To the Editor,
A recent contribution in this journal on a comparative investigation, performed between two different time intervals (April–September in 2020 and 2021), reported that the distribution of COVID-19 confirmed cases, hospitalization of positive patients and admission in the intensive care units (ICUs) in Italy, did not show any significant reduction in response to the many draconian restriction measures and/or the vaccination campaign (Coccia, 2022a). As a matter of fact, the evidence provided by this report suggested that the observed periodic differences of the indicated parameters within each year should be explained as a simple seasonality behavior of the COVID-19 pandemic in Italy (Coccia, 2022a). If true, the official narrative tale reported by the Italian press about the real effect of any well intended political measure on the pandemic in Italy,might be thoroughly revised. Aside from the possibility that this evidence should raise controversial opinions about the management of the pandemic emergency in the scientific community, as well as in the mainstream press, people might ask about the real effects of lockdown and social restrictions. Seasonality of virus widespread may be the only effective engine moving the different pandemic dynamics observed throughout an epidemiological survey, or, at least, we could say that seasonality is a major factor of epidemiological change to be greatly considered in any political decision.
Actually, some intriguing consideration went in the spotlight also during a recent investigation of us.
1. Introduction
In this paper we are going to demonstrate that also COVID-19 deaths, by using statistics and time-series calculations, are distributed following a seasonality pattern. The evidence that even COVID-19 deaths, besides cases, hospitalizations and crowding of ICUs, are distributed according a seasonality pattern, should confirm that the management of COVID-19 pandemic is a very complex issue, where environmental and social factors have to be considered as major components of any forecast investigation on the SARS-CoV2 incidence in the population.
If even COVID-19 behavior has a seasonal attitude likewise flu (Hoogeveen and Hoogeveen, 2021), the political intervention on pandemic emergency should have followed a seasonal rhythm. The paper by Coccia reports that fatality rate in 2021 is lower than 2020 (Coccia, 2022a), a result confirmed by our study, although, as detailed below, it is particularly difficult to achieve a smart conclusion that this reduction is undoubtedly caused by the effect of political measures.
In the paper by Coccia, the mean of confirmed COVID-19 cases, hospitalizations and admissions in ICUs in 2020 and in 2021, resulted equal, i.e. that no significant difference in the arithmetic mean was observed, contrarily to fatality rates, where a significant difference was yet reported (Coccia, 2022a). This evidence should suggest that COVID-19 deaths may be less homogeneously distributed within the two different observed periods respect to cases, hospitalizations and ICUs admissions. Data herein discussed retrieved different results, when deaths instead of mortality rates were investigated. We used data available for Italy at the European Center for Disease Prevention and Control (https://www.ecdc.europa.eu/en/cases-2019-ncov-eueea), and from the official Ministry of Health website, which were elaborated with descriptive statistics, Wilcoxon and Friedman tests (for p < 0.05) and a seasonality analysis, using methods described below.
Our research showed that, despite the general consideration that social restrictions, lockdown and vaccination, should reduce the rate of mortality of a population undergoing an infectious outbreak, deaths distribution, likewise some seasonal sickness such as flu (Coccia, 2022a; Hoogeveen and Hoogeveen, 2021), was directly associated with environmental causes and social habits. This may be particularly significant if deaths involved mainly frail individuals, such as elderly people, who represent a particularly high percentage of the resident population in Italy (22.4%), even exceeding the European Union (EU) average (20%) (Abbatecola and Antonelli-Incalzi, 2020). Elderly people are particularly susceptible to a seasonal-driven sickness.
Table 1 summarizes the statistical results on COVID-19 deaths reported from data provided by the European Center for Diseases Control (ECDC) in the periods from March 2020 to December 2020 and from March 2021 to December 2021. The huge variability in the reported data, which any statistical test may consider as outliers, may not support the robustness of statistics applied on their day-by-day distribution, reported by ECDC on the basis of RT-PCR positive swabs. Therefore, caution must be held.
Table 1.
Seasonality behavior of COVID-19 deaths in Italy in the years 2020–2021 (*).
| 2020 | 2021 | Notch box plot data | |||||
| month | sum | mean ± sd | month | sum | mean ± sd | 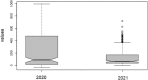 |
|
| 1 | March | 12,399 | 220.6 ± 315.1 | March | 11,647 | 366.9 ± 81.7 | |
| 2 | April | 15,539 | 500.2 ± 132.8 | April | 11,461 | 369.3 ± 107.4 | |
| 3 | May | 5448 | 155.6 ± 91.0 | May | 5321 | 157.5 ± 66.1 | |
| 4 | June | 1386 | 36.9 ± 24.4 | June | 1438 | 41.8 ± 24.0 | |
| 5 | July | 374 | 10.3 ± 6.9 | July | 497 | 14.2 ± 7.0 | |
| 6 | August | 342 | 6.0 ± 27.4 | August | 1158 | 32.0 ± 17.4 | |
| 7 | September | 411 | 12.7 ± 5.2 | September | 1700 | 55.2 ± 12.0 | |
| 8 | October | 2724 | 61.5 ± 75.4 | October | 1179 | 36.4 ± 11.0 | |
| 9 | November | 16,958 | 534.5 ± 173.5 | November | 1728 | 54.1 ± 18.7 | |
| 10 | December | 18,583 | 575.4 ± 167.3 | December | 3574 | 109.7 ± 34.7 | 2020 [48.45–131.55] median = 90; 2021 [52.81–77.19] median = 65 Wilcoxon test Z = −1.376 p = 0.16758 (n.s.); W = 14 (should be 8 for N = 10) p > 0.05 (n.s.) |
| Seasonality and statistical difference | ||
|---|---|---|
| Seasonal decomposition by Loess 2020 | Seasonal decomposition byLoess 2021 | Seasonality data |
 |
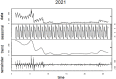 |
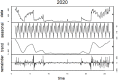
Test for seasonality of the time series 2020: χ2 = 34,661,991.56 p < 0.0001 Test for seasonality of the time series for 2021: χ2 = 3,902,764.02 p < 0.0001 |
| Wilcoxon test | ||
| Oct 2020–Oct 2021: p = 0.00714 Nov 2020–Nov 2021: p < 0.0001 Dec 2020–Dec 2021: p < 0.0001 Jan 2021–Jan 2022: p < 0.0001 Feb 2021–Feb 2022: p = 0.03318 | ||
| Friedman test | ||
| Mar–Dec 2020–2021 χ2 = 1.9236 p = 0.16546 Apr–Sept 2020–2021 χ2 = 11.5 p = 0.0007 | ||
Levene test on all series (1–10): F = 71.990786, p = 6.66134 × 10−16 (p < 0.0001); Levene test on series 2–7: F = 3.590507, p = 0.0588984 (p < 0.05, p = n.s.).
(*) data from ECDC https://www.ecdc.europa.eu/en/cases-2019-ncov-eueea.
Table 1 shows high standard deviations and variability in the expanded distribution March–December and moreover, the Levene's test assessed that the difference between the variances of some groups was big enough to be statistically significant (p = 6.66134 × 10−16, F = 71.990786). In this circumstance, the observed effect size F is large (0.34), so indicating that the magnitude of the difference between the averages is large as well. The η2 equals 0.11. It means that the group explains 10.6% of the variance from the average (similar to R2 in the linear regression). When the Levene's test was used for deaths distribution in the time range considered by Coccia, namely April–September, p-value > α and therefore H0 was accepted (Coccia, 2022a). This means that the averages of all groups considered to be equal. In other words, the difference between the variances of all the groups, was not big enough to be statistically significant (Table 1). If collected data are restricted to the time range April–September 2020–2021, therefore, the Levene's test showed that the difference between the variances of all the groups (p = 0.0588984, F = 3.590507), would suggest that the period investigated by the author is particularly homogeneous for the distribution of COVID-19 deaths (Coccia, 2022a), whereas this cannot be said for a time distribution ranging from March to December.
Furthermore, the distributions from March to December 2020 and 2021 have different medians (90 and 65, respectively) (see box plot in Table 1) but a Wilcoxon test reported that such arrays did not differ each other significantly (p = 0.16758, see Table 1).
At a first glance, the daily mean of the reported COVID-19 caused deceases on March 2020 was 220.6 ± 315.1 SD whereas on March 2021 was 366.9 ± 81.7 SD, showing an apparent increase in 2021, which yet resulted not significant at a Wilcoxon test (p = 0.7414). However, following the narrative tale held by politics and the mainstream, the draconian and strong lockdown performed in the period March–May 2020, should result in a marked reduction in deaths starting from June 2020. Actually, the daily mean of COVID-19 deceases in June 2020 was 36.9 ± 24.4 SD (March 2020 vs June 2020: Wilcoxon test p = 1.0256 × 10−6), so apparently supporting the thesis of a lockdown-caused success. However, when the same matched evaluation was reported for 2021, the result was perfectly comparable to 2020 (June 2021 = 41.8 ± 24.0 SD, Wilcoxon test March 2021 vs June 2021, p = 3.0029 × 10−11), i.e. that no difference was observed in the decease distribution between June 2020 and June 2021 (p = 0.87079).
The reduction in deaths observed in June 2020, as well as in June 2021, might not be, therefore, the result of lockdown and restriction measures, but much probably was caused by season-driven effects (comparison June 2020–June 2021 p = 0.87079), confirmed for other parameters by others (Coccia, 2022a).
Deaths distribution in 2020 and in 2021 may have a seasonal behavior, therefore.
Actually, Table 1 shows that, using a Seasonality Trend Loess (STL) decomposition (Cleveland et al., 1990), deaths should follow a seasonal behavior. COVID-19 deceases are strongly reduced during the warmest climatic periods, and a marked reduction in COVID-19 deaths occurred also at the end of 2021, for which we cannot state if caused by a massive vaccination campaign or by a strong reduction in the viral lethality, due to the prevalence in the variant of concern (VoC) B.1.1.529, known as Omicron (Nyberg et al., 2022). Actually, our data (Table 1) confirmed previous reported evidence showing a reduction in COVID-19 mortality between the first and the second wave (Coccia, 2021b).
Yet, seasonality, according to the Friedman test, can be reported for the distribution April–September 2020–2021 (Table 1).
Despite the number of deaths were sensitively reduced starting from October 2021, we are currently unable to elucidate if this reduction is due to vaccination or to the impact of the new VoC. To date, at March 21st, 2022, in Italy the SARS-CoV2 β-VoC, i.e. the B.1.351 beta variant, accounts on only 169 cases, whereas the SARS-CoV2 ο-VoC, i.e. the B.1.1.529 omicron variant, accounts on 16,628 cases (Mattiuzzi et al., 2022). Actually, on January 2022 a reduction of 5261 death (p < 0.0001) respect to January 2021 and a reduction of 914 deaths on February 2022 respect to February 2021 (p < 0.04) were observed. This reduction may be indifferently caused by the increased impact of the Omicron VoC, which is highly contagious but very poorly lethal or by the increase in the number of vaccinated subjects. Further insightful investigations are needed to address this point.
The seasonal behavior of COVID-19 deaths may even be separated from cases, as fatality rate exhibited a different statistical distribution (Coccia, 2022a). This may suggest that COVID-19 deaths were particularly sensitive to environmental factors in a way quite independent respect to confirmed cases, hospitalizations and ICUs admissions.
2. Discussion
The role of seasonality in COVID-19 pandemic is of utmost importance to approach a proper emergency management and improve the impact of political decisions on the viral widespread in the population. In our research, we have demonstrated that even COVID-19 deaths follow a seasonal pattern. A surprising temporal convergence between mortality in the first months of 2022 and 2021 can be outlined, despite in 2021 less vaccines were administered. This evidence supports the interpretation that COVID-19 deaths depend on unpredictable factors upon different environmental and periodic conditions.
Experts should wonder if social restrictions and lockdowns, as well as the mass vaccination campaign, resulted actually effective in reducing deaths due to SARS-CoV2 pandemic. Recent Cox models showed that the effectiveness of vaccination against deaths is 95% (Cocchio et al., 2022).
Virus endemism, starting with the quite complete replacement of the previous VoCs, i.e. beta (B 1.351, from South Africa), gamma (P1, from Brazil) and delta (B 1.617.2, from India), with omicron, (B 1.1.529 from South Africa) may explain the observed reduction in the COVID-19 epidemiological markers from the end of 2021 to the present (Khan et al., 2022, Micheli et al., 2022). If true, a reduction should be observed also in confirmed cases, if vaccines are a leading cause, otherwise we should consider the effect of the omicron VoC. Actually, in January 2022 confirmed COVID-19 cases increased of 4,411,567 units respect to January 2021 (p < 0.0001) and in February 2022 of 1,305,649 units respect to February 2021 (p < 0.0001). It is possible that the observed reduction in COVID-19 deaths may mostly derive from a much lesser lethality of the omicron VoC.
Elucidating the possible causes of the observed seasonality is far to be fully accomplished, despite some hypotheses were recently forwarded (Kharapiperis et al., 2021; Chirumbolo and Bjørklund, 2020; Coccia, 2021c; Kumar et al., 2021). A recent simulation using the Empirical Mode Decomposition (EEMD) method reported that warm seasons reduce the rate of mortality in the countries located at the Northern Hemisphere (Liu et al., 2021), an evidence that appears to support our reported data. The same distribution of data here reported, suggested us that any draconian political decision did not impacted significantly on the seasonality pattern, so to further assess for an expected success of the same restrictive and mandatory measures. This circumstance should confirm previously reported considerations about the poor effectiveness of lockdown and social restriction on the COVID-19 epidemiological phenomena (Coccia, 2021a). On the other hand, seasonal different climates should be included in the thorough evaluation of how COVID-19 pandemic evolves in a studied population, in order to improve any political decision. In this perspective, for example, epidemic modeling is possible by considering fluid dynamics simulations, able to foresee an airborne infection rate via the evaluation of SARS-CoV2 particles in patients’ saliva samples (Dbouk and Drikakis, 1994).
The summer “rapid disappearing” observed in our mortality data was reported also by other authors, who recommended to address political decisions mainly onto winter months, rather than throughout the whole year (Erren et al., 2021). Taking into consideration novel straightforward and sound approaches to address COVID-19 pandemic with the highest performance, is a leading matter of debate (Coccia, 2022b).
The complex effect of additive components in the epidemiological behavior of SARS-CoV2 infections and the involvements of further VoCs such as the B.1.1.7 and B.1351 from South Africa (beta-variants), should elucidate why on September 2021 the daily mean in COVID-19 deaths was significantly higher (55.2 ± 12.0 SD) than 2020 (12.7 ± 5.2 SD, p = 2.9376−11), despite reduction due to vaccination was yet expected, whereas on December and January the daily number of COVID-19 deaths was significantly lower than the previous year (Table 1), promoting, by contrast, the thesis that vaccination might have a relevant impact on the COVID-19 mortality rate, particularly in the latest months. Data so far reported seem to assess the hypothesis that deaths reduction was caused by environmental causes, seasonality and endemic variants. Any rising and falling down of the many COVID-19 epidemiological parameters, cannot be read with a simplistic language, anyway.
3. Conclusions
We have observed that, as confirmed cases, hospitalizations and ICUs admissions, also COVID-19 deaths have a seasonality pattern. Despite our paper has neither political nor controversy purposes, yet the data here presented compel the scientific community to expand the debate with experts and institutional responsibilities, professional boards and citizen representants, to approach a much more realistic and correct management of the SARS-CoV2 pandemic, which is going ahead with infectious contacts and debilitating chronic disorders, such as long-COVID (or also known as post-acute sequelae of COVID-19 (PASC)). The narrative tale performed so far would justify the management of the pandemic emergency by referring to changes in the punctual (day-by-day) data as a consequence of the different measures adopted.
By the contrast, politics should consider an intertwined network of environmental, social and epidemiological causes, along with new updating cultural habits, as the leading cause of the complex variability underneath the reporting of data about COVID-19 epidemiology, trying to thoroughly revise a too simplistic interpretation of the pandemic and lead a more outstanding overview of the health emergency.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abbatecola A.M., Antonelli-Incalzi R. Editorial: COVID-19 spiraling of frailty in older Italian patients. J. Nutr. Health Aging. 2020;24(5):453–455. doi: 10.1007/s12603-020-1357-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirumbolo S., Bjørklund G. The bimodal SARS-CoV-2 outbreak in Italy as an effect of environmental and allergic causes. J. Allergy Clin. Immunol. 2020 Aug;146(2):331–332. doi: 10.1016/j.jaci.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland R.B., Cleveland W.S., McRae J.E., Terpenning I. STL: a seasonal-trend decomposition procedure based on loess. J. Off. Stat. 1990;6:3–73. [Google Scholar]
- Cocchio S., Zabeo F., Facchin G., Piva N., Furlan P., Nicoletti M., Saia M., Tonon M., Mongillo M., Russo F., Baldo V. The effectiveness of a diverse COVID-19 vaccine portfolio and its impact on the persistence of positivity and length of hospital stays: the veneto Region's experience. Vaccines (Basel) 2022 Jan 11;10(1):107. doi: 10.3390/vaccines10010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia M. The relation between length of lockdown, numbers of infected people and deaths of COVID-19, and economic growth of countries: lessons learned to cope with future pandemics similar to COVID-19. Sci. Total Environ. 2021;775:145801. [Google Scholar]
- Coccia M. The impact of first and second wave of the COVID-19 pandemic in society: comparative analysis to support control measures to cope with negative effects of future infectious diseases. Environ. Res. 2021 Jun;197:111099. doi: 10.1016/j.envres.2021.111099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia M. Encyclopedia of COVID-19. Vol. 1. 2021. Pandemic prevention: lessons from COVID-19; pp. 433–444. [DOI] [Google Scholar]
- Coccia M. COVID-19 pandemic over 2020 (with lockdowns) and 2021 (with vaccinations): similar effects for seasonality and environmental factors. Environ. Res. 2022 Jan 13;208:112711. doi: 10.1016/j.envres.2022.112711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia M. Preparedness of countries to face COVID-19 pandemic crisis: strategic positioning and factors supporting effective strategies of prevention of pandemic threats. Environ. Res. 2022 Jan;203:111678. doi: 10.1016/j.envres.2021.111678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dbouk T., Drikakis D. Fluid dynamics and epidemiology: seasonality and transmission dynamics. Phys. Fluids. 1994;33(2) doi: 10.1063/5.0037640. 2021 Feb 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erren T.C., Lewis P., Morfeld P. Factoring in coronavirus Disease 2019 seasonality: experiences from Germany. J. Infect. Dis. 2021 Sep 17;224(6):1096. doi: 10.1093/infdis/jiab232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogeveen M.J., Hoogeveen E.K. Comparable seasonal pattern for COVID-19 and flu-like illnesses. One Health. 2021 Dec;13:100277. doi: 10.1016/j.onehlt.2021.100277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan K., Karim F., Cele S., San J.E., Lustig G., Tegally H., Rosenberg Y., Bernstein M., Ganga Y., Jule Z., Reedoy K., Ngcobo N., Miya Y., Mazibuko M., Mthabela N., Mhlane Z., Mbatha N., Giandhari J., Ramphal Y., Naidoo T., Manickchund N., Magula N., Karim S.A., Gray G., Hanekom W., von Gottberg A., Milo R., Gosnell B., Lessells R., de Oliveira T., Moore P., Moosa Y.S., Sigal A. Omicron infection of vaccinated individuals enhances neutralizing immunity against the Delta variant. medRxiv [Preprint] 2022 Jan 28 doi: 10.1101/2021.12.27.21268439. 2021.12.27.21268439. [DOI] [Google Scholar]
- Kharapiperis Christos, Kouklis Panos, Papastratos Stelios, Chasapi Anastasia, Danchin Antoine, Angelis Lefteris, Ozounis Christos. A strong seasonality pattern for covid-19 incidence rates modulated by UV radiation levels. Viruses. 2021;13(4):574–591. doi: 10.3390/v13040574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M., Mazumder P., Mohapatra S., Kumar Thakur A., Dhangar K., Taki K., Mukherjee S., Kumar Patel A., Bhattacharya P., Mohapatra P., Rinklebe J., Kitajima M., Hai F.I., Khursheed A., Furumai H., Sonne C., Kuroda K. A chronicle of SARS-CoV-2: seasonality, environmental fate, transport, inactivation, and antiviral drug resistance. J. Hazard Mater. 2021 Mar 5;405:124043. doi: 10.1016/j.jhazmat.2020.124043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Huang J., Li C., Zhao Y., Wang D., Huang Z., Yang K. The role of seasonality in the spread of COVID-19 pandemic. Environ. Res. 2021 Apr;195:110874. doi: 10.1016/j.envres.2021.110874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattiuzzi C., Henry B.M., Lippi G. COVID-19 vaccination and SARS-CoV-2 Omicron (B.1.1.529) variant: a light at the end of the tunnel? Int. J. Infect. Dis. 2022;118:167–168. doi: 10.1016/j.ijid.2022.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheli V., Bracchitta F., Rizzo A., Mancon A., Mileto D., Lombardi A., Stefanelli P., Gismondo M.R. First identification of the new SARS-CoV-2 Omicron variant (B.1.1.529) in Italy. Clin. Infect. Dis. 2022 Jan 21 doi: 10.1093/cid/ciab1044. ciab1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg T., Ferguson N.M., Nash S.G., Webster H.H., Flaxman S., Andrews N., Hinsley W., Bernal J.L., Kall M., Bhatt S., Blomquist P., Zaidi A., Volz E., Aziz N.A., Harman K., Funk S., Abbott S., COVID-19 Genomics UK (COG-UK) consortium. Hope R., Charlett A., Chand M., Ghani A.C., Seaman S.R., Dabrera G., De Angelis D., Presanis A.M., Thelwall S. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022 Mar 16;S0140–6736(22):462–467. doi: 10.1016/S0140-6736(22)00462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


