Abstract
Background
Airborne particulate matter (PM), a widespread air contaminant, is a complex mixture of solids and aerosols composed of particles suspended in the air. PM is associated with inflammatory responses and may worsen inflammatory skin diseases. However, the mechanisms through which PM affects atopic dermatitis (AD) remain unclear.
Objective
To establish an in vitro model that more accurately mimics AD using human keratinocyte (HaCaT), dermal fibroblast (HDF), and mast cell (HMC-1) and using this model to investigate the mechanism through which PMs affect AD.
Methods
An AD-like in vitro model was established by seeding HaCaT, HDF, and HMC-1 cells with recombinant human interleukin (IL)-1α and polyinosinic:polycytidylic acid. We confirmed the effect of PM on the inflammatory cytokine expression of a triple-cell culture model. SRM 1649b Urban Dust, which is mainly composed of polycyclic aromatic hydrocarbons, was used as the reference PM. The effects of PM on the expression levels of proinflammatory cytokines and skin barrier markers were assessed using quantitative real-time polymerase chain reaction and western blotting. Inflammatory cytokine levels were measured using an enzyme-linked immunosorbent assay.
Results
Interactions between various skin cell types were evaluated using a co-culture system. PM treatment increased mRNA and protein levels of the inflammatory cytokines IL-6, IL-1α, tumor necrosis factor-α, IL-4, and IL-1β and decreased the expression of the skin barrier markers filaggrin and loricrin.
Conclusion
Our results suggest that an in vitro triple-cell culture model using HaCaT, HDF, and HMC-1 cells may be reliable for obtaining more physiological, functional, and reproducible data on AD and skin barriers.
Keywords: Atopic dermatitis, Coculture techniques, Inflammation, Particulate matter
INTRODUCTION
Exposure to ambient particulate matter (PM) is a major global concern. The problem of air pollution has become prevalent worldwide, and interest in health problems caused by PM has also grown1,2. PM, a key constituent of air pollution, is known to increase the risk of cardiovascular, respiratory, nervous, and immune diseases3,4. Recently, the damaging effects of PM on the skin have been of great interest5,6. PM correlated not only with the exacerbation of inflammatory effects but also with the progression of inflammatory skin diseases7, atopic dermatitis (AD)8,9,10, acne, and allergic reactions11,12,13,14.
In vitro studies have evaluated the pro-inflammatory effects of PM, but the role of communication between the three types of skin cells is not well known15,16. In this regard, a few studies have shown that the interactions between human keratinocyte (HaCaT) and dermal fibroblast (HDF) lead to an enhanced response to PM and that this interaction is dependent on cellular cross-linking15,17,18.
Triple-cell culture systems have been used extensively to study the interactions between different cell populations and to understand cell-to-cell interactions19,20. In contrast, monoculture systems provide only the cell growth environment and are not able to imitate intercellular signaling factors16. Cell-to-cell interactions are controlled through direct intercellular contact with signaling molecules secreted by cells21. The interactions between HaCaT and HDF that have had direct contact with precipitated particles are important; however, they remain largely unknown21,22,23. We previously showed that the in vitro triple-cell culture method facilitates the co-culture of at least three cell populations with a high degree of cell-to-cell contact2,15,24. Therefore, in this study, we aimed to establish an AD-like in vitro model using a triple-cell culture system and use this model to investigate the mechanism through which PM affects AD.
MATERIALS AND METHODS
Preparation of particle matter samples
A standard diesel PM (SRM 1649b) with a diameter measuring less than 2.5 µm (PM2.5), issued by the National Institute of Standards and Technology (NIST, Gaithersburg, MD, USA), was purchased from Sigma-Aldrich (St. Louis, MO, USA). SRM 1649b suspensions were prepared in cell culture media at PM concentrations of 25 µg/cm2 and 50 µg/cm2. The suspended particles were sonicated for 60 minutes to prevent agglomeration. Experiments were performed within 1 hour of the PM preparation to avoid variability in PM components in the different fractions between replicates. The cells were exposed to the total PM suspensions at varying concentrations of 25~50 µg/cm2 for 24 hours. PM suspensions were prepared in media for bioactivity assays.
Reagents
Polyinosinic–polycytidylic acid sodium salt (Poly I:C) was purchased from Sigma-Aldrich, and recombinant human interleukin (IL)-1α and recombinant human IL-4 were purchased from PeproTech (Rocky Hill, NJ, USA). For western blot analysis, IL-4, IL-1β, IL-6, filaggrin (FLG), and loricrin (LOR) were purchased from Thermo Fisher Scientific Inc. (Waltham, MA, USA). Anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Human IL-4, IL-13, and enzyme-linked immunosorbent assay (ELISA) kits were purchased from R&D Systems (Minneapolis, MN, USA).
Cell culture
The HaCaT and HDF cell lines were purchased from the Korean Cell Line Bank (KCLB, Seoul, Korea). The human mast cell line (HMC-1) was purchased from Millipore (Carlsbad, CA, USA). HaCaT and HDF cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS) at 37℃ in a humidified 5% CO2 atmosphere. HMC-1 cells were maintained in Iscove’s Modified Dulbecco’s Medium (IMDM, Thermo Fisher Scientific Inc.) supplemented with 10% FBS, 1.2 mM α-thioglycerol (Sigma-Aldrich), 2 mM L-glutamine, penicillin (100 IU/ml), and streptomycin (50 µg/ml) in the same CO2 incubator. The medium for mast cells was changed every 3~4 days. The co-culture medium was prepared using a DMEM:IMDM mixture ratio of 3:1 (Table 1). Upon reaching confluence, the cells were treated with 0.05% trypsin in 0.53 mM EDTA and incubated for 3 to 5 minutes at 37℃. The cells were conditioned for at least 6 hours in serum-free medium before experimentation.
Table 1. Media used for cell cultures.
| Cell culture | Medium | Other | |
|---|---|---|---|
| Single cultures | |||
| HaCaT | DMEM | ||
| HDF | DMEM | ||
| HMC-1 | IMDM | Alpha-thioglycerol | |
| Co-cultures | |||
| HaCaT+HDF+HMC-1 | DMEM+IMDM, 3:1 | ||
HaCaT: human keratinocyte, HDF: human dermal fibroblast, HMC-1: human mast cell, DMEM: Dulbecco’s Modified Eagle’s Medium, IMDM: Iscove’s Modified Dulbecco’s Medium.
Poly I:C, IL-1α, and PM treatment of HaCaT, HDF, and HMC-1 cells
A total of 1×105 cells were seeded per well of a 96-well plate (2 ml triple-cell culture suspension per well) and incubated for 24 hours. The triple-cell cultures were pretreated with Poly I:C (10 µg/ml) and IL-1α (10 ng/ml). After incubation for 24 hours, the triple-cell culture models were treated with PM at concentrations of 25 µg/cm2 or 50 µg/cm2. The plates were incubated at 37℃ for 24 hours. The PM concentration was optimized according to those in previous studies.
Quantitative real-time polymerase chain reaction
Total RNA was extracted from cells using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. mRNA concentrations were measured using an ND-2000 spectrophotometer (Thermo Fisher Scientific Inc.), and purity was determined by measuring the A260/A280 ratio. cDNA was generated from 1 µg of purified RNA via reverse transcription using the RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Inc.) and incubated for 1 hour at 42℃. Quantitative real-time polymerase chain reaction (qRT-PCR) was performed using the QuantStudio 3 system (Applied Biosystems, Waltham, MA, USA) and a PowerUp SYBR Green Master Mix (Applied Biosystems). All data were normalized to those of the housekeeping gene GAPDH. Relative quantitation was performed using the 2–ΔΔCt method, according to the manufacturer’s instructions.
Western blot analysis
Triple-cell cultures were seeded onto 6-well culture plates, and cellular quiescence was induced at cell confluence via incubation in serum-free DMEM in 1% FBS for 12 hours. Growth-arrested cells were incubated with or without PM at various concentrations at 37℃. After 24 hours of incubation, the cells were washed with ice-cold phosphate-buffered saline. The collected cells were lysed with 1% Triton-X radioimmunoprecipitation assay (RIPA) buffer containing a protease and phosphatase inhibitor cocktail. Lysates were centrifuged at 16,000×g for 20 minutes at 4℃ to obtain the whole-cell extract. Protein concentration was determined using the bicinchoninic acid assay, according to the manufacturer’s instructions. Samples from the supernatant fractions (25 µg protein) were denatured and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis using a 15% separation gel. Proteins were then transferred onto nitrocellulose membranes, which were incubated successively at room temperature with 5% skim milk in Tris-buffered saline containing Tween-20 for 1 hour. Subsequently, the membranes were incubated with primary antibodies (1:2,000 dilution) overnight at 4℃ and horseradish peroxidase-conjugated secondary antibodies (1:3,000 dilution) for 1 hour at room temperature. After rinsing, the bands were visualized using the Ez-West Lumi Plus system (ATTO, Tokyo, Japan). The results were obtained using an image analyzer (Image Lab 3.0; Bio-Rad, Hercules, CA, USA) and quantified using ImageJ software (ImageJ; National Institutes of Health, Bethesda, MD, USA).
Enzyme-linked immunosorbent assay
The culture supernatant was incubated for 24 hours with PM, and the culture supernatants were collected and stored at –80℃. IL-4 and IL-13 released into the supernatant were measured using a human ELISA kit (R&D Systems) according to the manufacturer’s instructions.
Statistical analysis
We compared the resulting PM effects in the treatment groups and controls using one-way analysis of variance and Tukey’s multiple-comparison post hoc test. Differences between groups were considered significant at p<0.05, and statistical analyses were performed using GraphPad Prism 8.0 (GraphPad Software, Inc., CA, USA).
RESULTS
Morphology of co-cultures, and PM2.5 stimulates pro-inflammatory cytokine production in the triple-cell culture model
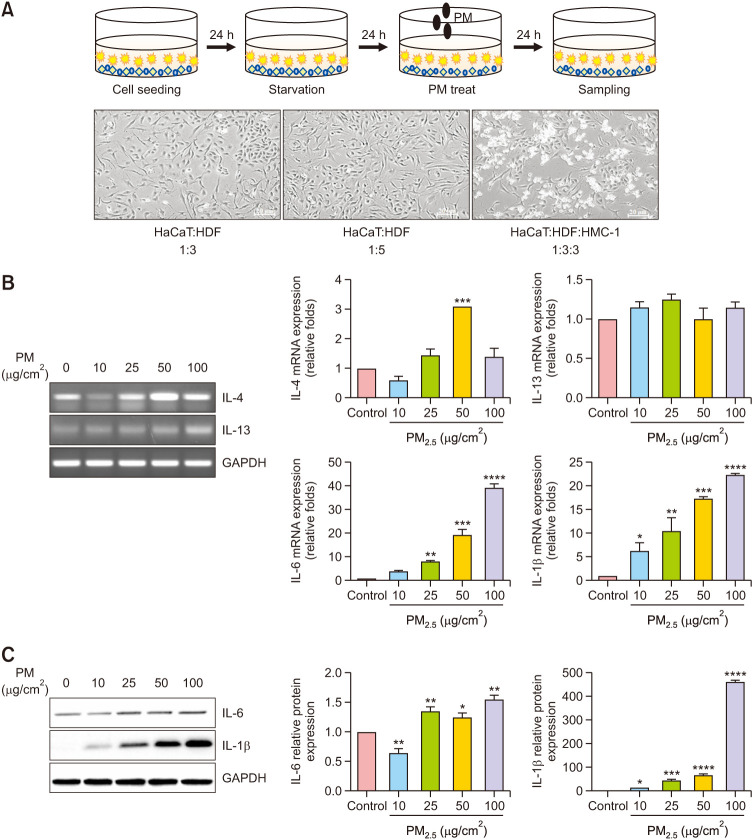
A co-culture system with three different cell types was created to evaluate the interactions among different cell types in the skin. Cultures of three different cell types, including HaCaT, HDF, and HMC-1, were used. Their morphology was further evaluated using microscopy, which revealed a homogenous distribution of the three cell types. The morphologies of the keratinocytes, fibroblasts, and mast cells examined using phase-contrast microscopy were as follows: keratinocytes and fibroblasts at a 1:3 ratio; keratinocytes and fibroblasts at a 1:5 ratio; and keratinocytes, fibroblasts, and mast cells at a 1:3:3 ratio (Fig. 1A). We conducted experiments on various cell ratios, of which cell proliferation and morphology were appropriate at 1:3:3 ratio, and inflammation was easily induced. The effects of PM on pro-inflammatory cytokine expression in a triple-cell culture model were confirmed. As shown in Fig. 1B, C, the models were treated with different PM concentrations (10, 25, 50, and 100 µg/cm2) for 24 hours to determine changes in mRNA and protein expression levels. Reverse transcription polymerase chain reaction (RT-PCR) was performed to determine the mRNA levels of IL-4 and IL-13. qRT-PCR and western blotting were performed to determine the mRNA and protein levels of IL-6 and IL-1β. The results showed that PM treatment increased the mRNA and protein expression levels of IL-6 and IL-1β in a dose-dependent manner in the triple-cell culture model when compared with the levels in the untreated controls. Expression levels were normalized to that of GAPDH. The protein levels showed significant differences between groups.
Fig. 1. Particulate matter (PM)-induced proinflammatory responses in the triple-cell culture model. (A) Schematic representation of the strategy of PM exposure in the triple-cell culture model. Morphology of co-cultured keratinocytes, fibroblasts, and mast cells examined using phase-contrast microscopy. The cells were serum-starved overnight and treated with PM (0, 10, 25, 50, and 100 µg/cm2) for 24 hours. (B) The mRNA expression levels of interleukin (IL)-4, IL-13, IL-6, and IL-1β are presented in a graphical form (the relative mRNA levels are presented as fold change compared with untreated cells). (C) The protein expression levels of IL-6 and IL-1β were determined using western blotting. Quantification of protein levels. Values are presented as mean±standard deviation (n=3). *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 compared with basal levels.
PM induces inflammatory responses in the AD-like in vitro model
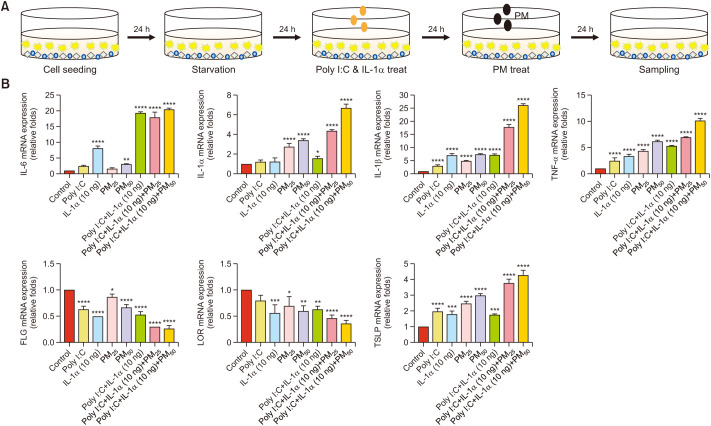
The AD-like in vitro model was established through treatment with Poly I:C (10 µg/ml) and IL-1α (10 ng/ml) in the triple-cell culture system. To confirm the effects of PM on the inflammatory responses in AD-like in vitro models, the cells were treated with PM (25 and 50 µg/cm2) for 24 hours following pre-treatment with Poly I:C and IL-1α for 24 hours (Fig. 2A). qRT-PCR was used to measure the mRNA expression levels of IL-6, IL-1β, IL-1α, tumor necrosis factor (TNF)-α, LOR, and FLG. Expression levels were normalized to that of GAPDH. The results showed that the mRNA expression of the inflammatory cytokines IL-6, IL-1β, IL-1α, and TNF-α increased, while that of the skin barrier markers FLG and LOR decreased (Fig. 2B). These results showed that there was a more significant increase in expression in the cytokine-stimulated groups than in the PM-only group.
Fig. 2. Direct co-culture system. (A) Schematic representation of the strategy of particulate matter (PM) and proinflammatory cytokines exposure with the atopic dermatitis (AD)-like in vitro model. AD-like in vitro models were pretreated with Poly I:C (10 µg/ml) and IL-1α (10 ng/ml) for 24 hours and subsequently incubated with PM (25 or 50 µg/cm2) for 24 hours. (B) The mRNA expression levels of IL-6, IL-1α, IL-1β, LOR, FLG, TSLP, and TNF-α are presented in a graphical form (the relative mRNA levels are presented as fold change compared with untreated cells). Values are presented as mean±standard deviation (n=3). Poly I:C: polyinosinic:polycytidylic acid, IL: interleukin, FLG: filaggrin, LOR: loricrin, TSLP: thymic stromal lymphopoietin. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 compared with basal levels.
Effects of PM on inflammation and the skin barrier in the AD-like in vitro model
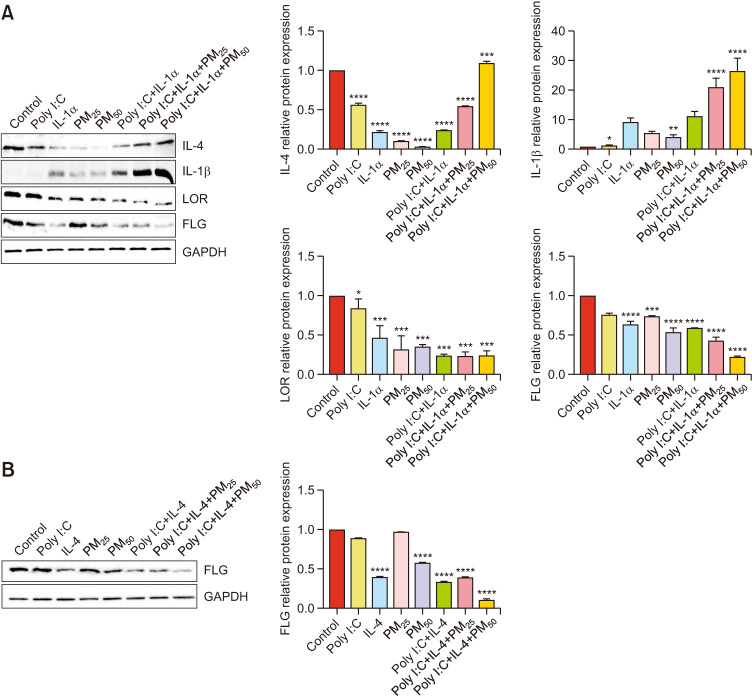
Western blotting was used to assess the effects of PM (25 and 50 µg/cm2) on the expression levels of proinflammatory cytokines and skin barrier markers. Cells were treated with PM for 24 hours following pretreatment with Poly I:C, IL-1α (Fig. 3A), and IL-4 (50 ng/ml) for 24 hours (Fig. 3B). PM treatment increased the protein levels of the inflammatory cytokines IL-4 and IL-1β in the triple-cell culture model and decreased the expression of the skin barrier markers FLG and LOR. The results additionally showed that there were significant differences between the different groups.
Fig. 3. Effect of particulate matter (PM) on the protein expression levels of pro-inflammatory cytokine expression confirmed in an atopic dermatitis-like in vitro model. Cells were pretreated with (A) Poly I:C, IL-1α, and (B) IL-4 (50 ng/ml) for 24 hours and subsequently incubated with PM for 24 hours. The protein expression levels of IL-4, IL-1β, LOR, and FLG were determined using western blotting. Quantification of protein levels. Values are presented as mean±standard deviation (n=3). Poly I:C: polyinosinic:polycytidylic acid, IL: interleukin, FLG: filaggrin, LOR: loricrin, TSLP: thymic stromal lymphopoietin. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 compared with basal levels.
Effects of PM on the protein levels of proinflammatory cytokines in the AD-like in vitro model
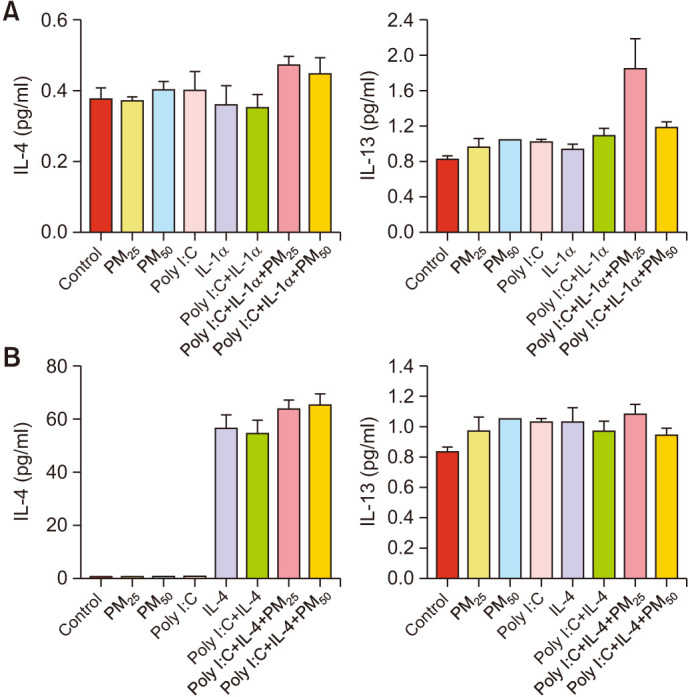
ELISA was performed to determine the protein levels of IL-4 and IL-13. PM treatment increased the protein levels of IL-4 and IL-13 in the triple-cell culture models when compared with those in the untreated controls (Fig. 4).
Fig. 4. Protein levels of IL-4 and IL-13 determined using an enzyme-linked immunosorbent assay. (A) Cells were pretreated with Poly I:C, IL-1α, and (B) IL-4 for 24 hours and subsequently incubated with particulate matter (PM) for 24 hours; samples were normalized to standard curves. Values are presented as mean±standard deviation (n=3). Poly I:C: polyinosinic:polycytidylic acid, IL: interleukin.
DISCUSSION
This study aimed to establish an AD-like in vitro model and evaluate an in vitro triple-cell culture model for the inflammatory effects after exposure to PM2.5. PM exposure has various adverse effects on the human skin, where the negative effects between PM exposure and the skin have been elucidated in studies on skin inflammation levels; skin cancer; and skin diseases, including atopic dermatitis, eczema, and psoriasis25,26.
In a previous study, a co-culture of keratinocytes and fibroblasts was used to evaluate PM-induced effects1, but in the present study, one more type of cell was added: mast cells. Mast cells expressing c-kit are crucial effector cells in allergic and anaphylactic responses. Higher numbers of mast cells are found in AD skin lesions than in non-lesioned skin. In AD, mast cells are suggested to be involved in immunopathology by detecting the surrounding environment27. A recent animal study suggested that mast cells are involved in controlling skin barrier function, which could be one of the pillars of AD28. In addition, Poly I:C and IL-1α were used as treatments in the triple-cell model to establish an AD-like in vitro model2,29,30,31.
The results showed that PM induced an increase in the expression of proinflammatory cytokines such as IL-1α, IL-1β, IL-6, IL-4, IL-13, and TNF-α in triple-cell cultures32. However, the paracrine effects of IL-4 and IL-13 did not differ significantly between the groups. Conversely, the expression of skin barrier markers FLG and LOR decreased. The cytokine secretion pattern after exposure to PM appeared to correspond to the in vivo effects related to PM previously shown33.
The most important finding of the present in vitro system is that the interaction between keratinocytes, fibroblasts, and mast cells leads to an amplified response to PM. These amplifications may mimic what occurs in the skin. Triple-cell cultures consisting of various cell types can mimic realistic interactions in human skin and is a more reliable evaluation model than traditional cell monocultures15. On our previous study33, the effects of air pollutants on atopic dermatitis were evaluated by treating keratinocytes with IL-4, IL-13 and PM. Comparison of mRNA and protein expression levels between the single cell model and triple co-culture model showed that there are significant differences in the expression of inflammatory and skin barrier markers in the triple co-culture model even without IL-4 or IL-13 cytokine treatment.
Furthermore, this AD-like in vitro triple cell model has strengths over conventional 3-dimensional (3D) skin equivalents in two aspects. First, if 3D skin equivalents are advantageous for seeing histological changes similar to those of real human skin, the triple cell model allows us to easily investigate the underlying mechanisms and examining quantitative changes of substances. Second, it is less expensive than the 3D skin equivalents, so more diverse research is possible. Still there are challenges in designing the micro-environment of the skin and the long-term culture in a triple cell model, and further research is needed in order to overcome these issues.
In conclusion, we developed a novel AD-like in vitro model that mimics the cell interactions within the skin. This model can lead to a better understanding of the different cellular mechanisms related to PM response. The AD-like in vitro model with keratinocytes, fibroblasts, and mast cells showed results consistent with the inflammation effects previously identified for PM. This novel system opens up the possibility of analyzing the effects of specific substances on cytokines levels and transduction signals in the human skin, allowing for a better understanding of the mechanisms involved in AD.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
FUNDING SOURCE: This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2019R1A2C1090226). This research was supported by the Chung-Ang University Research Scholarship Grants in 2020.
DATA SHARING STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Kim M, Kim JH, Jeong GJ, Park KY, Lee MK, Seo SJ. Particulate matter induces pro-inflammatory cytokines via phosphorylation of p38 MAPK possibly leading to dermal inflammaging. Exp Dermatol. 2019;28:809–815. doi: 10.1111/exd.13943. [DOI] [PubMed] [Google Scholar]
- 2.Wang G, Zhang X, Liu X, Zheng J, Chen R, Kan H. Ambient fine particulate matter induce toxicity in lung epithelial-endothelial co-culture models. Toxicol Lett. 2019;301:133–145. doi: 10.1016/j.toxlet.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 3.Cai C, Huang J, Lin Y, Miao W, Chen P, Chen X, et al. Particulate matter 2.5 induced arrhythmogenesis mediated by TRPC3 in human induced pluripotent stem cell-derived cardiomyocytes. Arch Toxicol. 2019;93:1009–1020. doi: 10.1007/s00204-019-02403-y. Erratum in: Arch Toxicol 2019;93:2711. [DOI] [PubMed] [Google Scholar]
- 4.Woo YR, Park SY, Choi K, Hong ES, Kim S, Kim HS. Air pollution and atopic dermatitis (AD): the impact of particulate matter (PM10) on an AD mouse-model. Int J Mol Sci. 2020;21:6079. doi: 10.3390/ijms21176079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang JH, Song CH, Ock SM, Park HJ. Particulate matter and skin. Korean J Fam Pract. 2014;4:116–121. [Google Scholar]
- 6.Ngoc LTN, Park D, Lee Y, Lee YC. Systematic review and meta-analysis of human skin diseases due to particulate matter. Int J Environ Res Public Health. 2017;14:1458. doi: 10.3390/ijerph14121458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krutmann J, Liu W, Li L, Pan X, Crawford M, Sore G, et al. Pollution and skin: from epidemiological and mechanistic studies to clinical implications. J Dermatol Sci. 2014;76:163–168. doi: 10.1016/j.jdermsci.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Ahn K. The role of air pollutants in atopic dermatitis. J Allergy Clin Immunol. 2014;134:993–999. doi: 10.1016/j.jaci.2014.09.023. discussion 1000. [DOI] [PubMed] [Google Scholar]
- 9.Kim EH, Kim S, Lee JH, Kim J, Han Y, Kim YM, et al. Indoor air pollution aggravates symptoms of atopic dermatitis in children. PLoS One. 2015;10:e0119501. doi: 10.1371/journal.pone.0119501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JH, Lee HS, Park MR, Lee SW, Kim EH, Cho JB, et al. Relationship between indoor air pollutant levels and residential environment in children with atopic dermatitis. Allergy Asthma Immunol Res. 2014;6:517–524. doi: 10.4168/aair.2014.6.6.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Annesi-Maesano I, Moreau D, Caillaud D, Lavaud F, Le Moullec Y, Taytard A, et al. Residential proximity fine particles related to allergic sensitisation and asthma in primary school children. Respir Med. 2007;101:1721–1729. doi: 10.1016/j.rmed.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 12.Brauer M, Hoek G, Smit HA, de Jongste JC, Gerritsen J, Postma DS, et al. Air pollution and development of asthma, allergy and infections in a birth cohort. Eur Respir J. 2007;29:879–888. doi: 10.1183/09031936.00083406. [DOI] [PubMed] [Google Scholar]
- 13.Seo S, Kim D, Min S, Paul C, Yoo Y, Choung JT. GIS-based association between PM10 and allergic diseases in Seoul: implications for health and environmental policy. Allergy Asthma Immunol Res. 2016;8:32–40. doi: 10.4168/aair.2016.8.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szyszkowicz M, Porada E, Searles G, Rowe BH. Ambient ozone and emergency department visits for skin conditions. Air Qual Atmos Health. 2012;5:303–309. [Google Scholar]
- 15.Alfaro-Moreno E, Nawrot TS, Vanaudenaerde BM, Hoylaerts MF, Vanoirbeek JA, Nemery B, et al. Co-cultures of multiple cell types mimic pulmonary cell communication in response to urban PM10. Eur Respir J. 2008;32:1184–1194. doi: 10.1183/09031936.00044008. [DOI] [PubMed] [Google Scholar]
- 16.Miranda-Azpiazu P, Panagiotou S, Jose G, Saha S. A novel dynamic multicellular co-culture system for studying individual blood-brain barrier cell types in brain diseases and cytotoxicity testing. Sci Rep. 2018;8:8784. doi: 10.1038/s41598-018-26480-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jia YY, Wang Q, Liu T. Toxicity research of PM2.5 compositions in vitro. Int J Environ Res Public Health. 2017;14:232. doi: 10.3390/ijerph14030232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim BE, Kim J, Goleva E, Berdyshev E, Lee J, Vang KA, et al. Particulate matter causes skin barrier dysfunction. JCI Insight. 2021;6:e145185. doi: 10.1172/jci.insight.145185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kletting S, Barthold S, Repnik U, Griffiths G, Loretz B, Schneider-Daum N, et al. Co-culture of human alveolar epithelial (hAELVi) and macrophage (THP-1) cell lines. ALTEX. 2018;35:211–222. doi: 10.14573/altex.1607191. [DOI] [PubMed] [Google Scholar]
- 20.Kook YM, Jeong Y, Lee K, Koh WG. Design of biomimetic cellular scaffolds for co-culture system and their application. J Tissue Eng. 2017;8:2041731417724640. doi: 10.1177/2041731417724640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Venter C, Niesler C. A triple co-culture method to investigate the effect of macrophages and fibroblasts on myoblast proliferation and migration. Biotechniques. 2018;64:52–58. doi: 10.2144/btn-2017-0100. [DOI] [PubMed] [Google Scholar]
- 22.Movia D, Bazou D, Prina-Mello A. ALI multilayered co-cultures mimic biochemical mechanisms of the cancer cell-fibroblast cross-talk involved in NSCLC MultiDrug resistance. BMC Cancer. 2019;19:854. doi: 10.1186/s12885-019-6038-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vis MAM, Ito K, Hofmann S. Impact of culture medium on cellular interactions in in vitro co-culture systems. Front Bioeng Biotechnol. 2020;8:911. doi: 10.3389/fbioe.2020.00911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wottrich R, Diabaté S, Krug HF. Biological effects of ultrafine model particles in human macrophages and epithelial cells in mono- and co-culture. Int J Hyg Environ Health. 2004;207:353–361. doi: 10.1078/1438-4639-00300. [DOI] [PubMed] [Google Scholar]
- 25.De Vuyst E, Salmon M, Evrard C, Lambert de Rouvroit C, Poumay Y. Atopic dermatitis studies through in vitro models. Front Med (Lausanne) 2017;4:119. doi: 10.3389/fmed.2017.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim JH, Kim M, Kim JM, Lee MK, Seo SJ, Park KY. Afzelin suppresses proinflammatory responses in particulate matter-exposed human keratinocytes. Int J Mol Med. 2019;43:2516–2522. doi: 10.3892/ijmm.2019.4162. [DOI] [PubMed] [Google Scholar]
- 27.Järvikallio A, Naukkarinen A, Harvima IT, Aalto ML, Horsmanheimo M. Quantitative analysis of tryptase- and chymase-containing mast cells in atopic dermatitis and nummular eczema. Br J Dermatol. 1997;136:871–877. [PubMed] [Google Scholar]
- 28.Sehra S, Serezani APM, Ocaña JA, Travers JB, Kaplan MH. Mast cells regulate epidermal barrier function and the development of allergic skin inflammation. J Invest Dermatol. 2016;136:1429–1437. doi: 10.1016/j.jid.2016.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eyerich K, Brown SJ, Perez White BE, Tanaka RJ, Bissonette R, Dhar S, et al. Human and computational models of atopic dermatitis: a review and perspectives by an expert panel of the International Eczema Council. J Allergy Clin Immunol. 2019;143:36–45. doi: 10.1016/j.jaci.2018.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gorr MW, Youtz DJ, Eichenseer CM, Smith KE, Nelin TD, Cormet-Boyaka E, et al. In vitro particulate matter exposure causes direct and lung-mediated indirect effects on cardiomyocyte function. Am J Physiol Heart Circ Physiol. 2015;309:H53–H62. doi: 10.1152/ajpheart.00162.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hennies HC, Poumay Y. Skin disease models in vitro and inflammatory mechanisms: predictability for drug development. Handb Exp Pharmacol. 2021;265:187–218. doi: 10.1007/164_2020_428. [DOI] [PubMed] [Google Scholar]
- 32.Ryu YS, Kang KA, Piao MJ, Ahn MJ, Yi JM, Hyun YM, et al. Particulate matter induces inf lammatory cytokine production via activation of NFκB by TLR5-NOX4-ROS signaling in human skin keratinocyte and mouse skin. Redox Biol. 2019;21:101080. doi: 10.1016/j.redox.2018.101080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bae YJ, Park KY, Han HS, Kim YS, Hong JY, Han TY, et al. Effects of particulate matter in a mouse model of oxazolone-induced atopic dermatitis. Ann Dermatol. 2020;32:496–507. doi: 10.5021/ad.2020.32.6.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.