Abstract
Tucaresol, a novel immunomodulator, was inactive against Leishmania donovani amastigotes in both peritoneal and bone marrow macrophages in vitro at concentrations between 100 and 1 μM, with toxicity to macrophages and parasites at 300 μM. However, against L. donovani in BALB/c mice at doses between 80 and 1.25 mg/kg of body weight administered once daily by the oral route during days 7 to 11 of infection, an optimal dose of 5 mg/kg produced a 43.8 to 62.4% suppression of liver amastigotes, with significantly reduced activity at the extremes of the dose range. This response was not related to levels of infection. No interaction with the standard pentavalent antimonial sodium stibogluconate (Pentostam) was observed during this period of infection. The optimum dose of 5 mg/kg was ineffective when administered during the first week of infection and was most effective against the liver infection when administered during weeks 2 to 3 of infection (42.3 to 46.8% inhibition) and against the splenic infection when administered during week 6 of infection (59.5% inhibition). The optimum dose of tucaresol against L. donovani in C57BL/6 mice was 5 mg/kg, which produced a 40.8 to 48.7% suppression of liver amastigotes when administered in a range of 80 to 1.25 mg/kg during days 7 to 11 of infection. The drug had no activity against L. donovani infections in C.B-17 scid mice when the same regimen was used.
Leishmaniasis is a complex of diseases with visceral, cutaneous, and mucocutaneous pathologies caused by up to 15 different species of the protozoan parasite Leishmania. The visceral form of the disease, caused by Leishmania donovani, Leishmania infantum, or Leishmania chagasi, can be potentially fatal if untreated. Visceral leishmaniasis (VL) is found in tropical and subtropical regions of the world and has a worldwide incidence of up to 500,000 cases (5; World Health Organization, Communicable Diseases Surveillance and Response [CSR]: Leishmaniasis Control [http://www.who.int/emc/diseases/leish/leisepidat.html]). Coinfections with L. infantum and human immunodeficiency virus (HIV) have been a growing problem in Mediterranean countries and have indicated that this parasite is also an opportunist (3). The current recommended drugs for the therapy of VL remain the pentavalent antimonials, sodium stibogluconate (Pentostam) and meglumine antimoniate (Glucantime) (9), which have been in clinical use for leishmaniasis for over 50 years. Other recommended drugs include amphotericin B, together with lipid formulations of this polyene antibiotic, and the aminoglycoside aminosidine (paromomycin) (9, 10). All the recommended drugs require parenteral administration and have other limitations that include cost, toxicity, variable efficacy, or restricted supplies. There is no effective treatment for immunosuppressed patients with L. infantum-HIV coinfection (3, 22).
In the search for new drugs for the treatment of leishmaniasis there has been a major emphasis on biochemical and molecular targets, for example, trypanothione reductase and cysteine proteases, and the identification of inhibitors by rational design or empirical screening (8, 39). The ability of Leishmania parasites to establish an infection in humans is dependent upon the adaptation of parasites to survive and multiply in the phagosomal compartment of macrophages as well as upon the development of host immunosuppression (12). The fine balance of this host-parasite interaction has suggested that immunostimulation is another rational approach to the treatment of leishmaniasis. The effects of immunomodulators on both VL and cutaneous leishmaniasis have been studied, either alone or in combination with chemotherapeutic drugs. These studies have included the endogenous biologicals gamma interferon (IFN-γ) (38) and granulocyte-macrophage colony-stimulating factor (7) in both experimental and clinical leishmaniasis and interleukin 12 (IL-12) (30) in experimental leishmaniasis, the bacterial and fungal derivatives muramyl dipeptide (2), trehalose dimycolate (19), and glucan (6), and the synthetic compounds levamisole (23), lipoidal amine CP-46,665-1 (1), cimetidine (20), tuftsin (18, 24), polyinosinic-polycytidylic acid (11), and imiquimod (14).
Tucaresol, an orally bioavailable immunopotentiatory drug, has been shown to enhance T-helper-cell activity, with the induction of increased IL-2 and IFN-γ levels in mice and humans (17, 36). The compound probably acts through the formation of a Schiff base on CD4+ T-cell surface amines which provides a costimulatory signal between antigen-presenting cells, such as macrophages, and T cells. Convergence of the tucaresol-mediated costimulatory signaling with T-cell receptor signaling occurs at the level of the mitogen-activated protein kinase ERK2 (16). Tucaresol has proved effective against experimental models of cytomegalovirus and murine colon adenocarcinoma and has been shown to be biologically active as an immunopotentiator, favoring a Th1 response in patients with malignant melanoma (29, 36). Tucaresol was well tolerated at doses of 400 mg/kg of body weight in phase I and II melanoma trials (29), and an elimination half-life of 1 week was shown in other studies (4). The costimulatory potential of tucaresol, as well as its ability to bias immunity toward a cell-mediated T-helper-cell response, suggests that it could prove to be of therapeutic benefit against chronic infectious diseases such as VL, which is characterized by its ability to induce T-cell anergy (26). In this study we describe the effect of tucaresol in murine models of L. donovani infection.
(This work was presented in part at the 39th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, Calif., 26 to 29 September 1999 [S. L. Croft, A. C. Smith, V. Yardley, and J. Rhodes, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1854, p. 730, 1999].)
MATERIALS AND METHODS
Parasites.
L. donovani (strain MHOM/ET/67/L82) was routinely maintained in golden hamsters (Mesocricetus auratus; Wright's strain; Charles River Ltd., Margate, United Kingdom) by passage every 6 to 8 weeks.
Compounds.
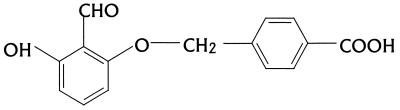
Tucaresol (4-[2-formyl-3-hydroxy-phenoxymethyl]benzoic acid) (Fig. 1) and sodium stibogluconate (Pentostam) were kindly provided by Glaxo Wellcome, Stevenage, United Kingdom.
FIG. 1.
Structure of tucaresol (4-[2-formyl-3-hydroxy-phenoxymethyl]benzoic acid).
In vitro studies.
Peritoneal exudate macrophages (PEMs) were isolated from female CD1 mice (Charles River Ltd.) and were maintained in 16-well Lab-tek (Nunc, Naperville, Ill.) tissue culture slides in RPMI 1640 medium (Gibco, Paisley, United Kingdom) with 10% heat-inactivated fetal calf serum at 37°C in a 5% CO2–air mixture. Bone marrow macrophages (BMMs) were derived from 8- to 10-week-old female BALB/c mice (Charles River Ltd.) as described previously (28). Briefly, bone marrow cells were eluted from the femur and were cultured for 8 days in Dulbecco modified Eagle medium with GlutaMAX I (Gibco) supplemented with 20% heat-inactivated fetal calf serum, 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 10% L-cell-conditioned medium as a source of colony-stimulating factor 1. Macrophages were rested for 2 days in the absence of colony-stimulating factor 1 before use in experiments. L. donovani amastigotes were isolated from an infected hamster spleen and were used to infect the macrophage cultures at a ratio of 10 amastigotes to 1 macrophage. Infected cultures were maintained in medium containing test compounds in either a three- or fivefold dilution series, with quadruplicate cultures at each concentration, for 5 days (35). Medium was replaced once with fresh medium containing drug on day 3. After the 5-day exposure, the slides were methanol fixed and Giemsa stained and the proportion of infected macrophages was determined in each chamber. The 50 and 90% effective doses (ED50s and ED90s, respectively) were determined by sigmoidal regression analysis with Xlfit software for Microsoft Excel.
In vivo studies.
Female BALB/c mice (Charles River Ltd.), C57BL/6 mice (Charles River Ltd.), and C.B-17 scid mice (from a breeding colony at the London School of Hygiene and Tropical Medicine) were infected intravenously, via the lateral tail vein, with 2 × 107 L. donovani amastigotes freshly isolated from the spleen of an infected hamster, followed by random sorting into groups of five. In initial and drug combination experiments, mice were dosed by the oral route once per day from days 7 to 11 of infection. In the time course studies separate groups of five mice were dosed for 5 consecutive days on either days 1 to 5, 7 to 11, 14 to 18, 21 to 25, 28 to 32, 35 to 40, 42 to 46, 49 to 53, or 56 to 60 of infection. Sodium stibogluconate, used either as a standard drug or in combination with tucaresol, was administered subcutaneously once a day for 5 consecutive days. In all regimens described, the mice were killed 3 days after the completion of treatment, livers and spleens were removed and weighed, and impression smears were prepared from a cut surface. Smears were methanol fixed and Giemsa stained (BDH, Poole, United Kingdom). Drug activity was determined by comparing the number of amastigotes per 500 liver cells or spleen cells times the organ weight (in milligrams) in mice from the treated and untreated groups. Data represent the mean ± standard error of the mean (SEM), with differences between values analyzed by a two-tailed Student's t test. ED50s were determined by sigmoidal regression analysis with Xlfit software for Microsoft Excel.
All studies were conducted by procedures approved under the United Kingdom Home Office Animals (Scientific Procedures) Act of 1986.
RESULTS
In vitro.
Tucaresol had no activity against L. donovani amastigotes in BMMs at 30 to 1 μM or in PEMs in the range of 100 to 1 μM but was toxic to both PEMs and parasites at 300 μM. In the same studies the standard drug sodium stibogluconate had an ED50 of 4.8 to 5.3 μg of SbV per ml and an ED90 of 11.3 to 13.4 μg of SbV per ml for parasites in PEMs, in line with previously reported results with this model (35).
In vivo.
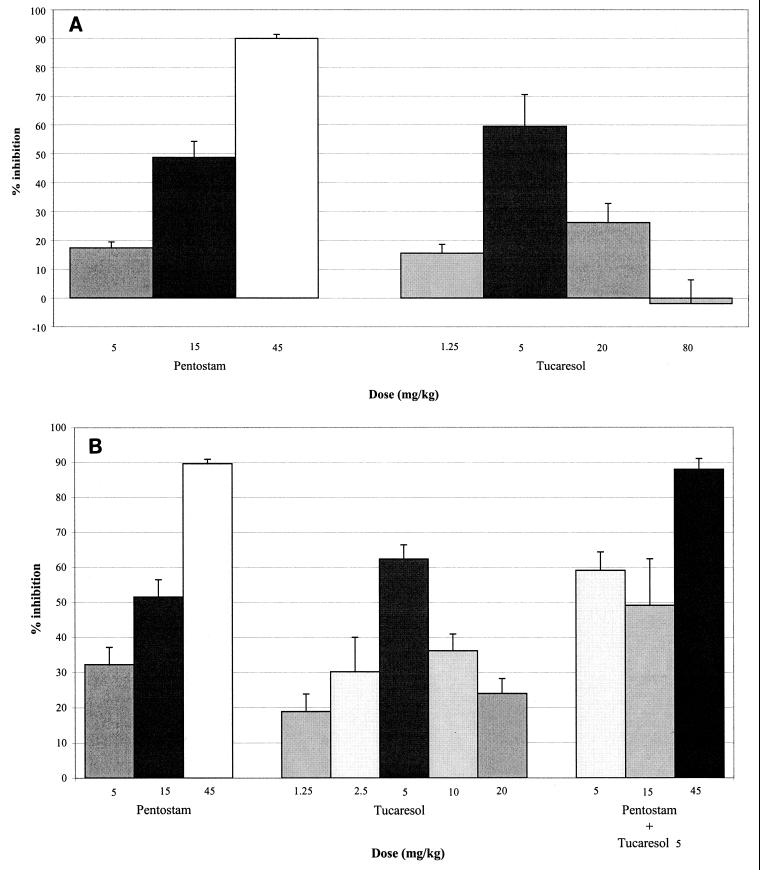
In initial studies with L. donovani-infected BALB/c mice, animals were dosed during days 7 to 11 of infection, as performed in standard chemotherapy studies. In two separate experiments, 20 mg of tucaresol per kg, the optimal dose reported by Rhodes et al. (36), resulted in 26.2 and 24.1% inhibition of liver amastigotes. In three further experiments that explored higher and lower doses of tucaresol, an optimum dose of 5 mg/kg was observed, with 43.8% (P < 0.05), 59.5% (P < 0.01) (Fig. 2A), and 62.4% (P < 0.01) (Fig. 2B) suppression of liver amastigotes and limited or no activity at the extremes of the dose range, 1.25 and 80 mg/kg. Although the levels of liver infection in control (untreated) mice varied by 50% between experiments (P < 0.05), from 8.9 × 105 ± 7.1 × 104 amastigotes to 1.7 × 106 ± 1.5 × 105 amastigotes, the levels of inhibition caused by 5 mg/kg were similar, at 59.5 and 62.4%, respectively. Evaluation of the activity of tucaresol against spleen amastigotes was not possible during days 7 to 11 of infection due to low parasite numbers.
FIG. 2.
(A) Dose-response activity of tucaresol and sodium stibogluconate (Pentostam) against L. donovani in BALB/c mice. Mice were dosed during days 7 to 11 of infection, and the inhibition of liver amastigotes was determined at day 14 postinfection. The 5-mg/kg dose of tucaresol results in a highly significant (P < 0.01) inhibition of liver amastigotes relative to the numbers in untreated controls (Student's t test). Data represent means ± SEMs derived from five mice per group. (B) Dose-response activity of tucaresol and sodium stibogluconate (Pentostam) alone and in combination against L. donovani in BALB/c mice. Mice were dosed during days 7 to 11 of infection, and the inhibition of liver amastigotes was determined at day 14 postinfection. The 5-mg/kg dose of tucaresol (Tucaresol 5) results in a highly significant (P < 0.01) inhibition of liver amastigotes relative to the numbers in untreated controls (Student's t test). Data represent means ± SEMs derived from five mice per group.
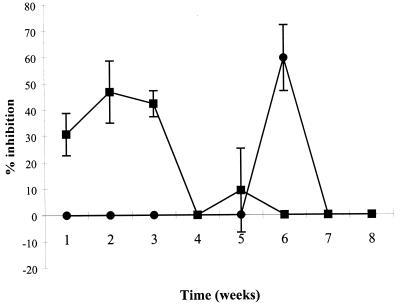
The effect of tucaresol on liver and spleen amastigote loads during the course of infection in BALB/c mice was determined at the previously determined optimum dose of 5 mg/kg. Groups of mice were separately treated during either week 1, 2, 3, 4, 5, 6, 7, or 8 of infection and were killed 3 days after the completion of dosing. The pattern of infection of L. donovani in BALB/c mice, with liver infections reaching a maximal level by weeks 4 to 8 postinfection and spleen parasite numbers increasing after weeks 4 to 8 of infection, has been reported elsewhere (21). In this study and in an earlier study with 20 mg/kg, tucaresol was inactive during the first week of infection. At 5 mg/kg, activity against the liver infection reached a maximum (46.8 and 42.3%, respectively) during weeks 2 to 3, whereas activity against the splenic infection reached a maximum (59.5%) during week 6 (Fig. 3).
FIG. 3.
Activity of 5 mg of tucaresol per kg against L. donovani infections in livers (■) and spleens (●) of BALB/c mice. Groups of mice were separately treated during either week 1, 2, 3, 4, 5, 6, 7, or 8 of infection and were killed 3 days after the completion of dosing. Data represent means ± SEMs derived from five mice per time point.
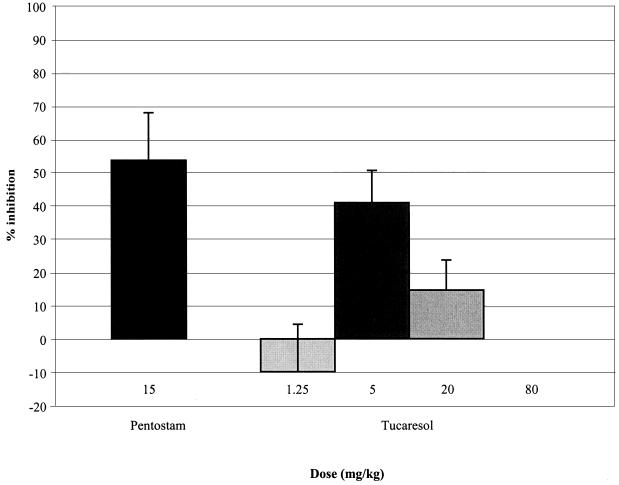
The activity of tucaresol administered during days 7 to 11 of infection was also ascertained in two other strains of mice. In separate experiments, within the range of 80 to 1.25 mg/kg, the maximum efficacy of tucaresol against liver infections in C57BL/6 mice was 40.8% (Fig. 4) and 48.7% at an optimal dose of 5 mg/kg. The compound showed no activity against liver or spleen infections in C.B-17 scid mice in the range of 80 to 1.25 mg/kg.
FIG. 4.
Dose-response activity of tucaresol and sodium stibogluconate (Pentostam) against L. donovani in C57BL/6 mice. Mice were dosed during days 7 to 11 of infection, and the inhibition of liver amastigotes was determined at day 14 postinfection. Data represent means ± SEMs derived from five mice per group.
In all studies, sodium stibogluconate was included as the standard control and showed a normal dose-response effect (Fig. 2A and B) with ED50s against L. donovani in the range of 10.9 to 16.8 mg of SBV per kg in BALB/c mice and 15.9 mg of SBV per kg in C57BL/6 mice, and limited activity in C.B-17 scid mice. Combinations of tucaresol at 5 mg of Sbv per kg with sodium stibogluconate at 45, 15, and 5 mg/kg during days 7 to 11 of infection in BALB/c mice showed no synergistic or additive effects (Fig. 2B).
DISCUSSION
VL in humans is characterized by downregulation of the Th2-associated immune response, which involves the action of IL-4 and IL-10, suppression of the secretion of IL-12, and possible inhibition of IFN-γ production and macrophage responsiveness to IFN-γ (15, 32, 37). The most detailed studies of the immune response have been determined with murine models of experimental VL, in which, in addition to disease-promoting Th2- and disease-suppressing Th1-associated immune responses, L. donovani has also been shown to bring about the downregulation of macrophage costimulatory molecules and major histocompatibility complex class II expression, which leads to poor T-cell and macrophage costimulation and parasite persistence (27, 32). Tucaresol could overcome this anergic state by acting as a donor for the naturally occurring T-cell ligands associated with the costimulatory activation of T cells (17, 36) to produce the IFN-γ necessary for macrophage activation and Leishmania killing. In this study we have demonstrated that tucaresol is active against experimental L. donovani infections at the low dose of 5 mg/kg in both the BALB/c (noncure haplotype) and the C57BL/6 (cure haplotype) murine models by oral administration. There was little difference in the activity profile for L. donovani in BALB/c mice (59.5 to 62.4%) (Fig. 2A and B) and C57BL/6 mice (40.8 to 48.7%) (Fig. 4), despite the different host genetic backgrounds and immunological responses to infection in these two murine models (25, 27). Interestingly, IFN-γ also produces a similar (50 to 60%) reduction in liver amastigote levels in BALB/c mice when administered alone (31), but unlike IFN-γ, tucaresol does not act in synergy or have an additive response with sodium stibogluconate. The T-cell-dependent action of sodium stibogluconate is well documented (27, 33, 37), with the synergy with IFN-γ possibly due to an alteration in the macrophage activation threshold (27).
The absence of activity in scid mice, which lack T and B cells (13), as well as the lack of activity against amastigotes in either elicited PEMs or naive BMMs in vitro, would seem to confirm that tucaresol is functioning as an immunomodulator. Further evidence is provided by the characteristic bell-shaped dose-response curve, a possible consequence of high tucaresol doses interfering with antigen-presenting cell–T-cell conjugation, also reported by Rhodes et al. (36). The difference in the activity of tucaresol against liver and spleen infections in BALB/c mice follows the pattern of T-cell responses in these two organs, as reported elsewhere (21). In particular, the absence of any antileishmanial activity during the early weeks of infection in the spleen is due to the slow development of infection in this organ, which reaches a maximal level only after 4 to 8 weeks postinfection. In contrast, liver parasite numbers increase quickly, reaching a maximum in the first 3 to 4 weeks (21). The optimal activity of tucaresol against liver and spleen infections in BALB/c mice (Fig. 3) reflects not only the development of the parasite burdens in these organs but also the development of parasite persistence and T-cell anergy (26). Potentially, this could have an effect upon the timing of tucaresol dosing postinfection, but it also suggests that tucaresol is acting more like a cytokine, such as IL-12 (27, 34), in which the timing of administration determines success or failure.
In conclusion, we have demonstrated the novel but limited activity of the immunomodulator tucaresol against experimental VL. As no interactions with the standard antileishmanial agent sodium stibogluconate were shown, further studies will need to explore combinations with other drugs, as well as establish the basis of the interaction with the immune system that leads to the killing of Leishmania parasites.
ACKNOWLEDGMENTS
This investigation received financial support from the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR).
REFERENCES
- 1.Adinolfi L E, Bonventre P F. Enhancement of Glucantime therapy of murine Leishmania donovani infection by a synthetic immunopotentiating compound (CP-46,665-1) Am J Trop Med Hyg. 1985;34:270–277. doi: 10.4269/ajtmh.1985.34.270. [DOI] [PubMed] [Google Scholar]
- 2.Adinolfi L E, Bonventre P F, Vander Pas M, Eppstein D A. Synergistic effect of Glucantime and a liposome-encapsulated muramyl dipeptide analog in therapy of experimental visceral leishmaniasis. Infect Immun. 1985;48:409–416. doi: 10.1128/iai.48.2.409-416.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvar J, Cañavate C, Gutiérrez-Solar B, Jiménez M, Laguna F, López-Vélez R, Molina R, Moreno J. Leishmania and human immunodeficiency virus coinfection: the first 10 years. Clin Microbiol Rev. 1997;10:298–319. doi: 10.1128/cmr.10.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arya R, Rolan P E, Wootton R, Posner J, Bellingham A J. Tucaresol increases oxygen affinity and reduces haemolysis in subjects with sickle cell anaemia. Br J Haematol. 1996;93:817–821. doi: 10.1046/j.1365-2141.1996.d01-1744.x. [DOI] [PubMed] [Google Scholar]
- 5.Ashford R W, Desjeux P, DeRaadt P. Estimation of population at risk of infection and number of cases of leishmaniasis. Parasitol Today. 1992;8:104–105. doi: 10.1016/0169-4758(92)90249-2. [DOI] [PubMed] [Google Scholar]
- 6.Avila J L, Biondo F, Monzon H, Convit J. Cutaneous leishmaniasis in mice: resistance to glucan immunotherapy, either alone or combined with chemotherapy. Am J Trop Med Hyg. 1982;31:53–59. doi: 10.4269/ajtmh.1982.31.53. [DOI] [PubMed] [Google Scholar]
- 7.Badaro R, Nascimento C, Carvalho J S, Badaro F, Russo D, Ho J L, Reed S G, Johnson W D, Jr, Jones T C. Recombinant human granulocyte-macrophage colony-stimulating factor reverses neutropenia and reduces secondary infections in visceral leishmaniasis. J Infect Dis. 1994;170:413–418. doi: 10.1093/infdis/170.2.413. [DOI] [PubMed] [Google Scholar]
- 8.Barrett M P, Coombs G H, Mottram J C. Recent advances in identifying and validating drug targets in trypanosomes and leishmanias. Trends Microbiol. 1999;7:82–88. doi: 10.1016/s0966-842x(98)01433-4. [DOI] [PubMed] [Google Scholar]
- 9.Berman J D. Human leishmaniasis: clinical, diagnostic, and chemotherapeutic developments in the last 10 years. Clin Infect Dis. 1997;24:684–703. doi: 10.1093/clind/24.4.684. [DOI] [PubMed] [Google Scholar]
- 10.Berman J D, Badaro R, Thakur C P, Wasunna K M, Behbehani K, Davidson R, Kuzoe F, Pang L, Weerasuriya K, Bryceson A D M. Efficacy and safety of liposomal amphotericin B (AmBisome) for visceral leishmaniasis in endemic developing countries. Bull W H O. 1998;76:25–32. [PMC free article] [PubMed] [Google Scholar]
- 11.Bhakuni V, Sigha U K, Dutta G P, Levy H B, Maheshwari R K. Killing of Leishmania donovani amastigotes by poly ICLC in hamsters. J Interferon Cytokine Res. 1996;16:321–325. doi: 10.1089/jir.1996.16.321. [DOI] [PubMed] [Google Scholar]
- 12.Bogdan C, Rollinghoff M. The immune response to Leishmania: mechanisms of parasite control and evasion. Int J Parasitol. 1998;28:121–134. doi: 10.1016/s0020-7519(97)00169-0. [DOI] [PubMed] [Google Scholar]
- 13.Bosma G C, Custer R P, Bosma M J. A severe combined immunodeficiency mutation in the mouse. Nature. 1983;301:527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- 14.Buates S, Matlashewski G. Treatment of experimental leishmaniasis with the immunomodulators imiquimod and S-28463: efficacy and mode of action. J Infect Dis. 1999;179:1485–1494. doi: 10.1086/314782. [DOI] [PubMed] [Google Scholar]
- 15.Cenini P, Berhe N, Hailu A, McGinnes K, Frommel D. Mononuclear cell subpopulations and cytokine levels in human visceral leishmaniasis before and after chemotherapy. J Infect Dis. 1993;168:986–994. doi: 10.1093/infdis/168.4.986. [DOI] [PubMed] [Google Scholar]
- 16.Chen H, Hall S, Heffernan B, Thompson N T, Rogers M V, Rhodes J. Convergence of Schiff base costimulatory signaling and TCR signaling at the level of mitogen-activated protein kinase ERK2. J Immunol. 1997;159:2274–2281. [PubMed] [Google Scholar]
- 17.Chen H, Hall S, Zheng B, Rhodes J. Potentiation of the immune system by Schiff base-forming drugs. Biodrugs. 1997;7:217–231. [Google Scholar]
- 18.Cillari E, Arcoleo F, Dieli M, D'Agostino R, Gromo G, Leoni F, Milano S. The macrophage-activating tetrapeptide tuftsin induces nitric oxide synthesis and stimulates murine macrophages to kill Leishmania parasites in vitro. Infect Immun. 1994;62:2649–2652. doi: 10.1128/iai.62.6.2649-2652.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen H. Induction of delayed-type sensitivity to Leishmania parasite in a case of leishmaniasis cutanea diffusa with BCG and cord-factor (trehalose-6-6′ dimycolate) Acta Dermatovener. 1979;59:547–549. [PubMed] [Google Scholar]
- 20.Coleman R E, Edman J D, Semprevivo L H. Effect of cimetidine and 2′-deoxyguanosine on the development of Leishmania mexicana in BALB/c mice. Trans R Soc Trop Med Hyg. 1988;82:232–233. doi: 10.1016/0035-9203(88)90425-7. [DOI] [PubMed] [Google Scholar]
- 21.Engwerda C R, Murphy M L, Cotterell S E, Smelt S C, Kaye P M. Neutralization of IL-12 demonstrates the existence of discrete organ-specific phases in the control of Leishmania donovani. Eur J Immunol. 1998;28:669–680. doi: 10.1002/(SICI)1521-4141(199802)28:02<669::AID-IMMU669>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 22.Gradoni L, Bryceson A, Desjeux P. Treatment of Mediterranean visceral leishmaniasis. Bull W H O. 1995;73:191–197. [PMC free article] [PubMed] [Google Scholar]
- 23.Grimaldi G F, Moriearty P L, Hoff R. Leishmania mexicana in C3H mice: BCG and levamisole treatment of established infections. Clin Exp Immunol. 1980;41:237–242. [PMC free article] [PubMed] [Google Scholar]
- 24.Guru P Y, Agrawal A K, Singha U K, Singhal A, Gupta C M. Drug targeting in Leishmania donovani infections using tuftsin-bearing liposomes as drug vehicles. FEBS Lett. 1989;245:204–208. doi: 10.1016/0014-5793(89)80222-4. [DOI] [PubMed] [Google Scholar]
- 25.Honore S, Garin Y J-F, Sulahian A, Gangneux J-P, Derouin F. Influence of the host and parasite strain in a mouse model of visceral Leishmania infantum infection. FEMS Immunol Med Microbiol. 1998;21:231–239. doi: 10.1111/j.1574-695X.1998.tb01170.x. [DOI] [PubMed] [Google Scholar]
- 26.Kaye P M. Costimulation and the regulation of antimicrobial immunity. Immunol Today. 1995;16:423–427. doi: 10.1016/0167-5699(95)80018-2. [DOI] [PubMed] [Google Scholar]
- 27.Kaye P M, Gorak M, Murphy M, Ross S. Strategies for immune intervention in visceral leishmaniasis. Ann Trop Med Parasitol. 1995;89:75–81. doi: 10.1080/00034983.1995.11813016. [DOI] [PubMed] [Google Scholar]
- 28.Kiderlen A F, Kaye P M. A modified colorimetric assay of macrophage activation for intracellular cytotoxicity against Leishmania parasites. J Immunol Methods. 1990;127:11–18. doi: 10.1016/0022-1759(90)90334-r. [DOI] [PubMed] [Google Scholar]
- 29.Kirkwood J M, Schuchter S, Donnelly S, Stover L, Drobins P, Whiteside T L, Burnham J P, Heitman C K, Johnston J M. A novel immunopotentiating agent, tucaresol: results from a multicenter, pilot study in patients with metastatic melanoma. Proc Am Assoc Cancer Res. 1997;38:402. [Google Scholar]
- 30.Murray H W. Endogenous interleukin-12 regulates acquired resistance in experimental visceral leishmaniasis. J Infect Dis. 1997;175:1477–1479. doi: 10.1086/516482. [DOI] [PubMed] [Google Scholar]
- 31.Murray H W, Berman J D, Wright D. Immunochemotherapy for Leishmania donovani infection: γ interferon plus pentavalent antimony. J Infect Dis. 1988;157:973–978. doi: 10.1093/infdis/157.5.973. [DOI] [PubMed] [Google Scholar]
- 32.Murray H W, Hariprashad J, Coffman R L. Behaviour of visceral Leishmania donovani in an experimentally induced T helper cell 2 (Th2)-associated response model. J Exp Med. 1997;185:867–874. doi: 10.1084/jem.185.5.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murray H W, Oca M J, Granger A M, Schreiber R D. Requirement for T cells and effect of lymphokines in successful chemotherapy for an intracellular infection. Experimental visceral leishmaniasis. J Clin Investig. 1989;83:1253–1257. doi: 10.1172/JCI114009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nabors G S, Afonso L C, Farrell J P, Scott P. Switch from a type 2 to a type 1 T helper cell response and cure of established Leishmania major infection in mice is induced by combined therapy with interleukin 12 and Pentostam. Proc Natl Acad Sci USA. 1995;92:3142–3146. doi: 10.1073/pnas.92.8.3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neal R A, Croft S L. An in-vitro system for determining the activity of compounds against the intracellular amastigote form of Leishmania donovani. J Antimicrob Chemother. 1984;14:463–475. doi: 10.1093/jac/14.5.463. [DOI] [PubMed] [Google Scholar]
- 36.Rhodes J, Chen H, Hall S R, Beesley J E, Jenkins D C, Collins P, Zheng B. Therapeutic potentiation of the immune system by co-stimulatory Schiff-base-forming drugs. Nature. 1995;377:71–75. doi: 10.1038/377071a0. [DOI] [PubMed] [Google Scholar]
- 37.Sundar S, Reed S G, Sharma S, Mehrotra A, Murray H W. Circulating T helper 1 (TH1) cell and TH2 cell-associated cytokines in Indian patients with visceral leishmaniasis. Am J Trop Med Hyg. 1997;56:522–525. doi: 10.4269/ajtmh.1997.56.522. [DOI] [PubMed] [Google Scholar]
- 38.Sundar S, Rosenkaimer F, Murray H W. Successful treatment of refractory visceral leishmaniasis in India using antimony plus interferon. J Infect Dis. 1994;170:659–662. doi: 10.1093/infdis/170.3.659. [DOI] [PubMed] [Google Scholar]
- 39.Wang C C. Validating targets for antiparasite chemotherapy. Parasitology. 1997;114:S31–S44. [PubMed] [Google Scholar]