Abstract
Background
Multiple myeloma (MM) is a malignant tumor originating from plasma cells in the bone marrow. The existing treatment methods can prolong the survival time of patients, but they still face the problems of myeloma relapse and refractory disease. Chimeric antigen receptor (CAR)-T cell therapy is a new cellular immunotherapy that can target and recognize antigens and kill tumor cells but the efficacy and safety data varied in different studies. We performed this systematic review and meta-analysis to understand its efficacy and safety.
Methods
Literature published from January 2015 to November 2021 was obtained by searching the keywords “CAR-T”, “CAR-T Cell”, and “Multiple Myeloma” by computer using the Embase, PubMed, Web of Science, and Cochrane library databases according to the PICOS (Participants, Interventions, Comparisons, Outcomes, Study type) criteria. The quality of the literature was assessed by the Joanna Briggs Institute (JBI) Critical Appraisal Tool for prevalence studies. The complete response rate, the incidence of cytokine release syndrome (CRS) above grade 3, and the overall incidence of adverse reactions were used as the outcome indicators. The pooled rates were performed and analyzed using the R language toolkit.
Results
A total of 10 studies including 353 study cases were included. Meta-analysis showed that the pooled complete response rate of CAR-T therapy in the treatment of MM was 0.55, 95% confidence interval (CI): (0.50, 0.60), the pooled incidence of CRS was 0.55, 95% CI: (0.50, 0.60), and the pooled incidence of serious adverse reactions was 0.92, 95% CI: (0.88, 0.95). Subgroup analysis was performed based on antigen types or costimulatory molecules, and there was no significant difference in the efficacy of CAR-T and the incidence of CRS between the two subgroups (P>0.05).
Conclusions
As a new immunotherapy strategy with great potential, CAR-T has a significant effect in the treatment of MM, but its safety needs to be further improved. The types of costimulatory molecules and CAR-T antigens can affect its efficacy and safety.
Keywords: Chimeric antigen receptor (CAR), cellular immunotherapy, multiple myeloma (MM)
Introduction
Multiple myeloma (MM) is a malignant tumor arising from plasma cells in the bone marrow, and has been classified by the World Health Organization (WHO) as a type of B-cell lymphoma. It is typically characterized by abnormal proliferation of plasma cells in the bone marrow, accompanied by excessive production of monoclonal immunoglobulins or light chains (M proteins). Patients are prone to concurrent multiple lytic lesions, hypercalcemia, anemia, and renal impairment due to inhibited production of normal immunoglobulins, as well as various bacterial infections (1). MM accounts for about 1% of malignant tumors and 13% of hematological tumors. The incidence of MM varies greatly in different races and regions. The incidence of MM in Western countries is about 5.6/100,000 (2). At present, MM cannot be completely cured. Although the application of proteasome inhibitors, immunomodulators, monoclonal antibodies, and autologous stem cell transplantation (ASCT) prolongs the survival time of patients, they still face the problems of myeloma relapse and refractory disease, and it is therefore urgent to find new treatments (3). Chimeric antigen receptor (CAR)-T cell therapy is a brand new cellular immunotherapy, and its mechanism is to transfect CAR into T lymphocytes and induce their expression on the cell surface so that T lymphocytes have the effect of targeting and recognizing antigens and killing tumor cells (4). CAR is a genetically modified transmembrane protein containing three parts: extracellular domain, transmembrane domain, and intracellular domain. At present, the successively developed antigens include cluster of differentiation antigen 19 (CD19), CD138, natural killer cell surface activating receptor D (NKG2D), signaling lymphocyte activation molecule family member 7 (SLAMF7), CD38, and B lymphocyte maturation antigen (BCMA), which shows the great potential of this treatment for MM (5). Although existing clinical study (6) has confirmed the efficacy of CAR-T therapy, most patients will develop nausea, vomiting, decreased white blood cell count, elevated transaminases, and other adverse reactions, and more severe adverse reactions even have the risk of disability and death. Cytokine release syndrome (CRS), a common toxicity during CAR-T treatment, is a systemic inflammatory response characterized by fever, rash, hypotension, tachycardia, respiratory distress, epilepsy, and organ failure, mostly caused by systemic immune disorders resulting from immunotherapeutic drugs (7). A previous meta-analysis (8) evaluated the efficacy and safety of CAR-T cell therapy, but the overall response rate and overall incidence of adverse reactions may not distinguish the different efficacy and safety profiles of different antigens. Also, the results of different studies varied. The pooled overall response rate was 85.2% [95% confidence interval (CI): 0.797–0.910] in the study (8), while in another single-arm study by Usmani et al. (9) the overall response rate was only 65.0% (95% CI: 0.480–0.790). So we conducted this analysis to fully explore the efficacy and safety of CAR-T cell therapy, and introduced the complete response rate, the incidence of CRS above grade 3, and the overall incidence of adverse reactions as the outcome indicators.
We present the following article in accordance with the MOOSE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-344/rc).
Methods
Inclusion of studies
We defined the inclusion criteria according to the PICOS criteria (Participants, Interventions, Comparisons, Outcomes, Study type).
Study type
As CAR-T therapy had been developed from the first generation to the second, third, and fourth generations, we limited the publication date of studies to within the past 5 years. We did not limit the language of the study. The study should be a single-arm prevalence survey study, and randomized controlled trials (RCTs), controlled studies, case studies, review studies, and conference minutes were excluded. We also excluded studies with the number of cases less than 10.
Participants
The study subjects were required to meet the characteristics of relapsed or refractory MM (RRMM), and studies with animals as the research objects were excluded.
Interventions
All patients were treated with proteasome inhibitors or immunomodulatory agents before CAR-T therapy, and we did not limit the types of target antigens in CAR-T therapy. They included one or more of CD19, CD38, CD138, and BCMA.
Outcomes
We counted complete response rate as the main efficacy indicator, and we only counted the stringent complete response (sCR). We took the incidence of CRS as the main safety indicator, but we only counted the number of patients with grade 3 or higher CRS. The incidence of any adverse reaction above grade 3 was used as a secondary safety outcome indicator based on the fact that almost all patients experience adverse reactions during CAR-T therapy (including hematological leukopenia, neutropenia, lung diseases such as pulmonary edema, gastrointestinal adverse reactions such as nausea, vomiting, diarrhea, and liver and kidney adverse reactions).
Literature search strategy
Embase, PubMed, Web of Science, and Cochrane library databases were searched by computer for literature published from January 2015 to November 2021. The search method was keyword rapid search. The input keywords were: “CAR-T”, “CAR-T Cell”, and “Multiple Myeloma”.
Selection of literature
Two researchers independently completed the screening and inclusion of literature. If there was inconsistency in this process, a third researcher was invited and agreement was reached through negotiation. After the initial search, we saved all the retrieved literature with “.enw” as the suffix, and articles were managed uniformly after being imported by Endnote X9 software. The software menu “References” → “find duplicates” allows the software to de-duplicate the retrieved literature. For the remaining literature, two researchers read the titles and abstracts. For literature with overlapping study contents, only one of them would be retained. When reading the titles and abstracts, preliminary screening was performed to remove the literature that obviously did not meet the inclusion requirements. For the remaining literature, we tried to obtain the full text, which could be obtained using the “Find full text” function of the software, or by manually searching from the internet. If the literature could not be obtained through the internet, we tried to contact the original author (via email) to obtain the original text. If this still failed, the literature was excluded. For the obtained literature, the two researchers read the full text to check whether the data were complete and excluded literature without data records or outcome indicators.
Data extraction
After obtaining the full text of the literature, the two researchers used a self-made data form to extract the information in the literature, as follows: (I) basic data of the literature: publication time, author, region; (II) characteristics of the study subjects: patient age, race, body mass index (BMI), monoclonal type, time since diagnosis, myeloma cell count, whether conditioning was performed, whether ASCT was performed, and the drugs taken; (III) characteristics of the literature intervention: number of participants, intervention time, and follow-up time; (IV) outcome data.
Literature quality assessment
The Joanna Briggs Institute (JBI) Critical Appraisal Tool for prevalence studies was used to analyze the quality of the included literature. The tool contains 9 items to evaluate the sampling, subjects, data collection and distribution of the literature. The maximum score was 9 points (each item devotes 1 points), and a score of more than 5 points was considered to be good quality. The higher the score, the better the literature quality and the less the bias.
Heterogeneity investigation and sensitivity analysis
We performed a subgroup analysis of the literature to determine the source of heterogeneity. If the results of both fixed effect model and random effect model showed similar, we considered the results stable.
Statistical methods
We used the R language development environment (R version 4.1.2 released by “The R Foundation for Statistical Computing”) to summarize and analyze the data of prevalence studies, and we used rates as the effect size measures, 95% CI as the confidence interval, and P<0.05 as statistically significant. For the heterogeneity among different studies, the I2 test and Q test were used for analysis. Heterogeneity was not statistically significant when I2<50% or P≥0.1, indicating that there was no (or acceptable) heterogeneity among the literature, otherwise it indicated that there was heterogeneity among the literature. If there was no statistical heterogeneity among the literature, the fixed effect model was used; if there was heterogeneity, the random effect model was used. Subgroups was introduced to investigate the heterogeneity. The analysis results were presented in forest plots, and publication bias was reported in a funnel plot.
Results
Literature screening results
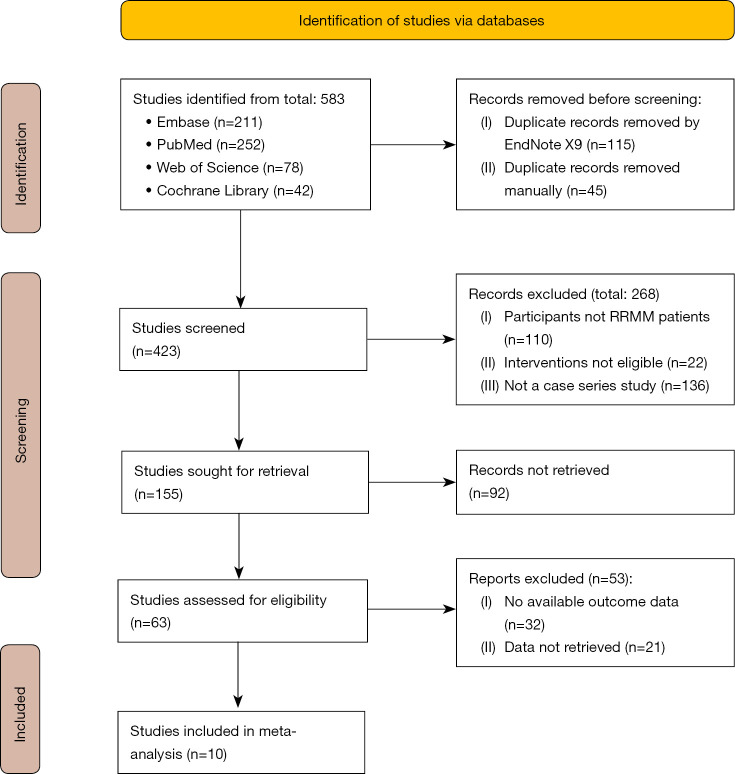
A total of 583 articles were initially searched, and 10 studies (10-19) were finally included, as shown in Figure 1.
Figure 1.
The study selection flow chart. RRMM, relapsed or refractory multiple myeloma.
Basic characteristics of the studies
The publication years of the studies included in this meta-analysis ranged from 2018 to 2021, with a total of 353 cases. The study subjects were all patients with RRMM, with an age range of 18–72 years. The minimum number of cases in the cohort was 10, and the maximum number was 97. Among them, all received second-generation CAR-T therapy, except 2 studies (13,16) in which patients received third-generation CAR-T therapy, as shown in Table 1.
Table 1. Basic characteristics, intervention measures, follow-up time, outcome indicators, and quality scores of the included literature.
| Author, year of publication | Age (years) | Sex (male:female) | Sample size | Antigen | Costimulatory motif | CAR-T dose (×106) | Median follow-up time | Outcomes | JBI score |
|---|---|---|---|---|---|---|---|---|---|
| Raje N et al. (10), 2019 | N/A | N/A | 33 | BCMA | 4-1BB | 50–800 | 9 mo | (I), (II), (III) | 6 |
| Mei H et al. (11), 2021 | 59 [49–72] | 11:12 | 23 | LCAR-B38M | 4-1BB | 1–4/kg | 6 mo | (I), (II), (III) | 7 |
| Zhao WH et al. (12), 2018 | 54 [27–72] | 34:23 | 57 | LCAR-B38M | CD28 | 0.07–2.1/kg | 8 mo | (I), (II), (III) | 7 |
| Yan Z et al. (13), 2019 | 58 [49.5–61] | 10:11 | 21 | BCMA + CD19 | 4-1BB | 1/kg | 18 mo | (I), (II), (III) | 8 |
| Brudno JN et al. (14), 2018 | N/A | N/A | 16 | BCMA | CD28 | 3–9/kg | 34 mo | (I), (II), (III) | 6 |
| He SL et al. (15), 2021 | 59 [44–70] | 35:24 | 59 | BCMA | CD3 + CD28 | 1.00 (0.50–6.00)/kg | 5 mo | (I) | 6 |
| Yan L et al. (16), 2021 | N/A | N/A | 10 | BCMA + CD19 | CD28 | 1/kg (CD19) + 3/kg (BCMA) | 20 mo | (I), (II), (III) | 7 |
| Deng H et al. (17), 2021 | 56 [44–70] | N/A | 20 | BCMA | CD3 | 2.21±0.39/kg | 7.3 mo | (I), (II), (III) | 8 |
| Berdeja JG et al. (18), 2021 | 60 [57–67] | 57:40 | 97 | BCMA | 4-1BB | 0.5–1/kg | 12 mo | (I), (II), (III) | 6 |
| Xu J et al. (2019) | N/A | N/A | 17 | LCAR-B38M | 4-1BB | 0.21–1.47/kg | 12 mo | (I), (II), (III) | 6 |
Outcomes: (I) response rate; (II) grade 3 or higher adverse event rate; (III) CRS rate. CAR, chimeric antigen receptor; JBI, The Joanna Briggs Institute; BCMA, B lymphocyte maturation antigen; CD19, differentiation antigen 19; CD28, differentiation antigen 28; CRS, cytokine release syndrome.
Meta-analysis results
Efficacy
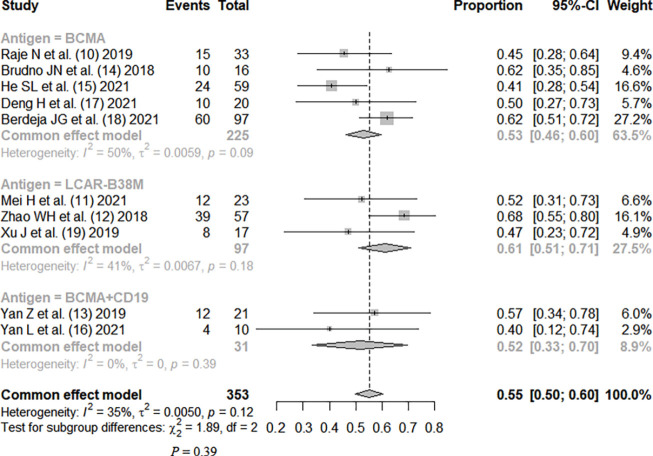
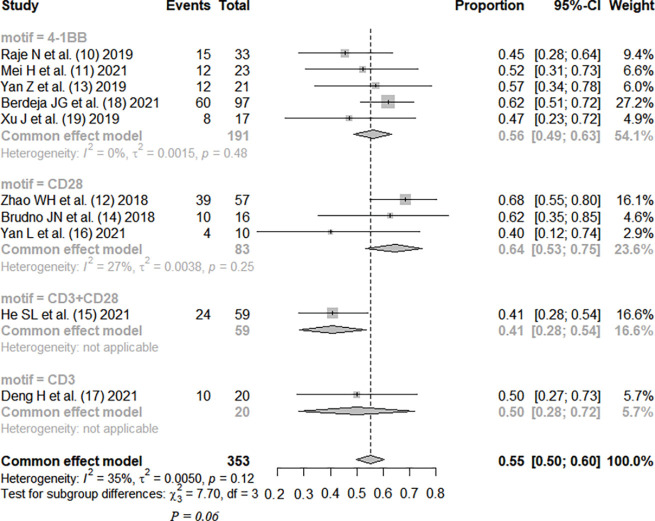
The complete response rate was reported in all studies, without significant heterogeneity between studies (I2=35%; P=0.12). The fixed-effects model was used. Meta-analysis results showed that the pooled complete response rate was 0.55, 95% CI: (0.50, 0.60). The patients were divided into three subgroups by CAR-T antigen type, without statistical significance (P=0.39). The patients were divided into four subgroups by costimulatory motif of CAR-T, with no statistical significance (P=0.06), as shown in Figures 2,3.
Figure 2.
Therapeutic efficacy of CAR-T therapy for RRMM (subgrouped by antigen) (10-19). CI, confidence interval; CAR, chimeric antigen receptor; RRMM, relapsed or refractory multiple myeloma.
Figure 3.
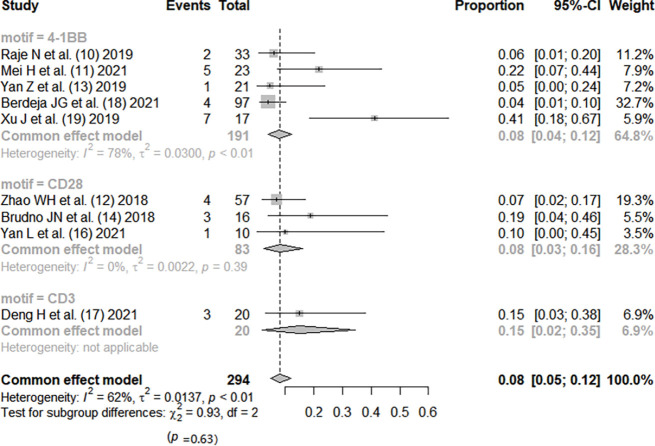
Therapeutic efficacy of CAR-T therapy for RRMM (subgrouped by motif) (10-19). CI, confidence interval; CAR, chimeric antigen receptor; RRMM, relapsed or refractory multiple myeloma.
Incidence of CRS
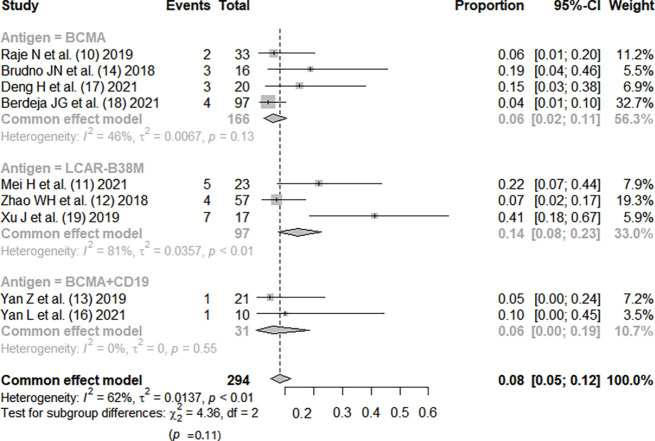
Except for the study by He et al. (15), the incidence rate of CRS was reported in all other studies (10-14,16-19). There was no significant heterogeneity between the studies (I2=35%; P=0.12). The fixed-effects model was used. Meta-analysis results showed that the pooed incidence of CRS was 0.55, 95% CI: (0.50, 0.60). Based on CAR-T antigen type, patients were divided into three subgroups, and the efficacy was not statistically significant (P=0.39). Based on the costimulatory motif of CAR-T, patients were divided into four subgroups, and the efficacy was not statistically significant (P=0.06), as shown in Figures 4,5.
Figure 4.
Summary of CRS incidence with CAR-T therapy for RRMM (subgrouped by antigen) (10-14,16-19). CI, confidence interval; CRS, cytokine release syndrome; CAR, chimeric antigen receptor; RRMM, relapsed or refractory multiple myeloma.
Figure 5.
Summary of CRS incidence during CAR-T therapy for RRMM (subgrouped by motif) (10-14,16-19). CI, confidence interval; CRS, cytokine release syndrome; CAR, chimeric antigen receptor; RRMM, relapsed or refractory multiple myeloma.
Incidence of serious adverse reactions
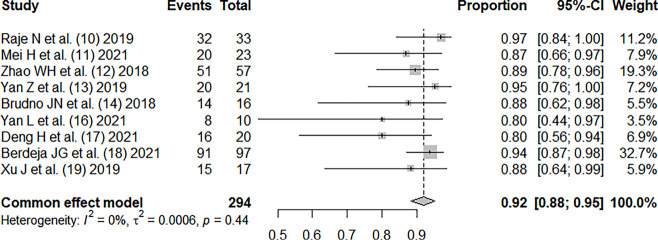
Except for the study by He et al. (15), all other studies (10-14,16-19) reported the incidence rate of serious adverse reactions. There was no significant heterogeneity between the studies (I2=0%; P=0.44), and the pooled incidence of serious adverse reactions was 0.92, 95% CI: (0.88, 0.95), as shown in Figure 6.
Figure 6.
Overall incidence of adverse reactions in RRMM patients treated with CAR-T therapy (10-14,16-19). CI, confidence interval; RRMM, relapsed or refractory multiple myeloma; CAR, chimeric antigen receptor.
Heterogeneity investigation and sensitivity analysis
In the summary of complete response rate, CRS rate, and incidence rate of adverse reactions, there was no significant heterogeneity between the studies. After subgrouping based on antigen and costimulatory motif, there was still no significant heterogeneity between the studies, indicating that the stability of the results was good.
Analysis of publication bias
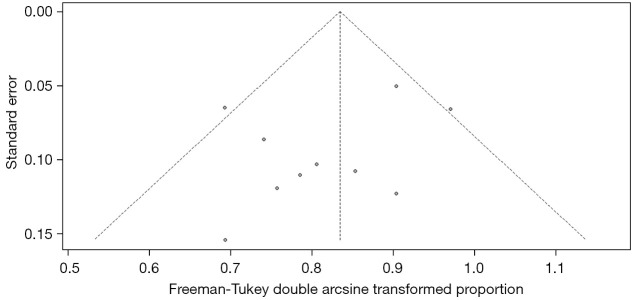
In the treatment efficacy of CAR-T therapy for RRMM, the funnel plot showed that the two sides were unevenly distributed, suggesting the presence of publication bias, as shown in Figure 7.
Figure 7.
Funnel plot of the therapeutic efficacy of CAR-T therapy for RRMM. CAR, chimeric antigen receptor; RRMM, relapsed or refractory multiple myeloma.
Discussion
CAR-T therapy has been developed to the fourth generation, and the structural composition of the first generation includes a single-chain variable antibody fragment, CD8 transmembrane domain, and CD3 intracellular activation domain, on which the second generation adds intracellular costimulatory molecules, the third generation allows the presence of a variety of preserved costimulatory molecules, and the fourth generation develops a structure that can release interleukin (IL)-12, enhancing the tumor-killing effect (20). Our meta-analysis included 8 studies of second-generation structure and 2 studies of third-generation structure, with a total of 353 patients. The results showed that the complete response rate of CAR-T therapy was 0.55, 95% CI: (0.50, 0.60), but the results also showed that the incidence rate of serious adverse reactions was as high as 0.92, 95% CI: (0.88, 0.95), and the incidence rate of grade 3 or higher CRS was 0.08, 95% CI: (0.05, 0.12). The studies were subgrouped by different antigens. The efficacy rankings of the three different antigens were LCAR-B38M > BCMA > BCMA + CD19, and the incidence of CRS was ranked as LCAR-B38M > BCMA > BCMA + CD19, but there was no significant difference among the three different groups (P>0.05). Ten studies were divided into three subgroups by different costimulatory molecule types (4-1BB, CD28, CD3), and the efficacy rankings of the three different costimulatory molecules were CD28 > 4-1BB > CD3, while the incidence of CRS was ranked as 4-1BB = CD28 < CD3, but there was no significant difference among the groups (P>0.05).
BCMA, also known as tumor necrosis factor (TNF) ligand superfamily member 17, is selectively expressed in MM cell lines and plays an important role in the proliferation and differentiation of malignant B lymphocytes in MM, making it an ideal antigenic target. However, there may be multiple subclonal phenotypes in the same patient that easily escape single antigen therapy (21). LCAR-B38M is a dual-BCMA epitope CAR-T therapy. Compared with single-BCMA epitope CAR-T (such as bb2121), LCAR-B38M has higher affinity to the target, reduced efficacy per unit dose, or reduced immune escape mechanism of a single antigen, which may be the reason why the efficacy of LCAR-B38M is higher than that of BCMA (22).
The difference in costimulatory molecules may also have an impact on efficacy. Costimulatory molecules play an immune role synergistically with T lymphocyte activation, proliferation, and differentiation. CD28 costimulatory molecules can produce strong stimulatory signals and can rapidly induce T lymphocyte activation and differentiate into effector memory phenotypes, while 4-1BB induces a relatively slow process of activated T lymphocytes and has a lower stimulation intensity, which can differentiate into a large number of effector T lymphocytes and central memory T lymphocytes (23). This may be the reason why CD28 costimulatory molecules are more effective than 4-1BB, but CD28 may also produce overstimulation for T lymphocytes, increasing the risk of CRS (24).
In addition to the relationship between CRS, adverse events, and the dose of CAR-T infusion, a study by Lee et al. (25) has shown that CRS is also closely related to the increased levels of serum IL-6, C-reactive protein (CRP), TNF-α, and other immune factors. Prophylactic treatment and immunosuppressive therapy with steroid hormones and anti-IL-6 receptor monoclonal antibodies (tocilizumab) are often used in clinical practice. The study by Garfall et al. (26) found that early ASCT reduced autoimmune reactions after CAR-T infusion, along with reducing tumor burden, and showed similar effects in controlling CRS.
BCMA antigen therapy in the study by Raje et al. (10), which was infused in groups based on escalating CAR-T infusion dose, showed a positive correlation between infusion dose and efficacy. It improved immunogenicity while increasing the dose, but also increased the safety risk. In the studies (11,12,19) using LCAR-B38M, no dose escalation was designed, and although the average dose of cell infusion was low, the efficacy was no worse than that of BCMA.
In this study, the third-generation CAR-T therapy (BCMA + CD19) did not show better efficacy than the second-generation therapy (BCMA), which was consistent with the conclusion of the study by Ge et al. (27). However, because only two third-generation CAR-T therapies were included in this study, more literature is needed to support the efficacy of third-generation CAR-T therapies. In this study, fourth-generation CAR-T therapies were not included. The results of the primary study by Feng et al. (28) showed that the proliferation activity, chemotactic ability, and durability of fourth-generation CAR-T therapies provided a strategy to overcome the defects of traditional CAR-T cells, such as low survival rate, poor durability, and inhibition by tumor microenvironment, but more clinical studies are needed to support it. In the study by Smith et al. (29), CAR-T therapy with BCMA antigen was combined with radiation therapy, which may become a new treatment for RRMM.
In this study, there was no significant heterogeneity between the studies. The JBI tool was used for quality assessment, and the quality of the included studies was good. However, the funnel plot showed that there may be some publication bias. The relevant studies still need to be supported by evidence from large-sample and higher quality studies.
Conclusions
In summary, CAR-T, as a new immunotherapy strategy with great potential, has a significant effect in the treatment of MM, but the safety needs to be further improved. The types of costimulatory molecules and CAR-T antigens can affect its efficacy and safety. Improving the CAR structure, preparing multi-target CAR-T, and increasing CAR-T costimulatory molecules will become future research topics of CAR-T in the treatment of MM.
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Reporting Checklist: The authors have completed the MOOSE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-344/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-344/coif). The authors have no conflicts of interest to declare.
(English Language Editor: C. Betlazar-Maseh)
References
- 1.Brigle K, Rogers B. Pathobiology and Diagnosis of Multiple Myeloma. Semin Oncol Nurs 2017;33:225-36. 10.1016/j.soncn.2017.05.012 [DOI] [PubMed] [Google Scholar]
- 2.Kazandjian D. Multiple myeloma epidemiology and survival: A unique malignancy. Semin Oncol 2016;43:676-81. 10.1053/j.seminoncol.2016.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moreau P. The future of therapy for relapsed/refractory multiple myeloma: emerging agents and novel treatment strategies. Semin Hematol 2012;49 Suppl 1:S33-46. 10.1053/j.seminhematol.2012.05.004 [DOI] [PubMed] [Google Scholar]
- 4.Zhao YL, Liu DY, Sun RJ, et al. Integrating CAR T-Cell Therapy and Transplantation: Comparisons of Safety and Long-Term Efficacy of Allogeneic Hematopoietic Stem Cell Transplantation After CAR T-Cell or Chemotherapy-Based Complete Remission in B-Cell Acute Lymphoblastic Leukemia. Front Immunol 2021;12:605766. 10.3389/fimmu.2021.605766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D, Wang J, Hu G, et al. A phase 1 study of a novel fully human BCMA-targeting CAR (CT103A) in patients with relapsed/refractory multiple myeloma. Blood 2021;137:2890-901. 10.1182/blood.2020008936 [DOI] [PubMed] [Google Scholar]
- 6.Chen W, Wang Y, Qi K, et al. Efficacy and Safety of Chimeric Antigen Receptor T-Cell Therapy for Relapsed/Refractory Immunoglobulin D Multiple Myeloma. Transplant Cell Ther 2021;27:273.e1-5. 10.1016/j.jtct.2020.12.017 [DOI] [PubMed] [Google Scholar]
- 7.Giavridis T, van der Stegen SJC, Eyquem J, et al. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat Med 2018;24:731-8. 10.1038/s41591-018-0041-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang L, Shen X, Yu W, et al. Comprehensive meta-analysis of anti-BCMA chimeric antigen receptor T-cell therapy in relapsed or refractory multiple myeloma. Ann Med 2021;53:1547-59. 10.1080/07853890.2021.1970218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Usmani SZ, Garfall AL, van de Donk NWCJ, et al. Teclistamab, a B-cell maturation antigen × CD3 bispecific antibody, in patients with relapsed or refractory multiple myeloma (MajesTEC-1): a multicentre, open-label, single-arm, phase 1 study. Lancet 2021;398:665-74. 10.1016/S0140-6736(21)01338-6 [DOI] [PubMed] [Google Scholar]
- 10.Raje N, Berdeja J, Lin Y, et al. Anti-BCMA CAR T-Cell Therapy bb2121 in Relapsed or Refractory Multiple Myeloma. N Engl J Med 2019;380:1726-37. 10.1056/NEJMoa1817226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mei H, Li C, Jiang H, et al. A bispecific CAR-T cell therapy targeting BCMA and CD38 in relapsed or refractory multiple myeloma. J Hematol Oncol 2021;14:161. 10.1186/s13045-021-01170-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao WH, Liu J, Wang BY, et al. A phase 1, open-label study of LCAR-B38M, a chimeric antigen receptor T cell therapy directed against B cell maturation antigen, in patients with relapsed or refractory multiple myeloma. J Hematol Oncol 2018;11:141. 10.1186/s13045-018-0681-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan Z, Cao J, Cheng H, et al. A combination of humanised anti-CD19 and anti-BCMA CAR T cells in patients with relapsed or refractory multiple myeloma: a single-arm, phase 2 trial. Lancet Haematol 2019;6:e521-9. 10.1016/S2352-3026(19)30115-2 [DOI] [PubMed] [Google Scholar]
- 14.Brudno JN, Maric I, Hartman SD, et al. T Cells Genetically Modified to Express an Anti-B-Cell Maturation Antigen Chimeric Antigen Receptor Cause Remissions of Poor-Prognosis Relapsed Multiple Myeloma. J Clin Oncol 2018;36:2267-80. 10.1200/JCO.2018.77.8084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He SL, Cheng YH, Wang D, et al. Anti-BCMA CAR-T Cell Therapy in Relapsed or Refractory Multiple Myeloma Patients with Impaired Renal Function. Curr Med Sci 2021;41:474-81. 10.1007/s11596-021-2373-7 [DOI] [PubMed] [Google Scholar]
- 16.Yan L, Qu S, Shang J, et al. Sequential CD19 and BCMA-specific CAR T-cell treatment elicits sustained remission of relapsed and/or refractory myeloma. Cancer Med 2021;10:563-74. 10.1002/cam4.3624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng H, Liu M, Yuan T, et al. Efficacy of Humanized Anti-BCMA CAR T Cell Therapy in Relapsed/Refractory Multiple Myeloma Patients With and Without Extramedullary Disease. Front Immunol 2021;12:720571. 10.3389/fimmu.2021.720571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berdeja JG, Madduri D, Usmani SZ, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet 2021;398:314-24. Erratum in: Lancet 2021;398:1216. 10.1016/S0140-6736(21)00933-8 [DOI] [PubMed] [Google Scholar]
- 19.Xu J, Chen LJ, Yang SS, et al. Exploratory trial of a biepitopic CAR T-targeting B cell maturation antigen in relapsed/refractory multiple myeloma. Proc Natl Acad Sci U S A 2019;116:9543-51. 10.1073/pnas.1819745116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen AD, Garfall AL, Stadtmauer EA, et al. B cell maturation antigen-specific CAR T cells are clinically active in multiple myeloma. J Clin Invest 2019;129:2210-21. 10.1172/JCI126397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu J, Ming X, Wang C, et al. Long event-free survival after anti-BCMA CAR-T cell treatment for relapsed and refractory multiple myeloma patients: Two case reports. Medicine (Baltimore) 2021;100:e25784. 10.1097/MD.0000000000025784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paíno T, Paiva B, Sayagués JM, et al. Phenotypic identification of subclones in multiple myeloma with different chemoresistant, cytogenetic and clonogenic potential. Leukemia 2015;29:1186-94. 10.1038/leu.2014.321 [DOI] [PubMed] [Google Scholar]
- 23.Ying Z, He T, Wang X, et al. Parallel Comparison of 4-1BB or CD28 Co-stimulated CD19-Targeted CAR-T Cells for B Cell Non-Hodgkin's Lymphoma. Mol Ther Oncolytics 2019;15:60-8. 10.1016/j.omto.2019.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao X, Yang J, Zhang X, et al. Efficacy and Safety of CD28- or 4-1BB-Based CD19 CAR-T Cells in B Cell Acute Lymphoblastic Leukemia. Mol Ther Oncolytics 2020;18:272-81. 10.1016/j.omto.2020.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014;124:188-95. 10.1182/blood-2014-05-552729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garfall AL, Stadtmauer EA, Hwang WT, et al. Anti-CD19 CAR T cells with high-dose melphalan and autologous stem cell transplantation for refractory multiple myeloma. JCI Insight 2018;3:e120505. Erratum in: JCI Insight 2019. 10.1172/jci.insight.120505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ge J, Zhao TT, Wan CY, et al. Comparison of single infusion of anti-BCMA versus combined infusion of anti-CD19 chimeric antigen receptor T cells for immune reconstruction in relapsed/refractory multiple myeloma. Zhonghua Xue Ye Xue Za Zhi 2021;42:733-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feng DD, Chen XH, Guo JJ, et al. Preliminary study of the fourth-generation CAR-T cells targeting CS1 in the treatment of refractory and recurrent multiple myeloma. Zhonghua Zhong Liu Za Zhi 2021;43:657-65. [DOI] [PubMed] [Google Scholar]
- 29.Smith EL, Mailankody S, Staehr M, et al. BCMA-Targeted CAR T-cell Therapy plus Radiotherapy for the Treatment of Refractory Myeloma Reveals Potential Synergy. Cancer Immunol Res 2019;7:1047-53. 10.1158/2326-6066.CIR-18-0551 [DOI] [PMC free article] [PubMed] [Google Scholar]