disorders. Eur J Neurosci 53, 281–297. [DOI] [PubMed] [Google Scholar]
- Bohineust A, Garcia Z, Corre B, Lemaitre F, and Bousso P. (2020). Optogenetic manipulation of calcium signals in single T cells in vivo. Nat Commun 11, 1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnefil V, Dietz K, Amatruda M, Wentling M, Aubry AV, Dupree JL, Temple G, Park HJ, Burghardt NS, Casaccia P, et al. (2019). Region-specific myelin differences define behavioral consequences of chronic social defeat stress in mice. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsini A, Zunszain PA, Thuret S, and Pariante CM (2015). The role of inflammatory cytokines as key modulators of neurogenesis. Trends Neurosci 38, 145–157. [DOI] [PubMed] [Google Scholar]
- Bottcher C, Fernandez-Zapata C, Snijders GJL, Schlickeiser S, Sneeboer MAM, Kunkel D, De Witte LD, and Priller J. (2020). Single-cell mass cytometry of microglia in major depressive disorder reveals a non-inflammatory phenotype with increased homeostatic marker expression. Transl Psychiatry 10, 310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böttcher C, Fernández-Zapata C, Snijders GJL, Schlickeiser S, Sneeboer MAM, Kunkel D, De Witte LD, and Priller J. (2020). Single-cell mass cytometry of microglia in major depressive disorder reveals a non-inflammatory phenotype with increased homeostatic marker expression. Translational psychiatry 10, 310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buljevac D, Hop WC, Reedeker W, Janssens AC, van der Meche FG, van Doorn PA, and Hintzen RQ (2003). Self reported stressful life events and exacerbations in multiple sclerosis: prospective study. BMJ 327, 646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushong EA, Martone ME, Jones YZ, and Ellisman MH (2002). Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci 22, 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai N, Revez JA, Adams MJ, Andlauer TFM, Breen G, Byrne EM, Clarke TK, Forstner AJ, Grabe HJ, Hamilton SP, et al. (2020). Minimal phenotyping yields genome-wide association signals of low specificity for major depression. Nat Genet 52, 437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Li LP, Wang Q, Wu Q, Hu HH, Zhang M, Fang YY, Zhang J, Li SJ, Xiong WC, et al. (2013). Astrocyte-derived ATP modulates depressive-like behaviors. Nat Med 19, 773–777. [DOI] [PubMed] [Google Scholar]
- Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, et al. (2006). Control of microglial neurotoxicity by the fractalkine receptor. Nature neuroscience 9, 917–924. [DOI] [PubMed] [Google Scholar]
- Carlyle BC, Duque A, Kitchen RR, Bordner KA, Coman D, Doolittle E, Papademetris X, Hyder F, Taylor JR, and Simen AA (2012). Maternal separation with early weaning: a rodent model providing novel insights into neglect associated developmental deficits. Dev Psychopathol 24, 1401–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathomas F, Bevilacqua L, Ramakrishnan A, Kronman H, Costi S, Schneider M, Chan KL, Li L, Nestler EJ, Shen L, et al. (2022). Whole blood transcriptional signatures associated with rapid antidepressant response to ketamine in patients with treatment resistant depression. Transl Psychiatry 12, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathomas F, Murrough JW, Nestler EJ, Han MH, and Russo SJ (2019). Neurobiology of Resilience: Interface Between Mind and Body. Biological psychiatry 86, 410–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang LC, Jamain S, Lin CW, Rujescu D, Tseng GC, and Sibille E. (2014). A conserved BDNF, glutamate- and GABA-enriched gene module related to human depression identified by coexpression meta-analysis and DNA variant genome-wide association studies. PLoS One 9, e90980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury S, Aurbach EL, Sharma V, Blandino P Jr., Turner CA, Watson SJ, and Akil H. (2014). FGF2 is a target and a trigger of epigenetic mechanisms associated with differences in emotionality: partnership with H3K9me3. Proc Natl Acad Sci U S A 111, 11834–11839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Desse S, Martinez A, Worthen RJ, Jope RS, and Beurel E. (2018). TNFalpha disrupts blood brain barrier integrity to maintain prolonged depressive-like behavior in mice. Brain, behavior, and immunity 69, 556–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang JJ, Cole SW, Bower JE, Irwin MR, Taylor SE, Arevalo J, and Fuligni AJ (2019). Depressive symptoms and immune transcriptional profiles in late adolescents. Brain Behav Immun 80, 163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Jeong B, Polcari A, Rohan ML, and Teicher MH (2012). Reduced fractional anisotropy in the visual limbic pathway of young adults witnessing domestic violence in childhood. Neuroimage 59, 1071–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Jeong B, Rohan ML, Polcari AM, and Teicher MH (2009). Preliminary evidence for white matter tract abnormalities in young adults exposed to parental verbal abuse. Biol Psychiatry 65, 227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffel DJ, Golden SA, Dumitriu D, Robison AJ, Janssen WG, Ahn HF, Krishnan V, Reyes CM, Han MH, Ables JL, et al. (2011). IkappaB kinase regulates social defeat stress-induced synaptic and behavioral plasticity. J Neurosci 31, 314–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffel DJ, Golden SA, Walsh JJ, Guise KG, Heshmati M, Friedman AK, Dey A, Smith M, Rebusi N, Pfau M, et al. (2015). Excitatory transmission at thalamo-striatal synapses mediates susceptibility to social stress. Nat Neurosci 18, 962–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington HE 3rd, Maze I, LaPlant QC, Vialou VF, Ohnishi YN, Berton O, Fass DM, Renthal W, Rush AJ 3rd, Wu EY, et al. (2009). Antidepressant actions of histone deacetylase inhibitors. J Neurosci 29, 11451–11460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Yang Y, Ni Z, Dong Y, Cai G, Foncelle A, Ma S, Sang K, Tang S, Li Y, et al. (2018). Astroglial Kir4.1 in the lateral habenula drives neuronal bursts in depression. Nature 554, 323–327. [DOI] [PubMed] [Google Scholar]
- Danielski LG, Giustina AD, Badawy M, Barichello T, Quevedo J, Dal-Pizzol F, and Petronilho F. (2018). Brain Barrier Breakdown as a Cause and Consequence of Neuroinflammation in Sepsis. Molecular neurobiology 55, 1045–1053. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, and Kelley KW (2008). From inflammation to sickness and depression: when the immune system subjugates the brain. Nature Reviews Neuroscience 9, 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Faria O Jr., Gonsalvez DG, Nicholson M, and Xiao J. (2019). Activity-dependent central nervous system myelination throughout life. J Neurochem 148, 447–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean DC 3rd, Planalp EM, Wooten W, Kecskemeti SR, Adluru N, Schmidt CK, Frye C, Birn RM, Burghy CA, Schmidt NL, et al. (2018). Association of Prenatal Maternal Depression and Anxiety Symptoms With Infant White Matter Microstructure. JAMA Pediatr 172, 973–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerens C, Stankoff B, Logak M, Anglade P, Allinquant B, Couraud F, Zalc B, and Lubetzki C. (1996). Induction of myelination in the central nervous system by electrical activity. Proc Natl Acad Sci U S A 93, 9887–9892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyama S, Bang E, Wohleb ES, Li XY, Kato T, Gerhard DM, Dutheil S, Dwyer JM, Taylor SR, Picciotto MR, et al. (2019). Role of Neuronal VEGF Signaling in the Prefrontal Cortex in the Rapid Antidepressant Effects of Ketamine. Am J Psychiatry 176, 388–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dion-Albert L, Cadoret A, Doney E, Kaufmann FN, Dudek KA, Daigle B, Parise LF, Cathomas F, Samba N, Hudson N, et al. (2022). Vascular and blood-brain barrier-related changes underlie stress responses and resilience in female mice and depression in human tissue. Nat Commun 13, 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiSabato DJ, Nemeth DP, Liu X, Witcher KG, O’Neil SM, Oliver B, Bray CE, Sheridan JF, Godbout JP, and Quan N. (2021). Interleukin-1 receptor on hippocampal neurons drives social withdrawal and cognitive deficits after chronic social stress. Mol Psychiatry 26, 4770–4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingues HS, Portugal CC, Socodato R, and Relvas JB (2016). Corrigendum: Oligodendrocyte, Astrocyte and Microglia Crosstalk in Myelin Development, Damage, and Repair. Front Cell Dev Biol 4, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue RJ, Muschamp JW, Russo SJ, Nestler EJ, and Carlezon WA Jr. (2014). Effects of striatal DeltaFosB overexpression and ketamine on social defeat stress-induced anhedonia in mice. Biol Psychiatry 76, 550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, and Lanctot KL (2010). A meta-analysis of cytokines in major depression. Biological psychiatry 67, 446–457. [DOI] [PubMed] [Google Scholar]
- Dudek KA, Dion-Albert L, Lebel M, LeClair K, Labrecque S, Tuck E, Ferrer Perez C, Golden SA, Tamminga C, Turecki G, et al. (2020). Molecular adaptations of the blood-brain barrier promote stress resilience vs. depression. Proc Natl Acad Sci U S A 117, 3326–3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, and Aghajanian GK (2012). Synaptic dysfunction in depression: potential therapeutic targets. Science 338, 68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, and Monteggia LM (2006). A neurotrophic model for stress-related mood disorders. Biol Psychiatry 59, 1116–1127. [DOI] [PubMed] [Google Scholar]
- Dumas AA, Borst K, and Prinz M. (2021). Current tools to interrogate microglial biology. Neuron 109, 2805–2819. [DOI] [PubMed] [Google Scholar]
- Dunlop BW, and Mayberg HS (2014). Neuroimaging-based biomarkers for treatment selection in major depressive disorder. Dialogues Clin Neurosci 16, 479–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore MR, Najafi AR, Koike MA, Dagher NN, Spangenberg EE, Rice RA, Kitazawa M, Matusow B, Nguyen H, West BL, et al. (2014). Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron 82, 380–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enache D, Pariante CM, and Mondelli V. (2019). Markers of central inflammation in major depressive disorder: A systematic review and meta-analysis of studies examining cerebrospinal fluid, positron emission tomography and post-mortem brain tissue. Brain, behavior, and immunity 81, 24–40. [DOI] [PubMed] [Google Scholar]
- Epstein J, Pan H, Kocsis JH, Yang Y, Butler T, Chusid J, Hochberg H, Murrough J, Strohmayer E, Stern E, et al. (2006). Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. Am J Psychiatry 163, 1784–1790. [DOI] [PubMed] [Google Scholar]
- Ernst C, Nagy C, Kim S, Yang JP, Deng X, Hellstrom IC, Choi KH, Gershenfeld H, Meaney MJ, and Turecki G. (2011). Dysfunction of astrocyte connexins 30 and 43 in dorsal lateral prefrontal cortex of suicide completers. Biol Psychiatry 70, 312–319. [DOI] [PubMed] [Google Scholar]
- Fan KQ, Li YY, Wang HL, Mao XT, Guo JX, Wang F, Huang LJ, Li YN, Ma XY, Gao ZJ, et al. (2019). Stress-Induced Metabolic Disorder in Peripheral CD4(+) T Cells Leads to Anxiety-like Behavior. Cell 179, 864–879 e819. [DOI] [PubMed] [Google Scholar]
- Fitzgerald E, Parent C, Kee MZL, and Meaney MJ (2021). Maternal Distress and Offspring Neurodevelopment: Challenges and Opportunities for Pre-clinical Research Models. Front Hum Neurosci 15, 635304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleshner M, Frank M, and Maier SF (2017). Danger Signals and Inflammasomes: Stress-Evoked Sterile Inflammation in Mood Disorders. Neuropsychopharmacology 42, 36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Thompson BM, Watkins LR, and Maier SF (2012). Glucocorticoids mediate stress-induced priming of microglial pro-inflammatory responses. Brain, behavior, and immunity 26, 337–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French HM, Reid M, Mamontov P, Simmons RA, and Grinspan JB (2009). Oxidative stress disrupts oligodendrocyte maturation. J Neurosci Res 87, 3076–3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman A, Homma D, Bloem B, Gibb LG, Amemori KI, Hu D, Delcasso S, Truong TF, Yang J, Hood AS, et al. (2017). Chronic Stress Alters Striosome-Circuit Dynamics, Leading to Aberrant Decision-Making. Cell 171, 1191–1205 e1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullana N, Gasull-Camos J, Tarres-Gatius M, Castane A, Bortolozzi A, and Artigas F. (2020). Astrocyte control of glutamatergic activity: Downstream effects on serotonergic function and emotional behavior. Neuropharmacology 166, 107914. [DOI] [PubMed] [Google Scholar]
- Furutani K, Ohno Y, Inanobe A, Hibino H, and Kurachi Y. (2009). Mutational and in silico analyses for antidepressant block of astroglial inward-rectifier Kir4.1 channel. Mol Pharmacol 75, 1287–1295. [DOI] [PubMed] [Google Scholar]
- Galea I, Bechmann I, and Perry VH (2007). What is immune privilege (not)? Trends in immunology 28, 12–18. [DOI] [PubMed] [Google Scholar]
- Galichet C, Clayton RW, and Lovell-Badge R. (2021). Novel Tools and Investigative Approaches for the Study of Oligodendrocyte Precursor Cells (NG2-Glia) in CNS Development and Disease. Front Cell Neurosci 15, 673132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher D, Siddiqui F, Fish J, Charlat M, Chaudry E, Moolla S, Gauthier-Fisher A, and Librach C. (2019). Mesenchymal Stromal Cells Modulate Peripheral Stress-Induced Innate Immune Activation Indirectly Limiting the Emergence of Neuroinflammation-Driven Depressive and Anxiety-like Behaviors. Biol Psychiatry 86, 712–724. [DOI] [PubMed] [Google Scholar]
- Garcia-Keller C, Carter JS, Kruyer A, Kearns AM, Hopkins JL, Hodebourg R, Kalivas PW, and Reichel CM (2021). Behavioral and accumbens synaptic plasticity induced by cues associated with restraint stress. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynes BN, Warden D, Trivedi MH, Wisniewski SR, Fava M, and Rush AJ (2009). What did STAR*D teach us? Results from a large-scale, practical, clinical trial for patients with depression. Psychiatr Serv 60, 1439–1445. [DOI] [PubMed] [Google Scholar]
- Giacci M, and Fitzgerald M. (2018). Oligodendroglia Are Particularly Vulnerable to Oxidative Damage After Neurotrauma In Vivo. J Exp Neurosci 12, 1179069518810004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson EM, Purger D, Mount CW, Goldstein AK, Lin GL, Wood LS, Inema I, Miller SE, Bieri G, Zuchero JB, et al. (2014). Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science 344, 1252304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, et al. (2010). Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science (New York, NY) 330, 841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girgenti MJ, Pothula S, and Newton SS (2020). Stress and Its Impact on the Transcriptome. Biol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girgenti MJ, Wang J, Ji D, Cruz DA, Traumatic Stress Brain Research, G., Stein MB, Gelernter J, Young KA, Huber BR, Williamson DE, et al. (2021). Transcriptomic organization of the human brain in post-traumatic stress disorder. Nat Neurosci 24, 24–33. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Curran JE, Winkler AM, Carless MA, Kent JW Jr., Charlesworth JC, Johnson MP, Goring HH, Cole SA, Dyer TD, et al. (2012). High dimensional endophenotype ranking in the search for major depression risk genes. Biological psychiatry 71, 6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg D. (2011). The heterogeneity of “major depression”. World Psychiatry 10, 226–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden SA, Christoffel DJ, Heshmati M, Hodes GE, Magida J, Davis K, Cahill ME, Dias C, Ribeiro E, Ables JL, et al. (2013). Epigenetic regulation of RAC1 induces synaptic remodeling in stress disorders and depression. Nat Med 19, 337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldmann T, Wieghofer P, Jordão MJ, Prutek F, Hagemeyer N, Frenzel K, Amann L, Staszewski O, Kierdorf K, Krueger M, et al. (2016). Origin, fate and dynamics of macrophages at central nervous system interfaces. Nature immunology 17, 797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein EZ, Pertsovskaya V, Forbes TA, Dupree JL, and Gallo V. (2021). Prolonged Environmental Enrichment Promotes Developmental Myelination. Front Cell Dev Biol 9, 665409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves CA, Leite MC, and Guerra MC (2010). Adipocytes as an Important Source of Serum S100B and Possible Roles of This Protein in Adipose Tissue. Cardiovasc Psychiatry Neurol 2010, 790431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshen I, and Yirmiya R. (2009). Interleukin-1 (IL-1): a central regulator of stress responses. Front Neuroendocrinol 30, 30–45. [DOI] [PubMed] [Google Scholar]
- Govindan RM, Behen ME, Helder E, Makki MI, and Chugani HT (2010). Altered water diffusivity in cortical association tracts in children with early deprivation identified with Tract-Based Spatial Statistics (TBSS). Cereb Cortex 20, 561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudmundsson P, Skoog I, Waern M, Blennow K, Palsson S, Rosengren L, and Gustafson D. (2007). The relationship between cerebrospinal fluid biomarkers and depression in elderly women. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry 15, 832–838. [DOI] [PubMed] [Google Scholar]
- Gundersen GA, Vindedal GF, Skare O, and Nagelhus EA (2014). Evidence that pericytes regulate aquaporin-4 polarization in mouse cortical astrocytes. Brain Struct Funct 219, 2181–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guneykaya D, Ivanov A, Hernandez DP, Haage V, Wojtas B, Meyer N, Maricos M, Jordan P, Buonfiglioli A, Gielniewski B, et al. (2018). Transcriptional and Translational Differences of Microglia from Male and Female Brains. Cell Rep 24, 2773–2783 e2776. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Hoptman MJ, Lim KO, Murphy CF, Klimstra S, Latoussakis V, Majcher-Tascio M, Hrabe J, Ardekani BA, and Alexopoulos GS (2008). Macromolecular white matter abnormalities in geriatric depression: a magnetization transfer imaging study. Am J Geriatr Psychiatry 16, 255–262. [DOI] [PubMed] [Google Scholar]
- Habek M, Brinar M, Brinar VV, and Poser CM (2006). Psychiatric manifestations of multiple sclerosis and acute disseminated encephalomyelitis. Clin Neurol Neurosurg 108, 290–294. [DOI] [PubMed] [Google Scholar]
- Hagemeyer N, Hanft K-M, Akriditou M-A, Unger N, Park ES, Stanley ER, Staszewski O, Dimou L, and Prinz M. (2017). Microglia contribute to normal myelinogenesis and to oligodendrocyte progenitor maintenance during adulthood. Acta Neuropathologica 134, 441–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallof-Bustrich H, and Di Benedetto B. (2019). Examining the Coverage of Blood Vessels by Astrocyte Endfeet in an Animal Model of Major Depressive Disorder. Methods Mol Biol 1938, 255–263. [DOI] [PubMed] [Google Scholar]
- Hamidi M, Drevets WC, and Price JL (2004). Glial reduction in amygdala in major depressive disorder is due to oligodendrocytes. Biol Psychiatry 55, 563–569. [DOI] [PubMed] [Google Scholar]
- Han RT, Kim RD, Molofsky AV, and Liddelow SA (2021). Astrocyte-immune cell interactions in physiology and pathology. Immunity 54, 211–224. [DOI] [PubMed] [Google Scholar]
- Hanson JL, Knodt AR, Brigidi BD, and Hariri AR (2015). Lower structural integrity of the uncinate fasciculus is associated with a history of child maltreatment and future psychological vulnerability to stress. Dev Psychopathol 27, 1611–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmann H, Gamradt S, Taenzer A, Nowacki J, Zain R, Patas K, Ramien C, Paul F, Wingenfeld K, Piber D, et al. (2018). Pro-inflammatory Monocyte Phenotype and Cell-Specific Steroid Signaling Alterations in Unmedicated Patients With Major Depressive Disorder. Front Immunol 9, 2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Nihonmatsu-Kikuchi N, Yu X, Ishimoto K, Hisanaga SI, and Tatebayashi Y. (2011). A novel, rapid, quantitative cell-counting method reveals oligodendroglial reduction in the frontopolar cortex in major depressive disorder. Mol Psychiatry 16, 1155–1158. [DOI] [PubMed] [Google Scholar]
- Heidt T, Sager HB, Courties G, Dutta P, Iwamoto Y, Zaltsman A, von zur Muhlen C, Bode C, Fricchione GL, Denninger J, et al. (2014). Chronic variable stress activates hematopoietic stem cells. Nature Medicine 20, 754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herculano-Houzel S. (2014). The glia/neuron ratio: how it varies uniformly across brain structures and species and what that means for brain physiology and evolution. Glia 62, 1377–1391. [DOI] [PubMed] [Google Scholar]
- Hernandez LM, Kim M, Hoftman GD, Haney JR, de la Torre-Ubieta L, Pasaniuc B, and Gandal MJ (2021). Transcriptomic Insight Into the Polygenic Mechanisms Underlying Psychiatric Disorders. Biol Psychiatry 89, 54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heye AK, Culling RD, Valdes Hernandez Mdel C, Thrippleton MJ, and Wardlaw JM (2014). Assessment of blood-brain barrier disruption using dynamic contrast-enhanced MRI. A systematic review. NeuroImage Clinical 6, 262–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodes GE, Kana V, Menard C, Merad M, and Russo SJ (2015a). Neuroimmune mechanisms of depression. Nat Neurosci 18, 1386–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodes GE, Ménard C, and Russo SJ (2016). Integrating Interleukin-6 into depression diagnosis and treatment. Neurobiology of stress 4, 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodes GE, Pfau ML, Leboeuf M, Golden SA, Christoffel DJ, Bregman D, Rebusi N, Heshmati M, Aleyasin H, Warren BL, et al. (2014). Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc Natl Acad Sci U S A 111, 16136–16141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodes GE, Pfau ML, Purushothaman I, Ahn HF, Golden SA, Christoffel DJ, Magida J, Brancato A, Takahashi A, Flanigan ME, et al. (2015b). Sex Differences in Nucleus Accumbens Transcriptome Profiles Associated with Susceptibility versus Resilience to Subchronic Variable Stress. J Neurosci 35, 16362–16376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokenson RE, Short AK, Chen Y, Pham AL, Adams ET, Bolton JL, Swarup V, Gall CM, and Baram TZ (2021). Unexpected Role of Physiological Estrogen in Acute Stress-Induced Memory Deficits. J Neurosci 41, 648–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes SE, Hinz R, Conen S, Gregory CJ, Matthews JC, Anton-Rodriguez JM, Gerhard A, and Talbot PS (2018). Elevated Translocator Protein in Anterior Cingulate in Major Depression and a Role for Inflammation in Suicidal Thinking: A Positron Emission Tomography Study. Biological psychiatry 83, 61–69. [DOI] [PubMed] [Google Scholar]
- Hong S, Dissing-Olesen L, and Stevens B. (2016). New insights on the role of microglia in synaptic pruning in health and disease. Current opinion in neurobiology 36, 128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issler O, van der Zee YY, Ramakrishnan A, Wang J, Tan C, Loh YE, Purushothaman I, Walker DM, Lorsch ZS, Hamilton PJ, et al. (2020). Sex-Specific Role for the Long Non-coding RNA LINC00473 in Depression. Neuron 106, 912–926 e915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata M, Ota KT, and Duman RS (2013). The inflammasome: pathways linking psychological stress, depression, and systemic illnesses. Brain, behavior, and immunity 31, 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson D, Kannis-Dymand L, Beaudequin DA, Schwenn P, Shan Z, McLoughlin LT, Lagopoulos J, and Hermens DF (2021a). Can measures of sleep quality or white matter structural integrity predict level of worry or rumination in adolescents facing stressful situations? Lessons from the COVID-19 pandemic. J Adolesc 91, 110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson D, Shan Z, Lagopoulos J, and Hermens DF (2021b). The role of adolescent sleep quality in the development of anxiety disorders: A neurobiologically-informed model. Sleep Med Rev 59, 101450. [DOI] [PubMed] [Google Scholar]
- Jana A, and Pahan K. (2007). Oxidative stress kills human primary oligodendrocytes via neutral sphingomyelinase: implications for multiple sclerosis. J Neuroimmune Pharmacol 2, 184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen R, Penninx BW, Madar V, Xia K, Milaneschi Y, Hottenga JJ, Hammerschlag AR, Beekman A, van der Wee N, Smit JH, et al. (2016). Gene expression in major depressive disorder. Molecular psychiatry 21, 339–347. [DOI] [PubMed] [Google Scholar]
- Janssen E, Köhler S, Geraets AFJ, Stehouwer CDA, Schaper NC, Sep SJS, Henry RMA, van der Kallen CJH, Schalkwijk CG, Koster A, et al. (2021). Low-grade inflammation and endothelial dysfunction predict four-year risk and course of depressive symptoms: The Maastricht study. Brain, behavior, and immunity. [DOI] [PubMed] [Google Scholar]
- John CS, Smith KL, Van’t Veer A, Gompf HS, Carlezon WA Jr., Cohen BM, Ongur D, and Bechtholt-Gompf AJ (2012). Blockade of astrocytic glutamate uptake in the prefrontal cortex induces anhedonia. Neuropsychopharmacology 37, 2467–2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S, Hong SI, Lee J, Peyton L, Baker M, Choi S, Kim H, Chang SY, and Choi DS (2020). Activation of Astrocytes in the Dorsomedial Striatum Facilitates Transition From Habitual to Goal-Directed Reward-Seeking Behavior. Biol Psychiatry 88, 797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner AA, Marchi N, Fazio V, Mayberg MR, Koltz MT, Siomin V, Stevens GH, Masaryk T, Aumayr B, Vogelbaum MA, et al. (2003). Serum S100beta: a noninvasive marker of blood-brain barrier function and brain lesions. Cancer 97, 2806–2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavalali ET, and Monteggia LM (2020). Targeting Homeostatic Synaptic Plasticity for Treatment of Mood Disorders. Neuron 106, 715–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh BS, and McCarthy KD (2015). Astrocyte calcium signaling: from observations to functions and the challenges therein. Cold Spring Harb Perspect Biol 7, a020404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim R, Healey KL, Sepulveda-Orengo MT, and Reissner KJ (2018). Astroglial correlates of neuropsychiatric disease: From astrocytopathy to astrogliosis. Prog Neuropsychopharmacol Biol Psychiatry 87, 126–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita M, Hirayama Y, Fujishita K, Shibata K, Shinozaki Y, Shigetomi E, Takeda A, Le HPN, Hayashi H, Hiasa M, et al. (2018). Anti-Depressant Fluoxetine Reveals its Therapeutic Effect Via Astrocytes. EBioMedicine 32, 72–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofuji P, and Araque A. (2021a). Astrocytes and Behavior. Annu Rev Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofuji P, and Araque A. (2021b). Astrocytes and Behavior. Annu Rev Neurosci 44, 49–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JW, and Wohleb ES (2021). How Stress Shapes Neuroimmune Function: Implications for the Neurobiology of Psychiatric Disorders. Biological psychiatry 90, 74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopec AM, Smith CJ, Ayre NR, Sweat SC, and Bilbo SD (2018). Microglial dopamine receptor elimination defines sex-specific nucleus accumbens development and social behavior in adolescent rats. Nature communications 9, 3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg G, Uhlemann R, Endres M, and Gertz K. (2019). Microglia, Monocytes, and the Recurrence of Anxiety in Stress-Sensitized Mice. Biol Psychiatry 85, e67–e68. [DOI] [PubMed] [Google Scholar]
- Kronman H, Torres-Berrio A, Sidoli S, Issler O, Godino A, Ramakrishnan A, Mews P, Lardner CK, Parise EM, Walker DM, et al. (2021). Author Correction: Long-term behavioral and cell-type-specific molecular effects of early life stress are mediated by H3K79me2 dynamics in medium spiny neurons. Nat Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudinova AY, Deak T, Hueston CM, McGeary JE, Knopik VS, Palmer RH, and Gibb BE (2016). Cross-species evidence for the role of interleukin-33 in depression risk. J Abnorm Psychol 125, 482–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Behen ME, Singsoonsud P, Veenstra AL, Wolfe-Christensen C, Helder E, and Chugani HT (2014). Microstructural abnormalities in language and limbic pathways in orphanage-reared children: a diffusion tensor imaging study. J Child Neurol 29, 318–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Gupta RC, Albert Thomas M, Alger J, Wyckoff N, and Hwang S. (2004). Biophysical changes in normal-appearing white matter and subcortical nuclei in late-life major depression detected using magnetization transfer. Psychiatry Res 130, 131–140. [DOI] [PubMed] [Google Scholar]
- Kuske JX, and Trainor BC (2021). Mean Girls: Social Stress Models for Female Rodents. Curr Top Behav Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labonte B, Engmann O, Purushothaman I, Menard C, Wang J, Tan C, Scarpa JR, Moy G, Loh YE, Cahill M, et al. (2017). Sex-specific transcriptional signatures in human depression. Nat Med 23, 1102–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lago N, Kaufmann FN, Negro-Demontel ML, Ali-Ruiz D, Ghisleni G, Rego N, Arcas-Garcia A, Vitureira N, Jansen K, Souza LM, et al. (2020). CD300f immunoreceptor is associated with major depressive disorder and decreased microglial metabolic fitness. Proc Natl Acad Sci U S A 117, 6651–6662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammertink F, Vinkers CH, Tataranno ML, and Benders M. (2020). Premature Birth and Developmental Programming: Mechanisms of Resilience and Vulnerability. Front Psychiatry 11, 531571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassmann H, and van Horssen J. (2016). Oxidative stress and its impact on neurons and glia in multiple sclerosis lesions. Biochim Biophys Acta 1862, 506–510. [DOI] [PubMed] [Google Scholar]
- Lauber K, Blumenthal SG, Waibel M, and Wesselborg S. (2004). Clearance of apoptotic cells: getting rid of the corpses. Molecular cell 14, 277–287. [DOI] [PubMed] [Google Scholar]
- Lautarescu A, Hadaya L, Craig MC, Makropoulos A, Batalle D, Nosarti C, Edwards AD, Counsell SJ, and Victor S. (2021). Exploring the relationship between maternal prenatal stress and brain structure in premature neonates. PLoS One 16, e0250413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautarescu A, Pecheva D, Nosarti C, Nihouarn J, Zhang H, Victor S, Craig M, Edwards AD, and Counsell SJ (2020). Maternal Prenatal Stress Is Associated With Altered Uncinate Fasciculus Microstructure in Premature Neonates. Biol Psychiatry 87, 559–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leday GGR, Vertes PE, Richardson S, Greene JR, Regan T, Khan S, Henderson R, Freeman TC, Pariante CM, Harrison NA, et al. (2018). Replicable and Coupled Changes in Innate and Adaptive Immune Gene Expression in Two Case-Control Studies of Blood Microarrays in Major Depressive Disorder. Biological psychiatry 83, 70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann ML, Cooper HA, Maric D, and Herkenham M. (2016). Social defeat induces depressive-like states and microglial activation without involvement of peripheral macrophages. J Neuroinflammation 13, 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann ML, Weigel TK, Elkahloun AG, and Herkenham M. (2017). Chronic social defeat reduces myelination in the mouse medial prefrontal cortex. Sci Rep 7, 46548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann ML, Weigel TK, Poffenberger CN, and Herkenham M. (2019). The Behavioral Sequelae of Social Defeat Require Microglia and Are Driven by Oxidative Stress in Mice. The Journal of neuroscience : the official journal of the Society for Neuroscience 39, 5594–5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin SG, and Godukhin OV (2017). Modulating Effect of Cytokines on Mechanisms of Synaptic Plasticity in the Brain. Biochemistry (Mosc) 82, 264–274. [DOI] [PubMed] [Google Scholar]
- Li H, Sagar AP, and Kéri S. (2018). Translocator protein (18kDa TSPO) binding, a marker of microglia, is reduced in major depression during cognitive-behavioral therapy. Progress in neuro-psychopharmacology & biological psychiatry 83, 1–7. [DOI] [PubMed] [Google Scholar]
- Li J, Seidlitz J, Suckling J, Fan F, Ji GJ, Meng Y, Yang S, Wang K, Qiu J, Chen H, et al. (2021). Cortical structural differences in major depressive disorder correlate with cell type-specific transcriptional signatures. Nat Commun 12, 1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, and Barres BA (2018). Microglia and macrophages in brain homeostasis and disease. Nature Reviews Immunology 18, 225–242. [DOI] [PubMed] [Google Scholar]
- Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Munch AE, Chung WS, Peterson TC, et al. (2017). Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddelow SA, Marsh SE, and Stevens B. (2020). Microglia and Astrocytes in Disease: Dynamic Duo or Partners in Crime? Trends Immunol 41, 820–835. [DOI] [PubMed] [Google Scholar]
- Liu J, Dietz K, DeLoyht JM, Pedre X, Kelkar D, Kaur J, Vialou V, Lobo MK, Dietz DM, Nestler EJ, et al. (2012). Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat Neurosci 15, 1621–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Dietz K, Hodes GE, Russo SJ, and Casaccia P. (2018). Widespread transcriptional alternations in oligodendrocytes in the adult mouse brain following chronic stress. Dev Neurobiol 78, 152–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Dupree JL, Gacias M, Frawley R, Sikder T, Naik P, and Casaccia P. (2016). Clemastine Enhances Myelination in the Prefrontal Cortex and Rescues Behavioral Changes in Socially Isolated Mice. J Neurosci 36, 957–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Likhtik E, Shereen AD, Dennis-Tiwary TA, and Casaccia P. (2020). White Matter Plasticity in Anxiety: Disruption of Neural Network Synchronization During Threat-Safety Discrimination. Front Cell Neurosci 14, 587053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Magri L, Zhang F, Marsh NO, Albrecht S, Huynh JL, Kaur J, Kuhlmann T, Zhang W, Slesinger PA, et al. (2015). Chromatin landscape defined by repressive histone methylation during oligodendrocyte differentiation. J Neurosci 35, 352–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Ge T, Leng Y, Pan Z, Fan J, Yang W, and Cui R. (2017). The Role of Neural Plasticity in Depression: From Hippocampus to Prefrontal Cortex. Neural Plast 2017, 6871089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Nemeth DP, McKim DB, Zhu L, DiSabato DJ, Berdysz O, Gorantla G, Oliver B, Witcher KG, Wang Y, et al. (2019). Cell-Type-Specific Interleukin 1 Receptor 1 Signaling in the Brain Regulates Distinct Neuroimmune Activities. Immunity 50, 317–333.e316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez J, and Bagot RC (2021). Defining Valid Chronic Stress Models for Depression With Female Rodents. Biol Psychiatry 90, 226–235. [DOI] [PubMed] [Google Scholar]
- Louveau A, Harris TH, and Kipnis J. (2015). Revisiting the Mechanisms of CNS Immune Privilege. Trends in immunology 36, 569–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgaard I, Osorio MJ, Kress BT, Sanggaard S, and Nedergaard M. (2014). White matter astrocytes in health and disease. Neuroscience 276, 161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz PE, Tanti A, Gasecka A, Barnett-Burns S, Kim JJ, Zhou Y, Chen GG, Wakid M, Shaw M, Almeida D, et al. (2017). Association of a History of Child Abuse With Impaired Myelination in the Anterior Cingulate Cortex: Convergent Epigenetic, Transcriptional, and Morphological Evidence. Am J Psychiatry 174, 1185–1194. [DOI] [PubMed] [Google Scholar]
- Makinodan M, Ikawa D, Yamamuro K, Yamashita Y, Toritsuka M, Kimoto S, Yamauchi T, Okumura K, Komori T, Fukami SI, et al. (2017). Effects of the mode of re-socialization after juvenile social isolation on medial prefrontal cortex myelination and function. Sci Rep 7, 5481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinodan M, Rosen KM, Ito S, and Corfas G. (2012). A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science 337, 1357–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi N, Rasmussen P, Kapural M, Fazio V, Kight K, Mayberg MR, Kanner A, Ayumar B, Albensi B, Cavaglia M, et al. (2003). Peripheral markers of brain damage and blood-brain barrier dysfunction. Restor Neurol Neurosci 21, 109–121. [PMC free article] [PubMed] [Google Scholar]
- Martin-Fernandez M, Jamison S, Robin LM, Zhao Z, Martin ED, Aguilar J, Benneyworth MA, Marsicano G, and Araque A. (2017). Synapse-specific astrocyte gating of amygdala-related behavior. Nat Neurosci 20, 1540–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiisen TM, Lehre KP, Danbolt NC, and Ottersen OP (2010). The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia 58, 1094–1103. [DOI] [PubMed] [Google Scholar]
- Matsusue Y, Horii-Hayashi N, Kirita T, and Nishi M. (2014). Distribution of corticosteroid receptors in mature oligodendrocytes and oligodendrocyte progenitors of the adult mouse brain. J Histochem Cytochem 62, 211–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayne SL, Hannan C, Davis M, Young JF, Kelly MK, Powell M, Dalembert G, McPeak KE, Jenssen BP, and Fiks AG (2021). COVID-19 and Adolescent Depression and Suicide Risk Screening Outcomes. Pediatrics 148. [DOI] [PubMed] [Google Scholar]
- McKim DB, Niraula A, Tarr AJ, Wohleb ES, Sheridan JF, and Godbout JP (2016). Neuroinflammatory Dynamics Underlie Memory Impairments after Repeated Social Defeat. The Journal of neuroscience : the official journal of the Society for Neuroscience 36, 2590–2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKim DB, Weber MD, Niraula A, Sawicki CM, Liu X, Jarrett BL, Ramirez-Chan K, Wang Y, Roeth RM, Sucaldito AD, et al. (2018). Microglial recruitment of IL-1beta-producing monocytes to brain endothelium causes stress-induced anxiety. Molecular psychiatry 23, 1421–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meares GP, Ma X, Qin H, and Benveniste EN (2012). Regulation of CCL20 expression in astrocytes by IL-6 and IL-17. Glia 60, 771–781. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Golembo NI, Nosarti C, Colvert E, Mota A, Williams SC, Rutter M, and Sonuga-Barke EJ (2009). Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: the English and Romanian Adoptees study pilot. J Child Psychol Psychiatry 50, 943–951. [DOI] [PubMed] [Google Scholar]
- Menard C, Pfau ML, Hodes GE, Kana V, Wang VX, Bouchard S, Takahashi A, Flanigan ME, Aleyasin H, LeClair KB, et al. (2017). Social stress induces neurovascular pathology promoting depression. Nat Neurosci 20, 1752–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menassa DA, and Gomez-Nicola D. (2018). Microglial Dynamics During Human Brain Development. Front Immunol 9, 1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mensch S, Baraban M, Almeida R, Czopka T, Ausborn J, El Manira A, and Lyons DA (2015). Synaptic vesicle release regulates myelin sheath number of individual oligodendrocytes in vivo. Nat Neurosci 18, 628–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BJ, Buckley P, Seabolt W, Mellor A, and Kirkpatrick B. (2011). Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biological psychiatry 70, 663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milosevic A, Liebmann T, Knudsen M, Schintu N, Svenningsson P, and Greengard P. (2017). Cell- and region-specific expression of depression-related protein p11 (S100a10) in the brain. J Comp Neurol 525, 955–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsonis CI, Zervas IM, Mitropoulos PA, Dimopoulos NP, Soldatos CR, Potagas CM, and Sfagos CA (2008). The impact of stressful life events on risk of relapse in women with multiple sclerosis: a prospective study. Eur Psychiatry 23, 497–504. [DOI] [PubMed] [Google Scholar]
- Miyata S, Koyama Y, Takemoto K, Yoshikawa K, Ishikawa T, Taniguchi M, Inoue K, Aoki M, Hori O, Katayama T, et al. (2011). Plasma corticosterone activates SGK1 and induces morphological changes in oligodendrocytes in corpus callosum. PLoS One 6, e19859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata S, Taniguchi M, Koyama Y, Shimizu S, Tanaka T, Yasuno F, Yamamoto A, Iida H, Kudo T, Katayama T, et al. (2016). Association between chronic stress-induced structural abnormalities in Ranvier nodes and reduced oligodendrocyte activity in major depression. Sci Rep 6, 23084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monai H, Ohkura M, Tanaka M, Oe Y, Konno A, Hirai H, Mikoshiba K, Itohara S, Nakai J, Iwai Y, et al. (2016). Calcium imaging reveals glial involvement in transcranial direct current stimulation-induced plasticity in mouse brain. Nat Commun 7, 11100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteggia LM, Luikart B, Barrot M, Theobold D, Malkovska I, Nef S, Parada LF, and Nestler EJ (2007). Brain-derived neurotrophic factor conditional knockouts show gender differences in depression-related behaviors. Biol Psychiatry 61, 187–197. [DOI] [PubMed] [Google Scholar]
- Mostafavi S, Battle A, Zhu X, Potash JB, Weissman MM, Shi J, Beckman K, Haudenschild C, McCormick C, Mei R, et al. (2014). Type I interferon signaling genes in recurrent major depression: increased expression detected by whole-blood RNA sequencing. Molecular psychiatry 19, 1267–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moura RP, Almeida A, and Sarmento B. (2017). The role of non-endothelial cells on the penetration of nanoparticles through the blood brain barrier. Prog Neurobiol 159, 39–49. [DOI] [PubMed] [Google Scholar]
- Mrdjen D, Pavlovic A, Hartmann FJ, Schreiner B, Utz SG, Leung BP, Lelios I, Heppner FL, Kipnis J, Merkler D, et al. (2018). High-Dimensional Single-Cell Mapping of Central Nervous System Immune Cells Reveals Distinct Myeloid Subsets in Health, Aging, and Disease. Immunity 48, 380–395.e386. [DOI] [PubMed] [Google Scholar]
- Murphy ML, and Frodl T. (2011). Meta-analysis of diffusion tensor imaging studies shows altered fractional anisotropy occurring in distinct brain areas in association with depression. Biol Mood Anxiety Disord 1, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy-Royal C, Johnston AD, Boyce AKJ, Diaz-Castro B, Institoris A, Peringod G, Zhang O, Stout RF, Spray DC, Thompson RJ, et al. (2020). Stress gates an astrocytic energy reservoir to impair synaptic plasticity. Nat Commun 11, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai J, Yu X, Papouin T, Cheong E, Freeman MR, Monk KR, Hastings MH, Haydon PG, Rowitch D, Shaham S, et al. (2021). Behaviorally consequential astrocytic regulation of neural circuits. Neuron 109, 576–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy C, Maitra M, Tanti A, Suderman M, Theroux JF, Davoli MA, Perlman K, Yerko V, Wang YC, Tripathy SJ, et al. (2020). Single-nucleus transcriptomics of the prefrontal cortex in major depressive disorder implicates oligodendrocyte precursor cells and excitatory neurons. Nat Neurosci 23, 771–781. [DOI] [PubMed] [Google Scholar]
- Nemes-Baran AD, White DR, and DeSilva TM (2020). Fractalkine-Dependent Microglial Pruning of Viable Oligodendrocyte Progenitor Cells Regulates Myelination. Cell reports 32, 108047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neniskyte U, and Gross CT (2017). Errant gardeners: glial-cell-dependent synaptic pruning and neurodevelopmental disorders. Nature reviews Neuroscience 18, 658–670. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, and Hyman SE (2010). Animal models of neuropsychiatric disorders. Nat Neurosci 13, 1161–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas RS, Wing MG, and Compston A. (2001). Nonactivated microglia promote oligodendrocyte precursor survival and maturation through the transcription factor NF-kappa B. The European journal of neuroscience 13, 959–967. [DOI] [PubMed] [Google Scholar]
- Nicholson M, Wood RJ, Fletcher JL, Gonsalvez DG, Hannan AJ, Murray SS, and Xiao J. (2020). Remodeling of pre-existing myelinated axons and oligodendrocyte differentiation is stimulated by environmental enrichment in the young adult brain. bioRxiv, 2020.2001.2021.914572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niklasson F, and Agren H. (1984). Brain energy metabolism and blood-brain barrier permeability in depressive patients: analyses of creatine, creatinine, urate, and albumin in CSF and blood. Biological psychiatry 19, 1183–1206. [PubMed] [Google Scholar]
- Notter T, Coughlin JM, Sawa A, and Meyer U. (2018). Reconceptualization of translocator protein as a biomarker of neuroinflammation in psychiatry. Mol Psychiatry 23, 36–47. [DOI] [PubMed] [Google Scholar]
- Nwaobi SE, Cuddapah VA, Patterson KC, Randolph AC, and Olsen ML (2016). The role of glial-specific Kir4.1 in normal and pathological states of the CNS. Acta Neuropathol 132, 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary LA, and Mechawar N. (2021). Implication of cerebral astrocytes in major depression: A review of fine neuroanatomical evidence in humans. Glia. [DOI] [PubMed] [Google Scholar]
- Ohno Y, Hibino H, Lossin C, Inanobe A, and Kurachi Y. (2007). Inhibition of astroglial Kir4.1 channels by selective serotonin reuptake inhibitors. Brain Res 1178, 44–51. [DOI] [PubMed] [Google Scholar]
- Otte C, Gold SM, Penninx BW, Pariante CM, Etkin A, Fava M, Mohr DC, and Schatzberg AF (2016). Major depressive disorder. Nat Rev Dis Primers 2, 16065. [DOI] [PubMed] [Google Scholar]
- Pantazatos SP, Huang YY, Rosoklija GB, Dwork AJ, Arango V, and Mann JJ (2017). Whole-transcriptome brain expression and exon-usage profiling in major depression and suicide: evidence for altered glial, endothelial and ATPase activity. Mol Psychiatry 22, 760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR 3rd, Lafaille JJ, Hempstead BL, Littman DR, and Gan WB (2013). Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 155, 1596–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena CJ, and Nestler EJ (2018). Progress in Epigenetics of Depression. Prog Mol Biol Transl Sci 157, 41–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH (2018). Microglia and major depression: not yet a clear picture. Lancet Psychiatry 5, 292–294. [DOI] [PubMed] [Google Scholar]
- Petzold GC, and Murthy VN (2011). Role of astrocytes in neurovascular coupling. Neuron 71, 782–797. [DOI] [PubMed] [Google Scholar]
- Pfau M, Lindner M, Goerdt L, Thiele S, Nadal J, Schmid M, Schmitz-Valckenberg S, Sadda SR, Holz FG, Fleckenstein M, et al. (2019a). Prognostic Value of Shape-Descriptive Factors for the Progression of Geographic Atrophy Secondary to Age-Related Macular Degeneration. Retina 39, 1527–1540. [DOI] [PubMed] [Google Scholar]
- Pfau ML, Menard C, Cathomas F, Desland F, Kana V, Chan KL, Shimo Y, LeClair K, Flanigan ME, Aleyasin H, et al. (2019b). Role of Monocyte-Derived MicroRNA106b approximately 25 in Resilience to Social Stress. Biol Psychiatry 86, 474–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham T, Jette N, Bulloch AGM, Burton JM, Wiebe S, and Patten SB (2018). The prevalence of anxiety and associated factors in persons with multiple sclerosis. Mult Scler Relat Disord 19, 35–39. [DOI] [PubMed] [Google Scholar]
- Phan KL, Orlichenko A, Boyd E, Angstadt M, Coccaro EF, Liberzon I, and Arfanakis K. (2009). Preliminary evidence of white matter abnormality in the uncinate fasciculus in generalized social anxiety disorder. Biol Psychiatry 66, 691–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planchez B, Surget A, and Belzung C. (2019). Animal models of major depression: drawbacks and challenges. J Neural Transm (Vienna) 126, 1383–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyakova M, Sander C, Arelin K, Lampe L, Luck T, Luppa M, Kratzsch J, Hoffmann K-T, Riedel-Heller S, Villringer A, et al. (2015). First evidence for glial pathology in late life minor depression: S100B is increased in males with minor depression. Frontiers in Cellular Neuroscience 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponomarev I, Rau V, Eger EI, Harris RA, and Fanselow MS (2010). Amygdala transcriptome and cellular mechanisms underlying stress-enhanced fear learning in a rat model of posttraumatic stress disorder. Neuropsychopharmacology 35, 1402–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW, and Furman SA (2011). The Early Development of the Autonomic Nervous System Provides a Neural Platform for Social Behavior: A Polyvagal Perspective. Infant Child Dev 20, 106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner J, Cha J, Roy AK, Peterson BS, Bansal R, Gustafsson HC, Raffanello E, Gingrich J, and Monk C. (2016). Alterations in amygdala-prefrontal circuits in infants exposed to prenatal maternal depression. Transl Psychiatry 6, e935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell ND, Sloan EK, Bailey MT, Arevalo JM, Miller GE, Chen E, Kobor MS, Reader BF, Sheridan JF, and Cole SW (2013). Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via beta-adrenergic induction of myelopoiesis. Proceedings of the National Academy of Sciences of the United States of America 110, 16574–16579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, and Drevets WC (2010). Neurocircuitry of mood disorders. Neuropsychopharmacology 35, 192–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz M, Masuda T, Wheeler MA, and Quintana FJ (2021). Microglia and Central Nervous System-Associated Macrophages-From Origin to Disease Modulation. Annual review of immunology 39, 251–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucak ML, Carroll KA, Kerr DA, and Kaplin AI (2007). Neuropsychiatric manifestations of depression in multiple sclerosis: neuroinflammatory, neuroendocrine, and neurotrophic mechanisms in the pathogenesis of immune-mediated depression. Dialogues Clin Neurosci 9, 125–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi S, and Medzhitov R. (2003). Toll-like receptors and their role in experimental models of microbial infection. Genes and immunity 4, 87–94. [DOI] [PubMed] [Google Scholar]
- Raison CL, Capuron L, and Miller AH (2006). Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends in immunology 27, 24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Hughes J, Stockmeier CA, Javier Miguel-Hidalgo J, and Maciag D. (2013). Coverage of blood vessels by astrocytic endfeet is reduced in major depressive disorder. Biol Psychiatry 73, 613–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Mahajan G, Maciag D, Sathyanesan M, Iyo AH, Moulana M, Kyle PB, Woolverton WL, Miguel-Hidalgo JJ, Stockmeier CA, et al. (2015). Oligodendrocyte morphometry and expression of myelin - Related mRNA in ventral prefrontal white matter in major depressive disorder. J Psychiatr Res 65, 53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, and Stockmeier CA (2013). Astrocyte pathology in major depressive disorder: insights from human postmortem brain tissue. Curr Drug Targets 14, 1225–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappeneau V, Blaker A, Petro JR, Yamamoto BK, and Shimamoto A. (2016). Disruption of the Glutamate-Glutamine Cycle Involving Astrocytes in an Animal Model of Depression for Males and Females. Front Behav Neurosci 10, 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards EM, Zanotti-Fregonara P, Fujita M, Newman L, Farmer C, Ballard ED, Machado-Vieira R, Yuan P, Niciu MJ, Lyoo CH, et al. (2018). PET radioligand binding to translocator protein (TSPO) is increased in unmedicated depressed subjects. EJNMMI research 8, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkin-Graboi A, Bai J, Chen H, Hameed WB, Sim LW, Tint MT, Leutscher-Broekman B, Chong YS, Gluckman PD, Fortier MV, et al. (2013). Prenatal maternal depression associates with microstructure of right amygdala in neonates at birth. Biol Psychiatry 74, 837–844. [DOI] [PubMed] [Google Scholar]
- Rocca MA, Barkhof F, De Luca J, Frisen J, Geurts JJG, Hulst HE, Sastre-Garriga J, Filippi M, and Group MS (2018). The hippocampus in multiple sclerosis. Lancet Neurol 17, 918–926. [DOI] [PubMed] [Google Scholar]
- Ross JM, Kim C, Allen D, Crouch EE, Narsinh K, Cooke DL, Abla AA, Nowakowski TJ, and Winkler EA (2020). The Expanding Cell Diversity of the Brain Vasculature. Front Physiol 11, 600767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudzki L, and Maes M. (2020). The Microbiota-Gut-Immune-Glia (MGIG) Axis in Major Depression. Mol Neurobiol 57, 4269–4295. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, et al. (2006). Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 163, 1905–1917. [DOI] [PubMed] [Google Scholar]
- Russo SJ, and Nestler EJ (2013). The brain reward circuitry in mood disorders. Nat Rev Neurosci 14, 609–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchet MD, and Gotlib IH (2017). Myelination of the brain in Major Depressive Disorder: An in vivo quantitative magnetic resonance imaging study. Sci Rep 7, 2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachis PN, Armstrong DL, Becker LE, and Bryan AC (1982). Myelination of the human vagus nerve from 24 weeks postconceptional age to adolescence. J Neuropathol Exp Neurol 41, 466–472. [DOI] [PubMed] [Google Scholar]
- Salim S. (2014). Oxidative stress and psychological disorders. Current neuropharmacology 12, 140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador AF, de Lima KA, and Kipnis J. (2021). Neuromodulation by the immune system: a focus on cytokines. Nat Rev Immunol 21, 526–541. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Treccani G, and Popoli M. (2012). Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology 62, 63–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders EA, and van Lieshout HB (1992). Psychiatric symptoms and mental changes as major features of multiple sclerosis. Clin Neurol Neurosurg 94 Suppl, S144–146. [DOI] [PubMed] [Google Scholar]
- Savitz J. (2020). The kynurenine pathway: a finger in every pie. Molecular psychiatry 25, 131–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz J, Frank MB, Victor T, Bebak M, Marino JH, Bellgowan PS, McKinney BA, Bodurka J, Kent Teague T, and Drevets WC (2013). Inflammation and neurological disease-related genes are differentially expressed in depressed patients with mood disorders and correlate with morphometric and functional imaging abnormalities. Brain, behavior, and immunity 31, 161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, and Stevens B. (2012). Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74, 691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinost D, Kwon SH, Lacadie C, Sze G, Sinha R, Constable RT, and Ment LR (2016). Prenatal stress alters amygdala functional connectivity in preterm neonates. Neuroimage Clin 12, 381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schipke CG, Heuser I, and Peters O. (2011). Antidepressants act on glial cells: SSRIs and serotonin elicit astrocyte calcium signaling in the mouse prefrontal cortex. J Psychiatr Res 45, 242–248. [DOI] [PubMed] [Google Scholar]
- Schmidt MV, Wang XD, and Meijer OC (2011). Early life stress paradigms in rodents: potential animal models of depression? Psychopharmacology (Berl) 214, 131–140. [DOI] [PubMed] [Google Scholar]
- Schneider H, Pitossi F, Balschun D, Wagner A, del Rey A, and Besedovsky HO (1998). A neuromodulatory role of interleukin-1beta in the hippocampus. Proceedings of the National Academy of Sciences of the United States of America 95, 7778–7783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter ML, Abdul-Khaliq H, Diefenbacher A, and Blasig IE (2002). S100B is increased in mood disorders and may be reduced by antidepressive treatment. Neuroreport 13, 1675–1678. [DOI] [PubMed] [Google Scholar]
- Schuh C, Wimmer I, Hametner S, Haider L, Van Dam AM, Liblau RS, Smith KJ, Probert L, Binder CJ, Bauer J, et al. (2014). Oxidative tissue injury in multiple sclerosis is only partly reflected in experimental disease models. Acta Neuropathol 128, 247–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seney ML, Glausier J, and Sibille E. (2021). Large-Scale Transcriptomics Studies Provide Insight Into Sex Differences in Depression. Biol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seney ML, Huo Z, Cahill K, French L, Puralewski R, Zhang J, Logan RW, Tseng G, Lewis DA, and Sibille E. (2018). Opposite Molecular Signatures of Depression in Men and Women. Biol Psychiatry 84, 18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo JH, Miyamoto N, Hayakawa K, Pham LD, Maki T, Ayata C, Kim KW, Lo EH, and Arai K. (2013). Oligodendrocyte precursors induce early blood-brain barrier opening after white matter injury. J Clin Invest 123, 782–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo JS, Park JY, Choi J, Kim TK, Shin JH, Lee JK, and Han PL (2012). NADPH oxidase mediates depressive behavior induced by chronic stress in mice. The Journal of neuroscience : the official journal of the Society for Neuroscience 32, 9690–9699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setiawan E, Wilson AA, Mizrahi R, Rusjan PM, Miler L, Rajkowska G, Suridjan I, Kennedy JL, Rekkas PV, Houle S, et al. (2015). Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA psychiatry 72, 268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Mittler BL, and Mintun MA (2002). The hippocampus and depression. Eur Psychiatry 17 Suppl 3, 300–305. [DOI] [PubMed] [Google Scholar]
- Shelton RC, Claiborne J, Sidoryk-Wegrzynowicz M, Reddy R, Aschner M, Lewis DA, and Mirnics K. (2011). Altered expression of genes involved in inflammation and apoptosis in frontal cortex in major depression. Mol Psychiatry 16, 751–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemer A, Erny D, Jung S, and Prinz M. (2015). Microglia Plasticity During Health and Disease: An Immunological Perspective. Trends in immunology 36, 614–624. [DOI] [PubMed] [Google Scholar]
- Siehl S, King JA, Burgess N, Flor H, and Nees F. (2018). Structural white matter changes in adults and children with posttraumatic stress disorder: A systematic review and meta-analysis. Neuroimage Clin 19, 581–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons JM, Winsky L, Zehr JL, and Gordon JA (2021). Priorities in stress research: a view from the U.S. National Institute of Mental Health. Stress 24, 123–129. [DOI] [PubMed] [Google Scholar]
- Simons M, and Nave KA (2015). Oligodendrocytes: Myelination and Axonal Support. Cold Spring Harb Perspect Biol 8, a020479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson S Jr., Tan H, Otahal P, Taylor B, Ponsonby AL, Lucas RM, Blizzard L, Valery PC, Lechner-Scott J, Shaw C, et al. (2016). Anxiety, depression and fatigue at 5-year review following CNS demyelination. Acta Neurol Scand 134, 403–413. [DOI] [PubMed] [Google Scholar]
- Slattery DA, Hudson AL, and Nutt DJ (2004). Invited review: the evolution of antidepressant mechanisms. Fundam Clin Pharmacol 18, 1–21. [DOI] [PubMed] [Google Scholar]
- Snijders G, Sneeboer MAM, Fernández-Andreu A, Udine E, Boks MP, Ormel PR, van Berlekom AB, van Mierlo HC, Bӧttcher C, Priller J, et al. (2020). Distinct non-inflammatory signature of microglia in post-mortem brain tissue of patients with major depressive disorder. Molecular psychiatry. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, and Vinters HV (2010). Astrocytes: biology and pathology. Acta Neuropathol 119, 7–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, Merali Z, and Anisman H. (1999). Variations of nucleus accumbens dopamine and serotonin following systemic interleukin-1, interleukin-2 or interleukin-6 treatment. Neuroscience 88, 823–836. [DOI] [PubMed] [Google Scholar]
- Spaas J, van Veggel L, Schepers M, Tiane A, van Horssen J, Wilson DM 3rd, Moya PR, Piccart E, Hellings N, Eijnde BO, et al. (2021). Oxidative stress and impaired oligodendrocyte precursor cell differentiation in neurological disorders. Cell Mol Life Sci 78, 4615–4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spijker S, Van Zanten JS, De Jong S, Penninx BW, van Dyck R, Zitman FG, Smit JH, Ylstra B, Smit AB, and Hoogendijk WJ (2010). Stimulated gene expression profiles as a blood marker of major depressive disorder. Biological psychiatry 68, 179–186. [DOI] [PubMed] [Google Scholar]
- Steiner J, Walter M, Gos T, Guillemin GJ, Bernstein HG, Sarnyai Z, Mawrin C, Brisch R, Bielau H, Meyer zu Schwabedissen L, et al. (2011). Severe depression is associated with increased microglial quinolinic acid in subregions of the anterior cingulate gyrus: evidence for an immune-modulated glutamatergic neurotransmission? Journal of neuroinflammation 8, 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwagen D, and Malenka RC (2006). Synaptic scaling mediated by glial TNF-alpha. Nature 440, 1054–1059. [DOI] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, et al. (2007). The classical complement cascade mediates CNS synapse elimination. Cell 131, 1164–1178. [DOI] [PubMed] [Google Scholar]
- Su L, Faluyi YO, Hong YT, Fryer TD, Mak E, Gabel S, Hayes L, Soteriades S, Williams GB, Arnold R, et al. (2016). Neuroinflammatory and morphological changes in late-life depression: the NIMROD study. The British journal of psychiatry : the journal of mental science 209, 525–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su S, Ohno Y, Lossin C, Hibino H, Inanobe A, and Kurachi Y. (2007). Inhibition of astroglial inwardly rectifying Kir4.1 channels by a tricyclic antidepressant, nortriptyline. J Pharmacol Exp Ther 320, 573–580. [DOI] [PubMed] [Google Scholar]
- Sugama S, Takenouchi T, Fujita M, Kitani H, and Hashimoto M. (2011). Cold stress induced morphological microglial activation and increased IL-1beta expression in astroglial cells in rat brain. J Neuroimmunol 233, 29–36. [DOI] [PubMed] [Google Scholar]
- Syed SA, Beurel E, Loewenstein DA, Lowell JA, Craighead WE, Dunlop BW, Mayberg HS, Dhabhar F, Dietrich WD, Keane RW, et al. (2018). Defective Inflammatory Pathways in Never-Treated Depressed Patients Are Associated with Poor Treatment Response. Neuron 99, 914–924 e913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan T, Wang W, Liu T, Zhong P, Conrow-Graham M, Tian X, and Yan Z. (2021). Neural circuits and activity dynamics underlying sex-specific effects of chronic social isolation stress. Cell Rep 34, 108874. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, Iwama H, Nishikawa T, Ichihara N, Kikuchi T, et al. (1997). Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science 276, 1699–1702. [DOI] [PubMed] [Google Scholar]
- Tanti A, Kim JJ, Wakid M, Davoli MA, Turecki G, and Mechawar N. (2018). Child abuse associates with an imbalance of oligodendrocyte-lineage cells in ventromedial prefrontal white matter. Mol Psychiatry 23, 2018–2028. [DOI] [PubMed] [Google Scholar]
- Teissier A, Le Magueresse C, Olusakin J, Andrade da Costa BLS, De Stasi AM, Bacci A, Imamura Kawasawa Y, Vaidya VA, and Gaspar P. (2020). Early-life stress impairs postnatal oligodendrogenesis and adult emotional behaviour through activity-dependent mechanisms. Mol Psychiatry 25, 1159–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tham MW, Woon PS, Sum MY, Lee TS, and Sim K. (2011). White matter abnormalities in major depression: evidence from post-mortem, neuroimaging and genetic studies. J Affect Disord 132, 26–36. [DOI] [PubMed] [Google Scholar]
- Torres-Berrio A, Issler O, Parise EM, and Nestler EJ (2019). Unraveling the epigenetic landscape of depression: focus on early life stress. Dialogues Clin Neurosci 21, 341–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Platas SG, Cruceanu C, Chen GG, Turecki G, and Mechawar N. (2014). Evidence for increased microglial priming and macrophage recruitment in the dorsal anterior cingulate white matter of depressed suicides. Brain Behav Immun 42, 50–59. [DOI] [PubMed] [Google Scholar]
- Turner MD, Nedjai B, Hurst T, and Pennington DJ (2014). Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 1843, 2563–2582. [DOI] [PubMed] [Google Scholar]
- Tye KM, Mirzabekov JJ, Warden MR, Ferenczi EA, Tsai HC, Finkelstein J, Kim SY, Adhikari A, Thompson KR, Andalman AS, et al. (2013). Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature 493, 537–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tynan RJ, Beynon SB, Hinwood M, Johnson SJ, Nilsson M, Woods JJ, and Walker FR (2013). Chronic stress-induced disruption of the astrocyte network is driven by structural atrophy and not loss of astrocytes. Acta Neuropathol 126, 75–91. [DOI] [PubMed] [Google Scholar]
- Tynan RJ, Naicker S, Hinwood M, Nalivaiko E, Buller KM, Pow DV, Day TA, and Walker FR (2010). Chronic stress alters the density and morphology of microglia in a subset of stress-responsive brain regions. Brain, behavior, and immunity 24, 1058–1068. [DOI] [PubMed] [Google Scholar]
- Vainchtein ID, Chin G, Cho FS, Kelley KW, Miller JG, Chien EC, Liddelow SA, Nguyen PT, Nakao-Inoue H, Dorman LC, et al. (2018). Astrocyte-derived interleukin-33 promotes microglial synapse engulfment and neural circuit development. Science 359, 1269–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainchtein ID, and Molofsky AV (2020). Astrocytes and Microglia: In Sickness and in Health. Trends Neurosci 43, 144–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasile F, Dossi E, and Rouach N. (2017). Human astrocytes: structure and functions in the healthy brain. Brain Struct Funct 222, 2017–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veronese M, Reis Marques T, Bloomfield PS, Rizzo G, Singh N, Jones D, Agushi E, Mosses D, Bertoldo A, Howes O, et al. (2018). Kinetic modelling of [(11)C]PBR28 for 18 kDa translocator protein PET data: A validation study of vascular modelling in the brain using XBD173 and tissue analysis. J Cereb Blood Flow Metab 38, 1227–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vialou V, Robison AJ, Laplant QC, Covington HE 3rd, Dietz DM, Ohnishi YN, Mouzon E, Rush AJ 3rd, Watts EL, Wallace DL, et al. (2010). DeltaFosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nat Neurosci 13, 745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake H, Lee PR, and Fields RD (2011). Control of local protein synthesis and initial events in myelination by action potentials. Science 333, 1647–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Zhang CJ, Martin BN, Bulek K, Kang Z, Zhao J, Bian G, Carman JA, Gao J, Dongre A, et al. (2017a). IL-17 induced NOTCH1 activation in oligodendrocyte progenitor cells enhances proliferation and inflammatory gene expression. Nat Commun 8, 15508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Holt LM, Huang HH, Sesack SR, Nestler EJ, and Dong Y. (2021). Astrocytes in cocaine addiction and beyond. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Jie W, Liu JH, Yang JM, and Gao TM (2017b). An astroglial basis of major depressive disorder? An overview. Glia 65, 1227–1250. [DOI] [PubMed] [Google Scholar]
- Westermair AL, Munz M, Schaich A, Nitsche S, Willenborg B, Munoz Venegas LM, Willenborg C, Schunkert H, Schweiger U, and Erdmann J. (2018). Association of Genetic Variation at AQP4 Locus with Vascular Depression. Biomolecules 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MR, Sharma P, Macdonald C, Pearce RKB, Hirsch SR, and Maier M. (2019). Axonal myelin decrease in the splenium in major depressive disorder. Eur Arch Psychiatry Clin Neurosci 269, 387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilton DK, Dissing-Olesen L, and Stevens B. (2019). Neuron-Glia Signaling in Synapse Elimination. Annual review of neuroscience 42, 107–127. [DOI] [PubMed] [Google Scholar]
- Wohleb ES, Franklin T, Iwata M, and Duman RS (2016). Integrating neuroimmune systems in the neurobiology of depression. Nature reviews Neuroscience 17, 497–511. [DOI] [PubMed] [Google Scholar]
- Wohleb ES, Hanke ML, Corona AW, Powell ND, Stiner LM, Bailey MT, Nelson RJ, Godbout JP, and Sheridan JF (2011). beta-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. The Journal of neuroscience : the official journal of the Society for Neuroscience 31, 6277–6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Powell ND, Godbout JP, and Sheridan JF (2013). Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. The Journal of neuroscience : the official journal of the Society for Neuroscience 33, 13820–13833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Terwilliger R, Duman CH, and Duman RS (2018). Stress-Induced Neuronal Colony Stimulating Factor 1 Provokes Microglia-Mediated Neuronal Remodeling and Depressive-like Behavior. Biological psychiatry 83, 38–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodburn SC, Bollinger JL, and Wohleb ES (2021). Synaptic and behavioral effects of chronic stress are linked to dynamic and sex-specific changes in microglia function and astrocyte dystrophy. Neurobiol Stress 14, 100312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray NR, Ripke S, Mattheisen M, Trzaskowski M, Byrne EM, Abdellaoui A, Adams MJ, Agerbo E, Air TM, Andlauer TMF, et al. (2018). Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet 50, 668–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Writing Committee for the Attention-Deficit/Hyperactivity, D., Autism Spectrum D, Bipolar D, Major Depressive D, Obsessive-Compulsive D, and Schizophrenia EWG, Patel Y, Parker N, Shin J, Howard D, et al. (2021). Virtual Histology of Cortical Thickness and Shared Neurobiology in 6 Psychiatric Disorders. JAMA Psychiatry 78, 47–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Z, Zhang K, Ren Q, Chang L, Chen J, and Hashimoto K. (2019). Increased expression of inwardly rectifying Kir4.1 channel in the parietal cortex from patients with major depressive disorder. J Affect Disord 245, 265–269. [DOI] [PubMed] [Google Scholar]
- Yamamuro K, Yoshino H, Ogawa Y, Makinodan M, Toritsuka M, Yamashita M, Corfas G, and Kishimoto T. (2018). Social Isolation During the Critical Period Reduces Synaptic and Intrinsic Excitability of a Subtype of Pyramidal Cell in Mouse Prefrontal Cortex. Cereb Cortex 28, 998–1010. [DOI] [PubMed] [Google Scholar]
- Yang Y, Cheng Z, Tang H, Jiao H, Sun X, Cui Q, Luo F, Pan H, Ma C, and Li B. (2017). Neonatal Maternal Separation Impairs Prefrontal Cortical Myelination and Cognitive Functions in Rats Through Activation of Wnt Signaling. Cereb Cortex 27, 2871–2884. [DOI] [PubMed] [Google Scholar]
- Yang Y, Zhang Y, Luo F, and Li B. (2016). Chronic stress regulates NG2(+) cell maturation and myelination in the prefrontal cortex through induction of death receptor 6. Exp Neurol 277, 202–214. [DOI] [PubMed] [Google Scholar]
- Yi Z, Li Z, Yu S, Yuan C, Hong W, Wang Z, Cui J, Shi T, and Fang Y. (2012). Blood-based gene expression profiles models for classification of subsyndromal symptomatic depression and major depressive disorder. PloS one 7, e31283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin W, Gallagher NR, Sawicki CM, McKim DB, Godbout JP, and Sheridan JF (2019). Repeated social defeat in female mice induces anxiety-like behavior associated with enhanced myelopoiesis and increased monocyte accumulation in the brain. Brain Behav Immun 78, 131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya R, Rimmerman N, and Reshef R. (2015). Depression as a microglial disease. Trends Neurosci 38, 637–658. [DOI] [PubMed] [Google Scholar]
- Yoshino Y, Roy B, Kumar N, Shahid Mukhtar M, and Dwivedi Y. (2021). Molecular pathology associated with altered synaptic transcriptome in the dorsolateral prefrontal cortex of depressed subjects. Transl Psychiatry 11, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Nagai J, and Khakh BS (2020). Improved tools to study astrocytes. Nat Rev Neurosci 21, 121–138. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Strachan E, Fowler E, Bacus T, Roy-Byrne P, and Zhao J. (2019). Genome-wide profiling of DNA methylome and transcriptome in peripheral blood monocytes for major depression: A Monozygotic Discordant Twin Study. Transl Psychiatry 9, 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann P, Bruckl T, Nocon A, Pfister H, Lieb R, Wittchen HU, Holsboer F, and Angst J. (2009). Heterogeneity of DSM-IV major depressive disorder as a consequence of subthreshold bipolarity. Arch Gen Psychiatry 66, 1341–1352. [DOI] [PubMed] [Google Scholar]