Abstract
Background
Widespread environmental contamination can directly interact with human immune system functions. Environmental effects on the immune system may influence human susceptibility to respiratory infections as well as the severity of infectious diseases, such as the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Furthermore, the efficacy of vaccines to respiratory diseases may be impacted by environmental exposures through immune perturbations. Given the quick pace of research about COVID-19 and associated risk factors, it is critical to identify and curate the streams of evidence quickly and effectively.
Objective
We developed this systematic evidence map protocol to identify and organize existing human and animal literature on high-priority environmental chemical classes (Per- and polyfluoroalkyl substances, pesticides, phthalates, quaternary ammonium compounds, and air pollutants) and their potential to influence three key outcomes: (1) susceptibility to respiratory infection, including SARS-CoV-2 (2) severity of the resultant disease progression, and (3) impact on vaccine efficacy. The result of this project will be an online, interactive database which will show what evidence is currently available between involuntary exposures to select environmental chemicals and immune health effects, data gaps that require further research, and data rich areas that may support further analysis.
Search and study eligibility
We will search PubMed for epidemiological or toxicological literature on select toxicants from each of the chemical classes and each of the three outcomes listed above.
Study appraisal and synthesis of methods
For each study, two independent reviewers will conduct title and abstract screening as well as full text review for data extraction of study characteristics. Study quality will not be evaluated in this evidence mapping. The main findings from the systematic evidence map will be visualized using a publicly available and interactive database hosted on Tableau Public.
Keywords: Per- and polyfluoroalkyl substances (PFAS), Pesticides, Phthalates, Quaternary Ammonium Compounds (QACs), Air pollutants, Respiratory virus susceptibility, Respiratory disease severity, Vaccine efficacy, SARS-CoV-2, COVID-19, Systematic evidence map
1. Introduction
1.1. Rationale
Exposures to environmental contaminants can adversely impact the immune system, which can subsequently increase susceptibility to viral infection and disease progression (Forrest 2011). Recently, the World Health Organization released estimates showing that two million lives and fifty-three million disability-adjusted-life-years were lost in 2019 due to chemical exposures (World Health Organization 2021). This highlights that chronic environmental exposures to industrial chemicals may be an important consideration in the management of the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2 or coronavirus) pandemic. A study of the 2002 SARS epidemic found associations between higher exposure to air pollution and increased risk of death (Cui et al., 2003, Pozzer et al., 2020). Further, studies of environmental contaminants have shown that common contaminant exposures, such as polyfluoroalkyl substances (PFAS) and methylmercury, can decrease the effectiveness of vaccines (Environmental Working Group, 2019, Grandjean et al., 2017). Thus, it is critical to identify environmental and chemical risk factors of: (1) susceptibility to coronavirus infection, (2) severity of the resultant disease progression for coronavirus disease 2019 (COVID-19), and (3) impaired COVID-19 vaccine efficacy.
Although the general public experiences widespread exposure to multiple environmental contaminants, the data and evidence on their potential to influence specific health effects is not well identified, organized, and curated (Ferguson et al. 2017). Systematic evidence maps are an emerging tool to support decision-making by identifying, organizing, and presenting the available health and toxicology related literature, and have been used to better understand the evidence base around environmental contaminants of emerging and growing concern (Ferguson et al., 2017, Pelch et al., 2019, Walker et al., 2018, Wolffe et al., 2020, Wolffe et al., 2019). Systematic evidence maps provide an accessible summary of available literature that can be easily updated. The best maps are interactive, taking the form of a database of references with associated meta-data that can be sorted, filtered, or searched on key aspects like exposures, research model, financial conflict of interest (COI), or health effects. These methods have been used by the Integrative Health Assessment Branch (IHAB) in the Division of the National Toxicology Program (DNTP) to develop interactive maps of the available human and animal evidence evaluating several exposures – health effect questions, including health effects of neonicotinoid pesticide exposures (Boyd et al. 2020). These maps have also been tested and demonstrated by The Endocrine Disruption Exchange (TEDX) to map the human, animal, and in vitro streams of evidence on PFAS (per- and poly- fluorinated alkyl substances) (Pelch et al. 2019). Systematic evidence maps are excellent at identifying the breadth of information along with areas that are data rich or data poor to direct further research or analysis activities. Unlike systematic reviews, these maps do not attempt to synthesize or integrate evidence to answer a detailed research question, but rather remove barriers associated with full systematic reviews, provide users with a tool to explore their own research questions, and identify emerging trends using a broad evidence base (James et al. 2016).
Given the rapid pace of research about COVID-19 and associated risk factors, it is critical to curate the streams of evidence quickly and effectively. Thus, in collaboration with the California Office of Environmental Health and Hazard Assessment (OEHHA) and DNTP, we propose systematically and transparently searching, curating, and mapping the evidence base around the impact of select chemical exposures on the respiratory and immune systems as it relates to direct and proxy measures of susceptibility to coronavirus infection, severity of COVID-19, and COVID-19 vaccine efficacy.
This primary objective of this systematic evidence map is to characterize the respiratory and immune impacts of select chemicals with the following exposure: chemicals commonly emitted from high polluting plants in the State of California and designated as high priority under CA AB2588 (California Air Resources Board, 2021a, California Air Resources Board, 2021b), chemicals currently considered high priority by the United States Environmental Protection Agency (US EPA) under the 2016 Toxic Substances Control Act (TSCA) (US Environmental Protection Agency 2021b), Quaternary Ammonium Compounds (QACs) (Biomonitoring California 2019), fluorinated chemicals, air pollutants, and high production volume pesticides (Pellizzari et al. 2019). The final list of chemicals was identified and prioritized through collaborative discussions between OEHHA, PRHE, and DNTP in order to address salient policy questions for the State of California.
The secondary objectives of this systematic evidence map are to identify indicators in studies such as assessment of early-life exposures, COI , analysis of chemical mixtures, and potential to inform health equity (including worker exposures). It is critical to understand the breadth of research on not just our primary objective of environmental contaminants and respiratory/immune system impacts, but with regard to populations or issues of interest.
1.1.1. Early-Life exposures
Biological factors such as age can significantly affect health impacts from chemical exposure. Fetuses, infants, and children are especially susceptible to reproductive and developmental toxicants because of critical windows of vulnerability during important stages of development. For example, the prenatal life stage can be the most sensitive to developmental and reproductive toxicants. (Bennett et al., 2016, Lanphear et al., 2005, US Environmental Protection Agency, 2017).
1.1.2. Conflict of Interest
Across several research areas, including tobacco, pharmaceutical, nutrition and chemical health effects research, even when controlling for methodological biases, studies with the presence of industry sponsorship or with authors with a financial COI are more likely to have study outcomes, including results and conclusions that favor the sponsor’s product than studies with no or other sources of sponsorship and COI.(Barnes and Bero, 1998, Bero et al., 2016, Huss et al., 2007, Lundh et al., 2017, Mandrioli et al., 2016, Yank et al., 2007) Thus, highlighting the impact of financial incentives on research. (Chartres et al., 2020, Mandrioli et al., 2016, Lundh et al., 2017).
1.1.3. Chemical mixtures
At present in the United States, chemicals are assessed for their risk and addressed through public policy using a chemical-by-chemical approach; however, assessing chemicals one at a time may lead to an underestimation of risk. As the U.S. population is not exposed to single chemicals through individual pathways, but instead to mixtures of multiple chemicals through multiple aggregate pathways, it is critical to understand how these chemicals may present human health hazards both individually as well as collectively (via compounding health hazards) (National Research Council 2008). Current scientific evidence shows that multiple chemical exposures acting collectively can elicit a wide range of toxic effects (e.g., endocrine disruption, developmental/neurological toxicity, and immune dysregulation). (National Research Council 2008). For example, endocrine disrupting chemicals such as phthalates may elevate risk of negative reproductive and developmental outcomes, and a recent study found that “elevated plasma-PFBA concentrations were associated with an increased risk of more severe course of COVID-19” (Grandjean et al. 2020). These chemicals directly interface with the circulating immune system upon human exposure; therefore, the downstream consequences of immune dysregulation remain an important research gap.
1.1.4. Health equity
Across a population, typically the highest environmental contaminant exposures, and subsequent health hazards, are to workers and communities near industrial facilities or contaminated sites (Mohai and Saha, 2015, National Research Council, 2009, US Environmental Protection Agency, 1999). There is substantial evidence that communities that experience higher levels of exposure to environmental contaminants as a result of industrial pollution (Schintler et al. 2020), are the same communities that must be prioritized due to their increased likelihood of susceptibility to coronavirus infection and severity of COVID-19. These are also the same communities that are impacted by fundamental causes of health disparities such as structural racism (Phelan and Link, 2015, Wilson et al., 2020). Current research on COVID-19 health disparities has found disproportionate impacts on Black communities, farmworkers, people experiencing homelessness, and incarcerated populations (Lee and Choi, 2020, Lewer et al., 2020, Millett et al., 2020, National Academies of Sciences, 2020, Rabin, 2020, Shen et al., 2018). Due to systemic racism and labor exploitation, workers from these communities are also overrepresented in low-wage essential work settings such as factories, grocery stores, and public transportation, putting them at increased risk of exposure to COVID-19 and other occupational hazards (McClure et al., 2020, US Centers for Disease Control and Prevention, 2021b).
The result of this project will be an online, interactive database which will show (1) what evidence is currently available between involuntary exposures to select environmental chemicals and immune impacts (susceptibility, severity, and vaccine efficacy) as well as impacts around populations of interest (the very young, communities that experience higher levels of exposure to environmental contaminants, workers) or issues of concern (chemical mixtures, conflict of interest), (2) data gaps which require further research, and (3) data rich areas that may support further analysis. Understanding and characterizing the impact of involuntary exposures to environmental contaminants is critical for policymakers designing public health interventions with regard to COVID-19. This systematic evidence map will be of immense use to environmental health researchers, funding agencies, and community groups as it will be accessible, interrogable, and can be updated as new studies are available.
1.2. Objectives
The objectives of this systematic evidence map are to:
-
1.
Identify and organize the available scientific research on the relationship between environmental contaminants and direct and proxy measures of coronavirus susceptibility, COVID-19 severity, and COVID-19 vaccine efficacy, as measured in human and animal studies. Further curate this information based on impacts to populations of interest (the very young, communities that experience higher levels of exposure to environmental contaminants, workers) or on issues of concern (chemical mixtures, conflict of interest).
-
2.
Present the literature in an interactive, web-based database which will directly connect users to the referenced studies.
-
3.
Identify data gaps and research or policy needs around COVID-19 and environmental contaminants.
-
4.
Publish a narrative summary of the systematic evidence map.
The protocol described here serves to document decisions made a priori regarding the conduct of the systematic evidence mapping. Any protocol updates or modifications will be documented as amendments to the original protocol as they take place.
2. Methods
This protocol has been prepared in accordance with the ENVINT PRISMA-SM-P report (available at (Environment International 2017)) and based on expert guidance from the developers of TEDX, including CK. We attempted to register the protocol in PROSPERO, however, it was not currently accepting registrations for scoping reviews, literature reviews, or mapping reviews.
2.1. Search strategy
We used the below outcome definitions to refine the search logic used in a recent NTP monograph on PFAS and immunotoxicity (US National Toxicology Program 2016). We will identify the relevant published peer-reviewed literature through searching the PubMed electronic database. The PubMed search will include chemical names and common synonyms as found through ChemIDPlus (including CASRN) (US National Institutes of Health 2021), Wikipedia (Wikipedia 2021), Common Chemistry (American Chemical Society 2021), EPA’s CompTox Database (US Environmental Protection Agency 2021c), and InChiKey (InChI Trust 2021), for the select contaminants as well as the key immune endpoints. Additional PFAS terms were added by incorporating terms from the search strings in the TEDX evidence map protocol (Pelch et al. 2019). Additional phthalate terms were added by including common secondary metabolites of our phthalates of interest and repeating the search as described above. We generated our final list of synonyms by removing duplicate terms, terms not found in PubMed, and terms that were overly broad. Searches were run on July 27, 2021, producing 9,951 studies (after de-duplication) and the final search strings can be found in Appendix 1. Given that the goals of the project are to develop a systematic evidence map that supports decision making on further research or analysis activities, the search was only conducted in PubMed as it has import compatibility within DistillerSR. Furthermore, PubMed is the database with the strongest biomedical focus for human and animal research that was judged most likely to have studies that address the project objectives. We recognize a single database is a limitation of this project. However, the choice of a single database was made based on the goals and timeline of the project to provide a categorized evidence base that can be used in inform decisions in the relatively short term (i.e., 12 months) as the impacts of SARS-CoV-2 and COVID-19 vaccination continue to change rapidly. The addition of other databases such as Scopus and Web of Science will be considered in potential future updates or expansions.
2.2. Eligibility criteria
A study’s eligibility is determined based on the PECO statement (Table 1 ). Eligible studies will contain primary research that investigates links between the listed chemicals and one of the specified immune outcomes. We will include human and animal evidence as defined below. We will tag mechanistic studies for future use. Studies that do not consider toxicological, health, or mechanistic information on a given chemical will be excluded after the title/abstract stage.
Table 1.
Populations, Exposures, Comparators, and Outcomes (PECO) Statement.
| PECO Element | Evidence |
|---|---|
| Population |
Human: Primary studies that quantitatively examine the association of our selected exposures on the related selected immune outcomes, in children and/or adults. Animal: Whole organism studies, including experimental and observational studies. |
| Exposure | Exposure to at least one of the priority chemicals listed in Table 2 below. Exposures may include, for example: biomarkers of exposure (e.g., urine, blood, or other specimens), modeling of potential exposures, and/or administered exposures. There are no limitations on the timing, route, level, or determination of estimated exposure. |
| Comparator | Humans, animals, organs, tissues, cell lines, or cellular components exposed to a lower level of the chemical/pollutant than the more highly exposed subjects or treatment groups, or vehicle-only treatment. |
| Outcome | Characteristics of immune impacts such as coronavirus susceptibility, COVID-19 severity, and COVID-19 vaccine efficacy. Primary outcomes: Humans:COVID-19 infection and diagnosis of or medication use for respiratory infections, diseases, and conditions that may increase coronavirus susceptibility to COVID-19 (influenza, severe acute respiratory syndrome, middle east respiratory syndrome, asthma, bronchitis, pneumonia, rhinitis, sinusitis, chronic obstructive pulmonary disease, cystic fibrosis, and lung function parameters). Cardiovascular conditions arising from the search will be included only into full text screening as a conservative measure for identifying potential respiratory outcomes not identified in the screening step. Upon full text review, studies with only cardiovascular outcomes (lacking respiratory outcomes) will be excluded. Prognosis and severity of known respiratory infections to COVID-19, influenza, severe acute respiratory syndrome, middle east respiratory syndrome, and accompanying cytokine storm.Indicators influencing vaccine efficacy (immunoglobulins/antibodies, and clinically confirmed immune modulation or immune suppression). Animals: Lung and lymph tissue histopathology and bioassays following experimental chemical exposures in animal models and validated animal models of respiratory conditions (pneumonia, chronic obstructive pulmonary disease, cystic fibrosis, and asthma). Co-exposure to chemicals and respiratory pathogen challenge; accompanying lung and lymph tissue histopathology and bioassays. Pathogen challenge, chemical co-exposures, and antibody production/response.Other applicable immune function assays (e.g., antibody response, natural killer cell activity). |
For human studies we will include primary experimental and observational studies including randomized controlled trials, cohort, case-control, cross-sectional, , case cross over, panel or case studies, time series analaysis, or other relevant designs that quantitatively examine the association of our selected exposures on the related selected immune outcomes, in children and/or adults. For animal studies we will include whole organism studies, including experimental and observational studies and ex vivo immune assays.
We will attempt to look for translated manuscripts of non-English studies if the title and abstract fit the PECO but will not include non-English language studies if there is no available translated manuscript. Conference abstracts, presentations, posters, and theses/dissertations will not be included.
2.3. Exposures
The chemicals prioritized for inclusion in this systematic evidence map were based on criteria determined with input from topic experts from OEHHA, PRHE, DNTP, and CK, in an effort to capture a diverse group of policy-relevant chemicals to the State of California, with a focus on substances with greater potential impact on the respiratory and immune systems. The final list represents priority chemicals to OEHHA such as: chemicals commonly emitted from high polluting plants in the State of California and designated as high priority under CA AB2588 (California Air Resources Board, 2021a, California Air Resources Board, 2021b), chemicals currently considered high priority by the US EPA under the 2016 TSCA (US Environmental Protection Agency 2021b), QACs (Biomonitoring California 2019), fluorinated chemicals, air pollutants, and high production volume pesticides (Pellizzari et al. 2019) (Table 1). Prioritization for chemical exposures of interest was determined by OEHHA and in collaboration with the authors to ensure that the map could be completed within the 1-year grant timeline. Ample studies have documented how particulate matter (PM2.5) is associated with various respiratory virus infections (Ciencewicki and Jaspers, 2007, Cohen et al., 2005, Mehta et al., 2013) and can modulate a person’s susceptibility to respiratory infections (Harrod et al., 2003, Michielsen et al., 2002). As this evidence map is focused on chemicals with less robust databases, we determined that PM2.5 is not currently a priority but will be assessed at a later date.
We conducted an initial search of priority chemicals identified by OEHHA using a single database (PubMed) on February 1, 2021, to determine the scale of studies. We generated a preliminary list of search terms for each chemical using the National Institutes of Health/National Library of Medicine (NIH/NLM) database ChemIDplus (US National Institutes of Health 2021), which was then used to inform conversations with OEHHA and ultimately determine a group of high priority chemicals with high exposures and high production volumes. Based on our preliminary search output, our systematic evidence map will be informative and yield many studies for multiple chemicals. (Appendix 1) The present map contains a balance of chemicals and will be well equipped to address research and policy gaps. The classes of our priority chemicals are below, and details of our final search strategy can be found in Section 2.1.
2.3.1. Per- and polyfluoroalkyl substances (PFAS)
Associations between PFAS and health outcomes have been mapped previously, particularly for long-chain PFAS such as PFOA and PFOS, which were reviewed by the US EPA, Agency for Toxic Substances and Disease Registry (ATSDR) and NTP (Agency for Toxic Substances and Disease Registry, 2021, US Environmental Protection Agency, 2021d, US National Toxicology Program, 2016). Additionally, TEDX mapped the human, animal, and in vitro streams of evidence for understudied PFAS (Pelch et al. 2019). This evidence map will also focus on understudied PFAS such as: Perfluorohexanoic acid (PFHxA), Perfluorohexanesulfonic acid (PFHxS), Perfluorobutanesulfonic acid (PFBS), Perfluorobutyric acid (PFBA), Perfluorononanoic acid (PFNA), Perfluorodecanoic acid (PFDA), and 8:2 fluorotelomer (Table 2 ). These chemicals are priority due to being detected in Biomonitoring California’s California Regional Exposure (CARE) cohort in Los Angeles (Biomonitoring California 2021), and their designation as chemicals of concern to OEHHA.
Table 2.
List of Chemicals prioritized* by the California Office of Environmental Health Hazard Assessment and included in the systematic evidence map.
| Priority | Abbreviation | Chemical Name (CASRN) |
|---|---|---|
| 1 | BBP + MBzP + MBP | Butyl benzyl phthalate (85–68-7) + Monobenzyl phthalate (2528–16-7) +Monobutyl phthalate (131–70-4) |
| PFBS | Perfluorobutanesulfonic acid (375–73-5) | |
| IMI | Imidacloprid (138261–41-3) | |
| CTAC | Hexadecyltrimethyl ammonium chloride (112–02-7) | |
| O3 | Ozone (10028–15-6) | |
| NO2 | Nitrogen Dioxide (10102–44-0) | |
| 2 | DBP + MBP | Dibutyl phthalate (84–74-2) + Monobutyl phthalate (131–70-4) |
| DEHP + MEHP | Diethylhexyl phthalate (117–81-7) + Monoethylhexyl phthalate(4376–20-9) | |
| PFHxS | Perfluorohexanesulfonic acid (355–46-4) | |
| PFNA | Perfluorononanoic acid (375–95-1) | |
| 3 | DiBP + MiBP | Diisobutyl phthalate (84–69-5) + Monoisobutyl phthalate (30833–53-5) |
| DCHP + MCHP | Dicyclohexyl phthalate (84–61-7) + Monocyclohexyl phthalate (7517–36-4) | |
| PFHxA | Perfluorohexanoic acid (307–24-4) | |
| PFDA | Perfluorodecanoic acid (335–76-2) | |
| 8:2 FT | 8:2 fluorotelomer (678–39-7) | |
prioritization based on multiple factors, including high-volume production, knowledge gaps, and urgency for policy action.
2.3.2. Pesticides
Imidacloprid (IMI) is a neonicotinoid pesticide prioritized by OEHHA and was chosen from a list of chemicals identified in a review from the National Institute of Health’s Environmental influences on Child Health Outcomes (ECHO) initiative (Pellizzari et al. 2019). This list was generated in order to prioritize chemicals which may adversely impact children’s health but have not been biomonitored nationwide. While there is a paucity of recent literature regarding IMI and our outcomes of interest in human epidemiological studies. Evidence of adverse immune effects in pigs and partridges through dietary IMI exposure has been demonstrated (Hernandez et al., 2018, Lopez-Antia et al., 2015). Further, other noenicitinoid insecticides have demonstrated negative impacts on lymphocyte production and macrophage function in rats and immune signaling in an in vitro model using a human monocytic cell line (Di Prisco et al., 2017, Shakthi Devan et al., 2015).
2.3.3. Phthalates
Phthalates are widely found in food, consumer products, and air and dust in the indoor environment. There is limited research on the possible associations among phthalates and SARS-CoV2, however, there exists evidence that prenatal phthalate exposure can increase allergies, infectious disease, and airway inflammation (Ait Bamai et al., 2018, Jahreis et al., 2018). Additionally, participants exposed to dibutyl phthalate had an increased early allergic response (Maestre-Batlle et al. 2020). Further, facemask use during the pandemic has also been demonstrated to result in increased phthalate esters exposure, such as di(2-ethylhexyl) phthalate (Jin et al. 2021). Phthalates need to be prioritized for understanding their ability to alter COVID-19 pathology, especially since there is an increase in exposure directly related to the pandemic.
Dibutyl phthalate (DBP), Diethylhexyl phthalate (DEHP), Diisobutyl phthalate (DIBP), Butyl benzyl phthalate (BBP), and Dicyclohexyl phthalate (DCHP) were chosen due to their designation as priority chemicals under AB 2588 or The Air Toxics “Hot Spots” Information and Assessment Act of 1987 (California Air Resources Board 2021a). This Act established a statewide program for the inventory of air toxics emissions from individual facilities and addresses public concerns that emissions from individual facilities might cause a local concentration of air toxics “Hot Spots” or an elevated risk of adverse health effects. They are also listed as high priority chemicals for consideration under TSCA, and are monitored by Biomonitoring CA The half-lives of phthalates are relatively short (<24 h) because of their rapid metabolism in humans (Hoppin et al. 2002). Modern epidemiology studies, therefore, predominantly assess the aforementioned phthalates through urinary excretion products for exposure assessment. As such, we will also focus on the major secondary monoester metabolites of each of these phthalates, which includes monobenzyl phthalate (MBzP), monobutyl phthalate (MBP), mono(2-ethylhexyl) phthalate (MEHP), mono-isobutyl phthalate (MiBP), and mono-cyclohexyl phthalate (MCHP).
2.3.4. Quaternary ammonium compounds (QACs)
The QAC hexadecyltrimethyl ammonium chloride (CTAC) was chosen based on its active usage in antimicrobials and disinfectants (US Environmental Protection Agency 2021e), as well as its statewide and national aggregate production volumes (Biomonitoring California 2019). QACs are a broad and diverse class of chemicals and chemical mixtures, and these compounds have a wide range of uses, including as pesticides, antimicrobials, disinfectants, antistatic agents, and corrosion inhibitors. As disinfectant use has increased due to the COVID-19 pandemic, concerns are rising about the impacts of widespread human exposure to QACs, and OEHHA has designated CTAC as a priority chemical (Biomonitoring California 2020). A recent comprehensive review was conducted to explore existing evidence of associations between QACs and respiratory effects in cleaning personnel (Clausen et al. 2020). This review reported evidence between QAC exposure and asthma while also highlighting animal and mechanistic evidence of potential inflammatory reaction and bronchoconstriction in response to inhalation (Clausen et al. 2020). A recent large cohort study in the Nurses’ Health Study II also reported that occupational exposure to QACs was associated with chronic obstructive pulmonary disease incidence among female nurses (Dumas et al. 2019). Based on these previous reviewed studies, QACs may be important chemicals for our COVID-19 related outcomes of interests via respiratory effects.
2.3.5. Air pollutants
Air pollution directly sensitizes the respiratory system and can cause inflammatory responses from immune and peripheral tissue (Grunig et al. 2014). Some highly common criteria air pollutants arising from industrial manufacturing sources and vehicle emissions include ozone (O3) and nitrogen dioxide (NO2) (US Centers for Disease Control and Prevention, 2021a, US Environmental Protection Agency, 2021a). Notably, these contaminants are also elevated directly as a result of wildfires or produced as secondary products through atmospheric reactions (Lipsett and Materna 2008).
2.4. Outcomes
The categorization effort of this systematic evidence map will cluster outcomes into three key health effect domains (direct and proxy measures of susceptibility to coronavirus and other respiratory pathogen infection, COVID-19 and other respiratory disease severity, and COVID-19 and other vaccine efficacy) and across two streams of evidence (human and animal). If studies address multiple outcomes, such as susceptibility and severity or severity and vaccine efficacy, they will be tagged under those outcomes in the final map. While we will collect mechanistic studies in our search, we do not have capacity to analyze them within the short-time frame and will tag them during title/abstract stage and analyze them at a later date.
Within each of these domains, we will contextualize data extraction based on the definitions outlined below. We limited our use of non-specific immunology terms (such as cytokines, chemokines, and other immune biomarkers) as they may result in an overly broad literature search output. We recognize the narrowness of the search as a potential limitation of this project; however, we specifically chose immune terms that were associated with the objectives of the review (coronavirus susceptibility, COVID-19 severity, and vaccine efficacy). A full list of search terms can be found in Appendix 1.
2.4.1. Human studies
2.4.1.1. Susceptibility to coronavirus and other respiratory pathogens
Given that coronavirus predominantly impacts the respiratory system in humans, in addition to assessing studies that directly evaluate susceptibility to coronavirus infection, we will leverage studies of other pathogens with similar physiological endpoints as a proxy for coronavirus susceptibility. We will evaluate literature on associations between selected environmental contaminants and the diagnosis of the following infectious diseases: Influenza, Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS). We will evaluate the literature for other pulmonary and respiratory system conditions which lead to increased susceptibility to coronavirus or higher risk for severe illness from COVID-19 as described by the Centers for Disease Control and Prevention (US Centers for Disease Control Prevention 2020), such as pneumonia, chronic obstructive pulmonary disease (COPD), cystic fibrosis, and asthma. We will also include studies of relevant pathogens that cause respective respiratory conditions. Across each of these outcomes, we will be inclusive of studies that assess medication use for a given phenotype related to these outcomes as a proxy measure. Additionally, while we are not explicitly searching for cardiovascular outcomes in our protocol, we recognize that many epidemiologic studies may couple cardiorespiratory outcomes together. Therefore, in the initial abstract screening phase, studies of cardiovascular outcomes that appear from our search string output will be included as a conservative approach to capture potential studies that focus on cardiorespiratory outcomes collectively.
2.4.1.2. COVID-19 or other respiratory disease severity
Upon confirmed diagnosis of coronavirus or the aforementioned proxy respiratory conditions, another outcome of interest is disease prognosis and severity in association with environmental contaminants. This encompasses tissue specific and peripheral immune biomarkers following known infection, comorbidities across the duration of the infection (e.g., chronic kidney disease, chronic obstructive pulmonary disease, heart disease, and diabetes), and risk of mortality from infection.
2.4.1.3. COVID-19 and other vaccine efficacy
We will evaluate proxy measures of vaccine efficacy by focusing directly on studies that have tested for differences in vaccine efficacy for previous vaccines used for our proxy respiratory pathogens. Additionally, we will explore studies which look at the impact of selected environmental contaminants on vaccine efficacy using proxies, such as perturbations in antibody concentrations, and immune modulations, such as alterations in the proportions of present immune cells.
In the present protocol, in vitro studies of human samples that are identified through the search will be tagged for future potential analysis and incorporation.
2.4.2. Animal models
The outcome domains defined above remain the foci for animal models. However, adjustments in the definitions of each domain are made for animal models, given that exposure and outcome assessments often occur experimentally rather than purely observationally or prospectively as in human studies.
2.4.2.1. Susceptibility to coronavirus and other respiratory pathogen infections
Following direct exposure to environmental contaminants, we will focus on studies that assess tissue specific histopathology (e.g., lung and lymph tissue). This encompasses immune specific bioassays (e.g., cytokines and immune cell surface markers) in target tissues. In instances where there are existing validated animal models of respiratory conditions (pneumonia, COPD, cystic fibrosis, and asthma), we will include studies that investigate environmental exposures in these model systems. We recognize that focusing on lung and lymph tissue limits inference on other organs potentially affected by the coronavirus. The final systematic evidence map will state this limitation so that users interpret findings appropriately and may build on this scope of work to explore additional tissues to address knowledge gaps that are not addressed in our systematic evidence map.
2.4.2.2. COVID-19 or other respiratory disease severity
In this outcome domain, we will focus on studies that specifically evaluate co-exposure to respiratory pathogen and antigen challenge and chemical exposures. We will focus on histopathology and bioassays at target tissues, in addition to the progression of the infection.
2.4.2.3. COVID-19 and other vaccine efficacy
We will include studies of animal models that directly evaluate vaccine injection and co-exposure to environmental contaminants. We will also focus on studies of chemical exposures and antibody production resulting from pathogen challenge or stimulation with antigen.
2.5. Data management
2.5.1. Management of literature updates and study flow diagram
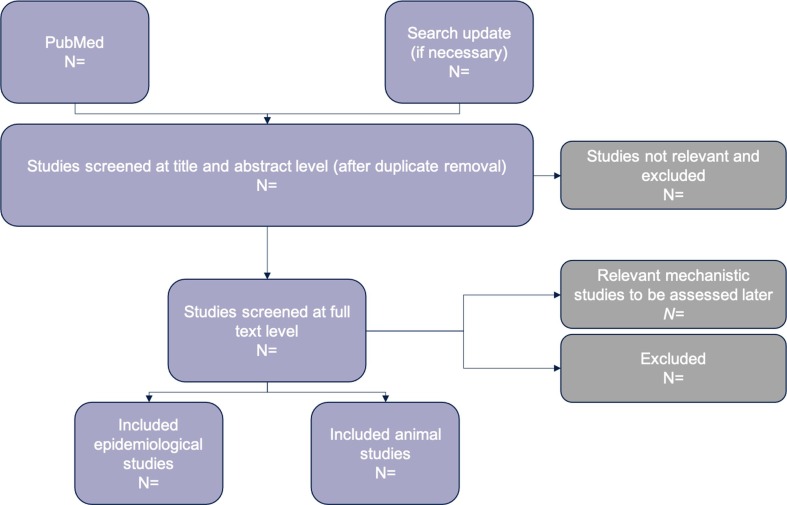
A study flow diagram will track the number of studies retrieved and processed in the review (Fig. 1 ). If for any reason the search needs to be updated, it will be repeated but limited by the date of the last search using the PubMed Advanced Search Builder’s “date-create” field. Any protocol updates or modifications will be documented on PROSPERO as amendments to the original registered protocol if PROSPERO begins accepting reviews.
Fig. 1.
Example study flow diagram to show how studies will proceed through the review.
The PubMed literature results will be imported to Endnote X9 (Clarivate Analytics; Philadelphia, Pennsylvania). Duplicate records will be resolved based on title and author fields, using the “Find Duplicates” feature. The updated literature records will all have a unique identification number that will carry through until the end of the project. These records will be uploaded to DistillerSR (Evidence Partners; Ottawa, Ontario, Canada). After upload, these records will be manually reviewed by multiple screeners at the title/abstract stage (SR, MA, NC, CC). Mechanistic studies will be tagged as such at the full text stage and extracted at a later date. The records that pass this level will then pass through the full-text data extraction screening phrase for human and animal studies. The DistillerSR extraction codes that form the foundation of our database will be exported into a.csv file. These database visualization will be built using TableauPrep (Tableau; Seattle, WA) and uploaded to Tableau Desktop Professional Edition vs 2018.3 (Tableau; Seattle, WA) for visualization and public use.
The final systematic evidence map, alongside supplementary background information, will be hosted on the Program on Reproductive Health and the Environment, University of California, San Francisco (PRHE, UCSF) website (prhe.ucsf.edu).
2.6. Selection and data collection processes
Title and abstract screening will be carried out in DistillerSR by four researchers (CC, MA, NC, SR). DistillerSR's artificial intelligence (AI) machine learning functionalities may be utilized to prioritize studies for title and abstract screening and full-text review, respectively. Title and abstract screening in DistillerSR will require one reviewer for inclusion to the next level and two independent reviewers for exclusion. In the event that an abstract is missing or there are discrepancies between the two reviewers which cannot be resolved by discussion, the default will be to move the reference forward for full text review. After abstract screening, there will be a full text screening step conducted by one reviewer and then quality control checked by a second reviewer. Data extraction and coding will be conducted by a single reviewer with a second reviewer confirming the accuracy and completeness of extracted and coded data. In the event of discrepancies between the two reviewers which cannot be resolved by discussion, a third reviewer will adjudicate the decision. In parallel with the previous step, AR and VW will use a subset of the human epidemiological data to develop, train, and pilot the semi-automated data extraction tool or Dexter that may be used to gain efficiencies in tagging or categorization for potential future updates or expansions. QA/QC of this model will be conducted by AR and VW using manual screening. As much of the extracted data collected will be free text, the final set of data will undergo a normalization step to ensure a level of coding control and data consistency.
In the case of missing information, we will attempt to contact the study author to request additional information. However, if unable to acquire it, missing information will be noted.
2.7. Data coding strategy
Data extraction from full-text studies will use structured forms in DistillerSR. Prior to commencing the extraction, DistillerSR structured forms will be piloted by the reviewers on a small set of studies to ensure the accuracy of the data extraction and potential compatibility with visualization in Tableau. In addition to basic study information and exposure and outcomes assessment, data extraction will be performed for the following information: authors, journal, reference information, year of publication, evidence streams (human, animal), conflict of interest statement (COI), funding statement, acknowledgements statement, author affiliations, contaminants(s) evaluated, the health outcome category/categories, if the study considered multiple contaminants, if a study considered mixtures of contaminants, if a study considered early life impacts, and if a study would be useful to health equity scholarship pursuant to California’s Statewide Plan to Promote Health Equity (Appendix 2) (California Department of Public Health 2015).
2.8. Data mapping method
Studies will be grouped and presented in a tiered manner collated by contaminant studied, evidence stream, and health outcome. The DistillerSR extraction codes that form the foundation of our database will be exported into a.csv file. These database visualization flows will be built using TableauPrep (Tableau; Seattle, WA) and extracted using Tableau Desktop Professional Edition vs 2018.3 (Tableau; Seattle, WA) for visualization. Finally, we will upload the database to Tableau Public for public use.
The systematic evidence map will additionally be hosted on PRHE’s website (prhe.ucsf.edu) via embedding Tableau Public. The systematic evidence map in Tableau Public will be interactive, list the final included studies, and contain adjustable filters to display information based on evidence streams, health outcomes, or environmental contaminants. Based on their search requirements, users can modify the interactive map to present only the papers relevant to their search. Users will be able to retrieve additional study details, including abstracts and be able to navigate externally to access the full PubMed article.
2.9. Study quality assessment
Study quality will not be assessed in this systematic evidence map.
2.10. Summary of results
The output of this project will be an interactive systematic evidence map and a narrative summary manuscript for peer review. The main goals of this manuscript will be to provide the overall results of the literature search, and to analyze the overall trends for these publications by year. The human evidence stream will be summarized by environmental contaminants evaluated, the frequency of different study types and locations of studies, the ranges of reported exposures as well as their different health outcomes. The animal evidence stream will be similarly summarized, but for observational and experimental studies. Additionally, the animal evidence stream will address the frequency of different species studied, as well as the different experimental facets around the timing, route, level of exposure and outcomes.
2.11. Conclusion
2.11.1. Implication for research
This online, interactive systematic evidence map will help identify what evidence is currently available between involuntary exposures to select environmental chemicals and our immune impacts (susceptibility, severity, and vaccine efficacy), data gaps which require further research, and data rich areas. This systematic evidence map will be of immense use to environmental health researchers, funding agencies, and community groups as it will be accessible, interrogable, and can be updated as new studies are available and with additional exposures of interest.
2.11.2. Implication for Policy/Management
Understanding and characterizing the impact of involuntary exposures to environmental contaminants is critical for policymakers designing public health interventions with regard to COVID-19. The creation of this systematic evidence map will support policymakers in identifying data gaps and working to fill them as well as data rich areas that would support current action and analysis. This map will also support policymakers in identifying health equity concerns pursuant to California’s Statewide Plan to Promote Health Equity (California Department of Public Health 2015). Finally, the approach from this systematic evidence map will inform prioritization for cumulative risk assessments of chemical mixtures in the future.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
Not applicable.
Competing interests
The authors declare they have no actual or potential competing financial interests.
Authors' contributions
SR, MA, and NC designed and wrote the review protocol. TW, CF, CK, AR, DG, and VW reviewed the protocol. SR, and MA wrote the search strategy and will undertake the literature search. SR, MA, CC, CF, and NC will conduct the title and abstract screening and full article screening for final study inclusion. SR, MA, CC, CF, and NC will conduct data collection and cleaning. NC and TW are guarantors. All authors will contribute to the final manuscript. All steps were conducted in consultation with OEHHA.
Funding
Financial support for this work was provided by the California Office of Environmental Health Hazard Assessment (OEHHA) Agreement #19-E0023.
CRediT authorship contribution statement
Swati D.G. Rayasam: Conceptualization, Methodology, Data curation, Writing – original draft, Writing – review & editing, Project administration. Max T. Aung: Conceptualization, Methodology, Data curation, Writing – original draft, Writing – review & editing, Project administration. Courtney Cooper: Methodology, Data curation. Carol Kwiatkowski: Writing – review & editing. Dori R. Germolec: Methodology, Writing – review & editing. Andrew A. Rooney: Methodology, Writing – review & editing. Vickie R. Walker: Methodology, Writing – review & editing. Chanese Forte: Methodology, Data curation, Writing – review & editing. Tracey J. Woodruff: Conceptualization, Writing – review & editing, Supervision, Funding acquisition. Nicholas Chartres: Conceptualization, Methodology, Writing – original draft, Writing – review & editing, Supervision, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to acknowledge Kathleen Durkin, Katie Pelch, Aditi Shah, and Michael Sholinbeck for their valuable contributions to the development of this review protocol. We would like to acknowledge Ryan Lanigan for his valuable contribution to support drafting the database visualization in Tableau.
Handling Editor: Adrian Covaci
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envint.2022.107230.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Agency for Toxic Substances and Disease Registry. Toxicological Profile for Perfluoroalkyls. in: Services U.D.o.H.a.H., ed; 2021. [PubMed]
- Ait Bamai Y., Miyashita C., Araki A., Nakajima T., Sasaki S., Kishi R. Effects of prenatal di(2-ethylhexyl) phthalate exposure on childhood allergies and infectious diseases: The Hokkaido Study on Environment and Children's Health. Sci Total Environ. 2018;618:1408–1415. doi: 10.1016/j.scitotenv.2017.09.270. [DOI] [PubMed] [Google Scholar]
- American Chemical Society. CAS Common Chemistry. 2021.
- Barnes D.E., Bero L.A. Why review articles on the health effects of passive smoking reach different conclusions. JAMA. 1998;279:1566–1570. doi: 10.1001/jama.279.19.1566. [DOI] [PubMed] [Google Scholar]
- Bennett, D., Bellinger, D.C., Birnbaum, L.S., Bradman, A., Chen, A., Cory-Slechta, D.A., Engel, S.M., Fallin, M.D., Halladay, A., Hauser, R., Hertz-Picciotto, I., Kwiatkowski, C.F., Lanphear, B.P., Marquez, E., Marty, M., McPartland, J., Newschaffer, C.J., Payne-Sturges, D., Patisaul, H.B., Perera, F.P., Ritz, B., Sass, J., Schantz, S.L., Webster, T.F., Whyatt, R.M., Woodruff, T.J., Zoeller, R.T., Anderko, L., Campbell, C., Conry, J.A., DeNicola, N., Gould, R.M., Hirtz, D., Huffling, K., Landrigan, P.J., Lavin, A., Miller, M., Mitchell, M.A., Rubin, L., Schettler, T., Tran, H.L., Acosta, A., Brody, C., Miller, E., Miller, P., Swanson, M., Witherspoon, N.O., (ACOG), A.C.o.O.a.G., Society, C.N., Society, E., Association, I.N., Environment, I.S.f.C.s.H.a.t., Epidemiology, I.S.f.E., Physicians, N.C.o.A.P.I., Association, N.H.M., Association, N.M. Project TENDR: Targeting Environmental Neuro-Developmental Risks The TENDR Consensus Statement. Environ Health Perspect 2016,124:A118-122. [DOI] [PMC free article] [PubMed]
- Bero L., Anglemyer A., Vesterinen H., Krauth D. The relationship between study sponsorship, risks of bias, and research outcomes in atrazine exposure studies conducted in non-human animals: Systematic review and meta-analysis. Environ Int. 2016;92–93:597–604. doi: 10.1016/j.envint.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biomonitoring California. Meeting of Scientific Guidance Panel for Biomonitoring California - Preliminary Screening of Quaternary Ammonium Compounds for Future Consideration as Potential Designated Chemicals. 2019.
- Biomonitoring California. Designated chemicals. 2020.
- Biomonitoring California. California Regional Exposure (CARE) Study. 2021.
- Boyd, W.A., Boyles, A.L., Blain, R.B., Skuce, C.R., Engstrom, A.K., Walker, V.R., Thayer, K.A., Rooney, A.A. NTP research report on the scoping review of potential human health effects associated with exposures to neonicotinoid pesticides: Research report 15. 2020. [PubMed]
- California Air Resources Board. AB2588 Air Toxics “Hot Spots”. 2021a.
- California Air Resources Board. CARB Pollution Mapping Tool. 2021b.
- California Department of Public Health. Portrait of promise: The California statewide plan to promote health and mental health equity. 2015.
- Chartres N., Fabbri A., McDonald S., Diong J., McKenzie J.E., Bero L. Association of food industry ties with findings of studies examining the effect of dairy food intake on cardiovascular disease and mortality: systematic review and meta-analysis. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-039036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciencewicki J., Jaspers I. Air pollution and respiratory viral infection. Inhal Toxicol. 2007;19:1135–1146. doi: 10.1080/08958370701665434. [DOI] [PubMed] [Google Scholar]
- Clausen, P.A., Frederiksen, M., Sejbæk, C.S., Sørli, J.B., Hougaard, K.S., Frydendall, K.B., Carøe, T.K., Flachs, E.M., Meyer, H.W., Schlünssen, V., Wolkoff, P. Chemicals inhaled from spray cleaning and disinfection products and their respiratory effects. A comprehensive review. Int J Hyg Environ Health 2020;229:113592. [DOI] [PubMed]
- Cohen A.J., Ross Anderson H., Ostro B., Pandey K.D., Krzyzanowski M., Künzli N., Gutschmidt K., Pope A., Romieu I., Samet J.M., Smith K. The global burden of disease due to outdoor air pollution. J Toxicol Environ Health A. 2005;68:1301–1307. doi: 10.1080/15287390590936166. [DOI] [PubMed] [Google Scholar]
- Cui Y., Zhang Z.F., Froines J., Zhao J., Wang H., Yu S.Z., Detels R. Air pollution and case fatality of SARS in the People's Republic of China: an ecologic study. Environ Health. 2003;2:15. doi: 10.1186/1476-069X-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Prisco G., Iannaccone M., Ianniello F., Ferrara R., Caprio E., Pennacchio F., Capparelli R. The neonicotinoid insecticide Clothianidin adversely affects immune signaling in a human cell line. Sci Rep. 2017;7:13446. doi: 10.1038/s41598-017-13171-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas O., Varraso R., Boggs K.M., Quinot C., Zock J.P., Henneberger P.K., Speizer F.E., Le Moual N., Camargo C.A. Association of Occupational Exposure to Disinfectants With Incidence of Chronic Obstructive Pulmonary Disease Among US Female Nurses. JAMA Netw Open. 2019;2 doi: 10.1001/jamanetworkopen.2019.13563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Environment International. PRISMA-P Report (modified) for Systematic Map Protocols Submitted to Environment International (Version 1.0). 2017.
- Environmental Working Group. PFAS Chemicals Harm the Immune System, Decrease Response to Vaccines, New EWG Review Finds. 2019.
- Ferguson A., Penney R., Solo-Gabriele H. A review of the field on children's exposure to environmental contaminants: A risk assessment approach. Int J Environ Res Public Health. 2017;14:265. doi: 10.3390/ijerph14030265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest B. Impact of environmental contamination on immunity: Implications for childhood vaccination policy. J Health Pollut. 2011;1:5–7. [Google Scholar]
- Grandjean P., Heilmann C., Weihe P., Nielsen F., Mogensen U.B., Timmermann A., Budtz-Jørgensen E. Estimated exposures to perfluorinated compounds in infancy predict attenuated vaccine antibody concentrations at age 5-years. J Immunotoxicol. 2017;14:188–195. doi: 10.1080/1547691X.2017.1360968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P., Timmermann C.A.G., Kruse M., Nielsen F., Vinholt P.J., Boding L., Heilmann C., Mølbak K. Severity of COVID-19 at elevated exposure to perfluorinated alkylates. PLoS ONE. 2020;15 doi: 10.1371/journal.pone.0244815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunig G., Marsh L.M., Esmaeil N., Jackson K., Gordon T., Reibman J., Kwapiszewska G., Park S.H. Perspective: ambient air pollution: inflammatory response and effects on the lung's vasculature. Pulm Circ. 2014;4:25–35. doi: 10.1086/674902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrod K.S., Jaramillo R.J., Rosenberger C.L., Wang S.Z., Berger J.A., McDonald J.D., Reed M.D. Increased susceptibility to RSV infection by exposure to inhaled diesel engine emissions. Am J Respir Cell Mol Biol. 2003;28:451–463. doi: 10.1165/rcmb.2002-0100OC. [DOI] [PubMed] [Google Scholar]
- Hernandez J., Volland A., Leyshon B.J., Juda M., Ridlon J.M., Johnson R.W., Steelman A.J. Effect of imidacloprid ingestion on immune responses to porcine reproductive and respiratory syndrome virus. Sci Rep. 2018;8:11615. doi: 10.1038/s41598-018-30093-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppin J.A., Brock J.W., Davis B.J., Baird D.D. Reproducibility of urinary phthalate metabolites in first morning urine samples. Environ Health Perspect. 2002;110:515–518. doi: 10.1289/ehp.02110515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huss A., Egger M., Hug K., Huwiler-Müntener K., Röösli M. Source of funding and results of studies of health effects of mobile phone use: systematic review of experimental studies. Environ Health Perspect. 2007;115:1–4. doi: 10.1289/ehp.9149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- InChI Trust. InChI and InChIKeys for Chemical Structures. 2021.
- Jahreis S., Trump S., Bauer M., Bauer T., Thurmann L., Feltens R., Wang Q., Gu L., Grutzmann K., Roder S., Averbeck M., Weichenhan D., Plass C., Sack U., Borte M., Dubourg V., Schuurmann G., Simon J.C., von Bergen M., Hackermuller J., Eils R., Lehmann I., Polte T. Maternal phthalate exposure promotes allergic airway inflammation over 2 generations through epigenetic modifications. J Allergy Clin Immunol. 2018;141:741–753. doi: 10.1016/j.jaci.2017.03.017. [DOI] [PubMed] [Google Scholar]
- James, K., Randall, N., 2016. Haddaway, N. A methodology for systematic mapping in environmental sciences. Environ Evid 5.
- Jin L., Griffith S.M., Sun Z., Yu J.Z., Chan W. On the Flip Side of Mask Wearing: Increased Exposure to Volatile Organic Compounds and a Risk-Reducing Solution. Environ Sci Technol. 2021;55:14095–14104. doi: 10.1021/acs.est.1c04591. [DOI] [PubMed] [Google Scholar]
- Lanphear B.P., Vorhees C.V., Bellinger D.C. Protecting Children from Environmental Toxins. PLoS Med. 2005;2 doi: 10.1371/journal.pmed.0020061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G.H., Choi K.C. Adverse effects of pesticides on the functions of immune system. Comp Biochem Physiol C Toxicol Pharmacol. 2020;235 doi: 10.1016/j.cbpc.2020.108789. [DOI] [PubMed] [Google Scholar]
- Lewer D., Braithwaite I., Bullock M., Eyre M.T., White P.J., Aldridge R.W., Story A., Hayward A.C. COVID-19 among people experiencing homelessness in England: a modelling study. Lancet Respir Med. 2020;8:1181–1191. doi: 10.1016/S2213-2600(20)30396-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsett M., Materna B. In Wildfire smoke: a guide for public health officials. California Office of Environmental Health Hazard. Assessment. 2008 [Google Scholar]
- Lopez-Antia A., Ortiz-Santaliestra M.E., Mougeot F., Mateo R. Imidacloprid-treated seed ingestion has lethal effect on adult partridges and reduces both breeding investment and offspring immunity. Environ Res. 2015;136:97–107. doi: 10.1016/j.envres.2014.10.023. [DOI] [PubMed] [Google Scholar]
- Lundh, A., Lexchin, J., Mintzes, B., Schroll, J.B., Bero, L. Industry sponsorship and research outcome. Cochrane Database Syst Rev 2017;2:MR000033. [DOI] [PMC free article] [PubMed]
- Maestre-Batlle D., Huff R.D., Schwartz C., Alexis N.E., Tebbutt S.J., Turvey S., Bolling A.K., Carlsten C. Dibutyl Phthalate Augments Allergen-induced Lung Function Decline and Alters Human Airway Immunology. A Randomized Crossover Study. Am J Respir Crit Care Med. 2020;202:672–680. doi: 10.1164/rccm.201911-2153OC. [DOI] [PubMed] [Google Scholar]
- Mandrioli D., Kearns C.E., Bero L.A. Relationship between Research Outcomes and Risk of Bias, Study Sponsorship, and Author Financial Conflicts of Interest in Reviews of the Effects of Artificially Sweetened Beverages on Weight Outcomes: A Systematic Review of Reviews. PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0162198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure E.S., Vasudevan P., Bailey Z., Patel S., Robinson W.R. Racial Capitalism Within Public Health-How Occupational Settings Drive COVID-19 Disparities. Am J Epidemiol. 2020;189:1244–1253. doi: 10.1093/aje/kwaa126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta S., Shin H., Burnett R., North T., Cohen A.J. Ambient particulate air pollution and acute lower respiratory infections: a systematic review and implications for estimating the global burden of disease. Air Qual Atmos Health. 2013;6:69–83. doi: 10.1007/s11869-011-0146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michielsen C., Zeamari S., Leusink-Muis A., Vos J., Bloksma N. The environmental pollutant hexachlorobenzene causes eosinophilic and granulomatous inflammation and in vitro airways hyperreactivity in the Brown Norway rat. Arch Toxicol. 2002;76:236–247. doi: 10.1007/s00204-002-0326-x. [DOI] [PubMed] [Google Scholar]
- Millett G.A., Jones A.T., Benkeser D., Baral S., Mercer L., Beyrer C., Honermann B., Lankiewicz E., Mena L., Crowley J.S., Sherwood J., Sullivan P.S. Assessing differential impacts of COVID-19 on black communities. Ann Epidemiol. 2020;47:37–44. doi: 10.1016/j.annepidem.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohai P., Saha R. Which came first, people or pollution? Assessing the disparate siting and post-siting demographic change hypotheses of environmental injustice. Environ Res Lett. 2015;10 [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine. Decarcerating correctional facilities during COVID-19: Advancing health, equity, and safety. Washtington, DC; 2020. [PubMed]
- National Research Council. Phthalates and cumulative risk assessment: The tasks ahead. 2008. [PubMed]
- National Research Council. Science and decisions: Advancing risk assessment. 2009. [PubMed]
- Pelch K.E., Reade A., Wolffe T.A.M., Kwiatkowski C.F. PFAS health effects database: Protocol for a systematic evidence map. Environ Int. 2019;130 doi: 10.1016/j.envint.2019.05.045. [DOI] [PubMed] [Google Scholar]
- Pellizzari, E.D., Woodruff, T.J., Boyles, R.R., Kannan, K., Beamer, P.I., Buckley, J.P., Wang, A., Zhu, Y., Bennett, D.H., (Environmental Influences on Child Health Outcomes). Identifying and prioritizing chemicals with uncertain burden of exposure: Opportunities for biomonitoring and health-related research. Environ Health Perspect 2019;127:126001. [DOI] [PMC free article] [PubMed]
- Phelan J., Link B. Is Racism a Fundamental Cause of Inequalities in Health? Annual Review of Sociology. 2015 [Google Scholar]
- Pozzer A., Dominici F., Haines A., Witt C., Münzel T., Lelieveld J. Regional and global contributions of air pollution to risk of death from COVID-19. Cardiovasc Res. 2020;116:2247–2253. doi: 10.1093/cvr/cvaa288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin R. Prisons Are Covid-19 Hotbeds. When Should Inmates Get the Vaccine? The. N.Y. Times. 2020 [Google Scholar]
- Schintler, L., Kulkarni, R., McNeely, C., Haynes, K. Environmental and occupational exposure to toxic industrial chemicals and COVID-19: An exploratory analysis of United States counties 2020.
- Shakthi Devan R.K., Prabu P.C., Panchapakesan S. Immunotoxicity assessment of sub-chronic oral administration of acetamiprid in Wistar rats. Drug Chem. Toxicol. 2015;38:328–336. doi: 10.3109/01480545.2014.966382. [DOI] [PubMed] [Google Scholar]
- Shen Y., Pellow D., Vazin J., Kime S., Levine M., McAlpine S., Ashby H. Environmental injustice behind bars: Toxic imprisonment. America. 2018 [Google Scholar]
- US Centers for Disease Control and Prevention. Air Quality - Air Pollutants. 2021a.
- US Centers for Disease Control and Prevention. Health Equity Considerations and Racial and Ethnic Minority Groups. 2021b.
- US Centers for Disease Control Prevention. People with certain medical conditions. 2020.
- US Environmental Protection Agency. Sociodemographic Data Used or Identifying Potentially Highly Exposed Populations. Washington, DC; 1999.
- US Environmental Protection Agency. NIEHS/EPA Children’s Environmental Health and Disease Prevention Research Centers Impact Report: Protecting Children’s Health Where They Live, Learn, and Play. 2017.
- US Environmental Protection Agency. Air Pollution Emissions Overview. 2021a.
- US Environmental Protection Agency. Chemicals Undergoing Risk Evaluation under TSCA. 2021b.
- US Environmental Protection Agency. CompTox Database. 2021c.
- US Environmental Protection Agency. Human Health Toxicity Assessments for GenX Chemicals 2021d.
- US Environmental Protection Agency. List N Tool: COVID-19 Disinfectants. 2021e.
- US National Institutes of Health. National Library of Medicine: ChemIDplus database. 2021.
- US National Toxicology Program. Monograph on immunotoxicity associated with exposure to perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS). Research Triangle Park, NC; 2016.
- Walker V.R., Boyles A.L., Pelch K.E., Holmgren S.D., Shapiro A.J., Blystone C.R., Devito M.J., Newbold R.R., Blain R., Hartman P., Thayer K.A., Rooney A.A. Human and animal evidence of potential transgenerational inheritance of health effects: An evidence map and state-of-the-science evaluation. Environ Int. 2018;115:48–69. doi: 10.1016/j.envint.2017.12.032. [DOI] [PubMed] [Google Scholar]
- Wikipedia. Wikipedia. 2021.
- Wilson S., Bullard R., Patterson J., Thomas S. Roundtable on the pandemics of racism, environmental injustice, and COVID-19 in America. Environ Justice. 2020;13:56–64. [Google Scholar]
- Wolffe T.A.M., Vidler J., Halsall C., Hunt N., Whaley P. A Survey of Systematic Evidence Mapping Practice and the Case for Knowledge Graphs in Environmental Health and Toxicology. Toxicol. Sci. 2020;175:35–49. doi: 10.1093/toxsci/kfaa025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolffe T.A.M., Whaley P., Halsall C., Rooney A.A., Walker V.R. Systematic evidence maps as a novel tool to support evidence-based decision-making in chemicals policy and risk management. Environ Int. 2019;130 doi: 10.1016/j.envint.2019.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . Switzerland; Geneva: 2021. Public health impact of chemicals: knowns and unknowns; 2019 data addendum. [Google Scholar]
- Yank V., Rennie D., Bero L.A. Financial ties and concordance between results and conclusions in meta-analyses: retrospective cohort study. BMJ. 2007;335:1202–1205. doi: 10.1136/bmj.39376.447211.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.