Abstract
Background and Aims:
Patients with Crohn’s disease (CD) treated with ustekinumab who experience inadequate response, or loss of response after standard induction and/or maintenance dosing may benefit from dose escalation. We conducted a systematic review and meta-analysis examining the effectiveness of re-induction and/or dose interval shortening of ustekinumab in patients with active CD despite standard induction and maintenance.
Methods:
Through a systematic literature search through March 31, 2021, we identified 15 cohort studies in 925 adults with CD with inadequate response or loss of response to standard dose ustekinumab, underwent dose escalation (re-induction and/or dose interval shortening to <8 weeks), and reported rates of achieving clinical response, corticosteroid-free clinical remission, endoscopic response and/or remission. We calculated pooled rates (with 95% confidence interval [CI]) using random effects meta-analysis and examined factors associated with response to dose escalation through qualitative synthesis of individual studies.
Results:
On meta-analysis, 55% (95% CI, 52–58%) patients with inadequate response or loss of response who underwent ustekinumab dose escalation achieved clinical response, with moderate heterogeneity (I2=57%). Approximately, 61% patients were able to achieve endoscopic response, including 29% who achieved endoscopic remission. Dose interval shortening alone recaptured response in 57% patients. No consistent factors associated with response to dose escalation were identified on qualitative synthesis.
Conclusion:
In real word settings, ustekinumab dose escalation was effective in achieving response in patients with CD with inadequate response, or loss of response to standard dose induction and/or maintenance therapy.
Keywords: pharmacokinetics, dose adjustment, therapeutic drug monitoring, biologics
Several clinical and biological factors influence the pharmacokinetics of biologic agents in patients with inflammatory bowel diseases (IBD), which in turn, influences clinical response to the biologic therapy. In patients with suboptimal clinical response or breakthrough symptoms, therapeutic drug monitoring may help identify patients with rapid clearance and low trough concentration. In these instances, biologic dose escalation is frequently utilized with variable success to recapture response. Real-world studies in patients with IBD treated with tumor necrosis factor-α (TNFα) antagonists suggest 20–40% may undergo dose escalation, due to loss of response1, 2 with approximately 60–80% patients recapturing response with dose escalation.3, 4 In a systematic review of 10 cohort studies of vedolizumab, Peyrin-Biroulet and colleagues observed pooled incidence rates of loss of response were 47.9 per 100 person-years of follow up; approximately 54% patients recaptured response with dose escalation.
Ustekinumab, a fully-humanized IgG monoclonal antibody directed towards p40, a subunit with shared homology between interleukin (IL)-12 and IL-23, was approved for moderate to severe Crohn’s Disease (CD) in September 2016. In the United States, standard ustekinumab dosing for CD is intravenous induction at ~6mg/kg, followed by subcutaneous maintenance therapy every 8 weeks. European labeling includes every 8- and 12-week subcutaneous maintenance regimens. Since its approval, real-world observational studies have confirmed effectiveness and safety of standard ustekinumab dosing regimens. In a systematic review of 38 studies of ustekinumab in patients with CD, 60% and 34% patients achieved clinical response and remission with induction therapy, and 42% and 31% maintained response and remission at 1 year of therapy, respectively.5 Similar to other biologics, there is inter-individual variability in drug clearance; higher serum trough concentrations have been observed in patients with clinical and endoscopic response, compared with non-responders.6 As a result, dose escalation, including intravenous re-induction and/or intensification to every 4–6 week subcutaneous dosing, have been utilized in patients with sub-optimal response with variable success.7–21
We conducted a systematic review with meta-analysis to evaluate the effectiveness of ustekinumab re-induction and/or dose intensification in recapturing response in patients with CD. We qualitatively synthesized factors associated with response to ustekinumab dose-escalation.
METHODS
This systematic review is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) standards and followed an a priori establish protocol (available upon request).22
Selection Criteria
We included cohort studies in (1) Patients: adult patients with active luminal CD with inadequate response, or experiencing loss of response, to standard intravenous induction and subcutaneous maintenance dosing with ustekinumab, at doses approved by Food and Drug Administration (FDA) and European Medical Agency (EMA), (2) Intervention: underwent dose escalation (re-induction and/or dose intensification [defined as dose interval shortening to <8 weeks]) and (3) Outcome: reported effectiveness in achieving clinical response. We excluded studies that only reported rates of loss of response and dose escalation without reporting effectiveness in recapturing response/remission, used non-standard induction and/or maintenance dosing prior to FDA/EMA approval, evaluated patients with perianal CD only or ulcerative colitis (given recency of its approval for this indication), or were conducted in pediatric populations.
Search Strategy
We conducted a comprehensive search of PubMed, Embase and Cochrane Database of Systematic Reviews from September 1, 2016 (date of regulatory approval of ustekinumab for CD) to March 31, 2021, using a combination of keywords and MeSH terms: (“ustekinumab”[Supplementary Concept] OR “ustekinumab”[All Fields]) AND ((“crohn disease”[MeSH Terms] OR (“crohn”[All Fields] AND “disease”[All Fields]) OR “crohn disease”[All Fields] OR (“crohn’s”[All Fields] AND “disease”[All Fields]) OR “crohn’s disease”[All Fields]) OR (“inflammatory bowel diseases”[MeSH Terms] OR (“inflammatory”[All Fields] AND “bowel”[All Fields] AND “diseases”[All Fields]) OR “inflammatory bowel diseases”[All Fields] OR (“inflammatory”[All Fields] AND “bowel”[All Fields] AND “disease”[All Fields]) OR “inflammatory bowel disease”[All Fields])). Two study investigators (JM, SS) independently reviewed the title and abstract of studies identified in the search, to exclude studies that did not address the research question of interest, based on pre-specified inclusion and exclusion criteria. The full text of the remaining articles was examined to determine whether it contained relevant information. Conflicts in study selection at this stage were resolved by consensus. We performed a recursive search of the bibliographies of these selected articles as well as published systematic reviews on this topic, to identify any additional studies. We also conducted a manual search of abstracts from major gastroenterology conferences (Digestive Disease Week, American College of Gastroenterology, Crohn’s and Colitis Congress, European Crohn’s and Colitis Organization Congress) from 2019–2021 to identify additional abstracts on the topic. We did not limit articles regardless of publication type or language. In case of multiple studies from the same center, we included the most comprehensive report on the question of interest.
Data Extraction and Risk of Bias Assessment
One author independently (JM) abstracted data using a standardized data collection form: (a) study characteristics – primary author, time period of study/year of publication, geographic location; (b) patient characteristics – age, sex, smoking, body mass index, IBD type and Montreal Classification, prior IBD-related surgeries, IBD therapies prior to initiation of ustekinumab, concomitant steroids and/or immunomodulators, and reasons for dose escalation (primary nonresponse without any symptom improvement, inadequate or partial response; secondary loss of response after initial improvement in symptoms; escalation in asymptomatic patients with ongoing endoscopic disease activity, or unspecified with active clinical disease); (c) outcome assessment – time to dose intensification, ustekinumab re-induction and/or interval shortening, definition and proportion of patients achieving clinical response, clinical remission, corticosteroid-free clinical remission, endoscopic response and remission, need for corticosteroids, and progression to surgery, with various ustekinumab dose intensification strategies (re-induction and/or dose escalation); see Table 1 for individual study definitions used. A second author (SS) independently reviewed the abstracted data for accuracy, and any discrepancies were addressed by a joint re-evaluation of the original article. The Newcastle-Ottawa scale (NOS) was used for critical assessment of the quality of each included study. The NOS evaluates nine characteristics of a study including four, one, and three items for sample selection, group comparison, and the outcome, respectively. For our analysis we excluded the group comparison. The typical scores range between 0 to 9, with our modification making the score range 0–7.23
Table 1.
Characteristics of included studies
| Study (Author) | Study Type | Maintenance Dosing | Reason for Dose Intensification and Criteria for Active Disease‡ |
Dose Intensification Strategy | Primary Outcome Definition | Secondary Outcome Definitions | Median Time to: Assessment/ Follow-up (weeks) |
|---|---|---|---|---|---|---|---|
| Biemanns*7 | Multicenter Prospective Cohort (12/2016–1/2019, Netherlands) |
90mg SubQ UST Q8 or Q12 week | NR | Interval shortening to q4w (44%); IV re-induction (40%); both interval shortening and re-induction (16%) | Corticosteroid free clinical remission: HBI ≤ 4 | Clinical Response: HBI reduction of at least 3 points | NR/NR |
| Fumery*11 | Multicenter Retrospective Cohort (10/2015–12/2018, French) |
90mg SubQ UST Q8 or Q12 week | Secondary loss of response (26%); inadequate response (74%) Active disease: HBI ≥ 4 and one objective sign of inflammation (CRP ≥ 5 mg/L and/or fecal calprotectin ≥ 250 μg/g and/or radiologic and/or endoscopic evidence of disease activity) |
Interval shortening to q4w (100%) | Clinical Remission: HBI ≤ 4; Endoscopic Remission: Absence of ulcerations | Clinical Response: HBI reduction of at least 3 points | 10/34 |
| Hudson15 | Single Center Retrospective Cohort (No time period reported, USA) | 90mg SubQ UST Q8 week | Secondary loss of response (100%) Active disease: NR |
IV re-induction (33%); both IV reinduction and interval shortening (67%) | Clinical Response: Undefined | NR | 16/NR |
| Kopylov17 | Multicenter Retrospective Cohort (No time period reported, Israel) |
90mg SubQ UST Q8 week* | Clinically active disease (100%) Active disease: HBI >4 or CDAI ≥ 150 |
Interval shortening to q4w or q6w (78%); IV re-induction (10%); both interval shortening and re-induction (12%) | Clinical Response: HBI reduction of at least 3 points or CDAI reduction of more than 70 | Clinical Remission: HBI ≤ 4 or CDAI ≤ 150; Endoscopic Response: Documented improvement from prior study; Endoscopic Remission: Mucosal Healing; Biochemical Remission: CRP normalization | 16/26 |
| Ollech18 | Single Center Retrospective Cohort (No time period reported, USA) | 90mg SubQ UST Q8 week | Clinically active disease (100%) Active disease: HBI >4, active endoscopic inflammation, CRP >5 mg/L, or FCP >250 ug/g |
Interval shortening to q4w (100%) | Clinical Remission: HBI ≤ 4 | Endoscopic Response: SES-CD, if available, or scale from normal/quiescent to mild, moderate, severe; Biochemical Remission: CRP normalization | 12/36 |
| Sedano20 | Single Center Prospective and Retrospective Cohort (5/2018–2/2020, Canada) | 90mg SubQ UST Q4 week | Clinically active disease (100%) Active disease: Active disease by PGA or HBI >4 |
IV re-induction (100%) | Clinical Remission: PGA Disease Severity score of 0 AND HBI ≤ 4 | Clinical Response: ≥50% reduction in symptoms by PGA Disease Severity score AND HBI reduction of at least 3 points; Endoscopic Response: Documented improvement from prior study; Endoscopic Remission: Absence of ulcers | 14.9/49 |
| Dalal10 | Single Center Retrospective Cohort (1/2016–1/2019, USA) | 90mg SubQ UST Q8 week | Secondary loss of response (75%) Escalation in patients in clinical remission: minimal but short-term symptom recurrence (8%); endoscopic activity (7%); low UST level (3%); elevated FCP (2%); radiologic Activity (2%); perianal Disease (2%); patient preference (1%) Active disease: HBI >4 |
Interval shortening to q4w (54%) or q6w (46%) | Clinical Remission: HBI ≤ 4 | Clinical Response: HBI reduction of at least 3 points; Endoscopic Response: Documented improvement from prior study; Biochemical Remission: CRP normalization | 48/74 |
| Haider13 | Single Center Retrospective Cohort (1/2016–10/2018, USA) | 90mg SubQ UST Q8 week | Clinically active disease (100%) Active disease: PGA |
Interval shortening to q4w (100%) | Clinical Response: PGA Disease Severity score decrease by >1 | Clinical Remission: PGA Disease Severity score of 0 | 29/78 |
| Johnson*16 | Multicenter Retrospective Cohort (12/2016–1/2019, USA) |
90mg SubQ UST Q8 week | Secondary loss of response (41%); Primary nonresponse (59%) Active disease: NR |
NR | Clinical Response: PGA Disease Severity score | NR | 48/55 |
| Ramaswamy19 | Single Center Retrospective Cohort (No dates or location reported) | 90mg SubQ UST Q8 week | Clinically active disease (100%) Active disease: HBI >4, active endoscopic or radiologic inflammation, CRP >5 mg/L, or FCP >250 ug/g |
Interval shortening to q4w (100%) | Clinical Response: HBI reduction of at least 3 points | NR | 12/NR |
| Bundschuh8 | Single Center Retrospective Cohort (4/2017–4/2018, USA) | 90mg SubQ UST Q8 week | Secondary loss of response (100%) Active disease: NR |
Interval shortening to q4w (82%) or q6w (18%) | Clinical Response: HBI reduction of at least 3 points | NR | NR/NR |
| Cohen9 | Single Center Retrospective Cohort (2016–2018, USA) | 90mg SubQ UST Q8 week | Clinically active disease (100%) Active disease: NR |
Interval shortening to q4w (100%) | NR | Clinical Response: Undefined; Endoscopic Response: Undefined; Endoscopic Remission: Undefined | NR/NR |
| Glass12 | Single Center Retrospective Cohort (No dates reported, USA) | 90mg SubQ UST Q8 week | Clinically active disease (100%) Active disease: NR |
Interval shortening to q4w (60%) or q6w (21%); IV re-induction (19%) | Clinical Response: Undefined but had to be off of corticosteroids | Endoscopic Response: Documented improvement from prior study; Biochemical Remission: Undefined | NR/18 |
| Heron14 | Multicenter Retrospective Cohort (1/2017–7/2018, Canadian) |
90mg SubQ UST Q4 week (89% of cohort, does not specify others) | Clinically active disease (100%) Active disease: NR |
IV re-induction (100%)** | Clinical Remission: HBI ≤ 4 (off corticosteroids) | Endoscopic Remission: SES-CD score of < 3† | 14/14 |
| Young21 | Single Center Retrospective Cohort (9/2016–10/2017, USA) | 90mg SubQ UST Q8 week | Clinically active disease (100%) Active disease: NR |
Interval shortening to q4w (52%) or q6w (24%); both interval shortening and re-induction (24%) | NR (Clinical response undefined) | NR | NR/25 |
[Abbreviations: SQ=subcutaneous, IV=intravenous, UST=ustekinumab, Q4w=every 4 weeks, HBI= Harvey-Bradshaw Index; PGA= Physician’s Global Assessment, CDAI= Crohn’s Disease Activity Index, SES-CD=Simple Endoscopic Score for Crohn’s Disease, CRP= C-reactive protein, NR=not reported]
Kopylov et al included patients with prior q12w maintenance only if they had previously been escalated to q8w before additional escalation
Allowed to decrease interval after IV re-induction
Reported as a combined outcome of complete clinical, biochemical, endoscopic remission
Those listed as active disease did not specify between escalation for secondary loss of response after initial response, inadequate or partial response
Outcome utilized to determine clinical response per study is underlined
Outcomes Assessed
The primary outcome was pooled proportion of patients who captured clinical response with ustekinumab dose-escalation (either intravenous re-induction and/or dose intensification). Secondary outcomes were: (a) proportion of patients achieving clinical remission, (b) corticosteroid-free clinical remission, and (c) achieving endoscopic response and/or remission, after ustekinumab dose escalation; and (d) proportion of patients who recaptured clinical response after initial response to standard dose ustekinumab. Rates of achieving these outcomes separately with intravenous re-induction alone and dose interval shortening to every 4 or 6 weeks alone were also abstracted, as available. We identified criteria for dose escalation and qualitatively synthesized factors associated with successful recapturing of clinical response with ustekinumab dose escalation.
Statistical Analysis
We used double arcsine transformation described by Stuart and Ord to calculate standardized proportions (and 95% confidence interval [CI]) followed by the random-effects meta-analysis of proportions described by DerSimonian and Laird to calculate pooled proportion of patients (and 95% confidence interval [CI]) achieving outcomes of interest.24, 25 We assessed heterogeneity between study-specific estimates using the inconsistency index (I2), and used cut-offs of 0–40%, 30–60%, 50–90% and 75–100% to suggest minimal, moderate, substantial and considerable heterogeneity, respectively.26 Small study effects (publication bias) was assessed visually using funnel plots, and statistically using Egger’s regression test.27 All analyses were performed using StatsDirect version 3.3.4 (StatsDirect, Merseyside, UK).
Data Availability Statement
The data underlying this article are available within the article. All data were obtained from previously published studies in public domain.
RESULTS
From a total of 1,525 unique studies and abstracts identified using the search strategy, we included 8 cohort studies and 7 conference abstracts encompassing a total 925 patients undergoing ustekinumab dose escalation. See Figure 1 for study selection flowchart.7–21
Figure 1.

Study selection flowchart
Characteristics and Quality of Included Studies
Table 1 describes study characteristics and Table 2 describes the characteristics of patients included in the studies. Median age ranged from 35–55 years of age with median disease duration ranging from 10–18 years. The majority of patients had ileocolonic disease (55%) with 28–32% of total cohort having disease complications of stricturing, fistulizing, or perianal disease. Approximately 90% had prior TNFα antagonist exposure; 393 (47%) were also exposed to vedolizumab. Overall, 137 (25%) and 207 (26%) were receiving concomitant immunomodulator (methotrexate or thiopurines) or corticosteroid therapy, respectively at the time of dose intensification. Average median of meantime to follow-up was 9.7 months with a range of medians, 3.7–17.3 months in 9 studies reporting this.10, 11, 13, 14, 16, 18, 20, 21 Mean time to dose intensification from initial induction was 39.8 weeks (range, 20–52.5 weeks) in the 8 studies reporting this metric.10, 11, 13, 17, 18, 20
Table 2.
Characteristics of patients with Crohn’s disease undergoing ustekinumab dose escalation
| Study | Age, median (IQR) | Sex (male, %) | Disease Duration, years (IQR) | Disease Location (I/C/IC/U, %) | Disease Complications (B2/B3/P, %) | Prior IBD Surgery (%) | Prior TNFα antagonist(%) | Prior VDZ (%) | Concurrent IMM (%) | Concurrent steroids (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Biemanns* 7 | 38 (29–52) | 40 | 12.3 (7.5–19.3) |
31/34/34/5 | 29/18/17 | 62 | 99 | 21 | 20 | 16 |
| Fumery* 11 | 35 (28–49) | 48 | 11.6 (7.3–20.2) |
NR | 28/37/47 | 49 | 99 | 55 | 27 | 29 |
| Hudson15 | -- | -- | -- | 0/0/100/0 | 0/50/0 | -- | -- | -- | -- | -- |
| Kopylov17 | 35 (26–49) | 55 | 10 (5–17) | 18/22/61/0 | 37/27/23 | 57 | 56** | 40** | 17 | 24 |
| Ollech18 | 36 (27–51) | 47 | 14.4 (7.1–23) | 13/15/70/4 | 41/29/10 | -- | 93† | 46† | -- | 41 |
| Sedano20 | 52 (33–66) | 33 | -- | 0/13/73/13 | 20/33/20 | 53 | 40 | 13 | -- | 53 |
| Dalal10 | 42 (NR) | 44 | 12.7–15.5 (NR) |
13/22/62/2 | 18/55/42 | 64 | 100 | 48 | -- | 24 |
| Haider13 | 39 (21–27) | 73 | 11.4 (2–24) | 27/7/67/20 | 33/20/20 | 47 | 100 | 53 | 67 | 13 |
| Johnson* 16 | 38 (NR) | 48 | -- | -- | -- | -- | 90 | -- | -- | -- |
| Ramaswamy19 | 55 (NR) | 45 | -- | -- | -- | -- | -- | -- | 65 | 39 |
| Bundschuh8 | 40 (24–61) | 56 | -- | -- | --/--/73 | 82 | 96 | -- | -- | 55 |
| Cohen9 | 39 (NR) | 44 | -- | --/--/52/-- | 57/--/-- | 54 | 95 | 47 | -- | -- |
| Glass12 | 38 (33–49) | 50 | 18 (10.2) | 13/23/62/2 | 20/23/63 | 65 | 92 | 74 | 31 | 25 |
| Heron14 | 35.5 | 54 | -- | -- | -- | -- | -- | -- | -- | -- |
| Young21 | -- | -- | -- | -- | 33/48/-- | -- | -- | -- | -- | -- |
[Abbreviations: IQR=interquartile range, SD= standard deviation, NR=not reported, I=ileal, C=colonic, IC=ileocolonic, U=upper gut, B2=stricturing, B3=penetrating, P=perianal, IBD=Inflammatory Bowel Disease, TNF=tumor necrosis factor, VDZ=vedolizumab, IMM=immunomodulator]
Percentages of baseline characteristics from larger total cohort than just those who received ustekinumab dose escalation
Kopylov et al reported prior biologics as: 33 patients with 1 prior biologic, 56 patients with 2 prior biologics, 46 patients with 3 prior biologics, 2 patients with 4 prior biologics, and 57 patients received both prior TNFα antagonists and vedolizumab. Though biologic type is not clearly reported, the synthesized results reported in the table are made under the reasonable assumption that most patients receive TNFα antagonist as first line therapy for moderate to severe Crohn’s Disease.
Ollech et al reported prior biologics as: 6 patients with one or no prior biologic, 6 patients with >1 prior biologic, 2 patients with prior vedolizumab exposure, and 3 biologic naïve patients. Though biologic type is not clearly reported, the synthesized results reported in the table are made under the reasonable assumption that most patients receive TNFα antagonist as first line therapy for moderate to severe Crohn’s Disease.
Among studies that reported outcomes in all ustekinumab-treated patients and included a subset of patients who underwent dose-escalation (and reported their outcomes separately, hence being eligible for inclusion in our analysis), approximately 20% patients underwent dose escalation (225/1095).7, 8, 13, 18, 19, 21 The majority of patients received dose-interval reduction to either every 4 or 6 weeks as the method of therapeutic intensification (83%); 12% received solely intravenous re-induction with continued standard dose subcutaneous maintenance and 5% received intravenous re-induction and dose interval reduction (Table 1). All patients had clinical, endoscopic, radiologic, and/or biochemical confirmation of active disease prior to dose escalation and these criteria, are included in Table 1. Reason for dose intensification was specified in all but one study.7 The majority specified only that patients had active disease, consisting of 49% of the total cohort.9, 12–14, 17–21 Approximately 18% of the total cohort received dose intensification for secondary loss of response after initial clinical response.8, 10, 11, 15, 16 One study reported patients who received dose intensification for inadequate or partial response contributing to less than 1% of the total cohort; they did not included a definition of primary nonresponse.11 Only Johnson et al specifically included patients with primary nonresponse, consisting of 14% of the total cohort.16 Dalal et al included patients escalated while in clinical remission for reasons such as active endoscopic, radiologic, or biochemical disease activity.10
Overall, the included studies were at moderate risk of bias, with all studies having well-defined outcomes, occurring at tertiary or specialized centers, and a mix of retrospective and prospective cohorts.
Recapturing clinical response with ustekinumab dose escalation
On meta-analysis of 925 patients with CD with inadequate response or loss of response to standard ustekinumab dosing, who underwent dose escalation of ustekinumab (intravenous re-induction and/or dose interval reduction), 55% (95% CI, 52–58%) captured clinical response, with moderate heterogeneity (I2=57%) (Figure 2A).7–21 In a subset of 524 patients from 9 studies who underwent dose interval shortening, 57% (95% CI, 51–63%) recaptured clinical response, with moderate heterogeneity (I2=33%) (Figure 2B).7–11, 13, 17–19 Where reported, 55% (95% CI, 49–61%) achieved clinical response with dose escalation to every 4 week dosing, and 54% achieved clinical response with dose escalation to every 6 week. In a smaller cohort of 44 patients who underwent intravenous re-induction without dose interval shortening, 25/44 (59%) recaptured clinical response.7, 17, 20, 21
Figure 2.

Pooled rate of clinical response with (A) dose escalation (reinduction and/or dose-interval shortening), or (B) maintenance dose-interval shortening of ustekinumab.
Corticosteroid-free clinical remission with ustekinumab dose escalation
On meta-analysis of 220 patients with CD who underwent dose escalation of ustekinumab, 40% (95% CI, 21–61%) patients achieved corticosteroid-free clinical remission, albeit with considerable heterogeneity (I2=88%) (Figure 3).7, 10, 11, 17 All patients in this cohort underwent ustekinumab interval shortening without intravenous re-induction. This outcome was not reported specifically for patients who underwent intravenous re-induction.
Figure 3.
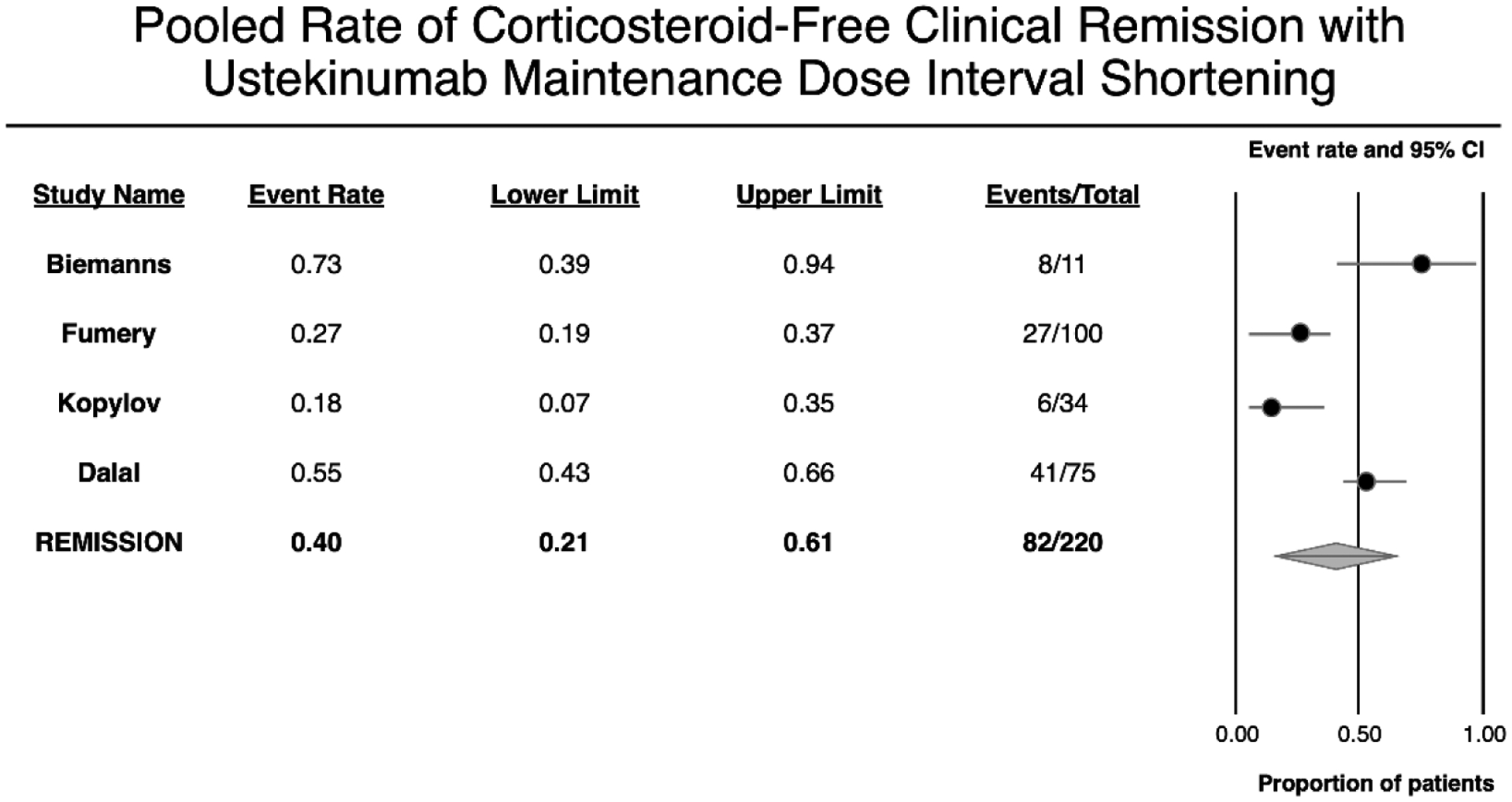
Pooled rate of corticosteroid-free clinical remission with maintenance dose-interval shortening of ustekinumab.
Endoscopic response and remission with dose intensification of ustekinumab
On meta-analysis of 126 patients with CD who underwent endoscopy before and after escalation of ustekinumab, 61% (95% CI, 52–69%) patients achieved endoscopic response, without heterogeneity (I2=0%) (Figure 4A).9, 10, 12, 17, 20 In a meta-analysis of 118 patients with CD who underwent endoscopy before and after escalation of ustekinumab, 29% (95% CI, 16–44%) patients achieved endoscopic remission, with substantial heterogeneity (I2=62%) (Figure 4B).9, 11, 17, 18, 20
Figure 4.


Pooled rate of (A) endoscopic response and (B) endoscopic remission, with dose escalation of ustekinumab.
Other outcomes
Biochemical remission with dose intensification of ustekinumab:
On meta-analysis of 256 patients with CD with elevated C-reactive protein (CRP) before escalation of ustekinumab, 21% (95% CI, 17–27%) patients achieved normalization of CRP (biochemical remission), without heterogeneity (I2=0%) (eFigure 1).10, 12, 17, 18 Fecal calprotectin response was reported in one study which found 2/17 patients achieving normalization of fecal calprotectin after ustekinumab dose intensification.10
eFigure 1.
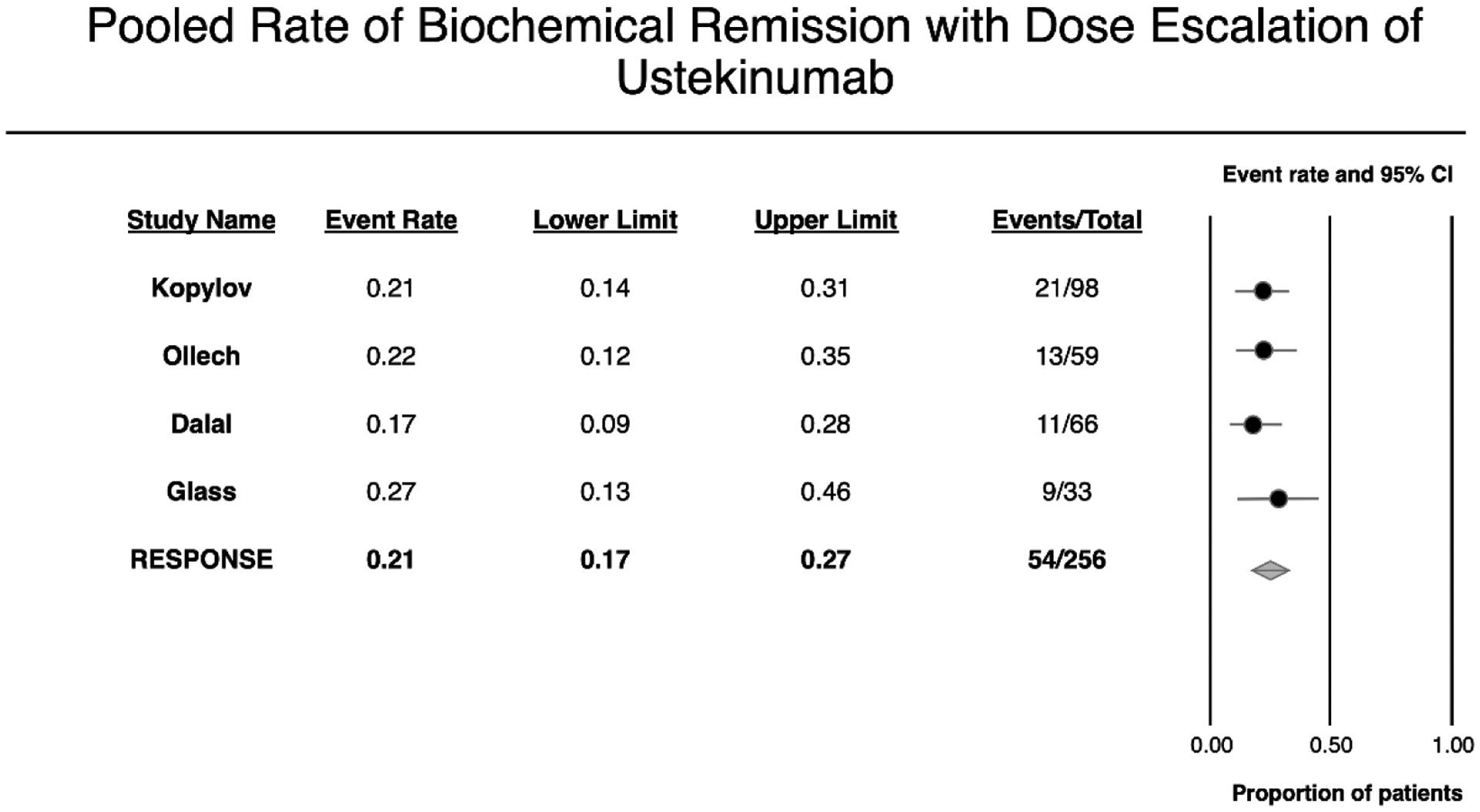
Pooled rate of biochemical remission with dose escalation of ustekinumab
Progression to surgery:
Six studies reported that with 11% patients (69/616) required surgical intervention despite ustekinumab dose escalation.10–12, 17, 18, 21
Factors associated with response to ustekinumab dose escalation
Five studies reported factors associated with recapturing response to ustekinumab dose escalation. Fumery and colleagues observed duration of ustekinumab therapy before dose escalation and reason for dose intensification (loss of response vs. incomplete response) may be associated with short-term clinical response on univariable analysis; however, these results were not significant on multivariable analysis.11 Similarly, Ollech and colleagues observed that patients with more severe disease were associated with lower probability of response to ustekinumab dose escalation; however, this was not significant on multivariable analysis.18 Dalal and colleagues observed that perianal disease (OR, 3.2; 95% CI, 1.0–9.9), clinically active disease based (Harvey Bradshaw Index >7; OR, 1.3; 95% CI, 1.1–1.6), and opiate use (OR, 3.4; 95% CI, 1.1–10.8) at time of dose escalation was associated with failure to achieve remission. Two studies did not report any significant factors associated with successful recapturing response.7, 17
DISCUSSION
A substantial proportion of patients treated with ustekinumab, particularly those with prior exposure to TNFα antagonists, may experience inadequate response, or loss of response after initial response. Ustekinumab dose escalation including re-induction and/or dose interval shortening is frequently pursued in these instances, though these strategies are frequently off-label in some jurisdictions. In this systematic review of 15 cohort studies of 925 ustekinumab-treated patients with CD who underwent dose escalation, we observed that more than 50% patients were able to capture response, including 40% patients who achieved corticosteroid-free clinical remission. Approximately 61% patients achieved endoscopic response, and 29% achieved endoscopic remission with dose escalation. Dose-interval shortening to every 4–6 weeks was the most frequent escalation strategy used in these studies. These findings suggest substantial benefit with dose escalation of ustekinumab in patients with refractory CD, in a subset of patients with partial response to therapy.
Several clinical and biological factors influence the pharmacokinetics of monoclonal antibodies, including age, sex, body mass index, burden of inflammation and albumin, leading to variability in drug clearance. Similar to TNFα antagonists, an exposure-response relationship has been suggested for ustekinumab with higher serum trough concentrations during maintenance associated with higher rates of clinical and endoscopic response. In post-hoc analyses of UNITI trials of CD, Adedokun and colleagues observed week 8 ustekinumab level >3.3μg/ml, and maintenance week 24 and 44 trough concentrations of >0.8–1.6μg/ml were associated with higher rates of clinical remission. Other studies have suggested ustekinumab concentration >4.5μg/ml may be associated with higher rates of clinical remission.28 These inter-individual differences in clearance of ustekinumab may explain why some patients are able to recapture response with dose escalation.
There was limited evaluation of factors associated with response to ustekinumab dose escalation. Most studies in the review did not report specific trough concentration cut-offs below which ustekinumab dose escalation was attempted or was successful. In one study, factors generally associated with worse prognosis including perianal disease, high clinical disease burden and opiate use were also associated with lower likelihood of response to ustekinumab dose escalation. Prior studies have identified that patients with prior primary non-response to TNFα antagonists may be less likely to respond to second-line biologics such as ustekinumab as compared with patients with secondary loss of response or intolerance to TNFα antagonists. In our review, >90% patients had prior exposure to TNFα antagonists, but reasons for failure of TNFα antagonists, and its impact on likelihood of response to ustekinumab dose escalation was not well-reported. Dulai and colleagues developed and validated a clinical decision support tool, based on active fistulizing disease, prior bowel surgery, active smoking, or prior TNFα antagonist exposure, associated with response to high-, intermediate- or low likelihood ustekinumab.29 In patients in the highest probability response group, ustekinumab trough concentrations were higher and speed of onset of response was faster. It is probable that patients in the low- or intermediate-probability group may be most likely to respond ustekinumab dose escalation.
There are important limitations to our systematic review as well as included studies. First, in retrospective cohort studies, indications for dose escalation, pattern of escalation and outcome measures assessing response to therapy were not systematically collected using standard definitions and variably reported in studies. This includes a significant difference in the nature of intervention – dose interval shortening was performed in 83% of cohort, whereas re-induction (without dose interval shortening) was performed in 12% of cohort. Second, there was limited systematic evaluation of factors associated with response to dose escalation, including trough concentration which may help identify patients most likely to benefit from escalation. Third, considerable heterogeneity was observed for several analyses, and we were limited in our ability to perform sub-group analyses to examine sources of heterogeneity. Fourth, all evidence is based on observational studies, and hence cause-effect relationship is difficult to infer. An ongoing clinical trial, POWER, examining the impact of intravenous re-induction of ustekinumab in patients with CD with secondary loss of response will further inform the magnitude of benefit (ClinicalTrials.gov Identifier: NCT03782376). Finally, our study focused only on ustekinumab dose escalation in patients with CD. Ustekinumab was approved for moderate to severely active ulcerative colitis in 2019, and emerging evidence suggests dose escalation may be warranted in some patients with ulcerative colitis.
In summary, in a meta-analysis of cohort studies, ustekinumab re-induction and/or dose interval shortening was effective in capturing response in patients with CD with inadequate response, or loss of response, to initial induction and/or maintenance therapy. Rates of capturing response are similar to what has been observed with dose escalation of TNFα antagonists and vedolizumab. This study helps to inform clinical practice and shared-decision making with patients while providing a synthesis of real-world evidence on ustekinumab dose escalation. However, the cost-effectiveness of such an approach has not been well-studied. Future studies evaluating predictors of response to dose escalation of ustekinumab are warranted.
Disclosures:
Dr. Meserve is supported by NIH/NIDDK (T32DK007202) Dr. Singh is supported by NIH/NIDDK (K23DK117058 and R03DK129631), IOIBD Operating Grant 2019, and the American College of Gastroenterology Junior Faculty Development Award (#144271).
Conflicts of Interest:
JM - None to declare
CM - Consulting fees from AbbVie, Amgen, AVIR Pharma Inc, Bristol Myers Squibb, Ferring, Fresenius Kabi, Janssen, McKesson, Mylan, Takeda, Pfizer, Roche, Alimentiv (formerly Robarts Clinical Trials Inc.); speaker’s fees from AbbVie, Amgen, AVIR Pharma Inc, Janssen, Takeda, and Pfizer; research support from Pfizer.
PSD - Research grants from Janssen, Takeda, Pfizer; Consulting for Abbvie, Janssen, Takeda, Pfizer, Gilead, BMS, Lily, Prometheus, Buhlmann; Scientific advisor for DigbiHealth
VJ - Received has received consulting fees from AbbVie, Eli Lilly, GlaxoSmithKline, Arena pharmaceuticals, Genetech, Pendopharm, Sandoz, Merck, Takeda, Janssen, Robarts Clinical Trials, Topivert, Celltrion; speaker’s fees from Takeda, Janssen, Shire, Ferring, Abbvie, Pfizer
SS - Research grants from AbbVie, Janssen
REFERENCES
- 1.Qiu Y, Chen BL, Mao R, et al. Systematic review with meta-analysis: loss of response and requirement of anti-TNFα dose intensification in Crohn’s disease. J Gastroenterol 2017;52:535–554. [DOI] [PubMed] [Google Scholar]
- 2.Ehrenberg R, Griffith J, Theigs C, et al. Dose Escalation Assessment Among Targeted Immunomodulators in the Management of Inflammatory Bowel Disease. J Manag Care Spec Pharm 2020;26:758–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Billioud V, Sandborn WJ, Peyrin-Biroulet L. Loss of response and need for adalimumab dose intensification in Crohn’s disease: a systematic review. Am J Gastroenterol 2011;106:674–84. [DOI] [PubMed] [Google Scholar]
- 4.Ma C, Huang V, Fedorak DK, et al. Adalimumab dose escalation is effective for managing secondary loss of response in Crohn’s disease. Aliment Pharmacol Ther 2014;40:1044–55. [DOI] [PubMed] [Google Scholar]
- 5.Honap S, Meade S, Ibraheim H, et al. Effectiveness and Safety of Ustekinumab in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Dig Dis Sci 2021. [DOI] [PubMed] [Google Scholar]
- 6.Hanauer SB, Sandborn WJ, Feagan BG, et al. IM-UNITI: Three-year Efficacy, Safety, and Immunogenicity of Ustekinumab Treatment of Crohn’s Disease. J Crohns Colitis 2020;14:23–32. [DOI] [PubMed] [Google Scholar]
- 7.Biemans VBC, van der Meulen-de Jong AE, van der Woude CJ, et al. Ustekinumab for Crohn’s Disease: Results of the ICC Registry, a Nationwide Prospective Observational Cohort Study. J Crohns Colitis 2020;14:33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bundschuh M, McDonough G, Kumar P, et al. 777 Effect of Ustekinumab Dose Escalation on Recapturing Clinical Response in Patients With Crohn’s Disease. Official journal of the American College of Gastroenterology | ACG 2019;114:S452. [Google Scholar]
- 9.Cohen E, Ha C, Syal G. 656 Ustekinumab Dose Escalation Is Effective in Treatment of Crohn’s Disease. Official journal of the American College of Gastroenterology | ACG 2019;114:S384–S385. [Google Scholar]
- 10.Dalal RS, Njie C, Marcus J, et al. Predictors of Ustekinumab Failure in Crohn’s Disease After Dose Intensification. Inflamm Bowel Dis 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fumery M, Peyrin-Biroulet L, Nancey S, et al. Effectiveness And Safety Of Ustekinumab Intensification At 90 Mg Every Four Weeks In Crohn’s Disease: A Multicenter Study. J Crohns Colitis 2020. [DOI] [PubMed] [Google Scholar]
- 12.Glass JAY, Petrasek J, et al. Gastroenterology. Gastroenterology 2020;158:960–961. [Google Scholar]
- 13.Haider SA, Yadav A, Perry C, et al. Ustekinumab dose escalation improves clinical responses in refractory Crohn’s disease. Therap Adv Gastroenterol 2020;13:1756284820959245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heron V, Panaccione N, Candido K, et al. Mo1895 – Efficacy of Intravenous Ustekinumab Re-Induction in Patients with Crohn’s Disease with a Loss of Response. Gastroenterology 2019;156:S–877. [Google Scholar]
- 15.Hudson J, Herfarth HH, Barnes EL. Letter: optimising response to ustekinumab therapy for patients with Crohn’s disease. Aliment Pharmacol Ther 2020;52:906. [DOI] [PubMed] [Google Scholar]
- 16.Johnson A, Dulai PS, et al. The Real-World Effectiveness of Ustekinumab in the Treatment of Crohn’s Disease. Conference Abstract. Digestive Disease Week 2021. 2021. [Google Scholar]
- 17.Kopylov U, Hanzel J, Liefferinckx C, et al. Effectiveness of ustekinumab dose escalation in Crohn’s disease patients with insufficient response to standard-dose subcutaneous maintenance therapy. Aliment Pharmacol Ther 2020;52:135–142. [DOI] [PubMed] [Google Scholar]
- 18.Ollech JE, Normatov I, Peleg N, et al. Effectiveness of Ustekinumab Dose Escalation in Patients With Crohn’s Disease. Clin Gastroenterol Hepatol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramaswamy PMH, et al. Efficacy of Dose Intensification of Ustekinumab in Crohn’s Disease. Gastroenterology 2020;158(6):S–956. [Google Scholar]
- 20.Sedano R, Guizzetti L, McDonald C, et al. Intravenous Ustekinumab Reinduction Is Effective in Prior Biologic Failure Crohn’s Disease Patients Already on Every-4-Week Dosing. Clin Gastroenterol Hepatol 2020. [DOI] [PubMed] [Google Scholar]
- 21.Young A, Tsistrakis S, Rubinov J, et al. Ustekinumab Dose Intensification Can Be Effective in Crohn’s Disease Patients Not Responding to Induction: 617. Official journal of the American College of Gastroenterology | ACG 2018;113:S351–S352. [Google Scholar]
- 22.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 2009;151:W65–94. [DOI] [PubMed] [Google Scholar]
- 23.Stang A Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- 24.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- 25.Kendall MG, Stuart A, Ord JK. Kendall’s advanced theory of statistics. New York: Oxford University Press, 1987. [Google Scholar]
- 26.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. Bmj 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sterne JA, Egger M, Smith GD. Systematic reviews in health care: Investigating and dealing with publication and other biases in meta-analysis. Bmj 2001;323:101–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Battat R, Kopylov U, Bessissow T, et al. Association Between Ustekinumab Trough Concentrations and Clinical, Biomarker, and Endoscopic Outcomes in Patients With Crohn’s Disease. Clin Gastroenterol Hepatol 2017;15:1427–1434.e2. [DOI] [PubMed] [Google Scholar]
- 29.Dulai P, Guizzetti L, Ma T, et al. 637 Clinical Prediction Model and Decision Support Tool for Ustekinumab in Crohn’s Disease. Official journal of the American College of Gastroenterology | ACG 2019;114:S373. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article are available within the article. All data were obtained from previously published studies in public domain.


