Abstract
Ischemic disease is among the deadliest and most disabling illnesses. Prominent examples include myocardial infarction and stroke. Most, if not all, underlying pathological changes, including oxidative stress, inflammation, and nutrient deprivation, are potent inducers of the integrated stress response (ISR). Four upstream kinases are involved in ISR signaling that sense a myriad of input stress signals and converge on the phosphorylation of serine 51 of eukaryotic translation initiation factor 2α (eIF2α). As a result, translation initiation is halted, creating a window of opportunity for the cell to repair itself and restore homeostasis. A growing number of studies show strong induction of the ISR in ischemic disease. Genetic and pharmacological evidence suggests that the ISR plays critical roles in disease initiation and progression. Here, we review the basic regulation of the ISR, particularly in response to ischemia, and summarize recent findings relevant to the actions of the ISR in ischemic disease. We then discuss therapeutic opportunities by modulating the ISR to treat ischemic heart disease, brain ischemia, ischemic liver disease, and ischemic kidney disease. Finally, we propose that the ISR represents a promising therapeutic target for alleviating symptoms of ischemic disease and improving clinical outcomes.
Subject terms: Protein folding, Cardiomyopathies
Facts
The integrated stress response is an evolutionarily conserved, adaptive process to cope with various intracellular and extracellular disturbances. Four upstream kinases sense perturbations and converge on eIF2α phosphorylation to inhibit protein translation.
Ischemic disease is one of the deadliest and most disabling illnesses affecting many tissues and organs, such as the heart, brain, liver, kidney, and limbs.
Most, if not all, underlying pathological adversaries of ischemic disease are potent inducers of the integrated stress response.
The integrated stress response is strongly activated under various ischemic disease conditions.
Pharmaceutical interventions have been explored to target the integrated stress response and mitigate injuries from ischemic disease.
Open questions
The identities of the upstream activators of the integrated stress response that are engaged under different ischemic disease conditions remain to be delineated.
The integrated stress response primarily suppresses protein translation initiation. However, whether the integrated stress response regulates other steps of protein translation, such as elongation, termination, and ribosome recycling, is not known.
Although the integrated stress response generally imposes global inhibition of protein translation, there may be targeting of particular groups of proteins yet to be identified under specific ischemic disease conditions.
Temporal control and amplitude of the integrated stress response in the setting of ischemic disease is incompletely understood. More work is warranted before therapeutic targeting of this pathway can be realized.
Some therapeutic reagents have been developed to target the integrated stress response. Their pharmacological dynamics and efficacy need to be further defined for individual ischemic diseases.
Introduction
Protein translation is the most crucial process in controlling protein homeostasis, which is therefore subjected to tight regulatory control [1]. Multiple steps are involved in protein translation, including initiation, elongation, termination, and recycling of ribosomes [2]. Under most conditions, initiation is the rate-limiting step of translation [3–5]. Thus, governing the initiation step is the most efficient means to control protein synthesis [1]. Mammalian/mechanistic target of rapamycin (mTOR) and the integrated stress response (ISR) are two key opposing signaling pathways that regulate protein synthesis by targeting the initiation step [6, 7]. mTOR signaling primarily responds to growth cues, such as nutrient availability and growth factor levels, to achieve a balance between anabolism and catabolism [8]. In contrast, the ISR is an adaptive signaling network that senses intracellular and extracellular stresses, halting new protein synthesis to favor the resolution of the initiating stress and restoring cellular homeostasis [9].
Ischemic disease occurs when blood vessels are constricted or occluded under various pathological conditions. Abrupt disruption of blood supply leads to deprivation of oxygen and nutrients either temporarily or permanently. Consequently, cell death and tissue damage ensue, which is primarily determined by the magnitude and duration of ischemic insult. Under ischemic conditions, protein translation is inhibited primarily to preserve energy for survival. Emerging evidence shows that the ISR is activated in ischemia, and modulation of protein translation through the ISR represents a promising approach to mitigate ischemic damage. Here, we provide an overview of recent findings relevant to the ISR and its role under ischemic stress. Furthermore, we discuss therapeutic opportunities by targeting the ISR to alleviate ischemic injury.
The integrated stress response (ISR)
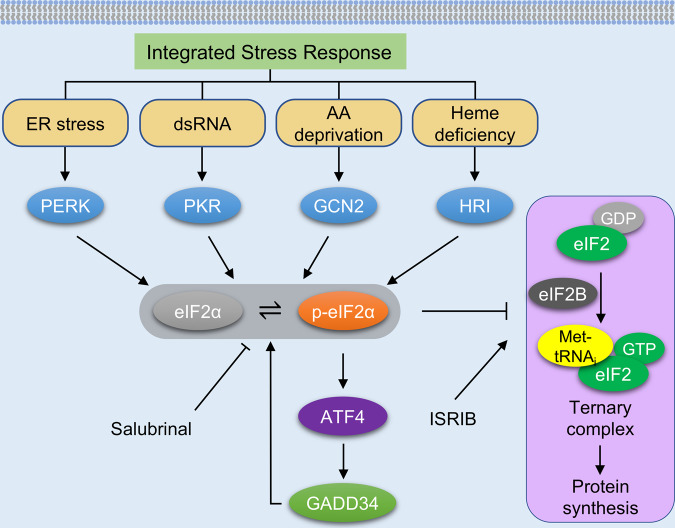
The ISR is an evolutionarily conserved pathway in response to a variety of internal and external perturbations that can stress eukaryotic cells [10]. At the core of the ISR is the phosphorylation of the α subunit of eukaryotic translation initiation factor 2 (eIF2α). Four upstream eIF2α kinases respond to diverse intracellular or extracellular stimuli (Fig. 1). Each of them has a unique domain to sense its respective activating stress condition: heme‐regulated inhibitor kinase (HRI, encoded by EIF2AK1) is activated by heme deficiency [11]; interferon-induced double‐stranded RNA‐dependent eIF2α kinase (PKR, encoded by EIF2AK2) is stimulated by viral infection [12]; PKR-like endoplasmic reticulum resident kinase (PERK, encoded by EIF2AK3) is triggered by endoplasmic reticulum (ER) protein-folding stress [13]; general control non‐derepressible 2 (GCN2, encoded by EIF2AK4) is activated by amino acid deprivation [14].
Fig. 1. The integrated stress response.
During the integrated stress response (ISR), four upstream eIF2α kinases are elicited by different stresses. PERK is primarily activated by the accumulation of unfolded proteins in the endoplasmic reticulum (ER). PKR senses double-stranded RNAs (dsRNAs). GCN2 is activated by amino acid deprivation. The absence of heme stimulates HRI in erythroid cells. eIF2α phosphorylation inhibits the activity of eIF2B, thereby suppressing the formation of translation ternary complex and attenuating protein synthesis. A few proteins related to the ISR are preferably translated, including ATF4. Further, GADD34, a downstream target of ATF4, forms a phosphatase complex with protein phosphatase 1 (PP1) and terminates the ISR signaling by dephosphorylating eIF2α, creating a regulatory feedback loop. Salubrinal enhances the ISR by inhibiting eIF2α dephosphorylating, while ISRIB suppresses the ISR by activating eIF2B. PERK PKR-like endoplasmic reticulum resident kinase, PKR interferon-induced double-stranded RNA-dependent eIF2α kinase, GCN2 general control non-derepressible 2, HRI heme-regulated inhibitor kinase, eIF2α α subunit of eukaryotic initiation factor 2, p-eIF2α phosphorylated eIF2α at serine 51, ATF4 activating transcription factor 4, GADD34 growth arrest and DNA-damage-inducible 34, eIF2 eukaryotic translation initiation factor 2, eIF2B eukaryotic translation initiation factor 2B, GDP guanosine diphospate, GTP guanosine triphosphate, Met-tRNAi translation initiator methionyl transfer RNA, ISRIB integrated stress response inhibitor.
Phosphorylation of eIF2α at serine 51 inhibits the guanine nucleotide exchange cycle of eIF2 catalyzed by eIF2B, which is required for the re-initiation of cap-dependent translation (Fig. 1). As a consequence, general protein translation is stalled and global protein synthesis is suppressed. Among all cellular processes, protein synthesis is the most ATP-consuming, typically accounting for 30–40% energy use [15]. Under conditions of stress, activation of the ISR inhibits protein translation and preserves energy for essential functions to support cell survival [16].
On the other hand, there is a small subset of mRNAs encoding proteins necessary for resolving stress which must be translated. These mRNAs, including ATF4 (activating transcription factor 4), are preferentially translated when the ISR is activated. A common feature exists in these genes, i.e., the presence of one or more upstream open reading frames (uORFs) which suppress the translation of downstream coding sequences under normal conditions. When eIF2α is phosphorylated, these uORFs are skipped, allowing the translation of downstream, protein-encoding full-length mRNAs. The proteins produced, such as ATF4, play essential roles in restoring cellular homeostasis under stress conditions [6]. ATF4 promotes the expression of protein phosphatase 1 regulatory subunit GADD34 [6]. GADD34 forms a complex with protein phosphatase 1 (GADD34–PP1), facilitating a negative feedback loop that ultimately terminates the ISR by dephosphorylating eIF2α. However, if the stress is not sufficiently mitigated, the ISR may eventually trigger apoptosis, thereby eliminating terminally damaged cells [10]. Taken as a whole, the ISR operates in a fine-tuned manner to regulate gene expression and protein synthesis, which together balance protein-folding capacity, maintain differentiation, and ensure cell viability.
Ischemia induces the ISR
Ischemia is a condition of hypoperfusion that occurs when the circulation is blocked by a thrombus, a plaque, a fat globule, or a gas bubble. Artery obstruction limits blood flow and causes either systemic or regional hypoxia. As a result, the O2-dependent ATP production by the electron transport chain (ETC) in mitochondria is impaired, requiring cells to depend on a less efficient process of anaerobic glycolysis to generate ATP. Delivery of substrates for glycolysis is likely also compromised due to arterial blockage. The ensuing energetic crisis causes further dysfunction of sodium-potassium pumps and ribosome detachment. Consequently, prolonged ischemia leads to cell death, tissue injury, and the establishment of permanent infarction. Ischemic disease can occur in multiple organs and tissues, such as the heart, brain, liver, kidney, and limbs [17].
Clinically, timely restoration of blood flow through occluded vessels with either interventional surgery or pharmaceutical thrombolysis is the best way to resolve tissue ischemia. Although it is essential to restore normal circulation as soon as possible to reduce ischemic damage, the process of tissue reperfusion per se can cause a different mode of tissue damage, termed ischemia/reperfusion injury. For example, cardiac ischemia/reperfusion leads to myocardial stunning, microvascular dysfunction/no-reflow, and cardiomyocyte death [18]. Despite extensive efforts from both basic and clinical researchers, the mechanisms of ischemia/reperfusion injury remain incompletely understood. While reactive oxygen species (ROS) generation plays a central role in ischemia/reperfusion damage, other pathological alterations such as calcium overload, endothelial dysfunction, DNA damage, mitochondrial injury, and inflammation may also contribute to disease progression.
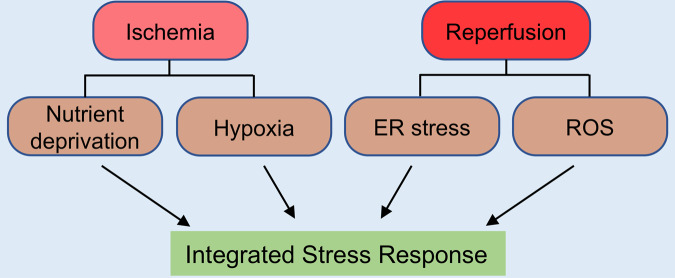
During ischemia, affected tissues suffer hypoxia and nutrition deprivation, both being potent inducers of ER stress and energetic crisis. ER stress can activate PERK, one of the three signaling transducers of the unfolded stress response (UPR) and an upstream kinase of the ISR. On the other hand, nutrient deprivation can stimulate GCN2 that senses ribosomal stalling caused by the presence of uncharged transfer RNAs (tRNA) due to amino acid deficiency. Therefore, ischemia is a bona fide inducer of the ISR via diverse mechanisms (Fig. 2). Pathological changes in response to reperfusion may differ from those that occur during ischemia. Disturbance of redox balance and excessive oxidative stress are central to reperfusion injury. Oxidative stress may damage mitochondria, resulting in the release of mitochondrial RNAs (mtRNAs), which can take on a double-stranded RNA (dsRNA) configuration in the cytosol capable of activating PKR, a central component of the interferon antiviral defense pathway and an upstream kinase of the ISR [19]. Therefore, although certain differences exist, both ischemia and ischemia/reperfusion present key stress signals that stimulate the ISR.
Fig. 2. Ischemia induces the ISR.
Both ischemia and ischemia/reperfusion induce the ISR. During ischemia, the affected tissue suffers from hypoxia and nutrient deprivation, which triggers ER stress, oxidative stress, and amino acid deprivation. The eIF2α kinases PERK, PKR, and GCN2 may be activated to elicit the ISR. Upon reperfusion, unfolded proteins may accumulate in the ER and reactive oxygen species (ROS) may be induced, which then activates PERK and PKR to stimulate the ISR.
The ISR in ischemic heart disease
Myocardial infarction is a leading cause of death worldwide [20]. Although revascularization by percutaneous coronary intervention (PCI) is essential to protect the heart from further ischemic damage, this process itself causes unwanted ischemia/reperfusion injury upon the restoration of coronary blood flow [21]. Studies have shown that the ISR is activated in ischemic heart disease. Castillero et al. found that eIF2α was phosphorylated at serine 51 following myocardial infarction in the mouse heart [22]. Similarly, Wang et al. showed that cardiac ischemia/reperfusion in rats resulted in eIF2α phosphorylation [23]. It is important to note that the treatment with an inhibitor of the serine/threonine protein phosphatase PP1 (PP1-12) increased eIF2α phosphorylation and substantially reduced ischemia/reperfusion-induced cardiac cell death, suggesting that the ISR is cardioprotective. In accord with these in vivo findings, in vitro studies using human fibroblasts and Hela cells demonstrated that eIF2α phosphorylation was elevated by exposure to either hypoxia or CoCl2 treatment, independent of HIF1-α (hypoxia-inducible factor 1-α) [24]. Importantly, PERK−/− mouse embryonic fibroblasts (MEFs), but not PKR−/− MEFs, manifested diminished eIF2α phosphorylation under hypoxia, identifying PERK as the relevant kinase under this condition [24]. Liu et al. found that hypoxia increased ROS and activated PERK in MEFs, which stimulated eIF2α phosphorylation [25]. Prevention of eIF2α phosphorylation using a non-phosphorylatable Ser51Ala mutant exacerbated cell death under hypoxia. This is also consistent with a protective role for PERK as the loss of PERK reduced cell survival following hypoxic stress [24]. Interestingly, the activation of PERK/eIF2α signaling diminished ROS production and enhanced cell survival during hypoxic cycling of cancer cells, which was also HIF1-α-independent [26]. GSK2606414 (or PERKi, a pharmacological inhibitor of PERK) decreased the tolerance of cancer cells to hypoxia [27]. Taken together, these findings suggest that the PERK-eIF2α axis of the ISR confers cellular protection under hypoxic conditions.
In contrast, there are several studies suggesting the opposite, being that inhibition of eIF2α phosphorylation is associated with cardioprotection under hypoxia. Pu et al. found that GCN2 and eIF2α expression was increased in the blood of patients with myocardial infarction/reperfusion [28]. Moreover, interfering with GCN2/eIF2α pathway in H9c2 cells under simulated ischemia/reperfusion (deprivation of both oxygen and glucose, followed by replenishing culture media and re-oxygenation) could inhibit inflammatory signaling, decrease oxidative stress, and reduce apoptosis [28]. Additionally, Yu et al. found that melatonin protected the heart from reperfusion injury by increasing reperfusion injury salvage kinase (RISK) and survivor activating factor enhancement (SAFE) pathways [29]. This was accompanied by suppressed eIF2α phosphorylation, although the involvement of the ISR in this process remained only speculative. The discrepancies between these findings may be due to differences in cell lines, stimuli, and hypoxia intensity and duration. More work is warranted to further dissect the role of the ISR in ischemic heart disease.
The ISR in brain ischemia
Under ischemic insult, not all organs demonstrate equal susceptibility, among which the brain likely represents the more susceptible one. Brain ischemia, a leading cause of disability worldwide [20], is caused by reduced blood flow to the brain. It can manifest either focally, due to blockage of specific arteries in the brain, or globally, due to cardiac arrest. The brain is the most oxygen-consuming organ in the body, whose energy supply is primarily provided by glucose oxidation [30]. During stroke, the core of the ischemic region sustains the most damaged due to hypoxia and nutrient deprivation. It is important to note that stroke causes minor damage in the border region surrounding the infarcted core due to ample collateral circulation. This makes the brain different than the heart during myocardial infarction, in which the border zone displays unique intermediate pathology. During cardiac arrest and resuscitation, the entire brain is subjected to a brief period of complete ischemia, followed by reperfusion. Brain ischemic damage manifests differently in selective areas. The hippocampus is more vulnerable to transient brain ischemia than other regions, and distinct responses are also observed in different hippocampal subregions [31]. The CA1 area is susceptible to transient ischemia, followed by reperfusion, but neuronal damage continues to occur far after the termination of the ischemia/reperfusion event.
Ischemic brain damage is accompanied by generalized and prolonged reduction of nascent protein synthesis capacity [32]. Suppression of protein synthesis has been recognized for 30 years as a hallmark of vulnerable brain neurons under ischemia/reperfusion [31, 33]. Previous studies showed that this suppression was primarily due to inhibition of the initiation step of translation that is controlled by eIF2α phosphorylation, the core factor of the ISR [31, 34, 35]. PERK has been shown to play a dominant role in the control of eIF2α phosphorylation during early reperfusion after transient global brain ischemia [36]. eIF2α phosphorylation was increased significantly in both vulnerable and non-vulnerable brain regions after 10 min of reperfusion following 10 min of cardiac arrest [37]. However, 4 h later, eIF2α phosphorylation persisted only in neurons in which cytochrome c could be detected in the cytoplasm, suggesting that only the most vulnerable neurons display sustained ISR. Activation of PERK and the UPR in the reperfused brain was shown to drive the transient increase in eIF2α phosphorylation [37]. In contrast, other groups have shown that PERK activation was independent of the increase in unfolded proteins in the ER following transient global brain ischemia [38], as PERK was still activated in response to ischemia despite that anisomycin was added to inhibit protein synthesis. Therefore, the precise upstream signals activating PERK in the ischemic brain remain to be determined.
Emerging evidence suggests that the PERK/eIF2α axis of the ISR may play a detrimental role in the ischemic brain. Transient middle cerebral artery occlusion induced both ER stress and autophagy in neurons [39]. Pretreatment with melatonin protected against ischemic damage by reducing ER stress, autophagy, and caspase cleavage. Other studies found that melatonin treatment protected the brain from I/R injury and was accompanied by attenuation in PERK activity [40]. Taken together, the PERK-eIF2α axis of the ISR may be the primary pathway triggering detrimental autophagy and apoptosis following ischemia/reperfusion, as inhibition of PERK signaling reduced ER stress-induced autophagy and alleviated ischemia/reperfusion injury [39]. Consistent with this, preconditioning protection downregulated PERK activation and caspase 12 cleavage in a focal cerebral ischemia/reperfusion rat model [41].
Additional upstream mechanisms may operate in conjunction with PERK to activate the ISR in the ischemic brain. GCN2 was also elevated after cerebral ischemia, and suppressing its activity was found to protect against ischemia/reperfusion injury by inhibiting FoxO3a and reducing oxidative stress and apoptosis [42]. These findings demonstrate that the ISR is induced through multiple pathways under brain ischemia, and may be an underlying mechanism of neuron loss and brain damage. However, most studies to date have demonstrated a correlative, rather than causative, relationship between ISR activation and ischemic brain injury. It therefore remains to be definitively established whether and when the ISR is protective or detrimental or both during brain ischemia.
The ISR in other ischemic diseases
Ischemia can also occur in the liver, kidney, and limbs. Hepatic ischemia/reperfusion damage is one of the leading causes of liver injury during liver transplantation [43]. In a pig liver ischemia/reperfusion model, Li et al. showed that phosphorylation of eIF2α was increased significantly compared to the sham group [44]. Consistently, downstream targets of the ISR, including ATF4 and CHOP, were elevated at both mRNA and protein levels in the liver. Caspase 12 is located in the ER and responds to ER stress. Viatoba et al. found that caspase 12 was activated after lethal liver ischemia/reperfusion in mice, while inhibition of the PERK-eIF2α axis of the ISR decreased apoptosis and mitigated liver reperfusion injury [45].
Renal ischemia/reperfusion is one of the most common causes of acute kidney injury [46]. Similar to other organs, renal eIF2α phosphorylation was significantly elevated 4 h after reperfusion [47]. At in vitro, previous studies showed that the PERK/eIF2α axis of the ISR functioned to limit the amount of cell injury after anoxia/recovery in cultured glomerular epithelial cells [48].
In the context of skeletal system ischemia, osteonecrosis of the femoral head in the hip joint is a severe orthopedic problem, primarily caused by ischemia/hypoxia. The PERK-eIF2α axis of the ISR was found to be activated in osteonecrosis of the femoral head, and prolonging this activation enhanced angiogenesis and bone healing [49]. Collectively, these findings demonstrate that ischemic insult to other organs also elicits the ISR mainly through PERK.
The ISR as a therapeutic target to treat ischemic disease
The ISR plays a critical role in the initiation and progression of ischemic damage, representing a promising therapeutic target. To lend further support, a recent study by Wang et al. showed that PERK conditional knockout mice had larger infarcts and worse neurological outcomes compared with controls in a cerebral ischemic model [50]. Consistent with this, overexpression of PERK with AAV9 viruses in isolated neonatal cardiomyocytes showed a protective effect against reperfusion injury [51].
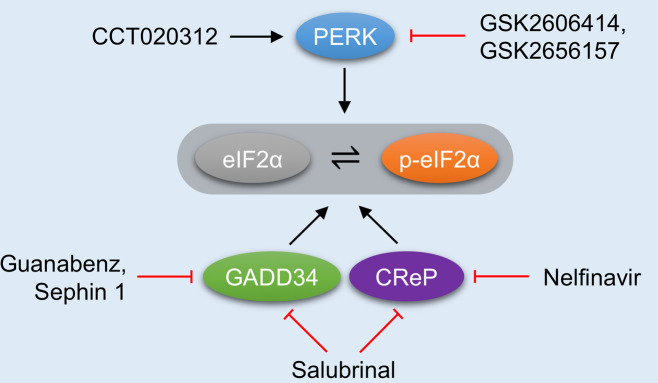
Several small molecules have been developed to modulate the ISR (Fig. 3). ISRIB (integrated stress response inhibitor) is a cell-permeable, selective ISR inhibitor that can bind eIF2B and promote its assembly, therefore counteracting the consequence of eIF2α phosphorylation [52, 53]. In a hypoxia model using breast cancer cells, the ISR was found to drive breast cancer plasticity and adaptation to hypoxia [54]. Treatment with ISRIB prevented the synthesis of plasticity-induced factors during hypoxic stress. ISR inhibition may therefore prove an effective therapy to control breast cancer and its metastasis. On the other hand, salubrinal, a PP1 inhibitor, prevented eIF2α dephosphorylation by impairing the formation of the GADD34–PP1 and CReP–PP1 complexes [10]. Salubrinal can act to maintain and sustain the ISR. Guanabenz, primarily known as an α2-adrenergic receptor agonist, can also act as a GADD34–PP1c inhibitor, similar to salubrinal [55]. However, due to guanabenz’s ability to activate α2-adrenergic receptors and potential adverse effects this could have on other cardiovascular processes, Sephin1, a derivative of guanabenz, was developed [56]. Sephin1 specifically binds GADD34, inhibiting the formation of the active binary GADD34-PP1 complex. In addition, Nelfinavir is an anti-HIV-1 drug in clinical use that has been shown to suppress eIF2α dephosphorylation and consequently activate the ISR [57]. Sephin1 and Nelfinavir have not yet been assessed in the settings of ischemic disease.
Fig. 3. Pharmacological modulation of the ISR as a therapeutic approach in ischemic disease.
Various therapeutic strategies have been developed to intervene the ISR. PERK inhibitors can relieve the unfolded protein response (UPR) and the ISR. Acute activation of PERK may activate the ISR and protect cells from reperfusion injury. Dephosphorylation of eIF2α may be targeted to either enhance or attenuate the ISR for therapeutic gain.
In addition to eIF2α, PERK also represents an attractive target to be exploited for therapeutic gain. CCT020312 is a small chemical that selectively activates PERK without affecting other two branches of the UPR [58]. In addition, GSK2606414 and GSK2656157 are highly potent, cell-permeable PERK inhibitors that target the ATP-binding region of PERK [59]. Another critical concept worth emphasizing is the therapeutic window in ischemic disease. The timing and amplitude of ISR activation may be distinct at different phases of ischemic disease. Therefore, the ISR may exert beneficial or detrimental effects on targeted organs depending upon temporal profile. It will be critical to define the detailed activation process of the ISR and more precisely identify individual contributions to disease pathophysiology. In the following section, we discuss the applications and outcomes of various ISR-targeting approaches designed to tackle the ischemic disease.
The ISR as a therapeutic target in ischemic heart disease
Although much effort has been focused on understanding the mechanisms of cardiac reperfusion injury and identifying novel therapeutic targets, limited consensus has been reached. Studies with pre-clinical animal models showed that ischemic pre-conditioning and various pharmacological interventions could reduce infarct size. However, necessary clinical studies to test these approaches are missing [60]. The ISR is emerging as a new target to mitigate cardiac ischemia/reperfusion injury (Fig. 4). Salubrinal has been shown to protect the heart from myocardial infarction in a rat model [61]. Salubrinal decreased cardiomyocyte apoptosis and infarct size by increasing eIF2α phosphorylation and reducing caspase 12 and CHOP expression. Moreover, salubrinal protected against hypoxia-induced cardiomyocyte death in vitro. The underlying mechanism of salubrinal’s mode of action relies on attenuation of misfolded protein synthesis and reduction in ER stress-induced apoptosis [62].
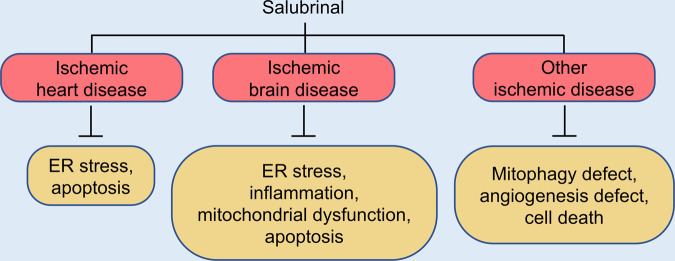
Fig. 4. Salubrinal as a therapeutic drug in ischemic disease.
Salubrinal may protect cells and tissues from ischemic disease via different mechanisms. In ischemic heart disease, salubrinal can attenuate ER stress and inhibit apoptosis. In brain ischemia, salubrinal can inhibit ER stress and inflammation and enhance mitochondrial function. As a result, apoptosis and necroptosis are suppressed. In other ischemic diseases, such as hepatic ischemia/reperfusion and osteonecrosis of the femoral head, salubrinal can stimulate mitophagy, angiogenesis, and cell differentiation, which together enhance cell survival and bone healing.
Furthermore, Liu et al. showed that myocardial function was significantly improved by salubrinal treatment in a myocardial infarction-induced rat model of heart failure [63]. In addition, Li et al. presented evidence, suggesting that salubrinal could alleviate ferroptosis and cell injury [64]. Beyond further delineating the molecular mechanisms and efficacy of salubrinal, future work may focus on other ISR-activating drugs, such as Sephin1. In addition, different effects of these drugs during ischemia and upon reperfusion need to be dissected in order to identify appropriate therapeutic windows for alleviating ischemic heart injury.
The ISR as a therapeutic target in brain ischemia
A significant effort from physicians and basic scientists has gone into the development of new ways to treat brain ischemia by neuroprotection, such as anti-apoptosis, anti-calcium overload, anti-inflammation, and anti-oxidative injury. The ISR may be a new therapeutic target in brain ischemia (Fig. 4). Recent studies using a global cerebral ischemia model showed that salubrinal treatment reduced ischemic damage by alleviating ER stress and enhancing the PERK-eIF2α branch of the ISR [65]. Furthermore, the neuroprotective effect of salubrinal on CA1 suggests distinct modes of action in different regions of the brain and a potential role for inflammation. A later study from the same group showed that acute treatment with salubrinal provided neuroprotection that could be observed even 7 days after insult [66]. Moreover, salubrinal and anti-inflammatory drugs had synergistic effects, suggesting that a proper combination of various agents may provide a more substantial neuroprotective effect. Additionally, salubrinal reduced the transcription of mixed lineage kinase domain-like pseudokinase (MLKL) in ischemia‐induced necroptosis in CA1 [67]. In a cardiac arrest model, salubrinal treatment was also found to improve neurological function 24 h after cardiopulmonary resuscitation by preserving mitochondria and stabilizing HIF1-α [68]. Although a growing number of studies support the notion that salubrinal treatment is neuroprotective in brain ischemia [50, 69, 70], controversies remain. For example, Gao et al. showed that salubrinal treatment blocked the activation of autophagy induced by ischemic preconditioning and attenuated neuroprotection [71]. More studies are therefore required to clarify the effect of salubrinal and modulation of the ISR in brain ischemia.
The ISR as a therapeutic target in other ischemic diseases
Salubrinal has also been studied in other ischemic organs besides the heart and brain (Fig. 4). In mice subjected to hepatic ischemia/reperfusion, salubrinal pretreatment upregulated Parkin and alleviated hepatocyte damage by enhancing mitophagy and quenching mitochondria-derived ROS [72]. In a surgery model of osteonecrosis of the femoral head, salubrinal treatment improved pathological symptoms by enhancing angiogenesis and bone healing [49]. This is consistent with a previous study showing that salubrinal treatment affected the differentiation of both osteoblasts and osteoclasts, suggesting that salubrinal may be developed as an anti-osteoporosis drug [73, 74]. Additionally, Huang et al. showed that salubrinal treatment protected chondrocytes from apoptosis and relieved osteoarthritis-like pathological changes on mandibular condylar cartilage under hypoxic stress [75]. Moreover, in a rat model of hypoxic pulmonary hypertension, salubrinal was found to prevent and reverse right ventricular remodeling and normalize gene expression and signaling pathways in the right ventricle exposed to hypobaric hypoxia [76]. Consistent with this, salubrinal treatment alleviated ER stress-induced apoptosis in a model of human pulmonary arterial endothelial cell hypoxia [77]. Collectively, the use of salubrinal to enhance the ISR may be explored to treat multiple forms of ischemic disease. However, it is important to note that most studies only tested salubrinal for its ability to enhance the ISR, which may be associated with adverse side effects. Better drugs with higher efficacy and specificity may be pursued in the future.
Despite that the ISR has received extensive attention due to its fundamental involvement in various disease conditions, our understanding remains incomplete. For example, although the four upstream kinases all phosphorylate eIF2α and attenuate translation initiation, individual contributions to the ISR under specific contexts may not be equal. Currently, most studies have focused on the PERK branch of the ISR, however, this should not be considered evidence that PERK is more important than the other three transducers. Comprehensive and direct comparison is necessary to conclusively address the individual roles of the four kinases under different conditions. Although eIF2α is the central mediator of the ISR, there are other downstream effectors of the four kinases such as Keap, calcineurin, diacylglycerol, FoxO1, p38, p53, and NFκB, which may also contribute to the final outcome of the ISR [78]. In addition, potential synergistic interactions between these signaling pathways and the ISR can be critical to mediate appropriate cellular responses. Moreover, the duration of ISR signaling is critical to pathological outcomes. Either too little or too much ISR activity may be detrimental. Targeting the ISR at the right time and magnitude is essential to ensure cell survival and avoid pathological consequences. Finally, although it is generally agreed that the ISR imposes global suppression of protein translation, it remains to be answered whether the ISR may selectively target specific proteins under distinct disease conditions.
Conclusions and future perspectives
During the ISR, four distinct upstream kinases sense diverse extracellular and intracellular perturbations. Consequently, translation initiation is inhibited, creating a window of opportunity for the cell to repair and restore homeostasis. The ischemic disease may impact multiple tissues and organs. Most, if not all, pathological events in ischemic disease are potent inducers of the ISR. Numerous studies have firmly established the relevance of the ISR in ischemic disease. Moreover, modulation of the ISR represents a promising approach to mitigate tissue damage and improve clinical outcomes. However, depending on the intensity and duration of stress, the ISR may have either cytoprotective or detrimental consequences. Therefore, caution needs to be exercised while moving forward to apply pre-clinical knowledge regarding the ISR for therapeutic gain. Further studies are warranted to elucidate the role of the ISR in ischemic disease and identify more specific and potent modulators of the ISR to treat ischemic disorders.
Acknowledgements
We thank the members of the Wang lab for valuable discussions. This work was supported by funding from the American Heart Association (14SDG18440002, 17IRG33460191, and 19IPLOI34760325 to ZVW; 20POST35210756 to XW; 19TPA34920001 to BAR), the American Diabetes Association (1-17-IBS-120 and 7-20-IBS-218 to ZVW), the National Institute of Health (HL137723 to ZVW; HD101006, HD087351, and HL147276 to BAR), and Agencia Nacional de Investigacion y Desarrollo (ANID), Chile (FONDAP 15130011 and FONDECYT 1200490 to SL).
Author contributions
GZ, SL and ZVW wrote the manuscript with the help from XW and BAR. All authors revised and approved the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Edited by G Melino
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sergio Lavandero, Email: slavander@uchile.cl.
Zhao V. Wang, Email: zhao.wang@utsouthwestern.edu
References
- 1.Jackson RJ, Hellen CU, Pestova TV. The mechanism of eukaryotic translation initiation and principles of its regulation. Nat Rev Mol Cell Biol. 2010;11:113–27. doi: 10.1038/nrm2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hershey JWB, Sonenberg N, Mathews MB. Principles of translational control. Cold Spring Harb Perspect Biol. 2019;11:a032607. doi: 10.1101/cshperspect.a032607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yan X, Hoek TA, Vale RD, Tanenbaum ME. Dynamics of translation of single mRNA molecules in vivo. Cell. 2016;165:976–89. doi: 10.1016/j.cell.2016.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu B, Eliscovich C, Yoon YJ, Singer RH. Translation dynamics of single mRNAs in live cells and neurons. Science. 2016;352:1430–5. doi: 10.1126/science.aaf1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morisaki T, Lyon K, DeLuca KF, DeLuca JG, English BP, Zhang Z, et al. Real-time quantification of single RNA translation dynamics in living cells. Science. 2016;352:1425–9. doi: 10.1126/science.aaf0899. [DOI] [PubMed] [Google Scholar]
- 6.Wek RC. Role of eIF2alpha kinases in translational control and adaptation to cellular stress. Cold Spring Harb Perspect Biol. 2018;10:a032870. doi: 10.1101/cshperspect.a032870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Proud CG. Phosphorylation and signal transduction pathways in translational control. Cold Spring Harb Perspect Biol. 2019;11:a033050. doi: 10.1101/cshperspect.a033050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–93. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11:619–33. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 10.Pakos-Zebrucka K, Koryga I, Mnich K, Ljujic M, Samali A, Gorman AM. The integrated stress response. EMBO Rep. 2016;17:1374–95. doi: 10.15252/embr.201642195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu L, Han AP, Chen JJ. Translation initiation control by heme-regulated eukaryotic initiation factor 2alpha kinase in erythroid cells under cytoplasmic stresses. Mol Cell Biol. 2001;21:7971–80. doi: 10.1128/MCB.21.23.7971-7980.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galabru J, Katze MG, Robert N, Hovanessian AG. The binding of double-stranded RNA and adenovirus VAI RNA to the interferon-induced protein kinase. Eur J Biochem. 1989;178:581–9. doi: 10.1111/j.1432-1033.1989.tb14485.x. [DOI] [PubMed] [Google Scholar]
- 13.Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–4. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- 14.Ravindran R, Loebbermann J, Nakaya HI, Khan N, Ma H, Gama L, et al. The amino acid sensor GCN2 controls gut inflammation by inhibiting inflammasome activation. Nature. 2016;531:523–7. doi: 10.1038/nature17186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu B, Qian SB. Translational reprogramming in cellular stress response. Wiley Interdiscip Rev RNA. 2014;5:301–15. doi: 10.1002/wrna.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu S, Henninger K, McGrath BC, Cavener DR. PERK regulates working memory and protein synthesis-dependent memory flexibility. PLoS One. 2016;11:e0162766. doi: 10.1371/journal.pone.0162766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, et al. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation. 2018;137:e67–e492. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 18.Verma S, Fedak PW, Weisel RD, Butany J, Rao V, Maitland A, et al. Fundamentals of reperfusion injury for the clinical cardiologist. Circulation. 2002;105:2332–6. doi: 10.1161/01.cir.0000016602.96363.36. [DOI] [PubMed] [Google Scholar]
- 19.Kim Y, Park J, Kim S, Kim M, Kang MG, Kwak C, et al. PKR senses nuclear and mitochondrial signals by interacting with endogenous double-stranded RNAs. Mol Cell. 2018;71:1051–63.e6. doi: 10.1016/j.molcel.2018.07.029. [DOI] [PubMed] [Google Scholar]
- 20.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 21.Hausenloy DJ, Yellon DM. Ischaemic conditioning and reperfusion injury. Nat Rev Cardiol. 2016;13:193–209. doi: 10.1038/nrcardio.2016.5. [DOI] [PubMed] [Google Scholar]
- 22.Castillero E, Akashi H, Najjar M, Ji R, Brandstetter LM, Wang C, et al. Activin type II receptor ligand signaling inhibition after experimental ischemic heart failure attenuates cardiac remodeling and prevents fibrosis. Am J Physiol Heart Circ Physiol. 2020;318:H378–h390. doi: 10.1152/ajpheart.00302.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu CL, He YY, Li X, Li RJ, He KL, Wang LL. Inhibition of serine/threonine protein phosphatase PP1 protects cardiomyocytes from tunicamycin-induced apoptosis and I/R through the upregulation of p-eIF2α. Int J Mol Med. 2014;33:499–506. doi: 10.3892/ijmm.2013.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koumenis C, Naczki C, Koritzinsky M, Rastani S, Diehl A, Sonenberg N, et al. Regulation of protein synthesis by hypoxia via activation of the endoplasmic reticulum kinase PERK and phosphorylation of the translation initiation factor eIF2alpha. Mol Cell Biol. 2002;22:7405–16. doi: 10.1128/MCB.22.21.7405-7416.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu L, Wise DR, Diehl JA, Simon MC. Hypoxic reactive oxygen species regulate the integrated stress response and cell survival. J Biol Chem. 2008;283:31153–1162. doi: 10.1074/jbc.M805056200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rouschop KM, Dubois LJ, Keulers TG, van den Beucken T, Lambin P, Bussink J, et al. PERK/eIF2 alpha signaling protects therapy resistant hypoxic cells through induction of glutathione synthesis and protection against ROS. Proc Natl Acad Sci USA. 2013;110:4622–7. doi: 10.1073/pnas.1210633110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cojocari D, Vellanki RN, Sit B, Uehling D, Koritzinsky M, Wouters BG. New small molecule inhibitors of UPR activation demonstrate that PERK, but not IRE1 alpha signaling is essential for promoting adaptation and survival to hypoxia. Radiother Oncol. 2013;108:541–7. doi: 10.1016/j.radonc.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Pu Y, Wu D, Lu X, Yang L. Effects of GCN2/eIF2alpha on myocardial ischemia/hypoxia reperfusion and myocardial cells injury. Am J Transl Res. 2019;11:5586–98. [PMC free article] [PubMed] [Google Scholar]
- 29.Yu L, Li B, Zhang M, Jin Z, Duan W, Zhao G, et al. Melatonin reduces PERK-eIF2alpha-ATF4-mediated endoplasmic reticulum stress during myocardial ischemia-reperfusion injury: role of RISK and SAFE pathways interaction. Apoptosis. 2016;21:809–24. doi: 10.1007/s10495-016-1246-1. [DOI] [PubMed] [Google Scholar]
- 30.Hofmeijer J, van Putten MJAM. Ischemic cerebral damage an appraisal of synaptic failure. Stroke. 2012;43:607–15. doi: 10.1161/STROKEAHA.111.632943. [DOI] [PubMed] [Google Scholar]
- 31.Krause GS, Tiffany BR. Suppression of protein synthesis in the reperfused brain. Stroke. 1993;24:747–55. doi: 10.1161/01.str.24.5.747. [DOI] [PubMed] [Google Scholar]
- 32.DeGracia DJ, Kumar R, Owen CR, Krause GS, White BC. Molecular pathways of protein synthesis inhibition during brain reperfusion: implications for neuronal survival or death. J Cereb Blood Flow Metab. 2002;22:127–41. doi: 10.1097/00004647-200202000-00001. [DOI] [PubMed] [Google Scholar]
- 33.White BC, Sullivan JM, DeGracia DJ, O’Neil BJ, Neumar RW, Grossman LI, et al. Brain ischemia and reperfusion: molecular mechanisms of neuronal injury. J Neurol Sci. 2000;179:1–33. doi: 10.1016/s0022-510x(00)00386-5. [DOI] [PubMed] [Google Scholar]
- 34.Burda J, Martin ME, Garcia A, Alcazar A, Fando JL, Salinas M. Phosphorylation of the alpha subunit of initiation factor 2 correlates with the inhibition of translation following transient cerebral ischaemia in the rat. Biochem J. 1994;302:335–8. doi: 10.1042/bj3020335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paschen W. Role of calcium in neuronal cell injury: which subcellular compartment is involved? Brain Res Bull. 2000;53:409–13. doi: 10.1016/s0361-9230(00)00369-5. [DOI] [PubMed] [Google Scholar]
- 36.Owen CR, Kumar R, Zhang P, McGrath BC, Cavener DR, Krause GS. PERK is responsible for the increased phosphorylation of eIF2alpha and the severe inhibition of protein synthesis after transient global brain ischemia. J Neurochem. 2005;94:1235–42. doi: 10.1111/j.1471-4159.2005.03276.x. [DOI] [PubMed] [Google Scholar]
- 37.Page AB, Owen CR, Kumar R, Miller JM, Rafols JA, White BC, et al. Persistent eIF2alpha(P) is colocalized with cytoplasmic cytochrome c in vulnerable hippocampal neurons after 4 h of reperfusion following 10-minute complete brain ischemia. Acta Neuropathol. 2003;106:8–16. doi: 10.1007/s00401-003-0693-2. [DOI] [PubMed] [Google Scholar]
- 38.Sanderson TH, Deogracias MP, Nangia KK, Wang J, Krause GS, Kumar R. Pkr-Like endoplasmic reticulum kinase (Perk) activation following brain ischemia is independent of unfolded nascent proteins. Neuroscience. 2010;169:1307–14. doi: 10.1016/j.neuroscience.2010.05.076. [DOI] [PubMed] [Google Scholar]
- 39.Feng D, Wang B, Wang L, Tanenbaum ME, Abraham N, Tao K, Huang L. Pre-ischemia melatonin treatment alleviated acute neuronal injury after ischemic stroke by inhibiting endoplasmic reticulum stress-dependent autophagy via PERK and IRE1 signalings. J Pineal Res. 2017;62:e12395. doi: 10.1111/jpi.12395. [DOI] [PubMed] [Google Scholar]
- 40.Lin YW, Chen TY, Hung CY, Tai SH, Huang SY, Chang CC, et al. Melatonin protects brain against ischemia/reperfusion injury by attenuating endoplasmic reticulum stress. Int J Mol Med. 2018;42:182–92. doi: 10.3892/ijmm.2018.3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu YQ, Chen W, Yan MH, Lai JJ, Tang N, Wu L. Ischemic preconditioning protects brain from ischemia/reperfusion injury by attenuating endoplasmic reticulum stress-induced apoptosis through PERK pathway. Eur Rev Med Pharm Sci. 2017;21:5736–44. doi: 10.26355/eurrev_201712_14020. [DOI] [PubMed] [Google Scholar]
- 42.Shi WZ, Tian Y, Li J. GCN2 suppression attenuates cerebral ischemia in mice by reducing apoptosis and endoplasmic reticulum (ER) stress through the blockage of FoxO3a-regulated ROS production. Biochem Biophys Res Commun. 2019;516:285–92. doi: 10.1016/j.bbrc.2019.05.181. [DOI] [PubMed] [Google Scholar]
- 43.de Rougemont O, Lehmann K, Clavien PA. Preconditioning, organ preservation, and postconditioning to prevent ischemia-reperfusion injury to the liver. Liver Transpl. 2009;15:1172–82. doi: 10.1002/lt.21876. [DOI] [PubMed] [Google Scholar]
- 44.Li H, Bai G, Ge Y, Zhang Q, Kong X, Meng W, et al. Hydrogen-rich saline protects against small-scale liver ischemia-reperfusion injury by inhibiting endoplasmic reticulum stress. Life Sci. 2018;194:7–14. doi: 10.1016/j.lfs.2017.12.022. [DOI] [PubMed] [Google Scholar]
- 45.Vilatoba M, Eckstein C, Bilbao G, Smyth CA, Jenkins S, Thompson JA, et al. Sodium 4-phenylbutyrate protects against liver ischemia reperfusion injury by inhibition of endoplasmic reticulum-stress mediated apoptosis. Surgery. 2005;138:342–51. doi: 10.1016/j.surg.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 46.Eltzschig HK, Eckle T. Ischemia and reperfusion-from mechanism to translation. Nat Med. 2011;17:1391–401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li J, Xu S, Zhu JB, Song J, Luo B, Song YP, et al. Pretreatment with cholecalciferol alleviates renal cellular stress response during ischemia/reperfusion-induced acute kidney injury. Oxid Med Cell Longev. 2019;2019:1897316. doi: 10.1155/2019/1897316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cybulsky AV, Takano T, Papillon J, Bijian K. Role of the endoplasmic reticulum unfolded protein response in glomerular epithelial cell injury. J Biol Chem. 2005;280:24396–403. doi: 10.1074/jbc.M500729200. [DOI] [PubMed] [Google Scholar]
- 49.Liu DQ, Zhang YL, LI XL, Li J, Yang S, Xing XX, et al. eIF2 alpha signaling regulates ischemic osteonecrosis through endoplasmic reticulum stress. Sci Rep. 2017;7:5062. doi: 10.1038/s41598-017-05488-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang YC, Li X, Shen YT, Lyu J, Sheng HX, Paschen W, et al. PERK (Protein kinase RNA-like ER kinase) branch of the unfolded protein response confers neuroprotection in ischemic stroke by suppressing protein synthesis. Stroke. 2020;51:1570–7. doi: 10.1161/STROKEAHA.120.029071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang J, Lu L, Chen S, Xie J, Lu S, Zhou Y, et al. PERK Overexpression-mediated Nrf2/HO-1 pathway alleviates hypoxia/reoxygenation-induced injury in neonatal murine cardiomyocytes via improving endoplasmic reticulum stress. Biomed Res. Int. 2020;2020:6458060. doi: 10.1155/2020/6458060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsai JC, Miller-Vedam LE, Anand AA, Jaishankar P, Nguyen HC, Renslo AR, et al. Structure of the nucleotide exchange factor eIF2B reveals mechanism of memory-enhancing molecule. Science. 2018;359:eaaq0939. [DOI] [PMC free article] [PubMed]
- 53.Zyryanova AF, Weis F, Faille A, Alard AA, Crespillo-Casado A, Sekine Y, et al. Binding of ISRIB reveals a regulatory site in the nucleotide exchange factor eIF2B. Science. 2018;359:1533–6. doi: 10.1126/science.aar5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jewer M, Lee L, Leibovitch M, Zhang G, Liu J, Findlay SD, et al. Translational control of breast cancer plasticity. Nat Commun. 2020;11:2498. doi: 10.1038/s41467-020-16352-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Neuber C, Uebeler J, Schulze T, Sotoud H, El-Armouche A, Eschenhagen T. Guanabenz interferes with ER stress and exerts protective effects in cardiac myocytes. PLoS One. 2014;9:e98893. doi: 10.1371/journal.pone.0098893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Das I, Krzyzosiak A, Schneider K, Wrabetz L, D’Antonio M, Barry N, et al. Preventing proteostasis diseases by selective inhibition of a phosphatase regulatory subunit. Science. 2015;348:239–42. doi: 10.1126/science.aaa4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Gassart A, Bujisic B, Zaffalon L, Decosterd LA, Di Micco A, Frera G, et al. An inhibitor of HIV-1 protease modulates constitutive eIF2α dephosphorylation to trigger a specific integrated stress response. Proc Natl Acad Sci USA. 2016;113:E117–126. doi: 10.1073/pnas.1514076113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stockwell SR, Platt G, Barrie SE, Zoumpoulidou G, Te Poele RH, Aherne GW, et al. Mechanism-based screen for G1/S checkpoint activators identifies a selective activator of EIF2AK3/PERK signalling. PLoS One. 2012;7:e28568. doi: 10.1371/journal.pone.0028568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Axten JM, Medina JR, Feng Y, Shu A, Romeril SP, Grant SW, et al. Discovery of 7-methyl-5-(1-{[3-(trifluoromethyl)phenyl]acetyl}-2,3-dihydro-1H-indol-5-yl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (GSK2606414), a potent and selective first-in-class inhibitor of protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK) J Med Chem. 2012;55:7193–207. doi: 10.1021/jm300713s. [DOI] [PubMed] [Google Scholar]
- 60.Heusch G, Gersh BJ. The pathophysiology of acute myocardial infarction and strategies of protection beyond reperfusion: a continual challenge. Eur Heart J. 2017;38:774–84. doi: 10.1093/eurheartj/ehw224. [DOI] [PubMed] [Google Scholar]
- 61.Li RJ, He KL, Li X, Wang LL, Liu CL, He YY. Salubrinal protects cardiomyocytes against apoptosis in a rat myocardial infarction model via suppressing the dephosphorylation of eukaryotic translation initiation factor 2alpha. Mol Med Rep. 2015;12:1043–9. doi: 10.3892/mmr.2015.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu CL, Li X, Hu GL, Li RJ, He YY, Zhong W, et al. Salubrinal protects against tunicamycin and hypoxia induced cardiomyocyte apoptosis via the PERK-eIF2alpha signaling pathway. J Geriatr Cardiol. 2012;9:258–68. doi: 10.3724/SP.J.1263.2012.02292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu Y, Wang J, Qi SY, Ru LS, Ding C, Wang HJ, et al. Reduced endoplasmic reticulum stress might alter the course of heart failure via caspase-12 and JNK pathways. Can J Cardiol. 2014;30:368–75. doi: 10.1016/j.cjca.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 64.Li WY, Li W, Leng Y, Xiong YH, Xia ZY. Ferroptosis is involved in diabetes myocardial ischemia/reperfusion injury through endoplasmic reticulum stress. DNA Cell Biol. 2020;39:210–25. doi: 10.1089/dna.2019.5097. [DOI] [PubMed] [Google Scholar]
- 65.Anuncibay-Soto B, Perez-Rodriguez D, Santos-Galdiano M, Font E, Regueiro-Purrinos M, Fernandez-Lopez A. Post-ischemic salubrinal treatment results in a neuroprotective role in global cerebral ischemia. J Neurochem. 2016;138:295–306. doi: 10.1111/jnc.13651. [DOI] [PubMed] [Google Scholar]
- 66.Anuncibay-Soto B, Perez-Rodriguez D, Santos-Galdiano M, Font-Belmonte E, Ugidos IF, Gonzalez-Rodriguez P, et al. Salubrinal and robenacoxib treatment after global cerebral ischemia. Exploring the interactions between ER stress and inflammation. Biochem Pharm. 2018;151:26–37. doi: 10.1016/j.bcp.2018.02.029. [DOI] [PubMed] [Google Scholar]
- 67.Font-Belmonte E, Ugidos IF, Santos-Galdiano M, Gonzalez-Rodriguez P, Anuncibay-Soto B, Perez-Rodriguez D, et al. Post-ischemic salubrinal administration reduces necroptosis in a rat model of global cerebral ischemia. J Neurochem. 2019;151:777–94. doi: 10.1111/jnc.14789. [DOI] [PubMed] [Google Scholar]
- 68.Zhang JC, Wang Y, Ju MJ, Song JQ, Zheng YJ, Lin SL, et al. Neuroprotective effect of the inhibitor salubrinal after cardiac arrest in a rodent model. Oxid Med Cell Longev. 2020;2020:7468738. doi: 10.1155/2020/7468738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sheng R, Liu XQ, Zhang LS, Gao B, Han R, Wu YQ, et al. Autophagy regulates endoplasmic reticulum stress in ischemic preconditioning. Autophagy. 2012;8:310–25. doi: 10.4161/auto.18673. [DOI] [PubMed] [Google Scholar]
- 70.Nakka VP, Gusain A, Raghubir R. Endoplasmic reticulum stress plays critical role in brain damage after cerebral ischemia/reperfusion in rats. Neurotox Res. 2010;17:189–202. doi: 10.1007/s12640-009-9110-5. [DOI] [PubMed] [Google Scholar]
- 71.Gao B, Zhang XY, Han R, Zhang TT, Chen C, Qin ZH, et al. The endoplasmic reticulum stress inhibitor salubrinal inhibits the activation of autophagy and neuroprotection induced by brain ischemic preconditioning. Acta Pharmacologica Sin. 2013;34:657–66. doi: 10.1038/aps.2013.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li Y, Ruan DY, Jia CC, Zheng J, Wang GY, Zhao H, et al. Aging aggravates hepatic ischemia-reperfusion injury in mice by impairing mitophagy with the involvement of the EIF2alpha-parkin pathway. Aging. 2018;10:1902–20. doi: 10.18632/aging.101511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.He L, Lee J, Jang JH, Sakchaisri K, Hwang J, Cha-Molstad HJ, et al. Osteoporosis regulation by salubrinal through eIF2alpha mediated differentiation of osteoclast and osteoblast. Cell Signal. 2013;25:552–60. doi: 10.1016/j.cellsig.2012.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hamamura K, Tanjung N, Yokota H. Suppression of osteoclastogenesis through phosphorylation of eukaryotic translation initiation factor 2 alpha. J Bone Min Metab. 2013;31:618–28. doi: 10.1007/s00774-013-0450-0. [DOI] [PubMed] [Google Scholar]
- 75.Huang ZW, Zhou M, Wang Q, Zhu MJ, Chen S, Li H. Mechanical and hypoxia stress can cause chondrocytes apoptosis through over-activation of endoplasmic reticulum stress. Arch Oral Biol. 2017;84:125–32. doi: 10.1016/j.archoralbio.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 76.He YY, Liu CL, Li X, Li RJ, Wang LL, He KL. Salubrinal attenuates right ventricular hypertrophy and dysfunction in hypoxic pulmonary hypertension of rats. Vasc Pharm. 2016;87:190–8. doi: 10.1016/j.vph.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 77.Chen A, Liu J, Zhu J, Wang X, Xu Z, Cui Z, et al. FGF21 attenuates hypoxiainduced dysfunction and apoptosis in HPAECs through alleviating endoplasmic reticulum stress. Int J Mol Med. 2018;42:1684–94. doi: 10.3892/ijmm.2018.3705. [DOI] [PubMed] [Google Scholar]
- 78.Guo J, Ren R, Sun K, He J, Shao J. PERK signaling pathway in bone metabolism: friend or foe? Cell Prolif. 2021;54:e13011. doi: 10.1111/cpr.13011. [DOI] [PMC free article] [PubMed] [Google Scholar]