Abstract
Aims:
To conduct a systematic review in order to comprehensively synthesize the findings from a diverse range of genetically informative studies on comorbid depression and type 2 diabetes.
Methods:
Database searches (1 January 2008 to 1 June 2020) in PubMed and EMBASE were conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines. Eligible reports employed any type of genetically informed design, including twin modelling, Mendelian randomization, genome-wide association studies, polygenetic risk scores, or linkage disequilibrium score regression. Searches generated 451 unique citations, and 16 manuscripts met the inclusion criteria.
Results:
The included studies addressed three aetiological models of the depression–diabetes relationship: uni- or bi-directional phenotypic causation; shared genetic liability; or gene–environment interaction. From these studies, there is modest evidence that type 2 diabetes is causally related to risk of developing depression, but much more limited evidence that depression is causally related to risk of diabetes. There is little evidence of shared genetic liability between depression and diabetes or of gene–environment interaction.
Conclusions:
Findings from genetically informed studies are mixed but provide some support for the uni- or bi-directional phenotypic model of depression and type 2 diabetes. Future studies should also explore the hypothesis that this relationship may be influenced by shared environmental risk factors. Findings can inform multifaceted approaches to diabetes prevention and care that reflect how psychosocial factors contribute to type 2 diabetes risk and outcomes.
1 |. INTRODUCTION
Depression and type 2 diabetes are two of the most common and costly health conditions worldwide.1,2 It is well documented that depression and diabetes co-occur, and that appropriate clinical management of this comorbidity is a core component of comprehensive diabetes management and depression treatment.3,4 Indeed, the Collaborative Care Model, which was initially applied to address the issue of comorbid depression–diabetes, today represents one of the most robust, evidence-based treatment models for medical–psychiatric comorbidity implemented in primary healthcare settings.5
In the past two decades, over two dozen longitudinal studies of depression and type 2 diabetes have been conducted. Most of these reports show that, while clinically identified type 2 diabetes is associated with the development or worsening of depressive symptoms, depression earlier in adulthood is also predictive of diabetes later in life6–8; that is, observational epidemiological studies suggest that this relationship is bi-directional: diabetes is associated with onset and worsening of depressive symptoms, and depressive symptoms are associated with onset of type 2 diabetes and poor glycaemic control among people with established disease.
Recent evidence, however, suggests more nuance is needed regarding this apparent bi-directional relationship. For example, if depression is indeed a cause of type 2 diabetes, it is expected that effective treatment for and/or recovery from depression would mitigate this risk. Although clinical trials of depression treatment do suggest that improving depressive symptoms is correlated with improvements in glycaemic control among people with type 2 diabetes,9 there is no compelling evidence from observational reports that antidepressant medication use reduces the risk of developing diabetes in normoglycaemic individuals.10,11 Also, if the mechanism underlying the depression–diabetes relationship is biological (e.g. systemic inflammation, alternations in the hypothalamic–pituitary–adrenal axis, it is expected that depression would be correlated with hyperglycaemia regardless of whether or not the diabetes had been clinically diagnosed. However, there is little evidence that depression is associated with measures of insulin resistance (e.g. homeostatic model assessment of insulin resistance), glucose tolerance, or elevated fasting glucose or HbA1c among people who do not have clinically identified diabetes.12–14 That is, depression is associated with the clinical diagnosis of type 2 diabetes, not the underlying hyperglycaemia that is indicated by that diagnosis. Together, these findings suggest that the conceptualization of the relationship between depression and type 2 diabetes as bi-directional (i.e. depression is a cause of diabetes and diabetes is a cause of depression) needs refinement.
Genetically informed study designs can provide a window into the salience of exposures that are hypothesized to play a causal role but are not amenable to randomized controlled trials (i.e. exposures such as depression or diabetes).15 This is particularly important for complex phenotypes such as depression and type 2 diabetes, both of which are polygenic in nature but are also correlated with a wide range of confounding variables (e.g. age, sex, socio-economic status). Moreover, genotype is determined prior to birth, which means that genetically informed designs can help disentangle life course relationships between depression and type 2 diabetes without interference from reverse-causality.
Genetically informative designs include ‘natural experiments’, such as comparing monozygotic and dizygotic twins to decompose the covariance of depression and diabetes into genetic vs environmental sources; these types of designs are useful to evaluate whether shared genetic liability is the underlying cause of comorbid depression–diabetes. As another example, Mendelian randomization studies use genotype as an instrumental variable (i.e. a variable that causes exposure but does not cause the outcome, except through the exposure); these types of designs are used to test uni- and bi-directional phenotypic causal hypotheses (e.g. diabetes as a risk factor for developing depression, or vice versa). Genome-wide association studies (GWAS), polygenetic risk scores, and cross-trait linkage disequilibrium score regression can be used to quantify genetic liability for polygenic phenotypes such as depression and/or type 2 diabetes; these quantities can also be used to test hypotheses about phenotypic causation. Finally, given that risk of both depression and type 2 diabetes are multifactorial in nature, studies on gene–environment interaction can help identify both high-risk subgroups and potential biological mechanisms underlying environmental risks. In this way, genetically informative study designs provide more robust evidence regarding potential aetiological relationships, including clarifying potential bi-directional relationships such as those that are hypothesized to underlie the comorbidity between depression and type 2 diabetes.
The goal of the present systematic review was to address the question ‘What is the nature of the association between depression and type 2 diabetes?’, by comprehensively synthesizing the findings from a diverse range of genetically informative studies published in the past decade. Understanding the nature of the depression–diabetes relationship has importance both for research efforts to understand the physiological correlates of depression and the psychosocial correlates of type 2 diabetes, and for efforts to promote mental health and prevent type 2 diabetes over the life course.
2 |. METHODS
2.1 |. Study inclusion and exclusion criteria
To be included, a study had to: 1) include a measure of both depression and type 2 diabetes status; 2) be published in a peer-reviewed journal; 3) be written in English; 4) include data from human participants; 5) present empirical comparative findings (no reviews, case studies or abstracts, and no studies limited to persons with depression or diabetes were eligible); and 6) use a genetically informed design (e.g. twin or family study, Mendelian randomization, GWAS, polygenetic risk scores, linkage disequilibrium score regression, gene–environment interaction or related design). Studies were excluded if they did not examine the relationship between depression and type 2 diabetes in a genetically informative manner (i.e. studies in which the genetic analysis did not in some way examine the relationship between the disorders or its potential mechanisms).
2.2 |. Search strategy
This literature review was conducted in accordance with the 2009 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.16 An experienced Health Sciences research librarian helped generate Medical Subject Headings (MeSH) terms based on two sentinel articles,17,18 which were used in the development of the main search strategy in PubMed. This strategy was then translated into EMBASE. The following search terms were used across databases: ‘depression’, ‘depressive disorder’, ‘diabetes mellitus’, ‘diabetes’, ‘genes’ and ‘genetic’. Limits imposed were ‘publication date from 1 January 2008 to 1 June 2020’, ‘English language’ and ‘human subjects’. In order to capture the full range of articles published within the 10-year span following the initial review, all searches were completed by 30 June 2020 and imported into the reference manager, Zotero (Zotero release 5.0.74; Center for History and New Media, Fairfax, VA, 2016). This review was not registered with PROSPERO because we could not conduct a quantitative meta-analysis due to the wide variety and non-comparable nature of the methodological approaches used in the included studies.
2.3 |. Data extraction
After entering the search terms in PubMed and EMBASE, one reviewer extracted articles and identified abstracts for review. Two reviewers (A.R., D.W.) independently applied the inclusion and exclusion criteria to the identified abstracts. Of the articles not excluded based on the abstract, two reviewers (A.R., D.W.) again independently applied the inclusion and exclusion criteria to the full article text. Concordance was assessed, and any discordant decisions were settled by consensus among all authors.
Next, the entire study team collaborated to design a manuscript abstraction tool, piloting the tool on four manuscripts to reach consensus regarding data abstraction procedures. The following was included in the abstraction tool: authorship; study design, sample size, source and composition; measurement of depression; measurement of diabetes; genetic assessment and analytical approach; and selected estimate and interpretation. Most studies reported more than one effect estimate. To provide a succinct summary of the overall conclusion from each report, in the tables we provide the effect estimate identified by the study authors as the most representative of their results. In instances of multiple representative findings, we selected the estimate with the lowest P value. Two authors (K.M.K., A.R.) then conducted a systematic review of the final manuscripts identified and completed the abstraction. In cases where manuscripts lacked sufficient information regarding the sample composition, researchers examined prior publications of those cohorts to abstract that information.
2.4 |. Ethics
This study was not submitted to an institutional review board as literature reviews do not involve research in human subjects.
3 |. RESULTS
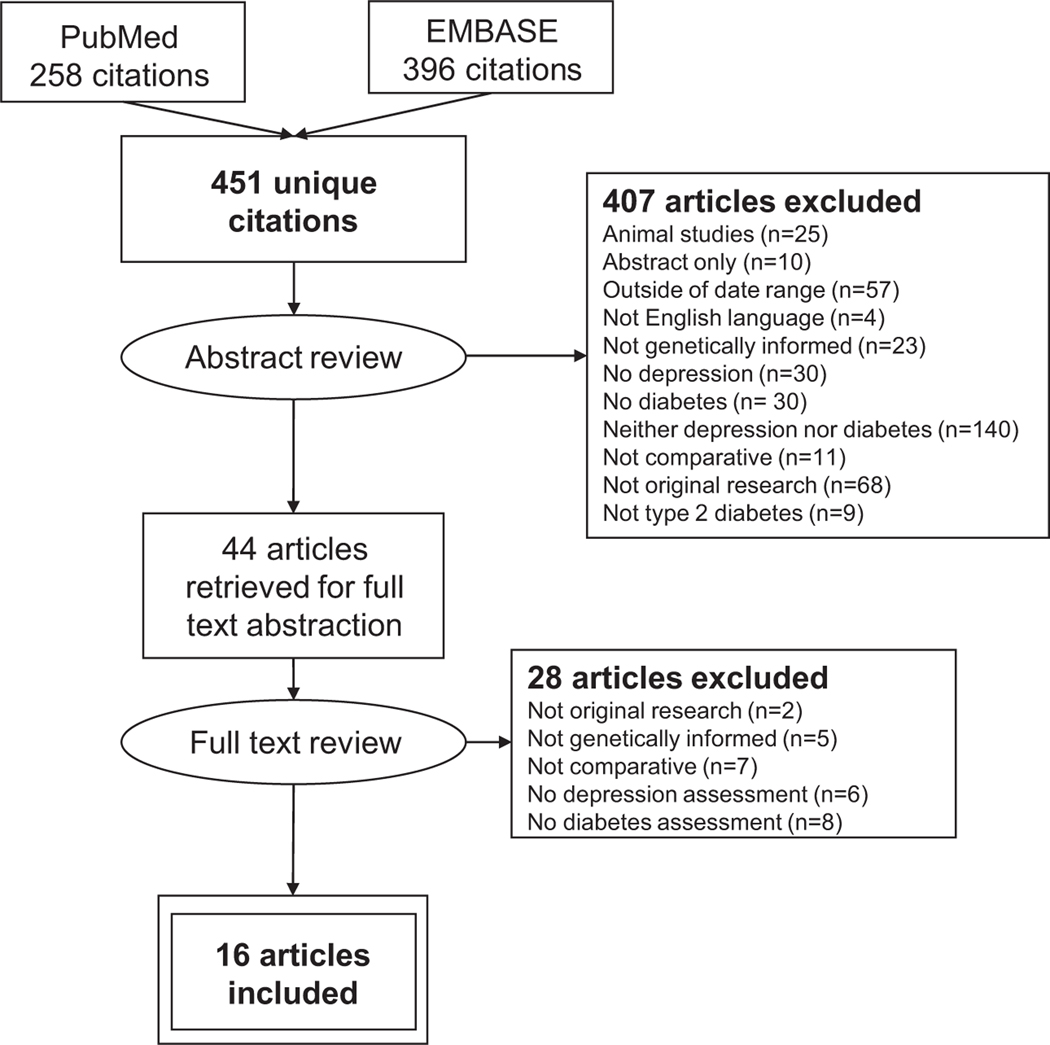
As shown in Figure 1, 451 unique citations were generated from the searches. Of these articles, 407 were excluded after independent review of manuscript abstracts. During abstract review, articles were primarily excluded because: they examined either depression or type 2 diabetes, but not both conditions; original research was not presented; or they fell outside the specified date range. Studies that did not use a comparative approach (i.e. were limited to people with type 2 diabetes or to people with depression) were excluded. After full-text review an additional 28 articles were excluded. During full-text review, articles were primarily excluded because they did not examine the relationship between diabetes and depression in a genetically informed manner.
FIGURE 1.
PRISMA flowchart of the study identification, selection and abstraction process.
The 16 included articles addressed three major hypotheses about the aetiological relationship between depression and type 2 diabetes: 1) direct phenotypic causation, i.e. the comorbidity between depression and diabetes occurs because depression causes type 2 diabetes and/or type 2 diabetes causes depression. These studies are summarized in Table 1; 2) shared genetic liability, i.e. the comorbidity between depression and type 2 diabetes is attributable to underlying genetic liability that is common to these two conditions. These studies are summarized in Table 2; 3) gene–environment interaction, i.e. the comorbidity between depression and type 2 diabetes is attributable to depression interacting with diabetes-specific genetic liability or diabetes interacting with depression-specific genetic liability. These studies are summarized in Table 3.
TABLE 1.
Direct phenotype causation: studies testing uni- or bi-directional phenotypic causation hypotheses of comorbid depression and type 2 diabetes
| First author (year) | Study design, sample source, size, and characteristicsa | Depression assessment | Diabetes assessment | Genetic assessment and analytical approach | Findings and interpretationb |
|---|---|---|---|---|---|
| Tang (2020)32 | Two-sample, bi-directional Mendelian randomization Summary statistics from multiple existing GWAS consortia: MD: UK Biobank + PGC N = 1 306 354 Type 2 diabetes: DIAGRAM N = 898 130 |
Lifetime MD UK Biobank GWAS defined MD as ever seeking treatment for ‘nerves, anxiety, tension or depression.’ PCG consists of multiple cohorts. All cohorts defined MD using DSM-IV criteria. |
DIAGRAM consists of multiple cohorts, type 2 diabetes defined by self-report of type 2 diabetes, diabetes medication use, or diagnosis in medical chart. | Two-sample, two-directional Mendelian randomization using summary data: 1. Genetic liability of MD predicting odds of type 2 diabetes 2. Genetic liability of type 2 diabetes predicting odds of MD |
Findings • Genetic liability for MD was associated with increased odds of type 2 diabetes (odds ratio: 1.26, 95% CI 1.10–1.43, P = 6×10−4). • Genetic liability for type 2 diabetes was not associated with odds of MD (odds ratio: 1.00, 95% CI 0.97–1.03, P = 0.948). Interpretation Findings are consistent with the hypothesis that MD causes type 2 diabetes, but not that type 2 diabetes causes MD. |
| Wehby (2018)26 | Longitudinal population-based cohort Data source: Health and Retirement Study N = 7338 100% non-Hispanic white 57% female Age 65+ years |
Current MD Instrument: CESD Type: Screening |
Genetic liability for type 2 diabetes using existing GWAS estimates. | Whole-genome association testing of type 2 diabetes PRS with MD |
Findings • No association between PRS of type 2 diabetes and depression (odds ratio: 0.97, 95% CI 0.82–1.16) or depressive symptoms (beta: −0.02, 95% CI −0.15 to 0.12). Interpretation Findings do not support the hypothesis that type 2 diabetes is causally related to risk of developing MD. |
| Xuan (2018)28 | Cross-sectional, population-based sample Data source: Risk Evaluation of Cancers in Chinese Diabetic Individuals (REACTION) Study N = 11 506 Mean age: 57 years 78% female 100% Asian |
Current MD Instrument: PHQ Type: Screening |
Fasting plasma glucose | Approach 1: PRS of 34 type 2 diabetes-associated SNPs. Approach 2: Used Mendelian randomization to assess the association between this PRS and MD. |
Findings • Approach 1: PRS type 2 diabetes was positively associated with depression (odds ratio: 1.21, 95% CI 1.07–1.37). • Approach 2: type 2 diabetes positively associated with odds of depression (odds ratio: 1.84, 95% CI 1.25–2.70). Interpretation Findings support the hypothesis that type 2 diabetes is causally related to risk of developing MD. |
| Clarke (2O17)20 | Cross-sectional, population-based sample Data source: Generation Scotland, N = 19,858 Age 18+ 59% female 99% European Summary statistics from multiple existing GWAS consortia: DIAGRAM, AGEN-T2D, SAT2D, MAT2D, T2D-GENES |
Lifetime MD Instrument: SCID: Type: Diagnostic |
Self-report type 2 diabetes, diabetes medication use, or diagnosis in medical chart. | Approach 1: Used multiple PRS for type 2 diabetes to assess the bi-directional relationship between type 2 diabetes and MD. Approach 2: Used Mendelian randomization to assess the association between 11- SNP type 2 diabetes PRS and MD. |
Findings • Approach 1: type 2 diabetes genetic liability was nominally associated with elevated risk of MD (type 2 diabetes PRS was associated with MD status at 3 out of 5 P value thresholds (beta: 0.007, P < 0.015; all betas nonsignificant after correcting for multiple testing). • Approach 2: Lower type 2 diabetes genetic liability was nominally associated with lower risk of MD (type 2 diabetes-protective SNP rs6808574 was also protective for MD: beta: −0.008, P = 0.02; non-significant after correcting for multiple testing). Interpretation Findings do not support the hypothesis that type 2 diabetes is causally related to risk of developing MD. |
| Wesolowska (2017) 27 | Cross-sectional, population-based sample Data source: Cardiovascular Risk in Young Finns N = 1217 Mean age: 43 years 58.9% female 100% Finnish |
Current MD Instrument: BDI Type: Screening |
Fasting plasma glucose | PRS of 35 type 2 diabetes-associated SNPs. Used Mendelian randomization to assess the association between this PRS and MD. |
Findings • Genetic liability for fasting glucose was inversely associated with depressive symptoms. PRS was positively associated with glucose (beta: 0.09, 95% CI 0.07, 0.12; P < 0.001) and negatively associated with depressive symptoms (beta: −0.04 95% CI −0.07, −0.005; P = 0.025). Interpretation Findings do not support the hypothesis that type 2 diabetes is causally related to risk of developing MD. |
| Mezuk (2015)17 | Cross-sectional population-based study of MZ and DZ twins Data source: Screening Across the Lifespan Twin (SALT) N = 31 043 100% Swedish Mean age: 59 years |
Lifetime MD Instrument: CIDI Type: Diagnostic |
Self-report of physician diagnosis of type 2 diabetes | Twin modelling with comparison of direction of causation models to Cholesky decomposition (shared genetic/environmental cause) |
Findings • MD was associated with risk of type 2 diabetes after accounting for genetic liability (hazard ratio for women: 1.74, 95% CI 1.09–2.79; hazard ratio for men: 1.08, 95% CI 0.70–1.67). • Type 2 diabetes was associated with risk of MD after accounting for genetic liability (hazard ratio for women: 1.49, 95% CI 1.04–2.12; hazard ratio for men: 1.21, 95% CI 0.83–1.78). Interpretation Findings are consistent with a bi-directional model of MD– type 2 diabetes. |
| Samaan (2015) | Cross-sectional, population-based sample Data source: EpiDREAM N = 17404 Mean age: 53 years 60.9% female 53.9% European, 18.9% Latin American, 15.8% South Asian, 7.2% African, 2.9 Native North American, 1.3% East Asian |
Past year MD Instrument: Structured interview Type: Diagnostic |
OGTT | PRS of 20 type 2 diabetes-associated SNPs. Assessed the relationship between this PRS with MD. |
Findings • OGTT-identified impaired fasting glucose, impaired glucose tolerance, and type 2 diabetes were not significantly associated with past year MD (significance for all relationships: 0.30 ⩽ P ⩽ 0.65). • Genetic risk for type 2 diabetes: 12 of the 20 type 2 diabetes SNPs were significantly associated with type 2 diabetes. • Genetic risk for type 2 diabetes and MD: The 20 SNPs and resulting PRS were not associated with MD (P ⩾ 0.09). Interpretation Findings do not support the hypothesis that type 2 diabetes is causally related to risk of developing MD. |
Abbreviations: AGEN-T2D, Asian Genetic Epidemiology Network Type 2 Diabetes; BDI, Beck Depression Inventory; CESD, Center for Epidemiologic Studies of Depression Scale; CIDI, Composite International Diagnostic Inventory; DIAGRAM, DIAbetes Genetics Replication And Meta-analysis Consortium; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders-IV; DZ, dizygotic (fraternal) twins; EpiDREAM, Diabetes REduction Assessment with ramipril and rosiglitazone Medication trial; GWAS, genome-wide association study; MAT2D, Mexican American Type 2 Diabetes Consortium; MD, major depression; MZ, monozygotic (identical) twins; OGTT, oral glucose tolerance test; PGC, Psychiatric Genetics Consortium; PHQ, Patient Health Questionnaire; PRS, polygenetic risk score; SALT, Screening Across the Lifespan Twin Study; SAT2D, South Asian Type 2 Diabetes Consortium; SCID, Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders; SNP, single nucleotide polymorphism; T2D-GENES, Type 2 Diabetes Genetic Exploration by Next-Generation Sequencing in Multi-Ethnic Samples Consortium.
Detailed sample characteristics for GWAS consortia are not provided as these samples are generally used to generate aggregate summary statistics rather than for individual-level analysis. These samples are all publicly available on their respective websites. Unless specified otherwise, these GWAS consortia samples consist primarily of individuals of European descent.
The quantitative measure of association provided in this table is the effect estimate each study’s authors indicated as the most representative of their conclusions; in instances where a study reported multiple representative estimates, this table reports the estimate with the lowest P value provided in the study.
TABLE 2.
Shared genetic liability: studies testing the common genetic liability hypothesis of comorbid depression and type 2 diabetes
| First author (year) | Study design, sample source, size, and characteristicsa | Depression assessment | Diabetes assessment | Genetic assessment and analytic approach | Findings and interpretationb |
|---|---|---|---|---|---|
| Wong (2019)29 | Cross-sectional, population-based sample Summary statistics from multiple existing GWAS consortia: MD PGC, N = 18 759 CONVERGE, N = 10 640 Type 2 diabetes MAGIC, N = 58 074 DIAGRAM, N = 110 452 |
Lifetime MD PGC consists of multiple cohorts. All cohorts defined MD using DSM-IV criteria. CONVERGE defined people with recurrent melancholic MD, most of whom had been hospitalized for MD. |
MAGIC: fasting insulin, fasting glucose, HOMA-p and HOMA-IR. DIAGRAM consists of multiple cohorts. Type 2 diabetes defined by self-report of type 2 diabetes, diabetes medication use, or diagnosis in medical chart. |
Approach 1: Cross-trait LDSR of MD and type 2 diabetes Approach 2: Used PRS for type 2 diabetes and MD to assess genetic overlap. Approach 3: Gene ontology and pathway analysis to identify shared genetic variants |
Findings • Approach 1: In PGC, cross-trait LDSR indicated no significant genetic correlation between MD and type 2 diabetes (rG: 0.032, P =0.757). In CONVERGE, cross-trait LDSR indicated significant inverse genetic correlation between MD and type 2 diabetes (rG: −0.232, P <0.009). • Approach 2: In PGC, PRS for MD were positively associated with type 2 diabetes (odds ratio: 1.01, P <0.0016). In CONVERGE, PRS for MD was nominally negatively associated with type 2 diabetes (odds ratio: 0.99, P <0.0052). • Approach 3: In PGC, MD-type 2 diabetes was associated with enrichment of immune-related pathways. In CONVERGE, inverse relation of MD-type 2 diabetes was associated with enrichment of oxytocin and brain-derived neurotropic factor. Interpretation Findings partially support the hypothesis that type 2 diabetes and MD have a shared genetic liability; findings suggest MD– type 2 diabetes relationship differs in Chinese population. |
| Haljas (2018)21 | Cross-sectional, population-based genetic consortium samples Summary statistics from multiple existing GWAS consortia: CHARGE: N = 51 258 DIAGRAM: N = 149 821 MAGIC: N = 46 186 |
Current MD Instruments: CESD, GDS, PHQ, MQ, and BDI Type: Screening |
DIAGRAM: Selfreport, registry data, physician’s diagnosis, and OGTT. MAGIC: fasting insulin, fasting glucose, HOMA-p and HOMA-IR. |
Approach 1: Cross-trait LDSR of MD with type 2 diabetes and glycaemic traits Approach 2: Bivariate GWAS to identify individual pleiotropic SNPs. |
Findings • Approach 1: No genetic correlation between SNPs associated with MD and those associated with type 2 diabetes (rG: −0.03 (SE 0.13), P =0.82). • Approach 2: Identified 17 SNPs with pleiotropic effects on both MD and type 2 diabetes, 11 of which were associated with both MD and type 2 diabetes in the same manner; remaining six were +MD and −TD or vice versa. Interpretation Findings partially support the hypothesis that type 2 diabetes and MD have a shared genetic liability. |
| Clarke (2017)20 | Cross-sectional, population-based sample Summary statistics from multiple existing GWAS consortia: Type 2 diabetes: DIAGRAM, N = 69 033 MD: PGC, N = 18 759 |
Lifetime MD PGC consists of multiple cohorts. All cohorts defined MD using DSM-IV criteria. |
DIAGRAM consists of multiple cohorts. Type 2 diabetes defined by self-report of type 2 diabetes, diabetes medication use, or diagnosis in medical chart. | Cross-trait LDSR of MD and type 2 diabetes |
Findings • Cross-trait LDSR indicated no significant genetic correlation between depression and type 2 diabetes (rG: 0.0278, P =0.79). Interpretation Findings do not support the hypothesis that type 2 diabetes and MD have a shared genetic liability. |
| Ji (2016)25 | Cross-sectional, population-based genetic consortium samples Summary statistics from multiple existing GWAS consortia: Type 2 diabetes: DIAGRAM, N = 149 821 MD: PGC, N = 18 759 |
Lifetime MD PGC consists of multiple cohorts. All cohorts defined MD using DSM-IV criteria. |
DIAGRAM consists of multiple cohorts. Type 2 diabetes defined by self-report of type 2 diabetes, diabetes medication use, or diagnosis in medical chart. | Functional annotations of genes associated with both MD and type 2 diabetes from existing GWAS. |
Findings 1. Identified 496 SNPs (marginally significant, P value = 1.0×10−7) associated with both MD and type 2 diabetes, significantly more than the number of SNPs that would be expected by chance. 2. 216 of these SNPs were in annotated genes, and functional analysis of these genes indicated they were enriched in pathways relate to immune responses, cell signalling, and lipid metabolism. Interpretation Findings support the hypothesis of a shared genetic liability for type 2 diabetes and MD. |
| Kan (2016)23 | Cross-sectional population-based samples of MZ and DZ twins Data sources: SALT and STAGE, N = 68 606 Mean age: 58 years Danish Twin Registry, N=95,403 Mean age: 60 years Race/ethnicity not specified |
Lifetime MD Instrument: Medical records (in/outpatient) Type: Diagnostic |
Medical record (in/outpatient) of type 2 diabetes | Bivariate twin modelling of type 2 diabetes and MD, including sex-specific models. |
Findings • Type 2 diabetes and MD have correlated genetic liability among women but not men (genetic correlation between type 2 diabetes and MD: rGMen: 0.06, 95% CI −0.13 to 0.25;rGwomen: 0.23, 95% CI 0.07–0.38). • In men: phenotypic correlation between type 2 diabetes and depression was 31% due to genetic factors and 69% due to unique environmental factors • In women: phenotypic correlation between type 2 diabetes and MD was 75% due to genetic factors and 25% due to unique environmental factors Interpretation Findings partially support the hypothesis that comorbidity of type 2 diabetes and MD have a shared genetic liability (sex-specific). |
| Bulik-Sullivan (2015) | Cross-sectional, population-based genetic consortium samples Summary statistics from multiple existing GWAS consortia: PGC: N=18,759 DIAGRAM: N=69,033 MAGIC: N=46,186 |
Lifetime MD PGC consists of multiple cohorts. All cohorts defined MD using DSM-IV criteria. |
DIAGRAM: Selfreport, registry data, physician’s diagnosis, and OGTT. MAGIC: Fasting insulin. |
Cross-trait LDSR of MD with type 2 diabetes and glycaemic traits |
Findings • No genetic correlation of MD with type 2 diabetes (rG: −0.04781 (SE 0.114), P =0.6751) • No genetic correlation of MD with HbA1c (rG: −0.2218 (SE 0.1602), P =0.1661). Interpretation Findings do not support the hypothesis that type 2 diabetes and MD have a shared genetic liability. |
| Mezuk (2015)17 | Cross-sectional population-based sample of MZ and DZ twins Data source: Screening Across the Lifespan Twin (SALT) Study N = 37 043 100% Swedish Mean age: 59 years |
Lifetime MD Instrument: CIDI Type: Diagnostic |
Self-report of physician type 2 diabetes diagnosis. | Bivariate twin modeling with MZ and DZ twins, including bivariate Cholesky decomposition and direction of causation models. |
Findings • No evidence of a shared genetic liability for MD and type 2 diabetes. Cross-twin, cross-trait correlations (rMZmen: −0.13, rDZmen: 0.06, rMZwomen: −0.04, rDZwomen: −0.06 were all non-significant). • Significant correlation between unique environmental influences associated with type 2 diabetes and those associated with MD (covariance in unique environmental factors between MD and type 2 diabetes (covE: 0.15; 95% CI 0.01 – 0.30). Interpretation Findings do not support the hypothesis that MD and type 2 diabetes have a shared genetic liability. Findings suggest that MD and type 2 diabetes share some environmental liability. |
| Scherrer (2011) | Cross-sectional population-based sample of MZ and DZ twins Data source: Vietnam Era Twin Study of Aging (VETSA) N = 1237 100% male 95.4% white, 4.0% black Age range: 52–60 years |
Current MD Instrument: SF-36 Type: Screening |
Self-report of type 2 diabetes and anti-diabetic medication and insulin use. | Bivariate twin modeling with MZ and DZ twins. |
Findings • No evidence of a shared genetic liability common to MD and type 2 diabetes (genetic correlation between MD and type 2 diabetes was r : 0.19, 95% CI 0–0.46). • No evidence that environmental influences, either shared or unique to each condition, influence comorbidity of MD-type 2 diabetes (unique environmental correlation between MD and type 2 diabetes was r : 0.09, 95% CI 0–0.45). Interpretation Findings do not support the hypothesis that type 2 diabetes and MD have a shared genetic liability. |
| Zeman (2010)24 | Cross-sectional study N=140 60.7% Female 100% Caucasian Mean age: 61.4 years |
Current MD diagnosis Instrument: Unspecified Type: Diagnostic using DSM-IV criteria |
Metabolic syndrome as defined by the International Diabetes Federation | Tested candidate SNPs for association with MD and metabolic syndrome. |
Findings • SNP within the BDNF gene was associated with depression (P <0.003) but not metabolic syndrome. No other SNPs were associated either depression or metabolic syndrome. Interpretation Findings do not support the hypothesis that type 2 diabetes and MD have a shared genetic liability. |
Abbreviations: BDI, Beck Depression Inventory; CESD, Center for Epidemiologic Studies of Depression Scale; CHARGE, Cohorts for Heart and Aging Research in Genomic Epidemiology; CIDI, Composite International Diagnostic Inventory; CONVERGE, China, Oxford, and Virginia Commonwealth University Experimental Research on Genetic Epidemiology; covE: Covariance of unique environment risk for MD and type 2 diabetes; DIAGRAM, DIAbetes Genetics Replication And Meta-analysis; DZ, dizygotic (fraternal) twins; GDS, Geriatric Depression Scale; GWAS, genome-wide association study; HOMA-IR, homeostatic model assessment of insulin resistance; LDSR, linkage disequilibrium score regression; MAGIC: Meta-Analyses of Glucose and Insulin-related traits Consortium; MD, major depression; MQ: Maastricht Depression Questionnaire; MZ, Monozygotic (identical) twins; OGTT, oral glucose tolerance test; PGC, Psychiatric Genomics Consortium; PHQ, Patient Health Questionnaire; PRS, polygenetic risk score; rG, genetic correlation; SCID, Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders; SALT, Screening Across the Lifespan Twin Study; SF-36, short-form 36 Mental Health Questionnaire; SNP, single nucleotide polymorphism; STAGE, Swedish Twin Studies of Adults: Genes and Environment; VETSA, Vietnam Era Twin Study of Aging.
Detailed sample characteristics for GWAS consortia are not provided as these samples are generally used as aggregate summary data rather than for individual-level analysis. These samples are all publicly available on their respective websites. Unless specified otherwise, these GWAS consortia samples consist primarily of individuals of European descent.
The quantitative measure of association provided in this table is the effect estimate each study’s authors indicated as the most representative of their conclusions; in instances where a study reported multiple representative estimates, this table reports the estimate with the lowest P value provided in the study.
TABLE 3.
Gene–environment interaction: studies testing gene–environment interaction hypotheses of comorbid depression and type 2 diabetes
| First author (year) | Study design, sample source, size, and characteristicsa | Depression assessment | Diabetes assessment | Genetic assessment and analytic approach | Findings and interpretationb |
|---|---|---|---|---|---|
| Haljas (2018)22 | Candidate gene interaction study Data source: Prevalence, Prediction and Prevention of Diabetes-Botnia (PPP-Botnia) Study N = 4455 Mean age: 50 years 53% female 100% Finnish |
Current MD Instrument: MHI-5 Type: Screening |
OGTT, HOMA-IR, insulin sensitivity index, insulin response, disposition index | Tested gene-environment interaction between SNP rs10830963 (an SNP in a melatonin receptor gene) and MD to predict glycaemic traits. |
Findings • Main effect of SNP rs10830963 on glycaemic traits (association with fasting glucose: beta: 0.174, 95% CI 0.142–0.206; P <0.001) • MDxSNP interaction was not statistically significant (MD*rs10830963 on fasting plasma glucose: beta: −0.004, 95% CI −0.040 to 0.033; P =0.572). Interpretation Findings do not support the hypothesis that candidate genes related to melatonin interact with MD to influence risk of type 2 diabetes or glycaemic traits. |
| Garasia (2017)30 | Cross-sectional and longitudinal analyses Data source: EpiDREAM N = 17 375 Mean age: 53 years 60.9% female 53.9% European, 18.9% Latin American, 15.8% South Asian, 7.2% African, 2.9 Native North American, 1.3% East Asian |
Past year MD Instrument: Structured interview Type: Diagnostic |
OGTT, impaired fasting glucose, Impaired glucose tolerance | Tested for interaction between a 20-SNP type 2 diabetes PRS and MD at predicting type 2 diabetes and related glycaemic traits. |
Findings • MD was not associated with prevalent type 2 diabetes (odds ratio: 1.00, 95% CI 0.88–1.13, P = 0.96) or incident type 2 diabetes (odds ratio: 1.22, 95% CI 1.01–1.47, P = 0.04) after Bonferroni correction. • Interaction between MD and type 2 diabetes-PRS in predicting prevalent type 2 diabetes was not significant (MD*PRS odds ratio: 1.05, 95% CI 1.01–1.10; P =0.026). • Interaction between MD and type 2 diabetes-PRS in predicting incident type 2 diabetes was not significant (MD*PRS Odds ratio: 1.01, 95% CI 0.94–1.07; P =0.86) Interpretation No evidence of gene-environment interaction between MD and genetic risk for type 2 diabetes in predicting diabetes or related outcomes. |
Abbreviations: EpiDREAM, Diabetes REduction Assessment with ramipril and rosiglitazone Medication trial; HOMA-IR, homeostatic model assessment of insulin resistance; MD, major depression; MHI, five-item Mental Health Inventory; OGTT, oral glucose tolerance test; PRS, polygenetic risk score; SNP, single nucleotide polymorphism.
Detailed sample characteristics for GWAS consortia are not provided as these samples are generally used as aggregate summary data rather than for individual-level analysis. These samples are all publicly available on their respective websites.
The quantitative measure of association provided in this table is the effect estimate each study’s authors indicated as the most representative of their conclusions; in instances where a study reported multiple representative estimates, this table reports the estimate with the lowest P value provided in the study.
3.1 |. Sample characteristics
As shown in the tables, most studies were conducted in analytical samples of predominantly white people of European ancestry,17–27 although two studies examined individuals of Asian ancestry28,29 and two (13%) used study populations of diverse racial/ethnic ancestry.30,31 Seven articles (44%) came from large (n >10 000) population-based studies.17,20,23,28–31 Smaller (n <10 000) population-based cohorts were used in four articles (26%).18,22,26,27 One study (7%) used data from a clinical sample (n=140).24 Several articles used data from large genetic consortia [i.e. 23 and Me, UK Biobank, the Psychiatric Genetics Consortium (PGC), the Meta-Analyses of Glucose and Insulin-related traits Consortium (MAGIC), Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE), DIAbetes Genetics Replication And Meta-analysis (DIAGRAM)].19–21,25,29,32
3.2 |. Assessment of depression and type 2 diabetes
Studies varied in measurement and timing (i.e. current vs lifetime) of depression. In five (31%) studies, depression status was determined using clinical diagnostic interviews [e.g. Composite International Diagnostic Interview (CIDI), Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders (SCID)]17,19,20,25,29; another three (20%) used an unspecified structured clinical interview.24,30,31 Six studies (40%) assessed depression using screening tools [e.g. the General Health Questionnaire (GHQ), Beck Depression Inventory (BDI)].18,21,22,26–28 Only one (7%) used depression diagnosis from inpatient/outpatient psychiatric services.23
In contrast to the varied approaches to the timing of depression, most studies assessed lifetime history of type 2 diabetes. In three studies (20%), diabetes status was only determined by self-report of diabetes-specific medication use or clinical diagnosis.17,18,20 Five studies (33%) assessed current diabetes status using the oral glucose tolerance test or fasting glucose levels,22,27,28,30,31 and one (7%) used criteria for metabolic syndrome.24 Four (25%) used a combination of methods including self-report, oral glucose tolerance test and medical records.19,21,25,29
3.3 |. Genetically informed analytical approach
The included studies used a variety of genetically informed approaches to assess the relationship between type 2 diabetes and depression. These included: twin modelling (n=3, 20%)17,18,23; Mendelian randomization (n=4, 27%)20,28,32,33; GWAS and polygenetic risk scores (n=6, 38%)20,26,28,29,31,33; and cross-trait linkage disequilibrium score regression (n=4, 25%).19–21,29 Several used a combination of these approaches [e.g. Clarke et al. (2017),20 Haljas et al. (2018)21 and Wong et al. (2019)29]. Two studies examined candidate genes selected on the basis of biological plausibility.22,24
3.4 |. Qualitative synthesis of findings
3.4.1 |. Direct phenotypic causation
Of the seven studies that examined the uni- or bi-directional phenotypic causation hypothesis, two reported at least one significant relationship,28,32 one reported suggestive evidence,20 three found no evidence to support the relationship,26,27,31 and one was inconclusive.17 The four studies that used a Mendelian randomization design to address this hypothesis20,28,32,33 reported mixed results. For example, Xuan et al. (2018)28 reported an association consistent with a causal effect of diabetes on odds of current depression. Clarke et al. (2017)20 did not find a significant effect of diabetes on depression. Contrary to expectations, Wesołowska et al. (2017)27 reported an inverse relationship between hyperglycaemia and risk of depression.27 Finally, Tang et al. (2020)32 examined the bi-directional relationship between depression and diabetes, finding that genetic liability for diabetes was unrelated to risk of depression, but that genetic liability for depression was related to elevated risk of type 2 diabetes.
3.4.2 |. Shared genetic liability
Of the nine studies that assessed genetic correlation between depression and type 2 diabetes, five reported no support for this hypothesis17–20,24 and four reported suggestive evidence of shared genetic liability.21,23,25,29 For example, Ji et al. (2016),25 when using a relaxed P value cut-off (1.0×10E-7), identified 496 single nucleotide polymorphisms (SNPs) associated with both depression and type 2 diabetes. Of these, 276 were located on annotated genes that could be included in an enrichment analysis to identify biological pathways associated with disease phenotypes, suggesting potential pathways that may be influenced by a shared genetic risk between depression and diabetes. Haljas et al. (2018)21 used two different analytical approaches across multiple genetic consortiums (DIAGRAM, MAGIC and CHARGE) and reported mixed findings. They found no evidence of a genetic correlation between diabetes and depression using linkage disequilibrium score regression; however, they identified several bivariate-significant SNPs with concordant direction of effect for both phenotypes (i.e. 11 SNPs associated with both depression and type 2 diabetes, and two SNPs associated with both depression and fasting glucose) using bivariate GWAS. Notably, Wong et al. (2019)29 reported a borderline significant negative genetic correlation between depression and type 2 diabetes within the COVERGE cohort.29
3.4.3 |. Gene–environment interaction
Finally, two studies22,30 examined hypotheses related to gene–environment interaction (i.e. interaction between diabetes polygenetic risk scores or candidate genes related to diabetes with depression); neither found support for their hypothesis.
4 |. DISCUSSION
Risk of depression and type 2 diabetes, like almost all health conditions, is a function of both genetic and environmental factors. This fact can be leveraged to test hypotheses about the nature of the co-occurrence of these conditions. The present systematic review sought to clarify the relationship between depression and type 2 diabetes by comprehensively synthesizing the findings from a diverse range of genetically informative studies. This review highlights the ways that genetic data have been applied to test hypotheses about the nature of relationships that are not amenable to experimentation. However, it is important to note that some hypotheses are more challenging to test than others, and that it is possible that the nature of the depression–diabetes relationship may change over the life course. As such, for some hypotheses about comorbid depression–type 2 diabetes there is genetically informed evidence of absence, but for others there is an absence of (genetically informed) evidence. This framework guides our summary, with emphasis on the findings from large cohorts or genetic consortia and those studies that used multiple analytical approaches, as these provide the most robust evidence regarding the nature of the depression–diabetes relationship.
4.1 |. Refinement of the conceptualization of comorbid depression–type 2 diabetes
While traditional observational studies generally support the hypothesis that the relationship between depression and type 2 diabetes is attributable to bi-directional phenotypic causation (i.e. depression increases risk of developing type 2 diabetes and vice versa), the evidence from genetically informed studies is less consistent. Although Mendelian randomization has not yet been widely applied to this question, the handful of existing studies reviewed in the present paper provide mixed evidence of a causal effect of type 2 diabetes on risk of depression. In addition, there is little evidence from genetically informed studies to indicate a causal effect of depression on risk of developing type 2 diabetes. However, this latter finding is not surprising given the relative dearth of replicated genetic variants for depression, the low proportion of phenotypic variance explained by current variants for depression,34 and the high potential for pleiotropy in said variants,35 which would violate requirements for Mendelian randomization. In terms of genetically informed studies of bi-directional phenotypic causation, this is a situation of absence of evidence, rather than evidence of absence.
Based on the literature summarized in the present review, there is little evidence that the comorbidity of depression and diabetes is driven from shared genetic liability. Specifically, genetic variants known to predict type 2 diabetes do not also appear to predict risk of depression. This interpretation has an important caveat, which is that depression is a heterogeneous phenotype defined by symptomology, not pathology. In addition, investigators use different measurement approaches (i.e. symptom scales vs diagnostic interviews, current vs lifetime) to operationalize depression status, which further complicates efforts to understand this comorbidity. As such, it may be that there are depressions, each with distinct pathologies, some of which are more related to metabolic disorders such as diabetes than others.36 Genetically informed designs have the potential to help refine the depression phenotype, including identification of clinically meaningful subtypes which may have a common liability with type 2 diabetes.37,38
Finally, twin studies of this comorbidity confirm that both depression and type 2 diabetes are influenced by genetic and environmental factors and provide evidence to suggest that individual-specific environmental factors may contribute to risk of both diseases. However, existing studies have yet to provide compelling evidence of specific gene–environment interaction interactions that contribute to this relationship.
In summary, existing genetically informative studies generally reject the hypothesis that comorbidity of depression and type 2 diabetes is driven by shared genetic liability, and leave open, but largely untested, hypotheses that this comorbidity is attributable to bi-directional causation. The role of shared environmental risk factors has also been generally unexplored. Figure 2 provides a heuristic illustration summarizing this evidence.
FIGURE 2.
Summary of evidence regarding comorbid depression–diabetes from this systematic review of genetically informative studies.
4.2 |. Insights about risk factors and mechanisms
The studies reviewed in the present paper suggest several shared physiological mechanisms have the potential to contribute both depression and type 2 diabetes. For example, the report by Ji et al. (2016) suggests that immune-inflammatory pathways, implicated in the aetiology of both type 2 diabetes39 and depression,40 may contribute to this comorbidity. One potential factor correlated with both depression and type 2 diabetes that is implicated in inflammatory pathways is diet quality. Nutrients obtained from food have pro- and anti-inflammatory properties, which have been associated with both type 2 diabetes and depression.41 Systemic inflammation is sensitive to gut microbiome–host interactions, and there is growing interest in exploring the potential of targeting the gut microbiome in both type 2 diabetes42 and depression.43
Stress exposure is another potential shared risk factor for both type 2 diabetes and depression. Stressful life events are an established risk factor for depression,44 and there is growing evidence that stress exposure is also related to risk of diabetes.45 In addition, stress prompts the use of self-regulatory coping behaviours, including behaviours known to increase diabetes risk such as a preference for high-fat/high-sugar foods, physical inactivity, tobacco use, and alcohol use.46 Obesity has reciprocal associations with diabetes and depression, in part due to the production of pro-inflammatory cytokines by adipose tissue.47 Finally, increasing physical activity and weight loss can prevent or delay onset and improve treatment outcomes for both depression and type 2 diabetes.48,49 In this way, stress exposure may directly increase risk of depression, and indirectly increase risk of diabetes by prompting use of behaviours that lead to insulin resistance, in addition to potentially directly increasing diabetes risk through physiological pathways, such as allostatic load.45 Finally, some antidepressant medications are associated with weight gain, which may provide another pathway linking depression and diabetes risk.11
4.3 |. Strengths and limitations
While we queried two large scientific publication data-bases, it is possible that eligible articles were excluded, particularly if they were published in a language other than English. Although we worked with a reference librarian to develop our search strategy, papers not indexed by our selected MeSH terms may have been missed. Publication bias may result in an underreporting of null findings. The genetic cohorts examined were overwhelmingly represented by individuals of white European ancestry. Finally, while a strength of this review is our inclusion of a wide range of genetically informed designs, the diversity of these approaches precluded us from meta-analysing the findings in a quantitative manner.
It is worth noting that several of the studies included in this review had methodological limitations. Genetically informed designs generally require larger samples than traditional observational approaches; however, several of the papers reviewed had relatively small samples18,24,27 or only examined select candidate genetic variants,22 both of which would result in underpowered studies. Additionally, some studies failed to demonstrate that their application of genetically informed methods satisfied the assumptions of those analyses. For example, Tang et al. (2020)32 and Clarke et al. (2017)20 both used Mendelian randomization to examine the effect of depression on diabetes using depression-related SNPs as genetic ‘instrumental variables’; however, neither analysis demonstrated that their genetic ‘instruments’ were only associated with the outcome (i.e. diabetes) through the exposure (i.e. depression), one of the core requirements of this approach. As a result, claims of identifying a causal (as opposed to correlational) effect should be tempered.
4.4 |. Clinical implications
The comorbidity of type 2 diabetes and depression necessitates a comprehensive approach to clinical care which recognizes and addresses both psychosocial and medical needs. This is consistent with clinical guidelines, which outline integration of psychosocial and behavioural factors in managing diabetes. This review suggests that future research should seek to identify specific shared environmental risk factors, with an emphasis on how those factors relate to depression and type 2 diabetes over the life course. This research can inform multifaceted approaches to diabetes prevention and care that reflect how psychosocial and behavioural factors contribute to type 2 diabetes risk.
What’s new?
Depression is often comorbid with type 2 diabetes.
There is little support for the hypothesis that comorbidity of depression and diabetes is driven by shared genetic liability.
There is modest evidence to support the bi-directional phenotypic causation explanatory model of this phenomenon.
Future research should explore the role of shared environmental risk factors as a contributor to comorbid depression–diabetes.
Comorbidity of depression and type 2 diabetes necessitates a comprehensive approach to clinical care which recognizes and addresses psychosocial factors.
Acknowledgments
Funding information
R.S.B. (NIMH T32-MH73553) and K.M.K. (T32-HG00040) are supported by training grants from the National Institutes of Health (NIH). Additional support for this work was provided by the NIH (NIMH K01-MH093642) and the American Diabetes Association (1–16-ICTS-082), both to B.M. These funders had no role in the study design, data collection, data analysis, manuscript preparation or decision to publish this paper.
Footnotes
COMPETING INTERESTS
None declared.
REFERENCES
- 1.American Diabetes Association. Economic Costs of Diabetes in the U.S. in 2012. Diabetes Care. 2013;36:1033–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donohue JM, Pincus HA. Reducing the societal burden of depression: a review of economic costs, quality of care and effects of treatment. Pharmacoeconomics. 2007;25:7–24. [DOI] [PubMed] [Google Scholar]
- 3.Sartorius N. Comorbidity of mental and physical disorders: a key problem for medicine in the 21st century. Acta Psychiatr Scand. 2018;137:369–370. [DOI] [PubMed] [Google Scholar]
- 4.Holt RIG, de Groot M, Lucki I, et al. NIDDK International Conference Report on Diabetes and Depression: Current Understanding and Future Directions. Diabetes Care. 2014;37:2067–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katon W, Unützer J, Wells K, et al. Collaborative depression care: history, evolution and ways to enhance dissemination and sustainability. Gen Hosp Psychiatry. 2010;32:456–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mezuk B, Eaton WW, Albrecht S, et al. Depression and type 2 diabetes over the lifespan: a meta-analysis. Diabetes Care. 2008;31:2383–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kan C, Silva N, Golden SH, et al. A systematic review and meta-analysis of the association between depression and insulin resistance. Diabetes Care. 2013;36:480–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nouwen A, Winkley K, Twisk J, et al. Type 2 diabetes mellitus as a risk factor for the onset of depression: a systematic review and meta-analysis. Diabetologia. 2010;53:2480–2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brieler JA, Lustman PJ, Scherrer JF, et al. Antidepressant medication use and glycaemic control in co-morbid type 2 diabetes and depression. Fam Pract. 2016;33:30–36. [DOI] [PubMed] [Google Scholar]
- 10.Ratliff S, Mezuk B. Depressive symptoms, psychiatric medication use, and risk of type 2 diabetes: results from the Health and Retirement Study. Gen Hosp Psychiatry. 2015;37:420–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salvi V, Grua I, Cerveri G, et al. The risk of new-onset diabetes in antidepressant users - A systematic review and meta-analysis. PLoS One. 2017;12:e0182088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nouwen A, Nefs G, Caramlau I, et al. Prevalence of depression in individuals with impaired glucose metabolism or undiagnosed diabetes: a systematic review and meta-analysis of the European Depression in Diabetes (EDID) Research Consortium. Diabetes Care. 2011;34:752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen S, Zhang Q, Dai G, et al. Association of depression with pre-diabetes, undiagnosed diabetes, and previously diagnosed diabetes: a meta-analysis. Endocrine. 2016;53:35–46. [DOI] [PubMed] [Google Scholar]
- 14.Mezuk B, Johnson-Lawrence V, Lee H, et al. Is ignorance bliss? Depression, antidepressants, and the diagnosis of prediabetes and type 2 diabetes. Health Psychol. 2013;32:254–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCaffery JM, Snieder H, Dong Y, et al. Genetics in psychosomatic medicine: research designs and statistical approaches. Psychosom Med. 2007;69:206–216. [DOI] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, et al. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mezuk B, Heh V, Prom-Wormley E, et al. Association between major depression and type 2 diabetes in midlife: findings from the Screening Across the Lifespan Twin Study. Psychosom Med. 2015;77:559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scherrer JF, Xian H, Lustman PJ, et al. A Test for Common Genetic and Environmental Vulnerability to Depression and Diabetes. Twin Res Hum Genet. 2011;14:169–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bulik-Sullivan B, Finucane HK, Anttila V, et al. An Atlas of Genetic Correlations across Human Diseases and Traits. Nat Genet. 2015;47:1236–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clarke T-K, Obsteter J, Hall LS, et al. Investigating shared aetiology between type 2 diabetes and major depressive disorder in a population based cohort. J Med Genet. 2017;174:227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haljas K, Amare AT, Alizadeh BZ, et al. Bivariate Genome-Wide Association Study of Depressive Symptoms With Type 2 Diabetes and Quantitative Glycemic Traits. Psychosom Med. 2018;80:242–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haljas K, Lahti J, Tuomi T, et al. Melatonin receptor 1B gene rs10830963 polymorphism, depressive symptoms and glycaemic traits. Ann Med. 2018;50:704–712. [DOI] [PubMed] [Google Scholar]
- 23.Kan C, Pedersen NL, Christensen K, et al. Genetic overlap between type 2 diabetes and depression in Swedish and Danish twin registries. Mol Psychiatry. 2016;21:903–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeman M, Jachymova M, Jirak R, et al. Polymorphisms of genes for brain-derived neurotrophic factor, methylenetetrahydrofolate reductase, tyrosine hydroxylase, and endothelial nitric oxide synthase in depression and metabolic syndrome. Folia Biol. 2010;56:19–26. [DOI] [PubMed] [Google Scholar]
- 25.Ji H-F, Zhuang Q-S, Shen L. Genetic overlap between type 2 diabetes and major depressive disorder identified by bioinformatics analysis. Oncotarget. 2016;7:17410–17414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wehby GL, Domingue BW, Wolinsky FD. Genetic Risks for Chronic Conditions: Implications for Long-term Wellbeing. J Gerontol A Biol Sci Med Sci. 2018;73:477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wesołowska K, Elovainio M, Hintsa T, et al. Fasting Glucose and the Risk of Depressive Symptoms: Instrumental-Variable Regression in the Cardiovascular Risk in Young Finns Study. Int J Behav Med. 2017;24:901–907. [DOI] [PubMed] [Google Scholar]
- 28.Xuan L, Zhao Z, Jia X, et al. Type 2 diabetes is causally associated with depression: a Mendelian randomization analysis. Front Med. 2018;12:678–687. [DOI] [PubMed] [Google Scholar]
- 29.Wong BC-F, Chau CK-L, Ao F-K, et al. Differential associations of depression-related phenotypes with cardiometabolic risks: Polygenic analyses and exploring shared genetic variants and pathways. Depress Anxiety. 2019;36:330–344. [DOI] [PubMed] [Google Scholar]
- 30.Garasia S, Samaan Z, Gerstein HC, et al. Influence of depression on genetic predisposition to type 2 diabetes in a multiethnic longitudinal study. Sci Rep. 2017;7:1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samaan Z, Garasia S, Gerstein HC, et al. Lack of association between type 2 diabetes and major depression: epidemiologic and genetic evidence in a multiethnic population. Transl Psychiatry. 2015;5:e618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang B, Yuan S, Xiong Y, et al. Major depressive disorder and cardiometabolic diseases: a bidirectional Mendelian randomisation study. Diabetologia. 2020;63:1305–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wesołowska K, Elovainio M, Hintsa T, et al. Type-2 diabetes and depressive symptoms: Results of applying a Mendelian randomization in the Cardiovascular Risk in Young Finns Study. Psychother Psychosom. 2015;84:77. [Google Scholar]
- 34.Wray NR, Ripke S, Mattheisen M, et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet. 2018;50:668–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cai N, Kendler KS, Flint J. Minimal phenotyping yields GWAS hits of low specificity for major depression. BioRxiv. 2018:440735.
- 36.Wray NR, Maier R. Genetic Basis of Complex Genetic Disease: The Contribution of Disease Heterogeneity to Missing Heritability. Curr Epidemiol Rep. 2014;1:220–227. [Google Scholar]
- 37.Lasserre AM, Strippoli M-PF, Glaus J, et al. Prospective associations of depression subtypes with cardio-metabolic risk factors in the general population. Mol Psychiatry. 2017;22:1026–1034. [DOI] [PubMed] [Google Scholar]
- 38.Milaneschi Y, Lamers F, Berk M, et al. Depression Heterogeneity and Its Biological Underpinnings: Toward Immunometabolic Depression. Biol Psychiatry. 2020;88:369–380. [DOI] [PubMed] [Google Scholar]
- 39.Sjöholm Å, Nyström T. Inflammation and the etiology of type 2 diabetes. Diabetes/Metabolism Research and Reviews. 2006;22:4–10. 10.1002/dmrr.568. [DOI] [PubMed] [Google Scholar]
- 40.Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16:22–34. 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bergmans RS, Malecki KM. The association of dietary inflammatory potential with depression and mental well-being among US adults. Prev Med. 2017;99:313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brunkwall L, Orho-Melander M. The gut microbiome as a target for prevention and treatment of hyperglycaemia in type 2 diabetes: from current human evidence to future possibilities. Diabetologia. 2017;60:943–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dinan TG, Cryan JF. Gut–brain axis in 2016: Brain–gut–microbiota axis — mood, metabolism and behaviour. Nat Rev Gastroenterol Hepatol. 2017;14:69–70. [DOI] [PubMed] [Google Scholar]
- 44.Kendler KS, Karkowski LM, Prescott CA, et al. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry. 1999;156:837–841. [DOI] [PubMed] [Google Scholar]
- 45.Hackett RA, Steptoe A. Type 2 diabetes mellitus and psychological stress - a modifiable risk factor. Nat Rev Endocrinol. 2017;13:547–560. [DOI] [PubMed] [Google Scholar]
- 46.Mezuk B, Ratliff S, Concha JB, et al. Stress, self-regulation, and context: Evidence from the Health and Retirement Survey. SSM Popul Health. 2017;3:455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luppino FS, de Wit LM, Bouvy PF, et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry. 2010;67:220–229. [DOI] [PubMed] [Google Scholar]
- 48.Martinsen EW. Physical activity in the prevention and treatment of anxiety and depression. Nordic J Psychiatry. 2008;62:25–29. [DOI] [PubMed] [Google Scholar]
- 49.Sigal RJ, Kenny GP, Wasserman DH, et al. Physical Activity/Exercise and Type 2 Diabetes. Diabetes Care. 2004;27:2518–2539. [DOI] [PubMed] [Google Scholar]