Abstract
Angiotensin‐converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) both inhibit the renin‐angiotensin system (RAS) but have different sites of action. Whether clinically meaningful differences exist is still debated. The authors set up a population‐based nationwide retrospective cohort study with at least 5 years of follow‐up based on the comprehensive French Health Insurance Database linked to the French hospital discharge database. Patients aged 50 or above, identified as ARB or ACE inhibitor new users in 2009 (at least one delivery during the year and no such delivery in 2008) were eligible. Exclusion criteria included history of cancer, cardiovascular disease, or chronic renal insufficiency. Main outcome measure was overall mortality. Secondary outcomes were cardiovascular deaths, major cardiovascular events, and major or other cardiovascular events. Out of 407 815 eligible patients, 233 682 (57%) were ARB users; two‐third had no previous exposure to antihypertensive drug. Based on propensity‐score based Cox model, ARB new user group had a better overall (HR: .878, 95%CI, .854 to .902), and cardiovascular (HR: .841, 95%CI, .800 to .84) survival and had a lower risk for major cardiovascular events (HR: .886, 95%CI, .868 to .905). Statistically significant quantitative interactions were detected with diabetes. Considering subgroup analyses, ARBs had a better survival than ACE inhibitors in nondiabetic patients.
Keywords: angiotensin receptor blockers, angiotensin‐converting enzyme inhibitors, cardiovascular diseases, mortality
1. INTRODUCTION
Hypertension is a worldwide health problem associated with mortality and cardiovascular (CV) morbidity, which are both further increased when diabetes is present. In primary prevention, angiotensin‐converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) are prescribed, with thiazide‐type diuretic and calcium channel blocker, as first line drug options in (nonblack) hypertensive patients, with diabetes or not. 1 , 2 , 3
Contrary to ARBs, ACE inhibitors have been shown to reduce mortality and CV morbidity in placebo‐controlled RCTs. 4 Meta‐analyses indicated that ACE inhibitors and ARBs have potentially different effects on mortality reduction among hypertensive patients with/without diabetes. 5 , 6 Another meta‐analysis comparing ACE inhibitors to ARBs in a head‐to‐head manner 7 in patients with uncontrolled hypertension found no reliable difference for total deaths. However, data mostly derived from the ONTARGET trial. 8
ACE inhibitors and ARBs both inhibit the renin‐angiotensin system (RAS) but have different sites of action. Whether clinically meaningful differences exist has been long debated. A possible biological rationale relies on bradykinin antagonism. Some differences in blood pressure (BP)‐dependent effects (magnitude of BP decrease), BP‐independent effects (reduction of oxidative stress and endothelial dysfunction, improvement in glucose metabolism, inhibition and stabilization of atherosclerotic plaque) may exist. 9
Focusing on primary prevention of cardiovascular events and death, and considering a potential weak difference if any and a needed long follow‐up, a head‐to‐head randomized comparison seems not appropriate. We therefore planned a large nationwide observational study to compare, in primary prevention, the effectiveness of ARBs versus ACE inhibitors. Our primary objective was overall mortality. Secondary objectives included cardiovascular mortality, major cardiovascular events (hemorrhagic stroke, ischemic stroke, myocardial infarction, or heart failure) along with revascularization procedures.
2. METHODS
2.1. Study design and setting
We set up a nationwide population‐based cohort with a 6‐year follow‐up period using French Health Insurance Data (SNDS). We identified through reimbursement claims a cohort of patients ≥50 years old who initiated in 2009 an ACE inhibitor or ARB regimen, free of overt cardiovascular disease and without ACE inhibitor or ARB in 2008. Patients were followed until the end of 2014, to collect information on major cardiovascular events or revascularization procedures or death.
2.2. Participants
Study population selection was based on the following inclusion criteria: age ≥ 50 years‐old; at least one delivery reimbursement in 2009 of ACE inhibitor or ARB (Anatomical Therapeutic Chemical (ATC) Classification System: C09Ax or C09Cx or C09Bx or C09Dx; Table S1). Exclusion criteria were: delivery reimbursement of a ACE inhibitor or ARB in 2008; simultaneous prescription (defined as deliveries within 30 days) for ACE inhibitors and ARB; no reimbursement for any health care in 2008; a diagnostic of cancer before the inclusion date, based on hospitalization or long‐term disease registration (Table S2); diagnostic of cardiovascular disease (i.e., ischemic stroke, hemorrhagic stroke, myocardial infarction, ischemic heart disease, heart failure, or peripheral vascular disease); or chronic renal insufficiency, before the inclusion date, based on hospitalization discharge diagnosis or long‐term disease registration (Table S3). Our goal was to identify a cohort patients treated in a primary prevention setting.
2.3. Data sources
SNDS links anonymously and comprehensively through a personal unique number a health reimbursement database (DCIR) to the French hospital discharge database (PMSI). SNDS contains basic data such as age and date of death. Data on drug delivery were extracted, which included strength per unit, number of units per pack for each drug as well as the date of prescription and dispensing. PMSI contains diagnostic codes (International Classification of Disease, 10th revision, ICD‐10), admission dates and medical acts (using common classification of medical acts, CCMA) for all hospitalizations either in public or private hospitals. This study was based on subjects affiliated to the French National Health Insurance general scheme, covering almost 50 million people. In SNDS there is no information on the reason why drugs are prescribed. There is no diagnosis coding for outpatient clinic visits in SNDS. However, hypertension represents very probably the main reason of those treatments after exclusion of patients presumed to have a coronary disease or cardiac heart failure. Blood pressure levels are not available in SNDS.
2.4. Variables
We classified patients according to some risk factors: diabetes mellitus (based on hospitalization discharge diagnosis, long‐term registration or drug reimbursed deliveries), lipid‐lowering drug use, and according to have previously receiving some types of drugs (assessed within a time frame of 12 months before the inclusion date, with at least two deliveries): antihypertensive drugs, specifically calcium channel blockers, beta‐blockers or thiazides or antithrombotic drugs, i.e., antiplatelet drugs or anticoagulants (Table S4). Our definition of “smoking” or “drinking” identified patients who had a hospitalization with a code for “Problems related to lifestyle” (Z72.0 Tobacco use), for “counseling and medical advice,” for “Mental and behavioral disorders due to psychoactive substance use,” or for disease related to alcohol abuse (e.g., K70 Alcoholic liver disease), or who were prescribed drugs used in nicotine or alcohol dependence (Table S4). Altogether, we defined a priori a three‐level variable: patients having diabetes and using lipid‐lowering drug; patients having only one of the above risk factors or using antithrombotic drug; patients with none of the above three conditions. Prespecified subgroups analyses were based on those variables along with age and sex. Age was dichotomized with a cut‐off at 65 years old. A complete list of codes and algorithms used to classify confounders and effect modifiers is provided in Table S4.
2.5. Study size
A meta‐analysis 5 showed a mortality incidence of 20 per 1000 patient‐years; we therefore anticipated around 10% of deaths within 5 years of follow‐up; 96 000 patients per group (1:1) were needed to detect a hazard ratio of 1.05 with 95% power at a two‐sided .05 alpha level.
2.6. Statistical methods
The first delivery of ACE inhibitor or ARB in 2009 has been tagged as the inclusion date. Treatment group was defined, as for an intention‐to‐treat analysis, on the first observed delivery of ACE inhibitor or ARB similar, without taking in account later switched (a subsequent dispensing of the alternative drug class, for instance ARB after stopping ACE inhibitor or vice versa) or stopped treatments. For descriptive purpose, the first medication order was queried to identify the ACE inhibitor or ARB molecule prescribed; based on strength per unit for each drug type (ATC 7‐digit code with same international nonproprietary name INN) we defined three dose levels (high, medium, low; Table S5); in addition, frequency of switching/stopping and timing (based on last dispensing date that preceded end‐of‐study date or censoring date) were reported.
The primary outcome was overall mortality, from first delivery to the death date or December 31, 2014. Secondary outcomes were: cardiovascular death (Table S6, using a strict and a broader definition), major cardiovascular events (myocardial infarction, ischemic stroke, intracranial hemorrhage, heart failure, considering the date of the first event, and death being considered as a competing risk), and major or other cardiovascular events (adding revascularization procedures (Table S7) to the above‐mentioned list). We used entry hospital date as the event date.
A weighted (Stabilized Inverse Probability of Treatment Weighting, SIPTW) propensity score‐based Cox model was used to calculate hazard ratios (HRs) between ARB and ACE, using ACE inhibitor users as the reference group.
The predicted probability of starting (new use) ARB versus ACE inhibitor given baseline variables was calculated using a logistic regression model. Eight covariates (sex, age as a continuous variable, diabetes, obesity, alcohol, smoking habit, renal disease, rheumatic disease, all assessed within a time frame of 12 months before the inclusion date) were forced in the model. Other covariates from a set of 24 variables, whenever their statistical level of significance was ≤.15 when checking association with mortality, were included and then selected using backward selection. The degree to which the propensity score was appropriately specified was ascertained through evaluation of common support, defined by overlapping distributions of propensity scores between exposure groups. In order to examine the changes in confounder distribution due to SIPTW, we calculated standardized biases for the original sample and for the sample following application of SIPTW.
Prespecified subgroups analyses were performed: we fit SIPTW Cox models stratified by age (> 65 years), sex, diabetes (further split as severe or not, long history or not), use of lipid‐lowering drug, first line antihypertensive drug use, and cardiovascular risk factor level. We added an interaction term in model to investigate whether there was a heterogeneity across strata.
Different ways for handling propensity score were planned as sensitivity analyses: (1) matching: each patient taking an ARB was matched to a patient taking an ACE inhibitor using 1:1 matching based on logit of the propensity score; A maximum caliper of .2 times the standard deviation of logit of the propensity score was used to ensure similarity of matched patients and q Cox model with the COVS(AGGREGATE) option was ran; (2) subclassification: a stratified (on five strata according to propensity score quintiles) Cox model was used with an interaction term for testing homogeneity across strata; (3) an SIPTW Cox model was ran on an on‐treatment population where patients were censored at the time they stopped (or switched) their initial drug. To further explore the association between drug regimen and outcomes, we defined, on this on‐treatment population, an indicator of adherence, the medication possession ratio (MPR) (Supplemental Materials, Expanded Materials & Methods S8): a stratified analysis on MPR values with an arbitrarily chosen threshold (≥80%) was conducted.
Because there is no information in SNDS on the reason why drugs are prescribed, and for some low dose of ARB or ACE inhibitor as a first line therapy, heart failure might have been the indication, we performed a post hoc analysis discarding those patients.
All statistical analyses used procedures available in SAS software version 9.4 (SAS Institute Inc, Cary, NC); all p‐values were nominal (i.e., without adjustment for multiple testing).
2.7. Bias
Thanks to SNDS, no loss of follow‐up and no missing data were anticipated. Exhaustive nationwide data also minimized selection bias. We identified first ACE inhibitor or ARB users and selected patients without clinically overt cardiovascular disease hence confounding by indication was minimized. In addition, we used propensity score to further reduce any difference in clinical characteristics. Cardiovascular events relied on hospital discharge diagnosis with detailed and unbiased information as ICD‐10 codes were recorded timely and prospectively; in addition, we thought misclassification on outcome was minimized and at least nondifferential as previous exposure to ACE inhibitor or ARB was not taken in account in the coding process. Lastly, seeking for death used a thorough approach through a dedicated algorithm (Table S9). We do acknowledge that smoking and drinking variables have a poor sensitivity, but there is no reason for a differential misclassification bias.
2.8. Data access and cleaning methods
Data access was operative in June 2021. Investigators had full access to the extracted database from SNDS, which was used to create the study population. Extracted database was stored locally in a dedicated and secured data center: extraction was performed by CNAMTS; csv data files were imported into MySQL database with a physical data model consistent to the SNDS original database design. Some metrics and visual tools were used to check data completeness and adequacy to expected data extraction: metrics included number of extracted patients (compared to expected number), stability of reimbursement frequencies over time in order to validate data completeness at a population level.
3. RESULTS
3.1. Participants
From 593 098 adults ≥ 50 years old with at least one delivery of an ACE inhibitor or an ARB in 2009 but not in 2008, 407 815 patients left after application of exclusion criteria (Figure 1). A total of 233 682 (57%) were ARB users. Among exclusion criteria, there was a simultaneous prescription for ACE inhibitors and ARB, which was observed for 7140 patients: ACE inhibitors and ARB were delivered concomitantly as mentioned on a single order for 428 (6%). There was also a vital status deemed unknown or unreliable (n = 462 patients) because a health care claim was observed more than 30 days after the recorded “death” date (n = 359) or two death dates were collected from two different sources with a difference exceeding one day (and exceeding 30 days for 60 patients).
FIGURE 1.
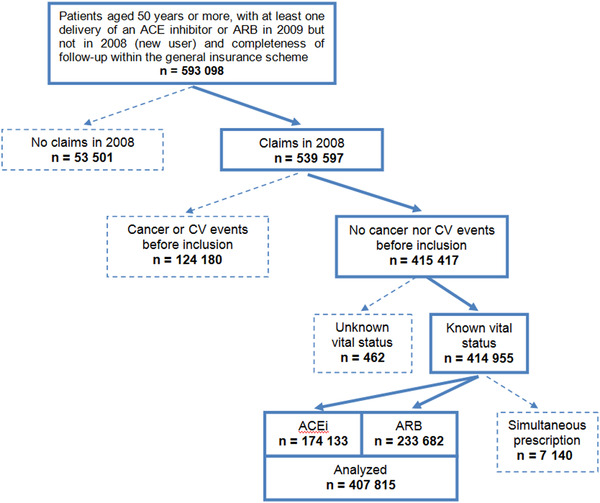
Patient selection diagram 73 457 patients had a hospitalization for cardiovascular events before inclusion, 37 104 a hospitalization for cancer, 1962 a hospitalization for chronic kidney disease, and 11 657 a hospitalization for two or three of the above conditions
3.2. Descriptive data
Table 1 displays summary statistics of the key covariates, stratified by ACE inhibitor or ARB group. ARB users were more likely to be women, younger, but less likely to have diabetes, comorbidities or cardiovascular risk factors, or to have prior antithrombotic drug orders. In both group, two‐third had no previous exposure to antihypertensive drug (thiazides, calcium channel blockers, or beta‐blockers) and were considered as first‐line users. In addition, there were differences in the prevalence of laboratory tests except for cholesterol measurement.
TABLE 1.
Baseline characteristics of angiotensin receptor blocker (ARB) (N = 233 682) and ACE inhibitor new users (N = 174 133) in a nationwide cohort in France, 2009, along with standardized differences (SD) before (un‐weighted) and following SIPTW (stabilized inverse probability of treatment weighting) application
| Characteristics | Unweighted | Weighted | |||||
|---|---|---|---|---|---|---|---|
| ACE | ARB | SD | ACE | ARB | SD | ||
| Female | 55.8 | 58.1 | .05 | 57.1 | 57.1 | .00 | |
| Age, years | 66.3 | 65.4 | .08 | 65.8 | 65.8 | .00 | |
| Co‐morbidities a | Diabetes | 20.7 | 15.4 | .14 | 17.6 | 17.6 | .00 |
| Obesity | 1.04 | .82 | .02 | .90 | .90 | .00 | |
| Alcohol | 1.29 | 1.07 | .02 | 1.14 | 1.15 | .00 | |
| Smoking habit | .72 | .52 | .02 | .60 | .60 | .00 | |
| Renal disease | .03 | .02 | .03 | .03 | .03 | .00 | |
| Rheumatic disease | .18 | .12 | .02 | .14 | .15 | .00 | |
| Potential confounders § | Dementia | .39 | .18 | .04 | .27 | .27 | .00 |
| Dementia treated | 6.17 | 5.99 | .01 | 6.16 | 6.16 | .00 | |
| Chronic pulmonary disease (except COPD) | .33 | .26 | .01 | .29 | .29 | .00 | |
| Hemiplegia or paraplegia | .12 | .06 | .02 | .09 | .09 | .00 | |
| Diabetes with chronic complications | .71 | .39 | .04 | .51 | .51 | .00 | |
| Liver disease (moderate or severe) | .05 | .03 | .05 | .04 | .04 | .00 | |
| Ischemic heart disease | .02 | .00 | .03 | .01 | .01 | .00 | |
| Atrial fibrillation | .91 | .49 | .05 | .68 | .69 | .00 | |
| COPD | .37 | .23 | .02 | .29 | .29 | .00 | |
| Drug use b | Prior delivery of beta‐blockers | 5.02 | 5.75 | .03 | 5.46 | 5.45 | .00 |
| Prior delivery of calcium channel blockers | 20.0 | 20.8 | .02 | 20.6 | 20.5 | .00 | |
| Prior delivery of thiazides | 14.1 | 12.2 | .06 | 13.1 | 13.1 | .00 | |
| Prior delivery of anti‐platelet drugs | 4.83 | 3.12 | .09 | 3.87 | 3.88 | .00 | |
| Prior delivery of anticoagulants | 5.53 | 3.54 | .10 | 4.40 | 4.42 | .00 | |
| Prior delivery of lipid lowering drugs | 38.4 | 34.7 | .08 | 36.3 | 36.3 | .00 | |
| Biological measures c | BUN observed | 68.9 | 65.7 | .07 | 67.1 | 67.1 | .00 |
| Potassium observed | 7.69 | 6.63 | .04 | 7.09 | 7.1 | .00 | |
| Cholesterol observed | 5.58 | 5.61 | .00 | 5.59 | 5.59 | .00 | |
| HDL observed | 60.8 | 59.4 | .03 | 60 | 60 | .00 | |
| HbA1c observed | 23.8 | 19.5 | .11 | 21.3 | 21.3 | .00 | |
Values are percentage or mean for age; within a timeframe of 12 months before the inclusion date (first dispensing of ARB or ACE inhibitor):
at least one hospitalization (ICD‐10 codes in Supplementary Materials);.
at least 1 delivery;.
at least one measurement.
Most prescribed ACE inhibitors (> 90%) were perindopril, ramipril, enalapril, and trandolapril; valsartan, irbesartan, candesartan, olmesartan, and telmisartan were the most (93%) ARB prescribed. Based on strength per unit for each drug type, patients ordered with high dose were 40 348 (23.2%) with an ACE inhibitor versus 45 336 (19.4%) with an ARB; for medium dose they were 73 062 (41.9%) with an ACE inhibitor versus 146 184 (62.5%) with an ARB and for low dose they were 60 723 (34.9%) with an ACE inhibitor versus 42 162 (18.0%) with an ARB; 53 999 (31.1%) were ordered a fixed combination with an ACE inhibitor and 86 151 (36.8%) with an ARB. Frequency of switching/stopping and timing is displayed in Supplementary Materials, Online Figure I. There was a better persistence with ARB whatever the initial dose level.
The person‐time at risk and the number of events for each outcome, stratified by treatment group, are presented in Table 2. Crude incidence rate was lower in ARB new user group. Other incidence rates for cardiovascular events were more or less similar except for heart failure and ischemic stroke. Kaplan–Meier survival curves were plotted for each outcome stratified by ACE inhibitor–ARB new user group (Figure 2).
TABLE 2.
Number of events, person‐time at risk (ITT‐like population) and incidence rate (per 100 person‐years) for each outcome, stratified by treatment group, angiotensin receptor blocker (ARB) (N = 233 682) and angiotensin‐converting enzyme (ACE) inhibitor new users (N = 174 133)
| ACE inhibitor | ARB | |||||
|---|---|---|---|---|---|---|
| Events, No | Person‐years at risk | Incidence rate | Events, No | Person‐years at risk | Incidence rate | |
| Death | 100228 | 948 043 | 1.08 | 10,310 | 1,280,649 | .80 |
| CV death (strict def.) | 1654 | 948 043 | .17 | 1716 | 1,280,649 | .13 |
| Other CV deaths (broad def.) | 1564 | 948 043 | .16 | 1314 | 1,280,649 | .10 |
| Major cardiovascular event | 9289 | 923 797 | 1.00 | 10 113 | 1 256 692 | .80 |
| Hemorrhagic stroke | 667 | 923 797 | .07 | 815 | 1 256 692 | .06 |
| Ischemic stroke | 2656 | 923 797 | .29 | 3006 | 1 256 692 | .24 |
| Heart failure | 4135 | 923 797 | .45 | 3823 | 1 256 692 | .30 |
| Myocardial infarction | 1831 | 923 797 | .20 | 2469 | 1 256 692 | .20 |
| Other cardiovascular events | 1574 | 942 830 | .17 | 2162 | 1 274 026 | .17 |
| CABG | 69 | 942 830 | .01 | 99 | 1 274 026 | .01 |
| PCA | 1300 | 942 830 | .14 | 1829 | 1 274 026 | .14 |
| PVD | 205 | 942 830 | .02 | 234 | 1 274 026 | .02 |
Abbreviations: CABG: coronary artery bypass graft surgery; PCA: percutaneous coronary angioplasty; and PVD: peripheral vascular disease.
First event within each composite outcome (major or other cardiovascular events) was recorded.
Cause of death was available for 19 122 patients (93.1%) out of 20 538.
FIGURE 2.
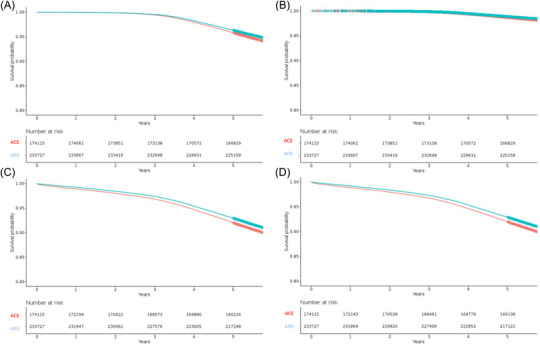
Kaplan–Meier weighted (SIPTW) survival curves for each outcome according to drug class: ACE inhibitor (dotted red line) and ARB new user group (solid blue line): mortality (A), cardiovascular death (B), major cardiovascular events (C), and major or other cardiovascular events (D)
The probability of ARB use (with ACE inhibitor as reference) was modeled using logistic regression (Table S10). Propensity score distribution by treatment group is displayed in Supplementary Materials, Online Figure II. There was a great deal of overlap in the propensity score between the two groups. Summary weighted statistics for important covariates for the two groups after SIPW are displayed in the last two columns of Table 1; of note, standardized differences are all very close to null.
3.3. Main results
They are displayed in Table 3. For all outcomes, HRs were consistently below unity thus favoring ARBs, suggesting that the ARB new user group had a better overall (HR: .878, 95%CI,.854 to .902), and cardiovascular (HR: .841, 95%CI, .800 to .84) survival and had a lower risk for major (or nonmajor) cardiovascular events (HR: .886, 95%CI, .868 to .905).
TABLE 3.
Outcome comparison for angiotensin receptor blocker (ARB) new users (N = 233 682) compared to angiotensin‐converting enzyme (ACE) inhibitor new users (N = 174 133), ITT‐like population (SIPTW or stratified analyses), or using 1:1 matching based on logit of the propensity score (169 865 pairs)
| Outcome | Crude HR (95%CI) | SIPTW HR (95%CI) | Stratified HR (95%CI) | Matched HR (95%CI) |
|---|---|---|---|---|
| Death | .740 (.720–.761) | .878 (.854–.902) | .853 (.830–.877) | .873 (.848–.898) |
| Cardiovascular death (broad def.) | .691 (.658–.727) | .841 (.800–.884) | .814 (.774–.855) | .835 (.793–.880) |
| Major cardiovascular events a | .763 (.747–.779) | .886 (.868–.905) | .865 (.847–.883) | .878 (.860–.897) |
| Major and other cardiovascular events b | .763 (.748–.779) | .886 (.868–.905) | .865 (.847–.883) | .879 (.860–.898) |
Hemorrhagic stroke, ischemic stroke, heart failure or myocardial infarction whichever came first;
above outcomes or coronary artery bypass graft, percutaneous coronary angioplasty or peripheral revascularization procedure whichever came first.
3.4. Analysis of subgroups and interactions
Results are summarized in Figure 3 for mortality and Online Figure III (Supplementary Materials) for composite secondary outcomes. Statistically significant quantitative interactions were detected: Relative hazards were substantially lower for ARB new users compared with ACE inhibitors new users for “first‐line” users, and for those patients without diabetes, or those with none of the following three conditions: diabetes, lipid‐lowering drug use, and antithrombotic drug use. For major cardiovascular events, interaction with diabetes appeared qualitative: ACE inhibitor being better than ARB in those patients with severe diabetes, ARB being better than ACE inhibitor in those patients with nonsevere diabetes or in nondiabetic patients.
FIGURE 3.
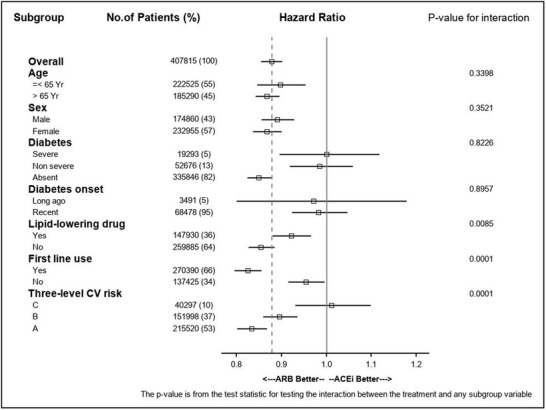
Analysis of subgroups and interactions for mortality (SIPTW Cox models). Severe diabetes if two (or more) oral antidiabetic drugs or insulin plus oral antidiabetic drug; long‐ago onset of diabetes if long‐term registration for more than 10 years; three‐level CV risk: patients having diabetes and using lipid‐lowering drug (C); patients having only one of the above risk factors or using antithrombotic drug (B); patients with none of the above three conditions (A)
3.5. Sensitivity analyses
Different ways in handling propensity scores led to very similar estimates (Table 3). Analyses among the on‐treatment population restricted to adherent patients (ARB new users (N = 135, 830), ACE inhibitor new users (N = 183,855), regularly treated with a MPR greater or equal to 80%) showed a HR (95%CI) for death equal to .674 (.628–.723). During the on‐treatment period, most patients were correctly adherent (MPR ≥80% for 78.0% of patients starting with ACE inhibitor and 78.7% of patients starting with ARB). Subgroups analyses and interaction testing yield similar observation that ITT‐like based analyses. Any attempt to make even more fair the comparison between ARB and ACE inhibitors (comparison for an even dose level) did not change the direction of risk estimates.
3.6. Post hoc analyses
Discarding those 35 517 patients starting an ARB or ACE inhibitor with a low dose (mostly, perindopril 2 mg or 2.5 mg, ramipril 1.25 mg, and enalapril 5 mg; or candesartan 4 mg, and valsartan 40 mg) as a first line therapy that might have been prompted by heart failure led to a weighted (SIPTW) estimate measure of association for overall survival (hazard ratio) of .916 (95%CI, .889 to .943), then still favoring ARB.
As an attempt to examine the role of residual confounding in our observational study that found an apparent relative risk (ARR) of .88 in favor of ARB, we set an association between an unmeasured confounder and death at 1.2 and its prevalence in the ACE inhibitor (reference) group at.10, .25, and .50. We observed that the “true” adjusted RR remains in favor of ARB for a prevalence in the ARB group of .00, .00, and .10, respectively. 10
4. DISCUSSION
4.1. Key results
In this population‐based cohort study, including 407 815 new users of ACE inhibitor or ARB in primary prevention, we observed over a 5‐year follow‐up that ARB users had a better survival and 11% decreased risk of major cardiovascular events when compared to ACE inhibitor. Considering subgroup analyses, ARBs had a better effectiveness as regard primary and secondary outcomes than ACE inhibitors in nondiabetic patients.
4.2. Strengths of the study
First, we used a population‐based nationwide new‐user cohort design which minimizes selection bias. We compared treated patients, i.e., who sought medical attention and subsequently had drug prescription. Drug claims are collected timely and prospectively; hence recall bias does not apply and misclassification on exposure is minimized.
Second, we thought confounding by indication was minimized by focusing on a primary prevention setting. We had comprehensive information (within a year) on previous drug prescriptions and hospitalization. In addition, we used several propensity score‐based methods to minimize at best confounding by indication and assess robustness of results. Consistency of risk estimation across several models and subgroups supports validity.
Third, we had no attrition bias, each patient being entirely followed up to death or end of study period; hence, all hospitalizations for cardiovascular disease were identified.
Finally, we employed statistical methods similar to those of randomized trials (similar to intention‐to‐treat principal) considering mortality and cardiovascular events as efficacy outcomes and putting forward a superiority hypothesis.
4.3. Limitations
The main limitation concerned variables which affect the treatment decision and outcomes not captured or poorly measured: dietary patterns, physical exercise, familial history of cardiovascular disease, highest level of education attained, or socio‐economic status; but when comparing drug users between each other, such healthy‐user effect is thought to be less problematic. We had indeed no outstanding information on some key behavioral risk factors (smoking habit, alcohol consumption) and no information at all on blood pressure level and body mass index; but we had noticed in Roy et al. study 11 that systolic blood pressure and body mass index variables were not strong predictors of ARB use compared to ACE inhibitor use (all standardized differences < .05). As proxies of smoking habit and alcohol consumption, we used hospitalization discharge diagnosis for COPD and liver disease and thought that misclassification bias if any was nondifferential. We cannot rule out residual confounding. Of note, all SIPTW standardized differences were very close to null and there was a great deal of overlap in the propensity score between the two groups.
Ascertainment of clinical outcomes from hospital discharge diagnosis may lead to some misclassification, but thought to be nondifferential. Obviously, this limit does not apply to the survival analysis.
The percentage of patients treated with anticoagulant (5.5%) is higher than the percentage of patients with atrial fibrillation (AF) which was very probably underestimated because the identification of AF in SNDS is challenging. The percentage of patients with antiplatelet drugs reflects the use of these drugs in France in high risk and diabetic patients despite a primary prevention setting.
Finally, intention‐to‐treat analysis focused on the effect of the initial treatment decision, but not on mechanisms. We reckoned we observed only what was delivered to patients (through pharmacy deliveries) and adherence was not accurately captured by our data. However, we estimated MPR and assessed persistence through last observed delivery of the initially prescribed drug. Taking in account an on‐treatment approach, censoring patients when stopped or switched their initial regimen, we attempted to assess whether ACE inhibitors and ARBs are equally effective among current/persistent users (on treatment analysis taking in account adherence through MPR). Even so, previously observed differences in survival remained significant.
4.4. Strengths and weaknesses in relation to other studies, discussing important differences in results
Our study obviously differed by design from clinical trials 12 , 13 , 14 but also in some important aspects of clinical setting and methodology to other observational studies. 15 , 16 , 17
In the very few head‐to‐head RCTs that compared ACE inhibitors and ARBs in hypertensivediabetic patients, no difference was found on death or clinical outcomes. Of note, data mostly derived from one large trial, ONTARGET, 8 which provided data for a subgroup of hypertensive 7 and diabetic patients; all other trials included 500 patients 11 or even less 12 , 13 hypertensive only 11 , 12 or also diabetic with early nephropathy, 13 with a history of cardiovascular disease 8 or not 12 ; main outcome was blood pressure control, 11 change in GFR 12 or albuminuria, and renal function. 13 All trials had a follow‐up of 5 years at most. 8 , 12 Of note, meta‐analysis of those trials 7 was largely driven by ONTARGET data. 8
Meta‐analyses all made indirect inference based on trials assessing effect of ACE inhibitors or ARBs versus placebo or control. A recent meta‐analysis 5 of 20 cardiovascular morbidity–mortality trials, conducted after year 2000, including mostly hypertensive patients (91% on average) compared active treatment with ACE inhibitor or ARB with control treatment: it showed a differential treatment effect on mortality between ACE inhibitors and ARBs through stratified subgroup analysis according to class of drug. Authors cautiously concluded that the finding should be considered as a post hoc observation. A similar conclusion for patients with diabetes (and mostly hypertensive) was supported by another meta‐analysis of data from 35 RCTs 6 : ARB did not significantly reduce the risk for CV deaths compared with control therapy but heterogeneity across trials was high (I2 = 61%). Of note, trials of ACE inhibitors and ARBs included patients with different characteristics (more patients with coronary or other vascular atherosclerotic disease in ACE inhibitors trials than in ARB trials). A metaregression analysis 18 pinpointed that the difference between ACE inhibitors and ARBs compared with placebo might be due to a higher placebo event rate in the ACE inhibitors trials (most of these trials were conducted a decade earlier than the ARB trials). A network meta‐analysis 19 concluded that no drug regimen was more effective than placebo for reducing all‐cause mortality in adults with diabetes and kidney disease.
As regards observational studies, no evidence of differences in rates of death, stroke, or coronary heart disease was found among 22 544 adults with hypertension from a large health system in Pennsylvania (USA) taking ACE inhibitors or ARBs and followed‐up over 4 years (11 000 persons‐years). Of note, 25% had diabetes, 17% coronary artery disease, 4% stroke, and 2% heart failure. No subgroup analysis according to diabetes or history of cardiovascular disease was provided. Incidence rates of outcomes were higher than in our study, probably reflecting a higher baseline risk level. 11
Based on Taiwan's National Health Insurance Research Database for the period 2000–2010, patients with newly diagnosed diabetes (88% were hypertensive, 20% had CAD, 5% had HF, and 2% had PVD) and newly treated with ACEIs (n = 21 436) or ARBs (n = 30 777) were identified. 15 After one‐to‐one matching by propensity score, no significant differences in risk of myocardial infarction (hazard ratio [HR] .92, 95%CI, .80 to 1.07), ischemic stroke (HR.95, 95%CI, .87 to 1.04), or all‐cause mortality (HR .95, 95%CI, .89 to 1.01) were found over a mean follow‐up period was 6.2 years. Subgroup analyses stratified by heart failure and coronary artery disease were consistent.
A population‐based retrospective cohort study (US claims database) of more than 87 000 insured Americans with diabetes (70% were hypertensive, 14% had a history of CVD, mostly IHD, 9.8% had diabetes‐related complications) found that ARB use relative to ACE inhibitor use was associated with a reduced risk of all‐cause mortality or hospitalization (composite primary outcome) with a maximum follow‐up of 6 years. 16 This finding was driven exclusively by reduced risk of hospitalization: relative risk for all‐cause mortality was .95 (95%CI, .65 to 1.40). Results were similar in patients with a baseline history of cardiovascular disease but no data were provided for patients without a baseline history of cardiovascular disease.
A recently published analysis from the REACH registry 17 reported that, in patients without established atherosclerosis (primary prevention), there was no difference between ARB or ACE inhibitors users for a composite of CV mortality, nonfatal myocardial infarction, nonfatal stroke or hospitalization for CV (RR = .98, 95%CI, .85 to 1.13), nor for all deaths (RR = 1.06, 95%CI, .86 to 1.31). Of note, in patient without diabetes, relative risk estimates were .88 (95%CI, .82 to .95) and .85 (95%CI, .74 to .97) for the composite outcome and all death respectively, in favor of ARB.
4.5. Interpretation
Our results do not support results from meta‐analyses suggesting, based on indirect evidence, that ACE inhibition leads to improved morbidity and mortality outcomes relative to ARBs. It is hard to draw parallel between previously published clinical trials or observational studies and our findings because we analyzed hypertensive patients (with diabetes or not) without clinically overt cardiovascular disease. Nevertheless, careful selection of patients (to focus on primary prevention) and relevant subgroup analyses, might have somehow disentangle some previously inconsistent or negative results: keeping in mind that our patients had no clinically overt cardiovascular disease, those without diabetes might have benefit to be ordered ARB.
4.6. Generalizability
Our nationwide French database mostly enrolled patients of Caucasian origin. No ethnic data were available and no conclusion could be drawn for black hypertensive patients.
4.7. Meaning of the study: possible explanations and implications for clinicians and policymakers
Patients taking ACE inhibitors were less persistent than ARB users, whatever the initial dose level. Early discontinuation could be related, at least in part, to adverse symptoms (cough). This might have underestimated a true beneficial effect of ACE inhibitor in intention‐to‐treat like analyses. These analyses may otherwise better represent real‐life practice and are more conservative. Nevertheless, the results were consistent with those from on‐treatment analyses. Finally, the hypothesis of better persistence/adherence as a plausible explanation for the observed result, if true, and otherwise unbiased, is not supported by our findings. It has been reported that ARBs are better tolerated than ACEIs and this could explain the highest adherence rate between all the antihypertensive classes in meta‐analysis. 20 Although subgroup analyses should be cautiously interpreted, any recommendation favoring ARB user might be restricted to low risk patients. Any firm conclusions as regards high‐risk patients, such as those with severe diabetes, cannot be drawn.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
Emmanuel Oger, Sandrine Kerbrat, Emmanuel Nowak, François Paillard, Pierre‐Yves Scarabin, and André Happe contributed to the design of the research. Emmanuel Oger, Sandrine Kerbrat, Emmanuel Nowak, and André Happe were involved in data management and implementation of the research. Emmanuel Oger, Sandrine Kerbrat, Emmanuel Nowak, and André Happe contributed to the analysis and writing of the manuscript first draft. François Paillard, and Pierre‐Yves Scarabin contributed to data interpretation and writing of final manuscript.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
This work was supported by a grant from the French National Public Drug Agency (ANSM, Agence Nationale de Sécurité du Médicament).
Oger E, Kerbrat S, Nowak E, Paillard F, Scarabin P‐Y, Happe A. Effectiveness of angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers on total and cardiovascular mortality and morbidity in primary prevention: A nationwide study based on French Health Insurance Data (SNDS). J Clin Hypertens. 2022;24:438–448. 10.1111/jch.14445
REFERENCES
- 1. James PA, Oparil S, Carter BL, et al. 2014 evidence‐based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507‐520. [DOI] [PubMed] [Google Scholar]
- 2. Williams B, Mancia G, Spiering W, et al. ESC Scientific Document Group. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021‐3104. [DOI] [PubMed] [Google Scholar]
- 3. Association AmericanDiabetes. Executive summary: standards of medical care in diabetes—2012. Diabetes Care. 2012;35:S4‐S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wright JM, Musini VM, Gill R. First‐line drugs for hypertension. Cochrane Database Syst Rev. 2018;4:CD001841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van Vark LC, Bertrand M, Akkerhuis KM, et al. Angiotensin‐converting enzyme inhibitors reduce mortality in hypertension: a meta‐analysis of randomized clinical trials of renin‐angiotensin‐aldosterone system inhibitors involving 158,998 patients. Eur Heart J. 2012;33:2088‐2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cheng J, Zhang W, Zhang X, et al. Effect of angiotensin‐converting enzyme inhibitors and angiotensin II receptor blockers on all‐cause mortality, cardiovascular deaths, and cardiovascular events in patients with diabetes mellitus: a meta‐analysis. JAMA Intern Med. 2014;174:773‐785. [DOI] [PubMed] [Google Scholar]
- 7. Li EC, Heran BS, Wright JM. Angiotensin converting enzyme (ACE) inhibitors versus angiotensin receptor blockers for primary hypertension. Cochrane Database Syst Rev. 2014;2014:CD009096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. ONTARGET Investigators , Yusuf S, Teo KK, Pogue J, et al. ONTARGET Investigators . Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547‐1559. [DOI] [PubMed] [Google Scholar]
- 9. Blood Pressure Lowering Treatment Trialists' Collaboration , Turnbull F, Neal B, Pfeffer M, et al. Blood pressure‐dependent and independent effects of agents that inhibit the renin‐angiotensin system. J Hypertens. 2007;25:951‐958. [DOI] [PubMed] [Google Scholar]
- 10. Schneeweiss S. Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiol Drug Safety. 2006;15:291‐303. [DOI] [PubMed] [Google Scholar]
- 11. Roy J, Shah NR, Wood GC, Townsend R, Hennessy S. Comparative effectiveness of angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers for hypertension on clinical end points: a cohort study. J Clin Hypertens (Greenwich). 2012;14:407‐414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bremner AD, Baur M, Oddou‐Stock P, Bodin F. Valsartan: long‐term efficacy and tolerability compared to lisinopril in elderly patients with essential hypertension. Clin Exp Hypertens. 1997;19:1263‐1285. [DOI] [PubMed] [Google Scholar]
- 13. Barnett AH, Bain SC, Bouter P, et al. Angiotensin‐receptor blockade versus converting‐enzyme inhibition in type 2 diabetes and nephropathy. N Engl J Med. 2004;351:1952‐1961. [DOI] [PubMed] [Google Scholar]
- 14. Lacourcière Y, Bélanger A, Godin C, et al. Long‐term comparison of losartan and enalapril on kidney function in hypertensive type 2 diabetics with early nephropathy. Kidney Int. 2000;58:762‐769. [DOI] [PubMed] [Google Scholar]
- 15. Shih CJ, Chu H, Ou SM, Chen YT. Comparative effectiveness of angiotensin‐converting enzyme inhibitors and angiotensin II receptor blockers on major adverse cardiac events in patients with newly diagnosed type 2 diabetes: a nationwide study. Int J Cardiol. 2015;199:283‐289. [DOI] [PubMed] [Google Scholar]
- 16. Padwal R, Lin M, Eurich DT. The comparative effectiveness of angiotensin‐converting enzyme inhibitors and angiotensin II receptor blockers in patients with diabetes. J Clin Hypertens (Greenwich). 2016;18:200‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Potier L, Roussel R, Elbez Y, et al. Angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers in high vascular risk. Heart. 2017;103:1339‐1346. [DOI] [PubMed] [Google Scholar]
- 18. Bangalore S, Fakheri R, Toklu B, Ogedegbe G, Weintraub H, Messerli FH. Angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers in patients without heart failure? Insights from 254,301 patients from randomized trials. Mayo Clin Proc. 2016;91:51‐60. [DOI] [PubMed] [Google Scholar]
- 19. Palmer SC, Mavridis D, Navarese E, et al. Comparative efficacy and safety of blood pressure‐lowering agents in adults with diabetes and kidney disease: a network meta‐analysis. Lancet. 2015;385:2047‐2056. [DOI] [PubMed] [Google Scholar]
- 20. Kronish IM, Woodward M, Sergie Z, Ogedegbe G, Falzon L, Mann DM. Meta‐analysis: impact of drug class on adherence to antihypertensives. Circulation. 2011;123:1611‐1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.


