Abstract
The objectives of the study were to review the articles to identify (a) the epidemiology of systemic lupus erythematosus (SLE) and coronavirus disease 2019 (COVID-19); (b) the clinical characteristics of SLE patients with COVID-19; (c) the treatment of COVID-19 in SLE patients; and (d) the impact of COVID-19 pandemic on SLE patients. PubMed was systematically reviewed for literature published from December 2019 to June 2021. Our search was limited to human studies, with language restriction of English. Studies were included if they reported COVID-19 in SLE patients. Our systematic review included 52 studies. The prevalence of COVID-19 infection ranged from 0.0% to 18.1% in SLE patients, and the hospitalisation rates ranged from 0.24% to 10.6%. COVID-19 infection is likely to mimic SLE flare. Hydroxychloroquine (HCQ) was ineffective in prevention of COVID-19, and SLE patients with COVID-19 faced difficulty in healthcare access, had financial constraints and suffered from psychological distress during the pandemic. The pandemic had a significant effect on mental and physical health. Adequate healthcare access, along with containment policies, social distancing measures and psychological nursing was required.
Keywords: SLE, COVID-19, SARS-CoV-2, Systematic Review
Introduction
The coronavirus disease 2019 (COVID-19) pandemic leads to serious illness in a considerable proportion of infected patients.1,2 As of 27 July 2021, the pandemic of COVID-19 has been responsible for more than 194 million infections and four million deaths globally.3 Some populations are at greater risk from poor outcomes related to this disease, including the elderly, male, obese people and individuals with any comorbidities, especially those with hypertension, cardiovascular disease or diabetes.4
Prognoses of COVID-19 vary widely between individuals. Systemic lupus erythematosus (SLE) patients are prone to COVID-19 infection on account of the aberrant immune responses inherent to the disease and the frequent treatment of immune-suppressants.5 Age over 60 years, male sex, Black and Asian ground, steroids, cyclophosphamide or rituximab and high disease activity were related to increased risk of poor consequences of COVID-19 in SLE patients.6
The purposes of the systematic review were to evaluate the epidemiology of SLE and COVID-19, to identify the clinical characteristics of SLE patients with COVID-19, to clarify the treatment of COVID-19 in SLE patients and to assess the impact of COVID-19 pandemic on SLE patients.
Methods
Search strategy
The review followed the Preferred reporting items for systematic reviews guidelines (PRISMA)7 for articles that revealed the relationship between COVID-19 and SLE. PubMed was searched to identify relevant articles from December 2019 to June 2021. The following search strategy was developed: (coronavirus[Title/Abstract] OR corona-virus[Title/Abstract] OR COVID[Title/Abstract] OR ‘COVID-19’[Mesh Terms] OR COVID-19[Title/Abstract] OR COVID-2019[Title/Abstract] OR severe acute respiratory syndrome coronavirus[Title/Abstract] OR severe acute respiratory syndrome coronavirus 2[Title/Abstract] OR 2019-nCoV[Title/Abstract] OR ‘SARS-CoV-2’[Mesh Terms] OR SARS-CoV-2[Title/Abstract] OR 2019nCoV[Title/Abstract]) AND (‘Lupus Erythematosus, Systemic’[Mesh Terms] OR Lupus Erythematosus, Systemic[Title/Abstract] OR systemic lupus erythematosus[Title/Abstract] OR SLE[Title/Abstract]). Our search was limited to human studies, with language restriction of English. We also assessed the reference lists for eligible studies.
Selection criteria
Studies were eligible if they reported relationship between COVID-19 and SLE. Observational studies (e.g. case reports and series, cross‐sectional studies, case–control studies and cohort studies), correspondence with relevant clinical data and randomised controlled trials were included. Comments, animal studies and in vitro studies were excluded. Studies were ineligible if the full texts were not available.
Study selection and data collection
Two independent reviewers conducted the phases of screening, eligibility and inclusion. They reviewed titles and abstracts for potential inclusion. Both reviewers inspected the full text for potential studies. Any discrepancy was resolved through consensus amongst review authors or through adjudication by an additional review author. Two authors tabulated outcome results from the included studies in detail, to examine and evaluate the potential patterns within the data. Quality assessment was performed using criteria adapted from the National Institutes of Health.8
Data synthesis
A narrative synthesis was performed within the following categories: (a) the epidemiology of SLE and COVID-19; (b) the clinical characteristics of SLE patients with COVID-19; (c) the treatment of COVID-19 in SLE patients; and (d) the impact of COVID-19 pandemic on SLE patients.
Results
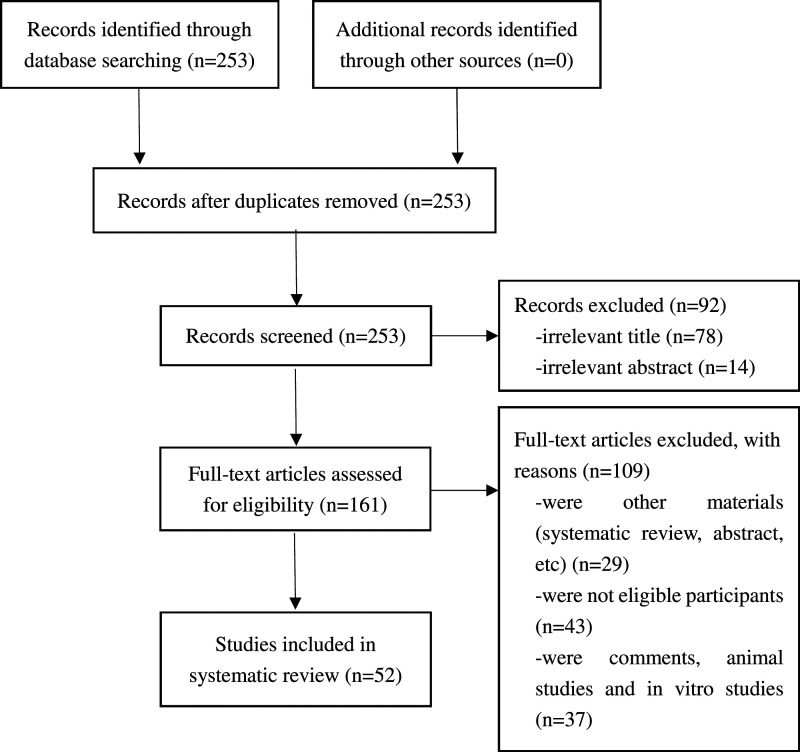
We initially yielded 253 literature, of which 52 met the eligibility criteria (Figure 1).9–60 The quality assessment of the 52 articles revealed a high quality in 19/52 (36.5%) studies. Characteristics and outcomes are shown in Table 1.
Figure 1..
Flowchart of literature search and study selection.
Table 1.
Characteristics and outcomes of included studies.
| Author | Study details | Results and conclusions | Strengths and limitations |
| Aguirre-Alastuey et al., 2021 | Case study | A 22-year-old-woman with SLE and aPL antibodies suffered from pulmonary thromboembolism during the COVID-19 infection. The authors made the hypothesis that COVID-19 could trigger the antiphospholipid syndrome | Small sample size |
| Ammitzbøll et al., 2021 | Patients with RA or SLE (n=405) and blood donors (n=513) | 206 SLE patients (91.3% females. Median age, 49.0 years [IQR, 38.3–60.1]. Median disease duration, 11.9 years [IQR, 6.0–24.0]), 199 RA patients (71.4% females. Median age, 62.5 years [IQR, 51.6–70.5]. Median disease duration, 14.0 years [IQR, 8.0–21.0]) and 513 blood donors (49.1% females. Median age, 47 years [IQR, 33–57 years]). Isolation was related to significant protection against COVID-19, but it often came at the cost of physical activity, increased pain, disease activity and depressive symptoms | |
| Cornet et al., 2021 | SLE patients using HCQ | 51.8% of patients experienced supply problems. 56.1% of patients underwent high anxiety due to the unavailability of HCQ. Even if normal supply resumed, 27.4% remained anxious | |
| Favalli et al., 2021 | 62 SLE patients between 25 Feb and 10 Apr | 91% were females. Mean age, 44.1 years. No cases had confirmed COVID-19, while 8 cases (including 5 on HCQ) had symptoms similar to COVID-19, rapidly recovering without additional therapy. Three patients contacted with COVID-19 cases and none developed symptoms of COVID-19 infection. No patients changed the medication and 93.5% had stable diseases. 95% of patients adopted strict preventive measures. The authors hypothesised that SLE did not increase the risk of COVID-19 | |
| Freeman-Beman et al., 2021 | Case study | An SLE patient with COVID-19 experienced a thrombotic event with concurrent recurrence of positive antiphospholipid antibodies | Small sample size |
| Goldman et al., 2021 | Non-COVID-19 patients received HCQ/CQ treatment (07/2014-9/2019) | HCQ/CQ was frequently used by patients with RA (n = 1,655, [51%]), SLE (n = 758, [24%]), SjS (n = 99, [3%]), arthritis or arthralgia (n = 93, [3%]) and cutaneous lupus erythematosus (n = 80, [2.5%]). CVAEs frequently occurred in patients with SLE (34% vs 22%, p < 0.01) | |
| Gracia-Ramos et al., 2021 | Case study | COVID-19 infection triggered the presentation or exacerbation of the autoimmune disease in SLE patients | Small sample size |
| Jung et al., 2021 | Patients with RA or SLE tested for SARS-CoV-2 (n = 2066) until 15 May 2020 | 90.9% of patients had RA and 14.5% had SLE. 31.4% (649/2066) used HCQ. 93.7% of HCQ users received 200–400 mg/day. 98.0% of HCQ users received HCQ over 3 months before the test. The infection rate in the HCQ users (2.3%, 15/649) was similar to that in the HCQ non-users (2.2%, 31/1417) (p = 0.860). After the attack, one HCQ non-users needed ICU admission, required ventilator treatment and died | |
| The authors hypothesised that prophylactic intake of HCQ at a standard dose was not effective in COVID-19 prevention | |||
| Marques et al., 2021 | IMRD patients with COVID-19 (n = 334) between 20 May 2020 and 24 Jul 2020 | SLE (32.9%) and RA (28.4%) were the most common rheumatic disease diagnoses. 28 patients died (11 had SLE, 4 had RA, 2 had axial spondyloarthritis, 5 had SSc and 6 had other diseases). SLE played a protective role for ICU admission (PR = 1.72, 95%CI 1.04 to 2.88, p = 0.036) | An observational multicentre prospective cohort |
| Mendel et al., 2021 | Experience of physicians from SLE referral centres | 31 responses (rate 74%) were received. 55% reported HCQ shortages in SLE patients during the pandemic, and 65% were contacted regarding this problem. The estimated proportion of SLE patients affected was 15% (IQR 5%–35%). The reasons for HCQ shortages were off-label prescribing and clinical trials of HCQ | Physician estimates from single tertiary centres |
| Rathi et al., 2021 | SLE patients (n = 1040) from an Indian inception cohort | 90% females. Mean age, 27.5 (SD, 19.1) years. Mean disease duration 1.25 years. Twenty-Four (2.3%) patients developed fever, but none had COVID-19.262 patients (25.2%) had difficulty in finance, with an average excess expenditure of more than $30 per month on healthcare. 378 patients (36%) complained that they were unable to get prescribed medicines because of lockdown, and of these, 40% of patients required to change the drug schedule. Nearly 54% of patients missed the follow-up visits and 37% reported problems in getting their investigations done because of lockdown. During the pandemic, 266 patients (25.5%) complained about worsening of symptoms SLE like joint pain, malar rash or oral ulcers | |
| Rentsch et al., 2021 | Adults first diagnosed with RA or SLE (n = 194 637) at least 6 months before 1 March 2020 | 30 569 (15·7%) patients received two or more prescriptions of HCQ (median number of prescriptions 5). HCQ users were younger and most were females. There were 547 deaths due to COVID-19, 70 were HCQ users. HCQ users had a standardised cumulative COVID-19 mortality of 0.23% (95% CI 0.18–0.29), while the standardised cumulative mortality among non-users was 0.22% (0.20–0.25). There was no relationship between HCQ use and COVID-19 mortality (HR = 1.03, 95% CI 0.80–1.33) | Large sample size |
| Slimani et al., 2021 | Case study | A case of SLE with APS developed a COVID‐19–related varicelliform rash | Small sample size |
| Sukhdeo et al., 2021 | Case study | An SLE child with COVID-19 presented with increased work of breathing and low oxygen saturation. The authors suggested that the clinical presentation described in paediatric SLE population with COVID-19 might be similar to those in adults | Small sample size |
| Zamani et al., 2021 | Case study | A 39-year-old with COVID-19 infection produced autoantibodies or developed the clinical characteristics of SLE | Small sample size |
| Zucchi et al., 2021 | SLE patients (n = 332) from a single Italian centre between Feb and Jul 2020 | 91.3% females. Median age, 47 years. Median duration of the disease, 17 years. 6 patients (1.8%) had confirmed COVID-19 infection, 3 of them (50.0%) needed hospitalisation, but none were sent to the ICU. There was a significant relationship between bDMARDs and COVID-19 (OR = 7.35, 95% Cl 1.42 to 37.87, p = 0.02). 36 patients (11.0%) discontinued medication for SLE due to physician’s suggestions or personal choice, and disease flare occurred in 8.1% (n = 27) of cases during the COVID-19 pandemic, all of which were not severe. There was a relationship between disease flare and treatment discontinuation. The authors hypothesised that treatment discontinuation might result in disease flare |
A single Italian centre |
| Alharthy et al., 2020 | Case study | A patient with SLE and end-stage kidney disease was admitted to ICU because of COVID-19. She was infected with COVID-19 despite the administration of HCQ. | Small sample size |
| Bonometti et al., 2020 | Case study | An 85-year-old woman with COVID-19 infection developed autoimmune antibodies, and she was diagnosed with SLE. The authors hypothesised that SLE was probably one of the possible chronic rheumatologic diseases triggered by COVID-19 |
Small sample size |
| Bozzalla Cassione et al., 2020 | A telemedicine project of SLE patients (n = 165) from Lombardy and Emilia-Romagna since 15 Feb 2020 | 84% females. Median age, 52.5 years. Median disease duration, 13 years. Fever and cough were common symptoms. 12 cases (7.2%) developed COVID-19, and 4 had confirmed infection, of which one was admitted to ICU. She had severe SLE and was on MMF, HCQ and oral prednisone before COVID-19. She was successfully discharged from ICU after initiation of methylprednisolone and non-invasive ventilation. All 11 remaining patients had mild disease courses and fully recovered. The authors concluded that the disease course was more likely to be mild and self-resolving |
|
| Cho et al., 2020 | Case study | Case 1 with stable SLE and COVID-19 infection developed severe thrombocytopenia. Case 2 with active lupus nephritis died from COVID-19 pneumonia. Case 3 with SLE, hypertensive heart disease and chronic kidney disease, and developed active lupus nephritis. All 3 cases had active SLE that required escalation of therapy |
Small sample size |
| Chuah et al., 2020 | Patients with SLE during the peak of the pandemic (from 18 Mar 2020 to 9 Jun 2020) | 18 cases with SLE required hospitalisation during the lockdown period and 6 were newly diagnosed with SLE. SLE hospitalisation rate fell by 65.4% during the pandemic relative to the same period in 2019, but hospitalised patients were likely to have more severe symptoms. Disease flare (38.9%) and infection (22.2%) were important causes of admission in SLE patients | Small sample size |
| El Aoud et al., 2020 | Case study | A COVID-19 patient presented with multiorgan involvement mimicking SLE and was successfully treated with glucocorticoids and tocilizumab | Small sample size |
| Fernandez-Ruiz et al., 2020 | Patients with SLE (n = 226) from a New York University lupus cohort, query of 2 hospital systems and referrals from rheumatologists | 41 SLE patients with COVID-19 (24 [58.5%] needed hospitalisation, 4 were admitted to ICU and 4 passed away), 42 had symptoms of COVID-19 (RT-PCR not performed) and 124 were asymptomatic (RT-PCR not performed). An exploratory logistic regression analysis identified nonwhite (OR = 7.78, 95%CI 1.13–53.58, p = 0.037) and presence of comorbidities (OR = 4.66, 95% CI 1.02–21.20, p = 0.047) as independent predictors of hospitalisation | A large and multi-racial/ethnic cohort |
| Freites Nuñez et al., 2020 | Patients with AIRD and symptomatic COVID-19 (n = 123) from Madrid, Spain (1 Mar 2020 to 24 Apr) | Of 8 SLE cases, 6 were non-admitted patients and 2 were admitted patients. SLE was not associated with hospital admission (OR = 0.4, 95%CI 0.07–2.08, p = 0.27) | A single centre research |
| Gartshteyn et al., 2020 | Case study | 18 SLE patients had confirmed (n = 10) or suspected COVID-19 (n = 8) infection. 89% females. Mean age, 41 years (SD 11). 50% Hispanic patients and 39% black patients. 61% had lupus nephritis. 15 received immunosuppressants, 7 received steroids and 13 received HCQ or CQ. Previous use of immunosuppressants before hospitalisation was not different between cases with mild and severe disease. 11 outpatients and 4 hospitalised patients improved without requiring supplemental oxygen. One patient with hypoxemic respiratory failure gradually improved while two patients remained critically ill | Small sample size |
| Gendebien et al., 2020 | 225 SLE patient | 92.9% female. Mean age of 51.7 (SD, 14.9) years. Infection was confirmed or suspected in 18 (8.0%) patients. There was no correlation between immunosuppressive drugs and COVID-19 infection or symptoms, but glucocorticoid dose was positively related to infection (OR = 1.57, p = 0.025), hospitalisation (OR = 4.39, p = 0.030), anosmia and ageusia (OR = 1.57, p = 0.025) and diarrhoea (OR = 1.75, p = 0.018). HCQ was ineffective in prevention of COVID-19 infection or suspicion (7.9% for HCQ users, 8.2% for HCQ non-users, p = 0.93) | |
| George et al., 2020 | Adults from the US with RA, PsA, AS and SLE (n = 1517) | 925 had RA, 299 had PsA, 185 had AS and 108 had SLE. Mean age (SD), 55.1 (11.7) years. 88.3% females. 89.5% White. Levels of COVID-19 concerns were high. Avoiding of physician’s office visits, laboratory testing and other testing were reported by 56.6%, 42.3% and 36.0% of participants, respectively, with higher rates in participants with SLE (all p < 0.05) | |
| Gianfrancesco et al., 2020 | Patients with COVID-19 and rheumatic disease (n = 600): 24 Mar 2020 to 20 Apr 2020 | Median age, 56 years (IQR, 45–67 years). 71% females. 38% had RA, 14% had SLE and 12% had PsA. In unadjusted analyses, patients who were hospitalised had SLE (17%) were more than those who were not hospitalised (11%). Compared with RA, SLE was not related to COVID-19 hospitalisation status in unadjusted (OR = 1.51, 95%CI 0.91–2.49) and adjusted (OR = 1.80, 95%CI 0.99–3.29) logistic regression models | Large descriptive studies |
| Guven et al., 2020 | Case study | A case with SLE with COVID-19 suffered from leptomeningeal involvement | Small sample size |
| Han et al., 2020 | Case study | SLE patients with the long-term intake of prednisone led to atypical infections, prolonged incubation periods and additional transmission of COVID-19 | Small sample size |
| He et al., 2020 | Case study | SLE patients with COVID-19 experienced a window period of asymptomatic infection after discharge | Small sample size |
| Holubar et al., 2020 | All adult patients with SLE (n = 120) from Montpellier University Hospital between 1 Feb and 24 Apr | Thirty-six (30.0%) had symptoms of COVID-19. The rate of SLE patients with symptoms of infection was similar in HCQ users (6.9%) and HCQ non-users (6.3%) | |
| Kondo et al., 2020 | Case study | A 58-year-old woman with SLE developed exacerbation of ITP triggered by COVID-19 | Small sample size |
| Leung et al., 2020 | Case study | A girl with SLE developed hyperpigmentation induced by HCQ | Small sample size |
| Mantovani Cardoso et al., 2020 | Case study | An SLE female with COVID-19 developed antiphospholipid antibodies and multiple deep venous thrombosis | Small sample size |
| Mathian et al., 2020 | COVID-19 patients with SLE under treatment with HCQ (n = 17): 29 Mar and 6 Apr | Median duration of HCQ therapy before COVID-19, 7.5 years (range, 0.5–29.8 years). Obesity (59%) and chronic kidney disease (47%) were frequently reported. 16 cases had quiescent SLE. 12 cases received prednisone and 7 received an immunosuppressant. The main symptoms were fever (100%), cough (82%), shortness of breath (82%), headache (59%), respiratory rate over 24 breaths per minute (53%), myalgia (47%) and diarrhoea (41%). Viral pneumonia occurred in 13 patients, of which 11 had respiratory failure and 5 had acute respiratory distress syndrome. Three cases had acute renal failure, with 2 cases needing haemodialysis. Median HCQ blood concentration in 8 cases was 648 ng/mL (range: 254–2095 ng/mL). Of 14 hospitalised cases, 7 were admitted to the ICU. 11 cases received oxygen therapy (5 nasal cannula, 1 high-flow nasal cannula and 5 invasive mechanical ventilation). One case required extracorporeal membrane oxygenation. 5 (36%) cases were discharged, 7 (50%) remained hospitalised and 2 (14%) died. HCQ lacked preventive effect on COVID-19 in SLE patients, at least serious COVID-19 |
Small sample size |
| Montero et al., 2020 | Patients with COVID-19 and underlying RMDs (n = 62): 4 Mar and 24 April 2020 | Mean age, 60.9 years. 42% men. 32% had RA, 26% had SpA/PsA, 6% had other inflammatory, 15% had SLE and 21% had other CTD. Patients who died were older, most were males and had more comorbidities, mainly DM and hypertension. 10 (16%) patients died, 9 required hospitalisation: 3 had RA, 2 had SLE, 1 had SpA and 4 had other rheumatic diseases. No relationship was found between SLE and more serious infection requiring hospitalisation (OR = 0.94, 95%CI 0.21–4.24). 10 (16%) patients died: 3 with RA, 2 with SLE, 1 with SpA and 4 with other rheumatic diseases | Small number from a single centre |
| Ning et al., 2020 | Case study | An SLE patient who took HCQ and prednisone was found to develop COVID-19 at the second visit, and was recovered from COVID-19 through intravenous immunoglobulin treatments and increased dose of corticosteroids | Small sample size |
| Pablos et al., 2020 | Observational matched cohort study with hospital confirmed COVID-19 rheumatic patients with chronic IA or CTDs (n = 228) from 5 centres | 65 (28.5%) patients had RA; 35 (15.4%) had PsA; 36 (15.8%) had SpA; 16 (7.0%) had SLE (Mean disease duration, 14.34 years [SD, 10.38]. 43.8% of the patients reported active rheumatic disease) | |
| Pablos et al., 2020 | A retrospective study with patients from rheumatology departments in Spain | SLE patients (n = 2253; 77% females; median age of cases, 51 years [IQR, 42–66 years]) had a prevalence of 0.62% (95%CI 0.34%–1.04%) | Large sample size |
| Plüß et al., 2020 | The current situation in SLE patients under the treatment of HCQ in Germany | 369 responses were included. 94% women. Almost all (95.8%) of respondents believed HCQ essential for the disease management. 70% complained about the unavailability of medications; 8.8% reduced their daily dose. 86.6% believed no benefit regarding COVID-19, and half of the patients realised increased vulnerability due to SLE. About 45% had supply problems, 44.4% had no problems and 10% stockpiled HCQ beforehand | |
| Raghavan et al., 2020 | Case study | A patient with COVID-19 triggered SLE flare | Small sample size |
| Ramirez et al., 2020 | SLE patients (n = 545) from three referral centres. Data from the survey were in comparison with those from general population | A total of 417 (77%) responders. 91% females. Over 60% of patients complained of symptoms of COVID-19 (myalgia [31%], rhinorrhoea [25%], fever [17%] and dry cough [16%]). 1.20% of SLE patients had confirmed COVID-19, in contrast to a 0.73% prevalence in the general population. The hospitalisation rate of infected patients was 0.24%, while the general population had a rate of 0.43%. New-onset fever, anosmia, cough, a history of neuropsychiatric SLE and contacting with COVID-19 patients were related to COVID-19 infection, as well as symptoms and lower compliance to protection. HCQ was ineffective in prevention of COVID-19.40 responders (10%) reduced or discontinued their drugs. The main cause was doctor’s suggestions. Medication shortage was complained by 36% and 17% of patients who reduced or discontinued HCQ or immunosuppressants, with 75% of these patients attributing this event to the COVID-19 pandemic | Descriptive report from questionnaire findings |
| Tee et al., 2020 | Patients with RA (n = 107) and SLE (n = 405) from May 19 to 26, 2020 | 95.5% females. Around 20% underwent moderate or severe impacts of COVID-19 outbreak. The mean IES-R score was higher in SLE compared with RA respondents. Stress, anxiety and depression were moderate to severe in 12.3% (mean stress subscale score, 10.11 [SD = 7.95]), 38.7% (mean anxiety subscale score, 6.79 [SD = 6.57]) and 27.7% (mean depression subscale score, 9.03 [SD = 8.77]) of patients. The risk factors for poor mental health included the comorbidities of hypertension and asthma; becoming a healthcare professional; and the specific symptoms, while the protective factors involved satisfaction with existing health information and wearing a mask | |
| Teh et al., 2020 | SLE patients with COVID-19 in Malaysia | There were five cases of SLE. Five were women. Mean age, 52.80 years (SD, 4.46). Mean disease duration, 13.20 years (SD, 3.92). All 5 cases received long-term treatment of HCQ. All patients had moderate to severe infection but were recovered well except one died patient. The authors concluded that background HCQ treatment for SLE patients was not effective for COVID-19 prevention. COVID-19 infection is likely to mimic SLE flare |
Small sample size |
| Tiendrébéogo et al., 2020 | Case study | Case 1 was diagnosed with SLE for 3 years. She was infected with COVID-19 in a consultation for headaches and arthromyalgia. Case 2 was an SLE patient with chronic renal failure and nephrotic syndrome. She had confirmed COVID-19 and was admitted to ICU. The authors concluded that SLE patients would be at risk of serious illness from infection, especially those with mutivisceral SLE damage |
Small sample size |
| Wallace et al., 2020 | 31 COVID-19 patients from University of Michigan rheumatology clinics between 1 Mar and 20 Apr 2020 | Five had SLE, four of whom received HCQ with a median duration of 7 (rang, 6–8) years. SLE patients might develop more severe manifestations of COVID-19 infection | |
| Wańkowicz et al., 2020 | 723 COVID-19 patients from 6 hospitals from 3 May 2020 to 17 May 2020 | 134 with SLE (88.81% females. Mean age, 38.34 [5.62] years old), 589 without SLE (46.69% females. Mean age, 39.71 [7.07] years old). COVID-19 patients with SLE had elevated symptoms of anxiety (OR = 3.683, 95%CI 2.271–5.974, p < 0.001), depression (OR = 4.183, 95%CI, 2.544–6.878, p < 0.001) and sleep disorders (OR = 6.781, 95%CI 2.968–15.492, p < 0.001) | |
| Wen et al., 2020 | LE patients and RA patients from Wuhan Union Hospital between 22nd Mar and 25 Mar 2020 | Of 338 patients diagnosed with LE/RA, 3 patients had severe COVID-19. Two SLE patients used HCQ were in a critical condition with COVID-19 and one RA patient without HCQ had severe pneumonia. One patient with SLE, concurrent with nephritis, died of respiratory failure due to infection. The attack rate in this study (3/338; 0.89%) appears to be higher than Wuhan’s overall infection rate (50,340/10,000,000; 0.46%). The COVID-19 infection rate in the immunosuppressive medications users was similar to that in the immunosuppressive medication non-users (p > 0.05). Thirty-two RA/LE patients discontinued previous treatments and 46.9% of them suspended treatment because of city lockdown for COVID-19. Medication withdrawal positively correlated with worse LE or RA clinical outcomes (outcome score in discontinuation due to COVID-19 group vs. continuation group: 2.86 ± 1.12 vs 1.82 ± 0.96; p < 0.001). In the COVID-19 group, treatment was interrupted after 4 weeks to several months, and the authors speculated that long-term interruption of immunosuppressive therapy would worsen their clinical outcomes | Small sample size |
| Widhani et al., 2020 | Patients diagnosed with autoimmune disease (n = 685) from Apr to May 2020 | Median age, 37 years old (IQR 29–45 years old). 93% females. Most of them had SLE (40.4%). Nearly all patients had great knowledge and practices about COVID-19. Adequacy of information (OR = 0.09, 95% CI 0.01–0.72) and steroid (OR = 0.29, 95% CI 0.11–0.76) or MMF/MPA use (OR = 15.68, 95% CI 1.93–127.31) were associated with cognition of the impact of pandemic on health. Visiting private clinic (OR = 0.45, 95% CI 0.26–0.78) and receiving HCQ/CQ sulfate (OR = 1.85, 95% CI 1.02–3.36) or sulfasalazine (OR = 8.94, 95% CI 1.07–74.87) were associated with concern that autoimmune conditions might make them more vulnerable to COVID-19. Work from home (OR = 2.61, 95% CI 1.06–6.45) was associated with concern that the symptoms would be more serious if infected with COVID-19. Living in Sumatra region (OR = 4.40, 95% CI 1.29–14.94) and getting HCQ/CQ sulfate (OR = 7.22, 95% CI 2.23–23.38) or MMF/MPA (OR = 5.66, 95% CI 1.86–17.23) were associated with perception that autoimmune drugs could prevent COVID-19. Adequate information (OR = 0.22, 95% CI 0.10–0.44), university education (OR = 0.60, 95% CI 0.38–0.95), private clinic visit (OR = 0.52, 95% CI 0.31–0.88) and HCQ/CQ sulfate use (OR = 1.99, 95% CI 1.32–3.01) were linked to perception of unavailability of medications during the pandemic | An online survey |
| Zen et al., 2020 | Patients with ARD (n = 916) from Italy: Apr 9th and Apr 25th | 397 had SLE (Mean age, 47.8 years [SD, 13.4]. 85.6% females. 84.8% remission. Prednisone > 7.5 mg/day [10.9%], mycophenolate [31.3%], antimalarial drugs [77%] and anti-BLYSS [11.8%] were mainly used. 18.9% has symptom compatible with COVID-19. The main signs and symptoms were fever > 37.5°C [10.1%], cough [10.8%] and sore throat [10.3%]), 182 had AAV, 176 had SSc, 111 had RA and 50 had IIM. 65 (7.1%) received SARS-CoV-2 testing, 2 (0.21%) were positive: One had SLE and one had SSc. Drug discontinuation occurred in 13 (3.3%) SLE patients, and of 5 unremitted patients, 3 patients had nephritis | A telephone survey |
| Zurita et al., 2020 | Case study | This case series reported 5 cases with COVID-19 who had used HCQ for SLE prior to infection, which suggested that HCQ lacked preventive effect on COVID-19 in SLE patients | Small sample size |
SLE: Systemic lupus erythematosus; aPL: Antiphospholipid antibodies; COVID-19: Coronavirus disease 2019; RA: Rheumatoid arthritis; HCQ: Hydroxychloroquine; CQ: Chloroquine; SjS: Sjogren’s syndrome; CVAEs: Cardiovascular adverse events; ICU: Intensive care unit; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; IMRD: Immune-mediated rheumatic disease; SSc: Systemic sclerosis; PR: Prevalence ratio; HR: Hazard ratio; APS, Antiphospholipid syndrome; bDMARDs, biological disease-modifying anti-rheumatic drugs; OR: Odds ratio; MMF: Mycophenolate mofetil; AIRD: autoimmune inflammatory rheumatic diseases; PsA: Psoriatic arthritis; AS: Ankylosing spondylitis; ITP: Immune thrombocytopenia; RMDs: Rheumatic and musculoskeletal diseases; SpA: Spondyloarthritis; DM: Diabetes mellitus; IA: Inflammatory arthritis; CTDs: Connective tissue diseases; IES-R: Impact of Events Scale-Revised; LE: Lupus erythematosus; MPA: Mycophenolic acid; ARD: autoimmune rheumatic diseases; Anti-BLYSS: anti-B lymphocytes stimulating factor; AAV: ANCA-associated vasculitis; IIM: Idiopathic inflammatory myopathies.
The epidemiology of SLE and COVID-19
Thirteen studies reported the epidemiology of SLE and COVID-19. The proportion of SLE patients varied from 7% to 32.9% in rheumatic patients infected with COVID-19.25,36,42 Relative to patients with other autoimmune diseases, SLE patients with COVID-19 infection appeared to develop more severe manifestations of COVID-19 infection, with higher rates of hospitalisation, invasive ventilation requirement or death.21,25,36,39,53 The prevalence of COVID-19 infection varied between 0.0% and 18.1% in SLE patients,13,18,19,41,45,59 and the hospitalisation rates ranged from 0.24% to 10.6%.19,45,59 A decline in SLE hospitalisation rate was found during the COVID-19 pandemic, and disease flare (38.9%) and infection (22.2%) were considered as the main reasons for hospitalisation in SLE patients.15
The clinical characteristics of SLE patients with COVID-19
The clinical characteristics of SLE patients with COVID-19 were reported in 23 studies. COVID-19 infection might mimic SLE flare, and sometimes could occur concurrently with SLE flare.14,17,29,30,40,46,51 The main clinical symptoms of COVID-19 infection in SLE patients were fever, cough, shortness of breath, headache, increased respiratory rate, myalgia, rhinorrhoea and diarrhoea.13,37,45,46 The clinical presentation described in paediatric SLE population with COVID-19 might be similar to those in adults, characterised by increased work of breathing and low oxygen saturation.49 Bozzalla Cassione et al.13 also reported that 11 of 12 SLE patients with COVID-19 are likely to have a mild and self-resolving disease course. However, patients with mutivisceral SLE damage would be at risk of severe COVID-19.52,55 A patient of SLE with COVID-19 could develop COVID‐19–related varicelliform rashes, leptomeningeal involvements, thrombotic events and immune thrombocytopenia.9,20,28,33,35,48 The infection of COVID-19 could trigger the presentation or exacerbation of SLE.12,27,44,57
The treatment of COVID-19 in SLE patients
Thirteen studies reported the treatment of COVID-19 in SLE patients. Previous use of immunosuppressants did not seem to influence COVID-19 infection or symptoms in SLE patients.22,23 Intravenous immunoglobulin treatments and increased dose of corticosteroids might be effective for patients with COVID-19 and SLE.23,40 According to the available evidence, hydroxychloroquine (HCQ) did not appear to prevent the occurrence of COVID-19.10,23,31,32,37,40,45,47,51,60 Cardiovascular adverse events were frequently reported in patients with SLE under HCQ or chloroquine (CQ) treatment,26 and paediatric SLE patients might develop hyperpigmentation induced by HCQ.34
The impact of COVID-19 pandemic on SLE patients
Fifteen studies reported the impact of COVID-19 pandemic on SLE patients. During this lockdown period, SLE patients experienced supply shortages, missed the doses of drugs, underwent financial constraints and spent more money on healthcare.38,45,46 Many patients faced problems of reduced care access, such as missing their scheduled follow-up visits and being unable to get their investigations.14,24,46 A few patients discontinued previous treatments, which might result in worse SLE clinical outcomes.11,45,55,58,59 Besides possible clinical effects, treatment discontinuation might cause psychological consequences including anxiety, concern and probably a sense of uncertainty about the future.16,24,43,56 Patients also displayed a high adherence to social distancing measures,18,56,58 but it often came at the cost of physical activity, increased pain, disease activity, anxiety, depression and sleep disorders.11,50,54,56
Discussion
This systematic review found that there was variation in infection rates and hospitalisation rates in SLE patients across the included studies. HCQ lacked preventive effect on COVID-19, and SLE patients with COVID-19 experienced supply shortages, missed the doses of drugs, underwent financial constraints and suffered from psychological distress during the pandemic.
The COVID-19 infection rates in SLE patients ranged from 0.0% to 18.1%. This wide variation might be attributed to prevention of contagion adopted by SLE patients. The strict implements of the precaution in public could greatly contain the outbreak.61 Social distancing measures along with handwashing and mask-wearing could play important roles in tackling COVID-1962. SLE patients were found to have higher overall attack rates of COVID-19 relative to the general population.45,55 This is possibly because of immune dysfunction, use of immunosuppressant agents, impairment from prior disease activity, presence of comorbidities and overexpression of angiotensin-converting enzyme two receptor.6
Disease flare was the main cause of hospitalisation in SLE patients.15 Treatment discontinuation was likely to result in SLE flare.59,63 The reasons for poor adherence in medications included concern about adverse effects, being distracted, life getting in the way, uncertainty or disagreement about the important role of medications and having personal reasons.64 Furthermore, HCQ shortage was frequently reported by SLE patients in our study. HCQ discontinuation or long-term dose reduction was always linked to increased risk of flare in SLE patients.65–67 Therefore, abrupt medication withdrawal must be assessed with caution based on infection risk and comorbidities of SLE patients.59
In our study, HCQ was not effective in COVID-19 prevention in SLE patients. Similarly, data from completed studies and randomised controlled trials suggest that HCQ does not work.68 Previous meta-analysis has proved that although HCQ treatment is safe, it could not decrease mortality or promote clinical/virological recovery in COVID-19 patients.69 Whereas the evidence supporting the HCQ treatment for COVID-19 is not clear, HCQ has shown great efficacy against immune-mediated diseases. Therefore, physicians and individuals should avoid misuse of HCQ for the prophylaxis of COVID-19.70
SLE is related to high health-care costs and great loss of productivity.71 Patients with SLE have an average excess expenditure of more than $30 per month on healthcare during the outbreak. Mental health problem was a concurrent epidemic of the pandemic.72 Mental problems were frequently reported during this period.73 While anxiety, depression and economic stressors are common phenomena around the world, specific behavioural responses are heavily influenced by government stances, misinformation, conspiratorialism and competing demands of resource scarcity.74
Our systematic review has several limitations. First, assumptions on the COVID-19 in SLE patients were mostly based on small sample sizes and thus should be treated with reserve. Second, studies not published in English and not reporting clinical information were excluded, so some studies on the subject may not have been identified.
In conclusion, SLE patients experience a great physical toll and emotional distress during the COVID-19 pandemic. Adequate medical services, containment policies and social distancing measures were essential to limit the effect of COVID-19 on SLE patients. Psychological approaches were also required during this period.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Humanities and Social Sciences of Ministry of Education Planning Fund (Project Number 20YJAZH007), Social and People’s Livelihood Technology in Nantong city-General Project (Project Number MS12019038), Postgraduate Research & Practice Innovation Program of Jiangsu Province (Project Number SJCX21_1480) and Nantong Science and Technology Plan Project (Project Number MSZ20020).
ORCID iD
Hong-Lin Chen https://orcid.org/0000-0003-0147-6863
References
- 1.Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020; 579: 270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. Jama 2020; 324: 782–793. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization . Situation reports. Weekly epidemiological update on COVID-19 - 27 July 2021. 2021.
- 4.Hu J, Wang Y. The clinical characteristics and risk factors of severe COVID-19. Gerontology 2021; 67: 255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spihlman AP, Gadi N, Wu SC, et al. COVID-19 and systemic lupus erythematosus: focus on immune response and therapeutics. Front Immunol 2020; 11: 589474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mason A, Rose E, Edwards CJ. Clinical management of Lupus patients during the COVID-19 pandemic. Lupus 2020; 29: 1661–1672. [DOI] [PubMed] [Google Scholar]
- 7.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Bmj 2009; 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Institutes of Health . Study Quality Assessment Tools. 2018.
- 9.Aguirre-Alastuey ME, Suárez-Díaz S, Rodríguez-Jerez F, et al. Venous thrombosis in a systemic lupus erythematosus patient with antiphospholipid antibodies coinciding with mild Covid-19. Lupus 2021; 30: 172–174. [DOI] [PubMed] [Google Scholar]
- 10.Alharthy A, Faqihi F, Nasim N, et al. COVID-19 in a patient with a flare of systemic lupus erythematosus: A rare case-report. Respir Med Case Rep 2020; 31: 101252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ammitzbøll C, Andersen JB, Vils SR, et al. Isolation, behavioral changes and low seroprevalence of SARS-CoV-2 antibodies in patients with Systemic Lupus Erythematosus or Rheumatoid arthritis. Arthritis Care Res (Hoboken) 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonometti R, Sacchi MC, Stobbione P, et al. The first case of systemic lupus erythematosus (SLE) triggered by COVID-19 infection. Eur Rev Med Pharmacol Sci 2020; 24: 9695–9697. [DOI] [PubMed] [Google Scholar]
- 13.Bozzalla Cassione E, Zanframundo G, Biglia A, et al. COVID-19 infection in a northern-Italian cohort of systemic lupus erythematosus assessed by telemedicine. Ann Rheum Dis 2020; 79: 1382–1383. [DOI] [PubMed] [Google Scholar]
- 14.Cho J, Kandane-Rathnayake R, Louthrenoo W, et al. COVID-19 infection in patients with systemic lupus erythematosus: Data from the Asia Pacific Lupus Collaboration. Int J Rheum Dis 2020; 23: 1255–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chuah SL, Teh CL, Wan Mohd Akbar SA, et al. Impact of COVID-19 pandemic on hospitalisation of patients with systemic lupus erythematosus (SLE): report from a tertiary hospital during the peak of the pandemic. Ann Rheum Dis 2020. [DOI] [PubMed] [Google Scholar]
- 16.Cornet A, Andersen J, Tani C, et al. Hydroxychloroquine availability during COVID-19 crisis and its effect on patient anxiety. Lupus Sci Med 2021; 8: e000496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El Aoud S, Morin C, Lorriaux P, et al. COVID-19 Presenting as Lupus Erythematosus-Like Syndrome. Disaster Med Public Health Prep 2020; 15: e12–e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Favalli EG, Gerosa M, Murgo A, et al. Are patients with systemic lupus erythematosus at increased risk for COVID-19?. Ann Rheum Dis 2021; 80: e25. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez-Ruiz R, Masson M, Kim MY, et al. Leveraging the United states epicenter to provide insights on COVID-19 in patients with systemic lupus erythematosus. Arthritis Rheumatol 2020; 72: 1971–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freeman-Beman L, Ratner S, Kabani N, et al. COVID-19 coagulopathy in a patient with systemic lupus erythematosus and antiphospholipid antibodies. J Clin Rheumatol 2021; 27: e60–e1. [DOI] [PubMed] [Google Scholar]
- 21.Freites Nuñez DD, Leon L, Mucientes A, et al. Risk factors for hospital admissions related to COVID-19 in patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis 2020; 79: 1393–1399. [DOI] [PubMed] [Google Scholar]
- 22.Gartshteyn Y, Askanase AD, Schmidt NM, et al. COVID-19 and systemic lupus erythematosus: a case series. Lancet Rheumatol 2020; 2: e452–e454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gendebien Z, von Frenckell C, Ribbens C, et al. Systematic analysis of COVID-19 infection and symptoms in a systemic lupus erythematosus population: correlation with disease characteristics, hydroxychloroquine use and immunosuppressive treatments. Ann Rheum Dis 2020; 80: e94. [DOI] [PubMed] [Google Scholar]
- 24.George MD, Venkatachalam S, Banerjee S, et al. Concerns, healthcare use, and treatment interruptions in patients with common autoimmune rheumatic diseases during the COVID-19 pandemic. J Rheumatol 2021; 48: 603–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gianfrancesco M, Hyrich KL, Al-Adely S, et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis 2020; 79: 859–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldman A, Bomze D, Dankner R, et al. Cardiovascular adverse events associated with hydroxychloroquine and chloroquine: A comprehensive pharmacovigilance analysis of pre-COVID-19 reports. Br J Clin Pharmacol 2021; 87: 1432–1442. [DOI] [PubMed] [Google Scholar]
- 27.Gracia-Ramos AE, Saavedra-Salinas M. Can the SARS-CoV-2 infection trigger systemic lupus erythematosus? A case-based review. Rheumatol Int 2021; 41: 799–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guven F, Ogul H, Turgut A, et al. Leptomeningeal involvement in a patient with systemic lupus erythematosus infected by COVID-19. Joint Bone Spine 2020; 87: 495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han Y, Jiang M, Xia D, et al. COVID-19 in a patient with long-term use of glucocorticoids: A study of a familial cluster. Clin Immunol 2020; 214: 108413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He F, Luo Q, Lei M, et al. Successful recovery of recurrence of positive SARS-CoV-2 RNA in COVID-19 patient with systemic lupus erythematosus: a case report and review. Clin Rheumatol 2020; 39: 2803–2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holubar J, Le Quintrec M, Letaief H, et al. Monitoring of patients with systemic lupus erythematosus during the COVID-19 outbreak. Ann Rheum Dis 2020; 80: e56. [DOI] [PubMed] [Google Scholar]
- 32.Jung SY, Kim MS, Kim MC, et al. Effect of hydroxychloroquine pre-exposure on infection with SARS-CoV-2 in rheumatic disease patients: a population-based cohort study. Clin Microbiol Infect 2021; 27: 611–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kondo Y, Kaneko Y, Oshige T, et al. Exacerbation of immune thrombocytopaenia triggered by COVID-19 in patients with systemic lupus erythematosus. Ann Rheum Dis 2020; 80: e77. [DOI] [PubMed] [Google Scholar]
- 34.Leung AK, McMillan T, Human A, et al. Hydroxychloroquine-induced hyperpigmentation in a 14-year-old female with systemic lupus erythematosus. Drugs Context 2020; 9: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mantovani Cardoso E, Hundal J, Feterman D, et al. Concomitant new diagnosis of systemic lupus erythematosus and COVID-19 with possible antiphospholipid syndrome. Just a coincidence? A case report and review of intertwining pathophysiology. Clin Rheumatol 2020; 39: 2811–2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marques CDL, Kakehasi AM, Pinheiro MM, et al. High levels of immunosuppression are related to unfavourable outcomes in hospitalised patients with rheumatic diseases and COVID-19: first results of ReumaCoV Brasil registry. RMD Open 2021; 7: e001461corr1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mathian A, Mahevas M, Rohmer J, et al. Clinical course of coronavirus disease 2019 (COVID-19) in a series of 17 patients with systemic lupus erythematosus under long-term treatment with hydroxychloroquine. Ann Rheum Dis 2020; 79: 837–839. [DOI] [PubMed] [Google Scholar]
- 38.Mendel A, Bernatsky S, Askanase A, et al. Hydroxychloroquine shortages among patients with systemic lupus erythematosus during the COVID-19 pandemic: experience of the systemic lupus international collaborating clinics. Ann Rheum Dis 2021; 80: 1–2. [DOI] [PubMed] [Google Scholar]
- 39.Montero F, Martínez-Barrio J, Serrano-Benavente B, et al. Coronavirus disease 2019 (COVID-19) in autoimmune and inflammatory conditions: clinical characteristics of poor outcomes. Rheumatol Int 2020; 40: 1593–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ning R, Meng S, Tang F, et al. A case of SLE with COVID-19 and multiple infections. Open Med (Wars) 2020; 15: 1054–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pablos JL, Abasolo L, Alvaro-Gracia JM, et al. Prevalence of hospital PCR-confirmed COVID-19 cases in patients with chronic inflammatory and autoimmune rheumatic diseases. Ann Rheum Dis 2020; 79: 1170–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pablos JL, Galindo M, Carmona L, et al. Clinical outcomes of hospitalised patients with COVID-19 and chronic inflammatory and autoimmune rheumatic diseases: a multicentric matched cohort study. Ann Rheum Dis 2020; 79: 1544–1549. [DOI] [PubMed] [Google Scholar]
- 43.Plüß M, Chehab G, Korsten P. Concerns and needs of patients with systemic lupus erythematosus regarding hydroxychloroquine supplies during the COVID-19 pandemic: results from a patient-centred survey. Ann Rheum Dis 2020; 80: e52. [DOI] [PubMed] [Google Scholar]
- 44.Raghavan S, Gonakoti S, Asemota IR, et al. a case of systemic lupus erythematosus flare triggered by severe coronavirus disease 2019. J Clin Rheumatol 2020; 26: 234–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramirez GA, Gerosa M, Beretta L, et al. COVID-19 in systemic lupus erythematosus: Data from a survey on 417 patients. Semin Arthritis Rheum 2020; 50: 1150–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rathi M, Singh P, Bi HP, et al. Impact of the COVID-19 pandemic on patients with systemic lupus erythematosus: Observations from an Indian inception cohort. Lupus 2021; 30: 158–164. [DOI] [PubMed] [Google Scholar]
- 47.Rentsch CT, DeVito NJ, MacKenna B, et al. Effect of pre-exposure use of hydroxychloroquine on COVID-19 mortality: a population-based cohort study in patients with rheumatoid arthritis or systemic lupus erythematosus using the OpenSAFELY platform. Lancet Rheumatol 2021; 3: e19–e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Slimani Y, Abbassi R, El Fatoiki FZ, et al. Systemic lupus erythematosus and varicella-like rash following COVID-19 in a previously healthy patient. J Med Virol 2021; 93: 1184–1187. [DOI] [PubMed] [Google Scholar]
- 49.Sukhdeo S, Negroponte E, Rajasekhar H, et al. Acute respiratory distress syndrome and COVID-19 in a child with systemic lupus erythematosus. Lupus 2021; 30: 836–839. [DOI] [PubMed] [Google Scholar]
- 50.Tee CA, Salido EO, Reyes PWC, et al. Psychological state and associated factors during the 2019 coronavirus disease (COVID-19) pandemic among filipinos with rheumatoid arthritis or systemic lupus erythematosus. Open Access Rheumatol 2020; 12: 215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Teh CL, Cheong YK, Wan Musa WR, et al. COVID-19 among Malaysian patients with systemic lupus erythematosus on hydroxychloroquine. Ann Rheum Dis 2021; 80: e69. [DOI] [PubMed] [Google Scholar]
- 52.Tiendrébéogo WJS, Kaboré F, Diendéré EA, et al. Case series of chronic inflammatory rheumatic disease patients infected by coronavirus disease 2019 (COVID-19). Case Rep Rheumatol 2020; 2020: 8860492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wallace B, Washer L, Marder W, et al. Patients with lupus with COVID-19: University of Michigan experience. Ann Rheum Dis 2021; 80: e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wańkowicz P, Szylińska A, Rotter I. Evaluation of mental health factors among people with systemic lupus erythematosus during the SARS-CoV-2 pandemic. J Clin Med 2020; 9: 2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wen J, Suo H, Zhang Y, et al. Risk of COVID-19 infection among lupus erythematosus patients and rheumatoid arthritis patients: a retrospective study in Hubei, China. Eur J Dermatol 2020; 30: 751–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Widhani A, Rengganis I, Susanto AJ, et al. Factors related to knowledge, perception, and practices towards COVID-19 among patients with autoimmune diseases: a multicenter online survey. Acta Med Indones 2020; 52: 214–226. [PubMed] [Google Scholar]
- 57.Zamani B, Moeini Taba SM, Shayestehpour M. Systemic lupus erythematosus manifestation following COVID-19: a case report. J Med Case Rep 2021; 15: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zen M, Fuzzi E, Astorri D, et al. SARS-CoV-2 infection in patients with autoimmune rheumatic diseases in northeast Italy: A cross-sectional study on 916 patients. J Autoimmun 2020; 112: 102502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zucchi D, Tani C, Elefante E, et al. Impact of first wave of SARS-CoV-2 infection in patients with Systemic Lupus Erythematosus: Weighting the risk of infection and flare. PLoS One 2021; 16: e0245274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zurita MF, Iglesias Arreaga A, Luzuriaga Chavez AA, et al. SARS-CoV-2 infection and COVID-19 in 5 patients in ecuador after prior treatment with hydroxychloroquine for systemic lupus erythematosus. Am J Case Rep 2020; 21: e927304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wei J, Guo S, Long E, et al. Why does the spread of COVID-19 vary greatly in different countries? Revealing the efficacy of face masks in epidemic prevention. Epidemiol Infect 2021; 149: e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Teslya A, Pham TM, Godijk NG, et al. Impact of self-imposed prevention measures and short-term government-imposed social distancing on mitigating and delaying a COVID-19 epidemic: A modelling study. Plos Med 2020; 17: e1003166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zeng X, Zheng L, Rui H, et al. Risk factors for the flare of systemic lupus erythematosus and its influence on prognosis: a single-center retrospective analysis. Adv Rheumatol 2021; 61: 43. [DOI] [PubMed] [Google Scholar]
- 64.Hardy C, Gladman DD, Su J, et al. Barriers to medication adherence and degree of nonadherence in a systemic lupus erythematosus (SLE) outpatient population. Rheumatol Int 2021; 41: 1457–1464. [DOI] [PubMed] [Google Scholar]
- 65.Peschken CA. Possible consequences of a shortage of hydroxychloroquine for patients with systemic lupus erythematosus amid the COVID-19 pandemic. J Rheumatol 2020; 47: 787–790. [DOI] [PubMed] [Google Scholar]
- 66.Canadian Hydroxychloroquine Study Group . A randomized study of the effect of withdrawing hydroxychloroquine sulfate in systemic lupus erythematosus. N Engl J Med 1991; 324: 150–154. [DOI] [PubMed] [Google Scholar]
- 67.Aouhab Z, Hong H, Felicelli C, et al. Outcomes of systemic lupus Erythematosus in patients who discontinue hydroxychloroquine. ACR Open Rheumatol 2019; 1: 593–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smit M, Marinosci A, Agoritsas T, et al. Prophylaxis for COVID-19: a systematic review. Clin Microbiol Infect 2021; 27: 532–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gupta T, Thakkar P, Kalra B, et al. Hydroxychloroquine in the treatment of coronavirus disease 2019: Rapid updated systematic review and meta-analysis. Rev Med Virol; 2021: e2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yazdany J, Kim AHJ. Use of hydroxychloroquine and chloroquine during the COVID-19 pandemic: what every clinician should know. Ann Intern Med 2020; 172: 754–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lau CS, Mak A. The socioeconomic burden of SLE. Nat Rev Rheumatol 2009; 5: 400–404. [DOI] [PubMed] [Google Scholar]
- 72.Castaldelli-Maia JM, Marziali ME, Lu Z, et al. Investigating the effect of national government physical distancing measures on depression and anxiety during the COVID-19 pandemic through meta-analysis and meta-regression. Psychol Med 2021; 51: 881–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu X, Zhu M, Zhang R, et al. Public mental health problems during COVID-19 pandemic: a large-scale meta-analysis of the evidence. Transl Psychiatry 2021; 11: 384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kendrick K, Isaac M. Overview of behavioural and psychological consequences of COVID 19. Curr Opin Psychiatry 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]