Abstract
Background:
Ivanir and Trobe have claimed that hypertropia (HT) that is greater in up than downgaze, or equal to it, is characteristic of decompensated congenital superior oblique (SO) palsy, and never present in ischemic, traumatic, or tumorous SO palsy1. The reliability of this claim was tested in patients with SO palsy confirmed by MRI demonstration of subnormal ipsilesional SO size.
Methods:
Quasi-coronal, surface coil MRI was performed in target-controlled central gaze to identify patients with unilateral reduction in SO cross section indicative of palsy. Nine patients gave unequivocal history or had markedly increased vertical fusional amplitudes indicative of congenital onset (mean age 38±16 years, standard deviation, SD). Seven patients had unequivocal acquired onset (age 47±14 years, symptom duration 5.4±4.8 years), including 2 with demonstrated trochlear Schwannoma and 5 with onset after severe head trauma. Fifteen patients had gradually progressive onset unequivocally not congenital yet not associated with any identifiable precipitating event (age 52±20 years, symptom duration 13±14 years).
Results:
Maximum SO cross section averaged 8.6±3.9mm2 in congenital palsy, not significantly different from 11.3±3.5mm2 in acquired palsy (P=0.08) either unequivocally or progressively acquired, but significantly less than about 19mm2 contralesionally in SO palsy (P<10-4). Although mean central gaze HT was greater at 20.6±8.0Δ in 9 cases of congenital than 22 acquired cases at 11.4±6.8Δ (P=0.002), HT was 8.4±16.3Δ less in up than down gaze in congenital SOP, and 3.7±11.2Δ less in acquired SO palsy. In congenital palsy, 33% of patients had HT greater in up than downgaze, while in 67% HT was greater in down gaze (by up to 42Δ). In acquired SO palsy, HT was greater in up than downgaze, or equal to it in 8 cases (36%, P=0.87, X2). In acquired SOP, HT was greater in up than down gaze in 37%, and greater in down than up gaze in 59% of cases. The HT was equal in up and central gazes in no congenital and three acquired cases of SO palsy. Trends were similar in unequivocal acquired and progressive acquired (non-congenital) SO palsy (P>0.4).
Conclusions:
Hypertropia is not characteristically greater in up than down gaze in congenital SO palsy proven by SO atrophy on MRI. In fact, average HT is greater in down than up gaze in both acquired and congenital palsy, sometimes strikingly so in the latter. The finding of HT greater in up than downgaze, or equal to it, does not reliably indicate that SO palsy is congenital, nor does maximum SO cross section.
Keywords: comitance, hypertropia, magnetic resonance imaging, strabismus, superior oblique palsy
INTRODUCTION
In neuroophthalmology, the diagnosis of superior oblique (SO) palsy carries implications for the neurological status of the patient’s brainstem, or of the trochlear (4th) cranial nerve that exclusively innervates the SO muscle. It is confounding that the diagnosis of “SO palsy” has often been broadly applied to a wide range of cases of hypertropia (HT) for which no other mechanistic diagnosis has been established2, insofar as half the cases captured in a strabismus clinic by such loose diagnosis are probably not caused by a neurological lesion at all3. For example, masquerading lesions such as displacement of the rectus muscle pulleys can mimic the incomitance caused by unilateral SO weakness4. The present report sought to avoid such confounding by considering only cases where there is deficiency of contractile function of the SO muscle, because the contractile apparatus of the muscle belly exhibits clear atrophy on magnetic resonance imaging (MRI) in both congenital and acquired5 SO palsy6,7. This approach is validated by the demonstration that experimental neurectomy of the subarachnoid trochlear nerve in monkey produces striking, stable atrophy of the SO belly within 5 weeks demonstrable both histologically and by MRI8, and that SO palsy in humans is also associated with MRI evidence of SO atrophy and loss of contractile thickening in infraduction9. I have observed this atrophy in patients as early as four weeks following acute SO denervation.
Even when the diagnosis of SO palsy is rigorously limited to disorders of the trochlear nerve causing contractile deficiency of the SO muscle, numerous causes must be considered. The congenital cranial dysinnervation disorders (CCDDs), such as congenital fibrosis of the extraocular muscles, often involve the trochlear nerve and thus include SO palsy10. Isolated congenital SO palsy itself therefore represents a limited CCDD11,12, and in a population-based study in Olmsted County, was the most common diagnosed cause of SO palsy even though most patients presented in adulthood13. Acquired SO palsy may result from trauma to the midbrain or trochlear nerve14, ischemia of the midbrain or trochlear nerve15, intrinsic or extrinsic compression of the trochlear nerve, demyelination16, abnormal transmission at the neuromuscular junction17, and even COVID-19 infection18. As a practitioner’s clinical experience accumulates, however, it becomes clear that there are many patients in whom historical and photographic evidence argue convincingly against congenital etiology, yet the apparently acquired and definitely progressive SO weakness cannot be attributed to any particular traumatic event or identifiable lesion or pathological process. This latter group of patients could be considered to have acquired, idiopathic, progressive SO palsy. It has been common practice to consider that this presentation represents progressive decompensation of congenital SO palsy first coming to clinical attention in adulthood19. No harm typically arises from diagnosing acquired, idiopathic, progressive SO palsy as “decompensated congenital” because of its typically benign course and associations. But a clinical dilemma arises when a patient presents with acute onset of vertical binocular diplopia due to incomitant HT in a clinical situation where one or more acute neurological lesions are under consideration. In such a situation, which is likely typical of inpatient or office neuro-ophthalmology consultative practice, it is important to distinguish benign congenital SO palsy from potentially more ominous SO palsy caused by an acute neurological lesion.
Vertical fusional amplitudes may be much larger than normal in some patients with congenital SO palsy20. Normal maximal vertical fusional amplitudes of typically 2–3Δ21,22 substantially smaller than horizontal fusional vergence amplitudes23, for example the typical average of about 35Δ for near convergence24. Extremely large vertical fusional amplitudes are typically considered evidence that SO palsy is congenital, although not all patients exhibiting them can relate a history of congenital symptoms25. The population-based study of Dosunmu et al. considered vertical fusional amplitudes of as little as 5Δ to be evidence of congenital onset of SO palsy13.
Ivanir and Trobe recently offered a solution to the clinical conundrum1. These authors claimed that HT greater in up than downgaze, or equal to it, is characteristic of decompensated congenital SO palsy, and absent in ischemic, traumatic, or tumorous SO palsy1. This criterion would be clinically very useful if reliable, but requires confirmation in a different patient population than that used to generate the recommendation. The current study was performed to test the Ivanir and Trobe criterion in a population of patients in whom SO had been confirmed by the MRI findings of unilateral SO palsy, and in whom detailed clinical histories were available to ascertain onset and etiology.
METHODS
The author has directed a prospective, IRB-approved study of strabismus conducted continuously for the past 32 years. This study has emphasized recruitment of healthy control subjects, as well as clinical patients diagnosed with strabismus who were willing to undergo detailed testing of eye movements, binocular alignment, and high resolution imaging of the orbits by MRI using surface coils and methods developed specifically for this purpose. Approximately 750 such strabismic volunteers contributed during the decades of the study, during which recruitment emphasized cyclovertical strabismus such as SO palsy and disorders of alignment that resemble SO palsy clinically. From the total cases were initially selected for the current analysis all 138 cases of unilateral or bilateral SO palsy from the past 25 years when MRI was stored in digital (rather than earlier film) format. From these 138 cases were selected all cases of unilateral atrophy of the SO belly in which both Hess screen analyses and complete sets of prism-cover measurements were available in diagnostic gaze positions, and excellent MRI image sets including quasi-coronal planes in each orbit imaged separately by the author using surface coils, during target fixation by the scanned eye. Clinical histories were available in all cases included. Cases were then excluded if there was a coexisting cranial nerve palsy, significant horizontal or vertical strabismus not typically associated with SO palsy, or prior strabismus or orbital surgery. These criteria were designed to assure that the study included selection of only clearly-defined SO palsy without confounding by other conditions or therapeutic manipulations.
Nine cases with mean age 38±16 (standard deviation, SD) years either gave unequivocal history of congenital onset of strabismus and head tilt, or exhibited the ability to intermittently fuse HT of at least 20Δ for a distance target, which is 10-fold greater than the typical upper limit of vertical fusional vergence for healthy young volunteers studied by the author26. Seven subjects of average age 47 ± 14 years had history or unequivocal historical evidence of acquired onset with symptom duration averaging 5.4 ± 4.8 years. The causes of these acquired palsies included trochlear Schwannoma in two cases and severe closed head trauma in five cases. Fourteen cases of mean age 52 ± 20 years had progressive onset of vertical diplopia that was unequivocally not congenital, yet not associated with any particular trauma or medical event, and symptom duration 13 ± 14 years.
Specialized, high resolution MRI of the orbits was performed in every case by the author himself. As principal investigator of the prospective study, the author himself personally directed the design of the surface coils and fiberoptic visual targets, developed the MRI pulse sequences, positioned the subjects in the scanner, personally operated the control console of the scanners in every case, interpreted the MRI images, and analyzed the images quantitatively. Each orbit was imaged separately using published methods9,27–29. The surface coils employed were investigational and not approved for routine clinical use. Imaging with T1 or T2-weighted sequences was in 2 mm thick, contiguous quasi-coronal planes perpendicular to the long axis of the orbit using a 10 cm square field of view and 256 × 256 matrix yielding 312 micron in-plane resolution. Patients monocularly fixated a target presented to the scanned eye. Imaging was performed in the supine position in most subjects, but in both the right and left lateral decubitus positions in a minority in whom ocular counter-rolling was under evaluation. Since decubitus positioning causes slight SO contraction during ipsilateral and slight relaxation during contralateral head tilting30, quantitative imaging data was averaged for the two body positions when supine imaging was not available. However, the functional effect of counter-rolling is so much smaller than the effect of SO denervation that this precaution would not have been necessary to confirm the presence of SO palsy4,9,31–33.
All patients included in this study had presented to the author, who is a faculty strabismus specialist at the (formerly Jules) Stein Eye Institute of the University of California, Los Angeles. Efforts were made to elicit the circumstances of onset of diplopia, head tilt, or visible strabismus, as well as known head trauma or other neurological event. Facial asymmetry was not used as a diagnostic criterion, since asymmetry in SO palsy has not in our hands proven to be specific34. All patients underwent complete ophthalmological history and examination, including evaluation of ocular versions, and measurement of binocular alignment using both prism-cover testing in horizontal and vertical diagnostic gaze positions at 4 m distance, and Hess screen testing at 50 cm (Clement Clark). Prism-cover measurements reported here represent maximum values revealed through dissociation through alternate covering of each eye, and so include intermittent HT sometimes compensated by enhanced fusional vergence in longstanding cases.
Patients who related a definite history of lifelong strabismus and head tilt, or who had such markedly enhanced fusional amplitude that they could fuse intermittent HT of a least 20Δ, were considered to have congenital SO palsy. Patients whose symptoms of vertical binocular diplopia had a clearly defined onset that was not congenital were considered to have “unequivocal acquired SO palsy” attributable temporally to a clearly defined pathological process. Pathologies causing definite acquired SO palsy included MRI-demonstrated trochlear Schwannoma in two cases for whom symptom onset was gradual, severe closed head trauma in a vehicular in four cases, and a severe football head injury in one case. Symptom onset was abrupt in traumatic cases, and gradually but progressive in trochlear Schwannoma.
The presence of unilateral SO palsy was confirmed by demonstration of unilateral atrophy of the SO muscle evidenced by reduction in maximum cross sectional area in quasi-coronal MRI planes. This atrophy was confirmed by examination of additional image planes anterior and posterior to the plane of maximum cross section. Figure 1 illustrates the MRI appearance of this unilateral atrophy in one case each of congenital and acquired SO palsy. Cross sectional areas were automatically determined in ImageJ after manual outlining using a digital cursor, and the maximum value was recorded for each orbit. Measured values were not corrected for SO path obliquity relative to the imaging plane. Statistical analysis was performed conventionally using paired and unpaired, two-sided Student t-testing.
Fig. 1.

Quasi-coronal MRI at mid-orbit demonstrating anatomical confirmation of right superior oblique (SO) palsy acquired from severe had trauma in a bicycle crash 14 years previously in a 27 year old man (top row, gadodiamide contrast), and congenitally in a 41 year old man (bottom row). IR – inferior rectus muscle. LPS – levator palpebrae superioris muscles. LR – lateral rectus muscle. MR – medial rectus muscle. SR – superior rectus muscle.
RESULTS
As anticipated based on patient selection, maximum SO cross section was significantly less in the hypertropic than in the fellow orbit (Fig. 2). Maximum SO cross section in the contralesional fellow orbit averaged about 19 mm2 and did not differ significantly among the congenital palsy, definite acquired palsy, and progressive acquired palsy groups. Cross section of the ipsilesional SO was highly significantly less than on the contralesional side at less than 13 mm2 for all three groups (P < 4×10−5). The congenitally palsied SO was insignificantly smaller at 8.6 ± 3.9 mm2 than in definitely acquired palsy at 12.3 ± 4.7 mm2 and in progressive acquired palsy at 10.8 ± 2.9 mm2. Thus, unilateral SO atrophy was established in all three patient groups, without statistically significant differences among them (P>0.1). When both subgroups of acquired SO palsy were pooled, mean ipsilesional SO cross section averaged 11.3 ± 3.5 mm2, not significantly different from ipsilesional SO cross section in congenital SO palsy (P = 0.078).
Fig. 2.
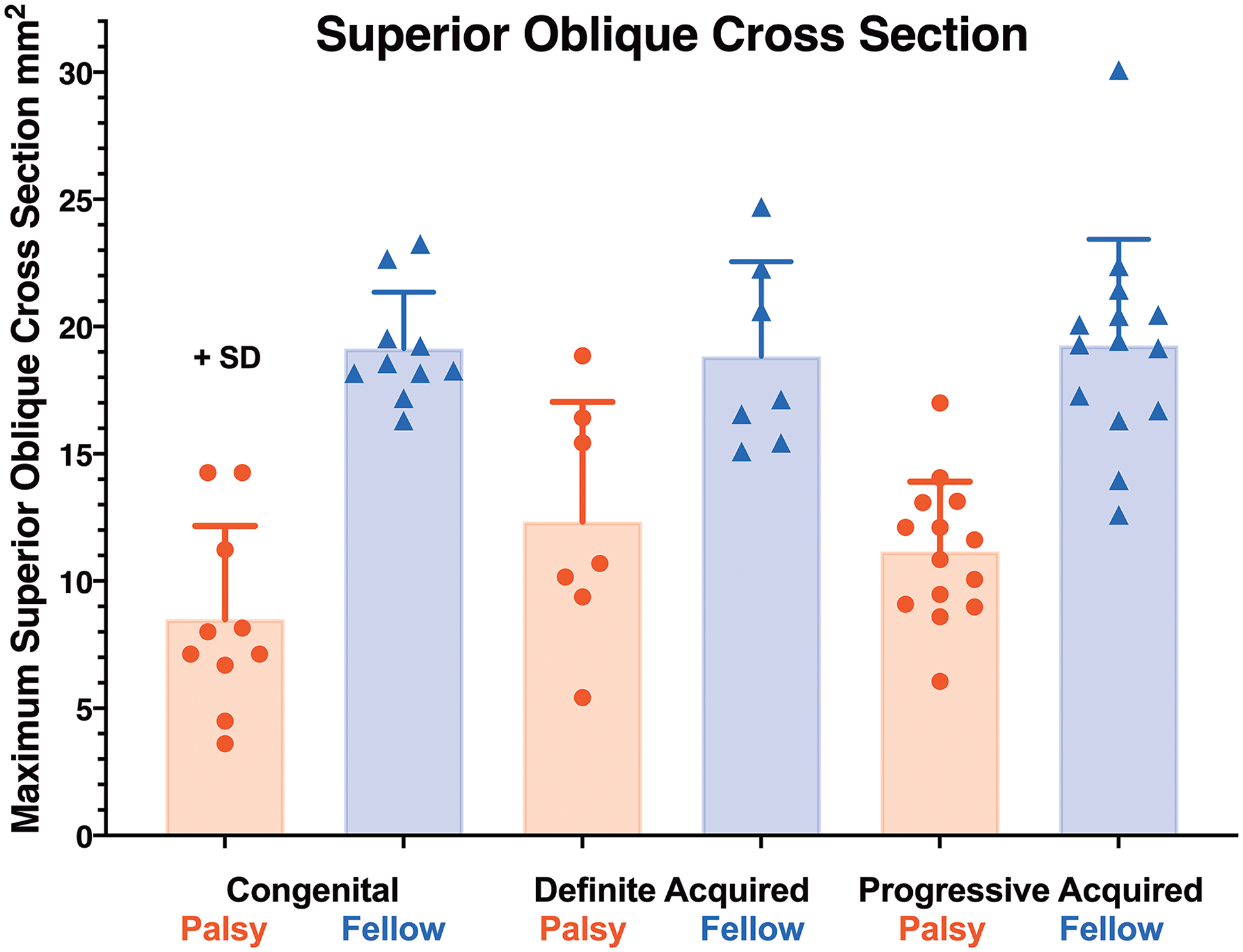
Mean maximum superior oblique (SO) muscle cross sectional area in congenital and acquired unilateral SO palsy. While cross section was highly significantly smaller in palsied than fellow eyes of all groups (P< 4×10−5), SO cross section was similar in congenital and unequivocal acquired palsy, here labeled “definite acquired” SO palsy for graphical clarity. SD – standard deviation.
Ipsilesional HT in down gaze was common in all three patient groups. This is exemplified by the Hess screen tests of the two patients with right SO atrophy illustrated in Fig. 1. Figure 3 is the Hess screen test for the 27 year old man whose SO palsy resulted from severe head trauma sustained 14 years previously in a bicycle crash, and whose MRI is shown in Fig. 1 (top). By Hess screen testing, the HT in this patient is greatest in the field of action of the right SO muscle, but greater at 6–7° in 30° direct up gaze than 4–5° in 30° direct down gaze. Prism-cover testing indicated right HT 15Δ in up gaze and 10Δ in down gaze for the patient. This example is particularly illustrative because it is inconsistent with the report of Ivanir and Trobe, who never found HT greater in up than down gaze in the 15 traumatic SO palsies in their series1.
Fig. 3.
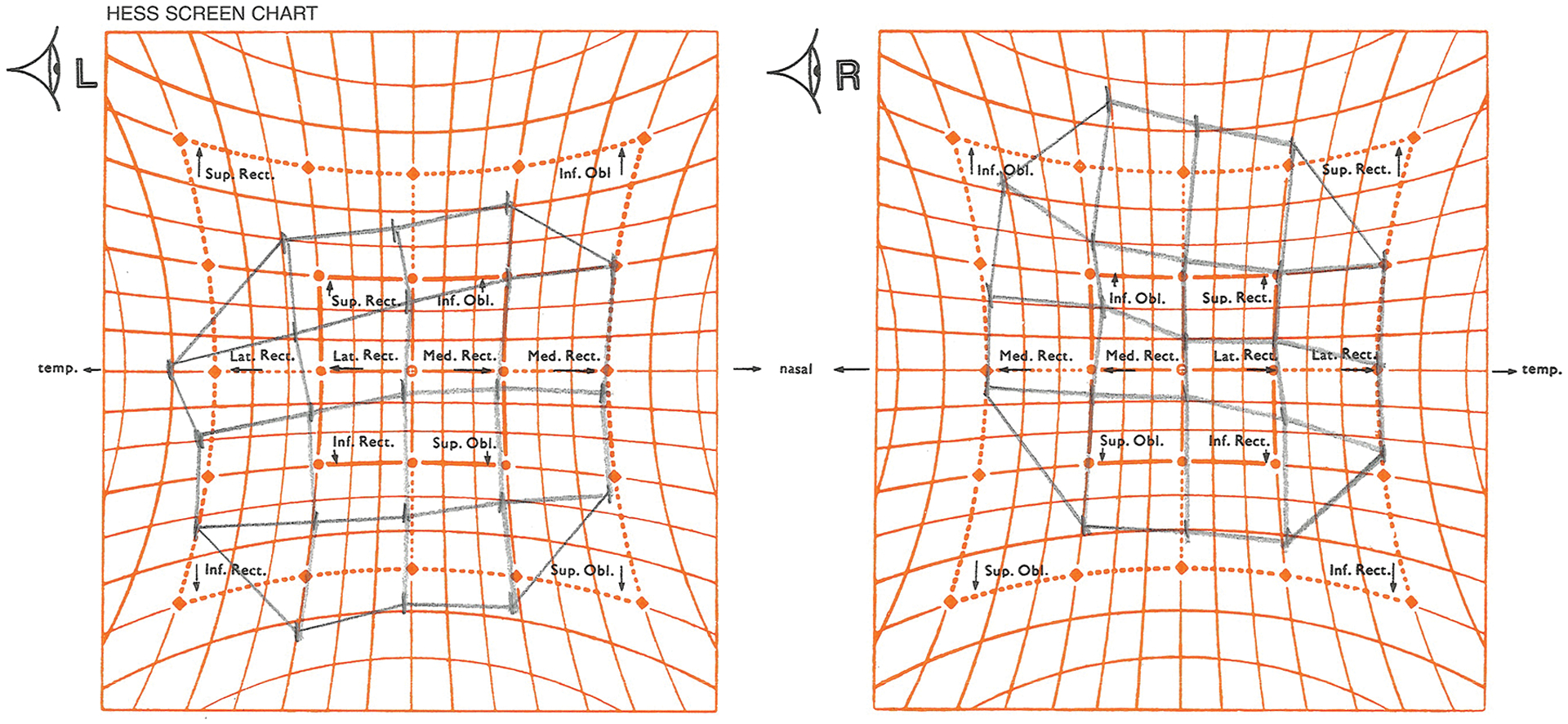
Hess screen test for patient in Fig. 1 top right superior oblique palsy with traumatically acquired in a bicycle crash. Note that the hypertropia is greater in 30° up than 30° down gaze, and is greatest in the field of action of the right inferior oblique muscle.
The foregoing case may be contrasted with Fig. 4, the Hess screen test for the 41 year old man with congenital right SO palsy whose orbits are illustrated in Fig. 1 (bottom). The right HT in this case is much larger in 30° down than 30° up gaze; in fact, the primary deviation is only about 1° in up gaze. Ivanir and Trobe found this pattern in only 28% of cases of congenital SO palsy1. In congenital palsy the Hess screen tests were generally consistent with prism-cover measurements, considering that the former were performed at 50 cm and the latter at 4 m. It is notable, however, that in only three cases of congenital SO palsy was the HT greater in up than down gaze; in the other six, the HT was greater in down than up gaze. In definite acquired SO palsy, Hess screen tests were qualitatively consistent with prism-cover measurements, although deviations were modestly larger with the Hess screen in three patients.
Fig. 4.

Hess screen test for 41 year old man with congenital right SO palsy. The primary deviation right hypertropia is much larger at about 28° during 30° downward fixation by the left eye, and only about 1° during 30° upward fixation by the left eye. The secondary deviation left hypotropia appreciated in the left plot is 9° during 30° upward fixation by the right eye, but far off scale during 30° downward fixation by the right eye.
For the convenience of the reader, the remainder of this report presents prism-cover measurements of HT. These prism-cover measurements are plotted for each patient in Fig. 5, where the red lines illustrate those cases in which HT in up gaze was greater than down gaze. It is obvious that the red line cases are not more common in congenital than acquired cases. Table 1 reports HT values for all three patient groups, but also pools both categories of acquired cases for comparison with congenital cases. Mean central gaze HT was significantly greater at 20.6±8.0Δ in congenital palsy than 11.4 ± 6.8Δ in the pooled group of all acquired palsy (P = 0.002), as well as significantly greater than 11.3 ± 5.9Δ in progressive acquired palsy (P = 0.004). On average, HT was 8.4 ± 16.3Δ less in up than down gaze in congenital and 3.7 ± 11.2Δ less in the pooled group of acquired SO palsy. Average HT was 4.8 ± 8.2Δ less in up than down gaze in definite acquired SO palsy, and 4.6 ± 11.7Δ less in progressive acquired SO palsy. Only three of 9 (33%) cases of congenital SO palsy met the Ivanir and Trobe1 criterion of HT greater in up than down gaze, or equal to it, claimed characteristic of congenital SO palsy. However, 9 of 22 cases (41%, P=0.69, X2) of acquired SO palsy met this criterion, including two of 7 definite acquired cases.
Fig. 5.
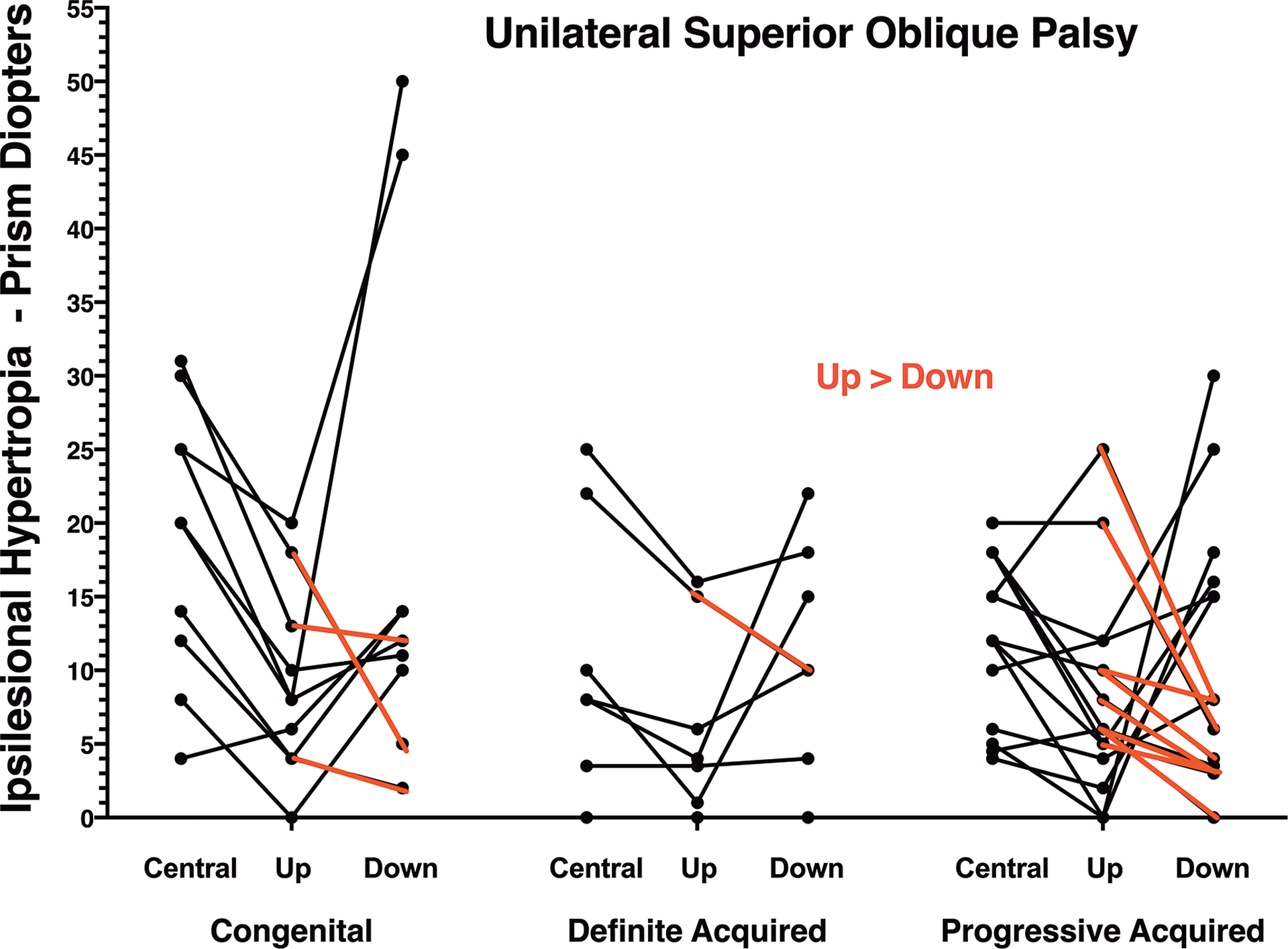
Hypertropia measured by prism-cover testing in unilateral superior oblique palsy. Cases in red represent hypertropia greater in up than down gaze.
Table 1.
Hypertropia (Δ) and Maximum Superior Oblique Cross Section (mm2) in Unilateral Superior Oblique Palsy
| Gaze Direction | Congenital SO Palsy | All Acquired | vs. Cong. | Unequivocal Acquired | vs. Cong. | Progressive Acquired | vs. Cong. | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | P | Mean | SD | P | Mean | SD | P | |
| Up | 9.4 | 6.6 | 7.6 | 6.7 | 0.234 | 6.5 | 6.5 | 0.387 | 6.9 | 5.3 | 0.640 |
| Central | 20.6 | 8.0 | 11.4 | 6.8 | 0.002 | 10.9 | 9.2 | 0.042 | 11.3 | 5.9 | 0.004 |
| Down | 17.9 | 17.2 | 11.3 | 8.2 | 0.151 | 11.3 | 7.7 | 0.365 | 11.5 | 9.0 | 0.220 |
| Max. SO Area | |||||||||||
| Palsy | 8.6 | 3.9 | 11.3 | 3.5 | 0.078 | 12.3 | 4.7 | 0.107 | 10.8 | 2.9 | 0.116 |
| Fellow | 19.2 | 2.3 | 19.1 | 3.8 | 0.871 | 18.8 | 3.7 | 0.795 | 19.1 | 4.2 | 0.980 |
It is notable that mean HT was not significantly greater in down than up gaze in any group with SO palsy (P > 0.06). Nor in any of the forgoing groups did mean central gaze HT significantly differ from HT in down gaze (paired t-testing P > 0.3).
DISCUSSION
In this group of patients with unequivocal SO palsy demonstrating significant atrophy of the ipsilesional SO belly, average HT was not characteristically greater in up than down gaze in congenital SOP. In fact, average HT was greater in down than up gaze in both acquired and congenital SOP, sometimes strikingly so in the latter. The finding of HT greater in up than downgaze, or equal to it, provided no useful clue regarding the congenital versus acquired character of SOP. Therefore it is not correct to state, as did Ivanir and Trobe, that “comparing the hypertropia in upgaze and downgaze improve(s) differential diagnosis and reduces the potential for unnecessary ancillary tests1.” Additional clinical data, particularly historical information, is required to ascertain the likelihood that SO palsy is congenital. However, since in this study the majority of patients with acquired SO palsy were idiopathic with gradual symptom onset, even historical information from a lucid and observant patient is likely to be ambiguous in many clinical cases. It is impossible to know if the group here termed “progressive acquired” SO palsy might not actually represent decompensation or progression of congenital causes; however, in this group the HT varied with vertical gaze similarly to patients with both unequivocal congenital and unequivocal acquired SO palsy. These findings also beg two important questions. First, why should the decompensation of congenital SO palsy be delayed typically into middle age, and not occur much earlier? Second, what is the cause or causes of the numerous cases of progressive acquired SO palsy included here? It is tempting to speculate that these idiopathic acquired, progressive cases might represent delayed onset of a CCDD, or possibly undiagnosed trochlear neuroma. These questions are ripe for future investigation, as the current study was not designed to address them.
Why did the current study reach a conclusion contrary to that of Ivanir and Trobe1, whose scholarship and clinical observations are of course not in dispute? One likely reason is clinical selection bias in the two patient samples. Ivanir and Trobe recruited during a 15 year interval from cases presumably evaluated by multiple examiners in a neuro-ophthalmology clinic. In contrast, the current study during a 25 year interval selected all eligible patients participating in a prospective study of strabismus who had been recruited from a strabismus clinic and examined by the author. While patient ages were similar in the two studies, in the Ivanir and Trobe study, 31 cases of ischemic and three cases of traumatic SO palsy resolved spontaneously, representing 29% of all cases they reported. None of the cases in the current report resolved spontaneously, and most went on to surgical repair. Probably most importantly, the average magnitude of central gaze HT in Ivanir and Trobe’s study was small: 7.1 ± 6.5Δ for decompensated congenital, 3.3 ± 1.9Δ for Ischemic, 2.5 ± 6.8Δ for traumatic, and 5.0 ± 4.9Δ for tumorous SO palsy1. In the current study, central gaze HT was more than twice as great, averaging 20.6 ± 8.0Δ in congenital cases, and 11.4 ± 6.8Δ in acquired cases. The patient populations in the two studies are therefore significantly dissimilar.
A second possible explanation for the present failure to replicate the findings of Ivanir and Trobe is the existence of conditions leading to overdiagnosis of SO palsy, and use of different diagnostic criteria in the two studies. When MRI evidence of the condition of the SO muscle is used as a gold standard, the 3-step test upon which Ivanir and Trobe, as well as most other authors13, have conventionally relied for diagnosis has been shown to be only 70% sensitive35 and 50% specific for actual SO palsy3. In our hands, no clinical features offer useful clues to the presence of SO atrophy in patients who have hypertropia that varies with head tilt3. More significantly, in 2009 the sagging eye syndrome (SES) was first described as a cause of small angle vertical strabismus in older adults, and was described in detail only in 201336. The mean HT in cyclovertical strabismus associated with SES is small, reportedly averaging 4.7Δ37,38 and 10Δ36 in different series. In the author’s experience, HT associated with SES may vary with head tilt. Since SES often progresses gradually, and produces HT with magnitude similar to acute SO palsy, SES might have been misdiagnosed as actual SO palsy, particularly during the historical period prior to first recognition of SES. During that period, the differential diagnosis of small acquired HT would likely have been limited to SO palsy and skew deviation. We have suggested that it is necessary to image the SO muscle itself to be certain that its chronic denervation is the cause of head-tilt dependent HT3. In the current series, maximum SO cross section did not differ significantly between cases of congenital and acquired SO palsy, which would preclude use of SO size as a distinguishing clinical feature in all except perhaps the most extreme cases (Fig. 2). Sato et al. reported that the volume ratio of the palsied to the fellow SO to be less in 9 congenital than 29 cases of acquired SO palsy7, but this difference was not statistically significant (P = 0.069), and so seems consistent with that current finding in 9 congenital cases that palsied SO cross section has an insignificant trend towards being smaller than in acquired palsy (P = 0.078). However, one could still confidently conclude that imaging that demonstrates complete SO aplasia unequivocally proves congenital onset39.
It may seem a weakness that the current study incorporates fewer patients that did Ivanir and Trobe1. However, it is a strength that the current study employed the rigorous criterion of SO atrophy to assure the correct diagnosis of unilateral SO palsy in every case, and employed uniform diagnostic measurements by both Hess screen testing and prism-cover measurements by the same examiner in all patients. Logic requires as few as one counterexample to demonstrate that a broad generalization is invalid, so the current sample size easily sufficies to support the conclusion that comparison of HT in upgaze and downgaze cannot distinguish congenital from acquired SO palsy. While it would have been convenient for clinicians if comparison of HT in various gaze positions were indeed reliable for this distinction, the criterion failed completely in the current sample of patients. Neuro-ophthalmologists should therefore not rely on this criterion. Additional clinical information can probably be helpful, especially if this includes reliable historical information. But even so, the gradual onset of many acquired cases, representing the majority in this series, may make it difficult even with excellent history to distinguish these from decompensated congenital cases, or from progression of a congenital cause such as a slowly growing benign neoplasm. Ascertainment of the cause and progression of some cases of SO palsy thus remains a clinical challenge.
Grant funding:
USPHS National Institutes of Health grants EY008313 and EY00331, and an Unrestricted Grant to the Department of Ophthalmology from Research to Prevent Blindness.
Footnotes
Conflict of Interest: None.
REFERENCES
- 1.Ivanir Y, Trobe JD. Comparing hypertropia in up and downgaze distinguishes congenital from acquired fourth nerve palsies. J Neuroophthalmol. 2017;37(4):365–368. [DOI] [PubMed] [Google Scholar]
- 2.Demer JL. Clarity of words and thoughts about strabismus. Am J Ophthalmol. 2001;132:757–759. [DOI] [PubMed] [Google Scholar]
- 3.Demer JL, Clark RA, Kung J. Functional imaging of human extraocular muscles in head tilt dependent hypertropia. Inv Ophthalmol Vis Sci. 2011;52(6):3023–3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suh SY, Le A, Clark RA, Demer JL. Rectus pulley displacements without abnormal oblique contractility explain strabismus in superior oblique palsy. Ophthalmology. 2016;123(6):1222–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horton JC, Tsai RK, Truwit CL, Hoyt WF. Magnetic resonance imaging of superior oblique muscle atrophy in acquired trochlear nerve palsy [letter]. Am J Ophthalmol. 1990;110:315–316. [DOI] [PubMed] [Google Scholar]
- 6.Ozkan S, Aribal ME, Sener EC, Sanac AS, Gurcan F. Magnetic resonance imaging in evaluation of congenital and acquired superior oblique palsy. J Pediatr Ophthalmol Strabismus. 1997;34:29–34. [DOI] [PubMed] [Google Scholar]
- 7.Sato M, Yagasaki T, Kora T, Awaya S. Comparison of muscle volume between congenital and acquired superior oblique palsies by magnetic resonance imaging. Jpn J Ophthalmol. 1998;42(6):466–470. [DOI] [PubMed] [Google Scholar]
- 8.Demer JL, Poukens V, Ying H, Shan X, Tian J, Zee DS. Effects of intracranial trochlear neurectomy on the structure of the primate superior oblique muscle. Invest Ophthalmol Vis Sci. 2010;51(7):3485–3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demer JL, Miller JM. Magnetic resonance imaging of the functional anatomy of the superior oblique muscle. Invest Ophthalmol Vis Sci. 1995;36(5):906–913. [PubMed] [Google Scholar]
- 10.Demer JL, Clark RA, Engle EC. Magnetic resonance imaging evidence for widespread orbital dysinnervation in congenital fibrosis of extraocular muscles due to mutations in KIF21A. Invest Ophthalmol Vis Sci. 2005;46:530–539. [DOI] [PubMed] [Google Scholar]
- 11.Botelho PJ, Giangiacomo JG. Autosomal-dominant inheritance of congenital superior oblique palsy. Ophthalmology. 1996;103(9):1508–1511. [DOI] [PubMed] [Google Scholar]
- 12.Bhola RM, Horne GV, Squirrell DM, Chan TK, Kumar D. Autosomal dominant congenital superior oblique palsy. Eye (Lond). 2001;15(Pt 4):479–484. [DOI] [PubMed] [Google Scholar]
- 13.Dosunmu EO, Hatt SR, Leske DA, Hodge DO, Holmes JM. Incidence and etiology of presumed fourth cranial nerve palsy: A population-based study. Am J Ophthalmol. 2018;185:110–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee J, Flynn JT. Bilateral superior oblique palsies. Br J Ophthalmol. 1985;69(7):508–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tamhankar MA, Biousse V, Ying GS, et al. Isolated third, fourth, and sixth cranial nerve palsies from presumed microvascular versus other causes: a prospective study. Ophthalmology. 2013;120(11):2264–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobson DM, Moster ML, Eggenberger ER, Galetta SL, Liu GT. Isolated trochlear nerve palsy in patients with multiple sclerosis. Neurology. 1999;53(4):877–879. [DOI] [PubMed] [Google Scholar]
- 17.Nair AG, Patil-Chhablani P, Venkatramani DV, Gandhi RA. Ocular myasthenia gravis: a review. Indian J Ophthalmol. 2014;62(10):985–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oliveira RMC, Santos DH, Olivetti BC, Takahashi JT. Bilateral trochlear nerve palsy due to cerebral vasculitis related to COVID-19 infection. Arq Neuropsiquiatr. 2020;78(6):385–386. [DOI] [PubMed] [Google Scholar]
- 19.Aoba K, Matsuo T, Hamasaki I, Hasebe K. Clinical factors underlying a single surgery or repetitive surgeries to treat superior oblique muscle palsy. Springerplus. 2015;4:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mudgil AV, Walker M, Steffen H, Guyton DL, Zee DS. Motor mechanisms of vertical fusion in individuals with superior oblique paresis. J AAPOS. 2002;6:145–153. [DOI] [PubMed] [Google Scholar]
- 21.Mottier ME, Mets MB. Vertical fusional vergences in patients with superior oblique muscle palsies. Am Orthoptic J. 1990;40:88–93. [Google Scholar]
- 22.von Noorden GK. Binocular Vision and Ocular Motility: Theory and Management of Strabismus. 4th ed. ed. St. Louis: Mosby; 1990. [Google Scholar]
- 23.Sharma K, Abdul-Rahim AS. Vertical fusional aplitude in normal adults. Am J Ophthalmol. 1992;114(5):636–637. [DOI] [PubMed] [Google Scholar]
- 24.Fray KJ. Fusional amplitudes: Developing testing standards. Strabismus. 2017;25(3):145–155. [DOI] [PubMed] [Google Scholar]
- 25.Demer JL, Clark RA. Functional anatomy of muscle mechanisms compensating vertical heterophoria. Am J Ophthalmol. 2021;221:137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Demer JL, Clark RA. Magnetic resonance imaging demonstrates compartmental muscle mechanisms of human vertical fusional vergence. J Neurophysiol. 2015;113(7):2150–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shin GS, Demer JL, Rosenbaum AL. High resolution dynamic magnetic resonance imaging in complicated strabismus. J Pediatr Ophthalmol Strabismus. 1996;33:282–290. [DOI] [PubMed] [Google Scholar]
- 28.Bhola R, Rosenbaum AL, Ortube MC, Demer JL. High-resolution magnetic resonance imaging demonstrates varied anatomic abnormalities in Brown syndrome. J AAPOS. 2004;9:438–448. [DOI] [PubMed] [Google Scholar]
- 29.Demer JL, Dusyanth A. T2 fast spin echo magnetic resonance imaging of extraocular muscles. J AAPOS. 2011;15(1):17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Demer JL, Clark RA. Magnetic resonance imaging of human extraocular muscles during static ocular counter-rolling. J Neurophysiol. 2005;94:3292–3302. [DOI] [PubMed] [Google Scholar]
- 31.Shin SY, Demer JL. Superior oblique extraocular muscle shape in superior oblique palsy. Am J Ophthalmol. 2015;59(6):1169–1179.e1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suh SY, Clark RA, Le A, Demer JL. Extraocular muscle compartments in superior oblique palsy. Invest Ophthalmol Vis Sci. 2016;57(13):5535–5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clark RA, Demer JL. Enhanced vertical rectus contractility by magnetic resonance imaging in superior oblique palsy. Arch Ophthalmol. 2011;129(7):904–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Velez FG, Clark RA, Demer JL. Facial asymmetry in superior oblique palsy and pulley heterotopy. J AAPOS. 2000;4:233–239. [DOI] [PubMed] [Google Scholar]
- 35.Manchandia A, Demer JL. Sensitivity of the three-step test in diagnosis of superior oblique palsy. J AAPOS. 2014;18(6):567–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chaudhuri Z, Demer JL. Sagging eye syndrome: Connective tissue involution as a cause of horizontal and vertical strabismus in older patients. JAMA Ophthalmol. 2013;131(5):619–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chaudhuri Z, Demer JL. Graded vertical rectus tenotomy for small-angle cyclovertical strabismus in sagging eye syndrome. Br J Ophthalmol. 2016;100(5):648–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chaudhuri Z, Demer JL. Long-term surgical outcomes in the sagging eye syndrome. Strabismus. 2018;26(1):6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chan TK, Demer JL. Clinical features of congenital absence of the superior oblique muscle as demonstrated by orbital imaging. J AAPOS. 1999;3:143–150. [DOI] [PubMed] [Google Scholar]


