Abstract
Background
Multiple patients with prostate cancer become resistant to castration therapies, which is termed castration-resistant prostate cancer (CRPC).
Purpose
The purpose of this review is to assess the status of efficacy (≥50% decline in prostate-specific antigen (PSA), progression-free survival (PFS), and overall survival (OS)) and safety (grade 3-4 adverse effects) of monoclonal antibodies in CRPC.
Data source
We searched databases including PubMed, Embase, Cochrane, Web of Science, and ClinicalTrials.gov.
Results
Hazard ratios of PFS and OS were 0.77 (95% CI = 0.69-0.87, I2 = 53%) and 0.98 (95% CI = 0.86-1.11, I2 = 40%), respectively, in the favor of monoclonal antibodies as compared to placebo. Risk ratio (RR) of >50% decline in PSA was 1.99 (95% CI = 0.97-4.08, I2 = 53%) in favor of monoclonal antibodies. Pooled incidence of >50% decline in PSA levels was 15% (95% CI = 0.1-0.23, I2 = 83%), 29% (95% CI = 0.14-0.51, I2 = 93%), 63% (95% CI = 0.49-0.76, I2 = 77%), and 88% (95% CI = 0.81-0.93, I2 = 0%) in single, two, three, and four-drug regimens, respectively.
Conclusion
Monoclonal antibodies are well tolerated and showed better PFS as compared to placebo. However, OS was only improved with ipilimumab. Denosumab delayed skeletal-related adverse events as compared to zoledronic acid. More multicenter double-blind clinical trials may be needed to confirm these results.
Keywords: castration-resistant prostate cancer, meta-analysis, systematic review, checkpoint inhibitors, monoclonal antibodies, prostate cancer
Introduction
Prostate cancer is the second most common cause of cancer deaths in men after lung cancer in the United States with both aggressive and slow-growing types identified. More than 20% of the newly diagnosed cases of cancer are prostate cancer [1]. The new cases and estimated deaths for prostate cancer reported in the US in 2019 were 174,650 and 31,620, respectively, with an increase in the trend seen in 2020 with 191,930 new cases and 33,330 estimated deaths [1,2]. Globally, 1,276,106 new cases were estimated in 2018. Developed countries have higher incidence probably due to better use of diagnostic testing [3].
The various modalities that continue to be the mainstay of treatment for prostate cancer are surgical (prostatectomy), hormonal (gonadotropin‐releasing hormone agonist or antagonist, androgen deprivation), and radiation (external beam radiotherapy, brachytherapy) [4-6]. However, surgical/chemical castration is required for most patients with metastatic disease. The progression of the carcinoma with or without metastasis despite castration therapy (androgen deprivation therapy) is termed as castrate-resistant or hormone-resistant cancer and is characterized by rising prostate-specific antigen (PSA) levels with castrate range of testosterone (<50 ng/dl or <1.7 nmol/l) [6-9].
Chemotherapy agents including taxanes, bisphosphonates, immunotherapy agents, and poly (ADP-ribose) polymerase-1 inhibitors have shown anti-tumor activity in patients with castration-resistant prostate cancer (CRPC). Taxane with prednisone is the most common treatment used for CRPC. Despite these treatment options, the prognosis and quality of life of these patients are very poor. There is still room for more combination therapies for the treatment of CRPC, especially for patients who do not tolerate and/or are refractory to first-line therapies [10-13].
In recent years, monoclonal antibodies have shown promising results in clinical trials. Monoclonal antibodies have been evaluated for their efficacy in CRPC due to their targeted action on various tumor factors that help control cancer progression [4]. The most common antibodies studied include bevacizumab (anti-vascular endothelial growth factor (VEGF)), which decreases angiogenesis and improves vessel penetration of cytotoxic agents like taxanes when used in combination [10,11]. Cixutumumab and ramucirumab act against insulin-like growth factor-1 receptor (IGF-1R)/vascular endothelial growth factor receptor (VEGFR) and can prevent tumor growth. Other monoclonal antibodies, including siltuximab, abituzumab, trastuzumab, and cetuximab, bind to interleukin-6, integrin alpha-V, human epidermal growth factor receptor 2 (HER2), and epidermal growth factor receptor (EGFR), respectively [12-15]. Checkpoint inhibitors including nivolumab (anti-programmed cell death protein 1 (PD-1)), pembrolizumab (anti-PD-1), and ipilimumab (anti-cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4)) are also tested in clinical trials for anti-tumor activity against CRPC [16,17]. While several of these immunotherapies are under evaluation in clinical trials, denosumab is the major monoclonal antibody approved by the FDA for metastatic bone lesions in CRPC [18].
The aim of this systematic review and meta-analysis is to assess the efficacy and safety of monoclonal antibodies alone or in combination with chemotherapy drugs in CRPC.
Materials and methods
In conducting this systematic review and meta-analysis, we followed a prespecified protocol registered on the International Prospective Register of Systematic Reviews (PROSPERO) (registration number: CRD42021230102). The protocol was made according to the guidelines established by Cochrane [19] and PRISMA-P (Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols) [20].
Search strategy
A literature search was performed on PubMed, Embase, Web of Science, Cochrane Library, and ClinicalTrials.gov with Medical Subject Heading (MeSH) and Emtree terms “monoclonal antibodies” and “castration-resistant prostate cancer.” The search was made from the inception of literature till March 20, 2021, by following the PICO framework (Appendix) [21].
Inclusion and exclusion criteria
We included all clinical trials that provided safety and efficacy data in clinical terms, i.e., objective response (OR), complete response (CR), partial response (PR), ≥50% decline in PSA, progression-free survival (PFS), overall survival (OS), and grade 3-4 adverse effects. We excluded all preclinical studies, case reports, meta-analyses, review articles, observation studies, and clinical studies irrelevant to the study question.
Study selection
Two researchers (WA and TAT) independently reviewed the articles identified through initial search and screened them based on inclusion and exclusion criteria. The differences were addressed by a third researcher (MAA).
Data extraction
Data were extracted by two authors (MS and MYA). The data were extracted for the characteristics of the study, baseline characteristics of participants, treatment drugs, efficacy measures, and toxicity (grade ≥ 3 adverse effects).
Risk of bias assessment
Two researchers (SR and SFB) assessed the risk of bias in randomized clinical trials (RCTs) selected for final inclusion by using the Risk of Bias 2 (RoB 2) tool for risk of bias assessment in RCTs [22]. The third researcher (MAA) addressed the differences.
Statistical analysis
The meta-analysis was performed using the “R” programming language. We used the “meta” package in R for our data analysis [23]. A random-effects model was used, irrespective of the heterogeneity, to keep our results consistent and applicable. All analyses used the DerSimonian-Laird estimator to calculate between-study variance. The risk ratios were pooled using the Mantel-Haenszel method. For studies with zero events in any of the arms, a continuity correction of 0.5 was used. Standard errors and other calculations were done using a 95% confidence interval. For pooling of the results, all the studies were included even if they have zero events in both arms. To estimate the heterogeneity, I2 was used.
Results
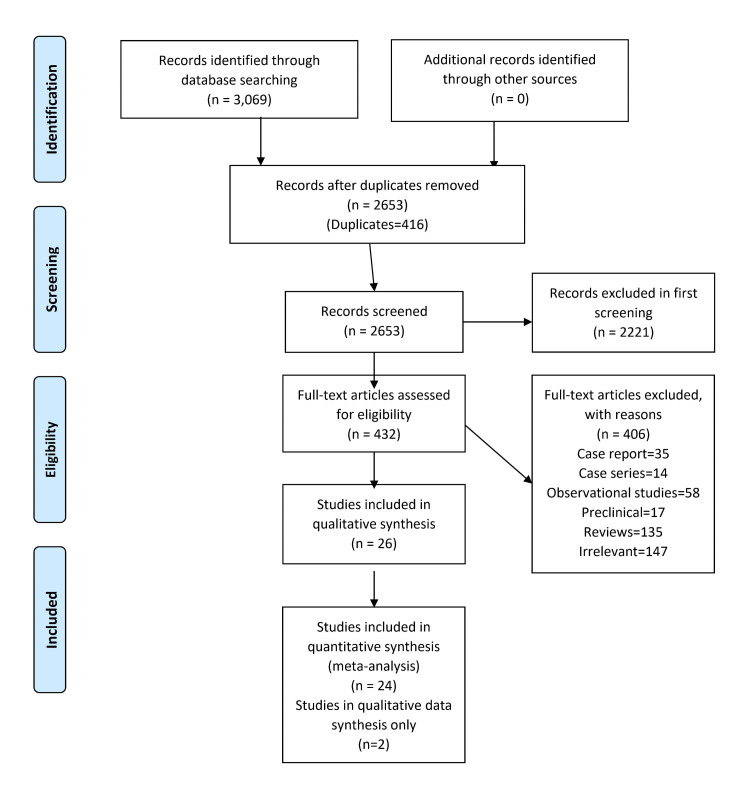
A total of 3,069 articles were identified with 424 articles from PubMed, 2,427 articles from Embase, 49 articles from Web of Science, 60 articles from Cochrane, and 109 articles from ClinicalTrials.gov. These articles were analyzed by the researchers and 416 articles were removed as duplicates. A total of 2,221 articles were excluded in the first screening based on exclusion criteria. Full texts of 432 articles were reviewed. Eight RCTs (N = 6,227) [13,24-30] and 18 non-randomized clinical trials (NRCTs, N = 920) [10,15,31-41] were included based on prespecified inclusion criteria (Figure 1).
Figure 1. Flow chart of literature search.
Risk of bias
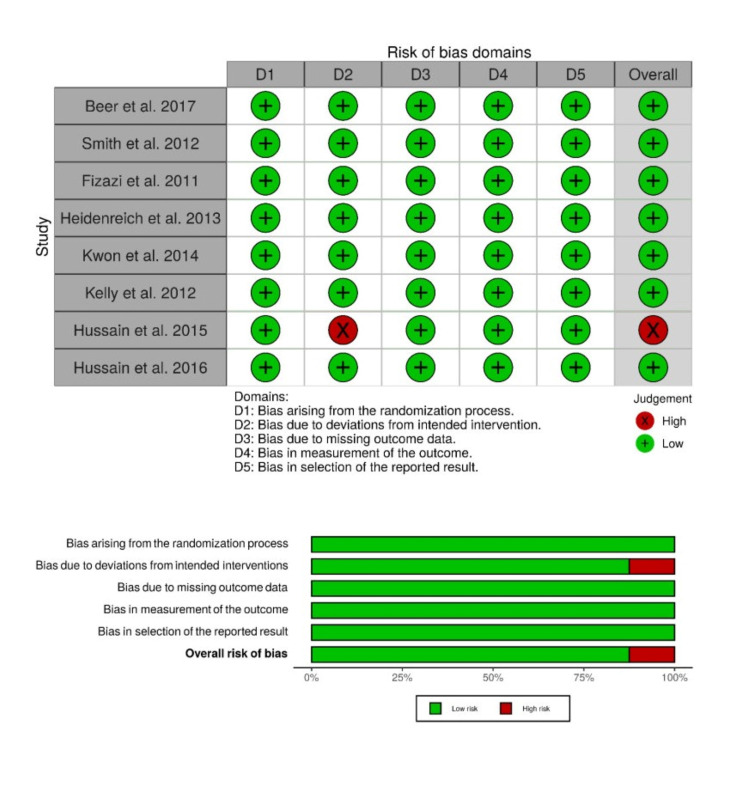
The risk of bias was low in double-blinded RCTs except for open-label RCT conducted by Hussain et al. (2015) [30] (Figure 2).
Figure 2. Risk of bias assessment with Risk of Bias 2 (RoB 2) tool.
Monoclonal antibodies vs. placebo
In six clinical trials (N = 4,194) [13,24-30], monoclonal antibodies were given to 2,225 participants while placebo was given to 1,969 participants. Standard of care (SOC) including luteinizing hormone-releasing hormone agonist/antagonist was given to 180 patients in the study by Hussain et al. [30]. The median ages of participants were ≥65 years in RCTs. Baseline characteristics of participants are given in Table 1.
Table 1. Baseline characteristics of trials.
NCT = National Clinical Trial; ECOG = Eastern Cooperative Oncology Group; PSA = prostate-specific antigen; CTLA-4 = cytotoxic T-lymphocyte-associated antigen-4; RANKL = receptor activator of nuclear factor kappa-B ligand; M = mitoxantrone; P = prednisone; XRT= radiation therapy; SOC = standard of care; VEGFR = vascular endothelial growth factor receptor; EGFR = epidermal growth factor receptor; PD-1 = programmed cell death protein 1; IL-6 = interleukin 6; IGF = insulin-like growth factor; HER2 = human epidermal growth factor receptor 2; VEGF-A = vascular endothelial growth factor A.
| Author, year | Phase | Trial NCT | Follow-up | N | Age | Treatment | ECOG score | Metastasis | Prior systemic therapy | Gleason score | Bone lesion | Median PSA |
| Randomized clinical trials | ||||||||||||
| Beer et al. (2017) [24] | III | NCT01057810 | 2-4 years | 400 | 70 (44-91) | Ipilimumab (anti-CTLA-4, 10 mg/Kg) | 0 = 75%, 1 = 25% | Bone = 78% | N/A | ≤7 = 47%, ≥8 = 48% | Yes = 78%, no = 21% | 41.2 (0.05-4,956) |
| 202 | 69 (42-92) | Placebo | 0 = 75%, 1 = 25% | Bone = 79% | N/A | ≤7 = 51%, ≥8 = 45% | Yes = 79%, no = 19% | 49.5 (0.01-9,297) | ||||
| Smith et al. (2012) [25] | III | NCT00286091 | N/A | 716 | 74·0 (67-80) | Denosumab (targets RANKL) (120 mg) | 0 = 71%, 1 = 29% | Non-metastatic | N/A | ≤7 = 60%, 8-10 = 30% | N/A | 12·2 (4·7-27·5) |
| 716 | 74·0 (67-80) | Placebo | 0 = 72%, 1 = 28% | Non-metastatic | N/A | ≤7 = 56%, 8-10 = 33% | N/A | 12·5 (4·9-28·5) | ||||
| Fizazi et al. (2011) [26] | III | NCT00321620 | 12.2 month | 950 | 71 (64-77) | Denosumab (targets RANKL) | 0-1 = 93% | Visceral metastasis = 17% | Recent chemotherapy = 14% | 2-6 = 18%, 7 = 29%, 8-10 = 41% | Skeletal event = 24% | 58·5 (18·2-225·6) |
| 11.2 month | 951 | 71 (66-77) | Zoledronic acid | 0-1 = 93% | Visceral metastasis = 19% | Recent chemotherapy = 14% | 2-6 = 19%, 7 = 29%, 8-10 = 43% | Skeletal event = 24% | 60·0 (19·8-202·2) | |||
| Heidenreich et al. (2013) [27] | II | N/A | 24 months | 66 | 68 (41, 83) | Docetaxel + prednisone + intetumumab (integrin α-V, 10 mg/kg) | 0 = 34, 1 = 30, 2 = 2 | Metastatic cancer | 57/66 | <7 = 31, >7 = 22 | N/A | N/A |
| 24 months | 65 | 68 (46, 82) | Docetaxel + prednisone + placebo | 0 = 31, 1 = 32, 2 = 2 | Metastatic cancer | 62/65 | <7 = 26, >7 = 25 | N/A | N/A | |||
| Kwon et al. (2014) [28] | III | NCT00861614 | 9.9 months | 399 | 69·0 (47-86) | Ipilimumab group (anti-CTLA-4, 10 mg/kg) | 0 = 168, 1 = 216, 2 = 3 | Bone events <5 = 276, >5 = 103 | N/A | <7 = 174, >7 = 192 | Bone <5 = 276, >5 = 103 | 138·5 (0-4576) |
| 9.3 months | 400 | 67·5 (45-86) | Placebo | 0 = 170, 1 = 220 | Bone events <5 = 253, >5 = 111 | N/A | <7 = 190, >7 = 187 | Bone <5 = 253, >5 = 111 | 176·5 (0-13768) | |||
| Kelly et al. (2012) [29] | III | N/A | 8 cycles | 524 | 68.8 | Bevacizumab (anti-VEGF-A, 15 mg/kg) + docetaxel | 0 = 57, 1 = 39, 2 = 4 | Metastatic cancer | N/A | N/A | N/A | N/A |
| 526 | 69.3 | Docetaxel | 0 = 55, 1 = 40, 2 = 5 | Metastatic cancer | N/A | N/A | N/A | N/A | ||||
| Hussain et al. (2015) [30] | II | NCT00683475 | N/A | 66 | 65 (48-88) | Cixutumumab (anti-IGF, 6 mg/kg) + M + P | 0 = 23, 1 = 38, 2 = 5 | Metastatic cancer | Docetaxel-pretreated | N/A | N/A | 133.45 (0.1-5530.0) |
| 66 | 68 (46-86) | Ramucirumab (VEGFR, 6 mg/kg) + M + P | 0 = 19, 1 = 41, 2 =6 | Metastatic cancer | Docetaxel-pretreated | N/A | N/A | 107.30 (2.2-5826.4) | ||||
| Hussain et al. (2016) [13] | II | NCT01360840 | 4.1 months | 60 | 69.5 (54-84) | Abituzumab (anti-CD-51, 750 mg) and SOC | 0 = 39, 1 = 18 | Metastasis = 57 | N/A | N/A | N/A | N/A |
| 4.2 months | 60 | 71.0 (53-88) | Abituzumab 1,500 mg and SOC | 0 = 34, 1 = 22 | Metastasis = 59 | N/A | N/A | N/A | N/A | |||
| 4.2 months | 60 | 71.0 (46-88) | Placebo and SOC | 0 = 32, 1 = 25 | Metastasis = 59 | N/A | N/A | N/A | N/A | |||
| Non-randomized clinical trials | ||||||||||||
| Vaishampayan et al. (2015) [31] | I | N/A | 4 weeks | 7 | 66-85 | Anti-CD3 x anti-HER2 bispecific antibody | 0-2 = 100% | Metastatic cancer | Hormones = 7, docetaxel = 1 | 6-9 | Present | N/A |
| Picus et al. (2011) [33] | II | N/A | 24 months | 77 | 69 (48-88) | Estramustine, docetaxel, and bevacizumab ( anti-VEGF-A) | 0-2 = 100% | Metastatic cancer | N/A | N/A | 86% | 123 ng/ml |
| Vaishampayan et al. (2014) [32] | II | N/A | 24 months | 30 | 67 (50-85) | Bevacizumab and satraplatin | N/A | Metastatic cancer | Docetaxel = 100% | 6 = 6%, 7 = 26%, 8-10 = 65% | 21 (68%) | 180.7 ng/ml (4.7-1,432.8 ng/ml) |
| McNeel et al. (2018) [34] | II | N/A | N/A | 26 | 73 (56-85) | Anti-tumor vaccine (+pembrolizumab-PD-1 inhibitor in 13) | <2 | Metastatic cancer | Radiation, chemo, abiraterone, enzalutamide | <7 = 8%, 7 = 19%, 8 = 19%, 9 = 54% | N/A | 24 (3-165) |
| Gross et al. (2017) [11] | Ib | NCT00574769 | 12 cycles + maintenance | 43 | 65 (50-79) | Docetaxel, bevacizumab, and everolimus | N/A | Bone = 88%, nodes = 44%, viscera = 19% | Abiraterone = 26%, orteronel = 7%, enzalutamide = 5% | N/A | Bone metastasis = 88% | 76.6 (0-1847) |
| Cathomas et al. (2012) [15] | II | NCT00728663 | 25.4 months | 38 | 68 (45-82) | Docetaxel + cetuximab (EGFR inhibitor, 400 mg/m2) | N/A | Bone = 89%, node = 63%, visceral = 34% | 1 regimen = 65%, 2 regimens = 26%, 3 regimens = 9% (docetaxel regimens) | N/A | Bone metastasis = 89% | 212 ng/ml (4.4-8,898) |
| Batra et al. (2020) [35] | I | NCT00916123 | N/A | 15 | 69 (49-80) | Docetaxel + J591 (177Lu-J591) | 0 = 40%, 1 = 53.3%, 2 = 6.7% | Bone = 93.3%, node = 60%, lung = 6.7% | Primary radiotherapy = 40%, salvage radiotherapy = 13.3%, prostatectomy = 46.7% | 6 = 13.3%, 7 = 40%, 8-10 = 40% | Bone metastasis = 93.3% | 84.32 ng/ml (17.2-776) |
| Madan et al. (2016) [36] | II | NCT00942578 | 47.5 months | 63 | 65.6 (51-82) | Lenalidomide with bevacizumab, docetaxel, and prednisone | 0 = 10, 1 = 50, 2 = 3 | Bone = 24, bone + nodes = 27, bone + visceral = 7 | N/A | ≤6 = 4, 7 = 15, 8 = 15, 9 = 23, 10 = 6 | Bone = 24 | 90.36 (0.14-3 520) |
| Slovin et al. (2013) [37] | I/II | NCT00323882 | N/A | 16 | 65 (53-76) | Ipilimumab (anti-CTLA-4, 10 mg/kg) | 0 = 10, 1 = 6, 2 = 0 | Metastatic cancer | 6 (38%) | N/A | 2.5 (1-12) | 132 (13-2581) |
| 34 | 66 (50-83) | Ipilimumab = 10 mg/kg + XRT | 0 = 9, 1 = 22, 2 = 0 | Metastatic cancer | 21 (62%) | N/A | 8 (1-15) | 120 (8-1314) | ||||
| Barata et al. (2019) [38] | I/II | NCT01083368 | N/A | 21 | 64 (53-82) | Temsirolimus and bevacizumab | 0 = 19%, 1 = 62%, 2 = 14% | Metastatic cancer | Docetaxel = 86%, mitoxantrone = 29%, ketoconazole = 24%, cabazitaxel = 10%, gemcitabine = 10% | <7 = 33%, >= 8 = 43% | 21 (100%) | 205.3 (11.1-1801.0) |
| Autio et al. (2020) [39] | I | NCT02265536 | N/A | 12 | 58-84 | LY3022855 | 0 = 33%, 1 = 58.3%, 2 = 8% | Metastatic cancer | Chemotherapy = 42% Abiraterone acetate/enzalutamide = 100% | N/A | 10/12 (83%) | N/A |
| Di Lorenzo et al. (2008) [40] | II | N/A | N/A | 20 | 66 (49-73) | Bevacizumab + docetaxel | N/A | Metastatic cancer | Docetaxel = 100%, mitoxantrone = 100%, vinorelbine = 65% | <7 = 8, >7 = 12 | Bone metastasis = 100% | 260 |
| Graff et al. (2020) [42] | II | NCT02312557 | 37 months | 28 | 72 (61-90) | Pembrolizumab (anti-PD-1, 200mg) + enzalutamide | 0 = 39%, 1 = 61% | Metastatic cancer | Docetaxel = 4, abiraterone = 10, enzalutamide = 28 | <7 = 1, 7 = 9, >7 =1 4 | Bone only = 13, bone and lymph nodes = 9 | 26.61 ng/ml (3.03-2502.75) |
| Francini et al. (2011) [43] | II | N/A | 11.3 months | 43 | 74 (58-82) | Docetaxel + bevacizumab + prednisone | 0 = 20.9%, 1-2 = 79% | Metastatic cancer | w-epirubicin + w-docetaxel = 21 3-w, docetaxel + prednisone = 15, w-docetaxel + prednisone = 7 | N/A | N/A | 78 (47-374) |
| Ning et al. (2010) [44] | II | N/A | 34 months | 60 | 66 (44-79) | Docetaxel, bevacizumab, thalidomide, prednisone | 0 = 13%, 1 = 80%, 2 = 7% | Metastatic cancer | N/A | <7 = 20 (33%), >8 = 39 (65%) | N/A | 99 (0.9-4,399) |
| Hudes et al. (2013) [41] | I | N/A | N/A | 39 | 66 (43, 82) | Docetaxel 75 mg/m2 + siltuximab (anti-IL-6, 6-12 mg/kg) | N/A | Metastatic cancer | N/A | 8 (5,10) | N/A | 57 (12, 1430) |
| Sharma et al. (2020) [16] | II | NCT02985957 | 11.9 months | 45 | 69 (48-85) | Nivolumab (anti-PD-1, 1 mg/kg) + ipilimumab (anti-CTLA-4, 3 mg/kg) | 0 = 26 (57.8%), 1 = 19 (42.2%) | M0 = 28 (62.2%), MI = 15 (33.3%) | Abiraterone = 66.7%, enzalutamide = 57.8%, bicalutamide = 55.6%, leuprolide = 60%, docetaxel = 11.1% | <7 = 35.5%, >7 = 60% | 0 = 20%, <4 = 13.3%, >4 = 66.7% | 59.5 ng/ml (93.3-1045) |
| 13.5 months | 45 | 65 (46-84) | Nivolumab 1 mg/kg + ipilimumab (3 mg/kg) | 0 = 25 (55.6) 1 = 20 (44.4%) | M0 = 22 (48.9%), MI = 20 (44.4%) | Abiraterone = 71.1%, enzalutamide = 62.2%, bicalutamide = 64.4%, leuprolide = 53.3%, docetaxel = 86.7%, cabazitaxel = 46.7% | 7 or less = 42.2%, 8 or more = 51.1% | 0 = 6.7%, <4 = 2.2%, >4 = 91.1% | 158.9 ng/ml (1.8-1348.7) | |||
| Antonarakis et al. (2020) [17] | II | NCT02787005 | 9.5 months | 133 PD-L1+ | 68 (48-85) | Pembrolizumab 200 mg | 0 = 36%, 1 = 53.4%, 2 = 10% | Metastatic cancer | No. of previous chemotherapy regimens: 1 = 183 (71%), 2 or more = 75 (29%) | 7 or less = 31.7%, 8 or more = 62%, unknown = 6.2% | Bone predominant = 59 | 115.5 (0.1-5000) |
| 7.9 months | 66 PD-L1- | 68 (53-84) | 116.1 (1.0-3583.0) | |||||||||
| 14.1 months | 59 | 71 (53-90) | 43.3 (0.1-2539.0) | |||||||||
Efficacy
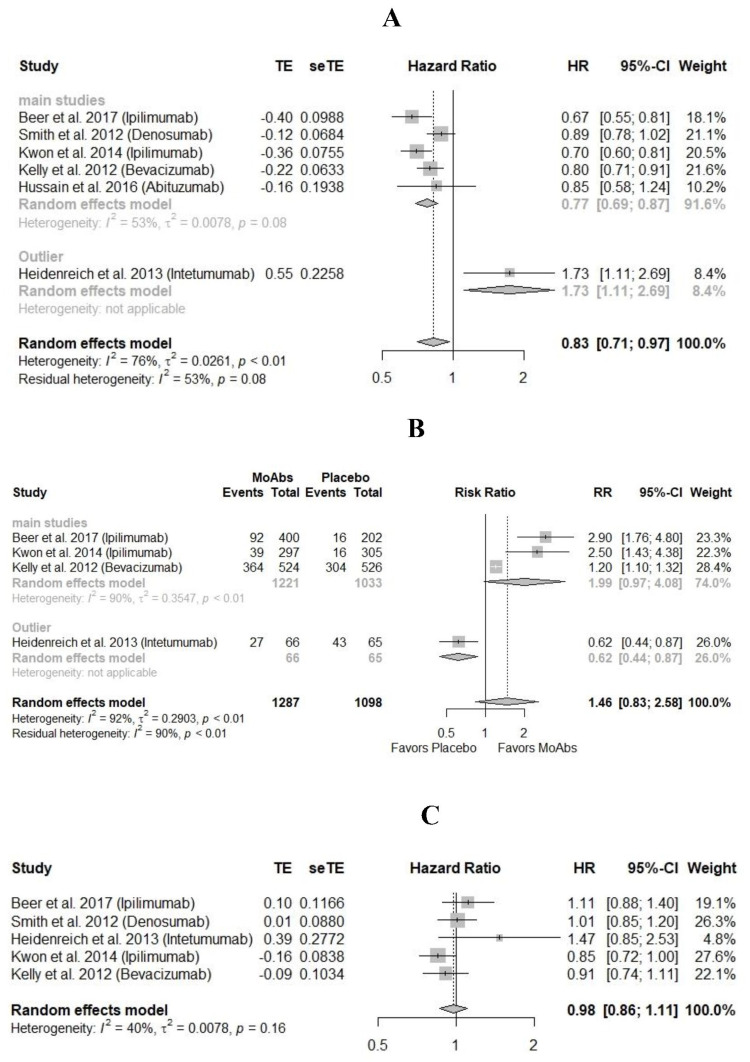
In RCTs with ipilimumab, denosumab, bevacizumab, and abituzumab (N = 4,063), pooled hazard ratio (HR) of PFS was 0.77 (95% CI = 0.69-0.87, I2 = 53) in favor of monoclonal antibodies as compared to placebo. HR of PFS for trial on intetumumab (N = 131) was 1.73 (95% CI = 1.11-2.69) in favor of placebo as compared to monoclonal antibodies (Figure 3A).
In RCTs with ipilimumab and bevacizumab (N = 2,254), the risk ratio (RR) of ≥50% decline in PSA was 1.99 (95% CI = 0.97-4.08, I2 = 53%) in favor of monoclonal antibodies as compared to placebo. While in the RCT with intetumumab, RR of ≥50% decline in PSA was 0.62 (95% CI = 0.44-0.87) in favor of placebo as compared to monoclonal antibodies (Figure 3B).
In RCTs with ipilimumab, denosumab, bevacizumab, and intetumumab (N = 4014), HR of overall survival was similar in monoclonal antibodies groups vs. placebo, i.e., 0.98 (95% CI = 0.86-1.11, I2 = 40%) (Figure 3C).
Figure 3. Comparison of efficacy in monoclonal antibodies vs. placebo.
(A) Hazard ratio of progression-free survival. (B) Risk ratio of ≥50% decline in prostate-specific antigen (PSA). (C) Hazard ratio of overall survival [24-30].
MoAbs = monoclonal antibodies; TE = treatment effect; seTE: standard error of treatment effect.
In RCT with denosumab (N = 1432), HRs of bone metastasis-free survival and first bone metastasis were statistically significant in favor of denosumab. HRs of bone metastasis-free survival and first bone metastasis were 0.85 (95% CI = 0.73-0.98) and 0.84 (95% CI = 0.71-0.98), respectively.
Safety
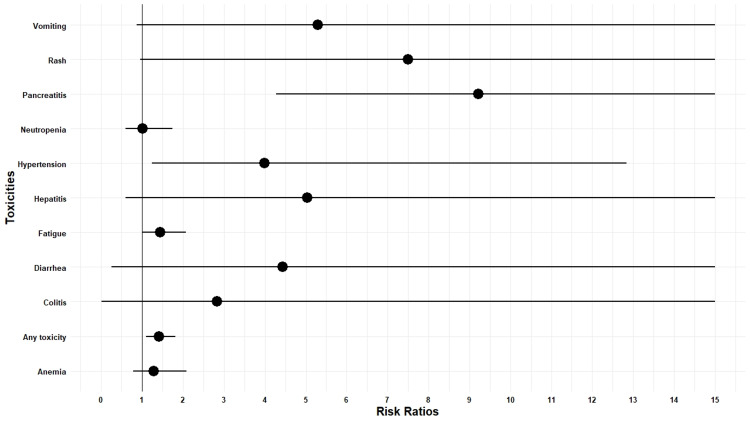
In RCTs, RRs of any ≥ grade 3 toxicity were 1.41 (CI = 1.10-1.82, I2 = 92%) in favor of placebo as compared to monoclonal antibodies. RRs of ≥ grade 3 adverse events, i.e., vomiting, rash, pancreatitis, neutropenia, hypertension, hepatitis, fatigue, diarrhea, colitis, and anemia, were 5.30 (95% CI = 0.87-32.36, I2 = 0), 7.50 (95% CI = 0.94-59.46, I2 = 0), 9.21 (95% CI = 4.27-19.85), 1.01 (95% CI = 0.58-1.74, I2 = 63.5%), 3.98 (95% CI = 1.23-12.84, I2 = 19.2%), 5.02 (95% CI = 0.58-42.95, I2 = 0), 1.44 (95% CI = 1.00-2.07, I2 = 22.8%), 4.42 (95% CI = 0.25-75.69, I2 = 81.1%), 2.82 (95% CI = 0.01-550.00, I2 = 84.6%), and 1.28 (95% CI = 0.79-2.08, I2 = 8.3%), respectively. Denosumab increased the incidence of ≥ grade 3 osteonecrosis of jaw in RCT 33/720 vs. 0/705 (Figure 4).
Figure 4. Plot of the risk ratio of ≥ grade 3 adverse events.
Denosumab vs. zoledronic acid
Fizazi et al. (2011) [26] compared denosumab vs. zoledronic acid for the treatment of CRPC (N = 1,904). HR of the first skeletal-related adverse event was 0.82 (95% CI = 0.71-0.95) in favor of denosumab as compared to zoledronic acid. The incidence of total skeletal-related events was 36% in the denosumab group vs. 41% in the zoledronic acid group. Radiation to bone was used in 19% of the people in the denosumab group vs. 21% in the zoledronic acid group. The incidence of adverse events was 97% each in both groups. Greater than or equal to grade 3 adverse events were 72% and 66% in denosumab and zoledronic acid groups, respectively. Osteonecrosis of the jaw was 1% in the zoledronic acid group vs. 2% in the denosumab group. Discontinuation of treatment due to adverse events was reported in 15% of participants in the zoledronic acid group and 17% in the denosumab group.
Cixutumumab vs. ramucirumab
Hussain et al. (2015) [30] compared cixutumumab vs. ramucirumab (N = 132). The median time to radiographic disease progression was 7.5 months (95% CI = 4.8-10.1) for patients on cixutumumab while it was 10.2 months (95% CI = 7.5-12.6) for patients on ramucirumab. Median OS was 10.8 months (95% CI = 6.5-13.0) for patients on cixutumumab while it was 13.0 months (95% CI = 9.5-16.0) for patients on ramucirumab. Decline >50% in PSA occurred in 18.5% of patients in the cixutumumab group and 21.4% of patients in the ramucirumab group. Among ≥ grade 3 adverse events, fatigue, diarrhea, dehydration, hypertension, neutropenia, and anemia were reported in 16.7% vs. 7.6%, 7.6% vs. 1.5%, 6.1% vs. 1.5%, 1.5% vs. 9.1%, 31.9% vs. 31.8%, and 3% vs. 10.6% of patients, respectively, in cixutumumab vs. ramucirumab groups.
Single-arm comparison of monoclonal antibody regimens
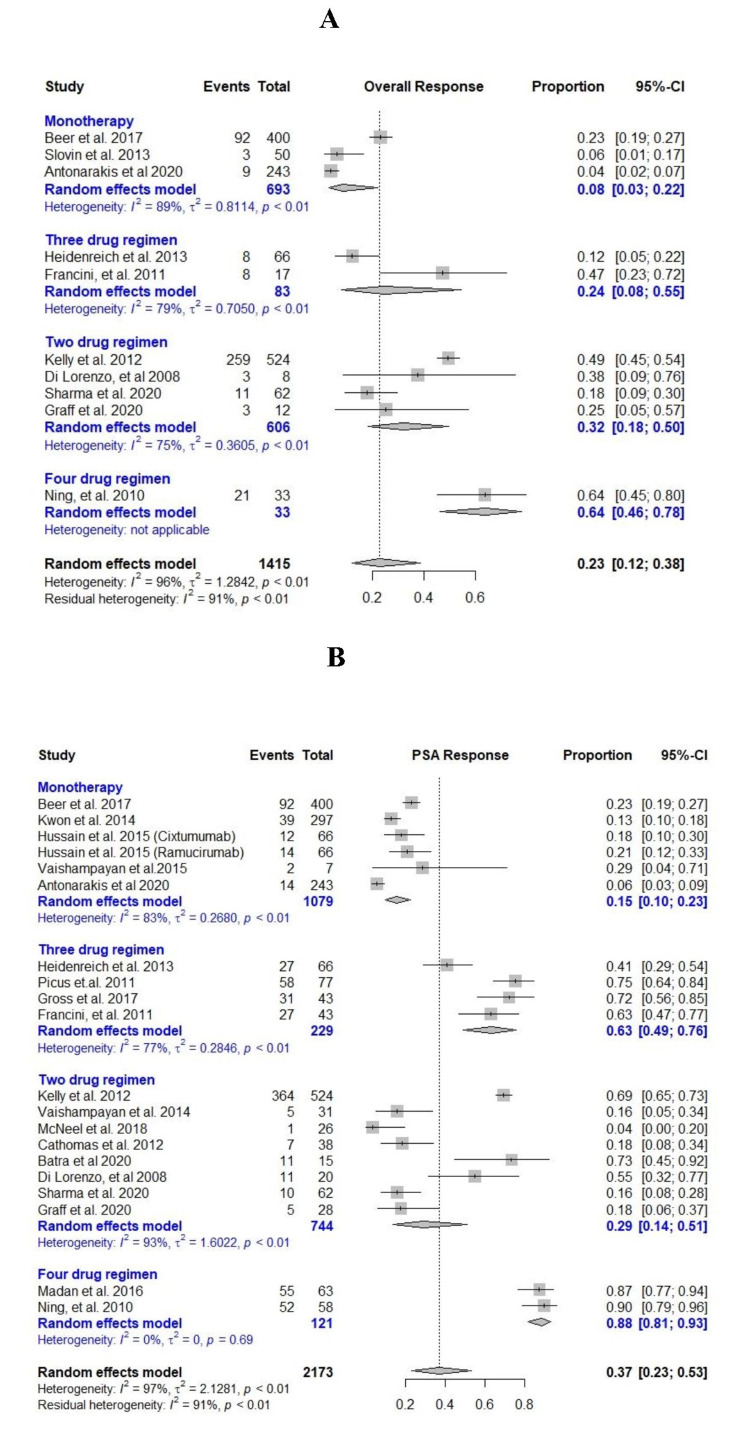
Ipilimumab, cixutumumab, ramucirumab, anti-CD3 x anti-HER2 bispecific antibody, and pembrolizumab were used as monotherapy in clinical trials (N = 1,129) [17,24,28,30,31]. Pooled incidences of OR and >50% decline in PSA were 8% (95% CI = 0.03-0.22, I2 = 89%) and 15% (95% CI = 0.1-0.23, I2 = 83%), respectively. Individual study results and pooled results are given in Figure 5. Median OS and PFS were 7.4-19.6 months and 2.1 months, respectively (Table 2).
Table 2. Survival rates and ≥ grade 3 adverse events in early phase trials.
PFS = progression-free survival; OS = overall survival.
| Author | Median PFS (months) | Median OS (months) | Any ≥ grade 3 | Diarrhea | Hypertension | Anemia | Neutropenia/lymphopenia | Colitis | Hepatitis | Fatigue | Rash | Vomiting |
| Monotherapy | ||||||||||||
| Vaishampayan et al. (2015) [31] | N/A | N/A | 5/7 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Antonarakis et al. (2020) [17] | 2.1 (2.1-2.2) | 9.6 (7.9-12.2) | 27 (10%) | 2 (<1%) | N/A | 2 (<1%) | N/A | 3 (1%) | 1 (<1%) | 3 (1%) | 0 | 0 |
| Slovin et al. (2013) [17] | N/A | 17.4 (11.5-24.7) | N/A | 4(8) | N/A | N/A | N/A | 8 (16) | 4 (8) | 3 (6) | 16 (32%) | 3 (6%) |
| Autio et al. (2020) [39] | N/A | N/A | 3 | N/A | N/A | N/A | N/A | N/A | N/A | 2 | N/A | 0 |
| Two-drug regimens | ||||||||||||
| Vaishampayan et al. (2014) [32] | 7.0 (4.7-8.5) | 11.2 (9.1-16.4) | N/A | 2/30 | 3/30 | 7/30 | 9/30 | N/A | N/A | 1/30 | N/A | N/A |
| McNeel et al. (2018) [34] | N/A | N/A | N/A | 1/26 | N/A | N/A | N/A | N/A | 1/26 | 1/26 | N/A | N/A |
| Cathomas et al. (2012) [15] | 2.8 (2.4-3.2) | 13.3 (7.3-15.4) | N/A | 1 (3%) | N/A | 1 (3%) | 3 (8%) | N/A | N/A | 4 (11%) | 2 (5%) | N/A |
| Batra et al. (2020) [35] | N/A | 18.4 (16.13-NR) | N/A | N/A | N/A | N/A | 11 (73.3%) | N/A | N/A | 1 (6.66%) | N/A | N/A |
| Di Lorenzo et al. (2008) [40] | 4 (2-6) | 9 (4-12.5) | 11/20 (55%) | N/A | N/A | 1/20 (5%) | 4 (20%) | N/A | N/A | N/A | N/A | 2 (10%) |
| Sharma et al. (2020) [16] | 5.5 (3.5-7.1) and 3.8 (2.1-5.1) | 19 (11.5-NE) and 15.2 (8.4-NE) | 43/85 | 8 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Graff et al. (2020) [42] | 3.8 (2.8-9.9) | 22.2 (14.7-28.4) | 19/28 (68%) | N/A | 3 (10.7%) | 1 (3.5%) | N/A | 2 (7.1%) | N/A | 1 (3.5%) | N/A | N/A |
| Barata et al. (2019) [38] | N/A | N/A | 9 (43%) | N/A | N/A | N/A | N/A | N/A | N/A | 5 (24%) | N/A | 1 (5%) |
| Hudes et al. (2013) [41] | N/A | N/A | 33/37 | 0 | N/A | 1 | 27/37 (73%) | N/A | N/A | N/A | N/A | N/A |
| Three-drug regimens | ||||||||||||
| Francini et al. (2011) [43] | N/A | N/A | 16/43 (37.2%) | N/A | N/A | 6 (13.9%) | 8 (18.6%) | N/A | N/A | 2 (4.6%) | N/A | N/A |
| Picus et al. (2011) [33] | 9.2 (7.5-10.9) | 24 (20.3-26.5 | N/A | N/A | 4/77 (5%) | N/A | 53/77 (69%) | N/A | N/A | 19 (24%) | N/A | N/A |
| Gross et al. (2017) [11] | 8.9 (7.4-10.6) | 21.9 (18.4-30.3) | N/A | N/A | 8 (19%) | N/A | 12 (28%) | N/A | N/A | 3 (7%) | N/A | N/A |
| Four-drug regimens | ||||||||||||
| Ning et al. (2010) [44] | 18.3 | 28.2 | N/A | 2/60 (3.33%) | 7/60 (11.6%) | 8/60 (13.3%) | 60/60 (100%) | N/A | N/A | 2/60 (3.33%) | N/A | N/A |
| Madan et al. (2016) [36] | 18.2 | 24.6 | N/A | 6 (10%) | N/A | 20 (32%) | 61 | N/A | N/A | 6 (11%) | N/A | N/A |
Figure 5. Meta-analysis of efficacy in single arms.
(A) Pooled overall response. (B) Pooled >50% prostate-specific antigen (PSA) response [10,17,24-35].
Bevacizumab + docetaxel, bevacizumab + satraplatin, anti-tumor vaccine + pembrolizumab, docetaxel + cetuximab, docetaxel + J591, bevacizumab + docetaxel, nivolumab + ipilimumab, and pembrolizumab + enzalutamide were two drug combination regimens used in clinical trials to treat CRPC (N = 744) [15,16,32,34,40,41]. Pooled incidences of OR and >50% decline in PSA were 32% (95% CI = 0.18-0.50, I2 = 75%) and 29% (95% CI = 0.14-0.51, I2 = 93%), respectively (Figure 5). Median OS and PFS were 9-19 months and 2.8-7 months, respectively (Table 2).
Intetumumab + docetaxel + prednisone, estramustine + docetaxel + bevacizumab, docetaxel + bevacizumab + everolimus, and docetaxel + bevacizumab + prednisone were the three-drug regimens used in clinical trials (N = 229) [10,27,33,40,43]. Pooled incidences of OR and >50% decline in PSA were 24% (95% CI = 0.08-0.55, I2 = 79%) and 63% (95% CI = 0.49-0.76, I2 = 77%), respectively (Figure 5). Median OS and PFS were 21.9-24 months and 9.2-8.9 months, respectively (Table 2).
Lenalidomide + bevacizumab + docetaxel + prednisone and docetaxel + bevacizumab + thalidomide + prednisone were the four-drug regimens used in clinical trials (N = 121) [36,44]. Pooled incidences of OR and >50% decline in PSA were 64% (95% CI = 0.46-0.78, I2 = 0%) and 88% (95% CI = 0.81-0.93, I2 = 0%), respectively (Figure 5). Median OS and PFS were 24.6-28.2 months and 18.2-18.3 months, respectively (Table 2).
Monoclonal antibodies with unfavorable results
Cixutumumab, figitumumab, carlumab, trastuzumab, LFA102, rilotumumab, and siltuximab did not show antitumor activity in early phase trials (Table 3) [12,14,45-50].
Table 3. Early phase trials on monoclonal antibodies with no anti-tumor activity.
MoAb = monoclonal antibody; IGF-1R = insulin-like growth factor-1 receptor; CRPC = castration-resistant prostate cancer; PSA = prostate-specific antigen; CTLA-4 = cytotoxic T-lymphocyte-associated antigen-4; PD-1 = programmed cell death protein 1; MCP-1 = monocyte chemotactic protein-1; IL-6 = interleukin 6; HER2 = human epidermal growth factor receptor 2.
| Author | Trial phase | Drug combination | Target of MoAB | Problem | Outcomes |
| McHugh et al. (2020) [45] | Phase I | Cixutumumab + temsirolimus | IGF-1R | Metastatic CRPC | The combination therapy had limited anti-tumor activity and a greater than expected toxicity |
| De Bono et al. (2014) [46] | Phase II | Figitumumab + docetaxel | IGF-1R | Metastatic CRPC | No significant PSA response. The combination not recommended by authors in Bono et al. |
| Boudadi et al. (2018) [48] | Phase II | Ipilimumab + nivolumab | CTLA-4, PD-1 | Metastatic CRPC | Anti-tumor activity was only seen in patients with AR-V7 isoform of the androgen receptor. Tumor activity was not seen in other patients |
| Pienta et al. (2013) [47] | Phase II | Carlumab | MCP-1 | Metastatic CRPC | Well tolerated but did not show anti-tumor activity as a single agent |
| Fizazi et al. (2012) [12] | Phase II | Siltuximab + mitoxantrone/prednisone | IL-6 | Metastatic CRPC | The drug combination was well tolerated, improvement in outcomes was not demonstrated |
| Ziada et al. (2004) (NCT00003740) [14] | Phase II | Trastuzumab | HER2 | CRPC | Well tolerated with no anti-tumor activity |
| Minami et al. (2020) (NCT01610050) [49] | Phase I | LFA102 | Anti-prolactin receptor | Metastatic CRPC | Well tolerated with no anti-tumor activity |
| Ryan et al. (2013) (NCT00770848) [50] | Phase I/II | AMG 102 (rilotumumab) | Hepatocyte growth factor | Resistant CRPC | Well tolerated with no anti-tumor activity |
Ongoing clinical trials and interim results of ongoing trials
Interim results of ongoing clinical trials on pembrolizumab, avelumab, atezolizumab, pasotuxizumab, and tremelimumab have shown promising results alone or in combination with chemotherapy [51-58]. Combinations are given in Table 4.
Table 4. Ongoing clinical trials and interim results of ongoing trials presented in conferences.
NCT = National Clinical Trial; DPP4 = dipeptidyl peptidase 4; HER2 = human epidermal growth factor receptor 2; PD-1 = programmed cell death protein 1; PD-L1 = programmed death-ligand 1; CTLA-4 = cytotoxic T-lymphocyte-associated antigen-4; IL-23 = interleukin 23; CRPC = castration-resistant prostate cancer; PSA = prostate-specific antigen; PSMA = prostate-specific membrane antigen.
| NCT/authors | No. of patients | Regimen | Target of antibody | Phase | Population | Outcome | Year of completion |
| Interim results of ongoing clinical trials | |||||||
| Gurney et al. (2019) (NCT02861573) [51] | 41 | Pembrolizumab + olaparib | PD-1 | Ib/II | Metastatic CRPC | PSA response 12%, well-tolerated | 2025 |
| Gurney et al. (2019) [51] | 72 | Pembrolizumab + docetaxel + prednisone | PD-1 | Ib/II | Metastatic CRPC | PSA response 31%, well-tolerated | 2025 |
| Gurney et al. (2019) [51] | 69 | Pembrolizumab + enzalutamide | PD-1 | Ib/II | Metastatic CRPC | PSA response 27%, well-tolerated | 2025 |
| Bryce et al. (2020) (NCT03409458) [52] | 14 | Avelumab + PT-112 | PD-L1 | I/II | Metastatic CRPC | Well tolerated with evidence of efficacy, PSA response 21% | 2021 |
| Aggarwal et al. (2020) (NCT03910660) [53] | 6 | BXCL701 (DPP4 inhibitor) + pembrolizumab | PD-1 | Ib | Metastatic CRPC | Well tolerated | 2022 |
| Patel et al. (2020) (NCT03406858) [54] | 33 | Pembrolizumab + HER2 bi-armed activated T cells | PD-1 | II | Metastatic CRPC | PSA response 2/6 patients, well-tolerated | 2021 |
| Dorff et al. (2020) (NCT03024216) [55] | 37 | Atezolizumab + sipuleucel-T | PD-L1 | I | Metastatic CRPC | Well tolerated with clinical activity | 2025 |
| Agarwal et al. (2020) (NCT03170960) [56] | 44 | Cabozantinib + atezolizumab | PD-L1 | Ib | Metastatic CRPC | Well tolerated with clinical activity | 2021 |
| Hummel et al. (2021) (NCT01723475) [57] | 47 | Pasotuxizumab, PSMA bispecific T-cell engager monotherapy | PSMA | I | Metastatic CRPC | Well tolerated with clinical activity | 2018 |
| Hotte et al. (2019) (NCT02788773) [58] | 52 | Durvalumab with or without tremelimumab | CTLA-4 + PD-L1 | II | Metastatic CRPC | No activity with durvalumab only, clinical activity reported with combination therapy | 2020 |
| Ongoing clinical trials | |||||||
| NCT03815942 | 23 | Nivolumab + ChAdOx1-MVA 5T4 vaccine | Anti-PD-1 | I/II | CRPC | Efficacy and safety (active, not recruiting) | 2021 |
| NCT04458311 | 55 | Tildrakizumab + abiraterone acetate | Anti-IL-23 | I/II | Metastatic CRPC | Efficacy and safety (recruiting) | 2024 |
| NCT03204812 | 27 | Durvalumab plus tremelimumab | Anti-PD-L1 and anti-CTLA-4 | II | Metastatic CRPC | Efficacy and safety (active, not recruiting) | 2021 |
| NCT04336943 | 30 | Durvalumab + olaparib | Anti-PD-L1 | II | Biochemically recurrent prostate cancer | Efficacy and safety (recruiting) | 2024 |
| NCT03910660 | 40 | Talabostat mesylate + pembrolizumab | Anti-PD-1 | I/II | Metastatic CRPC | Efficacy and safety (active, not recruiting) | 2022 |
| NCT04071236 | 24 | Radium Ra 223 + peposertib + avelumab | Anti-PD-L1 | I/II | Advanced metastatic CRPC | Efficacy and safety (recruiting) | 2023 |
| NCT04104893 | 30 | Pembrolizumab | Anti-PD-1 | II | Metastatic CRPC characterized by a mismatch repair deficiency or biallelic CDK12 inactivation | Efficacy and safety (recruiting) | 2023 |
| NCT02703623 | 198 | Abiraterone acetate, apalutamide, prednisone +/-ipilimumab | Anti-CTLA-4 | II | Metastatic CRPC | Efficacy and safety (active, not recruiting) | 2022 |
| NCT04159896 | 49 | ESK981 + nivolumab | Anti-PD-1 | II | Metastatic CRPC | Efficacy and safety (recruiting) | 2022 |
| NCT03367819 | 134 | Isatuximab + cemiplimab | Anti-CD-38 and Anti-PD-1 | I/II | Metastatic CRPC | Efficacy and safety (active, not recruiting) | 2021 |
| NCT03805594 | 43 | Lutetium Lu 177-PSMA-617 + pembrolizumab | Anti-PSMA + anti-PD-1 | I | Metastatic CRPC | Efficacy and safety (recruiting) | 2022 |
| NCT02499835 | 66 | Vaccine therapy + pembrolizumab | Anti-PD-1 | I/II | Metastatic CRPC | Efficacy and safety (active, not recruiting) | 2021 |
| NCT04471974 | 54 | Pembrolizumab + ZEN-3694 + enzalutamide | Anti-PD-1 | II | Metastatic CRPC | Efficacy and safety (recruiting) | 2025 |
| NCT04592237 | 120 | Cetrelimab + cabazitaxel + carboplatin + niraparib | Anti-PD-1 | II | Aggressive prostate cancer | Efficacy and safety (recruiting) | 2025 |
| NCT02312557 | 58 | Pembrolizumab + enzalutamide | Anti-PD-1 | II | Metastatic CRPC | Efficacy and safety (active, not recruiting) | 2022 |
| NCT03217747 | 184 | PF-04518600 + avelumab + utomilumab | Anti-OX40, anti-PDL1, and anti-CD137 | I/II | Patients with advanced malignancies | Efficacy and safety (recruiting) | 2023 |
| NCT02601014 | 15 | Ipilimumab + nivolumab | Anti-CTLA-4 and anti-PD-1 | II | AR-V7-expressing metastatic CRPC | Efficacy and safety (active, not recruiting) | 2022 |
| NCT04068896 | 90 | NGM120 | GFRAL antagonist blocking GDF15 | I | Metastatic CRPC | Efficacy and safety (recruiting) | 2021 |
| NCT03849469 | 242 | Pembrolizumab + XmAb22841 | Anti-PD-1 + anti-CTLA-4 | I | Selected advanced solid tumors (DUET-4) | Efficacy and safety (recruiting) | 2027 |
| NCT03517488 | 154 | XmAb20717 | Anti-PD-1/anti-CTLA-4 | I | Advanced solid tumors | Efficacy and safety (recruiting) | 2021 |
| NCT03454451 | 378 | CPI-006 + pembrolizumab | Anti CD73 + anti-PD-1 | I | Metastatic CRPC | Efficacy and safety (recruiting) | 2023 |
| NCT03330405 | 216 | Avelumab + talazoparib | Anti-PD-L1 | II | CRPC | Efficacy and safety (active, not recruiting) | 2021 |
| NCT04423029 | 260 | Nivolumab + DF6002 | Anti-PD-1 | I/II | Metastatic solid tumors | Efficacy and safety (recruiting) | 2024 |
| NCT03207867 | 376 | PDR001 + NIR178 | Anti-PD-1 | II | CRPC, solid tumors, and lymphoma | Efficacy and safety (recruiting) | 2021 |
| NCT03983954 | 45 | Naptumomab + durvalumab | Anti-5T4 and anti-PD-L1 | I | Solid tumor that is metastatic/advanced | Efficacy and safety (recruiting) | 2022 |
| NCT03970382 | 148 | Nivolumab | Anti-PD-1 | I | Locally advanced or metastatic solid tumors | Efficacy and safety (recruiting) | 2024 |
Discussion
Docetaxel is the most used chemotherapy-based treatment for metastatic CRPC as docetaxel improved OS, PFS, and PSA levels in RCT [59]. Among non-chemotherapy drugs, alpharadin, abiraterone, radium-223 dichloride, etc., showed improvement in survival rates with anti-tumor activity [60]. Among immunotherapies, sipuleucel-T extended OS without improving PFS [61]. However, these therapies are not curative, responses are rarely durable, and are poorly tolerated by some patients. Additional treatment options are needed for better outcomes. In RCTs, majorly monoclonal antibodies were used in combination with docetaxel or in patients refractory to docetaxel therapy. According to the pooled results, monoclonal antibodies improved PFS and PSA response as compared to placebo.
Checkpoint inhibitors, including PD-1, programmed death-ligand 1 (PD-L1), and CTLA-4 inhibitors, have shown efficacy in urothelial and other solid tumors [62-65]. However, the microenvironment of prostate cancer is more immunosuppressive as compared to other tumors [66,67]. Ipilimumab (CTLA-4 inhibitor) improved PFS and PSA levels in both trials, including docetaxel pre-treated and treatment naïve patients. It was well tolerated in both trials. OS was not prolonged on normal follow-up. However, long-term follow-up of five years showed better OS in the ipilimumab group as compared to placebo [68]. More trials are now conducted on combination therapy of ipilimumab. In the trial conducted by Boudadi et al. (2018) [48], 1 mg/kg of ipilimumab was used with nivolumab and anti-tumor activity was only reported in a small group of patients. However, according to the preliminary results of a trial by Sharma et al. (2020), 3 mg of ipilimumab with nivolumab showed anti-tumor activity in all subsets of patients and a large-scale phase II trial is in progress on ipilimumab + nivolumab in metastatic CRPC patients [16]. Another RCT is in progress to assess the efficacy and safety of ipilimumab in combination with abiraterone acetate, apalutamide, and prednisone. Ongoing clinical trials are also testing nivolumab in combination with ChAdOx1-MVA 5T4 vaccine, ESK981 (Pan-VEGFR/Tie2 tyrosine kinase inhibitor), and DF6002 (binds interleukin 12 (IL-12) receptor).
In a multicohort phase II trial by Antonarakis et al. (2020), pembrolizumab showed anti-tumor activity in docetaxel pretreated patients and the observed survival estimates are promising [17]. Although 5% of the patients showed OR, the response was durable. Pembrolizumab monotherapy was well tolerated, and no unexpected toxicities were reported. A combination of pembrolizumab with olaparib, enzalutamide, and docetaxel is tested in KEYNOTE-365, and the early results have shown anti-tumor activity of these combinations and are well tolerated [51]. According to the results of a phase II trial by Graff et al. (2020), pembrolizumab addition to enzalutamide showed anti-tumor activity in patients refractory to enzalutamide alone, and the response was durable [42]. Another trial was conducted on the addition of pembrolizumab to the anti-tumor DNA vaccine. The addition of pembrolizumab showed better results in terms of PSA decline, OR, and CD-8+ T cell infiltration into tumor lesions as compared to vaccination alone. More trials are in progress to assess the efficacy and safety of pembrolizumab in combination with dipeptidyl peptidase 4 (DPP4) inhibitor BXCL701, HER2 bi-armed activated T cells, talabostat mesylate, lutetium lu 177-PSMA-617, vaccine therapy, ZEN-3694 + enzalutamide, enzalutamide, XmAb22841, and CPI-006 (Table 4). Avelumab, atezolizumab, tremelimumab, cemiplimab, cetrelimab, XmAb20717, PDR001, and durvalumab are other checkpoint inhibitors that are getting tested alone and in combination therapy for the treatment of CRPC.
The anti-angiogenic drug, bevacizumab, also improved PFS and PSA levels without any improvement in OS. Bevacizumab was also tested in combination regimens. Among the combination regimens, the four-drug regimen of bevacizumab with docetaxel + thalidomide + prednisone and lenalidomide + docetaxel + prednisone showed the best efficacy outcomes, and toxicities were manageable (Figure 5 and Table 2). Early anti-tumor activity was reported with the addition of thalidomide and bevacizumab to docetaxel as compared to docetaxel alone. Bevacizumab in combination with satraplatin has shown promising results in early phase trials in docetaxel refractory patients. The addition of everolimus (mammalian target of rapamycin (mTOR) inhibitor) to docetaxel + bevacizumab did not show better outcomes as compared to docetaxel + bevacizumab in the early-phase trial.
Abituzumab improved the progression of the disease, but the results were not statistically significant. In our analysis, the trial with intetumumab was the outlier and intetumumab caused worsening in PFS or PSA levels. However, intetumumab did not increase adverse events as compared to placebo. Intetumumab might have interacted with docetaxel, resulting in lower efficacy.
Lack of improvement in OS despite changes in PFS and PSA levels might be due to the unique response of CRPC to these drugs. Also, the patients with metastatic CRPC are generally older than patients with other types of cancer, e.g., breast cancer and lung cancer, and comparatively more patients have bone metastasis [69-71]. Other possible explanations can be the unique mechanism of action of these drugs or flaws in trial designs. These drugs might show some improvement in OS if followed for longer durations. Further studies should be conducted on how to utilize the anti-tumor activity of these monoclonal antibodies.
Denosumab targets receptor activator of nuclear factor kappa-B ligand (RANKL) and is an anti-bone resorptive agent. It delayed skeletal-related adverse events as compared to zoledronic acid in patients with CRPC with bone metastasis in RCT. Zoledronic acid was proved better than a placebo in an RCT [72]. However, increased incidence of osteonecrosis of the jaw was associated with denosumab as compared to zoledronic acid. A meta-analysis showed similar results for denosumab in the prevention of skeletal-related adverse events as compared to zoledronic acid [73]. Moreover, an RCT by Smith et al. (2012) tested denosumab for the prevention of bone metastasis [25]. Denosumab significantly improved bone metastasis-free survival and time to first bone metastasis as compared to placebo. The major adverse event observed in the denosumab group was the osteonecrosis of the jawbone.
In a non-comparative randomized study, cixutumumab (IGF-1R inhibitor) and ramucirumab (VEGFR inhibitor) were used with mitoxantrone-prednisone. PFS in the cixutumumab group was similar to the projected value, while ramucirumab showed better PFS as compared to the projected value (6.7 months vs. 3.9 months). The incidence of adverse events was similar to expectations. Ramucirumab has shown improvement in OS in RCTs on other solid tumors [74]. Another trial by McHugh et al. (2020) has also shown no activity of cixutumumab with temsirolimus [45].
Among monoclonal antibodies, PD-1 inhibitors, PD-L1 inhibitors, and CTLA-4 inhibitors have the potential to become the drugs of the future for patients with prostate cancer. More multicenter randomized clinical trials should focus on finding the efficacy and appropriate combination of these medications. However, the role of monoclonal antibodies in prostate cancer is still debated.
Conclusions
Monoclonal antibodies were well tolerated and showed better outcomes in terms of PFS and >50% decline in PSA levels compared to placebo. However, OS was only improved with ipilimumab as compared to placebo on long-term follow-up of five years. Denosumab delayed skeletal-related adverse events as compared to zoledronic acid in CRPC with bone metastasis. Denosumab also delayed bone metastasis as compared to placebo in patients with metastatic CRPC. Pembrolizumab, avelumab, atezolizumab, pasotuxizumab, and tremelimumab have shown promising results in the early phase trials. More multicenter, double-blind clinical trials may be needed to confirm these results.
Appendices
Table 5. Keywords and search strings.
| P | I | C | O | S |
| "Prostatic Neoplasms, Castration-Resistant"[Mesh] | "Antibodies, Monoclonal"[Mesh] | |||
| Prostatic Neoplasms, Castration-Resistant Castration-Resistant Prostatic Neoplasm Prostatic Neoplasms, Castration-Resistant Androgen-Insensitive Prostatic Neoplasms Androgen Insensitive Prostatic Neoplasms Androgen-Resistant Prostatic Neoplasms Androgen Resistant Prostatic Neoplasms Prostatic Neoplasms, Hormone Refractory Hormone Refractory Prostatic Neoplasms Prostatic Neoplasms, Androgen-Independent Neoplasm, Androgen-Independent Prostatic Prostatic Neoplasm, Androgen-Independent Prostatic Neoplasms, Androgen Independent Prostatic Neoplasms, Androgen-Insensitive Androgen-Insensitive Prostatic Neoplasm Prostatic Neoplasms, Androgen Insensitive Prostatic Neoplasms, Androgen-Resistant Androgen-Resistant Prostatic Neoplasm Prostatic Neoplasm, Androgen-Resistant Prostatic Neoplasms, Androgen Resistant Androgen-Independent Prostatic Neoplasms Androgen Independent Prostatic Neoplasms Castration-Resistant Prostatic Neoplasms Castration-Resistant Prostatic Neoplasms Cancers, Castration-Resistant Prostatic Androgen-Insensitive Prostatic Cancer Androgen Insensitive Prostatic Cancer Androgen-Resistant Prostatic Cancer Androgen Resistant Prostatic Cancer Prostatic Cancer, Hormone Refractory Prostatic Cancer, Androgen-Independent Androgen-Independent Prostatic Cancers Prostatic Cancer, Androgen Independent Prostatic Cancers, Androgen-Independent Prostatic Cancer, Androgen-Insensitive Androgen-Insensitive Prostatic Cancers Cancer, Androgen-Insensitive Prostatic Cancers, Androgen-Insensitive Prostatic Prostatic Cancer, Androgen Insensitive Prostatic Cancers, Androgen-Insensitive Prostatic Cancer, Androgen-Resistant Androgen-Resistant Prostatic Cancers Cancer, Androgen-Resistant Prostatic Cancers, Androgen-Resistant Prostatic Prostatic Cancer, Androgen Resistant | Monoclonal Antibodies, Monoclonal Antibody, Antibody, Monoclonal | |||
| PubMed search string: (((((((((((((((((((((((((((((((((((((((((((((("Prostatic Neoplasms, Castration-Resistant"[Mesh]) OR (Prostatic Neoplasms, Castration-Resistant)) OR (Castration-Resistant Prostatic Neoplasm)) OR (Prostatic Neoplasms, Castration Resistant)) OR (Androgen-Insensitive Prostatic Neoplasms)) OR (Androgen Insensitive Prostatic Neoplasms)) OR (Androgen-Resistant Prostatic Neoplasms)) OR (Androgen Resistant Prostatic Neoplasms)) OR (Prostatic Neoplasms, Hormone Refractory)) OR (Hormone Refractory Prostatic Neoplasms)) OR (Prostatic Neoplasms, Androgen-Independent)) OR (Neoplasm, Androgen-Independent Prostatic)) OR (Prostatic Neoplasm, Androgen-Independent)) OR (Prostatic Neoplasms, Androgen Independent)) OR (Prostatic Neoplasms, Androgen-Insensitive)) OR (Androgen-Insensitive Prostatic Neoplasm)) OR (Prostatic Neoplasms, Androgen Insensitive)) OR (Prostatic Neoplasms, Androgen-Resistant)) OR (Androgen-Resistant Prostatic Neoplasm)) OR (Prostatic Neoplasm, Androgen-Resistant)) OR (Prostatic Neoplasms, Androgen Resistant)) OR (Androgen-Independent Prostatic Neoplasms)) OR (Androgen Independent Prostatic Neoplasms)) OR (Castration-Resistant Prostatic Neoplasms)) OR (Castration Resistant Prostatic Neoplasms)) OR (Cancers, Castration-Resistant Prostatic)) OR (Androgen-Insensitive Prostatic Cancer)) OR (Androgen Insensitive Prostatic Cancer)) OR (Androgen-Resistant Prostatic Cancer)) OR (Androgen Resistant Prostatic Cancer)) OR (Prostatic Cancer, Hormone Refractory)) OR (Prostatic Cancer, Androgen-Independent)) OR (Androgen-Independent Prostatic Cancers)) OR (Prostatic Cancer, Androgen Independent)) OR (Prostatic Cancers, Androgen-Independent)) OR (Prostatic Cancer, Androgen-Insensitive)) OR (Androgen-Insensitive Prostatic Cancers)) OR (Cancer, Androgen-Insensitive Prostatic)) OR (Cancers, Androgen-Insensitive Prostatic)) OR (Prostatic Cancer, Androgen Insensitive)) OR (Prostatic Cancers, Androgen-Insensitive)) OR (Prostatic Cancer, Androgen-Resistant)) OR (Androgen-Resistant Prostatic Cancers)) OR (Cancer, Androgen-Resistant Prostatic)) OR (Cancers, Androgen-Resistant Prostatic)) OR (Prostatic Cancer, Androgen Resistant)) AND ((((("Antibodies, Monoclonal"[Mesh]) OR (Monoclonal Antibodies)) OR (Monoclonal Antibody)) OR (Antibody, Monoclonal)) AND (((((((((((("Prostatic Neoplasms"[Mesh]) OR (Prostatic Neoplasms)) OR (Neoplasms, Prostate)) OR (Prostate Neoplasm)) OR (Neoplasms, Prostatic)) OR (Prostatic Neoplasm)) OR (Prostate Cancer)) OR (Prostate Cancers)) OR (Cancer of the Prostate)) OR (Prostatic Cancer)) OR (Prostatic Cancers)) OR (Cancer of Prostate))) = 424 | ||||
| Embase search string: ('castration resistant prostate cancer'/exp OR 'crpc (castration resistant prostate cancer)' OR 'castrate resistant prostate cancer' OR 'castration resistant prostate cancer' OR 'castration-resistant pc' OR 'castration-resistant pca' OR 'castration-resistant prostatic neoplasms' OR 'hormone refractory prostate cancer' OR 'prostatic neoplasms, castration-resistant') AND ('monoclonal antibody'/exp OR 'antibodies, monoclonal' OR 'antibodies, monoclonal, humanized' OR 'antibodies, monoclonal, murine derived' OR 'antibodies, monoclonal, murine-derived' OR 'antibody, monoclonal' OR 'clonal antibody' OR 'hybridoma antibody' OR 'monoclonal antibodies' OR 'monoclonal antibody') = 2,427 | ||||
| Web of Science: with keywords mentioned above = 49 | ||||
| Cochrane: with keywords mentioned above = 60 | ||||
| ClinicalTrials.gov: prostate cancer + monoclonal antibodies = 109 | ||||
| Total = 2,960 | ||||
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Cancer statistics, 2020. Siegel RL, Miller KD, Jemal A. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Cancer statistics, 2019. Siegel RL, Miller KD, Jemal A. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 3.Epidemiology of prostate cancer. Rawla P. World J Oncol. 2019;10:63–89. doi: 10.14740/wjon1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Epidemiology, staging and management of prostate cancer. Barsouk A, Padala SA, Vakiti A, et al. Med Sci (Basel) 2020;8:28. doi: 10.3390/medsci8030028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Mottet N, Bellmunt J, Bolla M, et al. Eur Urol. 2017;71:618–629. doi: 10.1016/j.eururo.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 6.EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Heidenreich A, Bastian PJ, Bellmunt J, et al. Eur Urol. 2014;65:467–479. doi: 10.1016/j.eururo.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Castration-resistant prostate cancer: AUA guideline. Cookson MS, Roth BJ, Dahm P, et al. J Urol. 2013;190:429–438. doi: 10.1016/j.juro.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Characterising the castration-resistant prostate cancer population: a systematic review. Kirby M, Hirst C, Crawford ED. Int J Clin Pract. 2011;65:1180–1192. doi: 10.1111/j.1742-1241.2011.02799.x. [DOI] [PubMed] [Google Scholar]
- 9.SEOM clinical guidelines for the treatment of metastatic prostate cancer (2017) Cassinello J, Arranz JÁ, Piulats JM, et al. Clin Transl Oncol. 2018;20:57–68. doi: 10.1007/s12094-017-1783-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bevacizumab. Kazazi-Hyseni F, Beijnen JH, Schellens JH. Oncologist. 2010;15:819–825. doi: 10.1634/theoncologist.2009-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Safety and efficacy of docetaxel, bevacizumab, and everolimus for castration-resistant prostate cancer (CRPC) Gross ME, Dorff TB, Quinn DI, Diaz PM, Castellanos OO, Agus DB. Clin Genitourin Cancer. 2017;16:0–21. doi: 10.1016/j.clgc.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Randomised phase II study of siltuximab (CNTO 328), an anti-IL-6 monoclonal antibody, in combination with mitoxantrone/prednisone versus mitoxantrone/prednisone alone in metastatic castration-resistant prostate cancer. Fizazi K, De Bono JS, Flechon A, et al. Eur J Cancer. 2012;48:85–93. doi: 10.1016/j.ejca.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 13.Differential effect on bone lesions of targeting integrins: randomized phase II trial of abituzumab in patients with metastatic castration-resistant prostate cancer. Hussain M, Le Moulec S, Gimmi C, Bruns R, Straub J, Miller K. Clin Cancer Res. 2016;22:3192–3200. doi: 10.1158/1078-0432.CCR-15-2512. [DOI] [PubMed] [Google Scholar]
- 14.The use of trastuzumab in the treatment of hormone refractory prostate cancer; phase II trial. Ziada A, Barqawi A, Glode LM, et al. Prostate. 2004;60:332–337. doi: 10.1002/pros.20065. [DOI] [PubMed] [Google Scholar]
- 15.Efficacy of cetuximab in metastatic castration-resistant prostate cancer might depend on EGFR and PTEN expression: results from a phase II trial (SAKK 08/07) Cathomas R, Rothermundt C, Klingbiel D, et al. Clin Cancer Res. 2012;18:6049–6057. doi: 10.1158/1078-0432.CCR-12-2219. [DOI] [PubMed] [Google Scholar]
- 16.Nivolumab plus ipilimumab for metastatic castration-resistant prostate cancer: preliminary analysis of patients in the CheckMate 650 trial. Sharma P, Pachynski RK, Narayan V, et al. Cancer Cell. 2020;38:489–499. doi: 10.1016/j.ccell.2020.08.007. [DOI] [PubMed] [Google Scholar]
- 17.Pembrolizumab for treatment-refractory metastatic castration-resistant prostate cancer: multicohort, open-label phase II KEYNOTE-199 study. Antonarakis ES, Piulats JM, Gross-Goupil M, et al. J Clin Oncol. 2020;38:395–405. doi: 10.1200/JCO.19.01638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denosumab treatment in the management of patients with advanced prostate cancer: clinical evidence and experience. Hegemann M, Bedke J, Stenzl A, Todenhöfer T. Ther Adv Urol. 2017;9:81–88. doi: 10.1177/1756287216686018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Hoboken, NJ: Wiley; 2019. Cochrane Handbook for Systematic Reviews of Interventions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. Liberati A, Altman DG, Tetzlaff J, et al. BMJ. 2009;339:0. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Utilization of the PICO framework to improve searching PubMed for clinical questions. Schardt C, Adams MB, Owens T, Keitz S, Fontelo P. BMC Med Inform Decis Mak. 2007;7:16. doi: 10.1186/1472-6947-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.RoB 2: a revised tool for assessing risk of bias in randomised trials. Sterne JA, Savović J, Page MJ, et al. BMJ. 2019;366:0. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 23.How to perform a meta-analysis with R: a practical tutorial. Balduzzi S, Rücker G, Schwarzer G. Evid Based Ment Health. 2019;22:153–160. doi: 10.1136/ebmental-2019-300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Randomized, double-blind, phase III trial of ipilimumab versus placebo in asymptomatic or minimally symptomatic patients with metastatic chemotherapy-naive castration-resistant prostate cancer. Beer TM, Kwon ED, Drake CG, et al. J Clin Oncol. 2017;35:40–47. doi: 10.1200/JCO.2016.69.1584. [DOI] [PubMed] [Google Scholar]
- 25.Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Smith MR, Saad F, Coleman R, et al. Lancet. 2012;379:39–46. doi: 10.1016/S0140-6736(11)61226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Fizazi K, Carducci M, Smith M, et al. Lancet. 2011;377:813–822. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.A randomized, double-blind, multicenter, phase 2 study of a human monoclonal antibody to human αν integrins (intetumumab) in combination with docetaxel and prednisone for the first-line treatment of patients with metastatic castration-resistant prostate cancer. Heidenreich A, Rawal SK, Szkarlat K, et al. Ann Oncol. 2013;24:329–336. doi: 10.1093/annonc/mds505. [DOI] [PubMed] [Google Scholar]
- 28.Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Kwon ED, Drake CG, Scher HI, et al. Lancet Oncol. 2014;15:700–712. doi: 10.1016/S1470-2045(14)70189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Randomized, double-blind, placebo-controlled phase III trial comparing docetaxel and prednisone with or without bevacizumab in men with metastatic castration-resistant prostate cancer: CALGB 90401. Kelly WK, Halabi S, Carducci M, et al. J Clin Oncol. 2012;30:1534–1540. doi: 10.1200/JCO.2011.39.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.A randomised non-comparative phase II trial of cixutumumab (IMC-A12) or ramucirumab (IMC-1121B) plus mitoxantrone and prednisone in men with metastatic docetaxel-pretreated castration-resistant prostate cancer. Hussain M, Rathkopf D, Liu G, et al. Eur J Cancer. 2015;51:1714–1724. doi: 10.1016/j.ejca.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phase I study of anti-CD3 x anti-Her2 bispecific antibody in metastatic castrate resistant prostate cancer patients. Vaishampayan U, Thakur A, Rathore R, Kouttab N, Lum LG. Prostate Cancer. 2015;2015:285193. doi: 10.1155/2015/285193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phase II trial of bevacizumab and satraplatin in docetaxel-pretreated metastatic castrate-resistant prostate cancer. Vaishampayan UN, Fontana J, Heilbrun LK, Smith D, Heath E, Dickow B, Figg WD. Urol Oncol. 2014;32:31–33. doi: 10.1016/j.urolonc.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.A phase 2 study of estramustine, docetaxel, and bevacizumab in men with castrate-resistant prostate cancer: results from Cancer and Leukemia Group B Study 90006. Picus J, Halabi S, Kelly WK, et al. Cancer. 2011;117:526–533. doi: 10.1002/cncr.25421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Concurrent, but not sequential, PD-1 blockade with a DNA vaccine elicits anti-tumor responses in patients with metastatic, castration-resistant prostate cancer. McNeel DG, Eickhoff JC, Wargowski E, Zahm C, Staab MJ, Straus J, Liu G. Oncotarget. 2018;9:25586–25596. doi: 10.18632/oncotarget.25387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phase I trial of docetaxel plus lutetium-177-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 (177Lu-J591) for metastatic castration-resistant prostate cancer. Batra JS, Niaz MJ, Whang YE, et al. Urol Oncol. 2020;38:848–816. doi: 10.1016/j.urolonc.2020.05.028. [DOI] [PubMed] [Google Scholar]
- 36.Phase II trial of docetaxel, bevacizumab, lenalidomide and prednisone in patients with metastatic castration-resistant prostate cancer. Madan RA, Karzai FH, Ning YM, et al. BJU Int. 2016;118:590–597. doi: 10.1111/bju.13412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase I/II study. Slovin SF, Higano CS, Hamid O, et al. Ann Oncol. 2013;24:1813–1821. doi: 10.1093/annonc/mdt107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Phase I/II study evaluating the safety and clinical efficacy of temsirolimus and bevacizumab in patients with chemotherapy refractory metastatic castration-resistant prostate cancer. Barata PC, Cooney M, Mendiratta P, Gupta R, Dreicer R, Garcia JA. Invest New Drugs. 2019;37:331–337. doi: 10.1007/s10637-018-0687-5. [DOI] [PubMed] [Google Scholar]
- 39.Immunomodulatory activity of a colony-stimulating factor-1 receptor inhibitor in patients with advanced refractory breast or prostate cancer: a phase I study. Autio KA, Klebanoff CA, Schaer D, et al. Clin Cancer Res. 2020;26:5609–5620. doi: 10.1158/1078-0432.CCR-20-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Combination of bevacizumab and docetaxel in docetaxel-pretreated hormone-refractory prostate cancer: a phase 2 study. Di Lorenzo G, Figg WD, Fossa SD, et al. Eur Urol. 2008;54:1089–1094. doi: 10.1016/j.eururo.2008.01.082. [DOI] [PubMed] [Google Scholar]
- 41.A phase 1 study of a chimeric monoclonal antibody against interleukin-6, siltuximab, combined with docetaxel in patients with metastatic castration-resistant prostate cancer. Hudes G, Tagawa ST, Whang YE, et al. Invest New Drugs. 2013;31:669–676. doi: 10.1007/s10637-012-9857-z. [DOI] [PubMed] [Google Scholar]
- 42.Pembrolizumab (pembro) plus enzalutamide (enza) for enza-resistant metastatic castration-resistant prostate cancer (mCRPC): KEYNOTE-199 cohorts 4-5. Graff JN, Antonarakis ES, Hoimes CJ, et al. J Clin Oncol. 2020;38:15. [Google Scholar]
- 43.Bevacizumab and weekly docetaxel in patients with metastatic castrate-resistant prostate cancer previously exposed to docetaxel. Francini F, Pascucci A, Francini E, et al. Prostate Cancer. 2011;2011:258689. doi: 10.1155/2011/258689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Phase II trial of bevacizumab, thalidomide, docetaxel, and prednisone in patients with metastatic castration-resistant prostate cancer. Ning YM, Gulley JL, Arlen PM, et al. J Clin Oncol. 2010;28:2070–2076. doi: 10.1200/JCO.2009.25.4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.A phase I trial of IGF-1R inhibitor cixutumumab and mTOR inhibitor temsirolimus in metastatic castration-resistant prostate cancer. McHugh DJ, Chudow J, DeNunzio M, et al. Clin Genitourin Cancer. 2020;18:171–178. doi: 10.1016/j.clgc.2019.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Phase II randomized study of figitumumab plus docetaxel and docetaxel alone with crossover for metastatic castration-resistant prostate cancer. de Bono JS, Piulats JM, Pandha HS, et al. Clin Cancer Res. 2014;20:1925–1934. doi: 10.1158/1078-0432.CCR-13-1869. [DOI] [PubMed] [Google Scholar]
- 47.Phase 2 study of carlumab (CNTO 888), a human monoclonal antibody against CC-chemokine ligand 2 (CCL2), in metastatic castration-resistant prostate cancer. Pienta KJ, Machiels JP, Schrijvers D, et al. Invest New Drugs. 2013;31:760–768. doi: 10.1007/s10637-012-9869-8. [DOI] [PubMed] [Google Scholar]
- 48.Ipilimumab plus nivolumab and DNA-repair defects in AR-V7-expressing metastatic prostate cancer. Boudadi K, Suzman DL, Anagnostou V, et al. Oncotarget. 2018;9:28561–28571. doi: 10.18632/oncotarget.25564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Phase I study of LFA102 in patients with advanced breast cancer or castration-resistant prostate cancer. Minami H, Ando Y, Tamura K, Tajima T, Isaacs R. Anticancer Res. 2020;40:5229–5235. doi: 10.21873/anticanres.14526. [DOI] [PubMed] [Google Scholar]
- 50.Targeted MET inhibition in castration-resistant prostate cancer: a randomized phase II study and biomarker analysis with rilotumumab plus mitoxantrone and prednisone. Ryan CJ, Rosenthal M, Ng S, et al. Clin Cancer Res. 2013;19:215–224. doi: 10.1158/1078-0432.CCR-12-2605. [DOI] [PubMed] [Google Scholar]
- 51.Pembrolizumab combination therapies in patients with metastatic castrate-resistant prostate cancer (mCRPC): cohorts A-C of the phase 1B/2 KEYNOTE-365 study. Gurney H, Massard C, Retz M, et al. https://onlinelibrary.wiley.com/doi/10.1111/ajco.13185 Asia-Pacific Journal of Clinical Oncology. 2019;15:31–32. [Google Scholar]
- 52.PT-112 in advanced metastatic castrate-resistant prostate cancer (mCRPC), as monotherapy or in combination with PD-L1 inhibitor avelumab: findings from two phase I studies. Bryce AH, Dronca RS, Costello BA, Infante JR, Ames TD, Jimeno J, Karp DD. J Clin Oncol. 2020;38:83. [Google Scholar]
- 53.Phase 1b study of BXCL701, a novel small molecule inhibitor of dipeptidyl peptidases (DPP), combined with pembrolizumab (pembro), in men with metastatic castration-resistant prostate cancer (mCRPC) Aggarwal RR, Costin D, O'Neill VJ, et al. J Clin Oncol. 2020;38:0. [Google Scholar]
- 54.Phase II trial of a novel immunotherapy combination of pembrolizumab and HER2 bi-armed activated T cells (BATs) in metastatic castrate resistant prostate cancer. Patel M, Lum LG, Deol A, et al. J Clin Oncol. 2020;38:97. [Google Scholar]
- 55.Assessing different sequencing regimens of atezolizumab (atezo) and sipuleucel-T (sipT) in patients who have asymptomatic or minimally symptomatic metastatic castrate-resistant prostate cancer. Dorff TB, Acoba JD, Pal SK, Scholz MC, Tamura DJ, Huang J, Rosser CJ. J Clin Oncol. 2020;38:141. [Google Scholar]
- 56.Cabozantinib (C) in combination with atezolizumab (A) in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC): results of cohort 6 of the COSMIC-021 study. Agarwal N, Loriot Y, McGregor BA, et al. J Clin Oncol. 2020;38:139. [Google Scholar]
- 57.Pasotuxizumab, a BiTE® immune therapy for castration-resistant prostate cancer: phase I, dose-escalation study findings. Hummel HD, Kufer P, Grüllich C, et al. Immunotherapy. 2021;13:125–141. doi: 10.2217/imt-2020-0256. [DOI] [PubMed] [Google Scholar]
- 58.CCTG IND 232: a phase II study of durvalumab with or without tremelimumab in patients with metastatic castration resistant prostate cancer (mCRPC) Hotte SJ, Winquist E, Chi KN, et al. Ann Oncol. 2019;30:885. [Google Scholar]
- 59.Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. Tannock IF, de Wit R, Berry WR, et al. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 60.Efficacy and safety of radium-223 dichloride in patients with castration-resistant prostate cancer and symptomatic bone metastases, with or without previous docetaxel use: a prespecified subgroup analysis from the randomised, double-blind, phase 3 ALSYMPCA trial. Hoskin P, Sartor O, O'Sullivan JM, et al. Lancet Oncol. 2014;15:1397–1406. doi: 10.1016/S1470-2045(14)70474-7. [DOI] [PubMed] [Google Scholar]
- 61.Sipuleucel-T immunotherapy for castration-resistant prostate cancer. Kantoff PW, Higano CS, Shore ND, et al. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 62.Efficacy and safety of pembrolizumab based therapies in triple-negative breast cancer: a systematic review of clinical trials. Ali MA, Aiman W, Shah SS, Hussain M, Kashyap R. Crit Rev Oncol Hematol. 2021;157:103197. doi: 10.1016/j.critrevonc.2020.103197. [DOI] [PubMed] [Google Scholar]
- 63.Immune checkpoint inhibitors, alone or in combination with chemotherapy, as first-line treatment for advanced non-small cell lung cancer. A systematic review and network meta-analysis. Dafni U, Tsourti Z, Vervita K, Peters S. Lung Cancer. 2019;134:127–140. doi: 10.1016/j.lungcan.2019.05.029. [DOI] [PubMed] [Google Scholar]
- 64.Pembrolizumab as second-line therapy for advanced urothelial carcinoma. Bellmunt J, de Wit R, Vaughn DJ, et al. N Engl J Med. 2017;376:1015–1026. doi: 10.1056/NEJMoa1613683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Sharma P, Retz M, Siefker-Radtke A, et al. Lancet Oncol. 2017;18:312–322. doi: 10.1016/S1470-2045(17)30065-7. [DOI] [PubMed] [Google Scholar]
- 66.Inherent and tumor-driven immune tolerance in the prostate microenvironment impairs natural killer cell antitumor activity. Pasero C, Gravis G, Guerin M, et al. Cancer Res. 2016;76:2153–2165. doi: 10.1158/0008-5472.CAN-15-1965. [DOI] [PubMed] [Google Scholar]
- 67.Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Sfanos KS, Bruno TC, Maris CH, et al. Clin Cancer Res. 2008;14:3254–3261. doi: 10.1158/1078-0432.CCR-07-5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Final analysis of the ipilimumab versus placebo following radiotherapy phase III trial in postdocetaxel metastatic castration-resistant prostate cancer identifies an excess of long-term survivors. Fizazi K, Drake CG, Beer TM, et al. Eur Urol. 2020;78:822–830. doi: 10.1016/j.eururo.2020.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. N Engl J Med. 2019;381:2020–2031. doi: 10.1056/NEJMoa1910231. [DOI] [PubMed] [Google Scholar]
- 70.Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. Sandler A, Gray R, Perry MC, et al. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 71.Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. Miller K, Wang M, Gralow J, et al. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 72.Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. Saad F, Gleason DM, Murray R, et al. J Natl Cancer Inst. 2004;96:879–882. doi: 10.1093/jnci/djh141. [DOI] [PubMed] [Google Scholar]
- 73.Denosumab versus zoledronic acid in the prevention of skeletal-related events in vulnerable cancer patients: a meta-analysis of randomized, controlled trials. Chen C, Li R, Yang T, et al. Clin Ther. 2020;42:1494–1507. doi: 10.1016/j.clinthera.2020.05.019. [DOI] [PubMed] [Google Scholar]
- 74.Assessing the risk-benefit profile of ramucirumab in patients with advanced solid tumors: a meta-analysis of randomized controlled trials. Effing SM, Gyawali B. EClinicalMedicine. 2020;25:100458. doi: 10.1016/j.eclinm.2020.100458. [DOI] [PMC free article] [PubMed] [Google Scholar]