Abstract
The ectoparasite Varroa destructor is the greatest threat to managed honey bee (Apis mellifera) colonies globally. Despite significant efforts, novel treatments to control the mite and its vectored pathogens have shown limited efficacy, as the host remains naïve. A prospective solution lies in the development of Varroa-resistant honey bee stocks, but a paucity of rigorous selection data restricts widespread adoption. Here, we characterise the parasite and viral dynamics of a Varroa-resistant honey bee stock, designated ‘Pol-line’, using a large-scale longitudinal study. Results demonstrate markedly reduced Varroa levels in this stock, diminished titres of three major viruses (DWV-A, DWV-B, and CBPV), and a two-fold increase in survival. Levels of a fourth virus that is not associated with Varroa—BQCV—do not differ between stocks, supporting a disruption of the transmission pathway. Further, we show that when decoupled from the influence of Varroa levels, viral titres do not constitute strong independent predictors of colony mortality risk. These findings highlight the need for a reassessment of Varroa etiology, and suggest that derived stocks represent a tractable solution to the Varroa pandemic.
Subject terms: Agroecology, Ecological epidemiology, Parasite host response, Applied microbiology, Pathogens
Introduction
Honey bee (Apis mellifera) colony losses constitute a severe and ubiquitous concern for both the migratory pollination industry, and modern agricultural security in general1–4. High annual mortality has become a pervasive facet of commercial pollination, despite significant scientific and legislative efforts to curtail it5,6. In the United States alone, current figures place 2018–2019 mortality at 37.5% for commercial beekeeping operations, rising substantially from a 13-year average of 28.8%7,8. The causes of this are myriad, and the interrelated nature of extraneous stressors upon colony health makes defining the principle interactions difficult9,10. Despite such inherent system complexity, however, the parasitic mite Varroa destructor repeatedly emerges as the single greatest driver of global colony losses1,11–13.
Varroa is associated with varied pathologies, including developmental disruption, immunosuppression, and behavioural alteration14–16. An extensive body of research demonstrates that the mite vectors and amplifies a plethora of viral pathogens17, several of which are strongly associated with colony mortality12,18,19, and the discovery of novel viral types is ongoing20. Additionally, recent work has shown that the process of Varroa feeding per se may cause significant damage, as these mites consume the honey bee hosts’ fat body—an organ with numerous vital functions12,21. Despite containment efforts, Varroa has continued its 50-year global expansion into formerly uninfested regions1,13,22,23 with concordant implications for managed and wild pollinators1,24. Simultaneously, established mite populations show increasing resistance to acaricide treatments, which currently constitute the last effective line of defence17,25–28. As such, the ongoing threat presented by this pandemic, and its rapid proliferation through industrialised agricultural systems29,30, is both immediate, and severe.
A promising, sustainable solution in the effort to mitigate Varroa, lies in the development of Varroa-resistant honey bee stocks17,31,32. This approach has advantages over other management strategies, in that it is both integrated, and less susceptible to the simple evolutionary adaptations that threaten chemical methods17,33,34. In natural A. mellifera populations, cases of resistance to Varroa mites have been widely reported35–37. Due to the devastating nature of infestation absent management, natural selection for colonies able to resist, or coexist, is rapid, and intense38. Survival mechanisms are varied, although there is some evidence to indicate convergent trait selection in isolated populations17,37. Notably however, many of the mechanisms that predispose colonies to Varroa-resistance in nature, such as smaller colony sizes and increased swarming frequencies38–40, are antithetical to the characteristics most valued in commercial beekeeping17. Indeed, the intensified nature of Varroa-host dynamics in managed operations41,42, combined with the evolutionary novelty of the association43, necessitates resistance mechanisms that are tailored to extreme conditions. Consequently, the selection of heritable resistance traits suited to commercial beekeeping, along with the maintenance of favourable colony-level characteristics, is a significant challenge for breeding efforts17,38.
Major programs to develop Varroa-resistant commercial stocks are ongoing38, with emerging support from marker-assisted selection (MAS)44,45, and ‘omics technologies46. Artificially selected traits, including Varroa-sensitive hygiene (VSH), in which workers remove mite-infested brood47–49; low mite population growth (low MPG), which comprises a suite of behaviours limiting Varroa reproduction50,51; and auto-/allo-grooming, the processes of removing and/or damaging phoretic mites52,53, have shown promise in commercial scenarios17,38,54. The adoption of resultant lines, however, has been limited54, ostensibly due to varying real-world efficacy31,32,55, a paucity of controlled large-scale trials with which to validate commercial viability38,56,57, and the challenges of scalability in breeding efforts58,59. These factors engender industry-wide inertia to the effective integration of Varroa-resistant honey bees, and subsequently, the globally managed A. mellifera population remains adaptively naïve—and thus acutely susceptible—to Varroa parasitism.
Here, we employ a controlled longitudinal analysis, to assess the performance of a novel and genetically distinct Varroa-resistant honey bee stock, ‘Pol-line’54,60. This stock is unique, in that it possesses the substantial mite-resistance of VSH behaviour61, combined with the desirable beekeeping characteristics of commercial Italian colonies; including large population size, substantial honey production, and docile temperament54. We utilised a year-long experimental design, comparatively analysing Pol-line and commercial Italian honey bees, in which all colonies were individually tracked, and parasite, pathogen, and health measures taken at key time points. Colonies were integrated into a migratory operation in the USA, focussing on almond pollination and honey production, and spanning the states of Mississippi, South Dakota, and California. This system is notable, as it constitutes one of the most intensive and stressful scenarios in modern beekeeping62,63, while simultaneously incorporating three distinct Köppen–Geiger climate zones64. As such, it provides a rigorous test of the functionality of colonies, and is not limited to localised applicability. The study design manipulated acaricide application, migration route, and colony management, to compare the performance of both stocks, while monitoring Varroa levels, and associated viral pathogen titres, with high fidelity.
In addition to assessing the functionality of Pol-line honey bees, we utilised our experimental system to examine the relative influences of Varroa and its associated viruses upon mortality in the field, along with the predictive utility of both for informing colony prognoses. While the influence of Varroa upon viral dissemination and amplification has been investigated extensively10,17, the specific impacts of Varroa feeding per se, versus those of the viruses that it transmits, remain poorly understood at the bipartite level12,17,29. This paucity of information arises from the difficulty of isolating either factor effectively, given their tightly coupled nature, and the requisite cost of controlled field studies1,12,65–67.
Current understanding places the RNA picornavirus Deformed wing virus (DWV)—specifically the master variants DWV-A68, and DWV-B69—as the prime viral threat to colony health70. Impetus for this is derived from the correlation that DWV exhibits with colony mortality, and the disseminative properties of the virus when vectored by Varroa23,71. In the presence of Varroa infestation, DWV can increase from latent levels of 6–13%, up to 100% prevalence in colonies, accompanied by a million-fold increase in viral titres within 3 years1,22. In tandem, there is evidence for the recombination of DWV strains, leading to more virulent hybrid forms, and crucially, selection for these forms when transmitted as a viral admixture, via Varroa feeding68,70,72. While the prevalence of DWV is well established, investigations of the role of viral diversity and propagation in determining pathogenicity have shown inconsistent results68,73–78, and surprisingly, some studies linking the virus to mortality do not account for the effect of Varroa levels on colony outcome73,79–81. The latter may be symptomatic of conceptualising Varroa as a viral vector first, and damaging agent second; however this view is inconsistent with both the mite’s biology12,17, and established large-scale datasets67,76,82,83.
Aside from the known Varroa-vectored viruses, certain other pathogens exhibit Varroa association, although their exact relationship with the mites is unclear17. One such case is the taxonomically unassigned RNA virus, Chronic bee paralysis virus (CBPV)84,85. Despite being one of the first described honey bee viruses86, CBPV is comparatively under-studied. However, it is known to cause mortality in adult bees84, to correlate with colony losses87–89, and has a wide host range86,90, with an increasing global incidence86,91. Notably, CBPV can exist at covert levels in healthy colonies, may increase in prevalence in the presence of Varroa parasitism92–94, and has been detected in Varroa90,95. A direct transmission pathway remains elusive, however84, and observational studies suggest variable association90,96.
Another potential Varroa associate is the RNA picornavirus Black queen cell virus (BQCV)17,97,98. BQCV is highly prevalent globally97, causes fatal infections in queen larvae99, and yet generally remains asymptomatic in adult workers98,99, showing limited association with colony losses100,101. As with CBPV, BQCV has been detected in Varroa95,102, and there is evidence for increased transmission to other hosts when the mites are present103. Notably however, while some studies have found that BQCV titres are influenced by Varroa infestation22,104, others indicate that the virus is decoupled from Varroa levels in colonies105–107, and conclusive proof of Varroa transmission is yet to be ascertained22. The former point is salient, as current understanding suggests that although Varroa-mediated transmission of BQCV may be feasible, its occurrence is, at most, rare98. Hence, quantifying this somewhat cryptic relationship will be of utility to the wider study of Varroa-virus dynamics.
Conflicting findings from etiological studies of DWV66,73,76,81,108, CBPV90,94,106,109, and BQCV17,95,105, exacerbated by a historically inaccurate research paradigm for Varroa feeding, which appears to have underestimated direct damage to the fat body12, exemplify the need for ongoing characterisation of Varroa-virus mediated colony mortality. Until this process is better understood, it remains an obstacle to effective treatment, because there is no clear consensus as to the relative importance of covariate factors1,12,66,110,111.
Thus, using the longitudinally tracked colonies in our experimental setup, we tested the influence of Varroa levels, and DWV-A, DWV-B, CBPV, and BQCV titres, on colony mortality. This constituted an assessment of viruses both tightly (DWV-A and DWV-B), and loosely (CBPV and BQCV), linked to Varroa parasitism. We then controlled for Varroa levels, grouping colonies by mite infestation strata, to characterise the relative additive predictive power of each virus in determining colony mortality, using an epidemiological approach. As such, we aimed to evaluate the performance of a novel Varroa-resistant honey bee stock, while simultaneously investigating the specific factor weightings of Varroa-mediated colony mortality.
Materials and methods
Colonies
Colony setup occurred prior to initiation of the study, between March and May 2017, in Mississippi, USA. Using established methods, queenless colony divisions, obtained from a large commercial beekeeping operation, were equalised to an average calculated population size of ~ 7000 workers112, and housed in 10-frame Langstroth hives (Table S1). After acclimatisation for 24–48 h, they each received an imminently emerging queen cell, containing a queen from one of two stocks, added to the same worker baseline. The stocks used consisted of an Italian ‘Commercial’ stock, propagated from collaborator established breeder queens, and thus representative of the industry standard, and the Varroa-resistant ‘Pol-line’ stock54. To ensure consistency, all queens were reared in the same ‘cell builder’ colonies, based at the USDA Honey Bee Breeding, Genetics and Physiology Laboratory, in Baton Rouge, Louisiana, USA. Colonies from each stock were held in independent apiaries, 80 km apart to maintain physical isolation; and to control genetic fidelity, virgin queens were open mated to drones of the same stock via drone saturation. Fourteen days after queen emergence, colonies were inspected, and mated queens were marked with paint on the thorax, to assist with identification, with white corresponding to Commercial, and blue to Pol-line. Colonies were allowed to acclimatise for six weeks before sampling began, and those that failed to achieve mating success, or had unacceptably high [≥ 3.0 ‘mites per hundred bees’ (MPHB)] Varroa levels, were removed, normalising the average between-stock Varroa difference to < 0.1 MPHB from the initiation of the study (Table S1). Each colony was then assigned a random ID number, for a total of 366 colonies; 193 with Commercial queens, and 173 with Pol-line queens. All colonies were provided with supplemental sucrose solution, and soya-based commercial pollen supplements ad libitum, in accordance with standard industry practices.
Experimental design
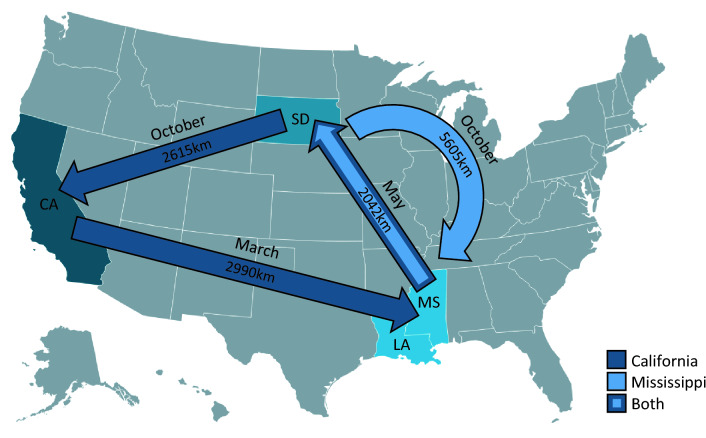
In order to evaluate the effects of migratory pollination procedures, colonies were randomly assigned to one of three migration route treatments. The migration treatments used were as follows; a ‘California’ group, consisting of 156 colonies; 80 Commercial and 76 Pol-line, moved to South Dakota in May, followed by California in October for overwintering and almond pollination, and back to Mississippi at the end of the study; a ‘Mississippi’ group, consisting of 156 colonies; 80 Commercial and 76 Pol-line, moved to South Dakota in May, and back to Mississippi via California in October for overwintering; and a ‘Stationary’ group, consisting of 54 colonies; 32 Commercial and 22 Pol-line, that remained in Mississippi for the duration of the study (Fig. 1). While in South Dakota, colonies were distributed across four apiaries, and in California and Mississippi, they were held in a single apiary. This practice mirrored standard industry protocol, and provided a more representative sample by accounting for localised differences in climate and forage.
Figure 1.
Migration routes used in the experimental setup. Arrows indicate travel routes, distances, and timings for each migration group (California, dark blue; Mississippi, light blue; both, dark and light blue). Choropleth map generated using Datawrapper (release v. 1.25.0).
In addition to migration route, colonies were divided by the frequency of acaricide treatment applied, to better elucidate the effects of varying infestation strata. Within each migration treatment, and stock, half of the colonies received a ‘high’ acaricide treatment, and half a ‘low’ acaricide treatment. Colonies in the high treatment group were treated twice, in September and December; while those in the low treatment group were treated only once, in December. All treatments were conducted using the acaricide amitraz.
Sampling
Colonies were sampled five times during the course of the study, in May, June, September, December, and February. For each colony, the queen was examined, and frames of workers, nectar, pollen, and brood were quantified visually by percentage cover113. Upon observation, if the queen did not have an existing mark, a single red paint mark was applied to her thorax, to indicate supersedure status. Then, ensuring that the queen was sequestered to prevent unintentional sampling, two samples of ~ 300 workers were taken in resealable bags; one being flash frozen with dry ice, and stored at − 80 °C for pathogen analyses; and the other being frozen with conventional ice, for Varroa analysis. To achieve a representative age sample, workers were removed from two brood frames, containing sealed and unsealed brood. Workers were evenly mixed using a 20 L bucket, before collection via a cup-measure, and subsequent assignation to one of the two sample types. The queen was then returned to the hive, along with any unsampled workers.
Varroa analysis
Varroa analyses were conducted for all colonies, at all five time points. To quantify the level of Varroa infestation in colonies, a detergent-wash method was employed114. The sample of bees was first placed in a mesh-partitioned cup with a solid base, to allow mite retrieval. Water was then added until the bees were submerged, and a surfactant-based detergent (Procter & Gamble) mixed in, ensuring an even coating. The lid of the cup was secured, and it was placed into a Model E5850 reciprocal shaker (Eberbach), to be agitated for a period of 60 min at 120 rpm. After agitation, the lower faction of the cup was removed, and the contents poured into a shallow tray, to count the number of mites falling through the mesh. This process was repeated until two consecutive zero counts were recorded, indicating that all mites had been removed. The bees were then separated and counted by tally counter, to produce an exact sample size. Finally, the total Varroa count was transformed to produce a standardised ‘mites per hundred bees’ (MPHB) value, as follows:
Pathogen analyses
Four specific viral targets were chosen: DWV-A, DWV-B, CBPV, and BQCV. Analyses were carried out on a randomised subset of 92 colonies from the California and Mississippi migration groups, divided evenly across stock, and survival outcome. Four major time points were selected: June, September, December, and February. All analyses were conducted using RT-qPCR, following previously established methods94,115.
RNA extraction
Per colony and time point, pools of 65 bees were randomly selected from the master samples of ~ 300, placed into 30 ml 19-6358Z bead tubes (Omni), and stored at − 80 °C, for homogenisation using a Bead Ruptor Elite (Omni). Immediately after homogenisation, 5 ml of homogenisation solution (Promega), and 4 ml sterile 1xPBS buffer, each at 4 °C, were added to sample tubes, before vortexing for 20 s using a Vortex-Genie 2 (Scientific Industries). 1.8 ml of each sample was then transferred to 2 ml tubes (Eppendorf), and centrifuged for 60 s at 5000 rpm, at 4 °C, using a 5430-R centrifuge (Eppendorf). Per sample, 400 μl of the resultant supernatant was then transferred to Maxwell RSC-48 cartridges (Promega), prepared in accordance with the manufacturer’s instructions, and RNA extractions were carried out using the Maxwell RSC simplyRNA tissue protocol (Promega) (Maxwell RSC simplyRNA Tissue Kit, AS1340, TM416, 2019).
Sample purity and yield were then calculated in duplicate, using a NanoDrop One microvolume UV–Vis spectrophotometer (Thermo Fisher Scientific), and appropriate RNA dilutions were completed to ensure sample standardisation to 100 ng/μl. Following standardisation, cDNA synthesis was achieved using QuantiTect Reverse Transcription Kits (Qiagen), utilising a T100 thermal cycler (Bio-Rad), running custom QuantiTect cDNA synthesis protocols (Qiagen) (QuantiTect Reverse Transcription Handbook, 2009). Resultant samples were stored at − 20 °C, prior to initiation of RT-qPCR analyses.
RT-qPCR
RT-qPCR was performed on 1 μl aliquots of each sample, in triplicate, in a total reaction volume of 10 μl, utilising SsoAdvanced Universal SYBR Green Supermix (Bio-Rad), on Multiplate 96-well optical PCR plates (Bio-Rad). The primers used for quantification of DWV-A116, DWV-B117, CBPV96, and BQCV115 have been reported previously, and validated for target specificity. For full sequence details, see (Table S2). Negative controls were included for each target, consisting of 1 μl of nuclease-free H2O (Promega), again run in triplicate. Additionally, to enable viral quantity mean determination, standard curves were produced for all targets, via tenfold serial dilutions of known viral quantities, covering 8 orders of magnitude. Linearity (r2), and reaction efficiency (E), were maintained at ≥ 0.990, and ≥ 92.5% respectively, for all assays (Table S2). All analyses were run on CFX Connect Real-Time PCR Detection Systems (Bio-Rad), using previously optimised thermal protocols (Table S3).
Data transformation
Samples were analysed in triplicate, to form a mean Ct value. Any triplicates with a standard deviation ≥ 1.0 were examined, and the divergent replicate removed, resulting in 26 technical replicate removals. If this failed to bring the standard deviation to < 1.0, the sample itself was replaced, and removed from further analyses. Ct means were then quantified against the standard curves for each target, to calculate absolute viral titres, using the following equation:
Due to the wide distribution of quantities inherent to viral replication dynamics118, these values were then log-transformed, to produce suitable data for further analyses. Where undetected values were present, a constant Ct mean of 45 was assumed, to allow for subsequent transformation of the data105.
Range of assessment factors
Analyses were broadly divided into measurements of colony survival, the extraneous influences of Varroa levels (MPHB), and viral titres (log10 viral quantity mean per μl cDNA) upon them, and how stock, mite treatment, and migration route modulated these interactions. Crucially, we examined both the general effect of Varroa levels and viral titres upon colony survival, and the predictive power of such measures at seasonally-relevant time points. We additionally determined the explanatory power of viral titres when colonies were matched for Varroa level, thus elucidating the relative additive influences of Varroa-transmissible viruses.
Colony and queen survival over time, population size, and honey production
First, the survival of colonies and queens over time, along with the population sizes of surviving colonies, and honey production, were compared between the two stocks using time series data collected throughout the course of the study. Colonies were classified as ‘dead’ when the worker frame-count dropped to < 1.0, and they were confirmed as vacant during any sequential sampling. This definition of colony death remained consistent across all further analyses. Queens were classified as ‘dead’ when the existing queen could not be found, and a new unmarked queen, or queen cells, were present, indicating supersedure119. Population sizes were compared using worker frame-counts from all colonies classified as ‘alive’, that is, those with ≥ 1.0 frames at the end of the study, in February. Honey production was quantified via net weight (kg) of honey extracted per colony during September, excluding colonies in the stationary migration group, for which extraction was not recorded.
Factors influencing colony survival
We then defined the influences of Varroa levels and viral titres upon colony survival, using data from all five and four time points, respectively. The effect of each factor, along with stock, mite treatment, migration route, and their concordant interactions, was quantified. By tracking individual colonies over time, in concert with their Varroa levels, and viral titres, we were able to determine factor effect sizes, and the interactions leading to death or survival in February. February was chosen as the time point of interest, as it fell immediately prior to almond pollination, and thus gave a representative, and commercially relevant, measure of colony strength going into this system. As viral titre measures were taken for a subset of colonies, separate analyses were conducted for these and Varroa measures, in order to preserve maximum sample sizes in each case. These data were then used to inform subsequent predictive analyses, based on Varroa levels and viral titres.
Predictive differences in Varroa levels and viral titres
To assess initial predictive differences in Varroa levels and viral titres, across survival outcomes and stocks, we linked survival status in February to two predictive time points: June, and September. Notably, the former of these encompassed the principle period of colony population growth, and the latter, of honey harvesting, prior to colony overwintering. These points constitute key junctures at which management decisions must be made, and thus are practically relevant as predictive benchmarks. In analyses, colonies were classified by stock, or their status as being either alive or dead in February. Varroa levels and viral titres were then compared between groups, thus representing a basic test of the usefulness of single time point data in defining outcomes.
Prognostic power of Varroa levels and viral titres
To quantify the epidemiological significance of Varroa levels and viral titres in determining colony survival, and therefore their predictive power, we conducted relative risk (RR) analyses for these factors, utilising colony death by February as the outcome of choice. RR is an established epidemiological measure, used to determine the probability of a given health outcome when exposed to a specific risk factor, and thus compare risk magnitudes120. RR analyses were conducted for both Varroa levels, and viral titres, again for the predictive time points of June and September. Significant interactions were further validated with attributable risk (AR) analyses, to provide a measure of effect size; and complementary Bayesian RR analyses, to visualise the risk probability distribution. Analyses were pooled across stocks, in order to better encompass the full range of infestation and infection strata. Additionally, they were stratified by Varroa level, to determine how changes in infestation severity influenced the risk of colony death.
We then isolated the additive effect of viral titres as a predictive factor, independent of Varroa. We considered this an important test of the stand-alone influence of Varroa-associated pathogens in Varroa-mediated colony loss, as ordinarily, these factors are difficult to decouple. To achieve this, a subset of 60 colonies were matched by September Varroa level (mean MPHB, < ± 0.1 between survival outcomes), and left unstratified in terms of viral titres. September was chosen as the key matching point for this analysis, as colonies showed the highest Varroa levels and viral titres at this stage, and thus these values were considered most relevant to overwinter survival outcomes. Colonies were subsequently divided into alive and dead cohorts, based on their status in February, to produce groups that differed in their survival outcomes, and viral titres, but that had tightly coupled Varroa level distributions. We then conducted RR analyses for this subset, examining viral titres in both June and September as predictive factors for colony survival, hence constituting a measure of viral effects, decoupled from Varroa.
Varroa model
To demonstrate the relationship between Varroa level and colony mortality, and generate a predictive model for treatment prognoses in both stocks, we pooled all colony data into Varroa infestation strata, derived from Varroa level in September, and assigned resultant percentage mortalities based on survival rates in each stratum. We then fit curve models to the data for the two stocks, thus enabling a predictive mortality outcome for the Varroa level and stock in question, based on an extensive empirical dataset.
Statistical analyses
For all pairwise comparison data in which analyses would assume a normal distribution, we performed Shapiro–Wilk tests to check for normality, and hence inform the application of appropriate statistical tests. In all cases, the data were not normally distributed, and thus independent sample Mann–Whitney U-tests were employed. For these analyses, we opted to report mean ranks, rather than medians, as based on descriptive statistics, we did not assume identical frequency distributions between sample groups. Due to the large sample sizes used, we also considered it important to include a measure of effect size, and this was achieved via Eta-squared (η2) post hoc tests121. Additionally, for analyses that were close to the threshold of significance (0.05–0.06), we cross-verified results by applying parametric methods to transformed data. This occurred in one case, and after transformation via reflection of the square root, the significance of the test was unaltered.
Cumulative colony and queen survival differences between stocks were assessed using mixed-effects Cox proportional hazards models, via the R packages ‘coxme’122 and ‘multcomp’123. The proportional hazards assumption was verified in each case by confirming independence between the Schoenfeld residuals and time, for both the fixed factors and model, using the R package ‘survival’124.
To assess differences between stocks in colony population size and honey production, we utilised generalised linear models (GLMs). The population size model used February as the response time point, while the honey production model used September. Model selection was based on AIC, and validated using omnibus tests, along with assessment of the deviance to degrees of freedom ratios.
To determine how Varroa levels and viral titres influenced colony survival outcome, and how stock, mite treatment, and migration route modulated these interactions, we employed generalised linear mixed models (GLMMs) with repeated measures. Separate models were generated for Varroa level and viral titre analyses, in order to maximise sample sizes, and minimise model complexity. Varroa level measurements were repeated over five time points, and viral titre measurements over four. Model selection was based on AIC, beginning with the full model and interactions. In all cases, model fit was validated via evaluation of the binned standardised residuals.
When testing the predictive power of Varroa levels and viral titres at single time points, we used relative risk (RR) analyses. All RR analyses made use of an ‘outgroup’ for the epidemiological factor in question, and then compared the mortality rate in this group to that of an ‘exposure’ group, to ascertain the relative change in risk. The exposure group in all viral titre analyses was defined as colonies with titres ≥ 1 × 107, a cut-off value indicative of an epidemiologically severe infection1,96,98, while the outgroup was composed of colonies with titres < 1 × 107. The exposure groups in the Varroa level analyses were stratified to cover the range of MPHB levels present in colonies, ≥ 1–2.5, > 2.5–5, > 5–7.5, and > 7.5, and the outgroup was maintained at < 1, thus providing a graded measure of the change in risk with increasingly severe infestations. RR values greater than one indicated an associated increase in mortality risk, while those less than one indicated an associated reduction. In accordance with standard interpretation, RR values were deemed not significant if the resultant 95% confidence intervals overlapped the value of one120. For significant RR values, we also calculated the AR, to demonstrate the magnitude of absolute effect size, as is recommended for binary epidemiological outcomes125. Significant risk probability distributions were then visualised via parallel Bayesian RR analyses, using the R package ‘brr’126. Across tests, we confirmed requisite sample sizes to provide a minimum power (1 − β) of 0.80, at an alpha (α) of 0.05, using standard deviation (σ) and mean difference (δ) values from the data. All statistical analyses were performed in PASS (release v. 21.0.2), SPSS (release v. 25.0.0.0), and R (release v. 4.0.2)127.
Colony and queen survival over time
We used mixed-effects Cox proportional hazards models to assess differences in colony and queen survival between the two stocks over the course of the study. These utilised stock, mite treatment, and migration route as fixed factor predictors, and colony ID as a random factor. As sampling occurred every two months, cumulative survival was updated on a bi-monthly basis, over a 10 month period.
Population size and honey production
The GLM assessing the effect of stock on colony population size used frame-count of surviving colonies in February as a gamma response variable with a log link, and stock, mite treatment, and migration route as fixed factor predictors. The GLM assessing the effect of stock on honey production used net honey weight in September as a gamma response variable with a log link, stock and mite treatment as fixed factor predictors, and omitted migration route as honey was extracted prior to transport.
Factors influencing colony survival
The GLMM assessing the effect of Varroa levels on colony survival used colony survival status in February as a binomial response variable with a probit link, Varroa MPHB, stock, mite treatment, and migration route as fixed factor predictors, their two-way interactions, and colony ID as a random factor. The GLMM assessing the effect of viral titres on colony survival used colony survival status in February as a binomial response variable with a logit link, log10 DWV-A titre, log10 DWV-B titre, log10 CBPV titre, log10 BQCV titre, stock, mite treatment, and migration route as fixed factor predictors, their two-way interactions, and colony ID as a random factor.
Predictive differences in Varroa levels and viral titres
Independent sample Mann–Whitney U-tests were utilised to compare differences in mean Varroa levels and viral titres, based on colony survival outcome and stock, for June and September time points.
Prognostic power of Varroa levels and viral titres
To quantify the relative predictive powers of Varroa levels and viral titres in June and September when determining colony mortality, and that of viral titres when matched for Varroa level, we used RR, and AR, analyses. To visualise risk probability distributions, we used Bayesian RR analyses, utilising a Poisson response, and the Jeffreys prior as an objective reference prior.
Varroa model
A curve estimation analysis was employed to fit curve equations to the Varroa model data, and curve selection was evaluated via the resultant model outputs, based on F-values.
Results
Colony and queen survival over time
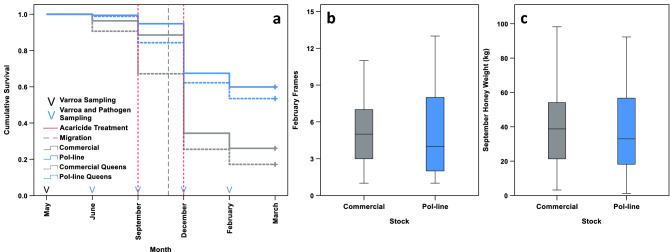
Percentage survival over time was significantly greater in Pol-line colonies when compared to Commercial colonies (Mixed-effects Cox proportional hazards model, effect of stock: hazard ratio = 0.408, χ2 = 122.800, d.f. = 1, N = 366, P < 0.001, percentage survivalPol-line = 59.884%, percentage survivalCommercial = 26.042%; Fig. 2a), and in colonies receiving the high mite treatment, as opposed to the low mite treatment (Mixed-effects Cox proportional hazards model, effect of mite treatment: hazard ratio = 0.516, χ2 = 25.192, d.f. = 1, N = 366, P < 0.001, percentage survivalhigh = 57.051%, percentage survivallow = 30.769%). Additionally, cumulative survival differed significantly between migration treatments (Mixed-effects Cox proportional hazards model, effect of migration route: χ2 = 10.854, d.f. = 2, N = 366, P = 0.004, percentage survivalStationary = 26.415%, percentage survivalCalifornia = 39.355%, percentage survivalMississippi = 50.000%). This difference was explained by higher survival in the California (Mixed-effects Cox proportional hazards model, Benjamini–Hochberg post hoc test: hazard ratio = 0.607, z = − 2.854, d.f. = 2, P = 0.004, percentage survivalCalifornia = 39.355%, percentage survivalStationary = 26.415%), and Mississippi (Mixed-effects Cox proportional hazards model, Benjamini–Hochberg post hoc test: hazard ratio = 0.570, z = − 3.206, d.f. = 2, P = 0.002, percentage survivalMississippi = 50.000%, percentage survivalStationary = 26.415%) migration groups, when compared to the Stationary migration group. Colony ID had a significant random effect (colony random effect: variance = 0.393, P < 0.001).
Figure 2.
(a) Survival proportions of colonies (solid lines), and queens (dashed lines), of each stock (Commercial, grey; Pol-line, blue), across the course of the study (NCommercial = 193, NPol-line = 173). Dashed vertical lines indicate the timings of management events (acaricide treatment, red; migration for overwintering, grey). Arrows designate the timings of sampling (Varroa sampling, black; Varroa and pathogen sampling, blue). Significant differences between stocks were present in colony survival (hazard ratio = 0.408, χ2 = 122.800, d.f. = 1, N = 366, P < 0.001), and queen survival (hazard ratio = 0.355, χ2 = 234.388, d.f. = 1, N = 366, P < 0.001). (b) Frames of adult bees in surviving colonies in February (NCommercial = 50, NPol-line = 103). Frame counts did not differ significantly between the two stocks (χ21 = 2.272, N = 153, P = 0.131). (c) Honey production in September (NCommercial = 141, NPol-line = 137). Honey production did not differ significantly between stocks (χ21 = 0.476, N = 278, P = 0.490).
Percentage queen survival over time was also significantly greater in Pol-line colonies when compared to Commercial colonies (Mixed-effects Cox proportional hazards model, effect of stock: hazard ratio = 0.355, χ2 = 234.388, d.f. = 1, N = 366, P < 0.001, percentage queen retentionPol-line = 88.350%, percentage queen retentionCommercial = 66.000%; Fig. 2a), marginally independent of mite treatment (Mixed-effects Cox proportional hazards model, effect of mite treatment: χ2 = 3.603, d.f. = 1, N = 366, P = 0.058), and migration route (Mixed-effects Cox proportional hazards model, effect of migration route: χ2 = 6.857, d.f. = 2, N = 366, P = 0.052). Colony ID again had a significant random effect (colony random effect: variance = 0.852, P < 0.001).
Population size and honey production
The population size of surviving colonies in February did not differ significantly between the two stocks (GLM, effect of stock: χ21 = 2.272, N = 153, P = 0.131; Fig. 2b), or mite treatments (GLM, effect of mite treatment: χ21 = 0.735, N = 153, P = 0.391), with migration route being the only significant factor (GLM, effect of migration route: χ22 = 8.935, N = 153, P = 0.011, mean frame-countStationary = 3.240, mean frame-countMississippi = 4.597, mean frame-countCalifornia = 5.614). Specifically, this difference was accounted for by higher frame counts in surviving colonies within the California migration group, when compared to those in the Stationary migration group (GLM, Holm-Bonferroni post hoc test: t = 3.154, d.f. = 1, P = 0.005, mean frame-countCalifornia = 5.614, mean frame-countStationary = 3.240). Similarly, honey production did not significantly differ between stocks (GLM, effect of stock: χ21 = 0.476, N = 278, P = 0.490; Fig. 2c), or mite treatments (GLM, effect of mite treatment: χ21 = 0.371, N = 278, P = 0.542).
Factors influencing colony survival
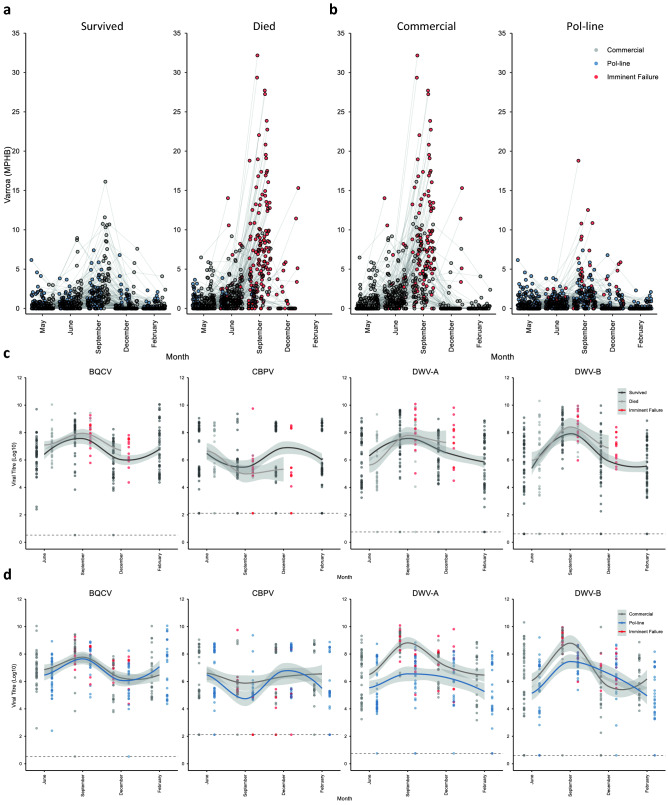
When examining the effect of Varroa level, survival status in February was significantly influenced by colony stock, mite treatment, migration route, and Varroa level (Fig. 3a,b) (Table 1). Additionally, significant interactions occurred between stock and mite treatment, and stock and Varroa level, indicating the importance of stock as a modulating factor (Fig. 3b; Table 1). The interaction between mite treatment and Varroa level was not significant (Table 1), suggesting that although Varroa level was reduced by mite treatment, this did not alter its underlying relationship with colony survival.
Figure 3.
(a,b) Varroa levels in colonies, divided by survival outcome (a), and stock (b), across the course of the study (NCommercial = 193, NPol-line = 173). Each point represents a single measure of Varroa for the corresponding month, and lines link repeated measures for the same colonies over time. Points are coloured based on stock (Commercial, grey; Pol-line, blue), and colonies that died by the following sampling point are highlighted (imminent failure, red). This latter designation is included to visualise the Varroa levels of colonies prior to failure. Varroa level had a significant influence upon colony survival outcomes (F1,373 = 53.124, P < 0.001). (c,d) Viral titres in colonies for each survival outcome (c), and stock (d), divided by target, across the course of the study (NCommercial = 46, NPol-line = 42). Points represent single month measures of viral titre for colonies, offset at each time point by survival outcome in February (survived, dark grey; died, light grey) (c), or stock (Commercial, grey; Pol-line, blue) (d). Colonies that were dead by the following sampling point are highlighted (imminent failure, red), and offset to their concordant group. This designation is included to visualise the viral titres of colonies prior to failure. Solid lines indicate LOESS (locally weighted scatterplot smoothing) moving averages, coloured according to their corresponding group. Shaded areas represent 95% confidence intervals. Dashed lines designate the baseline Ct detection limits for each target, after transformation. Viral tires of DWV-A (F1,66 = 1.026, P = 0.315), DWV-B (F1,85 = 0.206, P = 0.651), CBPV (F1,80 = 0.516, P = 0.475), and BQCV (F1,69 = 3.142, P = 0.081), did not have significant effects upon colony survival outcomes.
Table 1.
Summary statistics for GLMM assessing the effect of Varroa levels on colony survival.
| Type | Factor | Statistic | d.f | P |
|---|---|---|---|---|
| Fixed effect | Stock | F = 49.233 | 1, 371 | < 0.001 |
| Mite treatment | F = 24.385 | 1, 384 | < 0.001 | |
| Migration route | F = 3.337 | 2, 373 | 0.037 | |
| Varroa level | F = 53.124 | 1, 373 | < 0.001 | |
| Stock × Mite treatment | F = 17.502 | 1, 375 | < 0.001 | |
| Stock × Varroa level | F = 18.912 | 1, 360 | < 0.001 | |
| Mite Treatment × Varroa level | F = 1.042 | 1, 430 | 0.308 | |
| Fixed coefficient | Varroa level | Coefficient = 0.123 | 373 | < 0.001 |
| Pol-line × Varroa level | Coefficient = -0.095 | 360 | < 0.001 | |
| Holm-Bonferroni Post Hoc Test | Mite treatment: low–high | t = 5.940 | 378 | < 0.001 |
| Stock: commercial—Pol-line | t = 8.106 | 381 | < 0.001 | |
| Migration route: Stationary—Mississippi | t = 3.563 | 382 | 0.001 | |
|
Migration route: California—Mississippi |
t = 2.603 | 375 | 0.019 | |
| Low mite treatment: commercial—pol-line | t = 7.477 | 387 | < 0.001 | |
| High mite treatment: commercial—Pol-line | t = 1.401 | 386 | 0.162 | |
| Random effect | Colony ID | Z = 9.608 | – | < 0.001 |
Symbols INDICATE factor interactions (×), pairwise comparisons (−), and groupings (:), d.f. are calculated via the Welch–Satterthwaite approximation.
Specifically, when examining the influence of Varroa, colonies were significantly more likely to die during the course of the study, if they had higher Varroa levels (meandead = 2.698 MPHB, meanalive = 0.914 MPHB; Fig. 3a,b), were in the low mite treatment group (mean survivallow = 0.212, mean survivalhigh = 0.645), were of Commercial stock (mean survivalCommercial = 0.138, mean survivalPol-line = 0.666), and were in the Stationary or California, rather than Mississippi migration group (mean survivalStationary = 0.184, mean survivalCalifornia = 0.315, mean survivalMississippi = 0.541) (Table 1). Stock influenced the effect of mite treatment on survival, as Commercial colonies benefitted from an additional mite treatment to a greater extent than did Pol-line colonies, exemplified by their significantly reduced survival at low (mean survivalCommercial = 0.028, mean survivalPol-line = 0.625), but not at high mite treatment levels (mean survivalCommercial = 0.559, mean survivalPol-line = 0.724) (Table 1). Stock also influenced the effect of Varroa level on survival, as being of Pol-line stock significantly improved the prognosis for any given Varroa level (Fig. 3b; Table 1), leading to better survival outcomes across infestation strata. Colony ID had a significant random effect (Table 1), indicating a degree of unexplained inter-colony variability in survival outcomes, as would be expected.
In contrast to Varroa levels, there was no significant influence of DWV-A, DWV-B, CBPV, or BQCV titres upon colony survival status in February (Fig. 3c,d; Table 2). Additionally, when analysing the relationship between viral titres and survival, no effect of stock, mite treatment, or migration route was present, as colonies were selected based on an even distribution of stocks and migration routes across survival outcomes, to minimise any confounding effects upon viral dynamics (Table 2). As with the Varroa data, colony ID had a significant random effect (Table 2).
Table 2.
Summary statistics for GLMM assessing the effect of viral titres on colony survival.
| Type | Factor | Statistic | d.f | P |
|---|---|---|---|---|
| Fixed effect | Stock | F = 1.352 | 1, 64 | 0.249 |
| Mite treatment | F = 0.216 | 1, 67 | 0.644 | |
| Migration route | F = 0.135 | 1, 56 | 0.715 | |
| DWV-A titre | F = 1.026 | 1, 66 | 0.315 | |
| DWV-B titre | F = 0.206 | 1, 85 | 0.651 | |
| CBPV titre | F = 0.516 | 1, 80 | 0.475 | |
| BQCV titre | F = 3.142 | 1, 69 | 0.081 | |
| Random effect | Colony ID | Z = 4.185 | – | < 0.001 |
d.f. are calculated via the Welch–Satterthwaite approximation.
Predictive differences in Varroa levels and viral titres
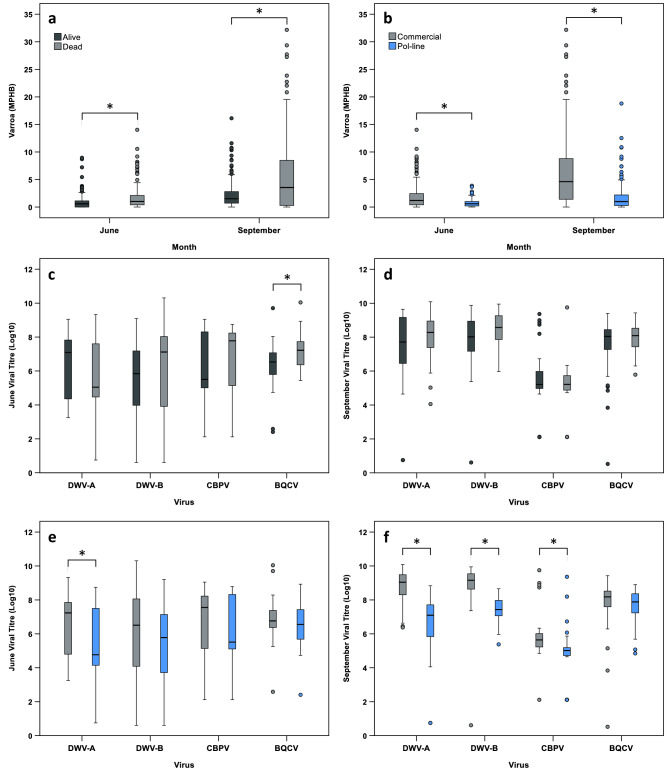
Varroa levels were significantly higher in colonies that died during the course of the study, when compared at both June (mean rankdead = 204.520, mean rankalive = 144.590), and September time points (mean rankdead = 201.890, mean rankalive = 119.550) (Fig. 4a; Table 3). Additionally, Pol-line colonies displayed significantly lower Varroa levels than Commercial colonies in both June (mean rankPol-line = 140.850, mean rankCommercial = 212.530) and September (mean rankPol-line = 106.440, mean rankCommercial = 215.200) (Fig. 4b; Table 3), indicating a predictive association between Varroa level, stock, and colony survival.
Figure 4.
(a,b) June and September differences in Varroa levels (June, NCommercial = 190, NPol-line = 167; September, NCommercial = 167, NPol-line = 157). (c–f) June and September differences in viral titres (June, NCommercial = 46, NPol-line = 42; September, NCommercial = 37, NPol-line = 39). Boxplots are coloured based on survival outcome in February (survived, dark grey; died, light grey) (a,c,d), and stock (Commercial, grey; Pol-line, blue) (b,e,f). Outliers (greater than 1.5 times the interquartile range from the median) are indicated with circles. Asterisks indicate significant differences between groups (η2 > 0.060, P < 0.05).
Table 3.
Summary statistics for Mann–Whitney U-tests comparing predictive differences in Varroa levels.
| Response | Factor | Statistic | N | η2 | P |
|---|---|---|---|---|---|
| Survival | June Varroa level | U = 20,811.000 | 152, 205 | 0.082 | < 0.001 |
| September Varroa level | U = 19,755.000 | 155, 169 | 0.193 | < 0.001 | |
| Stock | June Varroa level | U = 9,493.500 | 190, 167 | 0.120 | < 0.001 |
| September Varroa level | U = 4,308.000 | 167, 157 | 0.337 | < 0.001 |
Eta-squared values indicate factor effect sizes (small, η2 ≥ 0.010- < 0.060; intermediate, η2 ≥ 0.060- < 0.140; large, η2 ≥ 0.140).
Viral titres in June were not significantly different between colonies that died, or survived, for DWV-A (mean rankdead = 40.680, mean rankalive = 46.790), DWV-B (mean rankdead = 49.000, mean rankalive = 41.800), or CBPV (mean rankdead = 45.440, mean rankalive = 43.940) (Fig. 4c; Table 4). However, June BQCV titres were higher in colonies that died by February (mean rankdead = 53.200, mean rankalive = 39.280; Fig. 4c; Table 4). In September, there were no significant differences between colonies that died, or survived, for any of the viral titres measured (DWV-A: mean rankdead = 43.000, mean rankalive = 35.880; DWV-B: mean rankdead = 44.840, mean rankalive = 34.800; CBPV: mean rankdead = 36.960, mean rankalive = 39.400; BQCV: mean rankdead = 41.140, mean rankalive = 36.960; Fig. 4d; Table 4).
Table 4.
Summary statistics for Mann–Whitney U-tests comparing predictive differences in viral titres.
| Response | Factor | Statistic | N | η2 | P |
|---|---|---|---|---|---|
| Survival | June DWV-A titre | U = 781.500 | 55, 33 | 0.013 | 0.277 |
| June DWV-B titre | U = 1,056.000 | 55, 33 | 0.019 | 0.200 | |
| June CBPV titre | U = 938.500 | 55, 33 | 0.001 | 0.789 | |
| June BQCV titre | U = 1,194.500 | 55, 33 | 0.070 | 0.013 | |
| September DWV-A titre | U = 798.000 | 48, 28 | 0.021 | 0.175 | |
| September DWV-B titre | U = 849.500 | 48, 28 | 0.048 | 0.056 | |
| √-x (September DWV-B titre) | t = 1.951 | 48, 28 | 0.051 | 0.055 | |
| September CBPV titre | U = 629.000 | 48, 28 | 0.003 | 0.643 | |
| September BQCV titre | U = 746.000 | 48, 28 | 0.008 | 0.426 | |
| Stock | June DWV-A titre | U = 680.500 | 46, 42 | 0.065 | 0.017 |
| June DWV-B titre | U = 753.000 | 46, 42 | 0.036 | 0.075 | |
| June CBPV titre | U = 954.500 | 46, 42 | < 0.001 | 0.923 | |
| June BQCV titre | U = 780.500 | 46, 42 | 0.027 | 0.121 | |
| September DWV-A titre | U = 124.500 | 37, 39 | 0.506 | < 0.001 | |
| September DWV-B titre | U = 131.500 | 37, 39 | 0.495 | < 0.001 | |
| September CBPV titre | U = 300.000 | 37, 39 | 0.252 | < 0.001 | |
| September BQCV titre | U = 597.000 | 37, 39 | 0.022 | 0.196 |
Eta-squared values indicate factor effect sizes (small, η2 ≥ 0.010– < 0.060; intermediate, η2 ≥ 0.060– < 0.140; large, η2 ≥ 0.140).
In June, Pol-line colonies showed significantly lower titres of DWV-A than did Commercial colonies (mean rankPol-line = 37.700, mean rankCommercial = 50.710); however, there were no significant differences between stocks in DWV-B (mean rankPol-line = 39.430, mean rankCommercial = 49.130), CBPV (mean rankPol-line = 44.230, mean rankCommercial = 44.750), or BQCV titres (mean rankPol-line = 40.080, mean rankCommercial = 48.530) (Fig. 4e; Table 4). In September, viral titres were significantly lower in Pol-line colonies for DWV-A (mean rankPol-line = 23.190, mean rankCommercial = 54.640), DWV-B (mean rankPol-line = 23.370, mean rankCommercial = 54.450), and CBPV (mean rankPol-line = 27.690, mean rankCommercial = 49.890) (Fig. 4f; Table 4), as would be expected based on their correlation with Varroa levels (Fig. S1a–c). In contrast, however, September BQCV titres did not differ significantly between stocks (mean rankPol-line = 35.310, mean rankCommercial = 41.860; Fig. 4f; Table 4), and showed no correlation with Varroa levels (Fig. S1d).
Prognostic power of Varroa levels and viral titres
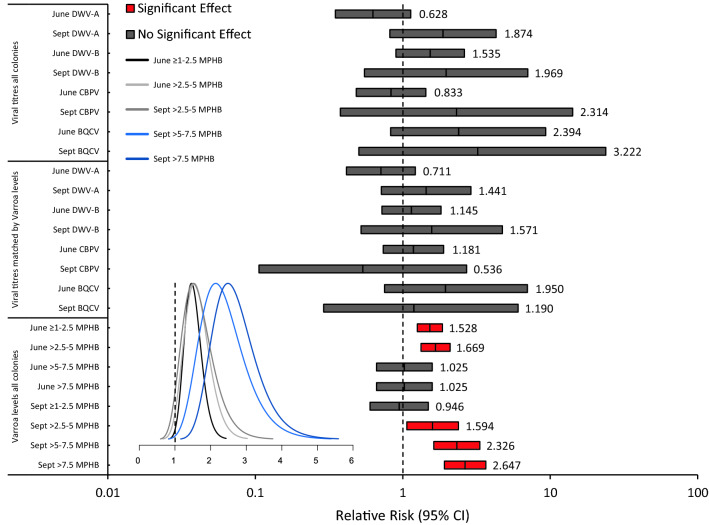
In June, Varroa levels significantly increased the relative risk of mortality by February if colonies fell into the ≥ 1–2.5 (RR, point estimate = 1.528, CI95% 1.256–1.859, AR = 0.238, P < 0.001; Fig. 5), or > 2.5–5 (RR, point estimate = 1.669, CI95% 1.330–2.059, AR = 0.303, P < 0.001; Fig. 5), MPHB infestation strata. However, the paucity of colonies with Varroa levels > 5 MPHB precluded significant prediction of mortality risk above this level (Fig. 5). In September, the initial risk threshold was higher, as Varroa levels began to significantly increase the relative risk of mortality only at > 2.5–5 (RR, point estimate = 1.594, CI95% 1.066–2.384, AR = 0.202, P = 0.024; Fig. 5), with the risk becoming sequentially greater at > 5–7.5 (RR, point estimate = 2.326, CI95% 1.624–3.333, AR = 0.431, P < 0.001; Fig. 5), and > 7.5 MPHB (RR, point estimate = 2.647, CI95% 1.916–3.658, AR = 0.536, P < 0.001; Fig. 5). Notably, no other factor significantly influenced the relative risk of mortality. For a list of all factors and risk probability distributions, see (Fig. 5).
Figure 5.
Relative mortality risks of Varroa levels (NCommercial = 193, NPol-line = 173), viral titres (NCommercial = 46, NPol-line = 42), and viral titres controlled for Varroa levels (NCommercial = 30, NPol-line = 30), as epidemiological exposure factors. Bars display relative risk point estimates, bounded by 95% confidence intervals, displayed on a log10 scale to accurately represent proportional change in risk. Colours indicate factor significance (RR, point estimate ± CI95% > 1.00, P < 0.05) as a predictor of mortality (significant effect, red; no significant effect, dark grey). Numbers provide exact relative risk point estimates. The inset displays posterior risk probability densities for significant factors, encompassing 100% credible intervals. Colours indicate factor (June ≥ 1–2.5 MPHB, black; June > 2.5–5 MPHB, light grey; September > 2.5–5 MPHB, dark grey; September > 5–7.5 MPHB, light blue; September > 7.5 MPHB, dark blue). For full details of exposure groupings, see “Materials and methods”.
Varroa model
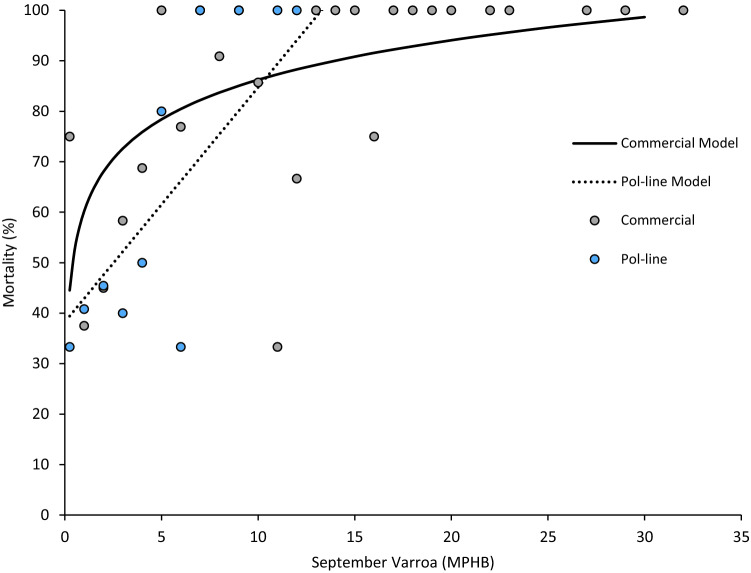
The relationship between Varroa level in September, and mortality, was explained by a logarithmic function in Commercial colonies (Curve estimation test, F1,24 = 12.851, R2 = 0.349, P < 0.001; Fig. 6), and a linear function in Pol-line colonies (Curve estimation test, F1,10 = 18.311, R2 = 0.647, P = 0.002; Fig. 6). At a threshold of 3 MPHB, high mortality was predicted for both Commercial (Curve estimation test, percentage mortalityCommercial = 72.618%; Fig. 6), and Pol-line colonies (Curve estimation test, percentage mortalityPol-line = 52.210%; Fig. 6), although this was substantially lower in the latter case. Interestingly, the two functions intersected at 10 MPHB, as despite considerable mortality, some commercial colonies were able to survive with very high infestation levels, as reflected in the reduced explanatory power of the commercial model (Fig. 6).
Figure 6.
Predictive relationship between September Varroa levels and colony mortality (NCommercial = 167, NPol-line = 157). Points are based on binned data from all colonies falling into a Varroa stratum, and concordant percentage mortality. Colours (Commercial, grey; Pol-line, blue), and line types (Commercial, solid; Pol-line, dashed), indicate stock. Lines are fit against the binned data for each stock (Commercial, R2 = 0.349, Pol-line, R2 = 0.647).
Discussion
Our results show that under migratory beekeeping conditions, Pol-line honey bees have significantly enhanced colony and queen survival when compared to a standard commercial Italian stock (Fig. 2a). Additionally, the productivity of Pol-line colonies appears equivalent to that of Commercial honey bees, as evidenced by comparable honey production and population sizes among surviving colonies (Fig. 2b,c). This improved performance is associated with lower Varroa levels (Figs. 3b, 4b), and concomitantly reduced titres of DWV-A, DWV-B, and CBPV in September (Fig. 3d, 4f). Notably, the multiplicative effect of Varroa levels on colony survival is significantly weaker in Pol-line colonies, leading to better survival prognoses at all but the highest infestation strata (Figs. 3b, 6; Table 1). An ostensible explanation for this is the constant removal of infested brood by Pol-line workers, as is characteristic of VSH behaviour48,54, and thus a subsequent dampening of Varroa growth dynamics and viral transmission. Consequently, Pol-line colonies that received only one mite treatment demonstrated survival rates empirically equivalent to Commercial colonies receiving two. Indeed, the benefit of additional mite treatments was generally greater for Commercial colonies, as the majority of Pol-line colonies naturally maintained Varroa levels below the recommended treatment threshold114. This has considerable implications both for improving colony survival, and reducing the escalating need for acaricide use27.
To our knowledge, these data constitute the fullest characterisation of improved functionality in a Varroa-resistant honey bee stock to date. Further, as the queens used in this study were mated via drone saturation, and the resultant colonies retained genetics for mite-resistance, our results are directly applicable to industrial implementation128. It should be noted that the present study comprises only a single year of data, and thus further work will be needed to validate Pol-line derivatives prior to commercial introduction. Encouragingly however, commercial field trials of untreated ‘Hilo’ bees60—a stock derived from Pol-line beginning in 2015—have shown superior overwinter survival, although slightly lower honey production, when compared to acaricide-treated commercial Italian, and Italian × Carniolan colonies (RGD, personal communication)129. Notably, allele frequency analyses place Pol-line and Hilo bees in a distinct genetic cluster, significantly differentiated from other commercial and research stocks currently used in the United States60. As such, our results serve as a promising empirical characterisation of the potential for derived Varroa-resistant stocks to fundamentally improve modern beekeeping.
The comparatively high mortality rates observed in this study7,8 are a consequence of two experimental design attributes. In order to assess the true resilience of colonies to Varroa, we necessarily included a minimal mite treatment group for each stock, resulting in significantly lower overall acaricide use than is standard in commercial operations. As such, Varroa levels were high, and its presence pervasive, with concordant implications for mite spillover and mortality prior to the second acaricide treatment—especially in the Commercial colonies. Additionally, industrial pollination operations place varied stresses on colonies, due to intensive transit, climatic extremes, high colony densities, and fluctuating local conditions57,63,130. The increased mortality levels observed in the stationary migration group exemplify this stochasticity, as colonies experienced a starvation event during the winter, leading to severe losses (Table 1). Such a result serves to highlight the importance of management regime and local stressors in influencing colony outcomes130, and is pertinent when considering distributed pollination systems. Notably, our results are not inconsistent with other commercial scenarios in which Varroa pressure is high, which is very often the case82,131. Accordingly, the present study constitutes a controlled but rigorous test, in a large-scale commercial operation, under significant parasite burden. In the case of stock validation, such a setup is preferable, as we wished to determine the continued efficacy of Pol-line’s outcrossed VSH traits54, and substantial Varroa pressure was required to do so effectively.
The findings from our assessment of Varroa, DWV-A, DWV-B, CBPV, and BQCV, and their influences upon colony mortality, were notable. In agreement with previous studies, Varroa levels were the single strongest predictor of colony mortality for both stocks, diverging as early as June, and peaking in September (Figs. 3, 4a,b; Tables 1, 3). Interestingly, the impacts of both early season and pre-winter Varroa levels on subsequent mortality, suggest that current treatment thresholds may not be conservative enough to curtail substantial losses (Figs. 3a, 6). Indeed, additional probit models support this assertion for the present data (Fig. S2). In contrast, titres of DWV-A, DWV-B, CBPV, and BQCV were not significant independent determinants of colony survival (Figs. 3c,d, 4c; Table 2). The only significant viral difference was in the case of June BQCV titres, which were higher in colonies that went on to die, however the effect size was intermediate (Table 4), and did not carry over into multivariate analyses, indicating a lack of causality.
While September titres of DWV-A, DWV-B, and BQCV were on average numerically greater in colonies that died, this difference was not significant when controlling for covariates, or when taken as a predictive measure, and levels of CBPV were in fact lower in colonies that were later lost (Figs. 3c, 4d; Table 2). DWV-A and DWV-B demonstrated broadly similar patterns across survival outcomes and stocks, except in December, in which DWV-B dropped to a greater extent in Commercial colonies than did DWV-A (Fig. 3d). The titres of DWV-A, DWV-B, and CBPV were significantly reduced in Pol-line colonies during September, and in the case of DWV-A, also during June; however BQCV showed no differences between stocks (Fig. 4e,f). This may be explained by the lower Varroa levels present in Pol-line colonies, with the greatest differences emerging in September (Figs. 3b, 4b; Table 3), and hence concomitantly reduced levels of directly and indirectly Varroa-associated pathogens (Figs. S1a–c). Notably, such a trend indicates that BQCV did not exhibit significant Varroa association, and is corroborated by its lack of direct correlation with September Varroa levels (Fig. S1d). This finding is pertinent to the etiological understanding of BQCV, as it suggests that despite potential replication in the mites102, and temporal correlations with Varroa invasion22, there is poor empirical evidence for an epidemiologically meaningful transmission pathway.
In contrast to DWV-A and DWV-B, the Varroa association of CBPV was comparatively weak (Fig. S1c). It is thus possible that stock-specific viral resistance mechanisms132,133, or destabilised immune function as a result of Varroa burdens134, played a role in the differences observed between stocks (Fig. 4f). Host–pathogen genotypic interactions are of interest in honey bee stock selection38, and may warrant further investigation in both Pol-line bees, and other mite-resistant varieties66,94. However, it will be important to separate such effects from pathogen opportunism, especially when stocks confer broadly improved colony health.
Taken together, our data suggest that DWV-A, DWV-B, and to a lesser extent CBPV titres, track Varroa levels, but are comparatively weaker predictors of colony survival outcome than Varroa itself. Relative and attributable risk analyses provided further insight into these results. When only colonies grouped by comparable Varroa levels were examined, differing titres of DWW-A, DWV-B, CBPV, and BQCV never constituted significant additive predictors of mortality risk (Fig. 5). Further, the only relative risk measures with a significant effect on mortality, belonged to those of Varroa levels alone (Fig. 5).
These findings are relevant to the understanding of DWV-A and DWV-B as causative agents of colony loss. Our data suggest that Varroa, as a factor, consistently has the greatest influence on mortality, likely as it encompasses both the effects of mechanical damage, via feeding, and the transmission of associated pathogens. While DWV and other viruses correlate with colony mortality, and have been shown both to kill, and sub-lethally influence bees65,135, it appears that when these effects are decoupled from their correlation with Varroa levels, their predictive power is significantly reduced. The crucial methodological and diagnostic implication of this, is that, at a minimum, compared to the viruses tested here, Varroa has significant additive power in predicting colony outcome. This additive effect may be explained by damage to the host’s fat body12,21, or additional latent pathogen transmission unaccounted for by single target measures17, and is corroborated by previous experimental evidence76,83,101,136. While such a trend does not dismiss the importance of Varroa-vectored viruses, it does suggest that the harm caused by Varroa feeding itself has been chronically underestimated, and implies that the established paradigm of Varroa-mediated colony loss may be incorrect. Notably, the comparatively diminished effect of viruses observed in our study, can be explained by the simultaneous use of a controlled mixed modelling approach, a commercially realistic sample size, and measures of viral titre only—rather than viral prevalence. The latter aspect is salient, as the potentially latent etiology of DWV infections137 necessitates a linkage between actual viral titres and mortality, especially if causative associations are to be made. It is true that Varroa-vectored viruses correlate with Varroa infestation levels, but the relationship is not monotonic1,22, and thus future studies linking these pathogens to mortality should include or control for Varroa levels, if they are to produce meaningful insight.
When considering colony losses, the relative weightings of DWV, or indeed any of the viruses vectored by Varroa, have never been effectively isolated from the effect of Varroa feeding per se12. It should be noted that, as demonstrated here, other datasets have found variable causative influences of viral titres upon bee health138, and colony mortality81,136. Further, to date, no studies have been able to decouple Varroa-virus mortality correlations from Varroa mortality correlations at the colony-level. Based on this, it is somewhat unclear as to why vectored viruses are assumed to be the prime, if not sole, causative agents of Varroa-mediated colony losses38,139, especially as the parasite’s mode of feeding necessitates organ damage12. Indeed, in certain cases, Varroa levels alone predict mortality, while viral titres fail to do so83,136. It is well established that by removing or controlling for Varroa mites, associated viruses may be managed indirectly111,112, thus, there is practical merit in focussing on Varroa, it being the upstream epidemiological factor. This is well-illustrated by the finding that while VSH behaviour can in fact lead to the spread of DWV via pupal cannibalism in the lab140, it nevertheless reduces DWV titres at the colony-level in the field (Figs. 3d, 4f), as it mitigates a more potent transmission pathway—that of Varroa feeding. In tandem with recent advances in the understanding of Varroa etiology12, a growing body of research also indicates that the mites themselves are not harmless vectors, but invasive epizootic parasites, that seriously damage their naïve hosts via destructive lipophagy. Alongside the present findings, this warrants serious revaluation of the relative importance of Varroa, and its transmitted viruses, in causing colony-level harm. Multifactorial approaches to understanding Varroa-virus dynamics, especially in relation to factor weightings, will be important in future epidemiological studies, as arguably, Varroa-mediated colony mortality still requires substantial mechanistic elucidation.
Our results demonstrate that Varroa-resistant honey bees present strong potential for reducing colony losses in commercial beekeeping operations. Incorporating the VSH trait into commercial stocks appears to engender both Varroa-resistance, and favourable colony productivity. Furthermore, we find that DWV-A, DWV-B, CBPV, and BQCV titres are inferior predictors of colony mortality when compared to Varroa levels alone. This implies that the relative damage caused by Varroa feeding per se, and the pathogens transmitted by this process, will require further investigation if effective mitigation methods are to be developed. In sum, our data suggest that a Varroa-centric control strategy, incorporating the adoption of resistant stocks, currently represents the most effective, sustainable, and technologically tractable solution to global honey bee losses.
Supplementary Information
Acknowledgements
The authors wish to thank David Dodge, Victor Rainey, Garrett Dodds, Dan Winfrey, Bob Cox, Hunter Martin, Nathalie Martin, and Josh Wales of the USDA ARS, Honey Bee Breeding, Genetics, and Physiology lab, for their technical support and valuable expertise; Hannah Penn, Sarah Lang, and Christopher Fellows from Louisiana State University, for field and laboratory support; and Kelvin Adee and the employees of Adee Honey Farms, without whom this work would not have been possible. The authors acknowledge support from the United States Department of Agriculture (USDA) National Institute of Food and Agriculture (NIFA) (https://nifa.usda.gov/) Grant 2017-69004-26515, ‘Minimizing the impact of Varroa mites and mite-borne pathogens on managed honey bees’. This Grant was awarded to K.B.H, R.D.G, M.S.-F and F.D.R. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The mention of trade names or commercial products in this publication is solely for the purpose of providing specific information, and does not imply recommendation or endorsement by the USDA. The USDA is an equal opportunity provider and employer.
Author contributions
T.A.O.-W., F.D.R., R.D.G., M.S.-F. and K.B.H. conceived the study, contributed to manuscript preparation, and were involved in data collection; T.A.O.-W. wrote the manuscript; T.A.O.-W. and P.G.T. conducted pathogen analyses; T.A.O.-W. carried out the statistical analyses. All authors edited the manuscript, and gave final approval for publication.
Data availability
The authors declare that all supporting data is available within the supplementary information.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-08643-w.
References
- 1.Martin SJ, et al. Global honey bee viral landscape altered by a parasitic mite. Science (80–) 2012;336:1304–1306. doi: 10.1126/science.1220941. [DOI] [PubMed] [Google Scholar]
- 2.Neumann P, Carreck NL. Honey bee colony losses. J. Apic. Res. 2010;49:1–6. [Google Scholar]
- 3.Ratnieks FLW, Carreck NL. Clarity on honey bee collapse? Science (80–) 2010;327:152–153. doi: 10.1126/science.1185563. [DOI] [PubMed] [Google Scholar]
- 4.Potts SG, et al. Safeguarding pollinators and their values to human well-being. Nature. 2016;540:220–229. doi: 10.1038/nature20588. [DOI] [PubMed] [Google Scholar]
- 5.Vanengelsdorp D, et al. A national survey of managed honey bee 2010–11 winter colony losses in the USA: Results from the bee informed partnership. J. Apic. Res. 2012;51:115–124. [Google Scholar]
- 6.Lee KV, et al. A national survey of managed honey bee 2013–2014 annual colony losses in the USA. Apidologie. 2015;46:292–305. [Google Scholar]
- 7.Kulhanek K, et al. A national survey of managed honey bee 2015–2016 annual colony losses in the USA. J. Apic. Res. 2017;56:328–340. [Google Scholar]
- 8.Bruckner, S. et al. Honey bee colony losses 2018–2019: Preliminary results. Bee Inf. (2019) https://beeinformed.org/results/honey-bee-colony-losses-2017-2018-preliminary-results/.
- 9.Nazzi F, Pennacchio F. Disentangling multiple interactions in the hive ecosystem. Trends Parasitol. 2014;30:556–561. doi: 10.1016/j.pt.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Francis RM, Nielsen SL, Kryger P. Varroa-virus interaction in collapsing honey bee colonies. PLoS One. 2013;8:25. doi: 10.1371/journal.pone.0057540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenkranz P, Aumeier P, Ziegelmann B. Biology and control of Varroa destructor. J. Invertebr. Pathol. 2010;103:S96–S119. doi: 10.1016/j.jip.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 12.Ramsey SD, et al. Varroa destructor feeds primarily on honey bee fat body tissue and not hemolymph. Proc. Natl. Acad. Sci. USA. 2019;116:1792–1801. doi: 10.1073/pnas.1818371116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin SJ. The role of Varroa and viral pathogens in the collapse of honeybee colonies: A modelling approach. J. Appl. Ecol. 2001;38:1082–1093. [Google Scholar]
- 14.Bowen-Walker PL, Gunn A. The effect of the ectoparasitic mite, Varroa destructor on adult worker honeybee (Apis mellifera) emergence weights, water, protein, carbohydrate, and lipid levels. Entomol. Exp. Appl. 2001;101:207–217. [Google Scholar]
- 15.Yang X, Cox-Foster DL. Impact of an ectoparasite on the immunity and pathology of an invertebrate: Evidence for host immunosuppression and viral amplification. Proc. Natl. Acad. Sci. USA. 2005;102:7470–7475. doi: 10.1073/pnas.0501860102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blanken LJ, van Langevelde F, van Dooremalen C. Interaction between Varroa destructor and imidacloprid reduces flight capacity of honeybees. Proc. R. Soc. B Biol. Sci. 2015;282:20151738. doi: 10.1098/rspb.2015.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Traynor KS, et al. Varroa destructor: A complex parasite, crippling honey bees worldwide. Trends Parasitol. 2020;36:592–606. doi: 10.1016/j.pt.2020.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Boecking O, Genersch E. Varroosis—the ongoing crisis in bee keeping. J. Verbraucherschutz Leb. 2008;3:221–228. [Google Scholar]
- 19.McMenamin AJ, Genersch E. Honey bee colony losses and associated viruses. Curr. Opin. Insect Sci. 2015;8:121–129. doi: 10.1016/j.cois.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 20.Levin S, et al. New viruses from the Ectoparasite Mite Varroa destructor infesting Apis mellifera and Apis cerana. Viruses. 2019;11:94. doi: 10.3390/v11020094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arrese EL, Soulages JL. Insect fat body: Energy, metabolism, and regulation. Annu. Rev. Entomol. 2010;55:207–225. doi: 10.1146/annurev-ento-112408-085356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mondet F, de Miranda JR, Kretzschmar A, Le Conte Y, Mercer AR. On the front line: Quantitative virus dynamics in honeybee (Apis mellifera L.) colonies along a new expansion front of the parasite Varroa destructor. PLoS Pathog. 2014;10:25. doi: 10.1371/journal.ppat.1004323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilfert L, et al. Honeybee disease: Deformed wing virus is a recent global epidemic in honeybees driven by Varroa mites. Science (80–) 2016;351:594–597. doi: 10.1126/science.aac9976. [DOI] [PubMed] [Google Scholar]
- 24.Santamaria J, et al. Evidence of Varroa-mediated deformed wing virus spillover in Hawaii. J. Invertebr. Pathol. 2017;151:126–130. doi: 10.1016/j.jip.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 25.Martin SJ, Elzen PJ, Rubink WR. Effect of acaricide resistance on reproductive ability of the honey bee mite Varroa destructor. Exp. Appl. Acarol. 2002;27:195–207. doi: 10.1023/a:1021675614116. [DOI] [PubMed] [Google Scholar]
- 26.Evans JD, Cook SC. Genetics and physiology of Varroa mites. Curr. Opin. Insect Sci. 2018;26:130–135. doi: 10.1016/j.cois.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 27.Rinkevich FD. Detection of amitraz resistance and reduced treatment efficacy in the Varroa Mite, Varroa destructor, within commercial beekeeping operations. PLoS One. 2020;15:1–12. doi: 10.1371/journal.pone.0227264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin SJ. Acaricide (pyrethroid) resistance in Varroa destructor. Bee World. 2004;85:67–69. [Google Scholar]
- 29.Grozinger CM, Flenniken ML. Bee viruses: Ecology, pathogenicity, and impacts. Annu. Rev. Entomol. 2019;64:205–226. doi: 10.1146/annurev-ento-011118-111942. [DOI] [PubMed] [Google Scholar]
- 30.Owen R. Role of human action in the spread of honey bee (Hymenoptera: Apidae) pathogens. J. Econ. Entomol. 2017;110:797–801. doi: 10.1093/jee/tox075. [DOI] [PubMed] [Google Scholar]
- 31.Rinderer TE, Harris JW, Hunt GJ, De Guzman LI. Breeding for resistance to Varroa destructor in North America. Apidologie. 2010;41:409–424. [Google Scholar]
- 32.Büchler R, Berg S, Le Conte Y. Breeding for resistance to Varroa destructor in Europe. Apidologie. 2010;41:393–408. [Google Scholar]
- 33.Eliash N, Mikheyev A. Varroa mite evolution: A neglected aspect of worldwide bee collapses? Curr. Opin. Insect Sci. 2020;39:21–26. doi: 10.1016/j.cois.2019.11.004. [DOI] [PubMed] [Google Scholar]
- 34.Beaurepaire AL, Krieger KJ, Moritz RFA. Seasonal cycle of inbreeding and recombination of the parasitic mite Varroa destructor in honeybee colonies and its implications for the selection of acaricide resistance. Infect. Genet. Evol. 2017;50:49–54. doi: 10.1016/j.meegid.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 35.Locke B. Natural Varroa mite-surviving Apis mellifera honeybee populations. Apidologie. 2016;47:467–482. [Google Scholar]
- 36.Oddie MAY, Dahle B, Neumann P. Norwegian honey bees surviving Varroa destructor mite infestations by means of natural selection. PeerJ. 2017;5:e3956. doi: 10.7717/peerj.3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oddie M, et al. Rapid parallel evolution overcomes global honey bee parasite. Sci. Rep. 2018;8:1–9. doi: 10.1038/s41598-018-26001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mondet F, et al. Honey bee survival mechanisms against the parasite Varroa destructor: A systematic review of phenotypic and genomic research efforts. Int. J. Parasitol. 2020;50:433–447. doi: 10.1016/j.ijpara.2020.03.005. [DOI] [PubMed] [Google Scholar]
- 39.Seeley TD. Life-history traits of wild honey bee colonies living in forests around Ithaca, NY, USA. Apidologie. 2017;48:743–754. [Google Scholar]
- 40.Loftus JC, Smith ML, Seeley TD. How honey bee colonies survive in the wild: Testing the importance of small nests and frequent swarming. PLoS One. 2016;11:1–11. doi: 10.1371/journal.pone.0150362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seeley TD, Smith ML. Crowding honeybee colonies in apiaries can increase their vulnerability to the deadly ectoparasite Varroa destructor. Apidologie. 2015;46:716–727. [Google Scholar]
- 42.Dynes TL, Berry JA, Delaplane KS, Brosi BJ, De Roode JC. Reduced density and visually complex apiaries reduce parasite load and promote honey production and overwintering survival in honey bees. PLoS One. 2019;14:1–16. doi: 10.1371/journal.pone.0216286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Di Pasquale G, et al. Influence of pollen nutrition on honey bee health: Do pollen quality and diversity matter? PLoS One. 2013;8:1–13. doi: 10.1371/journal.pone.0072016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guarna MM, et al. Peptide biomarkers used for the selective breeding of a complex polygenic trait in honey bees. Sci. Rep. 2017;7:1–10. doi: 10.1038/s41598-017-08464-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grozinger CM, Robinson GE. The power and promise of applying genomics to honey bee health. Curr. Opin. Insect Sci. 2015;10:124–132. doi: 10.1016/j.cois.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Techer MA, et al. Divergent evolutionary trajectories following speciation in two ectoparasitic honey bee mites. Commun. Biol. 2019;2:25. doi: 10.1038/s42003-019-0606-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harbo JR, Harris JW. Responses to Varroa by honey bees with different levels of Varroa sensitive hygiene. J. Apic. Res. 2009;48:156–161. [Google Scholar]
- 48.Tsuruda JM, Harris JW, Bourgeois L, Danka RG, Hunt GJ. High-resolution linkage analyses to identify genes that influence varroa sensitive hygiene behavior in honey bees. PLoS One. 2012;7:5. doi: 10.1371/journal.pone.0048276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spivak M, Danka RG. Perspectives on hygienic behavior in Apis mellifera and other social insects. Apidologie. 2020 doi: 10.1007/s13592-020-00784-z. [DOI] [Google Scholar]
- 50.Rinderer TE, et al. The release of ARS Russian honey bees. Am. Bee J. 2000;140:305–308. [Google Scholar]
- 51.Guzman LIDE, Rinderer TE, Frake AM. Growth of Varroa destructor (Acari: Varroidae) Populations in Russian Honey Bee (Hymenoptera: Apidae) Colonies. Ecol. Popul. Biol. 2007;100:187–195. [Google Scholar]
- 52.Morfin N, Given K, Evans M, Guzman-Novoa E, Hunt GJ. Grooming behavior and gene expression of the Indiana “mite-biter” honey bee stock. Apidologie. 2019;51:1–9. [Google Scholar]
- 53.Pritchard DJ. Grooming by honey bees as a component of varroa resistant behavior. J. Apic. Res. 2016;55:38–48. [Google Scholar]
- 54.Danka RG, Harris JW, Dodds GE. Selection of VSH-derived “Pol-line” honey bees and evaluation of their Varroa-resistance characteristics. Apidologie. 2016;47:483–490. [Google Scholar]
- 55.Guichard M, et al. Three decades of selecting honey bees that survive infestations by the parasitic mite Varroa destructor: Outcomes, limitations and strategy. Genet. Sel. Evol. 2020 doi: 10.1186/s12711-020-00591-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ibrahim A, Reuter GS, Spivak M. Field trial of honey bee colonies bred for mechanisms of resistance against Varroa destructor. Apidologie. 2007;38:67–76. [Google Scholar]
- 57.Danka RG, et al. Functionality of Varroa-resistant honey bees (Hymenoptera: Apidae) when used in migratory beekeeping for crop pollination. J. Econ. Entomol. 2012;105:313–321. doi: 10.1603/ec11286. [DOI] [PubMed] [Google Scholar]
- 58.Nielsen C, Lund M. The concept of business model scalability. Soc. Sci. Res. Netw. 2015;20:1–20. [Google Scholar]
- 59.Bixby M, et al. A bio-economic case study of Canadian Honey bee (Hymenoptera: Apidae) colonies: Marker-assisted selection (MAS) in queen breeding affects beekeeper profits. J. Econ. Entomol. 2017;110:816–825. doi: 10.1093/jee/tox077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saelao P, et al. Genome-wide patterns of differentiation within and among US commercial honey bee stocks. BMC Genom. 2020;21:704. doi: 10.1186/s12864-020-07111-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harbo JR, Harris JW. Resistance to Varroa destructor (Mesostigmata: Varroidae) when mite-resistant queen honey bees (Hymenoptera: Apidae) were free-mated with unselected drones. J. Econ. Entomol. 2001;94:1319–1323. doi: 10.1603/0022-0493-94.6.1319. [DOI] [PubMed] [Google Scholar]
- 62.Free JB. Insect Pollination of Crops. Academic Press; 1993. [Google Scholar]
- 63.Delaplane KS, Mayer DR, Mayer DF. Crop Pollination by Bees. Cabi; 2000. [Google Scholar]
- 64.Beck HE, et al. Present and future köppen-geiger climate classification maps at 1-km resolution. Sci. Data. 2018;5:1–12. doi: 10.1038/sdata.2018.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Benaets K, et al. Covert deformed wing virus infections have long-term deleterious effects on honeybee foraging and survival. Proc. R. Soc. B Biol. Sci. 2017;284:25. doi: 10.1098/rspb.2016.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thaduri S, Stephan JG, de Miranda JR, Locke B. Disentangling host-parasite-pathogen interactions in a varroa-resistant honeybee population reveals virus tolerance as an independent, naturally adapted survival mechanism. Sci. Rep. 2019;9:1–10. doi: 10.1038/s41598-019-42741-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kielmanowicz MG, et al. Prospective large-scale field study generates predictive model identifying major contributors to colony losses. PLoS Pathog. 2015;11:1–20. doi: 10.1371/journal.ppat.1004816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ryabov EV, et al. A virulent strain of deformed wing virus (DWV) of honeybees (Apis mellifera) prevails after Varroa destructor-mediated, or in vitro, transmission. PLoS Pathog. 2014;10:1004230. doi: 10.1371/journal.ppat.1004230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ongus JR, et al. Complete sequence of a picorna-like virus of the genus Iflavirus replicating in the mite Varroa destructor. J. Gen. Virol. 2004;85:3747–3755. doi: 10.1099/vir.0.80470-0. [DOI] [PubMed] [Google Scholar]
- 70.Dalmon A, et al. Evidence for positive selection and recombination hotspots in deformed wing virus (DWV) Sci. Rep. 2017;7:1–12. doi: 10.1038/srep41045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dainat B, Evans JD, Chen YP, Gauthier L, Neumanna P. Dead or alive: Deformed wing virus and varroa destructor reduce the life span of winter honeybees. Appl. Environ. Microbiol. 2012;78:981–987. doi: 10.1128/AEM.06537-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moore J, et al. Recombinants between Deformed wing virus and Varroa destructor virus-1 may prevail in Varroa destructor-infested honeybee colonies. J. Gen. Virol. 2011;92:156–161. doi: 10.1099/vir.0.025965-0. [DOI] [PubMed] [Google Scholar]
- 73.Kevill JL, et al. DWV-A lethal to honey bees (Apis mellifera): A colony level survey of DWV variants (A, B, and C) in England, Wales, and 32 States across the US. Viruses. 2019;11:25. doi: 10.3390/v11050426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tehel A, et al. The two prevalent genotypes of an emerging infectious disease, deformed wing virus, cause equally low pupal mortality and equally high wing deformities in host honey bees. Viruses. 2019;11:25. doi: 10.3390/v11020114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Posada-Florez F, et al. Deformed wing virus type A, a major honey bee pathogen, is vectored by the mite Varroa destructor in a non-propagative manner. Sci. Rep. 2019;9:12445. doi: 10.1038/s41598-019-47447-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Natsopoulou ME, et al. The virulent, emerging genotype B of Deformed wing virus is closely linked to overwinter honeybee worker loss. Sci. Rep. 2017;7:5242. doi: 10.1038/s41598-017-05596-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ryabov EV, et al. Dynamic evolution in the key honey bee pathogen deformed wing virus: Novel insights into virulence and competition using reverse genetics. PLoS Biol. 2019;17:e3000502. doi: 10.1371/journal.pbio.3000502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brettell LE, Martin SJ. Oldest Varroa tolerant honey bee population provides insight into the origins of the global decline of honey bees. Sci. Rep. 2017;7:1–7. doi: 10.1038/srep45953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mordecai GJ, et al. Superinfection exclusion and the long-term survival of honey bees in Varroa-infested colonies. ISME J. 2016;10:1182–1191. doi: 10.1038/ismej.2015.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Berthoud H, Imdorf A, Haueter M, Radloff S, Neumann P. Virus infections and winter losses of honey bee colonies (Apis mellifera) J. Apic. Res. 2010;49:60–65. [Google Scholar]
- 81.Cirkovic D, et al. Honey bee viruses in Serbian colonies of different strength. PeerJ. 2018;6:e5887. doi: 10.7717/peerj.5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rinderer TE, et al. Functionality of Varroa-resistant honey bees (Hymenoptera: Apidae) when used for Western US Honey production and almond pollination. J. Econ. Entomol. 2014;107:523–530. doi: 10.1603/ec13419. [DOI] [PubMed] [Google Scholar]
- 83.Smart M, Pettis J, Rice N, Browning Z, Spivak M. Linking measures of colony and individual honey bee health to survival among apiaries exposed to varying agricultural land use. PLoS One. 2016;11:0152685. doi: 10.1371/journal.pone.0152685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Seitz K, et al. A molecular clone of Chronic Bee Paralysis Virus (CBPV) causes mortality in honey bee pupae (Apis mellifera) Sci. Rep. 2019;9:16274. doi: 10.1038/s41598-019-52822-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ribière M, Olivier V, Blanchard P. Chronic bee paralysis: A disease and a virus like no other? J. Invertebr. Pathol. 2010;103:S120–S131. doi: 10.1016/j.jip.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 86.Gisder S, Genersch E. Viruses of commercialized insect pollinators. J. Invertebr. Pathol. 2017;147:51–59. doi: 10.1016/j.jip.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 87.Faucon J-P, et al. Honey bee winter mortality in France in 1999 and 2000. Bee World. 2002;83:14–23. [Google Scholar]
- 88.Antúnez K, Alessandro BD, Corbella E, Zunino P. Detection of Chronic bee paralysis virus and Acute bee paralysis virus in Uruguayan honeybees. J. Invertebr. Pathol. 2005;90:69–72. doi: 10.1016/j.jip.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 89.Amiri E, Meixner M, Büchler R, Kryger P. Chronic bee paralysis virus in honeybee queens: Evaluating susceptibility and infection routes. Viruses. 2014;6:25. doi: 10.3390/v6031188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Celle O, et al. Detection of Chronic bee paralysis virus (CBPV) genome and its replicative RNA form in various hosts and possible ways of spread. Virus Res. 2008;133:280–284. doi: 10.1016/j.virusres.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 91.Budge GE, et al. Chronic bee paralysis as a serious emerging threat to honey bees. Nat. Commun. 2020;11:2164. doi: 10.1038/s41467-020-15919-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ball BV, Allen MF. The prevalence of pathogens in honey bee (Apis mellifera) colonies infested with the parasitic mite Varroa jacobsoni. Ann. Appl. Biol. 1988;113:237–244. [Google Scholar]
- 93.Le Conte Y, Ellis M, Ritter W. Varroa mites and honey bee health: Can Varroa explain part of the colony losses? Apidologie. 2010;41:353–363. [Google Scholar]
- 94.De Guzman LI, Simone-Finstrom M, Frake AM, Tokarz P. Comb irradiation has limited, interactive effects on colony performance or pathogens in bees, Varroa destructor and wax based on two honey bee stocks. Insects. 2019;10:15. doi: 10.3390/insects10010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang S, et al. Occurrence of multiple honeybee viruses in the ectoparasitic mites Varroa spp. in Apis cerana colonies. J. Invertebr. Pathol. 2019;166:107225. doi: 10.1016/j.jip.2019.107225. [DOI] [PubMed] [Google Scholar]
- 96.Blanchard P, et al. Evaluation of a real-time two-step RT-PCR assay for quantitation of Chronic bee paralysis virus (CBPV) genome in experimentally-infected bee tissues and in life stages of a symptomatic colony. J. Virol. Methods. 2007;141:7–13. doi: 10.1016/j.jviromet.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 97.Spurny R, et al. Virion structure of black queen cell virus, a common honeybee pathogen. J. Virol. 2017;91:e02100–e2116. doi: 10.1128/JVI.02100-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Al Naggar Y, Paxton RJ. Mode of transmission determines the virulence of black queen cell virus in adult honey bees, posing a future threat to bees and apiculture. Viruses. 2020;12:535. doi: 10.3390/v12050535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Murray EA, et al. Viral transmission in honey bees and native bees, supported by a global black queen cell virus phylogeny. Environ. Microbiol. 2019;21:972–983. doi: 10.1111/1462-2920.14501. [DOI] [PubMed] [Google Scholar]
- 100.Desai SD, Currie RW. Effects of wintering environment and parasite-pathogen interactions on honey bee colony loss in north temperate regions. PLoS One. 2016;11:e0159615. doi: 10.1371/journal.pone.0159615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dainat B, Evans JD, Chen YP, Gauthier L, Neumann P. Predictive markers of honey bee colony collapse. PLoS One. 2012;7:25. doi: 10.1371/journal.pone.0032151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sabahi Q, Morfin N, Nehzati-Paghaleh G, Guzman-Novoa E. Detection and replication of deformed wing virus and black queen cell virus in parasitic mites, Varroa destructor, from Iranian honey bee (Apis mellifera) colonies. J. Apic. Res. 2020;59:211–217. [Google Scholar]
- 103.Loope KJ, Baty JW, Lester PJ, Wilson Rankin EE. Pathogen shifts in a honeybee predator following the arrival of the Varroa mite. Proc. R. Soc. B Biol. Sci. 2019;286:20182499. doi: 10.1098/rspb.2018.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hamiduzzaman MM, et al. Differential responses of Africanized and European honey bees (Apis mellifera) to viral replication following mechanical transmission or Varroa destructor parasitism. J. Invertebr. Pathol. 2015;126:12–20. doi: 10.1016/j.jip.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 105.Locke B, Forsgren E, Fries I, de Miranda JR. Acaricide treatment affects viral dynamics in Varroa destructor-infested honey bee colonies via both host physiology and mite control. Appl. Environ. Microbiol. 2012;78:227–235. doi: 10.1128/AEM.06094-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tentcheva D, et al. Prevalence and seasonal variations of six bee viruses in Apis mellifera L. and Varroa destructor mite populations in France. Appl. Environ. Microbiol. 2004;70:7185–7191. doi: 10.1128/AEM.70.12.7185-7191.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Barroso-Arévalo S, et al. High load of deformed wing virus and Varroa destructor infestation are related to weakness of honey bee colonies in Southern Spain. Front. Microbiol. 2019;10:1331. doi: 10.3389/fmicb.2019.01331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wu Y, Liu Q, Weiss B, Kaltenpoth M, Kadowaki T. Honey bee suppresses the parasitic mite Vitellogenin by antimicrobial peptide. Front. Microbiol. 2020;11:25. doi: 10.3389/fmicb.2020.01037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nguyen BK, et al. Effects of honey bee virus prevalence, Varroa destructor load and queen condition on honey bee colony survival over the winter in Belgium. J. Apic. Res. 2011;50:195–202. [Google Scholar]
- 110.Kevan PG, Hannan M, Ostiguy N, Guzman-novoa E. A summary of the Varroa-virus disease complex in honey bees. Am. Bee J. 2006;146:694–697. [Google Scholar]
- 111.Zhao Y, et al. The dynamics of deformed wing virus concentration and host defensive gene expression after Varroa mite parasitism in honey bees, Apis mellifera. Insects. 2019;10:16. doi: 10.3390/insects10010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dietemann V, Neumann P, Ellis JD. The COLOSS BEEBOOK, Volume I: Standard methods for Apis mellifera research. J. Apic. Res. 2013;52:25. [Google Scholar]
- 113.Rangel J, Seeley TD. Colony fissioning in honey bees: Size and significance of the swarm fraction. Insectes Soc. 2012;59:453–462. [Google Scholar]
- 114.Dietemann V, et al. Standard methods for varroa research. J. Apic. Res. 2013;52:1–54. doi: 10.3896/IBRA.1.52.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Simone-Finstrom M, Aronstein K, Goblirsch M, Rinkevich F, de Guzman L. Gamma irradiation inactivates honey bee fungal, microsporidian, and viral pathogens and parasites. J. Invertebr. Pathol. 2018;153:57–64. doi: 10.1016/j.jip.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 116.Boncristiani H, et al. Direct effect of acaricides on pathogen loads and gene expression levels in honey bees Apis mellifera. J. Insect Physiol. 2012;58:613–620. doi: 10.1016/j.jinsphys.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 117.Ryabov EV, et al. Recent spread of Varroa destructor virus-1, a honey bee pathogen, in the United States. Sci. Rep. 2017;7:1–10. doi: 10.1038/s41598-017-17802-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Brunetto MR, Colombatto P, Bonino F. Bio-mathematical models of viral dynamics to tailor antiviral therapy in chronic viral hepatitis. World J. Gastroenterol. 2009;15:531–537. doi: 10.3748/wjg.15.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tarpy DR, Vanengelsdorp D, Pettis JS. Genetic diversity affects colony survivorship in commercial honey bee colonies. Naturwissenschaften. 2013;100:723–728. doi: 10.1007/s00114-013-1065-y. [DOI] [PubMed] [Google Scholar]
- 120.Porta M. A Dictionary of Epidemiology. Oxford University Press; 2014. [Google Scholar]
- 121.Lenhard, W. & Lenhard, A. Calculation of effect sizes. Psychometrica. http://www.psychometrica.de/effect_size.html (2016). 10.13140/RG.2.2.17823.92329.
- 122.Therneau, T. M. coxme: Mixed effects cox models. R package version 2.2-16. (2020).
- 123.Hothorn T, Bretz F, Westfall P. Simultaneous inference in general parametric models. Biometr. J. 2008;50:346–363. doi: 10.1002/bimj.200810425. [DOI] [PubMed] [Google Scholar]
- 124.Therneau, T. M. A package for survival analysis in R. R package version 3.2-7. (2020).
- 125.Moher D, et al. CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. Int. J. Surg. 2012;10:28–55. doi: 10.1016/j.ijsu.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 126.Laurent, S. brr: Bayesian inference on the ratio of two poisson rates. R package version 1.0.0. (2015).
- 127.R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2020. [Google Scholar]
- 128.Hellmich RL, Waller GD. Preparing for Africanized honey bees: Evaluating control in mating apiaries. Am. Bee J. 1990;130:537–542. [Google Scholar]
- 129.Hilo Bees. Hilo bee field trials. https://www.hilobees.com/hilo-bee-field-trials.html (2021).
- 130.Simone-Finstrom M, et al. Migratory management and environmental conditions affect lifespan and oxidative stress in honey bees. Sci. Rep. 2016;6:32023. doi: 10.1038/srep32023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kefuss J, Vanpoucke J, Bolt M, Kefuss C. Selection for resistance to Varroa destructor under commercial beekeeping conditions. J. Apic. Res. 2015;54:563–576. [Google Scholar]
- 132.Khongphinitbunjong K, et al. Responses of Varroa-resistant honey bees (Apis mellifera L.) to Deformed wing virus. J. Asia. Pac. Entomol. 2016;19:921–927. [Google Scholar]
- 133.Khongphinitbunjong K, et al. Differential viral levels and immune gene expression in three stocks of Apis mellifera induced by different numbers of Varroa destructor. J. Insect Physiol. 2015;72:28–34. doi: 10.1016/j.jinsphys.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 134.Annoscia D, et al. Haemolymph removal by Varroa mite destabilizes the dynamical interaction between immune effectors and virus in bees, as predicted by Volterra’s model. Proc. R. Soc. B Biol. Sci. 2019;286:20190331. doi: 10.1098/rspb.2019.0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wells T, et al. Flight performance of actively foraging honey bees is reduced by a common pathogen. Environ. Microbiol. Rep. 2016;8:728–737. doi: 10.1111/1758-2229.12434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Clermont A, et al. Virus status, Varroa levels, and survival of 20 managed honey bee colonies monitored in luxembourg between the summer of 2011 and the spring of 2013. J. Apic. Sci. 2015;59:59–73. [Google Scholar]
- 137.McMenamin AJ, Flenniken ML. Recently identified bee viruses and their impact on bee pollinators. Curr. Opin. Insect Sci. 2018;26:120–129. doi: 10.1016/j.cois.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 138.Monchanin C, et al. Hazard of a neonicotinoid insecticide on the homing flight of the honeybee depends on climatic conditions and Varroa infestation. Chemosphere. 2019;224:360–368. doi: 10.1016/j.chemosphere.2019.02.129. [DOI] [PubMed] [Google Scholar]
- 139.Locke B, Forsgren E, De Miranda JR. Increased tolerance and resistance to virus infections: A possible factor in the survival of Varroa destructor-resistant honey bees (Apis mellifera) PLoS One. 2014;9:e99998. doi: 10.1371/journal.pone.0099998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Posada-Florez F, et al. Pupal cannibalism by worker honey bees contributes to the spread of deformed wing virus. Sci. Rep. 2021;11:8989. doi: 10.1038/s41598-021-88649-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that all supporting data is available within the supplementary information.