Abstract
Background
Triple-negative breast cancer (TNBC) is refractory and heterogeneous, comprising various entities with divergent phenotype, biology, and clinical presentation. As an aggressive subtype, Chinese TNBC patients with special morphologic patterns (STs) were restricted to its incidence of 10-15% in total TNBC population.
Methods
We recruited 89 patients with TNBC at Guangdong Provincial People’s Hospital (GDPH) from October 2014 to May 2021, comprising 72 cases of invasive ductal carcinoma of no-special type (NSTs) and 17 cases of STs. The clinical data of these patients was collected and statistically analyzed. Formalin-fixed, paraffin-embedded (FFPE) tumor tissues and matched blood samples were collected for targeted next-generation sequencing (NGS) with cancer-related, 520- or 33-gene assay. Immunohistochemical analysis of FFPE tissue sections was performed using anti-programmed cell death-ligand 1(PD-L1) and anti-androgen receptor antibodies.
Results
Cases with NSTs presented with higher histologic grade and Ki-67 index rate than ST patients (NSTs to STs: grade I/II/III 1.4%, 16.7%,81.9% vs 0%, 29.4%, 58.8%; p<0.05; Ki-67 ≥30%: 83.3% vs. 58.8%, p<0.05), while androgen receptor (AR) and PD-L1 positive (combined positive score≥10) rates were lower than of STs cases (AR: 11.1% vs. 47.1%; PD-L1: 9.6% vs. 33.3%, p<0.05). The most commonly altered genes were TP53 (88.7%), PIK3CA (26.8%), MYC (18.3%) in NSTs, and TP53 (68.8%), PIK3CA (50%), JAK3 (18.8%), KMT2C (18.8%) in STs respectively. Compared with NSTs, PIK3CA and TP53 mutation frequency showed difference in STs (47.1% vs 19.4%, p=0.039; 64.7% vs 87.5%, p=0.035).
Conclusions
In TNBC patients with STs, decrease in histologic grade and ki-67 index, as well as increase in PD-L1 and AR expression were observed when compared to those with NSTs, suggesting that TNBC patients with STs may better benefit from immune checkpoint inhibitors and/or AR inhibitors. Additionally, lower TP53 and higher PIK3CA mutation rates were also found in STs patients, providing genetic evidence for deciphering at least partly potential mechanism of action.
Keywords: Chinese breast cancer, triple negative breast cancer, special type, mutation landscape, PD-L1 (22C3)
Introduction
Triple-negative breast cancer (TNBC) is a special molecular subtype of breast cancer (BC) with unique biological and clinicopathologic characteristics, defined as estrogen receptor (ER)-, progesterone receptor (PR)-, and human epidermal growth factor receptor 2 (HER-2) - negative, representing 15-20% of BC cases (1, 2). Compared to other BC subtypes, TNBC is associated with a high histologic grade and strong invasiveness, lacking the opportunity for endocrine and targeted therapy due to the lack of corresponding targets (3). Chemotherapy is currently the main treatment, but its curative effect is unsatisfactory (4). At the histologic and genetic levels, TNBC represents a group of highly heterogeneous BCs, with the highest BRCA mutation rate among BC subtypes, particularly BRCA1 (5, 6). Moreover, invasive ductal carcinoma of no-special type (NSTs) also includes some special histologic subtypes with a special morphologic pattern (STs), such as medullary carcinoma, metaplastic carcinoma, and apocrine carcinoma (7–12). Although their immunophenotypes are triple-negative, their morphology, prognosis, and response to treatment are quite different (13). In recent years, TNBC has received extensive attention from a clinical and pathologic aspect. Targeted drugs for molecular typing and multiple signaling pathways have been extensively studied. In 2011, Lehmann et al. indicated the molecular classification of TNBC by DNA microarray firstly (14). However, for STs of TNBC, there are few studies on the combination of histologic examination results with genetic information, limited to its 10-15% incidence in TNBC.
At present, molecular-level research on BC has made great progress and gradually entered the era of precision treatment. This study aimed to identify the biological characteristics of the main TNBC histologic subtypes with genomic differences constituting important prognostic factors. The genomics of NSTs were compared to that of STs, and the panel, covering 520 cancer-related genes, was analyzed using capture-based ultra-deep targeted sequencing technology. A total of 89 TNBC cases were identified. The results of the genetic changes in the patients were combined with detailed histologic examination and PD-L1, AR test results for analysis.
Materials and Methods
Patient Selection
In this study, 89 hormone receptor (HR)-/HER2- patients with various tumor stages (stages I-IV), diagnosed at Guangdong Provincial People’s Hospital (GDPH) between October 2014 and May 2021, were enrolled. ER and PR expression in BC specimens were routinely evaluated at the Department of Pathology in GDPH, following the 2010 and 2013 American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) guidelines (15, 16). The HR status was determined as negative when immunohistochemistry (IHC) results of ER and PR were both <1%, while the HER2 status was determined as negative when IHC staining results were negative (0 to 1+) or equivocal (2+), and no amplification was identified by fluorescence in situ hybridization. This study was approved by the Ethics Committee of GDPH, and all patients provided written informed consent for translational research. Sequencing assays, blinded to the clinicopathologic parameters of the Clinical Laboratory Improvement Amendments-certified Burning Rock Biotech (Guangzhou, China), were performed.
Histologic and Clinicopathologic Criteria
In total, 89 patients’ clinicopathologic data were collected, comprising histologic subtypes, morphological features, age at diagnosis, tumor characteristics, Ki-67 index, tumor infiltrating lymphocytes (TILs), AR, and PD-L1 expression. Histological features of all available TNBC samples were centrally reviewed by the pathologists in GDPH.
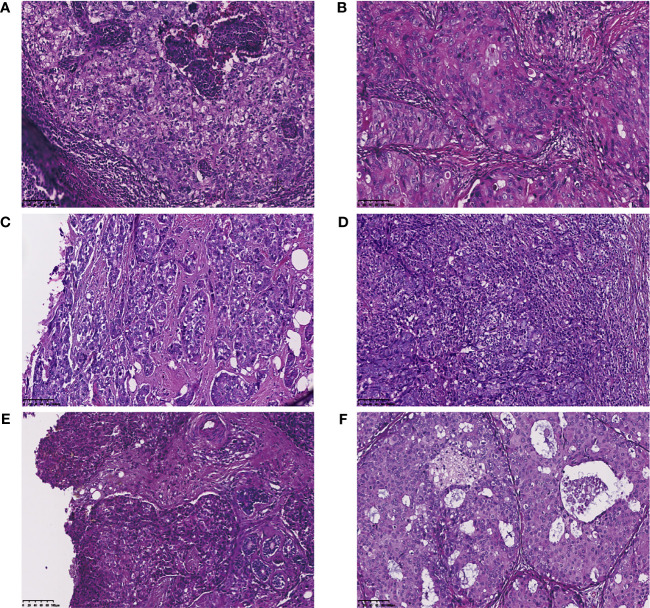
According to the 2012 and 2019 World Health Organization classification, BCs were classified as invasive ductal carcinoma NSTs and STs (special morphologic patterns, including metaplastic carcinoma, apocrine carcinoma, glycogen-rich clear cell carcinoma, and medullary carcinoma) ( Figure 1 ) (17–19), of which the predominant histologic subtype is the pattern with the highest percentage. Metaplastic carcinoma is defined as a carcinoma with squamous differentiation or spindle cell morphology (20). Apocrine carcinoma is defined as nuclear enlargement with prominent nucleoli and abundant, granular, eosinophilic cytoplasm (21). Glycogen-rich clear cell carcinoma is characterized by the presence of neoplastic cells with a glycogen-abundant clear cytoplasm (periodic acid-Schiff-positive, diastase-sensitive) (19). Medullary carcinoma always has the indistinct borders of the “pushing” type, giving the tumor a syncytial or sheet-like appearance. The tumor cells of this subtype are large and pleomorphic, with large nuclei, prominent nucleoli, and numerous mitoses. The prominent lymphoplasmacytic infiltrate at the periphery of the tumor is an important feature of medullary carcinoma (22).
Figure 1.
Invasive ductal carcinoma of no-special type and special morphologic pattern of triple negative breast cancers. (A) Invasive ductal carcinoma; (B) Apocrine carcinoma (Carcinoma with apocrine differentiation); (C) Metaplastic breast carcinoma; (D) Medullary carcinoma; (E) Glycogen-rich clear cell carcinoma; (F) Mixed type.
Immunohistochemistry
Immunohistochemical staining of formalin fixed, paraffin embedded (FFPE) tumor tissue sections were performed using anti-PD-L1 antibodies (clone 22C3, Cat#M3653, DAKO, 1:50 dilution; OrigiMed, Shanghai) and anti-AR antibodies (clone AR441, Mouse monoclonal, DAKO, 1:50 dilution, Gene Tech, Shanghai). All slides were counterstained with hematoxylin. PD-L1 expression was interpreted as a combined positive score (CPS), which was defined as the number of PD-L1-positive cells (including tumor cells, lymphocytes, and macrophages) divided by the total number of tumor cells, and then multiplied by 100. According to the 2021 National Comprehensive Cancer Network (NCCN) guidelines (23), the threshold for PD-L1 positivity was set at ≥10%. A positive AR status was defined as average of ≥1% positive tumor nuclei, which is the same cut-off follows the 2010 ASCO/CAP guidelines for ER and PR, frequently chosen by other studies.
Tumor-Infiltrating Lymphocytes Evaluation
Tumor infiltrating lymphocytes (TILs) were evaluated on hematoxylin and eosin (HE)-stained tissue sections at a magnification of ×20–40 with a ×10 ocular, according to the recommendations of an International Tumor Infiltrating Lymphocytes Working Group (24, 25). “High”TILs were defined as a tumor with 40-90% stromal TILs, presented as percentage of the stromal areas alone, and “Intermediate” TILs were defined as 10-40%, while “Low” TILs were defined as 0-10%.
Comprehensive Genomic Profiling
Next-generation sequencing library preparation and sequence data analyses were conducted according to a previous study (26, 27). Genomic profiling was performed using a panel of 520 cancer-related genes (OncoScreen Plus; Burning Rock Biotech, Guangzhou, China). Whole exons of 312 genes and critical exons, introns, and promoter regions of the remaining 208 genes were captured. Ten mL of peripheral blood was collected. Events were then classified as germline or somatic, depending on their presence in the matched normal set (white blood cells) of events.
Sequence Data Analysis
Sequence data were mapped to the human genome (hg19) using BWA aligner v.0.7.10. Local alignment optimization, variant calling, and annotation were performed using GATK v.3.2 and VarScan v.2.4.3. Variants were filtered using the VarScanfpfilter pipeline, with loci with depths less than 100 filtered out. At least five supporting reads were needed for insertions or deletions, while eight supporting reads were required for single-nucleotide variations (SNVs). According to the ExAC, 1000 Genomes, dbSNP, and ESP6500SI-V2 databases, variants with population frequencies over 0.1% were grouped as single-nucleotide polymorphisms (SNPs) and excluded from further analysis. The remaining variants were annotated using ANNOVAR and SnpEff v.3.6. DNA translocation analysis was performed using the Factera v.1.4.3.
Statistical Analysis
All data were summarized by frequency and percentage for categorical variables including mutation detection rate and distribution of mutation types. SPSS software version 26.0 (IBM Corp., Armonk, NY, USA) and R 3.5.1 (R Core Team, Vienna, Austria) were used for statistical analyses. Pearson’s chi-squared (χ2) test and Fisher’s exact test were used to test the pairwise correlation among clinicopathologic features, mutation detection rate, and distribution of mutation types. Statistical significance was set at p < 0.05.
Results
Clinicopathologic Features of the Patients
In total, tumor tissues of 89 patients with invasive TNBC were examined, which were divided into the NSTs (N=72) and STs (N=17) groups according to their histologic type ( Figure 1 ). The clinicopathologic features of the patients are summarized in Table 1 . All 89 patients were female, with a median age at diagnosis of 49 (range 22-81) years. The premenopausal status rate was 58.4% (of 52/89). The majority of patients included in this cohort presented with an early TNM stage. As shown in Table 1 , the NSTs group had a higher histologic grade (NST to ST: grade I, 1.4%; grade II, 16.7%; and grade III, 81.9% vs. grade I, 0%; grade II, 29.4%; and grade III, 58.8%; p<0.05) than the ST group according to the 8th edition of the American Joint Committee on Cancer (AJCC) staging system (18). No significant association was found between age at onset, menopausal status, lymph node status, tumor size, and TNM stage between the two groups.
Table 1.
Clinicopathologic characteristics of the GDPH TNBC cohort (N=89).
| Variables | TNBC | No-special Type | Special Type | p | |||
|---|---|---|---|---|---|---|---|
| N=89 | No. (%) | N=72 | No. (%) | N=17 | No. (%) | ||
| Age | 0.813 | ||||||
| [median, range] | 49 | [22-81] | 49 | [23-75] | 46 | [22-81] | |
| ≤50 years | 52 | 58.4 | 43 | 59.7 | 9 | 52.9 | |
| >50 years | 37 | 41.6 | 29 | 40.3 | 8 | 47.1 | |
| Menopausal status | 1 | ||||||
| Pre-menopause | 49 | 55.1 | 40 | 55.6 | 9 | 52.9 | |
| Post-menopause | 40 | 44.9 | 32 | 44.4 | 8 | 47.1 | |
| T-stage | 0.428 | ||||||
| T1 | 39 | 43.8 | 33 | 45.8 | 6 | 35.3 | |
| T2 | 40 | 44.9 | 30 | 41.7 | 10 | 58.8 | |
| T3 | 6 | 6.7 | 6 | 8.3 | 0 | 0.0 | |
| T4 | 4 | 4.5 | 3 | 4.2 | 1 | 5.9 | |
| N-stage | 0.604 | ||||||
| N0 | 48 | 53.9 | 39 | 54.2 | 9 | 52.9 | |
| N1 | 22 | 24.7 | 16 | 22.2 | 6 | 35.3 | |
| N2 | 13 | 14.6 | 11 | 15.3 | 2 | 11.8 | |
| N3 | 6 | 6.7 | 6 | 8.3 | 0 | 0.0 | |
| M-stage | 1 | ||||||
| M0 | 88 | 98.9 | 71 | 98.6 | 17 | 100.0 | |
| M1 | 1 | 1.1 | 1 | 1.4 | 0 | 1.0 | |
| Pathologic stage | 0.621 | ||||||
| I | 27 | 30.3 | 22 | 30.6 | 5 | 29.4 | |
| II | 42 | 47.2 | 32 | 44.4 | 10 | 58.8 | |
| III | 19 | 21.3 | 17 | 23.6 | 2 | 11.8 | |
| IV | 1 | 1.1 | 1 | 1.4 | 0 | 0.0 | |
| Histologic grade | 0.022# | ||||||
| I | 1 | 1.1 | 1 | 1.4 | 0 | 0.0 | |
| II | 17 | 19.1 | 12 | 16.7 | 5 | 29.4 | |
| III | 69 | 77.5 | 59 | 81.9 | 10 | 58.8 | |
| NA | 2 | 2.2 | 0 | 0.0 | 2 | 11.8 | |
| Ki-67(%) | 0.040# | ||||||
| [median, range] | 65 | [5-95] | 70 | [10-95] | 50 | [5-90] | |
| <14% | 7 | 7.9 | 3 | 4.2 | 4 | 23.5 | |
| ≥14% | 81 | 91.0 | 68 | 94.4 | 13 | 76.5 | |
| <30% | 18 | 20.2 | 11 | 15.3 | 7 | 41.2 | 0.048# |
| ≥30% | 70 | 78.7 | 60 | 83.3 | 10 | 58.8 | |
| NA | 1 | 1.1 | 1 | 1.4 | 0 | 0.0 | |
| AR | 0.002# | ||||||
| Positive (≥1%) | 16 | 18.0 | 8 | 11.1 | 8 | 47.1 | |
| Negative (<1%) | 59 | 66.3 | 50 | 69.4 | 9 | 52.9 | |
| NA | 14 | 15.7 | 14 | 19.4 | 0 | 0.0 | |
| TILs | 0.092 | ||||||
| Low (0-10%) | 36 | 40.4 | 27 | 37.5 | 9 | 52.9 | |
| Intermediate (10-40%) | 14 | 15.7 | 14 | 21.5 | 0 | 0.0 | |
| High (40-90%) | 32 | 36.0 | 24 | 33.3 | 8 | 47.1 | |
| NA | 7 | 7.9 | 7 | 9.7 | 0 | 0 | |
| Histologic type | <0.001# | ||||||
| IDC | 72 | 81.0 | 72 | 100.0 | 0 | 0.0 | |
| Mixed | 6 | 6.7 | 0 | 0.0 | 6 | 35.3 | |
| AC | 5 | 5.6 | 0 | 0.0 | 5 | 29.4 | |
| MC | 4 | 4.5 | 0 | 0.0 | 4 | 23.5 | |
| MBC | 1 | 1.1 | 0 | 0.0 | 1 | 5.9 | |
| GRCC | 1 | 1.1 | 0 | 0.0 | 1 | 5.9 | |
The p-value was calculated using the Pearson χ2 test and Fisher’s exact test; #, p-value<0.05. TNBC, triple-negative breast cancer; TILs, tumor-infiltrating lymphocyte; AR, androgen receptor; IDC, infiltrating ductal carcinoma; Mixed, mixed histologic; AC, apocrine Carcinoma; MC, medullary carcinoma; MBC, metaplastic breast carcinoma.
Ki-67 index data were available from 88 patients, with a median Ki-67 index of approximately 65% (ranging from 5% to 95%). In the NST group, the number of patients with a high Ki-67 index (≥30%) was significantly higher than that in the ST group (83.3% vs. 58.8%, p<0.05). Meanwhile, androgen receptor (AR) expression data were available from 75 patients, showing that the number of AR-positive NSTs patients was lower than that in the STs group (11.1% vs. 47.1%, p<0.01).
Furthermore, the PD-L1 IHC results of 67 qualified tumor samples were analyzed, including 52 NST and 15 ST cases ( Table 2 ). The percentage of PD-L1 positivity was 14.9% (10/67) in 67 patients with TNBC. Compared the expression levels of PD-L1 between the two groups, a PD-L1 score cut-off of 10 for positive was selected and found that the proportion of STs with PD-L1 CPS greater than or equal to 10 was higher than that of NSTs (33.3%; 9.6%, p=0.038). Considering PD-L1 as an immune biomarker, these results may suggest that ST TNBC patients might benefit from immunotherapy over NST TNBC patients. The stromal TILs for each case were also evaluated, and no significant differences were found between the two groups.
Table 2.
Characteristics of PD-L1 expression in the GDPH TNBC cohort (N=67).
| Variables | TNBC | No-special | Special | p | |||
|---|---|---|---|---|---|---|---|
| N=67 | No. (%) | N=52 | No. (%) | N=15 | No. (%) | ||
| PD-L1(cut-off=10) | 0.038# | ||||||
| Negative (<10) | 57 | 85.1 | 47 | 90.4 | 10 | 66.7 | |
| Positive (≥10) | 10 | 14.9 | 5 | 9.6 | 5 | 33.3 | |
p-value was calculated using the Pearson χ2 test and Fisher’s exact test; #, p-value<0.05.
Genomic Alteration Landscape of TNBC
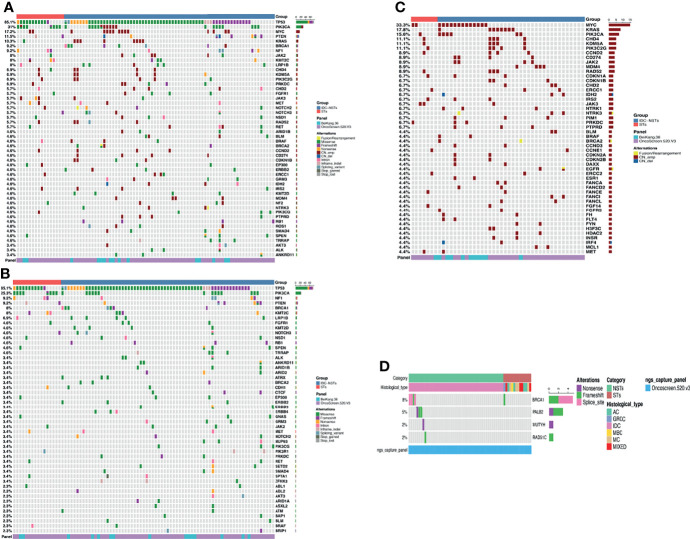
A total of 794 alterations, including 513 single-nucleotide variants (SNVs), 252 copy number (CN) amplifications, five copy number (CN) deletions, 14 insertions or deletions (Indels), nine fusions, and one large genomic rearrangement were detected in 285 genes from 89 patients. As shown in Figure 2 , TP53(85.1%) and PIK3CA (31%) were the top-ranked altered genes in this cohort, in which missense mutations were the predominant alterations. Further, alterations detected in the cohort included MYC (17.2%), PTEN (11.5%), and KRAS (10.3%). Besides, the vast majority of highest-frequency copy number alterations were seen in 43 patients (48.3%), including amplifications in MYC (33.3%), KRAS (17.8%), PIK3CA (15.6%), and CHD4 (11.1%). Based on the available data from 62 patients (paired white blood cells and tumor samples were sequenced using a panel consisting of 520 cancer-related genes, including 62 cancer susceptibility genes to interrogate germline and somatic alterations, respectively), the analysis revealed that 10 patients carried pathogenic germline mutations (10/62, 16.1%) for BRCA1 (5/62, 8.0%), PALB2 (3/62, 4.8%), MUTYH (1/62, 1.6%) and RAD51C (1/62, 1.6%). Except for one ST patient, all other patients carrying pathogenic germline mutations were of the NSTs subtype.
Figure 2.
The landscape of genetic alterations in TNBC. Top 50 genomic alterations are shown in the Oncoprint. Different colors denote different types of alterations and different clinicopathologic features. (A) Summary of the features of the genomic alteration of the 89 patients with TNBC. Tumor samples were grouped according to histologic types as: no-special type (NSTs, n = 72) and special type (STs, n = 17). The top bar shows the histologic type of each patient; the side bar (rows) summarizes the percentage of tumors with alterations in each gene (left) and alteration composition for each gene in the entire cohort (right). (B) Summary of the features of the genomic mutation of the 89 patients with TNBC. Tumor samples were grouped according to histologic types as: no-special type (NSTs, n = 72) and special type (STs, n = 17). The top bar shows the histologic type of each patient; the side bar (rows) summarizes the percentage of tumors with mutations in each gene (left), and the mutation composition for each gene in the entire cohort (right). (C) Summary of copy number variations and Fusion in the 45 patients with TNBC who carry copy number variations. Tumor samples were grouped according to histologic types as: no-special type (NSTs, n = 38) and special type (STs, n = 7). The top bar shows each patient’s histologic type; the side bar (rows) summarizes the percentage of tumors with variation in each gene (left) and alteration composition for each gene (right), in the entire cohort. (D) Summary of germline mutation of the 62 patients with TNBC. Tumor samples were grouped according to histologic types as: no-special type (NSTs, n = 48) and special type (STs, n = 14). The side bar (rows) summarizes the percentage of tumors with mutation in each gene (left) and alteration composition for each gene (right), in the entire cohort. TNBC, triple-negative breast cancer; NST, no-special type; ST, special type; indel, insertions or deletions; LGR, large genomic rearrangement; CN_amp, copy number amplification; CN_del, copy number deletion.
Differentially Mutated Genes Between TNBC NST and ST Patients
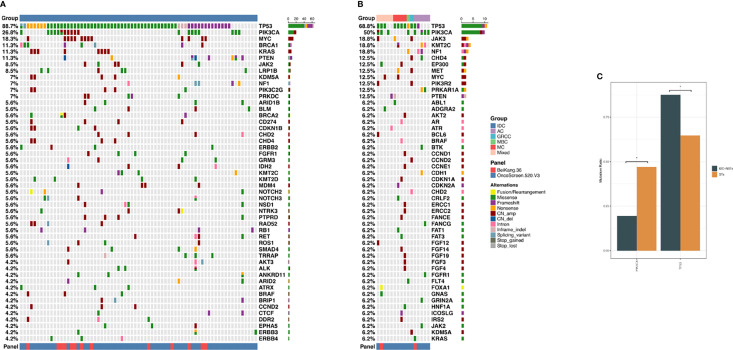
The differentially altered genes in TNBC NST and ST tumors were compared. As shown in Figure 3 , the top five altered genes were TP53 (88.7%), PIK3CA (26.8%), MYC (18.3%), BRCA1 (11.3%), and KRAS (11.3%) in NSTs, and TP53 (68.8%), PIK3CA (50%), JAK3 (18.8%), KMT2C (18.8%) and NF1 (18.8%) in STs. Considering mutations of oncogenic genes, TP53 (87.5%), PIK3CA (19.4%), PTEN (9.7%), and BRCA1 (8.3%) were most frequently mutated in NSTs, while TP53 (64.7%), PIK3CA (47.1%), and NF1 (11.8%) were the most frequently mutated in STs. TP53 and PIK3CA were found to be differentially mutated between NSTs and STs. The mutation frequency of TP53 was higher in TNBC NSTs than in STs, while PIK3CA mutations were more frequent in STs (87.5% vs. 64.7%, p=0.035; 19.4% vs. 47.1%, p=0.039, respectively). Moreover, BRCA1 somatic mutations were only identified in six (8.3%) NST TNBC patients. Copy number variant analysis of 38 NST patients and 7 STs patients revealed that MYC (34.2%), KRAS (21.1%), and PIK3CA (15.8%) were more frequently amplified in NSTs. In STs, however, the top-ranked copy number amplification genes were JAK3 (11.8%), MYC (11.8%), and PIK3R2 (11.8%).
Figure 3.
(A) Summary of genomic features of the 72 NST cases of TNBC patients. (B) Summary of genomic features of the 17 ST cases of TNBC patients. (C) Differentially mutated genes between NSTs and STs TNBC patients. The X-axis represents the specific genes. The Y-axis represents the percentage of samples with mutations in a specific gene. * indicated p values <0.05. TNBC, triple-negative breast cancer; NST, no-special type; ST, special type.
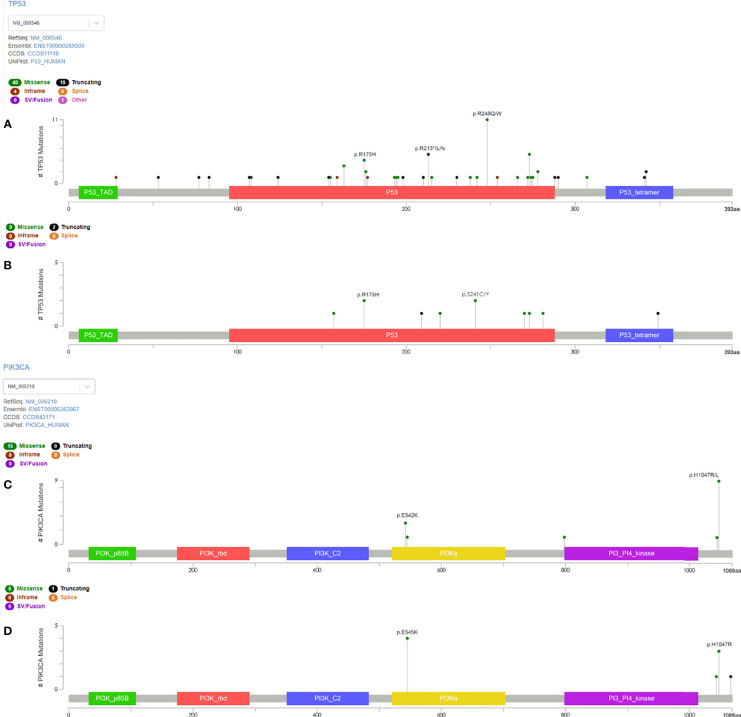
The alteration types and mutation sites of TP53 and PIK3CA in the TNBC-NST and ST groups were compared, studied, and are illustrated in Figures 3 , 4 . Sixty-three NST TNBC patients (63/72, 87.5%) and 11 STs patients (11/17, 64.7%) had TP53 mutations, which occurred in several exons (exons 4-10). Missense mutations were the dominant mutation form in both groups (39/63, 61.9%; 9/11, 81.8%), followed by frameshift and nonsense mutations (13/63, 20.6%; 6/63, 9.5%; 1/11, 9.1%; 1/11, 9.1%, respectively). The mutation sites of TP53 were also different between the two groups. Among NST TNBC patients, p.R248Q/W (11/63, 17.5%), p.R213*/fs/L (5/63, 7.9%), and p.R175H (4/63, 6.3%) accounted for the highest proportion of all mutation sites, whereas p.R175H and p.S241C/Y (2/11, 18.2%) mutation sites occupied the highest proportion, without p.R248 site-related mutations in the ST group.
Figure 4.
Lollipop diagram of the TP53 and PIK3CA domains with the mutation location identified in TNBC patients. Different types of mutations were colored by different colored dots, and each colored dot represents one mutation. The length of the lollipop represents the number of patients harboring a specific variant. (A) The type and location of TP53 mutation in NST patients. (B) The type and location of TP53 mutation in ST patients. (C) The type and location of PIK3CA mutation in NST patients. (D) The type and location of PIK3CA mutation in ST patients. TNBC, triple-negative breast cancer; NST, no-special type; ST, special type.
Regarding PIK3CA, a total of 24 mutations occurred in 22 patients, including 14 NST and eight ST patients; and one patient had double mutations in each group. PIK3CA mutations occurred in exons 10, 16, and 21 in the NSTs, and in exons 10 and 21 in ST patients. In both groups, missense mutations were the primary alteration type, followed by copy number amplification. In the NST group, p.H1047R (8/14, 57.1%) mutation sites accounted for the highest proportion, followed by p.E542K (3/14, 21.4%) substitutions. In the ST group, p. E542K (4/8, 50%) and p.H1047R (3/8, 37.5%) mutation sites accounted for the highest proportion.
Mutation Pathway Analysis in NST and ST Patients
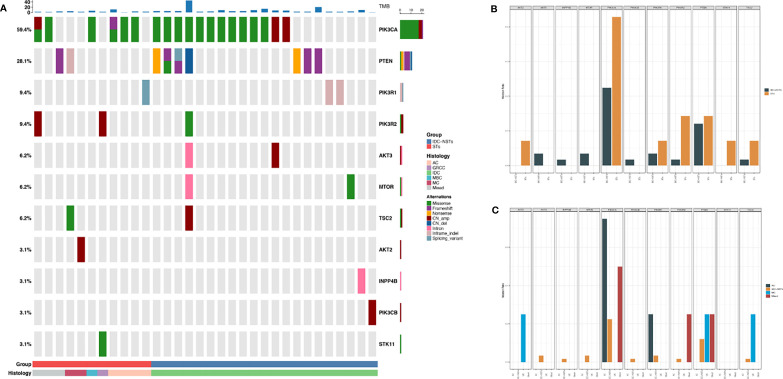
For further analysis, the molecular signatures database was used to analyze the distinct pathways that were affected by NSTs and STs. It revealed the major signaling pathways affected by genomic alterations in the ST patients as PI3K ( Figure 5 , 36.2% vs. 78.6%, p= 0.006). Although the presence of other signaling pathways in the two groups was diverse (cell cycle, HIPPO, TGF-beta, etc.), the statistical differences were insignificant. An in-depth pathway analysis within ST patients was performed, illustrating that patients with mixed histologic type presented lower PI3K pathway mutation levels than medullary carcinoma and apocrine carcinoma, but were still higher than NSTs. Mutations in genes involved in the PI3K pathway, detected in our cohort, included AKT2 (n=1), AKT3 (n=2), INPP4B (n=1), MTOR (n=2), PIK3CA (n=19), PIK3CB (n=1), PIK3R1 (n=3), PIK3R2 (n=3), PTEN (n=9), STK11 (n=1), and TSC2 (n=2). Alterations in AKT3 (n=2), INPP4B (n=1), MTOR (n=2), PIK3CA (n=6), PIK3CB (n=1), PIK3R1 (n=2), PIK3R2 (n=1), PTEN (n=7), and TSC2 (n=1) were detected in 58 patients with NST tumors. Five patients with NSTs had concurrent alterations in at least two genes. Meanwhile, AKT2 (n=1), PIK3CA (n=10), PIK3R1 (n=1), PIK3R2 (n=2), PTEN (n=2), STK11(n=1), and TSC2 (n=1) alterations were detected in 14 patients with ST tumors. Among these, three patients had at least two gene alterations.
Figure 5.
Kyoto Encyclopedia of Genes and Genomes analysis reveals distinct pathways in NSTs and STs tumors of TNBC. In total, 36.2% of the NSTs patients and 78.6% of the STs patients had at least one clinically relevant genomic alteration in genes involved in the PI3K-AKT signaling pathways, respectively. (A) Summary of the features of the PI3K-AKT signaling pathways genomic alteration of the 32 patients with TNBC. Tumor samples were grouped according to histologic types as: no-special type (IDC-NSTs, n=21) and special type (STs, n=11). (B) Comparison of the features of the PI3K-AKT signaling pathways genomic alteration between NSTs and STs TNBC patients. (C) Comparison of the features of the PI3K-AKT signaling pathways genomic alteration among STs TNBC patients. TNBC, triple-negative breast cancer; NST, no-special type; ST, special type; TMB, tumor mutation burden.
Collectively, these results indicate that NST and ST tumors are molecularly distinct based on the difference in the number and distribution of somatic alteration types, as well as the signaling pathways affected by these alterations.
Discussion
TNBC is clinically and molecularly heterogeneous and highly refractory and belongs to a mixed sub-category. It is a collective term for the remaining BCs after excluding ER, PR, and HER2-positive patients, accounting for 15%-20% of all BCs. In addition to the majority of invasive ductal carcinomas of NSTs, TNBC also includes a small number of STs, which have special morphological characteristics. According to the Cancer Genome Atlas (TCGA) and the Molecular Taxonomy of Breast Cancer International Consortium (METABRIC) cohorts, STs TNBC patients had poorer prognosis than NSTs ( Supplementary Figure 1 ). The Surveillance, Epidemiology, and End Results (SEER) database was utilized to determine the differences between TNBC-NSTs and STs, regarding survival, overall survival, and disease-specific survival rates (28). However, adjuvant therapy for TNBC NSTs and STs is indifferently represented by chemotherapy, without effective prognostic markers or therapeutic targets. To develop precision therapy, the molecular classification of TNBC is defined by DNA microarrays, gene sequence expression, mRNA expression, and IHC (29–31). This research explored the differences in clinicopathologic and molecular characteristics between the NSTs and the STs of Chinese patients with TNBC, looking for relevant markers that may be helpful in the development of precise and individualized systemic drugs for TNBC.
The 89 patients with TNBC were divided into two groups according to pathologic type for comparison. TNBC-NSTs and STs were similar in terms of the age of onset, tumor size, and TNM stage. Interestingly, the histologic grade and Ki-67 index of the NSTs were significantly higher than those of the STs. The histologic grades of invasive BCs are based on the differentiation of tumor cells, which is an important factor in predicting the prognosis of patients with BC and tumor aggressiveness. As confirmed by the NCCN guidelines, a higher histologic grade is an unfavorable factor for BC, which may require more aggressive adjuvant treatments. Ki-67 is a proliferation biomarker that is considered an independent predictive and prognostic factor for the management of BC (32, 33). BCs with high Ki-67 expression have proved to respond better to chemotherapy, and are associated with poor prognosis. Higher histologic grade and Ki-67 index of the NSTs may indicate that the prognosis of NSTs is worse than that of STs.
Immunotherapy with anti-PD-1/PD-L1 agents has emerged as a new treatment modality for TNBC in recent years (34, 35), introducing a novel therapeutic field. PD-L1 expression has been recognized as the most important biomarker for predicting the response to immunotherapy in cancers (36). Mittendorf et al. described that approximately 20%-30% of breast tumors express PD-L1, especially TNBC (37). In this study, the percentage of PD-L1 positivity was 14.9% in 67 TNBC patients. Compared with NSTs, the positive percentage of PD-L1 in STs was higher. These results suggest that ST patients may benefit more from treatment targeting the PD-L1 pathway. Besides, targeted therapy of AR has become another major hotspot in TNBC treatment. Research showed that STs patients presented significantly higher AR-positive rates than NSTs, which indicates that these patients are more likely to benefit more from AR inhibitors (38, 39). Multiple clinical studies have examined immunotherapy for TNBC. For advanced TNBC, based on the results of the Impassion130 and KEYNOTE-355 trials, Atezolizumab and Pembrolizumab have been approved in combination with chemotherapy as first-line treatments for PD-L1 positive advanced TNBC (40–42). For early TNBC, the results of KEYNOTE-522 trial set a new standard for early TNBC immunotherapy, and the combination chemotherapy of Pembrolizumab has been approved by the FDA for neoadjuvant therapy in patients with high-risk TNBC (43). Updates to the GeparNUEVO trial also confirmed the positive significance of neoadjuvant immunotherapy. To date, neither AR inhibitors (bicalutamide and enzalutamide) nor androgen biosynthetic inhibitor (abiraterone) exhibited significant anticancer activity against patients with AR positive metastatic TNBC (clinical benefit rates range from 19% to 35%) (44). Several clinical studies are under way.
For developing precision therapy, molecular information has some instructive significance. While the genomic landscape of TNBC is heterogeneous and complex, it was shown that Chinese patients with TNBC display a similar mutation spectrum to that reported in studies conducted in other countries, and TP53 (85.1%), PIK3CA (31%), and MYC (17.2%) are some of the most frequent alterations. Further studies performed to compare gene mutation profiling of NSTs to those of STs have found that the NSTs had higher frequencies of TP53 mutation than STs, while the PIK3CA mutation frequency was higher in the STs than in the NSTs. The tumor suppressor gene, TP53, is the most frequently mutated gene in human cancer (45, 46). Patients with BC with a somatic TP53 mutation have poor prognosis, which is consistent with the present research. Unfortunately, TP53 mutations are not presently targetable (47), and their predictive capacity for various therapies for BC has not been thoroughly examined. TP53 mutations are frequently found in BRCA1-associated human BCs (48–50). Except for one ST patient, six patients carrying BRCA1 mutations were found in NSTs in this study. Describing the mutation site of TP53 in the two groups separately, p.R248Q/W, p.R213*/fs/L, and p.R175H had the highest proportion in the NST group, while p.R175H and p.S241C/Y mutation sites had higher frequencies in the ST group. Although there are differences in the mutational profiling of TP53 between the two groups, the relevance of the mutation hotspots identified in the GDPH patients to the treatments for BC remains to be determined. Recently, Bert Vogelstein et al. successfully identified a bispecific single chain diabody (scDb) highly specific to the common TP53 mutations, and confirmed its mechanism of activating T cells to exert anti-tumor effects (51). Nevertheless, gene therapies, targeted tumor vaccines, and anti-cancer agents for TP53 alteration are still in the early stages of clinical trials, including APR-246, which is a targeted drug for three hot-spot mutations of the TP53 gene (R273, R175, and R248) (52, 53). Their effectiveness has not been proven in clinical trials. Therefore, authorities such as the United States Food and Drug Administration (FDA) have not approved any treatment for TP53 alteration yet. For TNBC patients who have BRCA defection or mutation, PARP inhibitors can block BRCA1/2-mediated homologous-recombinant based DNA double-strand break repair and promote tumor cell apoptosis. In the OlympiAD study, Olaparib, the PARP inhibitor had an improvement in median OS and 3-year survival rate compared with traditional chemotherapy for metastatic TNBC (54). This study led to olaparib’s approval from the FDA and EMA for the treatment of HER2-negative metastatic breast cancer patients with BRCA 1/2 mutations, opening the door to TNBC-targeted therapy. In 2021, Interim data from the OlympiA study were released, which suggesting that one-year adjuvant therapy with Olaparib alone can obviously reduce the risk of recurrence and death in HER2-negative early-stage breast cancer patients with gBRCA mutation (55). Olaparib has been accepted as the standard adjuvant therapy for high-risk HER2-negative early-stage breast cancer patients with gBRCA mutations.
From a molecular perspective, mutations in PIK3CA genes are found significantly more frequently in STs than in NSTs. Alteration types were dominated by missense mutations in both groups. Nevertheless, copy number amplification accounted for a larger proportion of NSTs than that in STs, indicating that the genome of the NSTs was unstable. Consistent with previous studies, the majority of PIK3CA mutations occurred in three hotspot sites, namely E542K and E545K, in the helical domain and H1047R, in the kinase domain (56, 57). In the NST group, p.H1047R (57.1%) mutation sites accounted for the highest proportion, while p.E542K (50%) mutation sites were the most frequently altered in the ST group. For HR-positive metastatic BC, the present study showed that the p.H1047R mutation might be a potential biomarker of sensitivity to everolimus, an mTOR inhibitor (58). Notably, the FDA-approved PI3K inhibitor, alpelisib, is highly recommended in PIK3CA-mutated HR-positive BC according to the most recently updated guidelines (23). Although the TNBC ST patients were negative for ERs and PRs, 50% of the ST patients still harbored PIK3CA mutations; thus, it is rational to assume the potential effect of alpelisib in the treatment strategy for the STs. Considering AKT as a downstream effector protein for PI3K, phase II trial LOTUS and PAKT tested highly selective oral pan-AKT inhibitors Ipatasertib and Capivasertib in combination with paclitaxel in the treatment of metastatic TNBC, with significant improvement in PFS (59, 60). In the FAIRLANE Phase II trial, adding Ipatasertib with neoadjuvant chemotherapy would significantly increase the pCR rate compared with adding placebo (61).
This study has several limitations. First, the relatively small number of cases in the two morphologic groups renders the analysis performed exploratory and hypothesis-generating. Secondly, the presence of somatic genetic alterations affecting 520 genes was surveyed. Hence, the possibility of additional differences between the NSTs and STs of TNBC being present if whole exome or whole genome sequencing was performed cannot be excluded. Incorporation of comprehensive genomic profiling into TNBC may shed light on potential therapeutic opportunities for both targeted drugs and immune checkpoint inhibitors. Additionally, prognostic data for patients with TNBC in the GDPH queue was not provided in this study. Although the higher Ki-67 index, histologic grades, and proportion of TP53 mutations suggested that the NST patients had poor prognoses, there was no reliable evidence to prove the relationship between the NSTs and STs. The STs have multiple pathological types, considering that some TNBC subtypes may exhibit different somatic cytogenetic changes. Resultantly, it is necessary to further study the genomic pattern of specific TNBC subgroups.
In conclusion, this study has shown significant differences in clinicopathologic features and gene signatures between Chinese NST and ST TNBC patients. The increased PD-L1 and AR expression, as well as elevated PIK3CA mutation frequency in the STs group implied that potential treatments might be available for these patients. Further studies with lager sample size and clinical outcomes data will be needed to explore the possibility to use immune checkpoint inhibitors, AR inhibitors and PIK3CA mutation-related treatments in TNBC patients with ST.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: NODE (http://www.biosino.org/node) with accession OEP002982 (http://www.biosino.org/node/project/detail/OEP002982).
Ethics Statement
The studies involving human participants were reviewed and approved by GDREC2014122H. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
NL, BC, and Y-ZL conceived, designed and supervised the study. Y-ZL, X-YL, J-LL, C-YR, L-ZW, F-RC, XL, L-PG, X-FQ, and YL collected the data, analyzed and interpreted them. YL, BC, X-YL, NL, G-CZ, J-GL, W-KX, CL, and HM wrote and revised the manuscript. All authors read and approved the final manuscript for submission.
Funding
This study received support from the Fundamental Research Funds for the Central Universities (y2syD2192230, BC); the National Natural Science Foundation of China (81902828, BC); High-level Hospital Construction Project of Guangdong Provincial People’s Hospital (DFJH201921, BC); Guangdong Medical Research Foundation (B2019039, BC); Natural Science Foundation of Guangdong Province (2016A030313768, NL; 2018A030313292), and Research Funds from Guangzhou Municipal Science and Technology Project (201707010418, NL).
Conflict of Interest
Authors X-FQ and YL are employed by OrigiMed Co. Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank all the patients and their families for participating in this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.830124/full#supplementary-material
Kaplan-Meier survival curves based on no-special type infiltrating ductal carcinoma and special types of triple negative breast cancer patients. (A) Disease free status of patients in the Cancer Genome Atlas cohort. (B) All patients in the Cancer Genome Atlas cohort. (C) All patients in the Molecular Taxonomy of Breast Cancer International Consortium cohort.
Immunohistochemical staining of programmed death-ligand 1(PD-L1) in triple- negative breast cancer (Magnification 200×). (A) Negative control of PD-L1 expression by immunohistochemistry. (B) Low PD-L1 expression in triple- negative breast cancer tumor samples. (C) High PD-L1 expression in triple- negative breast cancer tumor samples.
Hematoxylin and eosin staining of tumor-infiltrating lymphocytes (TILs) in triple-negative breast cancer (Magnification 200×). (A) Low-grade TILs. (B) Intermediate-grade TILs. (C) High-grade TILs.
References
- 1. Stagg J, Allard B. Immunotherapeutic Approaches in Triple-Negative Breast Cancer: Latest Research and Clinical Prospects. Ther Adv Med Oncol (2013) 5(3):169–81. doi: 10.1177/1758834012475152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Foulkes WD, Smith IE, Reis-Filho JS. Triple-Negative Breast Cancer. N Engl J Med (2010) 363(20):1938–48. doi: 10.1056/NEJMra1001389 [DOI] [PubMed] [Google Scholar]
- 3. Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-Negative Breast Cancer: Clinical Features and Patterns of Recurrence. Clin Cancer Res (2007) 13(15 Pt 1):4429–34. doi: 10.1158/1078-0432.Ccr-06-3045 [DOI] [PubMed] [Google Scholar]
- 4. Bianchini G, Balko JM, Mayer IA, Sanders ME, Gianni L. Triple-Negative Breast Cancer: Challenges and Opportunities of a Heterogeneous Disease. Nat Rev Clin Oncol (2016) 13(11):674–90. doi: 10.1038/nrclinonc.2016.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gass P, Lux MP, Rauh C, Hein A, Bani MR, Fiessler C, et al. Prediction of Pathological Complete Response and Prognosis in Patients With Neoadjuvant Treatment for Triple-Negative Breast Cancer. BMC Cancer (2018) 18(1):1051. doi: 10.1186/s12885-018-4925-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Caglevic C, Anabalón J, Soza C, Milla E, Gaete F, Carrasco AM, et al. Triple-Negative Breast Cancer: The Reality in Chile and in Latin America. Ecancermedicalscience (2019) 13:893. doi: 10.3332/ecancer.2019.893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Romero P, Benhamo V, Deniziaut G, Fuhrmann L, Berger F, Manié E, et al. Medullary Breast Carcinoma, a Triple-Negative Breast Cancer Associated With BCLG Overexpression. Am J Pathol (2018) 188(10):2378–91. doi: 10.1016/j.ajpath.2018.06.021 [DOI] [PubMed] [Google Scholar]
- 8. Niemeier LA, Dabbs DJ, Beriwal S, Striebel JM, Bhargava R. Androgen Receptor in Breast Cancer: Expression in Estrogen Receptor-Positive Tumors and in Estrogen Receptor-Negative Tumors With Apocrine Differentiation. Mod Pathol (2010) 23(2):205–12. doi: 10.1038/modpathol.2009.159 [DOI] [PubMed] [Google Scholar]
- 9. Budzik MP, Patera J, Sobol M, Czerw AI, Deptała A, Badowska-Kozakiewicz AM. Clinicopathological Characteristics of Metaplastic Breast Cancer - Analysis of the Basic Immunohistochemical Profile and Comparison With Other Invasive Breast Cancer Types. Breast (2019) 43:135–41. doi: 10.1016/j.breast.2018.12.004 [DOI] [PubMed] [Google Scholar]
- 10. Tray N, Taff J, Adams S. Therapeutic Landscape of Metaplastic Breast Cancer. Cancer Treat Rev (2019) 79:101888. doi: 10.1016/j.ctrv.2019.08.004 [DOI] [PubMed] [Google Scholar]
- 11. Reddy TP, Rosato RR, Li X, Moulder S, Piwnica-Worms H, Chang JC. A Comprehensive Overview of Metaplastic Breast Cancer: Clinical Features and Molecular Aberrations. Breast Cancer Res (2020) 22(1):121. doi: 10.1186/s13058-020-01353-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mills AM, EG C, MW S, MB C, Atkins KA. Pure Apocrine Carcinomas Represent a Clinicopathologically Distinct Androgen Receptor-Positive Subset of Triple-Negative Breast Cancers. Am J Surg Pathol (2016) 40(8):1109–16. doi: 10.1097/pas.0000000000000671 [DOI] [PubMed] [Google Scholar]
- 13. Weigelt B, Geyer FC, Reis-Filho JS. Histological Types of Breast Cancer: How Special Are They? Mol Oncol (2010) 4(3):192–208. doi: 10.1016/j.molonc.2010.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, et al. Identification of Human Triple-Negative Breast Cancer Subtypes and Preclinical Models for Selection of Targeted Therapies. J Clin Invest (2011) 121(7):2750–67. doi: 10.1172/jci45014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College Of American Pathologists Guideline Recommendations for Immunohistochemical Testing of Estrogen and Progesterone Receptors in Breast Cancer. J Clin Oncol (2010) 28(16):2784–95. doi: 10.1200/jco.2009.25.6529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for Immunohistochemical Testing of Estrogen and Progesterone Receptors in Breast Cancer (Unabridged Version). Arch Pathol Lab Med (2010) 134(7):e48–72. doi: 10.5858/134.7.e48 [DOI] [PubMed] [Google Scholar]
- 17. Tan PH, Ellis I, Allison K, Brogi E, Fox SB, Lakhani S, et al. The 2019 World Health Organization Classification of Tumours of the Breast. Histopathology (2020) 77(2):181–5. doi: 10.1111/his.14091 [DOI] [PubMed] [Google Scholar]
- 18. Tsuda H. General Rule Committee of the Japanese Breast Cancer S. Histological Classification of Breast Tumors in the General Rules for Clinical and Pathological Recording of Breast Cancer (18th Edition). Breast Cancer (2020) 27(3):309–21. doi: 10.1007/s12282-020-01074-3 [DOI] [PubMed] [Google Scholar]
- 19. Vranic S, Skenderi F, Beslagic V, Gatalica Z. Glycogen-Rich Clear Cell Carcinoma of the Breast: A Comprehensive Review. Appl Immunohistochem Mol Morphol (2020) 28(9):655–60. doi: 10.1097/pai.0000000000000850 [DOI] [PubMed] [Google Scholar]
- 20. Reis-Filho JS, Lakhani SR, Gobbi H, Sneige N. Metaplastic Carcinoma. In: Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van de Vijver MJ, editors. WHO Classification of Tumours of the Breast, vol. 2012 . Lyon: International Agency for Research on Cancer (IARC; (2012). p. 48–52. [Google Scholar]
- 21. O’Malley FE,V, Lakhani SR. Carcinomas With Apocrine Differentiation. In: Lakhani S, Ellis IO, Schnitt SJ, Tan PH, van de Vijver MJ, editors. WHO Classification of Tumours of the Breast, vol. 2012 . Lyon: International Agency for Research on Cancer (IARC; (2012). p. 53–4. [Google Scholar]
- 22. Russo J. Medullary Carcinoma. In: The Pathobiology of Breast Cancer. Switzerland: Springer Nature: (2016). p. 96. [Google Scholar]
- 23. Network NCC . Breast Cancer Version 4. Clinical Partice Guidelines in Oncology (NCCN Guidelines) (2021). Available at: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
- 24. Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, et al. The Evaluation of Tumor-Infiltrating Lymphocytes (TILs) in Breast Cancer: Recommendations by an International TILs Working Group 2014. Ann Oncol (2015) 26(2):259–71. doi: 10.1093/annonc/mdu450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Salgado R, L S, Denkert C, Badve S, Demaria S, Adams S, et al. International Immuno-Oncology Biomarker Working Group on Breast Cancer (2018). Available at: https://wwwtilsinbreastcancerorg/.
- 26. Zhang G, Wang Y, Chen B, Guo L, Cao L, Ren C, et al. Characterization of Frequently Mutated Cancer Genes in Chinese Breast Tumors: A Comparison of Chinese and TCGA Cohorts. Ann Transl Med (2019) 7(8):179. doi: 10.21037/atm.2019.04.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xiao W, Zhang G, Chen B, Chen X, Wen L, Lai J, et al. Characterization of Frequently Mutated Cancer Genes and Tumor Mutation Burden in Chinese Breast Cancer. Front Oncol (2021) 11:618767. doi: 10.3389/fonc.2021.618767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhao S, Ma D, Xiao Y, Jiang YZ, Shao ZM. Clinicopathologic Features and Prognoses of Different Histologic Types of Triple-Negative Breast Cancer: A Large Population-Based Analysis. Eur J Surg Oncol (2018) 44(4):420–8. doi: 10.1016/j.ejso.2017.11.027 [DOI] [PubMed] [Google Scholar]
- 29. Burstein MD, Tsimelzon A, Poage GM, Covington KR, Contreras A, Fuqua SA, et al. Comprehensive Genomic Analysis Identifies Novel Subtypes and Targets of Triple-Negative Breast Cancer. Clin Cancer Res (2015) 21(7):1688–98. doi: 10.1158/1078-0432.CCR-14-0432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jiang YZ, Ma D, Suo C, Shi J, Xue M, Hu X, et al. Genomic and Transcriptomic Landscape of Triple-Negative Breast Cancers: Subtypes and Treatment Strategies. Cancer Cell (2019) 35(3):428–40.e5. doi: 10.1016/j.ccell.2019.02.001 [DOI] [PubMed] [Google Scholar]
- 31. Zhao S, Ma D, Xiao Y, Li XM, Ma JL, Zhang H, et al. Molecular Subtyping of Triple-Negative Breast Cancers by Immunohistochemistry: Molecular Basis and Clinical Relevance. Oncologist (2020) 25(10):e1481–91. doi: 10.1634/theoncologist.2019-0982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen L, Kong X, Wang Z, Wang X, Fang Y, Wang J. Pre-Treatment Systemic Immune-Inflammation Index Is a Useful Prognostic Indicator in Patients With Breast Cancer Undergoing Neoadjuvant Chemotherapy. J Cell Mol Med (2020) 24(5):2993–3021. doi: 10.1111/jcmm.14934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Duffy MJ, Harbeck N, Nap M, Molina R, Nicolini A, Senkus E, et al. Clinical Use of Biomarkers in Breast Cancer: Updated Guidelines From the European Group on Tumor Markers (EGTM). Eur J Cancer (2017) 75:284–98. doi: 10.1016/j.ejca.2017.01.017 [DOI] [PubMed] [Google Scholar]
- 34. Curtis C, Shah SP, Chin SF, Turashvili G, Rueda OM, Dunning MJ, et al. The Genomic and Transcriptomic Architecture of 2,000 Breast Tumours Reveals Novel Subgroups. Nature (2012) 486(7403):346–52. doi: 10.1038/nature10983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Planes-Laine G, Rochigneux P, Bertucci F, Chretien AS, Viens P, Sabatier R, et al. PD-1/PD-L1 Targeting in Breast Cancer: The First Clinical Evidences Are Emerging. A Literature Review. Cancers (Basel) (2019) 11(7):1033. doi: 10.3390/cancers11071033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kurozumi S, Inoue K, Matsumoto H, Fujii T, Horiguchi J, Oyama T, et al. Clinicopathological Values of PD-L1 Expression in HER2-Positive Breast Cancer. Sci Rep (2019) 9(1):16662. doi: 10.1038/s41598-019-52944-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Buisseret L, Garaud S, de Wind A, Van den Eynden G, Boisson A, Solinas C, et al. Tumor-Infiltrating Lymphocyte Composition, Organization and PD-1/ PD-L1 Expression Are Linked in Breast Cancer. Oncoimmunology (2017) 6(1):e1257452. doi: 10.1080/2162402X.2016.1257452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lehmann BD, Pietenpol JA. Identification and Use of Biomarkers in Treatment Strategies for Triple-Negative Breast Cancer Subtypes. J Pathol (2014) 232(2):142–50. doi: 10.1002/path.4280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Traina TA, Miller K, Yardley DA, Eakle J, Schwartzberg LS, O'Shaughnessy J, et al. Enzalutamide for the Treatment of Androgen Receptor-Expressing Triple-Negative Breast Cancer. J Clin Oncol (2018) 36(9):884–90. doi: 10.1200/jco.2016.71.3495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Reddy SM, Carroll E, Nanda R. Atezolizumab for the Treatment of Breast Cancer. Expert Rev Anticancer Ther (2020) 20(3):151–8. doi: 10.1080/14737140.2020.1732211 [DOI] [PubMed] [Google Scholar]
- 41. Ahmed FS, Gaule P, McGuire J, Patel K, Blenman K, Pusztai L, et al. PD-L1 Protein Expression on Both Tumor Cells and Macrophages Are Associated With Response to Neoadjuvant Durvalumab With Chemotherapy in Triple-Negative Breast Cancer. Clin Cancer Res (2020) 26(20):5456–61. doi: 10.1158/1078-0432.Ccr-20-1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cortes J, Cescon DW, Rugo HS, Nowecki Z, Im SA, Yusof MM, et al. Pembrolizumab Plus Chemotherapy Versus Placebo Plus Chemotherapy for Previously Untreated Locally Recurrent Inoperable or Metastatic Triple-Negative Breast Cancer (KEYNOTE-355): A Randomised, Placebo-Controlled, Double-Blind, Phase 3 Clinical Trial. Lancet (2020) 396(10265):1817–28. doi: 10.1016/s0140-6736(20)32531-9 [DOI] [PubMed] [Google Scholar]
- 43. Schmid P, Cortes J, Pusztai L, McArthur H, Kümmel S, Bergh J, et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N Engl J Med (2020) 382(9):810–21. doi: 10.1056/NEJMoa1910549 [DOI] [PubMed] [Google Scholar]
- 44. Loibl S, Untch M, Burchardi N, Huober J, Sinn BV, Blohmer JU, et al. A Randomised Phase II Study Investigating Durvalumab in Addition to an Anthracycline Taxane-Based Neoadjuvant Therapy in Early Triple-Negative Breast Cancer: Clinical Results and Biomarker Analysis of GeparNuevo Study. Ann Oncol (2019) 30(8):1279–88. doi: 10.1093/annonc/mdz158 [DOI] [PubMed] [Google Scholar]
- 45. Petitjean A, Achatz MI, Borresen-Dale AL, Hainaut P, Olivier M. TP53 Mutations in Human Cancers: Functional Selection and Impact on Cancer Prognosis and Outcomes. Oncogene (2007) 26(15):2157–65. doi: 10.1038/sj.onc.1210302 [DOI] [PubMed] [Google Scholar]
- 46. Sirohi D, Devine P, Grenert JP, van Ziffle J, Simko JP, Stohr BA. TP53 Structural Variants in Metastatic Prostatic Carcinoma. PloS One (2019) 14(6):e0218618. doi: 10.1371/journal.pone.0218618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Akahane T, Kanomata N, Harada O, Yamashita T, Kurebayashi J, Tanimoto A, et al. Targeted Next-Generation Sequencing Assays Using Triplet Samples of Normal Breast Tissue, Primary Breast Cancer, and Recurrent/Metastatic Lesions. BMC Cancer (2020) 20(1):944. doi: 10.1186/s12885-020-07432-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chen B, Zhang G, Li X, Ren C, Wang Y, Li K, et al. Comparison of BRCA Versus Non-BRCA Germline Mutations and Associated Somatic Mutation Profiles in Patients With Unselected Breast Cancer. Aging (Albany NY) (2020) 12(4):3140–55. doi: 10.18632/aging.102783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Holstege H, Joosse SA, van Oostrom CT, Nederlof PM, de Vries A, Jonkers J. High Incidence of Protein-Truncating TP53 Mutations in BRCA1-Related Breast Cancer. Cancer Res (2009) 69(8):3625–33. doi: 10.1158/0008-5472.Can-08-3426 [DOI] [PubMed] [Google Scholar]
- 50. Manié E, Vincent-Salomon A, Lehmann-Che J, Pierron G, Turpin E, Warcoin M, et al. High Frequency of TP53 Mutation in BRCA1 and Sporadic Basal-Like Carcinomas But Not in BRCA1 Luminal Breast Tumors. Cancer Res (2009) 69(2):663–71. doi: 10.1158/0008-5472.Can-08-1560 [DOI] [PubMed] [Google Scholar]
- 51. Hsiue EH, Wright KM, Douglass J, Hwang MS, Mog BJ, Pearlman AH, et al. Targeting a Neoantigen Derived From a Common TP53 Mutation. Science (2021) 371(6533):eabc8697. doi: 10.1126/science.abc8697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Eriksson SE, Ceder S, Bykov VJN, Wiman KG. P53 as a Hub in Cellular Redox Regulation and Therapeutic Target in Cancer. J Mol Cell Biol (2019) 11(4):330–41. doi: 10.1093/jmcb/mjz005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shalom-Feuerstein R, Serror L, Aberdam E, Müller FJ, van Bokhoven H, Wiman KG, et al. Impaired Epithelial Differentiation of Induced Pluripotent Stem Cells From Ectodermal Dysplasia-Related Patients Is Rescued by the Small Compound APR-246/PRIMA-1met. Proc Natl Acad Sci USA (2013) 110(6):2152–6. doi: 10.1073/pnas.1201753109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Robson ME, Tung N, Conte P, Im SA, Senkus E, Xu B, et al. OlympiAD Final Overall Survival and Tolerability Results: Olaparib Versus Chemotherapy Treatment of Physician's Choice in Patients With a Germline BRCA Mutation and HER2-Negative Metastatic Breast Cancer. Ann Oncol (2019) 30(4):558–66. doi: 10.1093/annonc/mdz012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tutt ANJ, Garber JE, Kaufman B, Viale G, Fumagalli D, Rastogi P, et al. Adjuvant Olaparib for Patients With BRCA1- or BRCA2-Mutated Breast Cancer. N Engl J Med (2021) 384(25):2394–405. doi: 10.1056/NEJMoa2105215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Azizi Tabesh G, Izadi P, Fereidooni F, Emami Razavi AN, Tavakkoly Bazzaz J. The High Frequency of PIK3CA Mutations in Iranian Breast Cancer Patients. Cancer Invest (2017) 35(1):36–42. doi: 10.1080/07357907.2016.1247455 [DOI] [PubMed] [Google Scholar]
- 57. Deng L, Zhu X, Sun Y, Wang J, Zhong X, Li J, et al. Prevalence and Prognostic Role of PIK3CA/AKT1 Mutations in Chinese Breast Cancer Patients. Cancer Res Treat (2019) 51(1):128–40. doi: 10.4143/crt.2017.598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yi Z, Ma F, Liu B, Guan X, Li L, Li C, et al. Everolimus in Hormone Receptor-Positive Metastatic Breast Cancer: PIK3CA Mutation H1047R was a Potential Efficacy Biomarker in a Retrospective Study. BMC Cancer (2019) 19(1):442. doi: 10.1186/s12885-019-5668-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kim SB, Dent R, Im SA, Espié M, Blau S, Tan AR, et al. Ipatasertib Plus Paclitaxel Versus Placebo Plus Paclitaxel as First-Line Therapy for Metastatic Triple-Negative Breast Cancer (LOTUS): A Multicentre, Randomised, Double-Blind, Placebo-Controlled, Phase 2 Trial. Lancet Oncol (2017) 18(10):1360–72. doi: 10.1016/s1470-2045(17)30450-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schmid P, Abraham J, Chan S, Wheatley D, Brunt AM, Nemsadze G, et al. Capivasertib Plus Paclitaxel Versus Placebo Plus Paclitaxel As First-Line Therapy for Metastatic Triple-Negative Breast Cancer: The PAKT Trial. J Clin Oncol (2020) 38(5):423–33. doi: 10.1200/jco.19.00368 [DOI] [PubMed] [Google Scholar]
- 61. Oliveira M, Saura C, Nuciforo P, Calvo I, Andersen J, Passos-Coelho JL, et al. FAIRLANE, a Double-Blind Placebo-Controlled Randomized Phase II Trial of Neoadjuvant Ipatasertib Plus Paclitaxel for Early Triple-Negative Breast Cancer. Ann Oncol (2019) 30(8):1289–97. doi: 10.1093/annonc/mdz177 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Kaplan-Meier survival curves based on no-special type infiltrating ductal carcinoma and special types of triple negative breast cancer patients. (A) Disease free status of patients in the Cancer Genome Atlas cohort. (B) All patients in the Cancer Genome Atlas cohort. (C) All patients in the Molecular Taxonomy of Breast Cancer International Consortium cohort.
Immunohistochemical staining of programmed death-ligand 1(PD-L1) in triple- negative breast cancer (Magnification 200×). (A) Negative control of PD-L1 expression by immunohistochemistry. (B) Low PD-L1 expression in triple- negative breast cancer tumor samples. (C) High PD-L1 expression in triple- negative breast cancer tumor samples.
Hematoxylin and eosin staining of tumor-infiltrating lymphocytes (TILs) in triple-negative breast cancer (Magnification 200×). (A) Low-grade TILs. (B) Intermediate-grade TILs. (C) High-grade TILs.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: NODE (http://www.biosino.org/node) with accession OEP002982 (http://www.biosino.org/node/project/detail/OEP002982).