Abstract
Introduction
Long-acting somatostatin analogues such as lanreotide autogel (LAN) and octreotide long-acting release (OCT) are recommended as first-line treatment for patients with neuroendocrine tumors (NETs). However, only few real-world studies have compared the two medications. This retrospective, observational cohort study used a French claims database to compare patterns of use with LAN vs. OCT in patients with NETs.
Methods
Data on LAN and OCT patterns of use were obtained retrospectively from the National System of Health Data (SNDS), a national French claims database. Patients 18 years of age or older who initiated treatment for NETs between 2009 and 2016, and who received at least six subsequent dispensings of first-line LAN or OCT during the first year of treatment, were included. A subgroup analysis was performed on patients with gastroenteropancreatic (GEP)-NETs.
Results
Patients receiving LAN (n = 2327) vs. OCT (n = 2090) had greater median treatment duration (31.8 months vs. 22.1 months, respectively; p < 0.0001; log-rank test) and were less likely to discontinue treatment; adjusted hazard ratio (HR) 0.74 (95% confidence interval [CI] 0.69–0.80). In year 1, a significantly lower percentage of patients receiving LAN vs. OCT switched treatments (10.4% vs. 22.2%, respectively; p < 0.0001), received an average monthly dose per trimester above recommended dose (3.0% vs. 7.3%, respectively; p < 0.0001), and used rescue medication (3.1% vs. 10.0%, respectively; p < 0.0001). Dispensing of pancreatic enzymes was significantly higher in patients receiving LAN than OCT (16.4% vs. 13.9%, respectively). In the subgroup of patients with GEP-NETs, those receiving LAN (n = 1478) vs. OCT (n = 1278) had greater treatment duration and less treatment discontinuation, switching, dosage above the recommended dose, and rescue medication use, but no significant difference in dispensing of pancreatic enzymes or time to second-line treatment.
Conclusion
These real-world data suggest potential clinical and economic advantages of LAN over OCT in the management of patients with NETs in the French population.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-022-02060-1.
Keywords: GEP-NETs, Lanreotide, Neuroendocrine tumors, Octreotide, Real-world, Somatostatin analogues, SNDS
Key Summary Points
| Why carry out this study? |
| Neuroendocrine tumors (NETs) are rare malignancies that can have a substantial negative impact on patients’ health and quality of life. |
| Lanreotide autogel (LAN) and octreotide long-acting release (OCT), both long-acting somatostatin analogues, are first-line treatments for patients with NETs; however, only few studies have compared the two treatments. |
| This retrospective cohort study using the National System of Health Data (SNDS), a national French claims database, aimed to investigate the potential clinical and economic differences between LAN and OCT by evaluating patterns of use of first-line LAN and OCT and use of supplementary and second-line therapy. |
| What was learned from the study? |
| Patients treated with LAN had greater treatment duration and were less likely to discontinue treatment, switch to a different treatment, take a higher-than-recommended dose, or use rescue medication, compared with patients treated with OCT, suggesting potential clinical and economic advantages of LAN over OCT. |
| As a result of limitations inherent to database analyses, these findings should be further confirmed in controlled studies. |
Digital Features
This article is published with digital features, including a graphical abstract to facilitate understanding of the article. To view digital features for this article go to 10.6084/m9.figshare.19169915.
Introduction
Neuroendocrine tumors (NETs) are a group of rare malignancies arising from cells of the endocrine system [1, 2]. Most NETs (61%) are gastroenteropancreatic (GEP)-NETs and originate in the gastrointestinal system (small bowel, stomach, rectum, appendix) or pancreas [3, 4]. In Europe, the annual age-adjusted incidence rate of NETs is estimated to be 25 per 1,000,000 people (excluding lung NETs) [4]; however, the incidence of NETs is increasing, likely due in part to improvements in diagnostic techniques and increased awareness [2].
NETs can be functional or non-functional. Functional NETs cause specific symptoms related to the overproduction of hormones by the tumor, such as diarrhea, abdominal pain/cramping, and flushing, due to serotonin overproduction as part of carcinoid syndrome. These symptoms can have a considerable negative impact on patients’ overall health, health-related quality of life, and work life [5–8]. Non-functional NETs are not associated with specific symptoms related to hormone hypersecretion [9].
Long-acting somatostatin analogues (LA SSAs), such as lanreotide autogel (LAN) and octreotide long-acting release (OCT), are typically prescribed to treat NETs [10–13] and are approved as first-line treatments in this setting, with short-acting SSAs used as rescue therapy [14]. SSAs control the hormone-related symptoms associated with functional NETs and inhibit tumor growth [13, 15]. The phase 3 CLARINET study demonstrated that LAN was associated with significantly prolonged progression-free survival (PFS) compared with placebo [15]. In Europe, LAN 120 mg is currently indicated for the treatment of adult patients with unresectable, locally advanced or metastatic grade 1 and grade 2 GEP-NETs, while LAN 60–120 mg is recommended for the treatment of symptoms associated with NETs [16]. Results from the randomized, phase 3B PROMID study showed that OCT significantly lengthened time to tumor progression in patients with functionally active and inactive metastatic midgut NETs compared with placebo [17]. OCT 20 mg is currently recommended as the starting dose to treat functional GEP-NETs, with the dosage reduced to 10 mg or increased to 30 mg depending on the level of symptom control attained [18]. OCT 30 mg is additionally indicated for the treatment of patients with advanced NETs of the midgut or of unknown primary origin where non-midgut sites of origin have been excluded [18].
Clinical trials provide valuable information on drug efficacy and safety; however, strict inclusion and exclusion criteria can lead to results not being generalizable to the entire population with the disease. They also often do not include comparative efficacy, safety, or cost-effectiveness information to guide decisions across therapeutic options. Real-world evidence (RWE) provides insights into the effectiveness, tolerability, and cost of marketed products when used in clinical practice, and therefore can be useful to inform clinical decision-making [19]. While LAN and OCT are common treatments for NETs, there is currently little RWE on how their use in practice and clinical outcomes compare. Previous commercial claims database analyses in the USA and Canada have reported differences in patterns of use between LAN and OCT, with less above-label dose and rescue medication use reported among patients receiving LAN than OCT [20, 21]. However, these studies did not investigate differences in treatment persistence or time to second-line treatment, which are also important factors in assessing the effectiveness of therapies. Furthermore, commercial claims databases have limited generalizability because they do not include uninsured patients, or those with insurance through a social security system.
We previously performed an analysis of the National System of Health Data (Système National des Données de Santé [SNDS]; https://www.snds.gouv.fr/SNDS/Accueil), a national French claims database that covers around 99% of the population [22], and found that higher LA SSA doses and more syringes were used with OCT than LAN for the treatment of acromegaly and NETs, translating into higher overall treatment costs for patients receiving OCT [23]. With this current database analysis, we aimed to further investigate patterns of first-line use of LAN compared with OCT among patients with NETs in the French real-world setting, and to explore potential clinical and economic differences between the two treatments. Outcomes including dispensing and administration patterns, as well as use of supplementary and second-line therapy, were evaluated.
Methods
Study Design
This was a retrospective cohort study based on administrative claims data from the SNDS, a comprehensive French claims database [22]. The SNDS contains information on reimbursed outpatient healthcare, such as consultations, medical procedures (Common Classification System of Medical Acts [CCAM] codes), and prescription dispensing (Product identifier [CIP] or Anatomic Therapeutic Chemical [ATC] codes), as well as hospital stays and vital status.
All patients with NETs, including a subgroup of patients with GEP-NETs, who received LAN or OCT for the first time between 1 January 2009 and 31 December 2016 were identified using diagnostic codes and followed up until the end of 2017. GEP-NETs subgroup analyses were performed to examine the same outcomes in a more homogeneous population. The medical history of patients was documented from 1 January 2008 onwards. Initiation of treatment with an LA SSA was defined by the absence of dispensing of this drug during the preceding year. All included patients were followed up until 31 December 2017 in order to have at least a 1-year follow-up for each patient. Outcomes were compared between patients who received first-line LAN and those who received first-line OCT during the study period.
Participants
All diagnosis and drug codes used to define the study population can be found in the Supplementary Material. Patients were eligible for inclusion in the study if they had a NET, indicated by relevant International Classification of Diseases, Tenth Revision (ICD-10), Homogenous Patient Groups (GHM), CCAM, and/or biological test codes, and initiated LAN or OCT treatment between 2009 and 2016, indicated by no LA SSA treatment (LA SSA treatment identified using CIP and ATC codes) in the prior 12 months. In addition, eligible patients were required to be at least 18 years of age at the time of the first dispensing and have received at least six subsequent dispensings of first-line LAN or OCT (Table S1) during the first year of treatment. Patients with either “definite” or “probable” NET diagnosis were included (definitions provided in Table 1). Patients with acromegaly or thyrotroph adenoma were excluded on the basis of the following specific criteria: existence of an exemption from co-payment (ETM) for a long-term health condition (ALD) for acromegaly (corresponding to ICD-10 codes E22.0 or E16.8) or thyrotroph adenoma with hyperthyroidism (corresponding to ICD-10 codes D35.2 and E05), and/or hospitalization, with the main reason associated with the ICD-10 codes E22.0 or E16.8 (acromegaly) or D35.2 and E05 with or without E22.1 (thyrotroph adenoma with hyperthyroidism) and/or dispensing of pegvisomant, the growth hormone receptor antagonist, with or without cabergoline (a prolactin inhibitor; acromegaly) or dispensing of cabergoline (thyrotroph adenoma). Non-specific criteria excluding patients with acromegaly or thyrotroph adenoma included existence of an ETM for an ALD for an ICD-10 coded pathology associated with codes D35, D35.2, E22 or E05, and/or hospitalization with the main reason associated with the ICD-10 codes D35.2 or E05.8, and/or surgical intervention to remove the pituitary adenoma and/or at least five tests of the same hormone, among tests likely to be performed during the follow-up of acromegaly or thyrotroph adenoma. Patients who received only LAN 30 mg dispensings were also excluded from the study, as LAN 30 mg is a bimonthly formulation generally intended for short-term use and is not a recommended dose for longer-term tumor control or control of secretions in patients with NETs or GEP-NETs [24].
Table 1.
Criteria used to identify a definite or probable NETs diagnosis
| Definite NETs diagnosis | Probable NETs diagnosis |
|---|---|
| Had full insurance coverage for a long-term disease; or | Did not meet the diagnosis criteria for acromegaly or thyrotroph adenoma, and either had full healthcare coverage for a long-term disease or one hospitalization within 6 months of LA SSA treatment initiation linked to a metastatic cancer ICD-10 diagnosis code (Table S3); or |
| One cause of hospitalization linked to a NETs International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10) diagnosis code (Table S2) | Either underwent a medical liver procedure or had at least one hospitalization for liver metastasis within 6 months of LA SSA initiation, and had at least five blood or urinary tests of the same hormone, among tests likely to be performed during the follow-up of NETs (urine test for 5-hydroxindoleacetic acid, serum chromogranin A, serum gastrin, vasoactive intestinal peptide, serum insulin, free serum insulin; Table S4) |
This article does not contain any new studies with human or animal subjects performed by any of the authors; as such, informed consent of individuals was not required. The data set came from the France-based SNDS claims database.
Study Outcomes
In this exploratory analysis, the following outcomes were evaluated in patients receiving first-line LAN or OCT using SNDS claims data: treatment persistence, time to second-line treatment (GEP-NETs subgroup only), LA SSA treatment switching, average monthly dose per trimester above the recommended dose (as determined by national and international guidelines) [10–12], use of rescue medication, and dispensing of pancreatic enzyme replacement therapy (PERT).
Treatment Persistence and Time to Second-Line Treatment
Treatment persistence was evaluated by comparing probability of discontinuation between patients receiving LAN or OCT. Time to second-line treatment, which has been used as a surrogate for PFS in previous studies [25–27], was assessed on the basis of the probability of initiating a second-line cancer treatment at any time after the start of LA SSA treatment. This analysis was limited to the GEP-NETs subgroup, with initiation of second-line treatment identified on the basis of the occurrence of relevant ICD-10, ATC, or CCAM codes (Table S5). Patients who received cancer treatment prior to LA SSA initiation were not included in this analysis.
Treatment Switching
A treatment switch was defined as the initiation of a second LA SSA within a maximum of 12 months after the discontinuation of the first LA SSA. Analysis of treatment switching was stratified by time since treatment initiation, with the duration between the date of first LA SSA initiation and the initiation of a second LA SSA categorized as either “within 3 months” or “between 3 and 12 months.” The stratification by time interval accounted for the possibility that the main reasons for treatment switching within 3 months and between 3 and 12 months could be different (e.g., the main reason for early switching may be related to medication tolerance, while later switches are likely due to insufficient control of secretions).
An additional subgroup analysis of treatment switching was performed in the functional and non-functional NETs subgroups, as failure of an LA SSA (indicated by a treatment switch) in the non-functional NETs subgroup may suggest lack of medication tolerance or antitumor effectiveness, while failure in the functional NETs subgroup may additionally imply a lack of antisecretory effectiveness.
Dosing Above the Recommended Dose
Average monthly dose was estimated using the number of monthly LA SSA dispensings and the dosage of the dispensed drugs. To account for the fact that the number of monthly dispensings does not always correspond exactly to the number of doses received per month, the average monthly dose was based on the number of dispensings made over 3 months; averaging over a 3-month period reduced the impact of month-to-month variation in the number of dispensings. Table S6 lists the recommended monthly doses of LAN and OCT, by pathology. The proportions of patients who received an average monthly dose per trimester above the recommended dose of LAN or OCT were evaluated in the first 3 years of follow-up.
Rescue Medication Use and Dispensing of PERT
Rescue medication was defined as the use of a short-acting SSA, identified by the occurrence of relevant CIP or ATC codes (Table S7). Dispensing of PERT, a medication for pancreatic exocrine insufficiency (PEI), which can be caused by SSAs, was determined on the basis of the relevant ATC code (A09AA).
Statistical Analysis
Mean and standard deviation are presented for continuous variables (age), while frequency, including numbers and percentages of patients, is presented for categorical variables (sex, type of NETs, NET localization).
To determine the differences in outcomes between patients receiving LAN vs. OCT, the following tests were used: the Student’s t test for continuous, normally distributed variables, the Mann–Whitney test for continuous variables that were not normally distributed, and the chi-squared test for categorical variables. Kaplan–Meier survival analysis and the log-rank test were used to evaluate and compare median treatment duration and time to second-line treatment. In a multivariate analysis, the probabilities of discontinuing treatment and receiving second-line treatment were assessed using a Cox proportional hazards model, which provided hazard ratios (HRs) adjusted for age at LA SSA initiation, gender, presence of at least one comorbidity, and year of treatment initiation, and presence of metastatic disease at LA SSA initiation. For the GEP-NETs subgroup, HRs were also adjusted for GEP-NETs localizations. All tests were exploratory, two-tailed, and conducted at the α = 0.05 significance level, with all analyses performed using SAS® v9.4 software (Cary, NC, USA).
Results
Patient Disposition and Baseline Characteristics
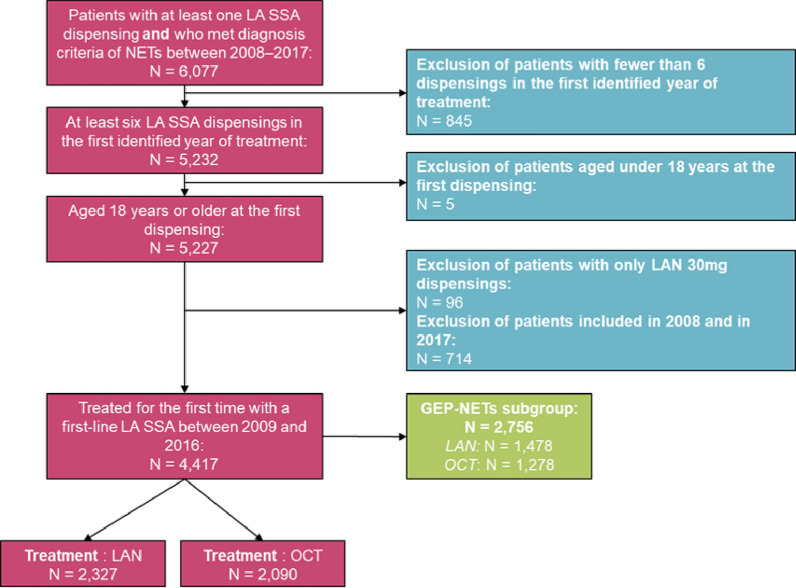
A total of 6077 patients in the SNDS database met the diagnostic criteria for NETs and had at least one LA SSA dispensing from the start of 2009 through the end of 2016. After the remaining eligibility criteria were applied, the final study population comprised 4417 patients with NETs, of whom 2327 (52.7%) initiated treatment with LAN and 2090 (47.3%) initiated treatment with OCT (Fig. 1). Within this population, 1546 (35.0%) had a definite NET diagnosis, and 2871 (65.0%) had a probable NET. In total, 2756 patients fit the criteria for inclusion in the GEP-NET subgroup, of which 1478 (53.6%) and 1278 (46.4%) initiated treatment with LAN and OCT, respectively (Fig. 1).
Fig. 1.
Patient disposition flowchart. LA SSA long-acting somatostatin analogue, LAN lanreotide autogel, NETs neuroendocrine tumors, OCT octreotide long-acting release
In the overall NETs population, age and sex were balanced between the LAN and OCT groups, and most patients in both groups had non-functional NETs (LAN 1434 [61.6%], OCT 1437 [68.8%]; Table 2). At LA SSA initiation, the proportion of metastatic NETs was slightly lower in the LAN group compared with the OCT group (75% vs. 78%, respectively; p = 0.007 [Table S8]). GEP-NETs were the most common NET localizations in both the LAN (1478 [63.5%]) and OCT (1278 [61.2%]) groups (Table 2). Within the GEP-NETs subgroup, the pancreas was the most common localization in both groups (LAN 625 [42.3%], OCT 485 [38.0%]; Table S9). In the overall population, median (quartile 1 [Q1]–Q3) follow-up time was relatively consistent between the LAN and OCT groups (2.8 [1.6–4.3] years and 3.0 [1.6–4.8] years, respectively). Follow-up duration in the GEP-NETs subgroup was 2.8 (1.7–4.4) years for the LAN group and 3.2 (1.7–5.0) years for the OCT group (Table 2).
Table 2.
Baseline patient characteristics
| Patient characteristics (N = 4417) | LAN (n = 2327) | OCT (n = 2090) | p value |
|---|---|---|---|
| Population, n (%) | 2327 (52.7) | 2090 (47.3) | |
| Male, n (%) | 1228 (52.8) | 1072 (51.3) | 0.3256a |
| Age, years, mean (SD) | 64.8 (12.2) | 64.4 (12.6) | 0.2722b |
| Type of NETs, n (%) | |||
| NETs with carcinoid syndrome | 299 (12.8) | 221 (10.6) | 0.0217a |
| Other functional NETS | 58 (2.5) | 51 (2.4) | 0.9983a |
| Non-functional NETs | 1434 (61.6) | 1437 (68.8) | < 0.0001a |
| NETs unclassifiable as functional or not | 536 (23.0) | 381 (18.2) | < 0.0001a |
| NET localization, n (%) | |||
| GEP-NET | 1478 (63.5) | 1278 (61.2) | 0.1118a |
| Lung | 107 (4.6) | 115 (5.5) | 0.1921a |
| Ovary/uterus | 59 (2.5) | 116 (5.6) | < 0.0001a |
| Other | 99 (4.3) | 105 (5.0) | 0.2523a |
| Multiple | 3 (0.1) | 2 (0.1) | 0.9999a |
| Unknown | 581 (25.0) | 474 (22.7) | 0.0809a |
| Follow-up time, years, median (Q1–Q3) | |||
| Overall population | 2.8 (1.6−4.3) | 3.0 (1.6−4.8) | – |
| GEP-NETs subgroup | 2.8 (1.7−4.4) | 3.2 (1.7−5.0) | – |
The localization of tumors causing a carcinoid syndrome (E340) or a metastatic disease (C77-C79) was established, if applicable, from the ICD-10 associated diagnostic code of carcinoid syndrome or metastatic tumor during the same hospital stay. The tumors searched were malignant primary neoplasms (C00-C75) and for carcinoid syndromes, in situ and benign neoplasms (D10-D36) were also searched. The following ICD-10 codes were not considered possible localizations of NETs: D18, C81 to C96, D33, C69 to C72, C46, C43, D03
GEP-NETs gastroenteropancreatic neuroendocrine tumors, ICD-10 International Classification of Diseases, Tenth Revision, LAN lanreotide autogel, NETs neuroendocrine tumors, OCT octreotide long-acting release
aChi-squared test
bStudent’s t test
cNo other localization of digestive NETs identified
Patient characteristics were additionally compared in patients with probable vs. definite NETs, to explore the rates of different types of tumors within those populations (Table 3). In 31.5% of probable NET cases, the primitive tumor (and therefore its localization) could not be identified in the database, as compared to 9.8% of cases for definite NETs diagnoses (Table 3). As a result of the differences in location and extension between definite and probable NETs, outcomes other than baseline characteristics were not compared between these groups.
Table 3.
Baseline patient characteristics according to the type of diagnostic criteria used (definite or probable)
| Patient characteristics (N = 4417) | Definite NETs (n = 1546) | Probable NETs (n = 2871) | p value |
|---|---|---|---|
| Population, n (%) | 1546 (35.0) | 2871 (65.0) | |
| Male, n (%) | 817 (52.9) | 1483 (51.7) | 0.4677a |
| Age, years, mean (SD) | 62.2 (13.1) | 66.0 (11.8) | < 0.001b |
| Type of NETs, n (%) | |||
| NETs with carcinoid syndrome | 520 (33.6) | 0c | |
| Other functional NETs | 109 (7.1) | 0c | |
| Non-functional NETs | 0 | 2871 (100.0) | |
| NETs unclassifiable as functional or not | 917 (59.3) | 0c | |
| NET localization, n (%) | |||
| GEP-NET | 1295 (83.8) | 1461 (50.9) | < 0.0001a |
| Lung | 56 (3.6) | 166 (5.8) | 0.0022a |
| Ovary/uterus | 0 (0.0) | 175 (6.1) | |
| Other | 44 (2.9) | 160 (5.6) | < 0.0001a |
| Multiple | 0 (0.0) | 5 (0.2) | |
| Unknown | 151 (9.8) | 904 (31.5) | < 0.0001a |
GEP-NET gastroenteropancreatic neuroendocrine tumor, NET neuroendocrine tumor
aChi-squared test
bStudent’s t test
cThe group of probable NETs did not include carcinoid tumors or other functional tumors because these tumors had either specific ICD-10 codes or identifiable biological assays in the database that have allowed definite identification of these tumors
Treatment Persistence
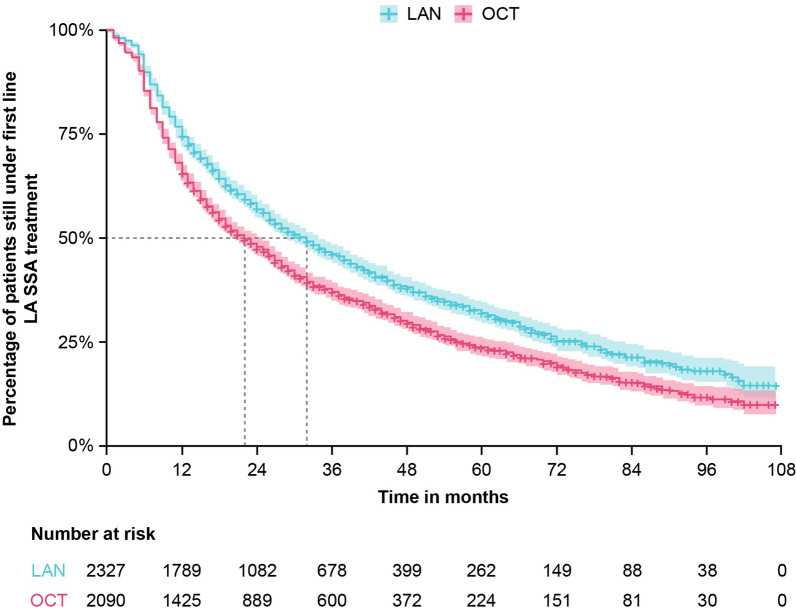
In the overall population, median treatment duration estimated from a Kaplan–Meier survival model was significantly longer for patients in the LAN group compared with patients in the OCT group (LAN 31.8 months [95% CI 29.1–34.0] vs. OCT 22.1 months [95% CI 20.1–24.5] months; log-rank p value < 0.0001; Fig. 2). After adjustment for covariates (age at LA SSA initiation, gender, presence of at least one comorbidity, year of treatment initiation, tumor localization, presence of metastasis at treatment initiation), patients in the LAN group were significantly less likely to discontinue treatment than patients in the OCT group (adjusted HR 0.74 [95% CI 0.69–0.80]; log-rank p value < 0.0001). Analysis of the GEP-NETs subgroup demonstrated a significantly longer median treatment duration for patients in the LAN group compared with patients in the OCT group (LAN 32.8 [95% CI 28.9–36.9] vs. OCT 23.1 [95% CI 20.1–25.9] months; log-rank p value < 0.0001; Fig. S1). On multivariate analysis, patients with GEP-NETs in the LAN group were also significantly less likely to discontinue treatment than patients in the OCT group (adjusted HR 0.7 [95% CI 0.7–0.8]; log-rank p value < 0.0001).
Fig. 2.
Treatment persistence of first-line LA SSA treatments in patients with NETs. Median (95% CI) treatment duration for LAN was 31.8 (29.1–34.0) months and for OCT it was 22.1 (20.1–24.5) months; p < 0.0001. Treatment persistence was estimated using Kaplan–Meier survival analysis; p value was calculated using the log-rank test. LAN lanreotide autogel, LA SSA long-acting somatostatin analogue, OCT octreotide long-acting release
Time to Second-Line Treatment (GEP-NETs Subgroup Only)
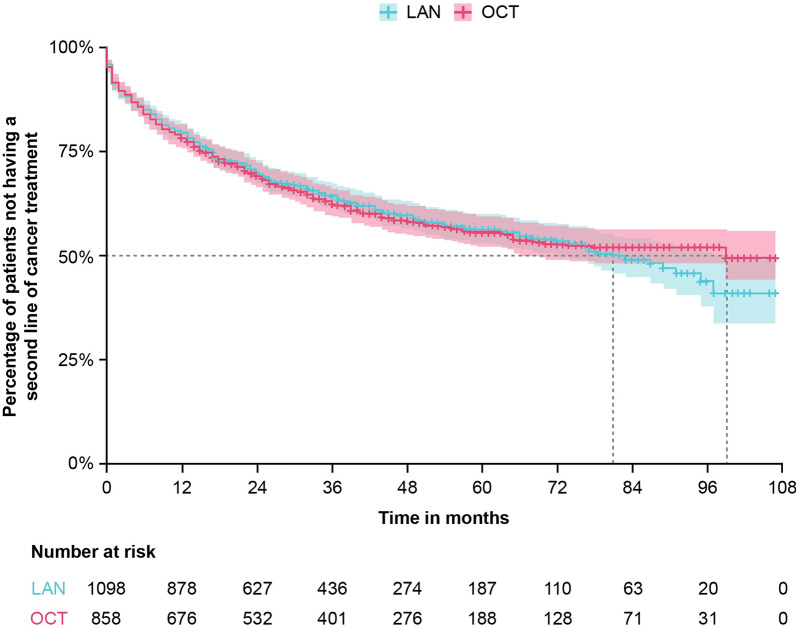
In the GEP-NETs subgroup, there was no significant difference in time to second-line treatment, estimated from a Kaplan–Meier survival model, for patients in the LAN group compared with patients in the OCT group (LAN 80.9 months [95% CI 69.9–97.0] vs. OCT 98.7 months [95% CI 65.8–NA]; log-rank p value = 0.97; Fig. 3). The results remained non-significant even after adjusting for covariates (adjusted HR 1.0 [95% CI 0.9–1.2]). This analysis was not performed in the overall study population.
Fig. 3.
Time to second-line treatment in patients with GEP-NETs. Median time until second-line treatment (95% CI) for LAN was 80.9 (69.9–97.0) months and for OCT was 98.7 (65.8–NA) months; p = 0.97. In patients treated with OCT, the upper bound of the confidence interval cannot be assessed because this median time is close to the maximum duration of patients’ follow-up. Treatment persistence was estimated using Kaplan–Meier survival analysis; p value was calculated using the log-rank test. GEP-NETs gastroenteropancreatic neuroendocrine tumors, LAN lanreotide autogel, SSA somatostatin analogue, OCT octreotide long-acting release
Treatment Switching
Throughout the total duration of treatment, a significantly lower percentage of patients in the LAN cohort vs. the OCT cohort switched to another LA SSA (LAN 11.6% vs. OCT 24.8%; p < 0.0001). Within the first 3 months of treatment, patients in the LAN cohort switched to another LA SSA at a significantly lower rate than patients in the OCT group (LAN 6.5% vs. OCT 11.6%; p < 0.0001). Between 3 and 12 months after treatment initiation, patients in the LAN group continued to switch to another LA SSA at a significantly lower rate (LAN 1.4% vs. OCT 2.8%; p = 0.0015; Table 4). In the GEP-NETs subgroup as well as the functional and non-functional NETs subgroups, a lower percentage of patients receiving LAN vs. OCT switched to another LA SSA within the first 3 months of treatment and between 3 and 12 months of treatment (Table 4).
Table 4.
Treatment switching in patients using LAN vs. OCT
| LAN (n = 2327) | OCT (n = 2090) | p value* | |
|---|---|---|---|
| All NETs (N = 4417) | n = 2327 | n = 2090 | |
| Switch to another LA SSA ≤ 3 months after initiation of 1st LA SSA, n (%) | 152 (6.5) | 242 (11.6) | < 0.0001 |
| Switch to another LA SSA between 3 and 12 months after initiation of 1st LA SSA, n (%) | 32 (1.4) | 58 (2.8) | 0.0015 |
| GEP-NETs (N = 2756) | n = 1478 | n = 1278 | |
| Switch to another LA SSA ≤ 3 months after initiation of 1st LA SSA, n (%) | 99 (6.7) | 165 (12.9) | < 0.0001 |
| Switch to another LA SSA between 3 and 12 months after initiation of 1st LA SSA, n (%) | 17 (1.2) | 37 (2.9) | 0.0016 |
| Functioning NETs (N = 629) | n = 357 | n = 272 | |
| Switch to another LA SSA ≤ 3 months after initiation of 1st LA SSA, n (%) | 29 (8.1) | 41 (15.1) | 0.0088 |
| Switch to another LA SSA between 3 and 12 months after initiation of 1st LA SSA, n (%) | 2 (0.5) | 13 (4.8) | 0.0015 |
| Non-functioning NETs (N = 2871) | n = 1434 | n = 1437 | |
| Switch to another LA SSA ≤ 3 months after initiation of 1st LA SSA, n (%) | 100 (7.0) | 150 (10.4) | 0.0013 |
| Switch to another LA SSA between 3 and 12 months after initiation of 1st LA SSA, n (%) | 20 (1.4) | 31 (2.2) | 0.1599 |
GEP-NETs gastroenteropancreatic neuroendocrine tumors, LAN lanreotide autogel, LA SSA long-acting somatostatin analogue, NETs neuroendocrine tumors, OCT octreotide long-acting release
*p values calculated using the chi-squared test
Average Monthly Dose per Trimester Above the Recommended Dose During the First 3 Years of Follow-Up
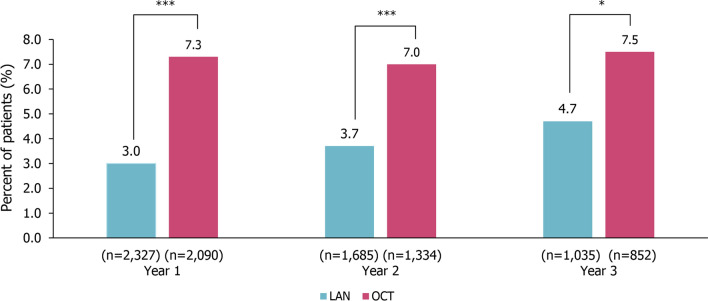
In year 1 of LA SSA treatment, significantly fewer patients in the LAN group compared with the OCT group had an average monthly dose per trimester above the recommended dose (LAN 3.0% vs. OCT 7.3%; p < 0.0001). The proportion of patients receiving an average monthly dose above the recommended dose remained significantly lower in patients receiving LAN vs. OCT in year 2 (LAN 3.7% vs. OCT 7.0%; p < 0.0001) and year 3 (LAN 4.7% vs. OCT 7.5%; p = 0.0149; Fig. 4). In the GEP-NETs subgroup, significantly fewer patients in the LAN group compared with the OCT group received an average monthly dose per trimester above the recommended dose in year 1 (LAN 2.9% vs. OCT 6.7%; p < 0.0001) and year 2 (LAN 3.7% vs. OCT 7.3%; p = 0.0005) but the difference was no longer significant in year 3 (LAN 5.7% vs. OCT 6.9%; p = 0.4697; Fig. S2).
Fig. 4.
Average monthly dose per trimester above the recommended dose in patients with NETs. ***p < 0.0001, *p < 0.05; p values calculated using the chi-squared test. LAN Lanreotide autogel, NETs neuroendocrine tumors, OCT octreotide long-acting release
Use of Rescue Medication
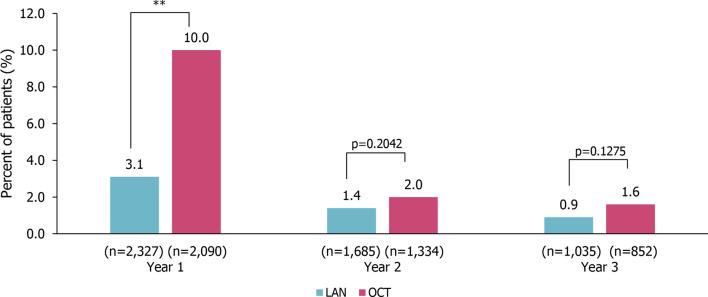
In year 1, patients in the LAN group had significantly lower use of rescue medication (short-acting SSA) compared with patients in the OCT cohort (LAN 3.1% vs. OCT 10.0%; p < 0.0001). This difference was no longer significant in year 2 (LAN 1.4% vs. OCT 2.0%; p = 0.2042) or year 3 (LAN 0.9% vs. OCT 1.6%; p = 0.1275) after treatment initiation (Fig. 5). In the GEP-NETs subgroup, patients in the LAN group compared with the OCT group had significantly lower use of rescue medication in year 1 (LAN 2.7% vs. OCT 9.5%; p < 0.0001), with no significant difference observed in year 2 (LAN 1.2% vs. OCT 2.0%; p = 0.1625), and year 3 (LAN 0.8% vs. OCT 1.9%; p = 0.0843; Fig. S3).
Fig. 5.
Use of rescue medication in the 3 years after treatment initiation in patients with NETs. Rescue medication was defined as a short-acting SSA. **p < 0.001; calculated using the chi-squared test. LAN lanreotide autogel, OCT octreotide long-acting release, SSA somatostatin analogue
Dispensing of Pancreatic Enzyme Replacement Therapy (PERT)
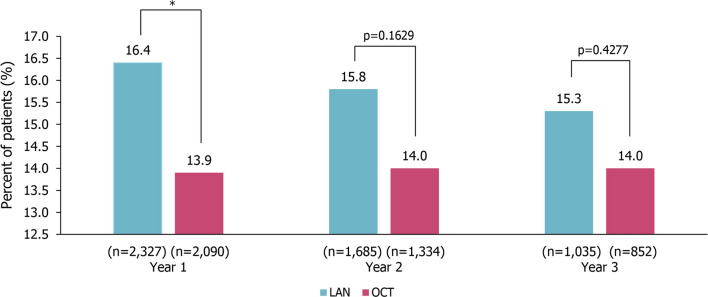
In the first year of treatment, dispensing of PERT was significantly higher in the LAN group than the OCT group (LAN 16.4% vs. OCT 13.9%; p = 0.0189). This difference was no longer significant in year 2 (LAN 15.8% vs. OCT 14.0%; p = 0.1629) or year 3 (LAN 15.3% vs. OCT 14.0%; p = 0.4277) after treatment initiation (Fig. 6). Conversely, in the GEP-NETs subgroup, there was no significant difference in the dispensing of PERT between patients in the LAN and OCT groups in year 1 (LAN 20.3% vs. OCT 17.9%; p = 0.1138), year 2 (LAN 18.9% vs. OCT 19.0%; p = 0.9834), or year 3 (LAN 18.0% vs. OCT 18.2%; p = 0.9327; Fig. S4).
Fig. 6.
Dispensing of PERT in the 3 years after treatment initiation in patients with NETs. *p < 0.05; calculated using the chi-squared test. LAN lanreotide autogel, NETs neuroendocrine tumors, OCT octreotide long-acting release, PERT pancreatic enzyme replacement therapy
Discussion
The objective of this database analysis was to explore patterns of use of first-line LAN compared with OCT through outcomes including dispensing and administration patterns, as well as use of supplementary or second-line therapy, among patients with NETs in the French real-world setting. Analyses were also performed in the subgroup of patients with GEP-NETs, which represents a population with less heterogeneity. Overall, patients treated with LAN had greater treatment persistence than patients receiving OCT, and a lower proportion switched to another LA SSA. In addition, significantly fewer patients treated with LAN had an average monthly dose per trimester above the recommended dose (during years 1–3 of treatment) or used rescue medication (during year 1 of treatment only) than those receiving OCT. However, dispensing of PERT was higher in the LAN group than the OCT group in the first year of treatment. The GEP-NETs subgroup analysis demonstrated greater treatment persistence, lower rates of treatment switching and patients with an average monthly dose per trimester above the recommended dose, and lower use of rescue medication in patients treated with LAN vs. OCT. Despite the consistency of these results (especially during the first year of treatment), and their potential economic impact, statistically significant differences between the LAN and OCT groups may be attributable to the large sample size. Whether these differences translate to clinically meaningful differences relevant to patients or physicians could not be determined from the data collected from the SNDS database.
Real-world studies that compare LAN and OCT treatment patterns in NETs are limited, particularly in the French population. An analysis of a US claims database (Symphony Health Solutions) that assessed median time to treatment discontinuation among 1086 patients with NETs did not find a statistically significant difference between the LAN and OCT groups (LAN 17.5 vs. OCT 19.2 months, p = 0.6) [28]. These results are not consistent with our finding that patients receiving LAN were significantly less likely to discontinue treatment than patients in the OCT group. However, in contrast to the previous study, we adjusted for potential confounders in a multivariate model, providing additional confidence in our results. In addition, in the USA LAN was approved for the treatment of GEP-NETs at the end of 2014 [29], and for the treatment of carcinoid syndrome in 2017 [30], while LAN was authorized for NETs symptom control in France in 2001 [31–33]. OCT, meanwhile, has been approved for NETs symptom control in the USA since 1988, while it was approved in France in 2004 [34–37]. The differing time frames for the approval of LAN and OCT treatment for NETs in France vs. the USA may help explain discrepancies between studies.
Notably, we did not find differences in the time to second-line treatment between patients receiving LAN and OCT in the GEP-NETs subgroup. Time to second-line treatment was used as a surrogate for PFS in this analysis, as PFS was not measurable because of the unavailability of imaging data necessary to apply RECIST criteria. Previous publications have estimated PFS using time to second-line treatment [25, 26]. Our findings indicate that there may not be a difference in time to disease progression in patients treated with LAN vs. OCT. However, as this analysis was limited to patients in the GEP-NETs subgroup, future studies would be needed to confirm this finding in patients with other types of NETs.
Patients with NETs, as well as those in the GEP-NETs subgroup, who received first-line OCT switched to another LA SSA at a significantly higher rate than patients who received first-line LAN, both within the first 3 months and between 3 and 12 months after treatment initiation. These results are consistent with a US claims analysis of 548 patients with metastatic GEP-NETs using the Pharmaceutical Research Associates Health Sciences US Outpatient Medical and Prescription Claims database. The results of this analysis showed that among patients who discontinued their index SSA during the follow-up period, the most common treatment among patients who discontinued OCT was LAN [20]. These findings suggest that LAN might have better effectiveness and tolerability and a better benefit–risk balance than OCT, although clinical and safety outcomes could not be directly measured in this analysis and would need to be confirmed in future studies.
Use of above-label dosage and rescue medication was found to be lower in patients receiving LAN compared with OCT. Our findings are consistent with the US claims analysis of patients with metastatic GEP-NETs, which reported that significantly fewer patients treated with LAN received a dose above the recommended dose than those treated with OCT (LAN 1.1% vs. OCT 12.7%; p < 0.01) [20]. A Canadian claims database analysis also used the IQVIA Private Drug Plan, Ontario Drug Benefit program, and Régie de l’assurance-maladie du Québec to evaluate rescue medication use in 908 patients treated with LAN or OCT [21]. Similar to our results, this study reported lower mean use of rescue medication in the LAN group compared with the OCT group; this difference, however, was greatest during the first months (mean difference, 0.2 claims/patient/year; p < 0.0001), likely due to variations in the pharmacokinetics of each product, after which the difference was only significant 5 and 6 months after treatment initiation [21]. Although we did not assess the use of rescue medication by month, this result supports our finding of significantly lower rescue medication use in the LAN cohort than in the OCT group in the first year after treatment initiation. OCT may be associated with greater use of healthcare resources than LAN, particularly in the first year of treatment. However, more evidence is needed to identify the factors driving differences in above-label use and rescue medication.
While our study did not directly assess economic outcomes, our previous SNDS analysis showed higher LA SSA doses and more syringe use with OCT than LAN, translating into an average additional treatment cost of €1304 per year for patients receiving OCT [23]. The Canadian claims analysis also reported lower total annual treatment costs for LAN than OCT ($27,829.35/patient [n = 373] vs. $31,255.49/patient [n = 530], respectively, p < 0.0001) [21]. These findings, and the lower use of above-label doses and rescue medication with LAN compared with OCT in the current study, suggest potential economic advantages of LAN over OCT.
Among all patients with NETs, we found greater dispensing of PERT in the first year of treatment in the LAN group than in the OCT group. Exocrine pancreatic insufficiency is a common adverse event of LA SSAs [38], and PERT can be administered to alleviate this [39]. Therefore, our results indicate that LAN may have a greater effect on pancreatic secretions than OCT, and exocrine pancreatic insufficiency may be more common in the LAN group than the OCT group in the first year of treatment. However, no differences between treatment groups were observed in years 2 or 3 of treatment, suggesting that differences in pancreatic function between patients receiving LAN or OCT may resolve after the first year of treatment. This non-significant result may also be explained by a lack of statistical power in years 2 and 3. For all 3 years, patients in the GEP-NETs subgroup received PERT at higher rates than patients in the overall study population for both LAN and OCT. Several possible explanations may account for this finding. Patients with GEP-NETs are often managed by gastroenterologists, who are generally more aware of PEI and therefore may be more likely to prescribe PERT; patients with resected pancreatic NETs are at an increased risk for PEI; any patients with previous digestive surgery/resection are at a high risk for diarrhea, which may be overdiagnosed as PEI, with those patients more likely to receive PERT.
This study analyzed a large, nationally representative database of patients with NETs; in contrast, previous real-world studies of LAN and OCT have used commercial or Medicare claims databases, which only capture a subset of a population. Because patients were followed longitudinally, we were also able to assess changes in outcomes over time. However, the results of this study should be interpreted in light of limitations inherent to claims database analyses. As the number of ICD-10 diagnostic codes available for accurate identification of NETs (mainly non-functional tumors or non-GEP-NETs) is very limited, cases of probable NETs were inferred on the basis of codes for metastatic cancer, liver procedures or metastases, and hormone level tests. It is possible that some patients with NETs were missed or incorrectly captured. Furthermore, it is likely that the clinical differences observed between NETs of definite and probable diagnosis are explained by the criteria used to identify these NETs in the database. Carcinoid tumors and functional tumors could be identified by ICD-10 codes and/or specific biological assays. By definition, these tumors are of definite diagnosis. Other NETs that could be identified in a probable and indirect manner were by definition metastatic malignancies identified within 6 months before or after initiation of long-term LA SSA. As the SNDS does not contain clinical data, stages and/or grades of NETs could not be compared between the two treatment groups, and data on the resectable/unresectable nature of NETs was not directly available in the database. Identification of all surgical procedures performed for each patient could not be determined as a result of multiple possible tumor locations. However, the metastatic stages of NETs can be identified from the diagnostic codes of hospital stays. This analysis was able to determine that the overall proportion of metastatic NETs was slightly lower for LAN vs. OCT (Table S8). The exposure and study outcomes were identified through administrative claims data; therefore, data quality issues such as missing data or coding errors were possible. The SNDS database does not include clinical information, such as laboratory test results, or information on patient risk factors (e.g., blood pressure, family history, dietary habits, tobacco use, alcohol use, body mass index), which limited the analyses that could be performed in this study. Further, the SNDS only contains information on healthcare reimbursement. Therefore, consumption of medical products that were prescribed, but not reimbursed, were not captured. It was also impossible to identify prescriptions that were prescribed by doctors, but not consumed by patients. While the results of this exploratory analysis suggest potential clinical and economic advantages of LAN over OCT in the management of NETs, specific controlled studies are needed to identify the factors driving differences between these two LA SSAs.
Conclusions
The results of this observational, claims-based cohort study suggest potential clinical and economic advantages of LAN over OCT in the management of NETs in the French population, adding to the body of RWE that has identified differences between these two drugs. These findings are subject to limitations inherent to database analyses and should be further explored in specific, controlled studies.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This study was sponsored by Ipsen, Boulogne-Billancourt, France. The study sponsor is also funding the journal’s Rapid Service and Open Access Fees.
Medical Writing and Editorial Assistance
Medical writing and editorial support were provided by Isabel Haber, BS, and Jenna Hebert, PhD, of Costello Medical, Boston, MA, USA, funded by Ipsen in accordance with Good Publication Practice guidelines (http://www.ismpp.org/gpp3).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Substantial contributions to study conception and design: Brooke Harrow, Francis Fagnani, Camille Nevoret, Xuan Mai Truong Thanh, Marie de Zélicourt, Louis de Mestier; substantial contributions to analysis and interpretation of the data: Brooke Harrow, Francis Fagnani, Camille Nevoret, Xuan Mai Truong Thanh, Marie de Zélicourt, Louis de Mestier; drafting the article or revising it critically for important intellectual content: Brooke Harrow, Francis Fagnani, Camille Nevoret, Xuan Mai Truong Thanh, Marie de Zélicourt, Louis de Mestier; final approval of the version of the article to be published: Brooke Harrow, Francis Fagnani, Camille Nevoret, Xuan Mai Truong Thanh, Marie de Zélicourt, Louis de Mestier.
Disclosures
Brooke Harrow is a former employee of Ipsen, and a current employee of Deciphera Pharmaceuticals; Francis Fagnani is an employee of CEMKA; Camille Nevoret is an employee of CEMKA; Xuan Mai Truong Thanh is an employee of Ipsen; Marie de Zélicourt is an employee of CEMKA; Louis de Mestier has received consulting fees from AAA, Ipsen, Keocyt, Novartis, Pfizer, SIRTex.
Compliance with Ethics Guidelines
This article does not contain any new studies with human or animal subjects performed by any of the authors; as such, informed consent of individuals was not required. The data set came from the France-based Système National des Données de Santé claims database. The formal authorization of access to the database was signed with CNAM (Caisse Nationale d’Assurance Maladie) on June 26, 2019 under the agreement N°TPS 301455.
Data Availability
Owing to the nature of this claims database analysis, Ipsen does not have access to individual participant data. The data sets analysed in this study are available through the National System of Health Data and the authors cannot share the data with any third parties or make the data publicly available owing to protections around the sharing of personal health data (General Data Protection Regulation, GDPR).
Footnotes
The original online version of this article was revised due to update of open access grants retrospectively.
Change history
3/26/2022
A Correction to this paper has been published: 10.1007/s12325-022-02127-z
Change history
10/31/2022
A Correction to this paper has been published: 10.1007/s12325-022-02335-7
References
- 1.Shah MH, Goldner WS, Benson AB, III, et al. Neuroendocrine and adrenal tumors, Version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19(7):839–868. doi: 10.6004/jnccn.2021.0032. [DOI] [PubMed] [Google Scholar]
- 2.Dasari A, Shen C, Halperin D, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3(10):1335–1342. doi: 10.1001/jamaoncol.2017.0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frilling A, Akerström G, Falconi M, et al. Neuroendocrine tumor disease: an evolving landscape. Endocr Relat Cancer. 2012;19(5):R163–R185. doi: 10.1530/ERC-12-0024. [DOI] [PubMed] [Google Scholar]
- 4.van der Zwan JM, Trama A, Otter R, et al. Rare neuroendocrine tumours: results of the surveillance of rare cancers in Europe project. Eur J Cancer. 2013;49(11):2565–2578. doi: 10.1016/j.ejca.2013.02.029. [DOI] [PubMed] [Google Scholar]
- 5.Beaumont JL, Cella D, Phan AT, et al. Comparison of health-related quality of life in patients with neuroendocrine tumors with quality of life in the general US population. Pancreas. 2012;41(3):461–466. doi: 10.1097/MPA.0b013e3182328045. [DOI] [PubMed] [Google Scholar]
- 6.Leyden JA-O, Pavlakis N, Chan D, et al. Patient-reported experience of the impact and burden of neuroendocrine tumors: oceania patient results from a large global survey. Asia Pac J Clin Oncol. 2018;14(3):256–263. doi: 10.1111/ajco.12785. [DOI] [PubMed] [Google Scholar]
- 7.Singh S, Granberg D, Wolin E, et al. Patient-reported burden of a neuroendocrine tumor (NET) diagnosis: results from the first global survey of patients with NETs. J Glob Oncol. 2017;3(1):43–53. doi: 10.1200/JGO.2015.002980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolin EM, Leyden J, Goldstein G, et al. Patient-reported experience of diagnosis, management, and burden of neuroendocrine tumors: results from a large patient survey in the United States. Pancreas. 2017;46(5):639–647. doi: 10.1097/MPA.0000000000000818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fazio N, de Braud F, Delle Fave G, Oberg K. Interferon-α and somatostatin analog in patients with gastroenteropancreatic neuroendocrine carcinoma: single agent or combination? Ann Oncol. 2007;18:13–19. doi: 10.1093/annonc/mdl144. [DOI] [PubMed] [Google Scholar]
- 10.Pavel M, O'Toole D, Costa F, et al. ENETS consensus guidelines update for the management of distant metastatic disease of intestinal, pancreatic, bronchial neuroendocrine neoplasms (NEN) and NEN of unknown primary site. Neuroendocrinology. 2016;103(2):172–185. doi: 10.1159/000443167. [DOI] [PubMed] [Google Scholar]
- 11.de Mestier L, Lepage C, Baudin E, et al. Digestive Neuroendocrine Neoplasms (NEN): French Intergroup clinical practice guidelines for diagnosis, treatment and follow-up (SNFGE, GTE, RENATEN, TENPATH, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, SFR) Dig Liver Dis. 2020;52(5):473–492. doi: 10.1016/j.dld.2020.02.011. [DOI] [PubMed] [Google Scholar]
- 12.Pavel M, Öberg K, Falconi M, et al. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31(7):844–860. doi: 10.1016/j.annonc.2020.03.304. [DOI] [PubMed] [Google Scholar]
- 13.Hofland J, Feelders RA, Brabander T, et al. Recent developments in the diagnosis and therapy of well-differentiated neuroendocrine tumours. Neth J Med. 2018;76(3):100–108. [PubMed]
- 14.Vinik AI, Wolin EM, Liyanage N, et al. Evaluation of lanreotide depot/autogel efficacy and safety as a carcinoid syndrome treatment (elect): a randomized, double-blind, Placebo-Controlled Trial. Endocr Pract. 2016;22(9):1068–1080. doi: 10.4158/EP151172.OR. [DOI] [PubMed] [Google Scholar]
- 15.Caplin ME, Pavel M, Ćwikła JB, et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371(3):224–233. doi: 10.1056/NEJMoa1316158. [DOI] [PubMed] [Google Scholar]
- 16.emc. Somatuline Autogel 60 mg, 90 mg, 120 mg solution for injection in a prefilled syringe. https://www.medicines.org.uk/emc/product/4808/smpc. Accessed 15 Mar 2021.
- 17.Rinke A, Müller H-H, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27(28):4656–4663. doi: 10.1200/JCO.2009.22.8510. [DOI] [PubMed] [Google Scholar]
- 18.emc. Sandostatin LAR 10 mg, 20 mg or 30 mg powder and solvent for suspension for injection. https://www.medicines.org.uk/emc/product/1038/smpc#gref. Accessed 15 Mar 2021
- 19.ISPOR. 2020 Top 10 HEOR TRENDS. https://www.ispor.org/docs/default-source/heor-resources/2020-top-10-heor-trends_v-online_00120191219.pdf?sfvrsn=9eebcb74_0. Accessed 19 Mar 2021.
- 20.Klink A, Feinberg B, Yu H-T, et al. Patterns of care among real-world patients with metastatic neuroendocrine tumors. Oncologist. 2019;24:1331–1339. doi: 10.1634/theoncologist.2018-0798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheung WY, Feuilly M, Laforty C, et al. A real-world observational study of somatostatin analogue use and costs in Canada. J Clin Oncol. 2020;38(4_suppl):608. doi: 10.1200/JCO.2020.38.4_suppl.608. [DOI] [Google Scholar]
- 22.Scailteux L-M, Droitcourt C, Balusson F, et al. French administrative health care database (SNDS): the value of its enrichment. Therapies. 2019;74(2):215–223. doi: 10.1016/j.therap.2018.09.072. [DOI] [PubMed] [Google Scholar]
- 23.Fagnani F, Feuilly M, de Zélicourt M et al. Lanreotide autogel and octreotide LAR treatment patterns: results from a nationwide French retrospective study. ENETS 2020.
- 24.Somatuline L.P. 30 mg extended-release suspension for injection (IM) powder and solvent—Summary of product characteristics. Ministere des Solidarites et de la Sante; 2021.
- 25.Lesén E, Granfeldt D, Berthon A, et al. Treatment patterns and survival among patients with metastatic gastroenteropancreatic neuroendocrine tumours in Sweden—a population-based register-linkage and medical chart review study. J Cancer. 2019;10(27):6876–6887. doi: 10.7150/jca.32381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benson AB, III, Broder MS, Cai B, et al. Real-world treatment patterns of gastrointestinal neuroendocrine tumors: a claims database analysis. World J Gastroenterol. 2017;23(33):6128–6136. doi: 10.3748/wjg.v23.i33.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen C-C, Parikh K, Abouzaid S, et al. Real-world treatment patterns, time to next treatment, and economic outcomes in relapsed or refractory multiple myeloma patients treated with pomalidomide or carfilzomib. J Manag Care Spec Pharm. 2017;23(2):236–246. doi: 10.18553/jmcp.2017.23.2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huynh L, Cai B, Cheng M, et al. Analysis of real-world treatment patterns, healthcare resource utilization, and costs between octreotide and lanreotide among patients with neuroendocrine tumors. Pancreas. 2019;48(9):1126–1135. doi: 10.1097/MPA.0000000000001403. [DOI] [PubMed] [Google Scholar]
- 29.Ipsen. Ipsen’s Somatuline® Depot® is the first therapy approved by the FDA in the United States for the treatment of patients with locally advanced or metastatic gastroenteropancreatic neuroendocrine tumors. IPSEN. https://www.ipsen.com/websites/Ipsen_Online/wp-content/uploads/2014/12/06185010/12-2014-corporate-PR-Approval-Somatuline-US-FINAL.pdf. Accessed 19 Mar 2021.
- 30.FDA. Highlights of prescribing information: somatuline depot (lanreotide) injection, for subcutaneous use. FDA; 2018.
- 31.Somatuline LP. 120 mg, solution injectable a liberation prolongee en seringue preremplie. Ministere Des Solidarites et de la Sante; 2021.
- 32.Somatuline LP. 90 mg, solution injectable a liberation prolongee en seringue preremplie. Ministeres Des Solidarites et de la Sante; 2021.
- 33.Somatuline LP. 60 mg, pre-filled syringe solution for injection. Ministere Des Solidarites et de la Sante; 2021.
- 34.Novartis Pharmaceuticals Corporation . Highlights of prescribing information: sandostatin LAR depot (octreotide acetate) for injectable suspension, for gluteal intramuscular use. East Hanover: Novartis Pharmaceuticals Corporation; 2021. [Google Scholar]
- 35.Sandostatine LP. 30 mg, poudre et solvant pour suspension injectable. Ministere des Solidarites et de la Sante; 2021.
- 36.Sandostatine LP. 10 mg, poudre et solvant pour suspension injectable. Ministere des Solidarites et de la Sante; 2021.
- 37.Sandostatin LP. 20 mg powder and solvent for suspension for injection. Ministere des Solidarites et de la Sante; 2021.
- 38.Lamarca A, McCallum L, Nuttall C, et al. Somatostatin analogue-induced pancreatic exocrine insufficiency in patients with neuroendocrine tumors: results of a prospective observational study. Exp Rev Gastroenterol Hepatol. 2018;12(7):723–731. doi: 10.1080/17474124.2018.1489232. [DOI] [PubMed] [Google Scholar]
- 39.Trang T, Chan J, Graham DY. Pancreatic enzyme replacement therapy for pancreatic exocrine insufficiency in the 21(st) century. World J Gastroenterol. 2014;20(33):11467–11485. doi: 10.3748/wjg.v20.i33.11467. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Owing to the nature of this claims database analysis, Ipsen does not have access to individual participant data. The data sets analysed in this study are available through the National System of Health Data and the authors cannot share the data with any third parties or make the data publicly available owing to protections around the sharing of personal health data (General Data Protection Regulation, GDPR).