Abstract
Purpose:
Sodium-glucose cotransporter-2 inhibitors (SGLT-2i) are increasingly being considered as first-line treatment for type 2 diabetes (T2D). The benefits of SGLT-2i from cardiovascular outcome trials may lead to preferential prescribing of SGLT-2i to patients at high cardiovascular risk, possibly causing confounding in non-randomized studies of SGLT-2i as first-line treatment. We assessed evolving imbalances in characteristics of patients starting SGLT-2i versus metformin as first-line monotherapy.
Methods:
Using claims data from two U.S. commercial health insurance and Medicare, we identified patients with T2D aged ≥18 years (>65 years in Medicare) initiating first-line SGLT-2i or metformin from 2013 through 2019. Standardized differences (SDs) for patient characteristics were assessed during four consecutive calendar time blocks (T1:4/2013–12/2014; T2:1/2015–6/2016; T3:7/2016–12/2017; and T4:1/2018–12/2019). We also estimated the propensity score of receiving SGLT-2i versus metformin within each time block and evaluated time trends in model discrimination with c-statistics.
Results:
We identified 9,113 initiators of first-line SGLT-2i and 810,348 initiators of first-line metformin. During T1, SGLT-2i initiators were younger (SD=−0.24) and less likely to have seen cardiologists (−0.07) with a similar prevalence of CVD (0.04) compared with metformin. During T4, patients were more balanced for age (−0.01). Cardiologist visits (0.08) and CVD (0.25) became more prevalent among SGLT-2i initiators.
Conclusions:
When comparing initiators of first-line SGLT-2i versus metformin, imbalances in patient characteristics evolved from 2013 through 2019, particularly channeling SGLT-2i to individuals at high cardiovascular risk. Evolving channeling in prescribing first-line SGLT-2i should be expected and accounted for in non-randomized comparative effectiveness research.
Keywords: Cardiovascular benefits, Channeling, First-line, SGLT-2i, Metformin, Type 2 diabetes
Purpose
The U.S. Food and Drug Administration (FDA) has recommended post-approval cardiovascular outcome trials (CVOTs) since 2008 to ensure the safety of new glucose-lowering drugs1 responding to the growing burden of cardiovascular disease (CVD) in type 2 diabetes (T2D) and the potential increase in cardiovascular risk with certain existing glucose-lowering drugs.2 Notably, sodium-glucose cotransporter-2 inhibitors (SGLT-2i) have demonstrated superiority to placebo in reducing the risk of cardiovascular events, including hospitalization for heart failure.3,4,5 Consequently, beginning in 2018, clinical guidelines in the U.S. have recommended SGLT-2i as a preferred second-line treatment for patients with T2D and CVD6,7,—further raising the question of whether SGLT-2i should be advanced to first-line treatment.8,9,
To our knowledge, one randomized controlled trial has been investigating a SGLT-2i versus metformin for cardiovascular outcomes among patients with T2D but without baseline CVD and is expected to complete in 2025.10 Therefore, non-randomized studies using real-world data could provide information on whether SGLT-2i may have greater cardiovascular benefits over metformin more timely than randomized clinical trials among both patients with and without existing CVD.11,12,13 While not benefitting from randomization, these non-randomized studies could achieve balance in patient characteristics, including those unmeasured, by adopting state-of-the-art pharmacoepidemiologic study designs, such as active-comparator and new-user.14,15 However, whether these designs can successfully achieve this balance is unknown when comparing first-line SGLT-2i with metformin because: (1) SGLT-2i are relatively new and typically used as second-line, whereas the established use of first-line metformin comes from more than 60 years of clinical experience16,17; (2) SGLT-2i are associated with considerably higher costs potentially coupled with restrictive drug coverage and formulary restrictions, which may limit the access to SGLT-2i for patients with lower socioeconomic status compared with the more affordable metformin18; (3) cardiovascular benefits may lead to preferential prescribing of SGLT-2i to patients at high cardiovascular risk19; and (4) SGLT-2i and metformin have different safety-related precautions, e.g., frequent genitourinary infections for SGLT-2i.
Therefore, we empirically examined potential imbalances in patient characteristics evolving over time comparing initiators of SGLT-2i as first-line T2D treatment versus metformin, using two commercial U.S. claims and Medicare databases.
Methods
Data Sources
We used data from two large commercial U.S. health insurance databases, Optum Clinformatics and IBM MarketScan, and Medicare fee-for-service. The commercial databases primarily represent individuals with employer-sponsored health insurance, Medicare Advantage, or Medicare Supplemental health insurance plans across the U.S. The Medicare database included individuals aged ≥65 years. The databases contained de-identified individual level, longitudinal information on baseline demographics, inpatient and outpatient diagnoses and procedures, and outpatient prescription dispensings recorded during billing of routine healthcare encounters. The study was approved by the Mass General Brigham Institutional Review Board, and licensing agreements were in place.
Study Population
We identified individuals who initiated SGLT-2i (canagliflozin, empagliflozin, or dapagliflozin) or metformin, both as monotherapy, between April 1, 2013 (consistent with the launch of SGLT-2i in the U.S.) and December 31, 2019 (December 31, 2018 for MarketScan and Medicare). We required no use of any antidiabetic drugs at any point prior to cohort entry and continuous health insurance enrollment with complete medical coverage and pharmacy benefits during 365 days before the date of treatment initiation, defined as cohort entry. Additional eligibility criteria were: age at cohort entry ≥18 years (>65 years for Medicare); at least one inpatient or outpatient diagnosis of T2D (ICD-9 diagnosis 250.x0 or 250.x2 through September 30, 2015, and ICD-10 diagnosis E11.xxx afterwards) at any point prior to or on cohort entry20,21; at least one prescription or a physician visit in both of two, six-month intervals (−365 days to −183 days and −182 days to −1 day) before cohort entry to reduce surveillance variability.22 We excluded patients who initiated more than one antidiabetic drug class on cohort entry and patients with a history of gestational or secondary diabetes, polycystic ovary syndrome, organ transplant, end-stage renal disease, HIV/AIDS, or nursing home admission in the preceding 365 days before cohort entry (Figure 1 and Supplementary Figure S1).
Figure 1. Flowchart of study cohort.
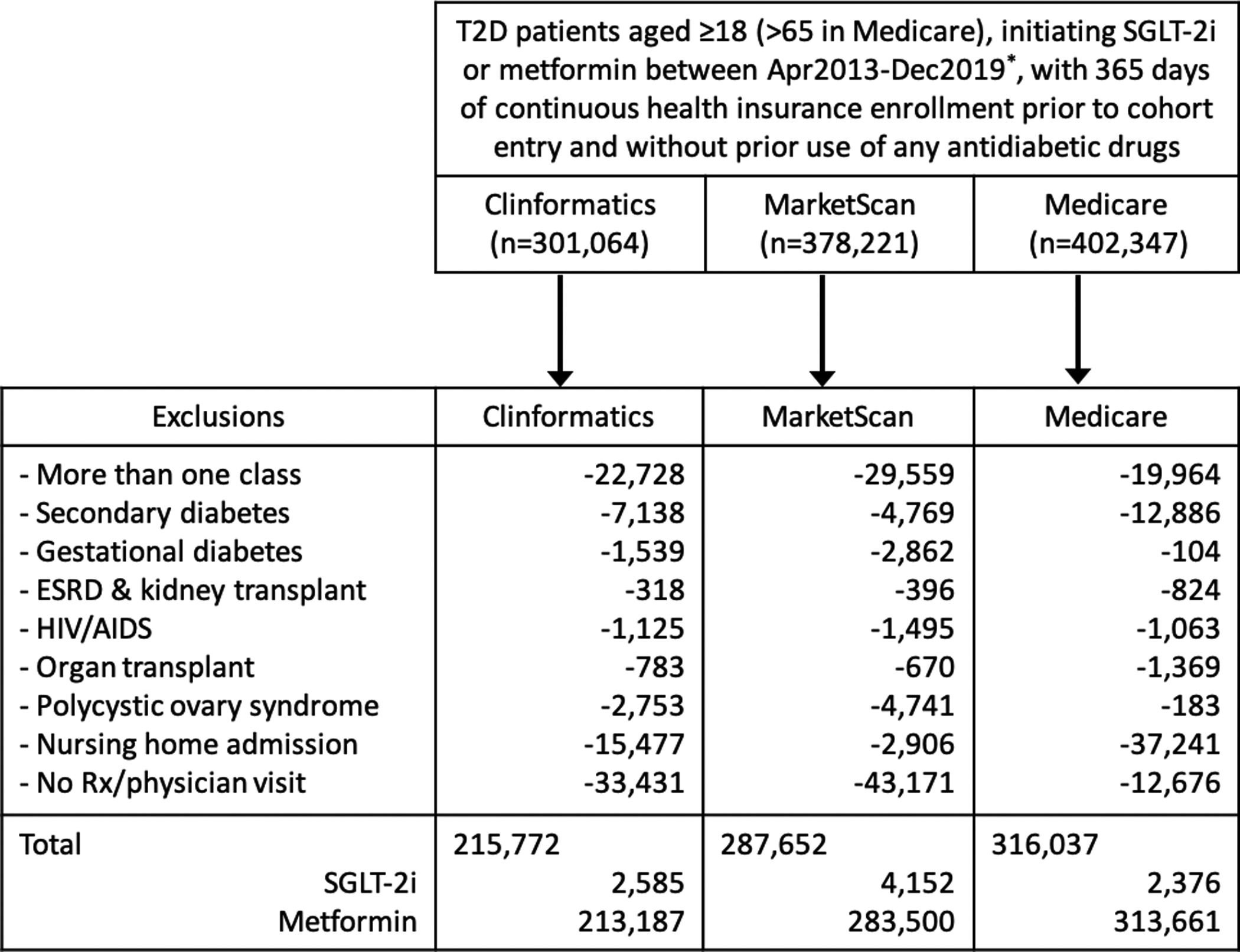
T2D: type 2 diabetes; ESRD: end-stage renal disease; HIV/AIDS: human immunodeficiency virus/acquired immune deficiency syndrome; Rx: prescription.
* Data range: Clinformatics (Apr 2013–Dec 2019) / MarketScan and Medicare (Apr 2013–Dec 2018)
Patient Characteristics
Patient characteristics were measured during the 365 days prior to or on cohort entry, including demographics, diabetes-related and other comorbidities, concomitant medications, and measures of healthcare utilization (Supplementary Table S1). We chose patient characteristics a priori based on subject matter knowledge regarding predictors of the cardiovascular outcomes, which would be used in a real-world study comparing first-line SGLT-2i versus metformin. Laboratory test results were available for approximately 15% of the population through linkage with national lab test provider chains.
Study Outcome
In this study, we used initiation of first-line SGLT-2i or metformin as the outcome in estimating the propensity scores. The associations between these treatment groups and cardiovascular outcomes were not investigated.
Statistical Analysis
To evaluate evolving imbalances in patient characteristics, the study period was stratified into four consecutive calendar time blocks (T1: 4/2013–12/2014, T2: 1/2015–6/2016, T3: 7/2016–12/2017, and T4: 1/2018–12/2019) (Figure 2). The cut point of the first time block (December 31, 2014) was chosen to examine patient characteristics in the early post-marketing period of SGLT-2i. The cut point of the second time block (June 30, 2016) was chosen to evaluate patient characteristics around the time when the first results from a pivotal CVOT of SGLT-2i were published in November 2015.3 The cut point of the third time block (December 31, 2017) coincided with the change in the U.S. clinical guideline, endorsing SGLT-2i as preferred second line treatment for patients with T2D and established CVD.6 These cut points resulted in time blocks of roughly equal length. Within each time block, we estimated standardized differences (SDs) comparing patient characteristics between the treatment groups and averaged SDs across databases weighted by the sample size of each database. Temporal trends in SDs were plotted over the four time blocks with a positive sign indicating a higher prevalence (or mean) among SGLT-2i initiators and a negative sign indicating a higher prevalence (or mean) among metformin initiators. The significance of trends in SDs was assessed using the least square method, assigning 0, 1, 2, and 3 to the four time blocks, approximately equal-sized.23 To summarize the overall imbalances in patient characteristics within each time block, we calculated the proportion of variables with |SD| >0.1, the threshold defining a meaningful imbalance regarding confounding a treatment effect association.24 Additionally, we computed database and time block-specific propensity score (PS) model c-statistics as a measure of discrimination.25 The PSs were estimated as a function of all pre-exposure patient characteristics except for laboratory values, which were not available for all patients. In a sensitivity analysis, we restricted the study population to patients with at least two years of continuous health insurance enrollment before cohort entry without use of any antidiabetic drugs. Analyses were performed using R v3.6.226 with analytic files generated using the Aetion Evidence Platform v4.10.27,28,29
Figure 2. Development timeline for SGLT-2i and study time blocks.
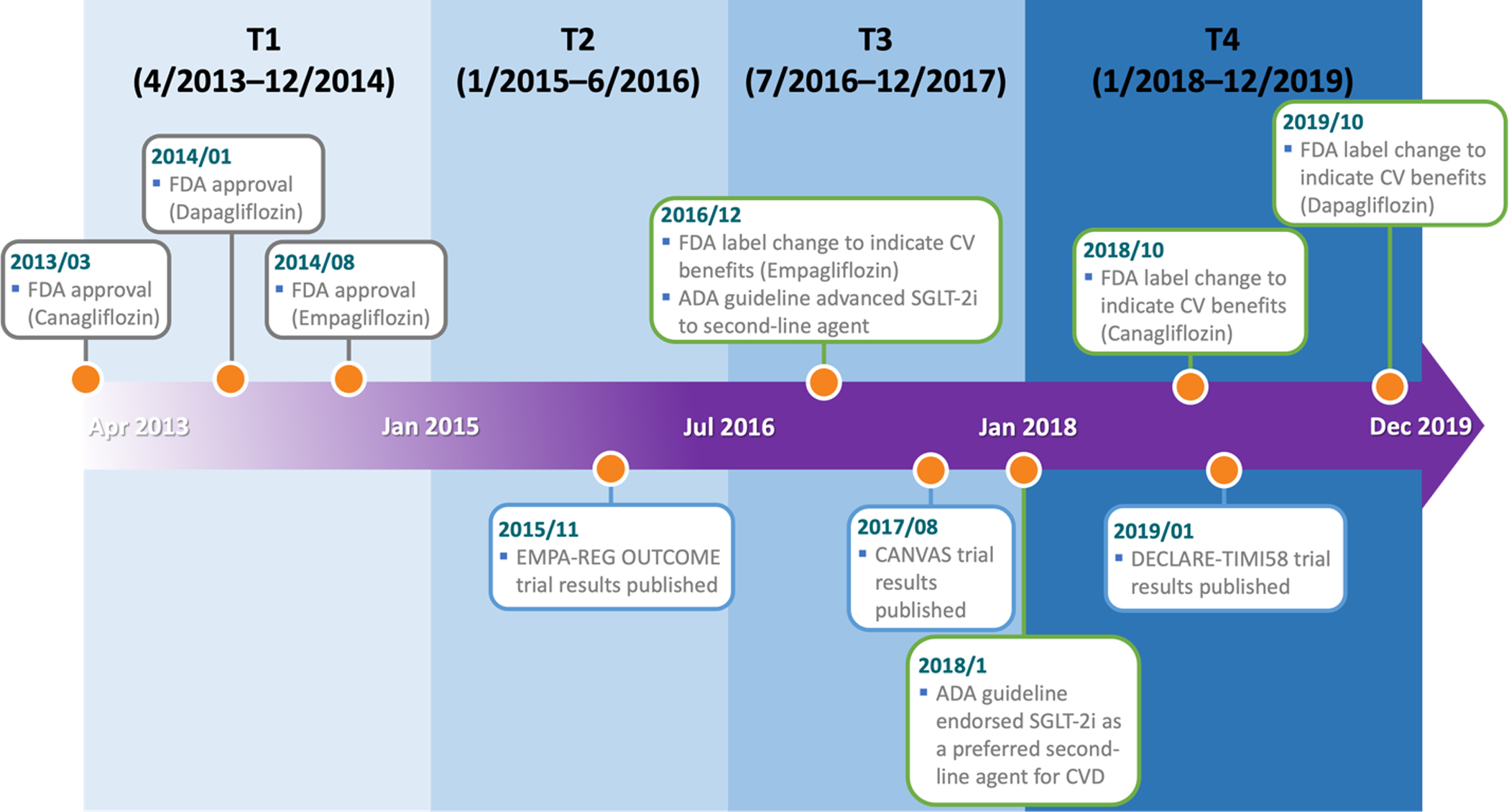
2013/03: FDA approval for canagliflozin (Invokana®) to treat T2D.
2014/01: FDA approval for dapagliflozin (Farxiga®) to treat T2D.
2014/08: FDA approval for empagliflozin (Jardiance®) to treat T2D.
2015/11: Publication of the EMPA-REG OUTCOME trial results for empagliflozin.
2016/12: FDA label change for empagliflozin to indicate cardiovascular benefits.
Advance of SGLT-2i to second-line agent by the American Diabetes Association (ADA).
2017/08: Publication of the CANVAS trial results for canagliflozin.
2018/01: ADA endorsement of SGLT-2i as a preferred second-line agent for patients with CVD.
2018/10: FDA label change for canagliflozin to indicate cardiovascular benefits.
2019/01: Publication of the DECLARE-TIMI 58 trial results for dapagliflozin.
2019/10: FDA label change for dapagliflozin to indicate cardiovascular benefits.
Results
We identified 9,113 initiators of first-line SGLT-2i and 810,348 initiators of first-line metformin between April 1, 2013 and December 31, 2019 (Figure 1). Descriptive statistics of pooled patient characteristics and the weighted average SDs are in Table 1, with a graphic presentation of trends in the SDs in Figure 3. Database-specific patient characteristics and SDs are in Supplementary Tables S2-S4.
Table 1.
Patient characteristics and standardized differences at the time of first-line SGLT-2i and metformin initiation (2013–2019). Values are percentage (%) unless otherwise specified.
| Apr2013-Dec2014 | Jan2015-Jun2016 | Jul2016-Dec2017 | Jan2018-Dec2019 | Linear trend (P) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SGLT-2i (n=1,875) | Metformin (n=271,143) | SD | SGLT2-i (n=2,765) | Metformin (n=228,142) | SD | SGLT2-i (n=2,390) | Metformin (n=173,647) | SD | SGLT2-i (n=2,083) | Metformin (n=137,416) | SD | ||
| Baseline characteristics | |||||||||||||
| Demographics | |||||||||||||
| Age (mean, std.dev) | 57.53 (9.04) | 64.14 (9.58) | −0.24 | 59.77 (8.92) | 64.21 (9.52) | −0.20 | 60.58 (9.29) | 62.64 (10.11) | −0.15 | 62.13 (10.57) | 62.24 (10.73) | −0.01 | 0.040 |
| Gender (Male) | 49.17 | 48.75 | 0.02 | 51.97 | 49.51 | 0.06 | 50.84 | 50.95 | 0.00 | 56.60 | 52.75 | 0.08 | 0.592 |
| Region* | |||||||||||||
| Northeast | 18.24 | 16.65 | 0.07 | 19.39 | 17.66 | 0.05 | 16.82 | 16.19 | 0.02 | 16.56 | 14.75 | 0.05 | 0.387 |
| South | 52.37 | 41.98 | 0.14 | 53.16 | 43.96 | 0.14 | 53.97 | 46.40 | 0.13 | 49.35 | 45.58 | 0.07 | 0.204 |
| Midwest | 17.28 | 23.21 | −0.13 | 14.79 | 21.11 | −0.17 | 15.73 | 20.70 | −0.14 | 18.10 | 21.34 | −0.08 | 0.342 |
| West | 12.11 | 18.16 | −0.12 | 12.66 | 17.28 | −0.09 | 13.47 | 16.71 | −0.08 | 15.99 | 18.34 | −0.07 | 0.067 |
| Medicare Advantage | 9.96 | 25.41 | −0.40 | 13.74 | 26.32 | −0.26 | 19.58 | 27.10 | −0.17 | 35.42 | 36.13 | −0.02 | 0.005 |
| Race (White) | 79.22 | 77.36 | 0.08 | 76.18 | 75.70 | 0.04 | 72.09 | 72.07 | 0.02 | 63.57 | 63.71 | 0.00 | 0.020 |
| Life-style risk factors | |||||||||||||
| Obesity or Overweight | 27.95 | 23.22 | 0.12 | 35.37 | 31.33 | 0.10 | 43.14 | 40.41 | 0.05 | 48.73 | 48.36 | 0.01 | 0.006 |
| Smoking | 8.05 | 12.55 | −0.06 | 12.30 | 15.25 | −0.04 | 15.86 | 17.55 | −0.03 | 20.16 | 20.05 | 0.00 | 0.040 |
| Comorbidities | |||||||||||||
| Diabetic Nephropathy | 2.40 | 1.74 | 0.09 | 3.47 | 2.69 | 0.07 | 5.65 | 4.12 | 0.08 | 9.07 | 5.31 | 0.14 | 0.315 |
| Diabetic Neuropathy | 7.36 | 4.23 | 0.22 | 7.85 | 5.13 | 0.14 | 8.62 | 5.90 | 0.12 | 11.09 | 6.24 | 0.17 | 0.499 |
| Diabetic Retinopathy | 2.03 | 1.11 | 0.10 | 2.03 | 1.09 | 0.09 | 1.17 | 1.02 | 0.02 | 3.65 | 1.43 | 0.14 | 0.892 |
| CVD** | 21.65 | 28.23 | 0.04 | 25.61 | 28.16 | 0.04 | 28.66 | 26.43 | 0.09 | 37.01 | 26.01 | 0.25 | 0.114 |
| Hyperlipidemia | 73.01 | 73.20 | 0.12 | 73.74 | 72.62 | 0.09 | 68.87 | 66.57 | 0.08 | 73.50 | 70.15 | 0.07 | 0.055 |
| Hypertension | 68.05 | 71.45 | 0.08 | 73.82 | 73.22 | 0.10 | 73.47 | 71.68 | 0.07 | 76.62 | 70.97 | 0.13 | 0.431 |
| CKD | 3.79 | 4.95 | 0.03 | 6.55 | 6.05 | 0.07 | 7.82 | 6.53 | 0.06 | 11.67 | 7.46 | 0.13 | 0.095 |
| COPD | 6.93 | 10.24 | −0.02 | 7.92 | 10.22 | −0.03 | 9.37 | 9.47 | 0.01 | 10.32 | 9.82 | 0.01 | 0.212 |
| History of Malignant Neoplasm | 7.04 | 10.14 | −0.02 | 8.93 | 10.23 | 0.01 | 8.45 | 9.43 | −0.02 | 9.79 | 8.96 | 0.03 | 0.367 |
| Physician specialties † | |||||||||||||
| Cardiologists | 2.77 | 5.33 | −0.07 | 3.25 | 5.21 | −0.07 | 4.18 | 5.00 | −0.03 | 7.06 | 5.20 | 0.08 | 0.111 |
| Endocrinologists | 4.16 | 2.24 | 0.09 | 3.47 | 2.41 | 0.07 | 3.39 | 2.43 | 0.06 | 4.08 | 2.25 | 0.11 | 0.690 |
| Internists | 46.83 | 64.05 | −0.37 | 45.35 | 65.65 | −0.42 | 44.52 | 66.68 | −0.45 | 45.13 | 67.02 | −0.45 | 0.073 |
| Nurse practitioners or physician assistants | 2.24 | 4.75 | −0.08 | 2.82 | 5.81 | −0.13 | 4.48 | 6.24 | −0.07 | 4.46 | 7.21 | −0.12 | 0.744 |
| Healthcare utilization | |||||||||||||
| Any recent hospitalization†† | 1.12 | 4.07 | −0.17 | 1.23 | 3.81 | −0.15 | 1.21 | 3.55 | −0.15 | 2.69 | 3.88 | −0.07 | 0.125 |
| Avg. length of hospitalizations (mean, std.dev) | 0.25 (1.43) | 0.49 (2.04) | −0.04 | 0.33 (1.66) | 0.47 (1.93) | −0.03 | 0.33 (1.37) | 0.44 (2.12) | −0.04 | 0.40 (1.50) | 0.47 (2.12) | −0.05 | 0.294 |
| Number of ED visits (mean, Std.dev) | 0.54 (1.40) | 0.57 (1.47) | −0.02 | 0.56 (1.43) | 0.58 (1.51) | −0.03 | 0.60 (1.66) | 0.64 (1.68) | −0.04 | 0.71 (1.84) | 0.72 (1.91) | −0.02 | 0.940 |
| Number of office visits (mean, std.dev) | 11.22 (9.78) | 9.76 (9.14) | 0.15 | 11.15 (9.81) | 9.65 (8.91) | 0.16 | 11.32 (10.23) | 9.84 (9.31) | 0.16 | 11.51 (9.89) | 9.86 (9.43) | 0.18 | 0.192 |
| Number of HbA1c test orders (mean, std.dev) | 1.70 (1.17) | 1.53 (1.08) | 0.26 | 1.79 (1.15) | 1.60 (1.08) | 0.25 | 1.83 (1.17) | 1.63 (1.04) | 0.20 | 1.90 (1.19) | 1.66 (1.04) | 0.22 | 0.233 |
| Brand/Generic ratio# (mean, std.dev) | −1.30 (1.20) | −1.61 (1.20) | 0.26 | −1.50 (1.19) | −1.79 (1.17) | 0.23 | −1.70 (1.21) | −1.92 (1.15) | 0.17 | −1.80 (1.19) | −2.01 (1.12) | 0.19 | 0.129 |
| Number of unique medications (mean, std.dev) | 10.47 (8.08) | 10.41 (7.44) | 0.09 | 10.38 (7.93) | 10.36 (7.53) | 0.05 | 10.47 (8.15) | 10.16 (7.49) | 0.06 | 11.18 (8.87) | 10.31 (7.76) | 0.10 | 0.740 |
| Copay for pharmacy cost ($, mean, std.dev) | 324.78 (429.14) | 354.29 (519.61) | 0.05 | 349.12 (516.60) | 336.45 (570.69) | 0.10 | 341.02 (624.02) | 302.06 (546.96) | 0.09 | 356.13 (588.57) | 292.89 (545.08) | 0.10 | 0.218 |
| Preventive healthcare service## | 70.93 | 75.22 | −0.01 | 72.77 | 75.90 | 0.00 | 73.10 | 75.21 | −0.03 | 75.37 | 75.52 | 0.00 | 0.983 |
| Concomitant medications | |||||||||||||
| ACE inhibitors or ARBs | 59.05 | 63.18 | −0.14 | 60.26 | 63.81 | −0.10 | 62.18 | 65.09 | −0.09 | 60.20 | 65.12 | −0.09 | 0.156 |
| Antithrombotic medications | 26.46 | 21.71 | 0.04 | 23.84 | 21.53 | 0.00 | 27.06 | 23.45 | 0.03 | 31.49 | 24.10 | 0.19 | 0.251 |
| Beta blockers | 44.29 | 45.51 | −0.09 | 43.89 | 44.99 | −0.05 | 45.44 | 46.53 | −0.04 | 48.91 | 45.98 | 0.06 | 0.073 |
| Calcium channel blockers | 29.53 | 31.85 | −0.09 | 30.84 | 31.47 | −0.04 | 30.49 | 32.04 | −0.04 | 28.32 | 32.16 | −0.03 | 0.111 |
| Loop diuretics | 14.48 | 14.65 | 0.00 | 18.27 | 14.38 | 0.04 | 14.50 | 15.72 | 0.02 | 19.01 | 15.90 | 0.10 | 0.137 |
| Statin | 58.77 | 66.49 | −0.19 | 60.02 | 67.41 | −0.15 | 65.77 | 70.12 | −0.11 | 64.55 | 71.08 | −0.09 | 0.005 |
| Thiazides | 10.61 | 15.04 | −0.08 | 11.39 | 14.79 | −0.09 | 11.59 | 14.50 | −0.08 | 11.52 | 14.55 | −0.09 | 0.544 |
| Laboratory values | |||||||||||||
| HbA1c§ (%; mean, std.dev) | 7.82 (2.02) | 7.37 (1.55) | 0.27 | 7.58 (1.54) | 7.32 (1.55) | 0.18 | 7.50 (1.71) | 7.33 (1.55) | 0.10 | 7.83 (1.90) | 7.27 (1.53) | 0.30 | 0.951 |
| Missing | 85.09 | 82.92 | −0.04 | 81.43 | 78.40 | −0.04 | 75.31 | 74.99 | −0.01 | 67.49 | 68.47 | −0.03 | 0.472 |
| eGFR¶ (mL/min/1.73m2; mean, std.dev) | 100.81 (17.48) | 99.45 (16.11) | 0.09 | 99.88 (19.40) | 98.67 (16.26) | 0.06 | 101.27 (16.91) | 98.50 (16.59) | 0.17 | 95.79 (19.56) | 97.46 (16.86) | −0.09 | 0.493 |
| Missing | 84.04 | 81.49 | −0.05 | 79.76 | 76.78 | −0.05 | 73.85 | 73.33 | 0.00 | 65.27 | 67.19 | −0.05 | 0.769 |
| LDL (mg/dl; mean, std.dev) | 97.98 (47.35) | 98.71 (44.11) | −0.01 | 101.67 (38.09) | 101.92 (40.92) | −0.04 | 99.23 (39.40) | 102.92 (40.78) | −0.09 | 88.03 (43.43) | 100.66 (40.35) | −0.30 | 0.082 |
| Missing | 84.89 | 82.43 | −0.03 | 82.26 | 78.64 | −0.02 | 76.23 | 75.81 | 0.00 | 69.71 | 70.75 | −0.03 | 0.896 |
| HDL (mg/dl; mean, std.dev) | 45.88 (16.27) | 45.83 (77.02) | 0.00 | 45.96 (11.95) | 46.47 (14.28) | −0.04 | 46.06 (13.92) | 46.93 (49.73) | −0.02 | 45.70 (13.74) | 46.11 (13.17) | −0.03 | 0.454 |
| Missing | 84.96 | 82.76 | −0.04 | 82.73 | 79.42 | −0.02 | 76.64 | 76.58 | −0.01 | 70.34 | 71.79 | −0.04 | 0.913 |
| Total cholesterol (mg/dl; mean, std.dev) | 183.37 (57.21) | 180.97 (56.83) | 0.05 | 185.31 (43.10) | 188.28 (45.96) | −0.08 | 181.94 (46.87) | 188.46 (45.77) | −0.14 | 178.25 (43.17) | 184.19 (44.08) | −0.13 | 0.111 |
| Missing | 84.70 | 82.73 | −0.05 | 82.52 | 79.12 | −0.02 | 76.23 | 76.12 | −0.01 | 69.90 | 71.11 | −0.03 | 0.584 |
| Triglyceride (mg/dl; mean, std.dev) | 175.67 (117.28) | 176.65 (155.89) | 0.00 | 174.07 (177.49) | 183.80 (160.77) | −0.07 | 172.98 (126.02) | 184.07 (180.21) | −0.07 | 209.34 (199.19) | 181.93 (159.59) | 0.14 | 0.446 |
| Missing | 84.83 | 82.79 | −0.04 | 82.57 | 79.43 | −0.03 | 76.23 | 76.37 | −0.01 | 69.96 | 71.29 | −0.04 | 0.643 |
SD: standardized difference; std.dev: standard deviation; HbA1c: hemoglobin A1c; CVD: cardiovascular disease; CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; ED: emergency department.
Northeast (CT, MA, ME, NH, NJ, NY, PA, RI, VT)
South (AL, AR, DC, DE, FL, GA, KY, LA, MD, MS, NC, OK, SC, TN, TX, VA, WV)
Midwest (IA, IL, IN, KS, MI, MN, MO, ND, NE, OH, SD, WI)
West (AK, AZ, CA, CO, HI, ID, MT, NM, NV, OR, UT, WA, WY)
Defined as a history of myocardial infarction, stable or unstable angina, other ischemic heart diseases, transient ischemic attack, stroke, atherosclerotic peripheral vascular disease, or heart failure.
Defined as specialist visits occurred within 7 days prior to cohort entry.
Defined as hospitalization occurred within 30 days prior to cohort entry.
Added 1 to both numerator and denominator, then log-transformed.
Defined as administration of bone mineral density (BMD) test, colonoscopy, fecal occult blood test, mammography, pap smear, prostate-specific antigen (PSA) test, flu vaccine, or pneumococcal vaccine.
Measured 180 days prior to or on cohort entry.
Estimated using the quadratic GFR equation: . If serum creatinine <0.8 mg/dL, use 0.8 for serum creatinine.
Figure 3.
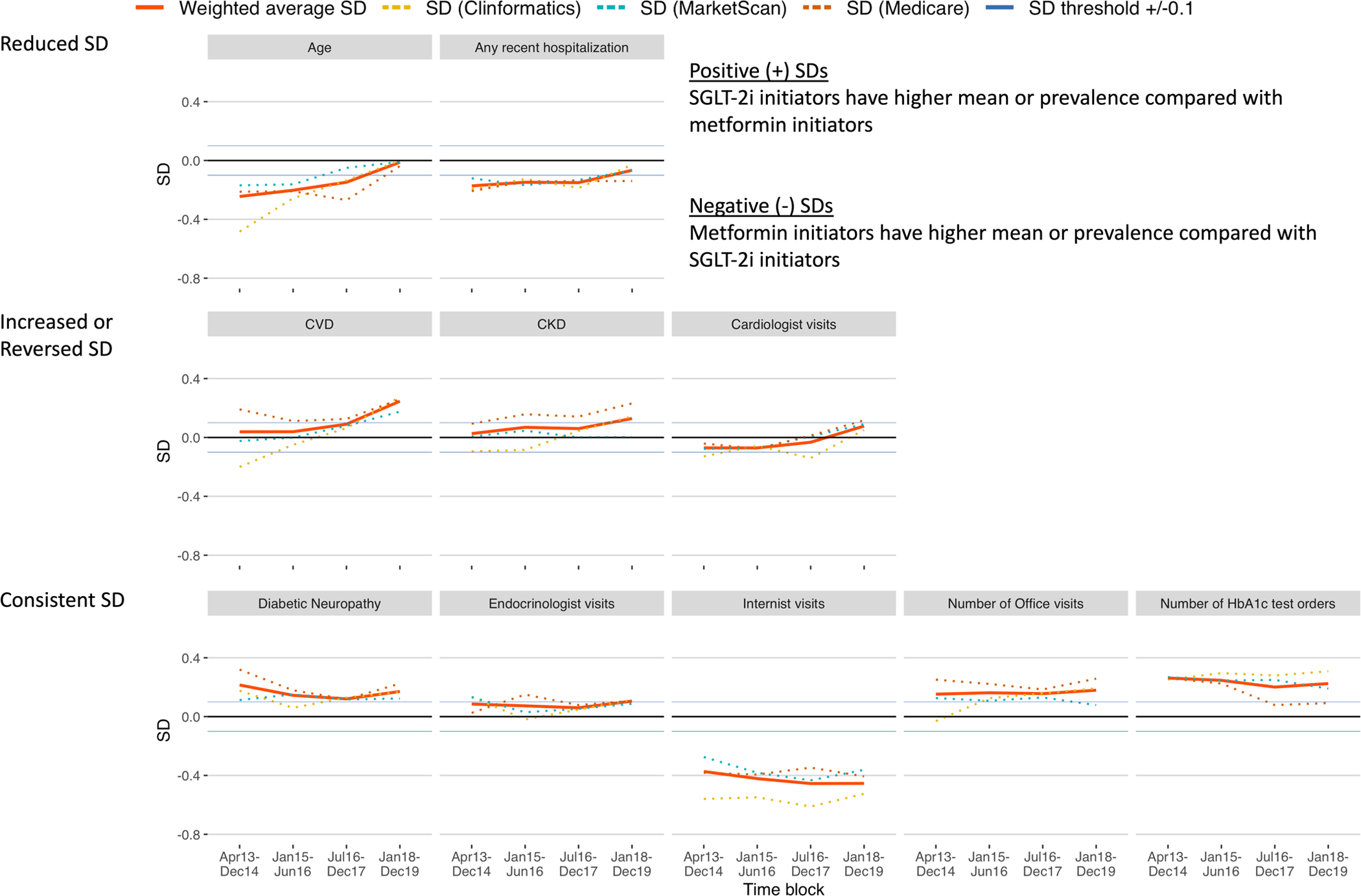
Trends of standardized differences (SDs) of selected patient characteristics, comparing first-line SGLT-2i versus metformin.
In the first time block (T1) when compared with metformin, SGLT-2i initiators were younger (SD=−0.24) and had similar burden of CVD (0.04) and CKD (0.03), while having prevalent diabetic neuropathy (0.22). SGLT-2i initiators were more likely to have seen endocrinologists (0.09), but less likely to have seen cardiologists (−0.07), internists (−0.37), or nurse practitioners or physician assistants (−0.08) compared with metformin. Additionally, SGLT-2i initiators were less likely to have recent hospitalizations (−0.17) and more likely to have office visits (0.15) or HbA1c test orders (0.26) compared with metformin.
In the last time block (T4) when compared with T1, initiators of SGLT-2i and metformin were more balanced for age (SD=−0.01; P-value for trend=0.04) and recent hospitalizations (−0.07; 0.13). However, imbalances in diabetic neuropathy (0.17; 0.50), visits to endocrinologists (0.11; 0.69), internists (−0.45; 0.07), and nurse practitioners or physician assistants (−0.12; 0.74), and frequency of office visits (0.18; 0.19) and HbA1c test orders (0.22; 0.23) continued. Notably, compared with T1, CVD (0.25; 0.11), CKD (0.13; 0.10), and visits to cardiologists (0.08; 0.11) became more prevalent among SGLT-2i initiators with marginally significant P-values due to the small number of time blocks (Table 1).
In a subset of the study population when compared with metformin, HbA1c was consistently higher among initiators of SGLT-2i, whereas LDL became more imbalanced with levels being lower in initiators of SGLT-2i, over the four time blocks; eGFR was lower among initiators of SGLT-2i in T4 (Table 1).
The time block-specific proportion of patient characteristics with |SD| >0.1 decreased from 41% (=16/39) in T1, 41% (=16/39) in T2, to 28% (=11/39) in T3, but increased to 41% (=16/39) in T4. This pattern of overall imbalances was mirrored by the PS model c-statistics, generally decreasing then leveling off over the study period (Figure 4).
Figure 4.
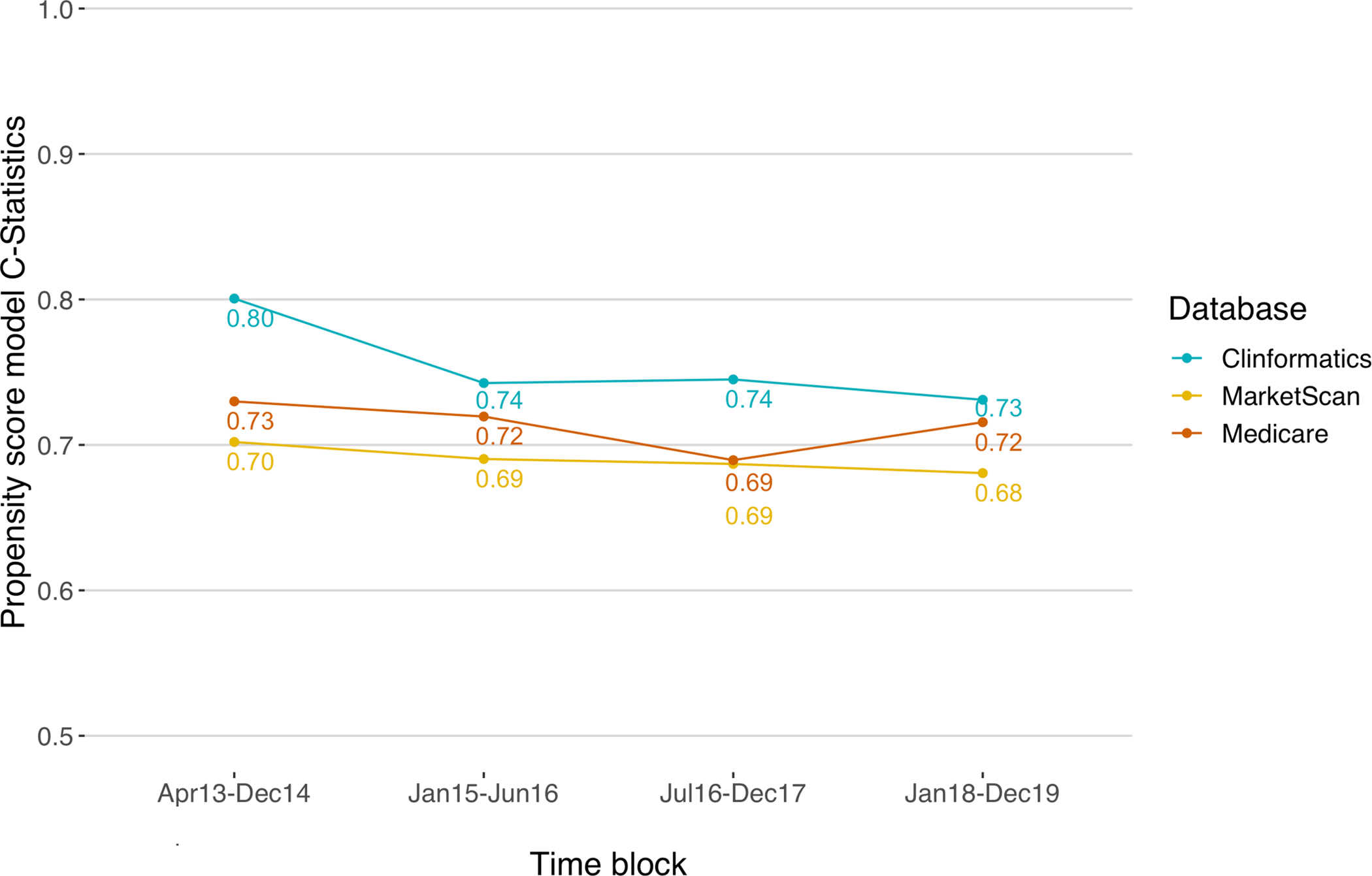
Trends of propensity score model c-statistics
When we restricted the analyses to individuals who had at least two years of continuous health insurance enrollment before cohort entry, trends in SDs remained consistent with the primary findings (Supplementary Table S5 and Figure S2).
Discussion and conclusions
This study demonstrated rapidly evolving imbalances in characteristics of adult patients initiating first-line SGLT-2i or metformin for T2D, captured in large U.S. commercial and federal insurance programs. From the introduction of SGLT-2i into the U.S. market in 2013, characteristics of patients initiating first-line SGLT-2i or metformin changed over the four calendar time blocks through 2019 with generally increasing prevalence for obesity, smoking, diabetic nephropathy, diabetic neuropathy, and CKD. In parallel, the overall imbalance in patient characteristics (the proportion of variables with |SD| >0.1) generally decreased over the same period. Consequently, the discrimination of the PS models decreased, suggesting increased equipoise between individuals initiating first-line SGLT-2i versus metformin. While this is encouraging, we found some noticeable imbalances between the exposure groups. These groups were consistently different in the prevalence of diabetic neuropathy, visits to endocrinologists or internists, and frequency of office visits or HbA1c test orders, while over time becoming similar regarding age and recent hospitalizations. Notably, CVD, CKD, and cardiologist visits became more prevalent among initiators of SGLT-2i over the study period, implying that benefits of SGLT-2i channeled to patients at high cardiovascular risk.
In the first time block (T1), SGLT-2i initiators were younger compared with metformin in keeping with previous findings that physicians might be more inclined to prescribe new drugs to younger patients.30 This imbalance in age lessened over the four time blocks with SGLT-2i initiators becoming increasingly older. In contrast to previous findings suggesting that physicians might be more inclined to prescribe new drugs to sicker patients31, we found that SGLT-2i initiators were healthier compared with metformin initiators as shown by the lower burden of non-diabetes-related comorbidities. However, SGLT-2i initiators had more advanced diabetes as shown by the higher burden of diabetic neuropathy.32 This suggests that, in the current study, the severity of diabetes might have been prioritized over general health status in prescribing new antidiabetic drugs for first-line T2D treatment, although SGLT-2i initiators might have had higher chances of diabetic neuropathy detection as the result of more frequent endocrinologist visits and overall better access to healthcare with consistently higher number of office visits and HbA1c test orders.
Patients initiating SGLT-2i were more likely to have endocrinologist visits at baseline and less likely to have internist or cardiologist visits compared with patients initiating metformin. Lack of familiarity with the newly approved SGLT-2i and compliance with clinical guidelines might have driven these visit patterns.33,34,35 While imbalances in visits to internists or endocrinologists remained consistent over the study period, visits to cardiologists became more common among SGLT-2i initiators. Aligning with this changing pattern in cardiologist visits, CVD and CKD were also increasingly prevalent among SGLT-2i initiators, suggesting channeling to patients at high cardiovascular risk possibly related to the demonstrated benefits of SGLT-2i in recent CVOTs3,4,5 and changes in treatment guidelines. In 2018, the American Diabetes Association endorsed SGLT-2i as a preferred second-line treatment for patients with T2D and CVD6, and the American College of Cardiology recommended SGLT-2i in addition to metformin for patients with atherosclerotic CVD or heart failure.36
The evolving channeling associated with the initiation of first-line SGLT-2i versus metformin has implications for comparative effectiveness and safety research with respect to confounding adjustment and statistical efficiency. We suggest: (1) ensuring tight matching on time to account for the evolving channeling over time in response to accumulating information on efficacy and safety and prescriber experience with SGLT-2i37; (2) considering excluding the time period immediately subsequent to the launch of SGLT-2i from the analysis due to the lack of adequate equipoise between treatment groups in the early phase of post-marketing; (3) estimating the PS and matching within subgroups that might be critical determinants of treatment choice, such as CVD status, to reduce residual confounding; and (4) using PS adjustment strategies maximizing efficiency, such as 1:N PS matching or PS fine stratification38,39, to address much lower initiation of SGLT-2i as first-line treatment compared with metformin.
This study has limitations. We cannot rule out the possibility that individuals with prior antidiabetic drug experience were included in the study cohort. A sensitivity analysis, requiring at least two years of continuous prior enrollment without any use of antidiabetic medications, showed results consistent with the primary findings reassuring that the analysis was robust toward the assumption of first-line use. Second, although we did not explicitly exclude patients with type 1 diabetes (T1D) diagnosis, it was highly unlikely that these patients were included in the study cohort because we only included patients with T2D diagnosis, and patients with T1D would not be expected to start oral anti-diabetic drugs. Finally, our findings may have limited generalizability to other populations including uninsured patients; however, our study cohort represented a wide-ranging population.
In conclusion, patient characteristics of first-line SGLT-2i initiators changed over time shifting to those with increased cardiovascular risk, in line with regulatory approvals and changes in clinical guidelines for SGLT-2i to reduce major cardiovascular outcomes. Evolving channeling of SGLT-2i as first-line should be expected and accounted for in non-randomized comparative effectiveness research.
Supplementary Material
Key points.
Cardiovascular benefits of SGLT-2i could channel to patients at high cardiovascular risk, possibly causing confounding in comparative effectiveness safety research of SGLT-2i.
From the introduction of SGLT-2i into the U.S. market in 2013 through 2019, the overall imbalance in patient characteristics comparing initiators of first-line SGLT-2i versus metformin generally decreased.
In the same period, CVD and cardiologist visits became more prevalent among first-line SGLT-2i initiators compared with metformin, implying evolving channeling.
Rapidly evolving channeling of first-line SGLT-2i should be expected and accounted for in non-randomized comparative effectiveness and safety research.
In this regard, we provide some suggestions regarding confounding adjustment and statistical efficiency.
Acknowledgments
The guarantor’s name:
Dr. HoJin Shin and Dr. Elisabetta Patorno
Funding/financial support:
This study was funded by the Division of Pharmacoepidemiology and Pharmacoeconomics, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA. Dr. Patorno was supported by a career development grant (K08AG055670) from the National Institute on Aging.
Conflict of Interest statement:
Dr. Shin has no conflict interest to disclose. Dr. Patorno is investigator of investigator-initiated grants to Brigham and Women’s Hospital from Boehringer Ingelheim, not directly related to the topic of the submitted work; Dr. Schneeweiss is investigator of investigator-initiated grants to Brigham and Women’s Hospital from Boehringer Ingelheim unrelated to the topic of this study. He is a consultant to Aetion Inc., a software manufacturer of which he owns equity. Dr. Glynn has received funding from grants to Brigham and Women’s Hospital from AstraZeneca, Kowa, Pfizer, and Novartis unrelated to the topic of this study. These interests were declared, reviewed, and approved by Brigham and Women’s Hospital and Partners HealthCare System in accordance with their institutional compliance policies.
Footnotes
Ethics Statement
The study was approved by the Mass General Brigham Institutional Review Board, and licensing agreements were in place. Access to the data and analytics infrastructure can be shared for relevant requests.
Reference to prior presentation of data:
We presented part of the results during the 36th virtual International Conference on Pharmacoepidemiology (ICPE), September 16–17, 2020.
References
- 1.Type 2 Diabetes Mellitus: Evaluating the Safety of New Drugs for Improving Glycemic Control. Diabetes Mellitus. Published online 2020:7. Accessed December 16, 2021. https://www.fda.gov/media/135936/download [Google Scholar]
- 2.Nissen SE, Wolski K. Effect of Rosiglitazone on the Risk of Myocardial Infarction and Death from Cardiovascular Causes. New England Journal of Medicine. 2007;356(24):2457–2471. doi: 10.1056/NEJMoa072761 [DOI] [PubMed] [Google Scholar]
- 3.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. New England Journal of Medicine. 2015;373(22):2117–2128. doi: 10.1056/NEJMoa1504720 [DOI] [PubMed] [Google Scholar]
- 4.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017;377(7):644–657. doi: 10.1056/NEJMoa1611925 [DOI] [PubMed] [Google Scholar]
- 5.Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. New England Journal of Medicine. 2019;380(4):347–357. doi: 10.1056/NEJMoa1812389 [DOI] [PubMed] [Google Scholar]
- 6.American Diabetes Association. 8. Pharmacologic Approaches to Glycemic Treatment. Dia Care. 2017;40(Supplement 1):S64–S74. doi: 10.2337/dc17-S011 [DOI] [PubMed] [Google Scholar]
- 7.Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2018;61(12):2461–2498. doi: 10.1007/s00125-018-4729-5 [DOI] [PubMed] [Google Scholar]
- 8.Inzucchi SE. Is It Time to Change the Type 2 Diabetes Treatment Paradigm? No! Metformin Should Remain the Foundation Therapy for Type 2 Diabetes. Diabetes Care. 2017;40(8):1128–1132. doi: 10.2337/dc16-2372 [DOI] [PubMed] [Google Scholar]
- 9.Verbrugge FH. Role of SGLT2 Inhibitors in Patients with Diabetes Mellitus and Heart Failure. Curr Heart Fail Rep. 2017;14(4):275–283. doi: 10.1007/s11897-017-0340-1 [DOI] [PubMed] [Google Scholar]
- 10.SGLT2 Inhibitor or Metformin as Standard Treatment of Early Stage Type 2 Diabetes (SMARTEST). ClinicalTrials.gov identifier: NCT03982381. Updated April 28, 2021. Accessed December 16, 2021. https://www.clinicaltrials.gov/ct2/show/NCT03982381 [DOI] [PubMed]
- 11.U.S. Food and Drug Administration. Framework for FDA’s Real-World Evidence Program. Published December 2018. Accessed December 16, 2021. https://www.fda.gov/media/120060/download
- 12.Schneeweiss S Improving therapeutic effectiveness and safety through big healthcare data. Clinical Pharmacology & Therapeutics. 2016;99(3):262–265. doi: 10.1002/cpt.316 [DOI] [PubMed] [Google Scholar]
- 13.Hernán MA, Robins JM. Using Big Data to Emulate a Target Trial When a Randomized Trial Is Not Available: Table 1. Am J Epidemiol. 2016;183(8):758–764. doi: 10.1093/aje/kwv254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patorno E, Patrick AR, Garry EM, et al. Observational studies of the association between glucose-lowering medications and cardiovascular outcomes: addressing methodological limitations. Diabetologia. 2014;57(11):2237–2250. doi: 10.1007/s00125-014-3364-z [DOI] [PubMed] [Google Scholar]
- 15.Patorno E, Garry EM, Patrick AR, et al. Addressing Limitations in Observational Studies of the Association Between Glucose-Lowering Medications and All-Cause Mortality: A Review. Drug Saf. 2015;38(3):295–310. doi: 10.1007/s40264-015-0280-1 [DOI] [PubMed] [Google Scholar]
- 16.White JR. A Brief History of the Development of Diabetes Medications. Diabetes Spectr. 2014;27(2):82–86. doi: 10.2337/diaspect.27.2.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cavaiola TS, Pettus JH. Management Of Type 2 Diabetes: Selecting Amongst Available Pharmacological Agents. In: Feingold KR, Anawalt B, Boyce A, et al. , eds. Endotext. MDText.com, Inc.; 2000. Accessed December 16, 2021. http://www.ncbi.nlm.nih.gov/books/NBK425702/ [Google Scholar]
- 18.Luo J, Feldman R, Rothenberger SD, Hernandez I, Gellad WF. Coverage, Formulary Restrictions, and Out-of-Pocket Costs for Sodium-Glucose Cotransporter 2 Inhibitors and Glucagon-Like Peptide 1 Receptor Agonists in the Medicare Part D Program. JAMA Netw Open. 2020;3(10):e2020969. doi: 10.1001/jamanetworkopen.2020.20969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petri H, Urquhart J. Channeling bias in the interpretation of drug effects. Statist Med. 1991;10(4):577–581. doi: 10.1002/sim.4780100409 [DOI] [PubMed] [Google Scholar]
- 20.Khokhar B, Jette N, Metcalfe A, et al. Systematic review of validated case definitions for diabetes in ICD-9-coded and ICD-10-coded data in adult populations. BMJ Open. 2016;6(8):e009952. doi: 10.1136/bmjopen-2015-009952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller DR, Safford MM, Pogach LM. Who Has Diabetes? Best Estimates of Diabetes Prevalence in the Department of Veterans Affairs Based on Computerized Patient Data. Diabetes Care. 2004;27(suppl 2):b10–b21. doi: 10.2337/diacare.27.suppl_2.B10 [DOI] [PubMed] [Google Scholar]
- 22.Glynn RJ, Knight EL, Levin R, Avorn J. Paradoxical Relations of Drug Treatment with Mortality in Older Persons: Epidemiology. 2001;12(6):682–689. doi: 10.1097/00001648-200111000-00017 [DOI] [PubMed] [Google Scholar]
- 23.Bernard A Fundamentals of Biostatistics. 8th ed. Brooks/Cole Cengage Learning; 2015. [Google Scholar]
- 24.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Statistics in Medicine. 2009;28(25):3083–3107. doi: 10.1002/sim.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glynn RJ, Schneeweiss S, Stürmer T. Indications for propensity scores and review of their use in pharmacoepidemiology. Basic Clin Pharmacol Toxicol. 2006;98(3):253–259. doi: 10.1111/j.1742-7843.2006.pto_293.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.RStudio Team (2020). RStudio: Integrated Development for R. RStudio, Inc., Boston, MA. Accessed December 16, 2021. http://www.rstudio.com/ [Google Scholar]
- 27.Software for real-world data analysis. Aetion, Inc. Accessed December 16, 2021. http://aetion.com [Google Scholar]
- 28.Wang S, Verpillat P, Rassen J, Patrick A, Garry E, Bartels D. Transparency and Reproducibility of Observational Cohort Studies Using Large Healthcare Databases. Clin Pharmacol Ther. 2016;99(3):325–332. doi: 10.1002/cpt.329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patorno E, Schneeweiss S, Gopalakrishnan C, Martin D, Franklin JM. Using Real-World Data to Predict Findings of an Ongoing Phase IV Cardiovascular Outcome Trial: Cardiovascular Safety of Linagliptin Versus Glimepiride. Diabetes Care. 2019;42(12):2204–2210. doi: 10.2337/dc19-0069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lublóy Á Factors affecting the uptake of new medicines: a systematic literature review. BMC Health Serv Res. 2014;14(1):469. doi: 10.1186/1472-6963-14-469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu YM, Kao Yang YH, Hsieh CR. The determinants of the adoption of pharmaceutical innovation: Evidence from Taiwan. Social Science & Medicine. 2011;72(6):919–927. doi: 10.1016/j.socscimed.2010.12.027 [DOI] [PubMed] [Google Scholar]
- 32.Bansal V, Kalita J, Misra UK. Diabetic neuropathy. Postgrad Med J. 2006;82(964):95–100. doi: 10.1136/pgmj.2005.036137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaduganathan M, Sathiyakumar V, Singh A, et al. Prescriber Patterns of SGLT2i After Expansions of U.S. Food and Drug Administration Labeling. Journal of the American College of Cardiology. 2018;72(25):3370–3372. doi: 10.1016/j.jacc.2018.08.2202 [DOI] [PubMed] [Google Scholar]
- 34.Vardeny O, Vaduganathan M. Practical Guide to Prescribing Sodium-Glucose Cotransporter 2 Inhibitors for Cardiologists. JACC: Heart Failure. 2019;7(2):169–172. doi: 10.1016/j.jchf.2018.11.013 [DOI] [PubMed] [Google Scholar]
- 35.Shin H, Schneeweiss S, Glynn RJ, Patorno E. Trends in First-Line Glucose-Lowering Drug Use in Adults With Type 2 Diabetes in Light of Emerging Evidence for SGLT-2i and GLP-1RA. Diabetes Care. 2021. Aug 1;44(8):1774–82. https://doi-org.ezp-prod1.hul.harvard.edu/10.2337/dc20-2926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Das SR, Everett BM, Birtcher KK, et al. 2018 ACC Expert Consensus Decision Pathway on Novel Therapies for Cardiovascular Risk Reduction in Patients With Type 2 Diabetes and Atherosclerotic Cardiovascular Disease. Journal of the American College of Cardiology. 2018;72(24):3200–3223. doi: 10.1016/j.jacc.2018.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bohn Justin. 2017. Summary Score-Based Confounding Control in Studies of New Drugs: Chapter 3. Conditioning on calendar time in studies of newly marketed drugs. Doctoral dissertation, Harvard T.H. Chan School of Public Health. Accessed December 16, 2021. http://nrs.harvard.edu/urn-3:HUL.InstRepos:34389885 [Google Scholar]
- 38.Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behavioral Research. 2011;46(3):399–424. doi: 10.1080/00273171.2011.568786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Desai RJ, Rothman KJ, Bateman BT, Hernandez-Diaz S, Huybrechts KF. A Propensity-score-based Fine Stratification Approach for Confounding Adjustment When Exposure Is Infrequent: Epidemiology. 2017;28(2):249–257. doi: 10.1097/EDE.0000000000000595 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


