Abstract
Background
The role of angiotensin-converting enzyme inhibitors (ACEi) and angiotensin II receptor blockers (ARB) in the pandemic context of coronavirus disease 2019 (COVID-19) continues to be debated. Patients with hypertension, diabetes mellitus, chronic renal failure, cerebro-cardiovascular disease, or chronic obstructive pulmonary disease (COPD), who often use ACEi/ARB, may be at risk of severe COVID-19. However, there are no data available on the association of ACEi/ARB use with COVID-19 severity in this population.
Methods
This study is an observational study of patients with a positive severe acute respiratory syndrome coronavirus 2 test and inpatient treatment at a healthcare facility, using the registry information of COVIREGI-JP. Our primary outcomes were in-hospital death, ventilator support, extracorporeal membrane oxygenation support, and intensive care unit admission. Out of the 6055 patients, 1921 patients with preexisting hypertension, diabetes mellitus, chronic renal failure, cerebro-cardiovascular disease, or COPD were enrolled.
Results
Factors associated with an increased risk of the primary outcomes were aging, male sex, COPD, severe renal impairment, and diabetes mellitus. No correlations were observed with ACEi/ARB, cerebro-cardiovascular diseases, or hypertension. Associated factors in male patients were aging, renal impairment, hypertension, and diabetes. In female patients, factors associated with an increased risk were aging, ACEi/ARB, renal impairment, and diabetes, whereas hypertension was associated with a lower risk of the primary outcomes.
Conclusions
Independent factors for the primary outcomes were aging, male sex, COPD, severe renal impairment, and diabetes, but not ACEi/ARB. Based on this registry data analysis, more detailed data collection and analysis is needed with the cooperation of multiple healthcare facilities.
Abbreviations: ACEi, angiotensin-converting enzyme inhibitors; ARB, angiotensin II receptor blockers; COVID-19, coronavirus disease 2019; COPD, chronic obstructive pulmonary disease; COVIREGI-JP, COVID-19 Registry Japan; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; ACE2, angiotensin-converting enzyme 2; ICU, intensive care unit; BMI, body mass index
Keywords: COVID-19, Disease outcome, Associated factors
Graphical abstract
Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, is mediated by angiotensin-converting enzyme 2 (ACE2) in host cells [1]. Since ACE inhibitors (ACEi) and angiotensin II receptor blockers (ARB) up-regulate the expression of ACE2 [[2], [3], [4]], they may increase the risk of and aggravate COVID-19. However, since ACE2 converts angiotensin II (1–8) to angiotensin (1–7) and exerts vasodilatory, fibrosis inhibitory, and anti-inflammatory effects via the Mas receptor, increases in the expression of ACE2 due to ACEi/ARB may suppress the aggravation of pneumonia [5]. Furthermore, the cytokine storm [[5], [6], [7]] and the promotion of thrombosis through endothelial damage and the cytokine storm [[8], [9], [10], [11]] may worsen COVID-19, and angiotensin II may enhance these effects via the AT1 receptor. Therefore, ACEi/ARB may suppress not only the cytokine storm [5], but also thrombus formation [12] by inhibiting the cytokine storm.
Previous studies reported relationships between ACEi/ARB and SARS-CoV-2 infection and COVID-19 aggravation; however, contradictory findings were obtained from a meta-analysis of these studies. One study reported that ARB prevented the aggravation of COVID-19 [13], whereas another showed that neither ACEi nor ARB played a role in SARS-CoV-2 infection or COVID-19 severity [14], and findings also widely varied in race- and region-dependent manners [15,16]. These studies were conducted in the USA, China, and Europe, and only limited information is currently available from Japan [17]. ACEi/ARB have been reported to not only improve the prognosis of patients with hypertension, heart failure, and myocardial infarction, but also exert urinary protein-reducing effects in patients with diabetes and chronic kidney disease [18,19], exert pneumonia risk-suppressive effect in chronic obstructive pulmonary disease (COPD) patients [20,21], and exert stroke-suppressive effects in hypertension/diabetic patients [22]. Therefore, subjects in the present study were hospitalized COVID-19 patients with hypertension, diabetes mellitus, chronic renal failure, cerebral and cardiovascular disease, or COPD as the underlying disease. Therefore, we herein conducted the present study using COVIREGI-JP, the COVID-19 registry Japan. Data from patients with hypertension, diabetes, severe renal impairment, cerebral and cardiovascular disease, and COPD as comorbidities before the onset of infection were extracted, and the involvement of ACEi/ARB was examined with combined primary outcomes consisting of in-hospital death, ventilator support, extracorporeal membrane oxygenation support, and intensive care unit (ICU) admission.
Methods
Study design and patients
This was an observational study. Healthcare facilities that voluntarily participated in COVIREGI-JP enrolled patients as previously described [23]. Inclusion criteria for COVIREGI-JP enrollment were as follows: (1) a positive SARS-CoV-2 test and (2) inpatient treatment at a healthcare facility. Study data were collected and managed using REDCap (Research Electronic Data Capture), a secure, Web-based data-capture application hosted at the JCRAC (Joint Center for Researchers, Associates and Clinicians) Data Center of the National Center for Global Health and Medicine [24].
Data set
We included patients enrolled in COVIREGI-JP hospitalized between January 26, 2020 and October 31, 2020 (Soft Frozen data are as of November 2, 2020) in the analysis. Patients with apparent input errors and unknown outcomes were excluded from the analysis.
Outcomes
Our primary combined primary outcomes consisted of in-hospital death, ventilator support, extracorporeal membrane oxygenation support, and ICU admission. Secondary outcomes were oxygen administration, disturbed consciousness, lower blood pressure (systolic blood pressure < 90 mmHg), diagnosis of pneumonia by computed tomography.
Statistical analysis
In the analysis of patient backgrounds, values are presented as the mean ± standard deviation (SD) for continuous variables, the total number or a percentage for dichotomous variables, and the median and interquartile range (IQR) for laboratory values. Comparisons of differences among groups were performed by the unpaired Student's t-test or Mann-Whitney U test for continuous variables and the chi-squared test for dichotomous variables where appropriate. We constructed a nonlinear mixed-effects univariate/multivariable logistic regression model using institutions as a random intercept to assess the relationship between the administration of ACEi/ARB and COVID-19 infection outcomes. Adjusted factors were as follows: history of cerebral and cardiovascular disease, COPD, severe renal impairment, hypertension, diabetes, age, and sex. We also conducted a sub-analysis of sex and the secondary outcomes. A p-value of less than 0.05 indicated a significant difference. Statistical analyses were performed with SAS version 9.4 (SAS Institute, Cary, NC, USA).
Ethics
The present study was approved by the National Center for Global Health and Medicine (NCGM) ethics review (NCGM-G-003494-0). All procedures performed in studies were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Results
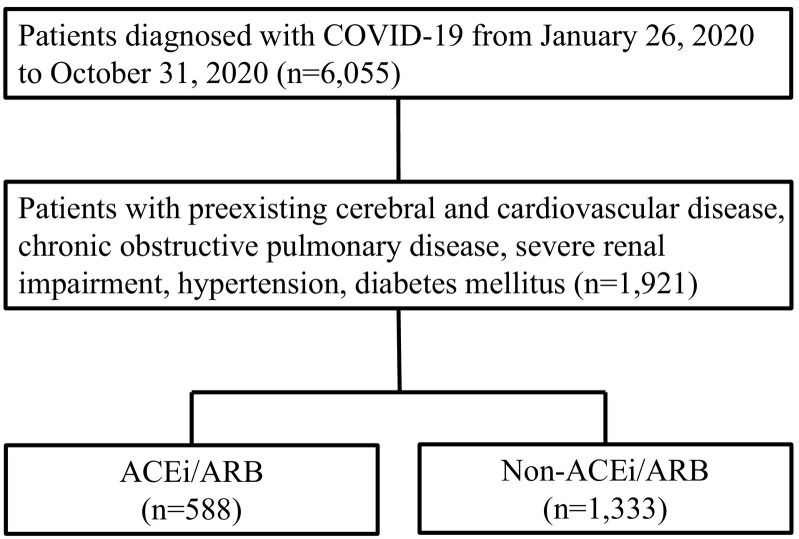
Table 1 shows the baseline characteristics of 1921 enrolled patients with preexisting cerebral and cardiovascular diseases, COPD, severe renal impairment defined as serum creatinine ≥3 mg/dl, renal replacement therapy, or renal transplantation, hypertension, and diabetes mellitus out of the 6055 patients diagnosed with COVID-19 (Fig. 1 ). The average age of patients was 68.5 years, males accounted for 65.2%, and body mass index (BMI) averaged 24.7. Regarding comorbidities at admission, 29.2% of patients had cerebral and cardiovascular diseases, 6.7% COPD, 3.7% severe renal impairment, 45.0% diabetes, and 57.0% hypertension. Among all patients, 4.2% were taking ACEi at admission and 29.0% ARB.
Table 1.
Clinical characteristics.
| Total | ACEi/ARB | Non-ACEi/ARB | P | |
|---|---|---|---|---|
| 1921 | 588 | 1333 | ||
| Age, years | 68.5 ± 15.0 | 69.3 ± 12.9 | 68.2 ± 15.9 | 0.123 |
| Male | 1252 (65.2%) | 373 (63.4%) | 879 (65.9%) | 0.288 |
| BMI, kg/m2 | 24.7 ± 5.3 | 25.4 ± 5.4 | 24.3 ± 5.1 | <0.001 |
| History of CCVD | 560 (29.2%) | 168 (28.6%) | 392 (29.4%) | 0.710 |
| COPD | 129 (6.7%) | 27 (4.6%) | 102 (7.7%) | 0.014 |
| Severe renal impairment | 72 (3.7%) | 18 (3.1%) | 54 (4.1%) | 0.293 |
| DM without three major complications | 735 (38.3%) | 182 (31.0%) | 553 (41.5%) | <0.001 |
| DM with three major complications | 130 (6.8%) | 44 (7.5%) | 86 (6.5%) | 0.407 |
| Hypertension | 1095 (57.0%) | 488 (83.0%) | 607 (45.5%) | <0.001 |
| Respiratory rate, /min | 20.1 ± 8.6 | 20.6 ± 9.8 | 19.8 ± 8.1 | 0.110 |
| Heart rate, /min | 87.2 ± 16.9 | 86.1 ± 16.2 | 87.7 ± 17.2 | 0.055 |
| Systolic blood pressure, mmHg | 132.3 ± 22.5 | 132.3 ± 23.1 | 132.3 ± 22.2 | 0.999 |
| Diastolic blood pressure, mmHg | 78.5 ± 15.5 | 77.8 ± 15.8 | 78.9 ± 15.3 | 0.161 |
| ACEi | 76 (4.2%) | 76 (12.9%) | 0 | <0.001 |
| ARB | 521 (29.0%) | 521 (88.3%) | 0 | <0.001 |
| White blood cell, 103/μl | 5.5 (4.3–7.3) | 5.5 (4.2–7.5) | 5.5 (4.3–7.3) | 0.960 |
| Neutrophils, % | 72.0 (63.0–81.0) | 72.7 (63.0–80.5) | 71.9 (62.8–81.1) | 0.853 |
| Lymphocytes, % | 19.0 (12.1–26.4) | 19.0 (12.4–25.8) | 19.0 (12.0–26.9) | 0.706 |
| Hemoglobin, g/dl | 13.6 (12.1–15.0) | 13.5 (12.1–14.7) | 13.7 (12.2–15.1) | 0.074 |
| Platelets, 103/μl | 169.0 (113.0–229.0) | 175.5 (119.0–227.0) | 166.0 (111.0–229.0) | 0.732 |
| Albumin, g/dl | 3.5 (3.0–3.9) | 3.5 (3.0–3.9) | 3.5 (3.0–3.9) | 0.772 |
| Aspartate aminotransferase, U/l | 33.0 (23.0–50.0) | 34.0 (23.0–50.0) | 33.0 (23.0–51.0) | 0.560 |
| Alanine aminotransferase, U/l | 26.0 (17.0–43.0) | 26.0 (17.0–41.0) | 26.0 (17.0–44.0) | 0.610 |
| Lactate dehydrogenase, U/l | 265.0 (212.0–365.0) | 267.0 (215.0–363.0) | 264.0 (211.0–365.0) | 0.819 |
| Creatine phosphokinase, U/l | 84.0 (50.0–151.0) | 93.0 (53.0–165.5) | 81.0 (49.0–142.0) | 0.007 |
| C-reactive protein, mg/dl | 4.0 (1.1–9.4) | 4.3 (1.1–9.1) | 3.9 (1.1–9.5) | 0.690 |
| Blood glucose, mg/dl | 126.0 (106.0–169.5) | 125.0 (105.0–167.0) | 126.0 (106.0–170.0) | 0.510 |
| Blood urea nitrogen, mg/dl | 16.0 (12.0–22.0) | 16.4 (12.3–22.6) | 15.5 (12.0–21.8) | 0.075 |
| Creatinine, mg/dl | 0.8 (0.7–1.1) | 0.9 (0.7–1.1) | 0.8 (0.7–1.0) | 0.004 |
| Sodium, mEq/l | 138.0 (135.0–140.0) | 138.0 (135.0–140.0) | 138.0 (135.0–140.0) | 0.496 |
| Potassium, mEq/l | 4.0 (3.7–4.3) | 4.0 (3.6–4.3) | 4.0 (3.7–4.3) | 0.228 |
Data are the mean ± SD, n (%), or a median (IQR).
Definition: severe renal impairment was defined as a serum creatinine level ≥ 3 mg/dl, renal replacement therapy, or renal transplantation.
Abbreviations: ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; CCVD, cerebral and cardiovascular diseases; COPD, chronic obstructive pulmonary disease; Cre, creatinine; DM, diabetes mellitus; SD, standard deviation; IQR, interquartile range.
Fig. 1.
Flowchart for the selection of study subjects.
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; COVID-19, coronavirus disease 2019.
The average respiratory rate at admission was 20/min, pulse rate 87/min, average systolic blood pressure 132 mmHg, and diastolic blood pressure 79 mmHg. The median leukocyte count at admission was 5500/μl, hemoglobin 13.6 g/dl, platelet count 169,000/μl, albumin 3.5 g/dl, aspartate aminotransferase 33.0 U/l, alanine aminotransferase 26.0 U/l, lactate dehydrogenase 265.0 U/l, creatine phosphokinase 84.0 U/l, C-reactive protein 4.0 mg/dl, blood glucose 126.0 mg/dl, blood urea nitrogen 16.0 mg/dl, creatinine 0.8 mg/dl, sodium 138.0 mEq/l, and potassium 4.0 mEq/l.
In comparisons with patients not receiving ACEi/ARB, those administered ACEi/ARB had a higher BMI, less frequent complications of COPD and uncomplicated diabetes, a higher incidence of hypertension, and higher creatine phosphokinase and creatinine levels. There were no significant differences between two groups regarding C-reactive protein and white blood cell count as markers of inflammation.
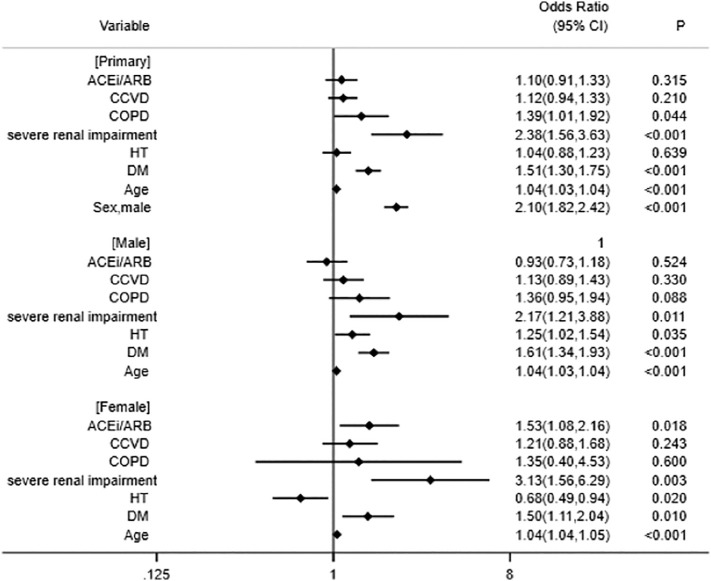
Factors associated with an increased risk of the primary outcomes were aging, male sex, COPD, severe renal impairment, and diabetes mellitus. No correlations were observed with the administration of ACEi/ARB, cerebral and cardiovascular diseases, or hypertension (Fig. 2 ).
Fig. 2.
Adjusted odd ratios with 95% confidence intervals (CI) for the primary outcome.
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CCVD, cerebral and cardiovascular diseases; COPD, chronic pulmonary obstructive disease; HT, hypertension; DM, diabetes mellitus.
In a stratified analysis by sex, factors associated with an increased risk of the primary outcomes in male patients were aging, severe renal impairment, hypertension, and diabetes (Fig. 2). No correlations were observed with the administration of ACEi/ARB, cerebral and cardiovascular diseases, or COPD (Fig. 2).
In female patients, factors associated with an increased risk of the primary outcomes were aging, the administration of ACEi/ARB, severe renal impairment, and diabetes (Fig. 2). No correlations were observed with cerebral and cardiovascular diseases or COPD, whereas hypertension was associated with a lower risk of the primary outcomes (Fig. 2).
Regarding secondary outcomes, the administration of ACEi/ARB did not correlate with hypoxemia requiring oxygen administration, disturbed consciousness, decreased blood pressure, or pneumonia (Table 2 ). Among the patient conditions at the time of discharge, there was information on the presence of tracheostomy and the presence of oxygen administration. Multivariable analysis revealed that taking ACEi/ARB was not an independent related factor for tracheostomy (OR 1.56, 95% CI 0.66–3.71, p = 0.31) and oxygen administration (OR 0.88, 95% CI 0.67–1.15, p = 0.33).
Table 2.
Secondary outcomes.
| Univariate |
Multivariable |
|||
|---|---|---|---|---|
| Oxygen administration | OR (95%CI) | p | OR (95%CI) | p |
| ACEi/ARB | 2.14 (1.66,2.75) | <0.001 | 1.23 (0.91,1.67) | 0.184 |
| Disturbed consciousness | ||||
| ACEi/ARB | 2.34 (1.73,3.13) | <0.001 | 1.30 (0.96,1.77) | 0.093 |
| Lower blood pressure | ||||
| ACEi/ARB | 0.77 (0.63,0.95) | 0.014 | 1.02 (0.80,1.31) | 0.862 |
| Pneumonia on CT | ||||
| ACEi/ARB | 1.61 (1.36,1.91) | <0.001 | 1.00 (0.81,1.23) | 0.975 |
Abbreviations: ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CT, computed tomograph.
Discussion
Independent factors for the primary outcomes were aging, male sex, COPD, severe renal impairment, and diabetes, but not the administration of ACEi/ARB. In the stratified analysis by sex, related factors in male patients were aging, severe renal impairment, hypertension, and diabetes. In female patients, aging, diabetes, and severe renal impairment, similar to male patients, as well as the administration of ACEi/ARB were positively associated with the primary outcomes, whereas hypertension was negatively associated with them.
The present study was conducted using data from COVIREGI-JP, a registry of SARS-CoV-2 infection in Japan. Subjects in the present study were hospitalized COVID-19 patients with hypertension, diabetes mellitus, chronic renal failure, cerebral and cardiovascular diseases, or COPD as the underlying disease. Multimorbidity is usually associated with the use of multiple medicines (polypharmacy). A marked difference between the present study and previous studies conducted in other countries is that ARB was selected for treatment more frequently than ACEi. ACE1 II genotype frequency is high in Japan, and the high frequency of coughing associated with the administration of ACEi is considered to be one of the reasons why ARB is more frequently prescribed than ACEi in Japan [25]. Since the frequency of ARB administration was higher than that of ACEi, a multivariable analysis was performed using ARB alone as an independent variable instead of ACEi/ARB. ARB was not an independent related factor for primary outcome [ARB: OR (95% CI) 1.08 (0.89,1.31), p = 0.460] as similar as the result of ACEi/ARB use.
In the multivariate analysis, the factors associated with an increased risk of the primary outcomes were aging, male sex, COPD, severe renal impairment, and diabetes. A previous study on 19,486 COVID-19 patients in the UK reported that chronic kidney disease, diabetes, aging, and male sex were associated with an increased risk of ICU admission [26], which is consistent with the present results, except for COPD. COPD was associated with a risk of aggravation in a meta-analysis focusing on the involvement of chronic respiratory disease in the aggravation of COVID-19 patients [27]. Therefore, it is necessary to examine whether COPD is one of the priority diseases from the viewpoint of prevention by vaccination and treatment as a high-risk for exacerbation group.
The multivariate analysis also revealed that ACEi/ARB did not correlate with the primary outcome in the present study. Since the spread of the novel coronavirus infection in China in 2019, a number of issues have been reported regarding the relationship between the renin-angiotensin (RA) system in the living body and COVID-19 [[1], [2], [3], [4], [5], [6]]. ACE2 has been shown to play an important role as a receptor in SARS-CoV-2 infection [1], and the administration of ACEi/ARB up-regulated the expression of ACE2 in various pathological animal models [[2], [3], [4]]. The administration of ACEi/ARB may increase the risk of infection. However, ACE2 cleaves Ang I into the inactivating peptide Ang (1–9) and Ang II into Ang (1–7), which exerts vasodilatory [28] and anti-inflammatory effects [29] by binding to the Mas receptor. Therefore, the up-regulated expression of ACE2 alternatively may contribute to protecting against the cardiovascular complications associated with COVID-19. Furthermore, SARS-CoV-2 infection down-regulated the expression of ACE2, resulting in an elevated plasma Ang II level through the suppression of Ang II metabolism, and a disintegrin and metalloprotease domain 17 (ADAM17) was activated through the increased binding of Ang II to AT1R receptors [6]. Activated ADAM17 promotes endothelial dysfunction by producing tumor necrosis factor-alpha/interleukin-6, which may result in acute respiratory distress syndrome (ARDS) [6]. One of the mechanisms contributing to severe pneumonia and respiratory failure in COVID-19 patients is the induction of ARDS by the so-called cytokine storm. However, ACEi/ARB have also been suggested to reduce the risk of aggravation by the cytokine storm.
Based on previous findings on the pathophysiological significance of ACEi/ARB, the relationship between the administration of ACEi/ARB and the risk of SARS-CoV-2 infection and COVID-19 aggravation has been the focus of research. The findings of a meta-analysis of these studies were contradictory; ARB was shown to prevent the aggravation of COVID-19 [13], whereas neither ACEi nor ARB was associated with the risk of infection or its aggravation [14]. Furthermore, race- and region-dependent differences were observed [15,16]. The subjects in the present study were patients with polymerase chain reaction-confirmed COVID-19; therefore, although the involvement of ACEi/ARB in the risk of infection currently remains unknown, their administration was not associated with the risk of progression to the primary outcomes.
A previous study reported that the risk of COVID-19 aggravation itself varies by race and region, and the involvement of the DD, ID, or II gene polymorphisms in ACE1 may one of the reasons for this disparity [30]. ACE1 II genotype frequency is higher in Asians, including Japanese, than in Europeans and Middle Eastern individuals, and the ACE1 II genotype has been associated with a lower risk of COVID-19 aggravation [30]. A gene polymorphism in ACE2 has also been suggested to contribute to the binding of ACE2 and SARS-CoV-2 [31], and possibly the risk of infection, which has not yet been confirmed. Although the detailed mechanism of the host response to SARS-CoV-2 is still insufficient, the immune system for viral invasion can be broadly divided into the innate immune response and the adaptive immune response. Innate immunity not only induces an inflammatory response and the production of interferon, but also has a role of initiating an adaptive immune response by antibodies and cytotoxic T lymphocytes for each specific virus [32]. Cytotoxic T lymphocytes recognize the viral epitope presented on human leukocyte antigen (HLA) on virus-infected cells and suppress virus infection by destroying the virus-infected cells. This HLA has a variety of alleles, and the frequency distribution of alleles differs depending on the region, ethnicity, and race. Using the SARS-Cov-2 peptide that can activate cytotoxic T lymphocytes as a probe, the relationship between epitope presentation by SARS-CoV-2 infection and activated T lymphocytes has been evaluated. South Asians frequently recognize CD8-specific epitopes, Europeans frequently recognize CD4-specific epitopes, and East Asians, Africans, and Oceanians have low ability to recognize both CD4/CD8 epitopes [32]. Although these findings do not directly prove the association with the onset and aggravation of COVID-19, they are considered to be one of the footholds for COVID-19 treatment including vaccine development.
Male sex was identified as an independent high-risk factor for the primary outcome, which is consistent with previous findings. However, the stratified analysis by sex revealed that the administration of ACEi/ARB was one of the independent high-risk factors for the primary outcome in an analysis of female patients only. A previous study investigated sex differences in the effects of ACEi/ARB, and found no significant differences [33]. The reason why the administration of ACEi/ARB was identified as a high-risk factor for the primary outcomes in female patients in the present study remains unclear. The univariate analysis showed that the administration of ACEi/ARB was a high-risk factor for the primary outcomes regardless of sex (male: OR 1.76, 95%CI 1.42–2.19, p < 0.001; female: OR 2.42 95%CI 1.77–3.31, p < 0.001); therefore, interactions between variables may have influenced the results of the multivariate analysis. A more detailed examination of a wide range of information, including comorbidities and drug exposure, is required in the future.
In addition to examining interactions between variables, sex differences in the immune response of COVID-19 patients need to be considered. A previous study demonstrated that the T-cell response of female patients was stronger and more persistent than that of male patients, and also that the weaker T-cell response in male patients correlated with a poor outcome [34]. Patients with COVID-19 had higher levels of innate immune cytokines and chemokines than healthy controls. In female patients, high levels of innate immune cytokines resulted in poor responsiveness to SARS-CoV-2 infection [34]. Although these findings suggest important differences in the baseline immune capacity between male and female patients in the early stages of SARS-COV-2 infection, the contribution of ACEi/ARB to the immune system has not yet been clarified.
ACE2 is a molecule that plays an important role in COVID-19 and the regulation of the RA system; however, since the ACE2 gene is on the X chromosome [35], sex differences may exist through the regulation of ACE2 expression. ACE2, which activates the Ang (1–7)/Mas receptor axis, plays a role in suppressing the activation of the RA system through the Ang II/AT1 receptor axis. Asian women have a markedly higher basal level of ACE2 than other ethnic groups [35]. In addition, a negative correlation was reported between the quantitative expression of ACE2 and severity of COVID-19 [35]. Although an ACE1/ACE2 imbalance is associated with the aggravation of COVID-19 [36] and ACEi/ARB may correct this imbalance [37], the present results suggest the opposite correlation in female patients only. The reason for this currently remains unclear, and it is important to confirm the reproducibility of this result and elucidate the underlying mechanism in the future.
The stratified analysis by sex revealed a relationship between hypertension and the primary outcomes. It was identified as a low-risk factor in female patients and a high-risk factor in male patients. In a clinical study with the aggravation of COVID-19 as an outcome, the hazard ratio of hypertension significantly changed in a regulator-dependent manner [38]. That study focused on the strong relationship between blood pressure and age, and examined the interaction between these variables. A strong interaction that hypertension is at high risk up to age 70 years and low risk beyond age 70 years was clarified, and it was reported that it may have contributed to the results. However, the reason for the negative correlation between hypertension and mortality in the elderly remains unclear. Further studies on a larger number of cases and wider range of information are needed.
Cerebro-cardiovascular disease was not a related factor for primary outcome in this study. Many reports have concluded that cardiovascular disease and stroke are the related factors of COVID-19 prognosis [[38], [39], [40], [41]], but cardiovascular disease, atrial fibrillation, and coexistence of heart failure are not associated with ICU admission [26]. When we evaluated the association with in-hospital death, which is one of the components of the primary outcome of this study, cerebro-cardiovascular disease has a significant positive association in multivariate analysis (OR 1.33, 95% CI 1.03–1.70, p = 0.028), similar to the results that were previously reported. It is known that the case fatality rate of COVID-19 varies greatly from 0 to 20% depending on the country [42]. The case fatality rate in Japan at the time of the data collection is 1.77% (October 31, 2020: 1765 fatalities / 99,959 infected), according to the public information of the Ministry of Health, Labor and Welfare (https://covid19.mhlw.go.jp/). Differences in case fatality rate among countries have been pointed out might be due to differences in the stage of spread of infection, differences in age and comorbidities, differences in ICU management, ventilator and extracorporeal membrane oxygenation support indications, and the case fatality rate in Japan is far from high. Therefore, it is possible that the maintenance and operation system of ICU, ventilator, extracorporeal membrane oxygenation, which can affect the prognosis, contribute to the low case fatality rate in Japan.
Limitations
The present study has several limitations. Although some confounders were corrected for, additional risk factors may not have been controlled. Furthermore, drugs other than ACEi/ARB and continuity of ACEi/ARB after admission were not examined, the frequency of administration of ACEi was low, and neither the type nor dose of ACEi/ARB was assessed. Moreover, the risk of infection was not investigated. Third, very severe renal dysfunction was targeted because the registry definition of renal dysfunction was more severe than the general definition of chronic kidney disease. Fourth, since the treatment method for COVID-19 has not been established and is not unified among institutions, it has not been examined as a related factor. Fifth, because ventilator support, extracorporeal membrane oxygenation support, and ICU admission are determined on a facility-by-facility basis, it cannot be denied that the differences among facilities may have affected the results. Another limitation is that the severity of comorbidities was not evaluated. Although the analysis of registry data is extremely important, the lack of several factors required for analysis needs efforts to collect and analyze more detailed data by multiple healthcare facilities.
Conclusions
Independent factors for the primary outcomes were aging, male sex, COPD, severe renal impairment, and diabetes, but not ACEi/ARB. In female patients, aging, diabetes, and severe renal impairment, similar to male patients, as well as ACEi/ARB were positively associated with the primary outcomes.
Funding
This study was funded by Health and Labour Sciences Research Grant, ‘Research for risk assessment and implementation of crisis management functions for emerging and re-emerging infectious diseases’, provided by the Japanese Ministry of Health, Labour, and Welfare (grand number 19HA1003) and by Japan Agency for Medical Research and Development (grant number; 20ek0210106h0003).
Declaration of competing interest
There is no conflict of interest in the present study.
Acknowledgments
We thank Chie Nishiguchi for their excellent assistance. We thank all the participating facilities for their care towards patients with COVID-19 and cooperation during data entry.
References
- 1.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ocaranza M.P., Godoy I., Jalil J.E., Varas M., Collantes P., Pinto M., et al. Enalapril attenuates downregulation of angiotensin-converting enzyme 2 in the late phase of ventricular dysfunction in myocardial infarcted rat. Hypertension. 2006;48:572–578. doi: 10.1161/01.HYP.0000237862.94083.45. [DOI] [PubMed] [Google Scholar]
- 3.Li Y., Zeng Z., Li Y., Huang W., Zhou M., Zhang X., et al. Angiotensin-converting enzyme inhibition attenuates lipopolysaccharide-induced lung injury by regulating the balance between angiotensin-converting enzyme and angiotensin-converting enzyme 2 and inhibiting mitogen-activated protein kinase activation. Shock. 2015;43:395–404. doi: 10.1097/SHK.0000000000000302. [DOI] [PubMed] [Google Scholar]
- 4.Klimas J., Olvedy M., Ochodnicka-Mackovicova K., Kruzliak P., Cacanyiova S., Kristek F., et al. Perinatally administered losartan augments renal ACE2 expression but not cardiac or renal mas receptor in spontaneously hypertensive rats. J. Cell. Mol. Med. 2015;19:1965–1974. doi: 10.1111/jcmm.12573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kreutz R., Algharably E.A.E., Azizi M., Dobrowolski P., Guzik T., Januszewicz A., et al. Hypertension, the renin-angiotensin system, and the risk of lower respiratory tract infections and lung injury: implications for COVID-19. Cardiovasc. Res. 2020;116:1688–1699. doi: 10.1093/cvr/cvaa097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirano T., Murakami M. COVID-19: a new virus, but a familiar receptor and cytokine release syndrome. Immunity. 2020;52:731–733. doi: 10.1016/j.immuni.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jonmarker S., Hollenberg J., Dahlberg M., Stackelberg O., Litorell J., Everhov Å.H., et al. Dosing of thromboprophylaxis and mortality in critically ill COVID-19 patients. Crit. Care. 2020;24:653. doi: 10.1186/s13054-020-03375-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chow J.H., Khanna A.K., Kethireddy S., Yamane D., Levine A., Jackson A.M., et al. Aspirin use is associated with decreased mechanical ventilation, intensive care unit admission, and in-hospital mortality in hospitalized patients with coronavirus disease 2019. Anesth. Analg. 2021;132:930–941. doi: 10.1213/ANE.0000000000005292. [DOI] [PubMed] [Google Scholar]
- 10.Joly B.S., Siguret V., Veyradier A. Understanding pathophysiology of hemostasis disorders in critically ill patients with COVID-19. Intensive Care Med. 2020;46:1603–1606. doi: 10.1007/s00134-020-06088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steckelings U.M., Sumners C. Correcting the imbalanced protective RAS in COVID-19 with angiotensin AT2-receptor agonists. Clin. Sci. (Lond.) 2020;134:2987–3006. doi: 10.1042/CS20200922. [DOI] [PubMed] [Google Scholar]
- 12.Wang Z., Yang Y., Liang X., Gao B., Liu M., Li W., et al. COVID-19 associated ischemic stroke and hemorrhagic stroke: incidence, potential pathological mechanism, and management. Front. Neurol. 2020;11 doi: 10.3389/fneur.2020.571996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pranata R., Permana H., Huang I., Lim M.A., Soetedjo N.N.M., Supriyadi R., et al. The use of renin angiotensin system inhibitor on mortality in patients with coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. Diabetes Metab Syndr. 2020;14:983–990. doi: 10.1016/j.dsx.2020.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caldeira D., Alves M., Gouveia E., Melo R., Silvério António P., Cunha N., et al. Angiotensin-converting enzyme inhibitors and angiotensin-receptor blockers and the risk of COVID-19 infection or severe disease: systematic review and meta-analysis. Int J Cardiol Heart Vasc. 2020;31:100627. doi: 10.1016/j.ijcha.2020.100627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alamer A.A., Almulhim A.S., Alrashed A.A., Abraham I. Mortality, severity, and hospital admission among COVID-19 patients with ACEI/ARB use: a meta-analysis stratifying countries based on response to the first wave of the pandemic. Healthcare (Basel) 2021;9:127. doi: 10.3390/healthcare9020127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patoulias D., Katsimardou A., Stavropoulos K., Imprialos K., Kalogirou M.S., Doumas M. Renin-angiotensin system inhibitors and COVID-19: a systematic review and meta-analysis. Evidence for significant geographical disparities. Curr. Hypertens. Rep. 2020;22:90. doi: 10.1007/s11906-020-01101-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsuzawa Y., Ogawa H., Kimura K., Konishi M., Kirigaya J., Fukui K., et al. Renin-angiotensin system inhibitors and the severity of coronavirus disease 2019 in Kanagawa, Japan: a retrospective cohort study. Hypertens. Res. 2020;43:1257–1266. doi: 10.1038/s41440-020-00535-8. [DOI] [PubMed] [Google Scholar]
- 18.Agha A., Amer W., Anwar E., Bashir K. Reduction of microalbuminuria by using losartan in normotensive patients with type 2 diabetes mellitus: a randomized controlled trial. Saudi J Kidney Dis Transpl. 2009;20:429–435. [PubMed] [Google Scholar]
- 19.Ye H., Huo Z., Ye P., Xiao G., Zhang Z., Xie C., et al. Comparative proteinuria management of different angiotensin-converting enzyme inhibitors or angiotensin receptor blockers for normotensive patients with CKD: a Bayesian network meta-analysis. Peer J. 2020;8 doi: 10.7717/peerj.8575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai C.C., Wang Y.H., Wang C.Y., Wang H.C., Yu C.J., Chen L. Comparative effects of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on the risk of pneumonia and severe exacerbations in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:867–874. doi: 10.2147/COPD.S158634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim J., Lee J.K., Heo E.Y., Chung H.S., Kim D.K. The association of renin-angiotensin system blockades and pneumonia requiring admission in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:2159–2166. doi: 10.2147/COPD.S104097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pai P.Y., Muo C.H., Sung F.C., Ho H.C., Lee Y.T. Angiotensin receptor blockers (ARB) outperform angiotensin-converting enzyme (ACE) inhibitors on ischemic stroke prevention in patients with hypertension and diabetes - a real-world population study in Taiwan. Int. J. Cardiol. 2016;215:114–119. doi: 10.1016/j.ijcard.2016.04.096. [DOI] [PubMed] [Google Scholar]
- 23.Matsunaga N., Hayakawa K., Terada M., Ohtsu H., Asai Y., Tsuzuki S., et al. Clinical epidemiology of hospitalized patients with COVID-19 in Japan: report of the COVID-19 REGISTRY JAPAN. Clin. Infect. Dis. 2021;73:e3677–e3689. doi: 10.1093/cid/ciaa1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishio K., Kashiki S., Tachibana H., Kobayashi Y. Angiotensin-converting enzyme and bradykinin gene polymorphisms and cough: a meta-analysis. World J. Cardiol. 2011;3:329–336. doi: 10.4330/wjc.v3.i10.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hippisley-Cox J., Young D., Coupland C., Channon K.M., Tan P.S., Harrison D.A., et al. Risk of severe COVID-19 disease with ACE inhibitors and angiotensin receptor blockers: cohort study including 8.3 million people. Heart. 2020;106:1503–1511. doi: 10.1136/heartjnl-2020-317393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gülsen A., König I.R., Jappe U., Drömann D. Effect of comorbid pulmonary disease on the severity of COVID-19: a systematic review and meta-analysis. Respirology. 2021;26:552–565. doi: 10.1111/resp.14049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sampaio W.O., Souza dos Santos R.A., Faria-Silva R., da Mata Machado L.T., Schiffrin E.L., Touyz R.M. Angiotensin-(1-7) through receptor mas mediates endothelial nitric oxide synthase activation via Akt-dependent pathways. Hypertension. 2007;49:185–192. doi: 10.1161/01.HYP.0000251865.35728.2f. [DOI] [PubMed] [Google Scholar]
- 29.Hammer A., Yang G., Friedrich J., Kovacs A., Lee D.H., Grave K., et al. Role of the receptor mas in macrophage-mediated inflammation in vivo. Proc. Natl. Acad. Sci. U. S. A. 2016;113:14109–14114. doi: 10.1073/pnas.1612668113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamamoto N., Ariumi Y., Nishida N., Yamamoto R., Bauer G., Gojobori T., et al. SARS-CoV-2 infections and COVID-19 mortalities strongly correlate with ACE1 I/D genotype. Gene. 2020;758 doi: 10.1016/j.gene.2020.144944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lippi G., Lavie C.J., Henry B.M., Sanchis-Gomar F. Do genetic polymorphisms in angiotensin converting enzyme 2 (ACE2) gene play a role in coronavirus disease 2019 (COVID-19)? Clin. Chem. Lab. Med. 2020;58:1415–1422. doi: 10.1515/cclm-2020-0727. [DOI] [PubMed] [Google Scholar]
- 32.Bose T., Pant N., Pinna N.K., Bhar S., Dutta A., Mande S.S. Does immune recognition of SARS-CoV2 epitopes vary between different ethnic groups? Virus Res. 2021;305 doi: 10.1016/j.virusres.2021.198579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dublin S., Walker R.L., Floyd J.S., Shortreed S.M., Fuller S., Albertson-Junkans L., et al. Renin-angiotensin-aldosterone system inhibitors and COVID-19 infection or hospitalization: a cohort study. Am. J. Hypertens. 2021;34:339–347. doi: 10.1093/ajh/hpaa168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takahashi T., Ellingson M.K., Wong P., Israelow B., Lucas C., Klein J., et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature. 2020;588:315–320. doi: 10.1038/s41586-020-2700-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen J., Jiang Q., Xia X., Liu K., Yu Z., Tao W., et al. Individual variation of the SARS-CoV-2 receptor ACE2 gene expression and regulation. Aging Cell. 2020;19 doi: 10.1111/acel.13168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gemmati D., Bramanti B., Serino M.L., Secchiero P., Zauli G., Tisato V. COVID-19 and individual genetic susceptibility/receptivity: role of ACE1/ACE2 genes, immunity, inflammation and coagulation. Might the double X-chromosome in females be protective against SARS-CoV-2 compared to the single X-chromosome in males? Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21103474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alexandre J., Cracowski J.L., Richard V., Bouhanick B. French Society of Pharmacology and Therapeutics (SFPT). Drugs acting on renin angiotensin system and use in ill patients with COVID-19. Therapie. 2020;75:319–325. doi: 10.1016/j.therap.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Bates C., Morton C.E., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li J., Wang X., Chen J., Zhang H., Deng A. Association of renin-angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for coronavirus disease 2019 (COVID-19) infection in Wuhan, China. JAMA Cardiol. 2020;5:825–830. doi: 10.1001/jamacardio.2020.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Docherty A.B., Harrison E.M., Green C.A., Hardwick H.E., Pius R., Norman L., et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study. BMJ. 2020;369 doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deng G., Yin M., Chen X., Zeng F. Clinical determinants for fatality of 44,672 patients with COVID-19. Crit. Care. 2020;24:179. doi: 10.1186/s13054-020-02902-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sorci G., Faivre B., Morand S. Explaining among-country variation in COVID-19 case fatality rate. Sci. Rep. 2020;10:18909. doi: 10.1038/s41598-020-75848-2. [DOI] [PMC free article] [PubMed] [Google Scholar]