Abstract
Glioblastomas (GBMs) are aggressive brain tumors that are resistant to chemotherapy and radiation. Bone morphogenetic protein (BMP) ligand BMP4 is being examine as a potential therapeutic for GBMs because it induces differentiation of cancer stem cells (CSC) to an astrocyte phenotype. ID1 is reported to promote self-renewal and inhibit CSC differentiation. In most cancers, ID1 is transcriptionally upregulated by BMP4 promoting invasion and stemness. This conflicting data brings into question whether BMP signaling is growth suppressive or growth promoting in GBMs. We utilized BMP inhibitors DMH1, JL5, and Ym155 to examine the role of BMP signaling on the growth of GBMs. DMH1 targets BMP type 1 receptors whereas JL5 inhibits both the type 1 and type 2 BMP receptors. Ym155 does not bind the BMP receptors but rather inhibits BMP signaling by inducing the degradation of BMPR2. We show that JL5, DMH1, and Ym155 decreased the expression of ID1 in SD2 and U87 cells. JL5 and Ym155 also decreased the expression of BMPR2 and its down-stream target inhibitor of apoptosis protein XIAP. JL5 treatment resulted in significant cell death and suppressed self-renewal to a greater extent than that induced by BMP4 ligand. The lysosome inhibitor chloroquine increases the localization of BMPR2 to the plasma membrane enhancing JL5 induced downregulation of ID1 and cell death in SD2 cells. We show that BMP signaling is growth promoting in GBMs. These studies suggest the need for development of BMP inhibitors and evaluation as potential therapeutic for GBMs.
Keywords: BMP, BMPR2, BMP inhibitors, Smad-independent, glioblastoma, cancer stem cells, ID1, cell survival, self-renewal
Introduction
Glioblastomas are highly aggressive brain tumors effecting approximately 12,000 people per year in the United States [1]. Brain tumors are the 3rd leading cause of cancer deaths between the ages of 15–39. Glioblastomas are universally fatal with mean survival of 14 months [2]. Glioblastomas are treated with the combination of radiation, chemotherapy, and surgery [2]. These dismal results make it clear new cancer therapeutics are needed for the treatment of malignant brain tumors.
Cancer cells within glioblastomas have characteristics similar to those of neural stem cells. Cancer stem cells (CSC) are tumor-initiating cells that self-renew and differentiate [3]. The glial cancer stem cell population is also resistant to chemotherapy and radiation [4]. The bone morphogenetic proteins (BMPs) are known to induce the differentiation of normal neural stem cells into astrocytes [5]. Studies have revealed that BMP ligands have a growth suppressive effect on glioblastoma CSCs [6–8]. The mechanisms by which BMP ligands suppress growth of glioblastomas are not fully elucidated. However, studies suggest that BMP4 may be mediated by inducing the differentiation of CSCs into astrocytes [6, 9].
BMPs are transcription activators of inhibitor of differentiation proteins, ID1–4. BMP ligands, BMP4 and BMP2, bind to type 1 BMP receptors (BMPR1), which are then activated by the BMP type 2 (BMPR2) receptors [10]. The BMPR1/BMPR2 complex then phosphorylates Smad-1/5 factor promoting its translocation to the nucleus inducing the transcription of ID1–4 [11–13]. BMP2/4 are potent activators of ID1 in many cancers including glioblastoma CSCs [14–16]. ID1 regulates cell invasion, proliferation, and self-renewal in many cancers including glioblastoma CSCs [14, 17–24].
BMP4 ligand is currently being evaluated as a potential therapy for glioblastomas. Interestingly, BMP4 has also been shown to induce quiescence of CSCs leading to chemotherapy resistance through its upregulation of ID1 [16]. How BMP4 suppresses glioblastomas while increasing ID1 expression, which is reported to enhance tumorigenesis, is not understood. BMP receptor inhibitors have been shown to inhibit growth of other cancer cell types through a process, which involves the downregulation of ID1 [14, 25, 26]. Gain of function mutations of alk2 occur in 25% of the rare pediatric brainstem tumor diffuse high-grade astrocytoma [27]. Gain of function mutations of alk2 occur in 95% of patients with Fibrodysplasia ossificans progressive (FOP), which is characterized by ectopic ossification of soft tissues [28, 29]. Laboratories have developed specific inhibitors of the type I BMP receptors to treat diseases with overactive BMP signaling. BMP inhibitors are expected to enter clinical trials shortly [30, 31]. BMP receptor inhibitors have not been evaluated in glioblastomas.
We hypothesized that inhibition of BMP receptor signaling will decrease ID1 expression and suppress growth of glioblastomas. Using BMP inhibitors, we show that following suppression of BMP signaling there is a significant decrease in the expression of ID1. BMP inhibitors significantly suppressed growth and self-renewal of glioblastoma cell lines compared to BMP4. These studies suggest that suppressing BMP signaling rather than inducing its activation may be a better strategy to suppress growth of glioblastomas.
Material and Methods
Cell culture and reagents
SD2 cells were derived from a primary glioblastoma that have been maintained in neural stem cell medium (Neurocult NS-A proliferation medium, Stemcell Technologies, WA, USA). SD2 cells were obtained from Roland H. Friedel (Department of Neurosurgery, Icahn School of Medicine at Mount Sinai, New York, NY) who authenticated and characterized the cell line [32]. The U87 MG cell line was purchased from ATCC and characterized genetically at COSMIC cancer database. U87 MG cells were cultured in EMEM medium (Sigma Aldrich, USA) supplemented with 10% FBS. For SD2 cells, plates were coated with 10ug/ml laminin (Sigma Aldrich, USA) for 4 hours at 37°. GBM2 and GBM3 are human primary undifferentiated grade IV glioblastoma cell lines obtained from Ramsey Foty [33, 34] (Department of Surgery, Rutgers Robert Wood Johnson Medical School). These cell lines were maintained in Eagles’ Minimal Essential Medium (EMEM)/10% fetal calf serum and antibiotics. Cell culture experiments performed in a mycoplasma free environment. JL5 was synthesized by Dr. Jacques Roberge and John Gilleran at Rutgers University, Molecular Design & Synthesis [35]. YM155 was purchased from Selleckchem (Houston, TX). Chloroquine and BMP4 were purchased from Sigma Aldrich, USA and R&D systems, USA, respectively.
Cell viability
For SD2 cell, 300,000 cells/well and for U87 cell 400,000 cells/well were seeded in duplicate into 6-well plates and grown overnight at 37° incubator with 5% CO2. The cells were then treated for the designated time period. After the treatment, SD2 cells were harvested with accutase and U87 cells were harvested with 0.05% trypsin. An automated Vi-CELL cell analyzer (Beckman Coulter, USA) was used to determine the percent dead cells and total number of live cells in each treatment group. Each experiment was replicated at least 4 times in our laboratory.
Immunofluorescence staining
SD2 and U87 cells were seeded overnight onto microscope cover glasses in a 6 well plate. Cells were treated with 2.5uM JL5, 20nM Ym155 and 80uM Chloroquine for designated period. Cells were then washed with PBS and fixed 4% formaldehyde. Permeabilized was performed with 0.5% triton-X. To determine BMPR2 on the plasma membrane, the permeabilization step was not performed. The cells were blocked with CAS-block (Life Technologies, USA) for 1h and then incubated with anti-BMPR2 antibody (Sigma-Aldrich, USA) for 1h at room temperature. This antibody recognizes an extracellular epitope of BMPR2. Then the cells were washed with PBS and incubated with Alexa Flour 488 conjugated secondary antibody at 1:100 dilution for 1h at room temperature. After washing with PBS, the nuclei were counterstained with DAPI (Sigma-Aldrich, USA) for 10 min. Fluorescent images were captured using an Nikon eclipse TE300 inverted epifluorescent microscope (60X oil lens) and a Cool Snap black and white digital camera. IP Lab imaging software was used to assign pseudo-color to each channel. ImageJ (NIH, USA) software was used to determine the mean fluorescence intensity and count BMPR2 positive cells.
Western blot analysis
Cells were seeded overnight in 6 well plates and then treated for the designated period. Total cellular protein was extracted using RIPA buffer and the protein concentration was determined by using Pierce BCA protein assay kit (ThermoFisher, USA). Equal amounts of proteins were loaded onto a polyacrylamide gel and separated by SDS-PAGE. The proteins were transferred overnight to a nitrocellulose membrane (Biorad, USA). The membranes were blocked for 1h using membrane blocking solution (Life Technologies, USA) and then incubated overnight with primary antibody at 4°C. The primary antibodies used were rabbit monoclonal anti-Id1 (Calbioreagents, San Mateo, CA), rabbit monoclonal XIAP, rabbit monoclonal beta III tubulin (Tuj1), rabbit monoclonal anti-pSmad 1/5 (Cell Signaling Technology, MA, USA), rabbit polyclonal anti-Smad 1/5 (Upstate Biotechnology, NY, USA), rabbit anti-actin, rabbit polyclonal anti-GAPDH (Sigma, St. Louis, MO), and mouse monoclonal anti-spectrin (EMD Millipore, CA, USA). Rabbit polyclonal SOX2 (Abcam, USA), mouse monoclonal anti-NeuN (Chemicon, USA), and mouse monoclonal anti-Nestin (Chemicon, USA).
3D-Sphere forming ability
To evaluate 3D-sphere forming ability, 1000 cells were plated per will in 12 well plates. To facilitate the sphere formation, the plate was not coated with laminin. Next, the cells were treated with DMSO, 40ng/ml BMP4 and 2.5uM of JL5 for 7 days then 4 20x images were taken per well using a Nikon eclipse TE300. Using ImageJ (NIH, USA) software the total number of spheres was counted. A histogram was plotted using average number of spheres in each treatment group.
Extraction of membrane proteins:
The membrane proteins were extracted using Mem-PER™ Plus Membrane Protein Extraction Kit (Thermo Scientific #89842) according to the manufacturer’s protocol. Briefly, 1 × 106 cells were grown overnight and then treated for 24 hours. After treatment, cells were collected by scraping, and then cell suspension was centrifuged at 300 × g for 5 minutes. The cell pellet was washed twice with Cell wash solution and centrifuged at 300 × g for 5 minutes. 100 μl of Permeabilization Buffer was added to the cell pellet and incubated for 10 minutes at 4°C. The permeabilized cells were then centrifuged at 16,000 × g for 15 minutes. The supernatant was collected as cytosolic proteins. Solubilization Buffer was added to the cell pellet and incubated for 30 minutes at 4°C. Solubilized membrane and membrane-associated proteins were collected after centrifuging the tubes 16,000 × g for 15 minutes at 4°C.
Statistical Analysis
The mean of the control group was compared to the mean of each treated group using a paired student t-test assuming unequal variances. Differences with p values <.05 were considered statistically significant.
RESULTS
JL5 treatment suppresses growth and downregulates ID1 expression of glioblastoma cells
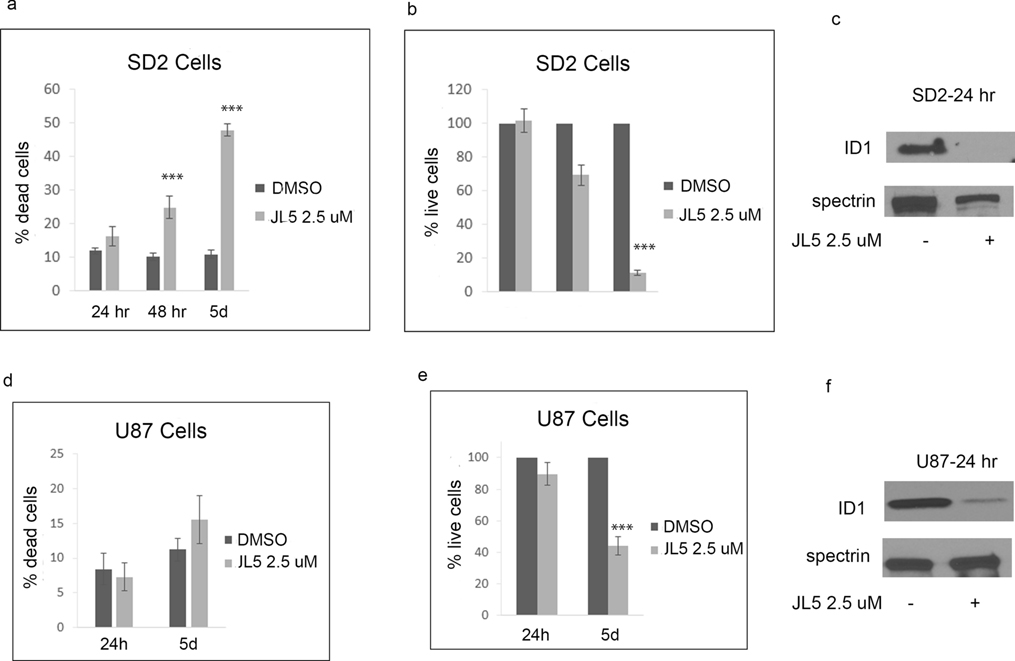
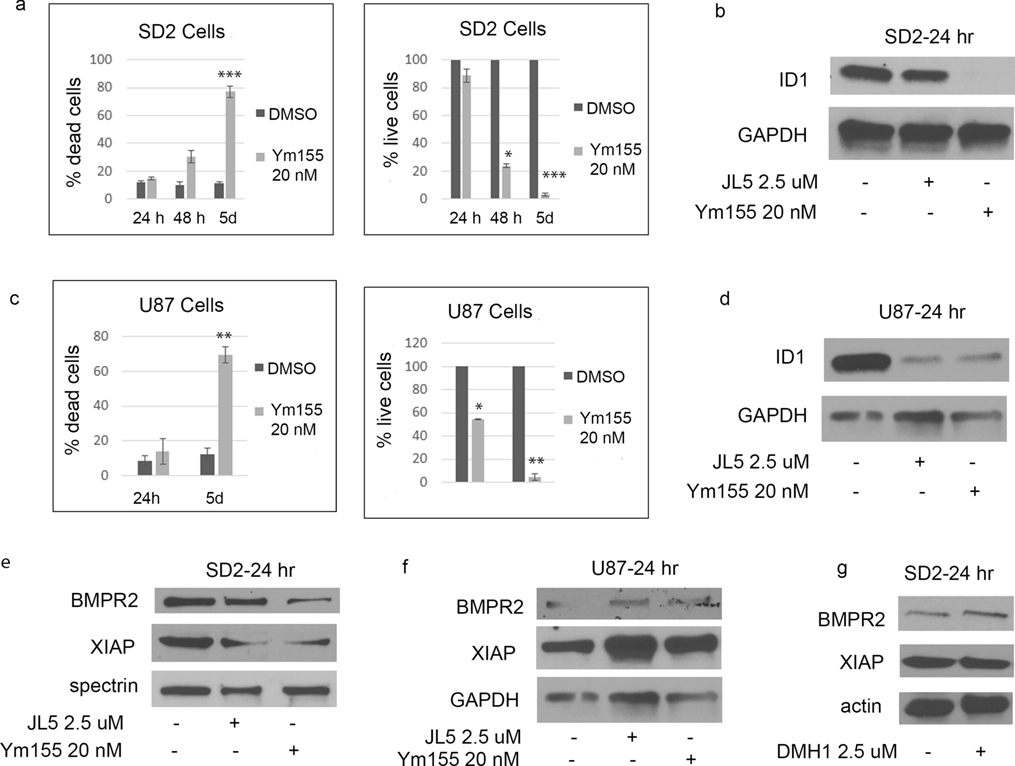
JL5 is a potent inhibitor of the BMP type 1 receptors alk2, alk3, and alk6 [35]. JL5 has some inhibition for BMPR2 but also suppresses BMPR2 signaling by causing its mislocalization to the cytoplasm [35, 36]. We have previously reported that JL5 is a more potent inhibitor of BMP signaling and growth suppression of lung cancer cells than inhibitors specific for BMPR1 (DMH1) [19, 35, 36]. The greater suppression of BMP signaling induced by JL5 is thought to be due to its ability to suppress BMPR2 smad-independent signaling. Here we show that JL5 treatment resulted in significant cell death and growth suppression of SD2 cells (Figure 1a–b). However, JL5 treatment of U87 cells, which are in a more differentiated state than SD2 cells, did not result in significant cell death, but appeared to significantly suppress growth (Figure 1d–e). JL5 significantly decreased the expression of ID1 in both SD2 and U87 cells (Figure 1c, f).
Figure 1. JL5 suppresses growth of glioblastoma cell lines and down-regulates the expression of ID1.
(a,b,d,e) SD2 and U87 cells were treated with JL5 for 5 days and the percentage of dead cells and number of live cells determined. Data represents the mean of 4 independent experiments. (c,f) Western blot analysis of SD2 and U87 cells treated with JL5 demonstrating a decrease in ID1 expression. *** p <0.0005 compared to control.
Effects of DMH1 on cell growth and ID1 expression.
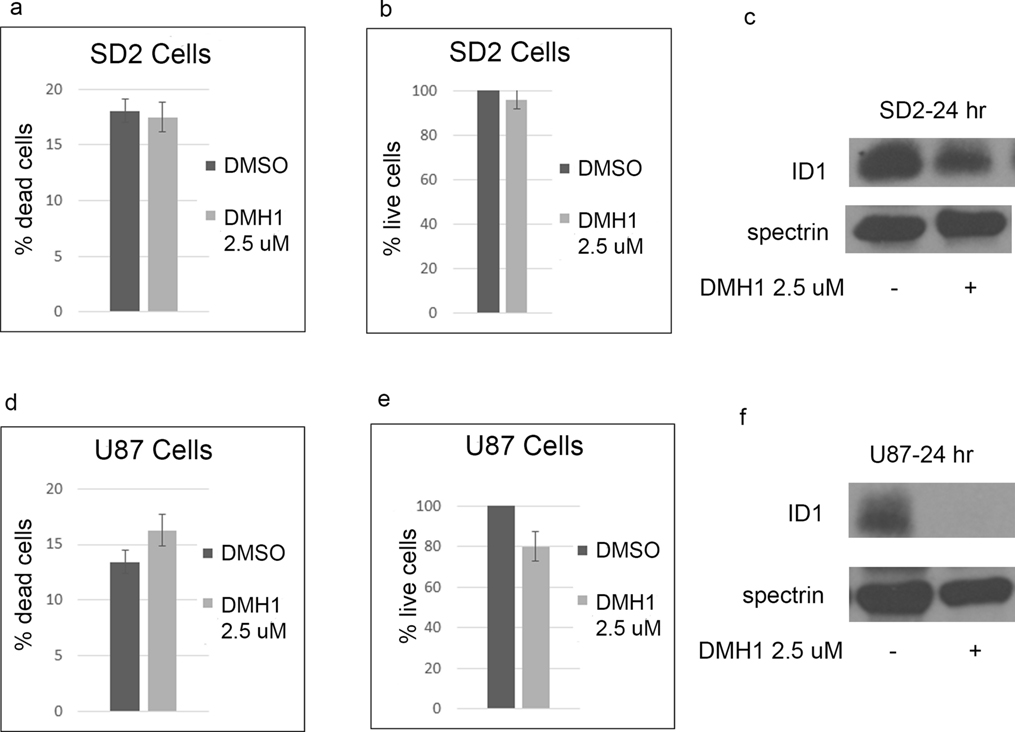
DMH1 is a selective inhibitor of the BMP type 1 receptors (alk2, alk3, and alk6) with no activity for BMPR2 [37]. In SD2 cells, DMH1 caused less growth suppression, cell death, and downregulation of Id1 than JL5 (Figure 2a–b). DMH1 also decreased ID1 expression in SD2 cells (Figure 2c). In U87 cells, DMH1 did suppress growth and caused a significant decrease in the expression of ID1 (Figure 2c–f).
Figure 2. Inhibitor of BMP type 1 receptor DMH1 decreases ID1 expression with little effect on cell survival.
(a,b,d,e) SD2 and U87 cells were treated with DMH1 for 5 days and the percentage of dead cells and number of live cells determined. Data represents the mean of 4 independent experiments. (c,f) Western blot analysis of SD2 and U87 cells treated with DMH1 demonstrating a decrease in ID1 expression, which was more in U87 cells.
Effects of JL5 and DMH1 on GBM2 and GBM3 cell lines
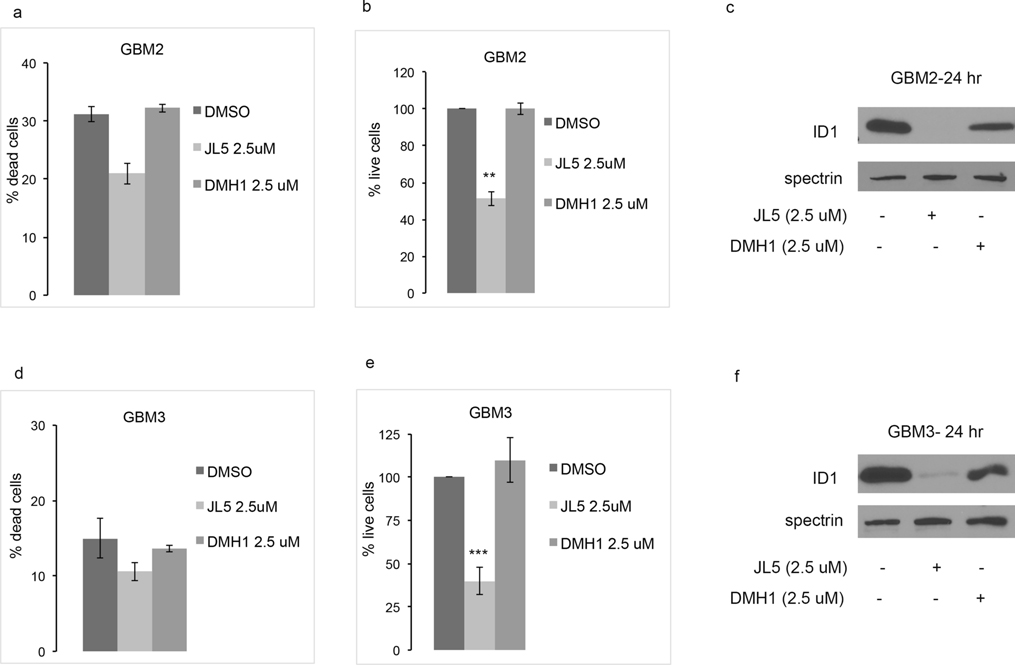
To further examine the effects of JL5 compared to DMH1, primary human glioblastoma cells lines GBM2 and GBM3 were treated with each compound at the same time. Neither of the compounds induced cell death (Figure 3a,d). JL5 caused significant growth suppression and downregulation of the expression of ID1 in both GBM2 and GBM3 cell lines (Figure 3). DMH1 had no significant effect on cell growth (Figure 3 b,e). Although DMH1 did suppress ID1 expression in both cell lines it was less compared to that of JL5 (Figure 3 c,f). Together, these studies show that BMP receptor small molecule inhibitors decrease ID1 expression suggesting BMP is an upstream regulator of ID1. Growth inhibition correlated with the degree of ID1 downregulation.
Figure 3.
JL5 decrease cell growth and ID1 expression in primary glioblastomas GBM2 and GBM3. (a,b,d,e) GBM2 and GBM2 glioblastoma cell lines were treated with JL5 or DMH1 for 5 days and the percentage of dead and live cells determined. Data represents the mean of 4 experiments for GBM3 and 2 experiments for GBM2. (C,F) Western blot analysis of GBMs treated with JL5 or DMH1 for 24 hr. **p<0.005, *** p <0.0005 compared to control.
Regulation of BMP4 on ID1 expression and cell growth
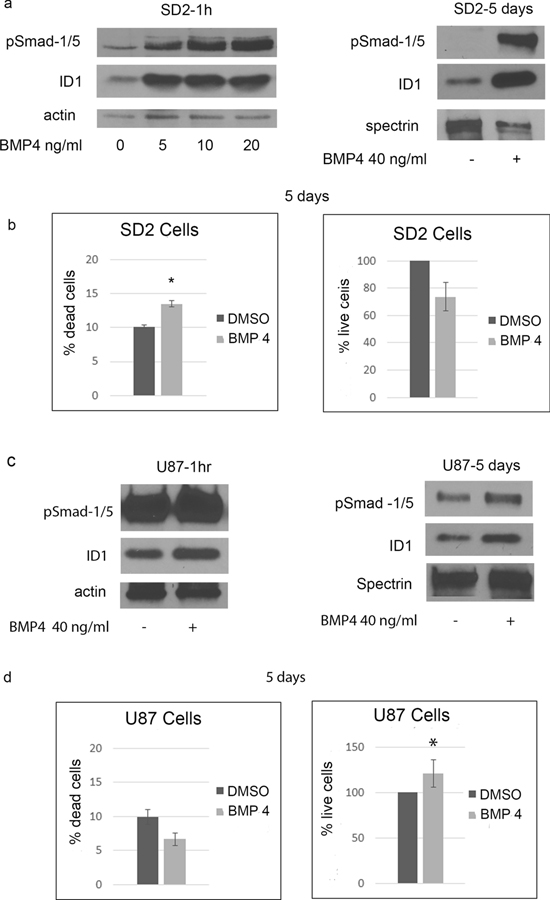
Next, we used BMP4 ligand to assess if BMP signaling is an upstream regulator of ID1 and exam ligands effects on cell growth. In SD2 cells, BMP4 activated Smad-1/5 and increased the expression of ID1 within 1 hour, and persisted for at least 5 days (Figure 4a). After 5 days of treating SD2 cells with BMP4 approximately 13% of the cells were dead (Figure 4b). In comparison, 50% of SD2 cells were dead after 5 days of treatment with JL5 (Figure 1a). The number of live cells was also decreased after 5 days but the change was not as significant as seen with JL5 (Figure 4b). In U87 cells, BMP4 also caused an increase in the activation of pSmad-1/5 and ID1 expression at 1 hour and 5 days but the increase was not as robust as that observed for SD2 cells (Figure 4c). However, the baseline phosphorylation of Smad-1/5 was higher in the U87 cells. Treating U87 cells with BMP4 for 5 days caused a significant increase in cell growth (Figure 4d).
Figure 4. BMP4 activates ID1 expression and suppresses growth of SD2 cells.
(a) Western blot analysis of SD2 cells treated with BMP4. (b) SD2 cells were treated with BMP4 for 5 days and percentage of dead cells and number of live cells determined. Data represents the mean of 4 independent experiments. (c) Western blot analysis of U87 cells treated with BMP4. (d) U87 cells were treated with BMP4 for 5 days and percentage of dead cells and number of live cells determined. Data represents the mean of 4 independent experiments. * p <0.05, compared to control.
Ym155 decreases ID1 and XIAP expression and induces cell death
Ym155 was originally reported to be a survivin inhibitor [38]. We found that Ym155 potently decreases BMP signaling and induces cell death of non-small cell lung carcinomas (NSCLC) cell lines [39]. Ym155 inhibition of BMP signaling is mediated by causing the mislocalization of BMPR2 to the cytoplasm and enhancing its degradation [39]. In both SD2 and U87 cells, Ym155 treatment results in significant cell death with few remaining live cells after 5 days (Figure 5a, c). In both cells lines, Ym155 decreased the expression of ID1 (Figure 5b, d). In SD2 but not in U87 cells, Ym155 decreased ID1 expression to a greater extent than JL5 (Figure 5b, d). Ym155 decreased the expression of BMPR2 and its’ down-stream target XIAP in SD2 cells similar to that of JL5 (Figure 5e). Neither Ym155 nor JL5 decreased the expression of BMPR2 or XIAP in U87 cells (Figure 5f). Inhibition of BMP type I receptors does not down-regulate XIAP in lung cancer cells [19, 36]. DMH1 also did not down-regulate XIAP in SD2 cells (Figure 5g). These studies suggest that Ym155 and JL5 but not DMH1 down-regulates BMPR2 Smad-independent signaling in SD2 cells.
Figure 5. Ym155 inhibits BMP signaling. J5 and Ym155 decrease XIAP expression in SD2 cells.
(a,c) SD2 and U87 cells were treated with Ym155 and the percentage of dead cells and number of live cells determined. Data represents the mean of 4 independent experiments. (b,d) Western blot analysis of SD2 and U87 cells treated with Ym155 or JL5 demonstrating a decrease in ID1 expression. (e,f) Western blot analysis of cells treated with JL5 and Ym155 showing a decrease the expression of XIAP in SD2 but not the U87 cells. (g) Western blot analysis showing DMH1 does not decrease expression of XIAP. * p <0.05, ** p <0.005, *** p <.0005 compared to control.
Ym155 and JL5 do not regulate differentiation
BMP4 signaling not only induces differentiation of NSC into astrocytes, it inhibits their differentiation into neurons [40]. We asked whether the suppression of BMP signaling with JL5 or Ym155 would promote neuronal differentiation in glioblastomas. SD2 cells have a higher expression of the NSC markers nestin and Sox2 in comparison to the U87 cells (Figure S1). SD2 cells also have a higher expression of the neuronal markers Tuj1 and NeuN in comparison to U87 cells (Figure S1). Other than a decrease in Sox2 in SD2 induced by Ym155, there was no significant change in any of the markers by Ym155 or JL5 in either the SD2 or U87 cells (Figure S1). Based on this study, we see no evidence that inhibition of BMP signaling promotes neuronal differentiation in glioblastomas.
Chloroquine increases BMPR2 cell membrane expression and enhances JL5 induced cell death
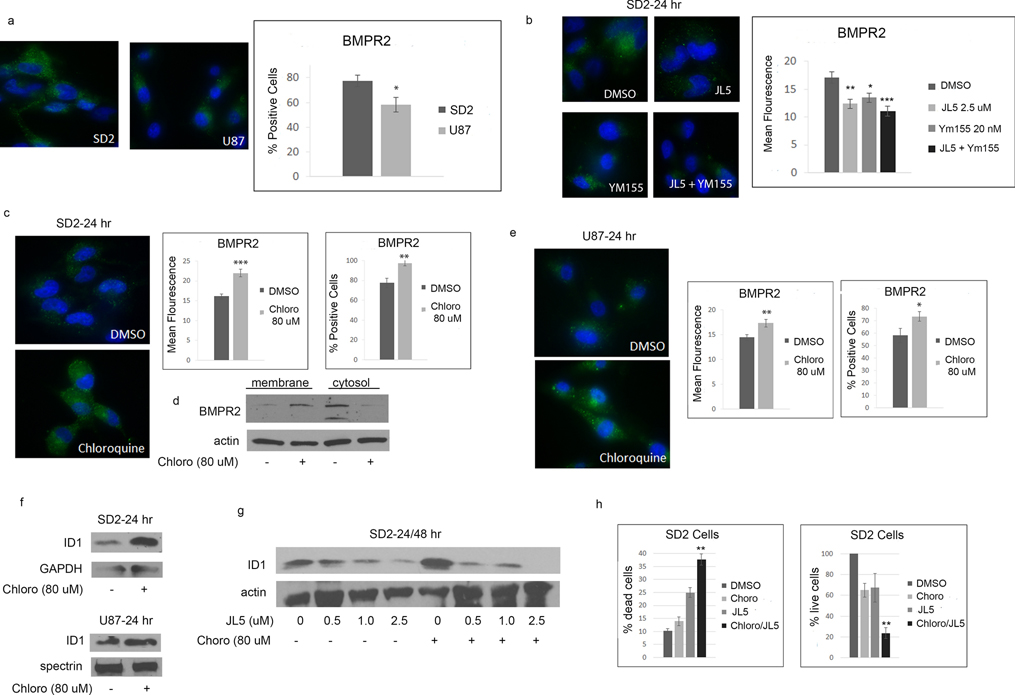
We previously reported in NSCLC cell lines that JL5 and Ym155 cause the mislocalization of BMPR2 to the cytoplasm with a corresponding decrease in the BMPR2 expressed on the plasma membrane [39]. Using an antibody recognizing the external epitope of BMPR2, we found that there was significantly fewer U87 cells expressed BMPR2 in comparison to SD2 cells (Figure 6a). Both JL5 and Ym155 decreased the expression of BMPR2 in SD2 cells (Figure 6b).
Figure 6. Chloroquine increases BMPR2 expression and enhances cell death induced by JL5.
(a) Representative immunofluorescent images of BMPR2 in untreated cells. Graphs represent the percentage of cells expressing BMPR2. Approximately 80 cells were counted from each cell line. (b) Representative immunofluorescent images of BMPR2 following treatment with JL5 and Ym155. Graphs represent the mean fluorescence of approximately 20 cells from each treatment group. (c,e) Representative immunofluorescent images of BMPR2 following treatment with chloroquine. Graphs represent the mean fluorescence of approximately 30 cells from each treatment group and the percentages of cells that express BMPR2. (d) Western blot of membrane and cytosol fraction of SD2 cells treated with chloroquine for 24 hours. (f) Western blot showing chloroquine increases the expression of ID1. (g) Western blot analysis showing that chloroquine enhances JL5 induced down-regulation of ID1. (h) Cell counts of SD2 cells pre-treated with either DMSO or Chloroquine for 24 hours, then treated again with either DMSO or JL5 for 48 hours. The data represents the mean of 4 independent experiments. * p <0.05, ** p <0.005, *** p <0.0005 compared to control. (g) ** p < 0.005 chloroquine + JL5 in comparison to JL5 alone.
Chloroquine decreases the lysosomal degradation of BMPR2 resulting in an increase in BMPR2 being expressed on the cell surface [41]. Chloroquine significantly increased the intensity and the number of cells expressing BMPR2 in both the SD2 and U87 cells (Figure 6c,e). Chloroquine also increased BMPR2 protein expression in the membrane fraction while decreasing expression in the cytosol (Figure 6d). Chloroquine also increased the expression of ID1 demonstrating an increase in BMP signaling (Figure 6f). SD2 cells pretreated with chloroquine were more responsive to JL5 as demonstrated by a greater decrease in the expression of ID1 (Figure 6g). The combination of JL5 with chloroquine increased the downregulation of Id1 and significantly enhanced cell death of SD2 cells compared to either compound alone (Figure 6h).
BMP inhibitors decrease self-renewal
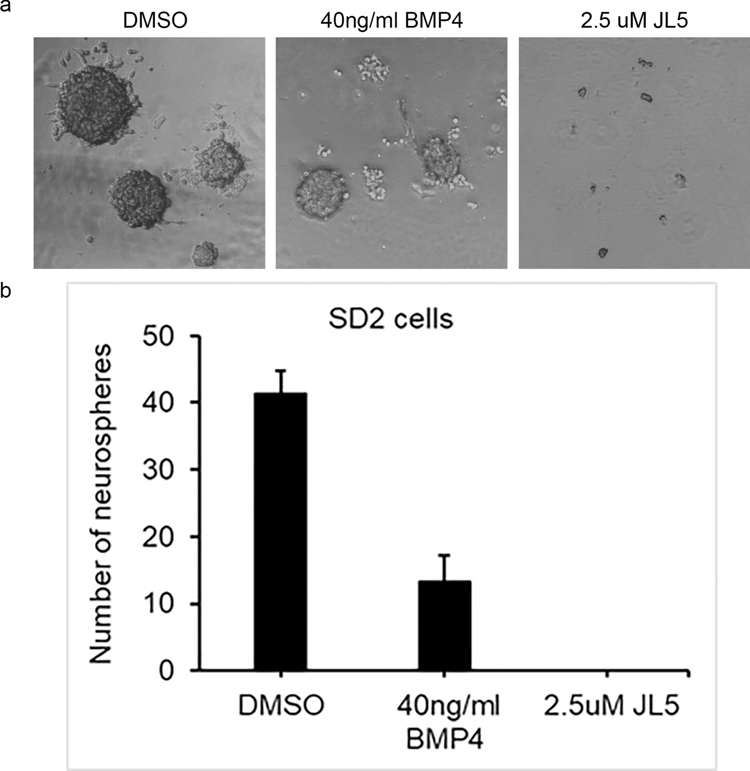
The ability to form spheres from single cells is commonly used to assess self-renewal of glioblastomas CSC populations. Similar to what has been previously reported, BMP4 ligand suppressed self-renewal of glioblastoma cells (Figure 7). Spheres formed following treatment with BMP4 were smaller and less in number (Figure 7). JL5 completely prevented sphere formation of glioblastoma cells (Figure 7). These data suggest that inhibition BMP signaling suppresses self-renewal more than its activation.
Figure 7. JL5 inhibits self-renew more than BMP4.
1000 SD2 cells were plated into 6 well plates and then treated with BMP4 or JL5 for 7 days. (a) Representative phase contrast images of sphere formation. (b) All the spheres in the 6 well plates were counted. Depicted is the mean number of spheres from 3 independent experiments.
Discussion
Terminally differentiating glial cancer stem cells into non-cycling astrocytes with BMP4 is a novel and intriguing therapeutic approach for glioblastoma. Initial studies showed that BMP4 reduced the number of glial tumor-initiating cells and effectively blocked tumor growth in mice after intracerebral grafting [6]. Subsequent studies have revealed challenges with BMP4 directed therapy [42]. Many patient-derived glioblastomas are resistant to the growth suppressive effects of BMP4 [43]. Of the glioblastomas that are responsive to BMP4, DNA methylation was incomplete and cells could re-enter the cell cycle when challenged with growth factors [44]. Activation of BMP signaling has also been shown to induce quiescence of glial cancer stem cells, which are resistant to radiation and temozolomide chemotherapy [16]. Other challenges include the ability to deliver BMP4 across the blood-brain barrier.
Adding to the complexity of BMP signaling in glioblastomas is its role in the regulation of ID1. ID1 enhances the tumorigenicity of several cancers including glioblastomas. Knockout of ID1 in glioblastomas decreased invasiveness, prevented tumor progression, and inhibited glial cancer stem cell self-renewal [45–47]. One study suggested that ID1 promoted self-renewal of glial cancer stem cells by suppressing the expression of BMPR2 [24]. In this paradigm, ID1 is an upstream regulator of BMP signaling. Multiple studies have shown that BMP signaling is an upstream transcriptional regulator of ID1 [11–13]. ID1 has been shown to be a direct target of BMP ligands in normal, cancer, and embryonic stem cells [15] [26] [11]. In our studies, suppression of BMP signaling with inhibitors significantly decreased ID1 expression without increasing the expression of BMPR2. In the present study, BMP ligands potently increased the expression of ID1 in glioblastoma cell lines indicating ID1 is a downstream target of BMP signaling. We conclude that BMP signaling is upstream of ID1 in glioblastomas and its suppression it likely contributing to the growth suppressive effects observed with BMP inhibition.
Glioblastoma consist of heterogenous cell populations consisting of cells with stem cell-like (CSC) properties as well are cells that are more differentiated [48]. CSC are tumor promoting and are more resistant to chemotherapy and radiation. Eradication of CSC are thought to be required to eliminate tumor burden. Non-CSC can acquire stem cell like properties depending on the microenvironment or in response to therapy [48]. ID1 promotes self-renewal of embryonic and hematopoietic stem cells [49] as well as CSC population in glioblastomas and other cancers [47, 50]. ID1 also regulates proliferation and invasiveness of cancer cells. Knockdown of ID1 in unselected cancer cell lines diminishes invasiveness and proliferation [45, 46]. These studies suggests that ID1 cancer promoting properties are not isolated only to the CSC population.
Our studies suggest that the inhibition of BMP signaling rather than its stimulation will have a greater effect suppressing growth and inducing cell death of glioblastoma, including its’ cancer stem cell population. Our studies have suggested that suppression of BMPR2 causes more cell death in cancer cells than the inhibition of BMP type 1 receptors [36]. BMP signaling has been shown in other cancers to stimulate pathways known to enhance survival. Some of these cell survival pathway are mediated by BMPR2 independent of BMPR1 and the activation of Smad-1/5. BMPR2 regulated pathways include the potent ant-apoptotic proteins XIAP and TAK1 [19, 51, 52]. One study suggested that inhibition of BMP type 1 receptor decreased oncogenic features of malignant astrocytes. Transgenic deletion of BMP 1A receptor (alk3) in astrocyte transformed with oncogenic Ras and deletion of p53 decreased oncogenic astrocytes proliferation, decreased migration, and decreased invasion [53]. Authors also report that DMH1 suppressed proliferation of oncogenic astrocytes more than BMP4 [53]. We cannot exclude that the cell death induced by JL5 was not mediated in part by other mechanism such as suppression of TGFß signaling. More potent and specific BMPR2 inhibitors are needed to address these important questions.
BMPR2 is cycled from the plasma membrane to the cytosol and back to the plasma membrane by recycling endosomes [54]. BMPR2 can also be trafficked to the lysosome for proteolytic degradation. Chloroquine increases lysosomal pH, which inhibits the degradation of cell surface protein including BMPR2 [41]. Chloroquine caused an increase expression of BMPR2 in SD2 and U87 cells with an increase in expression of ID1. Surprisingly, Chloroquine together with JL5 significantly enhanced cell death. These data suggest that the synergistic cell death induced by co-treatment was because the cells were more sensitive to BMP inhibition as a result of BMPR2 expression.
Conclusion
The studies presented here suggest that inhibition of BMP signaling is a potential therapeutic approach to treat glioblastomas. Our preliminary observations also suggest that synergistic cell death occurs with JL5 and chloroquine. Further investigation is needed with more specific BMP inhibitors in a larger number of glioblastomas both in vitro and in vivo tumor models to validate these findings.
Supplementary Material
Acknowledgements
This work was supported by grants from National Institute of Health (NIH) R01 CA225830 and 3R01CA225830-01S1, and Rutgers University and NIH, #U01HL150852.
Funding:
This work was funded by grants from National Institute of Health (NIH) R01 CA225830, 3R01CA225830-01S1, and HealthAdvance/REACH program, Rutgers University and NIH, #U01HL150852.
Abbreviations
- GBMs
glioblastomas
- CSC
cancer stem cells
- BMPs
bone morphogenetic proteins
- BMPR
bone morphogenetic protein receptor
- ID1
inhibitor of differentiation protein 1
- CO2
carbon dioxide
- PBS
phosphate buffered saline
- NIH
National Institute of Health
- XIAP
X-linked inhibitor of apoptosis protein
- TAK1
transforming growth factor-β-activated kinase 1
Footnotes
Conflict of interests:
The authors declare that they have no conflict of interest.
Institutional Approvals
The use of BMP inhibitors and cell lines have been approved by Rutgers University Institutional Biosafety Committee #13-424.
Publisher's Disclaimer: This AM is a PDF file of the manuscript accepted for publication after peer review, when applicable, but does not reflect post-acceptance improvements, or any corrections. Use of this AM is subject to the publisher’s embargo period and AM terms of use. Under no circumstances may this AM be shared or distributed under a Creative Commons or other form of open access license, nor may it be reformatted or enhanced, whether by the Author or third parties. See here for Springer Nature’s terms of use for AM versions of subscription articles: https://www.springernature.com/gp/open-research/policies/accepted-manuscript-terms
Data availability
Data will be made available on reasonable request
References
- 1.Blázquez-Medela AM, Jumabay M and Boström KI (2019) Beyond the bone: Bone morphogenetic protein signaling in adipose tissue. Obes Rev 20:648–658. doi: 10.1111/obr.12822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E and Mirimanoff RO (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–96. doi: 10.1056/NEJMoa043330 [DOI] [PubMed] [Google Scholar]
- 3.Chen J, Li Y, Yu TS, McKay RM, Burns DK, Kernie SG and Parada LF (2012) A restricted cell population propagates glioblastoma growth after chemotherapy. Nature 488:522–6. doi: 10.1038/nature11287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD and Rich JN (2006) Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444:756–60. doi: 10.1038/nature05236 [DOI] [PubMed] [Google Scholar]
- 5.Gross RE, Mehler MF, Mabie PC, Zang Z, Santschi L and Kessler JA (1996) Bone morphogenetic proteins promote astroglial lineage commitment by mammalian subventricular zone progenitor cells. Neuron 17:595–606. [DOI] [PubMed] [Google Scholar]
- 6.Piccirillo SG, Reynolds BA, Zanetti N, Lamorte G, Binda E, Broggi G, Brem H, Olivi A, Dimeco F and Vescovi AL (2006) Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature 444:761–5. doi: 10.1038/nature05349 [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez-Gomez P, Crecente-Campo J, Zahonero C, de la Fuente M, Hernandez-Lain A, Mira H, Sanchez-Gomez P and Garcia-Fuentes M (2015) Controlled release microspheres loaded with BMP7 suppress primary tumors from human glioblastoma. Oncotarget 6:10950–63. doi: 10.18632/oncotarget.3459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xi G, Best B, Mania-Farnell B, James CD and Tomita T (2017) Therapeutic Potential for Bone Morphogenetic Protein 4 in Human Malignant Glioma. Neoplasia 19:261–270. doi: 10.1016/j.neo.2017.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee J, Son MJ, Woolard K, Donin NM, Li A, Cheng CH, Kotliarova S, Kotliarov Y, Walling J, Ahn S, Kim M, Totonchy M, Cusack T, Ene C, Ma H, Su Q, Zenklusen JC, Zhang W, Maric D and Fine HA (2008) Epigenetic-mediated dysfunction of the bone morphogenetic protein pathway inhibits differentiation of glioblastoma-initiating cells. Cancer Cell 13:69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nickel J, Sebald W, Groppe JC and Mueller TD (2009) Intricacies of BMP receptor assembly. Cytokine Growth Factor Rev. 20:367–77. [DOI] [PubMed] [Google Scholar]
- 11.Hollnagel A, Oehlmann V, Heymer J, Ruther U and Nordheim A (1999) Id genes are direct targets of bone morphogenetic protein induction in embryonic stem cells. J Biol Chem 274:19838–45. [DOI] [PubMed] [Google Scholar]
- 12.Katagiri T, Imada M, Yanai T, Suda T, Takahashi N and Kamijo R (2002) Identification of a BMP-responsive element in Id1, the gene for inhibition of myogenesis. Genes Cells 7:949–60. doi: 573 [pii] [DOI] [PubMed] [Google Scholar]
- 13.Korchynskyi O and ten Dijke P (2002) Identification and functional characterization of distinct critically important bone morphogenetic protein-specific response elements in the Id1 promoter. J Biol Chem 277:4883–91. [DOI] [PubMed] [Google Scholar]
- 14.Langenfeld E, Deen M, Zachariah E and Langenfeld J (2013) Small molecule antagonist of the bone morphogenetic protein type I receptors suppresses growth and expression of Id1 and Id3 in lung cancer cells expressing Oct4 or nestin. Mol Cancer. 12:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clement JH, Marr N, Meissner A, Schwalbe M, Sebald W, Kliche KO, Hoffken K and Wolfl S (2000) Bone morphogenetic protein 2 (BMP-2) induces sequential changes of Id gene expression in the breast cancer cell line MCF-7. J Cancer Res Clin Oncol 126:271–9. [DOI] [PubMed] [Google Scholar]
- 16.Sachdeva R, Wu M, Johnson K, Kim H, Celebre A, Shahzad U, Graham MS, Kessler JA, Chuang JH, Karamchandani J, Bredel M, Verhaak R and Das S (2019) BMP signaling mediates glioma stem cell quiescence and confers treatment resistance in glioblastoma. Sci Rep 9:14569. doi: 10.1038/s41598-019-51270-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Langenfeld EM and Langenfeld J (2004) Bone morphogenetic protein-2 stimulates angiogenesis in developing tumors. Mol Cancer Res 2:141–9. [PubMed] [Google Scholar]
- 18.Langenfeld EM, Calvano SE, Abou-Nukta F, Lowry SF, Amenta P and Langenfeld J (2003) The mature bone morphogenetic protein-2 is aberrantly expressed in non-small cell lung carcinomas and stimulates tumor growth of A549 cells. Carcinogenesis 24:1445–54. Epub 2003 Jun 19. [DOI] [PubMed] [Google Scholar]
- 19.Augeri DJ, Langenfeld E, Castle M, Gilleran JA and Langenfeld J (2016) Inhibition of BMP and of TGFbeta receptors downregulates expression of XIAP and TAK1 leading to lung cancer cell death. Mol Cancer 15:27. doi: 10.1186/s12943-016-0511-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newman JH, Augeri DJ, NeMoyer R, Malhotra J, Langenfeld E, Chesson CB, Dobias NS, Lee MJ, Tarabichi S, Jhawar SR, Bommareddy PK, Marshall S, Sadimin ET, Kerrigan JE, Goedken M, Minerowicz C, Jabbour SK, Li S, Carayannopolous MO, Zloza A and Langenfeld J (2018) Novel bone morphogenetic protein receptor inhibitor JL5 suppresses tumor cell survival signaling and induces regression of human lung cancer. Oncogene. doi: 10.1038/s41388-018-0156-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Owens P, Pickup MW, Novitskiy SV, Giltnane JM, Gorska AE, Hopkins CR, Hong CC and Moses HL (2015) Inhibition of BMP signaling suppresses metastasis in mammary cancer. Oncogene 34:2437–49. doi: 10.1038/onc.2014.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le Page C, Puiffe ML, Meunier L, Zietarska M, de Ladurantaye M, Tonin PN, Provencher D and Mes-Masson AM (2009) BMP-2 signaling in ovarian cancer and its association with poor prognosis. J Ovarian Res. 2:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balboni AL, Hutchinson JA, DeCastro AJ, Cherukuri P, Liby K, Sporn MB, Schwartz GN, Wells WA, Sempere LF, Yu PB and DiRenzo J (2013) DeltaNp63alpha-mediated activation of bone morphogenetic protein signaling governs stem cell activity and plasticity in normal and malignant mammary epithelial cells. Cancer Res. 73:1020–30. doi: 10.1158/0008-5472.CAN-12-2862. Epub 2012 Dec 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin X, Jin X, Kim LJY, Dixit D, Jeon HY, Kim EJ, Kim JK, Lee SY, Yin J, Rich JN and Kim H (2018) Inhibition of ID1-BMPR2 Intrinsic Signaling Sensitizes Glioma Stem Cells to Differentiation Therapy. Clin Cancer Res 24:383–394. doi: 10.1158/1078-0432.ccr-17-1529 [DOI] [PubMed] [Google Scholar]
- 25.Langenfeld E, Hong CC, Lanke G and Langenfeld J (2013) Bone morphogenetic protein type I receptor antagonists decrease growth and induce cell death of lung cancer cell lines. PLoS One. 8:e61256. doi: 10.1371/journal.pone.0061256. Print 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langenfeld EM, Kong Y and Langenfeld J (2006) Bone morphogenetic protein 2 stimulation of tumor growth involves the activation of Smad-1/5. Oncogene 25:685–92. [DOI] [PubMed] [Google Scholar]
- 27.Fontebasso AM, Papillon-Cavanagh S, Schwartzentruber J, Nikbakht H, Gerges N, Fiset PO, Bechet D, Faury D, De Jay N, Ramkissoon LA, Corcoran A, Jones DT, Sturm D, Johann P, Tomita T, Goldman S, Nagib M, Bendel A, Goumnerova L, Bowers DC, Leonard JR, Rubin JB, Alden T, Browd S, Geyer JR, Leary S, Jallo G, Cohen K, Gupta N, Prados MD, Carret AS, Ellezam B, Crevier L, Klekner A, Bognar L, Hauser P, Garami M, Myseros J, Dong Z, Siegel PM, Malkin H, Ligon AH, Albrecht S, Pfister SM, Ligon KL, Majewski J, Jabado N and Kieran MW (2014) Recurrent somatic mutations in ACVR1 in pediatric midline high-grade astrocytoma. Nat Genet 46:462–6. doi: 10.1038/ng.2950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shore EM, Xu M, Feldman GJ, Fenstermacher DA, Cho TJ, Choi IH, Connor JM, Delai P, Glaser DL, LeMerrer M, Morhart R, Rogers JG, Smith R, Triffitt JT, Urtizberea JA, Zasloff M, Brown MA and Kaplan FS (2006) A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat Genet 38:525–7. doi: 10.1038/ng1783 [DOI] [PubMed] [Google Scholar]
- 29.van Dinther M, Visser N, de Gorter DJ, Doorn J, Goumans MJ, de Boer J and ten Dijke P (2010) ALK2 R206H mutation linked to fibrodysplasia ossificans progressiva confers constitutive activity to the BMP type I receptor and sensitizes mesenchymal cells to BMP-induced osteoblast differentiation and bone formation. J Bone Miner Res 25:1208–15. doi: 10.1359/jbmr.091110 [DOI] [PubMed] [Google Scholar]
- 30.Sanchez-Duffhues G, Williams E, Goumans MJ, Heldin CH and Ten Dijke P (2020) Bone morphogenetic protein receptors: Structure, function and targeting by selective small molecule kinase inhibitors. Bone 138:115472. doi: 10.1016/j.bone.2020.115472 [DOI] [PubMed] [Google Scholar]
- 31.Hoeman CM, Cordero FJ, Hu G, Misuraca K, Romero MM, Cardona HJ, Nazarian J, Hashizume R, McLendon R, Yu P, Procissi D, Gadd S and Becher OJ (2019) ACVR1 R206H cooperates with H3.1K27M in promoting diffuse intrinsic pontine glioma pathogenesis. Nat Commun 10:1023. doi: 10.1038/s41467-019-08823-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tejero R, Huang Y, Katsyv I, Kluge M, Lin JY, Tome-Garcia J, Daviaud N, Wang Y, Zhang B, Tsankova NM, Friedel CC, Zou H and Friedel RH (2019) Gene signatures of quiescent glioblastoma cells reveal mesenchymal shift and interactions with niche microenvironment. EBioMedicine 42:252–269. doi: 10.1016/j.ebiom.2019.03.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shannon S, Jia D, Entersz I, Beelen P, Yu M, Carcione C, Carcione J, Mahtabfar A, Vaca C, Weaver M, Shreiber D, Zahn JD, Liu L, Lin H and Foty RA (2017) Inhibition of glioblastoma dispersal by the MEK inhibitor PD0325901. BMC Cancer 17:121. doi: 10.1186/s12885-017-3107-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mehta M, Khan A, Danish S, Haffty BG and Sabaawy HE (2015) Radiosensitization of Primary Human Glioblastoma Stem-like Cells with Low-Dose AKT Inhibition. Mol Cancer Ther 14:1171–80. doi: 10.1158/1535-7163.mct-14-0708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Newman JH, Augeri DJ, NeMoyer R, Malhotra J, Langenfeld E, Chesson CB, Dobias NS, Lee MJ, Tarabichi S, Jhawar SR, Bommareddy PK, Marshall S, Sadimin ET, Kerrigan JE, Goedken M, Minerowicz C, Jabbour SK, Li S, Carayannopolous MO, Zloza A and Langenfeld J (2018) Novel bone morphogenetic protein receptor inhibitor JL5 suppresses tumor cell survival signaling and induces regression of human lung cancer. Oncogene 37:3672–3685. doi: 10.1038/s41388-018-0156-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.NeMoyer R, Mondal A, Vora M, Langenfeld E, Glover D, Scott M, Lairson L, Rongo C, Augeri DJ, Peng Y, Jabbour SK and Langenfeld J (2019) Targeting bone morphogenetic protein receptor 2 sensitizes lung cancer cells to TRAIL by increasing cytosolic Smac/DIABLO and the downregulation of X-linked inhibitor of apoptosis protein. Cell Commun Signal 17:150. doi: 10.1186/s12964-019-0469-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Engers DW, Frist AY, Lindsley CW, Hong CC and Hopkins CR (2013) Synthesis and structure-activity relationships of a novel and selective bone morphogenetic protein receptor (BMP) inhibitor derived from the pyrazolo[1.5-a]pyrimidine scaffold of dorsomorphin: the discovery of ML347 as an ALK2 versus ALK3 selective MLPCN probe. Bioorg Med Chem Lett. 23:3248–52. doi: 10.1016/j.bmcl.2013.03.113. Epub 2013 Apr 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakahara T, Kita A, Yamanaka K, Mori M, Amino N, Takeuchi M, Tominaga F, Hatakeyama S, Kinoyama I, Matsuhisa A, Kudoh M and Sasamata M (2007) YM155, a novel small-molecule survivin suppressant, induces regression of established human hormone-refractory prostate tumor xenografts. Cancer Res 67:8014–21. doi: 10.1158/0008-5472.Can-07-1343 [DOI] [PubMed] [Google Scholar]
- 39.Mondal A, NeMoyer R, Vora M, Napoli L, Syed Z, Langenfeld E, Jia D, Peng Y, Gilleran J, Roberge J, Rongo C, Jabbour SK and Langenfeld J (2021) Bone morphogenetic protein receptor 2 inhibition destabilizes microtubules promoting the activation of lysosomes and cell death of lung cancer cells. Cell Commun Signal 19:97. doi: 10.1186/s12964-021-00743-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diaz-Moreno M, Armenteros T, Gradari S, Hortiguela R, Garcia-Corzo L, Fontan-Lozano A, Trejo JL and Mira H (2018) Noggin rescues age-related stem cell loss in the brain of senescent mice with neurodegenerative pathology. Proc Natl Acad Sci U S A 115:11625–11630. doi: 10.1073/pnas.1813205115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dunmore BJ, Drake KM, Upton PD, Toshner MR, Aldred MA and Morrell NW (2013) The lysosomal inhibitor, chloroquine, increases cell surface BMPR-II levels and restores BMP9 signalling in endothelial cells harbouring BMPR-II mutations. Hum Mol Genet 22:3667–79. doi: 10.1093/hmg/ddt216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caren H, Beck S and Pollard SM (2016) Differentiation therapy for glioblastoma - too many obstacles? Mol Cell Oncol 3:e1124174. doi: 10.1080/23723556.2015.1124174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dalmo E, Johansson P, Niklasson M, Gustavsson I, Nelander S and Westermark B (2020) Growth inhibitory activity of bone morphogenetic protein 4 in human glioblastoma cell lines is heterogeneous and dependent on reduced SOX2 expression. Mol Cancer Res. doi: 10.1158/1541-7786.mcr-19-0638 [DOI] [PubMed] [Google Scholar]
- 44.Caren H, Stricker SH, Bulstrode H, Gagrica S, Johnstone E, Bartlett TE, Feber A, Wilson G, Teschendorff AE, Bertone P, Beck S and Pollard SM (2015) Glioblastoma Stem Cells Respond to Differentiation Cues but Fail to Undergo Commitment and Terminal Cell-Cycle Arrest. Stem Cell Reports 5:829–842. doi: 10.1016/j.stemcr.2015.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sachdeva R, Wu M, Smiljanic S, Kaskun O, Ghannad-Zadeh K, Celebre A, Isaev K, Morrissy AS, Guan J, Tong J, Chan J, Wilson TM, Al-Omaishi S, Munoz DG, Dirks PB, Moran MF, Taylor MD, Reimand J and Das S (2019) ID1 Is Critical for Tumorigenesis and Regulates Chemoresistance in Glioblastoma. Cancer Res 79:4057–4071. doi: 10.1158/0008-5472.can-18-1357 [DOI] [PubMed] [Google Scholar]
- 46.Guo P, Lan J, Ge J, Mao Q and Qiu Y (2013) ID1 regulates U87 human cell proliferation and invasion. Oncol Lett 6:921–926. doi: 10.3892/ol.2013.1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soroceanu L, Murase R, Limbad C, Singer E, Allison J, Adrados I, Kawamura R, Pakdel A, Fukuyo Y, Nguyen D, Khan S, Arauz R, Yount GL, Moore DH, Desprez PY and McAllister SD (2013) Id-1 is a key transcriptional regulator of glioblastoma aggressiveness and a novel therapeutic target. Cancer Res 73:1559–69. doi: 10.1158/0008-5472.Can-12-1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garnier D, Renoult O, Alves-Guerra MC, Paris F and Pecqueur C (2019) Glioblastoma Stem-Like Cells, Metabolic Strategy to Kill a Challenging Target. Front Oncol 9:118. doi: 10.3389/fonc.2019.00118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jankovic V, Ciarrocchi A, Boccuni P, DeBlasio T, Benezra R and Nimer SD (2007) Id1 restrains myeloid commitment, maintaining the self-renewal capacity of hematopoietic stem cells. Proc Natl Acad Sci U S A 104:1260–5. doi: 10.1073/pnas.0607894104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O’Brien CA, Kreso A, Ryan P, Hermans KG, Gibson L, Wang Y, Tsatsanis A, Gallinger S and Dick JE (2012) ID1 and ID3 regulate the self-renewal capacity of human colon cancer-initiating cells through p21. Cancer Cell. 21:777–92. doi: 10.1016/j.ccr.2012.04.036. [DOI] [PubMed] [Google Scholar]
- 51.Yamaguchi K, Nagai S, Ninomiya-Tsuji J, Nishita M, Tamai K, Irie K, Ueno N, Nishida E, Shibuya H and Matsumoto K (1999) XIAP, a cellular member of the inhibitor of apoptosis protein family, links the receptors to TAB1-TAK1 in the BMP signaling pathway. Embo j 18:179–87. doi: 10.1093/emboj/18.1.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiao G, Guo W, Ren T, Lu Q, Sun Y, Liang W, Ren C, Yang K and Sun K (2014) BMPR2 inhibition induced apoptosis and autophagy via destabilization of XIAP in human chondrosarcoma cells. Cell Death Dis 5:e1571. doi: 10.1038/cddis.2014.540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hover LD, Owens P, Munden AL, Wang J, Chambless LB, Hopkins CR, Hong CC, Moses HL and Abel TW (2016) Bone morphogenetic protein signaling promotes tumorigenesis in a murine model of high-grade glioma. Neuro Oncol 18:928–38. doi: 10.1093/neuonc/nov310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gleason RJ, Akintobi AM, Grant BD and Padgett RW (2014) BMP signaling requires retromer-dependent recycling of the type I receptor. Proc Natl Acad Sci U S A 111:2578–83. doi: 10.1073/pnas.1319947111 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on reasonable request