Abstract
Background.
The use of pediatric grafts for liver transplantation (LT) into adult recipients is rare, and reported outcomes are conflicting. The aim of this study is to evaluate the outcomes in adult recipients following LT with grafts from deceased pediatric donors.
Methods.
A retrospective study identifying adult LT between 2010 and 2020 using pediatric deceased donor liver grafts was conducted. Adults undergoing LT with deceased donor pediatric grafts (age ≤ 12) were identified and matched 1:2 with adults receiving adult grafts (age ≥ 18) based on recipient age (±10 y), model for end-stage liver disease (MELD) score at transplant (±5 points) and etiology of liver disease. To assess real liver size differences between the pediatric-donor and adult-donor groups, patients receiving a graft from a donor between 13 and 17 y were excluded from the main analysis and studied independently. Outcomes between the groups were compared. Complication rates were identified and graded using Clavien–Dindo classification. Graft and patient survival were assessed by Kaplan–Meier curves.
Results.
Twelve adult LT recipients with whole liver grafts from deceased pediatric donors were matched with 24 adult recipients of adult donors. Recipient age and MELD score were similar between groups. Recipients of pediatric grafts were more likely to be female (66.7% versus 16.7%, P = 0.007) and leaner (body mass index = 24.4 versus 29.9, P = 0.013). Alcohol-related cirrhosis was the most prevalent liver disease etiology in both groups (P = 0.96). There was no significant difference in length of stay, readmissions, early complications, or major complications between groups. Vascular and biliary complication rates were similar. Actuarial graft and patient survival at 1, 3, and 5 y were 100/100/100 versus 96/96/96 (P = 0.48).
Conclusions.
Excellent patient and graft survival is achievable with LT using young pediatric deceased donor grafts in smaller adult recipients. Outcomes are comparable with recipients of age and MELD-matched adult donors. Careful donor MELD-score recipient matching and close monitoring for potential biliary and vascular complications are crucial to achieve acceptable outcomes.
INTRODUCTION
Despite the growing volume of liver transplantation (LT) over the past decade, there remains a shortage of donor livers in the United States.1 In addition to the increased use of expanded criteria donors, an alternative option is the use of pediatric-donor livers. Although pediatric grafts should ideally be used for pediatric recipients, they may be allocated to adult recipients in certain situations when turned down by the pediatric recipient pool.2
There are insufficient data on the efficacy of pediatric liver grafts in adult recipients. Existing literature has generally found that when compared with those receiving adult-donor grafts, adults receiving pediatric-donor grafts experience similar rates of graft survival.2-6 However, some data have demonstrated an increased risk of early graft loss and mortality in these patients.3,7 Pediatric-to-adult liver transplantation is known to carry an increased risk of small-for-size syndrome secondary to inadequate liver volume.4 Pediatric age plays a significant role on liver size and is an important consideration when evaluating the impact of using a graft from a younger versus older pediatric donor in an adult recipient. Previous studies have used age <12 y old to characterize a pediatric donor.3 Evidence suggests that, similarly to the accepted graft-to-recipient weight ratio >0.8% for living donor transplantation, that cutoff may also be true for deceased donor liver transplantation.3 Pediatric liver grafts have also been found to be associated with a higher rate of vascular complications, particularly hepatic artery thrombosis (HAT).2,3,8
Because of the limited and conflicting evidence regarding its safety and efficacy, it is important to better understand the risks of using pediatric-donor grafts in adult recipients. The aim of our study is to evaluate the outcomes following LT of adult recipients receiving pediatric liver grafts at a single center. Furthermore, we subgroup pediatric donors into those <12 y old and 13–17 y old to assess the impact of using smaller size grafts in adult recipients.
MATERIALS AND METHODS
Study Design
A retrospective single-center case-control study was conducted on all consecutive adult patients who underwent LT with a whole liver graft from a deceased pediatric donor (≤12 y) between 2010 and 2020. Patients were identified by electronic medical record chart review. Records from pediatric LT recipients, adult recipients who underwent split LT, and retransplantations were excluded from the analysis. To assess real liver size differences between the pediatric-donor and adult-donor groups, decision to exclude patients receiving a graft from a donor between 13 and 17 y of age from the main analysis and study them independently was made.
Adult recipients receiving a graft from a pediatric-donor (pediatric-donor group) were matched in a 1:2 ratio to patients receiving a graft from an adult donor ≥18 y (adult-donor group) during the same period. The case-control matching was based on recipient age (±10 y), recipient model for end-stage liver disease (MELD) score at transplant (±5 points), and etiology of liver disease. Donor and graft characteristics, recipient demographic variables, surgical variables, postoperative outcomes and complications within 30-d and 1-y posttransplant were compared between the 2 groups.
Donor and Graft Data
The following donor characteristics were analyzed and compared between groups: age; gender; body mass index (BMI); cold ischemia time (CIT); warm ischemia time (rWIT), denoting recipient implantation time; and type of donor (donor after circulatory death or donor after brain death). In addition, percentage of grafts retrieved by another team were recorded for each group.
Recipient Data
Recipients were chosen by transplant surgeon based on appropriate recipient size and anatomy. The following recipient data were retrospectively collected: age; gender; BMI; native MELD score at transplant; time on waiting list; etiology of liver disease; pretransplant diagnosis of hepatocellular carcinoma (HCC); intraoperative variables; laboratory values posttransplant at 48 h, 7 d, and 1 mo; and postoperative complications within 30 d and within 1 y. Graft and patient survival at 1, 3, and 5 y was also evaluated.
The immunosuppression therapy was given following institutional protocols. In general, all patients received basiliximab and steroids for induction, followed by maintenance with calcineurin inhibitors and mycophenolate. Steroids were tapered to be discontinued by 3 mo posttransplant. Acute cellular rejection (ACR) rate during the first year was collected and analyzed. ACR was diagnosed by liver biopsy according to previously described criteria.9
Surgical Technique
The liver transplant technique used was surgeon preference/individual case dependent, following standardized techniques previously described.10-12 Briefly, most implants were performed using a piggy-back technique, given this technique adapts better to the vena cava size discrepancies encountered when implanting pediatric grafts into adult recipients. A side-to-side cavo-cavostomy was the preferred caval reconstruction technique. In most of the cases, reconstruction of the artery was performed using 7-0 prolene in a running fashion. A branch patch between recipient and donor arteries was used when needed to account for size discrepancies.
The size match between donor and recipient’s common bile duct was evaluated carefully. In most cases, bile duct reconstruction was performed with an end-to-end suture using running 6-0 polydioxanone suture. If a considerable mismatch was encountered, we either everted the mucosa of the larger bile duct to compensate for size discrepancy or the mucosa of the smaller duct was slightly opened longitudinally in the middle.
Postoperative Outcomes and Follow-up
Postoperative ultrasound (US) was performed at postoperative day (POD) 1, 3, and 5. All patients received aspirin 81 mg posttransplant once platelet count was >50 K/µL and deep vein thrombosis prophylaxis. Anticoagulation was based on individual assessments. In cases in which there was increased risk of HAT (ie, significant size mismatch), daily US for the first 5 d was performed. Postoperative outcomes were assessed by analysis of the following variables: length of hospital stay, intensive care unit stay, and readmission within the first month after LT. Complications at 30-d and 1-y posttransplant were collected and classified using the Clavien–Dindo classification system for surgical complications.13 Major complications were defined as those >3b. If >1 complication occurred in a patient, the more serious event was used for grading. Occurrence of primary nonfunction, vascular complications, and biliary complications were identified. Graft function after transplantation was assessed clinically and through biochemical markers measured at 48 h, 7 d, and 1 mo after discharge. Retransplantation rate and long-term outcomes were also analyzed by 1-, 3-, and 5-y graft and patient survival. Close follow-up was performed after LT in multidisciplinary clinic visits.
Statistical Analysis
Statistical analyses were carried out using IBM SPSS Statistics Version 26. Descriptive information is presented as median (interquartile range) for continuous variables and as frequencies with percentages for categorical variables. Continuous variables were compared using Student’s t or Mann–Whitney U tests, as appropriate. Categorical variables were compared using Pearson’s chi-square or Fisher’s exact test, as appropriate. For all analyses, 2-tailed P < 0.05 was considered statistically significant. Graft and patient survivals were calculated by the Kaplan–Meier method.
Analysis of Outcomes in Older Pediatric-Donors Versus Adult-Donors
Large variability on age ranges and pediatric age-subgroups exists throughout the literature.14,15 Nonetheless, classification of pediatric age-subgroups is often related to developmental stages, which plays a significant role on liver size in pediatric population.16,17 Therefore, to identify variations in outcomes according to the different pediatric-donor age subgroups, and assess the real impact of using smaller size grafts in adult recipients, an additional analysis was performed comparing outcomes after LT using a graft from an older pediatric donors (13–17 y) versus an adult donor. Actuarial graft and patient survival were also compared between groups.
Because of the retrospective nature of the study, it was deemed exempt from informed consent requirement by the Institutional Review Board of the University of Virginia Health System.
RESULTS
From January 2010 to July 2020, 762 liver transplants were performed at our institution. Among those, twelve adult recipients underwent LT with a whole liver graft from a deceased pediatric donor <12 y old and were matched 1:2 with 24 adult recipients receiving a graft from an adult donor (>18 y old). In the entire cohort, 23% (14/61) of the grafts were retrieved by another team. Among the pediatric group, 33% (4/12) were retrieved by another team, whereas 25% (6/24) were in the adult-donor group. In the older pediatric group, 16% (4/25) were retrieved by another team; however, 9 cases did not have that information.
Donor, Graft, and Perioperative Characteristics
Median donor age in the pediatric-donor group was 9.5 (6–11) y, whereas in the adult-donor group was 48 (29.5–57.5) y (P ≤ 0.001). Proportion of male donors was lower, although not significant, in the pediatric-donor group versus the adult-donor group (4 [33.3%] versus 13 [54.2%], P = 0.23). In concordance with the study groups definition, donors in the pediatric-donor group had lower BMI versus those in the adult-donor group (16.9 [16.1–17.8] versus 28.4 [24.1–31.7], respectively; P ≤ 0.001). CIT was significantly longer in the pediatric-donor group when compared with the adult-donor group (371 [323–512] min versus 314.4 [267.1-381.6, respectively; P = 0.04]). Similarly, rWIT was longer in the pediatric-donor group versus the adult-donor group (41.5 [38–7] versus 34 [30–41] min, respectively; P = 0.011). Proportion of donor after circulatory death donors was higher in the pediatric-donor, without reaching statistical significance (Table 1). Perioperative variables were similar between groups and can be found on Table S1 (SDC, http://links.lww.com/TXD/A412).
TABLE 1.
Donor and graft characteristics according to donor age
| Pediatric donor ≤12 (n = 12) | Adult donor (n = 24) | P | |
|---|---|---|---|
| Donor age | 9.5 (6–11) | 48 (29.5–57.5) | <0.001 |
| Donor male gender (%) | 4 (33.3) | 13 (54.2) | 0.23 |
| Donor BMI | 16.9 (16.1–17.8) | 28.4 (24.1–31.7) | <0.001 |
| CIT (min) | 371 (323–512) | 314.4 (267.1–381.6) | 0.04 |
| rWIT (min) | 41.5 (38.0–47.5) | 34 (30–41) | 0.011 |
| DCD | 3 (25) | 2 (8.3) | 0.17 |
Median (interquartile range).
BMI, body mass index; CIT, cold ischemia time; DCD, donation after circulatory death; rWIT, recipient warm ischemia time.
Recipient Preoperative Characteristics
Recipient age and MELD score at transplant (native) were similar between groups (Table 2). Waitlist time was considerably, but not statistically, shorter in the pediatric-donor group versus the adult-donor group (104.5 d [82.7–181] versus 201 d [47.5–317.2], respectively; P = 0.25). Proportion of male recipients was significantly lower in the pediatric-donor group when compared with the adult-donor group (4 [33%] versus 20 [83%], respectively; P = 0.007). Recipients in the pediatric-donor group had significantly lower BMI versus recipients in the adult-donor group (24 [22–31] versus 30 [28–35], respectively, P = 0.013). Etiologies of liver disease were similar between groups, with alcohol-related cirrhosis being the most common on both groups, followed by hepatitis C virus cirrhosis and nonalcoholic steatohepatitis (41.7%, 25% and 8.3% in the pediatric-donor group, respectively; and 50%, 25%, and 8.3% in the adult-donor group, respectively; P = 0.96). Incidence of HCC was found to be the same among groups (41.7%, P ≥ 0.9) (Table 2).
TABLE 2.
Preoperative characteristics of liver transplant recipients according to donor age
| Pediatric donor ≤ 12 (n = 12) | Adult donor (n = 24) | P | |
|---|---|---|---|
| Age at transplant | 58.5 (49.7–64.5) | 58 (51–63) | 0.78 |
| Male gender (%) | 4 (33.3) | 20 (83.3) | 0.007 |
| BMI | 24.4 (22.3–31.1) | 29.9 (28.0–35.4) | 0.013 |
| MELD score | 17 (10.5–24) | 17 (10.0–25.5) | 0.93 |
| Time on waitlist (d) | 104.5 (82.7–181.0) | 201 (47.5–317.2) | 0.25 |
| Etiology | 0.96 | ||
| Alcohol-related cirrhosis | 5 (41.7) | 12 (50) | |
| HCV | 3 (25) | 6 (25) | |
| NASH | 1 (8.3) | 2 (8.3) | |
| PSC ulcerative colitis | 1 (8.3) | 2 (8.3) | |
| Other | 2 (16.7) | 2 (8.3) | |
| HCC diagnosis yes/no | 5 (41.7) | 10 (41.7) | >0.9 |
| Multiorgan | 1 (8.3) | 1 (4.2) | 0.6 |
Median (interquartile range).
BMI, body mass index; MELD, model for end-stage liver disease; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; NASH, nonalcoholic steatohepatitis; PSC, primary sclerosing cholangitis.
Postoperative Outcomes
Intensive care unit stay and length of stay were similar between groups (Table 3). Likewise, readmission rate within the first 30 d posttransplant and complication rate in the first month were also similar. There were no cases of primary nonfunction in this cohort. No mortality cases were seen in the pediatric-donor group, whereas 1 (4.2%) patient in the adult-donor group died 2 mo after transplantation due to causes unrelated to the transplant. According to the Clavien–Dindo classification, major complications (>3b) within the first 30 d were higher in the pediatric-donor group, although not reaching statistical significance (4 [33.3%] in the pediatric-donor group versus 3 [12.5%] in the adult-donor group, respectively; P = 0.25). In the pediatric-donor group, these corresponded to 2 patients that developed HAT within the first week, both successfully managed with intraoperative thrombectomy, thrombolysis with Alteplase, and revision of the hepatic artery anastomosis. Both cases were identified by protocol US, without having clinically relevant changes. The first case was identified at POD 2. The patient is >3-y posttransplant and have had a remarkable recovery, without development of biliary complications or further vascular complications. The second case was identified at POD 3; since then, the patient developed an anastomotic bile duct stricture 4 mo posttransplant that was successfully managed with a stent placement during endoscopic retrograde cholangiopancreatography (ERCP). This patient has now recovered fully and is doing well with no further bile duct complications. The remaining 2 cases corresponded to 2 patients that required relaparotomy for evacuation of intra-abdominal hematomas. All patients fully recover from the aforementioned complications. In the adult-donor group, the three >3b grade complications were seen in 2 patients that underwent relaparotomy, 1 for evacuation of an intra-abdominal bleeding, and 1 patient for hepatic artery revision because of HAT concern. The artery was found to be kinked, and the anastomosis was successfully repaired. The remaining case was a patient with severe stenosis of the caval anastomosis, successfully managed with stent placement. Incidence of vascular complications within 30 d was similar between groups and corresponded to the cases previously discussed. Likewise, biliary complications within 30 d were similar among groups, with an incidence of 8.3% for both. Overall, bile duct strictures were higher, but not statistically significant, in the pediatric-donor group versus the adult-donor group (3 [25%] versus 4 [16.7%], respectively; P = 0.66). All cases were successfully managed with ERCP. Of note, in our cohort, significant bile duct size mismatch only occurred in 4 of the 12 adult recipients of a pediatric (<12 y old) graft. Additional postoperative outcomes and complications according to Clavien–Dindo classification are depicted in Tables 3 and 4, respectively.
TABLE 3.
Postoperative outcomes of liver transplant recipients according to donor age
| Pediatric donor ≤12 (n = 12) | Adult donor (n = 24) | P | |
|---|---|---|---|
| LOS (d) | 5 (4–8.5) | 6.5 (5–9) | 0.19 |
| Posttransplant ICU stay (d) | 1.7 (1.2–3.2) | 1 (1–2) | 0.3 |
| Readmission within 30 d | 2 (16.7) | 3 (12.5) | 0.73 |
| Complications within 30 d | 5 (41.7) | 7 (29.2) | 0.45 |
| >3b complications within 30 d | 4 (33.3) | 3 (12.5) | 0.13 |
| Complications within first year | 6 (50) | 9 (37.5) | 0.47 |
| Reoperation | 4 (33.3) | 2 (8.3) | 0.058 |
| Vascular complications within 30 d | 2 (16.7) | 2 (8.3) | 0.45 |
| HA stenosis | 2 (16.7) | 0 | 0.04 |
| HA thrombosis | 2 (16.7) | 1 (4.2) | 0.25 |
| PV stenosis | 0 | 0 | |
| PV thrombosis | 0 | 0 | |
| Biliary complications within 30 d | 1 (8.3) | 2 (8.3) | >0.9 |
| Biliary complications within first year | 3 (25) | 4 (16.7) | 0.66 |
| BD stricture | 3 (25) | 4 (16.7) | 0.66 |
| BD leak | 0 | 1 (4.2) | 0.47 |
| PNF | 0 | 0 | |
| New dialysis requirement | 0 | 2 (8.3) | 0.54 |
| Retransplant (%) | 0 | 0 | |
| Rejection within 1 y | 0 | 6 (25) | 0.058 |
| Recipient death | 0 | 1 (4.2) | 0.47 |
| 1 y mortality (%) | 0 | 1 (4.2) | 0.47 |
| 1-/3-/5-y graft survival (%) | 100/100/100 | 96/96/96 | 0.48 |
| 1-/3-/5-y patient survival (%) | 100/100/100 | 96/96/96 | 0.48 |
Median (interquartile range).
BD, bile duct; LOS, length of stay; ICU, intensive care unit; HA, hepatic artery; PV, portal vein; PNF, primary nonfunction.
TABLE 4.
Complications according to Clavien–Dindo classification
| Pediatric donor ≤12 (n = 12) | Adult donor (n = 24) | P | |
|---|---|---|---|
| 1 | 0 | 0 | 0.29 |
| 2 | 0 | 0 | |
| 3a | 3 (25) | 5 (20.8) | |
| 3b | 4 (33.3) | 3 (12.5) | |
| 4 | 0 | 0 | |
| 5 | 0 | 1 (4.2) |
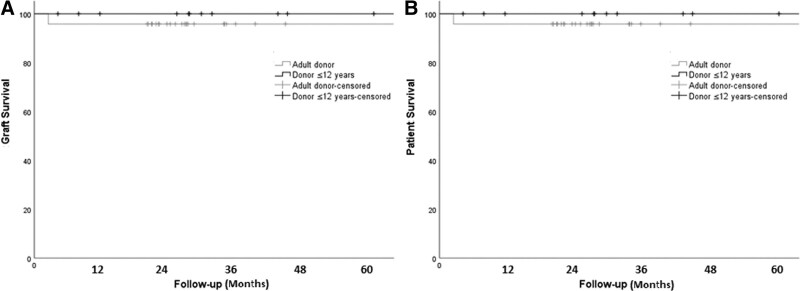
Actuarial graft and patient survival were similar between groups (Figure 1). One-, 3- and 5-y graft survival was 100% for all time periods in the pediatric-donor group versus 96% for all time periods in the adult-donor group (P = 0.48). Similarly, patient survival at 1, 3, and 5 y was 100%, 100%, and 100% in the pediatric-donor group versus 96%, 96%, and 96% in the adult-donor group, respectively; P = 0.48.
FIGURE 1.
Kaplan–Meier curves for graft and patient survival following liver transplantation with grafts from pediatric donors vs adult donors. A, Graft survival, P = 0.48, log rank (Mantel–Cox). B, Patient survival, P = 0.48, log rank (Mantel-Cox).
Outcomes in Older Pediatric-Donors (13–17 Y Old) Versus Adult-Donors
During the study period, 25 adults received a graft from a deceased pediatric donor between 13 and 17 y (older pediatric-donor group). Donor’s baseline characteristics followed the same pattern as in the main analysis, except for a more similar gender distribution between groups. In contrast to the main analysis, recipients’ BMI were similar among groups. Time on the LT waiting list was shorter, although not significant, in the older pediatric-donor group versus the adult-donor group (83 [25.5–241.5] d versus 201 [47.5–317.2] d, respectively; P = 0.22). Vascular and biliary complications were comparable between groups. Interestingly, in this subgroup analysis, 1-y mortality rates were higher, but not statistically significant, in the older pediatric-donor group, with an occurrence of 2 (8%) cases compared with 1 (4.2%) case in the adult-donor group; P = 0.57. Both cases in the older pediatric-donor group corresponded to patients with recurrent HCC with history of transarterial chemoembolization and chemotherapy pretransplant. One patient had 2 liver lesions measuring 2.8 cm and 2.1 cm, and the other had multiple liver lesions, with the largest measuring 2.8 cm. Unfortunately, explant pathology evidenced more severe disease, with macrovascular invasion and lymph node involvement for 1 patient, and large amounts of viable-appearing carcinoma without lymph node involvement for the other, respectively. The mortality case in the adult-donor group was previously discussed. No retransplants were found in this group. Graft and patient survival at 1, 3, and 5 y were comparable between groups (91%/86%/65% for the older pediatric-donor group versus 96%/96%/96% in the adult-donor group, P = 0.35) (Table S2, SDC, http://links.lww.com/TXD/A412).
DISCUSSION
Outcomes after adult LT using grafts from deceased pediatric donors remains poorly understood. During the study period, 37 adult recipients received a graft from a donor <18 y at our institution. Among those, 12 received a graft from a deceased pediatric donor ≤12 y, and 25 received a graft from a donor between 13 and 17 y. We found, although not statistically significant, that time on the waitlist was considerably shorter in the pediatric-donor group. Female recipients with normal BMI were predominant in the pediatric-donor group versus those receiving a graft from an adult donor. We found similar postoperative outcomes and comparable graft and patient survival among adults receiving a graft from a pediatric donor when compared with adult donors. Overall, vascular and biliary complications rates were also comparable.
The organ shortage and high mortality on the LT waitlist is forcing transplant teams to evaluate alternatives to lessen the burden of this organ demand -supply disparity. The use of pediatric-donor liver grafts in adult recipients is an option that may be considered if the organ is declined by the pediatric recipient pool. However, the latest Organ Procurement and Transplantation Network/Scientific Registry of Transplant Recipients report showed a decline from 11.7% to 7.7% on usage of pediatric livers for adult LT with no increase in the use of these grafts in the pediatric population.18 Nonetheless, recent data have suggested that pediatric liver grafts are not being adequately offered to potential pediatric recipients either,7 raising concerns regarding equitable allocation of organs in the United States. To overcome possible disparities, a recent policy change was proposed by United Network for Organ Sharing to direct pediatric-donor livers to pediatric candidates before adult candidates at the same level of medical urgency.19 Of note, this allocation policy was implemented after our study period; therefore, none of the adult recipients here mentioned were affected/favored by this change. This proposal, effective as of October 7, 2021, ensures a fair allocation to pediatric candidates, without taking out of the equation allocation to an adult candidate. This is particularly important because in many cases, there is no available pediatric candidates, and allocation to an adult is preferable than wasting an otherwise usable graft. The full allocation policy based on candidates characteristics, distance between transplant hospital from donor hospital, and donor/candidate blood type specifications can be found on https://optn.transplant.hrsa.gov/media/eavh5bf3/optn_policies.pdf, section 9.8 F, Table 9-12, 9-13.
In our practice, consideration to use these grafts are based on each individual case. However, we tend to use these technically more demanding grafts—when used in adult recipients—in patients who are closer to the hospital, with an anticipated easier/faster hepatectomy, low BMI, no previous abdominal surgeries, and no known portal vein thrombosis. This is to keep the CIT as short as possible. However, in our cohort, 23% of the grafts had initial allocation to another center, which then declined the graft, with subsequent offering of the organ to us. Because of the complexity of the technique and possible size mismatch, we tend to wait to start the recipient until the organ is at our institution and we are able to fully assess if it is suitable to be transplanted. In addition, sometimes the grafts were initially allocated to a pediatric recipient and ended up being declined by other centers because of size, resulting in a later organ offer for our team. This would explain the longer CIT observed in our study. Moreover, our results showed that the pediatric-donor group had a significantly lower proportion of male recipients compared with the adult-donor group. This finding is consistent with data from previous literature.2-5,8 Women are less likely to receive a liver transplant than men and have been found to disproportionately decline organs because of size mismatch as a result of small stature.20 It is not unexpected then that recipients of pediatric grafts are more likely to be female. Mean recipient BMI was also noted to be lower in the pediatric-donor group, supporting the idea of pairing smaller recipients for this particular group of donors in addition to consideration of the aforementioned characteristics.
Similar to our results, previous studies in the literature have found comparable graft survival and overall complication rates between pediatric-to-adult and adult-to-adult liver transplantation.2-6 However, pediatric-donor grafts have been found to be associated with an increased risk of vascular complications, particularly HAT.2,3,8,21 Pediatric grafts are thought to be associated with a higher risk of vascular complications caused by various factors including small vessel and graft size.2,4,22 Several studies have found an increased risk of HAT in adult recipients receiving pediatric grafts compared with those receiving adult grafts,2,3,8 whereas others have found no increased risk of vascular complications, including HAT.4,5 Our analysis revealed a slightly higher rate of 30-d vascular complications in the pediatric-donor group compared with the adult-donor group, although the difference was not statistically significant. Similarly, we found no statistical difference in HAT rates among adults receiving a graft from a pediatric donor versus those receiving a graft from an adult donor. Although, there were more HAT cases in the pediatric-donor group (2 [16.7%] versus 1 [4.2%], respectively) only 2 were found on a relatively small study group, accounting for a higher rate. Therefore, it is possible that the relatively small sample size of our study may have limited the significance of this finding, and results should be interpreted with caution. To note, in both HAT cases in the study group, the complication was managed successfully by redoing the anastomosis, and no significant long term complications were seen. Also, none of the recipients in our cohort required re-transplantation. In addition, it is important to mention that no HAT cases were found when evaluating adult recipients receiving a graft from an older pediatric donor.
The rate of biliary complications was similar between the groups in our data, which concurs with findings from similar single-center studies from the literature.5,6 An increased incidence of biliary complications has been related to HAT after liver transplantation, which can lead to ischemic cholangiopathy.23,24 In our cohort, out of the 2 patients in the pediatric-donor group with HAT, none have developed ischemic cholangiopathy to date. One of the recipients developed an anastomotic bile duct stricture at 4 mo posttransplant that was successfully managed with a stent placed during ERCP. This patient has now recovered successfully, without further biliary or vascular complications up to date. Although early HAT has been related to greater morbidity and mortality compared with late HAT,24 in our cohort, all HAT cases have a functioning graft at the moment.
Among other risk factors, higher donor age has been associated with the development of ACR.9 Late onset ACR is usually defined as cellular rejection occurring at 3–6 mo after transplantation and has been associated with graft loss, decreased patient survival, chronic rejection, and overall worse prognosis.9,25 In our cohort, no recipients in the pediatric-donor group developed ACR within the first year, compared with 25% of recipients developing ACR in the adult-donor group. Immunosuppressive therapy following institutional protocol was the same for both groups and involved basiliximab and steroids for induction followed by maintenance with calcineurin inhibitors and mycophenolate. Therefore, a potential benefit of using these types of grafts in adult recipients exists and should be taken into account when considering the use of a young pediatric-donor grafts versus an adult-donor graft. However, our results are limited because of our sample size and further analysis with bigger samples are needed.
Surgical technique is an important consideration for pediatric-to-adult liver transplantation. In our study, choice of technique was based on surgeon preference and anatomic characteristics. The majority of patients receiving a pediatric graft underwent caval reconstruction with the piggyback technique and side-to-side cavocaval anastomosis. This modified piggyback technique has been described in the literature and compared with the standard piggyback technique and end-to-side cavocavostomy.26 Studies have shown lower rates of intraoperative blood products, vascular complications, and reduced warm ischemia time with this technique in adult liver transplantation.27-28 A more recent study by Lee et al comparing side-to-side cavocavostomy with traditional piggyback has similarly found decreased intraoperative transfusion requirements and rates of temporary abdominal closure.29 However, there are no studies comparing these techniques in pediatric-to-adult liver transplantation. Overall, this technique offers many potential benefits when big size discrepancy is encountered between the grafts vena cava and the recipients vena cava or hepatic veins. However, chosen technique should be based on surgeons’ preference and level of comfort with each technique while taking into account grafts and recipients anatomical characteristics.
Finally, organ discard rate has been on the rise.18 Previous reports indicate that on average, a pediatric liver is declined once by a program before being used by another program for a pediatric recipient and is discarded after being declined by 9 consecutive programs.3 With evidence suggesting comparable outcomes in adult transplantation after using grafts from pediatric donors, consideration to use these grafts in well-selected recipients, such a smaller and leaner/lower BMI recipient, should be made if no pediatric recipient is suitable. There is potential to reduce waitlist time for these adult recipients, achieving excellent short and long-term outcomes, as seen in this report.
There are a number of limitations associated with our study. First, this was a single-center study with a restricted number of cases. Therefore, the small sample size limits the power of the study. However, we attempted to reduce this limitation by performing a matched case-control based on covariates considered important on graft and patient outcomes. In addition, the small sample size increases the likelihood of type II error skewing the results and decreasing the power of the study, another reason why these results should be interpreted with caution. Second, the retrospective nature of the study limited analysis of important variables that were not recorded (ie, graft weights, recipient dry weight) as well as the possibility to control for additional confounders among the study groups. However, detailed data collection and the scarcity of reports on the literature of pediatric deceased donors outcomes on adult liver transplantation are among the strengths of the present article. It is important to remark, that our main analysis included only pediatric donors ≤12 y, allowing for comparison of true size donors differences.
In conclusion, the use of pediatric-donor liver grafts in adult recipients is a viable option comparable to adult-donor grafts. Consideration to use these grafts could potentially reduce waitlist time for adult recipients, particularly small females, and reduce pediatric organ discard rates. Therefore, pediatric-to-adult liver transplantation represents a safe and efficacious option, in the event that a pediatric graft has been offered to and declined by all available pediatric recipients.
Supplementary Material
Footnotes
The authors declare no funding or conflicts of interest.
P.A.V. participated in data collection, writing of the article, analysis, interpretation of data, and drafting of the article. H.W. participated in data collection, writing of the article, and interpretation of data. C.D., C.A., Z.H., S.F., M.J.S., P.N., J.O., and S.P. participated in critical revision of the article for important, intellectual content. N.G. participated in conception and design of the article, writing of the article, analysis and interpretation of data, and drafting of the article. All authors approved the final version of the article.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
REFERENCES
- 1.Kirchner VA, Goldaracena N, Sapisochin G, et al. Current status of liver transplantation in North America. Int J Surg. 2020;82S:9–13. [DOI] [PubMed] [Google Scholar]
- 2.Emre S, Soejima Y, Altaca G, et al. Safety and risk of using pediatric donor livers in adult liver transplantation. Liver Transpl. 2001;7:41–47. [DOI] [PubMed] [Google Scholar]
- 3.Croome KP, Lee DD, Burns JM, et al. ; Mayo Clinic Collaborative in Transplant Research and Outcomes. Outcomes of liver transplantation with liver grafts from pediatric donors used in adult recipients. Liver Transpl. 2016;22:1099–1106. [DOI] [PubMed] [Google Scholar]
- 4.Lan C, Song JL, Yan LN, et al. Pediatric donor to adult recipients in donation after cardiac death liver transplantation: a single-center experience. Transplant Proc. 2017;49:1383–1387. [DOI] [PubMed] [Google Scholar]
- 5.Ju W, Li C, Zhang C, et al. Outcome of the use of paediatric donor livers in adult recipients: a single Chinese centre experience. Clin Res Hepatol Gastroenterol. 2019;43:148–154. [DOI] [PubMed] [Google Scholar]
- 6.Ghabril M, Dickson RC, Krishna M, et al. Liver transplantation using young pediatric donor grafts in adults with hepatitis C infection. Transplantation. 2009;87:1174–1179. [DOI] [PubMed] [Google Scholar]
- 7.Ge J, Hsu EK, Bucuvalas J, et al. Deceased pediatric donor livers: how current policy drives allocation and transplantation. Hepatology. 2019;69:1231–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fortunato AC, Pinheiro RS, Nacif LS, et al. Hepatic artery thrombosis in liver transplantation in adult recipients using pediatric deceased donors. Transplant Proc. 2020;52:1332–1335. [DOI] [PubMed] [Google Scholar]
- 9.Choudhary NS, Saigal S, Bansal RK, et al. Acute and chronic rejection after liver transplantation: what a clinician needs to know. J Clin Exp Hepatol. 2017;7:358–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Makowka L, Stieber AC, Sher L, et al. Surgical technique of orthotopic liver transplantation. Gastroenterol Clin North Am. 1988;17:33–51. [PMC free article] [PubMed] [Google Scholar]
- 11.Lin TS, Vishnu Prasad NR, Chen CL, et al. What happened in 133 consecutive hepatic artery reconstruction in liver transplantation in 1 year? Hepatobiliary Surg Nutr. 2019;8:10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karakoyun R, Romano A, Yao M, et al. Impact of hepatic artery variations and reconstructions on the outcome of orthotopic liver transplantation. World J Surg. 2020;44:1954–1965. [DOI] [PubMed] [Google Scholar]
- 13.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Job KM, Gamalo M, Ward RM. Pediatric age groups and approach to studies. Ther Innov Regul Sci. 2019;53:584–589. [DOI] [PubMed] [Google Scholar]
- 15.Contopoulos-Ioannidis DG, Seto I, Hamm MP, et al. Empirical evaluation of age groups and age-subgroup analyses in pediatric randomized trials and pediatric meta-analyses. Pediatrics. 2012;129:S161–S184. [DOI] [PubMed] [Google Scholar]
- 16.Calle-Toro JS, Back SJ, Viteri B, et al. Liver, spleen, and kidney size in children as measured by ultrasound: a systematic review. J Ultrasound Med. 2020;39:223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patzak M, Porzner M, Oeztuerk S, et al. ; EMIL Study Group. Assessment of liver size by ultrasonography. J Clin Ultrasound. 2014;42:399–404. [DOI] [PubMed] [Google Scholar]
- 18.Kwong AJ, Kim WR, Lake JR, et al. OPTN/SRTR 2019 Annual Data Report: Liver. Am J Transplant. 2021;2:208–315. [DOI] [PubMed] [Google Scholar]
- 19.UNOS OPTN. Available at https://unos.org/policy/liver-distribution/. Accessed December 13, 2021.
- 20.Nephew LD, Goldberg DS, Lewis JD, et al. Exception points and body size contribute to gender disparity in liver transplantation. Clin Gastroenterol Hepatol. 2017;15:1286–1293.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horvat N, Marcelino ASZ, Horvat JV, et al. Pediatric liver transplant: techniques and complications. Radiographics. 2017;37:1612–1631. [DOI] [PubMed] [Google Scholar]
- 22.Orlandini M, Feier FH, Jaeger B, et al. Frequency of and factors associated with vascular complications after pediatric liver transplantation. J Pediatr (Rio J). 2014;90:169–175. [DOI] [PubMed] [Google Scholar]
- 23.Kaldas FM, Korayem IM, Russell TA, et al. Assessment of anastomotic biliary complications in adult patients undergoing high-acuity liver transplant. JAMA Surg. 2019;154:431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujiki M, Hashimoto K, Palaios E, et al. Probability, management, and long-term outcomes of biliary complications after hepatic artery thrombosis in liver transplant recipients. Surgery. 2017;162:1101–1111. [DOI] [PubMed] [Google Scholar]
- 25.Nacif LS, Pinheiro RS, de Arruda Pécora RA, et al. Re-transplantation, higher creatinine levels in hepatitis C virus patients, and donor age are predictors of mortality in long-term analysis of late acute rejection in liver transplantation. Ann Transplant. 2017;22:9–16. [DOI] [PubMed] [Google Scholar]
- 26.Chan T, DeGirolamo K, Chartier-Plante S, et al. Comparison of three caval reconstruction techniques in orthotopic liver transplantation: a retrospective review. Am J Surg. 2017;213:943–949. [DOI] [PubMed] [Google Scholar]
- 27.Lerut JP, Molle G, Donataccio M, et al. Cavocaval liver transplantation without venovenous bypass and without temporary portocaval shunting: the ideal technique for adult liver grafting? Transpl Int. 1997;10:171–179. [DOI] [PubMed] [Google Scholar]
- 28.Navarro F, Le Moine MC, Fabre JM, et al. Specific vascular complications of orthotopic liver transplantation with preservation of the retrohepatic vena cava: review of 1361 cases. Transplantation. 1999;68:646–650. [DOI] [PubMed] [Google Scholar]
- 29.Lee TC, Dhar VK, Cortez AR, et al. ; Cincinnati Research on Outcomes and Safety in Surgery (CROSS). Impact of side-to-side cavocavostomy versus traditional piggyback implantation in liver transplantation. Surgery. 2020;168:1060–1065. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.