To the Editor:
The use of alternative tobacco products (ATPs) continues to expand even as cigarette smoking rates decline. Electronic cigarettes (e-cigarettes) and hookah water pipes (hookah) have emerged as two of the most popular ATPs (1). Both deliver nicotine and a mix of chemicals, including solvents that function as nicotine carriers, humectants, and/or flavoring chemicals to enhance the appeal. A key difference between these two ATPs is their emissions: e-cigarette liquids are heated to generate an inhalable mixture of particles and gases; hookah tobacco is burned/charred to generate nicotine-laden smoke. E-cigarette aerosols have been shown to contain fewer distinct toxicants than are typically measured in the smoke from tobacco combustion. However, carcinogens, respiratory irritants, and toxic metals have been detected in e-cigarette and hookah emissions (2, 3).
The upper respiratory epithelium (i.e., mouth and nose) are the first tissues exposed to inhaled pollutants. How inhaled toxicants affect these tissues often mirrors effects in the lower conducting airways and gas-exchange regions of the lung. Thus, upper respiratory mucosal tissues may serve as an easily accessible surrogate tissue to gauge pulmonary risk (4). However, inconsistent data from experimental, clinical, and epidemiological studies of ATP health consequences have made it challenging to reach a consensus on the potential health risks of these products.
In accordance with institutional review board-approved protocols (NYUSOM s17–01143 and s16–02226), 89 adults provided salivary and nasal samples as part of a larger study evaluating the air quality and health impacts of residential ATP use. Participants (aged 21–50) included never-smokers (n = 37), and current exclusive tobacco product users (defined as having used within the past 7 days): cigarette smokers (n = 16), hookah smokers (n = 16), and e-cigarette vapers (n = 20). Participants were asked to abstain from using their respective tobacco products for a minimum of 24 hours prior to sample collection; tobacco product verification and possession were confirmed during the study visit. Exhaled carbon monoxide confirmed combustible smoking status (hookah and cigarettes) and excluded potential individuals who were enrolled as exclusive e-cigarette users but who also used combustible tobacco products (i.e., were poly tobacco users). Saliva and nasal epithelial-lining fluid were collected using validated protocols (5, 6). A salivary cotinine ELISA (Salimetrics) confirmed smoking/nonsmoking status. A V Plex Proinflammatory Panel 1 (human) kit was used to assay salivary and nasal extracts for 10 proinflammatory cytokines (Meso-Scale Discovery).
Some notable differences between tobacco product users and cohort demographics were observed (Table E1 in the data supplement). The mean age of recruited cigarette smokers (37.1 years old) was approximately 5 years higher than that of vapers or hookah smokers (32.2 and 31.6 years old, respectively). Additionally, participant race/ethnicity seemed to influence product preference: most vapers were non-Hispanic whites. In contrast, most hookah smokers were Hispanic, while 50% of cigarette smokers were non-Hispanic Blacks. Annual household income also correlated with tobacco product preferences. More than half of vapers reported an annual household income ⩾$75,000; cigarette and hookah households were far more likely to report lower incomes. We evaluated upper respiratory inflammation in e-cigarette and hookah cohorts against two reference groups (nonsmokers and cigarette smokers), which served as negative and positive controls, respectively. Compared with both nonsmokers and cigarette smokers, nasal inflammation in ATP users was elevated, as evidenced by significantly increased cytokines in e-cigarette vapers and hookah smokers (Figure 1). Mean nasal-cytokine levels did not differ significantly between e-cigarette and hookah cohorts. Except for TNF-α, which was suppressed in cigarette smokers (Figure 1G), mean concentrations of nasal cytokines did not differ statistically between nonsmoking and cigarette-smoking cohorts. Unlike the ATP-specific inflammatory responses observed in nasal epithelial-lining fluid, we did not find any association between tobacco product use and oral inflammation (as assessed by salivary cytokines) (Table 1).
Figure 1.
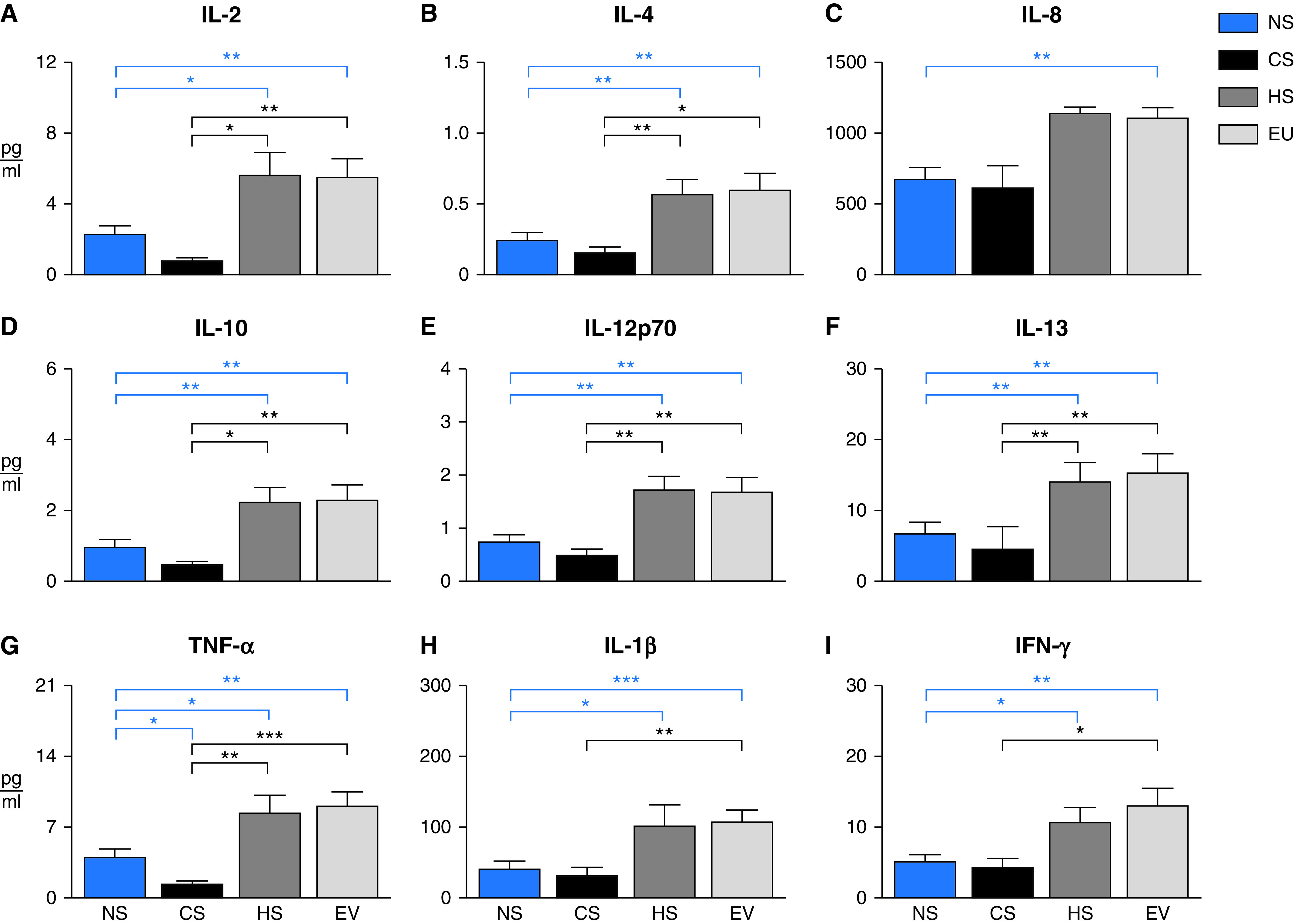
Aggregate nasal cytokine concentrations stratified by participant tobacco product status: adult nonsmokers (NS; blue, n = 35), cigarette smokers (CS; black, n = 13), hookah smokers (HS; dark gray, n = 13), and e-cigarette users (EU, light gray, n = 19). Data are expressed as cytokine group means (pg/ml) + SEM. Because nasal data failed normality tests, a Kruskal-Wallis test was used to identify mean differences. When P ⩽ 0.05, a post hoc Dunn’s multiple comparisons test was used to identify which groups were different from one another. Blue lines indicate when means were statistically different from NS reference group (i.e., negative control). Black lines indicate when means were statistically different from CS reference group (i.e., positive control). *P ⩽ 0.05, **P ⩽ 0.01, and ***P ⩽ 0.001.
Table 1.
Mean Salivary Cytokine and Cotinine Concentrations Stratified by Participant Tobacco Use
| Analyte | NS | CS | HS | EU | P value |
|---|---|---|---|---|---|
| IL-2 (pg/ml) | 1.1 ± 0.3 | 0.4 ± 0.1 | 0.5 ± 0.1 | 0.4 ± 0.1 | 0.92 |
| IL-4 (pg/ml) | 0.1 ± 0.03 | 0.1 ± 0.01 | 0.1 ± 0.01 | 0.1 ± 0.01 | 0.93 |
| IL-6 (pg/ml) | 1.4 ± 0.4 | 1.0 ± 0.4 | 0.6 ± 0.2 | 1.1 ± 0.5 | 0.83 |
| IL-8 (pg/ml) | 347.4 ± 94.0 | 82.3 ± 39.7 | 51.8 ± 23.1 | 21.2 ± 6.9 | 0.13 |
| IL-10 (pg/ml) | 0.6 ± 0.2 | 0.1 ± 0.03 | 0.1 ± 0.02 | 0.1 ± 0.02 | 0.74 |
| IL-12p70 (pg/ml) | 0.2 ± 0.06 | 0.2 ± 0.05 | 0.2 ± 0.06 | 0.2 ± 0.03 | 0.83 |
| IL-13 (pg/ml) | 2.6 ± 0.7 | 1.1 ± 0.4 | 1.3 ± 0.3 | 1.1 ± 0.3 | 0.89 |
| IL-1β (pg/ml) | 26.5 ± 6.8 | 23.8 ± 6.8 | 24.8 ± 9.9 | 14.6 ± 5.4 | 0.44 |
| IFN-γ (pg/ml) | 1.4 ± 0.3 | 1.9 ± 0.5 | 1.1 ± 0.4 | 1.6 ± 0.4 | 0.57 |
| TNF-α (pg/ml) | 1.8 ± 0.6 | 0.4 ± 0.10 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.86 |
| Cotinine (ng/ml) | N.D. | 23.8 ± 6.18**** | 2.88 ± 0.61† | 19.1 ± 4.57*** | <0.01 |
Definition of abbreviations: ATP = alternative tobacco product; CS = cigarette smoker; EU = e-cigarette user; HS = hookah smoker; N.D. = nondetectable (all samples below the lower limit of detection of 0.4 ng/ml); NS = nonsmoker.
Analyte aggregate means ± SEM by tobacco product use. Because salivary data failed normality tests, analytes were analyzed with nonparametric Kruskal-Wallis tests. When P ⩽ 0.05 (in bold), a Dunn’s multiple comparison test was performed post hoc to identify where statistically significant differences occurred.
Asterisks indicate when tobacco product differs from nonsmoking outcome (negative reference group). ***P ⩽ 0.001, and ****P ⩽ 0.0001.
Indicates when ATP groups differ from cigarettes (i.e., positive reference group).
All nonsmoking participants had nondetectable levels of salivary cotinine. Cotinine was modestly elevated in hookah smokers compared with nonsmokers (not statistically significant). Cotinine levels among vapers and cigarette smokers were significantly elevated (approximately 10 times higher than hookah smokers) (Table 1).
Here, we present data suggesting two popular ATPs, e-cigarettes and hookah water pipes, may represent a novel risk factor for nasal inflammation. The increase in nasal inflammation observed in ATP users was strikingly absent in cigarette smokers and points to the unique potential of these products to cause upper respiratory harm. When stratified by biological sex, nasal inflammation remained consistently elevated among hookah and e-cigarette cohorts (Table E1). One caveat: separating ATP cohort participants by sex often affected the power needed to detect statistically significant differences (Table E1). Interestingly, the fold-change of nasal inflammation was greater in male e-cigarette vapers (compared with vaping females, data not shown); this relative difference in effect size was attributed to sex-based differences in nasal inflammation of nonsmoking controls. These clinical data are consistent with previously reported in vitro findings of opposing inflammatory responses from e-cigarettes and cigarettes in nasal epithelial cells, reinforcing the potential for ATPs to elicit unique nasal harm (7).
Expected differences in product-specific tobacco-use patterns (daily cigarette/e-cigarette use versus less frequent hookah smoking sessions) were reflected in salivary cotinine levels, although cotinine did not correlate with either salivary or nasal inflammation. While saliva is a common and reliable matrix to quantify cotinine (and approximate recent nicotine consumption), no statistically significant changes in salivary cytokines were detected across tobacco cohorts. Together, these data suggest that saliva may not be an optimal matrix to evaluate ATP-related immune responses.
Several clinical studies have found that compared with nonsmokers, even acute ATP use can cause adverse pulmonary outcomes. While many ATP-linked pulmonary consequences mirror those observed in cigarette smokers (8, 9), an increasing number of studies are finding evidence of ATP-specific immunological responses in respiratory tissues (10). To this end, a recent study of tobacco-related inflammation implicated different pathways for inflammation related to e-cigarettes and cigarettes (11). Thus, the unique cytokine profiles seen here in ATP users’ nasal epithelium, but not saliva, suggest the nose may represent a uniquely important target tissue for the investigation of effects of e-cigarette and hookah emissions. While e-cigarette and hookah emissions have distinct chemical compositions, both contain sugar-based solvents, several of which are known respiratory irritants. Those solvents might explain the shared nasal inflammatory phenotype we observed (12–15). Alternatively, product-specific behaviors, such as nasal exhalation of ATP emissions, may be a significant influencer of nasal exposure, which could affect the tissue-specific responses we found.
While the elevated nasal inflammation observed among ATP users is intriguing and novel, the clinical significance of these modest changes remains unclear. However, chronic nasal inflammation is an independent risk factor of chronic rhinosinusitis (CRS), a disease characterized by persistent (⩾3 months) nasal and sinus inflammation. Many cytokines elevated in the nose of ATP users are secreted by Helper-T (Th) cells, including IL-12, IFN-γ, and TNF-α (Th1) and IL- 4, IL-5, IL-10, IL-13 (Th2). Not only have quantitative and functional disruptions of Th cell-type ratios been identified in patients diagnosed with rhinitis of multiple origins (16), including CRS (17), but overactive Th signaling has also been implicated in autoimmunity (Th1), allergy (Th2), and hypersensitivity (Th1 and Th2). Separately, nasal immune responses at both the gene and protein level, including Th1 cytokines, were attenuated in e-cigarette users inoculated with live-attenuated influenza virus (18). Whether increases in baseline nasal inflammation associated with ATP use in healthy adults increases the risk of rhinitis or augments response to infection remains to be seen.
Several study characteristics limit further interpretation of our findings. These include the relatively small sample size of the tobacco-product cohorts. The study’s cross-sectional nature prevents us from commenting on how previous tobacco history and current product use (i.e., frequency and brand) might influence the observed nasal inflammation. Additional limitations include approximate time from most recent tobacco product use and sample collection, allergic rhinitis status, and nasal corticosteroid use. Future work should validate these findings in a larger cohort, examining whether these ATPs trigger inflammation through shared biological pathways and exploring long-term health impacts of nasal-cytokine elevations. Our findings also highlight a need to expand the health endpoints assessed in ATP-toxicity studies. Understanding why some respiratory tissues, such as the nose, are more sensitive to ATP emissions will inform public health initiatives and substantiate clinical recommendations.
Footnotes
Supported by the National Institutes of Health/National Institute of Environmental Health Sciences (NIH/NIEHS P30 ES000260 and NIH/NIEHS R21 ES026996) and the National Heart, Lung, and Blood Institute (NIH/NHLBI R01 HL139239). E.K. was supported by NIH/NIEHS T32 ES007324.
Author Contributions: E.K., J.A.S., L.L., I.J., M.W., and T.G. conceived and designed the experiments. J.A.S., L.L., T.A.R., J.E., A.E., A.S., and S.K. were responsible for recruiting participants, conducting home visits, and collecting all participant data and samples. E.K. and J.H. performed cytokine and cotinine assays. E.K., J.H., K.F., M.E.R., G.G., and T.G. analyzed the data. E.K., M.E.R., I.J., M.W., and T.G. wrote and revised/edited the manuscript.
This letter has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1. Gilreath TD, Leventhal A, Barrington-Trimis JL, Unger JB, Cruz TB, Berhane K, et al. Patterns of alternative tobacco product use: emergence of hookah and e-cigarettes as preferred products amongst youth. J Adolesc Health . 2016;58:181–185. doi: 10.1016/j.jadohealth.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Elsayed Y, Dalibalta S, Abu-Farha N. Chemical analysis and potential health risks of hookah charcoal. Sci Total Environ . 2016;569-570:262–268. doi: 10.1016/j.scitotenv.2016.06.108. [DOI] [PubMed] [Google Scholar]
- 3. Hess CA, Olmedo P, Navas-Acien A, Goessler W, Cohen JE, Rule AM. E-cigarettes as a source of toxic and potentially carcinogenic metals. Environ Res . 2017;152:221–225. doi: 10.1016/j.envres.2016.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Harkema JR, Carey SA, Wagner JG. The nose revisited: a brief review of the comparative structure, function, and toxicologic pathology of the nasal epithelium. Toxicol Pathol . 2006;34:252–269. doi: 10.1080/01926230600713475. [DOI] [PubMed] [Google Scholar]
- 5. Shearston J, Lee L, Eazor J, Meherally S, Park SH, Vilcassim MR, et al. Effects of exposure to direct and secondhand hookah and e-cigarette aerosols on ambient air quality and cardiopulmonary health in adults and children: protocol for a panel study. BMJ Open . 2019;9:e029490. doi: 10.1136/bmjopen-2019-029490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rebuli ME, Speen AM, Clapp PW, Jaspers I. Novel applications for a noninvasive sampling method of the nasal mucosa. Am J Physiol Lung Cell Mol Physiol . 2017;312:L288–L296. doi: 10.1152/ajplung.00476.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rouabhia M, Piché M, Corriveau M-N, Chakir J. Effect of e-cigarettes on nasal epithelial cell growth, Ki67 expression, and pro-inflammatory cytokine secretion. Am J Otolaryngol . 2020;41:102686. doi: 10.1016/j.amjoto.2020.102686. [DOI] [PubMed] [Google Scholar]
- 8. Raad D, Gaddam S, Schunemann HJ, Irani J, Abou Jaoude P, Honeine R, et al. Effects of water-pipe smoking on lung function: a systematic review and meta-analysis. Chest . 2011;139:764–774. doi: 10.1378/chest.10-0991. [DOI] [PubMed] [Google Scholar]
- 9. Vardavas CI, Anagnostopoulos N, Kougias M, Evangelopoulou V, Connolly GN, Behrakis PK. Short-term pulmonary effects of using an electronic cigarette: impact on respiratory flow resistance, impedance, and exhaled nitric oxide. Chest . 2012;141:1400–1406. doi: 10.1378/chest.11-2443. [DOI] [PubMed] [Google Scholar]
- 10. Reidel B, Radicioni G, Clapp PW, Ford AA, Abdelwahab S, Rebuli ME, et al. E-cigarette use causes a unique innate immune response in the lung, involving increased neutrophilic activation and altered mucin secretion. Am J Respir Crit Care Med . 2018;197:492–501. doi: 10.1164/rccm.201708-1590OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ganesan SM, Dabdoub SM, Nagaraja HN, Scott ML, Pamulapati S, Berman ML, et al. Adverse effects of electronic cigarettes on the disease-naive oral microbiome. Sci Adv . 2020;6:eaaz0108. doi: 10.1126/sciadv.aaz0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Suber RL, Deskin R, Nikiforov I, Fouillet X, Coggins CRE. Subchronic nose-only inhalation study of propylene glycol in Sprague-Dawley rats. Food Chem Toxicol . 1989;27:573–583. doi: 10.1016/0278-6915(89)90016-1. [DOI] [PubMed] [Google Scholar]
- 13. Alexander DJ, Collins CJ, Coombs DW, Gilkison IS, Hardy CJ, Healey G, et al. Association of Inhalation Toxicologists (AIT) working party recommendation for standard delivered dose calculation and expression in non-clinical aerosol inhalation toxicology studies with pharmaceuticals. Inhal Toxicol . 2008;20:1179–1189. doi: 10.1080/08958370802207318. [DOI] [PubMed] [Google Scholar]
- 14. Viswam D, Trotter S, Burge PS, Walters GI. Respiratory failure caused by lipoid pneumonia from vaping e-cigarettes. BMJ Case Rep . 2018;2018:bcr-2018-224350. doi: 10.1136/bcr-2018-224350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wieslander G, Norbäck D, Lindgren T. Experimental exposure to propylene glycol mist in aviation emergency training: acute ocular and respiratory effects. Occup Environ Med . 2001;58:649–655. doi: 10.1136/oem.58.10.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ohta N, Sakurai S, Yoshitake H, Aoyagi M. Analysis of Th1, Th2, Tc1 and Tc2 cells in patients with allergic rhinitis. Clin Exp Allergy Rev . 2005;5:68–71. [Google Scholar]
- 17. Baba S, Kagoya R, Kondo K, Suzukawa M, Ohta K, Yamasoba T. T-cell phenotypes in chronic rhinosinusitis with nasal polyps in Japanese patients. Allergy Asthma Clin Immunol . 2015;11:33. doi: 10.1186/s13223-015-0100-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rebuli ME, Glista-Baker E, Hoffman JR, Duffney PF, Robinette C, Speen AM, et al. Electronic-cigarette use alters nasal mucosal immune response to live-attenuated influenza virus. A clinical trial. Am J Respir Cell Mol Biol . 2021;64:126–137. doi: 10.1165/rcmb.2020-0164OC. [DOI] [PMC free article] [PubMed] [Google Scholar]


