Author's summary
There is a fundamental trade-off that exists between ischemic and bleeding risk that must be considered in deciding the optimal strategy of dual antiplatelet therapy. Prasugrel-based de-escalation decreased the risk of net adverse clinical event (NACE) due to a reduction in bleeding in the HOST-REDUCE-POLYTECH-ACS trial. In non-ST-segment elevation acute coronary syndromes patients, prasugrel-based dose de-escalation from one-month post-percutaneous coronary intervention reduced the risk of NACE. In ST-elevation myocardial infarction (STEMI), de-escalation showed no benefit for NACE and a statistically insignificant but numerically higher rate of ischemic events. Our data raises caution about prasugrel dose reduction in higher thrombotic conditions.
Keywords: Acute coronary syndrome, Percutaneous coronary intervention, Prasugrel, ST elevation myocardial infarction, Non-ST elevated myocardial infarction
Abstract
Background and Objectives
De-escalation of dual-antiplatelet therapy through dose reduction of prasugrel improved net adverse clinical events (NACEs) after acute coronary syndrome (ACS), mainly through the reduction of bleeding without an increase in ischemic outcomes. Whether the benefits of de-escalation are sustained in highly thrombotic conditions such as ST-elevation myocardial infarction (STEMI) is unknown. We aimed to assess the efficacy and safety of de-escalation therapy in patients with STEMI or non-ST-segment elevation ACS (NSTE-ACS).
Methods
This is a pre-specified subgroup analysis of the HOST-REDUCE-POLYTECH-ACS trial. ACS patients were randomized to prasugrel de-escalation (5 mg daily) or conventional dose (10 mg daily) at 1-month post-percutaneous coronary intervention. The primary endpoint was a NACE, defined as a composite of all-cause death, non-fatal myocardial infarction, stent thrombosis, clinically driven revascularization, stroke, and bleeding events of grade ≥2 Bleeding Academic Research Consortium (BARC) criteria at 1 year.
Results
Among 2,338 patients included in the randomization, 326 patients were diagnosed with STEMI. In patients with NSTE-ACS, the risk of the primary endpoint was significantly reduced with de-escalation (hazard ratio [HR], 0.65; 95% confidence interval [CI], 0.48–0.89; p=0.006 for de-escalation vs. conventional), mainly driven by a reduced bleeding. However, in those with STEMI, there was no difference in the occurrence of the primary outcome (HR, 1.04; 95% CI, 0.48–2.26; p=0.915; p for interaction=0.271).
Conclusions
Prasugrel dose de-escalation reduced the rate of NACE and bleeding, without increasing the rate of ischemic events in NSTE-ACS patients but not in STEMI patients.
INTRODUCTION
The current guideline recommends potent P2Y12 inhibitor-based dual antiplatelet therapy (DAPT) as first line therapy in patients with acute coronary syndrome (ACS) after percutaneous coronary intervention (PCI).1),2),3) However, the beneficial anti-atherothrombotic effects of potent P2Y12 inhibitors are inevitably accompanied by an increased risk of bleeding.2),3),4) Thus, a fundamental trade-off exists between ischemic and bleeding risk that should be considered in deciding the potency and duration of DAPT.5),6),7),8)
Prasugrel-based de-escalation therapy significantly decreased the risk of net adverse clinical events (NACEs), mostly due to a significant reduction in bleeding in the HOST-REDUCE-POLYTECH-ACS trial.9) ST-elevation myocardial infarction (STEMI) represents a subgroup of patients with the highest milieu for thrombosis and thus de-escalation of potent P2Y12 therapy may increase the risk of thrombotic events.10) It remains to be seen whether the benefits seen in the HOST-REDUCE-POLYTECH-ACS trial are maintained in the STEMI subgroup and whether there is a differential effect of prasugrel de-escalation between non-ST-segment elevation ACS (NSTE-ACS) and STEMI. This analysis was a prespecified subgroup analysis of the HOST-REDUCE-POLYTECH-ACS trial and aimed to examine the efficacy and safety of de-escalation therapy compared with conventional therapy in patients with STEMI or NSTE-ACS.
METHODS
Ethical statement
An independent data and safety monitoring board reviewed the safety of the trial and had full access to the trial data. This study complied with the provisions of the Declaration of Helsinki 2013. The study protocol was approved by the ethics committees of Seoul National University Hospital Institutional Review Board (IRB) (1404-142-576), Presbyterian Medical Center IRB (2014-12-052), Pusan National University Yangsan Hospital IRB (03-2015-003), Hanyang University Seoul Hospital IRB (2014-10-027-001), Hanlim General Hospital IRB (2016-2), Chungbuk National University IRB (2014-10-007), Kangwon National University IRB (2015-08-009-001, 2016-06-008-001), Seoul Medical Center IRB (2014-073), Chosun Medical Center IRB (2016-02-005-002), Korea University Guro Hospital IRB (2015GR0751), Soonchunhyang University Cheonan Hospital IRB (2015-01-005), Ajou University Medical Center IRB (4-15-403), Dong-A University Hospital IRB (14-199, 16-195), Keimyung University Dongsan Medical Center IRB (2014-10-035-002), Korea University Anam Hospital IRB (MD16015), Seoul Boramae Hospital IRB (26-2014-133), Hallym University Sacred Heart Hospital IRB (2015-I022), Kyung Hee University Medical Center IRB (2017-07-049-003), Ilsan Paik Hospital IRB (3-1411-038), Wonkwang University Hospital IRB (201410-CTDV-033), Yeungnam University Hospital IRB (2014-01-506-003), Bucheon St. Mary’s Hospital IRB (PC14DIMV0078), Seoul National University Bundang Hospital IRB (E-1410/271-401), St. Vincent’s Hospital IRB (VC15DIMI0046), Gyeongsang National University Hospital IRB (2018-02-019-013), Ulsan University Hospital IRB (2014-10-011-002), National Health Insurance Service Ilsan Hospital IRB (2015-01-003-001), Kangdong Sacred Heart Hospital IRB (2014-01-060), Chungnam National University Hospital IRB (2017-06-045), Daegu Catholic University Medical Center IRB (15-004-L), Chonnam University Hospital IRB (2015-038), Pusan National University Hospital IRB (0-1412-007-024), and Kangnam Sacred Heart Hospital IRB (2014-10-142). All patients provided written informed consent.
Study design and population
This HOST-REDUCE-POLYTECH-ACS trial was an investigator-initiated, randomized, parallel-group, open-label, adjudicator-blinded, multicenter trial performed at 35 hospitals in South Korea. The detailed study protocols, subjects, and outcomes have been previously published.9),11),12) This study had a 2×2 factorial design testing 2 independent hypotheses and had 2 arms, a DAPT arm and a drug-eluting stent (DES) arm. The antiplatelet arm compared the prasugrel-based dose de-escalation therapy group (5 mg) with the conventional dose therapy group (10 mg), and the DES arm compared a durable polymer DES with an absorbable polymer DES. The main results have been previously published.9),12) The current study is a subgroup analysis of the HOST-REDUCE-POLYTECH-ACS trial. In the prasugrel randomization arm of the main trial, the prasugrel-based dose de-escalation therapy was compared with conventional dose therapy group in patients with STEMI or NSTE-ACS. Patients with ACS, aged at least 19 years with at least one culprit lesion in a native coronary artery eligible for stent implantation, were screened for participation in this trial. The major exclusion criteria were: patients with contraindication or hypersensitivity to heparin, aspirin, clopidogrel, prasugrel, ticagrelor, biolimus, everolimus, or contrast media; patients with major or active pathological bleeding; women of childbearing potential; a history of bleeding diathesis; the presence of non-cardiac comorbid conditions with life expectancy less than one year or conditions that might result in non-compliance with the protocol. All patients who were able to make an informed decision provided written consent for participation in the study before randomization. Patients who met the exclusion criteria for prasugrel (age ≥75 years, body weight <60 kg, or history of transient ischemic attack or stroke) were excluded from the antiplatelet randomization process.
The protocol recommended 300 mg aspirin and 60 mg prasugrel before undergoing PCI. Patients included in both randomized groups were administered aspirin 100 mg and prasugrel 10 mg for the first month. Then, patients in the de-escalation group received a reduced dose of 5 mg of prasugrel, while patients in the conventional dose group received the conventional dose of 10 mg daily. All patients were prescribed a daily dose of 100 mg aspirin indefinitely. DAPT was recommended for at least one year.
Definitions and outcomes
The definitions of clinical outcomes have been previously described.9) The primary endpoint was NACE, defined as a composite of all-cause death, non-fatal myocardial infarction (MI), stent thrombosis, clinically driven revascularization, stroke, and bleeding events of grade 2 or higher according to the Bleeding Academic Research Consortium (BARC) criteria at 1 year. The secondary endpoints were the efficacy outcomes (defined as cardiovascular death, MI, stent thrombosis, and ischemic stroke) and safety outcomes (bleeding events of BARC grade ≥2). Other secondary outcomes included individual elements of the primary endpoint, cardiac death, clinically driven target lesion revascularization, clinically driven target vessel revascularization, clinically driven non-target vessel revascularization, and bleeding events of BARC grade ≥3 at 1 year. Clinically driven revascularization was defined as repeat revascularization with a diameter stenosis ≥70%, or diameter stenosis ≥50% and if one of the following occurred: a history of recurrent angina pectoris, positive non-invasive test, or abnormal results of any invasive functional physiological test. All clinical outcomes followed the criteria provided by the Academic Research Consortium.13)
Statistical analysis
All numerical data are expressed as mean ± standard deviation for continuous variables and as percentages for categorical variables. For comparison among groups, the χ2 test or Fisher’s exact test was used for categorical variables and the unpaired Student’s t-test was used for continuous variables. If combined endpoints occurred in a patient, the first event was counted. The occurrence rate of time-dependent events was estimated using the Kaplan-Meier (K-M) method, and the clinical outcomes were compared using the log-rank test. Hazard ratios (HRs) and 95% confidence intervals (CIs) were generated using Cox proportional hazard models. Endpoints were analyzed on an intention-to-treat basis, then on a per-protocol basis. As the same treatment (10 mg prasugrel) was administered to both groups during the 4 weeks, prespecified 4 weeks landmark analysis was performed after excluding patients who experienced clinical events within 4 weeks after index PCI. A multivariable Cox regression model was used to identify independent predictors of the primary outcome. Analyses were performed using the following statistical packages: SPSS version 23.0 (IBM SPSS Statistics, Chicago, IL, USA) and R programming language version 3.5.0 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Baseline clinical, angiographic, and procedural characteristics
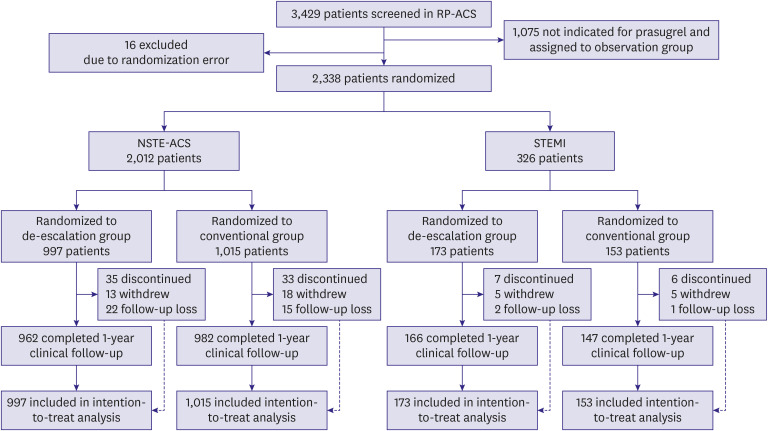
The flow of the study is shown in Figure 1. From September 2014 to December 2018, patients with ACS from 35 hospitals in South Korea were screened. Of the 3,429 patients screened for eligibility, 1,075 patients did not meet the core indication for full dose of prasugrel and were assigned to the observation group. Among the 2,338 patients included in the prasugrel randomization, 2,012 patients had the inclusion diagnosis of NSTE-ACS, and 326 patients had the inclusion diagnosis of STEMI. Among the 2,012 patients with NSTE-ACS, 997 patients were randomized to the de-escalation group, and 1,015 to the conventional group. Among the 326 patients with STEMI, 173 were randomized to the de-escalation group and 153 to the conventional group. Follow-up at 1 year was completed for 1,944 (96.6%) patients with NSTE-ACS and 313 (96.0%) patients with STEMI.
Figure 1. Study flow chart.
NSTE-ACS = non-ST-segment elevation acute coronary syndrome; RP-ACS = REDUCE-POLYTECH-ACS; STEMI = ST-elevation myocardial infarction.
The baseline characteristics of patients with NSTE-ACS and STEMI are summarized in Tables 1 and 2, Supplementary Tables 1 and 2. The baseline characteristics of those with NSTE-ACS are provided in Table 1. The NSTE-ACS group was 70.6% (n=1,421) unstable angina and 29.4% (n=591) non-ST-elevation myocardial infarction (NSTEMI). The mean age was 59.3 years, 88.9% of the subjects were males, and 41.5% had diabetes. Within the patients with NSTE-ACS, the 2 randomized groups were well balanced with respect to baseline characteristics, except for the prevalence of previous PCI and history of MI, which were higher in the conventional group.
Table 1. Baseline characteristics in NSTE-ACS patients.
| Total (n=2,012) | De-escalation (n=997) | Conventional (n=1,015) | p value | ||
|---|---|---|---|---|---|
| Age | 59.3±8.9 | 59.1±8.9 | 59.5±8.8 | 0.313 | |
| Age ≥75 | 2 (0.1) | 2 (0.2) | 0 (0) | 0.245 | |
| Age ≥65 | 644 (32.0) | 312 (31.3) | 332 (32.7) | 0.496 | |
| Male | 1,788 (88.9) | 889 (89.2) | 899 (88.6) | 0.671 | |
| Body mass index | 25.8±2.8 | 25.7±2.8 | 25.9±2.9 | 0.313 | |
| Left ventricular ejection fraction | 60.5±9.1 | 60.7±8.8 | 60.3±9.5 | 0.392 | |
| Hypertension | 1,294 (64.3) | 641 (64.4) | 653 (64.3) | 0.992 | |
| Diabetes mellitus | 835 (41.5) | 425 (42.6) | 410 (40.4) | 0.309 | |
| Dyslipidemia | 1,553 (77.2) | 772 (77.4) | 781 (76.9) | 0.795 | |
| Chronic kidney disease | 55 (2.7) | 25 (2.5) | 30 (3.0) | 0.538 | |
| Peripheral artery disease | 24 (1.2) | 16 (1.6) | 8 (0.8) | 0.092 | |
| Smoking status | 0.340 | ||||
| Never smoker | 896 (44.5) | 434 (43.5) | 462 (45.5) | ||
| Current smoker | 653 (32.5) | 339 (34.0) | 314 (30.9) | ||
| Ex-smoker | 463 (23.0) | 224 (22.5) | 239 (23.5) | ||
| Previous myocardial infarction | 76 (3.8) | 29 (2.9) | 47 (4.6) | 0.043 | |
| Previous PCI | 233 (11.6) | 99 (9.9) | 134 (13.2) | 0.022 | |
| Previous CABG | 19 (0.9) | 10 (1.0) | 9 (0.9) | 0.787 | |
| Previous cerebrovascular accident | 28 (1.4) | 14 (1.4) | 14 (1.4) | 0.962 | |
| Family history of CAD | 148 (7.4) | 67 (6.7) | 81 (8.0) | 0.279 | |
| Non ST-elevation myocardial infarction | 591 (29.4) | 308 (30.9) | 283 (27.9) | 0.138 | |
| Medication at discharge | |||||
| Aspirin | 1,984 (99.3) | 980 (99.0) | 1,004 (99.5) | 0.267 | |
| Clopidogrel | 160 (8.0) | 79 (8.0) | 81 (8.0) | 0.974 | |
| Prasugrel | 1,847 (92.4) | 916 (92.6) | 931 (92.3) | 0.768 | |
| BB | 1,033 (51.8) | 520 (52.6) | 513 (51.0) | 0.464 | |
| ACEI | 1,107 (55.6) | 567 (57.4) | 540 (53.7) | 0.095 | |
| Statin | 1,888 (94.7) | 941 (95.2) | 947 (94.1) | 0.270 | |
| CCB | 479 (24.0) | 258 (26.1) | 221 (22.0) | 0.031 | |
| Lab | |||||
| Hb | 14.4±1.6 | 14.4±1.6 | 14.4±1.6 | 0.927 | |
| Plt | 230.5±57.7 | 230.9±57.4 | 230.1±57.9 | 0.768 | |
| BUN | 16.5±8.7 | 16.7±10.0 | 16.4±7.2 | 0.361 | |
| Creatinine | 1.0±1.0 | 1.1±1.0 | 1.0±0.9 | 0.501 | |
| Total cholesterol | 172.7±44.6 | 173.4±45.3 | 172.2±43.8 | 0.564 | |
| LDL | 103.9±38.5 | 104.4±38.8 | 103.5±38.2 | 0.661 | |
| HDL | 43.3±10.8 | 43.2±10.9 | 43.4±10.7 | 0.594 | |
| TG | 155.6±109.7 | 156.7±4.0 | 154.5±101.5 | 0.671 | |
| Number of diseased vessels | 0.127 | ||||
| One vessel | 988/2,000 (49.4) | 500/992 (50.4) | 488/1,008 (48.4) | ||
| Two vessel | 598/2,000 (29.9) | 305/992 (30.7) | 293/1,008 (29.1) | ||
| Three vessel | 414/2,000 (20.7) | 187/992 (18.9) | 227/1,008 (22.5) | ||
| Multivessel disease | 1012/2,000 (50.6) | 492/992 (49.6) | 520/1,008 (51.6) | 0.373 | |
| Anticoagulant agent for PCI | |||||
| Unfractionated heparin | 385/2,012 (19.1) | 182/997 (18.3) | 203/1,015 (20.0) | 0.320 | |
| Enoxaparin | 151/2,012 (7.5) | 83/997 (8.3) | 68/1,015 (6.7) | 0.166 | |
| Glycoprotein IIb/IIIa inhibitor | |||||
| Abciximab | 9/2,012 (0.4) | 4/997 (0.4) | 5/1,015 (0.5) | 1.000 | |
| Tirofiban | 0/2,012 (0) | 0/997 (0) | 0/1,015 (0) | - | |
| Lesion complexity | |||||
| Multi-lesion intervention | 595/1,987 (29.9) | 289/986 (29.3) | 306/1,001 (30.6) | 0.540 | |
| Heavy calcification | 267/1,977 (13.5) | 123/977 (12.6) | 144/1,000 (14.4) | 0.239 | |
| Bifurcation lesion | 420/1,973 (21.3) | 211/976 (21.6) | 209/997 (21.0) | 0.722 | |
| Thrombotic lesion | 159/1,977 (8.0) | 84/977 (8.6) | 75/1,000 (7.5) | 0.370 | |
| ACC/AHA type B2/C lesion | 1,071/1,974 (54.3) | 539/975 (55.3) | 532/999 (53.3) | 0.366 | |
| In-stent restenosis lesion | 54/1,976 (2.7) | 27/977 (2.8) | 27/999 (2.7) | 0.934 | |
| IVUS use | 685/1,981 (34.6) | 337/981 (34.4) | 348/1,000 (34.8) | 0.834 | |
| Stent type | 0.723 | ||||
| Durable polymer-DES | 1,013/2,012 (50.3) | 498/997 (49.9) | 515/1,015 (50.7) | ||
| Absorbable polymer-DES | 999/2,012 (49.7) | 499/997 (50.1) | 500/1,015 (49.3) | ||
| Treated lesion number per person | 1.4±0.7 | 1.4±0.7 | 1.4±0.7 | 0.652 | |
| Stent number per person | 1.6±1.1 | 1.7±1.1 | 1.6±1.0 | 0.326 | |
| Total stent length (mm) | 42±31.4 | 42.2±32.8 | 41.8±30.0 | 0.775 | |
| Procedure success | 1,970/1,984 (99.3) | 975/984 (99.1) | 995/1,000 (99.5) | 0.270 | |
Values are presented as mean ± standard deviation or number (%).
ACC/AHA = American College of Cardiology/American Heart Association; ACEI = angiotensin-converting-enzyme inhibitor; BB = beta blocker; BUN = blood urea nitrogen; CABG = coronary artery bypass grafting; CAD = coronary artery disease; CCB = Calcium channel blocker; DES = drug-eluting stent; HDL = high density lipoprotein; Hb = hemoglobin; IVUS = intravascular ultrasound; LDL = low density lipoprotein; NSTE-ACS = non-ST-segment elevation acute coronary syndrome; PCI = percutaneous coronary intervention; Plt = platelet; TG = triglyceride.
Table 2. Baseline characteristics in STEMI patients.
| Total (n=326) | De-escalation (n=173) | Conventional (n=153) | p value | ||
|---|---|---|---|---|---|
| Age | 55.7±9.4 | 56.3±9.1 | 54.9±9.7 | 0.177 | |
| Age ≥75 | 0 (0) | 0 (0) | 0 (0) | - | |
| Age ≥65 | 59 (18.1) | 34 (19.7) | 25 (16.3) | 0.438 | |
| Male | 299 (91.7) | 161 (93.1) | 138 (90.2) | 0.439 | |
| Body mass index | 25.4±2.8 | 25.4±2.8 | 25.3±2.9 | 0.855 | |
| Left ventricular ejection fraction | 51.8±10.8 | 51.4±10.9 | 52.3±10.6 | 0.527 | |
| Hypertension | 182 (55.8) | 92 (53.2) | 90 (58.8) | 0.306 | |
| Diabetes mellitus | 155 (47.5) | 87 (50.3) | 68 (44.4) | 0.292 | |
| Dyslipidemia | 246 (75.5) | 118 (68.2) | 128 (83.7) | 0.001 | |
| Chronic kidney disease | 9 (2.8) | 5 (2.9) | 4 (2.6) | 1.000 | |
| Peripheral artery disease | 5 (1.5) | 4 (2.3) | 1 (0.7) | 0.376 | |
| Smoking status | 0.091 | ||||
| Never smoker | 86 (26.4) | 37 (21.4) | 49 (32.0) | ||
| Current smoker | 185 (56.7) | 104 (60.1) | 81 (52.9) | ||
| Ex-smoker | 55 (16.9) | 32 (18.5) | 23 (15.0) | ||
| Previous myocardial infarction | 14 (4.3) | 6 (3.5) | 8 (5.2) | 0.434 | |
| Previous PCI | 17 (5.2) | 8 (4.6) | 9 (5.9) | 0.610 | |
| Previous CABG | 2 (0.6) | 1 (0.6) | 1 (0.7) | 1.000 | |
| Previous cerebrovascular accident | 3 (0.9) | 0 (0) | 3 (2.0) | 0.102 | |
| Family history of CAD | 20 (6.1) | 11 (6.4) | 9 (5.9) | 0.858 | |
| Medication at discharge | |||||
| Aspirin | 313 (97.2) | 167 (97.7) | 146 (96.7) | 0.739 | |
| Clopidogrel | 16 (5.0) | 1 (0.6) | 15 (9.9) | <0.001 | |
| Prasugrel | 294 (91.3) | 163 (95.3) | 131 (86.8) | 0.006 | |
| BB | 241 (76.0) | 133 (79.2) | 108 (72.5) | 0.164 | |
| ACEI | 207 (65.3) | 107 (63.7) | 100 (67.1) | 0.523 | |
| Statin | 304 (95.6) | 162 (95.9) | 142 (95.3) | 0.809 | |
| CCB | 24 (7.5) | 5 (3.0) | 19 (12.8) | 0.001 | |
| Lab | |||||
| Hb | 15.0±1.7 | 15.0±1.6 | 14.9±1.7 | 0.601 | |
| Plt | 244.6±65.2 | 242.1±70.6 | 247.4±58.6 | 0.466 | |
| BUN | 15.8±5.1 | 16.0±5.2 | 15.7±5.0 | 0.545 | |
| Creatinine | 1.0±0.3 | 1.0±0.3 | 0.9±0.2 | 0.171 | |
| Total cholesterol | 190.3±47.8 | 188.1±50.8 | 192.7±44.4 | 0.403 | |
| LDL | 121.2±39.6 | 119.2±39.8 | 123.3±39.4 | 0.407 | |
| HDL | 44.1±16.9 | 44.3±19.6 | 43.8±13.5 | 0.824 | |
| TG | 170.0±130.6 | 167.5±130.8 | 172.7±130.9 | 0.739 | |
| Number of diseased vessels | 0.312 | ||||
| One vessel | 170/325 (52.3) | 85/172 (49.4) | 85/153 (55.6) | ||
| Two vessel | 93/325 (28.6) | 49/172 (28.5) | 44/153 (28.8) | ||
| Three vessel | 62/325 (19.1) | 38/172 (22.1) | 24/153 (15.7) | ||
| Multivessel disease | 155/325 (47.7) | 87/172 (50.6) | 68/153 (44.4) | 0.269 | |
| Anticoagulant agent for PCI | |||||
| Unfractionated heparin | 92/326 (28.2) | 48/173 (27.7) | 44/153 (28.8) | 0.839 | |
| Enoxaparin | 28/326 (8.6) | 10/173 (5.8) | 18/153 (11.8) | 0.054 | |
| Glycoprotein IIb/IIIa inhibitor | |||||
| Abciximab | 16/326 (4.9) | 10/173 (5.8) | 6/153 (3.9) | 0.438 | |
| Tirofiban | 1/326 (0.3) | 0/173 (0) | 1/153 (0.7) | 0.469 | |
| Lesion complexity | |||||
| Multi-lesion intervention | 69/324 (21.3) | 38/172 (22.1) | 31/152 (20.4) | 0.709 | |
| Heavy calcification | 27/321 (8.4) | 18/172 (10.5) | 9/149 (6.0) | 0.154 | |
| Bifurcation lesion | 42/321 (13.1) | 25/172 (14.5) | 17/149 (11.4) | 0.408 | |
| Thrombotic lesion | 147/321 (45.8) | 82/172 (47.7) | 65/149 (43.6) | 0.468 | |
| ACC/AHA type B2/C lesion | 223/321 (69.5) | 121/172 (70.3) | 102/149 (68.5) | 0.713 | |
| In-stent restenosis lesion | 5/322 (1.6) | 2/172 (1.2) | 3/150 (2.0) | 0.667 | |
| IVUS use | 90/322 (28.0) | 45/172 (26.2) | 45/150 (30.0) | 0.444 | |
| Stent type | 0.378 | ||||
| Durable polymer-DES | 164/326 (50.3) | 91/173 (52.6) | 73/153 (47.7) | ||
| Absorbable polymer-DES | 162/326 (49.7) | 82/173 (47.4) | 80/153 (52.3) | ||
| Treated lesion number per person | 1.3±0.6 | 1.3±0.6 | 1.2±0.5 | 0.338 | |
| Stent number per person | 1.5±0.9 | 1.6±1 | 1.4±0.7 | 0.114 | |
| Total stent length (mm) | 37.3±24.5 | 39.5±26.2 | 34.9±22.3 | 0.092 | |
| Procedure success | 322/324 (99.4) | 171/172 (99.4) | 151/152 (99.3) | 1.000 | |
Values are presented as mean ± standard deviation or number (%).
ACC/AHA = American College of Cardiology/American Heart Association; ACEI = angiotensin-converting-enzyme inhibitor; BB = beta blocker; BUN = blood urea nitrogen; CABG = coronary artery bypass grafting; CAD = coronary artery disease; CCB = calcium channel blocker; DES = drug-eluting stent; Hb = hemoglobin; HDL = high density lipoprotein; IVUS = intravascular ultrasound; LDL = low density lipoprotein; PCI = percutaneous coronary intervention; Plt = platelet; STEMI = ST-elevation myocardial infarction; TG = triglyceride.
Table 2 summarizes the baseline demographic and clinical characteristics of patients with STEMI and shows a balanced distribution between the 2 randomized groups, except for the prevalence of dyslipidemia. The mean age was 55.7 years, 91.7% of enrolled patients were male, and 47.5% had diabetes. Approximately half of the enrolled patients had multi-vessel disease. There were 42 (13.1%) bifurcation lesions, and 223 (69.5%) American College of Cardiology/American Heart Association type B2/C lesions. The stent type (durable polymer vs. absorbable polymer DES) was well distributed in both groups, and the mean number of implanted stents was 1.5.
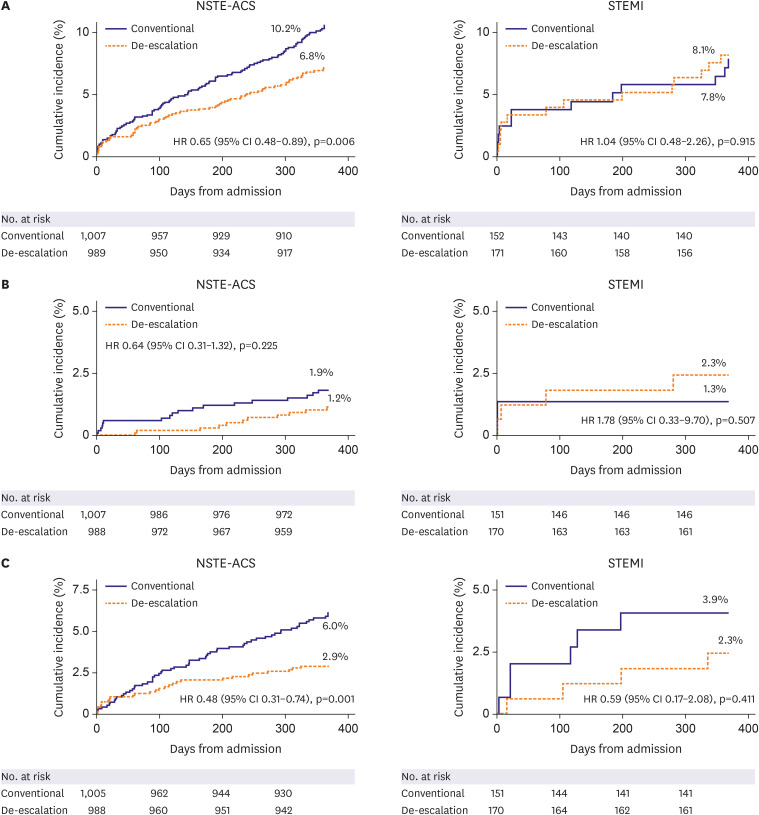
Clinical outcomes according to treatment strategy
In patients with NSTE-ACS, the occurrence of the primary endpoint was significantly lower in the de-escalation group (K-M estimates: 6.8% vs. 10.2%; HR, 0.65; 95% CI, 0.48–0.89; p=0.006 for de-escalation vs. conventional groups respectively, Table 3 and Figure 2). BARC grade 2 or higher-bleeding events occurred in 29 patients (2.9%) in the de-escalation group and 61 patients (6.0%) in patients in the conventional group (HR, 0.48; 95% CI, 0.31–0.74; p=0.001). Efficacy events occurred in 12 patients (1.2%) in the de-escalation group and 19 patients (1.9%) in the conventional group (HR, 0.64; 95% CI, 0.31–1.32; p=0.225). There were no significant differences in the incidence of other secondary endpoints between the 2 groups.
Table 3. Comparison of clinical outcomes in NSTE-ACS patients.
| Total (n=2,012) | De-escalation (n=997) | Conventional (n=1,015) | De-escalation vs. Conventional | p value | ||
|---|---|---|---|---|---|---|
| Net adverse clinical events* | 172 (8.5) | 68 (6.8) | 104 (10.2) | 0.65 (0.48–0.89) | 0.006 | |
| Efficacy events† | 31 (1.5) | 12 (1.2) | 19 (1.9) | 0.64 (0.31–1.32) | 0.225 | |
| Safety events | ||||||
| BARC ≥2 | 90 (4.5) | 29 (2.9) | 61 (6.0) | 0.48 (0.31–0.74) | 0.001 | |
| BARC ≥3 | 16 (0.8) | 8 (0.8) | 8 (0.8) | 1.01 (0.38–2.70) | 0.980 | |
| Target lesion failure‡ | 35 (1.7) | 17 (1.7) | 18 (1.8) | 0.96 (0.49–1.86) | 0.901 | |
| Death | 19 (0.9) | 7 (0.7) | 12 (1.2) | 0.59 (0.23–1.50) | 0.268 | |
| CV death | 10 (0.5) | 2 (0.2) | 8 (0.8) | 0.25 (0.05–1.19) | 0.082 | |
| Non-fatal myocardial infarction | 13 (0.6) | 5 (0.5) | 8 (0.8) | 0.63 (0.21–1.93) | 0.420 | |
| Stent thrombosis | 2 (0.1) | 0 (0) | 2 (0.2) | 0.02 (0–1,347.33) | 0.473 | |
| Repeat revascularization | 61 (3.0) | 29 (2.9) | 32 (3.2) | 0.92 (0.56–1.52) | 0.741 | |
| Revascularization related with target lesion | 24 (1.2) | 13 (1.3) | 11 (1.1) | 1.20 (0.54–2.68) | 0.657 | |
| Revascularization related with target vessel | 34 (1.7) | 18 (1.8) | 16 (1.6) | 1.14 (0.58–2.24) | 0.696 | |
| Non-target vessel PCI | 36 (1.8) | 16 (1.6) | 20 (2.0) | 0.81 (0.42–1.56) | 0.528 | |
| Stroke | 17 (0.8) | 9 (0.9) | 8 (0.8) | 1.14 (0.44–2.95) | 0.789 | |
| Ischemic stroke | 9 (0.4) | 5 (0.5) | 4 (0.4) | 1.27 (0.34–4.72) | 0.724 | |
| Hemorrhagic stroke | 8 (0.4) | 4 (0.4) | 4 (0.4) | 1.01 (0.25–4.05) | 0.987 | |
Values are presented as number (%) or hazard ratio (95% confidence interval).
BARC = Bleeding Academic Research Consortium; CV = cardiovascular; NSTE-ACS = non-ST-segment elevation acute coronary syndrome; PCI = percutaneous coronary intervention.
*Composite of all-cause death, non-fatal myocardial infarction, stent thrombosis, clinically driven revascularization, stroke, and BARC grade ≥2 bleeding. †Cardiac death, myocardial infarction, stent thrombosis, and ischemic stroke. ‡Includes cardiac death, target lesion revascularization, and target vessel myocardial infarction.
Figure 2. Primary endpoint in the intention-to-treat population at 1-year follow-up: (A) primary endpoint, (B) efficacy outcomes (cardiac death, myocardial infarction, stent thrombosis, and ischemic stroke), and (C) safety outcomes (BARC ≥2 bleeding events).
CI = confidence interval; HR = hazard ratio; NSTE-ACS = non-ST-segment elevation acute coronary syndrome; STEMI = ST-elevation myocardial infarction.
In contrast to the NSTE-ACS subgroup, there was no significant difference in the occurrence of the primary endpoint between the de-escalation group and conventional group in the STEMI patients (K-M estimates: 8.1% vs. 7.8%; HR, 1.04; 95% CI, 0.48–2.26; p=0.915 for de-escalation vs. conventional groups respectively; p for interaction=0.271, Table 4 and Figure 2). Numerically the rates of NACE were almost identical. Regarding the secondary endpoints, efficacy events occurred in 4 patients (2.3%) in the de-escalation group and 2 patients (1.3%) in the conventional group (HR, 1.78; 95% CI, 0.33–9.70; p=0.507). BARC grade 2 or higher-grade bleeding events occurred in 4 patients (2.3%) in the de-escalation group and in 6 patients (3.9%) in the conventional group (HR, 0.59; 95% CI, 0.17–2.08; p=0.411). There were no differences in the incidence of other secondary outcomes (Table 4). The per-protocol analysis showed similar results to the intention-to-treat analysis for the primary endpoint and the key secondary endpoints (Supplementary Figure 1).
Table 4. Comparison of clinical outcomes in STEMI patients.
| Total (n=326) | De-escalation (n=173) | Conventional (n=153) | De-escalation vs. Conventional | p value | ||
|---|---|---|---|---|---|---|
| Net adverse clinical events* | 26 (8.0) | 14 (8.1) | 12 (7.8) | 1.04 (0.48–2.26) | 0.915 | |
| Efficacy events† | 6 (1.8) | 4 (2.3) | 2 (1.3) | 1.78 (0.33–9.70) | 0.507 | |
| Safety events | ||||||
| BARC ≥2 | 10 (3.1) | 4 (2.3) | 6 (3.9) | 0.59 (0.17–2.08) | 0.411 | |
| BARC ≥3 | 1 (0.3) | 1 (0.6) | 0 (0) | 2.67 (0.14–389.71)‡ | 0.520 | |
| Target lesion failure§ | 5 (1.5) | 3 (1.7) | 2 (1.3) | 1.34 (0.22–8.01) | 0.749 | |
| Death | 5 (1.5) | 3 (1.7) | 2 (1.3) | 1.33 (0.22–7.98) | 0.753 | |
| CV death | 3 (0.9) | 1 (0.6) | 2 (1.3) | 0.45 (0.04–4.91) | 0.509 | |
| Non-fatal myocardial infarction | 2 (0.6) | 2 (1.2) | 0 (0) | 58.43 (0.001–5,217,105.23) | 0.484 | |
| Stent thrombosis | 2 (0.6) | 1 (0.6) | 1 (0.7) | 0.89 (0.06–14.17) | 0.932 | |
| Repeat revascularization | 11 (3.4) | 6 (3.5) | 5 (3.3) | 1.08 (0.33–3.53) | 0.901 | |
| Revascularization related with target lesion | 2 (0.6) | 2 (1.2) | 0 (0) | 58.43 (0.001–5,217,105.23) | 0.484 | |
| Revascularization related with target vessel | 3 (0.9) | 3 (1.7) | 0 (0) | 58.72 (0.01–645,922.98) | 0.391 | |
| Non-target vessel PCI | 8 (2.5) | 3 (1.7) | 5 (3.3) | 0.53 (0.13–2.23) | 0.388 | |
| Stroke | 1 (0.3) | 1 (0.6) | 0 (0) | 2.64 (0.14–385.02)‡ | 0.525 | |
| Ischemic stroke | 1 (0.3) | 1 (0.6) | 0 (0) | 2.64 (0.14–385.02)‡ | 0.525 | |
| Hemorrhagic stroke | 0 (0) | 0 (0) | 0 (0) | - | NA | |
Values are presented as number (%) or hazard ratio (95% confidence interval).
BARC = Bleeding Academic Research Consortium; CV = cardiovascular; NA = not available; PCI = percutaneous coronary intervention; STEMI = ST-elevation myocardial infarction.
*Composite of all-cause death, non-fatal myocardial infarction, stent thrombosis, clinically driven revascularization, stroke, and BARC grade ≥2 bleeding.
†Cardiac death, myocardial infarction, stent thrombosis, and ischemic stroke.
‡Model fitted by penalized maximum likelihood.
§Includes cardiac death, target lesion revascularization, and target vessel myocardial infarction.
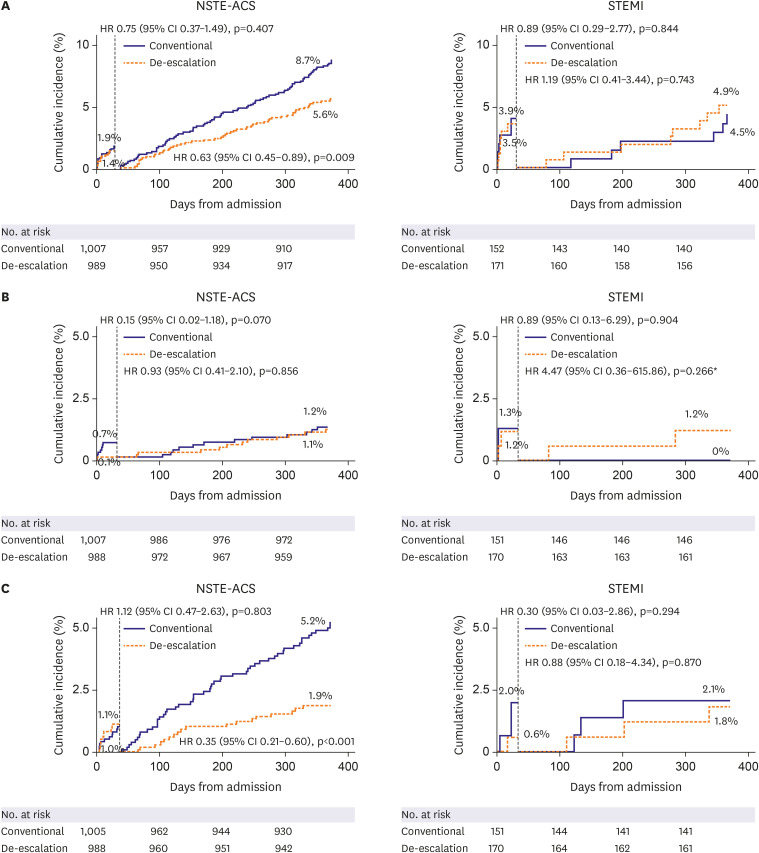
Landmark analysis
The results of the landmark analysis are shown in Figure 3. In patients with NSTE-ACS, the risk of the primary endpoint was similar between the 2 groups during the initial 4 weeks after the index procedure (1.4% vs. 1.9%; HR, 0.75; 95% CI, 0.37–1.49; p=0.407). However, beyond the first month, the curves diverged with a significantly lower occurrence in the de-escalation group (5.6% vs. 8.7%; HR, 0.63; 95% CI, 0.45–0.89; p=0.009). The risk of efficacy outcomes was similar between the 2 groups both before and after 4 weeks. The risk of BARC grade 2 or higher bleeding events was similar between the 2 groups before 4 weeks (1.1% vs. 1.0%; HR, 1.12; 95% CI, 0.47–2.63; p=0.803), whereas the risk of bleeding events was significantly lower in the de-escalation group than that in the conventional group after 4 weeks (1.9% vs. 5.2%; HR, 0.35; 95% CI, 0.21–0.60; p<0.001).
Figure 3. Prespecified landmark analysis at 4 weeks after index procedure: (A) primary endpoint, (B) efficacy outcomes (cardiac death, myocardial infarction, stent thrombosis, and ischemic stroke), and (C) safety outcomes (BARC ≥2 bleeding events).
CI = confidence interval; HR = hazard ratio; NSTE-ACS = non-ST-segment elevation acute coronary syndrome; STEMI = ST-elevation myocardial infarction.
*Model fitted by penalized maximum likelihood.
Like the primary analysis in STEMI patients, there was no beneficial effect of de-escalation for the primary outcome in the landmark analysis in patients with STEMI. The risk of the primary endpoint was similar between the 2 groups during the initial 4 weeks after the index procedure (3.5% vs. 3.9%; HR, 0.89; 95% CI, 0.29–2.77; p=0.844). However, the curves crossed after the first month with a statistically insignificant but numerically higher rates of the primary endpoint in the de-escalation group during the landmark analysis (4.9% vs. 4.5%; HR, 1.19; 95% CI, 0.41–3.44; p=0.743). The risk of efficacy outcomes was similar between the 2 groups during the initial 4 weeks after the procedure (1.2% vs. 1.3%; HR, 0.89; 95% CI, 0.13–6.29; p=0.904). After the initial 4 weeks, the efficacy outcomes were numerically higher in the de-escalation group (1.2% vs. 0%; HR, 4.47; 95% CI, 0.36–615.86; p=0.266). The risk of BARC grade 2 or higher bleeding was similar between the 2 groups both before and after 4 weeks. The results were consistent in the per-protocol analysis.
Independent predictors of net adverse clinical event
Multivariable Cox regression analysis showed that the independent predictors of the primary endpoint in the NSTE-ACS subgroup were high baseline creatinine level (serum creatinine concentration ≥2.0 mg/dL), and allocation to conventional therapy (Supplementary Table 3). However, in the STEMI subgroup, we were unable to identify any independent predictors of the primary endpoint. (Supplementary Table 4).
DISCUSSION
The current study evaluated the efficacy and safety of prasugrel-based de-escalation therapy in patients with STEMI or NSTE-ACS. Overall, although there was no statistically significant interaction for the effect of de-escalation according to subgroups, we found quite different results that may have clinical implications. In the NSTE-ACS patients, the results were mostly consistent with the overall results of the HOST-REDUCE-POLYTECH-ACS trial. Prasugrel-based dose de-escalation strategy from one-month post-PCI significantly reduced the risk of net clinical outcomes up to one year. The beneficial effects of de-escalation were mainly due to a decreased risk of bleeding and was not associated with an increase in ischemic events. In contrast, in the STEMI subgroup, there were no significant differences in the primary outcome between de-escalation and conventional therapy, with almost identical K-M estimates at one year. Further, primary analysis and landmark analysis showed no benefits of de-escalation in terms of bleeding and a slight trend toward worse ischemic outcomes for the de-escalation group in STEMI patients. These results suggest that prasugrel-based dose de-escalation could be a reasonable option in NSTE-ACS patients, whereas in highly thrombotic conditions such as STEMI, we need to be cautious in applying the main results of the HOST-REDUCE-POLYTECH-ACS trial.
Potent P2Y12 inhibitors, namely prasugrel and ticagrelor have been shown in large scale randomized trials to reduce the risk of ischemic outcomes in ACS patients.2),3) Several studies have shown that the more potent P2Y12 inhibitors might be associated with better results in patients with STEMI.14),15) In the TRITON-TIMI 38 trial, the HR for the primary outcome (death from cardiovascular causes, non-fatal MI, or non-fatal stroke) in the STEMI subgroup was left-shifted (greater relative benefit of the potent P2Y12 inhibitor; HR, 0.81 for the overall cohort, and 0.68 for the STEMI cohort, respectively).3),14),16) In the PLATO trial STEMI subgroup, there was greater benefit of ticagrelor for ischemic outcomes when compared with the benefit seen in the overall cohort (HR, 0.84 for the overall cohort and 0.67 for patients with a final diagnosis of STEMI, respectively).2),15),17) Further, in the TICO trial, which reported a significant benefit of ticagrelor monotherapy after 3-months of DAPT compared with continuing ticagrelor-based DAPT, there was no significant difference between the 2 groups in the STEMI subgroup.18),19) Taken together, previous trials suggest that intensification of antiplatelet therapy may be associated with greater benefit in patients with STEMI.
On the other hand, there is a fundamental trade-off that exists between ischemic and bleeding risk that needs to be considered in deciding the optimal duration or intensity of DAPT.5),6),7),8),20) Some recent trials have suggested benefit of de-escalation therapy. The HOST-REDUCE-POLYTECH-ACS trial studied dose reduction of the potent P2Y12 inhibitor prasugrel. In patients with ACS receiving PCI, a prasugrel based dose de-escalation strategy reduced the risk of net adverse events at 1 year compared to conventional therapy.9) The results were mainly driven by a significant reduction in bleeding events, without an increase in ischemic events. In patients who evaluated the PRU test at 1-month follow-up and 1-year follow-up, the percentage of patients within therapeutic range was higher in the de-escalation compared with the conventional group (61.7% vs. 31.7%, p<0.001).21) These results support the favourable outcomes seen in the de-escalation strategy over the conventional strategy. Another method of de-escalation is the early aspirin free strategy, which was studied in the TWILIGHT and TICO trials, both of which showed clinical benefit of the early ticagrelor monotherapy compared with continuation of DAPT.18),22) However, STEMI patients were excluded from the TWILIGHT trial and the results were neutral for STEMI patients in the TICO trial. Therefore, it remains uncertain whether de-escalation of antiplatelet therapy is beneficial in highly thrombotic situations such as STEMI. The data from the current sub-analysis showed no benefit of prasugrel de-escalation in STEMI patients with even a slight trend toward more ischemic events in the de-escalation group. Among the patients randomized, those with STEMI were 3.7 years younger than patients with NSTE-ACS, more likely to be males, more likely to be smokers, and had a higher frequency of diabetes mellitus. These are all characteristics that could be associated with an increased ischemic risk. The current analysis suggests that clinical outcomes maybe worse after de-escalation in those with a highly thrombotic milieu. Similar results were also observed in the SMART-DATE trial.23) In the overall trial, 6-month DAPT was non-inferior to 12-month or longer prolonged DAPT for the primary endpoint of major adverse cardiovascular events.23) However, in a post-hoc subgroup analysis of the risk of MI, prolonged DAPT appeared to be beneficial in the STEMI group. Finally the PEGASUS-TIMI 54 trial, which compared ticagrelor vs. placebo in stable patients with MI history of 1–3 years on top of conventional aspirin therapy, showed that in contrast to no difference observed for those with NSTEMI, ticagrelor significantly reduced the incidence of the primary outcome in patients with STEMI suggesting that these patients may need prolonged intensified antiplatelet therapy and that the de-escalation strategy might not be applicable in such patients.24)
There are several limitations of the current study. To maintain conventional prasugrel treatment of 10 mg daily in the first month after index PCI, patient with age >75, and body weight less than 60 kg were excluded from randomization. Most of the patients were males (89.3%), and the mean age of the enrolled subjects was younger (59 years) than in other trials. Therefore, there was only a small proportion of patients who were above 65 years of age and/or were female, both characteristics which increase the risk of atherothrombosis. Therefore, we should be careful in interpreting our results and to not over-generalize the results to all high-risk populations. Second, the HOST-REDUCE-POLYTECH-ACS study was not designed or powered for clinical endpoints in STEMI subjects alone, so there is a chance for type I error due to the multiple testing. The analysis of the STEMI subgroup was not prespecified and thus this analysis was post-hoc. Due to the small number of STEMI patients, the analysis was underpowered to provide reliable estimates of differences. Although hypothesis generating at best, we feel that the results of the current analysis raise important questions about whether de-escalation is an option that we should or should not consider for those with STEMI. Further large studies are warranted to evaluate the impact of prasugrel de-escalation and de-escalation therapy in general in patients with STEMI. Third, our study design was open-label, and thus, there is a possibility of patient self-reporting bias. However, adjudicators remained blinded to the treatment group, and the clinical outcomes were monitored and centrally adjudicated by an independent event adjudication committee. Fourth, this study was conducted only in the East Asian population. So, we should be cautious in extrapolating the current results to other ethnicities. Fifth, the number of STEMI patients was relatively small. Larger randomized controlled studies are warranted to confirm our principal findings. Finally, de-escalation was universal, and not based on any form of platelet function testing.
In conclusion, in STEMI patients, there was no benefit of prasugrel de-escalation for NACE and a statistically insignificant but numerically higher risk of ischemic events. Further large studies are warranted to evaluate the impact of prasugrel de-escalation in patients with STEMI.
Footnotes
Funding: This study was funded by Daiichi Sankyo, Boston Scientific, Terumo, Biotronik, Qualitech Korea, and Dio. This research was partially supported by a grant from Seoul National University Hospital (Research ID: 03-2021-0030). The funders of this study had no role in study design, collection of data and data analysis, or writing of the manuscript.
Conflict of Interest: Dr. Hyo-Soo Kim has received research grants or speaker’s fees from Daiichi Sankyo, Boston Scientific, Terumo, Biotronik, Dio, Medtronic, Abbott Vascular, Edwards Life Science, Amgen, and Behringer Ingelheim, outside of the submitted work. Dr. Kyung Woo Park reports speaker’s fees from Daiichi Sankyo, AstraZeneca, Sanofi, Bristol-Myers Squibb, Bayer, and Pfizer, outside of the submitted work. All other authors declare no competing interests.
Data Sharing Statement: The data generated in this study is available from the corresponding author(s) upon reasonable request.
- Conceptualization: Park KW, Koo BK, Kim HS.
- Methodology: Lee BK, Han JK, Yang HM, Kang HJ.
- Visualization: Hwang D, Kang J.
- Data curation: Won KB, Jeon DW, Han KR, Choi SW, Ryu JK.
- Funding acquisition: Kim HS.
- Writing - original draft: Ki YJ.
- Writing - review & editing: Park KW, Bae JW, Kim DB, Chae IH, Moon KW, Park HW, Jeong MH, Cha KS, Kim HS.
SUPPLEMENTARY MATERIALS
Baseline characteristics in NSTE-ACS patients
Baseline characteristics in STEMI patients
Independent predictors for the primary endpoint in NSTE-ACS patients
Independent predictors for the primary endpoint in STEMI patients
Kaplan-Meier cumulative event analysis of the primary endpoint and key secondary endpoints based on per-protocol analyses: (A) primary endpoint, (B) efficacy outcomes (cardiac death, myocardial infarction, stent thrombosis, and ischemic stroke), and (C) safety outcomes (BARC ≥2 bleeding events).
References
- 1.Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40:87–165. doi: 10.1093/eurheartj/ehy855. [DOI] [PubMed] [Google Scholar]
- 2.Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 3.Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–2015. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 4.Kang J, Park KW, Ki YJ, et al. Development and validation of an ischemic and bleeding risk evaluation tool in East Asian patients receiving percutaneous coronary intervention. Thromb Haemost. 2019;119:1182–1193. doi: 10.1055/s-0039-1688792. [DOI] [PubMed] [Google Scholar]
- 5.Kang J, Park KW, Palmerini T, et al. Racial differences in ischaemia/bleeding risk trade-off during anti-platelet therapy: individual patient level landmark meta-analysis from seven RCTs. Thromb Haemost. 2019;119:149–162. doi: 10.1055/s-0038-1676545. [DOI] [PubMed] [Google Scholar]
- 6.Ki YJ, Kang J, Park J, et al. Efficacy and safety of long-term and short-term dual antiplatelet therapy: a meta-analysis of comparison between Asians and non-Asians. J Clin Med. 2020;9:652. doi: 10.3390/jcm9030652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valgimigli M, Costa F, Lokhnygina Y, et al. Trade-off of myocardial infarction vs. bleeding types on mortality after acute coronary syndrome: lessons from the Thrombin Receptor Antagonist for Clinical Event Reduction in Acute Coronary Syndrome (TRACER) randomized trial. Eur Heart J. 2017;38:804–810. doi: 10.1093/eurheartj/ehw525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Costa F, van Klaveren D, James S, et al. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: a pooled analysis of individual-patient datasets from clinical trials. Lancet. 2017;389:1025–1034. doi: 10.1016/S0140-6736(17)30397-5. [DOI] [PubMed] [Google Scholar]
- 9.Kim HS, Kang J, Hwang D, et al. Prasugrel-based de-escalation of dual antiplatelet therapy after percutaneous coronary intervention in patients with acute coronary syndrome (HOST-REDUCE-POLYTECH-ACS): an open-label, multicentre, non-inferiority randomised trial. Lancet. 2020;396:1079–1089. doi: 10.1016/S0140-6736(20)31791-8. [DOI] [PubMed] [Google Scholar]
- 10.Franchi F, Rollini F, Angiolillo DJ. Antithrombotic therapy for patients with STEMI undergoing primary PCI. Nat Rev Cardiol. 2017;14:361–379. doi: 10.1038/nrcardio.2017.18. [DOI] [PubMed] [Google Scholar]
- 11.Lee JM, Jung JH, Park KW, et al. Harmonizing Optimal Strategy for Treatment of coronary artery diseases--comparison of REDUCtion of prasugrEl dose or POLYmer TECHnology in ACS patients (HOST-REDUCE-POLYTECH-ACS RCT): study protocol for a randomized controlled trial. Trials. 2015;16:409. doi: 10.1186/s13063-015-0925-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim HS, Kang J, Hwang D, et al. Durable polymer versus biodegradable polymer drug-eluting stents after percutaneous coronary intervention in patients with acute coronary syndrome: the HOST-REDUCE-POLYTECH-ACS trial. Circulation. 2021;143:1081–1091. doi: 10.1161/CIRCULATIONAHA.120.051700. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Garcia HM, McFadden EP, Farb A, et al. Standardized end point definitions for coronary intervention trials: the Academic Research Consortium-2 consensus document. Circulation. 2018;137:2635–2650. doi: 10.1161/CIRCULATIONAHA.117.029289. [DOI] [PubMed] [Google Scholar]
- 14.Montalescot G, Wiviott SD, Braunwald E, et al. Prasugrel compared with clopidogrel in patients undergoing percutaneous coronary intervention for ST-elevation myocardial infarction (TRITON-TIMI 38): double-blind, randomised controlled trial. Lancet. 2009;373:723–731. doi: 10.1016/S0140-6736(09)60441-4. [DOI] [PubMed] [Google Scholar]
- 15.Steg PG, James S, Harrington RA, et al. Ticagrelor versus clopidogrel in patients with ST-elevation acute coronary syndromes intended for reperfusion with primary percutaneous coronary intervention: a Platelet Inhibition and Patient Outcomes (PLATO) trial subgroup analysis. Circulation. 2010;122:2131–2141. doi: 10.1161/CIRCULATIONAHA.109.927582. [DOI] [PubMed] [Google Scholar]
- 16.Udell JA, Braunwald E, Antman EM, Murphy SA, Montalescot G, Wiviott SD. Prasugrel versus clopidogrel in patients with ST-segment elevation myocardial infarction according to timing of percutaneous coronary intervention: a TRITON-TIMI 38 subgroup analysis (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel-Thrombolysis In Myocardial Infarction 38) JACC Cardiovasc Interv. 2014;7:604–612. doi: 10.1016/j.jcin.2014.01.160. [DOI] [PubMed] [Google Scholar]
- 17.Velders MA, Abtan J, Angiolillo DJ, et al. Safety and efficacy of ticagrelor and clopidogrel in primary percutaneous coronary intervention. Heart. 2016;102:617–625. doi: 10.1136/heartjnl-2015-308963. [DOI] [PubMed] [Google Scholar]
- 18.Kim BK, Hong SJ, Cho YH, et al. Effect of ticagrelor monotherapy vs ticagrelor with aspirin on major bleeding and cardiovascular events in patients with acute coronary syndrome: the TICO randomized clinical trial. JAMA. 2020;323:2407–2416. doi: 10.1001/jama.2020.7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee SJ, Cho JY, Kim BK, et al. Ticagrelor monotherapy versus ticagrelor with aspirin in patients with ST-segment elevation myocardial infarction. JACC Cardiovasc Interv. 2021;14:431–440. doi: 10.1016/j.jcin.2020.11.036. [DOI] [PubMed] [Google Scholar]
- 20.Kupka D, Sibbing D. De-escalation of P2Y12 receptor inhibitor therapy after acute coronary syndromes in patients undergoing percutaneous coronary intervention. Korean Circ J. 2018;48:863–872. doi: 10.4070/kcj.2018.0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee HS, Park KW, Kang J, Han JK, Kim HS. De-escalation of prasugrel results in higher percentage of patients within optimal range of platelet reactivity. Thromb Haemost. 2021 doi: 10.1055/a-1496-7987. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Mehran R, Baber U, Sharma SK, et al. Ticagrelor with or without aspirin in high-risk patients after PCI. N Engl J Med. 2019;381:2032–2042. doi: 10.1056/NEJMoa1908419. [DOI] [PubMed] [Google Scholar]
- 23.Hahn JY, Song YB, Oh JH, et al. 6-month versus 12-month or longer dual antiplatelet therapy after percutaneous coronary intervention in patients with acute coronary syndrome (SMART-DATE): a randomised, open-label, non-inferiority trial. Lancet. 2018;391:1274–1284. doi: 10.1016/S0140-6736(18)30493-8. [DOI] [PubMed] [Google Scholar]
- 24.Bonaca MP, Bhatt DL, Cohen M, et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372:1791–1800. doi: 10.1056/NEJMoa1500857. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Baseline characteristics in NSTE-ACS patients
Baseline characteristics in STEMI patients
Independent predictors for the primary endpoint in NSTE-ACS patients
Independent predictors for the primary endpoint in STEMI patients
Kaplan-Meier cumulative event analysis of the primary endpoint and key secondary endpoints based on per-protocol analyses: (A) primary endpoint, (B) efficacy outcomes (cardiac death, myocardial infarction, stent thrombosis, and ischemic stroke), and (C) safety outcomes (BARC ≥2 bleeding events).